- 1Department of Urology and Urological Oncology, Multidisciplinary Regional Hospital in, Gorzów Wielkopolski, Poland
- 2Department and Clinic of Hematology, Oncology and Radiotherapy of the University of Zielona Góra, Multidisciplinary Regional Hospital in, Gorzów Wielkopolski, Poland
- 3General and Oncological Urology Clinic, University Hospital No. 1 Dr. Antoni Jurasz, Nicolaus Copernicus University in Toruń, Bydgoszcz, Poland
- 4Clinical Department of Nuclear Medicine with a PET/CT Laboratory of the University of Zielona Góra, Multidisciplinary Regional Hospital in, Gorzów Wielkopolski, Poland
Introduction: Radio-ligand targeted therapy is a new and promising concept of treatment Castration resistant prostate cancer (CRPC). Only a few radio-pharmaceutics were approved for usage in treating prostate cancer, among the multiple others tested. We aimed to review and summarize the literature on the therapeutic isotopes specific for PSMA.
Methods: We performed a scoping literature review of PubMed from January 1996 to December 2022.
Results: 98 publications were selected for inclusion in this review. The studies contained in publications allowed to summarize the data on pharmacokinetics, therapeutic effects, side effects and the medical use of 225Ac and 177Lu radionuclides. The review also presents new research directions for specific PSMA radionuclides.
Conclusion: Radioligand targeted therapy is a new and promising concept where Lu-177-PSMA-617 have promising outcomes in treatment according to standard of care.
1 Introduction
In men, prostate cancer (PC) is the most frequent cancer and the third leading cause of death, worldwide. Due to Prostate-Specific Antigen (PSA) testing, PC is diagnosed in early developmental stages when prostatectomy or radiotherapy can be performed with a successfully curative intent. However, in case of metastatic PC, the androgen deprivation therapy is part of the standard care. Nevertheless, in almost all cases, after an average of 2–3 years, the deprivation therapy may generate hormone-resistance, which is defined as the ability of PC to progress even when the testosterone level is at or below the castrate level. Castration-resistant prostate cancer (CRPC) is characterized by poor prognosis, which is reflected in a survival rate <3 years and with limited treatment modalities. In this case, the therapy includes chemotherapy drugs (e.g., docetaxel, cabazitaxel), inhibitors of androgen receptor signaling (ARSI) (e.g., acetate abiraterone, enzalutamide, and apalutamide), inhibitors of polymerase poli-ADP-ribose (PARP), autologous vaccine “Sipuleucel T”, and recently introduced immune checkpoint inhibitors (1–9). Metastatic CRCP (mCRPC) is a terminal disease, so more efficient measures are needed. Radio-ligand targeted therapy is a new and promising concept, where a radioisotope is bound to an antibodies or a small molecules are linked to the radionuclide and these conjugates recognize an antigen expressed on the membrane of the cancer cell.
The development of imaging methods over the years have led to implementation of new ways for PC treatment. Prostate-specific membrane antigen positron emission tomography (PET-PSMA) highlights cancer cells by absorption of radiation from a diagnostic isotope (i.e., 68Ga). That isotope connects with a prostate-specific membrane antigen (PSMA) on prostate gland cells or PC cells. Radionuclides therapy uses the abovementioned mechanism by replacing the diagnostic isotope with a therapeutic isotope. This isotope connects with cancer cell antigens (PSMA) and emits beta (i.e., lutetium-177, yttrium-90) or alpha (actinium-225) radiation. Cancer cells yield ionizing radiation with a minor impact on healthy cells. Only a few radio-pharmaceutics were approved for usage in treating prostate cancer, among the multiple others tested. The most recent radionuclide approved by the FDA for PC treatment is 177LuPSMA-617 (10, 11).
The following review aims to discuss and provide information about therapeutic isotopes specific for PSMA.
2 Molecular structure and biological function of the prostate-specific membrane antigen
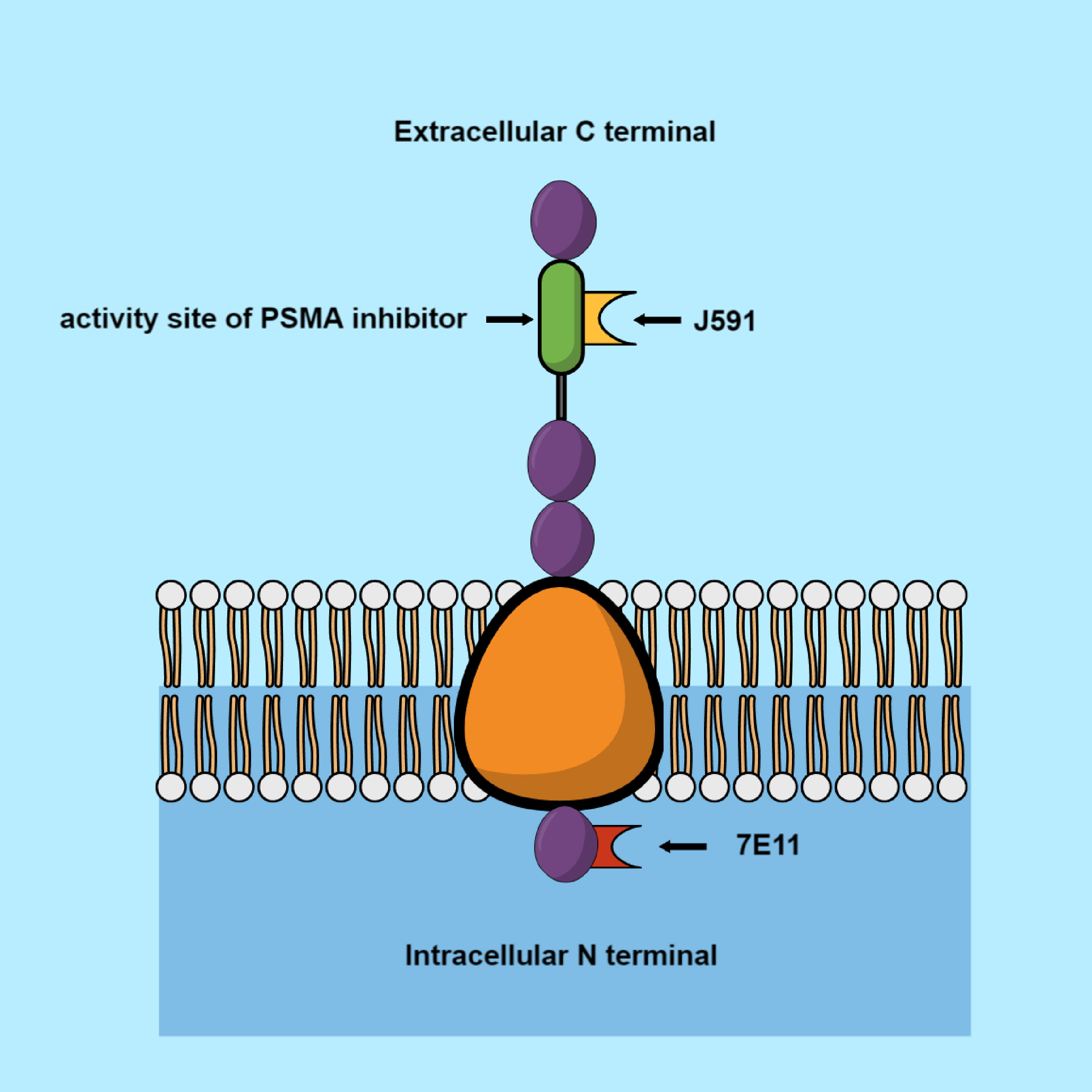
Glutamine carboxypeptidase II (GCPII), also known as PSMA, has been considered as a potential biomarker of PC, for many years. It is an enzyme coded by FOLH1 gene (folate hydrolase) localized on the short arm of chromosome 11 (11p11-p12) (12, 13). GCPII is identified mainly in prostate epithelium, the proximal tubules of the kidney, the jejunal brush border of the small intestine, and ganglia of the nervous system (14–16). Nevertheless, PSMA undergoes the strongest expression in prostate, where its frequency is >100 times than that in other tissues. In PC cells, PSMA is up-regulated 8–12 times in comparison to non-cancer cells. PSMA expression density on PC cells increases in correlation to the Gleason score for PC (Chang etal., 1999; Elgamal etal., 2000; Minner etal., 2011; Silver etal., 1997) and for CRPC (17). PSMA receptor is capable of internalization of ligands. This process allows the endocytosis of therapeutic isotopes into the intracellular space. Radionuclides that concentrated in cells, close to nearby nucleus, can cause DNA damage and effectively, its apoptosis of the cells by ionizing radiation. Because of this fact, PSMA was chosen as a biomarker in radionuclide therapy (18–20).
3 Antigens and “small particles” — ligands bounding with PSMA
Currently used ligands, selective to PSMA, may be divided into antibodies and small particles. The small particles are imaging ligands such as MIP-1095, PSMA-617, and PSMA-I&T. The well-known antibodies are 7E11-C5, J591, and PSMA-TTC.
The earliest evidence of evaluation of PSMA usefulness for radiotherapy was with 7E11-C5. This molecule targeted an epitope located in the intracellular domain of PSMA. With the help of this antibody, radionuclides of indium-111 and yttrium-90 were made (21, 22). Further studies resulted in the discovery of a monoclonal antibody (J591), in 1997. It was demonstrated that J591 induces PSMA internalization in cancer cells by connection with an external domain (Figure 1). The antibody was bound to the radionuclides via a chelating measure dodecane tetra acetic acid (DOTA). The following antibodies were used in these studies: indium-111, yttrium-90, 177Lu, and 225Ac (23–27). Another monoclonal antibody aimed at PSMA is PSMA-TTC (BAY 2315497). TTC is connected with an α-radiation emitter, thorium-227 (227 Th). PSMA-TTC was used in a phase I clinical study (NCT03724747), on patients with developing mCRPC (28). One of the most promising antibodies with a small molecular size (weighing almost two times less than J591, 80kDa vs. 150kDa) is IAB2M. It showed an accelerated plasma clearance, which reduced the amount of radioligand reaching the bone marrow, thereby preventing any severe hematological toxicity. However, the half-life of α-particles posed a problem; they were circulating inside the body, while emitting potentially hazardous ionizing radiation and damaging other organs (29, 30). PSMA-617 is the most frequently used α-particle. This is a small particle, created in Heidelberg (Germany), consisting of a chelator DOTA, which allows it to connect with the radionuclides (31).
The radioactive isotopes mentioned above are applicable in modern PC therapy and imaging.
4 Radionuclides used in aimed therapy of prostate cancer
A radionuclide is created by loading radioisotope into the chelator pocket which is bounded to the carrier (a small molecule, a whole antibody or its fragments). They are used in PC treatment after selective binding with PSMA.
The chelator is bound to the carrier (a small molecule, a whole antibody or its fragments), and the radionuclide is loaded into the chelator pocket.
Small molecules are modified PSMA inhibitors in which the structure of the molecule between the linking region and the radioactive metal chelator DOTA has been altered. Consequently, they do not exhibit homology to antibodies and they possess lower affinity in the nanomolar range than antibodies, so they bind less to PSMA in non-cancerous tissues. When combined with a radioactive element, they exert a cytotoxic effect on cancer cells (32, 33).
Noteworthy, small molecule is PSMA-I&T (imaging and therapy), introduced by Weineisen in 2014 as an additional PSMA ligand (34). This molecule has been utilized in both the diagnosis and treatment of advanced prostate cancer. To enhance its lipophilic effect and affinity for PSMA, a peptide linker unit has been incorporated. Furthermore, it has been coupled to 177Lu via DOTAGA.
Another, frequently used radioconjugate, known as MIP-1095, is a urea-based small particle designed for the PSMA radiolabeling with iodine I 131 (I131), and it possesses potential antineoplastic activity. Upon the administration of iodine I 131 MIP-1095, the MIP-1095 moiety selectively targets and binds to the extracellular domain of PSMA, thereby delivering cytotoxic iodine I 131 specifically to PSMA-expressing cancer cells (35).”Antibodies and small particles differ in their molecular structure and function and exhibit differences in kinetics and biodistribution. Accordingly, radionuclides oriented towards PSMA showed higher hematological toxicity when combined with antibodies, whereas when combined with small particles, non-hematological toxicity was observed, including nausea and xerostomia (36, 37). [177Lu] Lu-PSMA and [225Ac] 225Ac-PSMA are the most effective radionuclides available compared to other. Currently known radio pharmaceutics will be presented below.
5 Lutetium and actinium: elements which introduced new therapeutic possibilities aimed at PSMA
Iodine I 131 was mentioned in early clinical reports about the usage of radionuclides aimed at PSMA. This element was combined with a small particle MIP-1095, as a PSMA ligand. It showed acceptable pharmacological parameters. Its half-life was short and lasted for 8.02 days. Maximal size of the [I131] I-MIP-1095 was 2.4 micrometers, which caused its rapid internalization by cancer cells. Treatment efficacy was satisfactory, as it led to a 50% decrease in PSA concentration in 60% of the cured patients (38). Currently, it is not used because more efficient radionuclides have been invented. These efficient radionuclides cause less side effects, which will be discussed below. Currently, 225Ac and 177Lu are the most widely used radionuclides in PC treatment.
5.1 177 Lutetium
[177Lu] Lu-PSMA-617, also known as Vipivotide Tetraxetan or Pluvictotm are small particle radionuclides approved by FDA for treatment of hormone-resistant PC with metastases. This treatment is suggested for patients who have been treated with inhibitors of androgen receptor and undergone taxan-based therapy (39). The most significant radionuclide characteristics will be presented below.
5.1.1 Pharmacokinetics of Lu-177-PSMA-617
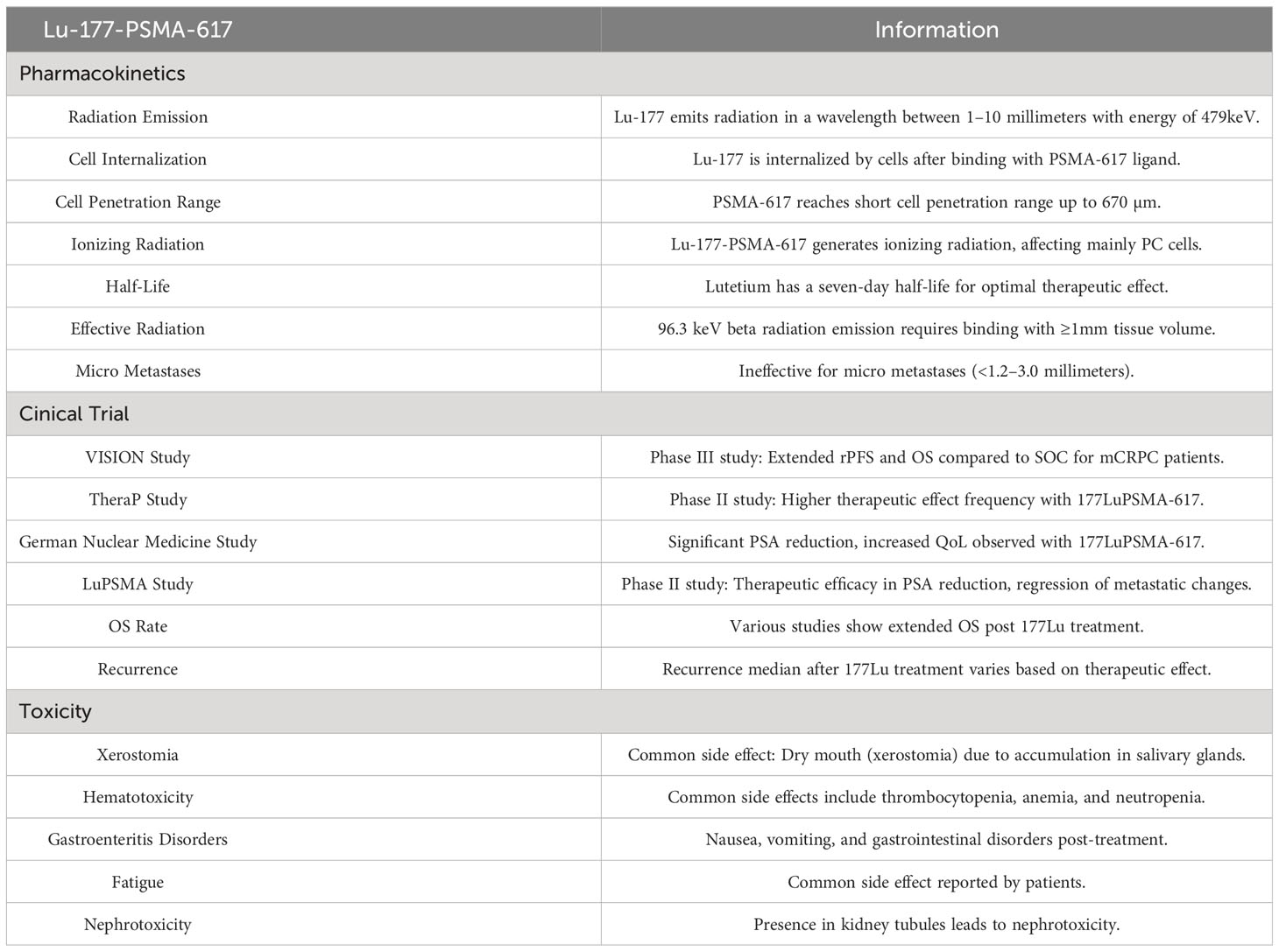
177Lu is an isotope which emits radiation in a wavelength between 1–10 millimeters. The emitted energy from the radiation is 479keV (range, 0.1–10 meV), in total. Lutetium is internalized by cells after PSMA binding with the PSMA-617 ligand. PSMA-617 reaches a short cell penetration range till 670 µm. Radionuclide Lu-PSMA-617 is able to generate ionizing radiation affecting only PC cells with a small impact on other cells due to its characteristics. A seven-day half-life of lutetium ensures an optimal therapeutic effect (40, 41). To initiate cancer cell apoptosis, 96.3 keV of beta radiation should be emitted to these cancer cells, which can be achieved by the binding of the lutetium isotope with at least 1 millimeter volume of tissue. As a result, 177Lu-PSMA-617 is ineffective for treating micro metastases, which are cancer sources <1.2–3.0 millimeters (42).
5.1.2 Clinical trials of 177 lutetium
Lutetium underwent many clinical trials, which resulted in a wide range of radionuclide treatment results. The most important studies on 177Lu were the phase III VISION and phase II TheraP study.
The VISION project measured the effectiveness of 177Lu PSMA-617 treatment on 831 patients with mCRPC. Results of this trial led to the 177Lu PSMA-617 RLT registration by FDA. rPFS (radiologic progression-free survival) and OS (overall survival) were assessed for patients treated with the radioligand 177Lu-PSMA-617, after applying SOC (standard of care) compared to patients who received only SOC. Lutetium treatment extended the rPFS up to 7–8 months, compared to that of patients after SOC who had an rPFS of 3–4 months. OS, after applying lutetium, also extended to 15.3 months, compared to 11.4 months after SOC (43).
In the TheraP study, 177Lu PSMA-617 treatment was compared with cabazitaxel therapy on 200 men with mCRPC. Studies measured the frequency of achieved therapeutic effect, which was defined as a 50% reduction in PSA concentration in blood serum compared to the output levels. The mentioned effect was achieved more frequently in cases treated with 177Lu-PSMA-617 than with cabazitaxel, 66% vs. 37% respectively (44).
German Nuclear Medicine Society conducted one of the largest retrospective clinical trials on 177Lu isotope usage. Results showed that use of 177Lu-PSMA-617 in PC treatment causes a significant decrease in PSA by 50% in the blood serum of 65 patients out of 145. Additionally, QoL (quality of life) rate increased by 60% due to the reduction of pain caused by cancer metastasis to bones (45–48).
“LuPSMA” conducted by Hoffman et al. is a prospective phase II trial. Results showed that lutetium treatment helped in achieving therapeutic efficacy. It was defined as a 50% reduction in PSA concentration in blood serum, which could be acquired in 57% of all cases. The trial included patients with visceral and lymph node cancer metastasis. Lutetium treatment induced an 82% regression rate of metastatic changes (according to Response Evaluation Criteria In Solid Tumors) in this group of patients (49).
OS rate after 177Lu treatment was measured in a few other studies. Violet et al. demonstrate the survival median and OS after lutetium treatment for 13 and 18 months, respectively (48). Gafity and Ahmadzadehfar also observed similar outcomes, after 12 and 13 months of treatment, respectively (10, 50).
Patients treated with 177Lu-PSMA also experienced a recurrence of the cancer. Recurrence median was 6–9 months. In cases where therapeutic effect of lutetium treatment, defined as a 50% reduction in PSA concentration, was achieved, the recurrence time was 8.3 months. In other cases, the recurrence time was 4.2 months. Other trials were conducted, where patients underwent lutetium nuclide treatment cycle. Therapeutic efficacy was achieved in 26–40% of the patients. Almost half of them regained a stable PSA level in approximately 12 months.
In conclusion, the mentioned trials show that 177Lu treatment causes positive therapeutic effects compared to SOC (standard of care) or with cabazitaxel treatment. It effectively extends OS and PFS, along with an increase in QoL.
5.1.3 Toxicity of 177Lu-PSMA-617 according to common terminology criteria for adverse events
Radionuclide treatment may entail some undesired side effects, which must be taken into consideration while making therapeutic decisions. The most frequent side effects of lutetium usage are presented below.
5.1.3.1 Xerostomia
177Lu PSMA-617 accumulated nonspecifically in the salivary glands (51). Salivary glands absorb the largest dosage of radiation among all organs (52). Occurrence of Xerostomia reaches 80–87%, but it is categorized as level 1, according to CTCAE (47). Applying protective therapies for salivary glands such as local cooling, PSMA inhibitors,and injection of botulinum toxin into glands is not significantly effective (53–55). High dosage of monosodium glutamate may decrease radioligand absorption by salivary glands; however, this is pre-clinical data and further research needs to be conducted (56). The lacrimal glands are also capable of radioligand absorption, but only a few cases of dry eye condition were observed.
5.1.3.2 Thrombopenia and anemia
Hematotoxicity of levels 3–4 after 177Lu PSMA-617 radioligand treatment is the most common side effect. Fourth level thrombopenia appears in 10–46% of all cured patients, anemia in 10%, and fourth level neutropenia in 6–26%. The lowest platelet count, as per morphology tests, was observed after 32 days of lutetium treatment. Lymphocytopenia (level 3–4) was one of the most common signs of hematotoxicity, as it was observed in 32% of the cases.
In a German multi-center study, a lesser percentage of anemia, thrombocytopenia, and leukopenia of level 3–4 were observed (10%, 4%, and 3%, respectively) (41). Further research is needed to identify the predictors of hematotoxicity after 177Lu-PSMA-617 radionuclide treatment. Thrombocytopenia seems to be the most common predictor of extensive bone marrow disease and limited marrow reserves, resulting from previous therapies.
Hematotoxicity, which results from the weak permeability of monoclonal antibodies in solids and their large size accompanied by slow elimination from the body, is a critical problem. Appropriate trials using small molecule PSMA inhibitors to limit this effect are in progress (57).
5.1.3.3 Gastroenteritis disorders
Nausea, vomiting, and gastrointestinal disorders usually appear in 50% of the cases, 12 hours after lutetium is given to a patient. It can be caused by an unidentified PSMA expression in the brush border of the proximal part of small intestine, especially duodenum and jejunum. Incidence of this undesired side effect is estimated to be 50%. To avoid this, patients may receive anti-inflammatory and anti-emetic drugs (48, 49, 58–63).
5.1.3.4 Fatigue
Fatigue is the most common side effect reported by patients and its incidence is 50% (64).
5.1.3.5 Nephrotoxicity
PSMA is present in the proximal kidney’s tubules. It leads to nephrotoxicity, as evident in a glomerular filtration rate (GFR) decrease of 11.5 ml after three months of 177Lu treatment (Table 1) (58, 59).
5.1.4 Qualification for lutetium treatment
For RLT PSMA treatment, patients should be chosen by a multidisciplinary uro-oncological team, including oncologists, radiotherapists, and urologists, specializing in PC treatment and nuclear medicine specialists.
Prostate Cancer Trials Working Group 3 (PCWG3) shows the qualification criteria for treatment (65): mCRPC with changes described in PET-PSMA, progression after application of at least one cycle of chemotherapy, progression after application of new androgen receptor-targeting agents (ARTA) (abirateron or enzalutamid), progression after application of Radium-223 or without medical indication for usage of this substance, recommendation for 177Lu PSMA-617 RLT in interdisciplinary proceeding.
5.1.5 Other studies
There are many clinical trials being conducted around the world. The preliminary findings of 177Lu-PSMA-617 trials are presented below.
5.2 177Lu-DOTAGA-PSMA-I&T
Isotope 177Lu, connected with small particle PSMA-I&T (imaging and therapy), is another currently used radionuclide. PSMA-I&T is used in imaging diagnostics as well as a carrier for therapeutic ligands in PC treatment.
5.2.1 177Lu-PSMA-I&T treatment effects
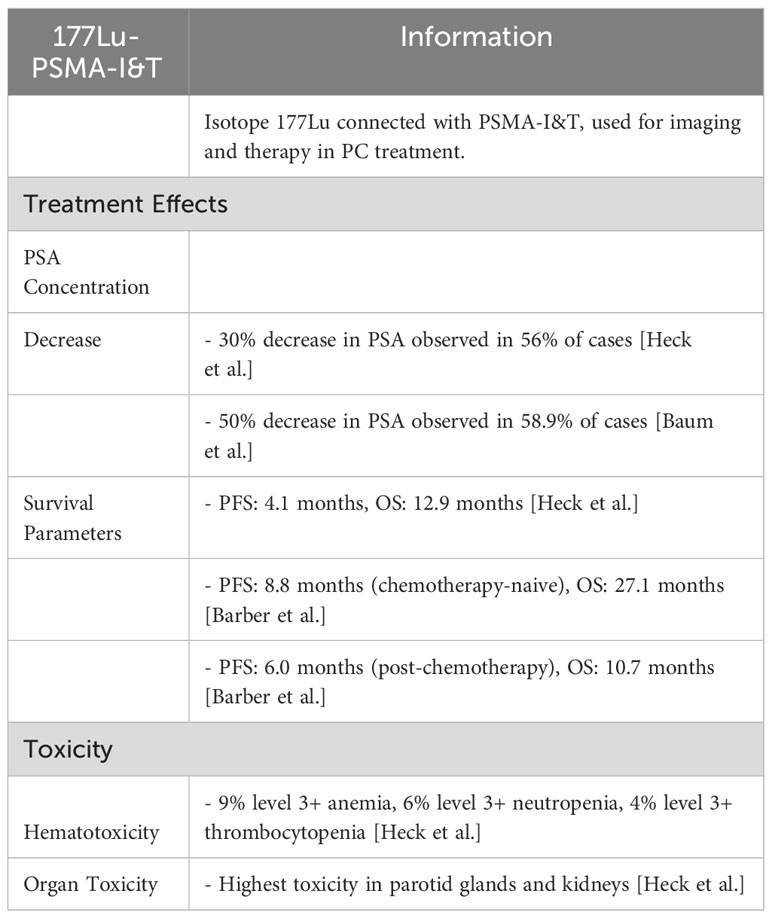
In a retrospective study conducted by Heck et al., a 30% decrease in PSA concentration was observed in 56% of cases treated with 177Lu-PSMA-I&T (66). Similarly, Baum et al. published a study exhibiting a 50% decrease in 58.9% of all cases. Heck et al. reported a 30% decrease in PSA concentration in 47% of the patients, whereas 38% patients showed more than a 50% decrease. In the trial results, PFS and OS parameters amounted to 4.1 and 12.9 months, respectively (60).
Barber et al. compared results of patients treated with 177Lu-PSMA-I&T and who underwent chemotherapy with those of chemotherapy naive patients. For patients who did not receive chemo-therapy, the median of lifetime PFS and OS amounted to 8.8 and 27.1 months and for patients after chemotherapy, it was 6.0 and 10.7 months, respectively (67).
OS rate in a cohort of chemotherapy-treated patients was longer than in the group where the primary treatment was based on taxanes.
5.2.2 Toxicity of 177Lu-PSMA- I&T
Data showing unwanted side effects of 177Lu-PSMA-I&T treatment are limited. The trial conducted by Heck et al. showed that 9% of the patients suffered from level 3+ anemia, 6% from level 3+ neutropenia, and 4% from level 3+ thrombocytopenia, after 177Lu-PSMA- I&T treatment. Organs which demonstrate the highest amounts of toxicity were the parotid glands and the kidneys (Table 2) (60).
5.3 177Lu-J591
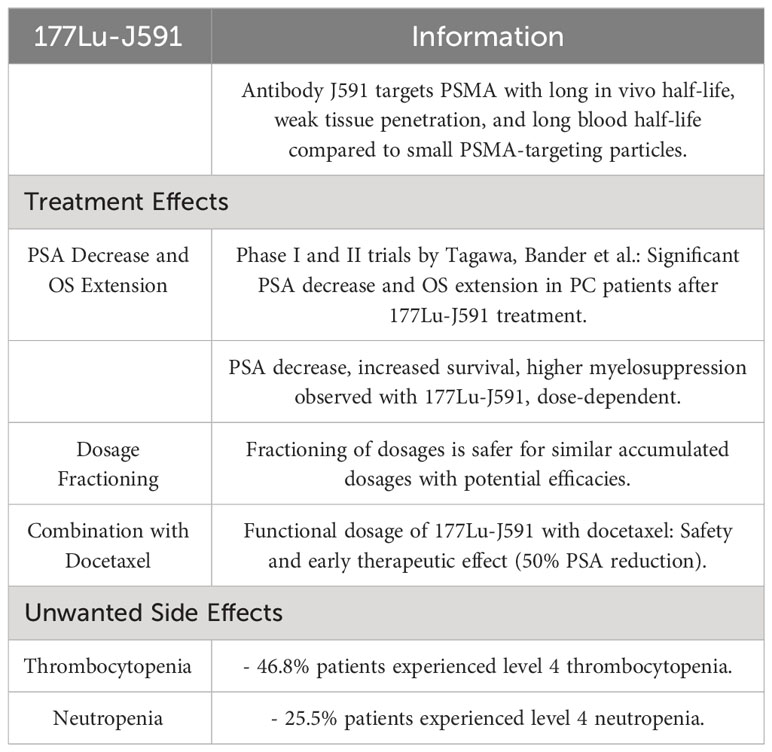
J591 is an antibody aimed at PSMA which has a long in vivo half-life, weak tissue penetration, and long blood half-life compared to the small particles aimed at PSMA (61). Phase I and II trials conducted by Tagawa, Bander et al. proved that a significant PSA decrease was achieved as well as an extension of OS in PC patients after 177Lu-J591 treatment (60, 61). It was shown that there is a decrease in PSA levels, increased survival rate, and higher myelosuppression, depending on the dosage of 177Lu-J591. Also, fractioning of dosages is safer in case of similar accumulated dosages with higher potential efficacies. Tests on the functional dosage of 177Lu-J591 connected with docetaxel were conducted. Patients were administered a standard dose of docetaxel, along with increasing fractionating doses of 177Lu-J591. A study demonstrated the safety of such a connection with early evidences of therapeutic effect, defined as 50% reduction of PSA concentration in blood serum in comparison with entry level (68).
Unwanted side effects reported as follows: 46.8% of the patients suffered from thrombocytopenia level 4 and 25.5% suffered from neutropenia level 4 (Table 3) (69).
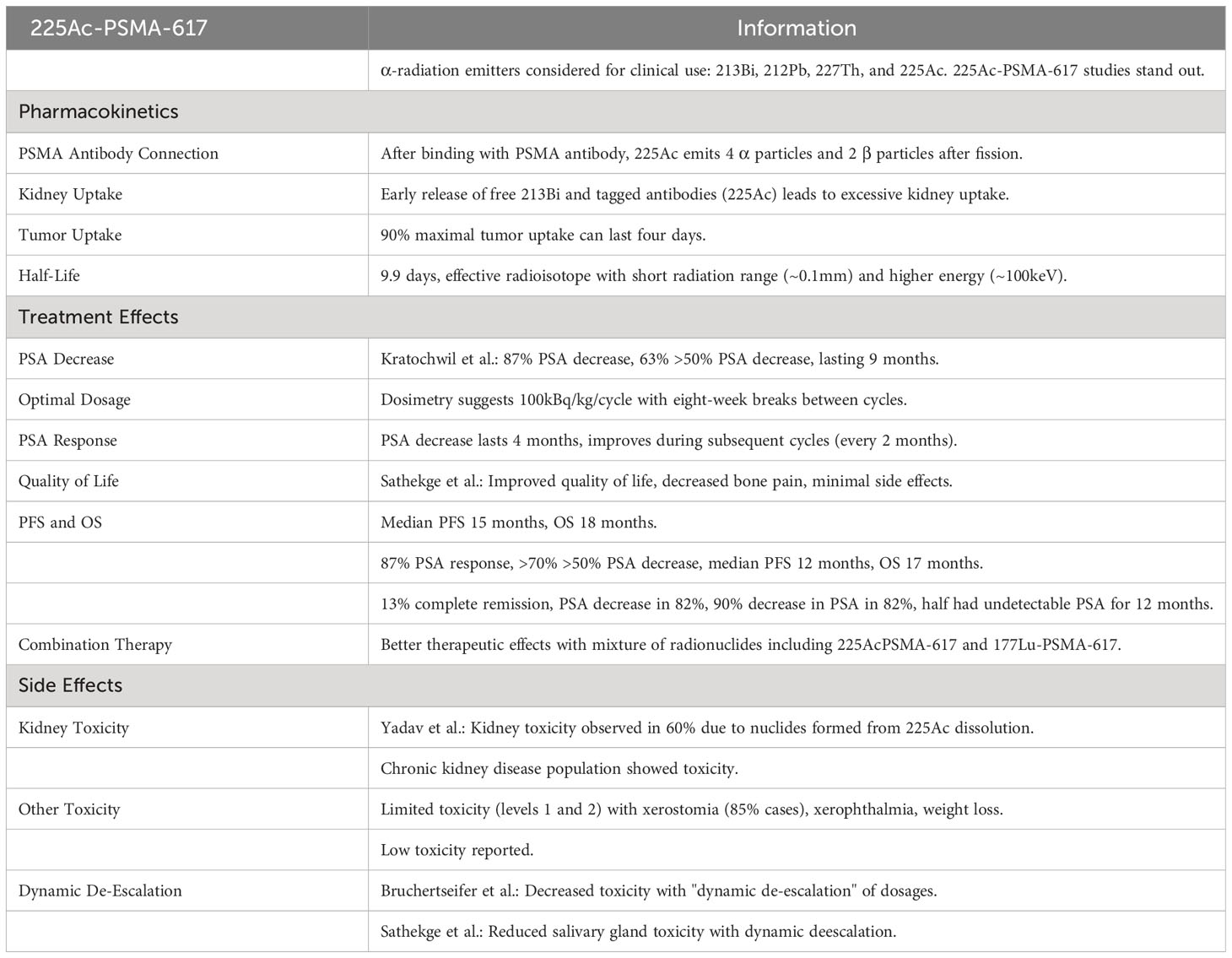
5.4 [225Ac] 225Ac-PSMA-617
From a limited number of α-radiation emitters, only 213Bi, 212Pb, 227Th and 225Ac were evaluated as promising candidates for clinical applications. Studies on these emitters are in progress, but data regarding 225Ac-PSMA-617 is the most impressive and will be the topic of the review survey.
5.4.1 Pharmacokinetics of 225Ac-PSMA-617
After connecting with the binding moiety of PSMA, 225Ac is internalized and works as an “in vivo nuclides generator”, emitting 4 α molecules and 2 β molecules after fission (70). Unfortunately, derivative nuclides are not connected with PSMA distribution in tissues and undergo natural biodistribution. It was demonstrated that the early release of free 213Bi along with circulating, tagged antibodies like 225Ac, causes excessive kidney uptake. Accumulation of 90% of maximal tumor uptake may last four days. Its half-life lasts for 9.9 days; it has a short range of radiation in human tissues, which makes it an effective radioisotope. α-emitters have a shorter range of penetration in tissues compared to β-emitters (<0.1mm) and a larger amount of energy (~100keV) (70).
5.4.2 Treatment effects of 225Ac-PSMA-617
Kratochwil et al. analyzed the effectiveness of PSMA 617 in 40 patients with mCRPC (71). Their results showed that PSA was decreased in 87% of cases after 225Ac-PSMA-617 treatment. 63% of the patients achieved more than a 50% PSA decrease in comparison to the entry level. PSA degression was obtained after nine months in the mentioned cases. Based on dosimetry studies, it was determined that 100kBq/kg/cycle is an optimal dosage for use in patients who undergo eight-week breaks between cycles. Effectively, PSA decrease was found to last four months and improved during the next cycles, which are applied every two months (72). Sathekge et al. demonstrated that most of patients after 225Ac-PSMA-617 treatment declared improved quality of life (with bone pains decreased) and minimal side effects (73). Another study from these authors revealed a PSA response in 87% of the patients, more than 70% of who reported a PSA decrease of more than 50%. The median PFS and OS was 15 and 18 months, respectively (74). Yadav et al. reported a >50% PSA decrease in 39% of Irish patients. Median PFS and OS was 12 and 17 months, respectively. A clinical trial at Saar University, Hamburg and in Bad Berka showed that 13% of treated patients come out with almost complete remission (75, 76). Another study showed that 82% of patients reported a 90% decrease in PSA, half of whom obtained undetectable PSA in their serum and remained in remission for next 12 months of therapy (73). Bruchertseifer et al. demonstrated better therapeutic effects when a mixture of radionuclides was applied, including 225Ac-PSMA-617 and 177Lu-PSMA-617 (77).
5.4.3 225Ac-PSMA-617 – side effects
Unwanted side effects of 225Ac treatment were studied by Yadav et al. They did not report toxicity in the bone marrow, whereas some toxicity was observed in kidneys (in 60% of cases, this is primarily caused by nuclides formed from decay of 225Ac, namely 213Bi), but only in a population with known chronic kidney disease (76). Side effects were limited to toxicity of levels 1 and 2 along with xerostomia (in 85% of cases), xerophthalmia, and weight loss in most cases. Very low toxicity has been demonstrated (78). Decrease of toxicity in Bruchertseifer et al. was achieved by “dynamic de-escalation” of dosages (77). The dynamic de-escalation protocol was also used in the Sathekge et al. study where decreased toxicity on the salivary glands was attained (Table 4) (75).
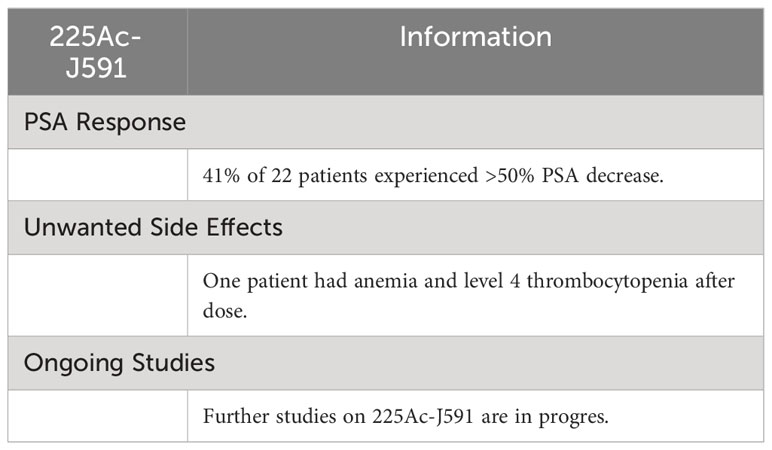
5.5 225Ac-J591
Results of phase I trial conducted by Tagawa et al. (NCT03276572) are available. They found that after injection of a single dose of 225Ac-J591 radio-therapeutics 41% out of 22 patients experienced more than a 50% decrease in PSA. One patient developed anemia and level 4 thrombocytopenia, after receiving the dose. Further studies on 225Ac-J591 are in progress (Table 5) (79).
6 Comparison of 225Ac and 177Lu
PSMA 225Ac causes more fractures in double-stranded DNA of cancer cells than those caused by 177Lu. This putative mechanism of action should result in better effectiveness. This strategy guarantees therapy optimization in cases with significant bone metastases. Shorter cumulative tissue explosion on β-radiation preserves the intact bone marrow. However, at the same time, toxicity in salivary glands is noticed. Due to this fact, in some centers it is preferred to administer 177Lu- PSMA to patients with soft tissue lesions, whereas 225Ac- PSMA is injected for high volume bone diseases. In preclinical studies, targeted α-therapy was more successful than therapy with β-emitters. [225Ac] Ac-PSMA-617 also demonstrated a high therapeutic efficacy in patients who experienced progression of PC after 177Lu-PSMA-617 treatment, which shows the high potential to concur resistance before β-emitter therapy (71, 72, 74).
7 New directions for specific PSMA radionuclides researches
CTT1403 is an organophosphoramidic peptidomimetic, which binds irreversibly with PSMA, allowing radionuclides to be quickly absorbed into the tumor. Because of its albumin binding component, CTT1403 has a long half-life inside blood, which leads to a higher amount of radionuclides build up around the tumor. CTT1403 reduces tumor mass and improves survival rate of animals with tumor cells transplanted (40). CTT1057 demonstrated biodistribution aimed at PSMA, with smaller exposition on kidneys and salivary glands (80). R2 is a PSMA ligand, examined in mice with PC and revealed high tumor uptake when it was marked [177Lu]. R2 is currently tested in PORter phase I/II research, which aims to test safety, tolerance, and dosimetry of radiation of [177Lu] Lu-PSMA-R2 in patients with mCRPC (NCT03490838) (81). Currently, other pre-clinical studies on different radionuclides are taking place: e.g., 149Tb-PSMA-617, 211At-Astatobenzamido-Ureido-Pentanedioic Acid, 213Bi-J591, 213Bi PSMA-I&T vs. JVZ008-nanobody, 225Ac-RPS-074, 227Th-PSMA-IgG, and 212Pb.
8 Discussion
CRCP is characterized by poor prognosis that is reflected in a poor survival rate and is a terminal disease. Radioligand targeted therapy is a new and promising concept, where a radioisotope is bound with an antibody or a small molecule ligand. The most recent FDA-approved radionuclide used in PC treatment is 177LuPSMA-617. Lu-177-PSMA-617 have promising outcomes in treatment according to standard of care. It effectively extends OS and PFS and increases the QoL. Radionuclide treatment may entail some undesired side effects. The most frequent side effects are xerostomia and hematotoxicity. From limited number of α emitters, 225Ac is the most impressive. Studies showed that PSA was lower in most cases after 225Ac-PSMA-617 treatment. Side effects were mostly limited to xerostomia, xerophthalmia, and weight loss. The most optimal approach seems to be the use of a combination of different radionuclides for maximum therapeutic effect. β− emitters are most commonly used for RLT, which have a low linear energy transfer (LET). The low deposited energy of β− emitters is only able to yield single-strand breaks of the DNA but can perform this over a longer range, which makes them especially suitable for larger tumors. Getting increasingly interesting for RLT, there are alpha emitters, which have a high LET and lower range; therefore, they are more suitable for smaller tumors and micrometastases. Administering alpha emitters at the onset of disease recurrence, when the maximum dose of beta radiation has been reached using 117Lu, could be a beneficial approach (33). There are multiple new directions for specific PSMA radionuclides research. Radioligand targeted therapy is a new and promising way of treatment of CRCP, which should be the subject of future clinical discussions and research.
Author contributions
PS and PP contributed to the conception and design of the review, drafting and reviewing of the manuscript, revisions/edits, and a review of references. All authors contributed to the article and approved the submitted version.
Funding
The authors declare that this study received funding from Department of Urology and Urological Oncology, Multidisciplinary Regional Hospital in Gorzów Wielkopolski, Poland and Department of General and Oncologic Urology, Antoni Jurasz University Hospital No. 1 in Bydgoszcz, Poland. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of the article, or the decision to submit it for publication.
Acknowledgments
Publication made possible in part by support from the Multidisciplinary Regional Hospital in Gorzów Wielkopolski, Poland and Antoni Jurasz University Hospital No. 1 in Bydgoszcz, Poland,
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Beer TM, Armstrong AJ, Rathkopf DE, Rathkopf DE, Loriot Y, Sternberg CN, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med (2014) 371:424–33. doi: 10.1056/NEJMoa1405095
2. de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med (2011) 364:1995–2005. doi: 10.1056/NEJMoa1014618
3. Kantoff PW, Higano CS, Shore ND, Bergerc ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resi_stant prostate cancer. N Engl J Med (2010) 363:411–22. doi: 10.1056/NEJMoa1001294
4. Parker C, Nilsson S, Heinrich D, Helle SI, O'Sullivan JM, Fosså SD, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med (2013) 369:213–23. doi: 10.1056/NEJMoa1213755
5. Petrylak DP, Tangen CM, Hussain MH, Lara PN Jr, Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mit_oxantrone and prednisonefor advanced refractory prostate cancer. N Engl J Med (2004) 351:1513–20. doi: 10.1056/NEJMoa041318
6. Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, et al. Abiraterone in metastatic prostate cancer without previouschemotherapy. N Engl J Med (2013) 368:138–48. doi: 10.1056/NEJMoa1209096
7. Scher HI, Fizazi K, Saad F, Taplin M-E, Sternberg CN, Miller K, et al. Increased survivalwithenzalutamide in prostate cancer after ch8. emotherapy. N Engl J Med (2012) 367:1187–97. doi: 10.1056/NEJMoa1207506
8. Smith M, Parker C, Saad F, Miller K, Tombal B, Sing Ng Q, et al. Addition of radium-223 toabirateroneacetateandprednisone or prednisolone in patients with castration_resistant prostate cancer and bone metastases (ERA 223): a randomised, double-blind, placebo_controlled, phase3 trial. Lancet Oncol (2019) 20:408–19. doi: 10.1016/S1470-2045(18)30860-X
9. Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med (2004) 351:1502–12. doi: 10.1056/NEJMoa040720
10. Ahmadzadehfar H, Wegen S, Yordanova A, Fimmers R, Kürpig S, Eppard E, et al. Overall survival and response pattern of castration-resistant metastatic prostate cancer to multiple cycles of radioligand therapy using [(177)Lu]Lu-PSMA-617. Eur J Nucl Med Mol Imaging (2017) 44:1448–54. doi: 10.1007/s00259-017-3716-2
11. Rahbar K, Bode A, Weckesser M, Avramovic N, Claesener M, Stegger L, et al. Radioligand therapy with 177Lu-PSMA-617 as a novel therapeutic option in patients with metastatic castration resistant prostate cancer. Clin Nucl Med (2016) 41:522–8. doi: 10.1097/RLU.0000000000001240
12. O'Keefe DS, Su SL, Bacich DJ, Horiguchi Y, Luo Y, Powell CT, et al. Mapping, genomic organization and promoter analysis of the human prostate-specific membrane antigen gene. Biochim Biophys Acta (BBA) - Gene Structure Expression (1998) 1443(1–2):113–27. doi: 10.1016/s0167-4781(98)00200-0
13. Pinto JT, Suffoletto BP, Berzin TM, Qiao CH, Lin S, Tong WP, et al. Prostate-specific membrane antigen: a novel folate hydrolase in human prostatic carcinoma cells. Clin Cancer Res (1996) 2(9):1445–51.
14. Barinka C, Sácha P, Sklenár J, Man P, Bezouska K, Slusher BS, et al. Identification of the N-glycosylation sites on glutamate carboxypeptidase II necessary for proteolytic activity. Protein Sci (2004) 13(6):1627–35. doi: 10.1110/ps.04622104
15. Sácha P, Zámecník J, Barinka C, Hlouchová K, Vícha A, Mlcochová P, et al. Expression of glutamate carboxypeptidase II in human brain. Neuroscience (2007) 144(4):1361–72. doi: 10.1016/j.neuroscience.2006.10.022
16. Mhawech-Fauceglia P, Zhang S, Terracciano L, Sauter G, Chadhuri A, Herrmann FR, et al. Prostate-specific membrane antigen (PSMA) protein expression in normal and neoplastic tissues and its sensitivity and specificity in prostate adenocarcinoma: an immunohistochemical study using mutiple [sic] tumour tissue microarray technique. Histopathology (2007) 50(4):472–83. doi: 10.1111/j.1365-2559.2007.02635.x
17. O'Keefe DS, Bacich DJ, Heston WD. Comparative analysis of prostate-specific membrane antigen (PSMA) versus a prostate-specific membrane antigen-like gene. Prostate (2004) 58(2):200–10. doi: 10.1002/pros.10319
18. Emmett L, Willowson K, Violet J, Shin J, Blanksby A, Lee J. 177 PSMA radionuclide therapy for men with prostate cancer: a review of the current literature and discussion of practical aspects of therapy. J Med Radiat Sci (2017) 64(1):52–60. doi: 10.1002/jmrs.227
19. Ranjan S. Lutetium-177 prostate-specific membrane antigen-617 theranostics: New therapeutic hope in metastatic castrate-resistant prostate cancer? Indian J Urol (2020) 36(3):227. doi: 10.4103/iju.IJU_193_2017
20. Fendler WP, Rahbar K, Herrmann K, Kratochwil C, Eiber M. 177Lu-PSMA radioligand therapy for prostate cancer. Chin J Nucl Med Mol Imaging (2019) 39(10):636–40. doi: 10.2967/jnumed.116.183194
21. Schuster DM, Nieh PT, Jani AB, Amzat R, Bowman FD, Halkar RK, et al. Anti-3-[(18)F]FACBC positron emission tomography-computerized tomography and (111)In-capromab pendetide single photon emission computerized tomography-computerized tomography for recurrent prostate carcinoma: results of a prospective clinical trial. J Urol. (2014) 191(5):1446–53. doi: 10.1016/j.juro.2013.10.065
22. Deb N, Goris M, Trisler K, Fowler S, Saal J, Ning S, et al. Treatment of hormone_refractory prostate cancer with 90Y-CYT-356 monoclonal antibody. Clin Cancer Res (1996) 2(8):1289–97.
23. Liu H, Moy P, Kim S, Xia Y, Rajasekaran A, Navarro V, et al. Monoclonal antibodies to the extracellular domain of prostate-specific membrane antigen also react with tumor vascular endothelium. Cancer Res (1997) 57(17):3629–34.
24. Liu H, Rajasekaran AK, Moy P, Xia Y, Kim S, Navarro V, et al. Constitutive and antibody-induced internalization of prostate-specific membrane antigen. Cancer Res (1998) 58(18):4055–60.
25. Vallabhajosula S, Kuji I, Hamacher KA, Konishi S, Kostakoglu L, Kothari PA, et al. Pharmacokinetics and biodistribution of 111In-and 177Lu-labeled J591 antibody specific for prostate-specific membrane antigen: prediction of 90Y-J591 radiation dosimetry based on 111In or 177Lu? J Nucl Med (2005) 46(4):634–41.
26. Milowsky MI, Nanus DM, Kostakoglu L, Vallabhajosula S, Goldsmith SJ, Bander NH. Phase I trial of yttrium-90—labeled anti—prostate-specific membrane antigen monoclonal antibody J591 for androgen-independent prostate cancer. J Clin Oncol (2004) 22(13):2522–31. doi: 10.1200/JCO.2004.09.154
27. Bander NH, Milowsky MI, Nanus DM, Kostakoglu L, Vallabhajosula S, Goldsmith SJ. Phase I trial of 177lutetium-labeled J591, a monoclonal antibody to prostate-specific membrane antigen, in patients with androgen-independent prostate cancer. J Clin Oncol (2005) 23(21):4591–601. doi: 10.1200/JCO.2005.05.160
28. Hammer S, Hagemann UB, Zitzmann-Kolbe S, Larsen A, Ellingsen C, Geraudie S, et al. Preclinical efficacy of a PSMA-targeted thorium-227 conjugate (PSMA-TTC), a targeted alpha therapy for prostate cancer. Clin Cancer Res (2020) 26(8):1985–96. doi: 10.1158/1078-0432.CCR-19-2268
29. Pandit-Taskar N, O’Donoghue JA, Beylergil V, Lyashchenko S, Ruan S, Solomon SB, et al. 89Zr-huJ591 immuno-PET imaging in patients with advanced metastatic prostate cancer. Eur J Nucl Med Mol Imaging (2014) 41:2093–105. doi: 10.1007/s00259-014-2830-7
30. Pandit-Taskar N, O’Donoghue JA, Ruan S, Lyashchenko SK, Carrasquillo JA, Heller G, et al. First-in-human imaging with 89Zr-Df-IAB2M anti-PSMA minibody in patients with metastatic prostate cancer: Pharmacokinetics, biodistribution, dosimetry, and lesion uptake. J Nucl Med (2016) 57:1858–64. doi: 10.2967/jnumed.116.176206
31. Benešová M, Schäfer M, Bauder-Wüst U, Afshar-Oromieh A, Kratochwil C, Mier W, et al. Preclinical evaluation of a tailor-made DOTA-conjugated PSMA inhibitor with optimized linker moiety for imaging and endoradiotherapy of prostate cancer. J Nucl Med (2015) 56(6):914–20. doi: 10.2967/jnumed.114.147413
32. Feldwisch J, Tolmachev V. Engineering of affibody molecules for therapy and diagnostics. Methods Mol Biol (2012) 899:103–26. doi: 10.1007/978-1-61779-921-1_7
33. Hennrich U, Eder M. [177Lu]Lu-PSMA-617 (PluvictoTM): The first FDA-approved radiotherapeutical for treatment of prostate cancer. Pharmaceuticals (2022) 15(10):1292. doi: 10.3390/ph15101292
34. Weineisen M, Schottelius M, Simecek J, Baum RP, Yildiz A, Beykan S, et al. 68Ga- and 177Lu-labeled PSMA I&T: optimization of a PSMA-targeted theranostic concept and first proof-of-concept human studies. J Nucl Med (2015) 56(8):1169–76. doi: 10.2967/jnumed.115.158550
35. National Cancer Institute. NCI thesaurus version 18.11d. Available at: https://ncit.nci.nih.gov/ncitbrowser/.
36. Kratochwil C, Haberkorn U, Giesel FL. Radionuclide therapy of metastatic prostate cancer. Semin Nucl Med (2019) 49(4):313–25. doi: 10.1053/j.semnuclmed.2019.02.003
37. Niaz MJ, Skafida M, Osborne J, Nanus D, Molina A, Thomas C, et al. Pd16–11 comparison of prostate-specific membrane antigen (Psma)-targeted radionuclide therapy (Trt) with lutetium-177 (177lu) via antibody J591 vs small molecule ligand psma-617. J Urology (2020) 203(Supplement 4):e367–e. doi: 10.1097/JU.0000000000000859.011
38. Afshar-Oromieh A, Haberkorn U, Zechmann C, Armor T, Mier W, Spohn F, et al. Repeated PSMA-targeting radioligand therapy of metastatic prostate cancer with (131)I_MIP-1095. Eur J Nucl Med Mol Imaging (2017) 44:950–9. doi: 10.1007/s00259-017-3665-9
39. Eiber M, Fendler WP, Rowe SP, Calais J, Hofman MS, Maurer T, et al. Prostate_specific membrane antigen ligands for imaging and therapy. J Nucl Med (2017) 58(Suppl. 2):67S–76S. doi: 10.2967/jnumed.116.186767
40. Powers E, Karachaliou GS, Kao C, Harrison MR, Hoimes CJ, George DJ, et al. Novel therapies are changing treatment paradigms in metastatic prostate cancer. J Hematol Oncol (2020) 13(1):144. doi: 10.1186/s13045-020-00978-z
41. Emmett L, Willowson K, Violet J, Shin J, Blanksby A, Lee J. Lutetium 177 PSMA radio_nuclide therapy for men with prostate cancer: a review of the current literature and discussion of practical aspects of therapy. J Med Radiat Sci (2017) 64(1):52–60. doi: 10.1002/jmrs.227
42. Ling X, Latoche JD, Choy CJ, Kurland BF, Laymon CM, Wu Y, et al. Preclinical dosim_etry, imaging, and targeted radionuclide therapy studies of Lu-177-labeled albumin-binding, PSMA-targeted CTT1403. Mol Imaging Biol (2020) 22(2):274–84. doi: 10.1007/s11307-019-01404-8
43. Sartor AO, Morris MJ, Messman R, Krause BJ. VISION: An international, prospective, open-label, multicenter, randomized phase III study of 177Lu-PSMA-617 in the treatment of patients with progressive PSMA-positive metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol (2020) 38(6_suppl):TPS259. doi: 10.1200/JCO.2020.38.6_suppl.TPS259
44. A trial of 177Lu_PSMA617 theranostic versus cabazitaxel in progressive meta_static castration resistant prostate cancer 2020. Available at: https://ClinicalTrials.gov/show/NCT03392428.
45. Fendler WP, Reinhardt S, Ilhan H, Delker A, Böning G, Gildehaus FJ, et al. Prelimi_nary experience with dosimetry, response and patient reported outcome after 177Lu-PSMA-617 therapy for metastatic castration-resistant prostate cancer. Oncotarget (2017) 8(2):3581. doi: 10.18632/oncotarget.12240
46. Herlemann A, Wenter V, Kretschmer A, Thierfelder KM, Bartenstein P, Faber C, et al. 68Ga-PSMA positron emission tomography/computed tomography provides accu_rate staging of lymph node regions prior to lymph node dissection in patients with prostate cancer. Eur Urol. (2016) 70(4):553–7. doi: 10.1016/j.eururo.2015.12.051
47. Rahbar K, Ahmadzadehfar H, Kratochwil C, Haberkorn U, Schäfers M, Essler M, et al. German multicenter study investigating 177Lu-PSMA-617 radioligand therapy in advanced prostate cancer patients. J Nucl Med (2017) 58(1):85–90. doi: 10.2967/jnumed.116.183194
48. Violet J, Sandhu S, Iravani A, Ferdinandus J, Thang SP, Kong G, et al. 177Lu-PSMA-617 theranostics in metastatic castration-resistant prostate cancer. J Nucl Med (2020) 61(6):857–65. doi: 10.2967/jnumed.119.236414
49. Hofman MS, Violet J, Hicks RJ, Ferdinandus J, Thang SP, Akhurst T, et al. [177Lu]- PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol (2018) 19(6):825–33. doi: 10.1016/S1470-2045(18)30198-0
50. Gafita A, Heck M, Rauscher I, Tauber R, Cala L, Franz C, et al. Early prostate-specific antigen changes and clinical outcome following (177)Lu-PSMA radionuclide treatment in patients with metastatic castration_resistant prostate cancer. J Nucl Med (2020) 61(10):1476–83. doi: 10.2967/jnumed.119.240242
51. Taieb D, Foletti JM, Bardies M, Rocchi P, Hicks RJ, Haberkorn U. PSMA-targeted radionuclide therapy and salivary gland toxi_city: why does it matter? J Nucl Med (2018) 59:74. doi: 10.2967/jnumed.118.207993
52. Violet J, Jackson P, Ferdinandus J, Sandhu S, Akhurst T, Iravani A, et al. Dosimetry of (177)Lu-PSMA-617 in metastatic castration-resistant prostate cancer: correlations between pre_therapeutic imaging and whole-body tumor dosimetry with treatment outcomes. J Nucl Med Off pub_lication Soc Nucl Med (2019) 60:517–23. doi: 10.2967/jnumed.118.219352
53. Kratochwil C, Giesel FL, Leotta K, Eder M, Hoppe-Tich T, Youssoufian H, et al. PMPA for nephroprotection in PSMA_targeted radionuclide therapy of prostate cancer. J Nucl Med (2015) 56:293–8. doi: 10.2967/jnumed.114.147181
54. Baum RP, Langbein T, Singh A, Shahinfar M, Schuchardt C, Volk GF, et al. Injection of botulinum toxin for preventing salivary gland toxicity after PSMA radioligand therapy: an empirical proof of a promising concept. Nucl Med Mol Imaging (2018) 52:80–1. doi: 10.1007/s13139-017-0508-3
55. Yilmaz B, Nisli S, Ergul N, Gursu RU, Acikgoz O, Cermik TF. Effect of external cooling on (177)Lu-PSMA uptake by the parotid glands. J Nucl Med (2019) 60:1388–93. doi: 10.2967/jnumed.119.226449
56. Rousseau E, Lau J, Kuo HT, Zhang Z, Merkens H, Hundal-Jabal N, et al. Monosodium glutamate reduces (68)Ga-PSMA-11 uptake in salivary glands and kidneys in a preclinical prostate cancer model. J Nucl Med (2018) 59:1865–8. doi: 10.2967/jnumed.118.215350
57. Maresca KP, Hillier SM, Femia FJ, Keith D, Barone C, Joyal JL , et al. A series of halogenated heterodimeric inhibitors of prostate specific membrane antigen (PSMA) as radiolabeled probes for targeting prostate cancer. JMed Chem (2009) 52:347–57. doi: 10.1021/jm800994j
58. Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and Malignant human tissues. Clin Cancer Res (1997) 3:81–5. doi: 10.2967/jnumed.116.178673
59. Hofman M, Violet JA, Hicks RJ, Ferdinandus J, Thang SP, Iravani A, et al. Results of a 50 patient single-center phase II prospective trial of Lutetium-177 PSMA-617 theranostics in metastatic castrate-resistant prostate cancer. J Clin Oncol (2019) 37:228. doi: 10.1200/JCO.2019.37.7_suppl.228
60. Heck MM, Tauber R, Schwaiger S, Retz M, D'Alessandria C, Maurer T, et al. Treatment outcome, toxicity, and predictive factors for radioligand therapy with (177)Lu-PSMA-I&T in metastatic castration-resistant prostate cancer. Eur Urology (2019) 75:920–6. doi: 10.1016/j.eururo.2018.11.016
61. Tagawa ST, Milowsky MI, Morris M, Vallabhajosula S, Christos P, Akhtar NH, et al. Phase II study of lutetium-177-labeled anti_prostate-specific membrane antigen monoclonal antibody J591 for metastatic castration-resistant prostate cancer. Clin Cancer Res (2013) 19:5182–91. doi: 10.1158/1078-0432.CCR-13-0231
62. Ahmadzadehfar H, Rahbar K, Kurpig S, Bögemann M, Claesener M, Eppard E, et al. Early side effects and first results of radioligand therapy with (177)Lu-DKFZ-617 PSMA of castrate_resistant metastatic prostate cancer: a two-centre study. EJNMMI Res (2015) 5:114. doi: 10.1186/s13550-015-0114-2
63. Yadav MP, Ballal S, Sahoo RK, Dwivedi SN, Bal C. Radioligand therapy with (177)Lu-PSMA for metastatic castration-resistant prostate cancer: a systematic review and meta-analysis. AJR Am J Roentgenol (2019) 213:275–85. doi: 10.2214/AJR.18.20845
64. Stone P, Richardson A, Ream E, Smith AG, Kerr DJ, Kearney N, et al. Cancer-related fatigue: Inevitable, unimportant and untrea_table? Results of a multi-centre patient survey. Ann Oncol (2000) 11:971–5. doi: 10.1023/A:1008318932641
65. Scher HI, Morris MJ, Stadler WM, Higano C, Basch E, Fizazi K, et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the prostate cancer clin_ical trials working group 3. J Clin Oncol (2016) 34:1402–18. doi: 10.1200/JCO.2015.64.2702
66. Heck MM, Retz M, D’Alessandria C, Rauscher I, Scheidhauer K, Maurer T, et al. Systemic radioligand therapy with 177Lu labeled prostate specific membrane antigen ligand for imaging and therapy in patients with metastatic castration resistant prostate cancer. J urology. (2016) 196(2):382–91. doi: 10.1016/j.juro.2016.02.2969
67. Barber TW, Singh A, Kulkarni HR, Niepsch K, Billah B, Baum RP. Clinical outcomes of 177lu_psma radioligand therapy in earlier and later phases of metastatic castration-resistant prostate cancer grouped by previous taxane chemotherapy. J Nucl Med (2019) 60(7):955–62. doi: 10.2967/jnumed.118.216820
68. Tagawa ST, Vallabhajosula S, Christos PJ, Jhanwar YS, Batra JS, Lam L, et al. Phase 1/2 study of fractionated dose lutetium-177–labeled anti–prostate-specific membrane antigen monoclonal antibody J591 (177Lu-J591) for metastatic castration-resistant prostate cancer. Cancer (2019) 125(15):2561–9. doi: 10.1002/cncr.32072
69. Batra JS, Niaz MJ, Whang YE, Sheikh A, Thomas C, Christos P, et al. Phase I trial of docetaxel plus lutetium-177-labeled anti–prostate-specific membrane antigen monoclonal antibody J591 (177Lu-J591) for metastatic castration-resistant prostate cancer. Urologic Oncol (2020) 38(11):848.e9–848.e16. doi: 10.1016/j.urolonc.2020.05.028
70. Morgenstern A, Apostolidis C, Kratochwil C, Sathekge M, Krolicki L, Bruchertseifer F. An overview of targeted alpha therapy with 225 actinium and 213 bismuth. Curr Radiopharm. (2018) 11(3):200–8. doi: 10.2174/1874471011666180502104524
71. Kratochwil C, Bruchertseifer F, Rathke H, Hohenfellner M, Giesel FL, Haberkorn U, et al. Targeted α-therapy of metastatic castration-resistant prostate cancer with 225Ac-PSMA-617: swimmer-plot analysis suggests efficacy regarding duration of tumor control. J Nucl Med (2018) 59(5):795–802. doi: 10.2967/jnumed.117.203539
72. Kratochwil C, Bruchertseifer F, Rathke H, Bronzel M, Apostolidis C, Weichert W, et al. Targeted alpha-therapy of metastatic castration-resistant prostate cancer with (225)Ac-PSMA-617: dosimetry estimate and empiric dose finding. J Nucl Med (2017) 58:1624–31. doi: 10.2967/jnumed.117.191395
73. Sathekge M, Bruchertseifer F, Knoesen O, Reyneke F, Lawal I, Lengana T, et al. 225Ac-PSMA-617 in chemotherapy-naive patients with advanced prostate cancer: a pilot study. Eur J Nucl Med Mol I (2019) 46(1):129–38. doi: 10.1007/s00259-018-4167-0
74. Sathekge M, Bruchertseifer F, Vorster M, Lawal I, Knoesen O, Mahapane J, et al. Predictors of overall and disease free survival in metastatic castration-resistant prostate cancer patients receiving 225Ac-PSMA-617 radioligand therapy. J Nucl Med (2019) 61(1):jnumed.119.229229. doi: 10.2967/jnumed.119.229229
75. Langbein T, Kulkarni H, Singh A, Lehmann C, Schuchardt C, Zhang J, et al. Combined PRLT using Ac-225 and Lu-177 labeled PSMA-617 (TANDEM-PRLT) in end-stage metastatic prostate cancer: A concept to reduce salivary gland toxicity? J Nucl Med (2019) 60(supplement 1):194. doi: 10.1055/s-0039-1683741
76. Kulkarni H, Zhang J, Langbein T, Schuchardt C, Singh A, Mueller D, et al. Radioligand therapy using combination of Ac-225 and Lu-177 labelled PSMA ligands for progressive end-stage metastatic prostate cancer: Effective trade-off between response and toxicity. J Nucl Med (2019) 60(supplement 1):464. doi: 10.1055/s-0039-1683560
77. Bruchertseifer F, Kratochwil C, Rathke H, Weis M, Verburg FA, Mottaghy F, et al. Optimizing the treat_ment regimen for targeted alpha therapy of mCRPC with 225Ac-PSMA_617. J Nucl Med (2019) 60:S1.
78. Yadav MP, Ballal S, Sahoo RK, Tripathi M, Seth A, Bal C. Efficacy and safety of 225Ac-PSMA-617 targeted alpha therapy in metastatic castration-resistant Prostate Cancer patients. Theranostics (2020) 10(20):9364–77. doi: 10.7150/thno.48107
79. Tagawa ST, Osborne J, Fernandez E, Thomas C, Niaz MJ, Ciriaco A, et al. Phase I dose-escalation study of PSMA-targeted alpha emitter 225Ac-J591 in men with metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol (2020) 38(Supplement 15):5560–0. doi: 10.1200/JCO.2020.38.15_suppl.5560
80. Behr SC, Aggarwal R, VanBrocklin HF, Flavell RR, Gao K, Small EJ, et al. Phase I study of CTT1057, an (18)F-labeled imaging agent with phosphoramidate core targeting prostate-specific membrane antigen in prostate cancer. J Nucl Med (2019) 60(7):910–6. doi: 10.2967/jnumed.118.220715
81. Muzio V, Ravasi L, Sacchetti L, Fugazza L, Bacot S, Debiossat M, et al, (Eds.). Assess_ment of in vivo biodistribution and treatment efficacy of Lu-177 PSMA-R2 and Lu_177-PSMA-617 on mice bearing prostate cancer tumors. In European journal of nuclear medicine and molecular imaging. New York, NY, United States: Springer One, New York Plaza, Suite 4600 (2019).
Keywords: radionuclide, PET-PSMA, 177Luthtet, 225Actinum, CRPC
Citation: Szponar P, Petrasz P, Brzeźniakiewicz-Janus K, Drewa T, Zorga P and Adamowicz J (2023) Precision strikes: PSMA-targeted radionuclide therapy in prostate cancer – a narrative review. Front. Oncol. 13:1239118. doi: 10.3389/fonc.2023.1239118
Received: 15 June 2023; Accepted: 18 September 2023;
Published: 16 November 2023.
Edited by:
Bekir Cinar, Clark Atlanta University, United StatesReviewed by:
Michael Epperly, University of Pittsburgh, United StatesGiulio Fracasso, University of Padova, Italy
Copyright © 2023 Szponar, Petrasz, Brzeźniakiewicz-Janus, Drewa, Zorga and Adamowicz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paweł Szponar, cGF3ZWxzenBvbmFyOTBAZ21haWwuY29t
†These authors have contributed equally to this work and share first authorship
 Paweł Szponar
Paweł Szponar Piotr Petrasz1†
Piotr Petrasz1† Piotr Zorga
Piotr Zorga Jan Adamowicz
Jan Adamowicz