
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol. , 27 September 2023
Sec. Genitourinary Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1240864
Purpose: Metastatic castration-resistant prostate cancer (mCRPC) is a lethal disease that imposes a major burden on patients and healthcare systems. Three structured literature reviews (treatment guidelines, treatment landscape, and human/clinical/patient burden) and one systematic literature review (economic burden) were conducted to better understand the disease burden and unmet needs for patients with late-stage mCRPC, for whom optimal treatment options are unclear.
Methods: Embase®, MEDLINE®, MEDLINE® In-Process, the CENTRAL database (structured and systematic reviews), and the Centre for Reviews and Dissemination database (systematic review only) were searched for English-language records from 2009 to 2021 to identify mCRPC treatment guidelines and studies related to the treatment landscape and the humanistic/economic burden of mCRPC in adult men (aged ≥18 years) of any ethnicity.
Results: In total, six records were included for the treatment patterns review, 14 records for humanistic burden, nine records for economic burden, three records (two studies) for efficacy, and eight records for safety. Real-world treatment patterns were broadly aligned with treatment guidelines and provided no optimal treatment sequencing beyond second line other than palliative care. Current post-docetaxel treatments in mCRPC are associated with adverse events that cause relatively high rates of treatment discontinuation or disruption. The humanistic and economic burdens associated with mCRPC are high.
Conclusion: The findings highlight a lack of treatment options with novel mechanisms of action and more tolerable safety profiles that satisfy a risk-to-benefit ratio aligned with patient needs and preferences for patients with late-stage mCRPC. Treatment approaches that improve survival and health-related quality of life are needed, ideally while simultaneously reducing costs and healthcare resource utilization.
Prostate cancer is the most commonly diagnosed cancer among men in developed countries and the second most commonly diagnosed cancer worldwide. Incidence rates are threefold higher in countries with a high human development index score than in those with a medium-to-low score (1–3). Prostate cancer accounted for approximately 6% of all cancer deaths in the United States (US) between 2012 and 2018 (4). Almost three-quarters of cases (73%) were diagnosed with localized (stage I–II) disease; 14% and 7% had regional spread (stage III) and distant metastases (stage IV) at diagnosis, respectively (4). In 2019, 29.2% of patients in England were diagnosed with stage I disease, with 12.9% having stage II, 21.2% having stage III, and 15.7% having stage IV (the stage was reported as “unknown” in 21%) (5). Despite being curable if diagnosed at an early, localized stage, metastatic prostate cancer is still incurable and will inevitably progress to become castration-resistant (6, 7).
Metastatic castration-resistant prostate cancer (mCRPC) is clinically challenging and lethal, with no curative treatment options (8, 9). The 5-year relative survival rate for distant metastatic prostate cancer in the US is currently 32.3% (4). In the United Kingdom (UK), the 5-year survival rate for stage IV prostate cancer is 49%, accounting for 14% of all cancer deaths in men (10). Beyond survival, health-related quality of life (HRQoL) and pain are highly relevant to patients with mCRPC (11). Up to 90% of patients have bone metastases, which are a clinically significant cause of morbidity that often result in severe bone pain, either directly due to metastatic disease or indirectly as a result of symptomatic skeletal events (SSEs) such as fracture and spinal-cord compression (2, 8).
Therapies for mCRPC have evolved greatly over the past decades. In addition to the introduction of androgen receptor pathway inhibitors (ARPIs) in both post-chemotherapy and chemotherapy-naïve settings, asymptomatic and minimally symptomatic patients can undergo treatment with the immunotherapy sipuleucel-T (US only) (12). While ARPIs have improved outcomes in mCRPC, primary or acquired resistance to these agents will ultimately develop in the majority of patients, limiting their effectiveness (7, 13). Docetaxel and cabazitaxel are options for chemotherapy, with a proven survival benefit in the mCRPC setting, and radium (Ra)-223 is a radiopharmaceutical approved for treatment of patients with symptomatic bone metastases and without visceral metastases (12, 14–17).
Recently, the poly (ADP-ribose) polymerase (PARP) inhibitors olaparib and rucaparib were approved as monotherapies in the US, and olaparib also in Europe for patients with mCRPC harboring germline or somatic mutations in DNA damage–repair (DDR) genes (18–21). Olaparib has also been approved by the FDA and EMA in combination with abiraterone for patients with prostate cancer and homologous recombination repair (HHR) mutations, especially BRCA2 (18, 20). In addition, the programmed death receptor 1 inhibitor pembrolizumab is approved in the US for patients with microsatellite instability-high tumors or genetic mutations consistent with Lynch syndrome (21, 22). These advances provide options for select groups of patients, and not all who are treated will benefit (23, 24). Established lines of evidence-based treatments beyond ARPIs and taxane chemotherapy in mCRPC are of limited clinical effectiveness. As such, recommendations in guidelines focus on first-line (1L) and second-line (2L) therapies, with few guidelines existing for treatments in the third-line (3L) setting and beyond (≥3L).
Combinations of ARPIs and PARP inhibitors include olaparib plus abiraterone (PROPEL) (25, 26), enzalutamide plus talazoparib (TALOPRO-2) (27, 28), niraparib plus abiraterone acetate (hereafter “abiraterone”; MAGNITUDE) (29), and enzalutamide with rucaparib (RAMP) (30). All combinations have shown associated benefits of median radiological progression-free survival (rPFS) in patients with mCRPC who harbor HHR mutations – the most frequently altered DDR genes in prostate cancer.
The purpose of this literature review was twofold: to reflect on the current treatment landscape for prostate cancer in the metastatic and castration-resistant setting, and to consider the economic burden of mCRPC. We aimed to identify the unmet needs for patients with late-stage mCRPC and explore the emerging treatment landscape.
Three separate structured literature reviews and one systematic literature review (one original and two updates) were conducted to identify publications relating to treatment guidelines, the treatment landscape, and the humanistic and economic burden of mCRPC.
Embase®, MEDLINE®, MEDLINE® In-Process, and CENTRAL databases were searched for the structured literature reviews; searches took place on June 28, 2019, and included records published between 2009 and 2019 (Supplemental Tables S1–S3). Embase®, MEDLINE®, MEDLINE® In-Process, and the Centre for Reviews and Dissemination database were searched for the systematic literature review for records from 2009 to 2019 using targeted keyword searches for each review; searches took place on September 16, 2019. To supplement the database searches: conference abstracts were searched for the period 2017–2019 from the American Society for Clinical Oncology (ASCO) and European Society for Medical Oncology (ESMO) for all of the reviews; abstracts from the Professional Society for Health Economics and Outcomes Research (ISPOR) and ISPOR Europe conferences were also searched for the economic burden systematic literature review. The National Institute for Health and Care Excellence (NICE) website was searched for the economic burden review. The bibliographies of included systematic reviews/meta-analyses were searched to identify potentially missing studies. Any data gaps were addressed via general internet searches (e.g., Google Scholar).
Updated searches were conducted using MEDLINE®, MEDLINE® In-Process, Embase®, The Health Technology Assessment (HTA) database, CENTRAL database, the Database of Abstracts of Reviews of Effects (DARE), and the Cochrane Database of Systematic Reviews. EconLit was also searched for the economic and HRQoL reviews. The NICE and Scottish Medicines Consortium (SMC) websites, as well as conference proceedings of ASCO, ESMO, and ISPOR, were searched. Further details are provided in the supplementary methods.
Embase®, MEDLINE®, MEDLINE® In-Process, and CENTRAL databases were searched for the systematic literature reviews; searches took place on April 6, 2021 (for clinical, economic, and HRQoL records), June 21, 2021 (for HRQoL records), and November 3, 2021 (Supplemental Tables S4–S9).
The population of interest was adult men (≥18 years of age) of any ethnicity with mCRPC. Only studies published in English were included. Although the economic systematic review was not restricted by geographic location (Supplemental Table S9), the following inclusion criteria were applied for the purposes of the present report: studies conducted in France, Germany, Italy, Spain, the UK, or the US in the previous 5 years (2017–2021); 2L therapy and beyond (≥2L) only; and studies with more than 100 participants (if reported) with data on total costs and/or incremental cost-effectiveness ratios (ICERs) or quality-adjusted life years (QALYs) (Supplemental Tables S4–S9). For the updated interventional (efficacy) systematic reviews (clinical), only approved/recommended therapies were included in this manuscript and only phase 3 trials were included in the search criteria. Full details of all inclusion/exclusion criteria for all of the searches are provided in the supplemental information.
For the structured literature review, first screening (titles and abstracts) and second screening (full text) were undertaken by a single reviewer followed by a quality check by a second independent reviewer (Supplemental Figure S1). Data were extracted by a single reviewer and verified by an independent reviewer. Any discrepancies between the two reviewers for both screening and data extraction were resolved by a third independent reviewer.
For the systematic literature reviews, first screening, second screening, and data extraction were conducted using a two-review process, whereby two independent reviewers performed the screening/data extraction and any discrepancies were resolved by a third reviewer (Supplemental Figure S1). The process was conducted in line with the requirements of NICE and in accordance with methodology established in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (31).
Quality assessments of the relevant randomized controlled trials and cost-effectiveness and HRQoL studies that were published in full-text were conducted using validated quality assessment tools (32–35).
In the initial structured review, a total of 130 records were included in the treatment landscape review, of which 37 reported treatment patterns for mCRPC in a real-world scenario and 25 reported safety outcomes, including two systematic reviews (Supplemental Figure S2). The searches and screens for each systematic review are described in Supplementary Figures S3–S5.
The searches and screens for each systematic review detailed in Supplemental Figure S2 yielded the following:
Interventional (efficacy) systematic literature review (Supplemental Figure S3)
1. A total of 9099 records were identified from the database
2. After screening by title/abstract, 1412 records were selected for full-text review
3. A total of 26 records from 20 original studies were selected for data extraction
4. A final total of three records (two original studies) were included in this manuscript
HRQoL systematic literature review (Supplemental Figure S4)
1. A total of 1767 records were identified using the Ovid platform
2. After screening by title/abstract, 294 records were selected for full-text review
3. A total of 98 records from 96 original studies were selected for data extraction
4. A final total of 14 records were included in the manuscript
Economic systematic literature review (Supplemental Figure S5)
1. A total of 3271 records were identified for economic evaluations from the database search
2. After screening by title/abstract, 456 records were selected for full-text review
3. A total of 74 records from 74 original studies were selected for data extraction
4. A final total of nine records were included in the manuscript
Based on the quality assessments that were conducted, no papers were excluded from the review. All papers were considered to be of appropriate quality for inclusion.
Six publications were included (two global, three US, and one Japan). Updated systematic searches were not conducted for treatment patterns (Table 1). Considering the limitations in these data, ARPIs, docetaxel, and Ra-223 were commonly prescribed as 1L and 2L treatments, whereas sipuleucel-T was prescribed at 2L only. In the US, abiraterone was the most frequent 1L treatment. At 2L, in Japan, enzalutamide was more frequently prescribed than abiraterone. Among the few patients who received 3L treatment, the most commonly prescribed agents were luteinizing hormone-releasing hormone (LHRH) agonists/antagonists, abiraterone, enzalutamide, docetaxel, and Ra-223. There was limited information about 1L treatment options. Across studies carried out in North America, Latin America, Europe, and Japan, the most commonly used therapies were ARPIs (6.2%–49.6%) (42–44), docetaxel (16.4%–26.9%) (42–44), sipuleucel-T (9.2%–18.6%) (42, 43), and Ra-223 (1.7%–2.7%) (42, 43). Cabazitaxel was used in <2.5% of patients (42–44). In the US, abiraterone was the most prevalent 1L treatment (42.5%–50.5%), followed by docetaxel (16.4%–26.9%), sipuleucel-T (9.2%–18.6%), and enzalutamide (10.3%–18.4%) (42, 43). In Japan, LHRH agonists/antagonists were prescribed in 43.6% of patients in the 1L setting, with antiandrogens, docetaxel, and steroids being given in 26.9%, 19.2%, and 18.7% of cases, respectively (44).
The most common 2L treatments were ARPIs (7.8%–37.7%), followed by docetaxel (13.2%–21.8%) and Ra-223 (1.5%–2.3%) (40, 42–44). Use of cabazitaxel was limited in the 2L setting (<3.5%) (42–44). In the US, ARPIs were the predominant 2L therapy, the most frequently prescribed being abiraterone (35.3%–37.7%) followed by enzalutamide (14.9%–22.6%) (42, 43). In one US study that included only patients who had received 1L docetaxel, 80.4% received an ARPI as a 2L treatment and 19.6% received cabazitaxel (41). Undertreatment in the ≥3L setting was reported in 56.1%–72.1% of patients in a US cohort (41). One global study noted that chemotherapy was more frequently prescribed as a 2L option in Latin America and in other countries, including Algeria, Iran, Pakistan, Saudi Arabia, Turkey, and United Arab Emirates (56.8% and 52.3%, respectively), compared with Europe (27.1%), as was combination chemotherapy and hormonal therapy (20.0%–20.9% vs. 6.6%); however, targeted therapy was more frequently prescribed in Europe (10.7% vs. 0.6%–1.7% in other countries) (36). In Japan, enzalutamide was a more popular choice (26.0%) than abiraterone (10.0%), and 24.2% of patients received antiandrogens, although the most common 2L therapy was LHRH (46.6%) (44). There appears to be no consensus regarding treatment sequencing.
A large proportion of patients do not receive treatment beyond 2L (69%–91%) (36, 41), which may explain the dearth of studies in this setting. Among those who receive 3L treatment, the most commonly prescribed agents are LHRH agonists/antagonists (41.2%), abiraterone (18.9%–33.7%), enzalutamide (13.6%–30.4%), docetaxel (12.2%–26.4%), and Ra-223 (2.2%–2.5%) (42, 43). A global study reported that the most frequent 3L treatment was hormonal therapies (50.6%), chemotherapy (32.1%), and palliative radiotherapy (18.5%) (36). Only one study, out of Japan, reported the use of LHRH agonists/antagonists, most frequently prescribed at 3L, followed by enzalutamide (30.4%) and docetaxel (26.4%) (44). In the US, abiraterone is the most common 3L treatment (21.2%–33.7%), followed by enzalutamide (13.6%–21.2%) and docetaxel (12.2%–17.3%); rates for all other named treatment options were <7% (42, 43). As with 1L and 2L, use of cabazitaxel is limited in the 3L setting globally.
Most pivotal phase 3 trials for currently approved and prescribed treatments (e.g., prostate-specific membrane antigen radioligand therapy [177Lu-PSMA-617], rucaparib, Ra-223, pembrolizumab) were published outside the scope of this systematic review (which only included papers published between 2017 and 2021). The clinical trials included in this review are the PROfound trial of olaparib versus enzalutamide plus abiraterone (two papers) and the CARD trial of cabazitaxel versus enzalutamide or abiraterone plus prednisone (Table 2).
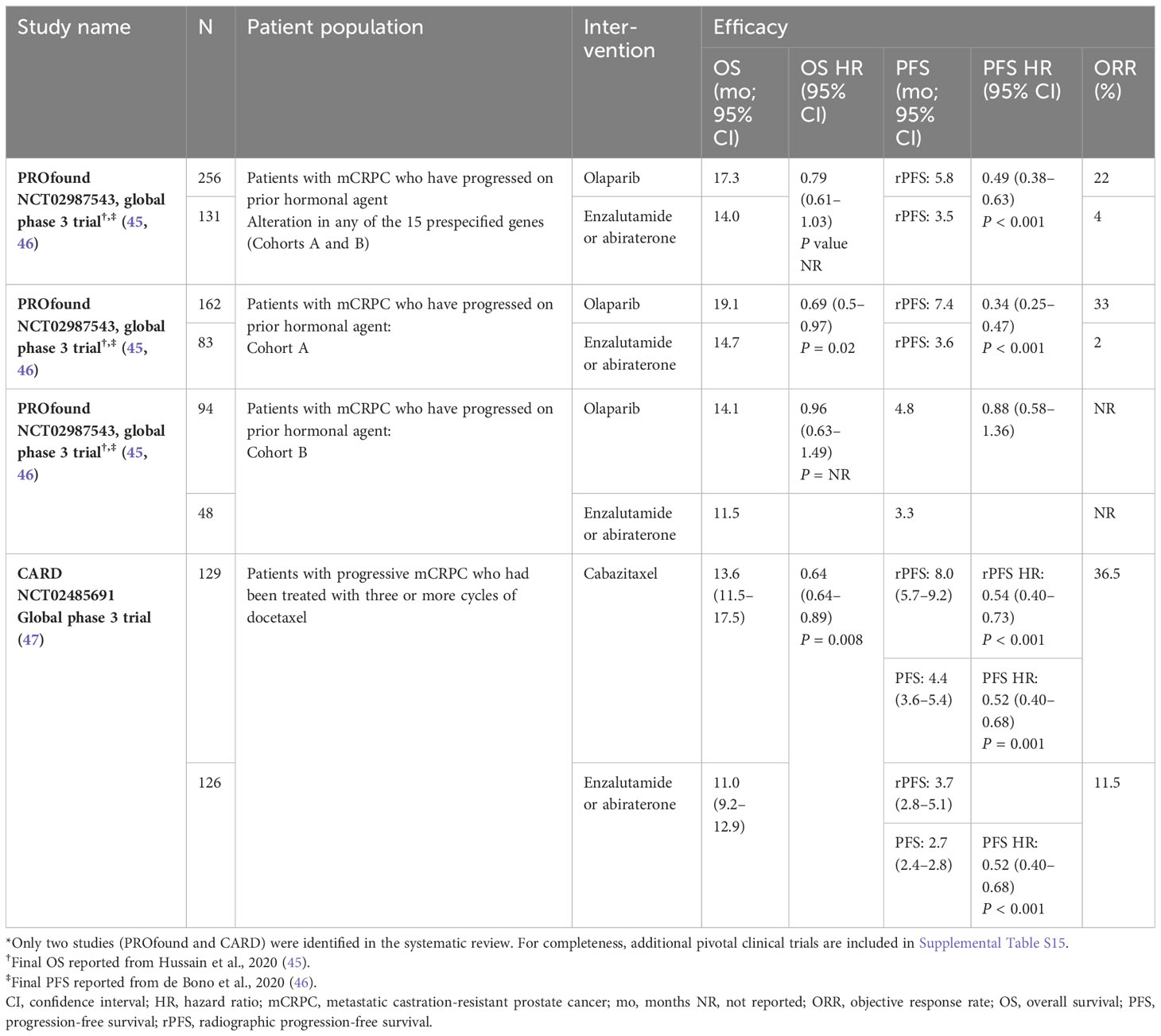
Table 2 Summary of efficacy of approved treatments for mCRPC (only studies identified in systematic review*).
Median overall survival (OS) of patients treated with abiraterone or enzalutamide ranged from 11.0 to 14.7 months (45–47). Median radiographic progression free survival (rPFS) or progression-free survival (PFS) of patients treated with abiraterone or enzalutamide ranged from 2.7 to 3.7 months (45–47). Median OS of patients treated with olaparib was 14.1–19.1 months, and median rPFS was 5.8–7.4 months (45, 46). Patients treated with cabazitaxel had median OS and rPFS durations of 13.6 and 8.0 months, respectively (47). The patient populations differed between these two trials: the PROfound study enrolled patients with mCRPC who had qualifying alterations in homologous recombination repair (HRR) genes and who had progressed after treatment with an ARPI (45), whereas patients in the CARD trial had been previously treated with docetaxel and an ARPI (47).
This report includes six real-world evidence studies from the structured review and three papers reporting two original clinical trials from the interventional systematic review (Table 3; Supplemental Table S14). Safety evidence from these studies is summarized below; however, due to the heterogeneity between study designs and patient populations, data must be interpreted with caution.

Table 3 Summary of included studies assessing safety of current approved/recommended mCRPC treatments.
The most commonly reported grade ≥3 treatment-emergent adverse events (TEAEs) experienced by patients treated with cabazitaxel (all previously treated with docetaxel) were hematological, comprising neutropenia (including febrile neutropenia), anemia, leukopenia, and thrombocytopenia. Diarrhea, vomiting, asthenia, renal failure, and septicemia/septic shock were also reported in real-world settings (49–51, 53). In a clinical trial in patients who had been pretreated with docetaxel and had progressed on an ARPI (47), additional common TEAEs of infection and musculoskeletal pain or discomfort were reported (Table 3) (49, 51, 53).
The most common toxicities of special interest in a Spanish real-world setting were fatigue, edema, hypertension, diarrhea, and vomiting (50). In elderly Italian patients treated with abiraterone, a grade 3 TEAE of liver toxicity was also reported in 2% of patients (Table 3) (52). In clinical trials of treatment with enzalutamide or abiraterone, the most commonly reported TEAEs were musculoskeletal pain or discomfort, fatigue or asthenia, infection, nausea, vomiting, and decreased appetite (Table 3) (45, 47). As expected, in the CARD trial there were no reports of febrile neutropenia with 2L ARPI treatment, compared with 3.2% of patients receiving 2L cabazitaxel (47).
Hematological and gastrointestinal TEAEs were the most frequently reported toxicities in patients who had received prior chemotherapy. Anemia was the most common grade 3/4 TEAE, and was four times more common in patients who had received prior chemotherapy compared with those who had not (8% vs. 2%) (37). In addition, grade 3/4 thrombocytopenia occurred at double the rate in patients who had previously received chemotherapy versus those who had not (4% vs. 2%).
Olaparib was associated with more grade ≥3 TEAEs and fewer serious adverse events (SAEs) than enzalutamide or abiraterone (Table 3). In the global randomized phase 3 PROfound trial of olaparib in patients with mCRPC who had progressed on an ARPI, the most common TEAEs were anemia, nausea, and fatigue or asthenia (45).
Studies reporting the impact of intervention included real-world, observational, prospective, retrospective, and cross-sectional studies, as well as surveys and randomized controlled trials. Across the included studies, HRQoL was assessed using multiple patient-reported outcome (PRO) measures. The application of additional inclusion criteria for this manuscript, limiting inclusion to full papers published in the last 5 years and with a study population of more than 100 receiving ≥2L therapy, yielded 14 papers reporting HRQoL endpoints (Table 4; Supplemental Figure 2), including the EuroQol five-dimension questionnaire (EQ-5D; five papers), Functional Assessment of Cancer Therapy-General/Prostate questionnaire (FACT-G/P; 11 papers), Brief Pain Inventory-Short Form questionnaire (BPI-SF; four papers), Short Form-36 questionnaire (SF-36; one paper), Brief Fatigue Inventory (BFI; two papers), Australian Quality of Life-8 dimension questionnaire (AQoL-8D; one paper), and European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ; one paper) (Table 4).
There were no clear trends for associations between improvement in HRQoL and mCRPC treatment. EQ-5D was either maintained or improved with treatment among populations that were diverse with respect to prior treatment history, irrespective of the treatment type (cabazitaxel, abiraterone, enzalutamide, docetaxel) (Table 4) (49, 55–58). Two studies demonstrated clinically important improvements or minimally important differences (change in visual analog scale score of ±7 points from baseline) after treatment with 2L cabazitaxel or 1L or 2L enzalutamide (49, 58).
Overall, HRQoL, as measured using FACT-G/P, was found to be either maintained or improved by treatments (including Ra-223, cabazitaxel, abiraterone, enzalutamide, docetaxel) in 55%–80% of patients (Table 4) (49, 58, 59, 61, 64, 65). Clinically relevant improvements in scores of FACT-P or its subscales, including physical well-being, social well-being, emotional well-being, functional well-being, and prostate-specific concerns, were also observed in some studies (Table 4) (58, 60, 65). There was some variation in the definitions of these improvements between the studies (ranging from ≥6 points to ≥16 points for FACT-P); the recommended range for clinically meaningful change is 6 to 10 points (67). For example, one registry-based study of heavily pretreated patients (including abiraterone and/or enzalutamide, docetaxel, cabazitaxel, or radiotherapy) with mCRPC noted a clinically meaningful improvement in FACT-P total score, defined as a change of 10 points from baseline in 31.4% of all patients and in 37.7% and 26.7% of those with and without pain at baseline, respectively, after treatment with Ra-223 (60). Another registry study of docetaxel-pretreated patients who received 2L therapies in Switzerland reported a low rate of improvement in the FACT-P total score of 23%, which they attributed potentially to the rather high threshold definition of a “clinically meaningful change” of ≥16 points (64). In yet another study evaluating patients from the PROSELICA (2L) and FIRSTANA (1L) trials, the threshold for a “definitive improvement from baseline” was ≥7 (65). In addition to different thresholds for clinically relevant findings, interpretation of the data should take into consideration the size, and the disease and treatment characteristics of the patient populations, as well as the widely varied treatments provided between these studies. SSEs are reported to have a substantial and negative impact on the HRQoL of patients with bone-metastatic mCRPC, with lower FACT-P functional well-being scores compared with non-SSE cohorts (Table 4) (2, 62).
Data for BPI-SF varied across studies, again likely due to differences between studies with regard to the patient population and design (Table 4). In a registry study of patients scheduled for Ra-223 treatment, 31.4% achieved a complete pain response (defined as a score of 0 on the BPI-SF “worst pain” item and no increase in daily use of analgesics). However, a clinically meaningful improvement in BPI-SF worst pain during treatment (defined as ≥30% change from baseline) was achieved by 49.5% of patients overall, and by 77.7% of those with pain at baseline (60). In the UK EXTREQOL trial, 40%–43% and 58%–65% of patients with moderate/severe pain at baseline saw clinically meaningful improvements (defined as a ≥2 point change in BPI-SF from baseline) in pain severity and interference, respectively, during systemic treatment for mCRPC (59). Findings were similar for the European PREMISE trial (observational study of enzalutamide in mCRPC), in which the same threshold for clinically meaningful change was used for pain severity, while that for pain interference was 1.25 (58). The UK EXTREQOL was the only study to use EORTC QLQ as a tool to assess humanistic burden. After 6 months of treatment, the mean change in global score from baseline was –1.01 (95% confidence interval [CI], –3.57 to 1.57; P = 0.44) (59).
Of the 456 full-text papers screened, 74 were included from 74 original studies, comprising 20 cost-effectiveness analysis (CEA)/cost-utility analysis studies, two budget impact models (BIMs), 15 HTA reports, and 37 studies reporting costs/healthcare resource use (HCRU). A total of nine records were finally included in this manuscript: eight papers and one HTA (Supplemental Figure S5; Tables 5, 6).
Most (n=6) of the papers were from the US perspective. Seven publications reported on the costs associated with mCRPC, including three HCRU papers and four CEA studies (Table 5), and six publications reported economic evaluations in mCRPC (Table 6). There was one BIM and one HTA that reported an economic evaluation in mCRPC from a UK (Scottish) perspective (Table 6).
Three HCRU studies, one observational study from each of Italy and Germany, one retrospective study from the US, and four CEA studies, all from the US perspective, were included, comparing the cost of abiraterone, enzalutamide, olaparib, best supportive care, docetaxel, and cabazitaxel (Table 5). Reflective of real clinical practice, the analysis by Restelli et al. (70) in Italy demonstrated the high economic cost of mCRPC. Annual direct medical costs ranged from €196.5 million to €228.0 million, representing ~0.2% of the financing of the Italian National Health Service in 2016 (70). Overall, the annual cost of 1L treatment in Italy is more than twice that for 2L at €136.9–160.3 million versus €59.7–67.8 million. In large part, this difference is probably reflecting the drop in patient numbers receiving 2L treatment (6497 vs. 3203) (70). Few studies have reported the cost of genetic testing. However Su D et al. (2020) reported a cost of $5800 for next-generation sequencing (73).
The total cost of mCRPC is also high in the US, as reported by cost-effectiveness modeling studies, with costs differing by treatment regimen and tumor characteristics. Costs for patients treated with olaparib, enzalutamide, or abiraterone increased substantially in the subgroup of patients with a tumor harboring at least one of three prespecified gene alterations (BRCA1, BRCA2, or ATM) versus those with at least one of 15 prespecified gene alterations (Table 5). This outcome was thought to be at least in part attributable to the greater number of patients that would need to be screened (and thus greater cost of screening) if only three specific gene alterations are sought compared with if any of 15 gene alterations are sought to identify patients eligible for treatment (73). In addition, since OS in patients with alterations in genes other than BRCA1, BRCA2, or ATM was comparable between those treated with olaparib versus standard of care (46), it could be that treating these patients with the relatively lower-cost olaparib would require fewer health resources toward similar outcomes (73).
The available data from direct comparisons have demonstrated that olaparib is generally more costly than abiraterone/enzalutamide, particularly when patients are screened only for mutations in BRCA1, BRCA2, or ATM (Table 5) (73, 74). Findings across studies indicate that the costs of treatment with enzalutamide and abiraterone are similar, and are lower than for cabazitaxel (69, 71, 72). Among these three treatment options, one study in the US concluded that enzalutamide was the preferred treatment option from a clinical and healthcare payer perspective. Over a lifetime (5-year horizon model), enzalutamide provided better life-years and QALY outcomes than either abiraterone + prednisone or cabazitaxel + prednisone at a lower cost than for abiraterone and higher than for cabazitaxel (US$109,213 vs. US$115,433 and US$85,377, respectively) in patients with post-docetaxel therapy resistance (71). Limitations of these findings include the different study designs, that all but two of the studies were conducted in the US, and that they were obtained in different patient populations with respect to sample number – either not reported (71, 72, 74) or ranging from 387 to 9700 (69, 70, 73) – and prior treatment history.
Six economic studies reporting ICERs and/or QALYs were included in this report: four CEA studies, one BIM, and one HTA (Table 6). All of the CEA studies were from the US, and the HTA was from the UK (SMC). One study found that enzalutamide provided greater QALYs than either abiraterone or cabazitaxel for patients with mCRPC and visceral involvement after docetaxel therapy resistance. Enzalutamide was cost-effective from a US payer perspective at 92% of the time with a willingness-to-pay (WTP) threshold of $100,000/QALY (71). Cabazitaxel was not cost-effective in patients enrolled in the CARD trial (72); however, this is based on only one study. According to the SMC, olaparib was cost-effective compared with both docetaxel and cabazitaxel in patients with mCRPC and BRCA1 or BRCA2 mutations (germline and/or somatic) who had progressed following prior therapy that included an ARPI (Table 6) (76). There were conflicting results for the cost-effectiveness of olaparib in the US: one study determined that olaparib was not cost-effective (in line with common WTPs in the US), with an ICER of $248,248 per QALY gained (Table 6) (74), whereas another study reported it was cost-saving, with a cost per QALY of $116,903 (73). However, the authors concluded that olaparib was a preferred option in patients with any of 15 prespecified genes (Table 6) (73).
The findings of these structured and systematic literature reviews demonstrate the challenges associated with the limited alternatives available for the treatment of mCRPC in the ≥2L setting. The data establish the existence of both a humanistic and economic burden in ≥2L mCRPC; patients are heavily affected in terms of HRQoL, side effects, and survival, as well as incurring substantial healthcare costs. That said, this burden is not limited to the ≥2L setting. Many patients do not receive treatment beyond 1L, and published data collected in the 2010s show that most patients with ≥2L mCRPC will receive only palliative care (77–80), contrasting starkly against the number of therapies being studied in phase 3 trials. There is a lack of data informing decisions around optimal treatment sequencing, including how clinicians and patients make these decisions while taking into account cost-effectiveness. Thus, there is a clear unmet need in patients with mCRPC who have been treated with at least one ARPI and at least one taxane. International collaboration is needed to standardize treatment sequencing to provide superior outcomes for these patients.
The information collected in this review regarding treatment patterns in mCRPC in the recent epoch before the introduction of PARP inhibitors in 2020 is generally confirmed by recently published real-world studies from the US, Australia, Europe, and Japan (9, 81–83). The ARPIs enzalutamide and abiraterone were the most common 1L systemic options for patients with mCRPC; the next most frequently used 1L systemic option was chemotherapy (primarily docetaxel), and then sipuleucel-T (9, 81, 83). Of note, one study reported at congress (thus not included in the review) noted undertreatment of patients in the US, with 38% of patients not receiving any 1L therapy (78). Similar findings regarding treatment patterns were reported in Canada and Australia (also congress reports), with the exception of sipuleucel-T; the most commonly used 1L options were ARPIs (26%–94%) and docetaxel (26%–30%) (84–86). In Spain, 64.2% of patients who had mCRPC with DDR gene mutations were treated in the 1L setting with abiraterone plus prednisone/enzalutamide (87).
The preferred 2L systemic option was chemotherapy in all countries except Japan, where either enzalutamide (83) or LHRH agonists/antagonists (44) were the more common options, and Spain, where ARPIs and chemotherapy were used equally often (83). In one study in Spain that was reported in a congress abstract, the most frequent choice of 2L therapy was abiraterone/enzalutamide (31.4%) or docetaxel (30.6%); however, 24.8% received no 2L therapy (87). Docetaxel is reportedly more commonly used as a 2L option in Canada (57%) compared with the US (6%–22%). Overall, these findings are consistent with National Comprehensive Cancer Network (NCCN) guidelines (12). Combination therapy with olaparib and abiraterone is currently being studied as a 1L option in the phase 3 PROpel trial, with recently published data demonstrating significantly improved rPFS over abiraterone alone in patients with mCRPC (25.0 vs. 16.4 months; HR 0.67; 95% CI 0.56–0.81), regardless of whether or not they harbored HRR gene mutations. OS data were immature in the planned interim analysis (40%); however, a trend toward improved OS with olaparib plus abiraterone versus abiraterone alone was reported (HR 0.83; 95% CI 0.66–1.03) (88). The HRs for time to first subsequent therapy or death and time to second progression or death (0.74 and 0.69, respectively), reported in an earlier primary analysis of PROpel, supported efficacy beyond first imaging-based progression (26). In August 2023, niraparib (not yet approved for prostate cancer) was approved in combination with abiraterone for the treatment of patients with deleterious or suspected deleterious BRCA1- or BRCA2-mutated mCRPC, based on the findings of the MAGNITUDE trial (NCT03748641) (89). This combination is also under investigation for the treatment of patients with deleterious HRR gene-mutated metastatic castration-sensitive prostate cancer (AMPLITUDE; NCT04497844) (89). It is also being studied in combination with cetrelimab (anti-programmed death 1 antibody) in patients with mCRPC, including the subpopulation with DDR gene defects (QUEST; NCT03431350) (90, 91), regardless of prior treatment history (except for prior PARP inhibition in AMPLITUDE).
There appears to be more variation regarding treatment sequencing, the importance of which has been emphasized in the updated Prostate Cancer Working Group recommendations (92, 93). There is currently no evidence-based guidance or consensus on the optimal sequence beyond 2L, and a lack of randomized trials to clarify the issue. According to a recent PubMed-based literature review of clinical studies on treatment sequencing and combinations in mCRPC, abiraterone followed by enzalutamide was the best sequential treatment in docetaxel-naïve patients, whereas enzalutamide was the most effective subsequent choice of treatment for patients who had previously failed on docetaxel (94). The findings of a phase 2 crossover study with HRQoL as an outcome measure support the use of abiraterone as a first subsequent therapy in patients with treatment-naïve mCRPC (95). In the CARD study, patients with mCRPC who had failed on docetaxel and either abiraterone or enzalutamide (before or after docetaxel), and then treated with 3L cabazitaxel experienced longer rPFS and OS than their counterparts who were treated in 3L with the other ARPI (i.e., abiraterone in those who had previously received enzalutamide, and vice versa) (47). The most common 1L-to-2L sequence identified in the present literature review was ARPI-to-chemotherapy, with other sequences being chemotherapy-to-ARPI, ARPI-to-ARPI, and chemotherapy-to-chemotherapy. Several trials are ongoing with ARPI plus androgen deprivation therapy (ADT) in combination with a third agent (“triplet therapies”) to treat metastatic hormone-sensitive prostate cancer (mHSPC), such as ENZAMET, ARASENS, and PEACE-1. Findings to date provide equivocal evidence that (at least for patients with high-volume mHSPC) OS, PFS, and various measures of PROs are improved by the addition of agents such as docetaxel, darolutamide, enzalutamide, and abiraterone to ADT (96–100). These findings may lead to changes in mCRPC sequencing. However, it should be noted that the timing of administration of the docetaxel-related regimen differed between the three trials (i.e., delivered before, during, or just after ARPI) (97, 98, 100). Furthermore, trials are underway to identify molecular biomarkers that predict response or primary resistance to particular treatments, which may be invaluable in the treatment decision-making process to optimize the patient’s therapeutic journey (101, 102). Biomarkers currently under study include: circulating androgen receptor gene status (assessed using liquid biopsy); alterations in phosphatase and tensin homolog protein and its pathway; immune biomarkers (e.g., programmed death ligand 1, sex-determining region Y-box 2; and DDR gene mutations (103–105). The multicenter, outcome-adaptive, biomarker-driven ProBio study is currently using a biomarker-driven platform to inform treatment decisions in men with mCRPC (102).
Geographic differences in the preferred treatment sequence were noted; for example, chemotherapy-to-ARPI was a more common 1L-to-2L sequence than ARPI-to-ARPI and chemotherapy-to-chemotherapy in Germany, Italy, and the UK, while chemotherapy-to-ARPI and ARPI-to-ARPI was administered to a similar degree in France and Spain (83). The most frequent 1L-to-2L treatment sequence in a large retrospective study in the US was abiraterone-to-enzalutamide, followed by enzalutamide-to-abiraterone (82), while in Ontario, Canada, the preference was to provide a subsequent (2L) treatment with a different mechanism of action (abiraterone or enzalutamide followed by docetaxel, with Ra-223 being the most common 3L treatment) (80). A retrospective, multicenter analysis of real-world data performed in Australia found that the most frequent subsequent systemic therapy was a carboplatin-based regimen, and that some patients also received rechallenge with an ARPI or docetaxel (106). However, it was also reported that choice of subsequent treatment did not impact survival outcomes; median OS did not differ according to subsequent systematic therapy (106). In contrast, in a single-center study, docetaxel rechallenge has shown meaningful anti-tumor activity in pretreated patients with mCRPC in the salvage setting (107).
Geographic differences in treatment sequencing preference may be attributable to a variety of country-, patient-, and physician-based factors, such as whether or not ARPI rechallenge is allowed, reimbursement rules, patient preference for oral versus intravenous agents, access and cost of newer agents, and the specialism of the treating physician (83, 108–110). Patients may live in regions without access to newer agents or where screening is not common and they are diagnosed at a later stage, rendering them ineligible for certain therapies (44, 108). Rechallenge with ARPIs is discouraged in ESMO and European Association of Urology (EAU) guidelines due to the potential for cross-resistance (101, 111, 112), but in the local guidelines for Germany, Italy, and Japan it is a suggested treatment option (83). Moreover, local reimbursement criteria may preclude rechallenge with a different ARPI – this is the case in Canada, with exceptions in the case of intolerance with the first ARPI or (in some provinces) if chemotherapy was used in between (“sandwich therapy”) (80, 113). According to one US study that compared therapy choice between oncologists and urologists, a significantly greater proportion of patients treated by oncologists were prescribed hormone therapy, chemotherapy, and radiation therapy; nonchemotherapy options were significantly more commonly prescribed by urologists than by oncologists (114). Another study from the US reported that in the 2L setting, oncologists were most likely to prescribe hormone therapy and chemotherapy, while for urologists the most commonly prescribed treatments were sipuleucel-T and hormone therapy (115). Other work from the US reported that urologists were increasingly prescribing oral therapies such as abiraterone and enzalutamide (116). Treatment options for mCRPC continue to evolve, and particularly in the 2L setting with the recent approval of several novel therapies such as PARP inhibitors (olaparib, rucaparib), immunotherapies (pembrolizumab), and 177Lu-PSMA-617. Although all of these new agents are now included in one or more of the recently updated NCCN, ESMO, EAU, and American Urological Association prostate cancer treatment guidelines, there is little real-world evidence regarding their use (12, 110, 111, 117). Olaparib and rucaparib are both approved in one or more countries (including the US) for the treatment of patients with HRR gene-mutated mCRPC who have progressed on ARPIs (olaparib) or in patients with mutations in BRCA1 or BRCA2 who have progressed on an ARPI and a taxane-based chemotherapy (rucaparib). Of note, NCCN guidelines do not recommend use of olaparib for patients harboring PPP2R2A mutations due to preliminary evidence of reduced efficacy in these patients (12). For both of these PARP inhibitors, gene mutations are confirmed using US Food and Drug Administration-approved companion diagnostics (18, 19). Among patients with mCRPC, only 11%–33% harbor germline mutations in DDR genes, including HRR genes such as BRCA1/BRCA2 (118, 119); therefore, the majority of patients are not eligible to receive them. Moreover, in a recent network analysis of the PROfound and CARD studies, it was noted that certain patient subgroups achieved reduced or no benefit with olaparib, including those harboring mutations in genes other than BRCA1 or BRCA2, compared with active standard-of-care agents (e.g., cabazitaxel) (120). Pembrolizumab is also approved in the US for the treatment of patients with mismatch-repair-deficient or microsatellite-instability-high solid tumors who have no satisfactory alternative (21), and 177Lu-PSMA-617 is approved for patients with PSMA-positive (determined using a PSMA imaging agent) mCRPC who have progressed on an ARPI and taxane-based therapy (121). It remains to be seen how these new agents will be incorporated into the treatment landscape in the real-world setting. Access to all therapy options has been shown to result in longer survival in patients with prostate cancer (122).
Most pivotal phase 3 trials for currently approved treatments for mCRPC were published prior to 2017 (Supplemental Table 15), and thus data for only two trials were included in this review: PROfound (olaparib vs. enzalutamide plus abiraterone) (45, 46) and CARD (cabazitaxel vs. enzalutamide or abiraterone plus prednisone) (47). Despite differences in the patient populations, both studies demonstrated improved survival with the study drug over abiraterone or enzalutamide. In PROfound, OS varied (according to the particular cohort) from 14.1 to 19.1 months with olaparib and from 11.5 to 15.1 months with an ARPI; rPFS was 5.8 versus 3.5 months (45, 46). In CARD, OS was 13.6 months with cabazitaxel versus 11.0 months with an ARPI, and PFS was 4.4 and 2.7 months, respectively (47). Based on these clinical trials, newer agents have been approved and brought into clinical practice (12, 112). Survival data for other approved treatments that were outside the scope of this review are provided in Supplemental Table S15.
The humanistic burden associated with mCRPC was high across the included studies, demonstrating that mCRPC is associated with poor HRQoL and imposing a significant burden on patients. This burden was worse for patients with SSEs, skeletal-related events, or bone metastases (2, 62). Although treatment was often associated with a delayed deterioration in HRQoL, no trends for one regimen over another emerged. Two studies (not identified in the current review) determined that patients treated with abiraterone reported less fatigue and cognitive impairment and had more favorable PROs compared with enzalutamide in both real-world and clinical trial settings (95, 123). However, a propensity score-matching study found that whilst there was no significant difference in the development of new comorbidities between the two agents, OS was better with enzalutamide; moreover, the overall Charlson Comorbidity Index score (and not its items) was a significant predictor of OS (124). There is increasing awareness that HRQoL is an important endpoint of value to both patients and healthcare providers. While the impact of treatments on many aspects of HRQoL in patients with localized disease is well established, there is less information on patients with advanced disease who are more likely to have poor performance status, are more frequently affected by pain due to bone metastases, and for whom the balance between longevity and quality of life is perhaps more important (125, 126). A recent systematic review of patient values, preferences, and expectations found that, while cancer progression or survival, pain, and fatigue were key considerations regarding treatment decisions, alleviation of pain was valued at the expense of survival benefits among symptomatic patients (127). PRO endpoints are included in clinical registration trials, as well as product labels of the latest interventions, and form an important part of disease management in patients with mCRPC (128, 129). However, the tools used to measure HRQoL in patients with mCRPC may not fully capture patients’ lived experiences of the burden of advanced prostate cancer (130–132). Some HRQoL instruments (e.g., EORTC QLQ-C30) are generic and have not been optimized to measure the specific burden of mCRPC; prostate cancer-specific tools (e.g., FACT-P) may capture a more accurate picture. It is therefore encouraging that a recent systematic review of trials on advanced prostate cancer conducted between 2011 and 2019 found that FACT-P was the most frequently adopted HRQoL tool (11/14 trials), followed by EQ-5D (6/14 trials) and EORTC QLQ-C30 (3/14 trials) (129). The evidence from that review demonstrated that in phase 3 trials, currently available systemic treatments (including ARPIs, Ra-223, and docetaxel) had a positive impact on HRQoL in patients with advanced cancer compared with standard ADT. Data for newer agents such as olaparib were available only for phase 2 trials, but nonetheless were also found to be beneficial (129). Moreover, some instruments are more meaningful at particular stages of the disease. Holmstrom et al. describe a conceptual model that characterizes patient experiences of living with mCRPC, indicating symptoms and impacts, such as fatigue, pain, urinary frequency, interference with daily activities, and frustration, that are not adequately assessed with currently available PRO instruments (133).
Further studies are required to determine the cost-effectiveness of the recently approved therapies, the optimal treatment sequence in patients who have progressed, and models that use data from real-world studies, not clinical trials. The economic burden to healthcare systems of patients with mCRPC is ongoing as patients are still progressing on currently available treatments.
There is a lack of overall consensus on genetic testing. US and European guidelines now recommend genetic testing (somatic and/or germline), especially in patients with advanced prostate cancer, and earlier genetic testing in patients who are high risk (including those with a relevant family history) (134, 135). In contrast, a post-meeting survey from the Asia-Pacific Advanced Prostate Cancer Consensus Conference revealed that biomarker testing is limited to patients who have progressed on multiple lines of therapy (136).
In summary, the lack of available data to inform the delivery of appropriate treatments beyond 2L to patients with mCRPC means that the optimal sequence remains undefined (85, 106, 137). The data have shown that options with alternative mechanisms of action may be preferred to avoid cross-resistance between ARPIs (80, 101). The treatment options for mHSPC are increasing, and future real-world studies assessing the impact of mHSPC treatment algorithms on subsequent mCRPC treatment alternatives are needed. In addition, there is a need for innovative treatment options in post-taxane and post-ARPI mCRPC that improve survival and HRQoL, as well as having a tolerable safety profile. Aligning and then switching from 1L to 2L mCRPC therapies in a timely manner will help to ensure patients receive the most benefit from a clinically efficacious agent. New treatment options for the ≥2L setting may help not only treatment sequencing optimization, but may also result in improvements to the humanistic and economic burden.
The literature search identified only seven HCRU/cost-effectiveness studies and six studies on treatment patterns from 2009 to 2021, among which five and three, respectively, originated in the US. In addition, only one study provided a cost associated with genetic testing (73). There is a clear need for studies analyzing both cost and treatment patterns across diverse geographic regions to provide a clear view of the barriers to treatment access.
The strengths of this review include the comprehensive search of recent literature over multiple databases for studies describing the global treatment landscape and disease burden associated with mCRPC. The methodology utilized both structured/targeted reviews and systematic literature reviews, and searches were repeated to ensure up-to-date information was captured. This strategy has allowed us to meet our objective of identifying unmet needs for patients with late-stage mCRPC, demonstrating the overall high disease burden, the need to establish optimal treatment sequencing, and the global need to develop novel treatments that are affordable, well tolerated, and can be implemented using a holistic approach.
One key limitation of this study is the heterogeneity between studies with regard to the characteristics of the patient population, study design, the tools used to measure PROs, and the terminology used to describe the therapies (e.g., enzalutamide and abiraterone are referred to variously in the literature as novel/new hormonal agents, ADT, ARPIs, and androgen receptor inhibitors). In addition, the search strategy was limited by the exclusion of some phase 2 or 3 trials of key approved therapies (olaparib, rucaparib, pembrolizumab), which means that the data on current treatment options may not be representative, and by the inclusion of only English-language publications from the past 5 years. Furthermore, it was not possible to explore the effect of race/ethnicity, since details of these were not provided in many of the identified studies (these are detailed in recent congress publications, and were therefore outside the scope of this review).
Finally, caution is needed when reviewing the cost-effectiveness data, since the therapies involved have different indications, eligible patient populations, and prior therapy requirements.
The findings outlined in this report highlight a lack of treatment options with novel mechanisms of action and more tolerable safety profiles that meet patient needs and preferences. Treatment approaches that improve survival and HRQoL are needed, ideally while simultaneously reducing costs and HCRU. The last decade has seen a renaissance in mCRPC treatment options, with several life-prolonging therapies becoming established. It is apparent from this literature review that treatment sequencing and patient follow up is not homogenous, resulting in varied patient outcomes by region, healthcare system, and physician. Further prospective studies should be conducted to define the optimal treatment sequence and time to change treatment. During their treatment journey, patients should expect to undergo germline and somatic genetic testing to allow strategic implementation of PARP inhibition or immune checkpoint inhibition with pembrolizumab. Further improvements are signaled by the advent of PSMA radioligand imaging, which promises to improve the diagnosis of prostate cancer. Moreover, its routine implementation in the clinic could provide a basis for selection of patients with mCRPC who may respond to novel PSMA-targeted radioligand therapy. All patients should also receive holistic care that provides psychosocial support, attention to physical function and frailty prevention, and caregiver support to ensure optimal outcomes for all patients and timely, informed treatment decisions.
OM and RG designed this study. GK contributed to the literature search, review, and data extraction. DL, SC, AM, OM, and RG contributed to manuscript drafting and manuscript revision. All authors have reviewed and approved the final version of this manuscript.
This literature review and manuscript were sponsored by Advanced Accelerator Applications (AAA), A Novartis Company.
Sheetal Sharma, formerly from Parexel International, developed the protocol and the original systematic and targeted literature reviews. Anna Forsythe, Sharada Harricharan, and Rozee Liu from Cytel updated the systematic reviews. Editorial and writing support was provided by Sarah Cocklin, PhD, and Sue Neville, MSc, of Parexel International, and was funded by Advanced Accelerator Applications (AAA), A Novartis Company.
Author SC reports honoraria from Clovis and Novartis; funding from Sanofi; acting as a speaker for AstraZeneca, Pfizer, and Janssen; and a consultant role with Clovis, Astellas Pharma, Bayer, Pfizer, Janssen, BeiGene, Remedy Bio, Telix, and Novartis. Author DL has received fees from Advanced Accelerator Applications (AAA) and Boston Scientific for consultancy work. Authors RG and OM are employees of Advanced Accelerator Applications (AAA), A Novartis Company. Author GK was an employee of Parexel International at the time of the original and updated literature reviews. Author AM reports honoraria, consulting/advisory, research funding, and/or travel/accommodations from Genentech, Janssen, Sanofi, AstraZeneca, Astellas Scientific and Medical Affairs Inc, Astellas Pharma, Janssen Oncology, Bayer, Clovis Oncology, Myovant Sciences, Advanced Accelerator Applications (AAA), Exelixis, Pfizer, Merck, Telix Pharmaceuticals, Blue Earth Diagnostics, Novartis, Myriad Genetics, Lantheus Medical Imaging, Seattle Genetics/Astellas, and Dendreon.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1240864/full#supplementary-material
1. Rawla P. Epidemiology of prostate cancer. World J Oncol (2019) 10(2):63–89. doi: 10.14740/wjon1191
2. Saad F, Fleshner NE, So A, Le Lorier J, Perrault L, Poulin-Costello M, et al. The burden of symptomatic skeletal events in castrate-resistant prostate cancer patients with bone metastases at three Canadian uro-oncology centres. Can Urol Assoc J (2018) 12(12):370–6. doi: 10.5489/cuaj.5053
3. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
4. National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Prostate Cancer, Bethesda, MD, USA (2022); USA. Available at: https://seer.cancer.gov/statfacts/html/prost.html.
5. Cancer Research UK. Prostate cancer incidence by stage statistics (2019). Available at: https://crukcancerintelligence.shinyapps.io/EarlyDiagnosis/.
6. Leslie SW, Soon-Sutton TL, Sajjad H, Siref LE. Prostate cancer. In: StatPearls. Treasure Island (FL: StatPearls Publishing (2022). Available at: https://www.ncbi.nlm.nih.gov/.
7. Dong L, Zieren RC, Xue W, de Reijke TM, Pienta KJ. Metastatic prostate cancer remains incurable, why? Asian J Urol (2019) 6(1):26–41. doi: 10.1016/j.ajur.2018.11.005
8. Frieling JS, Basanta D, Lynch CC. Current and emerging therapies for bone metastatic castration-resistant prostate cancer. Cancer Control (2015) 22(1):109–20. doi: 10.1177/107327481502200114
9. Shore ND, Laliberté F, Ionescu-Ittu RA-O, Yang L, Mahendran M, Lejeune D, et al. Real-world treatment patterns and overall survival of patients with metastatic castration-resistant prostate cancer in the US prior to PARP inhibitors. Adv Ther (2021) 38(8):4520–40. doi: 10.1007/s12325-021-01823-6
10. Cancer Research UK. Prostate Cancer Survival Statistics: Cancer Research UK (2022). Available at: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/prostate-cancer#heading-Two.
11. Shore ND. Radium-223 dichloride for metastatic castration-resistant prostate cancer: the urologist's perspective. Urology (2015) 85(4):717–24. doi: 10.1016/j.urology.2014.11.031
12. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Prostate Cancer Version 1 (2023). Available at: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.
13. Velho PI, Bastos DA, Antonarakis ES. New approaches to targeting the androgen receptor pathway in prostate cancer. Clin Adv Hematol Oncol (2021) 19(4):228–40.
14. Henríquez I, Roach M, Morgan TM, Bossi A, Gómez JA, Abuchaibe O, et al. Current and emerging therapies for metastatic castration-resistant prostate cancer (mCRPC). Biomedicines (2021) 9(9):1247. doi: 10.3390/biomedicines9091247
15. Berthold DR, Pond GR, Soban F, de Wit R, Eisenberger M, Tannock IF. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. J Clin Oncol (2008) 26(2):242–5. doi: 10.1200/JCO.2007.12.4008
16. de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels J-P, Kocak I, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet (2010) 376(9747):1147–54. doi: 10.1016/S0140-6736(10)61389-X
17. Parker CC, Coleman RE, Sartor O, Vogelzang NJ, Bottomley D, Heinrich D, et al. Three-year safety of radium-223 dichloride in patients with castration-resistant prostate cancer and symptomatic bone metastases from phase 3 randomized Alpharadin in Symptomatic Prostate Cancer Trial. Eur Urol (2018) 73(3):427–35. doi: 10.1016/j.eururo.2017.06.021
18. United States Food and Drug Administration. LYNPARZA® (olaparib) prescribing information (May 2023 update) (2023). Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/208558s025lbl.pdf.
19. United States Food and Drug Administration. RUBRACA® (rucaparib) prescribing information (June 2022 update) (2022). Available at: https://clovisoncology.com/pdfs/RubracaUSPI.pdf.
20. European Medicines Agency. LYNPARZA (olaparib) Summary of Product Characteristics (May 2023 update) (2023). Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/lynparza#product-information-section.
21. United States Food and Drug Administration. KEYTRUDA® (pembrolizumab) prescribing information (August 2022 update) (2022). Available at: https://www.merck.com/product/usa/pi_circulars/k/keytruda/keytruda_pi.pdf.
22. National Cancer Institute. FDA Approves Pembrolizumab for Tumors with Specific Genetic Features (2017). Available at: https://www.cancer.gov/news-events/cancer-currents-blog/2017/fda-pembrolizumab-genetic-features#:~:text=On%20May%2023%2C%20the%20Food,deficiency%20and%20high%20microsatellite%20instability.
23. Li H, Liu Z-Y, Wu N, Chen Y-C, Cheng Q, Wang J. PARP inhibitor resistance: the underlying mechanisms and clinical implications. Mol Cancer (2020) 19(1):107. doi: 10.1186/s12943-020-01227-0
24. Sun J-Y, Zhang D, Wu S, Xu M, Zhou X, Lu X-J, et al. Resistance to PD-1/PD-L1 blockade cancer immunotherapy: mechanisms, predictive factors, and future perspectives. Biomark Res (2020) 8(1):35. doi: 10.1186/s40364-020-00212-5
25. Clarke N, Wiechno P, Alekseev B, Sala N, Jones R, Kocak I, et al. Olaparib combined with abiraterone in patients with metastatic castration-resistant prostate cancer: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol (2018) 19(7):975–86. doi: 10.1016/S1470-2045(18)30365-6
26. Clarke NW, Armstrong AJ, Thiery-Vuillemin A, Oya M, Shore N, Loredo E, et al. Abiraterone and olaparib for metastatic castration-resistant prostate cancer. NEJM Evid (2022) 1(9):EVIDoa2200043. doi: 10.1056/EVIDoa2200043
27. Agarwal N, Azad A, Carles J, Fay AP, Matsubara N, Heinrich D, et al. TALAPRO-2: Phase 3 study of talazoparib (TALA) + enzalutamide (ENZA) versus placebo (PBO) + ENZA as first-line (1L) treatment in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol (2023) 41(6_suppl):LBA17–LBA. doi: 10.1200/JCO.2023.41.6_suppl.LBA17
28. Agarwal N, Azad A, Shore ND, Carles J, Fay AP, Dunshee C, et al. TALAPRO-2: A phase 3 randomized study of enzalutamide (ENZA) plus talazoparib (TALA) versus placebo in patients with new metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol (2021) 39(15_suppl):TPS5089–TPS. doi: 10.1200/JCO.2021.39.15_suppl.TPS5089
29. Chi KN, Sandhu S, Smith MR, Attard G, Saad M, Olmos D, et al. Niraparib plus abiraterone acetate with prednisone in patients with metastatic castration-resistant prostate cancer and homologous recombination repair gene alterations: second interim analysis of the randomized phase III MAGNITUDE trial. Ann Oncol (2023) 34(4):397–409. doi: 10.1016/j.annonc.2023.06.009
30. Rao A, Ryan CJ, Morris D, Assikis V, Jha G, Ablaza A-J, et al. Abstract 445: Genomic characteristics and response to rucaparib and enzalutamide in the phase 1b RAMP study of metastatic castration-resistant prostate cancer (mCRPC) patients. Cancer Res (2021) 81(13_Supplement):445. doi: 10.1158/1538-7445.Am2021-445
31. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (2021) 372:n71. doi: 10.1136/bmj.n71
32. Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess (2004) 8(36):1–158. doi: 10.3310/hta8360
33. NICE. Identification and selection of relevant studies. In: Single technology appraisal: User guide for company evidence submission template (2015). NICE, UK. Available at: https://www.nice.org.uk/Process/Pmg24/Chapter/Instructions-for-Companies.
34. Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ (1996) 313(7052):275–83. doi: 10.1136/bmj.313.7052.275
35. Efficace F, Bottomley A, Osoba D, Gotay C, Flechtner H, D'Haese S, et al. Beyond the development of health-related quality-of-life (HRQOL) measures: a checklist for evaluating HRQOL outcomes in cancer clinical trials–does HRQOL evaluation in prostate cancer research inform clinical decision making? J Clin Oncol (2003) 21(18):3502–11. doi: 10.1200/jco.2003.12.121
36. Akaza H, Procopio G, Pripatnanont C, Facchini G, Fava S, Wheatley D, et al. Metastatic castration-resistant prostate cancer previously treated with docetaxel-based chemotherapy: treatment patterns from the PROXIMA Prospective Registry. J Glob Oncol (2018) 4:1–12. doi: 10.1200/JGO.18.00009
37. Dizdarevic S, Petersen PM, Essler M, Versari A, Bourre JC, la Fougere C, et al. nterim analysis of the REASSURE (Radium-223 alpha Emitter Agent in non-intervention Safety Study in mCRPC popUlation for long-teRm Evaluation) study: patient characteristics and safety according to prior use of chemotherapy in routine clinical practice. Eur J Nucl Med Mol Imaging (2019) 46(5):1102–10. doi: 10.1007/s00259-019-4261-y
38. Harshman LC, Logue J, Sternberg CN, Sundar S, Schrijvers D, Schostak M, et al. First interim results of the radium-223 (Ra-223) REASSURE observational study: Analysis of patient (Pt) characteristics and safety by use of abiraterone and/or enzalutamide (Abi/Enza). Ann Oncol (2017) 28:v279–v80. doi: 10.1093/annonc/mdx370.024
39. Harshman LC, Sartor AO, Richardson T, Sylvester J, Song DY, Mantz C, et al. First interim results of the radium-223 (Ra-223) reassure observational study in metastatic castration-resistant prostate cancer (mCRPC): safety and baseline (BL) characteristics of U.S. patients (Pts) by prior/concomitant treatment (Tx). J Clin Oncol (2018) 36(Suppl 6):233. doi: 10.1200/JCO.2018.36.6_suppl.233
40. Higano CS, Zimberg SH, Dizdarevic S, Harshman LC, Logue J, Baldari S, et al. Patient (pt) characteristics and treatment patterns in the radium (Ra)-223 REASSURE observational study. J Clin Oncol (2017) 35(Suppl 15):5042. doi: 10.1200/JCO.2017.35.15_suppl.5042
41. Oh WK, Miao R, Vekeman F, Sung J, Cheng WY, Gauthier-Loiselle MD, et al. Patient characteristics and overall survival in patients with post-docetaxel metastatic castration-resistant prostate cancer in the community setting. Med Oncol (2017) 34(9):160. doi: 10.1007/s12032-017-1014-2
42. Wen L, Yao J, Valderrama A. Evaluation of treatment patterns and costs in patients with prostate cancer and bone metastases. J Manag Care Spec Pharm (2019) 25(3b):S1–S11. doi: 10.18553/jmcp.2019.25.3-b.s1
43. Wen L, Valderrama A, Costantino ME, Simmons S. Real-world treatment patterns in patients with castrate-resistant prostate cancer and bone metastases. Am Health Drug Benefits (2019) 12(3):142–9.
44. Uemura H, DiBonaventura M, Wang E, Ledesma DA, Concialdi K, Aitoku Y. The treatment patterns of castration-resistant prostate cancer in Japan, including symptomatic skeletal events and associated treatment and healthcare resource use. Expert Rev Pharmacoecon Outcomes Res (2017) 17(5):511–7. doi: 10.1080/14737167.2017.1300530
45. Hussain M, Mateo J, Fizazi K, Saad F, Shore N, Sandhu S, et al. Survival with olaparib in metastatic castration-resistant prostate cancer. N Engl J Med (2020) 383(24):2345–57. doi: 10.1056/NEJMoa2022485
46. de Bono J, Mateo J, Fizazi K, Saad F, Shore N, Sandhu S, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med (2020) 382(22):2091–102. doi: 10.1056/NEJMoa1911440
47. de Wit R, de Bono J, Sternberg CN, Fizazi K, Tombal B, Wülfing C, et al. Cabazitaxel versus abiraterone or enzalutamide in metastatic prostate cancer. N Engl J Med (2019) 381(26):2506–18. doi: 10.1056/NEJMoa1911206
48. Beardo P, Osman I, San Jose B, Llarena R, Congregado B, Campa J, et al. Safety and outcomes of new generation hormone-therapy in elderly chemotherapy-naive metastatic castration-resistant prostate cancer patients in the real world. Arch Gerontol Geriatr (2019) 82:179–85. doi: 10.1016/j.archger.2019.02.008
49. Carles J, Pichler A, Korunkova H, Tomova A, Ghosn M, El Karak F, et al. An observational, multicentre study of cabazitaxel in patients with metastatic castration-resistant prostate cancer previously treated with docetaxel (CAPRISTANA). BJU Int (2019) 123(3):456–64. doi: 10.1111/bju.14509
50. Gonzalez del Alba A, Puente J, Sala N, Mendez MJ, Pinto A, Sanchez AR, et al. Abiraterone acetate with prednisone and cabazitaxel as subsequent treatment after first-line docetaxel in metastatic castrate-resistant prostate cancer (mCRPC): Final efficacy and safety analysis of the CAPRO study. J Clin Oncol (2017) 35(Suppl 6):276. doi: 10.1200/JCO.2017.35.6_suppl.276
51. Fourrier-Réglat A, Oudard S, Fizazi K, Joly F, Tubach F, Rouyer M, et al. Use of cabazitaxel in treatment of metastatic castration-resistant prostate cancer (mCRPC): patient characteristics, safety and effectiveness in the FUJI cohort. (2018) 32(S1):PS1–015. Société Française de Pharmacologie et de Thérapeutiques; 12–14 June, 2018; Toulouse, France. Available at:https://onlinelibrary.wiley.com/doi/full/10.1111/fcp.12371.
52. Sirotova Z, Courthod G, Tartarone A, Caffo O, Maines F, Bertuccelli M, et al. Safety and efficacy of abiraterone acetate (AA) in patients aged 75 or more with metastatic castration-resistant prostate cancer (mCRPC) in both pre-chemotherapy or post-chemotherapy settings: Real-life experience from thirteen Italian centers. J Clin Oncol (2018) 36:abstr 209. doi: 10.1200/JCO.2018.36.6_suppl.209
53. Parente P, Ng S, Parnis F, Guminski A, Gurney H. Cabazitaxel in patients with metastatic castration-resistant prostate cancer: safety and quality of life data from the Australian early access program. Asia Pac J Clin Oncol (2017) 13(6):391–9. doi: 10.1111/ajco.12679
54. Summers N, Vanderpuye-Orgle J, Reinhart M, Gallagher M, Sartor O. Efficacy and safety of post-docetaxel therapies in metastatic castration-resistant prostate cancer: a systematic review of the literature. Curr Med Res Opin (2017) 33(11):1995–2008. doi: 10.1080/03007995.2017.1341869
55. Dearden L, Shalet N, Artenie C, Mills A, Jackson C, Grant L, et al. Fatigue, treatment satisfaction and health-related quality of life among patients receiving novel drugs suppressing androgen signalling for the treatment of metastatic castrate-resistant prostate cancer. Eur J Cancer Care (2019) 28(1):e12949. doi: 10.1111/ecc.12949
56. Fizazi K, Kramer G, Eymard J-C, Sternberg CN, de Bono J, Castellano D, et al. Quality of life in patients with metastatic prostate cancer following treatment with cabazitaxel versus abiraterone or enzalutamide (CARD): an analysis of a randomised, multicentre, open-label, phase 4 study. Lancet Oncol (2020) 21(11):1513–25. doi: 10.1016/S1470-2045(20)30449-6
57. Murasawa H, Sugiyama T, Matsuoka Y, Okabe T, Hino A, Tanaka N, et al. Health utility and health-related quality of life of Japanese prostate cancer patients according to progression status measured using EQ-5D-5L and FACT-P. Qual Life Res (2019) 28(9):2383–91. doi: 10.1007/s11136-019-02184-y
58. Payne H, Robinson A, Rappe B, Hilman S, De Giorgi U, Joniau S, et al. A European, prospective, observational study of enzalutamide in patients with metastatic castration-resistant prostate cancer: PREMISE. Int J Cancer (2022) 150(5):837–46. doi: 10.1002/ijc.33845
59. Jenkins V, Solis-Trapala I, Payne H, Mason M, Fallowfield L, May S, et al. Treatment experiences, information needs, pain and quality of life in men with metastatic castrate-resistant prostate cancer: Results from the EXTREQOL study. Clin Oncol (R Coll Radiol) (2019) 31(2):99–107. doi: 10.1016/j.clon.2018.11.001
60. Badrising SK, Louhanepessy RD, van der Noort V, Kieffer J, Coenen JLLM, Hamberg P, et al. Integrated analysis of pain, health-related quality of life, and analgesic use in patients with metastatic castration-resistant prostate cancer treated with Radium-223. Prostate Cancer Prostatic Dis (2022) 25(2):248–55. doi: 10.1038/s41391-021-00412-6
61. Guo F, Li G-H. Enzalutamide alleviates anxiety and depression as well as improves quality of life compared to bicalutamide in metastatic castration-resistant prostate cancer patients: a cohort study. Transl Cancer Res (2019) 8(5):1965–74. doi: 10.21037/tcr.2019.09.12
62. McKay RR, Haider B, Duh MS, Valdarrama A, Nakabayashi M, Fiorillo M, et al. Impact of symptomatic skeletal events on health-care resource utilization and quality oflife among patients with castration-resistant prostate cancer and bone metastases. Prostate Cancer Prostatic Dis (2017) 20(3):276–82. doi: 10.1038/pcan.2017.4
63. Saad F, Ivanescu C, Phung D, Loriot Y, Abhyankar S, Beer TM, et al. Skeletal-related events significantly impact health-related quality of life in metastatic castration-resistant prostate cancer: data from PREVAIL and AFFIRM trials. Prostate Cancer Prostatic Dis (2017) 20(1):110–6. doi: 10.1038/pcan.2016.62
64. Stenner F, Rothschild SI, Betticher D, Caspar C, Morant R, Popescu R, et al. Quality of life in second-line treatment of metastatic castration-resistant prostate cancer using cabazitaxel or other therapies after previous docetaxel chemotherapy: Swiss observational treatment registry. Clin Genitourin Cancer (2018) 16(1):e151–e9. doi: 10.1016/j.clgc.2017.08.003
65. Thiery-Vuillemin A, Fizazi K, Sartor O, Oudard S, Bury D, Thangavelu K, et al. An analysis of health-related quality of life in the phase III PROSELICA and FIRSTANA studies assessing cabazitaxel in patients with metastatic castration-resistant prostate cancer. ESMO Open (2021) 6(2):100089. doi: 10.1016/j.esmoop.2021.100089
66. Schultz NM, Caplan EO, Wilson S, Sheer R, Suehs BT. HSR19-109: The healthy days measure in a metastatic castration-resistant prostate cancer population treated with novel oral therapies. J Natl Compr Canc Netw (2019) 17(3.5):HSR19–109. doi: 10.6004/jnccn.2018.7197
67. Cella D, Nichol MB, Eton D, Nelson JB, Mulani P. Estimating clinically meaningful changes for the Functional Assessment of Cancer Therapy–Prostate: results from a clinical trial of patients with metastatic hormone-refractory prostate cancer. Value Health (2009) 12(1):124–9. doi: 10.1111/j.1524-4733.2008.00409.x
68. Kreis K, Horenkamp-Sonntag D, Schneider U, Zeidler J, Glaeske G, Weissbach L. Treatment-related healthcare costs of metastatic castration-resistant prostate cancer in Germany: a claims data study. Pharmacoecon Open (2021) 5(2):299–310. doi: 10.1007/s41669-020-00219-6
69. Schultz NM, Flanders SC, Wilson S, Brown BA, Song Y, Yang H, et al. Treatment duration, healthcare resource utilization, and costs among chemotherapy-naïve patients with metastatic castration-resistant prostate cancer treated with enzalutamide or abiraterone acetate: a retrospective claims analysis. Adv Ther (2018) 35(10):1639–55. doi: 10.1007/s12325-018-0774-1
70. Restelli U, Ceresoli GL, Croce D, Evangelista L, Maffioli LS, Gianoncelli L, et al. Economic burden of the management of metastatic castrate-resistant prostate cancer in Italy: a cost of illness study. Cancer Manag Res (2017) 9:789–800. doi: 10.2147/CMAR.S148323
71. Barqawi YK, Borrego ME, Roberts MH, Abraham I. Cost-effectiveness model of abiraterone plus prednisone, cabazitaxel plus prednisone and enzalutamide for visceral metastatic castration resistant prostate cancer therapy after docetaxel therapy resistance. J Med Econ (2019) 22(11):1202–9. doi: 10.1080/13696998.2019.1661581
72. Zhang P-F, Xie D, Li Q. Cost-effectiveness analysis of cabazitaxel for metastatic castration resistant prostate cancer after docetaxel and androgen-signaling-targeted inhibitor resistance. BMC Cancer (2021) 21(1):35. doi: 10.1186/s12885-020-07754-9
73. Su D, Wu B, Shi L. Cost-effectiveness of genomic test-directed olaparib for metastatic castration-resistant prostate cancer. Front Pharmacol (2020) 11:610601. doi: 10.3389/fphar.2020.610601
74. Li Y, Lin S, Zhong L, Luo S, Huang X, Huang X, et al. Is olaparib cost effective in metastatic castration-resistant prostate cancer patients with at least one favorable gene mutation in BRCA1, BRCA2 or ATM? Pharmacogenomics (2021) 22(13):809–19. doi: 10.2217/pgs-2021-0061
75. Flannery K, Drea E, Hudspeth L, Corman S, Gao X, Xue M, et al. Budgetary impact of cabazitaxel use after docetaxel treatment for metastatic castration-resistant prostate cancer. J Manag Care Spec Pharm (2017) 23(4):416–26. doi: 10.18553/jmcp.2017.23.4.416
76. Scottish Medicines Consortium. HTA assessment for olaparib 100mg and 150mg film-coated tablets (Lynparza®) SMC2366 (2021). Available at: www.olaparib-lynparza-final-september-2021-for-website.pdf.
77. Marteau F, Gimonet G, Gabriel S, Dinet J, Flinois A, LChJ Y. Epidemiology of patients with metastatic castrate resistant prostate cancer in Europe and Australia. Value Health (2014) 17(7):A619. doi: 10.1016/j.jval.2014.08.2188
78. Halwani A, Burningham Z, Rasmussen KM, Patil V, Narayanan S, Lin SW, et al. Practice patterns in metastatic castration-resistant prostate cancer (mCRPC): Evidence from the veterans health administration. Ann Oncol (2017) 28:v277–v8. doi: 10.1093/annonc/mdx370.021
79. George DJ, Sartor O, Miller K, Saad F, Tombal B, Kalinovský J, et al. Treatment patterns and outcomes in patients with metastatic castration-resistant prostate cancer in a real-world clinical practice setting in the United States. Clin Genitourin Cancer (2020) 18(4):284–94. doi: 10.1016/j.clgc.2019.12.019
80. Shayegan B, Wallis CJD, Malone S, Cagiannos I, Hamilton RJ, Ferrario C, et al. Real-world use of systemic therapies in men with metastatic castration resistant prostate cancer (mCRPC) in Canada. Urol Oncol (2022) 40(5):192.e1–.e9. doi: 10.1016/j.urolonc.2022.01.009
81. Anton AA-O, Pillai S, Semira MC, Wong S, Shapiro J, Weickhardt A, et al. Real-world first-line systemic therapy patterns in metastatic castration-resistant prostate cancer. BJUI Compass (2022) 3(3):201–13. doi: 10.1002/bco2.129
82. Malangone-Monaco E, Li W, Noxon V, Jiang S, Amin S, Ghate S, et al. Real-world treatment patterns among patients with metastatic castration-resistant prostate cancer (mCRPC) in the US. J Clin Oncol (2022) 40(Suppl 6):49. doi: 10.1200/JCO.2022.40.6_suppl.049
83. Leith A, Kim J, Ribbands A, Clayton E, Yang L, Ghate SR. Real-world treatment patterns in metastatic castration-resistant prostate cancer across Europe (France, Germany, Italy, Spain, and the United Kingdom) and Japan. Adv Ther (2022) 39(5):2236–55. doi: 10.1007/s12325-022-02073-w
84. Lahcene H, Aprikian AG, Vanhuyse M, Hu J, Bladou F, Cury F, et al. Impact of the introduction of novel hormonal agents on metastatic castration-resistant prostate cancer treatment choice. J Oncol Pharm Pract (2019) 26(2):293–305. doi: 10.1177/1078155219842329
85. Hotte S, Finelli A, Malone S, Shayegan B, So AI, Canil CM, et al. Real world patterns of treatment sequencing in Canada for metastatic castrate-resistant prostate cancer. J Clin Oncol (2018) 36:abstr 320. doi: 10.1200/JCO.2018.36.6_suppl.320
86. Pillai S, Semira MC, Pezaro C, Parente P, Tran B. Comparison of first-line treatment options for metastatic castrate resistant prostate cancer: A retrospective cohort study. Ann Oncol (2017) 28:x77–x8. doi: 10.1093/annonc/mdx662.002
87. Mendez-Vidal MJ, Lozano R, Castro E, Romero-Laorden N, Rodriguez-Vida A, Lainez N, et al. Treatment sequence in elderly metastatic castration-resistant prostate cancer (mCRPC) patients (pts) in a prospective cohort study. J Clin Oncol (2019) 37:abstr 5053. doi: 10.1200/JCO.2019.37.15_suppl.5053
88. Oya M, Armstrong AJ, Thiery-Vuillemin A, Shore N, Procopio G, Arslan C, et al. 157O - Biomarker analysis and updated results from the phase III PROpel trial of abiraterone (abi) and olaparib (ola) vs abi and placebo (pbo) as first-line (1L) therapy for patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). Ann Oncol (2022) 33:S1495–S502. doi: 10.1016/annonc/annonc1125
89. Rathkopf DE, Chi KN, Olmos D, Cheng HH, Agarwal N, Graff JN, et al. AMPLITUDE: a study of niraparib in combination with abiraterone acetate plus prednisone (AAP) versus AAP for the treatment of patients with deleterious germline or somatic homologous recombination repair (HRR) gene-altered metastatic castration-sensitive prostate cancer (mCSPC). J Clin Oncol (2021) 39(Suppl 6):TPS176. doi: 10.1200/JCO.2021.39.6_suppl.TPS176
90. Chi K, Fleshner N, Vincenzo Emanuele Chiuri S, Bruwaene SV, Hafron J, McNeel DG, et al. MP27-17 Niraparib with abiraterone acetate and prednisone for metastatic castration-resistant prostate cancer: results from the phase 2 QUEST study. J Urol (2022) 207(Suppl 5):e456. doi: 10.1097/JU.0000000000002570.17
91. ClinicalTrials.gov. A Study of Niraparib Combination Therapies for the Treatment of Metastatic Castration-Resistant Prostate Cancer (QUEST) (2022). Available at: https://clinicaltrials.gov/ct2/show/NCT03431350.
92. Teo MY, Rathkopf DE, Kantoff P. Treatment of advanced prostate cancer. Annu Rev Med (2019) 70:479–99. doi: 10.1146/annurev-med-051517-011947
93. Scher HI, Morris MJ, Stadler WM, Higano C, Basch E, Fizazi K, et al. Trial design and objectives for castration-resistant prostate cancer: Updated recommendations from the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol (2016) 34(12):1402–18. doi: 10.1200/jco.2015.64.2702
94. Chen M-k, Liang Z-j, Luo D-S, Xue K-y, Liao D-y, Li Z, et al. Abiraterone, orteronel, enzalutamide and docetaxel: Sequential or combined therapy? Front Pharmacol (2022) 13:843110. doi: 10.3389/fphar.2022.843110
95. Khalaf DJ, Sunderland K, Eigl BJ, Kollmannsberger CK, Ivanov N, Finch DL, et al. Health-related quality of life for abiraterone plus prednisone versus enzalutamide in patients with metastatic castration-resistant prostate cancer: Results from a phase II randomized trial. Eur Urol (2019) 75(6):940–7. doi: 10.1016/j.eururo.2018.12.015
96. Davis ID. Combination therapy in metastatic hormone-sensitive prostate cancer: is three a crowd? Ther Adv Med Oncol (2022) 14:17588359221086827. doi: 10.1177/17588359221086827
97. Smith MR, Hussain M, Saad F, Fizazi K, Sternberg CN, Crawford ED, et al. Darolutamide and survival in metastatic, hormone-sensitive prostate cancer. N Engl J Med (2022) 386(12):1132–42. doi: 10.1056/NEJMoa2119115
98. Davis ID, Martin AJ, Stockler MR, Begbie S, Chi KN, Chowdhury S, et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med (2019) 381(2):121–31. doi: 10.1056/NEJMoa1903835
99. Stockler MR, Martin AJ, Davis ID, Dhillon HM, Begbie SD, Chi KN, et al. Health-Related quality of life in metastatic, hormone-sensitive prostate cancer: ENZAMET (ANZUP 1304), an international, randomized phase III trial led by ANZUP. J Clin Oncol (2021) 40(8):837–46. doi: 10.1200/JCO.21.00941
100. Fizazi K, Foulon S, Carles J, Roubaud G, McDermott R, Fléchon A, et al. Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): a multicentre, open-label, randomised, phase 3 study with a 2×2 factorial design. Lancet (2022) 399(10336):1695–707. doi: 10.1016/S0140-6736(22)00367-1
101. Cattrini C, España R, Mennitto A, Bersanelli M, Castro E, Olmos D, et al. Optimal sequencing and predictive biomarkers in patients with advanced prostate cancer. Cancers (Basel) (2021) 13(18):4522. doi: 10.3390/cancers13184522
102. Crippa A, De Laere B, Discacciati A, Larsson B, Connor JT, Gabriel EE, et al. The ProBio trial: molecular biomarkers for advancing personalized treatment decision in patients with metastatic castration-resistant prostate cancer. Trials (2020) 21(1):579. doi: 10.1186/s13063-020-04515-8
103. Conteduca V, Mosca A, Brighi N, de Giorgi U, Rescigno P. New prognostic biomarkers in metastatic castration-resistant prostate cancer. Cells (2021) 10(1):193. doi: 10.3390/cells10010193
104. Fenor de la Maza MD, Chandran K, Rekowski J, Shui IM, Gurel B, Cross E, et al. Immune biomarkers in metastatic castration-resistant prostate cancer. Eur Urol Oncol (2022) S2588-9311(22):60–8. doi: 10.1016/j.euo.2022.04.004
105. Graf RP, Fisher V, Mateo J, Gjoerup OV, Madison RW, Raskina K, et al. Predictive genomic biomarkers of hormonal therapy versus chemotherapy benefit in metastatic castration-resistant prostate cancer. Eur Urol (2022) 81(1):37–47. doi: 10.1016/j.eururo.2021.09.030
106. Chazan G, Anton A, Wong S, Shapiro J, Weickhardt A, Azad A, et al. Beyond cabazitaxel: Late line treatments in metastatic castration resistant prostate cancer: A retrospective multicentre analysis. Asia Pac J Clin Oncol (2022) 18(6):642–9. doi: 10.1111/ajco.13732
107. Byeon S, Kim H, Kim J, Kwon M, Hur JY, Jeon HG, et al. Docetaxel rechallenge in metastatic castration-resistant prostate cancer: A retrospective, single-center study. Investig Clin Urol (2020) 61(6):588–93. doi: 10.4111/icu.20200214
108. Dasgupta P, Baade PD, Aitken JF, Ralph N, Chambers SK, Dunn J. Geographical variations in prostate cancer outcomes: A systematic review of international evidence. Front Oncol (2019) 9:238. doi: 10.3389/fonc.2019.00238
109. Uemura H, Ye D, Kanesvaran R, Chiong E, Lojanapiwat B, Pu Y-S, et al. United in Fight against prOstate cancer (UFO) registry: first results from a large, multi-centre, prospective, longitudinal cohort study of advanced prostate cancer in Asia. BJU Int (2020) 125(4):541–52. doi: 10.1111/bju.14980
110. Lew I. Managed care implications in castration-resistant prostate cancer. Am J Manag Care (2013) 19(18 Suppl):s376–81.
111. Mottet n, Cornford P, Van den Bergh RCN, Briers E, De Santis M, Gillessen S, et al. EAU – EANM – ESTRO – ESUR – ISUP – SIPG Guidelines on Prostate Cancer: European Association of Urology (2022). Available at: https://d56bochluxqnz.cloudfront.net/documents/full-guideline/EAU-EANM-ESTRO-ESUR-ISUP_SIOG-Guidelines-on-Prostate-Cancer-2022_2022-04-25-063938_yfos.pdf.
112. Parker C, Castro E, Fizazi K, Heidenreich A, Ost P, Procopio G, et al. Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol (2020) 31(9):1119–34. doi: 10.1016/j.annonc.2020.06.011
113. Woon DTS, Chandrasekar T, Aaron L, Basappa NS, Chi KN, Conter HJ, et al. Disparity in public funding of therapies for metastatic castrate-resistant prostate cancer across Canadian provinces. Can Urol Assoc J (2018) 12(10). doi: 10.5489/cuaj.5378
114. Engel-Nitz NM, Alemayehu B, Parry D, Nathan F. Differences in treatment patterns among patients with castration-resistant prostate cancer treated by oncologists versus urologists in a US managed care population. Cancer Manag Res (2011) 3:233–45. doi: 10.2147/CMAR.S21033
115. Gajra A, Hime Rupard S, Jeune-Smith Y, Feinberg B. Referral patterns and treatment preferences in patients with advanced prostate cancer: Differences between medical oncologists and urologists. J Clin Pathways (2021) 7(8):32–6. doi: 10.25270/jcp.2021.10.2
116. Caram MEV, Kaufman SR, Modi PK, Herrel L, Oerline M, Ross R, et al. Adoption of abiraterone and enzalutamide by urologists. Urology (2019) 131:176–83. doi: 10.1016/j.urology.2019.05.012
117. Lowrance WT, Breau R, Chou R, Chapin BF, Crispino T, Creicer R, et al. Advanced prostate cancer: AUA/ASTRO/SUO Guideline 2020. J Urol (2020) 205(1):14–29. doi: 10.1097/JU.0000000000001375
118. Messina C, Cattrini C, Soldato D, Vallome G, Caffo O, Castro E, et al. BRCA mutations in prostate cancer: Prognostic and predictive implications. J Oncol (2020) 2020:4986365. doi: 10.1155/2020/4986365
119. Scott RJ, Mehta A, Macedo GS, Borisov PS, Kanesvaran R, El Metnawy W. Genetic testing for homologous recombination repair (HRR) in metastatic castration-resistant prostate cancer (mCRPC): challenges and solutions. Oncotarget (2021) 12(16):1600–14. doi: 10.18632/oncotarget.28015
120. Wallis CJD, Klaassen Z, Jackson WC, Dess RT, Reichert ZR, Sun Y, et al. Olaparib vs cabazitaxel in metastatic castration-resistant prostate cancer. JAMA Network Open (2021) 4(5):e2110950. doi: 10.1001/jamanetworkopen.2021.10950
121. United States Food and Drug Administration. PLUVICTO® (lutetium Lu-vipivotide tetraxetan) prescribing information (2022). Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215833s000lbl.pdf.
122. Jazayeri SB, Srivastava A, Shore N. Review of second-generation androgen receptor inhibitor therapies and their role in prostate cancer management. Curr Opin Urol (2022) 32(3):283–91. doi: 10.1097/MOU.0000000000000984
123. Thiery-Vuillemin A, Poulsen MH, Lagneau E, Ploussard G, Birtle A, Dourthe LM, et al. Impact of abiraterone acetate plus prednisone or enzalutamide on patient-reported outcomes in patients with metastatic castration-resistant prostate cancer: Final 12-mo analysis from the observational AQUARiUS study. Eur Urol (2020) 77(3):380–7. doi: 10.1016/j.eururo.2019.09.019
124. Lin YT, Huang YC, Liu CK, Lee TS, Chen M, Chien YN. Treatment-emergent co-morbidities and survival in patients with metastatic castration-resistant prostate cancer receiving abiraterone or enzalutamide. Front Pharmacol (2021) 12:669236. doi: 10.3389/fphar.2021.669236
125. El-Amm J, Nassabein R, Aragon-Ching JB. Impact of abiraterone on patient-related outcomes in metastatic castration-resistant prostate cancer: current perspectives. Cancer Manag Res (2017) 9:299–306. doi: 10.2147/CMAR.S139305
126. Kretschmer A, van den Bergh RCN, Martini A, Marra G, Valerio M, Tsaur I, et al. Assessment of health-related quality of life in patients with advanced prostate cancer – current state and future perspectives. Cancers (Basel) (2022) 14(1):147. doi: 10.3390/cancers14010147
127. Connor MJ, Genie MG, Burns D, Bass EJ, Gonzalez M, Sarwar N, et al. A systematic review of patients’ values, preferences, and expectations for the treatment of metastatic prostate cancer. Eur Urol Open Sci (2022) 36:9–18. doi: 10.1016/j.euros.2021.10.003
128. Clark MJ, Harris N, Griebsch I, Kaschinski D, Copley-Merriman C. Patient-reported outcome labeling claims and measurement approach for metastatic castration-resistant prostate cancer treatments in the United States and European Union. Health Qual Life Outcomes (2014) 12(1):104. doi: 10.1186/s12955-014-0104-5
129. Kretschmer A, Ploussard G, Heidegger I, Tsaur I, Borgmann H, Surcel C, et al. Health-related quality of life in patients with advanced prostate cancer: a systematic review. Eur Urol Focus (2021) 7(4):742–51. doi: 10.1016/j.euf.2020.01.017
130. Sartor O, Flood E, Beusterien K, Park J, Webb I, MacLean D, et al. Health-related quality of life in advanced prostate cancer and its treatments: biochemical failure and metastatic disease populations. Clin Genitourin Cancer (2015) 13(2):101–12. doi: 10.1016/j.clgc.2014.08.001
131. Nussbaum N, George DJ, Abernethy AP, Dolan CM, Oestreicher N, Flanders S, et al. Patient experience in the treatment of metastatic castration-resistant prostate cancer: state of the science. Prostate Cancer Prostatic Dis (2016) 19(2):111–21. doi: 10.1038/pcan.2015.42
132. Morgans AK, van Bommel AC, Stowell C, Abrahm JL, Basch E, Bekelman JE, et al. Development of a standardized set of patient-centered outcomes for advanced prostate cancer: an international effort for a unified approach. Eur Urol (2015) 68(5):891–8. doi: 10.1016/j.eururo.2015.06.007
133. Holmstrom S, Naidoo S, Turnbull J, Hawryluk E, Paty J, Morlock R. Symptoms and impacts in metastatic castration-resistant prostate cancer: qualitative findings from patient and physician interviews. Patient (2019) 12(1):57–67. doi: 10.1007/s40271-018-0349-x
134. Prostate Cancer Foundation. Genetic Testing for Prostate Cancer (2023). Available at: https://www.pcf.org/patient-resources/family-cancer-risk/genetic-testing-prostate-cancer/.
135. Herberts C, Wyatt AW, Nguyen PL, Cheng HH. Genetic and genomic testing for prostate cancer: beyond DNA repair. Am Soc Clin Oncol Educ Book (2023) 43:e390384. doi: 10.1200/EDBK_390384
136. Chiong E, Murphy DG, Buchan NC, Chua MLK, Hakim L, Hamid AR, et al. Managing advanced prostate cancer in the Asia Pacific region: "Real-world" application of Advanced Prostate Cancer Consensus Conference 2019 statements. Asia Pac J Clin Oncol (2022) 18(6):686–95. doi: 10.1111/ajco.13722
Keywords: metastatic castration-resistant prostate cancer, treatment landscape, treatment pattern, burden of disease, cost, economic burden
Citation: Leaning D, Kaur G, Morgans AK, Ghouse R, Mirante O and Chowdhury S (2023) Treatment landscape and burden of disease in metastatic castration-resistant prostate cancer: systematic and structured literature reviews. Front. Oncol. 13:1240864. doi: 10.3389/fonc.2023.1240864
Received: 15 June 2023; Accepted: 09 August 2023;
Published: 27 September 2023.
Edited by:
Neal Shore, GenesisCare, United StatesReviewed by:
Nicolas Sayegh, The University of Utah, United StatesCopyright © 2023 Leaning, Kaur, Morgans, Ghouse, Mirante and Chowdhury. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Darren Leaning, ZC5sZWFuaW5nQG5ocy5uZXQ=
†Present address: Gagandeep Kaur, Medical Communications, Pharmacoevidence Pvt. Ltd., Mohali, Punjab, India
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.