- 1Department of Public Health, China Medical University, Taichung, Taiwan
- 2Department of Health Services Administration, China Medical University, Taichung, Taiwan
- 3Division of Gastroenterology and Hepatology, Department of Internal Medicine, Taichung Armed Forces General Hospital, Taichung, Taiwan
- 4Division of Gastroenterology and Hepatology, Department of Internal Medicine, Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan
- 5Department of Healthcare Administration, Asia University, Taichung, Taiwan
- 6Department of Medical Research, China Medical University Hospital, Taichung, Taiwan
- 7Center for Digestive Medicine, Department of Internal Medicine, China Medical University Hospital, Taichung, Taiwan
- 8School of Medicine, China Medical University, Taichung, Taiwan
Introduction: Multidisciplinary team care coordinates with medical teams to improve the quality of cancer care. This study explored multidisciplinary team care in hepatitis B or hepatitis C virus-related hepatocellular carcinoma patients from the time of diagnosis to the first-time treatment interval and investigated treatment outcomes and prognosis.
Methods: This retrospective cohort study included data from a nationwide population from 2007 to 2016. Data were collected from the Taiwan Cancer Registry Database, linked to the Taiwan National Health Insurance Research Database. Propensity score matching was applied at a ratio of 1:2 to reduce the selection bias. A multiple regression model with generalized estimating equations was used to analyze whether multidisciplinary team care affected the diagnosis-to-treatment interval. The stratified Cox proportional hazards model examined whether involvement in multidisciplinary team care influenced survival status.
Results: A total of 10,928 and 21,856 patients with hepatocellular carcinoma received multidisciplinary and non-multidisciplinary care, respectively. Participants with multidisciplinary care had a longer diagnosis-to-treatment interval but a lower risk of cumulative cancer death (HR=0.88, 95% CI:0.84-0.92). In patients with intermediate- to advanced-stage hepatocellular carcinoma, multidisciplinary team care has obvious benefits for improving survival.
Conclusion: Patients with hepatocellular carcinoma who participated in multidisciplinary team care had a longer diagnosis-to-treatment interval but a lower risk of cancer death. Patients with intermediate- to advanced-stage hepatocellular carcinoma who received multidisciplinary team care significantly benefited from this outcome. Hospitals should provide HCC patients with multidisciplinary team care to improve cancer care.
1 Introduction
Primary liver cancer, also known as hepatocellular carcinoma (HCC), has high incidence and mortality rates. According to the latest report from the International Agency for Research on Cancer (IARC), HCC is estimated to be the sixth leading cause of new cancer cases and the second leading cause of cancer-related deaths worldwide in 2020 (1). The latest Taiwan Cancer Registry Annual Report stated that HCC was the fifth leading cause of cancer (11,272 people per 100,000 person-years) and the second leading cause of cancer-related deaths (7,881 people per 100,000 person-years) in Taiwan in 2019 (2). The major risk factor for HCC is the gradual progression of chronic hepatitis to liver fibrosis and cirrhosis following hepatitis B virus (HBV) or hepatitis C virus (HCV) infection (3). About 80% of HCC cases are associated with chronic hepatitis B (CHB) or chronic hepatitis C infections (3). Other common risk factors for HCC include alcoholic hepatitis, non-alcoholic fatty liver disease, obesity, and diabetes (4). With liver injury or inflammation, liver cells respond to simultaneous regeneration and fibrosis. Liver fibrosis also regulates inflammatory cell activity in the liver. More than 80% of the patients diagnosed with HCC have cirrhosis (5). Liver dysfunction and worse tumor burden (multiple nodules or large tumors) are associated with a poorer prognosis for patients with HCC (6). Liver disease is usually insidious in onset, with inconspicuous symptoms; therefore, HCC has previously been diagnosed in the middle to late stages (7). Since HCC is always diagnosed at a non-early stage, treatment options are severely limited (6). There are no specific specialists who are sufficiently trained to meet the needs of this patient population (6).
Multidisciplinary team (MDT) care originated in a cancer care study that recommended integrated medical staff in each category and across specialists to provide consistent, high-quality individualized care (8). The purpose of MDT care is to help healthcare professionals make better medical decisions, improve patient outcomes, and optimize the quality of the healthcare system (9). MDT care is widely used for the clinical management of various cancers and chronic diseases (10–16). Some studies have reported that MDT care can extend the survival of late-stage non-small cell lung cancer and oral cavity cancer patients (17, 18), lower the mortality risk of colorectal, oral cavity, and esophageal cancer patients (19–21), decrease the frequency of emergency department visits of lung and colorectal cancer patients (22, 23), reduce the 14-day readmission rates of colorectal cancer patients (24), and lowering the relative risk of recurrence and death in breast cancer patients (25). However, the evidence that MDT care interventions are helpful in patient care is controversial (26, 27). Nonetheless, due to the complexity of HCC treatment, MDT care is emphasized to improve timely and fitting treatment guidelines and the overall survival of HCC patients (28). To improve the quality of cancer care, Taiwan’s Ministry of Health and Welfare (MOHW) promulgated the Cancer Control Act in 2003 and initiated the Complete Cancer Care Quality Improvement Project in 2005, which helped hospitals set up an MDT care meeting for cancer patients. The National Health Insurance (NHI) system is designed with a “Cancer Patient Treatment Planning and Consultation Fee” to encourage the establishment of MDT care plans (29). The Cancer Patient Treatment Planning and Consultation Fee is limited to one declaration for patients with a confirmed cancer or recurrence diagnosis according to the MDT treatment plan. Based on the Cancer Control Act and Regulations for Cancer Care Quality Assurance Measures, medical institutions follow the regulations to establish a committee and assign designated physicians to take charge of the cancer patient care tasks. The medical institutions must follow rigorous standards and pass the hospital evaluation, then get the qualification as a medical institution of cancer control (29). The MDT committee in each hospital abides by the regulations to hold a scheduled meeting with prescribed participating experts. Once a people diagnosed with cancer disease, the doctor may refer the patient to a cancer care team and implement MDT care. The MDT treatment plan was discussed and made based on the consensus of various specialists in the regular meetings. After the MDT meetings, the oncology nurse helps complete the medical record and upload the required information in the NHI system. Details regarding MDT care in Taiwan were described in our previous study (17, 21).
Studies using nationwide populations to explore MDT interventions for HBV- or HCV-associated HCC are lacking. This study aimed to explore the factors associated with MDT care in patients with HCC due to CHB or CHC, and to investigate the treatment outcomes and prognosis of patients with HCC who underwent MDT care. This study can be used as an important reference to improve cancer care and provide resources for health insurance policies.
2 Materials and methods
2.1 Study design and data sources
This was a retrospective cohort study of a national population. Data was sourced from the population-based Taiwan Cancer Registry Database (TCRD), which records information on all types of cancers diagnosed and treated in Taiwan with excellent quality and high completeness (97%). The records from the TCRD were linked with the Taiwan National Health Insurance Research Database (NHIRD) and the Cause of Death files obtained from the MOHW. The NHIRD is a comprehensive healthcare database that covers almost the entire population (up to 99.99%) of this country (30). This study was reviewed and approved by the Research Ethics Committee of China Medical University and Hospitals in Taichung, Taiwan (IRB number: CMUH110-REC3-227).
2.2 Study participants
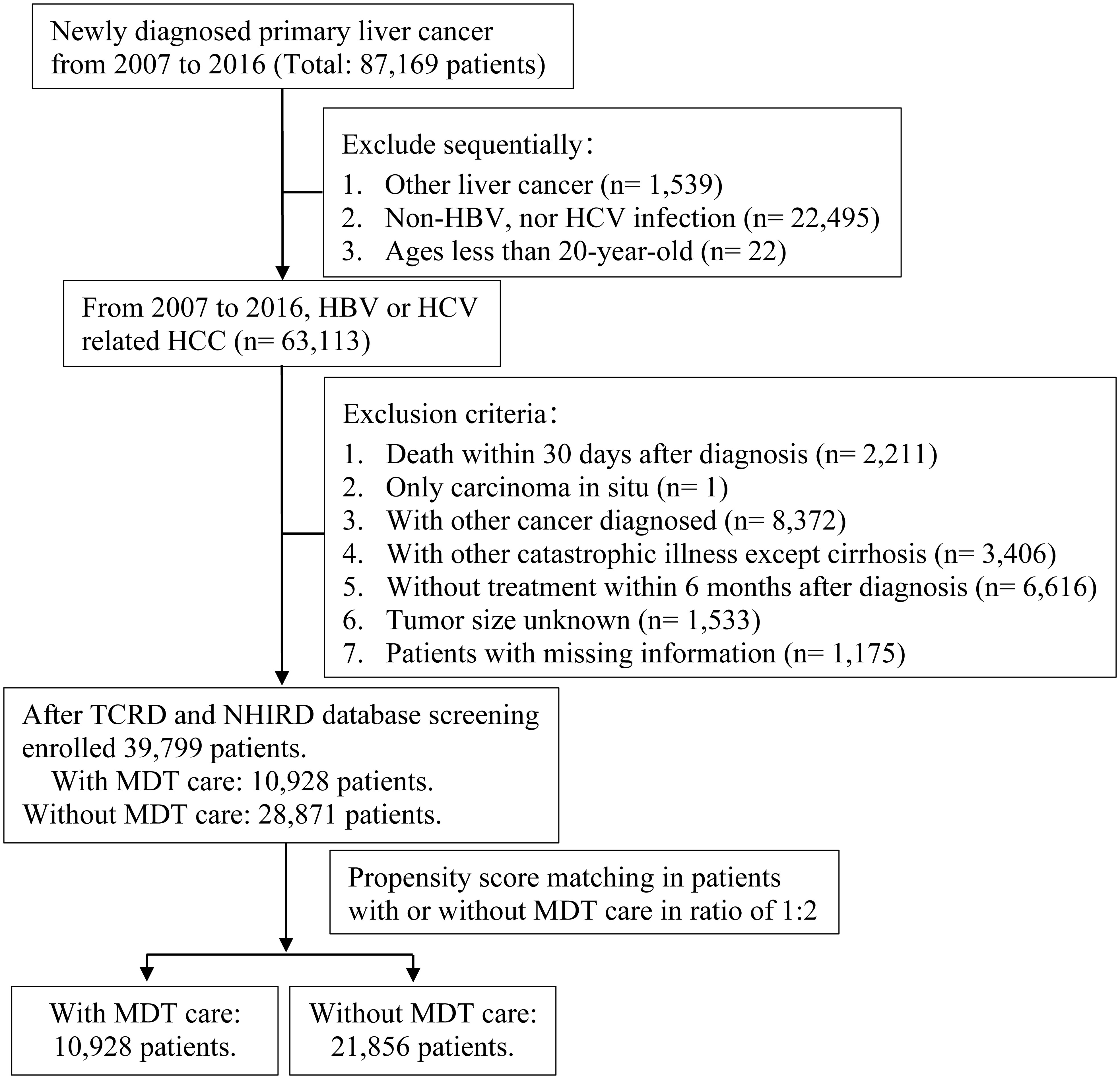
First, the authors applied TCRD to select all patients newly diagnosed with HCC from January 1, 2007, to December 31, 2016. We defined the study population as those aged ≥ 20 years with CHB or CHC. This study defined disease status based mainly on diagnosis codes according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) or International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM). The confirmed diagnosis of primary malignant neoplasm of the liver (hepatocellular carcinoma, HCC) was based on ICD-9-CM code:155.0 or ICD-10-CM code C22.0. Those who were infected with CHB used ICD-9-CM codes: 070.20, 070.21, 070.22, 070.23, 070.30, 070.31, 070.32, 070.33, V02.61 or ICD-10-CM codes: B18.0, B18.1, B19.1. Those who were infected with CHC used ICD-9-CM codes: 070.41, 070.44, 070.51, 070.54, 070.70, 070.71, V02.62 or ICD-10-CM codes: B18.2, B19.2, B19.21. This study assessed the survival status of HCC patients who received MDT care. The exclusion criteria were as follows:1) death within 30 days after diagnosis, 2) those who were later confirmed to have carcinoma in situ, 3) those diagnosed with other cancers, 4) those with other catastrophic illnesses, except for cirrhosis, 5) those without treatment within six months after diagnosis, and 6) those with missing relevant information. Whether a research subject had joined MDT care was based on the medical record, which declared a Cancer Patient Treatment Planning and Consultation Fee (47079 B). All included patients were followed up until death, loss to follow-up, or December 31, 2018, whichever occurred first. Ultimately, 39,799 patients were enrolled in this study. We used propensity score matching (PSM) to match the group of patients with HCC who received MDT care to those who did not at a ratio of 1:2. A flowchart of the screening process for study participants is shown in Figure 1.
2.3 Variable definitions and explanations
The typical characteristics of the patients with HCC were also examined. Age was defined as the age at which the study participants had a confirmed diagnosis of HCC. Participants’ socioeconomic status was based on their monthly salaries and grouped according to the National Health Insurance Administration. The environmental factor used was the degree of urbanization of the residential areas. The level of urbanization was established at seven degrees, from highly urbanized areas (level 1) to rural areas (level 7). Patients’ health status included comorbidity severity (Charlson Comorbidity Index [CCI]) and cirrhosis severity. According to the patient’s diagnosis, the comorbidity score of patients within two years before cancer diagnosis was calculated as an indicator of the severity of comorbidities in the study population. CCI was recorded along with Deyo’s Charlson Comorbidity index (31). The severity of cirrhosis was divided into no cirrhosis, mild cirrhosis, and severe cirrhosis in three groups. The confirmed diagnosis of cirrhosis was based on the ICD-9-CM codes 571.5, and ICD-10-CM codes K70.2, K70.30, K70.31, K74.1, K74.60, and K74.69. Patients who had a catastrophic cirrhosis illness belonged to the severe cirrhosis group; patients with only a diagnosis of cirrhosis were grouped into the mild cirrhosis group. Based on the NHI guidelines, the requirements for catastrophic illness of cirrhosis include cirrhosis complicated with 1) massive ascites that cannot be controlled, 2) esophageal varices or gastric varices with bleeding, or 3) hepatic encephalopathy or liver decompensation. This study excluded patients with other catastrophic illnesses because the catastrophic illness of cirrhosis was included in the classification of cirrhosis severity. The definition of ‘catastrophic illness’ aligns with the NHI definition, which includes 30 major disorders such as malignant neoplasm, chronic kidney disease requiring regular hemodialysis, respiratory failure requiring long-term mechanical ventilation, and liver cirrhosis with complications etc. (30). Whether patients received antiviral treatment for CHB or CHC is also an important factor affecting the prognosis of HCC, so the variable of antiviral treatment was divided into two groups: receiving antiviral therapy yes or no. Tumor factors included the tumor size and cancer stage. Tumor size according to the TCRD was recorded based on the maximum diameter of the tumor (if there were multiple nodules in one patient, only the diameter of the largest nodule was registered) and grouped into < 3 cm, 3-5 cm, and > 5 cm. Cancer staging was based on the Barcelona Clinic Liver Cancer (BCLC) classification and divided into BCLC stages 0, A, B, C, D, and unknown. The definitions of applied therapy were based on the relevant treatment codes as stated in the NHIRD, which were cross-comparisons with the therapy registered in the TCRD. A primary medical care facility was defined as a patient’s major care hospital. Medical institutions were divided into medical centers, regional hospitals, and district hospitals. Hospital ownership was allocated to public and nonpublic hospitals.
2.4 Outcomes and measurements
This study had two significant outcomes. The first outcome was diagnosis-to-treatment interval (DTI). DTI was defined as the time interval from the date of a confirmed diagnosis of HCC (date of imaging study or liver biopsy) to the date of the first course of treatment (surgery, local treatment, embolization, radiotherapy, or chemotherapy). Patients with HCC generally undergo an imaging assessment of therapy within three–six months of the first treatment. To clearly define the time frame of the first course of treatment, the treatment combination was defined as within six months after the confirmed diagnosis of HCC (32). The second outcome was cancer-related death. Cancer-related death was based on patient data from the Cause of Death file and compared with the NHIRD for validation.
2.5 Statistical analysis
Descriptive statistics were used to evaluate the univariate association between various variables (Table 1) and the status of participation in MDT care for patients with HCC. Statistics such as the number and percentage of each variable were calculated. DTI was expressed as the median and quartile.
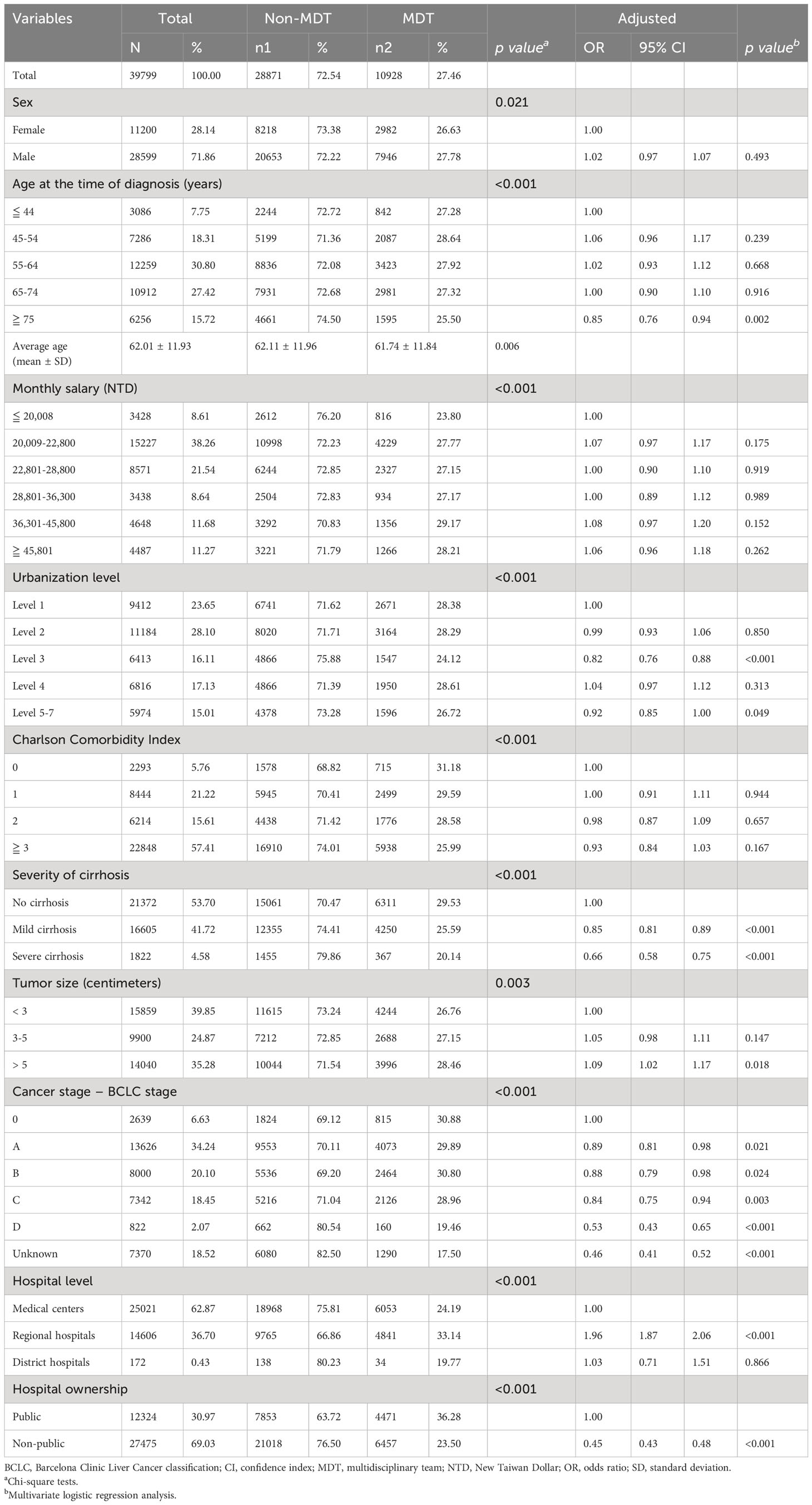
Table 1 Bivariate analysis and multivariate logistic regression analysis of hepatocellular carcinoma patient characteristics with or without multidisciplinary team care.
The study used variables, including sex, age at diagnosis, comorbidity severity (CCI), cirrhosis severity, antiviral therapy, tumor size, and tumor staging, to build a logistic regression model to calculate the propensity score for matching. Then, the propensity score using greedy nearest neighbor matching by digit without replacement was used to form a focus matching set to match the group of HCC patients who joined MDT care to those who did not receive MDT at a ratio of 1:2 to mitigate selection bias. The MDT and non-MDT patients have been matched in the same diagnosis year. The process executed the “best” match first, then the “next best” match in a classified sequence until no more matches could be made. Those with the highest digit match in the propensity score were considered the best matches. Each control sample was selected only once. The final matched-pair tests comprised tightly matched individual pairs and balanced control and case groups.
The Chi-square test was used to investigate whether patients with HCC were involved in MDT care and patient demographic characteristics (sex and age), socioeconomic status (monthly salary), urbanization level of the residence area, health condition, tumor characteristics (tumor size and cancer stage), and hospital characteristics (hospital level and hospital ownership). As DTI was not normally distributed, non-parametric statistics with the Kruskal-Wallis H test and Wilcoxon rank-sum test were used to analyze differences in DTI between patients with HBV- or HCV-induced HCC with or without MDT care intervention. The DTI time was then transformed into a natural log for further analysis. Multiple regression models with Generalized Estimating Equations were used for multivariate analysis to determine whether MDT care affected DTI. The results are presented as ratios with 95% confidence interval.
The log-rank test was employed as a bivariate analysis to evaluate the risk factors for cancer death after being involved in MDT. For the matched groups, the stratified Cox proportional hazards model was used to examine whether MDT care influenced the risk of death in patients with HCC when individual characteristics, socioeconomic status, environmental condition, health status, tumor features, and hospital characteristics were controlled (33). The results are presented as hazard ratios and 95% confidence intervals (CIs). The authors composed the survival curve of HCC patients with MDT versus non-MDT involvement at different BCLC stages after controlling for confounding variables, as listed above. All statistical analyses were performed using the SAS software (version 9.4, SAS Institute Inc., Cary, NC, USA). All tests were two-sided, and the level of statistical significance was set at p < 0.05.
3 Results
After participant enrollment, the total number of study participants was 39,799. A total of 10,928 patients with HBV- or HCV-related HCC underwent MDT (Table 1). Before matching, bivariate analysis showed significant differences in demographic characteristics (sex and age), socioeconomic factors (monthly salary), environmental factors (urbanization level), patient health status (CCI and severity of cirrhosis), tumor characteristics (tumor size, cancer stage), and medical institution characteristics (hospital level, hospital ownership) between patients with HCC participating in and those not participating in MDT (p < 0.05) (Table 1). After multivariate adjustment, HCC patients diagnosed at ≥ 75 years of age were 15% less likely to receive MDT care than those diagnosed at ≤ 44 years of age (OR 0.85, 95% CI 0.76-0.94). Increasing severity of cirrhosis and HCC stage at the time of diagnosis were associated with lower odds of receiving MDT care. However, increasing tumor size at diagnosis was associated with higher odds of receiving MDT care. Compared with those visiting medical centers, patients visiting regional hospitals were around two times more likely to receive MDT care. Patients at non-public hospitals were less likely to receive MDT (Table 1).
After PSM, 32,784 patients were enrolled in this study. After matching, bivariate analysis showed no significant differences in sex, age, monthly salary, CCI, severity of cirrhosis, antiviral therapy, tumor size, and cancer stage between the two groups (p > 0.05) (Table 2).
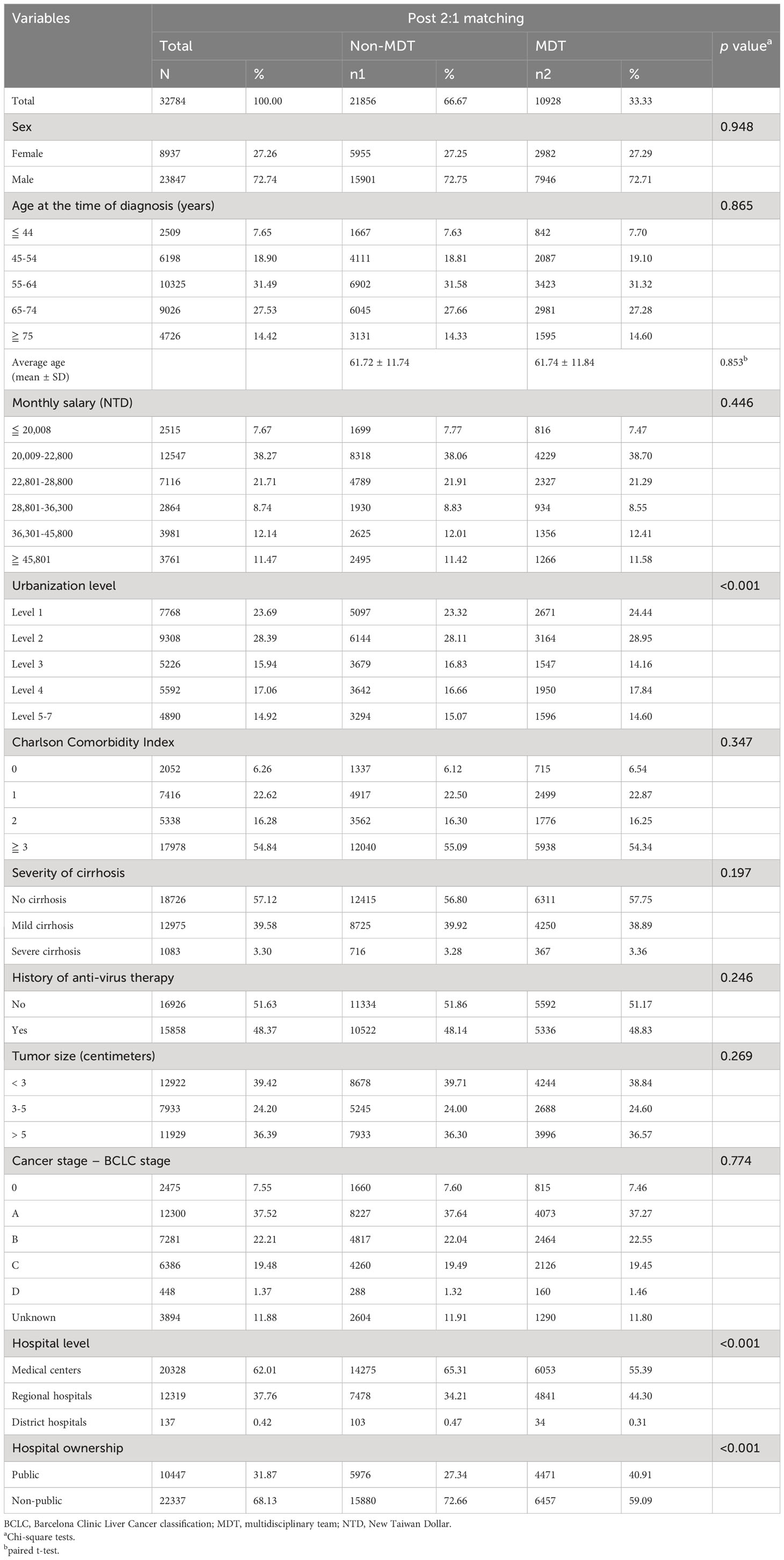
Table 2 Bivariate analysis of factors associated with HCC patients participating in MDT post-propensity score matching.
HCC patients who had participated in MDT care had a longer DTI than those who did not participate in MDT care (median:22 days vs. 20 days, p < 0.05) (Supplementary Table 1). Patients with no comorbidities or without cirrhosis had the shortest DTI. DTI was shorter in patients with larger tumors or more advanced tumors but was longer among patients who visited medical centers and public hospitals (Supplementary Table 1). We compared the DTI of liver cancer patients with different characteristics between MDT and non-MDT care groups. This revealed that patients with HCC who participated in MDT care had longer DTI for the most relevant factors. The average day of DTI was 29.55 ± 28.93 days in MDT care participants compared to 27.91 ± 29.65 days in non-MDT care participants (Supplementary Table 2). After controlling for the relevant variables, DTI increased by 1.24 times for HCC patients with MDT care than for those without MDT care (ratio = 1.24, 95% CI:1.18-1.32) (Table 3).
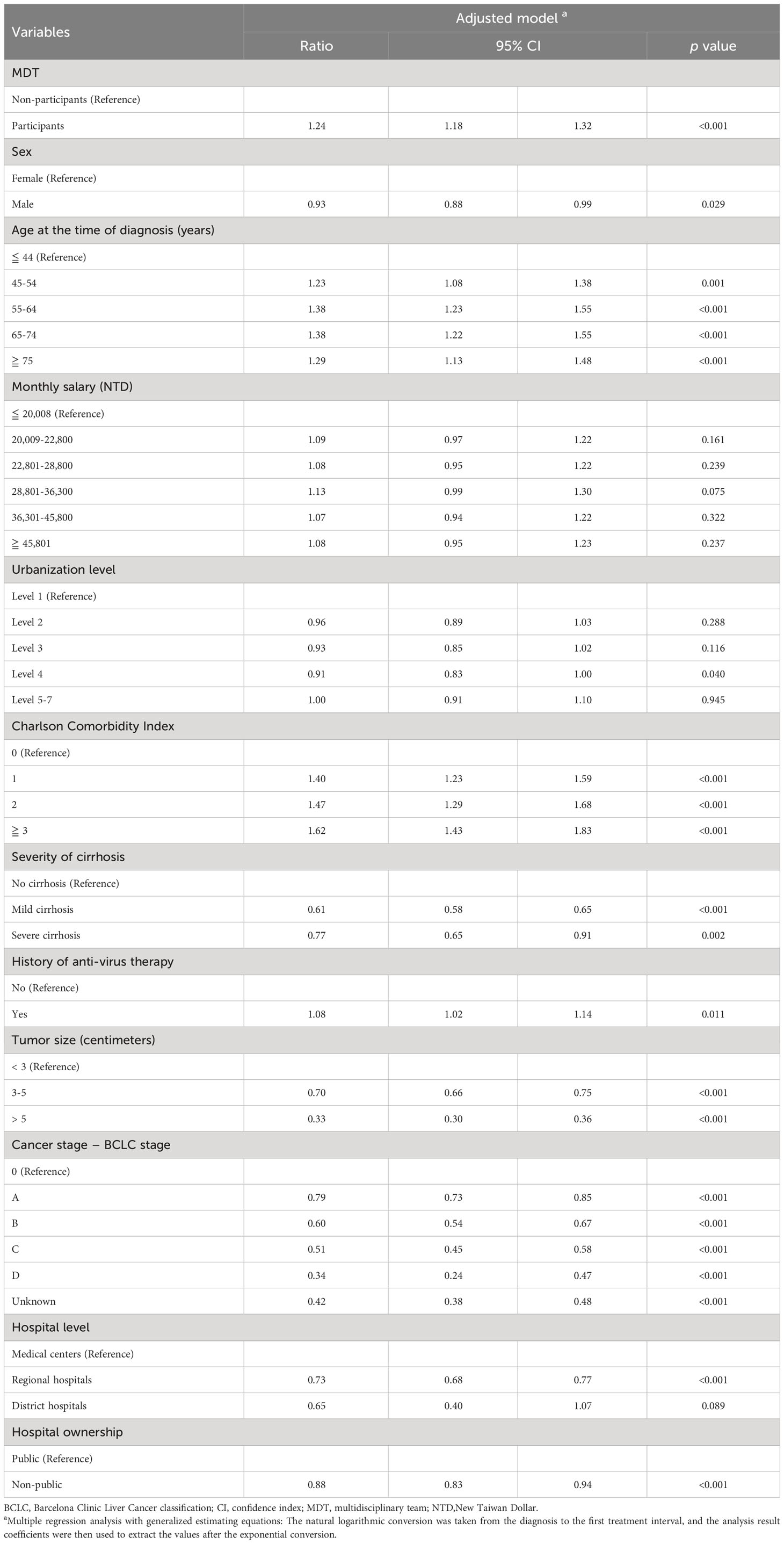
Table 3 Factors associated with diagnosis to the first treatment time interval in patients with HCC.
A log-rank test was used to analyze which factors were related to the decreased risk of cancer-related death in HBV- or HCV-related HCC patients after joining MDT care (Supplementary Table 3). By the end of the study, the total number of deaths among patients with HCC was 5,904 (54.03%) and 11,203 (51.26%) in the MDT and non-MDT care groups, respectively. However, the mortality of patients with HCC who did or did not participate in MDT care was not statistically significant (p = 0.206). Patients who lived in urbanization level 3 and received MDT care had a lower death rate than those who did not participate in MDT (51.58% vs. 52.49%, p = 0.034). Patients who visited medical centers (Death: MDT vs. non-MDT= 48.22% vs. 50.51%, p < 0.001) or public hospitals (Death: MDT vs. non-MDT= 48.62% vs. 50.87%, p = 0.002) with MDT care had a relatively lower death rate than those who did not (Supplementary Table 3).
After multivariate adjustment, patients who received MDT care had 12% higher survival than those who did not (HR=0.88, 95% CI:0.84-0.92) (Table 4). The receipt of anti-viral therapy for HBV- or HCV-related HCC was also associated with higher survival (lower risk of death). Increasing tumor size or a higher HCC stage were both independently associated with a higher risk of death (Table 4 and Supplementary Figure 1). HCC patients who received only surgical intervention or treatment combination with surgery within six months after diagnosis had better survival conditions (lower HR of cancer death) (Table 4).
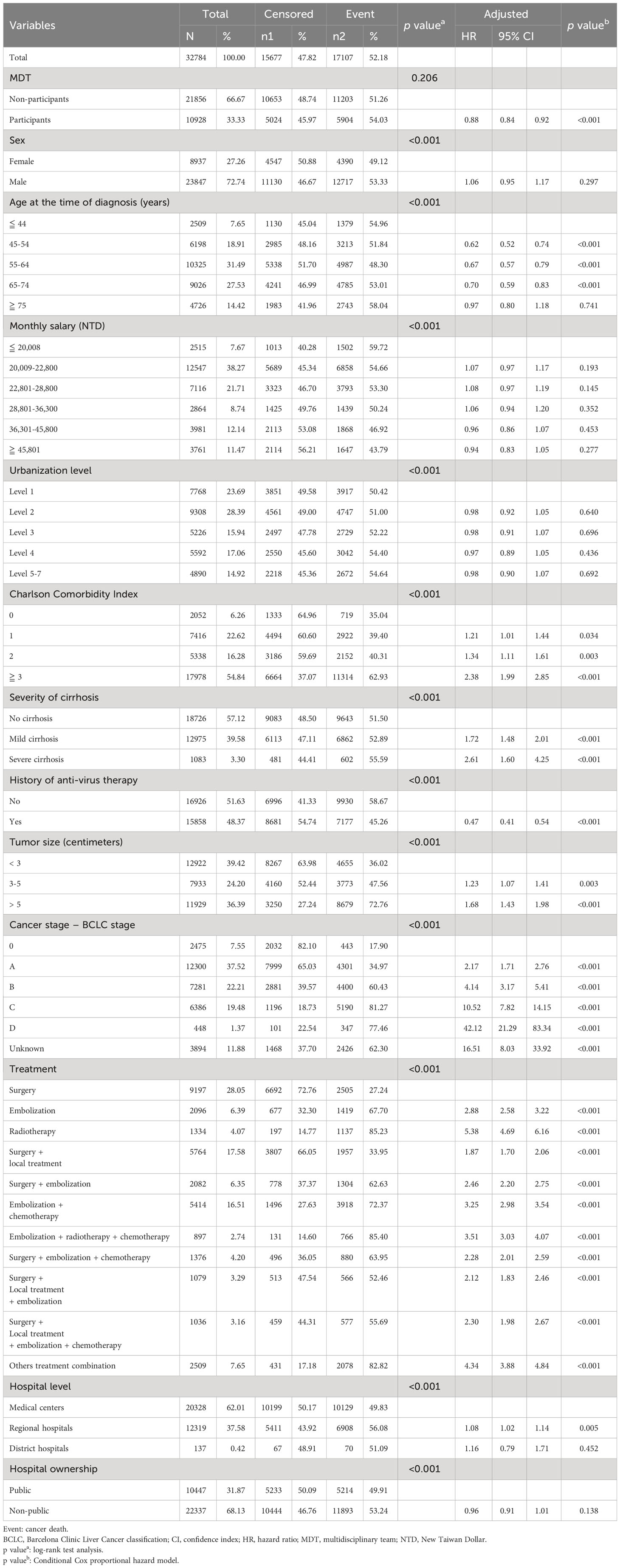
Table 4 Analysis of MDT participant status and various factors affecting cancer death in HCC patients.
On stratified analysis by the BCLC staging system for HCC, after controlling for all other variables, patients with Stage B who received MDT care had a 10% higher survival, and those with Stage C had a 18% higher survival than those without MDT care (Figure 2).
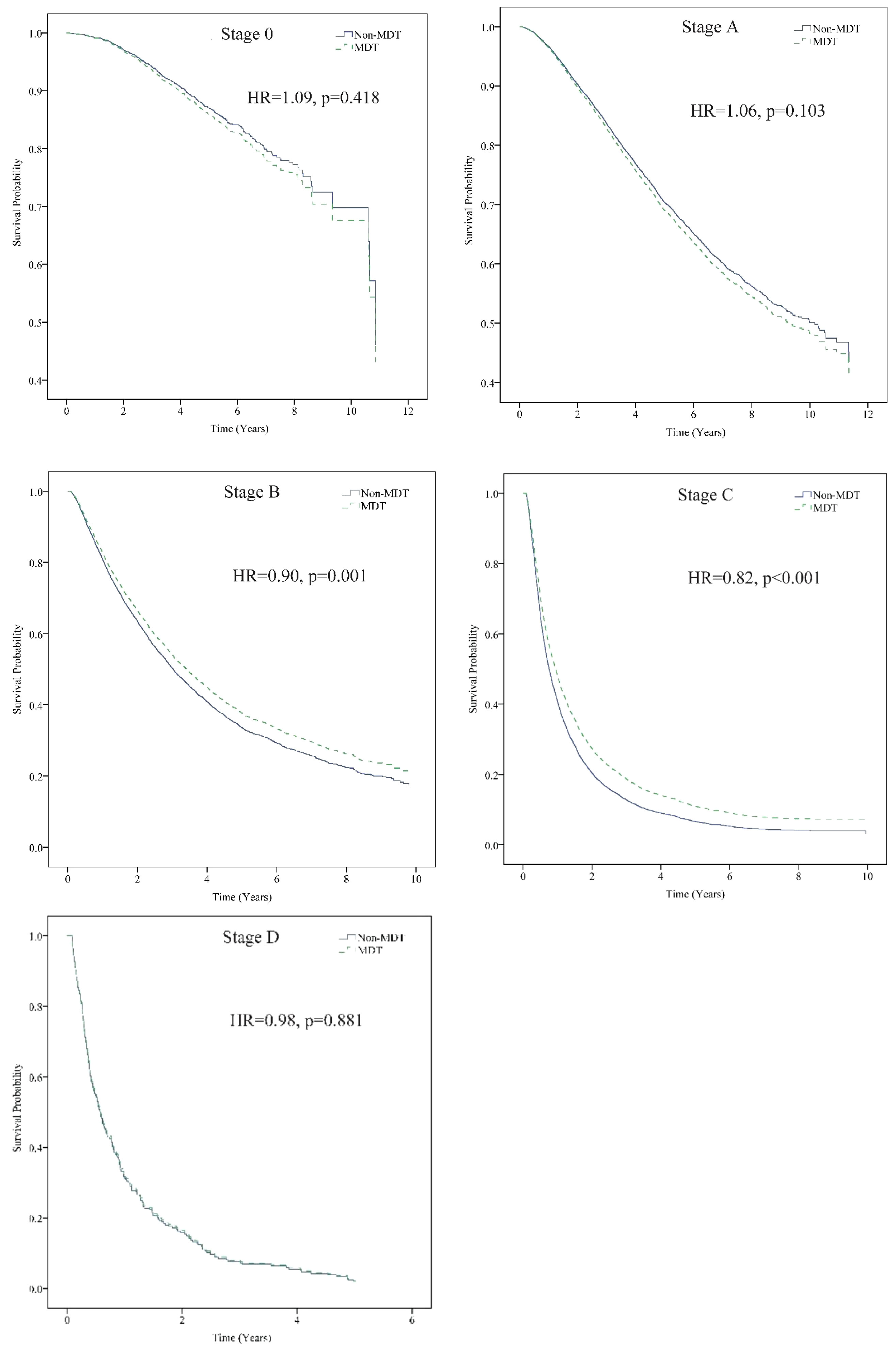
Figure 2 Stratification analysis of the patients according to BCLC stage. MDT care significantly reduced the risk of death for patients with BCLC stage B (Adj. HR = 0.90, p = 0.001) and stage C (Adj. HR = 0.82, p < 0.001) HCC. Event: cancer death. Stratification analysis was controlled for sex, age, monthly salary, urbanization level, CCI, cirrhosis severity, anti-viral therapy, tumor size, treatment method, hospital level, and hospital ownership. Adj. HR, adjusted hazard ratio; BCLC, Barcelona Clinic Liver Cancer classification; HCC, Hepatocellular carcinoma; MDT, multidisciplinary team. *The non-MDT group was the reference group. Cox proportional Hazard model.
4 Discussion
In this national population-based study with more than 10 years of coverage, we found that older patients, those with cirrhosis, a later stage of HCC, medical center visits, and non-public hospital visits were less likely to receive MDT care. Patients with HBV- or HCV-related HCC who had MDT care had prolonged DTI, but higher survival, while those with stages B and C also had a lower risk of cancer-related death (higher survival) when they received MDT care.
Yegin et al. compared surgical versus non-surgical treatment of HCC and found that treatment decisions were becoming more complex for increasing diversity and availability of treatment options. Therefore, comprehensive MDT care is required for HCC treatment (34). MDT care integrates the recommendations of medical experts to improve cancer care quality. However, this study found that old age, cirrhosis, late cancer stage, treatment in medical centers, and non-public hospitals were all factors contributing to a lower chance of participating in MDT care. Although the treatment options for HCC are varied and complex, this cancer staging system helps clinicians easily make treatment choices for HCC. The Taiwan Liver Cancer Association and Gastroenterological Society of Taiwan recommend using the BCLC staging system as the primary cancer staging and treatment guidance system (35). In Taiwan, hospital cancer care accreditation may compel hospitals, particularly medical centers and regional hospitals, to implement MDT care. Most district hospitals are non-public hospitals with limited capacity to implement MDT care.
After adjusting for correlated factors, patients with HBV- or HCV-related HCC who participated in MDT care had a longer time interval from diagnosis to first treatment than those who did not participate in MDT care. Studies have reported that demographic and socioeconomic factors may affect DTI (36, 37). Sharma et al. further pointed out that treatment modalities (radiation therapy) in medical centers and hospitals with large treatment volumes will extend DTI (37). Some researchers have shown that prolonged DTI is associated with poor cancer prognosis (37–40). However, clinical studies have reported no significant correlation between longer DTI and local tumor control, survival without distal metastasis, or overall survival in head and neck cancer (41). A systemic review article concluded that no significant association exists between longer DTI and poor prognosis in colorectal cancer (42). One population-based study revealed that early-stage HCC patients (AJCC stage I and II) who have prolonged DTI (> 60 days) would have lower survival rates (43). Taiwan’s MOHW has promulgated multiple rules to incentivize clinicians and people to strengthen the tracking and treatment of viral hepatitis. In a previous study in Taiwan, 78.46% of patients with HCC received early-stage cancer therapy within 30 days of diagnosis (43). Therefore, when MDT care requires regular meetings, the DTI is prolonged. In this study, patients with HBV- or HCV-related HCC who participated in MDT care had prolonged DTI compared to those who did not.
Several studies have reported that MDT care in patients with cancer has an improved survival rate or a decreased risk of cancer-related death (10, 15, 17–21, 24, 25). MDT care is used in patients with HCC to assist clinicians in making accurate diagnoses and treatment choices and prolonging or improving survival status (28, 44–48). In addition, studies have shown that MDT care increases the proportion of patients with HCC receiving appropriate therapy (5, 44–46, 49, 50). This nationwide population-based cohort study also found that HBV- and HCV-related HCC patients had a reduced risk of death after participating in MDT care. According to Chen et al., compared with the AJCC staging system, the BCLC staging system has a better long-term prognostic prediction for curative therapies, such as surgical treatment, in HCC patients (51). Most of the participants in this study whose therapy combination included surgery (62.73%) as the first treatment after diagnosis. Therefore, the BCLC staging system in this study has a better prognostic prediction than the AJCC staging system, which is commonly used for other cancers. Sinn et al. conducted a cohort study that enrolled 6,619 newly diagnosed HCC patients over nine years. It was concluded that the subgroup of patients with poor liver function (albumin-bilirubin grade 2 or 3), high alpha-fetoprotein (≥ 200 ng/mL), and intermediate to advanced HCC (BCLC stage B or C) who received MDT care had specific improvements in survival benefits (47). This study confirmed that HCC patients with BCLC stage B or C who participated in MDT care had a significantly higher survival rate.
This study used a nationwide population-based research database and PSM to eliminate selection bias; therefore, the sample was representative. However, the present study has some limitations. First, secondary data were used, and some personal information about detailed cancer treatment and disease prognosis could not be obtained. Second, some important clinical data influencing outcomes, such as portal hypertension, laboratory data, detailed Child-Turcotte-Pugh score, accurate tumor numbers, and each tumor’s actual size, were unavailable. This study employed cirrhosis severity to compensate for data limitations in assessing accurate liver function. Third, the members of the MDT (the actual members of medical experts), the method (direct meeting or virtual meeting), and the period of MDT meetings (the accurate frequency of MDT meetings, usually once a week in Taiwan) were different in every hospital; therefore, the study populations joining the exact model of MDT care were unknown. Even though there is a minor difference in MDT care in each hospital, the medical institute must follow the Act and Regulations to hold MDT conferences, maintain cancer care quality, and get cancer care qualifications. Fourth, we did not match variables that had less relation to disease outcome, such as urbanization level, hospital level, and hospital ownership. However, we have included those variables in the multivariate model to control them.
In conclusion, patients with HBV- or HCV-related HCC who participated in MDT care had longer DTI but a lower risk of cancer death. In patients with intermediate-to advanced-stage HCC (BCLC stage B or C), participation in MDT care significantly improved their outcomes. Hospitals should provide HCC patients with multidisciplinary team care to improve cancer care.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The research was conducted in accordance with the 1964 Declaration of Helsinki and its amendments and was reviewed and approved by the Research Ethics Committee of China Medical University and Hospitals, Taichung, Taiwan (IRB number: CMUH110-REC3-227). The need for informed consent was waived due to the use of anonymized secondary data.
Author contributions
Y-CT, C-YP, and W-CT contributed to the conception and design of the study. W-YC, P-TK, and W-CT collected the database and performed the statistical analysis. Y-CT and P-TK wrote the manuscript. All authors contributed to the manuscript revision read, and approved the submitted version.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by Taichung Armed Forces General Hospital (TCAFGH-D-111014) and supported by a grant (CMU111-ASIA-14) from China Medical University and Asia University.
Acknowledgments
We acknowledge the contributions of the Department of Medical Education and Research, Taichung Armed Forces General Hospital. We are grateful to the National Health Insurance Research Database, Cause of Death file, and the Taiwan Cancer Registry Database provided by the Science Center, Ministry of Health and Welfare, Taiwan. We are also grateful to the Health Data Science Center, China Medical University Hospital for providing administrative, technical, and funding support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1251571/full#supplementary-material
Abbreviations
BCLC, Barcelona Clinic Liver Cancer; CCI, Comorbidity severity; CHB, Chronic hepatitis B; Chronic hepatitis C; DTI, Diagnosis-to-treatment interval; HBV, Hepatitis B virus; HCV, Hepatitis C virus; HCC, Hepatocellular carcinoma; IARC, International Agency for Research on Cancer; MDT, Multidisciplinary team; MOHW, Ministry of Health and Welfare; NHI, National Health Insurance; NHIRD, National Health Insurance Research Database; PSM, Propensity score matching; TCRD, Taiwan Cancer Registry Database.
References
1. Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Pineros M, Znaor A, et al. Cancer statistics for the year 2020: an overview. Int J Cancer (2021) 149(4):778–89. doi: 10.1002/ijc.33588
2. Chu WCL PY, Lee WH, et al. Cancer Registry Annual Report, 2019 Taiwan: Health Promotion Administration ministry of health and welfare Taiwan (2022). Available at: https://www.hpa.gov.tw/Pages/Detail.aspx?nodeid=269&pid=14913.
3. Sinclair M, Roberts S, Kemp W, Knight V, Dev A, Gow P, et al. Epidemiology of hepatitis B-associated hepatocellular carcinoma in victoria. Intern Med J (2013) 43(5):501–6. doi: 10.1111/imj.12068
4. Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Primers (2021) 7(1):6. doi: 10.1038/s41572-020-00240-3
5. Serper M, Taddei TH, Mehta R, D’Addeo K, Dai F, Aytaman A, et al. Association of provider specialty and multidisciplinary care with hepatocellular carcinoma treatment and mortality. Gastroenterology (2017) 152(8):1954–64. doi: 10.1053/j.gastro.2017.02.040
6. Naugler WE, Alsina AE, Frenette CT, Rossaro L, Sellers MT. Building the multidisciplinary team for management of patients with hepatocellular carcinoma. Clin Gastroenterol Hepatol (2015) 13(5):827–35. doi: 10.1016/j.cgh.2014.03.038
7. Gish RG. Hepatocellular carcinoma: overcoming challenges in disease management. Clin Gastroenterol Hepatol (2006) 4(3):252–61. doi: 10.1016/j.cgh.2006.01.001
8. Calman K, Hine D. A policy framework for commissioning cancer services. (1995). Available at: http://www.surginet.org.uk/misc/interview/downloads/doh/cancerfr_CALMAN_HINE.pdf.
9. Leonard MW, Frankel AS. Role of effective teamwork and communication in delivering safe, high-quality care. Mt Sinai J Med (2011) 78(6):820–6. doi: 10.1002/msj.20295
10. Peng D, Cheng YX, Cheng Y. Improved overall survival of colorectal cancer under multidisciplinary team: a meta-analysis. BioMed Res Int (2021) 2021:5541613. doi: 10.1155/2021/5541613
11. Simmons A, McMahon L, Crosbie V, Carlson L. A multidisciplinary team approach to screening, assessment and early intervention for young people with type 1 diabetes and disordered eating behaviour. Clin Child Psychol Psychiatry (2021) 26(3):629–42. doi: 10.1177/13591045211013872
12. Wang S, Qian X. Effect of multidisciplinary team care on the management of cirrhotic patients with upper gastrointestinal bleeding: A retrospective cohort study. Ann Palliat Med (2021) 10(3):3050–8. doi: 10.21037/apm-21-85
13. Zhang H, Yu J, Wei Z, Wu W, Zhang C, He Y. The effect of multidisciplinary team discussion intervention on the prognosis of advanced colorectal cancer. J Cancer (2021) 12(11):3307–14. doi: 10.7150/jca.56171
14. Zhu S, Chen J, Ni Y, Zhang H, Liu Z, Shen P, et al. Dynamic multidisciplinary team discussions can improve the prognosis of metastatic castration-resistant prostate cancer patients. Prostate (2021) 81(11):721–7. doi: 10.1002/pros.24167
15. Shang C, Feng L, Gu Y, Hong H, Hong L, Hou J. Impact of multidisciplinary team management on the survival rate of head and neck cancer patients: A cohort study meta-analysis. Front Oncol (2021) 11:630906. doi: 10.3389/fonc.2021.630906
16. Hendriks JM, Jaarsma T. The multidisciplinary team approach in cardiovascular care. Eur J Cardiovasc Nurs (2021) 20(2):91–2. doi: 10.1093/eurjcn/zvaa005
17. Pan CC, Kung PT, Wang YH, Chang YC, Wang ST, Tsai WC. Effects of multidisciplinary team care on the survival of patients with different stages of non-small cell lung cancer: a national cohort study. PloS One (2015) 10(5):e0126547. doi: 10.1371/journal.pone.0126547
18. Tsai WC, Kung PT, Wang ST, Huang KH, Liu SA. Beneficial impact of multidisciplinary team management on the survival in different stages of oral cavity cancer patients: results of a nationwide cohort study in Taiwan. Oral Oncol (2015) 51(2):105–11. doi: 10.1016/j.oraloncology.2014.11.006
19. Hsu YH, Kung PT, Wang ST, Fang CY, Tsai WC. Improved patient survivals with colorectal cancer under multidisciplinary team care: A nationwide cohort study of 25,766 patients in Taiwan. Health Policy (2016) 120(6):674–81. doi: 10.1016/j.healthpol.2016.04.001
20. Wang YH, Kung PT, Tsai WC, Tai CJ, Liu SA, Tsai MH. Effects of multidisciplinary care on the survival of patients with oral cavity cancer in Taiwan. Oral Oncol (2012) 48(9):803–10. doi: 10.1016/j.oraloncology.2012.03.023
21. Huang YC, Kung PT, Ho SY, Tyan YS, Chiu LT, Tsai WC. Effect of multidisciplinary team care on survival of oesophageal cancer patients: A retrospective nationwide cohort study. Sci Rep (2021) 11(1):13243. doi: 10.1038/s41598-021-92618-w
22. Wang SM, Kung PT, Wang YH, Huang KH, Tsai WC. Effects of multidisciplinary team care on utilization of emergency care for patients with lung cancer. Am J Manag Care (2014) 20(8):e353–64.
23. Liao CM, Kung PT, Wang YH, Tsai WC. Effects of multidisciplinary team on emergency care for colorectal cancer patients: A nationwide-matched cohort study. Med (Baltimore) (2017) 96(23):e7092. doi: 10.1097/MD.0000000000007092
24. Lin WL, Sun JL, Chang SC, Tsai TC, Wu PH, Huang WT, et al. Effectiveness of the multidisciplinary team model in treating colorectal cancer. Gastroenterol Nurs (2018) 41(6):491–6. doi: 10.1097/SGA.0000000000000348
25. Tsai CH, Hsieh HF, Lai TW, Kung PT, Kuo WY, Tsai WC. Effect of multidisciplinary team care on the risk of recurrence in breast cancer patients: A national matched cohort study. Breast (2020) 53:68–76. doi: 10.1016/j.breast.2020.07.001
26. Bosch M, Faber MJ, Cruijsberg J, Voerman GE, Leatherman S, Grol RP, et al. Review article: effectiveness of patient care teams and the role of clinical expertise and coordination: A literature review. Med Care Res Rev (2009) 66(6 Suppl):5S–35S. doi: 10.1177/1077558709343295
27. Lamb BW, Brown KF, Nagpal K, Vincent C, Green JS, Sevdalis N. Quality of care management decisions by multidisciplinary cancer teams: A systematic review. Ann Surg Oncol (2011) 18(8):2116–25. doi: 10.1245/s10434-011-1675-6
28. Byrd K, Alqahtani S, Yopp AC, Singal AG. Role of multidisciplinary care in the management of hepatocellular carcinoma. Semin Liver Dis (2021) 41(1):1–8. doi: 10.1055/s-0040-1719178
29. Welfare MoHa. Regulations for cancer care quality assurance measures (2005). Available at: https://law.moj.gov.tw/LawClass/LawAll.aspx?pcode=L0070016.
30. Administration NHI. National health insurance annual report 2021-2022. Taiwan: Bilingual, Administration NHI (2022). Available at: https://www.nhi.gov.tw/English/Content_List.aspx?n=2BDB331B84E5BC43&topn=ED4A30E51A609E49.
31. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with icd-9-cm administrative databases. J Clin Epidemiol (1992) 45(6):613–9. doi: 10.1016/0895-4356(92)90133-8
32. Ikeda K, Kawamura Y, Kobayashi M, Kominami Y, Fujiyama S, Sezaki H, et al. Direct-acting antivirals decreased tumor recurrence after initial treatment of hepatitis C virus-related hepatocellular carcinoma. Dig Dis Sci (2017) 62(10):2932–42. doi: 10.1007/s10620-017-4739-z
33. Austin PC. A critical appraisal of propensity-score matching in the medical literature between 1996 and 2003. Stat Med (2008) 27(12):2037–49. doi: 10.1002/sim.3150
34. Yegin EG, Oymaci E, Karatay E, Coker A. Progress in surgical and nonsurgical approaches for hepatocellular carcinoma treatment. Hepatobiliary Pancreat Dis Int (2016) 15(3):234–56. doi: 10.1016/s1499-3872(16)60097-8
35. Surveillance g, Diagnosis g, Staging g, Surgery g, Local ablation g, group TTH, et al. Management consensus guideline for hepatocellular carcinoma: 2016 updated by the Taiwan liver cancer association and the gastroenterological society of Taiwan. J Formos Med Assoc (2018) 117(5):381–403. doi: 10.1016/j.jfma.2017.09.007
36. Mariscal M, Llorca J, Prieto-Salceda D, Palma S, Delgado-Rodriguez M. Determinants of the interval between diagnosis and treatment in patients with digestive tract cancer. Oncol Rep (2003) 10(2):463–7. doi: 10.3892/or.10.2.463
37. Sharma S, Bekelman J, Lin A, Lukens JN, Roman BR, Mitra N, et al. Clinical impact of prolonged diagnosis to treatment interval (Dti) among patients with oropharyngeal squamous cell carcinoma. Oral Oncol (2016) 56:17–24. doi: 10.1016/j.oraloncology.2016.02.010
38. Chen CP, Kung PT, Wang YH, Tsai WC. Effect of time interval from diagnosis to treatment for cervical cancer on survival: A nationwide cohort study. PloS One (2019) 14(9):e0221946. doi: 10.1371/journal.pone.0221946
39. Tsai CH, Kung PT, Kuo WY, Tsai WC. Effect of time interval from diagnosis to treatment for non-small cell lung cancer on survival: A national cohort study in Taiwan. BMJ Open (2020) 10(4):e034351. doi: 10.1136/bmjopen-2019-034351
40. Tsai WC, Kung PT, Wang YH, Huang KH, Liu SA. Influence of time interval from diagnosis to treatment on survival for oral cavity cancer: A nationwide cohort study. PloS One (2017) 12(4):e0175148. doi: 10.1371/journal.pone.0175148
41. Caudell JJ, Locher JL, Bonner JA. Diagnosis-to-treatment interval and control of locoregionally advanced head and neck cancer. Arch Otolaryngol Head Neck Surg (2011) 137(3):282–5. doi: 10.1001/archoto.2011.20
42. Molenaar CJL, Janssen L, van der Peet DL, Winter DC, Roumen RMH, Slooter GD. Conflicting guidelines: A systematic review on the proper interval for colorectal cancer treatment. World J Surg (2021) 45(7):2235–50. doi: 10.1007/s00268-021-06075-7
43. Tsai WC, Kung PT, Wang YH, Kuo WY, Li YH. Influence of the time interval from diagnosis to treatment on survival for early-stage liver cancer. PloS One (2018) 13(6):e0199532. doi: 10.1371/journal.pone.0199532
44. Agarwal PD, Phillips P, Hillman L, Lucey MR, Lee F, Mezrich JD, et al. Multidisciplinary management of hepatocellular carcinoma improves access to therapy and patient survival. J Clin Gastroenterol (2017) 51(9):845–9. doi: 10.1097/MCG.0000000000000825
45. Chang TT, Sawhney R, Monto A, Davoren JB, Kirkland JG, Stewart L, et al. Implementation of a multidisciplinary treatment team for hepatocellular cancer at a veterans affairs medical center improves survival. HPB (Oxford) (2008) 10(6):405–11. doi: 10.1080/13651820802356572
46. Duininck G, Lopez-Aguiar AG, Lee RM, Miller L, Dariushnia S, Wu C, et al. Optimizing cancer care for hepatocellular carcinoma at a safety-net hospital: the value of a multidisciplinary disease management team. J Surg Oncol (2019) 120(8):1365–70. doi: 10.1002/jso.25738
47. Sinn DH, Choi GS, Park HC, Kim JM, Kim H, Song KD, et al. Multidisciplinary approach is associated with improved survival of hepatocellular carcinoma patients. PloS One (2019) 14(1):e0210730. doi: 10.1371/journal.pone.0210730
48. Yopp AC, Mansour JC, Beg MS, Arenas J, Trimmer C, Reddick M, et al. Establishment of a multidisciplinary hepatocellular carcinoma clinic is associated with improved clinical outcome. Ann Surg Oncol (2014) 21(4):1287–95. doi: 10.1245/s10434-013-3413-8
49. Gashin L, Tapper E, Babalola A, Lai KC, Miksad R, Malik R, et al. Determinants and outcomes of adherence to recommendations from a multidisciplinary tumour conference for hepatocellular carcinoma. HPB (Oxford) (2014) 16(11):1009–15. doi: 10.1111/hpb.12280
50. Siddique O, Yoo ER, Perumpail RB, Perumpail BJ, Liu A, Cholankeril G, et al. The importance of a multidisciplinary approach to hepatocellular carcinoma. J Multidiscip Healthc (2017) 10:95–100. doi: 10.2147/JMDH.S128629
Keywords: hepatocellular carcinoma, multidisciplinary team, hepatitis B virus, hepatitis C virus, survival
Citation: Tseng Y-C, Kung P-T, Peng C-Y, Chou W-Y and Tsai W-C (2023) Effect of multidisciplinary team care on patient survival in chronic hepatitis B or C hepatocellular carcinoma. Front. Oncol. 13:1251571. doi: 10.3389/fonc.2023.1251571
Received: 20 July 2023; Accepted: 31 October 2023;
Published: 21 December 2023.
Edited by:
Ying Tang, Guangzhou University of Chinese Medicine, ChinaReviewed by:
Tianqi Gao, Guangzhou University of Chinese Medicine, ChinaKuo-Piao Chung, National Taiwan University, Taiwan
Paramita Dasgupta, Cancer Council Queensland, Australia
Copyright © 2023 Tseng, Kung, Peng, Chou and Tsai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wen-Chen Tsai, d2N0c2FpMjAxMUBnbWFpbC5jb20=; Yu-Chen Tseng, bG92ZXl1MDYxOUBnbWFpbC5jb20=
†These authors have contributed equally to this work
‡ORCID: Wen-Chen Tsai, orcid.org/0000-0002-9684-0789
 Yu-Chen Tseng1,2,3,4
Yu-Chen Tseng1,2,3,4 Pei-Tseng Kung
Pei-Tseng Kung Cheng-Yuan Peng
Cheng-Yuan Peng Wen-Chen Tsai
Wen-Chen Tsai