- 1David Geffen School of Medicine, University of California, Los Angeles/Translational Research in Oncology-United States Network, Los Angeles, CA, United States
- 2Eli Lilly and Company, Lilly Corporate Center, Indianapolis, IN, United States
- 3Eli Lilly and Company, Bengaluru, Karnataka, India
- 4Department of Thoracic Oncology, Airway Research Center North (ARCN), Member of the German Center for Lung Research (DZL), Lung Clinic Grosshansdorf, Großhansdorf, Germany
Introduction: In the REVEL trial, ramucirumab plus docetaxel demonstrated significant improvements in overall survival (OS), progression-free survival (PFS), and overall response rate (ORR) compared with placebo plus docetaxel for treatment of metastatic non-small cell lung cancer (NSCLC) that progressed during or after platinum-based chemotherapy. Since the approval of ramucirumab plus docetaxel, immune checkpoint inhibitors (ICIs), either as single agents or in combination with chemotherapy, have become the standard of care for first-line treatment of patients with advanced NSCLC. However, efficacy and safety data for ramucirumab plus docetaxel after prior ICI treatment from randomized controlled clinical studies are lacking.
Methods: Following Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines, a systematic literature review was performed. Electronic databases and select international oncology conference proceedings were searched. Studies published between 01 January 2014 and 01 July 2022, which evaluated 2 efficacy outcomes (and included at least 1 time-to-event endpoint) or safety outcomes of ramucirumab plus docetaxel in NSCLC that progressed after prior ICI treatment, were identified. Twelve studies were included in the analysis. Two treatment groups were selected: ramucirumab plus docetaxel after prior ICI ± chemotherapy (RAM + DTX ICI pre-treated) and ramucirumab plus docetaxel after prior chemotherapy only (RAM + DTX ICI naïve). OS, PFS, ORR, disease control rate (DCR), and safety data were extracted and descriptively summarized across both treatment groups.
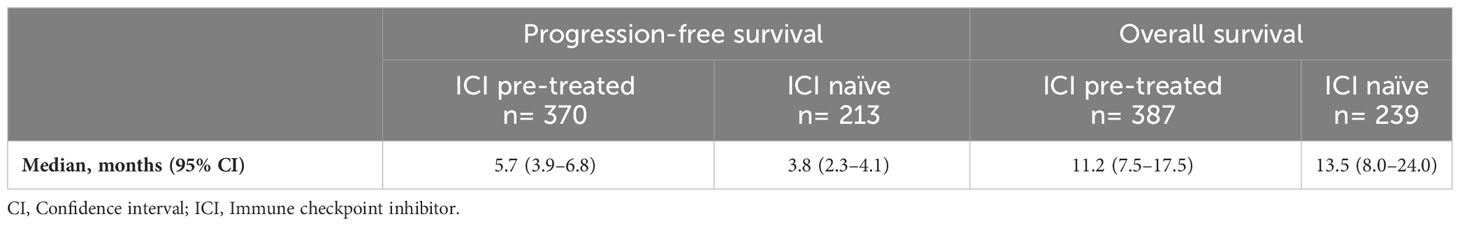
Results: The pooled weighted median PFS and median OS were 5.7 months (95% confidence interval [CI]: 3.9-6.8) and 11.2 months (95% CI: 7.5-17.5), respectively, in the RAM + DTX ICI pre-treated group and 3.8 months (95% CI: 2.3-4.1) and 13.5 months (95% CI: 8-24.0), respectively, in the RAM + DTX ICI naïve group. The ORR and DCR ranged from 20.9% to 60.0% and from 62.4% to 90.0%, respectively, in the RAM + DTX ICI pre-treated group and from 17.7% to 20.0% and from 57.1% to 75.0%, respectively, in the RAM + DTX ICI naïve group. The safety profile across studies was consistent between both treatment groups, and no new safety signals were reported.
Conclusions: Cumulatively, these results support the combination of ramucirumab plus docetaxel as an effective and safe subsequent therapy for the treatment of patients with metastatic NSCLC with disease progression irrespective of previous ICI treatment.
1 Introduction
The introduction of immune checkpoint inhibitors (ICIs) for the first-line treatment of patients with advanced non-small cell lung cancer (NSCLC) without driver alterations has dramatically improved clinical outcomes, with patients experiencing prolonged overall survival (OS) and durable responses compared with chemotherapy alone (1–6). Several ICIs, including anti-programmed death 1 (PD-1) or anti-programmed death ligand 1 (anti-PD-L1) antibodies, are currently recommended for the front-line treatment of metastatic NSCLC, either as single agents in select patient populations or in combination with chemotherapy or other immunotherapeutic agents (7, 8). However, approximately 50% of patients receive subsequent treatment upon progression during or after first-line treatment (9–12).
The current treatment guidelines for patients with metastatic NSCLC who experience disease progression after standard-of-care therapy in the first-line setting (7, 8) consist of single-agent chemotherapy or a combination of docetaxel with an antiangiogenic agent such as ramucirumab and nintedanib, or single agent anti-PD-(L)1 antibodies if not previously administered (13–17). Clinical outcomes with single-agent chemotherapy are modest. Treatment with docetaxel, in comparison to best supportive care, resulted in an overall response rate (ORR) of 7.1%, time to progression of 10.6 weeks, and median OS of 7.0 months (18). Similarly, treatment with gemcitabine, pemetrexed, or nab-paclitaxel demonstrated a median OS of 5.1 months (19), 8.3 months (20), and 8.5 months (21), respectively. Combination approaches with chemotherapy and antiangiogenic agents in the second-line setting have produced more favorable outcomes compared with chemotherapy alone.
Ramucirumab is a fully human immunoglobulin G1 monoclonal antibody that specifically binds to the vascular endothelial growth factor (VEGF) receptor-2 extracellular domain with high affinity, preventing binding of all VEGF ligands and subsequent receptor activation (22). In the phase 3 REVEL trial, the combination of ramucirumab plus docetaxel demonstrated a significant improvement in median OS (10.5 vs 9.1 months; hazard ratio [HR]: 0.86; P=0.023), median progression-free survival (median PFS, 4.5 vs 3.0 months; HR: 0.76; P<0.0001), and ORR (23% vs 14%; odds ratio: 1.89; P<0.0001) relative to docetaxel plus placebo in patients with stage IV NSCLC whose disease had progressed during or after first-line platinum-based chemotherapy (13). Importantly, ramucirumab plus docetaxel had a manageable safety profile and no detrimental impact on quality of life (13, 23). Based on these results, ramucirumab in combination with docetaxel received regulatory approval in 2014 in the United States and European Union for the treatment of patients with metastatic NSCLC with disease progression during or after platinum-based chemotherapy (24, 25). Additionally, second-line treatment with ramucirumab plus docetaxel resulted in an improvement of median PFS relative to docetaxel in other studies (26, 27), including in a randomized phase 2 trial that enrolled 160 Japanese patients with stage IV NSCLC (median PFS: 5.2 vs 4.2 months; HR: 0.83, 95% CI: 0.59-1.16) (28).
Studies have also demonstrated improved efficacy with other antiangiogenic agents in combination with chemotherapy in the second-line treatment of metastatic NSCLC. In the LUME-Lung 1 study, the combination of nintedanib, an antiangiogenic agent targeting 3 angiogenesis-related transmembrane receptors (14), with docetaxel resulted in statistically significant improvement in PFS, compared to docetaxel monotherapy. However, a statistically significant improvement in OS was observed only in the subgroup of patients with adenocarcinoma histology but not in the intention-to-treat population (13, 14). Hence, the approval of nintedanib in the European Union was restricted to NSCLC patients with adenocarcinoma histology who have undergone first-line chemotherapy (29).
The currently recommended treatment options for patients with NSCLC whose disease progressed during or after first-line treatment were investigated before the approval of ICIs in immunotherapy-naïve patients. Therefore, the results from these trials, including REVEL, do not optimally reflect the current patient population with disease progression after ICI treatment. Randomized controlled studies investigating the efficacy and safety of ramucirumab plus docetaxel in the post-immunotherapy setting are lacking. Nevertheless, the efficacy and safety of ramucirumab plus docetaxel in patients previously treated with ICIs have been reported in recent years, mostly from retrospective observational studies (30–44) and electronic health record studies (45–47). We conducted a systematic literature review (SLR) to consolidate the available evidence on the efficacy and safety of ramucirumab plus docetaxel when administered to patients with metastatic NSCLC previously treated with ICIs.
2 Methods
2.1 Search strategy
The SLR search, selection, and data extraction were conducted and reported using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 statement (48).
PubMed and EMBASE were searched to identify English-language manuscripts and abstracts submitted to select international oncology meetings (American Society of Clinical Oncology, European Society for Medical Oncology and World Conference on Lung Cancer) between 01 January 2014 and 01 July 2022. This literature search was completed on 11 July 2022. The following MeSH terms were used: (“non-small cell lung cancer” OR “NSCLC”) AND (“docetaxel” OR “DTX”) AND (“ramucirumab” OR “RAM”) AND (“immunotherapy” OR “Immune checkpoint inhibitor” OR “nivolumab” OR “pembrolizumab” OR “atezolizumab” OR “anti PD-1” OR “PD-L1”) (Supplementary Text 1.1).
The analysis included studies that evaluated at least 2 efficacy endpoints with at least one being a time-to-event endpoint (PFS or OS) of treatment with ramucirumab plus docetaxel in patients with advanced NSCLC who received prior ICI treatment. Studies reporting safety outcomes (irrespective of whether they reported efficacy outcomes) were also included. Two treatment groups were selected: ramucirumab plus docetaxel after prior ICI ± chemotherapy (RAM + DTX ICI pre-treated) and ramucirumab plus docetaxel after prior chemotherapy only (RAM + DTX ICI naïve).
The studies differed from each other on account of variations in several characteristics including sample size, lines of prior treatment, presence of driver alterations, PD-L1 expression, and patient performance status. Individual patient-level data was not available from any study. Therefore, data from the included studies were analyzed in a descriptive manner without formal statistical analysis. PFS, OS, ORR, disease control rate (DCR), and safety results were extracted and summarized for the 2 treatment groups.
2.2 Statistical analysis
Efficacy outcomes for each treatment group were pooled from the studies selected for efficacy analysis and compared descriptively. Estimates of median PFS and median OS from individual studies were pooled using the weighted median of medians, and approximate 95% confidence intervals (CIs) for weighted pooled medians were calculated using the wtd.quantile function from the Hmisc package of the R Statistical Software (v4.1.2; R Core Team 2021) (49). The weighted median of the study-specific medians was a pooled median estimate, where the weights were proportional to the number of patients in the study because sample sizes in the studies were independent of the individual study medians. ORR and DCR were summarized as ranges of percentages with 95% CIs from individual studies. For studies with available safety data, a descriptive comparison was performed for common adverse events (AEs) and AEs of special interest.
2.3 Assessment of bias
The Newcastle-Ottawa Scale was used to assess the risk of bias of studies comparing RAM + DTX ICI pre-treated versus RAM + DTX ICI naïve treatment groups (50). The Newcastle-Ottawa Scale includes 8 items within 3 categories: selection (4 items, 1 point each), comparability (1 item, up to 2 points), and outcome (3 items, 1 point each). The sum of points represents the methodologic quality of each study included in the SLR, with 9 points indicating the highest quality and 0 points the lowest quality. For studies that reported results from the RAM + DTX ICI pre-treated group only, the “quality assessment tool for before-after (pre-post) studies with no control group” outlined by the National Institutes of Health was used to assess the risk of bias (51). This tool comprises 12 questions, such as selection, reporting, or observer bias, with responses of yes, no, other, not reported (NR), not applicable, or cannot determine. All selected studies were free from the risk of bias as per the Newcastle-Ottawa Scale (Supplementary Table 1) and National Institutes of Health tools score (Supplementary Table 2). Two independent reviewers from Eli Lilly and Company assessed the risk of bias in the included studies for the RAM + DTX ICI pre-treated versus RAM + DTX ICI naïve groups. Disagreements were resolved by consensus with assistance from a third reviewer, also from Eli Lilly and Company.
3 Results
3.1 Search results
An initial literature search yielded 142 entries, with 23 results from PubMed and 119 results from EMBASE (Figure 1). Twelve of the 142 studies were included in the final analysis. Of these, 10 were retrospective observational studies (30–34, 36, 38–41) and 2 were prospective studies (43, 44). Among the 10 retrospective studies, 6 studies reported both safety and efficacy endpoints (30, 32–34, 39, 40) and 4 studies reported only efficacy endpoints (31, 36, 38, 41). Among the 10 studies included in the efficacy analysis, 5 compared outcomes between RAM + DTX ICI pre-treated and RAM + DTX ICI naïve patients (30–32, 38, 41) while the remaining 5 studies reported efficacy results only for RAM + DTX ICI pre-treated patients (33, 34, 36, 39, 40). Safety outcomes were extracted from 6 retrospective studies and 2 prospective studies (30, 32–34, 39, 40, 43, 44). Of the 2 prospective studies, one was a phase 2 randomized clinical trial that investigated the combination of pembrolizumab plus ramucirumab in patients whose disease had progressed during or after front-line ICI and platinum-based chemotherapy. The control arm, which comprised investigator’s choice standard-of-care therapy, included ramucirumab plus docetaxel among other agents. Safety data from the cohort of patients treated with ramucirumab plus docetaxel were used for the safety analysis reported in this SLR (44). The other prospective trial was a single-arm, multicenter, post marketing study, which reported safety and a time-to-event efficacy endpoint (12-month OS rate) in patients treated with ramucirumab plus docetaxel in the post-immunotherapy setting only (43).
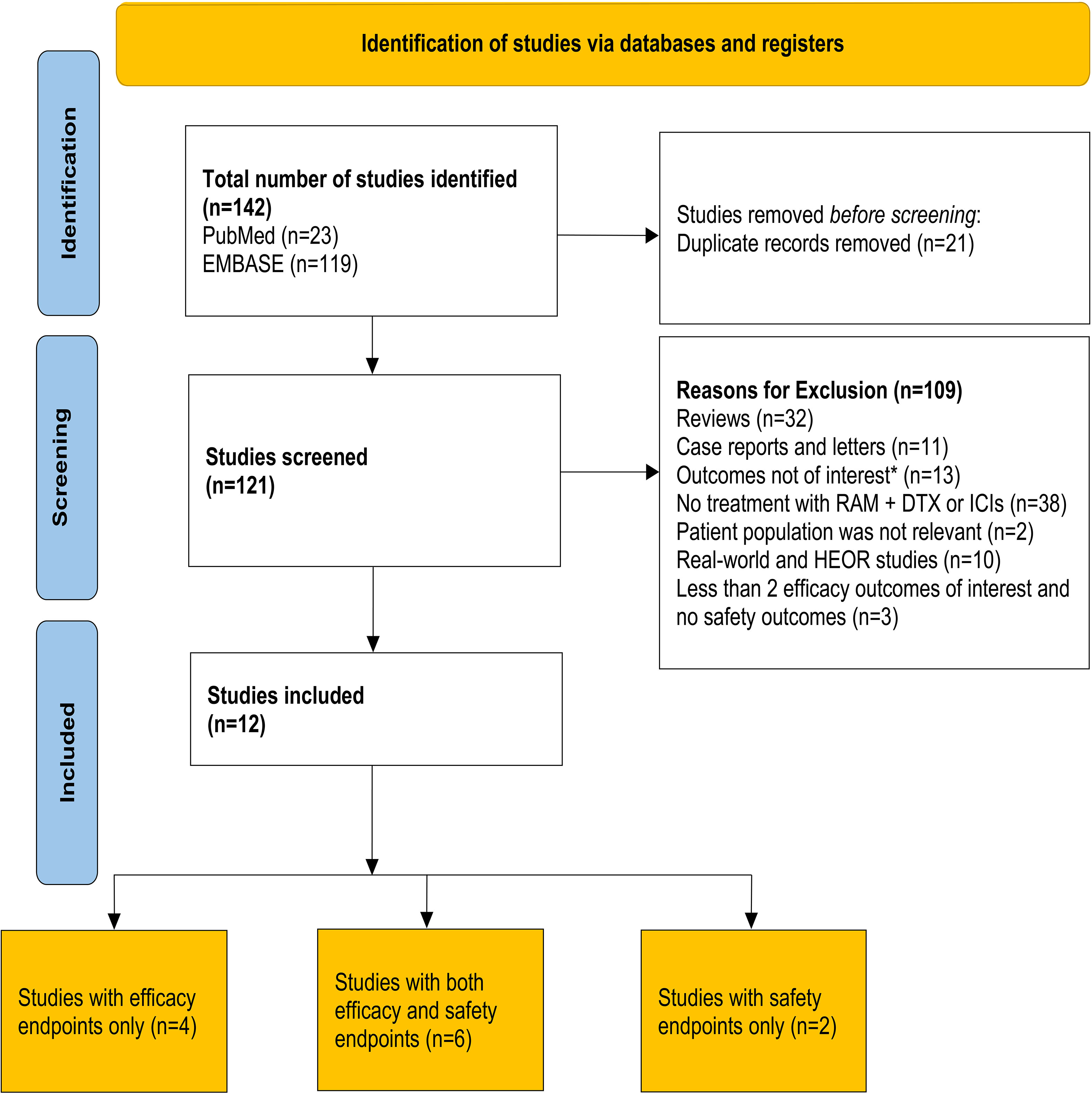
Figure 1 PRISMA flow diagram for article selection. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram for the systematic review, detailing the database searches and the number of studies screened and included for final analysis. *Outcomes that were not of interest included 12-month overall survival rate and time to treatment discontinuation. DTX, Docetaxel; HEOR, Health economics and outcomes research; ICI, Immune checkpoint inhibitor; n, Number of patients; RAM, Ramucirumab.
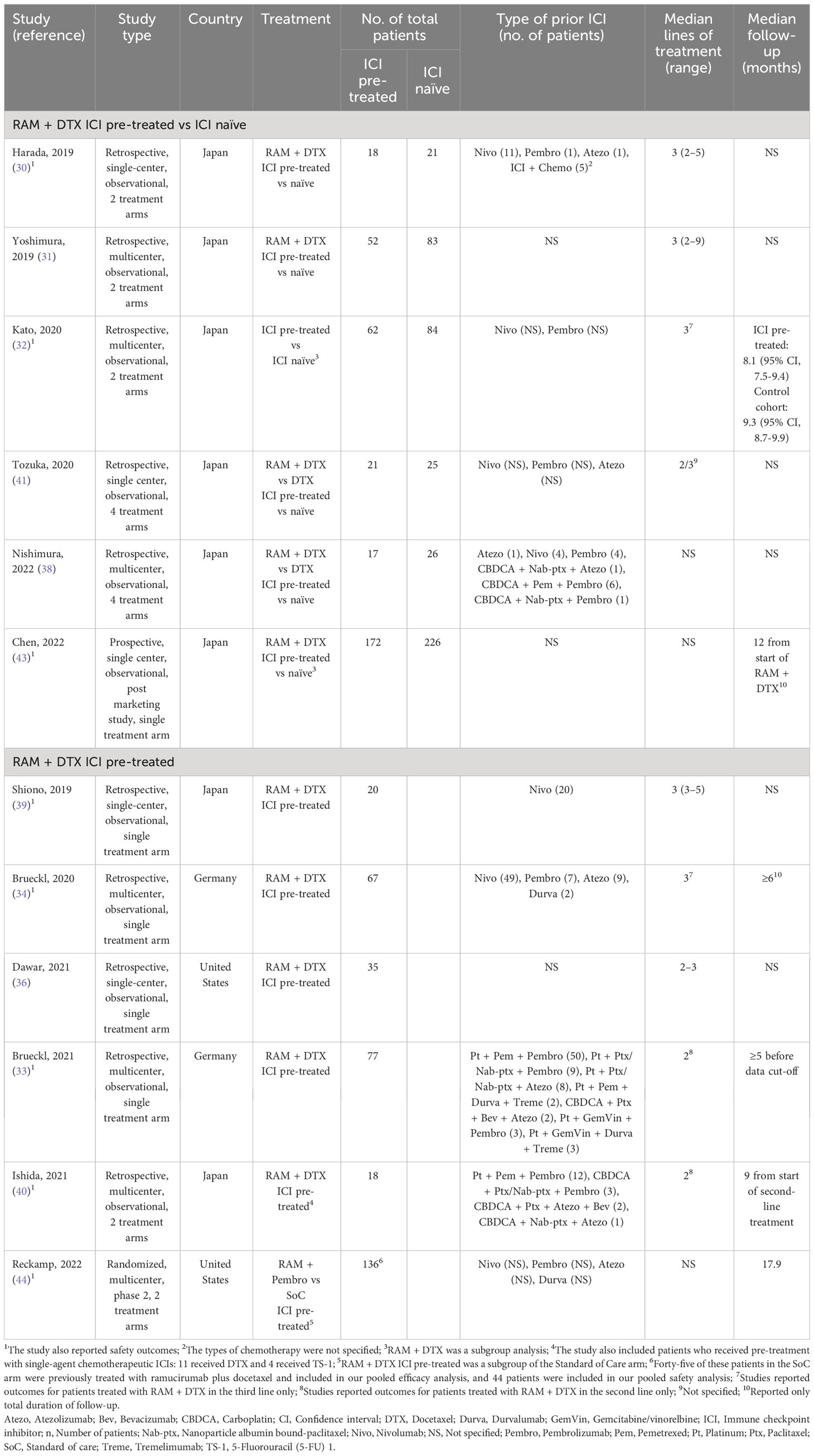
Eight studies were conducted in Japan where docetaxel doses ranged from 60 to 75 mg/m2 (30–32, 38–41, 43), while 2 studies each were conducted in Germany (33, 34), and in the United States (36, 44), where docetaxel is generally administered at a dose of 75-mg/m2.
Anti-PD-(L)1 agents use varied across studies, with nivolumab (21.7%), pembrolizumab (3.1%) and atezolizumab (2.8%), being the most frequently reported single-agent ICIs (61.0%) (30, 32, 34, 38, 39, 41). Chemoimmunotherapy combinations were used in 27.9% of patients (30, 33, 38, 40). The most commonly used combination of platinum-based chemotherapy and ICIs was platinum plus pemetrexed plus pembrolizumab (16.0%) (33, 40). Of the 12 studies, 4 included patients who had received only previous ICI monotherapy (32, 34, 39, 41), 2 included patients who had received only previous ICI in combination with chemotherapy (33, 40), 2 studies included patients who had received ICI in combination with chemotherapy or ICI monotherapy (30, 38) and the other 4 studies did not specify the type of immunotherapy used (31, 36, 43, 44). Of note, 2 studies that included patients who had received only previous ICI monotherapy investigated ramucirumab plus docetaxel in the third-line setting (32, 34), while both studies that included patients who had received only previous ICI in combination with chemotherapy investigated ramucirumab plus docetaxel in the second-line setting (Table 1) (33, 40). The other 8 studies did not specify the treatment line for ramucirumab plus docetaxel. Median follow-up was not reported for most studies but when reported ranged from 5.0 months (33) to 17.9 months (44).
3.2 Patient baseline characteristics
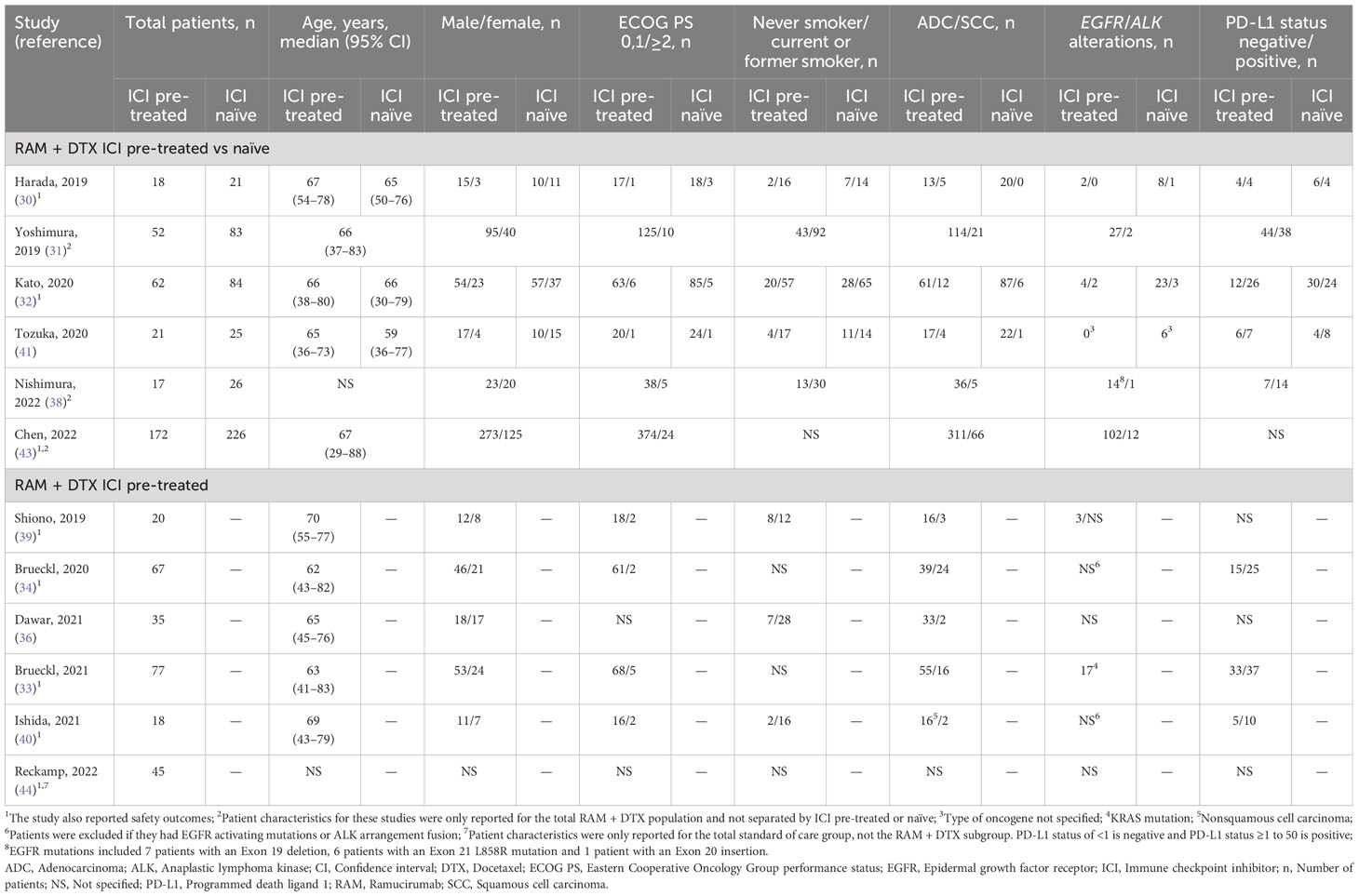
The median age of patients ranged from 59 to 70 years. Most patients had an Eastern Cooperative Oncology Group performance status score of 0 or 1 with an unknown PD-L1 status and wild-type epidermal growth factor receptor (EGFR). Patient characteristics across studies are summarized in Table 2.
3.3 Efficacy
Ten studies were included in the final efficacy analysis. From these studies, 387 pooled patients received ramucirumab plus docetaxel after ICI treatment (RAM + DTX ICI pre-treated) (30–34, 36, 38–41) and 239 pooled patients received ramucirumab plus docetaxel after chemotherapy only (RAM + DTX ICI naïve) (30–32, 38, 41). All included studies measured efficacy outcomes from the first day of treatment with ramucirumab plus docetaxel.
3.3.1 Progression-free survival
Of the 10 studies included in the efficacy analysis, median PFS was reported in 9 for RAM + DTX ICI pre-treated patients (30–34, 36, 39–41) and 4 of these 9 studies also reported median PFS in RAM + DTX ICI naïve patients (Figure 2A) (30–32, 41).
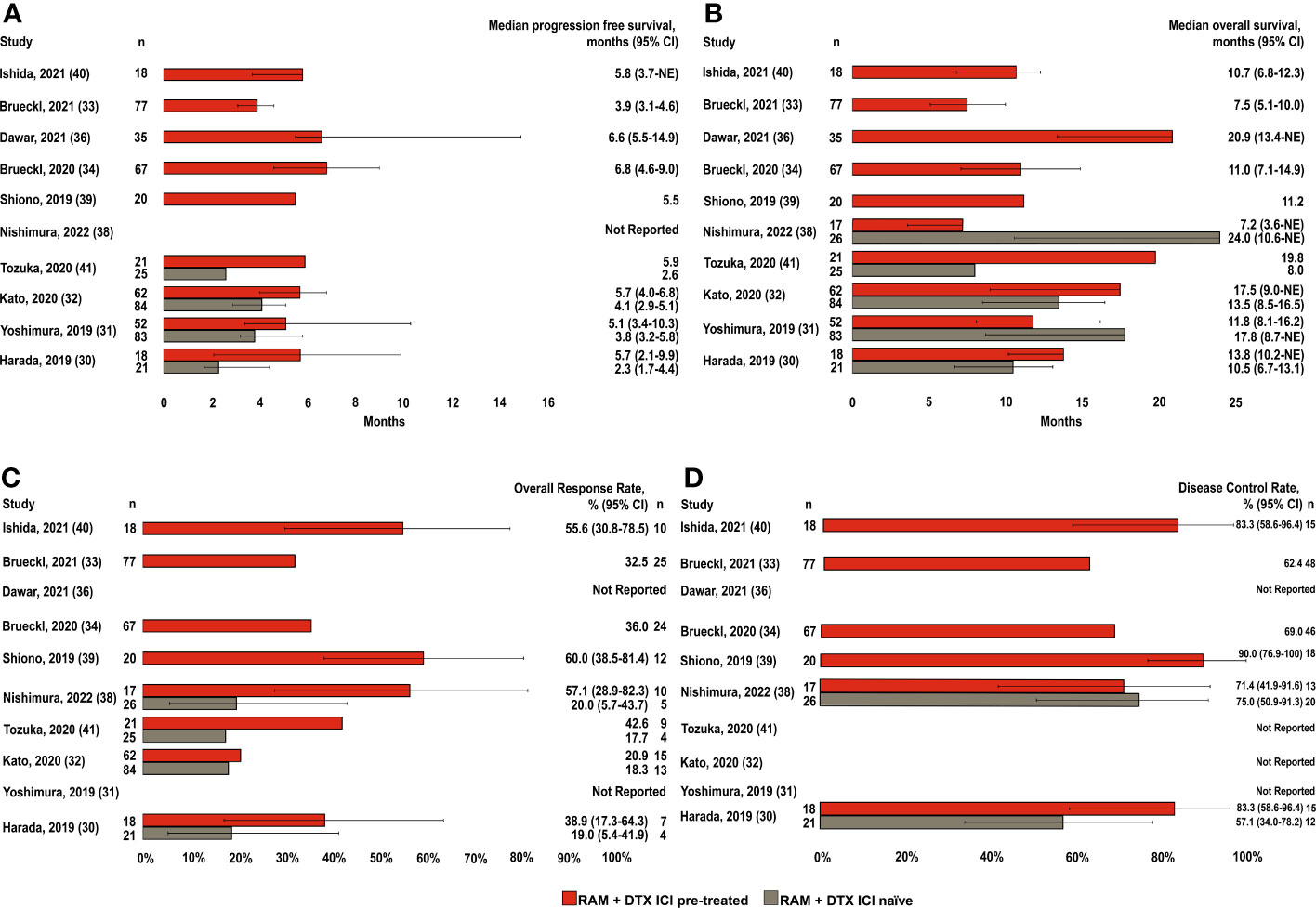
Figure 2 Efficacy endpoints across studies in RAM + DTX ICI pre-treated and ICI naïve patients. (A) Median progression-free survival; (B) Median overall survival; (C) Overall response rate; and (D) Disease control rate. Red bars indicate the RAM+DTX ICI pre-treated group and brown bars the RAM+DTX ICI naïve group. CI, Confidence interval; DTX, Docetaxel; ICI, Immune checkpoint inhibitor; n, Number of patients; NE, Not estimable; RAM, Ramucirumab.
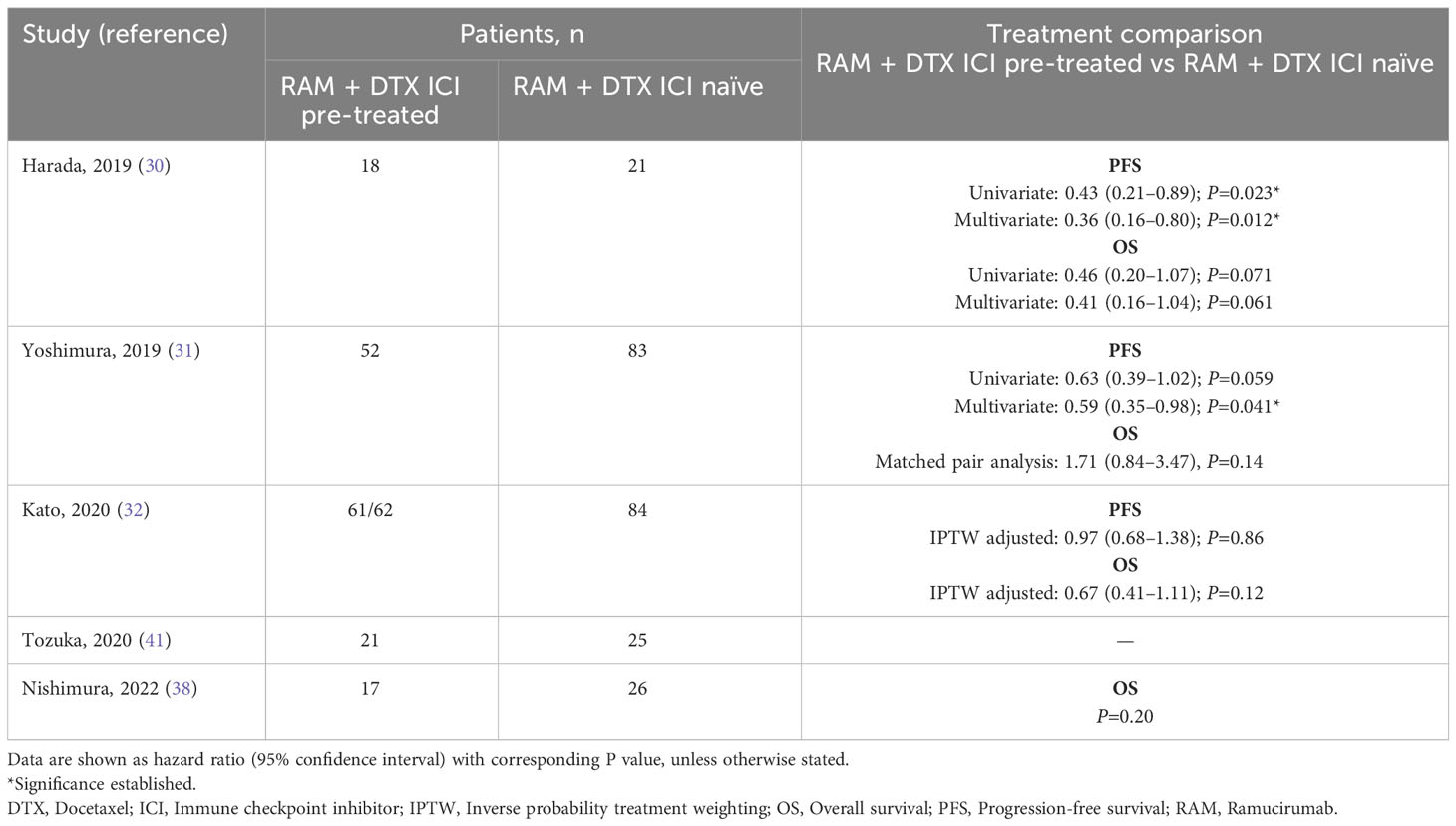
Of the 9 studies in RAM + DTX ICI pre-treated patients, 5 included patients in the RAM + DTX ICI pre-treated group only (33, 34, 36, 39, 40). Median PFS in these 5 studies ranged from 3.9 months (95% CI: 3.1-4.6) to 6.8 months (95% CI: 4.6-9.0) (33, 34). The other 4 studies reported median PFS across both RAM + DTX ICI pre-treated and RAM + DTX ICI naïve patients (30–32, 41). All 4 studies reported a trend toward improved median PFS for RAM + DTX ICI pre-treated patients (5.1 months [95% CI: 3.4-10.3] to 5.9 months [95% CI: Not reported (NR)]) compared with RAM + DTX ICI naïve patients (2.3 months [95% CI: 1.7-4.4] to 4.1 months [95% CI: 2.6-5.8]) (30–32, 41), with statistical significance established in 2 studies (P=0.012 and P=0.041) (Table 3) (30, 31).
The pooled weighted median PFS across all 9 studies was 5.7 months (95% CI: 3.9-6.8) in the RAM + DTX ICI pre-treated group and 3.8 months (95% CI: 2.3-4.1) in the RAM + DTX ICI naïve group (Table 4). The median PFS ranged from 3.9 months (95% CI: 3.1-4.6) (33) to 6.8 months (95% CI: 4.6-9.0) (34) in the RAM + DTX ICI pre-treated groups and from 2.3 months (95% CI: 1.7-4.4) (30) to 4.1 months (95% CI: 2.9-5.1) (32) in the RAM + DTX ICI naïve groups.
3.3.2 Overall survival
Median OS was reported in all 10 studies included in the efficacy analysis. Median OS for RAM + DTX ICI pre-treated patients were reported in all 10 studies (30–34, 36, 38–41), while only 5 studies reported median OS for RAM + DTX ICI naïve patients (Figure 2B) (30–32, 38, 41).
Of the 5 studies that included only RAM + DTX ICI pre-treated patients (33, 34, 36, 39, 40), median OS ranged from 7.5 months (95% CI: 5.1-10.0) to 20.9 months (95% CI: 13.4-not estimable [NE]) (33, 36). The other 5 studies reported outcomes for both RAM + DTX ICI pre-treated and RAM + DTX ICI naïve patients (30–32, 38, 41). Three of these 5 studies reported longer median OS in the RAM + DTX ICI pre-treated group (13.8 months [95% CI: 10.2-NE] to 19.8 months [95% CI: NR]) compared with the RAM + DTX ICI naïve group (8.0 months [95% CI: NR] to 13.5 months [95% CI: 8.5-16.5]), but statistical significance was not established in any of these studies (30, 32, 41). In the other 2 studies, a numerically longer median OS was observed in the RAM + DTX ICI naïve group (17.8 months [95% CI: 8.7-NE] and 24.0 months [95% CI: 10.6-NE]) compared with the RAM + DTX ICI pre-treated group (11.8 months [95% CI: 8.1-16.2] and 7.2 months [95% CI: 3.6-NE]), but this difference was not statistically significant in either study (P=0.14 and P=0.20, respectively) (Table 3) (31, 38).
The pooled weighted median OS across all 10 studies was 11.2 months (95% CI: 7.5-17.5) in the RAM + DTX ICI pre-treated group and 13.5 months (95% CI: 8.0-24.0) in the RAM + DTX ICI naïve group (Table 4). The median OS ranged from 7.2 months (95% CI: 3.6-NE) (38) to 20.9 months (95% CI: 13.4-NE) (36) in the RAM + DTX ICI pre-treated groups and from 8.0 months (95% CI: NR) (41) to 24.0 months (95% CI: 10.6-NE) (38) in the RAM + DTX ICI naïve groups.
3.3.3 Overall response rate
Of the 10 studies included in the efficacy analysis, ORRs were reported in 8 studies (Figure 2C) (30, 32–34, 38–41) in RAM + DTX ICI pre-treated patients (30, 32–34, 38–41), 4 of these studies also reported ORR in RAM + DTX ICI naïve patients (30, 32, 38, 41) studies (Figure 2C). In the 4 studies that reported outcomes with RAM + DTX in the ICI pre-treated groups only (33, 34, 39, 40), the ORR ranged from 32.5% (95% CI: NR) to 60.0% (95% CI: 38.5-81.4) (33, 39). In the 4 remaining studies, which reported results from both RAM + DTX ICI pre-treated and RAM + DTX ICI naïve groups (30, 32, 38, 41), the ORR was numerically higher in the RAM + DTX ICI pre-treated groups (38.9%, 20.9%, 57.1%, and 42.6%) compared with the respective RAM + DTX ICI naïve groups (19.0%, 18.3%, 20.0%, and 17.7%) (Figure 2C). Across all 8 studies, the ORR ranged from 20.9% (95% CI: NR) (32) to 60.0% (95% CI: 38.5-81.4) (39) in the RAM + DTX ICI pre-treated groups and from 17.7% (95% CI: NR) (41) to 20.0% (95% CI: 5.7-43.7) (38) in the RAM + DTX ICI naïve groups (Figure 2C).
3.3.4 Disease control rate
DCRs were reported in 6 of the 10 studies included in the efficacy analysis (30, 33, 34, 38–40). Four studies reported outcomes in the RAM + DTX ICI pre-treated groups alone (33, 34, 39, 40), whereas the remaining 2 studies reported DCRs in both the RAM + DTX ICI pre-treated and ICI-naïve groups (30, 38). Overall, the DCR ranged from 62.4% (95% CI: NR) to 90.0% (95% CI: 76.9-100.0) (33, 39) in the RAM + DTX ICI pre-treated groups and from 57.1% (95% CI: 34.0-78.2) to 75.0% (95% CI: 50.9-91.3) (30, 38) in the RAM + DTX ICI naïve groups (Figure 2D).
3.4 Safety
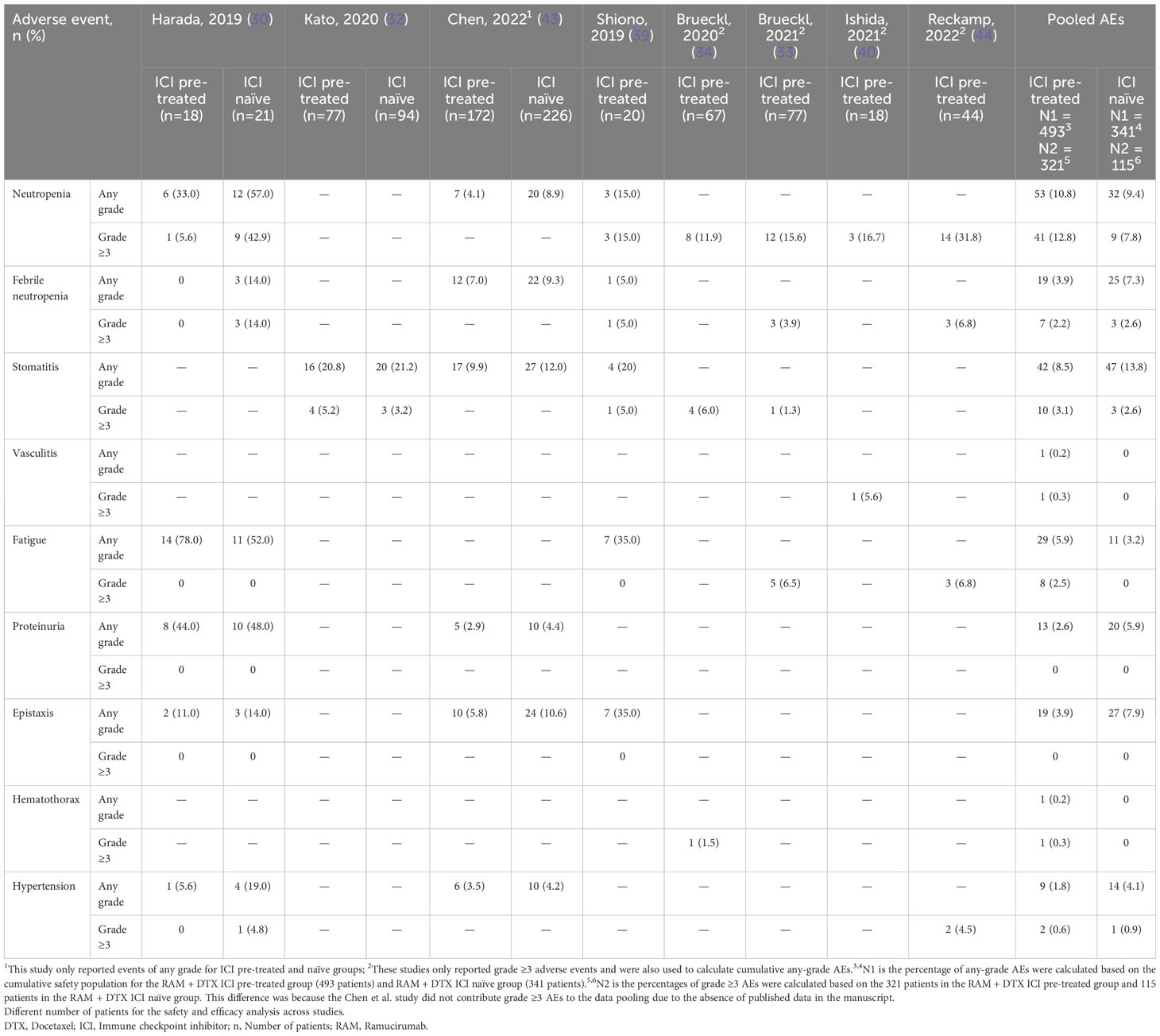
Safety data were derived from 8 studies for a total of 493 RAM + DTX ICI pre-treated patients and 341 RAM + DTX ICI-naïve patients (Table 5) (30, 32–34, 39, 40, 43, 44). All 8 studies included in the analysis reported any-grade AEs from the RAM + DTX ICI pre-treated group only (30, 32–34, 39, 40, 43, 44), and 3 studies reported any-grade AEs in both the RAM + DTX ICI naïve group and RAM + DTX ICI treated group (Table 5) (30, 32, 43).
Grade ≥3 AE data were derived from 7 studies for a total of 321 RAM + DTX ICI pre-treated patients and from 2 studies for a total of 115 patients in the RAM + DTX ICI naïve group (30, 32–34, 39, 40, 44). Of these 7 studies, 4 reported only grade ≥3 AEs (33, 34, 40, 44). For the purpose of this analysis, we focused on hematologic AEs, including neutropenia and febrile neutropenia; two nonhematologic AEs: fatigue and stomatitis; and AEs of special interest for antiangiogenic treatment, including bleeding events, hypertension, and proteinuria.
The percentage of any-grade AEs were calculated based on the cumulative safety population for the RAM + DTX ICI pre-treated group (493 patients) (30, 32–34, 39, 40, 43, 44) and RAM + DTX ICI naïve group (341 patients) (30, 32, 43), while the percentages of grade ≥3 AEs were calculated based on the 321 patients in the RAM + DTX ICI pre-treated group (30, 32–34, 39, 40, 44) and 115 patients in the RAM + DTX ICI naïve group (30, 32). This difference was because the Chen et al. study did not contribute grade ≥3 AEs to the data pooling due to the absence of published data in the manuscript (43). Neutropenia of any grade was reported in 53 (10.8%) patients in the RAM + DTX ICI pre-treated group (30, 33, 34, 39, 40, 43, 44) and in 32 (9.4%) patients in the RAM + DTX ICI naïve group (30, 43). Grade ≥3 neutropenia was reported in 41 (12.8%) patients in the RAM + DTX ICI pre-treated group (30, 33, 34, 39, 40, 44) and in 9 (7.8%) patients in the RAM + DTX ICI naïve group (Figure 3; Table 5) (30).
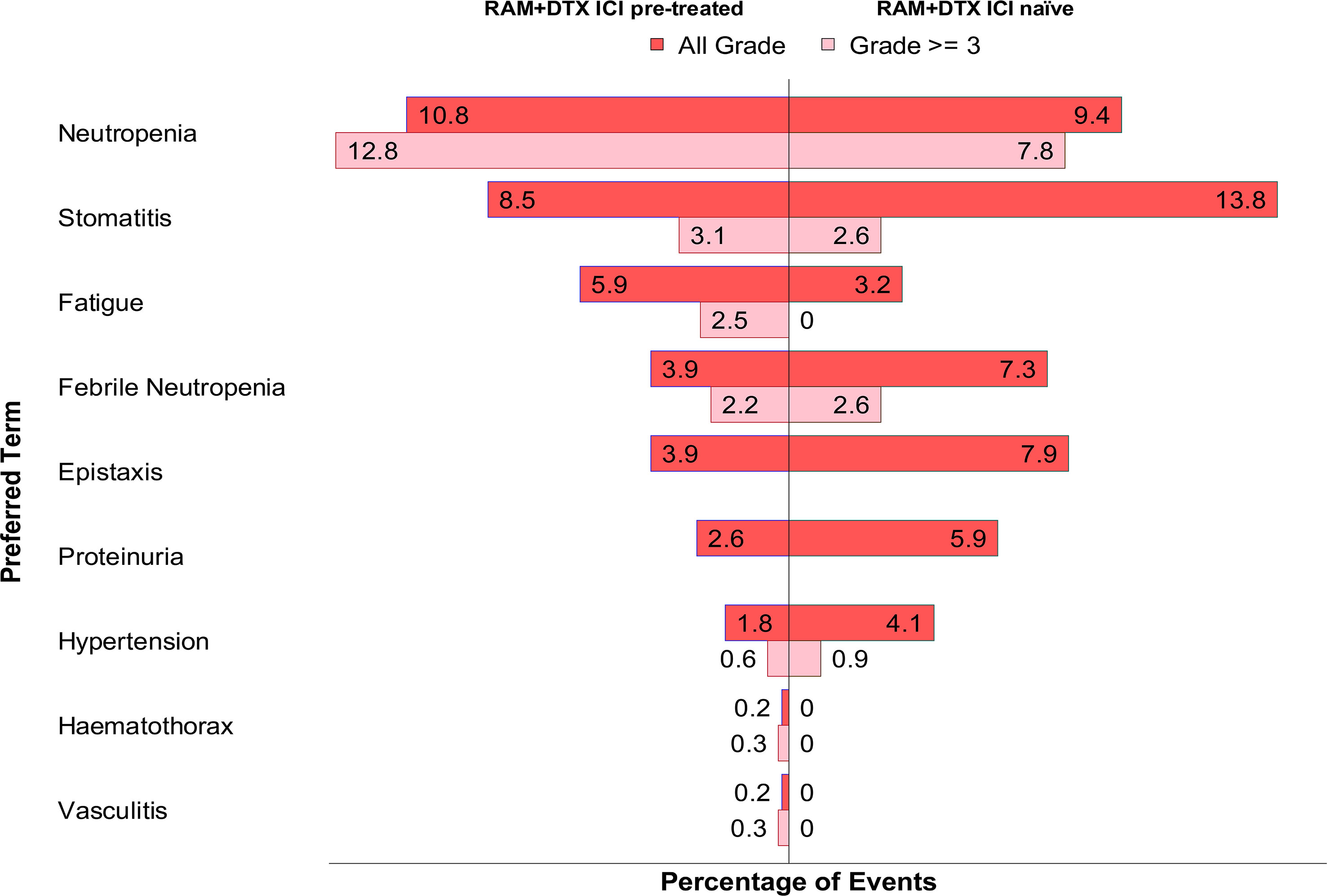
Figure 3 Pooled adverse events of any grade and grade ≥3 in RAM + DTX ICI pre-treated and naïve groups. The percentage of any-grade AEs were calculated based on the cumulative safety population for the RAM + DTX ICI pre-treated group (493 patients) and RAM + DTX ICI naïve group (341 patients), while the percentages of grade ≥3 AEs were calculated based on the 321 patients in the RAM + DTX ICI pre-treated group and 115 patients in the RAM + DTX ICI naïve group. This difference was because the Chen et al. study did not contribute grade ≥3 AEs to the data pooling due to the absence of published data in the manuscript. DTX, Docetaxel; ICI, Immune checkpoint inhibitor; RAM, Ramucirumab.
Febrile neutropenia of any grade was reported in 19 (3.9%) patients in the RAM + DTX ICI pre-treated group (30, 33, 39, 43, 44) and in 25 (7.3%) patients in the RAM + DTX ICI naïve group (30, 43). Grade ≥3 febrile neutropenia was reported in 7 (2.2%) patients in the RAM + DTX ICI pre-treated group (33, 39, 44) and in 3 (2.6%) patients in the RAM + DTX ICI naïve group (Figure 3; Table 5) (30).
The incidence of neutropenia of any grade in the RAM + DTX ICI pre-treated group was comparable to the RAM + DTX ICI naïve group in the two studies where both treatment groups were evaluated (Table 5). Similarly, the incidence and severity of febrile neutropenia were not higher in the RAM + DTX ICI pre-treated group (Table 5).
Stomatitis of any grade was reported in 42 (8.5%) patients in the RAM + DTX ICI pre-treated group (32–34, 39, 43) and in 47 (13.8%) patients in the RAM + DTX ICI naïve group (32, 43). Grade ≥3 stomatitis was reported in 10 (3.1%) patients in the RAM + DTX ICI pre-treated group (32–34, 39) and in 3 (2.6%) patients in the RAM + DTX ICI naïve group (Figure 3; Table 5) (32).
Fatigue of any grade was reported 29 (5.9%) patients in the RAM + DTX ICI pre-treated group (30, 33, 39, 44) and in 11 (3.2%) patients in the RAM + DTX ICI naïve group (30). Grade ≥3 fatigue was reported in 8 (2.5%) patients in the RAM + DTX ICI pre-treated group (33, 44), and no cases were reported in the RAM + DTX ICI naïve group (Figure 3; Table 5).
Among AEs of special interest, bleeding or epistaxis of any grade was reported in 19 (3.9%) patients in the RAM + DTX ICI pre-treated group (30, 39, 43) and in 27 (7.9%) patients in the RAM + DTX ICI naïve group (30, 43); no cases of grade ≥3 epistaxis were reported in either group.
The overall incidence of hypertension of any grade in the RAM + DTX ICI pre-treated and RAM + DTX ICI naïve groups was 9 (1.8%) and 14 (4.1%), respectively (30, 43, 44). The incidence of grade ≥3 hypertension in the RAM + DTX ICI pre-treated and RAM + DTX ICI naïve groups was 2 (0.6%) patients and 1 (0.9%) patient, respectively (Figure 3; Table 5).
Proteinuria of any grade was reported in 13 (2.6%) patients in the RAM + DTX ICI pre-treated group and in 20 (5.9%) patients in the RAM + DTX ICI naïve group (30, 43). No cases of grade ≥3 proteinuria were reported in either group (Figure 3; Table 5).
4 Discussion
The treatment landscape for patients with NSCLC that has progressed after front-line therapies has changed dramatically with the approval and availability of ICIs targeting coinhibitory molecules including anti-PD-1, anti-PD-L1, or anti-CTLA-4 (1–6). The introduction of ICIs in the first-line setting has resulted in significant improvement in efficacy outcomes; however, it has also generated a certain degree of complexity and uncertainty pertaining to the optimal treatment sequence for patients with disease progression during or after immunotherapy, mainly because the currently recommended treatment options for subsequent therapy, including the REVEL regimen, were investigated prior to the approval of ICIs for immunotherapy naïve patients (13, 14, 18–21). Thus, assessment of efficacy and safety for the combination of ramucirumab plus docetaxel after administration of ICIs provides important clinical information in support of its role in sequencing strategies after ICIs.
We analyzed the available information, derived mostly from retrospective observational studies, on the efficacy and safety of ramucirumab plus docetaxel in patients with metastatic NSCLC who had received prior ICIs with the aim of advancing the understanding of the clinical benefit and safety profile of ramucirumab plus docetaxel in the post-immunotherapy setting. To the best of our knowledge, we report the first SLR on the efficacy and safety of ramucirumab plus docetaxel in patients with NSCLC whose disease had progressed after treatment with ICIs either as single agents or in combination with chemotherapy.
We performed a weighted analysis of PFS and OS to adjust for the differing sample sizes across the studies included in the efficacy analysis. Based on this analysis, a numerically longer pooled weighted median PFS was observed with RAM + DTX ICI pre-treated patients compared with RAM + DTX ICI-naïve patients. The signal of improvement of median PFS was observed across all studies included in the efficacy analysis for the RAM + DTX ICI pre-treated group, although statistical significance for the treatment effect was reported in only 2 studies (30, 31). In contrast to PFS, the pooled weighted median OS was numerically longer in the RAM + DTX ICI naïve group compared with the RAM + DTX ICI pre-treated group. These results reflect the variations in median OS across the studies included in the pooled weighted analysis. Among the 5 studies that evaluated OS with ramucirumab plus docetaxel in both ICI pre-treated and ICI naïve groups, 3 reported an improvement in median OS in the RAM + DTX ICI pre-treated group compared with the ICI naïve group (30, 32, 41) while the remaining 2 studies reported numerically longer OS in the RAM + DTX ICI naïve group compared with the ICI pre-treated group (31, 38). These discrepancies in outcomes across studies are likely a result of several factors including imbalances in patient baseline and disease characteristics, differences in lines of treatment with ramucirumab plus docetaxel being administered in second line versus third and later lines, differences in timing between ICI administration and initiation of ramucirumab plus docetaxel therapy, and due to small sample sizes. It is also important to emphasize the contribution of variations in the proportion of patients with EGFR mutations across all the studies included in our pooled efficacy analysis. Thus, while 3 studies (30, 32, 38) included patients with mutant EGFR-positive lung adenocarcinoma who were treated with ramucirumab plus docetaxel, two others (34, 40) excluded patients who had EGFR or ALK alterations. In contrast, the remaining studies either reported mutation status for the total ramucirumab plus docetaxel treated populations and did not separate characteristics by ICI pre-treated or naïve (31, 43) or did not specify the type of oncogenic alteration (EGFR or ALK) (33, 36, 39, 41).
Only a few studies included in the efficacy analysis of this SLR included a control cohort of single-agent chemotherapy administered after ICIs (32, 38, 41). Therefore, a formal efficacy analysis of ramucirumab plus docetaxel relative to single-agent docetaxel after immunotherapy was not performed. However, in the retrospective observational study conducted by Kato et al, where a propensity score was used to correct for imbalances in patient characteristics across the ICI pre-treated and the ICI naïve cohorts, median OS in the subgroup of patients treated with single-agent docetaxel (n=102) or ramucirumab plus docetaxel (n=62) after anti-PD-1 treatment was 9.0 months and 17.5 months, respectively (32). Similarly, the retrospective study by Tozuka et al. in patients who had received prior anti-PD-(L)1 antibody therapy reported a median OS of 8.6 months in the cohort treated with single-agent docetaxel (n=18) and 19.8 months in the cohort treated with ramucirumab plus docetaxel (n=21). Additionally, a significant PFS improvement with ramucirumab plus docetaxel compared to docetaxel was reported (median PFS: 5.9 vs 2.8 months, HR: 0.43; 95% CI: 0.20-0.96; P=0.03) (41).
The combination of platinum-based chemotherapy and ICIs is currently the most widely used therapeutic option for the treatment of newly diagnosed patients with metastatic NSCLC without driver alterations (52). Results from real-world studies indicate that ramucirumab plus docetaxel is a widely used second-line therapy in NSCLC (53). Among the studies included in our analysis, only Brueckl et al. (2021) (33) and Ishida et al. (40) investigated the clinical benefit of second-line ramucirumab plus docetaxel after progression during or after chemoimmunotherapy. Of note, approximately two-thirds of patients in both studies had received prior treatment with pembrolizumab plus pemetrexed and platinum-based chemotherapy (33, 40). Both studies reported efficacy outcomes with ramucirumab plus docetaxel comparable to those reported in the REVEL trial with the exception of higher response rates. High response rates in the RAM + DTX ICI pre-treated group were also reported by other studies included in the efficacy analysis (30, 32–34, 38–41). Although OS is the gold standard to establish treatment efficacy, ORR represents an important clinical endpoint given the high symptom burden experienced by patients with lung cancers (54). Results from the VARGADO trial investigating the combination of nintedanib plus docetaxel in the post-ICI setting also reported improved ORR (58.0%) and median PFS (5.5 months) in patients with NSCLC receiving second-line nintedanib plus docetaxel after chemoimmunotherapy (55).
Prior response to immunotherapy and the timing between ICI treatment and initiation of the subsequent treatment may influence clinical responses to the next line of therapy. Among the studies included in the efficacy analysis of this SLR, few investigated the potential association between prior ICI treatment and improved efficacy outcomes with ramucirumab plus docetaxel. Prior treatment with ICIs was found to be an independent predictive factor for improvement in PFS with ramucirumab plus docetaxel by Harada et al. (30). Yoshimura et al. also demonstrated that prior immunotherapy was an independent prognostic factor for prolonged PFS, but not OS, with ramucirumab plus docetaxel (HR: 0.59; 95% CI: 0.35-0.98; P=0.041) (31). The potential association between response to prior immunotherapy and subsequent response to ramucirumab plus docetaxel was also investigated. Ishida et al. demonstrated that clinical benefit from prior ICI therapy, defined as a PFS of ≥8.8 months, was associated with a significantly longer PFS with ramucirumab plus docetaxel compared with the group of patients with a PFS of <8.8 months (HR: 0.12; 95% CI: 0.03-0.48; P=0.003) (40). The timing of administration of ramucirumab plus docetaxel after ICI treatment was investigated by Yoshimura et al. A trend suggesting prolonged PFS (HR: 0.54; 95% CI: 0.21-1.40; P=0.202) and OS (HR: 0.49; 95% CI: 0.22-1.10; P=0.079) was observed when ramucirumab plus docetaxel was administrated consecutively with ICI treatment (31). Taken together, these findings support the role of ramucirumab plus docetaxel in post-immunotherapy sequencing strategies.
The significant association between prior ICIs and favorable clinical outcomes with ramucirumab plus docetaxel may be explained, at least in part, by a role of ramucirumab in overcoming resistance to ICIs, a critical contributing factor that limits the efficacy of immune checkpoint blockade therapy in many patients (56, 57). Although several aspects of the underlying mechanisms responsible for resistance to ICIs have yet to be identified, emerging evidence supports a causal role of tumor extrinsic factors including immunosuppressive signals emanating from the tumor microenvironment (58). In addition to promoting angiogenesis, VEGF exerts important immunosuppressive effects on the tumor microenvironment. VEGF-induced abnormal neovascularization not only limits the access of tumor-directed cytotoxic T cells but also stimulates the recruitment of immunosuppressive cells, including myeloid-derived suppressor cells and regulatory T cells, and inhibits dendritic cell maturation, which ultimately leads to decreased activation of antigen-specific cytotoxic T cells (59–61). Therefore, targeting angiogenesis represents a rational approach to hinder immunosuppressive signals within the tumor microenvironment and restore antitumor cytotoxic T-cell responses. The immunomodulatory effects of ramucirumab may also explain the antitumor effects of ramucirumab combinations with anti-PD-(L)1 antibodies. Preclinical studies support this possibility. In a recently published study, the combination of DC101, a mouse surrogate of ramucirumab, with an anti-PD-1 antibody induced tumor regression and immunological memory in EMT6-LM2 and MC38 murine tumor models (62). Furthermore, the recently reported results from the phase 2 S1800A study of Lung-MAP demonstrated an improvement in OS (both median OS and HR), albeit no difference in ORR and PFS, with ramucirumab plus pembrolizumab compared with other standard-of-care options in patients with metastatic NSCLC whose disease had progressed on prior chemoimmunotherapy (63). Based on these results, a phase 3 trial investigating the combination of ramucirumab plus pembrolizumab in the same patient population is currently enrolling (NCT05633602) (64). An early efficacy signal has also been reported with ramucirumab in combination with atezolizumab in a heavily pre-treated NSCLC patient population (65), while another study investigating the combination of ramucirumab plus nivolumab in patients previously treated with ICIs and chemotherapy is currently ongoing (NCT03527108) (66).
A total of 11 studies were excluded from the efficacy analysis because they did not meet the inclusion criteria. However, results from these excluded studies also indicated that ramucirumab plus docetaxel was a safe and efficacious treatment after prior treatment with ICIs (35, 37, 42, 45–47, 67–70). Five of these were real-world studies that used electronic health record-derived databases were not included in the efficacy analysis because they did not meet the SLR inclusion criteria (45–47, 71) or they had a high percentage of missing data (e.g., tumor response assessment) and therefore had a high potential for misclassification and residual confounding bias (72). However, results from most of these studies suggest a trend towards improved efficacy outcomes with ramucirumab plus docetaxel administered after ICIs. In a small cohort of patients treated with second-line ramucirumab plus docetaxel after chemoimmunotherapy, real-world ORR and DCR were 37.5% and 75.0%, respectively (46). In an additional study by Clarke et al, ramucirumab plus docetaxel administered as a second- or third-line therapy after chemotherapy plus ICIs was associated with numerically higher real-world ORR (40.9% vs 30.4; P=0.21). Moreover, a statistically significant improvement in real-world DCR was observed when compared to ramucirumab plus docetaxel given after other non–ICIs (80.7% vs 54.4%; P<0.01) (45). Furthermore, in a cohort of patients treated with third-line ramucirumab plus docetaxel after ICI treatment, median real-world PFS (measured from the start of third-line ramucirumab plus docetaxel) was 3.6 months (range: 3.0-4.6 months) while median real-world OS (measured from the start of first-line therapy) was 19 months (range: 15.7-23.7 months) (47). The TREAT-LUNG study, a large retrospective observational study that collected data from 3 electronic health record-derived databases, investigated efficacy outcomes of second- or third-line therapy with ramucirumab plus docetaxel administered after prior chemotherapy plus ICIs. Study results showed that, after adjustment for baseline variables, differences in response rates, PFS, and OS were not statistically significant but trended in favor of ramucirumab plus docetaxel as observed in the REVEL trial (72). Taken together, with the limitations inherent to real-world data, these studies are supportive of a signal for increased activity of ramucirumab plus docetaxel in the post-immunotherapy setting.
A recently disclosed single-arm, multicenter, prospective phase 2 study of ramucirumab plus docetaxel following chemoimmunotherapy (SCORPION) in patients with metastatic NSCLC was not included in the SLR analysis as it was reported outside the window of literature search. This study demonstrated median PFS and median OS of 6.5 months and 17.5 months, respectively. ORR and DCR were 34.4% and 81.3%, respectively (73). No new safety signals were observed (73).
The descriptive safety analyses reported in this SLR support the conclusion that the select AEs observed with ramucirumab plus docetaxel administered after immunotherapy were similar in incidence and severity to those reported in the REVEL trial. Importantly, no new safety signals or additive toxicities emerged with ramucirumab plus docetaxel when administered after ICI treatment, including in the only controlled prospective study in the analysis (44). Notably, the retrospective study conducted by Harada et al. reported a higher incidence of pneumonitis in Japanese patients who had received ICIs before ramucirumab plus docetaxel; however, a causal relationship with ramucirumab plus docetaxel was not established (30). On the other hand, in the post marketing study by Chen et al, the incidence of pneumonitis of any grade in ICI-exposed and ICI-naïve patients was 1.2% and 0.9%, respectively (43). No cases of pneumonitis were observed or reported in the remaining studies. The safety analyses presented here must be interpreted with caution because most of the studies included in the safety analysis were retrospective and therefore underreporting of AEs cannot be ruled out.
Overall, the results presented in this SLR should be interpreted in the context of limitations inherent to the retrospective nature of most studies included in the analysis. To offset some limitations and decrease the heterogeneity of the data across studies, we included only studies free of bias that reported at least 2 efficacy endpoints with at least one being a time-to-event endpoint and performed weighted analyses to control for sample size. Despite the limitations, we believe that in the absence of controlled randomized clinical trials, the results present here provide valuable clinical information to complement current guideline recommendations for the use of ramucirumab plus docetaxel as a subsequent therapy in metastatic NSCLC.
In conclusion, the results of this SLR support the combination of ramucirumab plus docetaxel as an effective and safe subsequent therapy for the treatment of patients with metastatic NSCLC with disease progression regardless of prior treatment with ICIs.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
CV-G, MTR, SC, TP, and MR were involved in the conception and design of the work. EG, SC, and MR were involved in the acquisition of data for the work. SC, TP, and MR were involved in the analysis of the data for the work. MTR, SC, and TP were involved in drafting of the work. All authors were involved in the interpretation of the data for the work as well as critical revision to the work.
Funding
The study was funded by Eli Lilly and Company.
Acknowledgments
All authors were involved in the writing of the manuscript and its revisions. Sunoj Chacko Varughese and Karthik Chandrasekhar P from Eli Lilly and Company provided statistical support and assessed the risk of bias for the studies included in the analyses reported in this SLR. Preethi Govindarajan and Adrienne Schreiber from Syneos Health provided medical writing assistance and editorial support.
Conflict of interest
Author EG is a consultant and/or Advisor for AbbVie, ABL Bio, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Dracen Pharmaceutical, EMD Serono Inc, Eisai Co Ltd, Eli Lilly and Company, Gilead Sciences Inc, GlaxoSmithKline, Merck & Co, Natera, Novartis, Personalis, Regeneron Pharmaceuticals, Sanofi, Shionogi Inc, Xilio Therapeutics, and Zymeworks. He also reports Grants/Research Support from ABL Bio, AstraZeneca, Bristol Myers Squibb, Daiichi Sankyo, Dynavax Technologies, Eli Lilly and Company, EMD Serono Inc, Genentech, Gilead Sciences Inc, Iovance Biotherapeutics, Merck, Mirati Therapeutics, Neon Therapeutics and Novartis. He also reports sponsored Independent Medical Education from Daiichi Sankyo and Ipsen and travel from A2 Bio and Novartis. Authors CV-G, MTR, TP, and SC are all employees of Eli Lilly and Company with stock options. Author MR receives consulting fees, payment or honoraria, and support for attending meetings/travel from Amgen, AstraZeneca, BeiGene, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly and Company, Merck & Co, MSD, Mirati Therapeutics, Novartis, GlaxoSmithKline, Pfizer, and Roche. He is also on the advisory board or data safety monitoring board of Daiichi Sankyo and Sanofi.The authors declare that this study was funded by Eli Lilly and Company. Eli Lilly and Company had the following involvement in the study: data collection, data analysis, data interpretation, writing of the article, and decision to submit it for publication in collaboration with the authors.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1247879/full#supplementary-material
Abbreviations
AE, adverse event; CI, confidence interval; DCR, disease control rate; DTX, docetaxel; HR, hazard ratio; ICI, immune checkpoint inhibitor; NE, not estimable; NR, not reported; NSCLC, non-small cell lung cancer; ORR, overall response rate; OS, overall survival; PD-1, programmed death 1; PD-L1, programmed death ligand 1; PFS, progression-free survival; RAM, ramucirumab; SLR, systematic literature review; VEGF, vascular endothelial growth factor.
References
1. Gadgeel S, Rodríguez-Abreu D, Speranza G, Esteban E, Felip E, Dómine M, et al. Updated analysis from KEYNOTE-189: pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol (2020) 38(14):1505–17. doi: 10.1200/JCO.19.03136
2. Socinski MA, Nishio M, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, et al. IMpower150 final overall survival analyses for atezolizumab plus bevacizumab and chemotherapy in first-line metastatic nonsquamous NSCLC. J Thorac Oncol (2021) 16(11):1909–24. doi: 10.1016/j.jtho.2021.07.009
3. Paz-Ares L, Ciuleanu T-E, Cobo M, Schenker M, Zurawski B, Menezes J, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol (2021) 22(2):198–211. doi: 10.1016/S1470-2045(20)30641-0
4. Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim S-W, Costa EC, et al. Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N Engl J Med (2019) 381(21):2020–31. doi: 10.1056/NEJMoa1910231
5. Johnson ML, Cho BC, Luft A, Alatorre-Alexander J, Geater SL, Laktionov K, et al. Durvalumab with or without tremelimumab in combination with chemotherapy as first-line therapy for metastatic non–small-cell lung cancer: the phase III POSEIDON study. J Clin Oncol (2023) 41(6):1213–27. doi: 10.1200/JCO.22.00975
6. Gogishvili M, Melkadze T, Makharadze T, Giorgadze D, Dvorkin M, Penkov K, et al. Cemiplimab plus chemotherapy versus chemotherapy alone in non-small cell lung cancer: a randomized, controlled, double-blind phase 3 trial. Nat Med (2022) 28(11):2374–80. doi: 10.1038/s41591-022-01977-y
7. Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A, et al. Non–small cell lung cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw (2022) 20(5):497–530. doi: 10.6004/jnccn.2022.0025
8. Hendriks LE, Kerr KM, Menis J, Mok TS, Nestle U, Passaro A, et al. ESMO Guidelines Committee. Non-oncogene-addicted metastatic non-small-cell lung cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol (2023) 34(4):358–76. doi: 10.1016/j.annonc.2022.12.013
9. Arunachalam A, Li H, Bittoni MA, Camacho R, Cao X, Zhong Y, et al. Real-world treatment patterns, overall survival, and occurrence and costs of adverse events associated with second-line therapies for Medicare patients with advanced non small-cell lung cancer. Clin Lung Cancer (2018) 19(5):e783–99. doi: 10.1016/j.cllc.2018.05.016
10. Velcheti V, Hu X, Piperdi B, Burke T. Real-world outcomes of first-line pembrolizumab plus pemetrexed-carboplatin for metastatic nonsquamous NSCLC at US oncology practices. Sci Rep (2021) 11(1):9222. doi: 10.1038/s41598-021-88453-8
11. Davis KL, Goyal RK, Able SL, Brown J, Li L, Kaye JA. Real-world treatment patterns and costs in a US Medicare population with metastatic squamous non-small cell lung cancer. Lung Cancer (2015) 87(2):176–85. doi: 10.1016/j.lungcan.2014.11.002
12. Moro-Sibilot D, Smit E, de Castro Carpeño J, Lesniewski-Kmak K, Aerts J, Villatoro R, et al. Outcomes and resource use of non-small cell lung cancer (NSCLC) patients treated with first-line platinum-based chemotherapy across Europe: FRAME prospective observational study. Lung Cancer (2015) 88(2):215–22. doi: 10.1016/j.lungcan.2015.02.011
13. Garon EB, Ciuleanu T-E, Arrieta O, Prabhash K, Syrigos KN, Goksel T, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet (2014) 384:9944. doi: 10.1016/S0140-6736(14)60845-X
14. Reck M, Kaiser R, Mellemgaard A, Douillard J-Y, Orlov S, Krzakowski M, et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol (2014) 15(2):143–55. doi: 10.1016/S1470-2045(13)70586-2
15. Herbst RS, Garon EB, Kim D-W, Cho BC, Gervais R, Perez-Gracia JL, et al. Five year survival update from KEYNOTE-010: pembrolizumab versus docetaxel for previously treated, programmed death-ligand 1-positive advanced NSCLC. J Thorac Oncol (2021) 16(10):1718–32. doi: 10.1016/j.jtho.2021.05.001
16. Borghaei H, Gettinger S, Vokes EE, Chow LQM, Burgio MA, de Castro Carpeno J, et al. Five-year outcomes from the randomized, phase III Trials CheckMate 017 and 057: nivolumab versus docetaxel in previously treated non-small-cell lung cancer. J Clin Oncol (2021) 39(7):723–33. doi: 10.1200/JCO.20.01605
17. Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet (2017) 389(10066):25565. doi: 10.1016/S0140-6736(16)32517-X
18. Shepherd FA, Dancey J, Ramlau R, Mattson K, Gralla R, O’Rourke M, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol (2000) 18(10):2095–103. doi: 10.1200/JCO.2000.18.10.2095
19. Gridelli C, Perrone F, Gallo C, Rossi A, Barletta E, Barzelloni ML, et al. Single-agent gemcitabine as second-line treatment in patients with advanced non small cell lung cancer (NSCLC): a phase II trial. Anticancer Res (1999) 19(5c):4535–8.
20. Hanna N, Shepherd FA, Fossella FV, Pereira JR, De Marinis F, von Pawel J, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol (2004) 22(9):1589–97. doi: 10.1200/JCO.2004.08.163
21. Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med (2013) 369(18):1691–703. doi: 10.1056/NEJMoa1304369
22. Spratlin JL, Cohen RB, Eadens M, Gore L, Camidge DR, Diab S, et al. Phase I pharmacologic and biologic study of ramucirumab (IMC-1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2. J Clin Oncol (2010) 28(5):780–7. doi: 10.1200/JCO.2009.23.7537
23. Pérol M, Ciuleanu T-E, Arrieta O, Prabhash K, Syrigos KN, Goksel T, et al. Quality of life results from the phase 3 REVEL randomized clinical trial of ramucirumab-plusdocetaxel versus placebo-plus-docetaxel in advanced/metastatic non-small cell lung cancer patients with progression after platinumbased chemotherapy. Lung Cancer (2016) 93:95–103. doi: 10.1016/j.lungcan.2016.01.007
24. European Medicines Agency. Cyramza (ramucirumab): summary of product characteristics (2019). Available at: https://www.ema.europa.eu/en/documents/product-information/cyramza-epar-product-information_en.pdf (Accessed May 1, 2023).
25. Eli Lilly and Company. Cyramza (ramucirumab): US prescribing information, version 38 (2022). Available at: http://uspl.lilly.com/cyramza/cyramza.html (Accessed May 1, 2023).
26. Garon EB, Scagliotti GV, Gautschi O, Reck M, Thomas M, Iglesias Docampo L, et al. Exploratory analysis of front-line therapies in REVEL: a randomised phase 3 study of ramucirumab plus docetaxel versus docetaxel for the treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy. ESMO Open (2020) 5(1):e000567. doi: 10.1136/esmoopen-2019-000567
27. Paz-Ares LG, Pérol M, Ciuleanu TE, Kowalyszyn RD, Reck M, Lewanski CR, et al. Treatment outcomes by histology in REVEL: a randomized phase III trial of ramucirumab plus docetaxel for advanced non-small cell lung cancer. Lung Cancer (2017) 112:126–33. doi: 10.1016/j.lungcan.2017.05.021
28. Yoh K, Hosomi Y, Kasahara K, Yamada K, Takahashi T, Yamamoto N, et al. A randomized, double-blind, phase II study of ramucirumab plus docetaxel vs placebo plus docetaxel in Japanese patients with stage IV non-small cell lung cancer after disease progression on platinum-based therapy. Lung Cancer (2016) 99:186–93. doi: 10.1016/j.lungcan.2016.07.019
29. European Medicines Agency. Vargatef: summary of product characteristics (2019). Available at: https://www.ema.europa.eu/en/documents/product-information/vargatef-epar-product-information_en.pdf (Accessed March 6, 2023).
30. Harada D, Takata K, Mori S, Kozuki T, Takechi Y, Moriki S, et al. Previous immune checkpoint inhibitor treatment to increase the efficacy of docetaxel and ramucirumab combination chemotherapy. Anticancer Res (2019) 39(9):4987–93. doi: 10.21873/anticanres.13688
31. Yoshimura A, Yamada T, Okuma Y, Kitadai R, Takeda T, Kanematsu T, et al. Retrospective analysis of docetaxel in combination with ramucirumab for previously treated non-small cell lung cancer patients. Transl Lung Cancer Res (2019) 8(4):450–60. doi: 10.21037/tlcr.2019.08.07
32. Kato R, Hayashi H, Chiba Y, Miyawaki E, Shimizu J, Ozaki T, et al. Propensity score–weighted analysis of chemotherapy after PD-1 inhibitors versus chemotherapy alone in patients with non–small cell lung cancer (WJOG10217L). J Immunother Cancer (2020) 8(1):e000350. doi: 10.1136/jitc-2019-000350
33. Brueckl WM, Reck M, Rittmeyer A, Kollmeier J, Wesseler C, Wiest GH, et al. Efficacy of docetaxel plus ramucirumab as palliative second-line therapy following first-line chemotherapy plus immune-checkpoint-inhibitor combination treatment in patients with non-small cell lung cancer (NSCLC) UICC stage IV. Transl Lung Cancer Res (2021) 10(7):3093–105. doi: 10.21037/tlcr-21-197
34. Brueckl WM, Reck M, Rittmeyer A, Kollmeier J, Wesseler C, Wiest GH, et al. Efficacy of docetaxel plus ramucirumab as palliative third-line therapy following second-line immune-checkpoint-inhibitor treatment in patients with non-small-cell lung cancer stage IV. Clin Med Insights Oncol (2020) 14:1179554920951358. doi: 10.1177/1179554920951358
35. Offin M, Xu C, Jain H, Makhnin A, Hayes SA, Plodkowski AJ, et al. Efficacy of ramucirumab and docetaxel given either before or after immune checkpoint inhibitors in patients with lung cancers. In: 2019 American Society of Clinical Oncology Annual Meeting I. J Clin Oncol (2019) 37(15S):9078–8. doi: 10.1200/JCO.2019.37.15_suppl.9078
36. Dawar R, Gawri K, Rodriguez E, Thammineni V, Saul E, Lima Filho JOO, et al. P01.09 Improved outcomes with ramucirumab & docetaxel in metastatic non-small cell lung cancer after failure of immunotherapy. In: International Association for the Study of Lung Cancer 2020 World Conference on Lung Cancer Singapore, 2021 Jan 28-31; Singapore. J Thorac Oncol (2021) 16(3):S239–40. doi: 10.1016/j.jtho.2021.01.333
37. Grigg C, Reuland BD, Sacher AG, Yeh R, Rizvi NA, Shu CA. Clinical outcomes of patients with non-small cell lung cancer (NSCLC) receiving chemotherapy after immune checkpoint blockade. In: 2017 American Society of Clinical Oncology Annual Meeting I. J Clin Oncol (2017) 35(15S):9082–2. doi: 10.1200/JCO.2017.35.15_suppl.9082
38. Nishimura T, Fujimoto H, Okano T, Naito M, Tsuji C, Iwanaka S, et al. Is the efficacy of adding ramucirumab to docetaxel related to a history of immune checkpoint inhibitors in the real-world clinical practice? Cancers (Basel) (2022) 14(12):2970. doi: 10.3390/cancers14122970
39. Shiono A, Kaira K, Mouri A, Yamaguchi O, Hashimoto K, Uchida T, et al. Improved efficacy of ramucirumab plus docetaxel after nivolumab failure in previously treated non-small cell lung cancer patients. Thorac Cancer (2019) 10(4):775–81. doi: 10.1111/1759-7714.12998
40. Ishida M, Morimoto K, Yamada T, Shiotsu S, Chihara Y, Yamada T, et al. Impact of docetaxel plus ramucirumab in a second-line setting after chemoimmunotherapy in patients with non-small-cell lung cancer: a retrospective study. Thorac Cancer (2022) 13(2):173–81. doi: 10.1111/1759-7714.14236
41. Tozuka T, Kitazono S, Sakamoto H, Yoshida H, Amino Y, Uematsu S, et al. Addition of ramucirumab enhances docetaxel efficacy in patients who had received anti-PD-1/PD-L1 treatment. Lung Cancer (2020) 144:71–5. doi: 10.1016/j.lungcan.2020.04.021
42. Kawachi H, Tamiya M, Matsumoto K, Tamiya A, Yanase T, Tanizaki S, et al. Efficacy and safety of ramucirumab and docetaxel in previously treated patients with squamous cell lung cancer: a multicenter retrospective cohort study. Invest New Drugs (2022) 40(3):634–42. doi: 10.1007/s10637-022-01214-w
43. Chen Y, Nagaoka S, Katayose T, Sekine N. Safety and effectiveness of ramucirumab and docetaxel: a single-arm, prospective, multicenter, non-interventional, observational, post-marketing safety study of NSCLC in Japan. Expert Opin Drug Saf (2022) 21(5):691–8. doi: 10.1080/14740338.2022.2023127
44. Reckamp KL, Redman MW, Dragnev KH, Minichiello K, Villaruz LC, Faller B, et al. Phase II randomized study of ramucirumab and pembrolizumab versus standard of care in advanced non-small-cell lung cancer previously treated with immunotherapy-Lung-MAP S1800A. J Clin Oncol (2022) 40(21):2295–306. doi: 10.1200/JCO.22.00912
45. Clarke JM, Mathur R, Molife C, Batus M, Stefaniak VJ, Winfree KB, et al. Real-world tumor response (rwTR) to ramucirumab plus docetaxel (R+D) post platinum-based (Pt) and immune checkpoint inhibitor (ICI) therapy in advanced non-small cell lung cancer (aNSCLC) patients (pts). In: 2019 American Society of Clinical Oncology Annual Meeting I. J Clin Oncol (2019) 37(15S):e20725–5. doi: 10.1200/JCO.2019.37.15_suppl.e20725
46. Clarke JM, Mathur R, Molife C, Batus M, Stefaniak VJ, Winfree KB, et al. Real-world (rw) clinical outcomes for advanced/metastatic non-small cell lung cancer (aNSCLC) patients (pts) treated with second line (2L) ramucirumab plus docetaxel (R+D) post frontline (1L) platinum based chemotherapy plus immune checkpoint inhibitors (Pt + ICI). In: 2019 American Society of Clinical Oncology Annual Meeting I. J Clin Oncol (2019) 37(15S):e20727e20727. doi: 10.1200/JCO.2019.37.15_suppl.e20727
47. Clarke J, Stefaniak V, Batus M, Winfree K, Molife C, Cui Z, et al. P3.01-19 Sequencing of ramucirumab+docetaxel post-immune checkpoint inhibitors in advanced non-small cell lung cancer patients. In: International Association for the Study of Lung Cancer 19th World Conference on Lung Cancer; 2018 Sep 23-26; Toronto Canada. J Thorac Oncol (2018) 13(10):S874. doi: 10.1016/j.jtho.2018.08.1579
48. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (2021) 372:n71. doi: 10.1136/bmj.n71
49. McGrath S, Zhao X, Qin ZZ, Steele R, Benedetti A. One-sample aggregate data meta-analysis of medians. Stat Med (2019) 38(6):969–84. doi: 10.1002/sim.8013
50. Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Eur J Epidemiol (2011) 25:603–5.
51. National Heart, Lung and Blood Institute. Quality assessment tool for before-after (pre-post) studies with no control group (2021). Available at: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (Accessed February 20, 2023).
52. West HJ. Initial management of advanced non-small cell lung cancer lacking a driver mutation . UpToDate. Available at: https://www.uptodate.com/contents/initial-management-of-advanced-non-small-cell-lung-cancer-lacking-a-driver-mutation (Accessed May 1, 2023).
53. Divan HA, Bittoni MA, Krishna A, Carbone DP. Real-world treatment patterns and outcomes of patients with metastatic nonsquamous non-small cell lung cancer after progression on standard-of-care therapy in the United States. Lung Cancer (2023) 179:107–77. doi: 10.1016/j.lungcan.2023.107177
54. Batra A, Yang L, Boyne DJ, Harper A, Cheung WY, Cuthbert CA. Associations between baseline symptom burden as assessed by patient-reported outcomes and overall survival of patients with metastatic cancer. Support Care Cancer (2021) 29(3):1423–31. doi: 10.1007/s00520-020-05623-6
55. Grohé C, Gleiber W, Haas S, Losem C, Mueller-Huesmann H, Schulze M, et al. Nintedanib plus docetaxel after progression on immune checkpoint inhibitor therapy: insights from VARGADO, a prospective study in patients with lung adenocarcinoma. Future Oncol (2019) 15(23):2699–706. doi: 10.2217/fon-2019-0262
56. Tokaz MC, Baik CS, Houghton AM, Tseng D. New immuno-oncology targets and resistance mechanisms. Curr Treat Options Oncol (2022) 23(9):120118. doi: 10.1007/s11864-022-01005-8
57. Gide TN, Wilmott JS, Scolyer RA, Long GV. Primary and acquired resistance to immune checkpoint inhibitors in metastatic melanoma. Clin Cancer Res (2018) 24(6):1260–70. doi: 10.1158/1078-0432.CCR-17-2267
58. Labani-Motlagh A, Ashja-Mahdavi M, Loskog A. The tumor microenvironment: a milieu hindering and obstructing antitumor immune responses. Front Immunol (2020) 11:940. doi: 10.3389/fimmu.2020.00940
59. Gabrilovich D, Ishida T, Oyama T, Ran S, Kravtsov V, Nadaf S, et al. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood (1998) 92(11):4150–66. doi: 10.1182/blood.V92.11.4150
60. Gabrilovich DI, Chen HL, Girgis KR, Cunningham HT, Meny GM, Nadaf S, et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med (1996) 2(10):1096–103. doi: 10.1038/nm1096-1096
61. Khan KA, Kerbel RS. Improving immunotherapy outcomes with anti-angiogenic treatments and vice versa. Nat Rev Clin Oncol (2018) 15(5):310–24. doi: 10.1038/nrclinonc.2018.9
62. Li Y, Amaladas N, O’Mahony M, Manro JR, Inigo I, Li Q, et al. Treatment with a VEGFR-2 antibody results in intra-tumor immune modulation and enhances anti-tumor efficacy of PD-L1 blockade in syngeneic murine tumor models. PloS One (2022) 17(7):e0268244. doi: 10.1371/journal.pone.0268244
63. Reckamp KL, Redman MW, Dragnev KH, Minichiello K, Villaruz LC, Faller BA, et al. Overall survival from a phase II randomized study of ramucirumab plus pembrolizumab versus standard of care for advanced non–small-cell lung cancer previously treated with immunotherapy: Lung-MAP S1800A. J Clin Oncol (2022) 40(21):2295–306. doi: 10.1200/JCO.22.00912
64. Reckamp KL, Southwest Oncology Group Cancer Research Network. Ramucirumab plus pembrolizumab vs usual care for treatment of stage IV or recurrent non-small cell lung cancer following immunotherapy, Pragmatica-Lung Study (2023). Available at: https://clinicaltrials.gov/ct2/show/NCT05633602 (Accessed March 9, 2023).
65. Herzog BH, Waqar SN, Devarakonda S, Ward JP, Gao F, Govindan R, et al. Ramucirumab plus atezolizumab in patients with stage IV non-small cell lung cancer previously treated with immune checkpoint inhibitors. Lung Cancer (2022) 173:101–6. doi: 10.1016/j.lungcan.2022.09.011
66. Borghaei H, Fox Chase Cancer Centre. Nivolumab plus ramucirumab in patients with recurrent, advanced, metastatic NSCLC (2023). Available at: https://www.clinicaltrials.gov/ct2/show/NCT03527108 (Accessed March 9, 2023).
67. Tamura N, Horinouchi H, Sekine K, Matsumoto Y, Murakami S, Goto Y, et al. Efficacy of subsequent cytotoxic chemotherapy after nivolumab for patients with advanced non-small cell lung cancer. In: Abstract Book of the ESMO Asia Congress; 2017 Nov 17-19; Singapore. Ann Oncol (2017) 28(10S):x134. doi: 10.1093/annonc/mdx671.027
68. Matsumoto K, Tamiya A, Matsuda Y, Taniguchi Y, Atagi S, Kawachi H, et al. Impact of docetaxel plus ramucirumab on metastatic site in previously treated patients with non-small cell lung cancer: a multicenter retrospective study. Transl Lung Cancer Res (2021) 10(4):1642–52. doi: 10.21037/tlcr-20-1263
69. Kamiyoshihara M, Yazawa T, Igai H, Matsuura N, Ohsawa F, Iwashita H. Docetaxel and ramucirumab combination chemotherapy after nivolumab treatment for pretreated pulmonary squamous cell carcinoma: a successful case [in Japanese]. Gan To Kagaku Ryoho (2021) 48(2):211–3.
70. Usui Y, Sasada S, Kirita K, Watanabe S, Tsuchiya Y, Nakamura M. A case of lung adenocarcinoma that responded to ramucirumab and docetaxel after rapid tumor growth after administration of pembrolizumab [in Japanese]. Haigan (2021) 61(7):970–4. doi: 10.2482/haigan.61.970
71. Molife C, Hess LM, Cui ZL, Li XI, Beyrer J, Mahoui M, et al. Sequential therapy with ramucirumab and/or checkpoint inhibitors for non-small-cell lung cancer in routine practice. Future Oncol (2019) 15(25):2915–31. doi: 10.2217/fon-2018-0876
72. Pennel NA, Clarke J, Liu SV, Gutierrez M, Batus M, Bauman J, et al. CO154 Ramucirumab+docetaxel post immune checkpoint inhibitors (ICIS) and platinum-based chemotherapy (CHEMO) in advanced or metastatic non-small cell lung cancer (ANSCLC): learnings from the Treat-Lung Observational study. Value Health (2022) 25(7):S333. doi: 10.1016/j.jval.2022.04.247
73. Matsuzawa R, Morise M, Ito K, Hataji O, Takahashi K, Kuwatsuka Y, et al. 46P Multi-center, phase II study of docetaxel (DTX) plus ramucirumab (RAM) following platinum-based chemotherapy plus ICIs in patients with NSCLC: SCORPION study. In: Abstract Book of the European Lung Cancer Congress (ELCC) 2023; 2023 Mar 29-Apr 1. J Thorac Oncol (2023) 18(4S):S68. doi: 10.1016/S1556-0864(23)00300-3
Keywords: non-small cell lung cancer, ramucirumab, docetaxel, immune checkpoint inhibitors, antiangiogenic therapy
Citation: Garon EB, Visseren-Grul C, Rizzo MT, Puri T, Chenji S and Reck M (2023) Clinical outcomes of ramucirumab plus docetaxel in the treatment of patients with non-small cell lung cancer after immunotherapy: a systematic literature review. Front. Oncol. 13:1247879. doi: 10.3389/fonc.2023.1247879
Received: 26 June 2023; Accepted: 10 August 2023;
Published: 04 September 2023.
Edited by:
Liyun Shi, Nanjing University of Chinese Medicine, ChinaReviewed by:
Walid Shalata, Soroka Medical Center, IsraelTakaaki Sasaki, Asahikawa Medical University, Japan
Copyright © 2023 Garon, Visseren-Grul, Rizzo, Puri, Chenji and Reck. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Martin Reck, TS5SZWNrQGx1bmdlbmNsaW5pYy5kZQ==
 Edward B. Garon
Edward B. Garon Carla Visseren-Grul2
Carla Visseren-Grul2 Maria Teresa Rizzo
Maria Teresa Rizzo Tarun Puri
Tarun Puri Suresh Chenji
Suresh Chenji Martin Reck
Martin Reck