
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol. , 22 December 2023
Sec. Breast Cancer
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1249160
A correction has been applied to this article in:
Corrigendum: Aromatase inhibitors: the journey from the state of the art to clinical open questions
 Daniele Generali1,2*
Daniele Generali1,2* Rossana Berardi3
Rossana Berardi3 Michele Caruso4
Michele Caruso4 Marina Cazzaniga5,6
Marina Cazzaniga5,6 Ornella Garrone7
Ornella Garrone7 Ida Minchella8
Ida Minchella8 Ida Paris9
Ida Paris9 Carmine Pinto10
Carmine Pinto10 Sabino De Placido11
Sabino De Placido11Breast cancer is a major cause of death among females. Great advances have been made in treating this disease, and aromatase inhibitors (AIs) have been recognized as the cornerstone. They are characterized by high efficacy and low toxicity. The authors reviewed the available literature and defined state-of-the-art AI management. This study was designed to assist clinicians in addressing the need to equally weigh patients’ needs and disease control rates in their everyday clinical practice. Today, AIs play a central role in the treatment of hormone receptor-positive breast cancer. In this study, an expert panel reviewed the literature on the use of AIs, discussing the evolution of their use in various aspects of breast cancer, from pre- and postmenopausal early breast cancer to metastatic breast cancer, along with their management regarding efficacy and toxicity. Given the brilliant results that have been achieved in improving survival in everyday clinical practice, clinicians need to address their concerns about therapy duration and the adverse effects they exert on bone health, the cardiovascular system, and metabolism. Currently, in addition to cancer treatment, patient engagement is crucial for improving adherence to therapy and supporting patients’ quality of life, especially in a selected subset of patients, such as those receiving an extended adjuvant or combination with targeted therapies. A description of modern technologies that contribute to this important goal is provided.
In 2018, GLOBOCAN showed that breast cancer accounted for approximately 2.1 million diagnoses and 630,000 deaths in 185 countries (1). In 2020, the same source reported a higher incidence of the disease, accounting for approximately 2.3 million diagnoses and 690,000 deaths worldwide (2).
A perfect balance between the various approaches should be achieved when treating breast cancer. Surgery, neoadjuvant and adjuvant chemotherapy, endocrine therapy, targeted therapy, and other methods are available in the therapeutic armamentarium of modern oncologists. Specifically, endocrine therapy with selective estrogen receptor (ER) modulators (SERMs), such as tamoxifen (TAM) and aromatase inhibitors (AIs), is currently central to the treatment of hormone receptor-positive breast cancer (3–6).
To describe the “state of the art” on using AIs, the panel searched Pubmed with the keywords “breast cancer, aromatase inhibitors, bone loss, cardiotoxicity, drug adherence.” The authors believe that it is mandatory to include meta-analyses, reviews, systematic reviews, and randomized controlled trials published in the last 15 years, focusing on the results achieved with the use of AIs with particular attention to their toxicity profiles and keeping an eye on the management of patients undergoing extended AI therapy.
The first report on the use of an antiestrogenic drug in patients with advanced breast cancer was published in 1971 (7). This approach was first approved in the United Kingdom (1973) and later in the United States (1977). TAM was, at that time, the first endocrine agent named SERM, which was able to function as an antagonist of the ER in breast cancers. Aminoglutethimide is a nonselective AI used in postmenopausal patients, which demonstrated the ability to achieve a 90% reduction in circulating estrogen levels. Its clinical use was approved in 1980; however, it did not show any difference regarding efficacy compared with TAM in randomized controlled trials (8–10). Second-generation AIs (such as formestane, fadrozole, and vorozole) were intended to overcome aminoglutethimide side effects, leading to the development of drugs such as 4-hydroxyandrostenedione; however, they still lack selectivity (11). The third and last generation of AIs includes both steroidal (exemestane) and nonsteroidal (anastrozole and letrozole) drugs, with greater specificity and fewer side effects (12).
The exemestane, letrozole, and anastrozole mechanisms of action reside in the inhibition of the aromatase enzyme, which can drive the conversion of androgens into estrogens through the “aromatization pathway.” In postmenopausal women, the breast tissue is enhanced by the intratumoral production of estrogens. The inhibition of estrogen conversion is one of the main ways to suppress breast cancer relapses (13).
The drugs are considered long-acting based on their pharmacokinetic dosing interval, as their half-life is estimated to last over 42 h in patients with breast cancer (13, 14). They block the CYP19A1 chain by inhibiting its active site, resulting in loss of electron transfer. This blockade prevents the conversion of androgens into estrogens (15). Moreover, this class of drugs has negligible effects on blood cortisol, aldosterone, and thyroxine blood levels (13). All are oral and have been approved by the regulatory agencies active in each country for treating breast cancer in routine clinical practice.
Historically, patients with ER-positive breast cancer treated with TAM for 5 years had a decreased risk of death by approximately half during pharmacological treatment. This risk increases to about one-third at 15 years (16). AIs impede the conversion of androgens to estrogens; therefore, they cannot be adopted in premenopausal women unless they are exposed to ovarian function suppression (OFS) (17). In contrast, in postmenopausal women, AIs reduce the serum estrogen levels, thereby inhibiting ER-positive breast cancer cell stimulation (Figure 1).
The literature offers various trials that compare AIs and TAM in adjuvant endocrine therapy (AET) (18–26). The Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) meta-analysis was released in 2015 to clarify the relative benefits of AIs vs. TAM and the outcomes of scheduling these two drugs differently during the 5 years of AET (27). The authors included data from nine trials with 35,129 females enrolled and randomized to AIs and TAM. During this meta-analysis, all the trials had already demonstrated the ability of AIs to lower recurrence rates when compared with TAM. Thus, this paper aims to establish AIs’ role in decreasing breast cancer and all-cause mortality. The authors observed that 5 years of endocrine therapy with AIs can lessen breast cancer relapses by about one-third during years 5 to 9, as does 5 years of TAM. Moreover, when comparing TAM given for 2 years followed by AIs with TAM given for 2–4 years, the relapse rates diminish significantly. The authors hypothesized that AIs’ superiority over TAM was greater when patients had previously been treated with TAM. The reduction in breast cancer mortality with AIs compared to TAM appeared to be slight but persistent during years 0–4 and 5–9. This paper’s key findings consist mainly of the proportional risk reduction of approximately 30% in recurrence observed in the AIs vs. TAM comparison period and the proportional risk reduction of about 15% in mortality rate reported in the first 10 years. A 5-year course of TAM treatment can lower disease relapses by approximately half in years 0–4 and by approximately one-third in the following 5 years. Moreover, it decreases mortality rate by about 30% during the first decade and beyond (16). AIs administered for 5 years in the absence of any endocrine therapy lower relapse rates by about two-thirds while patients are actively on treatment and by about one-third in the following 5 years and reduce the mortality rate by approximately 40% throughout the first decade and perhaps beyond. Moreover, all the trials commencing endocrine treatment with an AI showed, collectively, a highly significant drop of 30% in recurrence during years 0 to 1, confirming the superiority of AIs over TAM (28). AIs are effective for lowering breast cancer relapses even in premenopausal women undergoing OFS (29). The literature details various relevant clinical trials comparing AIs with TAM in premenopausal women treated with OFS or ablation (30–33), and more recently, the EBCTCG published a meta-analysis to obtain a better definition of the risks and benefits of AIs vs. TAM in this patient setting (34). Trials for this paper were eligible if they randomly assigned premenopausal women affected by ER-positive operable breast cancer to receive an AI plus OFS vs. TAM plus OFS. ER-negative/PR-positive women were excluded from the data analysis because of uncertainties surrounding endocrine therapy’s efficacy in these patients. The primary outcomes were any recurrence (locoregional, distant, or contralateral metastasis from new primary breast cancer), breast cancer mortality, recurrence-free death, and all-cause mortality. The incidence and site of the second primary tumor and bone fractures were the secondary outcomes. The authors found that there was a decreased rate of recurrence for women receiving AIs compared to those receiving TAM, and the main gain was observed in years 0–4 with a significant drop in the relapse rate; a loss of benefits in years 5–9 was also observed. The 5-year absolute risk of relapse was 3.2% lower in the group of patients undergoing AIs with a similar absolute difference in 10-year relapse rates (14.7% in the AIs group and 17.5% in the TAM group). Distant recurrence appeared to reduce when AIs were given; however, at a median follow-up time of 8 years, the authors noted no significant difference in all-cause and breast cancer mortality. When the authors considered only trials for premenopausal women, few non-breast cancer deaths occurred (0.9% in the AIs group vs. 0.7% in the TAM group), most of which were due to a second primary tumor. Regarding secondary outcomes, the authors found that AIs caused a higher rate of fractures than TAM (6.4% vs. 5.1%). Regarding toxicity from AIs and TAM, each trial showed that they were similar for premenopausal and postmenopausal women; those receiving AIs showed higher osteoporosis rates, and those assigned to TAM suffered from endometrial abnormalities more frequently.
In summary, the results of this meta-analysis suggest that when AIs are initiated instead of TAM in premenopausal women, in addition to OFS, the absolute recurrence risk can be decreased by 3% at 5 and 10 years. In postmenopausal women, approximately 70% of all breast cancers are hormone receptor-responsive and candidates for endocrine therapy (35).
The benefits of endocrine therapy with AIs versus TAM in this patient setting have been solidly demonstrated in the literature (28), and other attempts to confirm these findings have led to the publication of different trials focusing on the superiority of AIs over TAM. The BIG 1-98 trial (18, 35–40) is a four-arm, phase III, double-blind, randomized trial comparing adjuvant letrozole versus TAM administered for 5 years in postmenopausal patients. This study was designed to describe these two drug sequences (2 years of one treatment followed by 3 years of the other). In 2011, the median follow-up (8.1 years) showed that letrozole administered as monotherapy provided a significant benefit regarding disease-free survival (DFS), overall survival (OS), distant recurrence-free interval (DRFI), and breast cancer-free interval (BCFI) compared with monotherapy with TAM (18). More recently, the BIG 1-98 long-term follow-up study (BIG 1-98 LTFU), which was an extension of the BIG 1-98 trial, provided an update on annual survival, disease status, and long-term adverse effects in women enrolled in this trial at a median follow-up time of 12.6 years (35). The primary endpoint was DFS; in contrast, the other endpoints included OS, DRFI, and invasive BCFI. The authors also examined breast cancer mortality and provided split analyses for monotherapy comparisons (letrozole vs. TAM) and sequential therapy comparisons versus TAM monotherapy. Consistent conclusions can be drawn regarding adjuvant AI therapy in postmenopausal patients and their long follow-up periods. The authors found that the DFS showed a relative risk reduction of 9% in patients receiving letrozole; the same was reported in a 10-year analysis of anastrozole in the ATAC trial (41). Other endpoints, compared with the previous analysis, showed slightly decreased hazard ratios (HRs) in favor of the letrozole arm, which could be due to non-breast events at such a higher median population age. Moreover, for the monotherapy comparison, 25.2% of patients receiving TAM selectively crossed over to letrozole, and 39.5% of patients receiving TAM selectively crossed over for the four-arm comparison. This event could have contributed to the improved outcome found in patients assigned to receive TAM and attenuated the benefits of letrozole. The authors even stated that they could not find any determining difference between the study arms when looking at myocardial infarction rates, osteoporosis or fracture, and cerebrovascular events at the long-term follow-up. However, they found a reduced thromboembolic event rate in the TAM group. In summary, this study demonstrated that initial letrozole can offer continued and slightly attenuated benefits compared with TAM in postmenopausal women with hormone-responsive early breast cancer (35). Regarding patients with locally advanced and metastatic endocrine-responsive postmenopausal breast cancer, a meta-analysis of phase III randomized controlled trials comparing first-line endocrine therapy with third-generation AIs and TAM was reported in the literature. This study examined OS and addressed whether the progression-free survival (PFS) benefit of AI therapy results from a reduction in de novo resistance or a delay in acquired resistance to endocrine therapy. It included data from four large trials, all designed with TAM administered in the control arm and different AIs (exemestane, anastrozole, and letrozole) administered in the experimental arm. Every trial was reviewed for the clinical benefit rate, duration of clinical benefit (DoCB), PFS, and OS. The authors observed that AIs enabled more patients to achieve clinical benefits (CB) than TAM. The DoCB appeared slightly higher for AIs but did not significantly differ from TAM. In contrast, the PFS was statistically significantly different between the two groups in favor of AIs. Finally, even after excluding letrozole from the data, OS did not significantly differ between the two arms. The study concludes that, in the first-line setting, the choice of an AI instead of TAM has a significant clinical benefit as it increases the duration of tumor control by prolonging the PFS (12).
Despite the widely demonstrated success of adjuvant endocrine therapy given for 5 years, it is also recognized that hormone-responsive breast cancer can be linked to a prolonged recurrence risk after the first 5 years of therapy (42). More than 50% of relapses occur after the first 5 years of treatment (43). Recently, many clinical trials have focused on evaluating the optimal duration and efficacy of endocrine therapy (44–52). It has been demonstrated that 10 years of adjuvant TAM can lower both recurrence risk and mortality in patients with breast cancer over time, thanks to its major impact between the 9th and 10th years of follow-up (53, 54). However, there has been extensive debate in the scientific community regarding using AIs as an extended therapy, and their optimal duration has not yet been established (55, 56). Clinical trials in recent years led to the publication of conflicting data and non-definitive conclusions. Specifically, trials such as MA.17, MA.17R, and 6a demonstrated a DFS benefit (45, 56, 57), with a particular improvement in OS in the MA.17 trial, in patients diagnosed with node-positive diseases and in those previously treated with TAM for 5 years. However, these results were not fully confirmed by the latest published randomized trials (47, 49, 51). Among these studies, only MA.17 was able to show that both DFS and OS improved in a statistically significant manner, especially in high-risk patients (those with larger tumors and/or positive nodes). The efficacy and toxicity of extended AI therapy in patients with hormone receptor-positive breast cancer have also been investigated in two subsequently published meta-analyses (58, 59). The first analysis (58) included eight studies (45, 47–51, 56, 57) in which endocrine therapy was employed: letrozole was investigated in four studies, anastrozole in three, and exemestane in one. Most of these studies evaluated the effects of extending AI therapy for up to 5 years (Tables 1, 2). The authors found improved DFS at a median follow-up time of 64.1 months, and major benefits were observed in patients with positive nodes. In addition to this increase in DFS rates, extending the therapy with AIs did not show any benefit regarding OS. This meta-analysis revealed a general increase of 22% in DFS, and this benefit appeared to be greater in patients with node positivity. The authors’ statement seems to be in accordance with the MA.17 results and with the DATA trial (49) post-hoc subgroup analysis, which again demonstrated that patients with node positivity benefit from an extension of AI therapy. Moreover, although the authors failed to find a significant improvement in OS, all trials included in the study showed a significant decrease in recurrence risk, a benefit in the breast cancer-free interval, and a 28% reduction in the cumulative risk of developing distant relapses (58). The second meta-analysis (59) included eight clinical trials (47, 49, 51, 52, 56, 57, 60, 61), most of which were the same as those previously discussed but focused on local and distant recurrence, contralateral tumors, non-breast cancer-related death, and toxicity. Most studies have evaluated the effects of extending AI therapy in patients who have already completed 5-year endocrine AI regimens. The data on DFS and OS were the same as those used in a previous meta-analysis. The pooled analysis demonstrated that the risk of death from any cause and non-breast cancer-related causes did not decrease when the AI was extended. Local and distant recurrences did not improve significantly. With regard to toxicity, the study reported an increased rate of osteoporosis, bone pain, bone fractures, hypertension, cardiovascular events, arthralgia, and myalgia, which are recognized as typical of this drug class. The authors of this meta-analysis concluded that extended AI therapy in postmenopausal women can provide better outcomes for recurrence rate; in contrast, the toxicity rates suggest that it should be chosen based on patient and disease characteristics. Moreover, owing to the carryover effect of endocrine therapy, which produces an absolute survival benefit that increases over time and becomes significant, especially after 10 years, it is clear that the follow-up period should be adequate to evaluate the real benefit of extended adjuvant endocrine therapy (59). Another matter of debate is the optimal duration of extended endocrine therapy for breast cancer. A meta-analysis (42) examined the optimal duration of extended AI therapy in postmenopausal patients who had already completed their 5-year regimens. The authors classified the included studies according to the total endocrine therapy extension time, assigning nine randomized controlled trials to three classes. The first class grouped the patients according to 10 years vs. 5 years of therapy (AERAS, MA.17, NSABP B-42, and NSABP B-33). The second group included studies comparing 7 to 8 years and 5 years of treatment (ABCSG 6a, DATA, and GIM4 LEAD). The last class grouped trials examined 10 years vs. 7–8 years of therapy (ABCSG-16 and IDEAL). This central meta-analysis demonstrated that DFS improved when endocrine therapy was extended, especially when the extension time frames were 5–10 and 5 –7 to 8 years. However, when prolonging therapy from 7 to 8 years to 10 years, the DFS did not improve. Regarding OS, the results showed that extended therapy was not associated with a lower risk of death from any cause, regardless of the time frame of the extension. When drugs are prolonged from 5 to 10 years, the risk of bone fracture, osteopenia/osteoporosis, bone pain, myalgia, joint stiffness, and alopecia increases. The following conclusions can be drawn from the subgroup analysis. Patients affected by node-positive, hormone receptor-positive, or tumors > 2 cm in size treated only with TAM or sequential TAM-AIs for 5 years may significantly benefit from the extension of endocrine therapy as soon as they are at a higher risk of recurrence (62). The extension of AI therapy in such patients for 2 years, rather than 5 years, seems to be the right choice to maximize the benefits without increasing the toxicity (63).
Cancer cells frequently present with cell cycle abnormalities, considered potential therapeutic targets. Cyclin-dependent kinases (CDKs) are cell cycle transition and cell division-governing regulatory enzymes that involve the tumor suppressor retinoblastoma (Rb) protein as a regulator of cellular proliferation. The CDK4/6-Rb pathway is often present in ER-positive breast cancer, as estrogens promote the evolution from the G1 to the S phase. This pathway is considered a key mediator of endocrine resistance (64). The binding mechanism between estrogen and its alpha receptor promotes cyclin D1 transcription, CDK4/6 activation, and Rb phosphorylation, immediately leading to cell cycling. Targeting and inhibiting CDK4/6 causes the cell to stop its cycle in the G1 phase, resulting in lower cell viability (65). Palbociclib, ribociclib, and abemaciclib represent the current armamentarium for developing highly selective oral CDK4/6 inhibitors. Various clinical trials performed in both metastatic breast cancer (mBC) and early breast cancer (eBC) patient settings (66–81) have extensively investigated these drugs, showing an impressive impact on outcomes (82). The combined use of CDK4/6 inhibitors and adjuvant endocrine therapy represents the most significant advancement in managing both advanced mBC and eBC, and its development has dramatically changed the therapeutic scenario for this disease. Patients can be exposed to this class of drugs for a long time, and the median PFS is approximately 28 months. Drug selection is an important aspect in this patient setting (83). The literature includes systematic reviews and meta-analyses that explore and compare the toxicity and tolerability profiles of palbociclib, ribociclib, and abemaciclib toxicity and tolerability profiles (83–85). When choosing one drug over another in everyday clinical practice, the right decision should be tailored to every patient and driven by comparative toxicity studies. A recent meta-analysis published in 2020 compared ribociclib and abemaciclib to palbociclib and quantified the treatment-related side effects for each endocrine therapy regimen (AIs or fulvestrant). The analysis revealed that ribociclib presented higher grade 3 to 4 nausea and vomiting than palbociclib but a non-significant lower grade 3 to 4 hematological toxicity, independent of the underlying endocrine therapy. Abemaciclib demonstrated higher grade 1v2 and 3 to 4 diarrhea and grade 1 to 2 nausea and vomiting, with lower grade 3 to 4 neutropenia than palbociclib. When combined with AIs, abemaciclib resulted in a lower percentage of grade 3–4 fatigue than palbociclib. Treatment discontinuation was similar for palbociclib and ribociclib; however, the rate was higher in patients who received abemaciclib. Moreover, the association rate between grade 3 to 4 diarrhea and abemaciclib and palbociclib was higher in patients receiving fulvestrant than in those receiving AIs. The authors stated that the differences in inhibitor-specific toxicity could be explained by the pharmacological features of the three drugs. Abemaciclib had the highest activity against CDK4 and CDK6; in contrast, palbociclib and ribociclib had similar potencies against CDK4 and CDK6. However, ribociclib is usually associated with higher levels of unbound blood. Abemaciclib more specifically binds CDK4; on the other hand, palbociclib has an equivalent specificity. If CDK6 appears to be the predominant CDK in hematopoietic cells, abemaciclib may cause a lower rate of neutropenia because of its favorable hematopoietic inhibition. Moreover, its action against CDK7 and CDK4 may partially explain its increased intestinal toxicity.
The authors conclude that it is likely that the “modulable” inhibition of CDK4 and CDK6 in the three drugs is more associated with their toxicity than their efficacy (83). Little is known about AI-induced musculoskeletal symptoms (AIMSS), one of the most detrimental adverse effects experienced by patients receiving endocrine therapy. In a recent review, the authors summarized all available data on this adverse effect analyzed in randomized phase III trials evaluating AI monotherapy or combined with CDK4/6 inhibitors in patients with metastasis. They focused on the additive influence of CDK4/6 inhibitors on AIMSS and found that the incidence of arthralgia decreased in patients who received combined therapy. Myalgia was also reduced by the addition of CDK4/6 inhibitors, which was consistent with the results observed for arthralgia. Regarding bone pain, the combination therapy resulted in a 2.9%–8.5% rate of incidence compared to 7%–32.9% reported for patients receiving AIs as monotherapy. Palbociclib presented with bone pain more commonly than ribociclib or abemaciclib. Overall, patients with metastases receiving CDK4/6 inhibitors had fewer adverse events than those receiving AI monotherapy. The authors also examined the back pain rate and observed that it manifested mostly in patients treated with AI monotherapy compared to those receiving combination therapy. Data from this review suggest that combining CDK4/6 inhibitors with a pre-existing AI therapy regimen tends to lessen the incidence of AIMSS; however, more data, both in adjuvant and metastatic settings, are required to clarify these findings (85). The literature demonstrates that besides the proven efficacy of the association between AIs and CDK4/6 inhibitors, these drugs have a safe toxicity profile that allows their wide use in patients with eBC and mBC. The literature can support everyday clinical practice and the need to choose one drug over another; however, further data to support the day-to-day decision-making processes is required.
As indicated above, AIs are a cornerstone for both pre- and postmenopausal women with hormone receptor-positive breast cancers. During adjuvant endocrine therapy, no significant decrease in health-related quality of life has been reported in several large-scale trials (86–88). Despite these data, early disruption is frequent in different research and clinical practice settings, ranging from 30% to 70% (89). The reasons for the discontinuation of endocrine therapy are usually multifactorial. The data suggest that up to 30% of patients do not adhere to AI therapy because of its adverse effects. Multifactorial barriers hampering correct adherence are usually associated with patient-, physician-, medication-, and system-related variables, and all of these factors are usually variously combined in women with a growing number of breast cancer survivors. Adherence to endocrine therapy is fundamental, as it can improve patient outcomes and survival curves (89, 90). Patients who disrupt their AI treatment early are exposed to an increased risk of all-cause mortality, cancer death, and recurrence because they do not fully receive the intended therapy-related benefits (91). Most women who fail to adhere to their endocrine treatment discontinue therapy during the first 6 months because the most severe toxicities related to AIs tend to occur within this period (92–94) (Table 3).
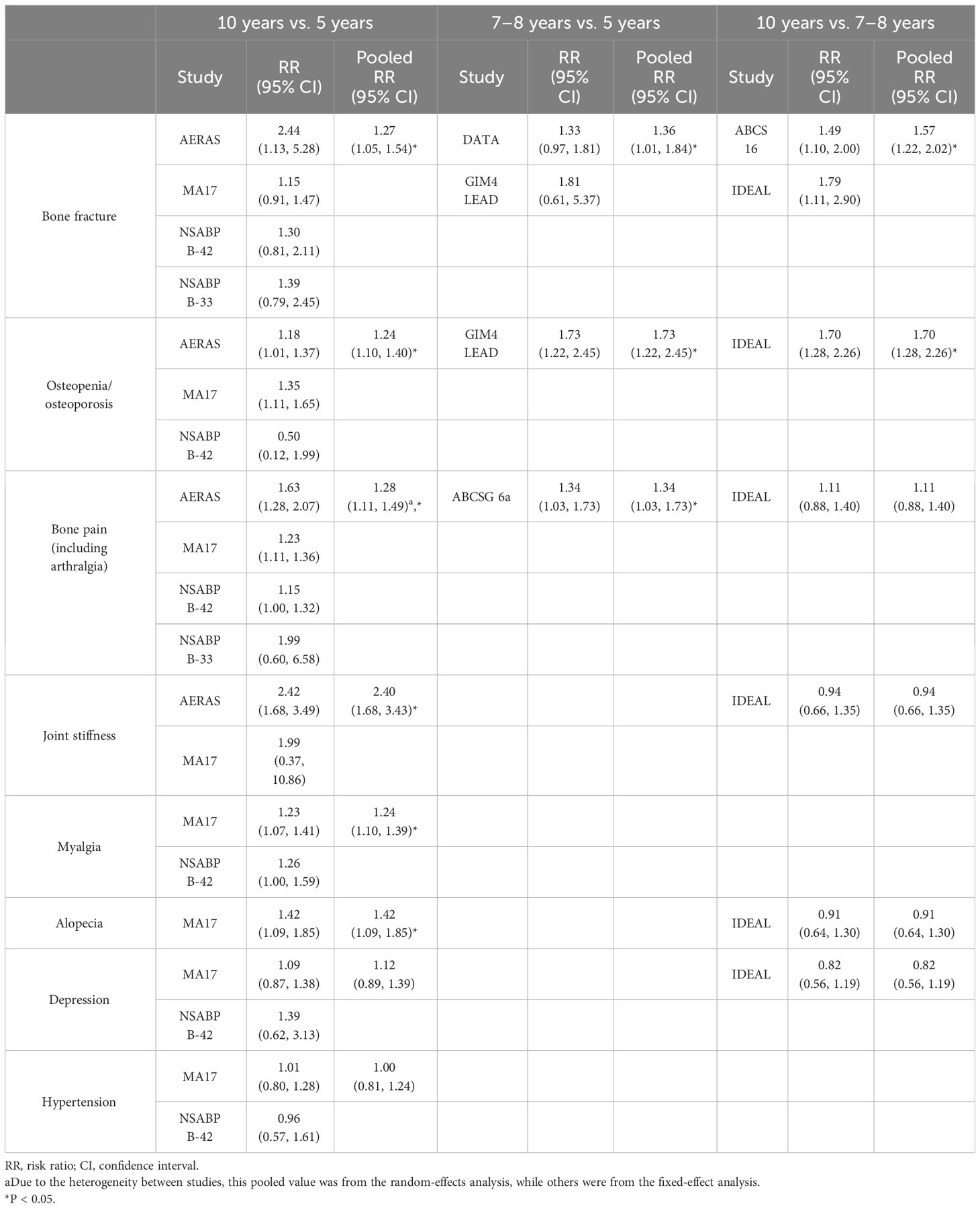
Table 3 Common adverse events in patients undergoing therapy with aromatase inhibitors in an extended and non-extended setting and their incidence pooled risk ratio.
Concerning endocrine therapy, one of the most significant adverse effects that play a fundamental role in the quality of life of this group of patients is bone loss. As soon as the fracture risk increases, a practical approach is mandatory to avoid worsening bone quality and quantity, which leads to fragility. Endocrine therapy works by directly or indirectly removing the effect of estradiol on the breast tissue. The same effect is exerted on the bone tissue, leading to bone loss (95, 96). A position paper published in 2017 by seven international bone and cancer societies thoroughly examined AI-induced bone loss (AIBL) (97). This produced an algorithm for AIBL management based on clinical risk factors and bone mineral density (BMD). More recently, interesting meta-analyses on bone health in patients treated with endocrine therapy clarified the state of the art of the issue. In a review by Waqas et al. (98), data published in 2017 on the relationship between AI therapy and bone health were analyzed. The authors included novel fracture risk assessment tools, such as the trabecular bone score and vertebral fracture assessment (VFA), to update the clinical management strategy defined in a position paper published in 2017. They even attempted to address bone loss and fracture risk in premenopausal women. The authors suggest that all women receiving AIs and/or OFS should undergo dual-energy X-ray absorptiometry scan (DEXA) and biochemical testing with a meticulous medical history and physical examination to rule out further secondary factors capable of causing osteoporosis. VFA, together with DEXA, could be part of the screening or follow-up for all postmenopausal and osteopenic premenopausal patients on endocrine therapy. The authors even advise against using the conventional fracture risk assessment tool, FRAX, as its role is still not well established and validated in women under 40, and it can underestimate the fracture risk. They stated that physicians should stress the role of physical exercise in women undergoing endocrine therapy, along with an adequate intake of calcium and vitamin D. According to the current ESMO guidelines, every woman starting AIs and/or OFS should undergo a fracture risk assessment using conventional risk factors and BMD measurement with DEXA and VFA. All women presenting a BMD score of 2.0 SD or more than two risk factors (prior fragility fractures, parental history of hip fracture, diabetes, BMI 20 kg/m2, rheumatoid arthritis, recurrent falls, use of glucocorticoids for more than 3 months, current smoking, and alcohol consumption) should be offered anti-resorptive therapy. Regarding follow-up, when no antiresorptive therapy has started, DEXA should be repeated yearly after AI initiation and every 2 years in patients on antiresorptive medication. The recommended antiresorptive therapy extension should be based on the extension of endocrine therapy and specific absolute fracture risk. When extended adjuvant endocrine therapy is considered, it may be necessary to consider whether or not to start anti-resorptive drugs based on an overall evaluation of individualized risk-to-benefit ratios and potential side effects. It can be affirmed that as breast cancer develops into a chronic disease, bone loss and fracture risk in patients receiving endocrine therapy will require special attention. Cardiotoxicity is a major concern in the prescription of AIs. Several adjuvant randomized controlled trials comparing AIs with TAM have shown that the risk of developing a cardiovascular disease increases with AIs (99–101). In contrast, previous clinical studies have indicated a favorable cardiovascular effect of TAM, specifically in reducing cholesterol and low-density lipoprotein blood levels and increasing high-density lipoprotein (101–106). Therefore, the AI-associated increased risk of cardiovascular events could be interpreted in light of the cardioprotective effects of TAM. An interesting systematic review and meta-analysis was published in 2017 to determine whether AI therapy is associated with a higher risk of cardiovascular events. This study included 19 randomized clinical trials. The authors stated that, given the fact that TAM is associated with a 33% decrease in the risk of cardiovascular events in all trials, including those comparing TAM to placebo or no treatment, it could be concluded that the cardioprotective effect of TAM explains the increased risk observed in randomized controlled trials comparing AIs to TAM. MA.17 and MA.17R supported this finding in an extended adjuvant setting; the authors declared no association between AIs and cardiovascular events or ischemic heart disease (45, 56).
In recent years, several attempts have been made to enhance adherent patient behavior regarding endocrine treatments. Constantly improving and using user-friendly technology can certainly help patients and physicians maintain contact with each other. Moreover, because it is easy to use and widespread, a technology that enables a quick response could be helpful for clinicians in ameliorating symptoms and obtaining fast answers.
Basic and advanced technologies have been used in studies and trials designed to improve adherence to endocrine therapy.
In 2018, Graetz et al. (107) conducted a pilot randomized controlled trial to analyze the use, feasibility, and short-term effects of a web-based application specifically released for patients with breast cancer.
The app communicated the onset of adverse symptoms and AI adherence through built-in alerts sent to the patient’s care team. Using their own enabled devices (smartphones and computers), patients can share real-time health information with their team of oncologists. This study found that this app, which provides reminders for real-time reporting of AI adherence and treatment-related side effects, was feasible and improved short-term adherence. The use of the app decreased when it worked without weekly reminders, and this finding was particularly frequent among black participants, younger women, and those with lower literacy levels. Adherence interventions are necessary for this sector of the population. Moreover, in patients using the app with weekly reminders, the authors reported a smaller increase in symptom burden, suggesting that they may have received adequate management due to the app-based real-time reports. Finally, from the clinicians’ point of view, the staff reported that participating in the initiative had a minimal impact on the daily workflow, suggesting that this patient-centered way of working could be widely implemented without heavily impacting the everyday burden of clinical work.
Two other randomized controlled trials (108, 109) investigated the feasibility of employing simple text messaging technology to limit the early discontinuation of breast cancer endocrine therapy. The first study found that a biweekly unidirectional message sent to patients undergoing endocrine treatment for 36 months did not affect adherence compared with usual care. This finding was perhaps because the project did not actively engage patients and was insufficient to produce a behavioral change (108).
Another randomized trial used a mobile phone text message reminder system (SMS) to achieve two primary objectives: to evaluate whether SMS ameliorates drug adherence compared to standard care in patients undergoing AI therapy and to assess whether SMS actively affects the blood levels of androstenedione, estradiol, and estrone. The authors found that, at 6 months, the percentage of adherence in the SMS group was higher (72,4%) than that in the standard care group (59.5%); however, at 1 year, the value for the SMS group was not significantly higher than that in the standard care group (68.9% and 65.8%, respectively). Moreover, they observed that the androstenedione blood level measured at 1 year was comparable in both groups, and the estrone level was higher in the SMS group; however, this difference was not statistically significant. Estradiol levels were not significantly lower in the SMS group than in the standard care group. They concluded that even though the SMS reminder system appeared to have high acceptability among patients, it showed only a significant short-term effect in improving medication adherence (109).
Further research needs to be conducted on this topic, focusing on how technology can help clinicians and patients manage their adherence to endocrine therapy, thereby paving the way for better and longer therapeutic responses. On the other side, it is important to prevent endocrine overtreatment especially in older patients. An interesting genomic approach has been reported to select the ultralow-risk 70-gene signature patients as candidates for treatment de-escalation (110).
Currently, AIs are the cornerstone in the management of breast cancer in every patient setting (pre- and postmenopausal, eBC, and mBC). Their use is widespread, as the literature has fully demonstrated their efficacy in improving survival and their low toxicity profile. Adverse effects on bone health, the cardiovascular system, and metabolism can be easily handled with dedicated network paths in which patients feel completely managed by experts and are globally followed up on every aspect of their disease. Greater efforts should be made to improve adherence to endocrine therapy, especially in a selected subset of patients undergoing extended adjuvant therapy because of the high risk of relapse after 5 years. Modern technology can help physicians; however, reaching the optimal point remains a long way off. The more patients follow endocrine therapy, the more we can achieve longer and better responses.
Conceptualization, DG; writing, original draft preparation, Ethos srl.; writing, review, and editing, Ethos srl; supervision, DG. All the authors have read, reviewed, and agreed to the published version of the manuscript.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Editorial assistance was provided by Ethos through an unconditional grant by Accord. Accord had no role in the drafting and review of the manuscript.
The authors thank Ethos srl for editorial support and Barbara Tolu for medical writing assistance.
DG declares grants and/or presentations at congresses and/or participation in scientific boards organized by Novartis, Pfizer, Lilly, Istituto Gentili, Accord, Roche. RB declares grants and/or presentations at congresses and/or participation in scientific boards organized by AZ, BI, Novartis, MSD, Otsuka, Lilly, Roche, Amgen, GSK, Eisai. OG declares advisory role for Eisai, MSD, Seagen, Gilead and honoraria from Pierre Fabre, Novartis, Eli-Lilly IP has received grants for presentations at congresses and participation in scientific boards from: Novartis, Lilly, Pfizer, Gentili, Seagen, Eisai, Astra Zeneca, Gilead, Pierre Fabre. SP has received personal fees presentations at congresses and/or participation in scientific boards/speaker/meetings from Novartis, Roche, Bristol Myers Squibb, AstraZeneca, Pfizer, Lilly, Eisai, Seagen, Daiichi Sankyo, MSD, Exact Sciences, Gilead.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AET, adjuvant endocrine therapy; AI, aromatase inhibitors; AIBL, aromatase inhibitors-induced bone loss; AIMSS, aromatase inhibitor-induced musculoskeletal symptoms; BCFI, breast cancer-free interval; BMD, bone mineral density; CB, clinical benefit; CBR, clinical benefit rate; CDK, cyclin-dependent kinase; DEXA, dual energy X-ray bone absorptiometry scan; DFS, disease-free survival; DoCB, duration of clinical benefit; eBC, early breast cancer; EBCTCG, Early Breast Cancer Trialists’ Collaborative Group; ER, estrogen receptor; HDL, high density lipoprotein; HRs, hazard ratios; HRQoL, health-related quality of life; LDL, low density lipoprotein; LTFU, long-term follow-up; mBC, metastatic breast cancer; OFS, ovarian function suppression; OS, overall survival; Rb, retinoblastoma; SERMs, selective estrogen receptor modulators; TAM, tamoxifen; TBS, trabecular bone score; VFA, vertebral fracture assessment.
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68:394–424. doi: 10.3322/caac.21492
2. Arnold M, Morgan E, Rumgay H, Mafra A, Singh D, Laversanne M, et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast (2022) 66:15–23. doi: 10.1016/j.breast.2022.08.010
3. Yu KD, Wu J, Shen ZZ, Shao ZM. Hazard of breast cancer-specific mortality among women with estrogen receptor-positive breast cancer after five years from diagnosis: implication for extended endocrine therapy. J Clin Endocrinol Metab (2012) 97:E2201–9. doi: 10.1210/jc.2012-2423
4. Petrelli F, Coinu A, Cabiddu M, Ghilardi M, Lonati V, Barni S. Five or more years of adjuvant endocrine therapy in breast cancer: a meta-analysis of published randomised trials. Breast Cancer Res Treat (2013) 140:233–40. doi: 10.1007/s10549-013-2629-4
5. Burstein HJ, Temin S, Anderson H, Buchholz T, Davidson NE, Jelmon KE, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American society of clinical oncology clinical practice guideline focused update. J Clin Oncol (2014) 32:2255–69. doi: 10.1200/JCO.2013.54.2258
6. Kennecke HF, Olivotto IA, Speers C, Norris B, Chia SK, Bryce C, et al. Late risk of relapse and mortality among postmenopausal women with estrogen responsive early breast cancer after 5 years of tamoxifen. Ann Oncol (2007) 18:45–51. doi: 10.1093/annonc/mdl334
7. Cole MP, Jones CT, Todd ID. A new anti-oestrogenic agent in late breast cancer. An early clinical appraisal of ICI46474. Br J Canc (1971) 25:270–5. doi: 10.1038/bjc.1971.33
8. Smith IE, Harris AL, Morgan M, Ford HT, Gazet JC, Harmer CL, et al. Tamoxifen versus aminoglutethimide in advanced breast carcinoma: a randomized cross-over trial. Br Med J (Clin Res Ed) (1981) 283:1432–4. doi: 10.1136/bmj.283.6304.1432
9. Lipton A, Harvey HA, Santen RJ, Boucher A, White D, Bernath A, et al. A randomized trial of aminoglutethimide versus tamoxifen in metastatic breast cancer. Cancer (1982) 50:2265–8. doi: 10.1002/1097-0142(19821201)50:11<2265::AID-CNCR2820501106>3.0.CO;2-#
10. Gale KE, Andersen JW, Tormey DC, Mansour EG, Davis TE, Horton J, et al. Hormonal treatment for metastatic breast cancer: an Eastern Cooperative Oncology Group phase III trial comparing aminoglutethimide to tamoxifen. Cancer (1994) 73:354–61. doi: 10.1002/1097-0142(19940115)73:2<354::AID-CNCR2820730220>3.0.CO;2-J
11. Bajetta E, Zilembo N, Buzzoni R, Noberasco C, Celio L, Bighisao E. Efficacy and tolerability of 4-hydroxyandrostenedione (4-OHA) as first-line treatment in post-menopausal patients with breast cancer after adjuvant therapy. Canc Treat Rev (1993) 19:31–6. doi: 10.1016/0305-7372(93)90005-C
12. Robertson JFR, Paridaens RJ, Lichfield J, Bradbury I, Campbell C. Meta-analyses of phase 3 randomised controlled trials of third generation aromatase inhibitors versus tamoxifen as first-line endocrine therapy in postmenopausal women with hormone receptor-positive advanced breast cancer. Eur J Cancer (2021) 145:19–28. doi: 10.1016/j.ejca.2020.11.038
13. Bhatnagar AS. The discovery and mechanism of action of letrozole. Breast Cancer Res Treat (2007) 105 Suppl 1:7–17. doi: 10.1007/s10549-007-9696-3
14. Schieweck K, Bhatnagar AS, Batzl C, Lang M. Anti-tumor and endocrine effects of non-steroidal aromatase inhibitors on estrogen-dependent rat mammary tumors. J Steroid Biochem Mol Biol (1993) 44(4-6):633–6. doi: 10.1016/0960-0760(93)90270-7
15. Nabholtz JM. Long-term safety of aromatase inhibitors in the treatment of breast cancer. Ther Clin Risk Manage (2008) 4(1):189–204. doi: 10.2147/TCRM.S1566
16. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet (2011) 378:771–84. doi: 10.1016/S0140-6736(11)60993-8
17. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Ovarian ablation in early breast cancer: overview of the randomised trials. Lancet (1996) 348:1189–96. doi: 10.1016/S0140-6736(96)05023-4
18. Regan MM, Neven P, Giobbie-Hurder A, Goldhirsch A, Ejlersten B, Mauriac L, et al. Assessment of letrozole and tamoxifen alone and in sequence for postmenopausal women with steroid hormone receptor-positive breast cancer: the BIG 1-98 randomized clinical trial at 8.1 years median follow-up. Lancet Oncol (2011) 12:1101–08. doi: 10.1016/S1470-2045(11)70270-4
19. Dubsky PC, Jakesz R, Mlineritsch B, Postlberger S, Samonigg H, Kwasny W, et al. Tamoxifen and anastrozole as a sequencing strategy: a randomized controlled trial in postmenopausal patients with endocrine-responsive early breast cancer from the Austrian breast and colorectal cancer study group. J Clin Oncol (2012) 30:722–28. doi: 10.1200/JCO.2011.36.8993
20. van de Velde CJH, Rea D, Seynaeve C, Putter H, Hasemburg A, Vannetzel JM, et al. Adjuvant tamoxifen and exemestane in early breast cancer (TEAM): a randomised phase 3trial. Lancet (2011) 377:321–31. doi: 10.1016/S0140-6736(10)62312-4
21. Coombes RC, Hall E, Gibson LJ, Paridaens R, Jassem J, Delozier T, et al. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med (2004) 350:1081–92. doi: 10.1056/NEJMoa040331
22. The ATAC (Arimidex, Tamoxifen Alone or in Combination) Trialists’ Group. Anastrozole alone or in combination with tamoxifen vs tamoxifen alone for adjuvant treatment of postmenopausal women with early-stage breast cancer. Cancer (2003) 98:1802–10. doi: 10.1002/cncr.11745
23. Kaufmann M, Jonat W, Hilfrich J, Aidtmann H, Gademann G, Zuna I, et al. Improved overall survival in postmenopausal women with early breast cancer after anastrozole initiated after treatment with tamoxifen compared with continued tamoxifen: The ARNO 95 study. J Clin Oncol (2007) 25:2664–70. doi: 10.1200/JCO.2006.08.8054
24. Boccardo F, Rubagotti A, Puntoni M, Guglielmini P, Amoroso D, Fini A, et al. Switching to anastrozole vs continued tamoxifen treatment of early breast cancer: preliminary results of the Italian tamoxifen anastrozole trial. J Clin Oncol (2005) 23:5138–47. doi: 10.1200/JCO.2005.04.120
25. Dowsett M, Cuzick J, Wale C, Howell T, Houghton J, Baum M. Retrospective analysis of time to recurrence in the ATAC trial according to hormone receptor status: a hypothesis-generating study. J Clin Oncol (2005) 23:7512–17. doi: 10.1200/JCO.2005.01.4829
26. Goss PE, Ingle JN, Pritchard KI, Ellis MJ, Sledge JW, Budd T, et al. Exemestane versus anastrozole in postmenopausal women with early breast cancer: NCIC CTG MA.27—a randomized controlled Phase III trial. J Clin Oncol (2013) 11:1398–404. doi: 10.1200/JCO.2012.44.7805
27. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomized trials. Lancet (2015) 386:1341–52. doi: 10.1016/S0140-6736(15)61074-1
28. Masuda N, Sagara Y, Kinoshita T, Iwata H, Nakamura S, Yanagita Y, et al. Neoadjuvant anastrozole versus tamoxifen in patient receiving goserelin for premenopausal breast cancer (STAGE<. A double blind randomized phase 3 trial. Lancet Oncol (2012) 13:345–52. doi: 10.1016/S1470-2045(11)70373-4
29. Gnant M, Mlineritsch B, Schippinger W, Luschin-Ebengreuth G, Poslberger S, Menzel C, et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med (2009) 360:679–91. doi: 10.1056/NEJMoa0806285
30. Gnant M, Mlineritsch B, Stoeger H, Luschin-Ebengreuth G, Knauer M, Moik M, et al. Zoledronic acid combined with adjuvant endocrine therapy of tamoxifen versus anastrozole plus ovarian function suppression in premenopausal early breast cancer: final analysis of the Austrian Breast and Colorectal Cancer Study Group Trial 12. Ann Oncol (2015) 26:313–20. doi: 10.1093/annonc/mdu544
31. Pagani O, Regan MM, Walley BA, Fleming GF, Colleoni M, Lang I, et al. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med (2014) 371:107–18. doi: 10.1056/NEJMoa1404037
32. Francis PA, Pagani O, Fleming GF, Walley BA, Colleoni M, Lang I, et al. Tailoring adjuvant endocrine therapy for premenopausal breast cancer. N Engl J Med (2018) 379:122–37. doi: 10.1056/NEJMoa1803164
33. Perrone F, De Laurentiis M, De Placido S, Orditura M, Cinieri S, Riccardi F, et al. Adjuvant zoledronic acid and letrozole plus ovarian suppression in premenopausal breast cancer: HOBOE phase 3 randomized trial. Eur J Cancer (2019) 118:178–86. doi: 10.1016/j.ejca.2019.05.004
34. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Aromatase inhibitors versus tamoxifen in premenopausal women with oestrogen receptor positive early-stage breast cancer treated with ovarian suppression: a patient-level meta-analysis of 7030 women from four randomized trials. Lancet Oncol (2022) 23:382–92. doi: 10.1016/S1470-2045(21)00758-0
35. Ruhstaller T, Giobbie Hurder A, Colleoni M, Jensen MB, Ejlertsen B, de Azambuja E, et al. Adjuvant letrozole and tamoxifen alone or sequentially for postmenopausal women with hormone receptor–positive breast cancer: long-term follow-up of the BIG 1-98 trial. JCO (2019) 37(2):105–14. doi: 10.1200/JCO.18.00440
36. Breast International Group (BIG) 1-98 Collaborative, Thürlimann B, Keshaviah A, Coates AS, Mouridsen H, Mauriac L, Forbes JF, et al. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med (2005) 353:2747–57. doi: 10.1056/NEJMoa052258
37. Breast International Group (BIG) 1-98 Collaborative, Coates AS, Keshaviah A, Thurlimann B, Mouridsen H, Mauriac L, Frobes JF, et al. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: Update of study BIG 1-98. J Clin Oncol (2007) 25:486–92. doi: 10.1200/JCO.2006.08.8617
38. Breast International Group (BIG) 1-98 Collaborative Group, Mouridsen H, Giobbie-Hurder A, Goldhirsch A, Thurlimann B, Paridaens R, et al. Letrozole therapy alone or in sequence with tamoxifen in women with breast cancer. N Engl J Med (2009) 361:766–76. doi: 10.1056/NEJMoa0810818
39. Giobbie-Hurder A, Price KN, Gelber RD, International Breast Cancer study group, BIG 1-98 collaborative group, et al. Design, conduct, and analyses of Breast International Group (BIG) 1-98: A randomized, double-blind, phase-III study comparing letrozole and tamoxifen as adjuvant endocrine therapy for postmenopausal women with receptor-positive, early breast cancer. Clin Trials (2009) 6:272–87. doi: 10.1177/1740774509105380
40. Regan MM, Price KN, Giobbie-Hurder A, Thurlimann B, Gelber RD, International Breast Cancer Study Group, et al. Interpreting Breast International Group (BIG) 1-98: A randomized, double-blind, phase III trial comparing letrozole and tamoxifen as adjuvant endocrine therapy for postmenopausal women with receptor-positive, early breast cancer. Breast Cancer Res (2011) 13:209. doi: 10.1186/bcr2837
41. Cuzick J, Sestak I, Baum M, Buzdar A, Howell A, Dowsett M, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol (2010) 11:1135–41. doi: 10.1016/S1470-2045(10)70257-6
42. Chen J, Power D, Lu Y, Zhang T, Ouyang Z, Sun Q. Optimal duration of endocrine therapy with extended aromatase inhibitors for postmenopausal patients with hormone receptor−positive breast cancer: a meta−analysis. Breast Cancer (2021) 28:630–43. doi: 10.1007/s12282-020-01196-8
43. Burstein HJ, Prestrud AA, Griggs JJ, Temin S. American Society of Clinical Oncology clinical practice guideline: update on adjuvant endocrine therapy or women with hormone receptor-positive breast cancer. J Clin Oncol (2010) 28(23):3784–96. doi: 10.1200/JCO.2009.26.3756
44. Mann BS, Johnson JR, Kelly R, Sridhara R, Williams G, Pazdur R. Letrozole in the extended adjuvant treatment of postmenopausal women with history of early-stage breast cancer who have completed 5 years of adjuvant tamoxifen. Clin Cancer Res (2005) 11(16):5671–7. doi: 10.1158/1078-0432.CCR-05-0354
45. Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer Inst (2005) 97(17):1262–71. doi: 10.1093/jnci/dji250
46. Del Mastro L, Mansutti M, Bisagni G, Ponzone R, Durando A, Amaducci L, et al. Benefit from letrozole as extended adjuvant therapy after sequential endocrine therapy: A randomized, phase III study of Gruppo Italiano Mammella (GIM). J Clin Oncol (2019) 37(15_suppl):504. doi: 10.1200/JCO.2019.37.15_suppl.504
47. Mamounas EP, Bandos H, Lembersky BC, Jeong JH, Geyer CE Jr, Rastogi P, et al. Use of letrozole after aromatase inhibitor-based therapy in postmenopausal breast cancer (NRG Oncology/NSABP B-42): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol (2019) 20(1):88–99. doi: 10.1016/S1470-2045(18)30621-1
48. Mamounas EP, Jeong JH, Wickerham DL, Smith RE, Ganz PA, Land SR, et al. Benefit from exemestane as extended adjuvant therapy after 5 years of adjuvant tamoxifen: intention-to-treat analysis of the National Surgical Adjuvant Breast and Bowel Project B-33 trial. J Clin Oncol (2008) 26(12):1965–71. doi: 10.1200/JCO.2007.14.0228
49. Tjan-Heijnen V, van Hellemond I, Peer P, Swinkels A, Smorenburg C, Sangen M, et al. Extended adjuvant aromatase inhibition after sequential endocrine therapy (DATA): a randomised, phase 3 trial. Lancet Oncol (2017) 18(11):1502–11. doi: 10.1016/S1470-2045(17)30600-9
50. Gnant M, Steger G, Fitzal F, Mlineritsch B, Manfreda D, Tausch C, et al. A prospective randomized multi-center phase-III trial of additional 2 versus additional 5 years of anastrozole after initial 5 years of adjuvant endocrine therapy—results from 3484 postmenopausal women in the ABCSG-16 trial 2017 San Antonio Breast Cancer Symposium. Cancer Res (2018) 78(4 Suppl):Abstract nr GS3-01. doi: 10.1158/1538-7445.SABCS17-GS3-01
51. Blok EJ, Kroep JR, Meershoek-Klein Kranenbarg E, Dijm-de Carpentier M, Putter H, van den Bosch J, et al. Optimal duration of extended adjuvant endocrine therapy for early breast cancer; results of the IDEAL Trial (BOOG 2006–05). J Natl Cancer Inst (2018) 110(1):40–8. doi: 10.1093/jnci/djx134
52. Ohtani S, Iijima K, Higaki K, Sato Y, Hozumi Y, Hasegawa Y, et al. A prospective randomized multi-center open-label phase III trial of extending aromatase-inhibitor adjuvant therapy to 10 years—results from 1697 postmenopausal women in the N-SAS BC 05 trial: Arimidex extended adjuvant randomized study (AERAS) 2018 San Antonio Breast Cancer Symposium. Cancer Re (2019) 79(4 Suppl):abstract nr GS3-04. doi: 10.1158/1538-7445.SABCS18-GS3-04
53. Davies C, Pan H, Godwin J, Gray R, Arriagada R, Raina V, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet (2013) 381(9869):805–16. doi: 10.1016/S0140-6736(12)61963-1
54. Gray RG, Rea D, Handley K, Bowden SJ, Perry P, Earl HM, et al. aTTom: long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6,953 women with early breast cancer. J Clin Oncol (2013) 31(15_suppl):5–5. doi: 10.1200/jco.2013.31.18_suppl.5
55. Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Niksic M, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet (2018) 391:1023–75. doi: 10.1016/S0140-6736(17)33326-3
56. Goss PE, Ingle JN, Pritchard KI, Robert NJ, Muss H, Gralow J, et al. Extending aromatase-inhibitor adjuvant therapy to 10 years. N Engl J Med (2016) 375:209–19. doi: 10.1056/NEJMoa1604700
57. Jakesz R, Greil R, Gnant M, Schmid M, Kwasny W, Kubista E, et al. Austrian breast and colorectal cancer study group. Extended adjuvant therapy with anastrozole among postmenopausal breast cancer patients: results from the randomized Austrian breast and colorectal cancer study group trial 6a. J Natl Cancer Inst (2019) 99(24):1845–53. doi: 10.1093/jnci/djm246
58. Corona SP, Roviello G, Strina C, Milani M, Madaro S, Zanoni D, et al. Efficacy of extended aromatase inhibitors for hormone receptors positive breast cancer: A literature-based meta-analysis of randomized trials. Breast (2019) 46:19–24. doi: 10.1016/j.breast.2019.04.004
59. Qian X, Li Z, Ruan G, Tu C, Ding W. Efficacy and toxicity of extended aromatase inhibitors after adjuvant aromatase inhibitors−containing therapy for hormone−receptor−positive breast cancer: a literature−based meta−analysis of randomized trials. Breast Cancer Res Treat (2020) 179:275–85. doi: 10.1007/s10549-019-05464-w
60. Gnant M, Steger G, Fitzal F, Mlineritsch B, Manfreda D, Tauch C, et al. A prospective randomized multi-center phase-III trial of additional 2 versus additional 5 years of anastrozole after initial 5 years of adjuvant endocrine therapy—results from 3,484 postmenopausal women in the ABCSG-16 trial. Cancer Res (2018) 78:1. doi: 10.1158/1538-7445.SABCS17-GS3-01
61. Zdenkowski N, Forbes JF, Boyle FM, Kannourakis G, Gill PG, Bayliss E, et al. Observation versus late reintroduction of letrozole as adjuvant endocrine therapy for hormone receptorpositive breast cancer (ANZ0501 LATER): an open-label randomised, controlled trial. Ann Oncol (2016) 27(5):806–12. doi: 10.1093/annonc/mdw055
62. Richman J, Dowsett M. Beyond 5 years: enduring risk of recurrence in oestrogen receptor-positive breast cancer. Nat Rev Clin Oncol (2019) 16(5):296–311. doi: 10.1038/s41571-018-0145-5
63. Gnant M, Fitzal F, Rinnerthaler G, Steger GG, Greil-Ressler S, Balic M, et al. Duration of adjuvant aromatase-inhibitor therapy in postmenopausal breast cancer. N Engl J Med (2021) 385:395–405. doi: 10.1056/NEJMoa2104162
64. Piezzo M, Chiodini P, Riemma M, Cocco S, Caputo R, Cianniello D, et al. Progression-free survival and overall survival of CDK 4/6 inhibitors plus endocrine therapy in metastatic breast cancer: A systematic review and meta-analysis. Int J Mol Sci (2020) 21:6400. doi: 10.3390/ijms21176400
65. Spring LM, Wander SA, Zangardi M, Bardia A. CDK 4/6 inhibitors in breast cancer: current controversies and future directions. Curr Oncol Rep (2019) 21(3):25. doi: 10.1007/s11912-019-0769-3
66. Turner NC, Huang Bartlett C, Cristofanilli M. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J.Med (2015) 373:1672–3. doi: 10.1056/NEJMc1510345
67. Verma S, Bartlett CH, Schnell P, De Michele AM, Loi S, Ro J, et al. Palbociclib in combinationWith fulvestrant inWomenWith hormone receptor-positive/HER2-negative advanced metastatic breast cancer: detailed safety analysis from a multicenter, randomized, pla-cebo-controlled, phase III study (PALOMA-3). Oncologist (2016) 21:1165–75. doi: 10.1634/theoncologist.2016-0097
68. Turner NC, Slamon DJ, Ro J, Bondarenko I, Im SA, Masuda N, et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J.Med (2018) 379:1926–36. doi: 10.1056/NEJMoa1810527
69. Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl.J Med (2016) 375:1738–48. doi: 10.1056/NEJMoa1609709
70. Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann Oncol (2018) 29:1541–7. doi: 10.1093/annonc/mdy155
71. Syed YY. Ribociclib: first global approval. Drugs (2017) 77:799–807. doi: 10.1007/s40265-017-0742-0
72. Im SA, Lu YS, Bardia A, Harbeck N, Colleoni M, Franke F, et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med (2019) 381:307–16. doi: 10.1056/NEJMoa1903765
73. Slamon DJ, Neven P, Chia S, Fashing PA, De Laurentiis M, Im SA, et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin Oncol (2018) 36:2465–72. doi: 10.1200/JCO.2018.78.9909
74. Sledge GW, Toi M, Neven P, Sohn J, Inoue K, Pivot X, et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol (2017) 35:2875–84. doi: 10.1200/JCO.2017.73.7585
75. Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S, Huober J, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol (2017) 35:3638–46. doi: 10.1200/JCO.2017.75.6155
76. Johnston S, Martin M, Di Leo A, Im SA, Awada A, Forrester T, et al. MONARCH 3 final PFS: A randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer (2019) 5:5. doi: 10.1038/s41523-018-0097-z
77. Dickler MN, Tolaney SM, Rugo HS, Cortes J, Dieras V, Patt D, et al. MONARCH 1, A phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory HR. Clin Cancer Res (2017) 23:5218–24. doi: 10.1158/1078-0432.CCR-17-0754
78. Johnston SRD, Harbeck N, Hegg R, Toi M, Martin M, Shao ZM, et al. Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR1, HER22, node-positive, high-risk, early breast cancer (monarchE). J Clin Oncol (2020) 38:3987–98. doi: 10.1200/JCO.20.02514
79. Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): A randomised phase 2 study. Lancet Oncol (2015) 16:25–35. doi: 10.1016/S1470-2045(14)71159-3
80. Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med (2016) 375:1925–36. doi: 10.1056/NEJMoa1607303
81. Rugo HS, Finn RS, Dieras V, Ettl J, Lipatov O, Joy AA, et al. Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up. Breast Cancer Res Treat (2019) 174:719–29. doi: 10.1007/s10549-018-05125-4
82. Schettini F, Giudici F, Giuliano M, Cristofanilli M, Arpino G, Del Mastro L, et al. Overall survival of CDK4/6-inhibitor–based treatments in clinically relevant subgroups of metastatic breast cancer: systematic review and meta-analysis. J Natl Cancer Inst (2020) 112(11):1089–97. doi: 10.1093/jnci/djaa071
83. Desnoyers A, Nadler MB, Kumar V, Saleh R, Amir E. Comparison of treatment-related adverse events of different Cyclin-dependent kinase 4/6 inhibitors in metastatic breast cancer: A network meta-analysis. Cancer Treat Rev (2020) 90:102086. doi: 10.1016/j.ctrv.2020.102086
84. Costa R, Costa RB, Talamantes SM, Elenowsky I, Peterson J, Kaplan J, et al. Meta-analysis of selected toxicity endpoints of CDK4/6 inhibitors: Palbociclib and ribociclib. Breast (2017) 35:1–7. doi: 10.1016/j.breast.2017.05.016
85. Andrikopoulou A, Fiste O, Liontos M, Dimopoulos MA, Zagouri F. Aromatase and CDK4/6 inhibitor-Induced musculoskeletal symptoms: A systematic review. Cancers (Basel). (2021) 13:465. doi: 10.3390/cancers13030465
86. Fallowfield L, Cella D, Cuzick J, Francis S, Locker G, Howell A. Quality of life of postmenopausal women in the Arimidex, Tamoxifen, Alone or in Combination (ATAC) adjuvant breast cancer trial. J Clin Oncol (2004) 22:4261–71. doi: 10.1200/JCO.2004.08.029
87. Fallowfield LJ, Bliss JM, Porter LS, Price MH, Sowdon CF, Jones SE, et al. Quality of life in the intergroup exemestane study: A randomized trial of exemestane versus continued tamoxifen after 2 to 3 years of tamoxifen in postmenopausal women with primary breast cancer. J Clin Oncol (2006) 24:910–17. doi: 10.1200/JCO.2005.03.3654
88. Whelan TJ, Goss PE, Ingle JN, Pater JL, Tu D, Pritchard K, et al. Assessment of quality of life in MA.17: A randomized, placebo controlled trial of letrozole after 5years of tamoxifen in postmenopausal women. J Clin Oncol (2005) 23:6931–40. doi: 10.1200/JCO.2005.11.181
89. Murphy CC, Bartholomew LK, Carpentier MY, Bluethmann SM, Vernon SW. Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: A systematic review. Breast Cancer Res Treat (2012) 134:459–78. doi: 10.1007/s10549-012-2114-5
90. Hershman DL, Shao T, Kushi LH, Buono D, Tsai WY, Fehrenbacher L, et al. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat (2011) 126(2):529–37. doi: 10.1007/s10549-010-1132-4
91. Makubate B, Donnan PT, Dewar JA, Thompson AM, McCowan C. Cohort study of adherence to adjuvant endocrine therapy, breast cancer recurrence and mortality. Br J Cancer (2013) 108(7):1515–24. doi: 10.1038/bjc.2013.116
92. Henry NL, Azzouz F, Desta Z, Li L, Nguyen AT, Lemler S, et al. Predictors of aromatase inhibitor discontinuation as a result of treatment-emergent symptoms in early-stage breast cancer. J Clin Oncol (2012) 30(9):936–42. doi: 10.1200/JCO.2011.38.0261
93. Henry NL, Gile JT, Ang D, Mohan M, Dadabhoy D, Robarge J, et al. Prospective characterization of musculoskeletal symptoms in early-stage breast cancer patients treated with aromatase inhibitors. Breast Cncer Res Treat (2008) 111(2):365–72. doi: 10.1007/s10549-007-9774-6
94. Zivian MT, Salgado B. Side effects revisited: women’s experiences with aromatase inhibitors. San Francisco: Breast Cancer Action (2008).
95. Kyvernitakis I, Kostev K, Hadji P. The tamoxifen paradox-influence of adjuvant tamoxifen on fracture risk in pre- and post-menopausal women with breast cancer. Osteoporos Int (2018) 29:2557–64. doi: 10.1007/s00198-018-4642-2
96. Stumpf U, Kostev K, Kyvernitakis J, Bocker W, Hadji P. Incidence of fractures in young women with breast cancer - a retrospective cohort study. J Bone Oncol (2019) 18:100254. doi: 10.1016/j.jbo.2019.100254
97. Hadji P, Aapro MS, Body JJ, Gnant M, Brandi LM, Reginster JY, et al. Management of Aromatase Inhibitor-Associated Bone Loss (AIBL) in postmenopausal women with hormone sensitive breast cancer: Joint position statement of the IOF, CABS, ECTS, IEG, ESCEO IMS, and SIOG. J Bone Oncol (2017) 7:1–12. doi: 10.1016/j.jbo.2017.03.001
98. Waqas K, Ferreira JL, Tsourdi E, Body JJ, Hadji P, Zillikens MC. Updated guidance on the management of cancer treatment-induced bone loss (CTIBL) in pre-and postmenopausal women with early-stage breast cancer. Journ Bone Oncol (2021) 28:100355. doi: 10.1016/j.jbo.2021.100355
99. Colleoni M, Giobbie-Hurder A, Regan MM, Thurlimann B, Mouridsen H, Mauriac L, et al. Analyses adjusting for selective crossover show improved overall survival with adjuvant letrozole compared with tamoxifen in the BIG 1-98 study. J Clin Oncol (2011) 29:1117–24. doi: 10.1200/JCO.2010.31.6455
100. Bliss JM, Kilburn LS, Coleman RE, Forbes JF, Coates AS, Jones SE, et al. Disease-related outcomes with long-term follow-up: an updated analysis of the intergroup exemestane study. J Clin Oncol (2012) 30:709–17. doi: 10.1200/JCO.2010.33.7899
101. Dewar JA, Horobin JM, Preece PE, Tavendale R, Tunstall-Pedoe H, Wood RA. Long term effects of tamoxifen on blood lipid values in breast cancer. BMJ (1992) 305:225–26. doi: 10.1136/bmj.305.6847.225
102. Bruning PF, Bonfrer JM, Hart AA, de Jong-Bakker M, Linders D, van Loon J, et al. Tamoxifen, serum lipoproteins and cardiovascular risk. Br J Cancer (1988) 58:497–99. doi: 10.1038/bjc.1988.248
103. Cuzick J, Allen D, Baum M, Barrett J, Clark G, Kakkar V, et al. Long term effects of tamoxifen. Biological effects of Tamoxifen Working Party. Eur J Cancer (1992) 29A:15–21. doi: 10.1016/0959-8049(93)90568-z
104. Grainger DJ, Schoeld PM. Tamoxifen for the prevention of myocardial infarction in humans: preclinical and early clinical evidence. Circulation (2005) 112:3018–24. doi: 10.1161/CIRCULATIONAHA.104.531178
105. Guetta V, Lush RM, Figg WD, Waclawiw MA, Cannor RO 3rd. Effects of the antiestrogen tamoxifen on low-density lipoprotein concentrations and oxidation in postmenopausal women. Am J Cardiol (1995) 76:1072–73. doi: 10.1016/S0002-9149(99)80302-6
106. Love RR, Wiebe DA, Feyzi JM, Newcomb PA, Chappell RJ. Effects of tamoxifen on cardiovascular risk factors in postmenopausal women after 5 years of treatment. J Natl Cancer Inst (1994) 86:1534–39. doi: 10.1093/jnci/86.20.1534
107. Graetz I, McKillop CN, Stepanski E, Vidal GA, Anderson JN, Schwartzberg LS. Use of a web-based app to improve breast cancer symptom management and adherence for aromatase inhibitors: A randomized controlled feasibility trial. Cancer Surviv (2018) 12(4):431–40. doi: 10.1007/s11764-018-0682-z
108. Ershman DL, Unger JM, Hillyer GC, Moseley A, Arnold KB, Dakhil SR, et al. Randomized trial of text messaging to reduce early discontinuation of adjuvant aromatase inhibitor therapy in women with early-stage breast cancer: SWOG S1105. J Clin Oncol (2020) 38:2122–29. doi: 10.1200/JCO.19.02699
109. Tan EH, Wong ALA, Tan CC, Wong P, Tan SH, Ang LEW, et al. Improving medication adherence with adjuvant aromatase inhibitor in women with breast cancer: A randomised controlled trial to evaluate the effect of short message service (SMS) reminder. Breast (2020) 53:77–84. doi: 10.1016/j.breast.2020.06.012
Keywords: aromatase inhibitors, bone loss, cardiotoxicity, drug adherence, breast cancer
Citation: Generali D, Berardi R, Caruso M, Cazzaniga M, Garrone O, Minchella I, Paris I, Pinto C and De Placido S (2023) Aromatase inhibitors: the journey from the state of the art to clinical open questions. Front. Oncol. 13:1249160. doi: 10.3389/fonc.2023.1249160
Received: 28 June 2023; Accepted: 30 November 2023;
Published: 22 December 2023.
Edited by:
Maritha J Kotze, Tygerberg Hospital, South AfricaReviewed by:
Olesya A Kharenko, Syantra Inc, CanadaCopyright © 2023 Generali, Berardi, Caruso, Cazzaniga, Garrone, Minchella, Paris, Pinto and De Placido. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniele Generali, ZGdlbmVyYWxpQHVuaXRzLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.