- 1Multidisciplinary Treatment Cancer Center, Kurume University Hospital, Kurume, Japan
- 2Division of Gastroenterology, Department of Medicine, Kurume University School of Medicine, Kurume, Japan
- 3Department of Surgery, Kurume University School of Medicine, Kurume, Japan
- 4Department of Otolaryngology, Head and Neck Surgery, Kurume University School of Medicine, Kurume, Japan
- 5Department of Diagnostic Pathology, Kurume University Hospital, Kurume, Japan
Introduction: Expression of the NTRK gene is rare in solid tumors but is highly prevalent in salivary gland secretory carcinomas. Here, we report a case of a complete response to entrectinib in a patient with NTRK fusion gene-positive parotid carcinoma.
Case description: The patient was a 44-year-old man who underwent total left parotidectomy and left cervical lymph node dissection for a left parotid tumor at 24 years of age. The histopathological diagnosis was mammary analog secretory carcinoma. Postoperatively, the patient received only radiation therapy. Sixteen years after the surgery, the patient became aware of a mass in the left parotid region. A close examination revealed local recurrence and multiple cervical lymph node metastases. S-1 monotherapy was started as chemotherapy but was discontinued 3 years later because of disease progression. As there was no standard treatment, a comprehensive genomic profiling test using a next-generation sequencer was performed, and the ETV6-NTRK3 fusion gene was identified. Entrectinib, an NTRK inhibitor, was immediately administered at a dose of 600 mg/day. The local recurrence rapidly shrank grossly from the beginning of treatment, and a complete response was observed 6 months later. However, creatinine levels exhibited an increase at week 68 of treatment; consequently, entrectinib dosage was lowered to 400 mg/day, leading to an immediate improvement in creatinine levels. Entrectinib was associated with additional side effects, including dysgeusia, fatigue, dizziness, and weight gain, all of which were also alleviated by the reduction in entrectinib dose. Thirty months after treatment initiation, the patient maintained a complete response and continued to receive entrectinib.
Conclusion: The NTRK fusion gene should always be checked in the presence of salivary gland secretory carcinoma.
1 Introduction
In 2010, Skalova et al. reported a histological type of salivary gland carcinoma that resembled the secretory carcinoma (SC) of the mammary gland, known as mammary analog secretory carcinoma (MASC) (1). Recently, MASC was established as a new histologic type in the World Health Organization Classification of Head and Neck Tumors (4th edition) as SC (2). SCs are low-grade salivary gland tumors that grow relatively slowly (1, 3, 4); however, there is currently no effective drug therapy. Recently, entrectinib, an inhibitor of tropomyosin receptor kinase (TRKs), has been reported to be effective in treating solid tumors with NTRK fusion genes (5). The ETV6-NTRK3 fusion gene has been identified in several SCs (1, 6, 7). Additionally, in Japan, a comprehensive genomic profiling (CGP) test using next-generation sequencing for solid tumors for which there is no standard treatment was covered by insurance in June 2019.
Herein, we report a case of a complete response to entrectinib in a patient with NTRK fusion gene-positive parotid carcinoma.
2 Case description
A 44-year-old man, in January 20XX (24 years old at that time), underwent left parotid pretreatment and cervical dissection for parotid carcinoma. A histopathological diagnosis of MASC was established. Postoperatively, the patient was treated with radiation therapy, but no drug therapy was administered, and the patient was followed up. In April 20XX+16, 16 years after the initial surgery, the patient visited our otolaryngology clinic because of a palpable mass in the left parotid region. After close examination, the patient was diagnosed with local recurrence and cervical lymph node metastasis. However, complete resection of the lymph nodes proved to be challenging. Unfortunately, there were no recommend drug therapies available for effective treatment. In response to the patient’s request for oral treatment, oral S-1 therapy was initiated. However, local recurrence gradually increased and lung metastasis appeared in October 20XX+19; therefore, S-1 oral therapy was discontinued, and a biopsy of cervical lymph node metastasis was performed simultaneously.
The pathological diagnosis of the biopsied tissue was SC, which was similar to that at the time of the initial surgery. Immunostaining for S-100, MUC4, and Pan-Trk showed widely positive results (Figure 1A), while immunostaining for mammaglobin revealed partially positive result. Subsequently, fluorescence in situ hybridization (FISH) with an ETV6 (12p13) break-apart probe (Vysis ETV6 Break Apart FISH Probe Kit, Abbott) using a previously reported method (8) revealed split signals in the nuclei (Figure 1B). These findings supported the diagnosis of SC. In Japan, the use of TRK inhibitors requires ETV6-NTRK3 fusion analysis using next-generation sequencing. Finally, a CGP test using FoundationOne® CDx (Foundation Medicine, Cambridge, MA, USA) was performed, and the ETV6-NTRK3 fusion gene was identified.
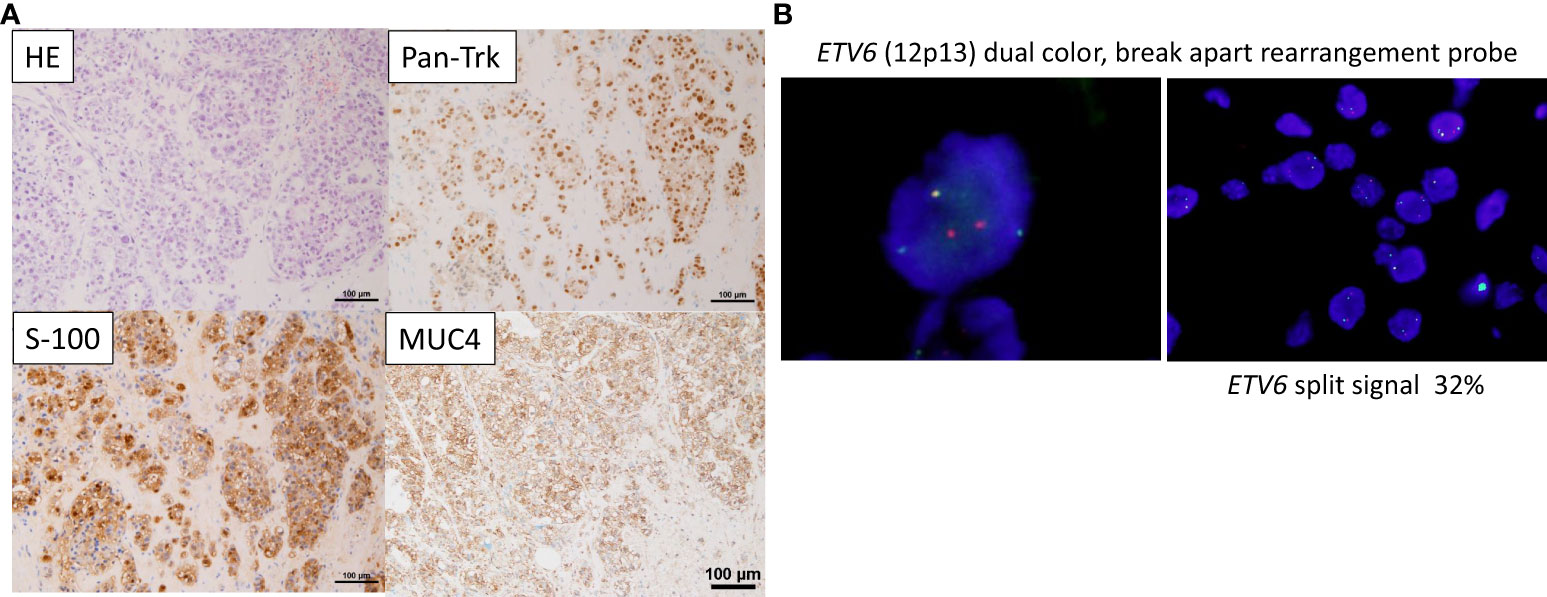
Figure 1 Pathological findings (A) On Hematoxylin & Eosin (HE) staining, tumor cells with well-defined nucleoli and a pale eosinophilic, partially vacuolated cytoplasm resemble a microcystic form, presenting a histology similar to the microcystic form of acinic cell carcinoma. Immunostaining showing that Pan-Trk and S-100, and MUC4 are widely expressed. (B) Fluorescence in situ hybridization (FISH) using the ETV6 break apart probe examined for the presence of yellow (red/green fusion) or green/red fluorescent signals in the tumor cells. Yellow signals are negative, and separate red and green signals are positive. ETV6 split signal of 32% is seen (cutoff, 10%).
The patient was started on entrectinib therapy (600 mg/day) in January 20XX +20. The patients’ pretreatment blood test results are shown in Supplementary Table 1; there were no problematic findings. However, the patient had a recurrent tumor approximately 7 cm in length in the left parotid region, which had partially self-disintegrated and showed effusion; therefor, he lived with a gauze over his neck at all times. After treatment, the local recurrence in the left parotid region shrank rapidly and completely disappeared 6 months later (Figure 2, upper row). Computed tomography (CT) findings also showed that the local recurrent lesion had shrunk deeply after 2 months of treatment and completely disappeared after 6 months of treatment (Figure 2, lower row). Simultaneously, the pulmonary metastases disappeared, and the CT images revealed a complete response.
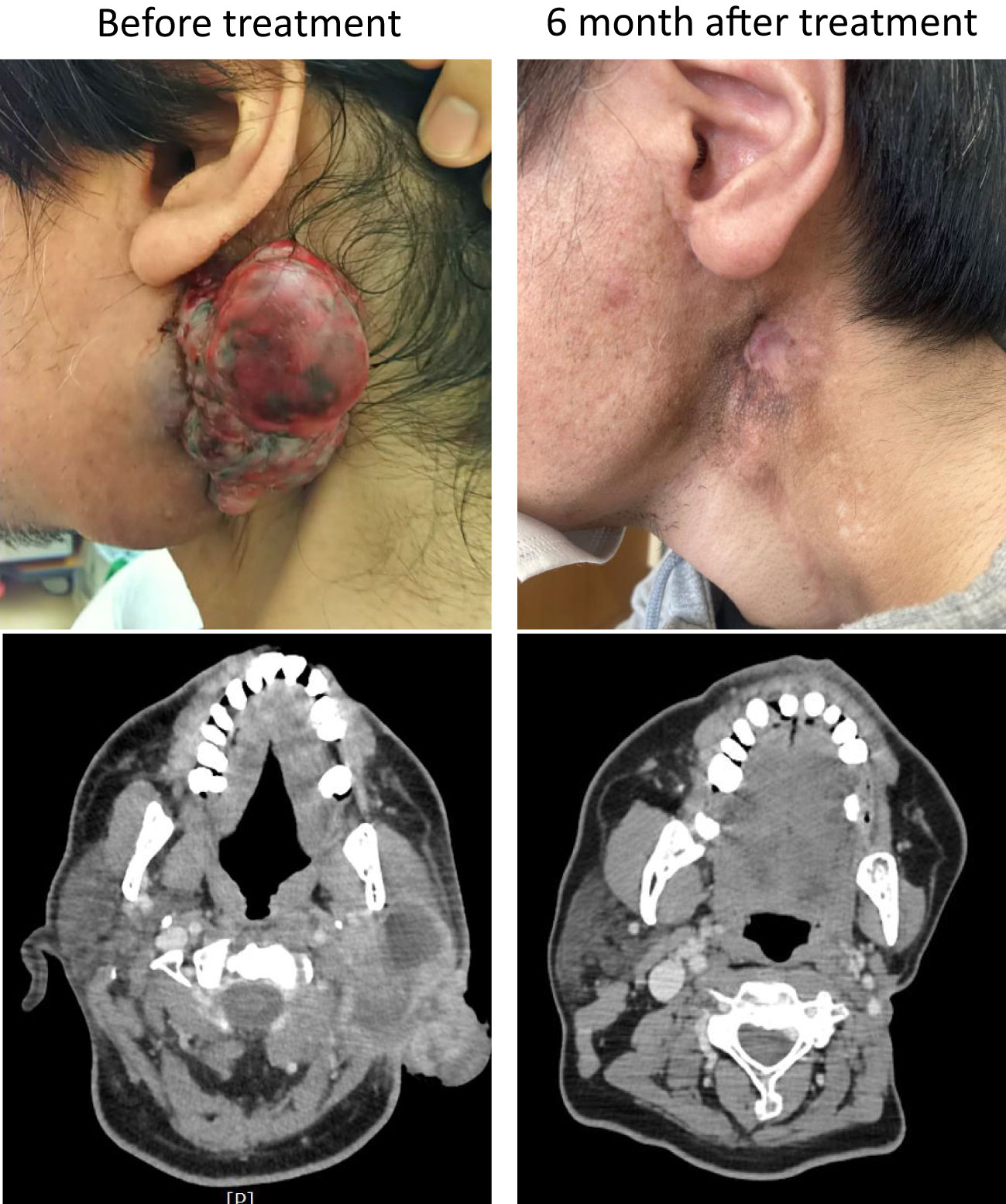
Figure 2 Gross and computed tomography (CT) images. The upper panels show the gross findings of the local recurrence site before and after treatment, and the lower panels show the CT images of the same site before and after treatment.
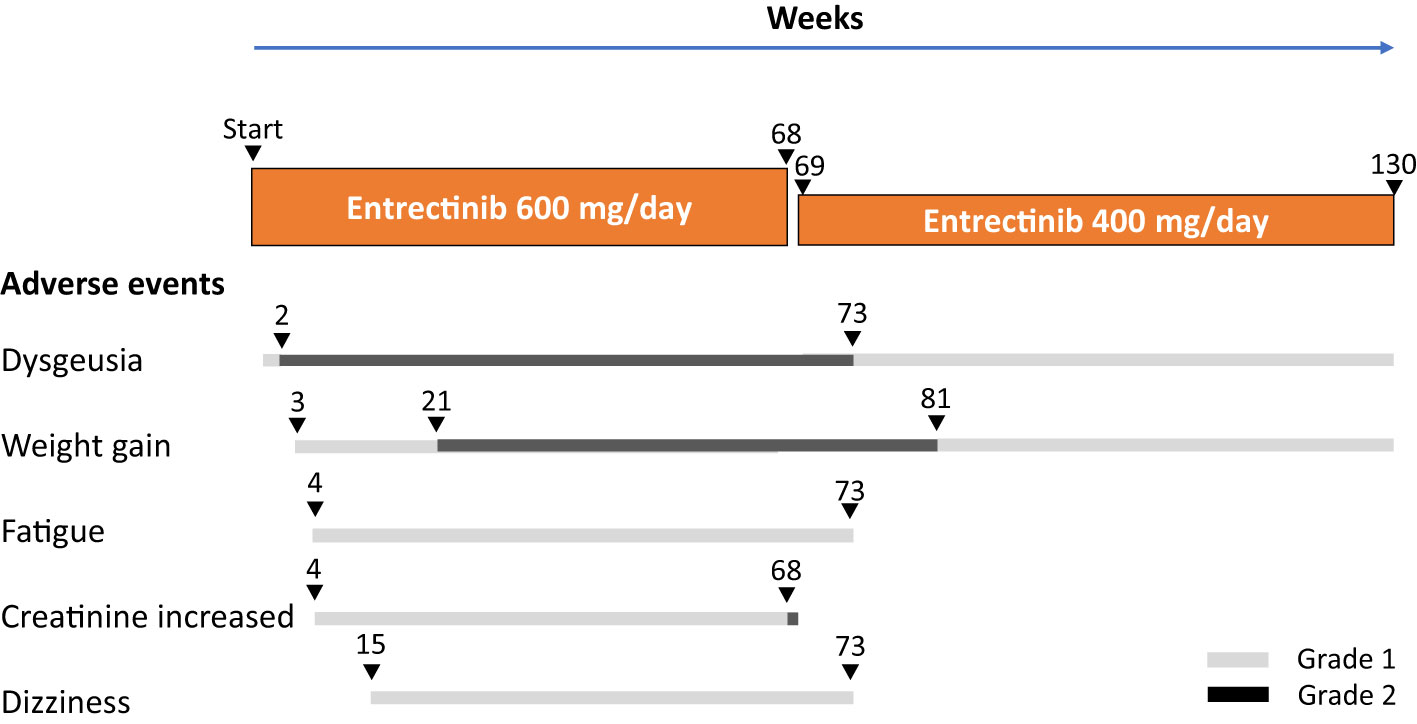
Adverse events experienced during entrectinib treatment and the patients’ clinical course are illustrated in Figure 3. Adverse events with entrectinib included grade 1 (CTCAE version 5.0) dysgeusia on day 4 of treatment, which worsened to grade 2 by week 2; however, the oral intake was good and tolerable. In addition, grade 1 weight gain was observed at week 3 of treatment, grade 1 fatigue and creatinine levels increased at week 4, grade 1 dizziness was observed at week 15, and grade 2 weight gain increased at week 21, all within acceptable limits; entrectinib was continued at a dose of 600 mg/day. However, an increase in grade 2 creatinine levels was observed at week 68 of treatment. Entrectinib was withdrawn for 1 week, and creatinine levels immediately improved to baseline values; therefore, entrectinib was reduced to 400 mg/day and continued from April 20XX+21. After the entrectinib dose reduction, all adverse events decreased, and the patient was able to go about his daily life, including work, without any problems.
Thirty months after the start of treatment, the patient is still receiving entrectinib therapy, and complete resolution of the recurrent lesions has been sustained on imaging and gross examination.
3 Discussion
The mean age of 91 patients with MASC was reported to be 44.7 years (range: 14–77 years), with 70% occurring in the parotid gland (9). The clinical course of salivary gland SC is characterized by a moderate risk of local recurrence, as well as a low risk of distant metastasis. Moreover, SC is generally considered a low-grade cancer with an overall good prognosis (1, 3, 4). However, some patients may develop local or distant metastases (10). Specifically, the clinical course of SC has been reported to have a moderate risk of local recurrence (15%) and lymph node metastasis (20%) and a low risk of distant metastasis (5%) (1, 11). The present case is considered a typical course of SC because of the local recurrence 20 years after surgery and subsequent slow enlargement.
The pathological diagnosis of SC is usually made using Hematoxylin & Eosin staining and immunostaining (S-100, mammaglobin, and Pan-Trk). Furthermore, to identify cases where NTRK mutation is not evident, the presence of the ETV6-NTRK3 fusion gene is required, which can be confirmed through FISH or reverse transcription polymerase chain reaction (1, 6, 7). In particular, the usefulness of MUC4 for SC and Pan-Trk for ETV6-NTRK3 fusion in immunostaining has recently been reported. NTRK fusion genes are expressed in 0.3–1.6% of all solid tumors, but the expression rate varies widely among cancer types (12, 13). Among them, salivary gland SCs are known to have a high rate (80–100%) of NTRK fusion gene expression, most of which are ETV6-NTRK3 fusion genes (7, 14).
Recently, the rearrangement of the ETV6-NTRK3 gene was identified as a therapeutic target (15, 16). The safety and antitumor activity of entrectinib, a potent oral inhibitor of the tyrosine kinases TRKA/B/C, ROS1, and ALK, have been demonstrated in several clinical trials in patients with advanced or metastatic solid tumors harboring genetic rearrangements of NTRK1-3, ROS1, or ALK, regardless of the histological type (17, 18). In an integrated analysis of phase 1-2 trials (STARTRK-1, STARTRK-2, and ALKA-372-001) of solid tumors with the NTRK fusion gene, the response rate to the TRK inhibitor entrectinib was 57%, and the median progression-free survival was 11.2 months (5). Another TRK inhibitor, larotrectinib, is also effective in the treatment of solid tumors with the NTRK fusion gene (19) and has been covered by insurance in Japan since March 2021, following insurance coverage of entrectinib. Adverse events of entrectinib in this patient included dysgeusia, fatigue, dizziness, weight gain, and creatinine increase, all of which were reported in the integrated analysis of phase 1-2 trial (5), and a reduction in the dose of entrectinib reduced these symptoms. This patient has been continuing entrectinib therapy 30 months after the start of treatment and has been living comfortably.
Thus, recognition of SC and testing for ETV6-NTRK3 gene rearrangements are critical for patient care. Although there has already been a case report on the sustained efficacy of entrectinib in SC with the ETV6-NTRK3 gene fusion (20), the number remains small. This case showed a typical clinical presentation of SC in the parotid gland, and complete response was maintained after 30 months of entrectinib treatment. However, without insurance coverage for CGP testing, the patient would not have benefited from entrectinib. Ultimately, our plan is to continue administering entrectinib as long as the therapeutic effect is sustained and side effects remain tolerable.
Data availability statement
The datasets presented in this article are not readily available because of ethical/privacy restrictions. Requests to access the datasets should be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
EM and KM provided patient care and wrote the manuscript. TO and HU were responsible for patient surgery and genetic diagnosis. JA and AK were responsible for pathology. SN, TT, and YS participated in data acquisition and critical revisions. FF and TK supervised this case report. All authors discussed the results and contributed to the final manuscript.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1247435/full#supplementary-material
Abbreviations
MASC, mammary analog secretory carcinoma; SC, secretory carcinoma; CGP, comprehensive genomic profiling; FISH, fluorescence in situ hybridization; TRK, tropomyosin receptor kinase.
References
1. Skalova A, Vanecek T, Sima R, Laco J, Weinreb I, Perez-Ordonez B, et al. Mammary analogue secretory carcinoma of salivary glands, containing the etv6-ntrk3 fusion gene: A hitherto undescribed salivary gland tumor entity. Am J Surg Pathol (2010) 34(5):599–608. doi: 10.1097/PAS.0b013e3181d9efcc
2. El-Naggar AK. Editor's perspective on the 4th edition of the who head and neck tumor classification. J Egypt Natl Canc Inst (2017) 29(2):65–6. doi: 10.1016/j.jnci.2017.03.003
3. Chiosea SI, Griffith C, Assaad A, Seethala RR. Clinicopathological characterization of mammary analogue secretory carcinoma of salivary glands. Histopathology (2012) 61(3):387–94. doi: 10.1111/j.1365-2559.2012.04232.x
4. Bishop JA, Yonescu R, Batista D, Eisele DW, Westra WH. Most nonparotid "Acinic cell carcinomas" Represent mammary analog secretory carcinomas. Am J Surg Pathol (2013) 37(7):1053–7. doi: 10.1097/PAS.0b013e3182841554
5. Doebele RC, Drilon A, Paz-Ares L, Siena S, Shaw AT, Farago AF, et al. Entrectinib in patients with advanced or metastatic ntrk fusion-positive solid tumours: integrated analysis of three phase 1-2 trials. Lancet Oncol (2020) 21(2):271–82. doi: 10.1016/S1470-2045(19)30691-6
6. Ito Y, Ishibashi K, Masaki A, Fujii K, Fujiyoshi Y, Hattori H, et al. Mammary analogue secretory carcinoma of salivary glands: A clinicopathologic and molecular study including 2 cases harboring etv6-X fusion. Am J Surg Pathol (2015) 39(5):602–10. doi: 10.1097/PAS.0000000000000392
7. Skalova A, Vanecek T, Simpson RH, Laco J, Majewska H, Baneckova M, et al. Mammary analogue secretory carcinoma of salivary glands: molecular analysis of 25 etv6 gene rearranged tumors with lack of detection of classical etv6-ntrk3 fusion transcript by standard rt-pcr: report of 4 cases harboring etv6-X gene fusion. Am J Surg Pathol (2016) 40(1):3–13. doi: 10.1097/PAS.0000000000000537
8. Kawahara A, Taira T, Abe H, Takase Y, Kurita T, Sadashima E, et al. Diagnostic utility of phosphorylated signal transducer and activator of transcription 5 immunostaining in the diagnosis of mammary analogue secretory carcinoma of the salivary gland: A comparative study of salivary gland cancers. Cancer Cytopathol (2015) 123(10):603–11. doi: 10.1002/cncy.21594
9. Sethi R, Kozin E, Remenschneider A, Meier J, VanderLaan P, Faquin W, et al. Mammary analogue secretory carcinoma: update on a new diagnosis of salivary gland Malignancy. Laryngoscope (2014) 124(1):188–95. doi: 10.1002/lary.24254
10. Majewska H, Skalova A, Stodulski D, Klimkova A, Steiner P, Stankiewicz C, et al. Mammary analogue secretory carcinoma of salivary glands: A new entity associated with etv6 gene rearrangement. Virchows Arch (2015) 466(3):245–54. doi: 10.1007/s00428-014-1701-8
11. Chiosea SI, Griffith C, Assaad A, Seethala RR. The profile of acinic cell carcinoma after recognition of mammary analog secretory carcinoma. Am J Surg Pathol (2012) 36(3):343–50. doi: 10.1097/PAS.0b013e318242a5b0
12. Okamura R, Boichard A, Kato S, Sicklick JK, Bazhenova L, Kurzrock R. Analysis of ntrk alterations in pan-cancer adult and pediatric Malignancies: implications for ntrk-targeted therapeutics. JCO Precis Oncol (2018) 2018:1–20. doi: 10.1200/PO.18.00183
13. Gouda MA, Nelson BE, Buschhorn L, Wahida A, Subbiah V. Tumor-agnostic precision medicine from the aacr genie database: clinical implications. Clin Cancer Res (2023) 29(15):1–8. doi: 10.1158/1078-0432.CCR-23-0090
14. Bishop JA, Yonescu R, Batista D, Begum S, Eisele DW, Westra WH. Utility of mammaglobin immunohistochemistry as a proxy marker for the etv6-ntrk3 translocation in the diagnosis of salivary mammary analogue secretory carcinoma. Hum Pathol (2013) 44(10):1982–8. doi: 10.1016/j.humpath.2013.03.017
15. Tognon CE, Somasiri AM, Evdokimova VE, Trigo G, Uy EE, Melnyk N, et al. Etv6-ntrk3-mediated breast epithelial cell transformation is blocked by targeting the igf1r signaling pathway. Cancer Res (2011) 71(3):1060–70. doi: 10.1158/0008-5472.CAN-10-3096
16. Chi HT, Ly BT, Kano Y, Tojo A, Watanabe T, Sato Y. Etv6-ntrk3 as a therapeutic target of small molecule inhibitor pkc412. Biochem Biophys Res Commun (2012) 429(1-2):87–92. doi: 10.1016/j.bbrc.2012.10.087
17. Drilon A, Li G, Dogan S, Gounder M, Shen R, Arcila M, et al. What hides behind the masc: clinical response and acquired resistance to entrectinib after etv6-ntrk3 identification in a mammary analogue secretory carcinoma (Masc). Ann Oncol (2016) 27(5):920–6. doi: 10.1093/annonc/mdw042
18. Drilon A, Siena S, Ou SI, Patel M, Ahn MJ, Lee J, et al. Safety and antitumor activity of the multitargeted pan-trk, ros1, and alk inhibitor entrectinib: combined results from two phase I trials (Alka-372-001 and startrk-1). Cancer Discov (2017) 7(4):400–9. doi: 10.1158/2159-8290.CD-16-1237
19. Hong DS, DuBois SG, Kummar S, Farago AF, Albert CM, Rohrberg KS, et al. Larotrectinib in patients with trk fusion-positive solid tumours: A pooled analysis of three phase 1/2 clinical trials. Lancet Oncol (2020) 21(4):531–40. doi: 10.1016/S1470-2045(19)30856-3
Keywords: NTRK fusion gene, ETV6-NTRK3 fusion gene, secretory carcinoma, salivary gland carcinoma, parotid gland cancer, entrectinib, next-generation sequencing, case report
Citation: Moriyama E, Nagasu S, Tanaka T, Shimotsuura Y, Ono T, Umeno H, Akiba J, Kawahara A, Fujita F, Kawaguchi T and Miwa K (2023) Case Report: A case of complete response to entrectinib in NTRK fusion gene-positive parotid gland cancer. Front. Oncol. 13:1247435. doi: 10.3389/fonc.2023.1247435
Received: 26 June 2023; Accepted: 19 July 2023;
Published: 04 August 2023.
Edited by:
Takumi Kumai, Asahikawa Medical University, JapanReviewed by:
Rong-Hui Xia, Shanghai Jiao Tong University, ChinaMichihisa Kono, Dana–Farber Cancer Institute, United States
Copyright © 2023 Moriyama, Nagasu, Tanaka, Shimotsuura, Ono, Umeno, Akiba, Kawahara, Fujita, Kawaguchi and Miwa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Keisuke Miwa, bWl3YV9rZWlzdWtlQG1lZC5rdXJ1bWUtdS5hYy5qcA==
 Etsuko Moriyama1,2
Etsuko Moriyama1,2 Toshimitsu Tanaka
Toshimitsu Tanaka Fumihiko Fujita
Fumihiko Fujita Takumi Kawaguchi
Takumi Kawaguchi Keisuke Miwa
Keisuke Miwa