
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol., 24 August 2023
Sec. Gastrointestinal Cancers: Colorectal Cancer
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1240013
Poorly cohesive duodenal carcinoma mixed with signet ring cell carcinoma is very rare, and no cases have been reported. When distant metastasis occurs, it is very easy to be misdiagnosed. We report the first case of a 52-year-old man with poorly cohesive carcinoma of the duodenum mixed with signet ring cell carcinoma with systemic metastasis. The process of its diagnosis and differential diagnosis is highlighted.
Primary duodenal cancer is rare, accounting for only 0.3%–0.5% of cancers of the digestive tract (1). Duodenal adenocarcinoma is the most common type of duodenal cancer. In a study of duodenal cancer, the proportion was subdivided into adenocarcinoma 87%, mucinous adenocarcinoma 7%, and signet ring cell carcinoma 1% (2). According to the fifth edition of the WHO Classification of Tumors of the Digestive System (3), among tumors of the small intestine and ampullary region, poorly cohesive carcinoma (PCC) is classified as adenocarcinoma of the small intestine not otherwise specified (SBAs-NOS). Among the subtypes of poorly cohesive small intestinal carcinomas, PCC not otherwise specified (PCC-NOS) consisting of less than 10% signet ring cells accounts for the majority, while PCC-NOS and signet ring cell carcinomas with 10%–90% signet ring cell components are relatively rare (4, 5). More than 96% of signet ring cell cancers occur in the stomach, with the remainder occurring primarily in the breast, gallbladder, pancreas, bladder, and intestine (6, 7).
Duodenal carcinoma is relatively rare, and poorly cohesive duodenal carcinoma mixed with signet ring cell carcinoma with systemic metastasis is very rare. It has not been fully elucidated in many aspects and is easy to be misdiagnosed. Therefore, this article will summarize the diagnosis and treatment process of poorly cohesive duodenal carcinoma mixed with signet ring cell carcinoma, so as to let clinicians have more understanding of the disease.
A 52-year-old man had persistent cough for 1 month due to novel coronavirus infection, and the anticough drugs were not effective. Chest computed tomography (CT) performed at another hospital suggested the possibility of left lung cancer, with enlarged lymph nodes in both lungs and mediastinum. He came to our hospital for further treatment. Physical examination on admission revealed stable respiratory rate, regular rhythm, normal and symmetrical auscultation breath sounds, and no obvious rales and wheezing. Scattered patchy skin macules were seen on the trunk, chest, abdomen, back, and lower limbs. Multiple lymph nodes were palpable on the left supraclavicular and bilaterally in the groin.
After the patient’s admission, contrast-enhanced CT of the chest and abdomen, tumor markers for lung cancer, tumor markers for gastrointestinal tract, and gastrointestinal endoscopy were performed to further confirm the situation. Gastroscopy showed a mass in the descending duodenum (Figure 1). Contrast-enhanced CT of the chest and abdomen (Figure 2) was considered to be lymphoma, and the possibility of sarcoidosis and lung cancer could not be excluded. The specific findings were as follows: multiple lymph nodes in the left clavicle region, mediastinum, bilateral hilar lung, diaphragmatic crus, portal vein space, abdominal cavity, retroperitoneal cavity and bilateral groin, partial enlargement. The submucosal wall of the descending duodenum was scattered and thickened. Lymphoma was considered in all these cases, but sarcoidosis was not excluded. Bronchoscopic biopsy was performed to exclude lung cancer if necessary.
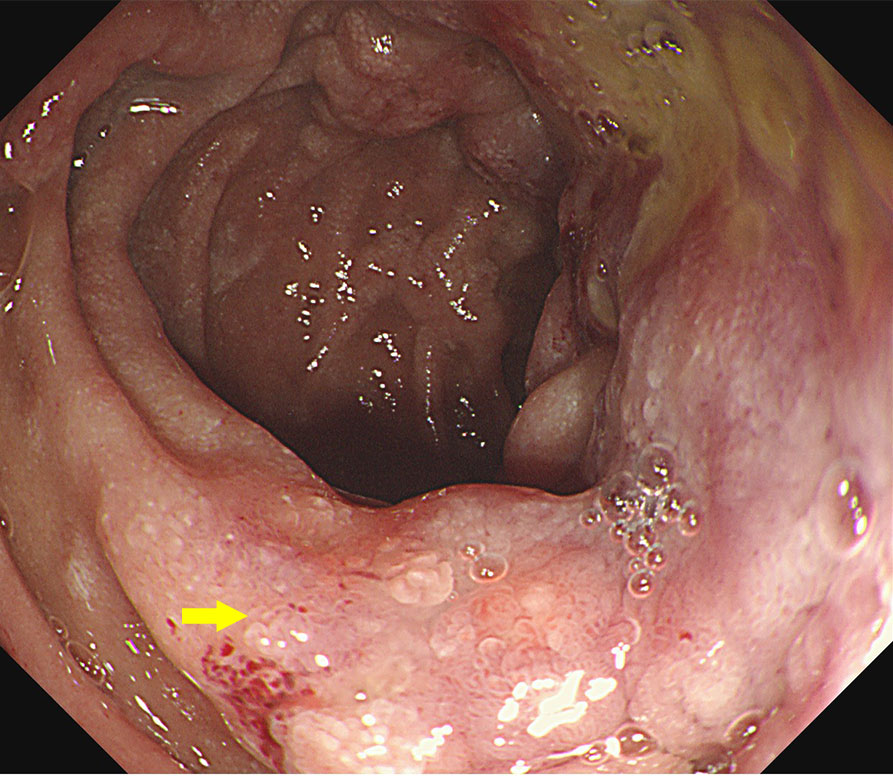
Figure 1 Endoscopic images at the time of the first admission. The descending duodenum was seen with thickening of the intestinal wall and surrounding ulceration.
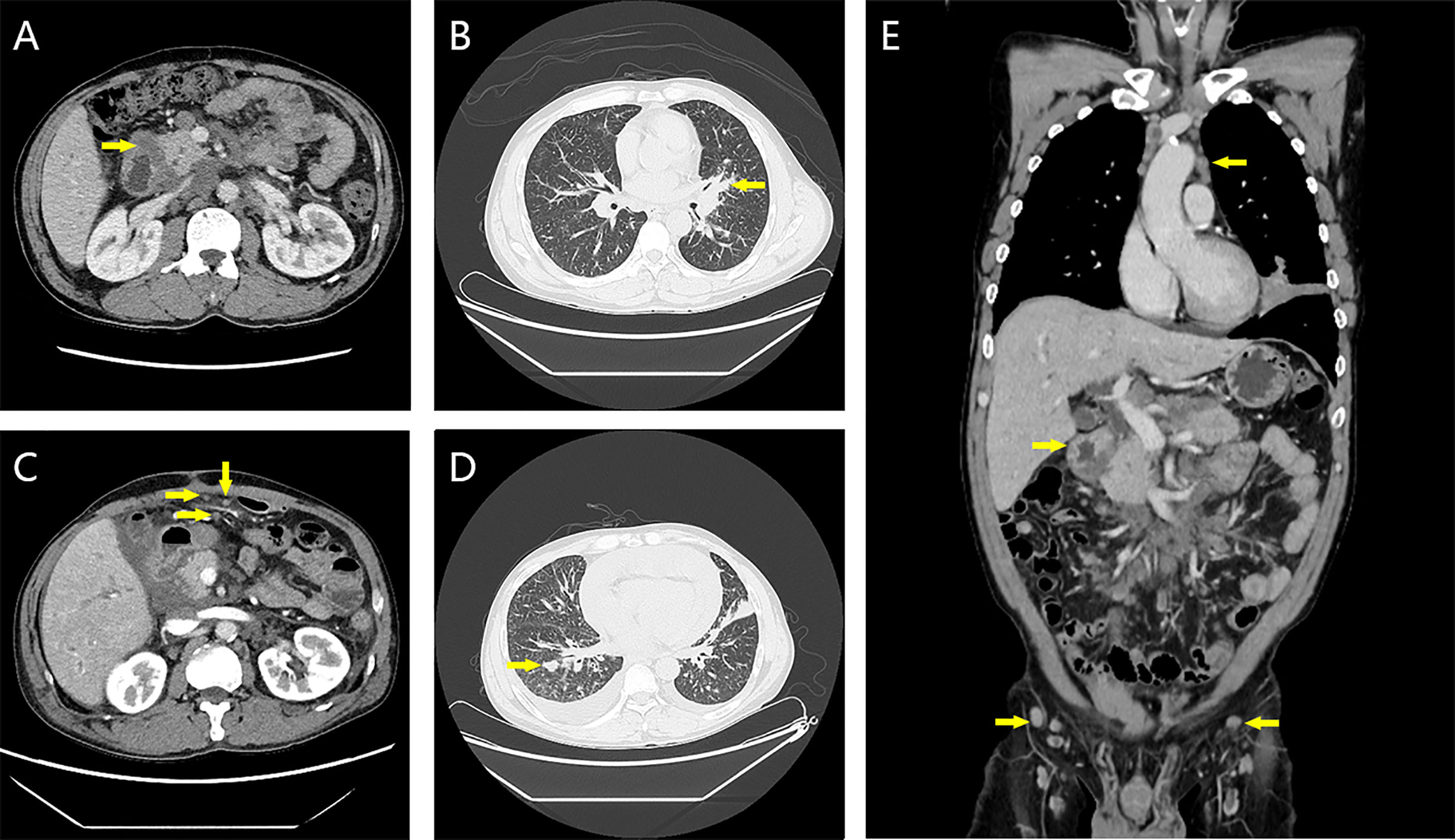
Figure 2 Contrast-enhanced CT images at first admission and 2 weeks after three cycles of chemotherapy. (A) The wall of the descending duodenum was thickened at the first admission. (B) Bronchus occlusion in the lingual segment of the left upper lobe of the lung on the first admission. (C) Irregular thickening of partial peritoneum, omentum, and mesentery at 2 weeks after the third cycle of chemotherapy. (D) New solid nodule in the right lung 2 weeks after the third cycle of chemotherapy. (E) On the first admission, thickening of the intestinal wall of the descending duodenum and multiple mediastinal and inguinal lymphadenopathy were seen.
Multiple tumor markers were elevated: carcinoembryonic antigen (CEA), 2,687 ng/mL; neuron-specific enolase (NSE), 29.58 ng/mL; squamous cell carcinoma antigen (SCC), 6.2 ng/mL; cytokeratin 19 fragment assay (CYFRA21-1), 37.49 ng/mL; carbohydrate antigen (CA)125, 43.15 U/mL; CA72-4, 600 U/mL; CA19-9, 472.4 U/mL. See Table 1 for the complete spelling of the abbreviations
Because of the uncertainty of the diagnosis, a multidisciplinary consultation was conducted, including input from medical oncology, imaging, intervention, endoscopy, and intensive care units. First, fiberoptic bronchoscopy was performed to obtain tissue biopsy of the left upper bronchus, and puncture biopsy of the left lung mass was performed to determine the condition of the left lung mass. Second, biopsy of the left supraclavicular lymph node was performed. Third, biopsy of duodenal mass was taken under a gastroscope to confirm the situation. Fourth, positron emission tomography (PET) and computed tomography (CT) were performed to confirm the general condition.
PET-CT was the first to show the results. The extent of the tumor and lymph node was the same as that of CT, and lymphoma was also considered.
The biopsy results (Figure 3) indicate the presence of PCC in various locations: ①Descending duodenum: Mucus and a few signet ring cells were observed, consistent with PCC (including signet ring cell carcinoma). ②Left upper bronchus: Diagnosed as PCC. Combined with immunohistochemistry, the possibility of gastrointestinal metastasis was considered. ③Left lung mass: Presence of mucus and signet ring cell mass. Combined with immunohistochemical phenotype and clinical history, the tumor was consistent with lung metastasis of PCC of the digestive tract (including signet ring cell carcinoma). ④Left supraclavicular lymph node: Considered to be metastatic with poorly cohesive cancer.
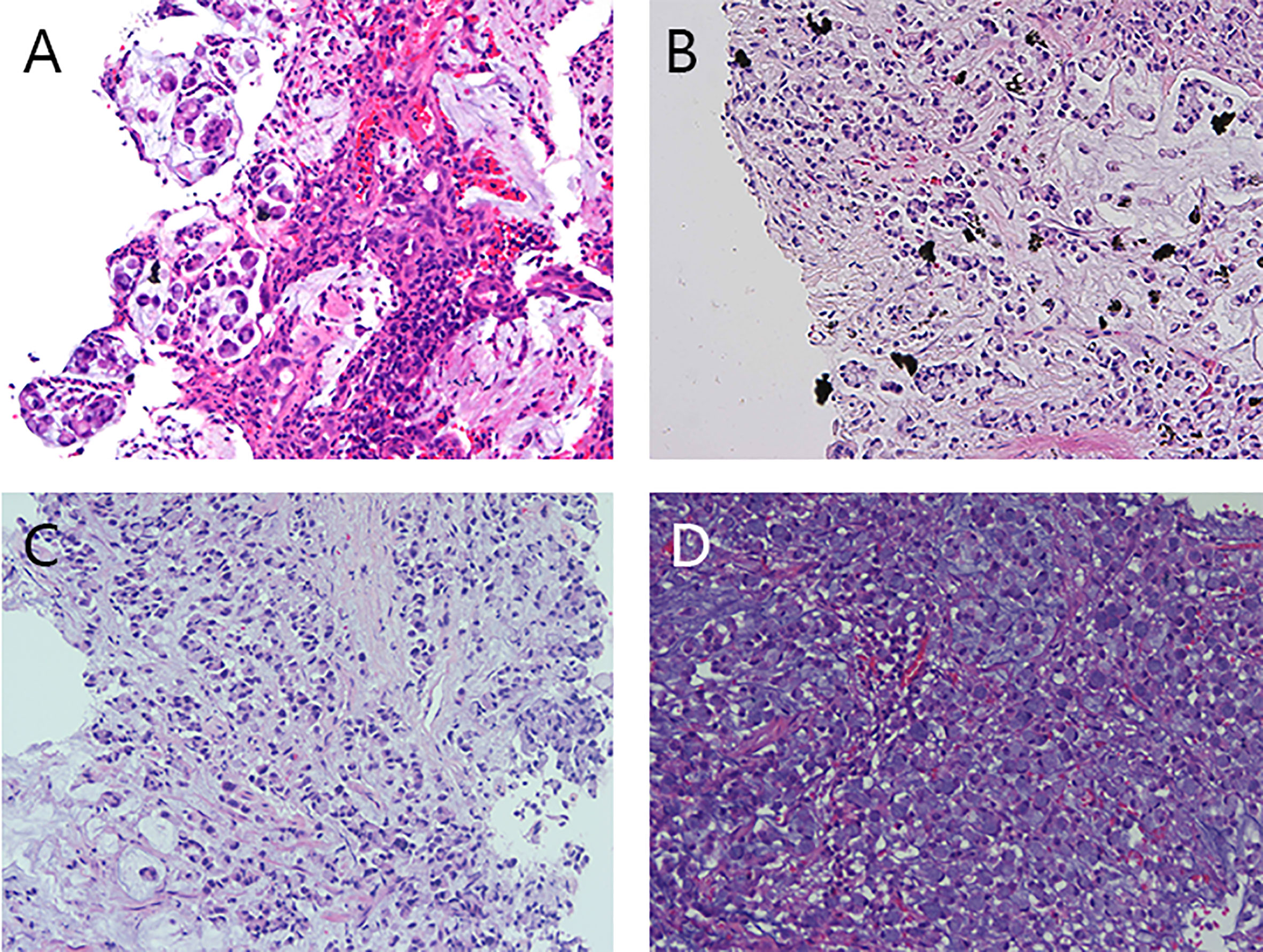
Figure 3 Preoperative pathological images of the descending duodenal mass, left lung mass, left upper bronchus, and left supraclavicular lymph node. (A) The pathological image of the mass in the descending duodenum showed mucus and a small amount of signet ring cells. (B) Pathological image of the left lung mass, showing mucus and signet ring cell mass. (C) Pathological image of the left upper bronchus, poorly cohesive carcinoma. (D) Left supraclavicular lymph node, lymph node cancer metastasis, poorly cohesive carcinoma was considered.
Immunohistochemical results were shown in Figure 4 and Table 2.
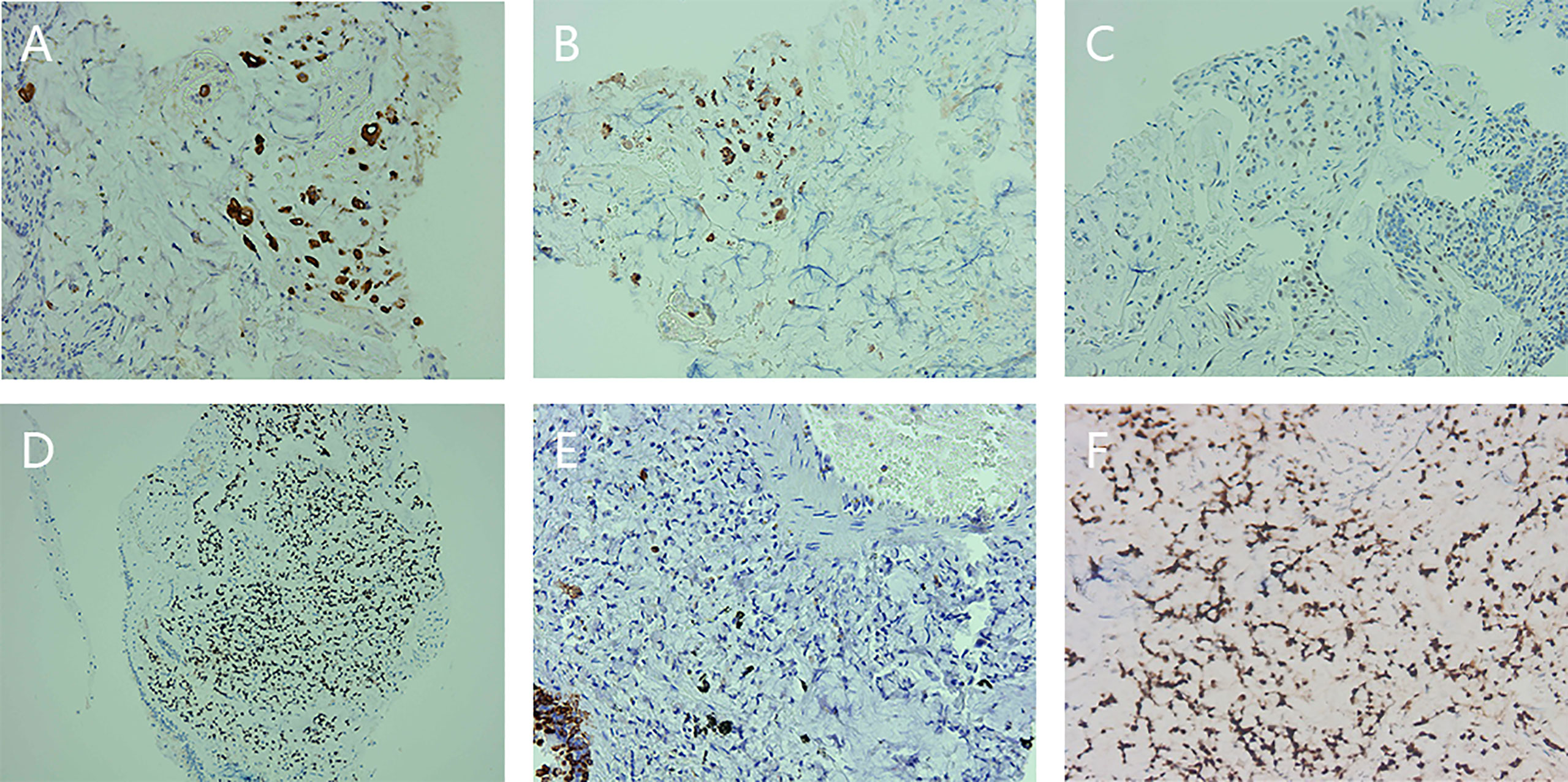
Figure 4 Preoperative immunohistochemical staining of descending duodenum, left lung mass, and left upper bronchus. (A) Immunohistochemical staining for cytokeratin (CK) expression in the descending duodenum. (B) Immunohistochemical staining for CK20 expression in the descending duodenum. (C) Immunohistochemical staining of P53 expression in the descending duodenum. (D) Immunohistochemical staining of caudal-type homeobox transcription factor 2 (CDX2) expression in the left lung mass. (E) Immunohistochemical staining of CK7 expression in the left lung mass. (F) Immunohistochemical staining for CDX2 expression in the left upper bronchus.
Based on the imaging and pathological and immunohistochemical results, the diagnosis of poorly cohesive duodenal carcinoma mixed with signet ring cell carcinoma with systemic metastasis was confirmed.
Considering the late stage and no possibility of radical resection, the patient and his family decided to undergo palliative bypass surgery to prevent further ampullary and duodenal obstruction. The specific plan was choledochojejunostomy, cholecystectomy, and Roux-en-y gastrojejunostomy.
The patient received three cycles of mFOLFOX6 (oxaliplatin 85 mg/m2, calcium folinate 400 mg/m2, fluorouracil 400 mg/m2, fluorouracil 2,400 mg/m2) chemotherapy after surgery. During this period, the patient’s left lung metastases and some lymph nodes shrank, and his cough resolved. The tumor progressed 2 weeks after cycle 3, with chest tightness, shortness of breath, cough, and expectoration. CT showed the possibility of bronchial spread, which was slightly progressive. The systemic lymph nodes were enlarged and increased, part of the peritoneum, omentum, and mesentery were irregularly thickened, implantation metastasis was considered, the bilateral pleurae were irregularly thickened, metastasis could not be excluded, and bilateral pleural effusion and pericardial effusion were massive. After supportive care, the patient received two cycles of FOLFIRI (irinotecan 180 mg/m2, calcium folinate 400 mg/m2, fluorouracil 400 mg/m2, fluorouracil 2,400 mg/m2) chemotherapy. The multidisciplinary consultation suggested that a large panel gene test should be performed to provide the basis for the next medication, but the patient with poor economic ability and short life expectancy refused our suggestion. Unfortunately, the tumor progressed rapidly, and the patient died of heart failure 35 days after cycle 2 of the FOLFIRI chemotherapy regimen.
Duodenal adenocarcinoma is the most common pathological type of duodenal cancer. It is most commonly found in the second part of the duodenum, followed by the third and fourth parts, and the first part, especially the duodenal bulb, is very rare (8–10). Our case of tumor was in the second part.
The pathological feature of PCC is that the PCC pattern accounts for more than 50% of the tumor (4). There are three subtypes of PCC in the small intestine. If the signet ring cells account for more than 90%, it is classified as signet ring cell carcinoma. If the signet ring cell component accounts for 10%–90%, it is classified as PCC and signet ring cell carcinoma not otherwise specified. Most PCCs in the small intestine consist of less than 10% signet ring cells, which are designated as PCC-NOS (4, 5). In our case, radical resection was not performed, only biopsy results were available, and the specific proportion of signet ring cell components was unknown, so it was difficult to accurately classify. However, combined with multiple pathological and immunohistochemical results, and the advice of pathologists, we considered the diagnosis of PCC mixed with signet ring cell carcinoma, that is, the proportion of signet ring cell component is 10%–90%.
Duodenal adenocarcinoma is usually difficult to diagnose. When symptoms appear, most of them are at an advanced stage, and the opportunity for radical resection is lost. Abdominal pain is the most common symptom of duodenal adenocarcinoma; other symptoms include nausea, vomiting, fatigue, weakness, and weight loss (8). Anemia, intestinal obstruction, and jaundice are advanced symptoms (8). However, the present case presented with cough as the first symptom, elevated amylase but no jaundice, and no abdominal pain so far, which undoubtedly increases the difficulty of diagnosis.
In terms of diagnosis and differential diagnosis, this case was mainly differentiated from lung cancer, lymphoma, sarcoidosis, and ampullary carcinoma in the actual process. In the presence of the possibility of multiple diseases, we first consider that all lesions and symptoms are caused by a single disease. Therefore, differential diagnosis is very important.
Endoscopy is the examination of choice for duodenal cancer and enables simultaneous visualization and biopsy (7, 8). Enhanced CT and magnetic resonance imaging (MRI) can be used to assess the depth of tumor invasion, regional lymph node invasion, and distant metastasis, which are important for determining tumor resectability as well as for treatment planning (7, 8, 11). PET and CT combined (PET-CT) have become important advanced imaging techniques in oncology, which are used in tumor staging, treatment response assessment, restaging, and longitudinal recurrence monitoring (12). They are also the most sensitive and accurate methods for staging distant metastasis (13). In this case, lung cancer, lymphoma, and sarcoidosis had been considered on imaging examinations. However, endoscopic biopsy and immunohistochemistry finally confirmed PCC mixed with signet ring cell carcinoma of the duodenum.
However, for duodenal carcinoma with distant metastasis, identification of the primary lesion and differential diagnosis require more examination methods, such as pathology, immunohistochemistry, and tumor markers. Thyroid transcription factor 1 (TTF-1) is a key single marker for lung adenocarcinoma (14). TTF-1, cytokeratin (CK)7, and CK20 can be used to distinguish primary and secondary lung adenocarcinomas, and the combination of TTF-1 negative, CK7 negative, and CK20 positive is highly correlated with adenocarcinoma of digestive tract origin (15). The immunohistochemistry of the left lung mass in our case belonged to this combination, so the primary tumor was considered to be of gastrointestinal origin.
Both duodenal carcinoma and ampullary carcinoma belong to periampullary carcinoma. It is often difficult to distinguish them based on morphology alone, but immunohistochemistry can help us to identify them. CK17, mucin 4 (MUC4), and MUC1 have been found to be expressed in the pancreaticobiliary type, whereas CK20, MUC2, and caudal-type homeobox transcription factor 2 (CDX2) are expressed in the intestinal type (16). In our case, CK20 and CDX2 were positive in the descending duodenum, left upper bronchus, and left lung mass, and MUC2 was also positive in the left lung mass. Therefore, ampullary carcinoma was excluded and duodenal carcinoma was considered. Endoscopy also revealed a mass in the lateral wall of the descending duodenum. Immunohistochemistry of different tumors is shown in Table 3.
Lymphoma can present with extranodal involvement (18). Invasion of the lungs then may cause cough, difficulty breathing, and other symptoms (19). Invasion of the digestive tract will cause gastrointestinal mass, abdominal pain, and other symptoms (20). When the skin is invaded, skin lesions occur (21). Our case had multiple lymph node enlargement, skin lesions, and pulmonary and duodenal masses. Therefore, lymphoma needs to be considered. Sarcoidosis is a noncaseating necrotizing granulomatous disease that can involve every organ, often involving the lungs, bilateral hilar lymph nodes, skin, and other organs (22). The patient had a left pulmonary mass, bilateral hilar lymphadenopathy, and rash. Therefore, sarcoidosis is also under consideration. The diagnosis of lymphoma and sarcoidosis is based on biopsy, and in addition to morphology, there are also typical immunophenotypes and molecular lesions to aid the diagnosis. Judging from the results of the biopsy, the diagnosis of lymphoma and sarcoidosis can be ruled out in our case.
The detection of tumor markers in the blood has certain reference values for tumor screening and diagnosis. Tumor markers in the gastrointestinal tract mainly include CEA, CA19-9, CA125, and CA72-4, of which CEA has the highest sensitivity (23–26), and tumor markers in lung cancer include CEA, CYFRA21-1, SCC, and NSE (27). In our case, tumor markers were elevated in both the gastrointestinal tract and the lung, indicating that the tumor markers were nonspecific and cannot be relied on alone to determine the origin of the primary tumor.
In addition, supraclavicular lymph node metastasis also plays a role in distinguishing the origin of the tumor. Metastasis of the left supraclavicular lymph node (Virchow lymph node) is associated with abdominal tumors, whereas the right supraclavicular lymph node is generally considered to be of thoracic origin (28). In this case, the left supraclavicular lymph node metastasis was found, which also confirmed that the primary lesion was in the gastrointestinal tract to a certain extent, and excluded the diagnosis of lung cancer.
The only possible cure for duodenal cancer is radical resection (29). Pancreaticoduodenectomy is considered the primary treatment option for eligible patients who can undergo radical tumor resection, while for palliative surgery, gastrointestinal and biliary bypass surgery is most commonly performed (7, 29). In our case, the cancer was advanced and incurable, so palliative bypass surgery was chosen.
The pathogenesis of small bowel cancer appears to be similar to that of colorectal cancer (30), and the treatment of small bowel adenocarcinoma has historically been based on the treatment strategies of colorectal cancer (5). In duodenal cancer, fluorouracil plus oxaliplatin is the most commonly used systemic chemotherapy regimen, with response rates ranging from 34% to 42%, median progression-free survival ranging from 6.9 to 8.2 months, and median overall survival ranging from 17.8 to 22.2 months. Irinotecan, cisplatin, and gemcitabine have also been reported. However, the response rate, median progression-free survival, and median overall survival were all relatively low (11). However, signet ring cell carcinoma can reduce the sensitivity to chemotherapy, and there is no literature to show the effect of systemic chemotherapy on duodenal signet ring cell carcinoma. The progression-free survival of our case was only 2 months, confirming to some extent the high malignancy and low sensitivity to chemotherapy of signet ring cell carcinoma. Previous studies did not recommend Her-2 and Ras gene testing in patients with duodenal cancer but did recommend testing for microsatellite instability (MSI) and mismatch repair (MMR) proteins and pembrolizumab monotherapy for MSI-high and Mismatch repair deficiency (dMMR) patients with duodenal cancer (11).
There are several important points in the diagnostic process in this case that should be noted. First, although abdominal pain is the most common symptom of duodenal adenocarcinoma, when metastatic disease develops, the first symptom may be caused by metastatic disease. Secondly, when considering a pulmonary malignant tumor, it is necessary to determine whether the tumor is primary or secondary. Finally, poorly cohesive duodenal carcinoma mixed with signet ring cell carcinoma is very rare, and it is easy to be misdiagnosed when the tumor is metastatic. The diagnosis often requires a comprehensive combination of endoscopy, imaging, pathology, immunohistochemistry, tumor markers, and even the location of supraclavicular lymph nodes.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Written informed consent was obtained from the individual for the publication of any potentially identifiable images or data included in this article.
ST and XL were the patient’s physicians and collected the patient’s data. ST reviewed the literature and completed the first draft of the article. AW reviewed the article and made revisions. All authors contributed to the article and approved the submitted version.
This work was supported by Guangzhou Royallee Cancer Center.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Alwmark A, Andersson ÅC, Lasson A. Primary carcinoma of the duodenum. Ann Surg (1980) 191(1):13–8. doi: 10.1097/00000658-198001000-00003
2. Buchbjerg T, Fristrup C, Mortensen MB. The incidence and prognosis of true duodenal carcinomas. Surg Oncol (2015) 24(2):110–6. doi: 10.1016/j.suronc.2015.04.004
3. Lokuhetty D, White VA, Watanabe R, Cree IA, World Health O, International Agency for Research on C. Digestive System Tumours. Fifth. Lyon: International Agency for Research on Cancer (2019).
4. Vanoli A, Guerini C, Grillo F, Klersy C, Fassan M, Arpa G, et al. Poorly cohesive carcinoma of the nonampullary small intestine: A distinct histologic subtype with prognostic significance. Am J Surg Pathol (2022) 46(4):498–508. doi: 10.1097/PAS.0000000000001821
5. Tedaldi G, Guerini C, Angeli D, Furlan D, Libera L, Lenti MV, et al. Molecular landscape and association with crohn disease of poorly cohesive carcinomas of the nonampullary small bowel. Am J Clin Pathol (2023) 159(4):315–24. doi: 10.1093/ajcp/aqac161
6. Tung SY, Wu CS, Chen PC. Primary signet ring cell carcinoma of colorectum: an age- and sex-matched controlled study. Am J Gastroenterol (1996) 91(10):2195–9.
7. Ye N, Bao X, Zhao X, Wang B. Signet-ring cell carcinoma of the duodenal bulb presenting with gastrointestinal hemorrhage: a case report and literature review. BMC Gastroenterol (2022) 22(1):226. doi: 10.1186/s12876-022-02267-0
8. Cloyd J, George E, Visser B. Duodenal adenocarcinoma: Advances in diagnosis and surgical management. World J Gastri Surg (2016) 8(3):212–21. doi: 10.4240/wjgs.v8.i3.212
9. Ross R, Hartnett N, Bernstein L, Henderson B. Epidemiology of adenocarcinomas of the small intestine: is bile a small bowel carcinogen? Bri J Cancer (1991) 63(1):143–5. doi: 10.1038/bjc.1991.29
10. Goldner B, Stabile B. Duodenal adenocarcinoma: why the extreme rarity of duodenal bulb primary tumors? Am Surg (2014) 80(10):956–9. doi: 10.1177/000313481408001010
11. Nakagawa K, Sho M, Fujishiro M, Kakushima N, Horimatsu T, Okada KI, et al. Clinical practice guidelines for duodenal cancer 2021. J Gastroenterol (2022) 57(12):927–41. doi: 10.1007/s00535-022-01919-y
12. Koppula BR, Fine GC, Salem AE, Covington MF, Wiggins RH, Hoffman JM, et al. PET-CT in clinical adult oncology: III. Gastrointestinal Malignancies. Cancers (2022) 14(11):2668. doi: 10.3390/cancers14112668
13. Gao G, Gong B, Shen W. Meta-analysis of the additional value of integrated 18FDG PET-CT for tumor distant metastasis staging: comparison with 18FDG PET alone and CT alone. Surg Oncol (2013) 22(3):195–200. doi: 10.1016/j.suronc.2013.06.004
14. Yatabe Y, Dacic S, Borczuk AC, Warth A, Russell PA, Lantuejoul S, et al. Best practices recommendations for diagnostic immunohistochemistry in lung cancer. J Thorac Oncol (2019) 14(3):377–407. doi: 10.1016/j.jtho.2018.12.005
15. Su YC, Hsu YC, Chai CY. Role of TTF-1, CK20, and CK7 immunohistochemistry for diagnosis of primary and secondary lung adenocarcinoma. Kaohsiung J Med Sci (2006) 22(1):14–9. doi: 10.1016/S1607-551X(09)70214-1
16. Bakshi N, Dhawan S, Nundy S, Rao S, Chopra P, Bhalla S. Role of immunohistochemistry in the subtyping of periampullary adenocarcinoma. Int J Surg Pathol (2019) 27(6):598–608. doi: 10.1177/1066896919837606
17. Higgins RA, Blankenship JE, Kinney MC. Application of immunohistochemistry in the diagnosis of non-Hodgkin and Hodgkin lymphoma. Arch Pathol Lab Med (2008) 132(3):441–61. doi: 10.5858/2008-132-441-AOIITD
18. Thomas AG, Vaidhyanath R, Kirke R, Rajesh A. Extranodal lymphoma from head to toe: part 2, the trunk and extremities. AJR Am J Roentgenol (2011) 197(2):357–64. doi: 10.2214/AJR.11.6738
19. Chien CC, Lee HS, Lin MH, Hsieh PP. Primary extranodal natural killer/T-cell lymphoma of bronchus and lung: A case report and review of literature. Thorac Cancer (2016) 7(1):140–4. doi: 10.1111/1759-7714.12254
20. Olszewska-Szopa M, Wróbel T. Gastrointestinal non-Hodgkin lymphomas. Adv Clin Exp Med: Off Organ Wroclaw Med Univ (2019) 28(8):1119–24. doi: 10.17219/acem/94068
21. Morris SL. Skin lymphoma. Clin Oncol (Royal Coll Radiol (Great Britain)) (2012) 24(5):371–85. doi: 10.1016/j.clon.2012.02.007
22. Valeyre D, Brauner M, Bernaudin JF, Carbonnelle E, Duchemann B, Rotenberg C, et al. Differential diagnosis of pulmonary sarcoidosis: a review. Front Med (2023) 10:1150751. doi: 10.3389/fmed.2023.1150751
23. Cao H, Zhu L, Li L, Wang W, Niu X. Serum CA724 has no diagnostic value for gastrointestinal tumors. Clin Exp Med (2023). doi: 10.1007/s10238-023-01025-0
24. Gao Y, Wang J, Zhou Y, Sheng S, Qian SY, Huo X. Evaluation of serum CEA, CA19-9, CA72-4, CA125 and ferritin as diagnostic markers and factors of clinical parameters for colorectal cancer. Sci Rep (2018) 8(1):2732. doi: 10.1038/s41598-018-21048-y
25. Shimada H, Noie T, Ohashi M, Oba K, Takahashi Y. Clinical significance of serum tumor markers for gastric cancer: a systematic review of literature by the Task Force of the Japanese Gastric Cancer Association. Gastric Cancer: Off J Int Gastric Cancer Assoc Japanese Gastric Cancer Assoc (2014) 17(1):26–33. doi: 10.1007/s10120-013-0259-5
26. Zhu L, Kim K, Domenico DR, Appert HE, Howard JM. Adenocarcinoma of duodenum and ampulla of Vater: clinicopathology study and expression of p53, c-neu, TGF-alpha, CEA, and EMA. J Surg Oncol (1996) 61(2):100–5. doi: 10.1002/(SICI)1096-9098(199602)61:2<100::AID-JSO3>3.0.CO;2-G
27. Bi H, Yin L, Fang W, Song S, Wu S, Shen J. Association of CEA, NSE, CYFRA 21-1, SCC-ag, and proGRP with clinicopathological characteristics and chemotherapeutic outcomes of lung cancer. Lab Med (2022) 54(4):372–9. doi: 10.1093/labmed/lmac122
28. Fernández Aceñero M, Caso Viesca A, Díaz Del Arco C. Role of fine needle aspiration cytology in the management of supraclavicular lymph node metastasis: Review of our experience. Diagno Cytopatho (2019) 47(3):181–6. doi: 10.1002/dc.24064
29. Solej M, D’Amico S, Brondino G, Ferronato M, Nano M. Primary duodenal adenocarcinoma. Tumori (2008) 94(6):779–86. doi: 10.1177/030089160809400601
Keywords: duodenal carcinoma, poorly cohesive carcinoma, signet ring cell carcinoma, systemic metastasis, case report
Citation: Tang S, Li X and Wu A (2023) Poorly cohesive duodenal carcinoma mixed with signet ring cell carcinoma with systemic metastasis: a case report and literature review. Front. Oncol. 13:1240013. doi: 10.3389/fonc.2023.1240013
Received: 14 June 2023; Accepted: 01 August 2023;
Published: 24 August 2023.
Edited by:
Antonio Mario Scanu, University of Sassari, ItalyReviewed by:
Ludovico Carbone, University of Siena, ItalyCopyright © 2023 Tang, Li and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aiguo Wu, d2FndHl6QHNpbmEuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.