- 1Department of Sarcoma, Peritoneal and Rare Tumours (SPRinT), Division of Surgery and Surgical Oncology, National Cancer Centre Singapore, Singapore, Singapore
- 2Department of Sarcoma, Peritoneal and Rare Tumours (SPRinT), Division of Surgery and Surgical Oncology, Singapore General Hospital, Singapore, Singapore
- 3SingHealth Duke-NUS Oncology Academic Clinical Program, Duke-NUS Medical School, Singapore, Singapore
- 4SingHealth Duke-NUS Surgery Academic Clinical Program, Duke-NUS Medical School, Singapore, Singapore
- 5Department of Peritoneal Cancer Surgery and Pathology, Beijing Tsinghua Changgung Hospital, Beijing, China
- 6Department of Gastrointestinal Surgery, Ghent University Hospital, Ghent, Belgium
- 7Laboratory of Applied Human Genetics, Division of Medical Sciences, National Cancer Centre Singapore, Singapore, Singapore
- 8Institute of Molecular and Cell Biology, ASTAR Research Entities, Singapore, Singapore
Editorial on the Research Topic
Translational research in the diagnosis and development of therapeutics for peritoneal surface malignancies
Peritoneal surface malignancies (PSM) refer to a heterogenous group of primary and metastatic cancers that can arise from intraperitoneal (e.g. gastrointestinal, gynecological) or extraperitoneal (e.g. lung, breast) organs (1). The structure of healthcare systems in most countries are organ-centric, leading to a phenomenon where PSM are often managed as an end-stage disease by each subspeciality. Accurate diagnosis of PSM and complete surgical extirpation are fraught with challenges (2). Coupled with a duet of biologically advanced disease and suboptimal knowledge and expertise in dealing with this disease, prognosis of patients with PSM is uniformly poor in many countries.
Fortunately, with the recognition of PSM as a treatable phenomenon that transcends multiple intraperitoneal and extraperitoneal organs, two major organisations (International Society for the Study of Pleura and Peritoneum (ISSPP) and The Peritoneal Surface Oncology Group International (PSOGI)) were set up to amalgamate the surgical and medical expertise to combat this disease collectively (3, 4). Clinical guidelines are continually being updated and management algorithms constantly debated to reach clinical consensus by the above mentioned consortia. However, the future of PSM management as in all other diseases lies in devising novel diagnostic and therapeutic strategies. Progressing translational research in this arena hence remains the cornerstone to improve patient outcome. It is with this vision that we started this Research Topic, with the aim to highlight areas of deficiencies and continual scientific research that can feed into clinical algorithms for PSM management.
Helderman et al. performed a pragmatic study to examine the effects of hyperthermia and the duration of intra-peritoneal chemotherapy in vivo. The authors demonstrated that the efficacy of intraperitoneal chemotherapy (oxaliplatin and mitomycin C) in in vitro and in vivo models is dependent on the temperature of the perfusate and treatment duration. Hyperthermic chemotherapy applied at 41-42° C for 90 minutes improved drug uptake, induced apoptosis and decreased proliferation compared to lower temperatures and shorter duration without increasing the toxicity in normal tissue. With the failure of the PRODIGE 7 study (5) to demonstrate superiority of the hyperthermic chemotherapy in improving patient outcome compared to control, we eagerly await the results of the GECOP-MMC trial (Clinicaltrials.gov identifier: NCT05250648), which studies the effect of hyperthermic mitomycin C in reducing tumour recurrence after cytoreductive surgery and hyperthermic intra-peritoneal chemotherapy (HIPEC) (6). A positive result from this randomised controlled study will be testament to the importance of translational research to inform appropriate trial design.
2 other studies by Valenzuela-Molina et al. and Löke et al. explore the biology of PSM and attempt to combine treatment planning software with an atomically accurate 3D-printed phantom of a female peritoneum. The elegant study by Valenzuela-Molina et al. demonstrated low intratumoral oxygen levels in psudomyxoma peritonei samples with consequent HIF-1α levels. This could pave the way for potential novel therapeutic strategies in regulation of the hypoxia pathway in PSM. Löke et al., on the other hand, combined software simulations with experimental validation to define the use of a thermal module to account for forced convection during HIPEC. Several experimental conditions including catheter setups, inflow temperatures and flow rates were considered and compared to simulations to determine the accuracy of the treatment planning software. This study demonstrated the potential of harnessing software simulation to guide and evaluate treatment strategies by optimising HIPEC treatments.
This series would not be complete without defining the effect of the genomic landscape of PSM on patient outcome. Nguyen et al. explored the tumour molecular signatures of peritoneal metastases across multiple histological subtypes and correlated this with progression-free survival of patients. While the sample size is limited, the authors identified AGAP5 as a potential prognostic gene among other putative driver genes identified. This study complements multiple other genome wide studies, which define the effect of the genomic, transcriptomic and epigenomic landscape on patient outcome and the development of novel therapeutics (7, 8).
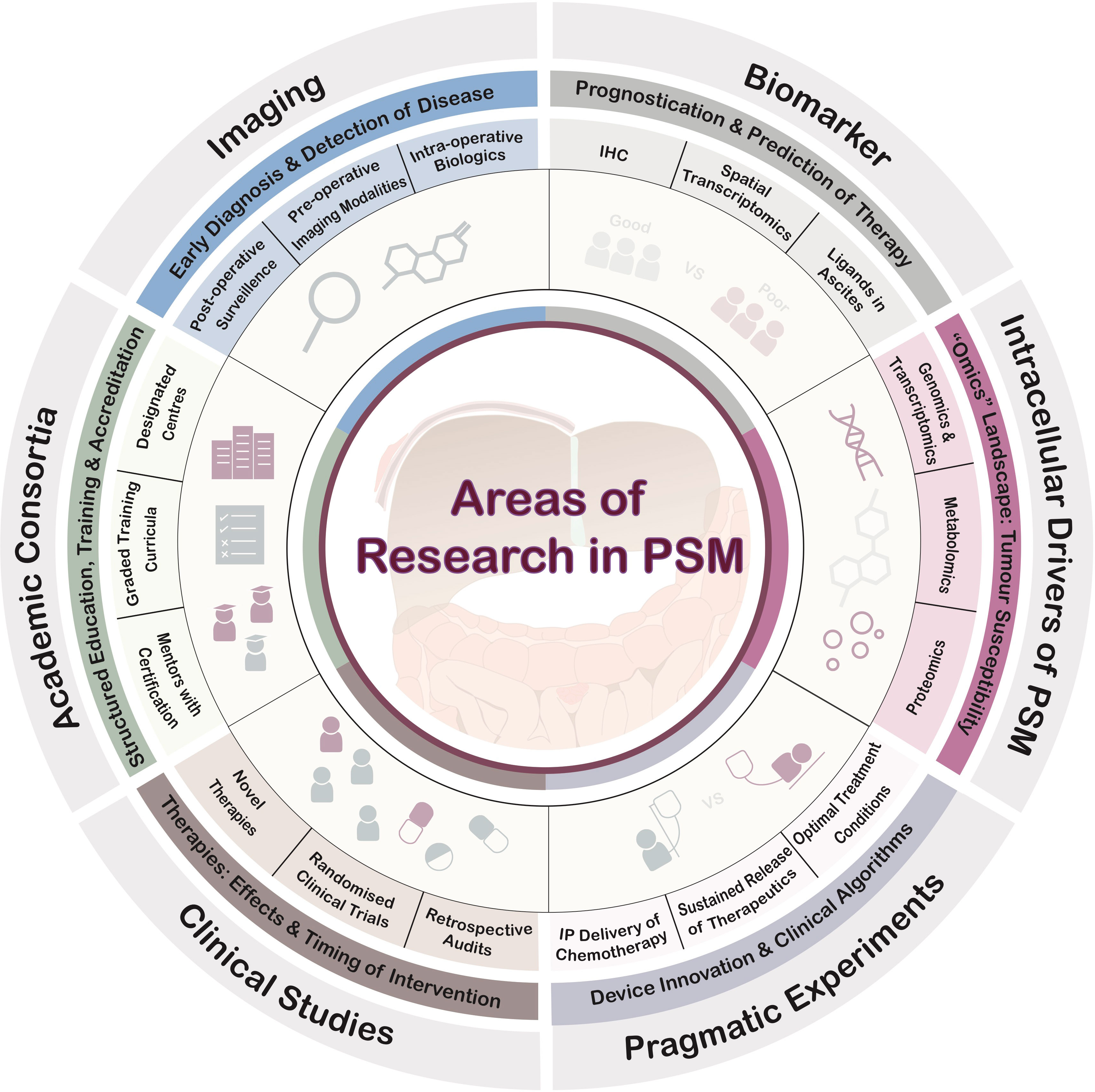
This short series of articles in this Research Topic highlights multiple arenas of future translational research to improve PSM management. Conceptually, the areas of research needed are summarised in Figure 1. Beyond the areas covered in these series, fundamental research into the tumour microenvironment that inform diagnostics, detection and therapeutics are also critical milestones for the advancement of care for PSM patients (9–13).
Finally, it is only with the tripartite development of clinical guidelines championed by consortia (e.g. ISSPP, PSOGI), translational research to move the needle for novel interventions, and rigorous testing via clinical studies, that care for PSM could improve substantially in the near future.
Author contributions
CSC, YL, WC and C-AJO wrote, reviewed and approved the manuscript for submission.
Funding
C-AJO is supported by the National Medical Research Council Clinician Scientist-Individual Research Grant (MOH-CIRG21jun-0005) and Clinician Scientist Award (INV category) (MOH-CSAINV22jul-0005). All funding sources had no role in the writing of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Cortés-Guiral D, Hübner M, Alyami M, Bhatt A, Ceelen W, Glehen O, et al. Primary and metastatic peritoneal surface malignancies. Nat Rev Dis Primers (2021) 7:91. doi: 10.1038/s41572-021-00326-6
2. Rubino MS, Abdel-Misih RZ, Bennett JJ, Petrelli NJ. Peritoneal surface malignancies and regional treatment: a review of the literature. Surg Oncol (2012) 21:87–94. doi: 10.1016/j.suronc.2010.12.001
3. ISSPP. International society for the study of pleura and peritoneum . Available at: https://isspp.org/ (Accessed May 2, 2023).
4. PSOGI. The 13th international congress on peritoneal surface malignancies . Available at: https://psogicongress2023.com/ (Accessed May 2, 2023).
5. Quénet F, Elias D, Roca L, Goéré D, Ghouti L, Pocard M, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol (2021) 22:256–66. doi: 10.1016/S1470-2045(20)30599-4
6. Pereira F, Serrano A, Manzanedo I, Pérez-Viejo E, González-Moreno S, González-Bayón L, et al. GECOP-MMC: phase IV randomized clinical trial to evaluate the efficacy of hyperthermic intraperitoneal chemotherapy (HIPEC) with mytomicin-c after complete surgical cytoreduction in patients with colon cancer peritoneal metastases. BMC Cancer (2022) 22:536. doi: 10.1186/s12885-022-09572-7
7. Gwee YX, Chia DKA, So J, Ceelen W, Yong WP, Tan P, et al. Integration of genomic biology into therapeutic strategies of gastric cancer peritoneal metastasis. J Clin Oncol (2022) 40(24):2830. doi: 10.1200/JCO.21.02745
8. Lenos KJ, Bach S, Moreno LF, ten Hoorn S, Sluiter NR, Bootsma S, et al. Molecular characterization of colorectal cancer related peritoneal metastatic disease. Nat Commun (2022) 13:4443. doi: 10.1038/s41467-022-32198-z
9. Hendrikson J, Liu Y, Ng WH, Lee JY, Lim AH, Loh JW, et al. Ligand-mediated PAI-1 inhibition in a mouse model of peritoneal carcinomatosis. Cell Rep Med (2022) 3:100526. doi: 10.1016/j.xcrm.2022.100526
10. Isobe A, Sawada K, Kinose Y, Ohyagi-Hara C, Nakatsuka E, Makino H, et al. Interleukin 6 receptor is an independent prognostic factor and a potential therapeutic target of ovarian cancer. PloS One (2015) 10:e0118080. doi: 10.1371/journal.pone.0118080
11. Yasumoto K, Koizumi K, Kawashima A, Saitoh Y, Arita Y, Shinohara K, et al. Role of the CXCL12/CXCR4 axis in peritoneal carcinomatosis of gastric cancer. Cancer Res (2006) 66:2181–7. doi: 10.1158/0008-5472.CAN-05-3393
12. Bekes I, Friedl TWP, Köhler T, Möbus V, Janni W, Wöckel A, et al. Does VEGF facilitate local tumor growth and spread into the abdominal cavity by suppressing endothelial cell adhesion, thus increasing vascular peritoneal permeability followed by ascites production in ovarian cancer? Mol Cancer (2016) 15:13. doi: 10.1186/s12943-016-0497-3
Keywords: translational, therapeutics, peritoneal surface malignancies, diagnosis, research
Citation: Chia CS, Li Y, Ceelen W and Ong C-AJ (2023) Editorial: Translational research in the diagnosis and development of therapeutics for peritoneal surface malignancies. Front. Oncol. 13:1232993. doi: 10.3389/fonc.2023.1232993
Received: 01 June 2023; Accepted: 26 June 2023;
Published: 10 July 2023.
Edited and Reviewed by:
Francesco Giovinazzo, Agostino Gemelli University Polyclinic (IRCCS), ItalyCopyright © 2023 Chia, Li, Ceelen and Ong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chin-Ann Johnny Ong, am9obm55Lm9uZy5jLmFAc2luZ2hlYWx0aC5jb20uc2c=
 Claramae Shulyn Chia
Claramae Shulyn Chia Yan Li
Yan Li Wim Ceelen
Wim Ceelen Chin-Ann Johnny Ong
Chin-Ann Johnny Ong