
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol., 26 September 2023
Sec. Surgical Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1241561
This article is part of the Research TopicInnovations in Surgical OncologyView all 20 articles
 D. Wagner*†
D. Wagner*† V. Wienerroither†
V. Wienerroither† M. Scherrer
M. Scherrer M. Thalhammer
M. Thalhammer F. Faschinger
F. Faschinger A. Lederer
A. Lederer H. M. Hau
H. M. Hau R. Sucher
R. Sucher P. Kornprat
P. KornpratIntroduction: Sarcopenia is defined as a decline in muscle function as well as muscle mass. Sarcopenia itself and sarcopenic obesity, defined as sarcopenia in obese patients, have been used as surrogates for a worse prognosis in colorectal cancer. This review aims to determine if there is evidence for sarcopenia as a prognostic parameter in colorectal liver metastases (CRLM).
Methods: PubMed, Embase, Cochrane Central, Web of Science, SCOPUS, and CINAHL databases were searched for articles that were selected in accordance with the PRISMA guidelines. The primary outcomes were overall survival (OS) and disease-free survival (DFS). A random effects meta-analysis was conducted.
Results: After eliminating duplicates and screening abstracts (n = 111), 949 studies were screened, and 33 publications met the inclusion criteria. Of them, 15 were selected after close paper review, and 10 were incorporated into the meta-analysis, which comprised 825 patients. No significant influence of sarcopenia for OS (odds ratio (OR), 2.802 (95% confidence interval (CI), 1.094–1.11); p = 0.4) or DFS (OR, 1.203 (95% CI, 1.162–1.208); p = 0.5) was found, although a trend was defined toward sarcopenia. Sarcopenia significantly influenced postoperative complication rates (OR, 7.905 (95% CI, 1.876–3.32); p = 0.001) in two studies where data were available.
Conclusion: Existing evidence on the influence of sarcopenia on postoperative OS as well as DFS in patients undergoing resection for CRLM exists. We were not able to confirm that sarcopenic patients have a significantly worse OS and DFS in our analysis, although a trend toward this hypothesis was visible. Sarcopenia seems to influence complication rates but prospective studies are needed.
Colorectal cancer (CRC), with 1.8 million new cases diagnosed per year, has been found to be the fourth most incident cancer worldwide. It accounts for the second-most cancer-related deaths worldwide, which means 800,000 CRC-related deaths annually (1, 2).
Colorectal liver metastases (CRLM) are present in approximately 15% of CRC patients at the time of the primary diagnosis, and another 16% of patients develop CRLM throughout their 5-year follow-up after CRC treatment (3, 4). With a 5-year survival of about 16% of overall CRLM patients, this number increases in resectable situations to up to 50% (5).
Various risk factors for the development of CRC have been defined. One of the major risk factors appointed by the World Health Organization is obesity with a body mass index (BMI) above 30 kg/m2 for adults (6, 7). It has been well described, and the rise of obesity in all industrial nations worldwide might also contribute to the higher incidences of CRC in these industrial populations (8, 9).
For obese patients, worse overall survival as well as higher incidences of CRLM and worse outcomes after CRLM resection has been shown, rebutting the “obesity paradox,” which had been described earlier, stating that moderate obesity might even be protective for patients sustaining CRC (10, 11). However, there is still limited high-quality evidence on the real impact of obesity on perioperative as well as long-term outcomes after resection for CRLM.
Sarcopenia has been used as a surrogate for muscle wasting in previous years and is defined as a progressive and generalized skeletal muscle disorder that is associated with an increased likelihood of adverse outcomes (12, 13). It comprises not only a decline in muscle mass but also a decline in muscle function. Generally, sarcopenia has been found to be present in about 38% of cancer patients at the time of presentation; in CRC patients, about 39% of patients have been described as sarcopenic (14, 15). In metastatic CRC patients, even 44% have been identified in studies as having sarcopenia. On top of over a third of patients being sarcopenic at the time of diagnosis of CRC, treatment of CRC with chemotherapy often leads to a significant reduction in muscle mass on top of already prevalent sarcopenia, leading to CRLM patients, who usually receive chemotherapy prior to resection, offering an even worse premise for a potential resection to the individual patient (16, 17).
Unfortunately, sarcopenia is defined very heterogeneously in the present literature. The definition is mainly based on measures of muscle mass and/or muscle density on computed tomography (CT) imaging. Commonly used measures that have been described are the skeletal muscle index (SMI), the total psoas area (TPA), or the Hounsfield Average Calculation (HUAC). All of these parameters are measured on single cross-sectional CT images of the abdomen at the level of the transverse processes of the third lumbar vertebra (L3), normalized for height. The HUAC reflecting the muscle density is measured using the Hounsfield Units of the psoas muscles in the described images and normalizing the measures for the psoas muscles area. Low muscle density has been used as an indicator for intramuscular adipose tissue content (IMAC) and therefore poorer muscle quality (18–20).
Sarcopenia in obese patients—known as sarcopenic obesity—has emerged in recent years as an additional and sometimes more precise prognostic tool as these patients seem to be highly prone to complications. Sarcopenic obesity has been attributed to poor oncologic as well as surgical prognosis (21, 22).
We aimed to perform a systematic review of sarcopenia in the setting of colorectal liver metastases. The presented review was registered in the PROSPERO database (https://www.crd.york.ac.uk/prospero, ID: 432501).
The search for this review was performed according to the preferred reporting items for systematic reviews and meta-analysis (PRISMA) guidelines (23). The Cochrane Register of Controlled Trials (CENTRAL), Embase, the Web of Science, Medline, and Google Scholar were screened for the search string: “colorectal neoplasms” [MeSH Terms] OR “colorectal neoplasms” [Title/Abstract] OR “colorectal cancer” [Title/Abstract] OR “colorectal carcinoma” [Title/Abstract] OR “colorectal tumor” [Title/Abstract] OR “colorectal adenocarcinoma” [Title/Abstract]) AND (“liver neoplasms” [MeSH Terms] OR “liver neoplasms” [Title/Abstract] OR “liver metastases” [Title/Abstract] OR “hepatic metastases” [Title/Abstract] OR “metastatic liver disease” [Title/Abstract])) AND (“sarcopenia” [MeSH Terms] OR “sarcopenia” [Title/Abstract] OR “muscle wasting” [Title/Abstract] OR “muscle loss” [Title/Abstract] OR “muscle atrophy” [Title/Abstract]. The search was carried out on the 22nd day of May 2023 by Scherrer M and Wagner D.
Only original studies investigating humans were included in the analysis. Studies were only included if they reported outcomes of patients aged 18 years and above who underwent liver resection with curative intent for colorectal liver metastases or if specific outcomes for sarcopenic patients were reported. Studies that reported a defined outcome as recurrence, disease-free survival, or overall survival were included in further analysis.
Only studies that reported the exact outcome as the main objective, defined as the influence of sarcopenia on patients’ survival and/or recurrence, were selected for further analysis.
We excluded case series, case reports, reviews, or editorials, as well as experimental research. Only studies written in English were considered for evaluation. Studies reporting from the same databases were limited to the most recent report. We also included outcomes that did not report in numbers and/or missing odds ratios or the possibility of deriving these odds ratios from reported numbers.
The authors F.F., S.M., and W.D. independently screened titles and abstracts to determine their eligibility for inclusion. Full texts were selected and screened upon identification after abstract reading.
Eligible studies were selected for further assessment and data extraction. Data were extracted into a database. Data selected included author, publication date, country, number of participants, median age, methods of sarcopenia assessment, preoperative therapy if reported, including chemotherapy, operation method, type of liver resection, and follow-up (duration, reported overall survival or disease-free survival or both as well as perioperative outcomes).
The quality assessment of the included studies was performed using the Quality in Prognosis Instrument (QUIPS) by three observers (D.W., M.S., and F.F.). The included 10 studies were analyzed using the instrument. The risk of bias was considered low if less than two items were rated as “low risk” or “moderate risk” in the respective assessment categories. Risk of bias was rated “high risk” if more than one item was rated high risk in the respective category (24).
The statistical analysis was performed using SPSS Version 26.0 (SPSS Inc, Chicago, IL, USA). To compare the combined effects of hazard ratios (HR) and/or odds ratios (OR), an inverse approach was applied using 95% confidence intervals (CI) for survival and other outcome data. Heterogeneity was assessed using a random effects model and a C2 test with a p-value of < 0.1 being considered significant. To assess the quantity of heterogeneity, I2 statistics were used with a cutoff value of 50%, and odds ratios defined the difference of dichotome variables in the pooled studies (25).
The initial search led to 949 studies. After the removal of duplicates (n = 838), 111 studies were screened, and a further 78 studies were excluded after abstract screening. The full publications of the remaining 33 studies were screened, and 15 were selected for inclusion into a close assessment. After an independent full-paper review by two investigators, five studies were excluded (no reporting of endpoints n = 2, reporting of endpoints not in inclusion criteria n = 3). The PRISMA Flow Chart is depicted in Figure 1.
So our search derived 10 studies with 1,619 participants that were included in the analysis. Studies were published between 2012 and 2022 and were published by Asian (n = 3), European (n = 6), and one US American study group (26–35). All studies were retrospective in nature. Databases for the reports had been compiled in a median of 108 months (range: 48–132 months) and mostly included all recipients who underwent CRLM resection in the respective centers and who had undergone preoperative imaging via CT scans, including the lumbar vertebral area at the level of L3. Only Yang et al. included patients who had undergone neoadjuvant treatment and therefore only investigated a limited number of patients who had undergone hepatic resection in their respective centers (33). The characteristics of the included studies are compiled in Table 1.
Six of the included studies reported primary tumor location (26–28, 31, 32, 34), and seven reported neoadjuvant and/or adjuvant chemotherapy as confounders (26–29, 33–35). All patients included underwent liver resection, whereas only five studies stated the resection technique (26, 27, 31, 33, 35), and only one reported the operation method in detail (35).
Sarcopenia was identified to be prevalent in 825 patients in all studies. Baseline characteristics were outlined in nine of 10 studies included in the analysis according to nonsarcopenic and sarcopenic patients. In four of them, baseline characteristics differed significantly in age, BMI, adipose tissue, tumor markers, tumor location, and the neutrophil-to-lymphocyte ratio (26, 28, 29, 34). Yang et al. also focused on the progression of sarcopenia after neoadjuvant chemotherapy (33).
Confounders assessed along with sarcopenia differed widely throughout the studies. All studies used age, BMI, tumor stage with TNM classification, ASA status, and gender as confounding variables. The outcome assessment in nine studies was defined as overall survival as well as disease-free survival (26–32, 34). Both were defined homogeneously throughout the nine studies as overall patient survival being the patient survival in the respective follow-up and disease-free survival being the recurrence-free survival in the respective follow-up. Only three studies (Runkel et al., Bajric et al., and Peng et al.) used postoperative morbidity and mortality as combined outcome endpoints, defining patients’ 30-day morbidity using Clavien–Dindo classification (26, 31, 35). Of them, only Bajric and Peng et al. could be used for meta-analysis, as the difference between the sarcopenic and nonsarcopenic patients was not stated in Runkel et al., neither in number nor in statistical form (26, 31).
In the quality assessment using the QUIPS chart, five studies were rated as having an overall low risk for bias, three had a moderate bias risk and two showed a high risk for bias in the category study participation/selection of study participants and assessment for confounders. All results are compiled in Table 2.
The definition of sarcopenia was very heterogeneous throughout the studies. Most of the included studies used the skeletal muscle index (SMI) to define sarcopenic patients (n = 7); four of these studies defined sarcopenia according to the established cutoffs by Prado et al., and two defined sarcopenia using the cutoff values defined by Martin et al. Only one study used statistical stratification to define the cutoffs and used the lowest quartile of their own patient set as a definition for sarcopenia. The other three studies used the total psoas area (TPA) with a cutoff derived by statistical stratification as a definition for sarcopenia, and only two studies incorporated muscle attenuation (i.e., intramuscular adipose tissue) in their primary definition for sarcopenia using the Hounsfield Units as a surrogate for muscle density.
The studies reported outcomes for a total of 825 patients. Sarcopenic patients therefore comprised 51% of overall patients. Sarcopenia assessment was heterogeneous throughout the studies, with most studies using SMI and cutoffs established by Prado et al. but not all.
Regression analysis of all aggregated data showed that sarcopenia was associated with postoperative overall survival, but no significance was reached due to selective outcome reporting (OR, 2.802 (95% CI, 1.094–1.11); p = 0.4, Figure 2). In the subgroup analysis of studies that reported the influence of neoadjuvant therapy vs. studies that did not, no significant influence on overall survival was observed, although the death rate recorded as events influenced overall survival, especially in patients with neoadjuvant therapy (OR, 2.802 (95% CI, 1.094–1.11); p = 0.4, Figure 3). No heterogeneity was found between the studies (I2 = 0.28; p = 0.6).
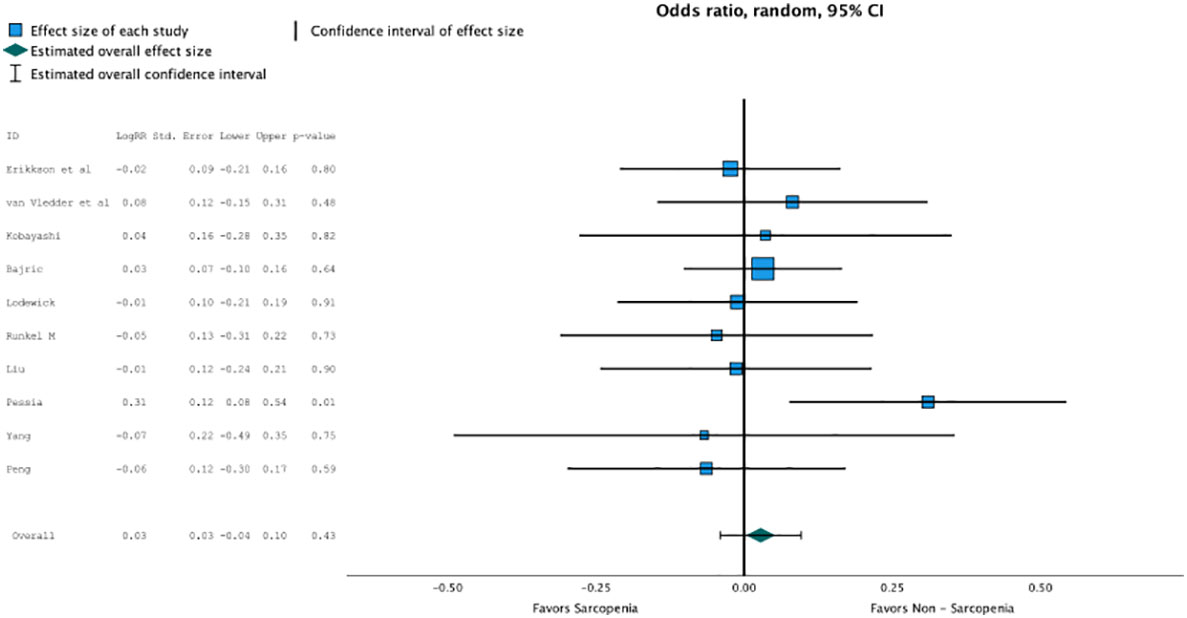
Figure 2 Regression analysis of all aggregated data showed that sarcopenia was associated with post operative overall survival, but no significance was reached due to selective outcome reporting (OR 2.802, CI95%1.094-1.11, p=0.4).
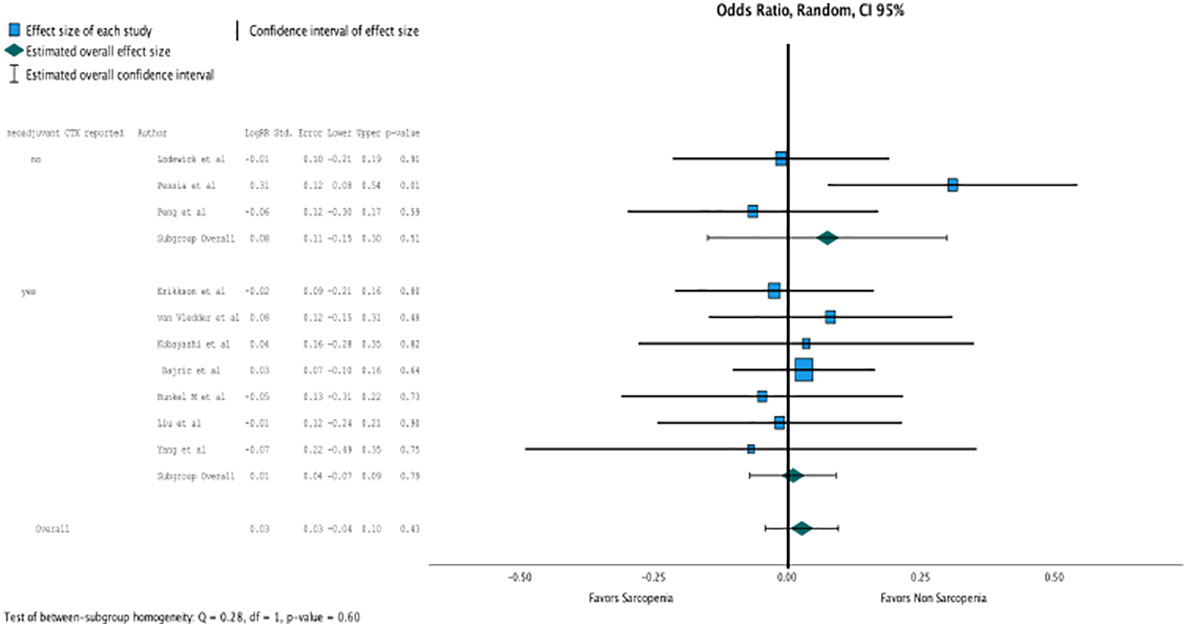
Figure 3 In the subgroup analysis of studies that reported the influence of neoadjuvant therapy vs. studies that did not, no significant influence on overall survival was observed. (OR 2.802, CI95% 1.094-1.11, p=0.4).
Concerning disease-free survival, which was reported in five studies, nonsarcopenia seemed better predictive but did not reach statistical significance due to heterogeneous reporting (OR, 1.203 (95% CI, 1.162–1.208); p = 0.5; Figure 4).
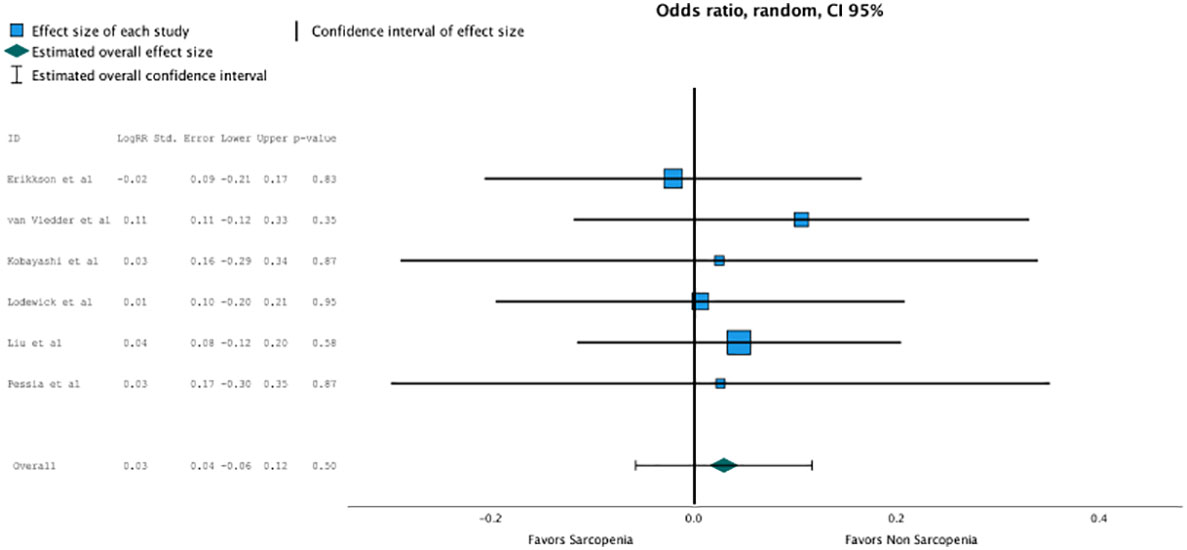
Figure 4 Non sarcopenia seemed better predictive for disease free survival but did not reach statistical significance due heterogenous reporting (OR 1.203, CI95% 1.162-1.208, p=0.5).
Only two studies reported data on postoperative complications according to sarcopenia. The meta-analysis between these studies showed a high association of sarcopenia with postoperative complications (OR, 7.905 (95% CI, 1.876–3.32); p = 0.001; Figure 5).
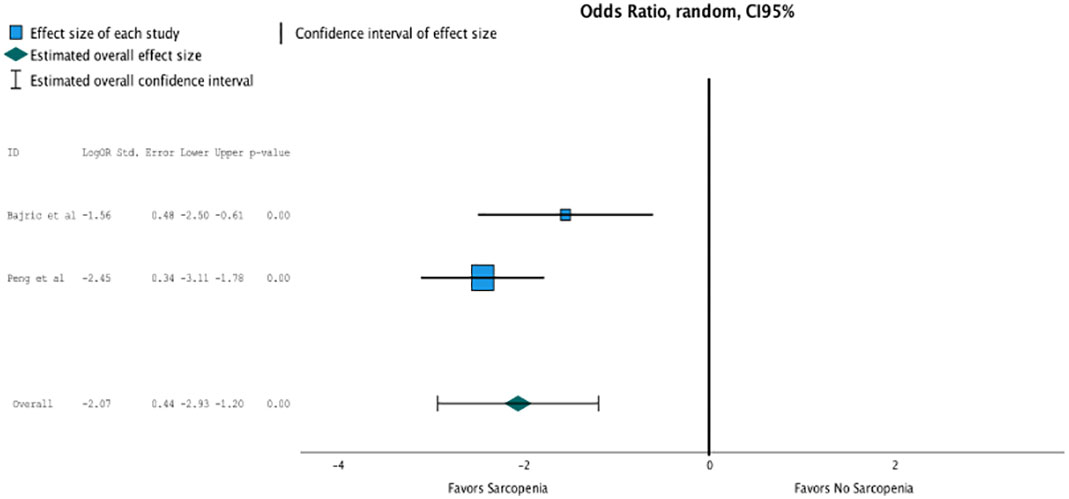
Figure 5 Only two studies reported data on postoperative complications according to sarcopenia. The meta analysis between these studies showed a high association of sarcopenia with postoperative complications (OR 7.905, CI95% 1.876-3.32, p=0.001).
To the best of our knowledge, this is the first meta-analysis that deals with the influence of sarcopenia on the outcome of patients who undergo liver resection for colorectal liver metastases (CRLM) alone. CRLM patients have been incorporated into previous meta-analyses (36, 37). However, due to the unique nature of CRLM and its rising incidence, preoperative assessment is more and more valued in this patient cohort (38). CRLMs are resected in up to 50% of cases. Recent guidelines for liver resection suggest incorporating prehabilitation into their recommendations for preoperative care of patients who undergo liver resection (39). Appropriate sarcopenia diagnosis and knowledge about the impact of sarcopenia on these patients might lead to optimization of preoperative patient care through optimization of prehabilitation and therefore contribute to better postoperative outcomes (19).
Sarcopenia is usually referred to as loss of muscle mass with loss of performance and impaired muscle strength (12, 13, 18). Performing a whole frailty or sarcopenia assessment is time-consuming. This fact often leads to the assessment being omitted or replaced by preoperative image analysis (40, 41). The definition of sarcopenia on preoperative images is still very heterogeneous, both through the working groups and the existing studies (39).
Our review not only confirmed this, but we were also able to display the heterogeneity systematically. Only 40% of the included studies used the same definition for sarcopenia (26, 27, 33, 35), even though 70% of the included studies used the same parameter to assess sarcopenia (p = 0.05). This not only is a significant difference; it also depicts the priority most clinicians usually have when it comes to sarcopenia assessment—to do it fast. This again stresses the high need in the clinical setting to have a readily available parameter. Williams et al. recently stressed this need from a perioperative patient management point of view (42). Until a concise and easily assessed parameter is available, sarcopenia will still be treated as a research parameter, although it is associated with dose-limiting toxicity in chemotherapy in other cancer patients (43). This was partially confirmed in the report by Yang et al., who clearly showed that patients who undergo neoadjuvant therapy do experience more pre- and postoperative sarcopenia and concomitantly higher morbidity and mortality rates (33).
Previous meta-analyses have found an association between overall survival and sarcopenia in patients who undergo loco-regional treatment for CRLM (44).
Although a trend toward sarcopenia was associated with lower overall survival rates in our patients, definitive significance was not observed among the included studies. Some studies have already shown this association. For example, Levolger et al. found poorer overall survival in patients undergoing resection of gastrointestnal malignancies.
Unfortunately, in this study, no report exists from an association of CRLM patients. In colorectal patients after resection, sarcopenia has been associated with poorer overall survival in studies by Trejo-Avila et al. This association was not as prominent in our meta-analysis, but it was observed (17).
Trejo-Avila et al. also performed a subanalysis on CRLM resection and postoperative complications. They found only a trend in association. Although we were only able to incorporate two studies into the meta-analysis, our association between sarcopenia and postoperative outcome was more prominent as compared to this previous study (26, 31).
Our analysis did not include studies that incorporated mixed populations due to their heterogenic nature in planning. This might explain this difference in one of our main findings compared to a recent meta-analysis incorporating all liver tumors (37, 45).
However, an association between sarcopenia and postoperative complications, according to Clavien–Dindo, has been reported in different resected tumors—for example, after gastrectomy (OR, 2.17 (95% CI, 1.53–3.08)) (16) or for colorectal cancer (OR, 1.82 (95% CI, 1.36–2.44)) (46). Similar patients with sarcopenia also showed limited survival in patients with pancreatic cancer (OR, 1.80 (95% CI, 1.42–2.29)) or esophageal cancer, as well as an association with higher complication rates (47).
After evaluation of the studies, we feel that the association between sarcopenia and worse postoperative outcomes after CRLM resection is clinically relevant and needs to be evaluated in prospective studies, including prehabilitation protocols prior to liver resection.
Why the association between postoperative complications and sarcopenia is so prominent in our meta-analysis can only be hypothesized. This might also be due to the fact that often multiple metastases are resected in one operation in CRLM patients. All studies that reported postoperative complications in our analysis detailed the complications, with biliary complications and bleeding complications the most prominent of the two studies that could be included in the meta-analysis (26, 31).
There is increased evidence that preoperative prehabilitation in the form of dietary supplements, protein supplementation, and exercise can improve muscle mass, function, and quantity (48). Studies showed that postoperative outcome was improved in patients who underwent resection for gastric cancer, and this is currently under prospective evaluation in these patients (49, 50). Also, chemotherapy tolerance as well as efficacy was ameliorated with the improvement of sarcopenia in recent reports (14, 47, 51).
However, our analysis has several limitations that need to be addressed. All the included studies were retrospective. Until now, no prospective analysis and follow-up of sarcopenic patients who undergo liver resection for CRLM exists. Despite the number of initially screened studies being high, only a limited number of studies could be selected for systematic review, and hence the quality of the analysis might be different in a higher study number setting. Also, the included studies were published over a relatively long period of time, between 2011 and 2023. This was also reflected in the quality of reporting of endpoints and in the quality assessment, with the studies that were reported recently showing lower risks for bias as compared to studies that had been reported earlier. All studies only measured sarcopenia using CT scans, whereas muscle performance seems to be crucial to defining real sarcopenia.
In conclusion, our meta-analysis showed that addressing sarcopenia seems to be beneficial for patients undergoing CRLM resections. A prospective study incorporating sarcopenia as muscle mass and muscle status and incorporating prehabilitation should be performed to accurately assess the value of sarcopenia in the setting of CRLM treatment with and without resection.
DW, PK, VW, HH, RS: conzeptuatlization of review. DW, MS, FF, VW: search string, literature review, study selection. DW, MS, VW: QUIPS analysis. MS, FF: data extraction. DW, MS: meta analysis. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
CRC, colorectal cancer; CRLM, colorectal liver metastases; OS, overall survival; DFS, disease-free survival.
1. Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Prz Gastroenterol (2019) 14(2):89–103. doi: 10.5114/pg.2018.81072
2. Arhin N, Ssentongo P, Taylor M, Olecki EJ, Pameijer C, Shen C, et al. Age-standardised incidence rate and epidemiology of colorectal cancer in Africa: a systematic review and meta-analysis. BMJ Open (2022) 12(1):e052376. doi: 10.1136/bmjopen-2021-052376
3. Horn SR, Stoltzfus KC, Lehrer EJ, Dawson LA, Tchelebi L, Gusani NJ, et al. Epidemiology of liver metastases. Cancer Epidemiol (2020) 67:101760. doi: 10.1016/j.canep.2020.101760
4. McNally SJ, Parks RW. Surgery for colorectal liver metastases. Dig Surg (2013) 30(4-6):337–47. doi: 10.1159/000351442
5. Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg (2006) 244(2):254–9. doi: 10.1097/01.sla.0000217629.94941.cf
6. Ma Y, Yang Y, Wang F, Zhang P, Shi C, Zou Y, et al. Obesity and risk of colorectal cancer: a systematic review of prospective studies. PloS One (2013) 8(1):e53916. doi: 10.1371/journal.pone.0053916
7. Bluher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol (2019) 15(5):288–98. doi: 10.1038/s41574-019-0176-8
8. Arroyo-Johnson C, Mincey KD. Obesity epidemiology worldwide. Gastroenterol Clin North Am (2016) 45(4):571–9. doi: 10.1016/j.gtc.2016.07.012
9. Stefan N, Haring HU, Hu FB, Schulze MB. Metabolically healthy obesity: epidemiology, mechanisms, and clinical implications. Lancet Diabetes Endocrinol (2013) 1(2):152–62. doi: 10.1016/S2213-8587(13)70062-7
10. Petrelli F, Cortellini A, Indini A, Tomasello G, Ghidini M, Nigro O, et al. Association of obesity with survival outcomes in patients with cancer: A systematic review and meta-analysis. JAMA Netw Open (2021) 4(3):e213520. doi: 10.1001/jamanetworkopen.2021.3520
11. Mintziras I, Miligkos M, Wachter S, Manoharan J, Maurer E, Bartsch DK. Reply letter to: “Response to: Sarcopenia and sarcopenic obesity are significantly associated with poorer overall survival in patients with pancreatic cancer: Systematic review and meta-analysis”. Int J Surg (2019) 66:101–2. doi: 10.1016/j.ijsu.2019.02.017
12. Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet (2019) 393(10191):2636–46. doi: 10.1016/S0140-6736(19)31138-9
13. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing (2019) 48(1):16–31. doi: 10.1093/ageing/afy169
14. Pamoukdjian F, Bouillet T, Levy V, Soussan M, Zelek L, Paillaud E. Prevalence and predictive value of pre-therapeutic sarcopenia in cancer patients: A systematic review. Clin Nutr (2018) 37(4):1101–13. doi: 10.1016/j.clnu.2017.07.010
15. Surov A, Pech M, Gessner D, Mikusko M, Fischer T, Alter M, et al. Low skeletal muscle mass is a predictor of treatment related toxicity in oncologic patients. A meta-analysis Clin Nutr (2021) 40(10):5298–310. doi: 10.1016/j.clnu.2021.08.023
16. Xie H, Wei L, Liu M, Liang Y, Yuan G, Gao S, et al. Prognostic significance of preoperative prognostic immune and nutritional index in patients with stage I-III colorectal cancer. BMC Cancer (2022) 22(1):1316. doi: 10.1186/s12885-022-10405-w
17. Trejo-Avila M, Bozada-Gutierrez K, Valenzuela-Salazar C, Herrera-Esquivel J, Moreno-Portillo M. Sarcopenia predicts worse postoperative outcomes and decreased survival rates in patients with colorectal cancer: a systematic review and meta-analysis. Int J Colorectal Dis (2021) 36(6):1077–96. doi: 10.1007/s00384-021-03839-4
18. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing (2010) 39(4):412–23. doi: 10.1093/ageing/afq034
19. Sayer AA, Cruz-Jentoft A. Sarcopenia definition, diagnosis and treatment: consensus is growing. Age Ageing (2022) 51(10). doi: 10.1093/ageing/afac220
20. Beaudart C, McCloskey E, Bruyere O, Cesari M, Rolland Y, Rizzoli R, et al. Sarcopenia in daily practice: assessment and management. BMC Geriatr (2016) 16(1):170. doi: 10.1186/s12877-016-0349-4
21. Dieli-Conwright CM, Courneya KS, Demark-Wahnefried W, Sami N, Lee K, Buchanan TA, et al. Effects of aerobic and resistance exercise on metabolic syndrome, sarcopenic obesity, and circulating biomarkers in overweight or obese survivors of breast cancer: A randomized controlled trial. J Clin Oncol (2018) 36(9):875–83. doi: 10.1200/JCO.2017.75.7526
22. Baracos VE, Arribas L. Sarcopenic obesity: hidden muscle wasting and its impact for survival and complications of cancer therapy. Ann Oncol (2018) 29(suppl_2):ii1–9. doi: 10.1093/annonc/mdx810
23. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev (2015) 4(1):1. doi: 10.1186/2046-4053-4-1
24. Hayden JA, van der Windt DA, Cartwright JL, Cote P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med (2013) 158(4):280–6. doi: 10.7326/0003-4819-158-4-201302190-00009
25. Leeflang MM. Systematic reviews and meta-analyses of diagnostic test accuracy. Clin Microbiol Infect (2014) 20(2):105–13. doi: 10.1111/1469-0691.12474
26. Bajric T, Kornprat P, Faschinger F, Werkgartner G, Mischinger HJ, Wagner D. Sarcopenia and primary tumor location influence patients outcome after liver resection for colorectal liver metastases. Eur J Surg Oncol (2022) 48(3):615–20. doi: 10.1016/j.ejso.2021.09.010
27. Eriksson S, Nilsson JH, Strandberg Holka P, Eberhard J, Keussen I, Sturesson C. The impact of neoadjuvant chemotherapy on skeletal muscle depletion and preoperative sarcopenia in patients with resectable colorectal liver metastases. HPB (Oxford) (2017) 19(4):331–7. doi: 10.1016/j.hpb.2016.11.009
28. Kobayashi A, Kaido T, Hamaguchi Y, Okumura S, Shirai H, Kamo N, et al. Impact of visceral adiposity as well as sarcopenic factors on outcomes in patients undergoing liver resection for colorectal liver metastases. World J Surg (2018) 42(4):1180–91. doi: 10.1007/s00268-017-4255-5
29. Liu YW, Lu CC, Chang CD, Lee KC, Chen HH, Yeh WS, et al. Prognostic value of sarcopenia in patients with colorectal liver metastases undergoing hepatic resection. Sci Rep (2020) 10(1):6459. doi: 10.1038/s41598-020-63644-x
30. Lodewick TM, van Nijnatten TJ, van Dam RM, van Mierlo K, Dello SA, Neumann UP, et al. Are sarcopenia, obesity and sarcopenic obesity predictive of outcome in patients with colorectal liver metastases? HPB (Oxford) (2015) 17(5):438–46. doi: 10.1111/hpb.12373
31. Peng PD, van Vledder MG, Tsai S, de Jong MC, Makary M, Ng J, et al. Sarcopenia negatively impacts short-term outcomes in patients undergoing hepatic resection for colorectal liver metastasis. HPB (Oxford) (2011) 13(7):439–46. doi: 10.1111/j.1477-2574.2011.00301.x
32. Pessia B, ROmano L, Carlei F, Lazzari S, Vicentini V, Giuliani A, et al. Preoperative sarcopenia predicts survival after hepatectomy for colorectal metastases: a prospective observational study. Eur Rev Med Pharmacol Sci (2021) 25(18):5619–24.
33. Yang YR, Shi CS, Chang SW, Wu YY, Su YL, Lin GP, et al. The impact of sarcopenia on overall survival in patients with pan-RAS wild-type colorectal liver metastasis receiving hepatectomy. Sci Rep (2023) 13(1):6911. doi: 10.1038/s41598-023-33439-x
34. van Vledder MG, Levolger S, Ayez N, Verhoef C, Tran TC, Ijzermans JN. Body composition and outcome in patients undergoing resection of colorectal liver metastases. Br J Surg (2012) 99(4):550–7. doi: 10.1002/bjs.7823
35. Runkel M, Diallo TD, Lang SA, Bamberg F, Benndorf M, Fichtner-Feigl S. The role of visceral obesity, sarcopenia and sarcopenic obesity on surgical outcomes after liver resections for colorectal metastases. World J Surg (2021) 45(7):2218–26. doi: 10.1007/s00268-021-06073-9
36. Thormann M, Omari J, Pech M, Damm R, Croner R, Perrakis A, et al. Low skeletal muscle mass and post-operative complications after surgery for liver Malignancies: a meta-analysis. Langenbecks Arch Surg (2022) 407(4):1369–79. doi: 10.1007/s00423-022-02541-5
37. O’Connell RM, O’Neill M, Ríordáin MGO, Súilleabháin CBO, O’Sullivan AW. Sarcopaenia, obesity, sarcopaenic obesity and outcomes following hepatic resection for colorectal liver metastases: a systematic review and meta-analysis. HPB (Oxford) (2022) 24(11):1844–53. doi: 10.1016/j.hpb.2022.07.003
38. Bernardi L, Roesel R, Vagelli F, Majno-Hurst P, Cristaudi A. Imaging based body composition profiling and outcomes after oncologic liver surgery. Front Oncol (2022) 12:1007771. doi: 10.3389/fonc.2022.1007771
39. Joliat GR, Kobayashi K, Hasegawa K, Thomson JE, Padbury R, Scott M, et al. Guidelines for perioperative care for liver surgery: enhanced recovery after surgery (ERAS) society recommendations 2022. World J Surg (2023) 47(1):11–34. doi: 10.1007/s00268-022-06732-5
40. Chen F, Chi J, Liu Y, Fan L, Hu K. Impact of preoperative sarcopenia on postoperative complications and prognosis of gastric cancer resection: A meta-analysis of cohort studies. Arch Gerontol Geriatr (2022) 98:104534. doi: 10.1016/j.archger.2021.104534
41. Chianca V, Albano D, Messina C, Gitto S, Ruffo G, Guarino S, et al. Sarcopenia: imaging assessment and clinical application. Abdom Radiol (NY) (2022) 47(9):3205–16.
42. Williams DGA, Molinger J, Wischmeyer PE. The malnourished surgery patient: a silent epidemic in perioperative outcomes? Curr Opin Anaesthesiol (2019) 32(3):405–11. doi: 10.1097/ACO.0000000000000722
43. Bozzetti F. Chemotherapy-induced sarcopenia. Curr Treat Options Oncol (2020) 21(1):7. doi: 10.1007/s11864-019-0691-9
44. Levolger S, van Vledder MG, Muslem R, Koek M, Niessen WJ, de Man RA, et al. Sarcopenia impairs survival in patients with potentially curable hepatocellular carcinoma. J Surg Oncol (2015) 112(2):208–13. doi: 10.1002/jso.23976
45. Waalboer RB, Meyer YM, Galjart B, Olthof PB, van Vugt JLA, Grunhagen DJ, et al. Sarcopenia and long-term survival outcomes after local therapy for colorectal liver metastasis: a meta-analysis. HPB (Oxford) (2022) 24(1):9–16. doi: 10.1016/j.hpb.2021.08.947
46. Kelly KN, Iannuzzi JC, Rickles AS, Monson JR, Fleming FJ. Risk factors associated with 30-day postoperative readmissions in major gastrointestinal resections. J Gastrointest Surg (2014) 18(1):35–43; discussion -4. doi: 10.1007/s11605-013-2354-7
47. Capurso G, Pecorelli N, Burini A, Orsi G, Palumbo D, Macchini M, et al. The impact of nutritional status on pancreatic cancer therapy. Expert Rev Anticancer Ther (2022) 22(2):155–67. doi: 10.1080/14737140.2022.2026771
48. Lee JA, Young S, O’Connor V, DiFronzo LA. Safety and efficacy of an enhanced recovery protocol after hepatic resection. Am Surg (2020) 86(10):1396–400. doi: 10.1177/0003134820964492
49. Wada Y, Nishi M, Yoshikawa K, Takasu C, Tokunaga T, Nakao T, et al. Preoperative nutrition and exercise intervention in frailty patients with gastric cancer undergoing gastrectomy. Int J Clin Oncol (2022) 27(9):1421–7. doi: 10.1007/s10147-022-02202-z
50. Tully R, Loughney L, Bolger J, Sorensen J, McAnena O, Collins CG, et al. The effect of a pre- and post-operative exercise programme versus standard care on physical fitness of patients with oesophageal and gastric cancer undergoing neoadjuvant treatment prior to surgery (The PERIOP-OG Trial): Study protocol for a randomised controlled trial. Trials (2020) 21(1):638. doi: 10.1186/s13063-020-04311-4
Keywords: colorectal liver metastases, colorectal cancer, liver metastases, overall survival, disease free survival, sarcopenia
Citation: Wagner D, Wienerroither V, Scherrer M, Thalhammer M, Faschinger F, Lederer A, Hau HM, Sucher R and Kornprat P (2023) Value of sarcopenia in the resection of colorectal liver metastases—a systematic review and meta-analysis. Front. Oncol. 13:1241561. doi: 10.3389/fonc.2023.1241561
Received: 16 June 2023; Accepted: 07 August 2023;
Published: 26 September 2023.
Edited by:
Beatrice Aramini, University of Bologna, ItalyCopyright © 2023 Wagner, Wienerroither, Scherrer, Thalhammer, Faschinger, Lederer, Hau, Sucher and Kornprat. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: D. Wagner, RG9yaXMud2FnbmVyQG1lZHVuaWdyYXouYXQ=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.