- 1Second Department of Pathology, National and Kapodistrian University of Athens School of Medicine, “Attikon” University Hospital, Haidari, Greece
- 2Third Department of Obstetrics and Gynecology, National and Kapodistrian University of Athens School of Medicine, “Attikon” University Hospital, Haidari, Greece
Perivascular epithelioid cell tumors are very rare mesenchymal neoplasms arising in various locations, such as the female genital tract, kidney, lung, prostate, bladder, pancreas, soft tissues, and bone. They possess a unique immunophenotype, co-expressing myogenic and melanocytic markers; molecular findings include mutations of tuberous sclerosis complex and translocations of transcription factor E3, a member of the microphthalmia transcription factor gene family. We herewith report a uterine collision tumor consisting of a perivascular epithelioid cell tumor and a moderately differentiated endometrial endometrioid carcinoma in a patient with genetically proven tuberous sclerosis; two leiomyomas were also found in contact with the tumor. Although two such cases one with a benign and another with a malignant perivascular epithelioid cell tumor have previously been reported, ours is, to our knowledge, the first reported in a tuberous sclerosis patient.
Introduction
Perivascular epithelioid cell tumors (PEComas) are very rare mesenchymal tumors, including some previously reported entities, such as renal and hepatic angiomyolipomas, lymphangioleiomyomatosis, pulmonary and extrapulmonary clear cell (“sugar”) tumors, abdominopelvic sarcoma of perivascular epithelioid cells, clear cell myomelanocytic tumor of the falciform ligament/ligamentum teres, and other similar tumors in different sites (1–4). Most PEComas are sporadic, arising more frequently in women than men, and exhibit a wide age range (5). A subset of cases, less than 10%, are linked to the tuberous sclerosis (TS) complex, also known as Bourneville-Pringle disease (6). PEComas arise in several locations, including the kidney, lung, prostate, bladder, pancreas, soft tissues, and bone (7–9). The most common location in the female genital tract is the uterine corpus, the uterine cervix, vagina, vulva, broad ligament, retroperitoneum, and ovary being less frequently involved (7). Histologically, PEComas are composed of cells with epithelioid and/or spindle cell morphology, clear to granular cytoplasm, and a centrally located, round to oval or elongated nucleus. Those cells stain with both myogenic and melanocytic immunohistochemical markers (8).
Leiomyomas are the most common benign mesenchymal neoplasms, whereas endometrioid endometrial carcinoma is the most common type of corpus uteri carcinoma, accounting for approximately 80% of all endometrial carcinomas (10, 11).
Collision tumors are two or more separate primary tumors in the same anatomic location without histological admixture or the existence of an intermediate cell population zone. They may be benign or malignant, primary or secondary in any combination. In some cases, they are the first manifestation of an occult disease (12, 13).
We herewith describe a unique case of uterine PEComa and endometrioid carcinoma with the coexistence of uterine leiomyomas in a patient with the underlying genetic background of TSC and review the literature.
Materials and methods
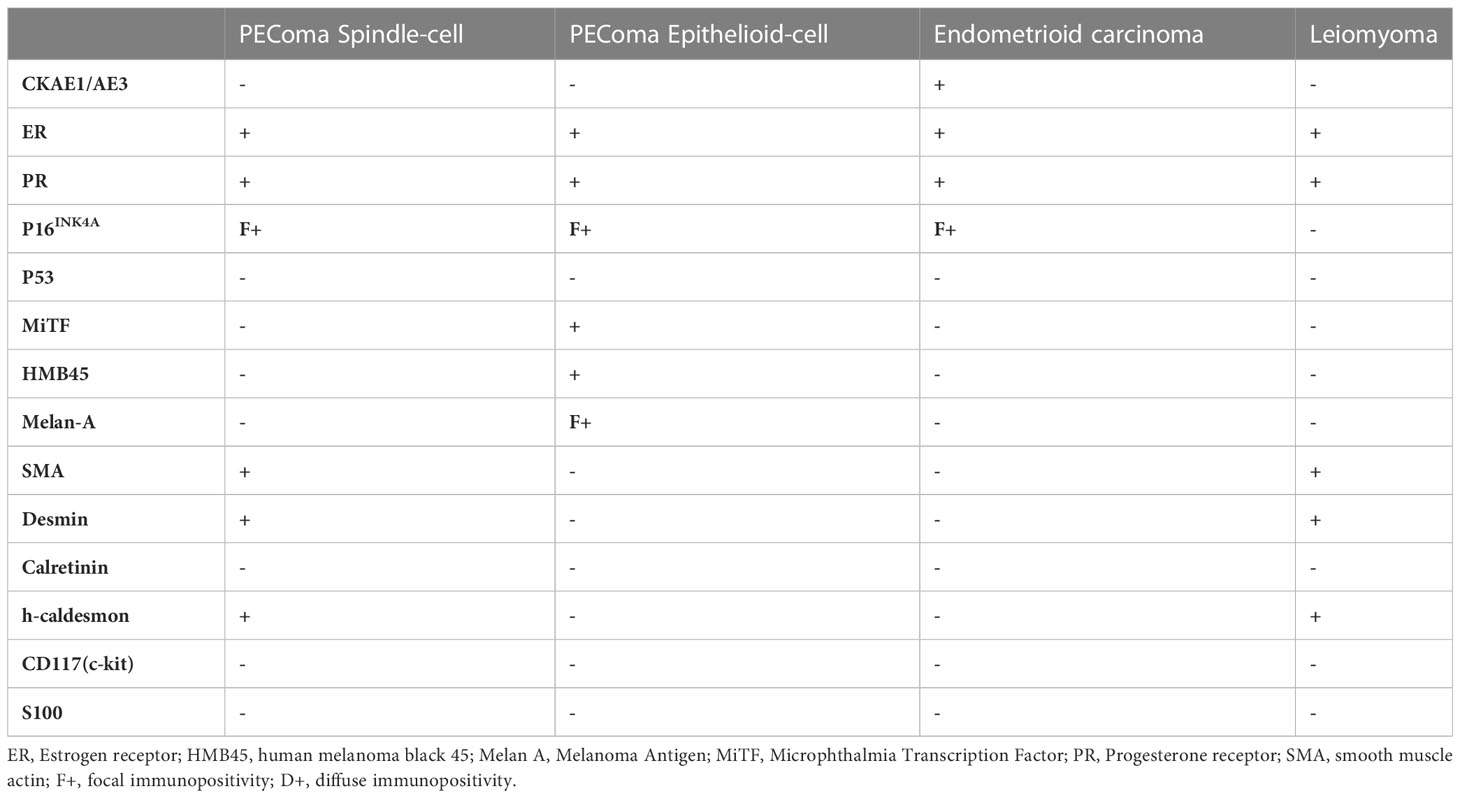
The surgical specimens underwent fixation in 10% buffered formalin. Formalin-fixed, paraffin-embedded blocks, were sectioned (4 μm thick) and the histological slides were stained with hematoxylin-eosin and immunohistochemical markers. The following antibodies were employed: anti-: CKAE1/AE3 (mouse monoclonal AE1/AE3 clone, Dako), ER (rabbit monoclonal EP1 clone, Dako), PR (mouse monoclonal PgR 636 clone, Dako), p16ink4a (mouse monoclonal JC2 clone, Zytomed), p53 (mouse monoclonal DO7 clone, Dako), MITF (mouse monoclonal C5/D5 clone, Cell Marque), HMB45 (mouse monoclonal HMB45 clone, Dako), Melan-A (mouse monoclonal A103 clone, Dako), SMA (mouse monoclonal 1A4 clone, Dako), desmin (mouse monoclonal D33 clone, Zytomed), calretinin (mouse monoclonal DAK-Calret 1 clone, Dako), h-caldesmon (mouse monoclonal h-CD clone, Dako), CD117 (rabbit polyclonal, Dako), S100 (rabbit polyclonal, Dako) MLH1 (mouse monoclonal ES05, DAKO), PMS2 (rabbit monoclonal EP51, DAKO), MSH2 (mouse monoclonal FE11, DAKO), and MSH6 (rabbit monoclonal EP49, DAKO) and β-catenin (rabbit monoclonal E247, Thermoscientific). Diaminobenzidine-chromogen substrate was used to visualize the staining and hematoxylin was used as counterstain. The patient has provided written informed consent. All procedures performed in the current study were approved by IRB (EΣ121/28-03-2023) in accordance with the 1964 Helsinki declaration, and its later amendments.
Case report
A 45-year-old patient with genetically proven TS (heterozygous for the R1743Q mutation in exon 40 of TSC2) exhibiting recurrent epileptic seizures along with a history of previous left nephrectomy due to angiomyolipoma was admitted due to vaginal bleeding. Abdominal ultrasound revealed multiple hypoechoic nodules on the right kidney consistent with angiomyolipoma. Abdominal computed tomography (CT) showed enlargement of the uterus and a 3.7 cm large tumor in contact with the left parametrium. A moderately differentiated endometrioid carcinoma was diagnosed in an endometrial curettage specimen, following which the patient underwent total abdominal hysterectomy with bilateral salpingo-oöphorectomy. A solid, gray-white, 6.5 cm large lesion within the uterine corpus was found on gross examination, with two smaller, 1 cm and 0.4 cm large nodules abutting to it. Microscopic examination of the lesion revealed a FIGO grade 2 endometrioid adenocarcinoma (Figures 1A, B) invading the left parametrium. The carcinomatous cells exhibited strong diffuse immunopositivity for CKAE1/AE3 (Figure 1C), Estrogen receptor (ER, Figure 1D), and Progesterone receptor (PR, Figure 1E). Immediately abutting the carcinoma was a mesenchymal neoplasm consisting mainly (around 90% of the tumor volume) of epithelioid (Figures 1A, F) and focally (an estimated 10% of the tumor volume) spindle (Figures 1A, I) cells arranged in sheets or nests, with a clear or eosinophilic cytoplasm, lacking significant cytological atypia or nuclear pleomorphism; thin, delicate vessels were found in between the tumor cells. The mitotic count was up to 1/50 High Power Field (HPF, x400 magnification). No necrosis or lymphovascular invasion was documented. The epithelioid cells were immunostained for melanocytic glycoprotein 100 [Human Melanoma Black-45 (HMB-45), Figure 1G] and Microphthalmia Transcription Factor (MiTF, Figure 1H), as well as focally for Melan-A, whereas Desmin decorated spindle cells (Figure 1J). SMA (Figure 1K), h-caldesmon, ER, and PR stains were positive in both epithelioid and spindle cells. A diagnosis of perivascular epithelioid cell tumor (PEComa) was therefore made. Both smaller nodules were consistent with leiomyomas (Figure 1L). Detailed immunohistochemical results are displayed in Table 1. A pathological stage pT3a was assigned for the endometrial carcinoma. The postoperative course was uneventful. The patient was discharged at the 6th postoperative day. The multidisciplinary tumor board opted for adjuvant radiation therapy, brachytherapy for the endometrioid carcinoma, and long-term follow-up for the PEComa. Patient underwent external beam radiotherapy with a total dose of 5000 cGy and vaginal brachytherapy as a boost after EBRT. The patient is alive 19 months postoperatively, with no sign of recurrence (normal range of CA-125, no signs on ultrasound examination and CT scan of recurrence). The timeline with relevant data from the episode of care is displayed in Figure 2.
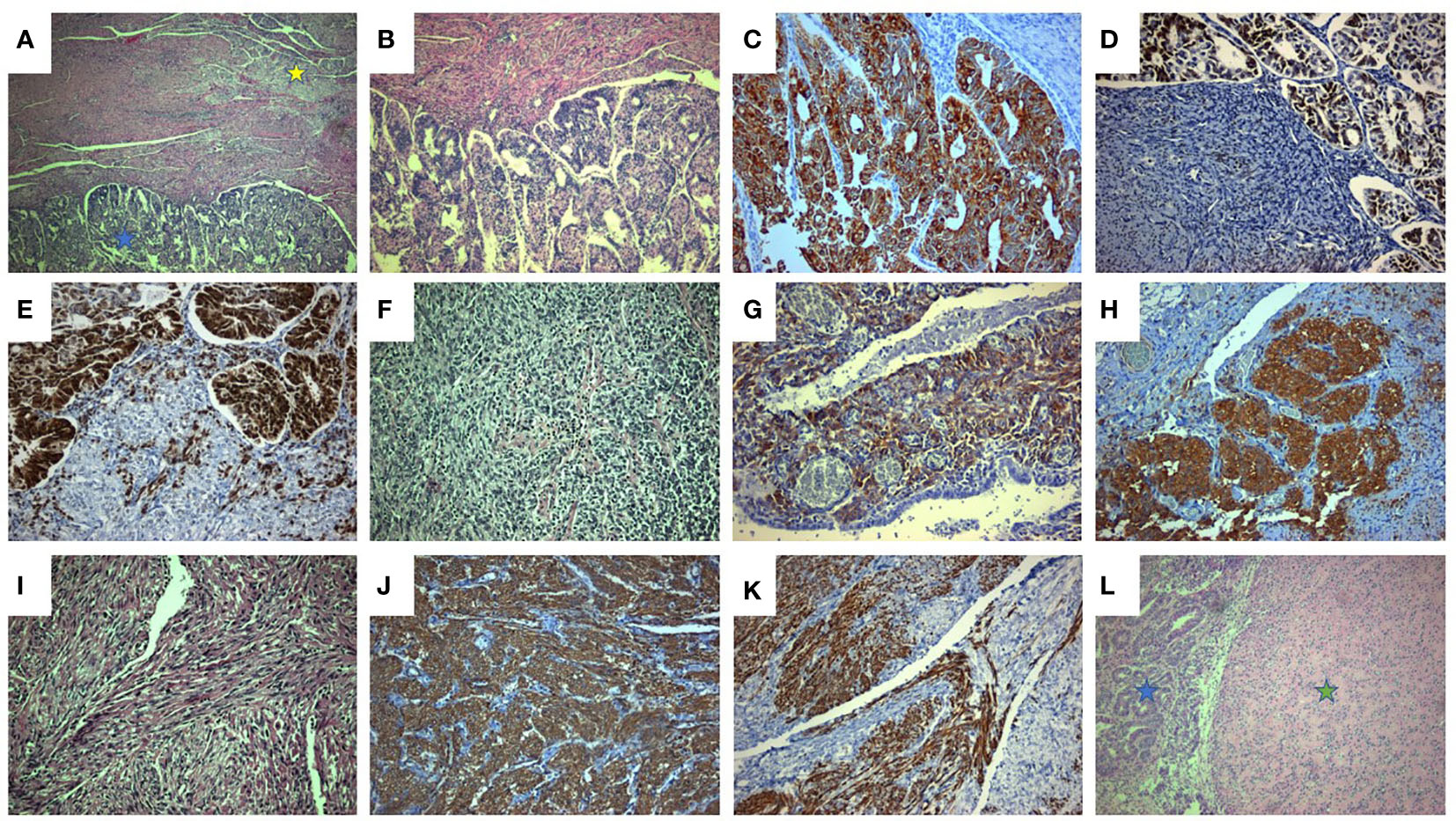
Figure 1 Coexistence of uterine endometrioid adenocarcinoma along with PEComa and leiomyoma. (A) Uterine endometrioid carcinoma (blue asterisk) associated with PEComa (yellow asterisk) (H&E x40). (B) The endometrioid carcinoma, displays mainly tubular and cribriform and in a lesser degree (estimated at 10%) solid architecture (solid architecture not shown). (H&E x 100). (C-E). Endometrioid carcinoma: diffuse and intense CKAE1/AE3 (C, x200), ER (D, x200), and PR (E, x200) immunostaining. (F-H). PEComa: epithelioid component (F, neoplastic cells with epithelioid morphology and clear cell cytoplasm, H&E x200) exhibiting strong HMB45 (G, x200) and MiTF (H, x200) immunostaining. (I-K). PEComa: spindle cell component (I, neoplastic cells with spindle cell morphology and clear cell cytoplasm, H&E, x200) exhibiting strong Desmin (J, x200) and SMA (K, x200) immunostaining. (L). Uterine leiomyoma (green asterisk) associated with endometrioid carcinoma (blue asterisk) (H&E, x100).
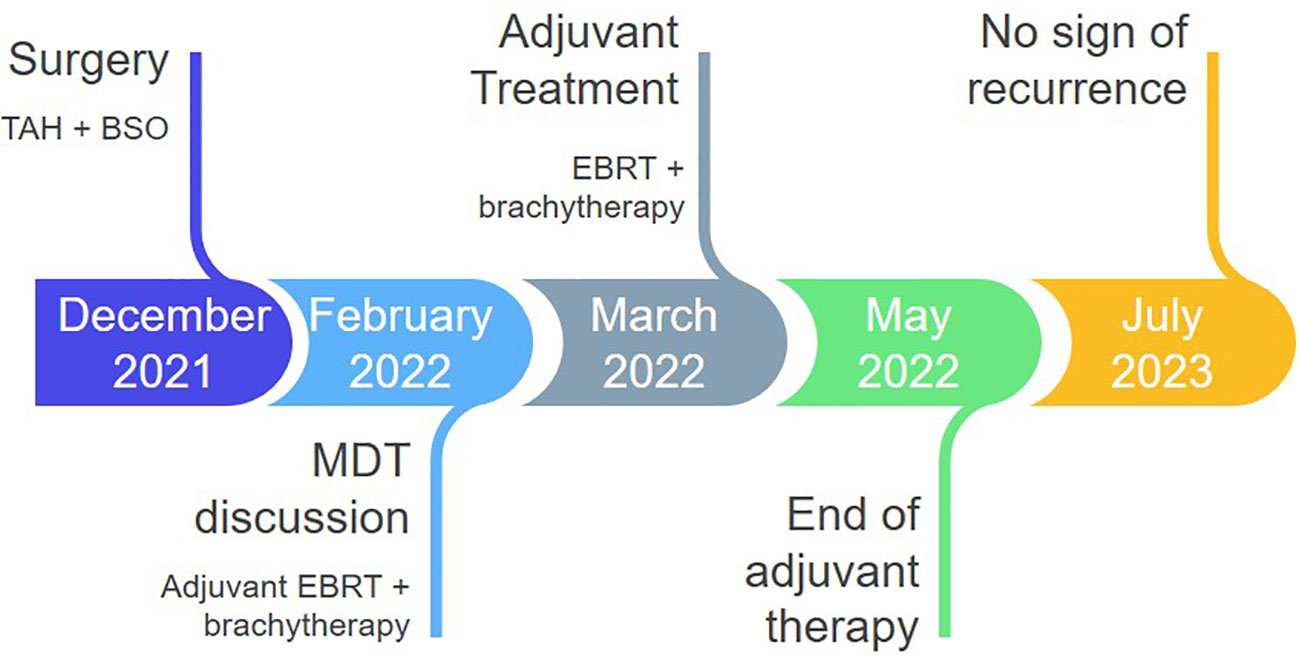
Figure 2 Timeline of postoperative management. TAH, total abdominal hysterectomy; BSO, bilateral salpingo-oophorectomy; MDT, multidisciplinary team board; EBRT, external beam radiotherapy.
Discussion
PEComas of the female genital tract present in patients in their fifth to sixth decades, usually with abnormal uterine bleeding and/or abdominal pain, although unusual presentations have been reported (7). These are extremely rare mesenchymal neoplasms, with approximately 100 cases reported, most frequently in the uterus (6, 7). PEComas are highly associated with tuberous sclerosis (TS), a rare autosomal dominant disease linked with mutations in either TSC1 or TSC2 genes, that map to chromosome loci 9q34 and 16p13.3 respectively (14, 15). TSC1 and TSC2 encode for hamartin and tuberin, respectively; both proteins form a complex which negatively regulates cell cycle progression through inhibition of mTOR (mammalian target of rapamycin) signaling (16). Inactivating mutations in either gene results in identical clinical manifestations. Our case was heterozygous for the R1743Q mutation in exon 40 of TSC2. Patients with TS harbor more frequent mutations in TSC2 than TSC1, with exons 37 and 38 having a higher mutation frequency, whereas exon 40 is less frequently mutated (17). Translocations of transcription factor E3 (TFE3), a member of the Microphthalmia Transcription Factor (MiTF) gene family located in the short arm of chromosome X, have also been detected in PEComas (18, 19). Besides, missense and silent TP53 mutations have been found in epithelioid PEComas, which may be associated with a more aggressive behavior (20); moreover, RAD51B fusions were detected in tumors displaying brisk mitotic activity and aggressive clinical behavior (6, 21).
Patients with TS exhibit tumors affecting multiple organs, including periungual fibromas, cardiac rhabdomyomas, giant-cell astrocytomas, chordomas, and PEComas. The most common renal manifestation is PEComa, including angiomyolipoma. Additionally, many patients exhibit neural manifestations like seizures and cognitive disorders. Non-neoplastic manifestations are also seen, such as cutaneous shagreen patches (22).
Histologically, PEComas may display pushing or invasive borders. Tumor cells can be epithelioid and/or spindled, with clear to eosinophilic granular cytoplasm (6, 23). There is variable nuclear atypia and mitotic count; tumor necrosis or lymphovascular invasion may be observed. Tumor vasculature may exhibit various forms, such as thin and delicate vessels, thin and ectatic vessels, thick-walled vessels, or hemangiopericytomatous-like vessels (7, 23). Uterine PEComas may rarely show morphological features akin to lymphangioleiomyomatosis (6, 24).
Immunohistochemically, PEComas express myomelanocytic markers with variable extent and intensity of staining. HMB-45, Melan-A and MiTF are variably expressed, often displaying only minimal staining (7). Smooth muscle markers tend to stain diffusely and strongly in spindle cells, whereas melanocytic markers are likewise expressed in epithelioid cells (25). Cathepsin K is often expressed strongly and diffusely, while PNL2, a novel melanocytic marker, displays variable cytoplasmic immunostaining among PEComas (26, 27).
Preoperative diagnosis of these tumors is problematic because a wide range of tumors, including leiomyoma, low-grade endometrial stromal sarcoma, uterine tumor resembling ovarian sex cord tumors (UTROSCT), other metastatic sarcomas such as a gastrointestinal stromal tumor, as well as melanoma, are included in the differential diagnosis. Besides, since this entity is very rare, its diagnosis is challenging. Leiomyomas may cause the most challenging differential diagnostic problems because they show morphologic features similar to PEComas. The presence of perinuclear vacuoles, diffuse eosinophilic cytoplasm without granularity, thick-walled vessels, and lack of thin and delicate vasculature characteristic of PEComas may provide helpful diagnostic clues. Immunohistochemically, leiomyomas may focally decorate for both melanocytic markers and Cathepsin K (7). Low-grade endometrial stromal sarcoma morphology is similar to proliferative phase endometrium. Gastrointestinal stromal tumors may also resemble PEComas, in which the expression of CD34, CD117, and DOG-1 is diagnostically useful. UTROSCTs display a morphological variability, showing small nests, broad trabeculae, anastomosing cords, glomeruloid or cystic structures, retiform or sertoliform tubules, and prominent Leydig-like cells. Immunohistochemically they may express markers of sex cord (calretinin, inhibin, and CD99), smooth muscle differentiation (SMA, desmin, and caldesmon) and melanocytic (Melan-A) differentiaton. In addition, they may sometimes express epithelial markers or CD10 (28). Melanoma may show different growth patterns, with highly pleomorphic cells displaying macronucleoli; nevertheless, melanoma cells do not express myogenic markers (7).
Three different diagnostic algorithms have been proposed to assess the biological potential of PEComas. Based on modified gynecology-specific criteria (6), soft tissue and gynecologic PEComas are classified into three categories, namely benign, of uncertain malignant potential, and malignant, based on size, type of tumor front (pushing or infiltrative), nuclear grade, mitotic count, necrosis, and vascular invasion (18). The other two approaches classify PEComas of the female genital tract into uncertain malignant potential and malignant classes based on the assessment of gross size, nuclear features, necrosis, vascular invasion, and mitotic rate with the algorithm proposed by Schoolmeester et al. (23) requiring four criteria to classify as malignant. In contrast, the algorithm proposed by Bennett et al. (6) requires three or more criteria to qualify for malignancy. Our case is stratified as uncertain malignant potential according to modified gynecology-specific criteria.
Molecular studies have recognized four distinct subtypes of endometrial carcinomas: copy number-high, copy number-low, microsatellite instability hypermutated and polymerase ϵ (POLE) ultramutated (29, 30). Also nuclear expression of β-catenin is usually related with CTNNB1 mutations that are associated with an adverse prognosis in patients with low grade, low risk endometrial carcinoma (31). This classification assists in patient risk stratification and management. Immunohistochemical staining on formalin-fixed paraffin embedded tissue blocks for mismatch repair system proteins (MMR) p53 and β-catenin can be used as surrogate markers and aid in molecular classification (32, 33). In our case nuclear expression of all four MMR (MLH1+/MSH2+/PMS2+/MSH6+) on immunohistochemical evaluation of both tumor components (carcinoma and PEComa) was retained. Besides, the carcinoma expressed cytoplasmic β-catenin as well as focal/faint p53 which is considered negative.
We found two previously reported similar cases with the coexistence of uterine PEComa, endometrioid carcinoma, and uterine leiomyoma (34, 35) (Table 2). Case 1 (Choi et al.) concerned a malignant PEComa, whereas in case 2 (Gao et al.), the PEComa had benign features. To our knowledge, ours is the first case reported in a patient with genetically proven tuberous sclerosis.
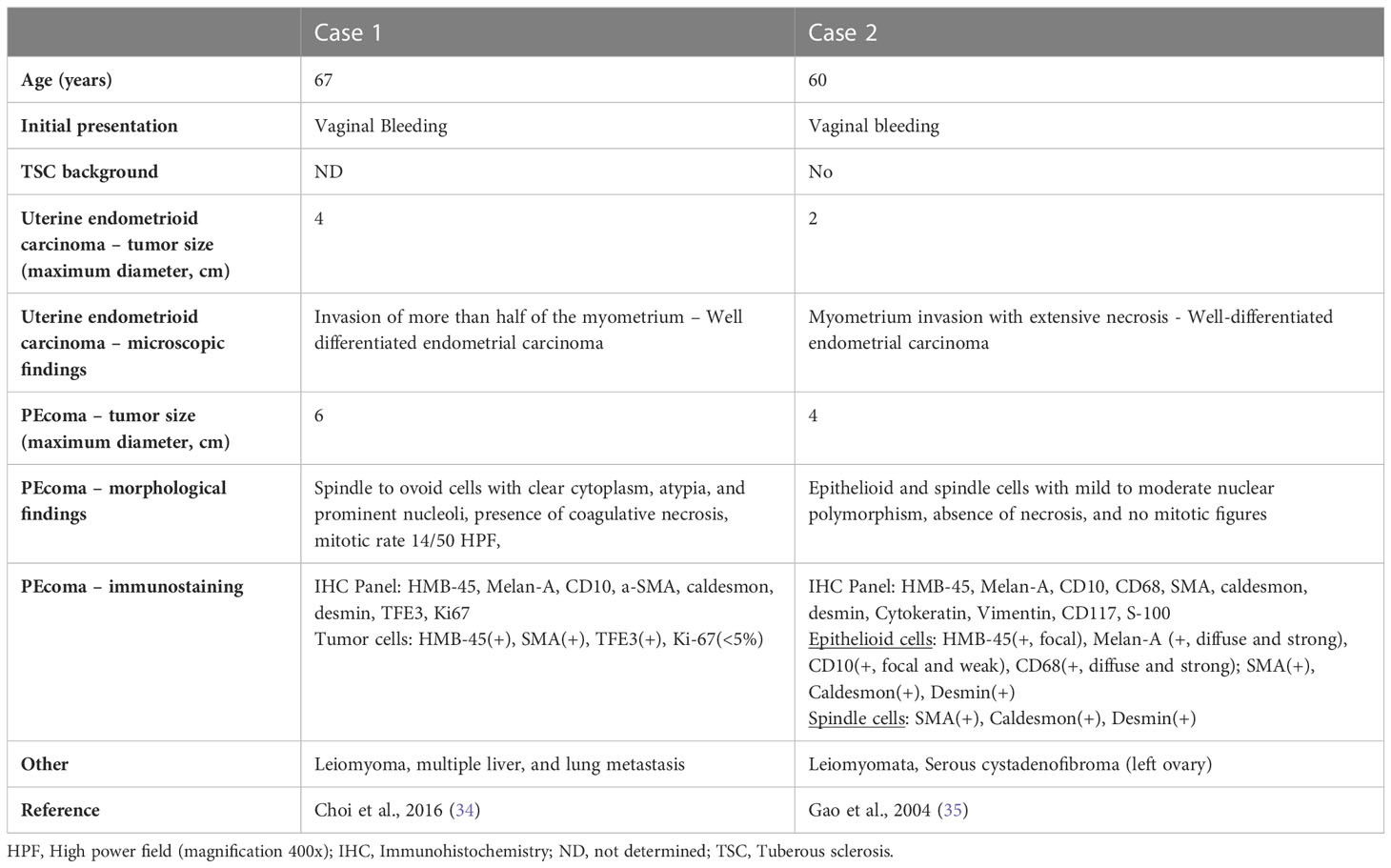
Table 2 Clinical and histopathological characteristics of two cases with synchronous uterine endometrial carcinoma and PEcoma along with leiomyoma.
Currently, surgery constitutes the treatment of choice for PEComas, with hysterectomy (with or without bilateral salpingo-oöphorectomy); a long-term follow-up is mandatory due to the possibility of local recurrence or metastasis, usually to the lung, even several years post-operation (9, 19, 21, 36–38). Cases with oligometastatic disease are handled with surgery when feasible. Systemic chemotherapy has so far shown poor results (39), in contrast to targeted therapies, especially with mTOR and VEGFR inhibitors, which have been promising (38–40). The role of adjuvant radiotherapy has not been determined (39). Given the small number of cases, the optimal therapeutic approach for uterine PEComas is debated.
Conclusions
In summary, we hereby report a collision tumor composed of PEComa, endometrioid endometrial carcinoma, and two leiomyomas in a patient with genetically proven tuberous sclerosis, the first case reported, to our best knowledge. Furthermore, we review the relevant literature focusing on the differential diagnostic issues and the diagnostic criteria of malignancy and discuss the molecular findings and treatment options for PEComas.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were approved by the Attikon Hospital Ethics Committee (EΣ121/28-03-2023) and conducted in accordance with the 1964 Helsinki Declaration, and its later amendments, and the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
Conceptualization: NK; ISP; Data curation: CK; A-RG; Formal analysis: A-II; AF; Funding acquisition: NV; Investigation: A-RG.; Methodology: A-RG; A-II; Project administration: IGP; NV Resources: CK; A-II.; Software: CK; A-RG; Supervision: IGP; NV; Validation: AF; CK; Visualization: AF; A-RG; Writing - original draft: NK; ISP; Writing - review & editing: NK; ISP; CK; A-RG; A-II; AF; IGP; NV.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Thway K, Fisher C. PEComa: morphology and genetics of a complex tumor family. Ann Diagn pathol (2015) 19(5):359–68. doi: 10.1016/j.anndiagpath.2015.06.003
2. Hornick J, Fletcher C. PEComa: what do we know so far? Histopathology (2006) 48(1):75–82. doi: 10.1111/j.1365-2559.2005.02316.x
3. Tazelaar HD, Batts KP, Srigley JR. Primary extrapulmonary sugar tumor (PEST): a report of four cases. Modern Pathol (2001) 14(6):615–22. doi: 10.1038/modpathol.3880360
4. Folpe AL, Goodman ZD, Ishak KG, Paulino AF, Taboada EM, Meehan SA, et al. Clear cell myomelanocytic tumor of the falciform ligament/ligamentum teres: a novel member of the perivascular epithelioid clear cell family of tumors with a predilection for children and young adults. Am J Surg pathol (2000) 24(9):1239–46. doi: 10.1097/00000478-200009000-00007
5. Vang R, Kempson RL. Perivascular epithelioid cell tumor (PEComa') of the uterus: a subset of HMB-45-positive epithelioid mesenchymal neoplasms with an uncertain relationship to pure smooth muscle tumors. Am J Surg pathol (2002) 26(1):1–13. doi: 10.1097/00000478-200201000-00001
6. Bennett JA, Braga AC, Pinto A, Van de Vijver K, Cornejo K, Pesci A, et al. Uterine PEComas: a morphological, immunohistochemical, and molecular analysis of 32 tumors. Am J Surg pathol (2018) 42(10):1370. doi: 10.1097/PAS.0000000000001119
7. Bennett JA, Oliva E. Perivascular epithelioid cell tumors (PEComa) of the gynecologic tract. Genes Chromosomes cancer. (2021) 60(3):168–79. doi: 10.1002/gcc.22908
8. Martignoni G, Pea M, Reghellin D, Zamboni G, Bonetti F. Perivascular epithelioid cell tumor (PEComa) in the genitourinary tract. Adv anatomic pathol (2007) 14(1):36–41. doi: 10.1097/PAP.0b013e31802e0dc4
9. Natella V, Merolla F, Giampaolino P, Bifulco G, Mainenti PP, Insabato L. A huge Malignant perivascular epithelioid cell tumor (PEComa) of the uterine cervix and vagina. Pathol Res practice. (2014) 210(3):186–8. doi: 10.1016/j.prp.2013.10.003
10. Rabban JT, Gilks CB, Malpica A, Matias-Guiu X, Mittal K, Mutter GL, et al. Issues in the differential diagnosis of uterine low-grade endometrioid carcinoma, including mixed endometrial carcinomas: recommendations from the International Society of Gynecological Pathologists. Int J Gynecological Pathol (2019) 38(1 Suppl 1):S25–39. doi: 10.1097/PGP.0000000000000512
11. Oliva E. Practical issues in uterine pathology from banal to bewildering: the remarkable spectrum of smooth muscle neoplasia. Modern Pathol (2016) 29(Suppl 1):S104–20. doi: 10.1038/modpathol.2015.139
12. Koufopoulos N, Zacharatou A, Gouloumis AR, Papadimitriou N, Tomos P, Foukas PG, et al. Metastatic thyroid osteosarcoma with concomitant multifocal papillary carcinoma presenting as a collision tumor. Cureus (2021) 13(6):e15425. doi: 10.7759/cureus.15425
13. Koufopoulos N, Goudeli C, Pigadioti E, Balalis D, Manatakis DK, Antoniadou F, et al. Synchronous colonic adenocarcinoma and metastatic lobular carcinoma in a colectomy specimen: A rare finding. Cureus (2018) 10(8):e3207. doi: 10.7759/cureus.3207
14. Consortium ECTS. Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell (1993) 75(7):1305–15. doi: 10.1016/0092-8674(93)90618-z
15. Slegtenhorst MV, Hoogt RD, Hermans C, Nellist M, Janssen B, Verhoef S, et al. Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science (1997) 277(5327):805–8. doi: 10.1126/science.277.5327.805
16. Huang J, Manning BD. The TSC1–TSC2 complex: a molecular switchboard controlling cell growth. Biochem J (2008) 412(2):179–90. doi: 10.1042/BJ20080281
17. Rosset C, Netto CBO, Ashton-Prolla P. TSC1 and TSC2 gene mutations and their implications for treatment in Tuberous Sclerosis Complex: a review. Genet Mol Biol (2017) 40:69–79. doi: 10.1590/1678-4685-gmb-2015-0321
18. Folpe AL, Mentzel T, Lehr HA, Fisher C, Balzer BL, Weiss SW. Perivascular epithelioid cell neoplasms of soft tissue and gynecologic origin: a clinicopathologic study of 26 cases and review of the literature. Am J Surg pathol (2005) 29(12):1558–75. doi: 10.1097/01.pas.0000173232.22117.37
19. Acosta AM, Adley BP. Predicting the behavior of perivascular epithelioid cell tumors of the uterine corpus. Arch Pathol Lab Med (2017) 141(3):463–9. doi: 10.5858/arpa.2016-0092-RS
20. Bing Z, Yao Y, Pasha T, Tomaszewski JE, Zhang PJ. p53 in pure epithelioid PEComa: an immunohistochemistry study and gene mutation analysis. Int J Surg pathol (2012) 20(2):113–20. doi: 10.1177/1066896912441829
21. Gennatas C, Michalaki V, Kairi PV, Kondi-Paphiti A, Voros D. Successful treatment with the mTOR inhibitor everolimus in a patient with perivascular epithelioid cell tumor. World J Surg Oncol (2012) 10:181. doi: 10.1186/1477-7819-10-181
22. Cardis MA, DeKlotz CMC. Cutaneous manifestations of tuberous sclerosis complex and the paediatrician's role. Arch Dis Childhood. (2017) 102(9):858–63. doi: 10.1136/archdischild-2016-312001
23. Schoolmeester JK, Howitt BE, Hirsch MS, Dal Cin P, Quade BJ, Nucci MR. Perivascular epithelioid cell neoplasm (PEComa) of the gynecologic tract: clinicopathologic and immunohistochemical characterization of 16 cases. Am J Surg pathol (2014) 38(2):176–88. doi: 10.1097/PAS.0000000000000133
24. Torres VE, Bjornsson J, King BF, Kumar R, Zincke H, Edell ES, et al. Extrapulmonary lymphangioleiomyomatosis and lymphangiomatous cysts in tuberous sclerosis complex. Mayo Clinic Proc (1995) 70(7):641–8. doi: 10.4065/70.7.641
25. Folpe AL, Kwiatkowski DJ. Perivascular epithelioid cell neoplasms: pathology and pathogenesis. Hum pathol (2010) 41(1):1–15. doi: 10.1016/j.humpath.2009.05.011
26. Valencia-Guerrero A, Pinto A, Anderson WJ, Trevisan G, Nucci MR, Hirsch MS. PNL2: A useful adjunct biomarker to HMB45 in the diagnosis of uterine perivascular epithelioid cell tumor (PEComa). Int J gynecological Pathol (2020) 39(6):529–36. doi: 10.1097/PGP.0000000000000653
27. Gulavita P, Fletcher CDM, Hirsch MS. PNL2: an adjunctive biomarker for renal angiomyolipomas and perivascular epithelioid cell tumours. Histopathology (2018) 72(3):441–8. doi: 10.1111/his.13369
28. Koufopoulos N, Boutas I, Dimas D, Kontogeorgi A, Dimitrakakis C. Plexiform tumorlet in a wOman with postmenopausal endometrioid endometrial carcinoma taking tamoxifen for breast cancer: A case report. Case Rep Women's Health (2022) 36:e00459. doi: 10.1016/j.crwh.2022.e00459
29. Levine DA. Mike 1 CGARNGscBIGGGSBCKLESASCL, Heather WUiSLKCDDFRFLK-VJMMDOLMS, California UoS, 22 Laird Peter W. 22 Shen Hui 22 JHBSBBMSLPHTJTJVDBDJWDJ, 23 IfSBRSMSI. Integrated genomic characterization of endometrial carcinoma. Nature (2013) 497(7447):67–73. doi: 10.1038/nature12113
30. McConechy MK, Ding J, Cheang MC, Wiegand KC, Senz J, Tone AA, et al. Use of mutation profiles to refine the classification of endometrial carcinomas. J pathol (2012) 228(1):20–30. doi: 10.1002/path.4056
31. Kurnit KC, Kim GN, Fellman BM, Urbauer DL, Mills GB, Zhang W, et al. CTNNB1 (beta-catenin) mutation identifies low grade, early stage endometrial cancer patients at increased risk of recurrence. Modern Pathol (2017) 30(7):1032–41. doi: 10.1038/modpathol.2017.15
32. Talhouk A, McConechy MK, Leung S, Li-Chang HH, Kwon J, Melnyk N, et al. A clinically applicable molecular-based classification for endometrial cancers. Br J cancer. (2015) 113(2):299–310. doi: 10.1038/bjc.2015.190
33. Kim G, Kurnit KC, Djordjevic B, Singh C, Munsell MF, Wang W-L, et al. Nuclear β-catenin localization and mutation of the CTNNB1 gene: a context-dependent association. Modern Pathol (2018) 31(10):1553–9. doi: 10.1038/s41379-018-0080-0
34. Choi YJ, Hong JH, Kim A, Kim H, Chang H. A case of Malignant PEComa of the uterus associated with intramural leiomyoma and endometrial carcinoma. J Pathol Trans Med (2016) 50(6):469–73. doi: 10.4132/jptm.2016.04.20
35. Gao Z, Bhuiya T, Anderson A. Perivascular epithelioid cell tumour (PEComa) of the uterus associated with Malignant neoplasm of the female genital tract. J obstetrics gynaecol (2004) 24(5):600–4. doi: 10.1080/01443610410001722905
36. Cossu A, Paliogiannis P, Tanda F, Dessole S, Palmieri G, Capobianco G. Uterine perivascular epithelioid cell neoplasms (PEComas): report of two cases and literature review. Eur J gynaecological Oncol (2014) 35(3):309–12.
37. Van Nguyen JM, Ghandehari H, Parra-Herran C, Vicus D. Uterine rupture: an unusual presentation of a uterine perivascular epithelioid cell tumor (PEComa). Int J Gynecological Cancer. (2020) 30(12):2008–11. doi: 10.1136/ijgc-2020-001837
38. Pecorino B, Scibilia G, Galia A, Scollo P. A very rare case of uterine PEComa HMB45 negative: primitive or relapse? Ital J gynaecol obstetrics (2015) 27(3):111. doi: 10.14660/2385-0868-024
39. Musella A, De Felice F, Kyriacou AK, Barletta F, Di Matteo FM, Marchetti C, et al. Perivascular epithelioid cell neoplasm (PEComa) of the uterus: A systematic review. Int J surgery. (2015) 19:1–5. doi: 10.1016/j.ijsu.2015.05.002
Keywords: uterine PEComa, endometrioid carcinoma, collision tumor, tuberous sclerosis, case report
Citation: Koufopoulos N, Pateras IS, Koratzanis C, Gouloumis A-R, Ieronimaki A-I, Fotiou A, Panayiotides IG and Vrachnis N (2023) Uterine collision tumor (PEComa and endometrioid carcinoma) in a tuberous sclerosis patient: a case report. Front. Oncol. 13:1244261. doi: 10.3389/fonc.2023.1244261
Received: 22 June 2023; Accepted: 21 July 2023;
Published: 09 August 2023.
Edited by:
Paolo Scollo, Kore University of Enna, ItalyReviewed by:
Liliana Mereu, University of Catania, ItalyMartina Ferrara, Cannizzaro Hospital, Italy
Giuseppe Angelico, Scientific Institute for Research, Hospitalization and Healthcare (IRCCS), Italy
Copyright © 2023 Koufopoulos, Pateras, Koratzanis, Gouloumis, Ieronimaki, Fotiou, Panayiotides and Vrachnis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nektarios Koufopoulos, a291Zm9uZWt0YXJAeWFob28uY29t
†These authors have contributed equally to this work
 Nektarios Koufopoulos
Nektarios Koufopoulos Ioannis S. Pateras
Ioannis S. Pateras Christos Koratzanis2
Christos Koratzanis2 Alina-Roxani Gouloumis
Alina-Roxani Gouloumis Alexandros Fotiou
Alexandros Fotiou