
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
EDITORIAL article
Front. Oncol., 20 July 2023
Sec. Pharmacology of Anti-Cancer Drugs
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1248272
This article is part of the Research TopicAnti-Cancer Drug Delivery: Lipid–Based NanoparticlesView all 6 articles
Editorial on the Research Topic
Anti-cancer drug delivery: lipid–based nanoparticles
Cancer continues to pose significant challenges that require extensive attention and efforts from the scientific community. The battle against cancer encompasses the development of effective and safe therapeutic approaches. However, achieving this balance is highly complex for anticancer therapies, as they often exhibit intense intrinsic cytotoxicity, affecting both cancerous and healthy cells and resulting in substantial toxicity that limits their clinical utility. A promising strategy to address this challenge involves the selective guidance of therapeutic agents to the cancer site, minimizing off-target effects. Nanotechnology offers powerful tools to engineer smart and targeted therapeutics that preferentially accumulate in cancerous tissues (1). This preferential localization is achieved through the Enhanced Permeation and Retention (EPR) effect, first reported by Prof. Hiroshi Maeda in 1984 (2). The EPR effect leverages the leaky vasculature in tumor regions, enabling enhanced infiltration of nanotherapeutics and localizing their therapeutic effects, which is commonly described as “passive targeting “. On the other hand, nanotechnologists may also employ “active targeting” by modifying nanoparticle surfaces with homing ligands that selectively recognize cancer cells (3). Both passive and active targeting strategies are keys for success of nanoparticle-based drug delivery systems, and serve as a justification for the development of nanotherapeutics. Extensive literature exists on various types of nanoparticles and nanomaterials with potential applications as drug delivery systems.
This Research Topic specifically focuses on lipid-based nanoparticles, which have emerged as one of the most extensively utilized nanotechnology platforms in drug delivery (4). Lipid nanoparticles was the first nanotherapeutic to secure FDA approval of Doxil® in 1995 (Figure 1) which is a delivery system for the anti-cancer drug doxorubicin (5). Recently, lipid-based nanoparticles have played a pivotal role in combating the global COVID-19 pandemic by facilitating the delivery of mRNA vaccines (6, 7). The successful clinical implementation of lipid-based nanoparticles in this context underscores their significant and growing presence in the field of drug delivery (Figure 1).
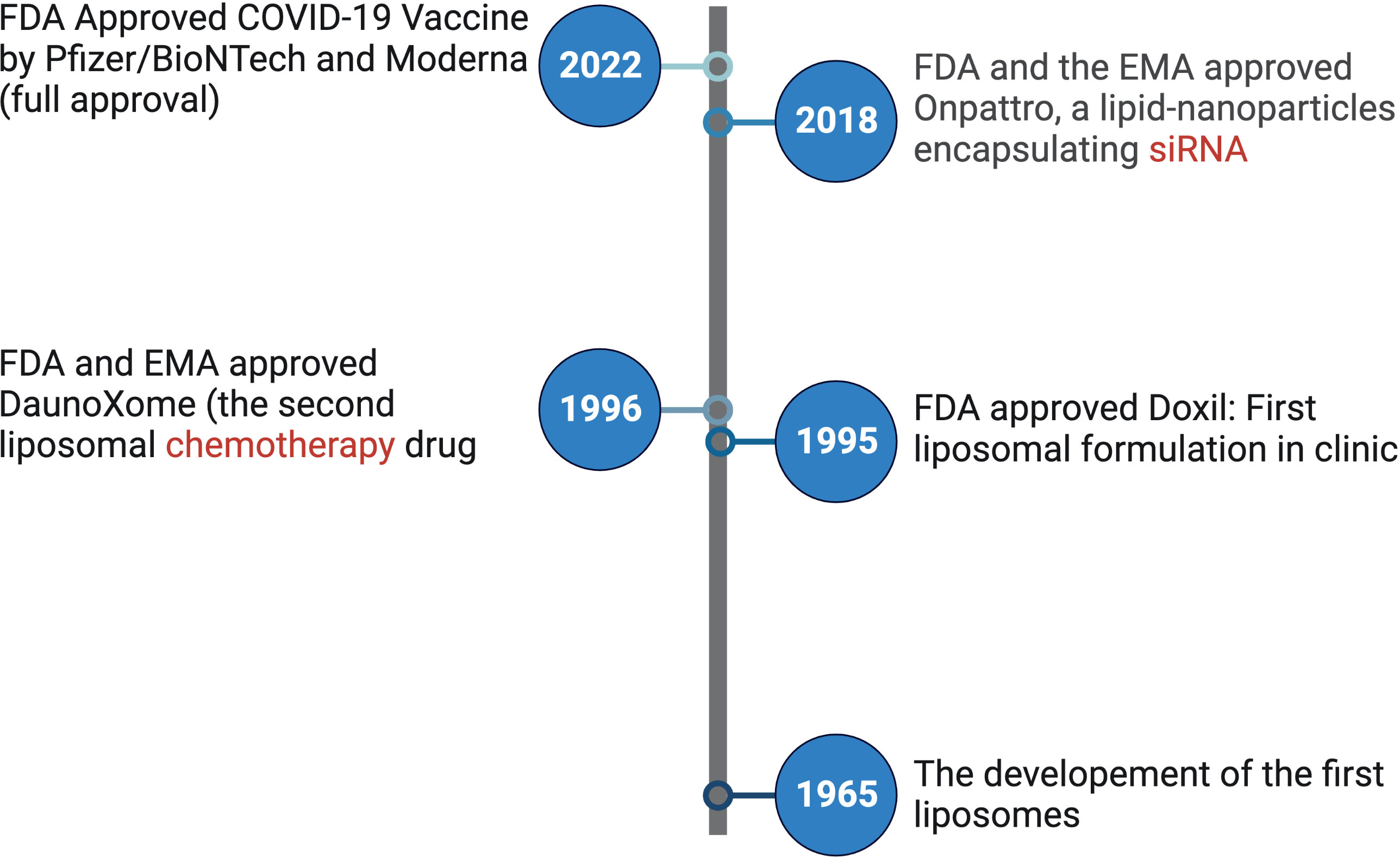
Figure 1 Timeline of selected important milestones for lipid nanoparticle-based therapeutic development as labeled. LNP, lipid nanoparticles; siRNA, small interfering RNA; FDA, United States Food and Drug Administration; EMA, European Medicines Agency.
Our Research Topic combines both clinical and preclinical evaluations of lipid-based nanoparticles and presents crucial findings that contribute to our collective understanding of lipidic nanoparticles in human, animal models, and cell cultures. Zhou et al. investigated the pharmacokinetics (PK) and safety profile of paclitaxel liposomes in patients with non-small cell lung cancer using population PK models. Their three-compartment model accurately described PK as well as exposure-safety relationship of paclitaxel liposome. The most important observation of this study was the probable association of neutropenia with higher exposure of paclitaxel liposomes. This is an invaluable source of information for clinicians for optimizing the clinical dosage of paclitaxel liposomes. Li et al. evaluated the bioequivalence and safety of generic and brand pegylated liposomal doxorubicin in breast cancer patients. Their multicenter crossover study confirmed the bioequivalence and comparable safety of the generic liposomal formulation to the reference product (Caelyx®). These results promote the momentum of generic nanotherapeutics which should ultimately widen the use of nanomedicine in the clinic.
Liver inflammation is associated with hepatocellular carcinoma (HCC) development; triiodothyronine (T3), being an anti-inflammatory drug may inhibit the hepatocarcinogenesis. Sun et al. reported the effective inhibition of hepatocarcinogenesis using T3-loaded liposomes via regulating the Inflammatory Microenvironment. This observation was confirmed in vitro and in rat model and the molecular mechanism was explored and reported. The highlight of the study was the evidence showing selective absorption of lipo-T3 by hepatic macrophases and remarkable reduction of drug associated side effects. Arsiwala et al. examined how low-intensity focused ultrasound (LiFUS)-mediated disruption of the blood-tumor barrier (BTB) affects the outcomes of survival of mice with brain metastases from triple-negative breast cancer (TNBC) when combined with a pegylated liposomal nanotherapeutics that circulates in the body for an extended period. Their results indicate that the combination of LiFUS and Doxil significantly increased the survival and slowed tumor progression in mice with TNBC brain metastases due to prolonged activity of doxorubicin within the tumor lesions. Finally, Hegde et al. provided the therapeutic applications of lipid-based nanoplatforms in central nervous system (CNS) tumors, with a focus on brain targeting, imaging, and immunotherapy. There critical analysis of the available literature concluded that Lipid-based nanoplatforms hold promise for precise and effective treatment of CNS tumors, with potential for revolutionizing cancer therapy through enhanced drug delivery and immunotherapy
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
The authors would like to thank to the journal (Frontiers in Oncology) for providing us this opportunity to organize the research topic on “Anti-Cancer Drug Delivery: Lipid–Based Nanoparticles”. We thank all the authors who contributed in this topic collection. We are also, very grateful to all the reviewers who participated in the whole manuscript review process.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Domingues C, Santos A, Alvarez-Lorenzo C, Concheiro A, Jarak I, Veiga F, et al. Where is nano today and where is it headed? A review of nanomedicine and the dilemma of nanotoxicology. ACS Nano (2022) 16:9994–10041. doi: 10.1021/acsnano.2c00128
2. Fang J, Nakamura H, Maeda H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Delivery Rev (2011) 63:136–51. doi: 10.1016/j.addr.2010.04.009
3. Alkilany AM, Zhu L, Weller H, Mews A, Parak WJ, Barz M, et al. Ligand density on nanoparticles: A parameter with critical impact on nanomedicine. Adv Drug Deliv Rev (2019) 143:22–36. doi: 10.1016/j.addr.2019.05.010
4. Tenchov R, Bird R, Curtze AE, Zhou Q. Lipid nanoparticles─From liposomes to mRNA vaccine delivery, a landscape of research diversity and advancement. ACS Nano (2021) 15:16982–7015. doi: 10.1021/acsnano.1c04996
5. Barenholz Y. Doxil® — The first FDA-approved nano-drug: Lessons learned. J Controlled Release (2012) 160:117–34. doi: 10.1016/j.jconrel.2012.03.020
6. Khurana A, Allawadhi P, Khurana I, Allwadhi S, Weiskirchen R, Banothu AK, et al. Role of nanotechnology behind the success of mRNA vaccines for COVID-19. Nano Today (2021) 38:101142–2. doi: 10.1016/j.nantod.2021.101142
Keywords: lipid-based nanoparticles, nanotherapeutics, drug delivery systems, liposomal formulations, cancer
Citation: Alkilany AM, Elhissi A, Alshaer W, Kunwar A and Giri J (2023) Editorial: Anti-cancer drug delivery: lipid-based nanoparticles. Front. Oncol. 13:1248272. doi: 10.3389/fonc.2023.1248272
Received: 27 June 2023; Accepted: 10 July 2023;
Published: 20 July 2023.
Edited and Reviewed by:
Olivier Feron, Université catholique de Louvain, BelgiumCopyright © 2023 Alkilany, Elhissi, Alshaer, Kunwar and Giri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alaaldin M. Alkilany, YWxraWxhbnlAcXUuZWR1LnFh; Amit Kunwar, a2FtaXRAYmFyYy5nb3YuaW4=; Jyotsnendu Giri, amdpcmlAYm1lLmlpdGguYWMuaW4=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.