- 1Department of Breast and Thyroid Surgery, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 2Department of Head and Neck Surgery, The First Hospital of Jiaxing, Zhejiang, China
Introduction: The efficacy and safety of adjuvant capecitabine in early-stage triple-negative breast cancer remains undefined. A meta-analysis was conducted to elucidate whether capecitabine-based regimens could improve survival in early-stage triple-negative breast cancer (TNBC).
Methods: The current study searched Medline, Embase, Cochrane Library, Web of Science, and ClinicalTrials.gov proceedings up to 2023.9. Disease-free survival (DFS), overall survival (OS), and grade 3–4 adverse events (AEs) were assessed. Extracted or calculated hazard ratios (HRs) and odds ratios (ORs) with 95% confidence intervals (CIs) were pooled.
Results: The capecitabine-based regimens showed significant advantages in DFS (HR = 0.81, 95% CI: 0.73–0.90; P <.001) and OS (HR = 0.75, 95% CI: 0.65–0.87; P <.001) from 12 randomized controlled trials (RCTs) with 5,390 unselected participants. Subgroup analysis of DFS showed analogous results derived from patients with lymph node negative (HR = 0.68, 95% CI: 0.50–0.92; P = .006) and capecitabine duration no less than six cycles (HR = 0.73; 95% CI: 0.62-0.86; P <.001). Improvement of DFS in the addition group (HR = 0.77, 95% CI: 0.68–0.87; P <.001) and adjuvant setting (HR = 0.79, 95% CI: 0.70–0.89; P <.001) was observed. As to safety profile, capecitabine was associated with more frequent stomatitis (OR = 5.05, 95% CI: 1.45–17.65, P = .011), diarrhea (OR = 6.11, 95% CI: 2.12–17.56; P =.001), and hand–foot syndrome (OR = 31.82, 95% CI: 3.23–313.65, P = .003).
Conclusions: Adjuvant capecitabine-based chemotherapy provided superior DFS and OS to early-stage TNBC. The benefits to DFS in selected patients with lymph node negative and the addition and extended duration of capecitabine were demonstrated.
Introduction
Triple-negative breast cancer (TNBC) is characterized by aggressiveness, heterogeneity, and a higher relapse tendency. For histopathological diagnosis, it pertains to an estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) negative breast cancer (BC) subtype. Anthracycline- and/or taxane-based standard chemotherapies have substantially improved survival outcomes (1, 2). However, the 10-year recurrence risk in early-stage TNBC remains approximately 20%–40% (3, 4). Accordingly, new drug-incorporated strategies should be explored for further clinical development.
Capecitabine is an oral fluoropyrimidine prodrug with high efficacy and favorable tolerability for advanced BC (5). It is not the standard chemotherapy for early BC. There were also some conflicting survival data (6–17). Recently, two meta-analyses were performed to explore the RCTs of capecitabine effect, but TNBCs were treated as a subgroup in these studies (18, 19). To further determine the influence of adjuvant capecitabine on early-stage TNBC, we incorporated prospective randomized controlled trials (RCTs) in full text, performed a meta-analysis to get robust conclusions.
Methods
Search strategy
This meta-analysis complied with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (20). Online databases Medline, Embase, Cochrane Library, and ClinicalTrials.gov were searched until September 17, 2023. Queries included the MeSH terms “breast cancer,” “breast neoplasm,” “triple-negative breast cancer,” “triple-negative breast neoplasms,” and the keywords “capecitabine” and “Xeloda.”.
Selection criteria
Inclusion criteria were as follows: (a) phase III RCTs of early BC involving TNBC; (b) RCTs contained a comparison of capecitabine-based chemotherapy against capecitabine-free regimens; (c) available HRs with 95% CIs for DFS and/or OS. RCTs published other than English were excluded.
Data extraction
Two reviewers (XL, JB) extracted data by search strategy independently. Discordance would be resolved by consensus. Information captured from RCTs included the following: trial name, authors, update year, study design, TNBC patients, baseline characteristics, chemotherapy schedules, median follow-up period, survival results (HRs and 95% Cis for DFS and/or OS), and grade 3–4 adverse events (AEs).
Quality assessment
The quality of studies was independently assessed using the Cochrane Risk Of Bias Assessment Tool (CROBAT), which consists of “random sequence generation,” “allocation concealment,” “blinding of participants and personnel,” “blinding of outcome assessment,” “incomplete outcome data,” “selective reporting,” and “other bias” (Figure S1). Publication bias was evaluated by Funnel plot and Egger’s regression asymmetry test (21) (Figures S2–S4).
Statistical analysis
By the generic inverse variance method, the HRs and 95% CIs for DFS and/or OS in TNBC were pooled (22).The odds ratios (ORs) of grade 3–4 AEs were weighted and estimated. In addition, current analysis followed the intention-to-treat principle. We conducted subgroup analyses in accordance with (a) nodal status, (b) capecitabine duration, (c) adding or replacing capecitabine in chemotherapy, (d) adjuvant or neoadjuvant chemotherapy, (e) dosage of capecitabine, (f) the TNBC proportion, (g) combined chemotherapy regimen, (h) sequential or concomitant capecitabine, (i) study region, (j) menopausal status, (k) tumor size, (l) histological grade, (m) basal or non-basal subtype, and (n) Ki-67 status. Heterogeneity among RCTs was evaluated by the Cochran Q statistics and I2 test (23). When P < 0.10 or I2 > 50%, we utilized the random-effect model. Otherwise, the fixed-effect model was used. Sensitivity analysis was conducted to assess the stability. Analyses were two-tailed with Stata 17.0 software.
Results
Characteristics of studies
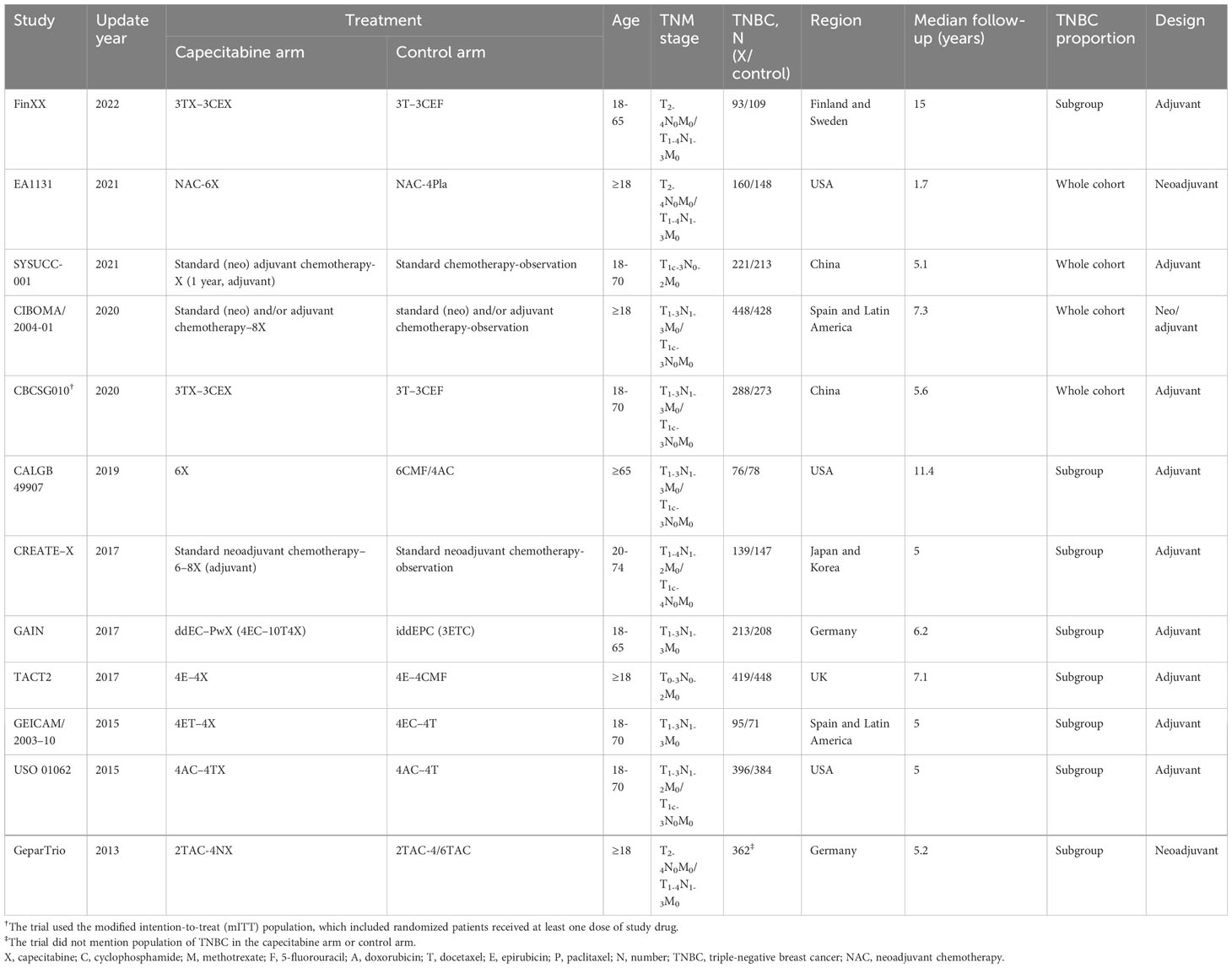
There were 12 RCTs with 5,390 TNBC patients who met the predefined criteria (Table 1; Figure 1) (6–17). The FinXX and CALGB 49907 trials, reported RFS rather than DFS; however, RFS was arguably the same definition as DFS (6, 11). Four studies reported early-stage TNBC only (7–10); nevertheless, the others incorporated all BC subtypes. The CBCSG010 trial conducted the modified intention-to-treat analysis, which might amplify the benefits, yet the dropouts were too small to yield a positive result (10).
There was no significant publication bias from the Funnel plot or Egger’s test (Figures S2–S4). Sensitivity analysis indicated that no certain trial affected the pooled results (Figures S5, S6).
Efficacy
Pooled analysis
Due to the absence of heterogeneity (P = .187; I2 = 26.2%), we utilized the fixed-effect model to calculate the pooled DFS (HR = 0.81, 95% CI: 0.73–0.90; P <.001). It also corresponded to significant improvement in OS for the capecitabine group (HR = 0.75, 95% CI: 0.65–0.87; P <.001). The forest plots of DFS and OS are shown in Figure 2.
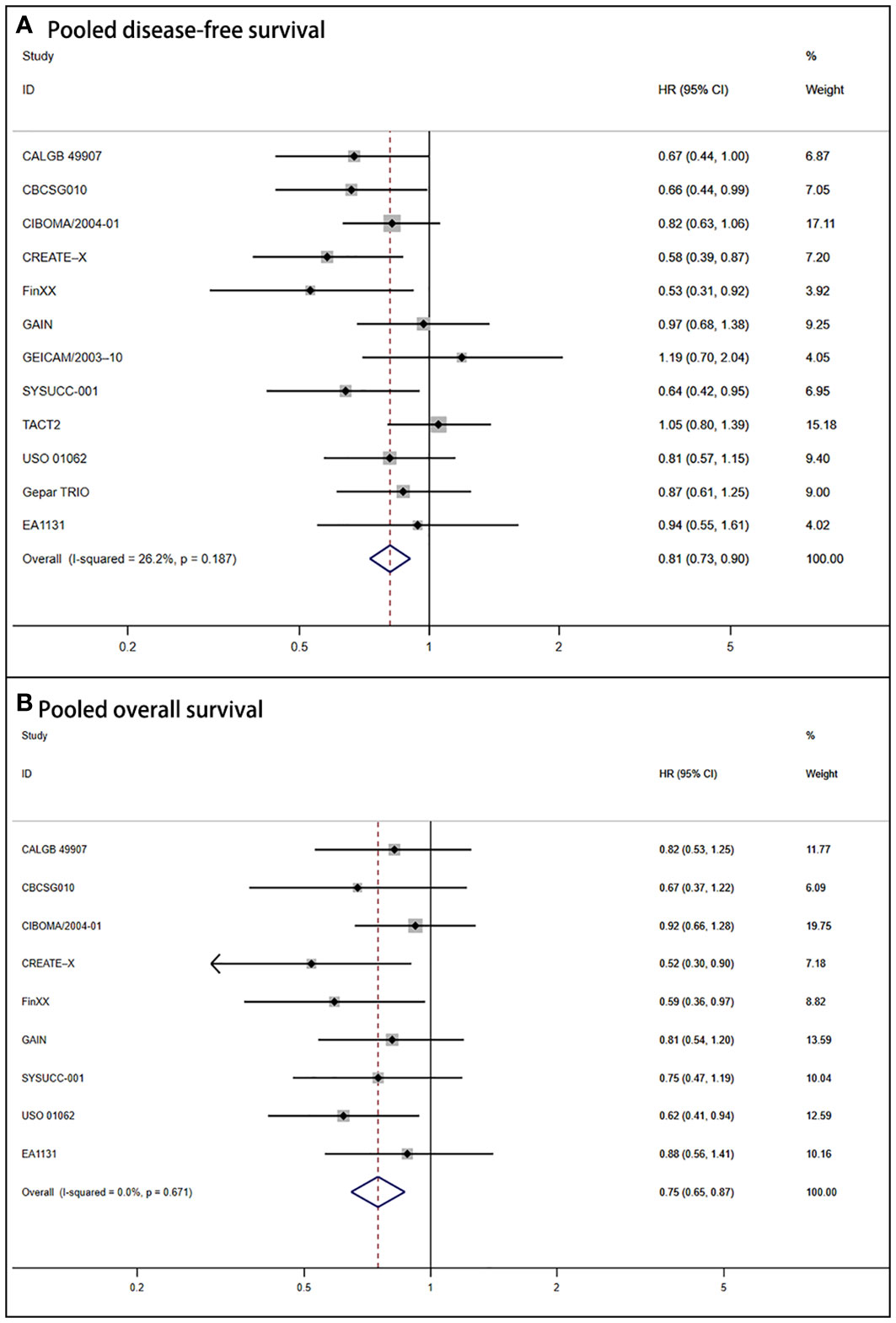
Figure 2 Forest plot of pooled disease-free survival and overall survival for capecitabine-based regimens in early triple-negative breast cancer (A) Shows the results for pooled disease-free survival. (B) Shows the outcomes for pooled overall survival.
Subgroup analysis
The nodal status subgroup analysis reached controversial outcomes (Figure 3A). DFS was statistically superior for women with lymph nodes negative (HR = 0.68, 95% CI: 0.50–0.92; P = .006) to positive (HR = 0.87, 95% CI: 0.72–1.05; P =.248). According to capecitabine duration, there were advantages of DFS (HR = 0.71, 95% CI: 0.60–0.84; P <.001) for patients who received capecitabine for at least 6 cycles (7, 9, 11, 12). For those with shorter duration (<6 cycles), no statistical significance was found (HR = 0.88, 95% CI: 0.73–1.01; P = .073) (Figure 3B) (6, 10, 13–17). The addition regimens improved DFS (HR = 0.77, 95% CI: 0.68–0.87; P<.001) (Figure 3C). In consideration of trials that TNBC acting as adjuvant chemotherapy, a greater DFS was determined (HR = 0.79, 95% CI: 0.70–0.89; P <.001) (Figure 3D) (6, 8–16).
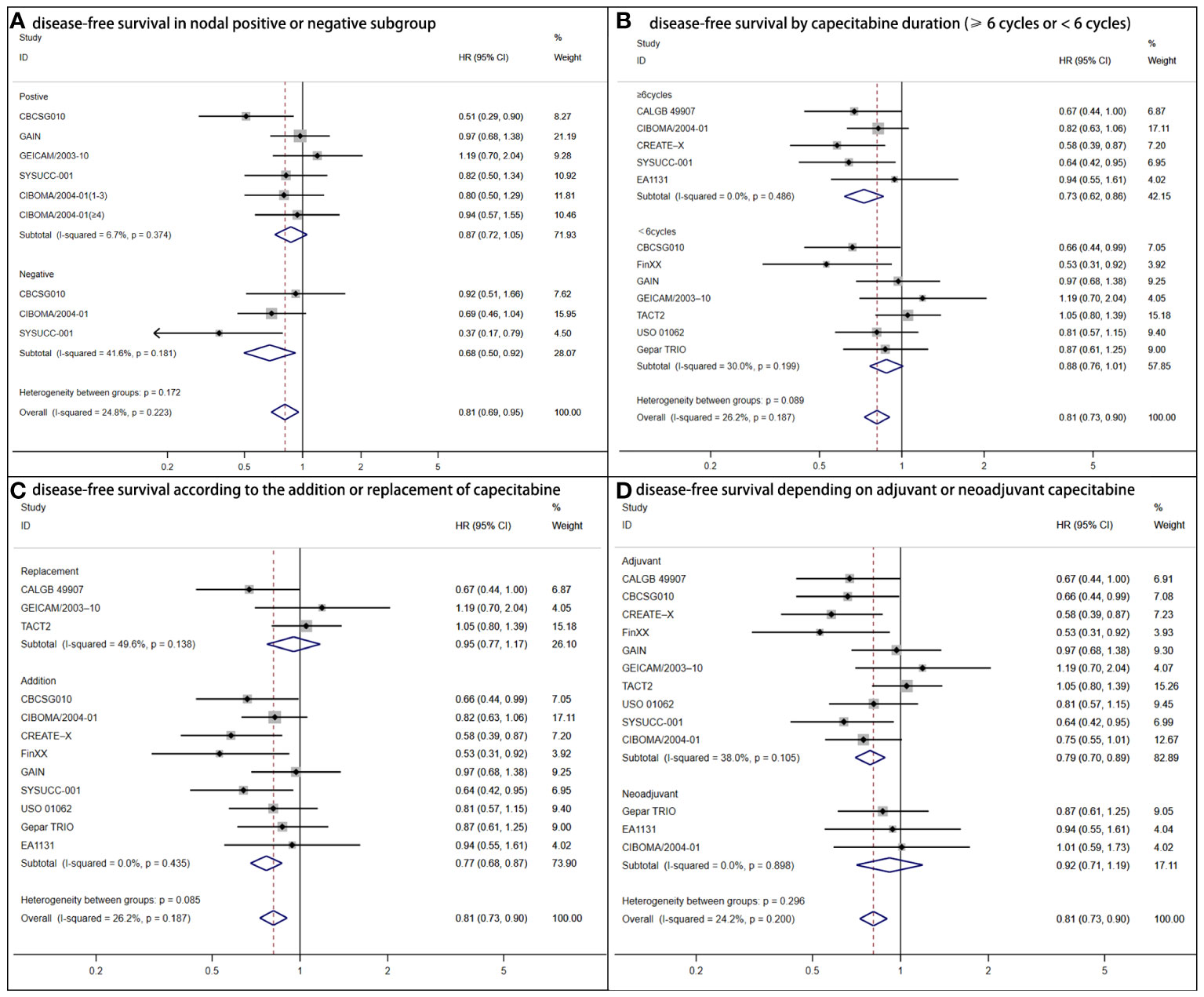
Figure 3 Forest plot of disease-free survival for adjuvant capecitabine in subgroups with heterogeneous results. (A) Shows disease-free survival in the nodal positive or negative subgroup. CIBOMA/2004-01(1-3) denotes subgroup with one to three positive lymph nodes. CIBOMA/2004-01(≥4) is defined as subset with no less than four positive nodes. (B) Shows disease-free survival by capecitabine duration (≥6 cycles or <6 cycles). (C) Shows disease-free survival according to the addition or replacement of capecitabine. (D) Shows disease-free survival depending on capecitabine accounting for adjuvant or neoadjuvant chemotherapy.
As shown in Table 2, DFS was superior in the anthracycline and taxane arm (HR = 0.83, 95% CI: 0.71–0.97; P =.020) and non-basal subtype group (HR = 0.47, 95% CI: 0.31–0.71; P <.001). We observed no statistical significance in either menopause status. However, subgroup analysis for DFS yielded positive effects by capecitabine dosage (<1,000 mg or ≥1,000 mg), TNBC proportion (as subgroup or whole cohort), capecitabine sequence (in sequential or concurrent), study region (America–Europe or Asia), tumor size (≤2 cm or >2 cm), histological grade (1–2 or 3), and Ki-67 status (<30% or ≥30%).
Safety and tolerability
The safety profile included hematologic side effects, gastrointestinal events, general disorders, skin and subcutaneous disorders, nervous system disorders, investigations, musculoskeletal and connective disorders, vascular disorders, and other disorders (grades 3–4) (Table 3). It was shown in four-RCT-specified TNBC (7–10). The pooled results indicated higher frequencies of stomatitis (OR = 5.05, 95% CI: 1.45–17.65; P =.011), diarrhea (OR = 6.11, 95% CI: 2.12–17.56; P =.001), and hand–foot syndrome (OR = 31.82, 95% CI: 3.23–313.65; P =.003) for capecitabine in early TNBC women.
Discussion
There were several RCTs with conflicting results about capecitabine in early TNBC. Recently, the EA1131 and SYSUCC-001 trials provided updated outcomes with details about patient characteristics and treatment strategies (7, 8). In addition, the FinXX trial updated overall survival on the basis of approximately 15-year follow-up of the patients (6). Therefore, it is reasonable to reevaluate the influence of capecitabine. As for metastatic TNBC, capecitabine had low response rates and limited activity in trials (23, 24). Selecting appropriate patients may enhance treatment effects, since the mechanisms remained unclear.
Focusing on the association of capecitabine and early TNBC, there were two meta-analyses recently (18, 19). However, meta-analysis from Xun et al. did not extract data from the TACT2 trial, which should be included as well (14, 18). In addition, the GAIN and GEICAM/2003-10 trials only consisted node-positive patients; in other words, they should be incorporated to the nodal status subgroup analysis as well (13, 15). Zhou et al. included trials more rigorously, so subgroup analysis performed with less information (19).
We conducted a comprehensive meta-analysis of 12 RCTs (full text) exploring the efficacy and safety of capecitabine-based chemotherapy. It significantly improved DFS and OS among 5,390 TNBC patients. Considering subgroup analysis, the survival benefits were observed in lymph node negative status, the addition, extended duration, and adjuvant setting of capecitabine.
Patients with nodal negative results had a superior DFS. Regarding the node-positive arm, the CBCSG010 trial reported an advantage for DFS (10), whereas four RCTs (SYSUCC-001, CIBOMA/2004-01, GAIN, GEICAM/2003-10) did not (8, 9, 13, 15). In addition, there were three arms of nodal status (0, 1–3, and ≥4) in the CIBOMA/2004-01 trial (9). However, no more details were available for lymph node positive status (1–3, 4–9, ≥10, without metastasis to internal mammary chain or infraclavicular/supraclavicular region). Therefore, we could not further determine the influence of different positive-node stages.
Metastasis of lymph node contributes to higher risk for TNBC. We speculated that capecitabine would confer further extended survival on node-positive patients. Nevertheless, the result was paradoxical. It might be strong distinction of capecitabine on micro-metastatic and overt lesions. Targeting the immune escape metastasis mechanism, dormant tumor cells with a lower proliferation index were more sensitive to an antimetabolite drug. Similar to capecitabine, it is a DNA synthesis inhibitor (25). Biomarkers determining which TNBC subtype favored most are also needed; for example, the PAM50 non-basal molecular subtype and the BRCA1-like DNA copy number (26, 27). Extrapolation from studies of the last decade with long-term follow-up should be cautiously applied. The RCTs of individual survival benefits of capecitabine for node-positive patients are still needed.
Adjuvant capecitabine added to the anthracycline and taxane regimens had survival advantages for early TNBC, in favor of the synergism of docetaxel and capecitabine in preclinical models (28). The results verified the rationale of combination chemotherapy (29). Patients derived greater DFS from extended capecitabine duration (≥6 cycles), which means capecitabine could modulate antitumor immune and anti-angiogenesis properties through metronomic therapy (30).
Our study has limitations. First, populations with various characteristics contributed to the heterogeneity. The definition of ER-negative in the CBCSG010 and CREAT-X studies (<10%) did not align with others (<1%) (10, 12). Second, the diverse chemotherapy regimens confounded the results and decreased robustness. Third, given no individual patient data available, the details (age and intrinsic subtype) were incomplete, so it was hard to assess and select the subpopulation.
Conclusion
This meta-analysis showed that capecitabine-based regimens significantly improved both DFS and OS in early-stage TNBC. There was a substantial improvement for DFS in the groups with lymph node negative status, the adjuvant, addition, and longer duration (≥6 cycles) of capecitabine to standard chemotherapy.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
Design: JB and XL. Data collection: JB, XY, YP. Data analysis: JB, XL. Writing-original draft: JB. Writing-review and editing: XW, XL. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (no. 82003135) and the Natural Science Foundation of Chongqing (cstc2021jcyj-msxmX0147).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1245650/full#supplementary-material
References
1. Sonnenblick A, Piccart M. Adjuvant systemic therapy in breast cancer: quo vadis? Ann Oncol (2015) 26(8):1629–34. doi: 10.1093/annonc/mdv108
2. Gadi VK, Davidson NE. Practical approach to triple-negative breast cancer. J Oncol practice (2017) 13(5):293–300. doi: 10.1200/JOP.2017.022632
3. Early Breast Cancer Trialists' Collaborative G, Peto R, Davies C, Godwin J, Gray R, Pan HC, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet (2012) 379(9814):432–44. doi: 10.1016/S0140-6736(11)61625-5
4. Ali AM, Ansari JAK, El-Aziz NMA, Abozeed WN, Warith AMA, Alsaleh K, et al. Triple negative breast cancer: A tale of two decades. Anti-cancer Agents medicinal Chem (2017) 17(4):491–9. doi: 10.2174/1871520616666160725112335
5. Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. New Engl J Med (2006) 355(26):2733–43. doi: 10.1056/NEJMoa064320
6. Joensuu H, Kellokumpu-Lehtinen PL, Huovinen R, Jukkola-Vuorinen A, Tanner M, Kokko R, et al. Adjuvant capecitabine in combination with docetaxel, epirubicin, and cyclophosphamide for early breast cancer: the randomized clinical finXX trial. JAMA Oncol (2017) 3(6):793–800. doi: 10.1001/jamaoncol.2016.6120
7. Mayer IA, Zhao F, Arteaga CL, Symmans WF, Park BH, Burnette BL, et al. Randomized phase III postoperative trial of platinum-based chemotherapy versus capecitabine in patients with residual triple-negative breast cancer following neoadjuvant chemotherapy: ECOG-ACRIN EA1131. J Clin Oncol (2021) 39(23):2539–51. doi: 10.1200/JCO.21.00976
8. Wang X, Wang SS, Huang H, Cai L, Zhao L, Peng RJ, et al. Effect of capecitabine maintenance therapy using lower dosage and higher frequency vs observation on disease-free survival among patients with early-stage triple-negative breast cancer who had received standard treatment: the SYSUCC-001 randomized clinical trial. Jama (2021) 325(1):50–8. doi: 10.1001/jama.2020.23370
9. Lluch A, Barrios CH, Torrecillas L, Ruiz-Borrego M, Bines J, Segalla J, et al. Phase III trial of adjuvant capecitabine after standard neo-/adjuvant chemotherapy in patients with early triple-negative breast cancer (GEICAM/2003-11_CIBOMA/2004-01). J Clin Oncol (2020) 38(3):203–13. doi: 10.1200/JCO.19.00904
10. Li J, Yu K, Pang D, Wang C, Jiang J, Yang S, et al. Adjuvant capecitabine with docetaxel and cyclophosphamide plus epirubicin for triple-negative breast cancer (CBCSG010): an open-label, randomized, multicenter, phase III trial. J Clin Oncol (2020) 38(16):1774–84. doi: 10.1200/JCO.19.02474
11. Muss HB, Polley MC, Berry DA, Liu H, Cirrincione CT, Theodoulou M, et al. Randomized trial of standard adjuvant chemotherapy regimens versus capecitabine in older women with early breast cancer: 10-year update of the CALGB 49907 trial. J Clin Oncol (2019) 37(26):2338–48. doi: 10.1200/JCO.19.00647
12. Masuda N, Lee SJ, Ohtani S, Im YH, Lee ES, Yokota I, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. New Engl J Med (2017) 376(22):2147–59. doi: 10.1056/NEJMoa1612645
13. von Minckwitz G, Mobus V, Schneeweiss A, Huober J, Thomssen C, Untch M, et al. German adjuvant intergroup node-positive study: a phase III trial to compare oral ibandronate versus observation in patients with high-risk early breast cancer. J Clin Oncol (2013) 31(28):3531–9. doi: 10.1200/JCO.2012.47.2167
14. Cameron D, Morden JP, Canney P, Velikova G, Coleman R, Bartlett J, et al. Accelerated versus standard epirubicin followed by cyclophosphamide, methotrexate, and fluorouracil or capecitabine as adjuvant therapy for breast cancer in the randomised UK TACT2 trial (CRUK/05/19): a multicentre, phase 3, open-label, randomised, controlled trial. Lancet Oncol (2017) 18(7):929–45. doi: 10.1016/S1470-2045(17)30404-7
15. Martin M, Ruiz Simon A, Ruiz Borrego M, Ribelles N, Rodriguez-Lescure A, Munoz-Mateu M, et al. Epirubicin plus cyclophosphamide followed by docetaxel versus epirubicin plus docetaxel followed by capecitabine as adjuvant therapy for node-positive early breast cancer: results from the GEICAM/2003-10 study. J Clin Oncol (2015) 33(32):3788–95. doi: 10.1200/JCO.2015.61.9510
16. O'Shaughnessy J, Koeppen H, Xiao Y, Lackner MR, Paul D, Stokoe C, et al. Patients with slowly proliferative early breast cancer have low five-year recurrence rates in a phase III adjuvant trial of capecitabine. Clin Cancer Res (2015) 21(19):4305–11. doi: 10.1158/1078-0432.CCR-15-0636
17. von Minckwitz G, Blohmer JU, Costa SD, Denkert C, Eidtmann H, Eiermann W, et al. Response-guided neoadjuvant chemotherapy for breast cancer. J Clin Oncol (2013) 31(29):3623–30. doi: 10.1200/JCO.2012.45.0940
18. Xun X, Cao Q, Hong P, Rai S, Zhou Y, Liu R, et al. Efficacy and safety of capecitabine for triple-negative breast cancer: A meta-analysis. Front Oncol (2022) 12:899423. doi: 10.3389/fonc.2022.899423
19. Zhou W, Cao Y, Gou P, Zeng X, Hu X, Lin Z, et al. Additional adjuvant capecitabine in early breast cancer patients: a meta-analysis of randomized controlled trials. Future Oncol (2021) 17(35):4993–5002. doi: 10.2217/fon-2020-1131
20. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. Bmj (2009) 339:b2700. doi: 10.1136/bmj.b2700
21. DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials (2015) 45(Pt A):139–45. doi: 10.1016/j.cct.2015.09.002
22. Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Institute (1959) 22(4):719–48.
23. Kaufman PA, Awada A, Twelves C, Yelle L, Perez EA, Velikova G, et al. Phase III open-label randomized study of eribulin mesylate versus capecitabine in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol (2015) 33(6):594–601. doi: 10.1200/JCO.2013.52.4892
24. Twelves C, Cortes J, Vahdat L, Olivo M, He Y, Kaufman PA, et al. Efficacy of eribulin in women with metastatic breast cancer: a pooled analysis of two phase 3 studies. Breast Cancer Res Treat (2014) 148(3):553–61. doi: 10.1007/s10549-014-3144-y
25. Schilsky RL. Biochemical and clinical pharmacology of 5-fluorouracil. Oncology (1998) 12(10 Suppl 7):13–8.
26. Asleh K, Lluch A, Goytain A, Barrios C, Wang XQ, Torrecillas L, et al. Triple-negative PAM50 non-basal breast cancer subtype predicts benefit from extended adjuvant capecitabine. Clin Cancer Res (2023) 29(2):389–400. doi: 10.1158/1078-0432.CCR-22-2191
27. de Boo LW, Jozwiak K, Joensuu H, Lindman H, Lauttia S, Opdam M, et al. Adjuvant capecitabine-containing chemotherapy benefit and homologous recombination deficiency in early-stage triple-negative breast cancer patients. Br J Cancer (2022) 126(10):1401–9. doi: 10.1038/s41416-022-01711-y
28. Sawada N, Ishikawa T, Fukase Y, Nishida M, Yoshikubo T, Ishitsuka H. Induction of thymidine phosphorylase activity and enhancement of capecitabine efficacy by taxol/taxotere in human cancer xenografts. Clin Cancer Res (1998) 4(4):1013–9.
29. Fujimoto-Ouchi K, Tanaka Y, Tominaga T. Schedule dependency of antitumor activity in combination therapy with capecitabine/5'-deoxy-5-fluorouridine and docetaxel in breast cancer models. Clin Cancer Res (2001) 7(4):1079–86.
Keywords: capecitabine, triple-negative breast cancer, chemotherapy, survival, adverse events
Citation: Bai J, Yao X, Pu Y, Wang X and Luo X (2023) Capecitabine-based chemotherapy in early-stage triple-negative breast cancer: a meta-analysis. Front. Oncol. 13:1245650. doi: 10.3389/fonc.2023.1245650
Received: 23 June 2023; Accepted: 03 October 2023;
Published: 25 October 2023.
Edited by:
Sharon R. Pine, University of Colorado Anschutz Medical Campus, United StatesReviewed by:
Karl Reinhard Aigner, MEDIAS Burghausen Clinic, GermanyMd. Mustafizur Rahman, Khulna University, Bangladesh
Copyright © 2023 Bai, Yao, Pu, Wang and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoyi Wang, d3h5dHNmQHNpbmEuY29t; Xinrong Luo, eGlucm9uZ2x1b0Bob3NwaXRhbC5jcW11LmVkdS5jbg==
 Jie Bai1
Jie Bai1 Xinrong Luo
Xinrong Luo