- 1Division of Thoracic Medicine, Department of Internal Medicine, Chang Gung Memorial Hospital at Linkou, Taoyuan, Taiwan
- 2Department of Medicine, College of Medicine, Chang Gung University, Taoyuan, Taiwan
- 3Department of Pulmonary and Critical Care, Buddhist Tzu Chi General Hospital, New Taipei City, Taiwan
- 4Division of Thoracic Oncology, Department of Respiratory and Critical Care Medicine, Chang Gung Memorial Hospital, Chiayi County, Taiwan
- 5Department of Respiratory Care, Chang Gung University of Science and Technology, Chiayi County, Taiwan
- 6Division of Pulmonary & Critical Care Medicine, Kaohsiung Chang Gung Memorial Hospital, Kaohsiung, Taiwan
- 7Division of Hematology-Oncology, Department of Internal Medicine, Chang Gung Memorial Hospital at Linkou, Taoyuan, Taiwan
- 8Department of Internal Medicine, Taoyuan Chang Gung Memorial Hospital, Taoyuan, Taiwan
- 9Department of Respiratory Therapy, College of Medicine, Chang Gung University, Taoyuan, Taiwan
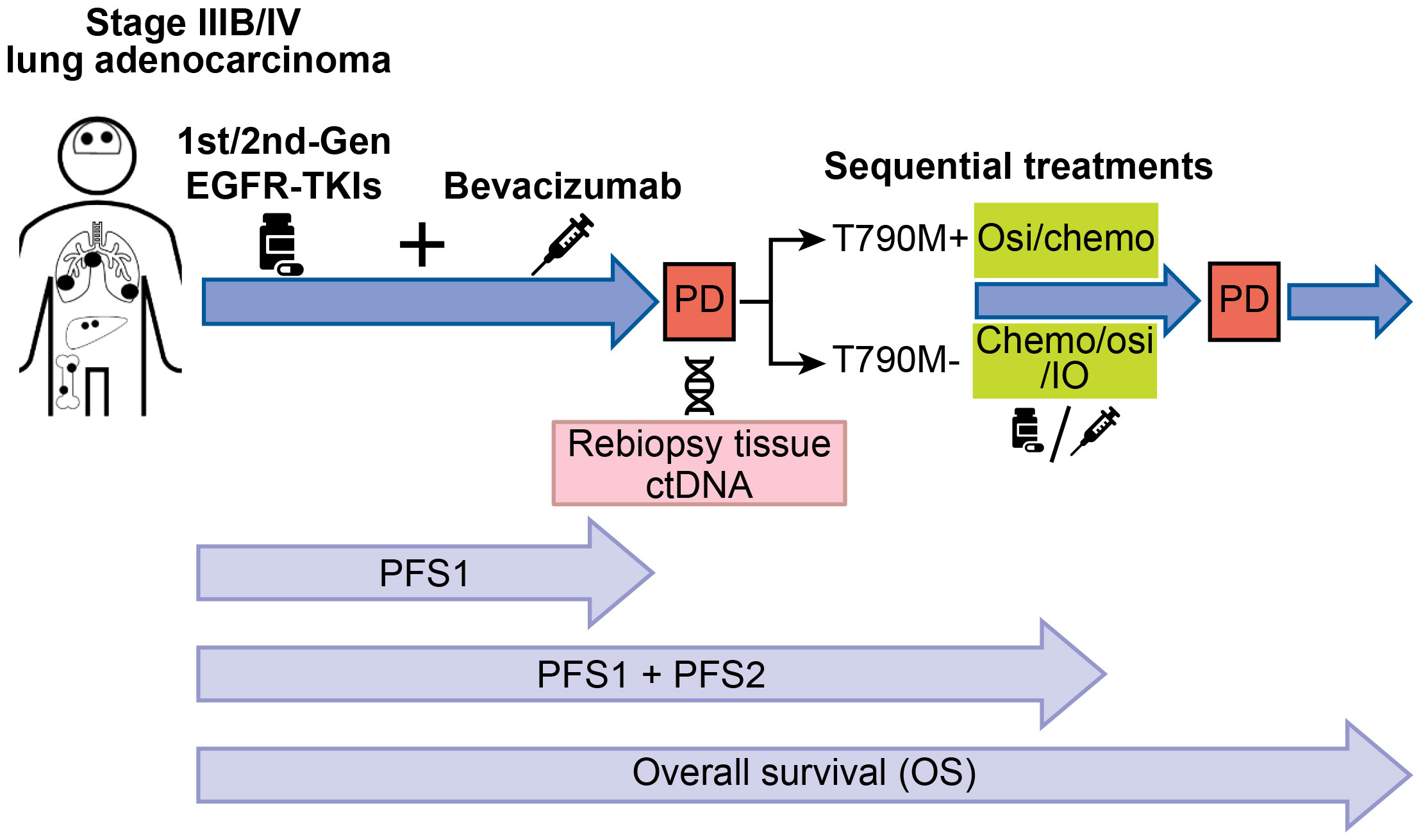
Introduction: The clinical outcomes of sequential treatment of advanced epidermal growth factor receptor (EGFR)-mutated non-small cell lung cancer (NSCLC) patients with first-line bevacizumab combined with 1st/2nd-generation EGFR-TKIs are unclear. Thus, we aimed to analyze the outcomes of these patients.
Methods: Between January 2015 and December 2020, data for 102 advanced EGFR-mutated lung adenocarcinoma patients receiving first-line bevacizumab combined with erlotinib or afatinib followed by treatments at multiple institutions were retrospectively analyzed. All patients with progressive disease (PD) after first-line therapy underwent secondary T790M mutation detection.
Results: The secondary T790M mutation positive rate of all study patients was 57.9%. First-line erlotinib use and progression-free survival (PFS) after first-line therapy > 12 months were positively associated with the T790M mutation (P <0.05). The response rates (RRs) to second-line treatments were 51.7% and 22.7% for the osimertinib and nonosimertinib groups, respectively (P = 0.001). The median PFS associated with second-line osimertinib and nonosimertinib therapy was 13.7 and 7.1 months, respectively (hazard ratio (HR) = 0.38; 95% confidence interval (CI), 0.23–0.63; P< 0.001). Patients with a secondary T790M mutation receiving second-line osimertinib treatment had a median overall survival (OS) of 54.3 months, and the median OS was 31.9 months for non-T790M-mutated patients receiving second-line nonosimertinib treatments (HR = 0.36; CI: 0.21–0.62, P < 0.001).
Conclusion: The majority of acquired resistance to first-line bevacizumab combined with 1st/2nd-generation EGFR-TKIs is associated with the T790M mutation. Sequential osimertinib treatment in patients with positive secondary T790M mutation is associated with better outcomes among these patients.
1 Introduction
The epidermal growth factor receptor (EGFR) and its downstream signaling pathway play crucial roles in the tumorigenesis of human non-small cell lung cancer (NSCLC) (1, 2). EGFR mutations account for the majority of oncogenic driver mutations in East Asian lung adenocarcinoma patients, and the incidence rate ranges from 40 to 55% (3, 4). The exon 19 deletion (in-frame deletions within exon 19) and L858R (a point mutation at codon 858 within exon 21 by leucine-to-arginine substitution) are the two most frequent (approximately 90%) EGFR mutations in lung adenocarcinoma (1–4). First- and second-generation EGFR tyrosine kinase inhibitors (TKIs), such as erlotinib and afatinib, have been demonstrated to be effective for treating advanced NSCLC harboring exon 19 deletion or L858R EGFR mutations (60-80% objective response rate (RR) and 10-14 months progression-free survival (PFS)) in several prospective clinical trials (5–7). Therefore, erlotinib and afatinib have been used as standard first-line therapies for advanced NSCLC harboring EGFR mutations worldwide.
The vascular endothelial growth factor receptor (VEGFR) signaling pathway has been reported to be involved in tumor growth and progression in various cancer cells (8, 9). Vascular endothelial growth factor (VEGF) is the ligand of VEGFR, and a previous study showed that EGFR-mutated NSCLC cells had increased VEGF protein expression levels compared with wild-type EGFR NSCLC cells (10). Another previous study showed that increased VEGF mRNA expression in plasma and tumor stroma was associated with resistance to EGFR-TKIs, and combined EGFR-TKIs and VEGF inhibitors had synergistic antitumor effects in an NSCLC mouse model (11). Bevacizumab is a humanized monoclonal antibody targeting VEGF and is used as an angiogenesis agent in anticancer therapies (8, 12). The efficacy of bevacizumab in combination with erlotinib or afatinib for the treatment of untreated advanced EGFR-mutated lung adenocarcinoma has been explored in several previous pivotal clinical trials and clinical studies (13–17). In these previous studies, the combination of erlotinib or afatinib with bevacizumab was demonstrated to have an objective RR of 80% and PFS of 13~24 months (13–17). Therefore, combination therapies have been suggested as a first-line therapeutic option for advanced lung adenocarcinoma patients with EGFR mutations.
The secondary T790M EGFR mutation is the most frequent cause of acquired resistance to first- and second-generation EGFR-TKIs (40%~60%) (18, 19). Osimertinib is a third-generation EGFR-TKI with active targeting of the T790M mutation and was shown to have promising efficacy (71% RR and 10.1 months PFS) in a pivotal clinical trial (AURA3 trial) (20). Therefore, osimertinib has been approved as a therapy for advanced NSCLC patients with secondary T790M mutation with progressive disease (PD) after first- or second-generation EGFR-TKI therapies.
The secondary T790M EGFR mutation appears in advanced lung adenocarcinoma with acquired resistance due to prior bevacizumab treatment combined with erlotinib or afatinib, and osimertinib is administered as a subsequent therapy for T790M-positive patients (15, 16). However, the clinical factors associated with the appearance of a positive T790M mutation in patients receiving first-line combination therapy remain unclear. Thus, we sought to analyze the survival outcomes of patients receiving first-line bevacizumab combined with erlotinib or afatinib followed by sequential systemic therapies (e.g., osimertinib or chemotherapy) after acquired resistance to first-line combination therapy.
2 Materials and methods
2.1 Patients and EGFR mutations
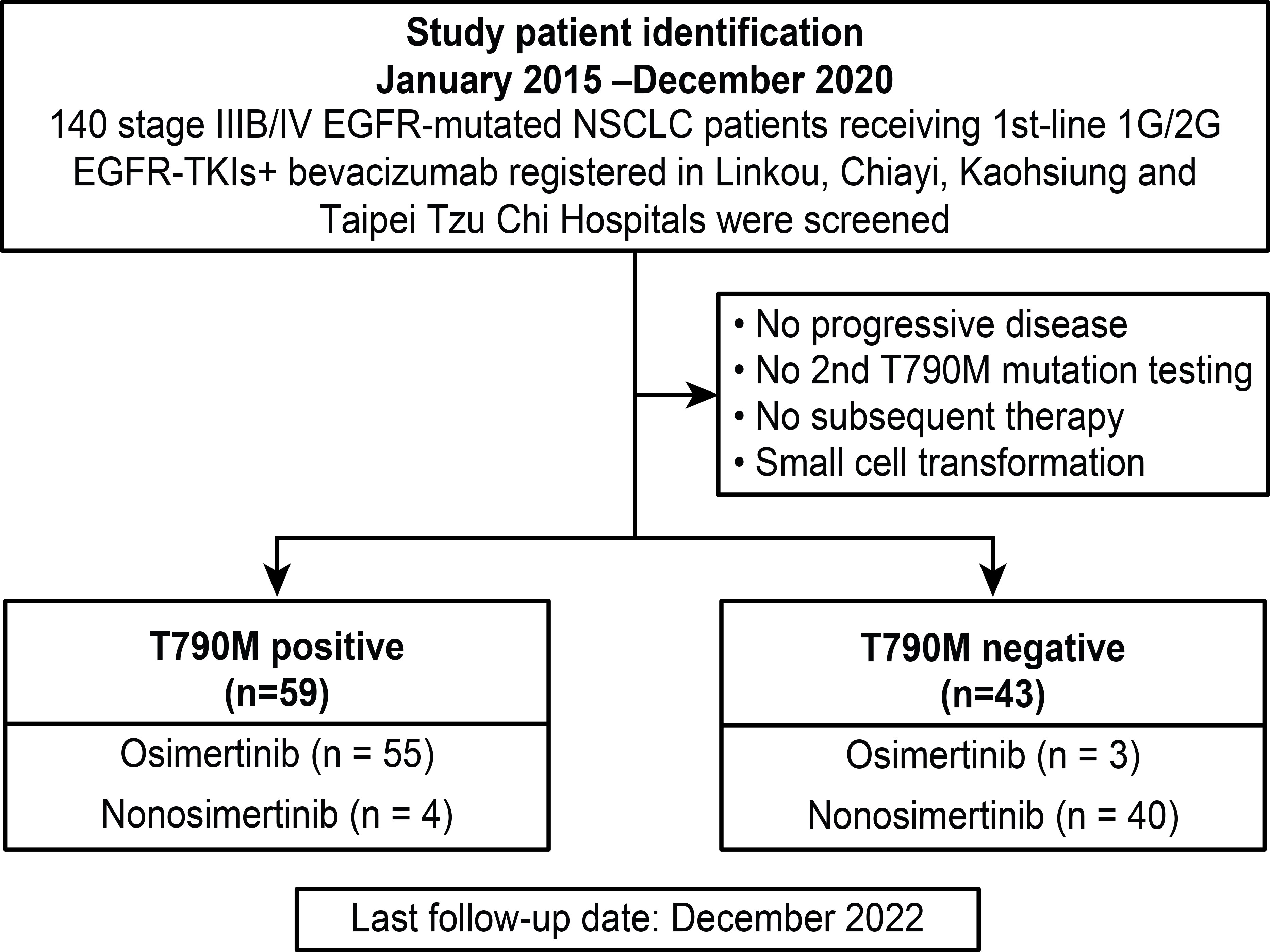
Data from all study patients were retrospectively retrieved from the cancer center database of Linkou, Kaohsiung, Chiayi Chang-Gung Memorial hospitals (CGMHs) and Taipei Tzu Chi Hospital. Between January 2015 and December 2020, 140 advanced lung adenocarcinoma patients with EGFR mutations receiving bevacizumab combined with first- or second-generation EGFR-TKIs as first-line therapy were screened. The inclusion criteria were as follows: (1) patients with primary EGFR mutations without de novo T790M; (2) patients with PD after first-line therapy; (3) patients with secondary EGFR T790M mutation tests; and (4) patients receiving subsequent systemic therapies. The exclusion criteria were as follows: (1) no PD after first-line therapy; (2) no tests to detect secondary EGFR T790M mutation; (3) no subsequent systemic therapy administered; and (4) small cell transformation. The summary of study subject screening is summarized in Figure 1.
Amplified refractory mutation system–Scorpion (ARMS/S) assays or next-generation sequencing (NGS) was used to detect primary EGFR mutations and secondary T790M mutations in patients with PD after first-line therapy. The NGS panel used in this study was the same as that described in a previous study (16).
2.2 Treatment response, survival evaluation, and follow-up
The baseline stages at initial diagnosis of all subjects were determined by computed tomography (CT) with contrast medium enhancement, fluorodeoxyglucose (FDG)-positron emission tomography (PET), and magnetic resonance imaging (MRI) of the brain. All study patients underwent whole-body CT scans every 3 to 4 months to evaluate treatment responses. Additional imaging studies such as sonogram, plain films, MRI and FDG-PET were ordered by physicians based on their need for assistance in disease status assessment.
Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. was used to assess treatment responses. The treatment responses were classified as complete response (CR), partial response (PR), stable disease (SD), and PD. The length of PFS was defined from the treatment start date to the date of first PD images detected or last follow-up. The length of overall survival (OS) was defined from the starting date of first-line therapy to the date of mortality recorded. For patients who survived through the time point of last follow-up (December 31, 2022), the OS was censored at the last recorded clinical visit date.
2.3 Statistical analysis
Categorical variables of study subjects are presented as quantitative variables, and age is presented as the mean ± standard deviation (SD). Cox regression with univariate and multivariate analyses was used to determine the clinical factors associated with the T790M mutation rates. PFS and OS were estimated by Kaplan–Meier survival curves, and two-sided P values were considered statistically significant when they were smaller than 0.05. IBM SPSS Statistics version 22.0 (SPSS Corp., Chicago, IL, USA) was used to perform univariate and multivariate Cox regression analyses. The PFS and OS survival curves were generated by using GraphPad Prism (Version 5.0; GraphPad Software, San Diego, CA, USA).
3 Results
3.1 Baseline patient clinical characteristics and information on sequential treatments
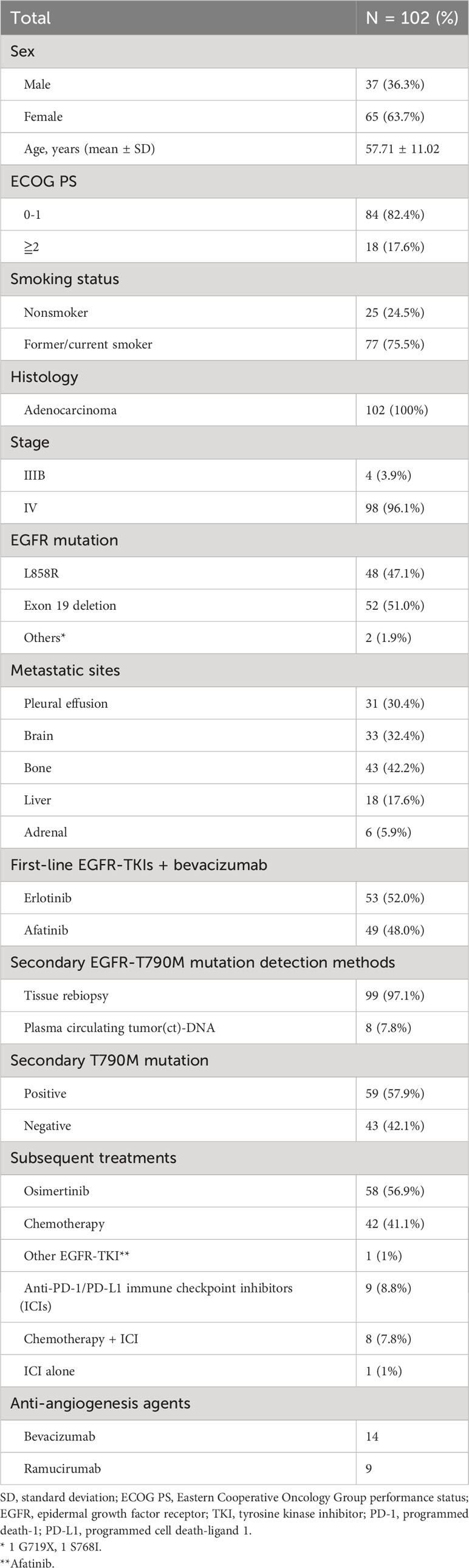
All baseline clinical characteristics of the 102 study patients are shown in Table 1. Ninety-nine (97.1%) patients underwent tissue rebiopsy, and 8 (7.8%) patients had plasma circulating tumor (ct)-DNA liquid biopsy for secondary T790M mutation tests. Five (4.9%) patients had both tissue rebiopsy and ctDNA tests, and 3 (2.9%) patients had ctDNA tests only. Among the 5 (4.9%) patients with both tissue rebiopsy and ctDNA assessment, all the rebiopsy tissues were tested by using NGS, and according to the NGS results, 4 (3.9%) patients were negative for the T790M mutation, and the other 1 (1%) was positive. All five (4.9%) patients were negative for the T790M mutation based on ctDNA tests. In the 3 (2.9%) patients who underwent ctDNA testing alone, 2 (1.9%) were positive for the T790M mutation, and 1 (1%) was negative. In the 59 (57.9%) patients positive for the T790M mutation, 55 (53.9%) were administered osimertinib, 3 (2.9%) received platinum-based doublet chemotherapy, and 1 (1%) was switched from first-line erlotinib to afatinib and continued to receive bevacizumab as 2nd-line therapy. In 43 (42.1%) patients negative for the T790M mutation, 3 (2.9%) received osimertinib, 39 received chemotherapy-based therapy, and 1 patient received single pembrolizumab (anti-programmed death-1 (PD-1) inhibitor) as 2nd-line therapy. Twenty-three (22.5%) patients received antiangiogenic agents, including bevacizumab and ramucirumab (anti-VEGFR inhibitor), as second-line therapy. Four (3.9%) patients received second-line osimertinib with continuation of bevacizumab, and 6 (5.9%) patients received ramucirumab combined with osimertinib as second-line therapy. Ten (9.8%) patients received bevacizumab combined with chemotherapy, and 4 (3.9%) of the 10 (9.8%) patients received bevacizumab combined with chemotherapy and atezolizumab (anti-programmed cell death-ligand 1 (PD-L1) inhibitor). Three (2.9%) patients received chemotherapy combined with ramucirumab.
3.2 Clinical factors associated with secondary EGFR T790M mutation after first-line bevacizumab combined with 1st-/2nd-generation EGFR-TKIs
The clinical factors associated with secondary T790M mutation after first-line therapy in this study were analyzed by using univariate and multivariate Cox regression (Table 2). In univariate analysis, the primary exon 19 deletion mutation had a trend of a higher secondary T790M mutation-positive rate than the primary L858R mutation, but no statistical significance was achieved. First-line bevacizumab combined with erlotinib had a significantly higher secondary T790M mutation-positive rate than bevacizumab combined with afatinib. In addition, a longer PFS (> 12 months) while on first-line treatment had a significantly higher T790M mutation rate than a shorter PFS (≦12 months). The multivariate analysis showed that first-line erlotinib use (vs. afatinib, odds ratio: 2.734, 95% confidence interval (CI): 1.144–6.531, P = 0.029) and longer PFS while on first-line therapy (vs. ≤12 months PFS, odds ratio: 2.958, 95% CI: 1.142–7.661, P = 0.025) were independent predictive factors associated with secondary T790M mutation. The clinical information comparison between patients treated with first-line afatinib plus bevacizumab and erlotinib plus erlotinib is shown in Supplementary Table S1.
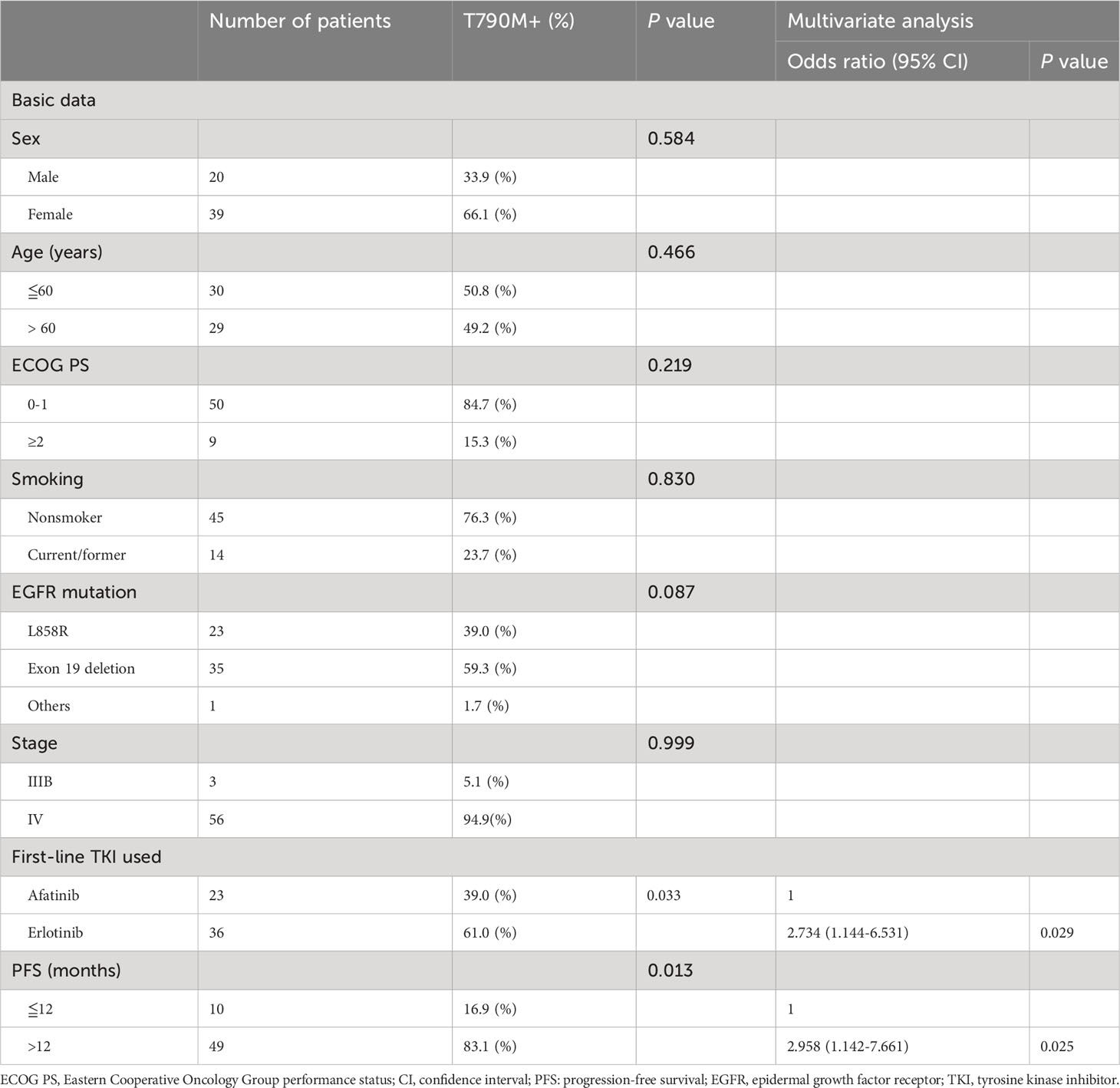
Table 2 Univariate and multivariate analyses of predictors associated with acquired T790M mutation (n=59).
3.3 Analysis of PFS and OS between the two first-line therapy groups
The PFS and OS of the 2 first-line therapies were analyzed by Kaplan–Meier survival curves. There was no significant difference in the median PFS of first-line bevacizumab combined with erlotinib and first-line bevacizumab combined with afatinib (19.6 vs. 18.7 months, hazard ratio (HR) = 1.05; CI: 0.52–1.15, P = 0.201) (Figure 2A). Patients with different primary EGFR mutations (L858R and exon 19 deletion) were divided into 2 groups to analyze the PFS associated with first-line therapies. In L858R-mutated patients, the median PFS was 18.4 and 21.3 months for the bevacizumab combined with erlotinib group and bevacizumab combined with erlotinib group, respectively (HR = 1.05; CI: 0.59–1.87, P = 0.874) (Figure 2B). For patients with primary exon 19 deletion mutations, the median PFS of the first-line bevacizumab combined with erlotinib group was significantly higher than that of the first-line bevacizumab combined with afatinib group (20.7 vs. 13.9 months, HR = 0.53; CI: 0.29–0.94, P = 0.031) (Figure 2C). Regarding OS, no significant difference was noted between the 2 groups of patients receiving first-line bevacizumab combined with erlotinib and first-line bevacizumab combined with afatinib (median OS: 49.4 VS. 42.6 months, HR = 0.841; CI: 0.51–1.38, P = 0.470) (Figure 2D). Patients with baseline brain metastasis were analyzed, and the results are shown in Supplementary Figure S1. The treatment response rate of first-line combination therapy was 84.8%, and median PFS was 14.7months in patients with baseline brain metastasis (Supplementary Figures S1A, B). The median OS of patients with baseline brain metastasis was 34.3 months (Supplementary Figure S1C).
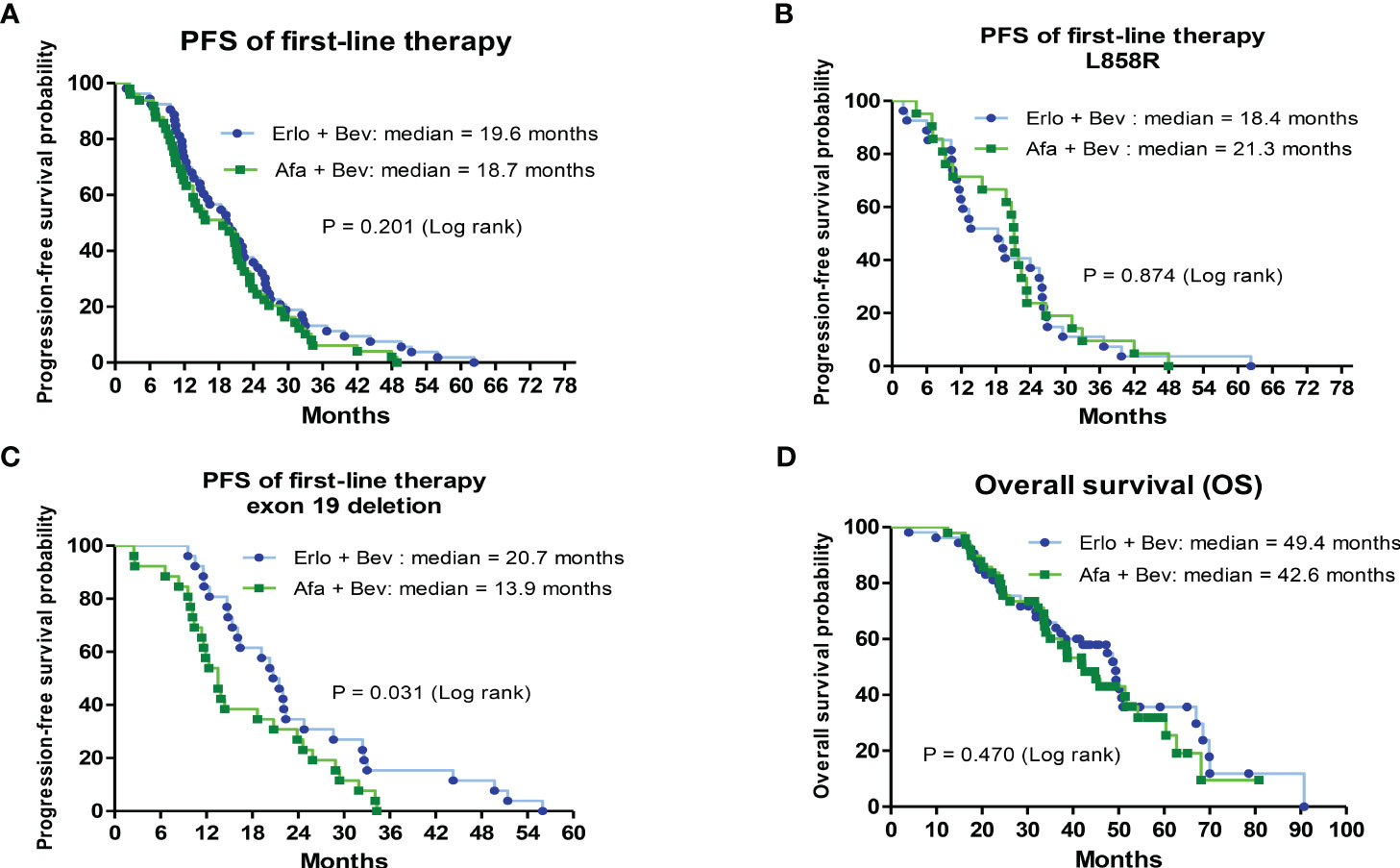
Figure 2 Analysis of progression-free survival (PFS) for first-line treatments and overall survival (OS) for first-line treatments by Kaplan–Meier survival curves. (A) Comparison of median PFS between bevacizumab combined with erlotinib or afatinib (HR = 1.05; 95% CI, 0.52–1.15; P= 0.201). (B) The median PFS between bevacizumab combined with erlotinib or afatinib in primary L858R-mutated patients (HR = 1.05; 95% CI, 0.59–1.87; P= 0.874). (C) The median PFS between bevacizumab combined with erlotinib or afatinib in primary exon 19 deletion-mutated patients (HR = 0.53; 95% CI, 0.29–0.94; P= 0). (D) The median OS between bevacizumab combined with erlotinib or afatinib (HR = 0.841; 95% CI, 0.51–1.38; P= 0.470).
3.4 Treatment outcomes of patients with different T790M mutation statuses and subsequent treatments
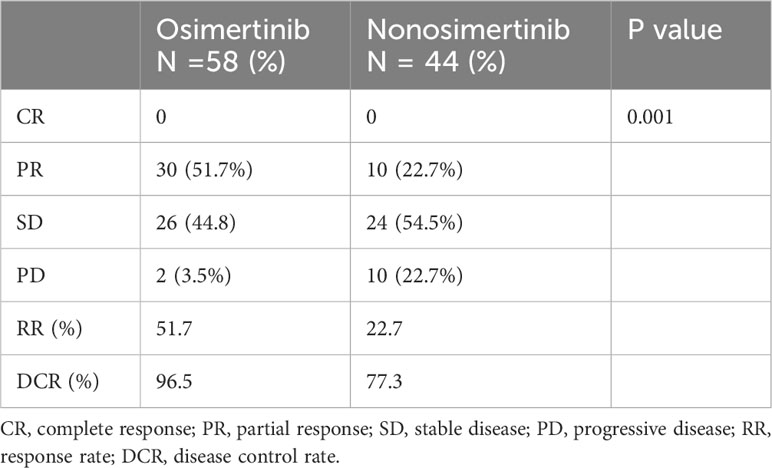
Most secondary T790M mutation-positive patients (56 of 59 = 94.9%) who received osimertinib as second-line therapy were divided into osimertinib and nonosimertinib groups for comparison. Second-line osimertinib treatment had a significantly higher objective RR than nonosimertinib therapy (51.7% vs. 22.7%, P = 0.001) (Table 3). All 3 patients who underwent liquid biopsy alone received osimertinib as subsequent treatments, and all patients had SD to osimertinib therapy. The PFS of the 3 patients ranged from 6.37 to 22.17 months. The patient who was T790M negative in liquid biopsy had a 14.83 PFS on osimertinib therapy.
Patients with secondary T790M mutation and second-line therapy had a significantly longer median PFS than those without T790M mutation (15.4 vs. 7.1 months, HR = 0.37; CI: 0.22–0.61, P < 0.001) (Figure 3A). The median PFS of those who received second-line osimertinib therapy was significantly longer than that of those who received nonosimertinib therapy (13.7 vs. 7.1 months, HR = 0.38; CI: 0.23–0.63, P < 0.001) (Figure 3B). The length of PFS of patients who received first-line plus second-line treatments (PFS1 + PFS2) was evaluated. Patients with a secondary T790M mutation had a significantly longer median PFS (1 + 2) than those without a T790M mutation (40.2 vs. 25.3 months, HR = 0.39; CI: 0.24–0.65, P < 0.001) (Figure 3C). Patients with a secondary T790M mutation who received 2nd-line osimertinib had a significantly longer median PFS (1 + 2) than those without a T790M mutation who received nonosimertinib therapy (41.8 vs. 25.9 months, HR = 0.39; CI: 0.23–0.65, P < 0.001) (Figure 3D).
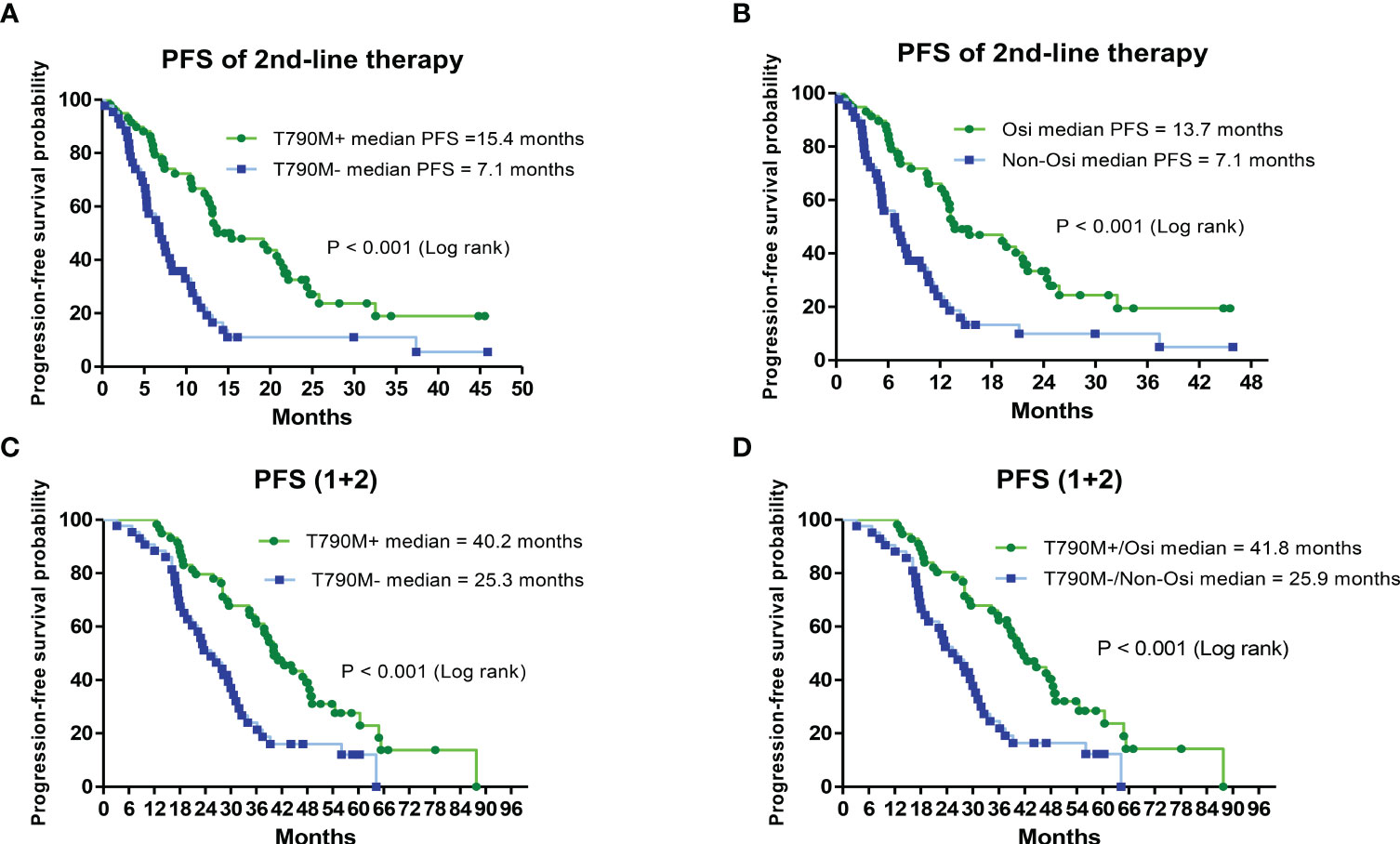
Figure 3 Analysis of progression-free survival (PFS) of second-line and first-line plus second-line (PFS1 + 2) therapies by Kaplan–Meier survival curves. (A) Comparison of PFS of second-line treatments between T790M mutation-positive and T790M mutation-negative patients (HR = 0.37; 95% CI, 0.22–0.61; P< 0.001). (B) Comparison of PFS between second-line osimertinib and nonosimertinib treatments (HR = 0.38; 95% CI, 0.23–0.63; P< 0.001). (C) Comparison of PFS (1 + 2) between T790M mutation-positive and T790M mutation-negative patients (HR = 0.39; 95% CI, 0.24–0.65; P< 0.001). (D) Comparison of PFS (1 + 2) between second-line osimertinib and nonosimertinib treatments (HR = 0.39; 95% CI, 0.23–0.65; P< 0.001).
We further analyzed the OS of patients with different secondary EGFR T790M mutation statuses and subsequent treatments. Patients with a secondary T790M mutation had a significantly longer median OS than those without a T790M mutation (54.3 vs. 33.5 months, HR = 0.34; CI: 0.19–0.59, P < 0.001) (Figure 4A). Patients with secondary T790M mutation who received osimertinib as subsequent treatment had a significantly longer median OS than those without T790M mutation who received a nonosimertinib subsequent therapy (54.3 VS. 31.9 months, HR = 0.36; CI: 0.21–0.62, P < 0.001) (Figure 4B).
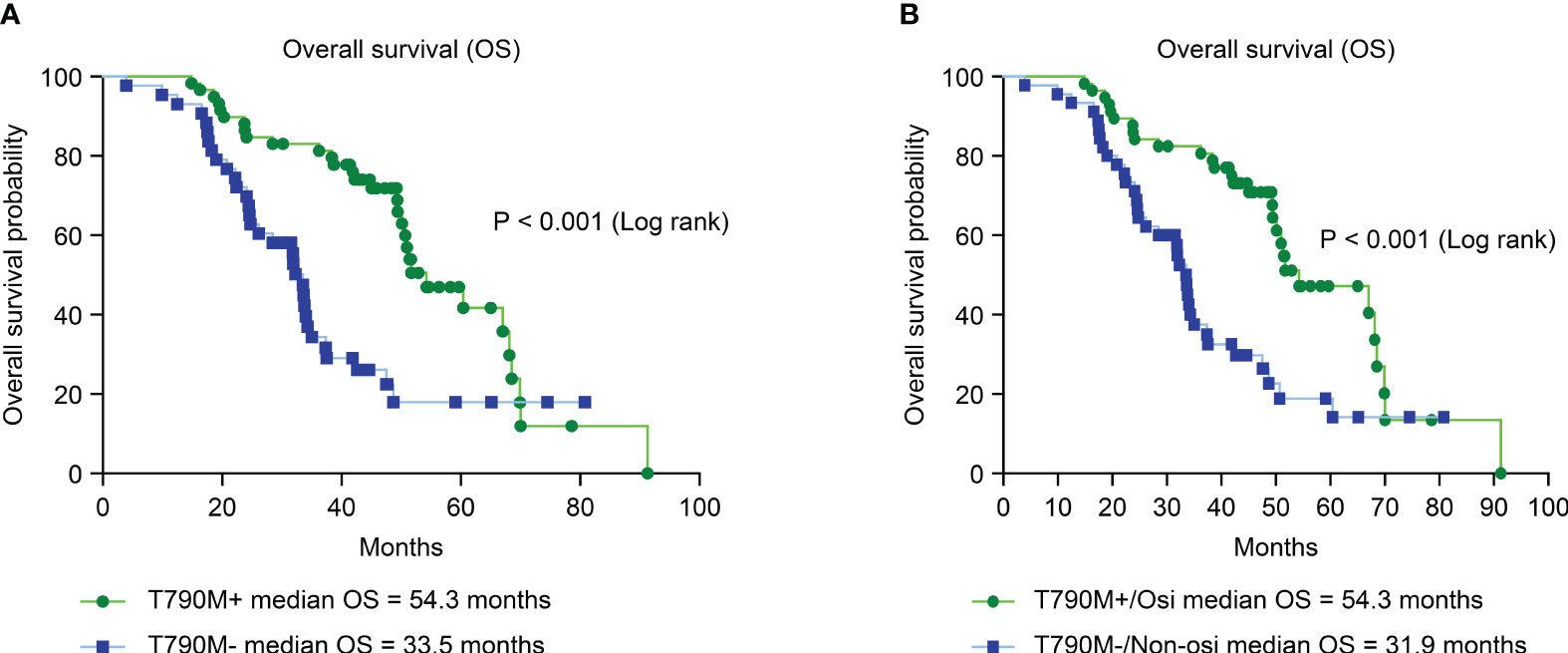
Figure 4 Analysis of overall survival (OS) between different T790M mutation statuses and second-line treatments by Kaplan–Meier survival curves. (A) Comparison of OS between T790M mutation-positive and T790M mutation-negative patients (HR = 0.34; 95% CI, 0.19–0.59; P< 0.001). (B) Comparison of OS between second-line osimertinib in T790M-positive and nonosimertinib in T790M-negative patients (HR = 0.36; 95% CI, 0.21–0.62; P< 0.001).
4 Discussion
The results of this study provide some important clinical information regarding sequential treatments for advanced EGFR-mutated NSCLC patients receiving first-line bevacizumab combined with 1st/2nd-generation EGFR-TKIs. First, the secondary T790M mutation rate after PD in this study was 57.9%. Second, the use of erlotinib in first-line therapy and PFS > 12 months were identified as independent predictive factors associated with higher secondary T790M mutation rates. Third, T790M-mutated patients receiving subsequent osimertinib had a significantly better treatment response and longer PFS than those without the T790M mutation receiving nonosimertinib therapy. In addition, T790M-mutated patients receiving subsequent osimertinib had significantly longer OS than those without the T790M mutation.
The acquired T790M mutation rate in this study was 57.9% and was consistent with that reported in previous studies (18, 21, 22). In contrast to previous studies, all the patients in this study received bevacizumab in addition to 1st/2nd-generation EGFR-TKIs, whereas most patients in previous studies received EGFR-TKI-alone therapies (18, 21, 22). The results of our study indicated that bevacizumab in addition to 1st/2nd-generation EGFR-TKIs does not alter the mechanism of acquired resistance in advanced primary EGFR-mutated NSCLC. Some previous studies showed that prior afatinib therapy was associated with a lower secondary T790M mutation-positive rate when compared with first-generation EGFR-TKIs, and these results were similar to those in our study (23, 24). Some previous studies showed that prior afatinib therapy was not associated with a lower secondary T790M mutation-positive rate and that this rate was even higher than that for first-generation EGFR-TKIs (25, 26). Although there are differences among our study and previous studies, the acquired T790M mutation rates after afatinib therapy in previous studies ranged from 30-50% (22–26). In addition, the small sample sizes in these studies may have led to different statistical significances among these studies. A long PFS of prior EGFR-TKI therapy (> 12 months) was identified as a predictive factor associated with acquired T790M mutation positive rates in previous studies, which was similar to the result in this study (22–26). A previous study showed that prolonging afatinib therapy in EGFR-mutated NSCLC by adding bevacizumab led to a positive acquired T790M mutation conversion, and the results in the same study suggested that prolonging afatinib therapy may induce the clonal selection of acquired T790M-mutated NSCLC cells (27). This clonal selection hypothesis may explain why long PFS of prior 1st/2nd-generation EGFR-TKI therapies is associated with an increased secondary T790M mutation rate. In the analysis of this study, bevacizumab combined with erlotinib had a significantly longer median PFS than bevacizumab combined with afatinib among patients with exon 19 deletion mutations. In addition, more patients in the bevacizumab combined with erlotinib treatment group had a longer PFS (> 12 months) than those in the bevacizumab combined with afatinib group (40 (75.5%) vs. 32 (65.3%)). Our study mainly focused on the rebiopsy results and subsequent therapies, and the study patients were retrospectively selected by selection criteria. Therefore, selection bias may lead to statistical significance in the median PFS between the first-line afatinib and erlotinib combined with bevacizumab groups in patients with exon 19 deletion mutations. Taken together, long treatment PFS is suggested to be the main factor associated with the occurrence of secondary T790M mutation, not afatinib and erlotinib therapies.
In the results of a previous clinical trial (AURA, NCT01802632), osimertinib had a 21% RR and a median PFS of 2.8 months for treating T790M mutation-negative patients with acquired resistance to prior EGFR-TKIs. The results of the AURA trial indicated that osimertinib is less effective in T790M-negative patients than in those with secondary T790M mutations after resistance to prior EGFR-TKI treatments (21). Before osimertinib was approved by the United States Food and Drug Administration (U.S. FDA, November 2015), platinum-based chemotherapy was the suggested subsequent treatment for patients who had PD after 1st/2nd-generation EGFR-TKI therapies (28, 29). Although osimertinib was approved for advanced NSCLC with acquired T790M mutation, chemotherapy has remained the clinically preferred subsequent treatment for T790M-negative patients with PD after 1st/2nd-generation EGFR-TKI therapies; furthermore, drugs targeting mutations other than T790M are still under investigation in clinical trials (29). Although immunotherapy, such as PD1/PD-L1 immune checkpoint inhibitors (ICIs), has been shown to improve the survival of advanced NSCLC patients without driver mutations (30), the survival benefit of immunotherapy is still very limited for advanced EGFR-mutated NSCLC patients (29, 30).
Osimertinib has been widely used as a late-line therapy for T790M-mutated NSCLC patients based on the results of AURA serial trials (20, 21, 31). In the survival analysis of the NEJ026 trial, patients treated with osimertinib in second-line or later-line therapies had significantly longer OS than those without osimertinib therapy after bevacizumab plus erlotinib or erlotinib alone treatment (32). A previous study also showed that T790M-mutated NSCLC patients receiving subsequent osimertinib therapy had significantly longer OS than those without acquired T790M mutation and subsequent osimertinib therapy. The same study showed that the use of 1st-generation or 2nd-generation EGFR-TKIs in first-line therapies did not affect OS (22). The results of our study are compatible with those shown in 2 previous clinical studies (22, 32). Taken together, these results indicated that the acquired T790M mutation is a key factor associated with OS in advanced EGFR-mutated NSCLC patients receiving 1st-generation or 2nd-generation EGFR-TKIs as first-line therapies.
Osimertinib is suggested as a first-line therapy for advanced EGFR-mutated NSCLC patients because a pivotal clinical trial (FLAURA) showed that osimertinib had a median PFS of 18.9 months and OS of 38.6 months, which were significantly longer than those of comparator therapies (10.2 months of median PFS and 31.8 months of median OS) (33). The median OS associated with first-line osimertinib in the FLAURA trial was 38.6 months (33). In the FLAURA trial (33), patients in the comparator arm received gefitinib or erlotinib alone treatments, whereas all patients in our study received bevacizumab in addition to erlotinib or afatinib.
A previous prospective trial (RELAY) demonstrated that erlotinib combined with ramucirumab had a significantly longer median PFS than erlotinib combined with placebo in untreated advanced EGFR-mutated NSCLC patients (19.4 vs. 12.4 months). Erlotinib combined with ramucirumab has been suggested as a first-line therapy choice for advanced EGFR-mutated NSCLC based on the results of the RELAY trial (34). However, patients with baseline brain metastasis were excluded by the RELAY trial, and the efficacy of ramucirumab combined with erlotinib in EGFR-mutated NSCLC patients with brain metastasis was not clear (34). In the NEJ026 study, 32% of study patients had baseline brain metastasis in the erlotinib combined with bevacizumab and erlotinib alone arms (14). A previous study also reported that bevacizumab in addition to EGFR-TKIs was more effective for brain metastasis control and prevention of the progression of brain metastasis than EGFR-TKI treatment alone in NSCLC with EGFR mutations (35). In addition, some previous studies reported that systemic administration of bevacizumab was effective for the control of NSCLC-related malignant pleural effusion (36). In this study, approximately 30% of patients had baseline brain metastasis and malignant pleural effusion. Regarding the concern regarding metastatic sites and study populations in previous studies, bevacizumab in combination with 1st-/2nd- EGFR-TKIs would be considered as first-line therapy for metastatic EGFR-mutated NSCLC patients.
In the NEJ026 clinical trial, patients who received osimertinib as second-line therapy had a median OS of approximately 50 months, and those who did not receive osimertinib treatments as second-line therapy had a median survival of approximately 40 months (32). In another retrospective clinical study (GioTag study), advanced EGFR-mutated NSCLC patients receiving first-line afatinib followed by osimertinib had a median OS of 37.6 months and 44.8 months in Asian patients (37, 38). A previous study also showed that patients receiving 1st/2nd-generation EGFR-TKIs followed by osimertinib had a median OS over 50 months (22). In this study, patients with acquired T790M mutations receiving subsequent osimertinib had a median OS of 54.3 months, which was compatible with the results of previous studies (22, 32, 37, 38). Together, these results suggest that the OS of advanced EGFR-mutated NSCLC patients who received bevacizumab in combination with 1st/2nd-generation EGFR-TKIs or afatinib alone followed by second-line osimertinib is not inferior to that of patients who received first-line osimertinib therapy. In addition, the median PFS of first-line bevacizumab combined with erlotinib or afatinib in advanced EGFR-mutated NSCLC patients shown by previous studies seems not inferior to first-line osimertinib (13–16). Therefore, bevacizumab combined with erlotinib or afatinib may be a choice of first-line therapy for advanced EGFR-mutated NSCLC patients other than osimertinib.
Rebiopsy, either liquid biopsy or direct tissue biopsy, for secondary T790M detection is recommended as standard care for advanced EGFR-mutated NSCLC with acquired resistance to 1st/2nd-generation EGFR-TKIs (21–26). Most patients in our study underwent tissue rebiopsy or liquid biopsy alone, and only a few (5 = 4.9%) patients underwent both procedures for tests. The second-line osimertinib therapy in this study had a 51.7% RR and a median PFS of 13.7 months, and these results indicated that the T790M mutation testing results were reliable in our study. Some previous studies have suggested that both liquid and tissue rebiopsy be performed for NGS tests because repeated biopsy by liquid or tissue increased the T790M detection rate and may also detect other genomic alterations for optimal subsequent treatment (39–41). In our study, all 5 patients who underwent both liquid and tissue rebiopsies were T790M-negative in liquid biopsy, and one was T790M-positive in tissue rebiopsy. This result indicated that a repeated tissue biopsy converts T790M-negative to T790M-positive results in some patients and is compatible with the findings of previous studies (39–41). In the 3 patients who underwent liquid biopsy alone, 2 had T790M-positive results, and 1 was T790M-negative. Although repeated rebiopsy has been recommended to increase the diagnostic accuracy and T790M positive rate, most patients received only one tissue rebiopsy. The main concerns regarding why patients did not receive repeated biopsies include personal acceptance, procedure-related adverse events, and tumor site procedure-unapproachable tumor sites such as tiny distant metastases (42, 43). Taken together, these findings explain why most patients have a low willingness to undergo repeated tissue rebiopsy in real-world clinical practice.
Small cell lung cancer transformation is a rare (<5%) acquired resistance to previous EGFR-TKI treatment (44). In this study, 2 patients had small cell transformation according to tissue rebiopsy and were excluded from further analysis. For advanced EGFR-mutated NSCLC patients who experienced small cell transformation after previous EGFR-TKI treatments, chemotherapy with platinum-based regimens combined with etoposide is recommended if the patient has acceptable performance status (44). For the 3 patients who underwent liquid biopsies only in this study, all of them were controlled by subsequent osimertinib treatments. Small cell transformation has also been reported as a resistance mechanism to prior osimertinib therapy in a previous study (44). According to the clinical treatment response to osimertinib in the 3 patients who underwent liquid biopsies only, the possibility of small cell transformation was very low, and the 3 patients were still included in this study.
Some limitations of this study should be clarified. First, the study population was East Asian, and whether the secondary T790M mutation rate and outcomes in other ethnic populations are similar to our results is unclear. A recent phase III clinical trial (BEVERLY) investigating the combination of bevacizumab with erlotinib for the treatment of advanced EGFR-mutated NSCLC recruited study patients mainly in European countries (45). In this trial, 24 (49%) patients in the bevacizumab combined with erlotinib arm were reported to receive osimertinib as second-line therapy, but information on the acquired T790M mutation and outcomes was not available (45). Second, the first-line EGFR-TKIs administered in this study were erlotinib and afatinib, and no patients in this study received gefitinib (1st-generation) or dacomitinib (2nd-generation) as first-line treatments. Future studies may be needed to analyze the clinical outcomes of advanced EGFR-mutated NSCLC patients receiving first-line bevacizumab combined with gefitinib or dacomitinib. Finally, the use of multiple genomic alteration detection methods, such as NGS, in NSCLC with acquired resistance to previous bevacizumab combined with erlotinib therapy increases in clinical practice, and resistant genomic alterations other than T790M, such as MET, HER2 or BRAF, can be detected by NGS (29). Targeted therapies for the abovementioned genomic alterations have been developed and explored in clinical trials (29), and patients who receive new targeted therapies may have improved outcomes in the future.
5 Conclusion
Our study clearly demonstrated the clinical perspective regarding sequential treatments with first-line bevacizumab combined with 1st/2nd-generation EGFR-TKIs in advanced lung adenocarcinoma patients harboring EGFR mutations. Secondary T790M mutation detection tests and optimal use of osimertinib may yield favorable survival outcomes.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This multicenter observational study was approved by the institutional review board (IRB) of the Chang Gung Medical Foundation (no. 202000137B0 and no. 202100379B0) and Taipei Buddhist Tzu Chi General Hospital (no. 09-X-002). Obtaining consent from study subjects was waived by the IRB because of the retrospective nature of this study. All patients in this study received standard cancer care and treatments following the protocol of Chang Gung Medical Foundation and Taipei Buddhist Tzu Chi General Hospital cancer centers. All treatment and evaluation procedures were performed in accordance with the Helsinki Declaration. No identifiable subjective information, such as personal ID or birthday, was presented in this manuscript.
Author contributions
Conception and design: P-CH, C-YH, C-TY. Acquisition of data: P-CH, C-YH, YL, S-HL, L-CC, H-WK, C-CW. Analysis and interpretation of data: P-CH, C-YH, YL, S-HL, L-CC, C-EW, SK, J-SJ, AH, H-WK, C-CW. Writing of the manuscript: P-CH, and C-TY. Review and revision of the manuscript: L-CC, C-EW, SK, H-WK, C-TY. Administrative and funding support: P-CH, C-YH, C-TY. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Taiwan Ministry of Science and Technology (MOST) (grant no. 111-2628-B-182-011– and 111-2628-B-182A-003- to P-CH).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1249106/full#supplementary-material
References
1. Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer (2007) 7:169–81. doi: 10.1038/nrc2088
2. Zhou H, Geng F, Chen Y, Du J, Zhang X, Liu B, et al. The mineral dust-induced gene mdig regulates angiogenesis and lymphangiogenesis in lung adenocarcinoma by modulating the expression of VEGF-A/C/D via EGFR and HIF-1α signaling. Oncol Rep (2021) 45(5):60. doi: 10.3892/or.2021.8011
3. Hsu PC, Jablons DM, Yang CT, You L. Epidermal growth factor receptor (EGFR) pathway, yes-associated protein (YAP) and the regulation of programmed death-ligand 1 (PD-L1) in non-small cell lung cancer (NSCLC). Int J Mol Sci (2019) 20(15):3821. doi: 10.3390/ijms20153821
4. Wei J, Meng P, Terpstra MM, van Rijk A, Tamminga M, Scherpen F, et al. Clinical value of EGFR copy number gain determined by amplicon-based targeted next generation sequencing in patients with EGFR-mutated NSCLC. Target Oncol (2021) 16(2):215–226y. doi: 10.1007/s11523-021-00798-2
5. Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small cell lung cancer (EURTAC): A multicenter, open-label, randomized phase 3 trial. Lancet Oncol (2012) 13:239–46. doi: 10.1016/S1470-2045(11)70393-X
6. Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Final overall survival results from a randomized, phase III study of erlotinib versus chemotherapy as first-line treatment of EGFR mutation-positive advanced non-small cell lung cancer (OPTIMAL, CTONG-0802). Ann Oncol (2015) 26:1877–83. doi: 10.1093/annonc/mdv276
7. Yang JC, Wu YL, Schuler M, Sebastian M, Popat S, Yamamoto N, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomized, phase 3 trials. Lancet Oncol (2015) 16(2):141–51. doi: 10.1016/S1470-2045(14)71173-8
8. Bi J, Dixit G, Zhang Y, Devor EJ, Losh HA, Newtson AM, et al. Advantages of tyrosine kinase anti-angiogenic cediranib over bevacizumab: cell cycle abrogation and synergy with chemotherapy. Pharm (Basel) (2021) 14(7):682. doi: 10.3390/ph14070682
9. Shiau JP, Wu CC, Chang SJ, Pan MR, Liu W, Ou-Yang F, et al. FAK regulates VEGFR2 expression and promotes angiogenesis in triple-negative breast cancer. Biomedicines (2021) 9(12):1789. doi: 10.3390/biomedicines9121789
10. Reinmuth N, Jauch A, Xu EC, Muley T, Granzow M, Hoffmann H, et al. Correlation of EGFR mutations with chromosomal alterations and expression of EGFR, ErbB3 and VEGF in tumor samples of lung adenocarcinoma patients. Lung Cancer (2008) 62:193–201. doi: 10.1016/j.lungcan.2008.03.011
11. Naumov GN, Nilsson MB, Cascone T, Briggs A, Straume O, Akslen LA, et al. Combined vascular endothelial growth factor receptor and epidermal growth factor receptor (EGFR) blockade inhibits tumor growth in xenograft models of EGFR inhibitor resistance. Clin Cancer Res (2009) 15(10):3484–94. doi: 10.1158/1078-0432.CCR-08-2904
12. Yang Y, Wang L, Li X, Zhang S, Yu J, Nie X, et al. Efficacy and safety of bevacizumab combined with EGFR-TKIs in advanced non-small cell lung cancer: A meta-analysis. Thorac Cancer (2022) 13(1):31–7. doi: 10.1111/1759-7714.14214
13. Seto T, Kato T, Nishio M, Goto K, Atagi S, Hosomi Y, et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced nonsquamous non-small cell lung cancer harboring EGFR mutations (JO25567): An open-label, randomized, multicenter, phase 2 study. Lancet Oncol (2014) 15:1236–44. doi: 10.1016/S1470-2045(14)70381-X
14. Saito H, Fukuhara T, Furuya N, Watanabe K, Sugawara S, Iwasawa S, et al. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced nonsquamous non-small cell lung cancer (NEJ026): Interim analysis of an open-label, randomized, multicenter, phase 3 trial. Lancet Oncol (2019) 20:625–35. doi: 10.1016/S1470-2045(19)30035-X
15. Wang CC, Chiu LC, Tung PH, Kuo SC, Chu CH, Huang AC, et al. A real-world analysis of patients with untreated metastatic epidermal growth factor receptor (EGFR)-mutated lung adenocarcinoma receiving first-line erlotinib and bevacizumab combination therapy. Oncol Ther (2021) 9(2):489–503. doi: 10.1007/s40487-021-00152-6
16. Lee SH, Lin YC, Chiu LC, Ju JS, Tung PH, Huang AC, et al. Comparison of afatinib and erlotinib combined with bevacizumab in untreated stage IIIB/IV epidermal growth factor receptor-mutated lung adenocarcinoma patients: a multicenter clinical analysis study. Ther Adv Med Oncol (2022) 14. doi: 10.1177/17588359221113278
17. Ishikawa N, Ninomiya T, Kozuki T, Kuyama S, Inoue K, Toshihide, et al. Afatinib (Afa) + bevacizumab (Bev) versus afatinib alone as first-line treatment of patients with EGFR-mutated advanced nonsquamous NSCLC: Primary analysis of the multicenter, randomized, phase II study—AfaBev-CS study. J Clin Oncol (2022) 16_suppl:9112–2. doi: 10.1200/JCO.2022.40.16_suppl.9112
18. Seto T, Nogami N, Yamamoto N, Atagi S, Tashiro N, Yoshimura Y, et al. Real-world EGFR T790M testing in advanced non-small cell lung cancer: A prospective observational study in Japan. Oncol Ther (2018) 6(2):203–15. doi: 10.1007/s40487-018-0064-8
19. Buder A, Hochmair MJ, Filipits M. The allele frequency of EGFR mutations predicts survival in advanced EGFR T790M-positive non-small cell lung cancer patients treated with Osimertinib. Target Oncol (2021) 16(1):77–84. doi: 10.1007/s11523-020-00781-3
20. Mok TS, Wu Y-L, Ahn M-J, Garassino MC, Kim HR, Ramalingam SS, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med (2017) 376(7):629–40. doi: 10.1056/NEJMoa1612674
21. Jänne PA, Yang JC, Kim DW, Planchard D, Ohe Y, Ramalingam SS, et al. AZD9291 in EGFR inhibitor-resistant non-small cell lung cancer. N Engl J Med (2015) 372(18):1689–99. doi: 10.1056/NEJMoa1411817
22. Hsu PC, Chang JW, Chang CF, Huang CY, Yang CT, Kuo CS, et al. Sequential treatment in advanced non-small cell lung cancer harboring EGFR mutations. Ther Adv Respir Dis (2022) 16. doi: 10.1177/17534666221132731
23. Huang AC, Huang CH, Ju JS, Chiu TH, Tung PH, Wang CC, et al. First- or second-generation epidermal growth factor receptor tyrosine kinase inhibitors in a large, real-world cohort of patients with non-small cell lung cancer. Ther Adv Med Oncol (2021) 13. doi: 10.1177/17588359211035710
24. Hsieh PC, Wu YK, Huang CY, Yang MC, Kuo CY, Tzeng IS, et al. Comparison of T790M acquisition after treatment with first- and second-generation tyrosine-kinase inhibitors: A systematic review and network meta-analysis. Front Oncol (2022) 12:869390. doi: 10.3389/fonc.2022.869390
25. Wu SG, Chiang CL, Liu CY, Wang CC, Su PL, Hsia TC, et al. An observational study of acquired EGFR T790M-dependent resistance to EGFR-TKI treatment in lung adenocarcinoma patients in Taiwan. Front Oncol (2020) 4:10:1481. doi: 10.3389/fonc.2020.01481
26. Lin YT, Chen JS, Liao WY, Ho CC, Hsu CL, Yang CY, et al. Clinical outcomes and secondary epidermal growth factor receptor (EGFR) T790M mutation among first-line gefitinib, erlotinib and afatinib-treated non-small cell lung cancer patients with activating EGFR mutations. Int J Cancer (2019) 144(11):2887–96. doi: 10.1002/ijc.32025
27. Hata A, Katakami N, Kaji R, Yokoyama T, Kaneda T, Tamiya M, et al. Does afatinib plus bevacizumab combination therapy induce positive conversion of T790M in previously negative patients? Oncotarget (2018) 9(78):34765–71. doi: 10.18632/oncotarget.26192
28. Hsu PC, Liu CY, Li SH, Huang SH, Wang CL, Kuo SCH, et al. Efficacy of platinum-based combination chemotherapy in advanced lung adenocarcinoma harboring sensitive epidermal growth factor receptor (EGFR) mutations with acquired resistance to first-line EGFR tyrosine kinase inhibitor (TKI). Cancer Treat Res Commun (2016) 9:48–55. doi: 10.1016/j.ctarc.2016.07.005
29. Magios N, Bozorgmehr F, Volckmar AL, Kazdal D, Kirchner M, Herth FJ, et al. Real-world implementation of sequential targeted therapies for EGFR-mutated lung cancer. Ther Adv Med Oncol (2021) 13. doi: 10.1177/1758835921996509
30. Digkas E, Tabiim AJ, Smith D, Valachis A. Randomized versus real-world evidence on the efficacy and toxicity of checkpoint inhibitors in cancer in patients with advanced non-small cell lung cancer or melanoma: A meta-analysis. Target Oncol (2022) 17(5):507–15. doi: 10.1007/s11523-022-00901-1
31. Yang JC, Ahn MJ, Kim DW, Ramalingam SS, Sequist LV, Su WC, et al. Osimertinib in pretreated T790M-positive advanced non-small cell lung cancer: AURA study phase II extension component. J Clin Oncol (2017) 35(12):1288–96. doi: 10.1200/JCO.2016.70.3223
32. Kawashima Y, Fukuhara T, Saito H, Furuya N, Watanabe K, Sugawara S, et al. Bevacizumab plus erlotinib versus erlotinib alone in Japanese patients with advanced, metastatic, EGFR-mutant non-small cell lung cancer (NEJ026): overall survival analysis of an open-label, randomized, multicenter, phase 3 trial. Lancet Respir Med (2022) 10(1):72–82. doi: 10.1016/S2213-2600(21)00166-1
33. Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med (2020) 382(1):41–50. doi: 10.1056/NEJMoa1913662
34. Nakagawa K, Garon EB, Seto T, Nishio M, Ponce Aix S, Paz-Ares L, et al. Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small cell lung cancer (RELAY): a randomized, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol (2019) 20(12):1655–69. doi: 10.1016/S1470-2045(19)30634-5
35. Feng PH, Chen KY, Huang YC, Luo CS, Wu SM, Chen TT, et al. Bevacizumab reduces S100A9-positive MDSCs linked to intracranial control in patients with EGFR-mutant lung adenocarcinoma. J Thorac Oncol (2018) 13(7):958–67. doi: 10.1016/j.jtho.2018.03.032
36. Zhao J, Liu N, Zhang B, He X, Ma Q, et al. The role of angiogenesis in Malignant pleural effusion: from basic research to clinical application. Am J Cancer Res (2022) 12(11):4879–91.
37. Hochmair MJ, Morabito A, Hao D, Yang CT, Soo RA, Yang JC, et al. Sequential afatinib and osimertinib in patients with EGFR mutation-positive non-small cell lung cancer: updated analysis of the observational GioTag study. Future Oncol (2019) 15(25):2905–14. doi: 10.2217/fon-2019-0346
38. Hochmair MJ, Morabito A, Hao D, Yang CT, Soo RA, Yang JC, et al. Sequential afatinib and osimertinib in patients with EGFR mutation-positive non-small cell lung cancer: final analysis of the GioTag study. Future Oncol (2020) 16(34):2799–808. doi: 10.2217/fon-2020-0740
39. Chiang CL, Huang HC, Shen CI, Luo YH, Chen YM, Chiu CH. PostProgression survival in secondary EGFR T790M-mutated non-small cell lung cancer patients with and without Osimertinib after failure of a previous EGFR TKI. Target Oncol (2020) 15(4):503–12. doi: 10.1007/s11523-020-00737-7
40. Popat S, Jung HA, Lee SY, Hochmair MJ, Lee SH, Escriu C, et al. Sequential afatinib and osimertinib in patients with EGFR mutation-positive NSCLC and acquired T790M: A global noninterventional study (UpSwinG). Lung Cancer (2021) 162:9–15. doi: 10.1016/j.lungcan.2021.09.009
41. Leonetti A, Sharma S, Minari R, Perego P, Giovannetti E, Tiseo M. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br J Cancer (2019) 121(9):725–37. doi: 10.1038/s41416-019-0573-8
42. Hasegawa T, Sawa T, Futamura Y, Horiba A, Ishiguro T, Marui T, et al. Feasibility of rebiopsy in non-small cell lung cancer treated with epidermal growth factor receptor-tyrosine kinase inhibitors. Intern Med (2015) 54(16):1977–80. doi: 10.2169/internalmedicine.54.4394
43. Kim TO, Oh IJ, Kho BG, Park HY, Chang JS, Park CK, et al. Feasibility of rebiopsy and EGFR mutation analysis in patients with non-small cell lung cancer. Thorac Cancer (2018) 9(7):856–64. doi: 10.1111/1759-7714.12762
44. Sato Y, Saito G, Fujimoto D. Histologic transformation in lung cancer: when one door shuts, another opens. Ther Adv Med Oncol (2022) 14. doi: 10.1177/17588359221130503
45. Piccirillo MC, Bonanno L, Garassino MC, Esposito G, Dazzi C, Cavanna L, et al. Addition of bevacizumab to erlotinib as first-line treatment of patients with EGFR-mutated advanced nonsquamous NSCLC: the BEVERLY multicenter randomized phase 3 trial. J Thorac Oncol (2022) 17(9):1086–97. doi: 10.1016/j.jtho.2022.05.008
Keywords: epidermal growth factor receptor mutation, tyrosine kinase inhibitor, bevacizumab, lung adenocarcinoma, T790M, osimertinib
Citation: Hsu P-C, Huang C-Y, Lin Y-C, Lee S-H, Chiu L-C, Wu C-E, Kuo SC-H, Ju J-S, Huang AC-C, Ko H-W, Wang C-C and Yang C-T (2023) Sequential treatment in advanced epidermal growth factor receptor-mutated lung adenocarcinoma patients receiving first-line bevacizumab combined with 1st/2nd-generation EGFR-tyrosine kinase inhibitors. Front. Oncol. 13:1249106. doi: 10.3389/fonc.2023.1249106
Received: 28 June 2023; Accepted: 14 September 2023;
Published: 03 October 2023.
Edited by:
Yusuke Okuma, National Cancer Center Hospital, JapanReviewed by:
Akihiro Yoshimura, Kyoto Prefectural University of Medicine, JapanYuping Li, Wenzhou Medical University, China
Copyright © 2023 Hsu, Huang, Lin, Lee, Chiu, Wu, Kuo, Ju, Huang, Ko, Wang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cheng-Ta Yang, eWFuZzE5NDZAYWRtLmNnbWgub3JnLnR3
 Ping-Chih Hsu
Ping-Chih Hsu Chun-Yao Huang3
Chun-Yao Huang3 Yu-Ching Lin
Yu-Ching Lin Chiao-En Wu
Chiao-En Wu Scott Chih-Hsi Kuo
Scott Chih-Hsi Kuo Ho-Wen Ko
Ho-Wen Ko Chin-Chou Wang
Chin-Chou Wang