- 1Early Detection, Prevention and Infections Branch, International Agency For Research On Cancer (IARC), Lyon, France
- 2Department of Cancer Epidemiology, National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences, Beijing, China
We review developments in molecular triaging options for women who test positive for high-risk human papillomavirus (hrHPV) on self-collected samples in the context of cervical cancer elimination. The World Health Organization (WHO) recommends hrHPV screening as the primary test for cervical screening due to its high sensitivity compared to other screening tests. However, when hrHPV testing is used alone for treatment decisions, a proportion of women of childbearing age receive unnecessary treatments. This provides the incentive to optimize screening regimes to minimize the risk of overtreatment in women of reproductive age. Molecular biomarkers can potentially enhance the accuracy and efficiency of screening and triage. HrHPV testing is currently the only screening test that allows triage with molecular methods using the same sample. Additionally, offering self-collected hrHPV tests to women has been reported to increase screening coverage. This creates an opportunity to focus health resources on linking screen-positive women to diagnosis and treatment. Adding an additional test to the screening algorithm (a triage test) may improve the test’s positive predictive value (PPV) and offer a better balance of benefits and risks for women. Conventional triage methods like cytology and visual inspection with acetic acid (VIA) cannot be performed on self-collected samples and require additional clinic visits and subjective interpretations. Molecular triaging using methods like partial and extended genotyping, methylation tests, detection of E6/E7 proteins, and hrHPV viral load in the same sample as the hrHPV test may improve the prediction of cervical intraepithelial neoplasia grade 2 or worse (CIN2+) and invasive cancer, offering more precise, efficient, and cost-effective screening regimes. More research is needed to determine if self-collected samples are effective and cost-efficient for diverse populations and in comparison to other triage methods. The implementation of molecular triaging could improve screening accuracy and reduce the need for multiple clinical visits. These important factors play a crucial role in achieving the global goal of eliminating cervical cancer as a public health problem.
1 Introduction
The World Health Organization (WHO) recommends cervical screening using the high-risk human papillomavirus (hrHPV) test in the general population as well as among women living with HIV (WLWH) (1). With a sensitivity and specificity of 94% (95% CI 89%–97%) and 88% (95% CI 84%–92%), respectively, the test accuracy of hrHPV is better than other screening tests, such as cytology or visual inspection with acetic acid (VIA), for detecting cervical intraepithelial neoplasia grade two or worse (CIN2+) (2, 3). Used alone for screening and treatment decisions, hrHPV testing reduces more cervical cancer-related deaths than these other screening tests (4). Additionally, self-collected and clinician-obtained sampling achieve a similar accuracy when using clinically validated polymerase chain reaction (PCR) for hrHPV DNA detection (3). Offering self-collected hrHPV tests to women has been reported to increase screening coverage (3, 5–8), and allows available health system resources to focus on the effective diagnosis and treatment of the estimated 10% of the general population who may be hrHPV positive (3, 4). Self-sampling strategies were particularly valuable in the COVID-19 context (9).
Ideally, a screening algorithm aims to balance sensitivity and specificity since low specificity may lead to a high proportion of false positives and women being unnecessarily treated (4, 10, 11). Minimizing harm from cervical screening is an important consideration, particularly in young women, due to the potential for adverse reproductive outcomes following precancer treatment (12). As new testing modalities emerge, refined treatment thresholds may be considered. For example, new techniques of histological classification use p16 staining to distinguish CIN2 which is high- and low-grade, reducing unnecessary treatment (13). In settings with sound infrastructure and high adherence to follow-up, treatment thresholds of CIN3 are being investigated among women of childbearing age (14–17). Moreover, the availability of molecular tests enables the detection of a woman’s cancer risk at an earlier phase compared to previous methods. This advancement may allow an extended monitoring period; however, surveillance required for this management is not widely available, especially in low-resource settings.
Screening algorithm fundamentally differ in high and low- and middle-income countries (HIC and LMIC). Generally in HICs, women are only treated when CIN2+ is histologically confirmed and close surveillance is possible for the remaining high-risk women. In LMIC it is more common for treatment to be based on the estimated risk of CIN2+ following one or more screening tests. The tests used in these different screening algorithm fulfil different purposes. Furthermore, in many LMICs, screening and treatment occur on the same day to avoid the transportation barriers and programmatic limitations of arranging the recall of patients. Molecular markers may improve the estimation of cervical cancer risk, which is especially useful for implementing same-day treatment. Molecular biomarkers are also attractive for screening and triage because the automated testing process relies less on training and subjective interpretation (18). Additionally, molecular testing can be performed on clinician-collected and self-collected specimens used for hrHPV testing, removing the need for additional clinic visits. In this review, we describe the advances in molecular triaging options for women testing positive on self-collected samples, highlighting current research gaps and potential future developments in this field.
2 HPV testing on self-collected samples in the context of cervical cancer elimination
Cervical cancer is almost completely preventable by vaccination and screening, but worldwide, over 300,000 women die yearly, and 90% live in LMICs (19). These deaths are largely attributable to the inequalities that exist in implementing primary and secondary prevention measures across and within countries (20). The WHO resolution to eliminate cervical cancer aims to reduce global inequalities relating to cervical cancer incidence and mortality (21). This is supported by modelling studies which show that all countries can achieve elimination by the end of the century (22). Elimination is dependent on achieving the following targets; 90% of girls fully vaccinated with the HPV vaccine by the age of 15, 70% of women need screening with a high-performance test (i.e. hrHPV testing) at least twice in their life by the ages of 35 and 45 years, and treating 90% of women who have cervical precancerous lesions or cervical cancer (23). Only 30% of LMICs have implemented national HPV vaccination programs, while they already exist in 80% of HIC (24, 25). Nevertheless, cervical screening will continue to be the most important method of prevention for many decades, because the effects of hrHPV vaccination on cervical cancer incidence and mortality are not immediate (22). Currently, most countries use cytology or VIA as the primary screening test, which are subjective tests (20). The accuracy of these tests can vary significantly depending on the quality of facilities, practitioner training, and quality assurance measures in place (2, 4, 26, 27).
Following the WHO recommendation and the recent European Council recommendations to introduce hrHPV testing for cervical screening, many HICs are transitioning to hrHPV testing and offering self-collection, especially for women who dont participate in screening (28). Given that the overall agreement between DNA hrHPV testing in self-collected versus clinician-collected samples is good (kappa 0.72, 95%CI 0.7–0.8) (8) and samples for self-collected hrHPV testing is well accepted by women in a range of different contexts (3, 8, 29), it is logical to offer this option in screening regimes. A recent modelling study found that self-collection could improve program effectiveness and increase uptake in the general population with only a small compromise in accuracy (7). The COVID-19 pandemic put an unprecedented strain on health services, increasing screening coverage inequalities (9, 30). Self-sampling for hrHPV may offer more efficient and inclusive screening with greater coverage to mitigate the covid-induced delays, reducing the load on the healthcare services (8, 31, 32). The uptake of self-sampling has been especially effective when self-sampling kits are sent directly to women’s homes or offered door-to-door by a health worker (32).
Including a triage test for hrHPV-positive women may improve accuracy of screening and reduce the number of false positives. This is particularly crucial for WLHV due to the high likelihood of hrHPV positivity, and in cases where treatment is based on estimated risk rather than histologically proven CIN2+. In countries where available, cytology triage is currently used after primary hrHPV testing, and studies have shown that this approach can reduce unnecessary treatment and potential harm to women of childbearing age. However, self-collected vaginal swabs are not suitable for evaluating cervical cytology, which limits the benefits of self-collection. WHO recommends triaging hrHPV-positive women with VIA in settings where ensuring quality-assured cytology would be difficult; however, VIA also requires stringent quality assurance to be effective (1, 33). Besides being significantly less sensitive and having all the limitations of a subjective test, VIA triaging also requires an additional clinic visit. Molecular triage may offer a more precise alternative to cytology or VIA triage (7).
3 HPV carcinogenesis as the basis for selecting molecular triaging tests
The integration of hrHPV DNA into the host genome is a necessary cause of cervical cancer, leading to the development of malignant cells and immune evasion (34). The process of HPV carcinogenesis begins when hrHPV enters the basal cell layer of the cervical epithelium through microscopic abrasions. Detecting higher levels of hrHPV DNA may suggest a greater risk of carcinogenesis and researchers are now exploring the value quantifying the viral DNA present in cervical samples (35–37). After entering the host cells, the viral DNA integrates into the host DNA, with seven genes expressed in early stages of gene expression, and two genes expressed in later stages. The activation of early genes, E6 and E7, produces vital proteins that result in cellular transformations which may lead to cancer. The E6 gene inhibits p53, enabling the virus to evade apoptosis and accumulate genetic mutations (38, 39). The E7 gene inhibits pRb, causing deregulation of cellular proliferation (38, 39). Although hundreds of HPV genotypes are known, persistent infection with 12 of these are associated with cervical cancer (40–42). Genotypes 16 and 18 have been detected in around 70% of cervical cancer cases, while the risk associated with other hrHPV genotypes is notably lower. This discrepancy in risk is the foundation for using partial and extended genotyping as a molecular triage test. DNA methylation is another molecular option for triage testing. In humans, this test most commonly identifies the addition of a methyl group to the fifth position of the cytosine preceeding guanines (CpG) to form 5-methylcytosine (43–45). DNA methylation of HPV viral genes can also occur following hrHPV infection and cervical neoplasia (43–46). Methylation may change the expression or function of genes but it does not change the genetic code.44 Over 100 genes that serve as methylation biomarkers in humans have been tested. In histological samples of CIN2+ and cervical cancer, consistent hypermethylation has been observed in genes such as CADM1, MAL, MIR-124-2, FAM19A4, POU4F3, EPB41L3, PAX1, and SOX1 (18, 46, 47). DNA hypermethylation in the late genes of the hrHPV virus is believed to play a major role in persistent infections. This is because it prevents E2 binding and results in increased E6 and E7 expression (18, 39). Consequently, these changes allow the virus to evade host defence mechanisms and allow the identification of persistent hrHPV infections with the potential to progress to cancer (18, 43, 48). Quantitative methylation-specific PCR and pyrosequencing are the most common methods used for DNA methylation testing. They require a minimal amount of DNA and are highly reproducible (47).
4 Evidence supporting available methods of molecular triage
Most hrHPV infections are transient; only 10% of acute hrHPV infections progress to CIN2+ or cervical cancer (49). In some settings, the PPV of hrHPV detection tests can be as low as 5-10% to detect CIN3+ lesions (50). The low PPV of hrHPV testing is also associated with a higher risk of overtreatment and low PPV of hrHPV testing are also associated with a higher risk of over-treatment (3, 26). This is even more problematic in WLWH because hrHPV test positivity may exceed 50% in this high-risk population (51). While more women require additional testing to identify high-grade lesions, colposcopies are inconvenient for women and costly to the health system (3, 26). Using a molecular test for self-collected samples that test positive for hrHPV can reduce the need for women to be recalled for further testing, improving program efficiency and cost-effectiveness. Different molecular triaging options may include (i) partial genotyping, (ii) methylation tests, (iii) hrHPV mRNA detection, (iv) detection of E6/E7 proteins or (v) hrHPV viral load quantification. Additionally, these options may be used in combination.
4.1 Partial and extended hrHPV genotyping
4.1.1 Rationale
Advances in commercially available molecular methods have expanded the ability to detect and characterize hrHPV genotypes beyond the research setting (52–55). This has created new possibilities for clinical applications, including risk stratification based on hrHPV genotype as a triage method. The idea of risks stratification based on hrHPV genotype was first reported in 2003 when IARC investigators suggested that women who tested positive for HPV 16, 18, and 45 merits closer surveillance than women infected with other hrHPV genotypes (56). Though most commercially available tests validated for screening detect HPV 16 and 18, certain tests allow the detection of additional common high-risk types (e.g., HPV 45; HPV 31 etc.) in combination or separately. Healthcare providers can tailor interventions to the individual’s risk profile by identifying specific high-risk genotypes, ensuring appropriate surveillance, follow-up, or treatment. These measures may improve cervical cancer prevention and control efforts.
4.1.2 Evidence
Systematic reviews have consistently shown that different hrHPV genotypes are associated with varying risks of CIN2+, and based on this, partial HPV genotyping has already been integrated into national screening guidelines in several countries, including the United States, Canada, Australia, and several European countries (41, 55–60). This is also supported by clinical studies that demonstrate the effectiveness of partial HPV genotyping as a triage test compared with cytology (55, 61–65). For example, the relative risks of CIN2+ following a screening regime of partial HPV genotyping in HPV-positive women in a Japanese cervical screening program was 19.5% (95% CI 12.4–29.4) in women infected with HPV16/18 compared to 5.6% (95% CI 3.1–10.0) in women infected with all 12 high-risk HPV genotypes (61). Another large-scale study among 9,526 women in rural China found that triage strategies among HPV-positive women using HPV16/18 genotyping improved the PPV for detection of CIN2+ by four times. In recent studies, extended hrHPV genotyping beyond HPV16/18 has also demonstrated the potential to improve risk stratification (55, 63–65). For example, in a large US study of 27,037 women with normal cytology, the Onclarity HPV Assay was used. This study found that extended genotyping stratified risk for CIN2+ among women 25+ years with normal cytology and that HPV16 and HPV 31 had the highest risk for CIN2+ (11.6% and 12.1%, respectively) (64). However, using partial genotyping as a triage test may lead to a drop in the sensitivity to 0.71 (95% CI-0.65–0.76), which may be more pronounced with extended genotyping (66, 67).
4.1.3 Implementation considerations
Performing hrHPV genotyping requires access to an appropriate validated test platform that provides genotyping information (at least for HPV 16/18). As discussed earlier, HPV 16/18 as a triage test has to be combined with additional testing (like cytology or VIA for those positive for other hrHPV types), for which the women need to be recalled (Figure 1).
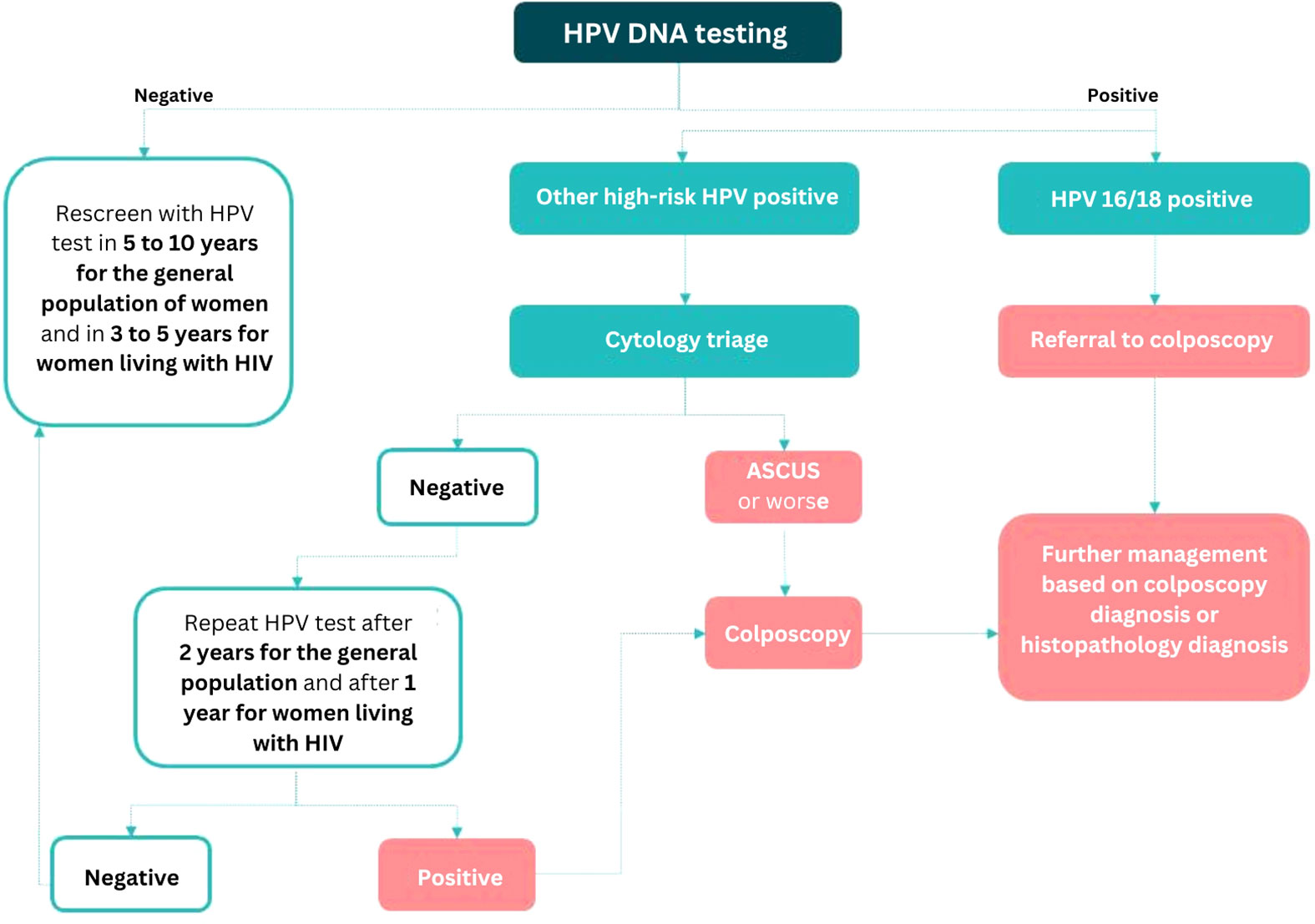
Figure 1 Triaging algorithm for hrHPV-positive women using a combination of partial genotyping and cytology based on the WHO guidelines [Source: Joshi S, Basu P, Lucas E (2023). Using HPV tests for cervical screening and managing hrHPV-positive women – a practical online guide: IARC CancerBase No. 18 [Internet]. Lyon, France: International Agency for Research on Cancer. Available from: https://screening.iarc.fr/atlasHPV.php, accessed on 25 May 2023.
4.2 DNA methylation of human and viral genes
4.2.1 Rationale
Gene expression and function is affected by DNA methylation. By analyzing the methylation patterns of specific human genes, it is possible to identify early molecular changes associated with the development of CIN2+ lesions (18). Incorporating HPV viral gene methylation provides valuable information on viral activity and persistence. As a triage test, the detection of DNA methylation in both human and viral genes has been found to improve the accuracy of detecting clinically significant precancerous lesions (43).
4.2.2 Evidence
A systematic review of 43 studies demonstrated the association between DNA methylation of several human genes (CADM1, MAL, MIR, EPB41L3, PAX1, SOX1, FAM19A4, and POU4F3) and hrHPV L1/L2 with increasing CIN grade (18, 45). Hypermethylation of these genes is more likely in women with CIN3 compared to CIN1, and they are nearly universally positive in cervical cancer (18). The combination of human and viral gene methylation analysis shows promise for improving triage performance compared to other methods such as hrHPV genotyping and cytology. When used to triage hrHPV+ women, DNA methylation had higher specificity than cytology (relative specificity 1.25, 95% CI 0.99-1.59) and higher sensitivity than HPV16/18 genotyping (relative sensitivity 1.22, 95% CI 1.05-1.42) (18). A study using the DNA-methylation test S5 found the positivity thresholds can be adjusted to alter sensitivity and specificity and tailor the test to the infrastructure capacity in different settings (44). An optimal sensitivity of 96.2% (95% CI 94.3–98.2) was reported (44). Hypermethylation of hrHPV L1/L2 genes has also been assessed separately as biomarkers for CIN2+ and cervical cancer and has better predictive capacity compared with other HPV viral methylation targets with a pooled sensitivity of 77% (95% CI 63%–87%), and specificity 64% (95% CI 55%–71%) (45). However, new targets for HPV methylation have not been thoroughly investigated through whole genome methylation. More broad investigation of methylation patterns may lead to improved triage when compared to hrHPV genotyping, cytology, or their combination (68).
4.2.3 Implementation considerations
While the evidence suggests that DNA methylation of human and viral genes holds promise for improving triage in cervical cancer screening, few tests have been commercialized to date. There is no agreement on which target gene or combination of genes would be ideal. Successful implementation would require further investigation on optimal assays, larger studies on clinical effectiveness, validation studies in diverse populations and cost-effectiveness evaluation.
4.3 hrHPV mRNA detection
4.3.1 Rationale
hrHPV mRNA testing specifically detects the presence of viral gene expression, indicating active viral replication and the potential for disease progression (69). When compared to DNA testing, mRNA testing is anticipated to offer more precise detection of persistent infections. This is because mRNA testing targets more advanced stages of the hrHPV pathogenetic pathway. By focusing on viral gene expression, it may be possible to enhance the specificity and PPV of triage tests, which can ultimately reduce the need for unnecessary follow-up procedures for women with temporary hrHPV infections.
4.3.2 Evidence
Detecting hrHPV E6/E7 mRNAs can predict CIN2+ (70, 71). In a large cross-sectional study, it was found that E6/E7 mRNA had high sensitivity (94.4%, 95% CI 89.1–97.3) (72). However, it generates too many positive results for triage and is recommended only for screening in the general population by WHO. There are few studies that examine the accuracy of hrHPV mRNA in self-collected samples or among WLHIV.
4.3.3 Implementation considerations
Due to a paucity of data, especially from longitudinal studies, the WHO has not yet recommended hrHPV mRNA test to be used for self-sampling.
4.4 E6/E7 oncoprotein detection
4.4.1 Rationale
The HPV E6 and E7 oncoproteins have a major role in the cervical carcinogenesis, acting as the primary drivers of HPV oncogenic activity (73, 74). As described earlier, they can prevent natural defence against unregulated cell proliferation (such as apoptosis) by deactivating host proteins involved in tumour suppression (such as p53 or pRb) (75–77). Moreover, these oncoproteins have a synergic action which allows them to increase genomic instability and cell mutations, therefore, driving the progression to invasive cancer (78, 79). Detecting these viral oncoproteins could thus mark active and persistent HPV-driven lesions with great potential for carcinogenesis rather than lesions more likely to regress.
4.4.2 Evidence
The OncoE6 Cervical Test detects elevated levels of oncoprotein E6 expressed by HPV16 and HPV18. Across several studies assessing its accuracy, heterogeneous sensitivity estimates were reported for high-grade cervical disease detection, ranging from 54–80%, with specificity estimates ranging from 78-95% when used to triage hrHPV-positive women (accuracy results for CIN2+ were similar to those of CIN3+) (80–84). The OncoE6 Cervical test was also evaluated in a study conducted in Africa to triage hrHPV-positive WLWH and showed a sensitivity of 58.3% (95% CI 30.4–86.2) with a 94.2% (95% CI 91.3–97.2) specificity (85). To improve on this low sensitivity, the OncoE6E7 Cervical test now includes six additional hrHPV types (HPV 45, 31, 33, 35, 52, and 58). One study from China reported promising results using this newly developed triage test showing a higher sensitivity than OncoE6 testing (100% vs 80%) significant difference in specificity (86% vs 92%) for CIN3+ detection, although the study was not adequately powered (83). The benefit of expanding oncoprotein expression to include more HPV genotypes was further established in another multicenter study from Greece and Germany, though this study was limited in sample size (86).
4.4.3 Implementation considerations
Oncoprotein testing has the advantage of being a simple qualitative test based on an immunochromatographic lateral flow format. Results from oncoprotein testing are usually obtained within 3-4 hours, conferring a potential point-of-care use. However, further evaluation studies are required with adequate sample size.
4.5 hrHPV viral load estimation
4.5.1 Rationale
The rationale behind viral load estimation lies in the hypothesis that persistent hrHPV infections with higher viral loads are more likely to progress to CIN2+ lesions and, ultimately, to cervical cancer. Viral load testing could offer a semi-quantitative measure of hrHPV infection and may infer increased viral replication.
4.5.2 Evidence
A large study among 39,728 hrHPV+ women found significantly higher detection of CIN2+ with increasing measure of viral load (35). Of great clinical importance, viral load testing identified more than half of CIN2+ lesions missed by colposcopy triage and the level of hrHPV viral load was directly linked to the severity of cervical lesions (35). A threshold of ten relative light units/control (RLU/CO) or higher indicated a suitable criterion for immediate colposcopy, while a viral load between 1 and 10 RLU/CO could be an indication for reflex cytology. This approach optimizes sensitivity and specificity of the test results while managing referral rates effectively (36). A Chinese study involving 2051 women positive for hrHPV on the Cobas4800 test evaluated the role of using cycle threshold (Ct) values for risk stratification. The observed CIN3+ incidence in women with low Ct value (≤ 33.2 for all high-risk types and ≤ 29.6 for high-risk types other than HPV 16) was nine-fold higher than that in HPV-positive women with higher Ct values (87).
4.5.3 Implementation considerations
Currently available PCR assays cannot measure hrHPV viral load, making their implementation in routine clinical settings challenging. Some hrHPV tests like Cobas4800 and Xpert HPV use real-time PCR and indirectly estimate viral loads through Ct values. Ct values indicate the number of cycles needed to detect hrHPV DNA. A low Ct value corresponds to a high viral load, while a high Ct value corresponds to a low viral load. However, only HC2 routinely reports RLU levels, and one must manually extract the Ct values for Cobas or Xpert HPV tests. Further research is required to replicate performance results and comprehend the potential use of different RLU and Ct value cutoffs. In the context of self-sampling, the RLU’s limited performance in detecting CIN2+ implies that it has limited utility as a surrogate measure of hrHPV viral load (3).
5 Gaps and future research
Cervical screening is shifting towards objective molecular tests rather than subjective ones like cytology and VIA. Based on the lessons learned from the COVID-19 pandemic, self-collected hrHPV tests are becoming more common, but recalling positive cases for triage tests undermines the purpose of self-collection which is to make screening more accessible and acceptable to women. Further research is needed to better stratify hrHPV-positive women to minimize recalls for colposcopy and treatment to those with the greatest risk of developing cervical cancer.
Self-collected hrHPV testing with molecular triage is promising but requires further research. However, all five options described in this review require robust comparative and longitudinal studies to understand their efficacy in clinically relevant contexts. Studies also need to consider test generalizability and evaluation in different populations. Especially of interest are vaccinated women, women over 65 years who could exit screening programs, WLWH, and women who are receiving post-treatment hrHPV tests (test of cure).
Diverse population data is lacking when reviewing the evidence for partial and extended HPV genotyping. Most of the evidence is derived from specific geographic regions. Where this data is easily available. Further research could be done to understand the potential to stratify the risk in sub-groups of the population, for example, vaccinated women and WLHIV. Evidence on the cost-effectiveness of HPV partial or extended genotyping on self-collected samples and comparisons with other triage methods are essential to inform decision-making and resource allocation.
Longitudinal studies with extended follow-up periods will be useful to assess the predictive value of oncoprotein E6 and E7 in a triage capacity from both self-collected and clinician-obtained samples. None of the evidence cited above includes estimates of test accuracy which are obtained for self-collected HPV samples. So far, the test has been tested in a limited number of geographic settings. There is also a paucity of data to evaluate the clinical utility of oncoprotein E6 and E7 in specific populations, for example, WLHIV. The cost-effectiveness of incorporating E6 and E7 triage using self-collected samples would also need further evaluations in different geographic settings and populations before implementation could be recommended.
The use of DNA methylation as a triage tool is in early stages and still evolving. Currently, the commercially available methylation tests focus on human genes, and experimental tests evaluate viral genes. There remains potential to discover more optimal CpG sites for detecting CIN2+ lesions. Whole genome methylation analysis may provide insight into the breadth of variation in HPV methylation across the genome and enable the identification of new relevant targets. To date, studies evaluating accuracy are very heterogenous and associated with moderate to high risk of bias (45). It is important to consider the differences in study population, their eligibility criteria, the different sampling techniques and tests, as well as the difference in reference verification standards. Testing using self-collected samples and more extensive validation of methylation testing in different geographical settings is required because ethnicity plays an essential role in the epigenome and, consequently, one’s methylation profile. Most studies have been done in Caucasian (43%) or Asian (49%) populations. Whole genome methylation analyses would also enable the identification of common and different cervical cancer-specific markers that may be relevant in different geographical populations, for example, women in sub-Saharan Africa with the highest incidence of cervical cancer worldwide. Detection of hrHPV in first-void urine samples will also be an exciting opportunity to improve screening participation. This is supported by studies finding that it can be as accurate as hrHPV testing on self-collected vaginal samples (88, 89). Some studies are also looking at methylation markers in urine. Testing for hrHPV testing in self-collected vaginal or urine samples and combining a well-validated methylation triage test using the same sample could support the cervical cancer elimination efforts.
Currently, there is no data on the accuracy of testing hrHPV viral load from self-collected samples. However, this may be a consideration in the future. Exploring different thresholds for detection may be relevant for determining the clinical utility of hrHPV viral load as a triage marker. Consistency in sample collection, evaluating and comparing different hrHPV quantification methods, and interpretation criteria is essential to ensure reliable and comparable results. Addressing these gaps in evidence will require further research and studies specifically designed to evaluate each triage test using self-collected samples, comparing it with other triage methods, assessing long-term clinical outcomes, considering cost-effectiveness, and including diverse population sub-sets.
Author contributions
PB conceptualized the review, contributed to writing and provided critical review and editing. KT, LD, AB and FZ contributed to writing, reviewing and editing. All authors approved the submitted version.
Funding
KT is supported by a Postdoc. Mobility Fellowship from the Swiss National Science Foundation (P500PM_210933).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer/World Health Organization.
References
1. WHO guideline for screening and treatment of cervical precancer lesions for cervical cancer prevention. Available at: https://www.who.int/publications/i/item/9789240030824 (Accessed July 24, 2021).
2. Mustafa RA, Santesso N, Khatib R, Mustafa AA, Wiercioch W, Kehar R, et al. Systematic reviews and meta-analyses of the accuracy of HPV tests, visual inspection with acetic acid, cytology, and colposcopy. Int J Gynecology Obstetrics (2016) 132(3):259–65. doi: 10.1016/j.ijgo.2015.07.024
3. Arbyn M, Smith SB, Temin S, Sultana F, Castle P. Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: updated meta-analyses on behalf of the Collaboration on Self-Sampling and HPV Testing. BMJ (2018) 363:4823. doi: 10.1136/bmj.k4823
4. Ronco G, Dillner J, Elfström KM, Tunesi S, Snijders PJ, Arbyn M, et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomized controlled trials. Lancet (2014) 383(9916):524–32. doi: 10.1016/S0140-6736(13)62218-7
5. Zhao XL, Xu XQ, Duan XZ, Rezhake R, Hu SY, Wang Y, et al. Comparative performance evaluation of different HPV tests and triaging strategies using self-samples and feasibility assessment of thermal ablation in ‘colposcopy and treat’ approach: A population-based study in rural China. Int J Cancer (2020) 147(5):1275–85. doi: 10.1002/IJC.32881
6. Poli UR, Muwonge R, Bhoopal T, Lucas E, Basu P. Feasibility, acceptability, and efficacy of a community health worker-driven approach to screen hard-to-reach periurban women using self-sampled HPV detection test in India. JCO Glob Oncol (2020) 6(6):658–66. doi: 10.1200/GO.20.00061
7. Smith MA, Hall MT, Saville M, Brotherton JML, Simms KT, Lew JB, et al. Could HPV testing on self-collected samples be routinely used in an organized cervical screening program? A modelled analysis. Cancer Epidemiol Biomarkers Prev (2021) 30(2):268–77. doi: 10.1158/1055-9965.EPI-20-0998
8. Nishimura H, Yeh PT, Oguntade H, Kennedy CE, Narasimhan M, Topp SM. HPV self-sampling for cervical cancer screening: a systematic review of values and preferences. BMJ Glob Health (2021) 6:3743. doi: 10.1136/bmjgh-2020-003743
9. Ginsburg O, Basu P, Kapambwe S, Canfell K. Eliminating cervical cancer in the COVID-19 era. Nat Cancer (2021) 2:2. doi: 10.1038/s43018-021-00178-9
10. Castle P, Feldman S, Perkins RB. The next generation of cervical cancer screening: should guidelines focus on best practices for the future or current screening capacity? J Low Genit Tract Dis (2018) 22(2):91–6. doi: 10.1097/LGT.0000000000000378
11. Arbyn M, Ronco G, Anttila A, Meijer CJ, Poljak M, Ogilvie G, et al. Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer. Vaccine (2012) 30(SUPPL.5):F88–99. doi: 10.1016/J.VACCINE.2012.06.095
12. Kyrgiou M, Koliopoulos G, Martin-Hirsch P, Arbyn M, Prendiville W, Paraskevaidis E. Obstetric outcomes after conservative treatment for intraepithelial or early invasive cervical lesions: Systematic review and meta-analysis. Lancet. (2006) 367(9509):489–98. doi: 10.1016/S0140-6736(06)68181-6
13. Darragh TM, Colgan TJ, Cox JT, Heller DS, Henry MR, Luff RD, et al. The lower anogenital squamous terminology standardization project for HPV-associated lesions: background and consensus recommendations from the college of american pathologists and the american society for colposcopy and cervical pathology. Arch Pathol Lab Med (2012) 136(10):1266–97. doi: 10.5858/arpa.LGT200570
14. Silver MI, Gage JC, Schiffman M, Fetterman B, Poitras NE, et al. Clinical outcomes after conservative management of cervical intraepithelial neoplasia grade 2 (CIN2) in Women Ages 21-39 Years. Cancer Prev Res (2018) 11(3):165–70. doi: 10.1158/1940-6207.CAPR-17-0293/254531/AM/CLINICAL-OUTCOMES-AFTER-CONSERVATIVE-MANAGEMENT-OF
15. Wilkinson TM, Sykes PHH, Simcock B, Petrich S. Recurrence of high-grade cervical abnorMalities following conservative management of cervical intraepithelial neoplasia grade 2. Am J Obstet Gynecol (2015) 212(6):769. doi: 10.1016/j.ajog.2015.01.010
16. Skorstengaard M, Lynge E, Suhr J, Napolitano G. Conservative management of women with cervical intraepithelial neoplasia grade 2 in Denmark: a cohort study. BJOG (2020) 127(6):729–36. doi: 10.1111/1471-0528.16081
17. Godfrey MAL, Nikolopoulos M, Garner JE, Adib TR, Mukhopadhyay D, Rains JS, et al. Conservative management of cervical intraepithelial neoplasia grade 2 (CIN2) in women under 30 years of age: A cohort study. Eur J Obstetrics Gynecology Reprod Biol (2018) 228:267–73. doi: 10.1016/j.ejogrb.2018.07.018
18. Kelly H, Benavente Y, Pavon MA, De SS, Mayaud P, Lorincz AT. Performance of DNA methylation assays for detection of high-grade cervical intraepithelial neoplasia (CIN2+): a systematic review and meta-analysis. Br J Cancer (2019) 121(11):954. doi: 10.1038/S41416-019-0593-4
19. Arbyn M, Weiderpass E, Bruni L, de Sanjosé S, Saraiya M, Ferlay J, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health (2020) 8(2):e191–203. doi: 10.1016/S2214-109X(19)30482-6
20. Bruni L, Serrano B, Roura E, Alemany L, Cowan M, Herrero R, et al. Cervical cancer screening programmes and age-specific coverage estimates for 202 countries and territories worldwide: a review and synthetic analysis. Lancet Glob Health (2022) 10(8):e1115–27. doi: 10.1016/S2214-109X(22)00241-8
21. World Health Organisation. Global strategy to accelerate the elimination of cervical cancer as a public health problem and its associated goals and targets for the period 2020 – 2030. United Nations Gen Assembly (2020) 2(1):1–3.
22. Simms KT, Steinberg J, Caruana M, Smith MA, Lew JB, Soerjomataram I, et al. Impact of scaled-up human papillomavirus vaccination and cervical screening and the potential for global elimination of cervical cancer in 181 countries, 2020-99: a modelling study. Lancet Oncol (2019) 20(3):394–407. doi: 10.1016/S1470-2045(18)30836-2
23. Lemp JM, De Neve JW, Bussmann H, Chen S, Manne-Goehler J, Theilmann M, et al. Lifetime prevalence of cervical cancer screening in 55 low- and middle-income countries. JAMA (2020) 324(15):1532. doi: 10.1001/JAMA.2020.16244
24. Brisson M, Kim JJ, Canfell K, Drolet M, Gingras G, Burger EA, et al. Impact of HPV vaccination and cervical screening on cervical cancer elimination: a comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet (2020) 395(10224):575–90. doi: 10.1016/S0140-6736(20)30068-4
25. Bruni L, Saura-Lázaro A, Montoliu A, Brotons M, Alemany L, Diallo MS, et al. HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010–2019. Prev Med (Baltim) (2021) 106399. doi: 10.1016/j.ypmed.2020.106399
26. Kelly H, Jaafar I, Chung M, Michelow P, Greene S, Strickler H, et al. Diagnostic accuracy of cervical cancer screening strategies for high-grade cervical intraepithelial neoplasia (CIN2+/CIN3+) among women living with HIV: A systematic review and meta-analysis. EClinicalMedicine (2022) 53:101645. doi: 10.1016/J.ECLINM.2022.101645
27. Mezei AK, Armstrong HL, Pedersen HN, Campos NG, Mitchell SM, Sekikubo M, et al. Cost-effectiveness of cervical cancer screening methods in low- and middle-income countries: A systematic review. Int J Cancer (2017) 141(3):437–46. doi: 10.1002/IJC.30695
28. General Secretariat of the European Council. Council Recommendation on strengthening prevention through early detection: A new EU approach on cancer screening replacing Council Recommendation. e: 2022/0290(NLE), 14770/22 (2022). Available at: https://data.consilium.europa.eu/doc/document/ST-14770-2022-INIT/en/pdf (Accessed May 10, 2023).
29. Arbyn M, Verdoodt F, Snijders PJF, et al. Accuracy of human papillomavirus testing on self-collected versus clinician-collected samples: A meta-analysis. Lancet Oncol (2014) 15(2):172–83. doi: 10.1016/S1470-2045(13)70570-9
30. Arbyn M, Bruni L, Kelly D, Verhoef VM, Suonio E, Dillner L, et al. Tackling cervical cancer in Europe amidst the COVID-19 pandemic. Lancet Public Health (2020) 5(8):e425. doi: 10.1016/S2468-2667(20)30122-5
31. Hawkes D, Keung MHT, Huang Y, McDermott TL, Romano J, Saville M, et al. Self-collection for cervical screening programs: from research to reality. Cancers (Basel) (2020) 12(4). doi: 10.3390/cancers12041053
32. Yeh PT, Kennedy CE, De Vuyst H, Narasimhan M. Self-sampling for human papillomavirus (HPV) testing: a systematic review and meta-analysis. BMJ Glob Health (2019) 4:1351. doi: 10.1136/bmjgh-2018-001351
33. Baena A, Mesher D, Salgado Y, Martínez S, Villalba GR, Amarilla ML, et al. Performance of visual inspection of the cervix with acetic acid (VIA) for triage of HPV screen-positive women: results from the ESTAMPA study. Int J Cancer (2023) 152(8):1581. doi: 10.1002/IJC.34384
34. Moscicki AB, Schiffman M, Burchell A, Albero G, Giuliano AR, Goodman MT, et al. Updating the natural history of human papillomavirus and anogenital cancers. Vaccine (2012) 30(SUPPL.5):F24–33. doi: 10.1016/j.vaccine.2012.05.089
35. Basu P, Muwonge R, Mittal S, Banerjee D, Ghosh I, Panda C, et al. Implications of semi-quantitative HPV viral load estimation by Hybrid capture 2 in colposcopy practice. J Med Screen (2016) 23(2):104–10. doi: 10.1177/0969141315606483
36. Zhou Y, Shi X, Liu J, Zhang L. Correlation between human papillomavirus viral load and cervical lesions classification: A review of current research. Front Med (2023) 10:1111269. doi: 10.3389/fmed.2023.1111269.
37. Sergievsky Center GH, Kuhn L, Saidu R, Tergas A, Moodley J, Persing D, et al. Clinical evaluation of modifications to a human papillomavirus assay to optimize its utility for cervical cancer screening in low-resource settings: a diagnostic accuracy study. Articles Lancet Glob Health (2020) 8:296–304. doi: 10.1016/S2214-109X(19)30527-3
38. Snijders PJF, Steenbergen RDM, Heideman DAM, Meijer CJLM. HPV-mediated cervical carcinogenesis: Concepts and clinical implications. J Pathology (2006) 208(2):152–64. doi: 10.1002/path.1866
39. Mirabello L, Yeager M, Yu K, et al. HPV16 E7 genetic conservation is critical to carcinogenesis. Cell (2017) 170(6):1164–1174.e6. doi: 10.1016/j.cell.2017.08.001
40. Clifford GM, Smith JS, Plummer M, Muñoz N, Franceschi S. Human papillomavirus types in invasive cervical cancer worldwide: A meta-analysis. Br J Cancer (2003) 88(1):63–9. doi: 10.1038/sj.bjc.6600688
41. Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: A meta-analysis update. Int J Cancer. (2007) 121(3):621–32. doi: 10.1002/ijc.22527
42. Publication of IARC handbooks of cancer prevention volume 18: cervical cancer screening – IARC. Available at: https://www.iarc.who.int/news-events/publication-of-iarc-handbooks-of-cancer-prevention-volume-18-cervical-cancer-screening/ (Accessed December 26, 2022).
43. Lorincz AT. Virtues and weaknesses of DNA methylation as a test for cervical cancer prevention. Acta Cytol (2016) 60(6):501–12. doi: 10.1159/000450595
44. Banila C, Lorincz AT, Scibior-Bentkowska D, Clifford GM, Kumbi B, Beyene D, et al. Clinical performance of methylation as a biomarker for cervical carcinoma in situ and cancer diagnosis: A worldwide study. Int J Cancer (2022) 150(2):290–302. doi: 10.1002/IJC.33815
45. Bowden SJ, Kalliala I, Veroniki AA, Arbyn M, Mitra A, Lathouras K, et al. The use of human papillomavirus DNA methylation in cervical intraepithelial neoplasia: A systematic review and meta-analysis. EBioMedicine (2019) 50:246–59. doi: 10.1016/J.EBIOM.2019.10.053
46. Bordignon V, Di Domenico EG, Trento E, D'Agosto G, Cavallo I, Pontone M, et al. How human papillomavirus replication and immune evasion strategies take advantage of the host DNA damage repair machinery. Viruses (2017) 9(12):390. doi: 10.3390/V9120390
47. Clarke MA, Gradissimo A, Schiffman M, Lam J, Sollecito CC, Fetterman B, et al. Human Papillomavirus DNA methylation as a biomarker for cervical precancer: Consistency across 12 genotypes and potential impact on management of HPV-positive women. Clin Cancer Res (2018) 24(9):2194. doi: 10.1158/1078-0432.CCR-17-3251
48. Kremer WW, Steenbergen RDM, Heideman DAM, Kenter GG, Meijer CJLM. The use of host cell DNA methylation analysis in the detection and management of women with advanced cervical intraepithelial neoplasia: a review. BJOG (2021) 128(3):504–14. doi: 10.1111/1471-0528.16395
49. Wentzensen N, Von Knebel Doeberitz M. Biomarkers in cervical cancer screening. Dis Markers (2007) 23(4):315. doi: 10.1155/2007/678793
50. Basu P, Mittal S, Banerjee D, Singh P, Panda C, Dutta S, et al. Diagnostic accuracy of VIA and HPV detection as primary and sequential screening tests in a cervical cancer screening demonstration project in India. Int J Cancer (2015) 137(4):859–67. doi: 10.1002/IJC.29458
51. Gilles C, Konopnicki D, Rozenberg S. The recent natural history of human papillomavirus cervical infection in women living with HIV: A scoping review of meta-analyses and systematic reviews and the construction of a hypothetical model. HIV Med (2023). doi: 10.1111/HIV.13490
52. Johnson LG, Saidu R, Mbulawa Z, Williamson AL, Boa R, Tergas A, et al. Selecting human papillomavirus genotypes to optimize the performance of screening tests among South African women. Cancer Med (2020) 9(18):6813. doi: 10.1002/CAM4.3329
53. Egli-Gany D, Spaar Zographos A, Diebold J, Masserey Spicher V, Frey Tirri B, Heusser R, et al. Human papillomavirus genotype distribution and socio-behavioural characteristics in women with cervical precancer and cancer at the start of a human papillomavirus vaccination programme: the CIN3+ plus study. BMC Cancer (2019) 19(1). doi: 10.1186/S12885-018-5248-Y
54. Arbyn M, Simon M, Peeters E, Xu L, Meijer CJLM, Berkhof J, et al. list of human papillomavirus assays suitable for primary cervical cancer screening. Clin Microbiol Infection (2020) 27(8):1083–95. doi: 10.1016/J.CMI.2021.04.031
55. Rohner E, Edelman C, Sanusi B, Schmitt JW, Baker A, Chesko K, et al. Extended HPV genotyping to compare HPV type-distribution in self and provider-collected samples for cervical cancer screening. Cancer Epidemiol Biomarkers Prev (2020) 29(12):2651. doi: 10.1158/1055-9965.EPI-20-0674
56. Clifford GM, Smith JS, Aguado T, Franceschi S. Comparison of HPV type distribution in high-grade cervical lesions and cervical cancer: a meta-analysis. Br J Cancer (2003) 89(1):101–5. doi: 10.1038/SJ.BJC.6601024
57. Demarco M, Egemen D, Raine-Bennett TR, Cheung LC, Befano B, Poitras NE, et al. A study of partial human papillomavirus genotyping in support of the 2019 ASCCP risk-based management consensus guidelines. (2020) 24(2):144–7. doi: 10.1097/LGT.0000000000000530.
58. Massad LS, Einstein MH, Huh WK, Katki HA, Kinney WK, Schiffman M, et al. updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis (2012) 17(5 SUPPL.1). doi: 10.1097/LGT.0B013E318287D329
59. Clifford GM, Gonçalves MAG, Franceschi S. Human papillomavirus types among women infected with HIV: a meta-analysis. AIDS (2006) 20(18):2337–44. doi: 10.1097/01.aids.0000253361.63578.14
60. de Sanjosé S, Diaz M, Castellsagué X, Clifford G, Bruni L, Muñoz N, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis (2007) 7(7):453–9. doi: 10.1016/S1473-3099(07)70158-5
61. Aoyama-Kikawa S, Fujita H, Hanley SJB, Kasamo M, Kikuchi K, Torigoe T, et al. Comparison of human papillomavirus genotyping and cytology triage, COMPACT Study: Design, methods and baseline results in 14 642 women. Cancer Sci (2018) 109(6):2003–12. doi: 10.1111/CAS.13608
62. Hanley SJB, Fujita H, Aoyama-Kikawa S, Kasamo M, Torigoe T, Matsuno Y, et al. Evaluation of partial genotyping with HPV16/18 for triage of HPV positive, cytology negative women in the COMPACT study. J Gynecol Oncol (2021) 32(6):86. doi: 10.3802/jgo.2021.32.e86
63. Schiffman M, Hyun N, Raine-Bennett TR, et al. A cohort study of cervical screening using partial HPV typing and cytology triage. Int J Cancer (2016) 139(11):2606–15. doi: 10.1002/IJC.30375
64. Stoler MH, Wright TC, Parvu V, Yanson K, Cooper CK, Andrews J. Stratified risk of high-grade cervical disease using onclarity HPV extended genotyping in women, ≥25 years of age, with NILM cytology. Gynecol Oncol (2019) 153(1):26–33. doi: 10.1016/j.ygyno.2018.12.024
65. Wright TC, Stoler MH, Parvu V, Yanson K, Cooper C, Andrews J. Risk detection for high-grade cervical disease using Onclarity HPV extended genotyping in women, ≥21 years of age, with ASC-US or LSIL cytology. Gynecol Oncol (2019) 154(2):360–7. doi: 10.1016/J.YGYNO.2019.05.012
66. Arbyn M, Xu L, Verdoodt F, Cuzick J, Szarewski A, Belinson JL, et al. Genotyping for human papillomavirus types 16 and 18 in women with minor cervical lesions: A systematic review and meta-analysis. Ann Intern Med (2017) 166(2):118–27. doi: 10.7326/M15-2735
67. Song F, Yan P, Huang X, Wang C, Du H, Qu X, et al. Roles of extended human papillomavirus genotyping and multiple infections in early detection of cervical precancer and cancer and HPV vaccination. BMC Cancer (2021). doi: 10.1186/s12885-021-09126-3
68. Cook DA, Krajden M, Brentnall AR, Gondara L, Chan T, Law JH, et al. Evaluation of a validated methylation triage signature for human papillomavirus positive women in the HPV FOCAL cervical cancer screening trial. Int J Cancer (2019) 144(10):2587–95. doi: 10.1002/IJC.31976
69. WHO guideline for screening and treatment of cervical precancer lesions for cervical cancer prevention, second edition: use of mRNA tests for human papillomavirus (HPV) Web Annex. Evidence-to-decision framework for mRNA testing for HPV ii (2021). Available at: http://apps.who.int/bookorders (Accessed May 22, 2023).
70. Nascimento NPG, Gally TB, Borges GF, Campos LCG, Kaneto CM. Systematic review of circulating MICRORNAS as biomarkers of cervical carcinogenesis. BMC Cancer (2022) 22(1):862. doi: 10.1186/S12885-022-09936-Z
71. Derbie A, Mekonnen D, Woldeamanuel Y, Van Ostade X, Abebe T. HPV E6/E7 mRNA test for the detection of high grade cervical intraepithelial neoplasia (CIN2+): a systematic review. Infect Agent Cancer. (2020). doi: 10.1186/S13027-020-0278-X
72. Rossi PG, Carozzi FM, Ronco G, Allia E, Bisanzi S, Gillio-Tos A, et al. p16/ki67 and E6/E7 mRNA accuracy and prognostic value in triaging HPV DNA-positive women. JNCI J Natl Cancer Institute (2021) 113(3):292. doi: 10.1093/JNCI/DJAA105
73. Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell (2011) 144(5):646–74. doi: 10.1016/J.CELL.2011.02.013
74. Mesri EA, Cesarman E, Boshoff C. Kaposi’s sarcoma and its associated herpesvirus. Nat Rev Cancer (2010) 10(10):707–19. doi: 10.1038/nrc2888
75. Mesri EA, Feitelson MA, Munger K. Human viral oncogenesis: a cancer hallmarks analysis. Cell Host Microbe (2014) 15(3):266–82.
76. Boccardo E, Lepique AP, Villa LL. The role of inflammation in HPV carcinogenesis. Carcinogenesis (2010) 31(11):1905–12. doi: 10.1093/CARCIN/BGQ176
77. Moody CA, Laimins LA. Human papillomavirus oncoproteins: pathways to transformation. Nat Rev Cancer (2010) 10(8):550–60. doi: 10.1038/NRC2886
78. Yeo-Teh NSL, Ito Y, Jha S. Molecular sciences high-risk human papillomaviral oncogenes E6 and E7 target key cellular pathways to achieve oncogenesis. Int J Mol Sci (2018) 19(6):1706. doi: 10.3390/ijms19061706
79. Mcbride AA, Warburton A. The role of integration in oncogenic progression of HPV-associated cancers. PLoS Pathog (2017) 13(4):e1006211. doi: 10.1371/journal.ppat.1006211
80. Ferrera A, Valladares W, Cabrera Y, de la Luz Hernandez M, Darragh T. Performance of an HPV 16/18 E6 oncoprotein test for detection of cervical precancer and cancer. Int J Cancer (2019) 145(8):2042–50. doi: 10.1002/IJC.32156
81. Yu L, Jiang M, Qu P, Wu Z, Sun P, Xi M, et al. Clinical evaluation of human papillomavirus 16/18 oncoprotein test for cervical cancer screening and HPV positive women triage. Int J Cancer (2018) 143(4):813–22. doi: 10.1002/IJC.31368
82. Zhang Q, Dong L, Hu S, Feng R, Zhang X, Pan Q, et al. Risk stratification and long-term risk prediction of E6 oncoprotein in a prospective screening cohort in China. Int J Cancer (2017) 141(6):1110–9. doi: 10.1002/IJC.30807
83. Rezhake R, Hu SY, Zhao S, Xu XQ, Zhao XL, Zhang L, et al. Eight-type human papillomavirus E6/E7 oncoprotein detection as a novel and promising triage strategy for managing HPV-positive women. Int J Cancer (2019) 144(1):34–42. doi: 10.1002/IJC.31633
84. Valdez M, Jeronimo J, Bansil P, Qiao YL, Zhao FH, Chen W, et al. Effectiveness of novel, lower cost molecular human papillomavirus-based tests for cervical cancer screening in rural China. Int J Cancer (2016) 138(6):1453–61. doi: 10.1002/IJC.29877
85. Ndizeye Z, Menon S, Van Geertruyden JP, Sauvaget C, Jacquemyn Y, Bogers JP, et al. Performance of OncoE6TM Cervical Test in detecting cervical precancer lesions in HIV-positive women attending an HIV clinic in Bujumbura, Burundi: a cross-sectional study. BMJ Open (2019) 9(9):e029088. doi: 10.1136/BMJOPEN-2019-029088
86. Agorastos T, Chatzistamatiou K, Moysiadis T, Kaufmann AM, Skenderi A, Lekka I, et al. Human papillomavirus E7 protein detection as a method of triage to colposcopy of HPV positive women, in comparison to genotyping and cytology. Final results PIPAVIR study. Int J Cancer (2017) 141(3):519–30. doi: 10.1002/IJC.30761
87. Zhang Y, Du H, Xiao A, Zhang W, Wang C, Huang X, et al. Verification of the association of the cycle threshold (Ct) values from HPV testing on Cobas4800 with the histologic grades of cervical lesions using data from two population-based cervical cancer screening trials. Infect Agent Cancer (2021) 17:27. doi: 10.1186/s13027-022-00440-4
88. Pathak N, Dodds J, Zamora J, Khan K. Accuracy of urinary human papillomavirus testing for presence of cervical HPV: systematic review and meta-analysis. BMJ (2014). doi: 10.1136/BMJ.G5264
Keywords: Cervical screening, molecular tests, triage, self-collection, cervical cancer elimination
Citation: Taghavi K, Zhao F, Downham L, Baena A and Basu P (2023) Molecular triaging options for women testing HPV positive with self-collected samples. Front. Oncol. 13:1243888. doi: 10.3389/fonc.2023.1243888
Received: 23 June 2023; Accepted: 28 August 2023;
Published: 22 September 2023.
Edited by:
Anthony Taylor, University of Leicester, United KingdomReviewed by:
Missaoui Nabiha, University of Sousse, TunisiaMirella Fortunato, Azienda Sanitaria Ospedaliera S.Croce e Carle Cuneo, Italy
Copyright © 2023 Taghavi, Zhao, Downham, Baena and Basu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katayoun Taghavi, dGFnaGF2aWtAaWFyYy53aG8uaW50
 Katayoun Taghavi
Katayoun Taghavi Fanghui Zhao
Fanghui Zhao Laura Downham
Laura Downham Armando Baena1
Armando Baena1