- 1Department of Cardiologic, Vascular and Thoracic Sciences, and Public Health, University of Padua, Padua, Italy
- 2Soft-Tissue, Peritoneum and Melanoma Surgical Oncology Unit, Veneto Institute of Oncology (IOV), Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS), Padua, Italy
- 3Veneto Tumor Registry, Azienda Zero, Padua, Italy
- 4Department of Surgery, Oncology and Gastroenterology (DISCOG), University of Padua, Padua, Italy
- 5Pathology and Cytopathology Unit, Department of Medicine-DIMED, University of Padua, Padua, Italy
Background: Costs related to the care of melanoma patients have been rising over the past few years due to increased disease incidence as well as the introduction of innovative treatments. The aim of this study is to analyse CMM cost items based on stage at diagnosis, together with other diagnostic and prognostic characteristics of the melanoma.
Methods: Analyses were performed on 2,647 incident cases of invasive CMM that were registered in 2015 and 2017 in the Veneto Cancer Registry (RTV). Direct melanoma-related costs per patient were calculated for each year ranging from 2 years before diagnosis to 4 years after, and were stratified by cost items such as outpatient services, inpatient drug prescriptions, hospital admissions, hospice admissions, and emergency room treatment. Average yearly costs per patient were compared according to available clinical-pathological characteristics. Lastly, log-linear multivariable analysis was performed to investigate potential cost drivers among these clinical-pathological characteristics.
Findings: Overall, the average direct costs related to melanoma are highest in the first year after diagnosis (€2,903) and then decrease over time. Hospitalization costs are 8 to 16 times higher in the first year than in subsequent years, while the costs of outpatient services and inpatient drugs decrease gradually over time. When stratified by stage it is observed that the higher expenditure associated with more advanced stages of CMM is mainly due to inpatient drug use.
Conclusion: The results of the present study show that grouping patients according to tumour characteristics can improve our understanding of the different cost items associated with cutaneous malignant melanoma. CMM patients experience higher costs in the first year after diagnosis due to higher hospitalization and outpatient services. Policy makers should consider overall and stage-specific annual costs when allocating resources for the management of CMM patients.
1 Introduction
Cutaneous malignant melanoma (CMM) is among some of the most common cancers in young adults (age 20-39) (1–3). Even though mortality due to cutaneous melanoma has been significantly decreasing over the past decade, its incidence has continued to rise at a steady pace (4). In the United States, the rate of new cases has more than tripled since 1975, and in 2021 cutaneous melanoma accounted for 5.6% of all new cancer cases (5, 6).This increase in overall CMM rate, as well as the introduction of innovative therapies for its treatment, means that CCM is a rising public health issue with a significant financial and social burden (7–9). However, health care pathways for CMM patients vary widely depending on their cancer stage (7, 10). While most thin melanomas can often be cured with simple wide excision, more advanced forms require complex treatments, implying quite a different burden of health care resource consumption (11, 12). In an effort to ensure that all patients receive the most effective therapies, policy-makers need to address the issue of sustainability of our healthcare systems as a whole (13–15).
There are many recent studies focusing on direct CMM costs, but few of them have analysed the differences in real-world costs associated with a comprehensive health care pathway covering all the stages of the disease (6, 14–17). Furthermore, very few studies have been conducted in Europe on the stage-specific direct health care costs of CMM (some that have done so include Lyth et al. and Buja et al.) (15–17).
This study aims to analyse the real-world direct costs related to a population-based CMM cohort of patients. Analyses will involve both overall costs as well as item-specific costs, stratifying the analyses by the cancer stage at diagnosis, patient survival, as well as other tumour characteristics, with a four-year follow-up after diagnosis.
2 Methods
2.1 Context
The Italian public healthcare system is managed regionally, providing universal coverage at the point of delivery, which is largely free of charge and primarily supported by general taxation (18). Its policies are grounded on the fundamental values of universality, free access, freedom of choice, pluralism in provision, and equity.
Veneto is an Italian region located in the North-eastern area of the country, with a population of 4.9 million residents and a mean age of 54.4 years. Considering the latest available socio-economic indicators, the Veneto region has a per capita gross domestic product (GDP) of 33,800 euros, with an unemployment rate of 5.8%, and 29.3% of Veneto inhabitants aged 30-34 have a university degree (11, 19).
In 2015, the Veneto Oncology Network (ROV) detailed the complete procedures for the clinical management of CMM patients in a document based on the current national and international literature (20–23). It defines standardized clinical care pathways from diagnosis to end-of-life care, as well as a set of indicators to assess the quality of care by evaluating the consistency between recommendations and real-world clinical practice (24).
2.2 Clinical and cost data
This population-based cohort study includes all 2015 and 2017 incident cases of cutaneous malignant melanoma recorded by the Veneto Cancer Registry (RTV) in the cutaneous malignant high-resolution regional cancer registry, which provides demographic coverage of the entire region on a two-year basis.
The RTV recording procedures rely on various informative sources such as pathology reports, clinical charts, death certificates, and health service administrative records. The following variables were extracted by high resolution registry for the present study: age, sex, 8th edition AJCC TNM stage at diagnosis, and CMM histological subtypes.
The cost analysis was conducted from a health system perspective. Data on visits to outpatient clinics, specialist services, drug prescriptions, hospital or hospice admissions, treatments at the emergency department, and the use of medical devices were obtained from the regional administrative subject-level databases (see below). The cost of any diagnostic or therapeutic (surgical or other) interventions was based on the reimbursement rates established by the Veneto Regional Authority. For cost assessment we considered the following sources:
● The Outpatient database, which contains the information on all medical procedures (i.e., specialist visits, laboratory and radiological tests, radiotherapy sessions, etc.), excluding inpatient care, delivered at outpatient facilities under NHS funding. These procedures are valued at the rate stated in the Tariff Nomenclature for outpatient services (TNOS), which is a detailed formulary of medical procedures for outpatients. These services are categorised within the cost item labelled as “Outpatient services”.
● The Hospital Admissions database, which includes the diagnosis-related group (DRG) for each admission, valued at the rate indicated in the Tariff Nomenclature for inpatient services (TNIP), which is a formulary covering all hospital activities, including day hospital admissions, preliminary pre-operative exams, and sentinel lymph node staging procedures. All services provided during hospitalization, whether in a day hospital or inpatient setting, are categorised under the cost item “Hospitalization”.
● Regional databases of outpatient drug prescriptions and in-hospital drug consumption, which record the costs of all medical therapies, encompassing dosage details. Also included are “high-cost” chemotherapeutic agents (i.e., interferon, pembrolizumab, nivolumab, vemurafenib, Osimertinib, dabrafenib, trametinib, ipilimumab, binimetinib, temozolomide, fotemustine, paclitaxel, melphalan) and all associated supportive therapies. The cost item associated with them is referred to as “Inpatient drugs”.
● The Emergency Department Admissions database, which records the cost of each admission as the sum of all medical procedures undertaken. The data generated is allocated to the cost item classified as “Emergency department visit”.
● The Hospice database, which records the length of stay. The corresponding cost item is classified as “Hospice”.
These distinct databases and their cost categorizations contribute to a more comprehensive analysis of healthcare expenditure patterns in the studied context, which is the Italian healthcare system.
Each patient was linked via a unique and anonymous identification code to all administrative data (see the cost assessment sources list above). All costs sustained from two years before melanoma diagnosis up to four years after diagnosis were included. The average real-world costs per year per patient (total and per single item of expenditure) were calculated and stratified by the following clinical variables: site of primary tumour, histological phenotype, and TNM stage at initial cancer assessment.
2.3 Statistical analysis
Differences in total costs, item expense distribution (comparing < 64 vs. 65-year-old patients), TNM stage, CMM histology subtype (i.e., nodular versus non-nodular), and survival time were also assessed by stratifying cost data by these variables.
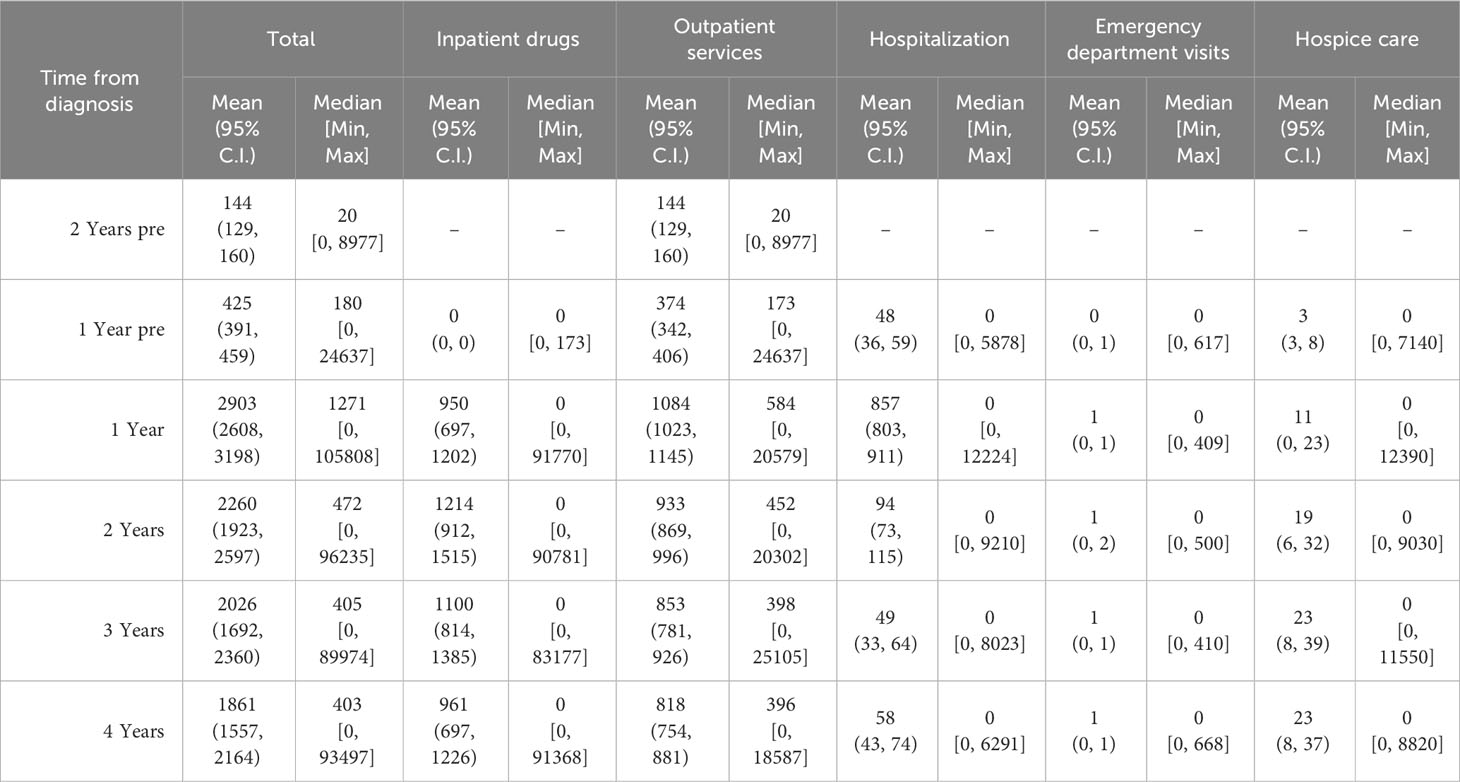
Costs are summarized as survival-weighted means and medians, 95% confidence intervals for mean and minimum–maximum intervals are also provided.
Survival-weighted costs were calculated (for each year and for the entire period observed) by dividing the sum of total incurred costs by the observed person-time, i.e., the sum of total time (days of the year) in which each patient was alive, and then transformed to person-years.
A log-linear multivariable analysis was performed to investigate potential cost drivers among the available clinic-pathological characteristics. The mean person-years melanoma-specific costs sustained from diagnosis to 4 years after diagnosis defined the output variables of four different models adapted to the data. Costs were transformed to the logarithmic in order to manage the skewed distribution and meet linear regression assumptions. The independent variables considered were age, sex, TNM stage at diagnosis, mitotic count, ulceration, regression, growth phase, TILs, together with tumour location, histology subtype, and time to death.
Results were deemed statistically significant at the p < 0.05 level. Data preparation, analyses and visualizations were conducted using R (25) and Python (26).
3 Results
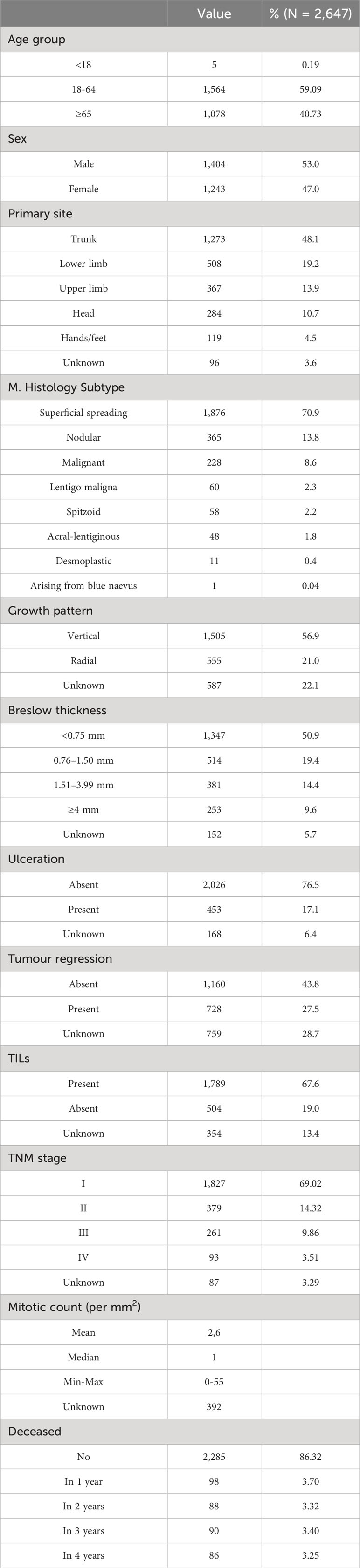
A total of 2,647 cutaneous malignant melanoma incident cases were included in this study, of which 1,279 cases were diagnosed in 2015 (48.3%) and 1,368 in 2017 (51.7%). The mean age at diagnosis was 59.7 (SD ± 16.2) years. The overall survival at 4 years was 86.32%. Less than 15% of the patients presented with an advanced TNM stage at diagnosis (9.9% in stage III, 3.5% in stage IV). The sample characteristics are summarized in Table 1.
Figure 1 indicates how melanoma-related expenditures change based on tumour stage at diagnosis, with stage I CMM costing much less than stage IV. The increase in expenditure associated with inpatient drug use is the main driver of these different melanoma stage-related costs, followed by outpatient service costs. Lastly, hospice costs appear to primarily involve stage IV CMMs, while the impact of emergency department visits on total costs are negligible for all tumour stages.
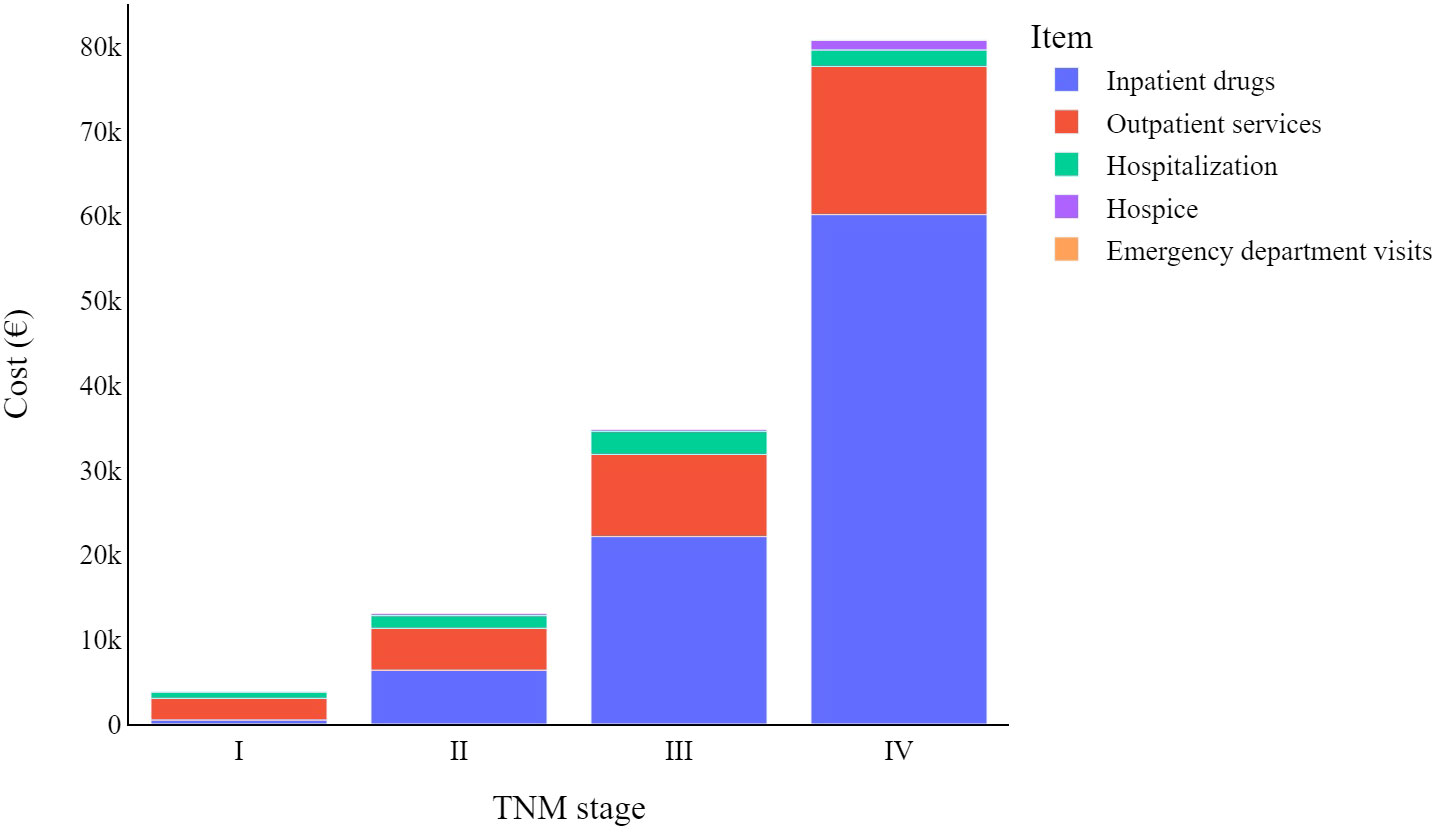
Figure 1 Total melanoma specific item cost (€) trends by TNM stage at diagnosis: from diagnosis to four years later.
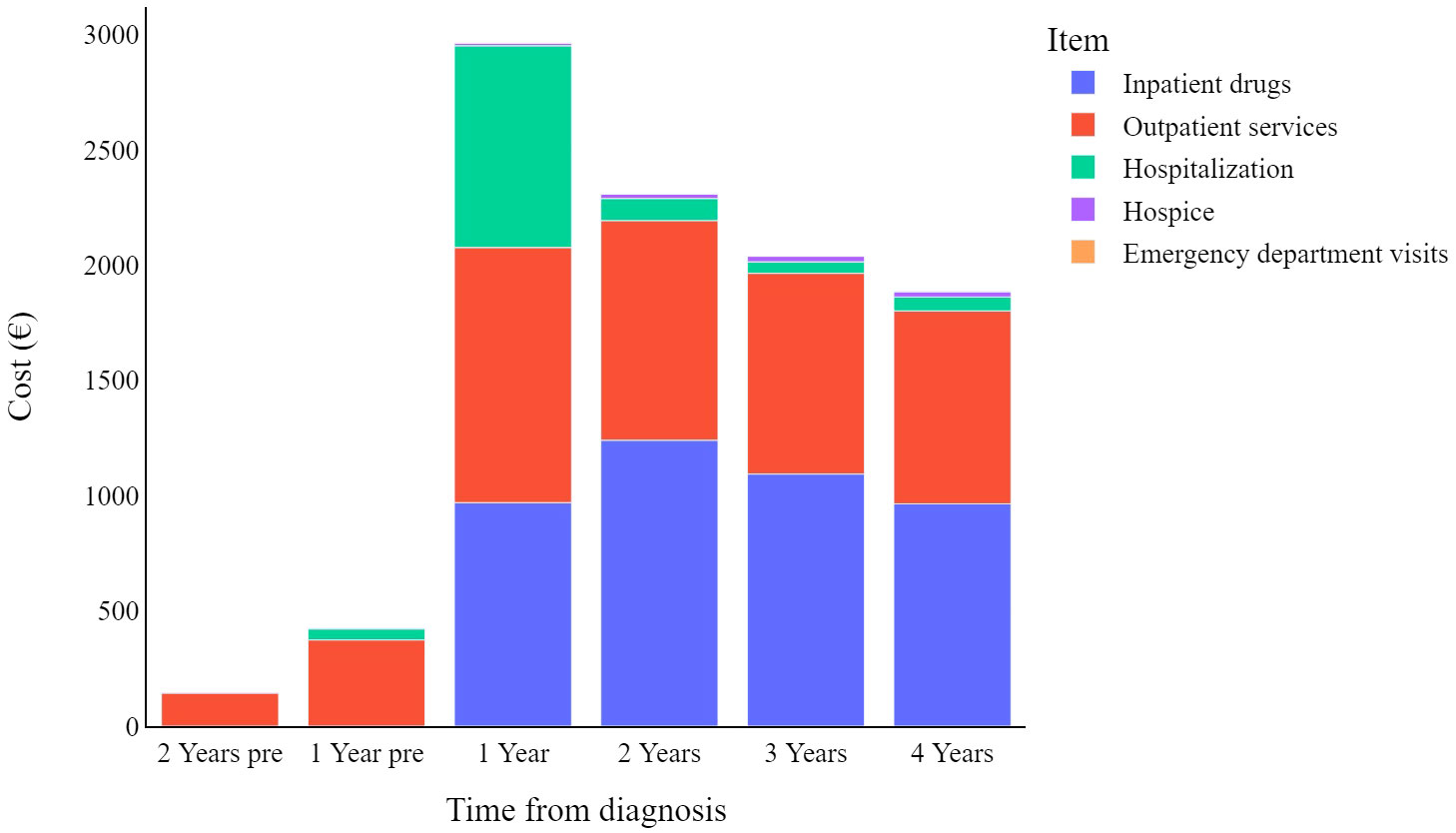
Figure 2 displays the mean weighted direct melanoma-related costs per item per year, covering the period from 2 years prior to melanoma diagnosis until 4 years post-diagnosis. The mean value presented in Table 2 represents the annual relative weight of each cost item within the total expenditure for CMM care, offering an immediate overview of the annual expenses across all categories. The data show that overall costs peak in the first year (€2,903, 95% C.I. [2,608-3,198]), and then decrease over time. Breaking it down further, in the first year hospitalization costs have a proportionally similar impact to that of inpatient drugs and outpatient services (€857, €950, and €1,084 respectively), while in subsequent years hospitalization costs are less impactful.
Stratifying patients by disease stage at diagnosis (Figure 3) highlights the yearly relative weight of each cost-item in the overall expenditure for CMM care. Hospitalization contributes proportionally more to the total annual cost in the first year after diagnosis particularly for stage I CMM, while outpatient services have a proportionally similar impact on annual costs, and inpatient drugs show more variation in stage/year specific costs.
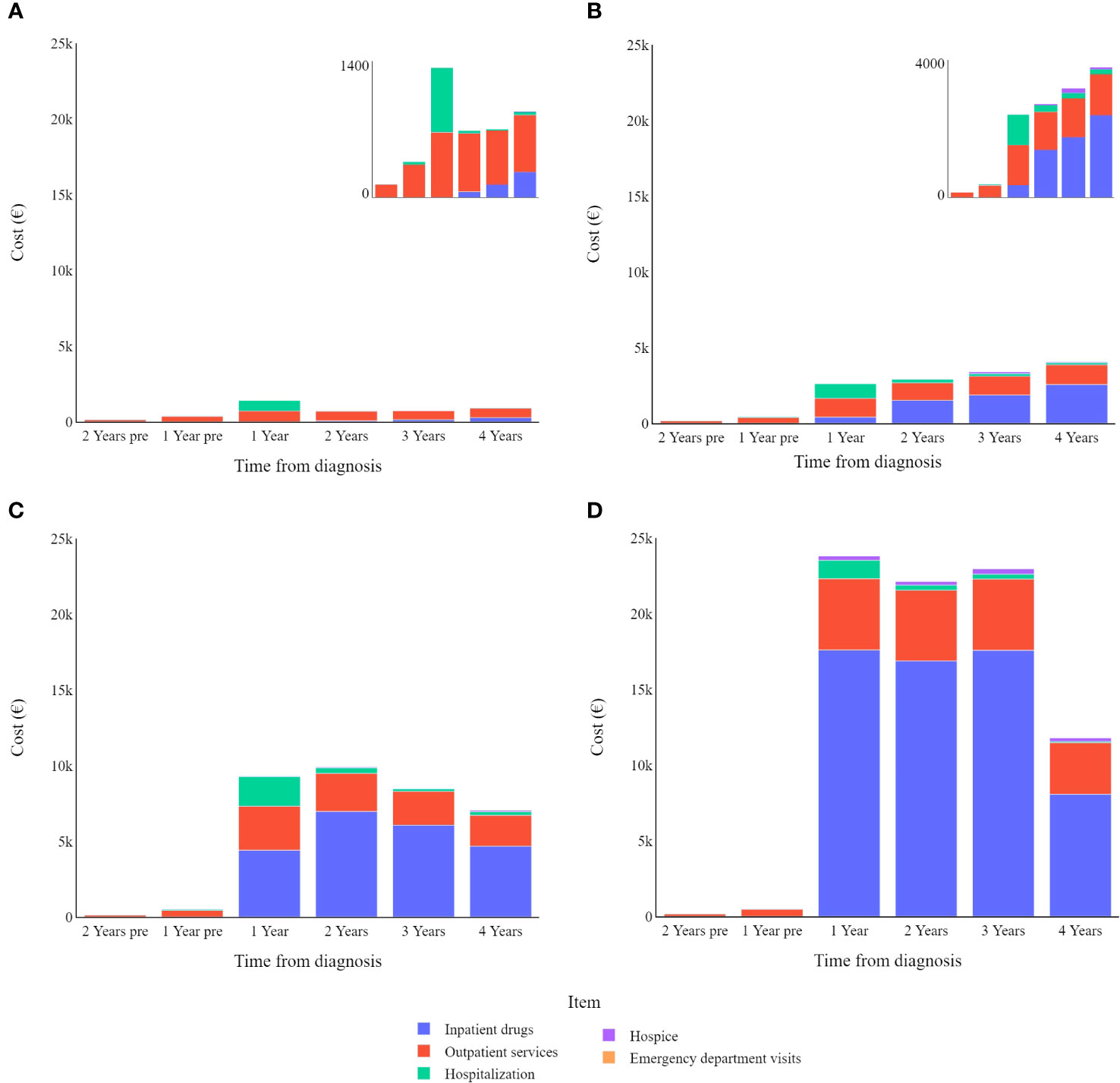
Figure 3 Melanoma specific item cost (€) trends by tumour stage (differentiated scales): (A) in stage I; (B) in stage II; (C) in stage III; (D) in stage IV.
Table 3 represents data on direct total costs of melanoma over a four-year period post-diagnosis, categorized by expense item and stratified by stage, age, sex, survival time, histological subtype, and overall costs.
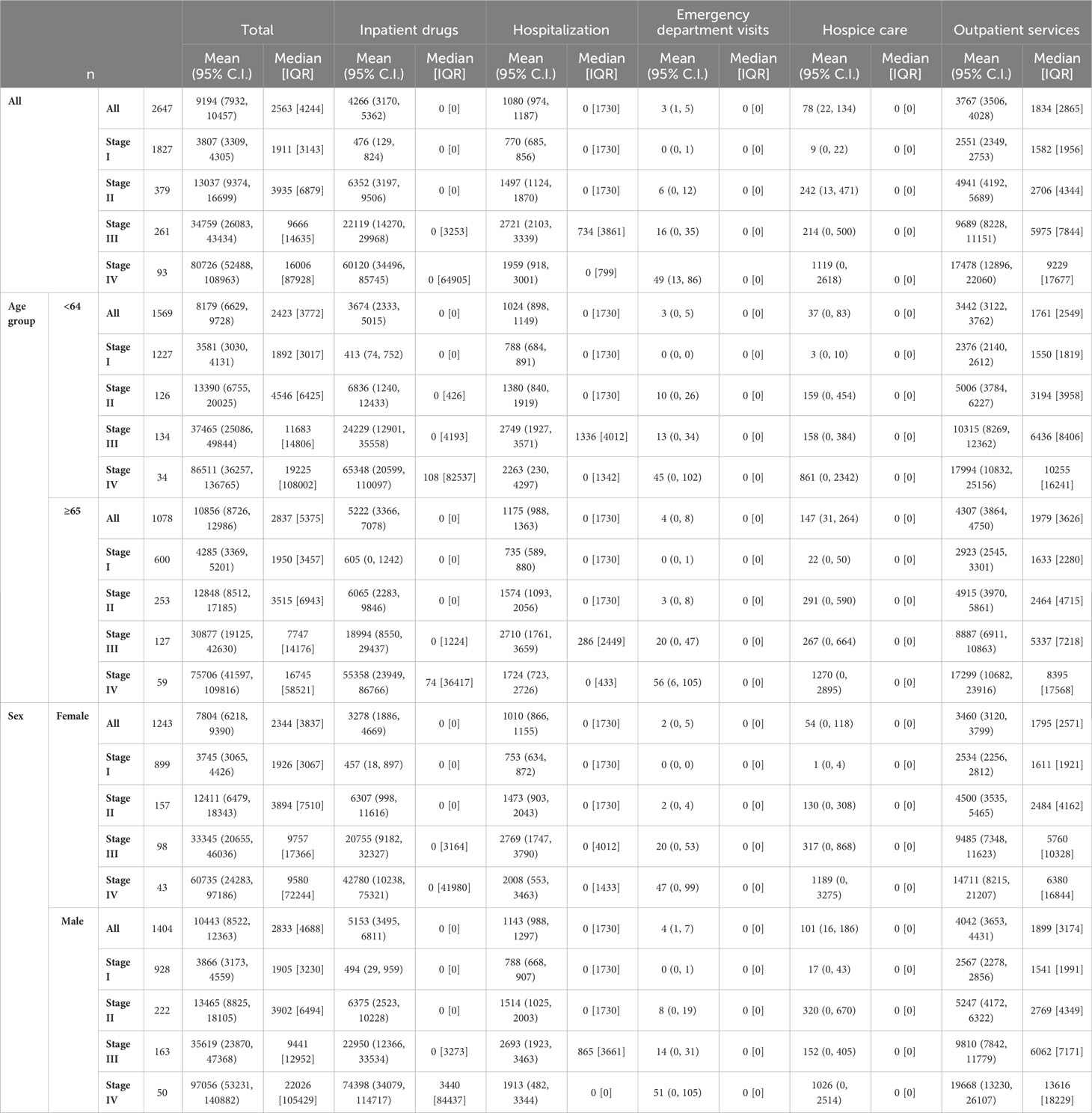
Table 3 Direct total costs of melanoma (€) in the four years after diagnosis by expense item stratified by stage for age, sex, survival time, histological subtype and overall.
Figure 4 shows that patients that survived less than four years have an annual expenditure mean of €11,827, compared to a mean of €1,648 for those who survived longer than the observation period.
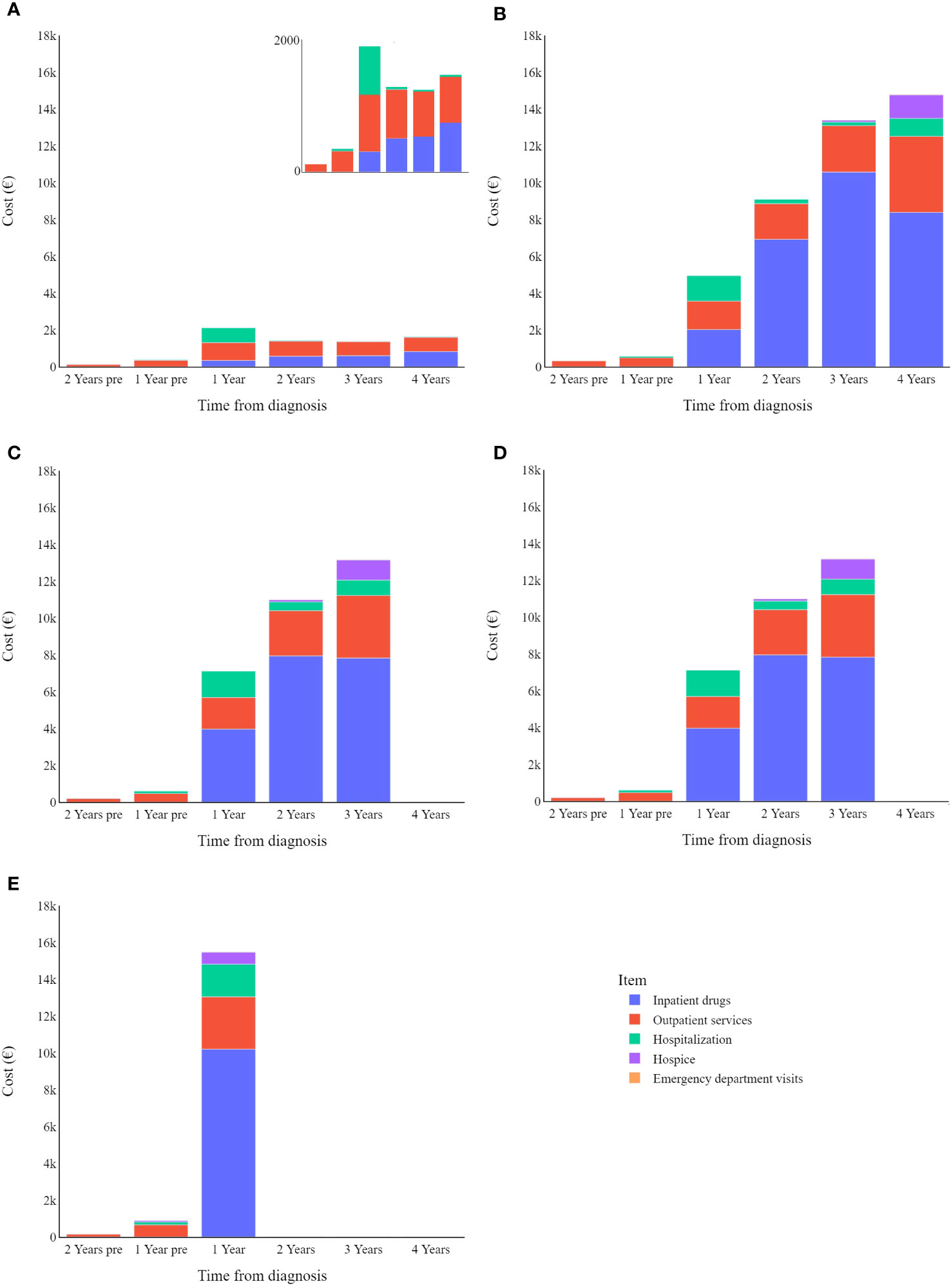
Figure 4 Melanoma specific item cost (€) trends by survival: (A) in survived; (B) in deceased in 4 years; (C) in deceased in 3 years; (D) in deceased in 2 years; (E) in deceased in 1 years.
Lastly, multivariable regression analysis (Table 4) shows that TNM stage at diagnosis and growth pattern were independently associated with cost changes, both for overall costs as well as cost per year for each of the four years following diagnosis. On the other hand, mitotic count, tumour regression, and primary tumour location were significantly associated with the year-specific cost but not when considering specific years. Lastly, the time to death in the index year resulted in statistically significant changes in costs.
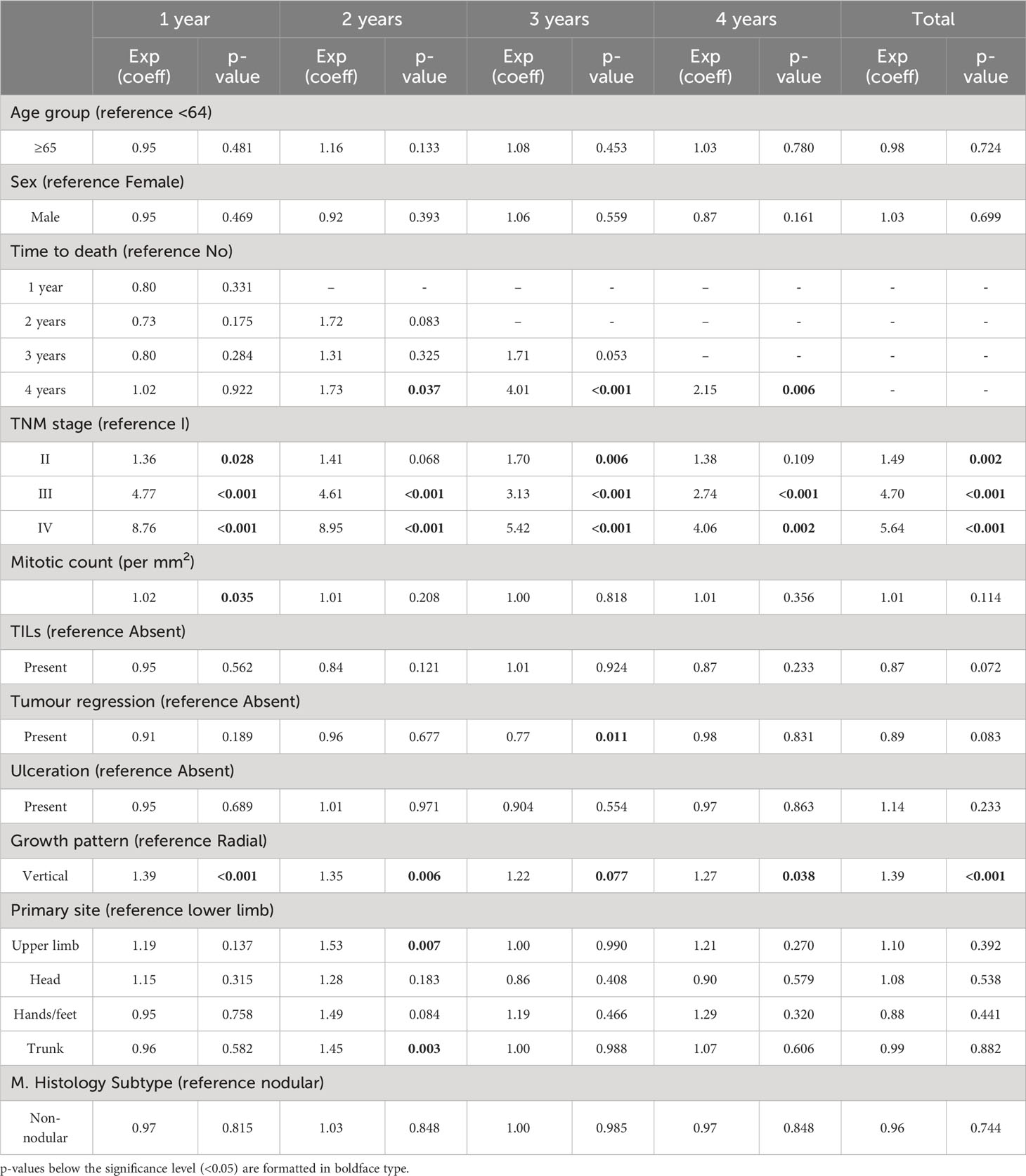
Table 4 Log-linear regression analysis of the association between person-years melanoma-specific direct costs (at specific times from diagnosis as well as covering the whole observed period) and age, sex, time to death, TNM stage at diagnosis, mitotic count, TILs, tumour regression, ulceration, growth phase, tumour location, and histology subtype.
4 Discussion
The current study indicated that cost items associated with cutaneous malignant melanoma can be better understood by grouping patients according to their tumour characteristics. Specific cost items contribute differently to the total cost of care for the patient over time and are also related to the clinical and anatomopathological characteristics of the tumour.
4.1 Yearly cost trends after diagnosis
Overall, analysing all data together, CMM patients tend to have higher costs in the first year after diagnosis, which then gradually decrease in subsequent years. As shown in the stage-specific sub analysis, this is only true when considering all CMM patients together, regardless of the stage of the disease at diagnosis (Figure 3).
In a previous publication analysing direct health care costs in patients with cutaneous malignant melanoma, Lyth and colleagues also reported a similar decrease in mean costs over time, both overall and depending on the clinical stage at diagnosis (15). Our data further reveal specific trends, by grouping costs into cost items (e.g., hospitalization, outpatient services, inpatient drugs, etc.). It should be noted here that under the Italian NHS, the reported costs of care are equal to the sum of the individual fees for each medical procedure provided to the patient, which are fixed amounts defined a priori by regional regulations. Thus, it can be observed that hospital-related costs peak in the first year after diagnosis and then gradually decrease over the years, suggesting that hospitalization is mainly considered at the beginning of treatment. In contrast, hospice-related spending shows the opposite trend, as patients with CMM could benefit from such care towards the end of their lives. The reason why hospital drug costs peak two years after diagnosis and then decline in subsequent years deserves to be investigated in future studies, as there is no obvious explanation either from the scientific literature or from analysis of the clinical care pathway established by regional guidelines (27). Finally, spending associated with outpatient services and emergency department visits does not seem to follow any obvious pattern that can be analysed.
4.2 Overall costs based on stage
As is known, advanced tumour stages are associated with higher costs of care (14, 15, 28, 29). Indeed, patients in our cohort that had stage IV CMMs were associated with costs that were more than 20 times higher than those with stage I (€80,726 vs. €3,807). Two other studies also reported melanoma-related costs of care according to tumour stage and time at diagnosis – these 2016 studies were carried out by Serra-Arbeloa et al. (14) and Lyth et al. (15). They drew data from the official Spanish national health system reports and the Swedish cancer registry (respectively). A comparison of first-year melanoma treatment costs shows that for stage I melanoma patients, we in Italy show the lowest first-year costs (€1,401), compared to the Spanish (€3,103) and the Swedes (€2,670). For stage IV patients, the Swedes reported the lowest expenditure (€29,291), followed by our present study (€30,762), with the Spanish reporting significantly higher expenditure than both (€88,268).
4.3 Cost items by stage at diagnosis
In most cases, the main cost driver is inpatient drugs, however this is not the case for stage I CMMs, which instead show higher costs related to outpatient services. The relative impact of hospitalization is greater for lower cancer stages because other cost items are relatively limited in these early stages of the disease. Even though it should be noted that hospitalizations are (in absolute value) costlier for more advanced melanomas, this drops slightly for stage IV melanomas.
As stated by Cullison C.S. and Bordeaux J.S. in a letter to the editor, excision of primary cutaneous melanoma is a low-risk operation usually associated with few complications, therefore hospitalization is not an expected consequence (30). While patients with stage I CMM often recover after the first tumour removal, more advanced melanoma patients face higher recurrence rates, which may influence the course of their disease in subsequent years and thus also increase costs (8, 31–33). Indeed, Leenaman and colleagues reported a 47% recurrence rate for stage III melanoma patients, compared to 29% for stage II and 8% for stage I (33). In stage IV advanced metastatic cutaneous malignant melanomas, surgical or other ablative treatments are limited to palliative care or as a complementary treatment in a combined approach to oligometastases (20, 23, 34–38).
Other costs four years from diagnosis differ widely among melanoma stages. Data highlight how the use of inpatient drugs heavily influences the cost of care for CMM, moving from an average cost of just over €6,000 for stage II melanomas to nearly €50,000 for stage IV melanomas. It should be noted that emergency department visits are always a minor factor in the total cost associated with CMM care, always accounting for less than 1% of the total expenditure.
4.4 Costs near the end of life
Overall, data show that death increases healthcare costs associated with CMM, where patients who survive beyond the observation period show substantially lower annual expenditure than patients who succumb to their disease. Specifically, patients who died during the observation period had the highest overall costs in their last year of life. In a recent literature review, Pisu and colleagues (39) summarized healthcare expenditure related to cancer care for multiple tumour types, stating that the initial and end-of-life phases are associated with the highest expenditure (both direct and out-of-pocket costs), while costs over the middle phases are generally lower. In an older systematic review covering articles from 1990-2011, Guy and colleagues (28) analysed the cost of melanoma care, highlighting that healthcare costs tend to be highest in the terminal phase and lowest in the interim phase (largely according to two studies conducted in the US). Consistently our present study indicates that end-of-life costs are highest and increased by hospice care (peaking in the last year of the patients’ lives) as well as hospitalization-related costs (which are higher than those seen in intermediate phases).
4.5 Other anatomo-pathological factors
Other factors can also significantly influence costs associated with CMM care (both overall and per year in the four years following diagnosis), such as tumour growth patterns. As hypothesized several decades ago, tumour progression in melanomas goes through successive stages of growth, from radial to vertical to metastatic disease (40–42). Invasion of the lower layers of the dermis has been associated with increased tumour aggressiveness, also linked by some authors to the expression of specific biological markers by the tumour (42). Consequently, it can be argued that the higher costs of vertically growing CMMs may be a consequence of the higher intensity of care required due to the more aggressive behaviour of these tumours (14, 43). However, this direct association between tumour growth pattern, treatment intensity and overall cost of care needs specific investigation to test its validity.
4.6 Limitations and strengths
The present study comes with the typical limitations and strengths seen in cohort studies that are based on regional, linked administrative databases. Even if a defined clinical pathway is common between different healthcare facilities in a region, the simple fact of including subjects that were treated in different hospitals could have distorted the results, not to mention the effect of dataset inaccuracies or missing values. Furthermore, due to the structure of these databases, our study could not explore the indirect costs of cutaneous malignant melanoma, thus limiting the goal of the research. Further studies are also needed to analyse cost differences after recent changes to the CMM treatment paradigm and the introduction of immunotherapies.
In contrast, the main strength of this study lies in its population-based design, which enables it to provide information on costs retrieved from real-world clinical practice, while minimizing the risk of selection bias that can occur with independently collected data.
5 Conclusion
Indeed, policy makers could greatly benefit from the analysis of CMM-related expenditure over time. In fact, a rational and judicious allocation of healthcare expenditures should account for the direct costs incurred in patient care pathways accordantly with results evidenced by health economic research. Unfortunately, researches based on expenditure determinants are still scarce. With better knowledge, they would be able to consider specific costs (i.e., overall, stage specific, and item specific costs, plus how they change over time) when allocating resources for CMM patients, in order to provide the best management of the disease and the best quality of care.
Data availability statement
The data supporting this study’s findings are held by the Veneto Epidemiological Registry and were used under license for this work, but they are not available to the general public. The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author and subject to authorization from the Veneto Epidemiological Registry (Veneto Regional Authority).
Ethics statement
The studies involving humans were approved by Veneto Oncological Institute’s Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
Conceptualization, AB; methodology, CC; software, CC; formal analysis, CC; investigation, CC; data curation, CC; visualization, CC; writing - original draft preparation, AB and AM; writing - review and editing, AB, AM and CC; supervision, AB; project administration, AB; All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research has received “Current Research 2023” funds from the Italian Ministry of Health to cover publication costs.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Melanoma Skin Cancer Statistics. Available at: https://www.cancer.org/cancer/melanoma-skin-cancer/about/key-statistics.html.
2. Rastrelli M, Tropea S, Rossi CR, Alaibac M. Melanoma: epidemiology, risk factors, pathogenesis, diagnosis and classification. In vivo (2014) 28(6):1055–11.
3. Pavri SN, Clune J, Ariyan S, Narayan D. Malignant melanoma: beyond the basics. Plast Reconstructive Surgery. (2016) 138(2):330e–40e. doi: 10.1097/PRS.0000000000002367
4. SEER*Explorer Application. Available at: https://seer.cancer.gov/statistics-network/explorer/application.html?site=53&data_type=1&graph_type=2&compareBy=sex&chk_sex_3=3&chk_sex_2=2&rate_type=2&race=1&age_range=1&stage=101&advopt_precision=1&advopt_show_ci=on&hdn_view=0&advopt_display=2#fnote_source.
5. SEER. Melanoma of the Skin - Cancer Stat Facts (2022). Available at: https://seer.cancer.gov/statfacts/html/melan.html.
6. Benčina G, Buljan M, Šitum M, Stevanović R, Benković V. Health and economic burden of skin melanoma in Croatia – cost-of-illness study. Acta Dermatovenerol Croat (2017) 25(1):1–7.
7. Buja A, Rivera M, De Polo A, Zorzi M, Baracco M, Italiano I, et al. Differences in direct costs of patients with stage I cutaneous melanoma: A real-world data analysis. Eur J Surg Oncol (2020) 46(6):976–81. doi: 10.1016/j.ejso.2020.02.017
8. Sullivan R, Peppercorn J, Sikora K, Zalcberg J, Meropol NJ, Amir E, et al. Delivering affordable cancer care in high-income countries. Lancet Oncol (2011) 12(10):933–80. doi: 10.1016/S1470-2045(11)70141-3
9. Hofmarcher T, Lindgren P, Wilking N, Jönsson B. The cost of cancer in Europe 2018. Eur J Cancer. (2020) 129:41–9. doi: 10.1016/j.ejca.2020.01.011
10. Mancuso F, Lage S, Rasero J, Díaz-Ramón JL, Apraiz A, Pérez-Yarza G, et al. Serum markers improve current prediction of metastasis development in early-stage melanoma patients: a machine learning-based study. Mol Oncol (2020) 14(8):1705–18. doi: 10.1002/1878-0261.12732
11. Buja A, Rugge M, De Luca G, Zorzi M, De Toni C, Cozzolino C, et al. Malignant melanoma: direct costs by clinical and pathological profile. Dermatol Ther (Heidelb). (2022) 12(5):1157–65. doi: 10.1007/s13555-022-00715-z
12. Conic RZ, Cabrera CI, Khorana AA, Gastman BR. Determination of the impact of melanoma surgical timing on survival using the National Cancer Database. J Am Acad Dermatol (2018) 78(1):40–46.e7. doi: 10.1016/j.jaad.2017.08.039
13. Laudicella M, Walsh B, Burns E, Smith PC. Cost of care for cancer patients in England: evidence from population-based patient-level data. Br J Cancer. (2016) 114(11):1286–92. doi: 10.1038/bjc.2016.77
14. Serra-Arbeloa P, Rabines-Juárez ÁO, Álvarez-Ruiz MS, Guillén-Grima F. Cost of Cutaneous Melanoma by Tumor Stage: A descriptive analysis. Actas Dermo-Sifiliográficas (English Edition). (2017) 108(3):229–36. doi: 10.1016/j.adengl.2017.02.006
15. Lyth J, Carstensen J, Synnerstad I, Lindholm C. Stage-specific direct health care costs in patients with cutaneous Malignant melanoma. J Eur Acad Dermatol Venereology. (2016) 30(5):789–93. doi: 10.1111/jdv.13110
16. Krensel M, Schäfer I, Augustin M. Cost-of-illness of melanoma in Europe - a modelling approach. J Eur Acad Dermatol Venereol. (2019) 33:34–45. doi: 10.1111/jdv.15308
17. Grange F, Mohr P, Harries M, Ehness R, Benjamin L, Siakpere O, et al. Economic burden of advanced melanoma in France, Germany and the UK: a retrospective observational study (Melanoma Burden-of-Illness Study). Melanoma Res (2017) 27(6):607–18. doi: 10.1097/CMR.0000000000000372
18. Ferré F, de Belvis AG, Valerio L, Longhi S, Lazzari A, Fattore G, et al. Italy: health system review. Health Syst Transit (2014) 16(4):1–168.
19. Regione del Veneto. Regione del Veneto - Sistema Statistico Regionale. Il Rapporto Statistico – il Veneto si Racconta, il Veneto si confronta (Italy: Regione del Veneto) (2022). Available at: https://statistica.regione.veneto.it/index_indicatori.jsp?indic=Pil_20220126.
20. Alaibac M, Baiocchi C, Bassetto F, Sileni VC, Pian PD, Montesco MC, et al. Regione del Veneto - Aggiornamento del PDTA per pazienti affetti da melanoma. Rete Oncol Veneta (2019), 1–79.
21. Associazione Italiana di Oncologia Medica (AIOM). Associazione Italiana Radioterapia e Oncologia clinica, Intergruppo melanoma integrato, Società Italiana di Chirurgia Oncologica. In: Linee guida Melanoma (2020). Available at: https://www.aiom.it/wp-content/uploads/2020/10/2020_LG_AIOM_Melanoma.pdf.
22. National Comprehensive Cancer Network. (2023). NCCN guidelines for patients melanoma. Available at: https://www.nccn.org/patients/guidelines/content/PDF/melanoma-patient.pdf.
23. National Institute for Health and Care Excellence. Melanoma: assessment and management (2015). Available at: https://www.nice.org.uk/guidance/ng14.
24. Buja A, Rugge M, De Luca G, Zorzi M, Cozzolino C, Vecchiato A, et al. Clinical performance indicators for monitoring the management of cutaneous melanoma: a population-based perspective. Melanoma Res (2022) 32(5):353. doi: 10.1097/CMR.0000000000000841
25. R: The R Foundation. (2023). Available at: https://www.r-project.org/foundation/.
26. Python.org. Welcome to Python.org (2023). Available at: https://www.python.org/psf-landing/.
27. Rete Oncologica Veneta. Aggiornamento del PDTA per pazienti affetti da Melanoma (2019). Available at: https://salute.regione.veneto.it/web/rov/pdta-melanoma.
28. Guy GP, Ekwueme DU, Tangka FK, Richardson LC. Melanoma treatment costs: A systematic review of the literature, 1990–2011. Am J Prev Med (2012) 43(5):537–45. doi: 10.1016/j.amepre.2012.07.031
29. Krensel M, Schäfer I, Augustin M. Cost-of-illness of melanoma in Europe – a systematic review of the published literature. J Eur Acad Dermatol Venereol. (2019) 33(3):504–10. doi: 10.1111/jdv.15315
30. Cullison CR, Tripathi R, Bordeaux JS. Identifying sociodemographic factors associated with hospitalization following primary excision of cutaneous melanoma. J Am Acad Dermatol (2022) 86(3):681–3. doi: 10.1016/j.jaad.2021.02.070
31. Joyce KM. Surgical management of melanoma. In: Ward WH, Farma JM editors. Cutaneous melanoma: etiology and therapy [Internet]. Brisbane (AU): Codon Publications (2017) Chapter 7:91–100.
32. Yushak M, Mehnert J, Luke J, Poklepovic A. Approaches to high-risk resected stage II and III melanoma. Am Soc Clin Oncol Educ Book. (2019) 39):e207–11. doi: 10.1200/EDBK_239283
33. Leeneman B, Franken MG, Coupé VMH, Hendriks MP, Kruit W, Plaisier PW, et al. Stage-specific disease recurrence and survival in localized and regionally advanced cutaneous melanoma. Eur J Surg Oncol (2019) 45(5):825–31. doi: 10.1016/j.ejso.2019.01.225
34. O’Neill CH, McMasters KM, Egger ME. Role of surgery in stage IV melanoma. Surg Oncol Clinics North America. (2020) 29(3):485–95. doi: 10.1016/j.soc.2020.02.010
35. Michielin O, van Akkooi ACJ, Ascierto PA, Dummer R, Keilholz U. Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol (2019) 30(12):1884–901. doi: 10.1093/annonc/mdz411
36. Deutsch GB, Kirchoff DD, Faries MB. Metastasectomy for stage IV melanoma. Surg Oncol Clinics North America. (2015) 24(2):279–98. doi: 10.1016/j.soc.2014.12.006
37. Caudle AS, Ross MI. Metastasectomy for stage IV melanoma: for whom and how much? Surg Oncol Clinics North America (2011) 20(1):133–44. doi: 10.1016/j.soc.2010.09.010
38. Knackstedt T, Knackstedt RW, Couto R, Gastman B. Malignant melanoma: diagnostic and management update. Plast Reconstructive Surgery. (2018) 142(2):202e–16e. doi: 10.1097/PRS.0000000000004571
39. Pisu M, Henrikson NB, Banegas MP, Yabroff KR. Costs of cancer along the care continuum: What we can expect based on recent literature: Cancer Costs Along the Care Continuum. Cancer (2018) 124(21):4181–91. doi: 10.1002/cncr.31643
40. Clark W Jr, From L, Bernardino EA, Mihm MC. The histogenesis and biologic behavior of primary human Malignant melanomas of the skin. Cancer Res (1969) 29(3):705–27.
41. Byers HR, Bhawan J. Pathologic parameters in the diagnosis and prognosis of primary cutaneous melanoma. Hematology/Oncology Clinics North America. (1998) 12(4):717–35. doi: 10.1016/S0889-8588(05)70020-4
42. Alonso SR, Ortiz P, Pollán M, Pérez-Gómez B, Sánchez L, Acuña MJ, et al. Progression in cutaneous Malignant melanoma is associated with distinct expression profiles. Am J Pathol (2004) 164(1):193–203. doi: 10.1016/S0002-9440(10)63110-0
Keywords: melanoma, direct costs, specific costs, cost of illness, health economics, healthcare assessment, healthcare services research
Citation: Buja A, Cozzolino C, Zanovello A, Geppini R, Miatton A, Zorzi M, Manfredi M, Bovo E, Del Fiore P, Tropea S, dall’Olmo L, Rossi CR, Mocellin S, Rastrelli M and Rugge M (2023) Cost items in melanoma patients by clinical characteristics and time from diagnosis. Front. Oncol. 13:1234931. doi: 10.3389/fonc.2023.1234931
Received: 27 July 2023; Accepted: 20 October 2023;
Published: 08 November 2023.
Edited by:
Sapna Patel, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Grainne Crealey, Clinical Costing Solutions, United KingdomFaramarz Samie, Columbia University, United States
Copyright © 2023 Buja, Cozzolino, Zanovello, Geppini, Miatton, Zorzi, Manfredi, Bovo, Del Fiore, Tropea, dall’Olmo, Rossi, Mocellin, Rastrelli and Rugge. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ruggero Geppini, cnVnZ2Vyby5nZXBwaW5pQHN0dWRlbnRpLnVuaXBkLml0
 Alessandra Buja
Alessandra Buja Claudia Cozzolino
Claudia Cozzolino Anna Zanovello1,2
Anna Zanovello1,2 Ruggero Geppini
Ruggero Geppini Andrea Miatton
Andrea Miatton Manuel Zorzi
Manuel Zorzi Mariagiovanna Manfredi
Mariagiovanna Manfredi Paolo Del Fiore
Paolo Del Fiore Saveria Tropea
Saveria Tropea Simone Mocellin
Simone Mocellin Marco Rastrelli
Marco Rastrelli Massimo Rugge
Massimo Rugge