- 1Center for Observational and Real-World Evidence (CORE), Merck & Co., Inc., Rahway, NJ, United States
- 2Center for Head & Neck Oncology, Dana-Farber Cancer Institute, Boston, MA, United States
by Black CM, Hanna GJ, Wang L, Ramakrishnan K, Goto D, Turzhitsky V and Hair GM (2023) Front. Oncol. 13:1160144. doi: 10.3389/fonc.2023.1160144
Error in Figure/Table
In the published article, there was an error in Table 1 as published. The mean (SD) for the 1L pembrolizumab + chemotherapy column for CPS<1 should be 18 (16.7), not 18 (6.7) as originally shown. Further, we used the terminology, CPS<1% in the first column and the correct terminology is CPS<1. The corrected Table 1 and its caption appear below.
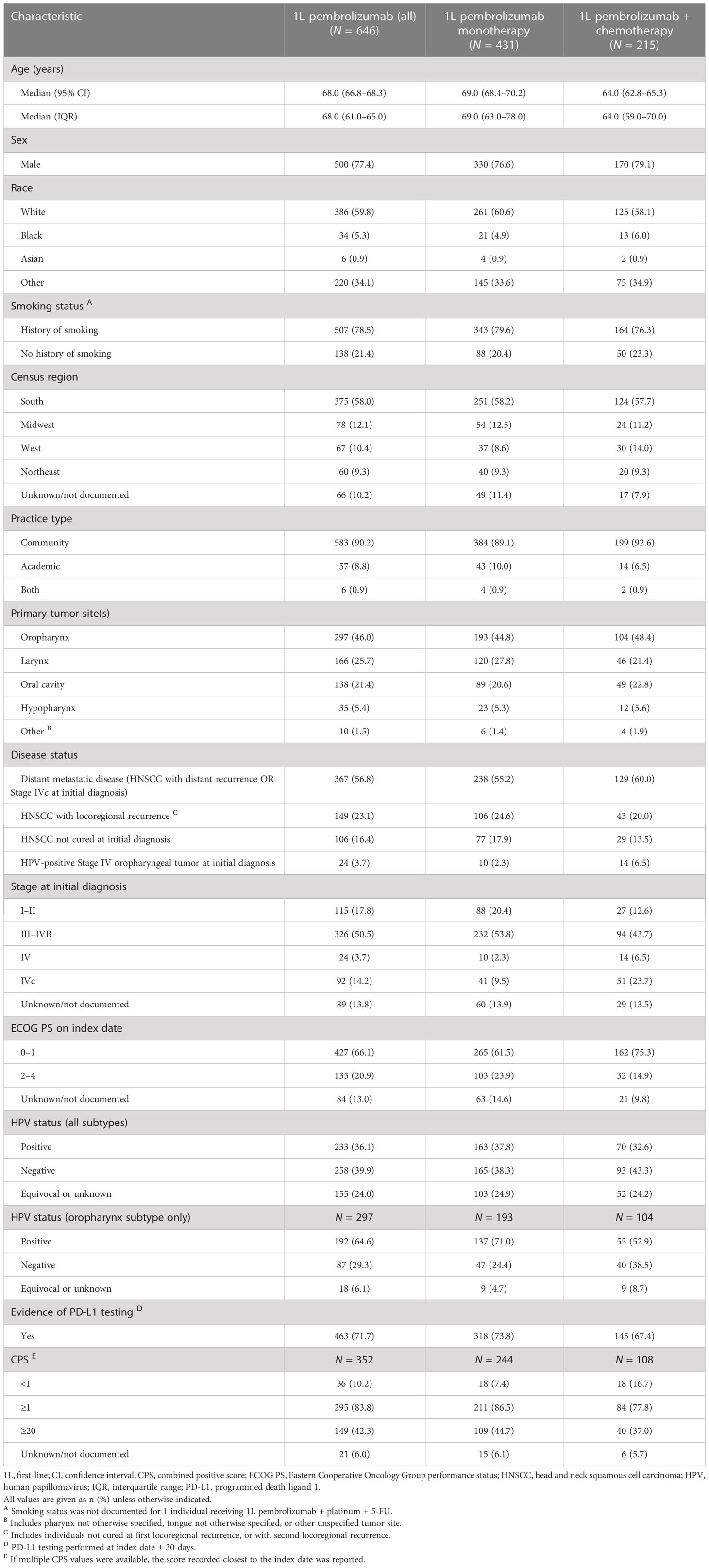
Table 1 Baseline demographic and clinical characteristics of individuals receiving 1L pembrolizumab monotherapy or pembrolizumab plus chemotherapy.
In the published article, there was an error in Table 2 as published. We used the terminology CPS (referent: <1%) in column one and the correct terminology is CPS (referent: <1). The corrected Table 2 and its caption appear below.
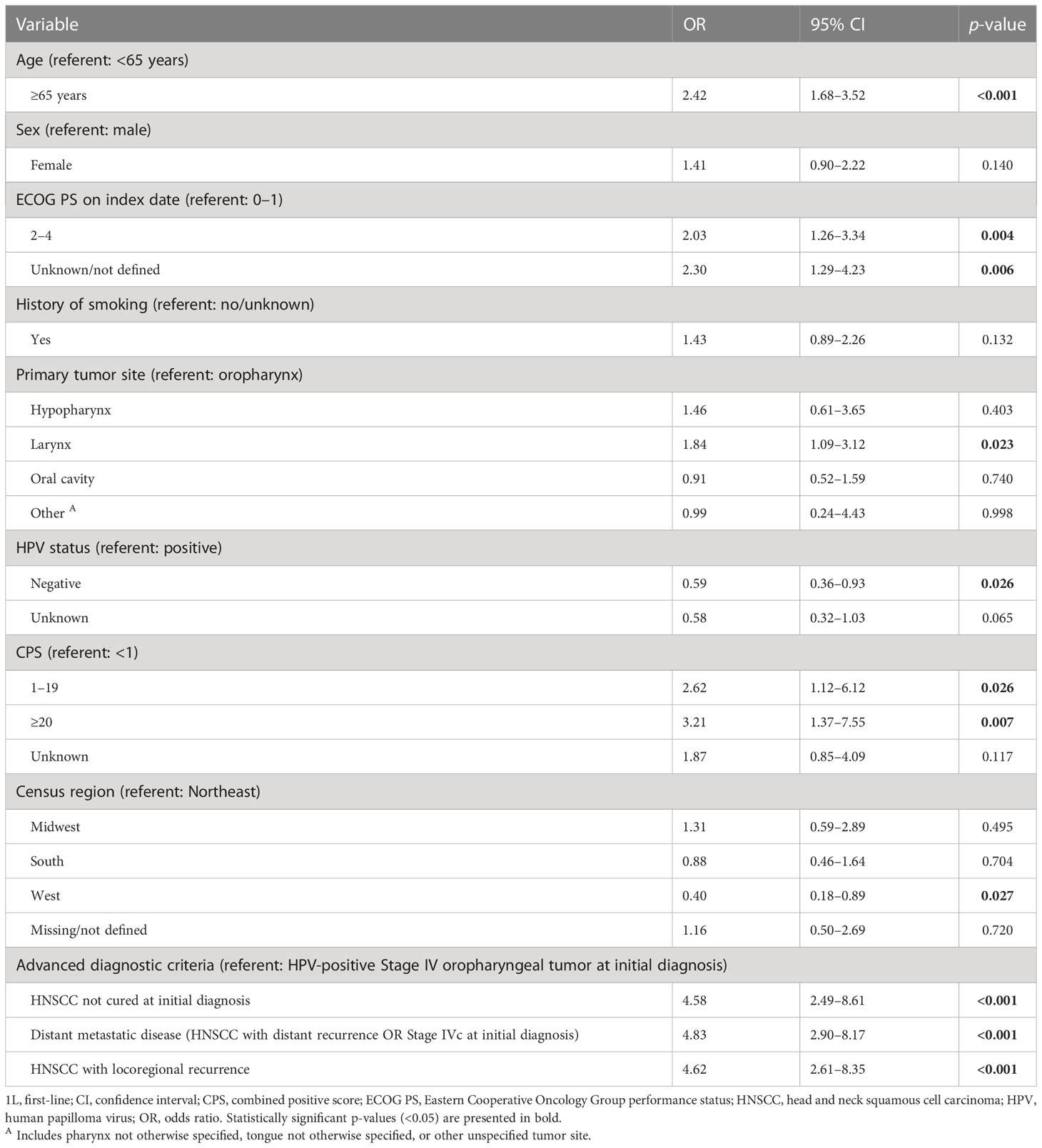
Table 2 Factors associated with use of 1L pembrolizumab monotherapy versus pembrolizumab plus chemotherapy: stepwise logistic regression model.
Text Correction
In the published article, there was an error to the text in Results, Study population, where "CPS<1" was referred to as "CPS<1%".
This sentence previously stated: “A CPS of <1% was recorded for 10.2% of this latter group, while 83.8% had a score of ≥1 and 42.3% had a score of ≥20.”
The corrected sentence appears below:
“A CPS of <1 was recorded for 10.2% of this latter group, while 83.8% had a score of ≥1 and 42.3% had a score of ≥20.”
The authors apologize for these errors and state that these does not change the scientific conclusions of the article in any way. The original article has been updated.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: head and neck squamous cell carcinoma, antineoplastic agents, immunological, antibodies, Kaplan-Meier estimate, patient outcomes, real-world observational study, treatment patterns
Citation: Black CM, Hanna GJ, Wang L, Ramakrishnan K, Goto D, Turzhitsky V and Hair GM (2023) Corrigendum: Real-world treatment patterns and outcomes among individuals receiving first-line pembrolizumab therapy for recurrent/metastatic head and neck squamous cell carcinoma. Front. Oncol. 13:1240947. doi: 10.3389/fonc.2023.1240947
Received: 17 June 2023; Accepted: 30 June 2023;
Published: 17 July 2023.
Edited and Reviewed by:
Fernanda Visioli, Federal University of Rio Grande do Sul, BrazilCopyright © 2023 Black, Hanna, Wang, Ramakrishnan, Goto, Turzhitsky and Hair. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christopher M. Black, Q2hyaXN0b3BoZXIuYmxhY2syQG1lcmNrLmNvbQ==
 Christopher M. Black
Christopher M. Black Glenn J. Hanna2
Glenn J. Hanna2