
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 19 September 2023
Sec. Genitourinary Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1249683
Objectives: Literature regarding experience with 3D laparoscopy about prostatectomy has remained scanty, and this could be related to the rise of robotic assisted laparoscopic surgery. This study aimed to perform a systemic review and meta-analysis to evaluate the perioperative, functional, and oncologic outcomes between 3D and 2D laparoscopic radical prostatectomy (LRP).
Methods: We systematically searched the PubMed, Embase, and Cochrane Library databases for studies that compared perioperative, functional, or oncologic outcomes of both 3D and 2D LRP. The Newcastle-Ottawa Scale (NOS) tool and Jadad scale were used to assess the risk of bias in the included studies. Review Manager 5.3 was used for the meta-analysis.
Results: Seven studies with a total of 542 patients were included in the analysis. Among them, two were RCTs. There was no difference between groups in terms of preoperative characteristics. Anastomosis time, hospital day, and overall complication rates were similar in 3D than 2D group. However, operative time [mean difference (MD) -36.96; 95% confidence interval [CI] -59.25 to -14.67; p = 0.001], blood loss (MD -83.5; 95% CI -123.05 to -43.94; p <0.0001), and days of drainage (MD -1.48; 95% CI -2.29 to -0.67; p = 0.0003) were lower in 3D LRP. 2D and 3D LRP showed similarity in the positive surgical margin (PSM) rate and biochemical recurrence (BCR) rate at 3, 6, and 12months postoperatively. Additionally, there was no significant differences in continence and potency recovery rate between two group except higher continence rate of 3D LRP at 3 months.
Conclusion: Current evidence shows that 3D LRP offers favorable outcomes compared with 2D LRP, including operative time, blood loss, days of drainage, and early continence. However, there was no conclusive evidence that 3D LRP was advantaged in terms of oncologic and functional outcomes (except for continence rate at 3 months).
Systematic review registration: The study has been registered on the International Prospective Register of Systematic Reviews (PROSPERO: CRD42023426403).
Prostate cancer (PCa) is the most commonly diagnosed cancer except lung cancer and the fifth leading cause of cancer death in males around the world, accounting for 14.1% (1,414,259) of total new cancer cases and 6.8% (375,304) of total cancer deaths in males in 2020 (1, 2). Since Schuessler first conducted laparoscopic radical prostatectomy (LRP) in 1992 (3), LRP has become a standard treatment for organ confined prostate cancer (4). However, widespread application of LRP has been restricted by some limitations, including two-dimensional (2D) vision, limited degrees of freedom, lacked stereoscopic perception, and a steep learning curve.
In this context, three-dimensional (3D) laparoscopic surgery is gradually emerging. The development of 3D laparoscopic technology attempts to overcome the 2D vision of traditional laparoscopic surgery. Previously, 3D systems made surgeons more prone to fatigue and harmful to the eyes (2, 5). These limitations hampered the widespread application of 3D laparoscopic technology in clinics. With the improvement of surgical navigation and augmented reality, 3D laparoscopic technology has taken an important step towards clinical practice (6). New-generation 3D laparoscopic technology had high-definition stable images, increasing the comfort of glasses and reducing the fatigue of surgeons (7) Nowadays, 3D laparoscopy is becoming increasingly popular worldwide.
On the other side, literature regarding experience with 3D laparoscopy about prostatectomy has remained scanty, and this could be related to the rise of robotic assisted laparoscopic surgery.
This study aimed to perform a systemic review and meta-analysis to evaluate the perioperative, functional, and oncologic outcomes between 3D and 2D LRP.
The systematic review and meta-analysis were based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (8).
Eligibility criteria were formulated using the specific population, intervention, comparison, outcomes, and study design (PICOS) framework. This review included studies that met the following criteria: (P): patients with organ confined PCa; (I): undergoing 3D LRP; (C): in which traditional 2D LRP was performed as comparator; (O): perioperative, oncologic, and functional outcomes; and (S): retrospective and prospective cohort studies.
Case series, surveys, letters, editorial comments, reviews, and animal studies were not included. In addition, studies without original data and articles in languages other than English were excluded.
A systematic search was conducted using Embase, PubMed, and the Cochrane Library. The search terms used were: (Prostatectomy OR Prostatectomies OR Prostatectomy, Suprapubic OR Prostatectomies, Suprapubic OR Suprapubic Prostatectomies OR Suprapubic Prostatectomy OR Prostatectomy, Retropubic OR Prostatectomies, Retropubic OR Retropubic Prostatectomies OR Retropubic Prostatectomy) AND 3D.
The search results were limited to humans. Studies published between January 1, 2010, and April 1, 2023, were included. Articles were reviewed by two authors (S.H and D.X) according to a priori inclusion/exclusion criteria. Any conflicts about eligibility were resolved between the two authors and any disagreements were resolved by a third party (W.T). Studies that meet our PICOS criteria were included.
The authors extracted data from the seven included studies. Data extracted included study characteristics (first author, year of publication, country, study design, number of participants), baseline demographics [age, body mass index (BMI), and prostate-specific antigen (PSA)], perioperative (operative time, anastomosis time, number anastomosis stitches, blood loss, hospital day, days of drainage/catheterization, complications), oncologic (PSM, BCR-free), and functional (urinary continence and potency) outcomes.
The Newcastle–Ottawa Scale (NOS) (9, 10) was used to assess the quality of non-RCT. The NOS checklist includes three quality parameters: population selection (4 points), comparability of cohorts (2 points), and assessment of outcome for cohort studies (3 points). Each study received a score ranging from 0 to 9. Studies with a score of 7 or higher were considered high-quality articles. And a risk of bias assessment was conducted on RCTs using the Jadad scale (11). A total score of 1–2 was considered low quality and 3–5 was considered high quality.
The meta-analysis included retrospective and prospective cohort studies and was performed using Review Manager 5.3 (Cochrane Collaboration, Oxford, UK). We pooled clinical effect estimates using the mean difference (MD), relative risk (RR), and their respective 95% CIs. The statistical significance level was set at p < 0.05. The Mantel–Haenszel effects model and inverse-variance effects model were used to combine the trials. We calculated and depicted forest plots with a 95% CI. The I2 test and Cochran’s Q test were used to assess the heterogeneity. Statistical heterogeneity was indicated by p < 0.1 in the Cochran’s Q test and I2 > 50% in the I2 test. If heterogeneity existed, a random effect model was adopted; otherwise, a fixed effect model was adopted. I2 values of 25%, 50%, and 75% indicate low, moderate, and high levels of inconsistency, respectively (12). Further sensitivity analyses were conducted to reduce heterogeneity and confirm the reliability of our findings.
Our initial research identified 311 articles, of which 7 were selected for further analysis. Figure 1 depicts the search process (PRISMA flowchart). Three studies were prospective (13–15), and four were retrospective (16–19). Two of them were RCTs (13, 15). Among the 542 patients included in the meta-analysis, 300 (55.4%) and 242 (44.6%) were 2D and 3D LRP, respectively. Among 7 articles included, all studies reported results on perioperative outcomes and complications (13–19), 4 studies on urinary continence (14–16, 19), 3 studies on potency (14, 15, 19), 4 studies on PSM (14–16, 19), and 3 articles on BCR (14, 15, 19). Table 1 provides an overview of the patients and details of our study population. All the included studies are of high quality.
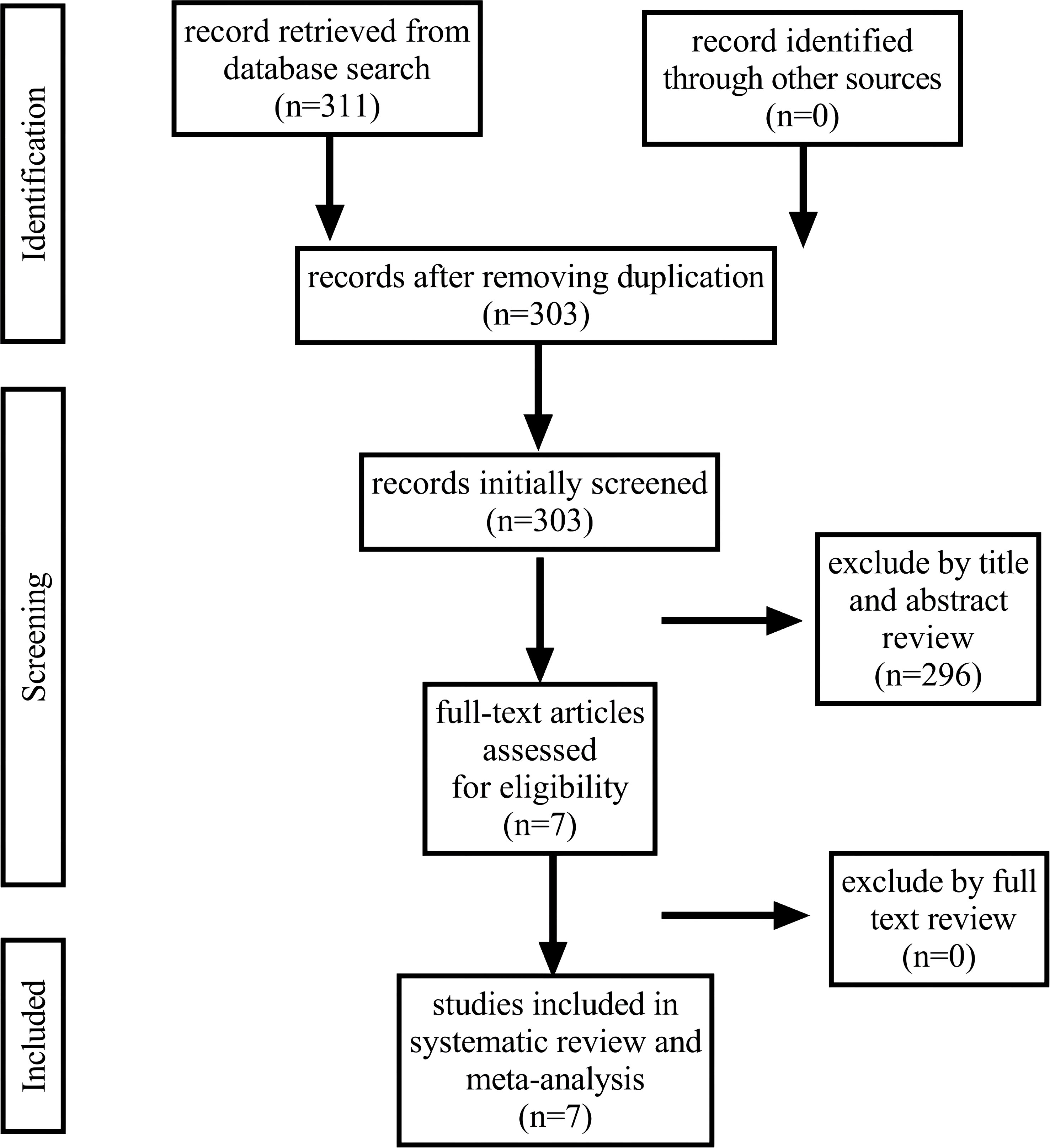
Figure 1 Flowchart illustrating the major steps of the review process in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement.
Table 2 presents the results of operative time, anastomosis time, number anastomosis stitches, blood loss, hospital stay, days of drainage/catheterization, and overall complications from the studies comparing 2D and 3D LRP.
Figure 2 depicts the pooled results of overall perioperative outcomes and complications. Meta-analysis demonstrates that the pooled estimates of operative time (MD -83.5; 95% CI -123.05 to -43.94; p <0.0001), blood loss (MD -83.5; 95%CI -123.05 to -43.94; p <0.0001), and days of drainage (MD -1.48; 95% CI -2.29 to -0.67; p = 0.0003) were lower in 3D LRP than those in 2D LRP (Figures 2A-C). No statistically significant differences were found in terms of anastomosis time, hospital day (Figures 2D, E).
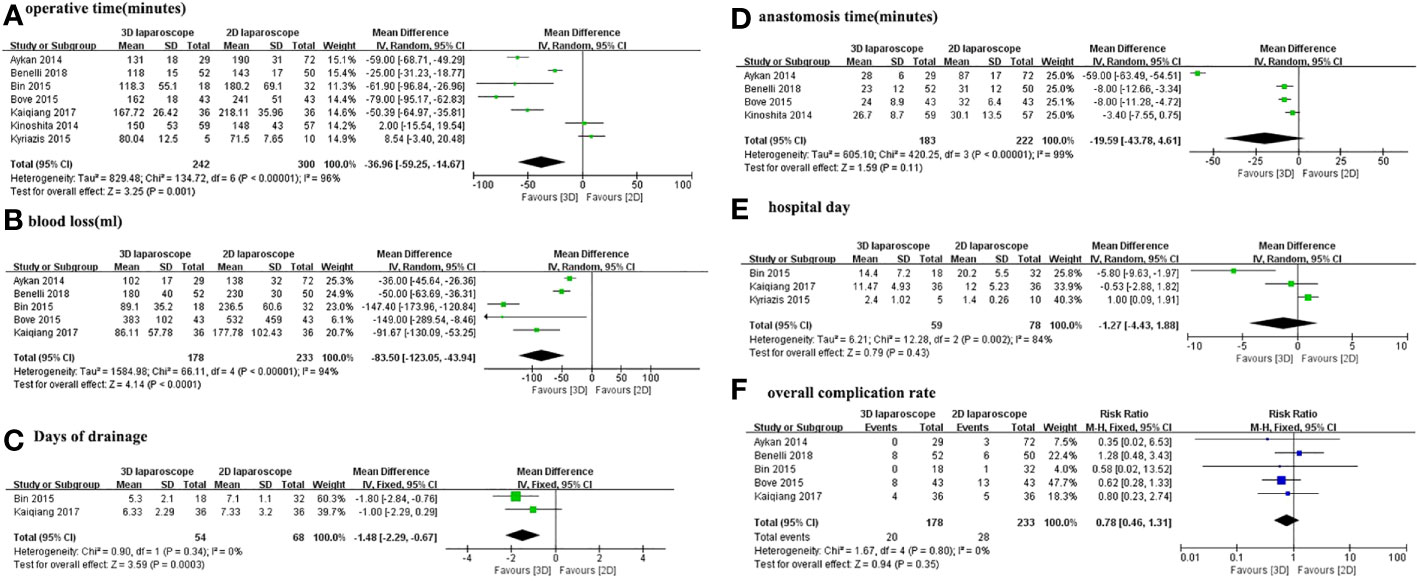
Figure 2 Forest plot comparing the perioperative outcomes of 3D and 2D LRP. (A) Operative time; (B) Anastomosis time; (C) Blood loss; (D) Hospital day; (E) Days of drainage; (F) Overall complication rate.
Moreover, the overall complication rates were 11.2% (20 out of 178 cases) for 3D LRP and 12% (28 of 233 cases) for 2D LRP, respectively. Pooled results from five studies showed no significant differences in the overall complication rates (RR 0.78; 95% CI 0.46 to 1.31; p=0.35) (Figure 2F).
The PSM and BCR-free rates for 3D and 2D LRP are presented in Table 3. Figure 3 depicts the pooled results of oncological outcomes. Meta-analysis demonstrates that there were no statistically significant differences between 3D and 2D LRP group concerning PSM and BCR-free at 3, 6, and 12months (Figure 3). Two studies used PSA>0.2 ng/ml to define BCR (14, 19) and two studies did not define the cutoff used (15, 16).
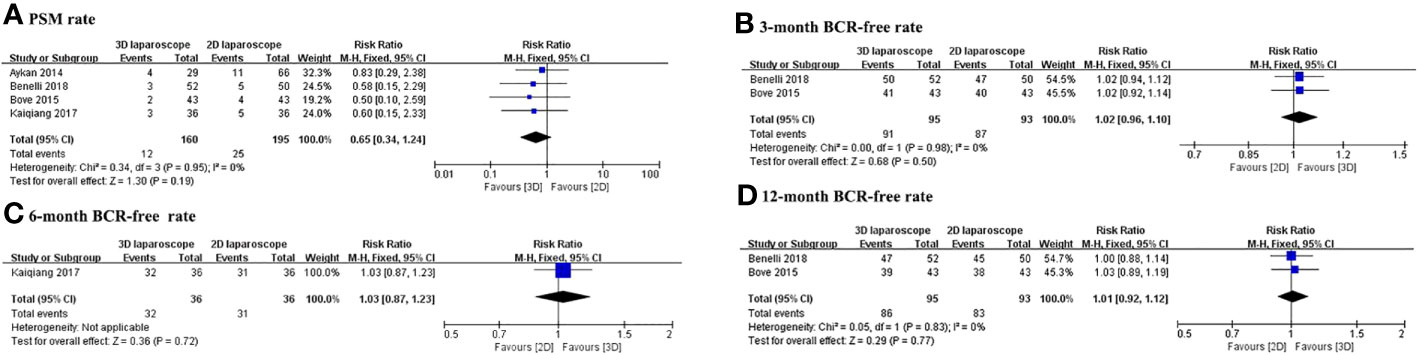
Figure 3 Forest plot comparing the oncologic outcomes of 3D and 2D LRP. (A) Positive surgical margin rate; BCR-free rate at 3 (B), 6 (C), and 12 (D) months.
The continence and potency recovery rates for 3D and 2D LRP are presented in Table 4. Figure 4 depicts the pooled results of functional outcomes. The overall urinary continence results at 3, 6, and 12 months were available from four studies with a pooled RR of 1.20 (95% CI 1.05 to 1.35; p=0.0007), 1.03 (95% CI 0.91 to 1.17; p=0.64), and 1.06 (95% CI 0.97 to 1.17; p=0.21), respectively. The urinary continence rate at 3 months was higher for 3D LRP than for 2D LRP (Figure 4A). No difference was found between two groups regarding continence rate at 6 and 12 months (Figures 4B, C). Two studies defined patient’s continence as no use of any pads (14, 15), and other studies defined it as the use of 1 pad or less per day (16, 19).
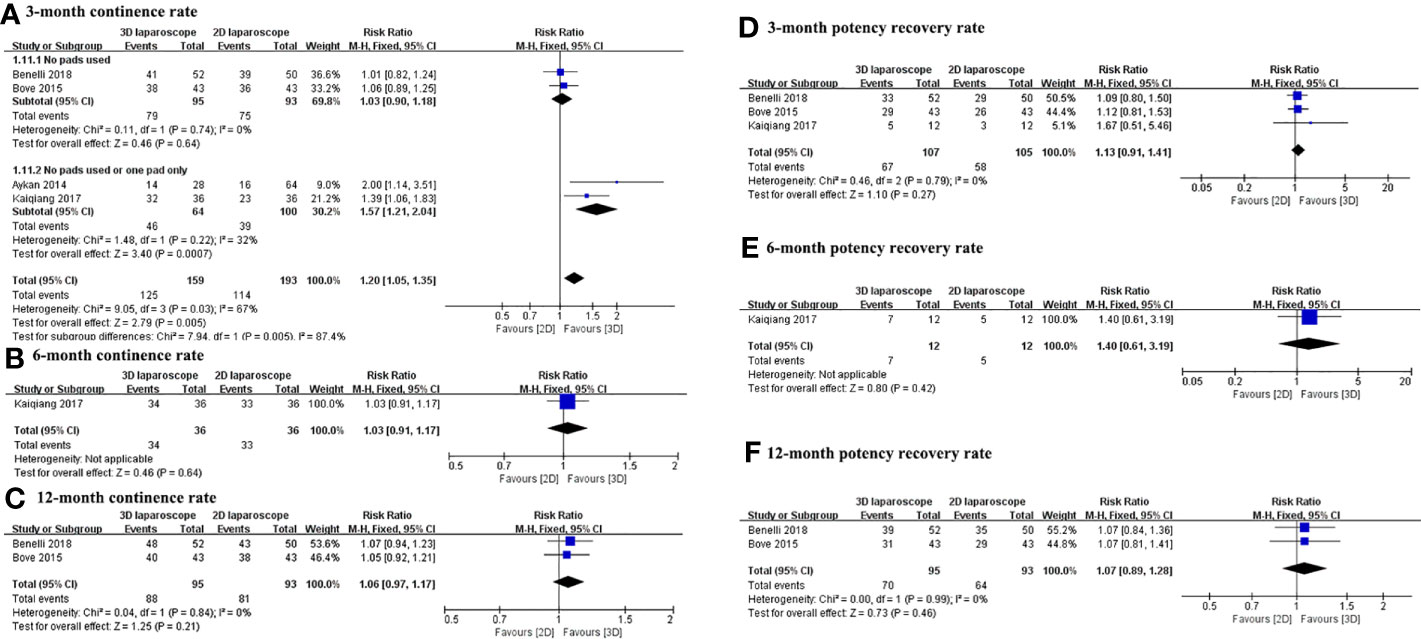
Figure 4 Forest plot comparing the functional outcomes of 3D and 2D LRP. Urinary continence rate at 3 (A), 6 (B), and 12 (C) months; potency recovery rate at 3 (D), 6 (E), and 12 (F) months.
There was no difference between groups in terms of potency recovery rate at 3, 6, and 12 months (Figures 4D-F). Three studies defined potency recovery as an IIEF-6 score≥17, IIEF-5 score≥16, and IIEF-5 score≥17, respectively (14, 15, 19).
Moderate to high heterogeneity among studies was found for most of perioperative outcomes. Low heterogeneity was found in oncological and functional outcomes. However, estimating low heterogeneity for these outcomes should be cautious, because von Hippel PT has proven that I2 has a substantial bias when the number of included studies is too small (20). Furthermore, although there was heterogeneity among the included studies, this is not surprising given differences in surgical technique, medical equipment, and ethnicity.
We were unable to assess publication bias because the testing ability was insufficient when there were 10 or fewer studies (21, 22).
With the assistance of 3D imaging systems, surgeons can perform complex laparoscopic surgeries with greater precision, flexibility, and effectiveness. Descazeaud et al. assumed that robotic-assisted radical prostatectomy (RARP) may become the standard technique for localized prostate cancer (23). However, there are regional differences in the use of robotic-assisted laparoscopic surgery compared to traditional laparoscopic surgery (24). In fact, RARP is only common in developed countries such as Europe and America, and most regions still rely on LRP as the primary surgical treatment for PCa. Due to the drawbacks such as inducing fatigue and damaging visual acuity in surgical practice, the adoption of 3D LRP has been slow in clinical settings. The latest advances in 3D laparoscopic technology have significantly improved the performance, precision, and hand-eye coordination of laparoscopic surgery, while providing greater depth perception during the surgical process and minimizing dizziness for surgeons (25, 26). Therefore, in recent years, there has been a resurgence in 3D laparoscopic surgery. According to a meta-analysis of general surgery procedures, it was determined that 3D laparoscopy provides superior surgical efficacy compared to 2D laparoscopy (27). To the best of our knowledge, no systematic reviews provide conclusive priority of 3D over 2D LRP in terms of their perioperative, oncologic, and functional outcomes. After considering all comparative studies, the present meta-analysis revealed that 3D LRP offers favorable outcomes compared with 2D LRP, including operative time, blood loss, days of drainage, and early continence. However, there was no conclusive evidence that 3D LRP was advantaged in terms of oncological and functional outcomes (except for continence rate at 3 months).
Our pooled analysis showed that operative time was lower in 3D LRP than those in 2D LRP. And no statistically significant difference was found between the two groups in terms of anastomosis time. A randomized comparative study indicated that 3D LRP may have limited advantages over 2D LRP only in terms of shortened operative time (13). However, in their study, Bove et al. (14), Benelli et al. (15), and Aykan et al (16) showed significantly shorter operative time and anastomosis time. Moreover, in Aykan et al.’s study, the variance in operation time and anastomosis time were almost perfectly aligned between the two groups (16). Authors speculated that the improvement in surgical duration could be primarily attributed to the facilitation of urethrovesical anastomosis. It was typically considered a most critical and time-consuming step in RP (28). With regard to blood loss, our research findings suggested that 3D LRP can significantly reduce blood loss. Lower blood loss may be attributed to magnified 3D view, which enables surgeons to better visualize vascular anatomy and perform more precise maneuvers (15, 16).
Hospital day was similar in 3D than 2D group. Due to variations in economic healthcare systems and healthcare insurance policies, hospitalization durations differ across different countries. In addition, the occurrence of postoperative complications, even mild ones, can significantly affect the length of hospital stay. A patient with urinary leakage due to anastomotic disruption caused by clot occlusion of an indwelling urinary catheter resulted in a hospitalization period approximately twice as long as other patients (17).
Concerning safety outcomes, there was no statistically significant difference between 3D and 2D group in terms of the overall complication rate. As is well known, postoperative complications are closely related to the safety of the surgery. Schmitges et al. compared the incidence of complications in minimally invasive radical prostatectomy between early (2001–2005) and late (2006–2007) study years and found that complication rates after surgery decreased over time (29). Authors analyzed that these observations may be associated with improved expertise and a higher proportion of sophisticate surgeons. Similarly, Bove et al. concluded that experience of the surgeon can also affect the complication rates to some extent (14).
Respecting oncological outcomes, PSM rate was similar between the two groups. In Bove et al.’s study (14), there was no significant difference in PSM rate between 3D and 2D LRP. On the contrary, when the cases were grouped by pathological stage, there was a significant difference in the rate of PSM occurrence between the two groups of patients with pT2c/pT3 disease. However, Huang et al. (30) conducted a meta-analysis that yielded results entirely contrary to those of Bove et al., despite the former’s comparison of RARP and LRP. It is undeniable that values of PSM rate in ≥pT3 tumors became higher than those in T2 tumors. Furthermore, PSM was also associated with surgeon’s experience, tumor stage, PSA level, and Gleason score.
With regard to BCR-free rates at 3, 6, and 12months, there was no statistically significant difference between 3D and 2D LRP group. PSM is associated with an increased likelihood of BCR and the need for adjuvant therapy (31). Yossepowitch et al. (32) reported that having positive margins is linked to a twofold increase in the risk of experiencing a biochemical relapse. In theory, tumors that are staged later should have a higher BCR rate owing to patients with a later stage of pathological staging have a higher PSM rate. Unfortunately, there is currently no study comparing BCR in pT2c and pT3 tumors in the included studies. Therefore, more studies are needed to clarify the relationship between BCR and tumor stage.
The recovery of urinary continence is the most important factor affecting the quality of life after radical prostatectomy (33), and it is also the most concerning issue for patients. The concept of continence is not always consistent. In one study defining continence as the use of 1 pad or less per day, Aykan et al. (16) concluded that 3D LRP was associated with higher early continence rates in comparison with 2D LRP. However, in another more recent RCT, Bove et al. (14) defined continence as no use of any pads and reported that no statistically significant difference was found in terms of overall continence rate. In our meta-analysis, the urinary continence rate at 3 months was higher for 3D LRP than for 2D LRP. The pooled RR for urinary continence rate at 6 and 12 months were 1.03 (95% CI 0.91 to 1.17; p=0.64) and 1.06 (95% CI 0.97 to 1.17; p=0.21), respectively. Although them did not reach a statistically significant difference, the trend is favorable to the 3D LRP group.
Similarly, there was no conclusive evidence that 3D LRP was advantaged in terms of the recovery of potency. However, the recovery of sexual dysfunctions after RP is a difficult outcome to evaluate and compare. Having a comprehensive discussion with the patients about preoperative erectile function, the actual incidence of postoperative erectile dysfunction (ED), and the concept of drug-assisted or spontaneous erection is a key issue in understanding ED prevention and promoting satisfactory erectile function recovery after RP (34). When comparing the functional outcomes of 2D and 3D LRP, it is necessary to pay attention to some key issues. First, we can come up with different outcomes when using different method to assess continence and potency. Second, the recovery of EF is influenced by many factors, including the patient’s age, preservation of sexual nerves, type of surgery, surgical techniques, and surgeon surgical skill (34). Third, collecting functional outcome data through a questionnaire survey may lead to errors in the study. Patients may not fully understand the questions or be unwilling to answer, which may result in inaccurate data. Overall, caution should be exercised when evaluating and comparing functional outcomes between 2D and 3D LRP.
This review has several limitations. First, this study was the lack of data on oncological and functional outcomes. The results showed that there were no statistically significant differences between 3D and 2D LRP group concerning oncologic and functional outcomes. However, these results were based on a limited number of studies and need to be confirmed in future studies. Second, studies included in this meta-analysis were retrospective or prospective cohort studies, which may have inherent biases. Third, the number of surgeons and their level of experience in surgery are not comparable between the included studies.
In conclusion, 3D LRP showed some advantages over 2D LRP in terms of perioperative outcomes, but there were no significant differences in oncologic and functional outcomes (except for continence rate at 3 months). However, due to the limited number and quality of the included studies, further studies are needed to validate these findings.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
TW had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: TW and HS. Acquisition of data: HS and XD. Analysis and interpretation of data: XD. Drafting of the manuscript: HS and TW. Critical revision of the manuscript for important intellectual content: HS, XD, and TW. Statistical analysis: HS. Supervision: TW. All authors contributed to the article and approved the submitted version.
This work was supported by the City of Nanchong Strategic Cooperation with the Local Universities Foundation of Technology (20SXQT0305, 18SXHZ0321), the Application and Basic Research Program of the Sichuan Science and Technology Department (2020YJ0185, 2022NSFSC0804), the Primary Health Development Research Center of Sichuan Province Program (SWFZ21-C-98), and the Medical Research Project of the Sichuan Medical Association (S21061).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Bhayani SB, Andriole GL. Three-dimensional (3d) vision: does it improve laparoscopic skills? An assessment of a 3d head-mounted visualization system. Rev Urol (2005) 7(4):211–4.
3. Schuessler WW, Schulam PG, Clayman RV, Kavoussi LR. Laparoscopic radical prostatectomy: initial short-term experience. Urology (1997) 50(6):854–7. doi: 10.1016/s0090-4295(97)00543-8
4. Mottet N, van den Bergh RCN, Briers E, Van den Broeck T, Cumberbatch MG, De Santis M, et al. Eau-Eanm-Estro-Esur-Siog guidelines on prostate cancer-2020 update. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol (2021) 79(2):243–62. doi: 10.1016/j.eururo.2020.09.042
5. Mueller MD, Camartin C, Dreher E, Hänggi W. Three-dimensional laparoscopy. Gadget or progress? A randomized trial on the efficacy of three-dimensional laparoscopy. Surg Endosc (1999) 13(5):469–72. doi: 10.1007/s004649901014
6. Volonté F, Pugin F, Bucher P, Sugimoto M, Ratib O, Morel P. Augmented reality and image overlay navigation with osirix in laparoscopic and robotic surgery: not only a matter of fashion. J Hepato Biliary Pancreatic Sci (2011) 18(4):506–9. doi: 10.1007/s00534-011-0385-6
7. Buchs NC, Volonte F, Pugin F, Toso C, Morel P. Three-dimensional laparoscopy: A step toward advanced surgical navigation. Surg Endosc (2013) 27(2):692–3. doi: 10.1007/s00464-012-2481-3
8. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The prisma 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical Res ed) (2021) 372:n71. doi: 10.1136/bmj.n71
9. Ottawa Hospital Research Institute. Coding manual for cohort studies (2021). Available at: http://www.ohri.ca/programs/clinical_epidemiology/nos_manual.pdf (Accessed Apr 06, 2023).
10. Ottawa Hospital Research Institute. Newcastle-Ottawa quality assessment scale cohort studies (2021). Available at: http://www.ohri.ca/programs/clinical_epidemiology/nosgen.pdf (Accessed Jan 03, 2022).
11. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controlled Clin Trials (1996) 17(1):1–12. doi: 10.1016/0197-2456(95)00134-4
12. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical Res ed) (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
13. Kinoshita H, Nakagawa K, Usui Y, Iwamura M, Ito A, Miyajima A, et al. High-definition resolution three-dimensional imaging systems in laparoscopic radical prostatectomy: randomized comparative study with high-definition resolution two-dimensional systems. Surg Endosc (2015) 29(8):2203–9. doi: 10.1007/s00464-014-3925-8
14. Bove P, Iacovelli V, Celestino F, De Carlo F, Vespasiani G, Finazzi Agrò E. 3d vs 2d laparoscopic radical prostatectomy in organ-confined prostate cancer: comparison of operative data and pentafecta rates: A single cohort study. BMC Urol (2015) 15(1):12. doi: 10.1186/s12894-015-0006-9
15. Benelli A, Varca V, Rosso M, Peraldo F, Gregori A. 3D versus 2D laparoscopic radical prostatectomy for organ confined prostate cancer: Our experience. J Clin Urol (2019) 12(3):186–191. doi: 10.1177/2051415818800536.
16. Aykan S, Singhal P, Nguyen DP, Yigit A, Tuken M, Yakut E, et al. Perioperative, pathologic, and early continence outcomes comparing three-dimensional and two-dimensional display systems for laparoscopic radical prostatectomy–a retrospective, single-surgeon study. J Endourol (2014) 28(5):539–43. doi: 10.1089/end.2013.0630
17. Kyriazis I, Özsoy M, Kallidonis P, Vasilas M, Panagopoulos V, Liatsikos E. Integrating three-dimensional vision in laparoscopy: the learning curve of an expert. J Endourol (2015) 29(6):657–60. doi: 10.1089/end.2014.0766
18. Xu B, Liu N, Jiang H, Chen SQ, Yang Y, Zhang XW, et al. [3d versus 2d laparoscopic radical prostatectomy for the treatment of prostate cancer]. Zhonghua nan ke xue = Natl J Androl (2015) 21(10):904–7.
19. Tang KQ, Pang SY, Bao JM, Lei CY, Tan WL. [Three-dimensional versus two-dimensional imaging systems in laparoscopic radical prostatectomy for prostate cancer: A retrospective cohort study]. Nan fang yi ke da xue xue bao = J South Med Univ (2017) 37(1):1–5.
20. von Hippel PT. The heterogeneity statistic I(2) can be biased in small meta-analyses. BMC Med Res Method (2015) 15:35. doi: 10.1186/s12874-015-0024-z
21. Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol (2000) 53(11):1119–29. doi: 10.1016/s0895-4356(00)00242-0
22. Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ (Clinical Res ed) (2006) 333(7568):597–600. doi: 10.1136/bmj.333.7568.597
23. Descazeaud A, Peyromaure M, Zerbib M. Will robotic surgery become the gold standard for radical prostatectomy? Eur Urol (2007) 51(1):9–11. doi: 10.1016/j.eururo.2006.10.007
24. Yu HY, Hevelone ND, Lipsitz SR, Kowalczyk KJ, Hu JC. Use, costs and comparative effectiveness of robotic assisted, laparoscopic and open urological surgery. J Urol (2012) 187(4):1392–8. doi: 10.1016/j.juro.2011.11.089
25. Kong SH, Oh BM, Yoon H, Ahn HS, Lee HJ, Chung SG, et al. Comparison of two- and three-dimensional camera systems in laparoscopic performance: A novel 3d system with one camera. Surg Endosc (2010) 24(5):1132–43. doi: 10.1007/s00464-009-0740-8
26. Sinha RY, Raje SR, Rao GA. Three-dimensional laparoscopy: principles and practice. J Minimal Access Surg (2017) 13(3):165–9. doi: 10.4103/0972-9941.181761
27. Cheng J, Gao J, Shuai X, Wang G, Tao K. Two-dimensional versus three-dimensional laparoscopy in surgical efficacy: A systematic review and meta-analysis. Oncotarget (2016) 7(43):70979–90. doi: 10.18632/oncotarget.10916
28. van Velthoven R, Ahlering TE, Skarecky DW, Huynh L, Clayman RV. Technique for laparoscopic running urethrovesical anastomosis: the single knot method. Urology (2020) 145:331–2. doi: 10.1016/j.urology.2020.04.026
29. Schmitges J, Trinh QD, Abdollah F, Sun M, Bianchi M, Budäus L, et al. A population-based analysis of temporal perioperative complication rates after minimally invasive radical prostatectomy. Eur Urol (2011) 60(3):564–71. doi: 10.1016/j.eururo.2011.06.036
30. Huang X, Wang L, Zheng X, Wang X. Comparison of perioperative, functional, and oncologic outcomes between standard laparoscopic and robotic-assisted radical prostatectomy: A systemic review and meta-analysis. Surg Endosc (2017) 31(3):1045–60. doi: 10.1007/s00464-016-5125-1
31. Meeks JJ, Eastham JA. Radical prostatectomy: positive surgical margins matter. Urol Oncol (2013) 31(7):974–9. doi: 10.1016/j.urolonc.2011.12.011
32. Yossepowitch O, Briganti A, Eastham JA, Epstein J, Graefen M, Montironi R, et al. Positive surgical margins after radical prostatectomy: A systematic review and contemporary update. Eur Urol (2014) 65(2):303–13. doi: 10.1016/j.eururo.2013.07.039
33. Sanda MG, Dunn RL, Michalski J, Sandler HM, Northouse L, Hembroff L, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. New Engl J Med (2008) 358(12):1250–61. doi: 10.1056/NEJMoa074311
Keywords: prostate cancer, radical prostatectomy, 3D laparoscopy, technology, meta-analysis
Citation: Shuai H, Duan X and Wu T (2023) Comparison of perioperative, oncologic, and functional outcomes between 3D and 2D laparoscopic radical prostatectomy: a systemic review and meta-analysis. Front. Oncol. 13:1249683. doi: 10.3389/fonc.2023.1249683
Received: 29 June 2023; Accepted: 05 September 2023;
Published: 19 September 2023.
Edited by:
Angelo Naselli, MultiMedica Holding SpA (IRCCS), ItalyReviewed by:
Giacomo Maria Pirola, IRCCS MultiMedica, ItalyCopyright © 2023 Shuai, Duan and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tao Wu, YWxoYXdraW5nQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.