
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol., 01 September 2023
Sec. Gynecological Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1232409
This article is part of the Research TopicLymph Node Assessment in Cervical CancerView all 9 articles
Background: Numerous studies have investigated the significance of pretreatment C-reactive protein (CRP) levels for determining the prognosis of cervical cancer (CC). The results of these studies, however, have been inconsistent. The present meta-analysis, therefore, focused on identifying the exact relationship of CRP levels with CC prognoses.
Methods: We searched the following databases from their inception until April 18, 2023: PubMed; Web of Science; Embase; and Cochrane Library. From the search results, we estimated the significance of CRP levels in determining the prognosis of CC, based on combined hazard ratios (HRs) and relevant 95% confidence intervals (CIs).
Results: The present meta-analysis included 12 studies, encompassing 2,204 patients. Based on combined data, an increased CRP level was significantly related to an unfavorable overall survival (OS) of patients with CC (HR = 1.63; 95% CI = 1.36–1.95; P < 0.001). Moreover, an increased CRP level was significantly associated with shortened progression-free survival (PFS) in patients with CC (HR = 1.68; 95% CI = 1.39–2.03; P < 0.001). According to the subgroup and sensitivity analyses, CRP level was a reliable factor in determining CC prognoses.
Conclusion: Based on the results of our present analyses, increased CRP levels were significant predictors of poor OS and PFS in patients with CC. CRP level, therefore, could be an independent and inexpensive factor for determining the prognosis of patients with CC in clinical settings.
Systematic review registration: INPLASY, identifier INPLASY202360074.
Cervical cancer (CC) ranks the fourth most common cancer in women; besides, it is also the fourth frequent cause of cancer death among women globally (1). There were 604,127 new cases of CC and 6 341,831 deaths due to CC in 2020 worldwide (1, 2). The primary etiological factor for CC is chronic human papillomavirus (HPV) infection (3, 4), and surgical resection is the preferred treatment for patients diagnosed with International Federation of Gynecology and Obstetrics (FIGO) stages ≤ IIA, while chemoradiation is recommended for patients diagnosed with higher stages (3, 5). It is estimated, however, that 20–25% of patients with CC will experience a recurrence after completing their primary treatment, and that locally advanced CC has a poor 5-year survival rate (6). To some extent, the lack of efficient prognostic markers may have a correlation to poor CC survival. Therefore, establishing reliable and easily available biomarkers for determining the prognosis of patients with CC would aid the development of treatment strategies.
A growing body of evidence suggests that inflammation is involved in the pathogenesis and development of solid tumors (7, 8). When infection, tissue injury, trauma, neoplastic growth, or surgery interferes with the homeostasis of an organism, an acute-phase response (APR) is immediately triggered (9). C-reactive protein (CRP), a sensitive and well-recognized systemic inflammatory marker produced by the liver in response to factors such as interleukin (IL)-6, IL-1, along with tumor necrosis factor-α (TNF-α) (10). CRP is an acute phase reactant that is widely considered to be a marker of both acute and chronic inflammation (11). CRP has several well-defined functions, including acting as a pattern recognition receptor with calcium-sensitive binding pockets for ligands expressing phosphocholine (PC) moieties (12). CRP can also bind to activated cell membranes in which the PC groups on phospholipids become accessible when diacylphospholipids are hydrolyzed into monoacylphospholipids (12). In addition to being found in serum, CRP has recently been found to exist in an array of cyclic pentameric discs. The effects of CRP on the behavior of cells and the microenvironment are, therefore, dependent on its structural configuration. Pentameric CRP (pCRP) binds to PC on the surface of the cell, causing it to dissociate into its distinct monomeric isoform (mCRP), which subsequently reduces its aqueous solubility (13). It has been suggested that CRP levels are an effective factor in determining the prognosis of various cancers, including esophageal cancer (14), renal cell carcinoma (RCC) (15), prostate cancer (16), and gastric cancer (17). The relationship between CRP levels and survival outcomes in patients with CC has been extensively investigated; however, no consistent results have been reported thus far (18–29). Elevated CRP levels have been reported to be a significant prognostic factor for CC in some studies (18, 20, 22), while others have demonstrated a non-significant association between CRP levels and CC prognosis (19, 24). Given these inconsistent results, we aimed to comprehensively search relevant articles in the present meta-analysis to quantitatively identify the true relationship of CRP levels and CC prognoses.
The present meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (30), and was registered with the International Platform of Registered Systematic Review and Meta-analysis Protocols (INPLASY) platform. The registration number of this meta-analysis on INPLASY is INPLASY202360074.
The present meta-analysis did not require the involvement of an institutional review board or ethics committee, as all of the data acquired were derived from previously published studies.
In the present study, we thoroughly searched the PubMed, Web of Science, Embase, and Cochrane Library databases from their inception until April 18, 2023, for the following keywords/search terms: (C-reactive protein OR CRP OR c-reactive protein) AND (cervical cancer OR cervical carcinoma OR uterine cervix cancer OR cervical neoplasm OR cervix cancer). Only English studies were considered. After the initial search, the references from each of the included articles were manually searched to identify additional relevant articles.
The inclusion criteria were as follows: (i) CC diagnosis confirmed based on pathology; (ii) studies explored the between serum CRP levels and any survival outcomes of patients with CC; (iii) available hazard ratios (HRs) and associated 95% confidence intervals (CIs) for prognoses from studies or calculable data based on the information provided in the articles; (iv) a cut-off value was defined with which to determine low/high CRP levels; and (v) studies were published in English. The exclusion criteria were as follows: (i) articles formatted as reviews, meeting abstracts, case reports, comments, or letters; (ii) patients with new infections, or chronic infection diseases, autoimmune diseases, organ dysfunctions, hematologic diseases, and patients with another type of tumor; (iii) articles involved animal studies; and (iv) duplicate articles.
Two researchers (SY and ZZ) independently screened all eligible studies and extracted the information required from each, which included the following: first author; publication year; country; sample size; study duration; age; FIGO stage; treatment; CRP level cut-off; cut-off determination method; survival endpoints; follow-up; survival analysis type; and HRs with 95% CIs. All disagreements were resolved by reaching a consensus after discussion with a third reviewer (LS). The overall survival (OS) was selected as the primary outcome, and progression-free survival (PFS) as the secondary outcome. Additionally, we utilized the Newcastle-Ottawa Scale (NOS) score to evaluate the quality of the included articles (31). The NOS evaluates studies based on 3 factors cohort selection; comparability; and results. A NOS score ≥ 6, based on a 0–9 point scale, suggests high-quality research.
Pooled HRs and 95% CIs were calculated to estimate the significance of CRP levels in determining the prognosis for patients with CC. In general, a combined HR > 1 with a 95% CI not overlapping 1 indicated a significant association with poor prognosis, while a combined HR < 1 with a 95% CI not overlapping 1 indicated a better prognosis. Inter-study heterogeneity was evaluated using Cochran’s Q-test (32) and I2 statistics (33). I2 was used to quantify the degree of heterogeneity among the studies, as follows: I2 < 25%, low degree; 25–75%, moderate degree; and > 75%, high degree of heterogeneity (33, 34). To analyze the pooled data, we used two different computational models, based on the traits of the included studies, and the cut-off for significant heterogeneity was set at I2 > 50% (35–38). When high heterogeneity was determined based on I2 > 50% and Q-test P < 0.10, and a random-effects model (REM; DerSimonian-Laird method) was used (39); otherwise, a fixed-effects model (FEM; Mantel-Haenszel method) was used (40). Subgroup analyses of OS and PFS were conducted to identify possible sources of heterogeneity, and we conducted a sensitivity analysis by removing one article at a time, in order, to evaluate the robustness of the combined results. Funnel plots and Begg’s test (41) were utilized to evaluate publication bias. All statistical analyses were conducted using Stata software version 12.0 (Stata Corporation, College Station, TX, USA), with P < 0.05 indicating a significant difference.
During the initial literature search, 850 relevant articles were identified, and after the removal of duplicate studies, 692 studies remained (Figure 1). After title and abstract screening, 658 articles were excluded because they were irrelevant studies or animal studies, the full texts of the remaining 34 articles were read, and 22 articles were further eliminated, for the following reasons: irrelevance to CRP (n = 10); non-available survival analysis data (n = 9); irrelevance to CC (n = 1); no identified cut-off value (n = 1); or overlapping cases (n = 1). In total, we included 12 articles in the present study, encompassing 2,204 cases (Figure 1, Table 1) (18–29).
Table 1 summarizes the basic characteristics of the present study. All of the eligible studies were published between 2007 and 2023, with 7 published in China (20, 22–25, 28, 29), 2 in Austria (18, 21), 2 in Japan (19, 27), and 1 in Turkey (26). Sample sizes ranged from 32 to 460 (median, 182) participants. Of the 12 included studies, 5 recruited patients diagnosed with FIGO stages I–IV (18, 20–22, 26), 2 with stages I–II (23, 24), and 2 with stages I–III (25, 28), while 3 included recurrent/metastatic tumor cases (19, 27, 29). The threshold CRP value was 2.6–10 (median, 5.55) mg/L, and 8 articles determined the cut-off value using receiver operating characteristic (ROC) analysis (19–22, 24, 26, 28, 29), while 4 studies utilized the median value of that individual study (18, 23, 25, 27). Of the 12 articles, 11 mentioned the significance of CRP in predicting OS (18–28), and 6 investigated the association between CRP and PFS in patients with CC (18, 20, 23, 24, 28, 29). HRs, together with the relevant 95% CIs, were collected through univariate regression in 9 articles (19, 20, 22, 24–28) and multivariate analysis in 4 (18, 21, 23, 29). The NOS scores all ranged from 7–9 (median, 8), indicating high quality articles (Table 1).
A total of 11 articles, encompassing 2,089 patients (18–28), presented data on the relationship between the CRP value and OS in patients with CC. REM was utilized in these articles because significant heterogeneity was detected (I2 = 71.6%; P < 0.001). In Table 2 and Figure 2, as seen based on the pooled data, increased CRP expression was significantly tied to extremely poor OS in patients with CC (HR = 1.63; 95% CI = 1.36–1.95; P < 0.001). Subgroup analyses were conducted based on various factors. As seen in Table 2, subgroup analysis results indicated that elevated levels of serum CRP were still a significant marker for predicting OS in patients with CC regardless of sample size, country, FIGO stage, treatment, CRP cut-off, cut-off determination, or survival analysis type (all, P < 0.05).
Of the 12 included articles, 6 studies, encompassing 1,415 patients (18, 20, 23, 24, 28, 29), provided data on the role of CRP values in predicting the PFS of patients with CC. The FEM was utilized with these articles, due to their low level of heterogeneity (I2 = 22.9%; P = 0.262; Figure 3). In line with our aforementioned analysis, however, increased CRP levels were significantly correlated with shortened PFS in patients with CC (HR = 1.68; 95% CI = 1.39–2.03; P < 0.001; Figure 3, Table 3). Similar to what we observed with the subgroup analysis for OS, an increased CRP level was significant in predicting PFS regardless of sample size, country, FIGO stage, treatment, CRP cut-off, cut-off determination, or survival analysis type (P < 0.05; Table 3).
The impact of each article on the combined results was analyzed by performing a sensitivity analysis, which revealed stable outcomes for both OS (Figure 4A) and PFS (Figure 4B).
Begg’s test and funnel plots were used to assess publication bias. As shown in Figure 5, symmetry was observed in the funnel plots. For Begg’s test, the resulting P-value for OS was 0.082, while that for PFS was 0.452, indicating the absence of significant publication bias in the present study.
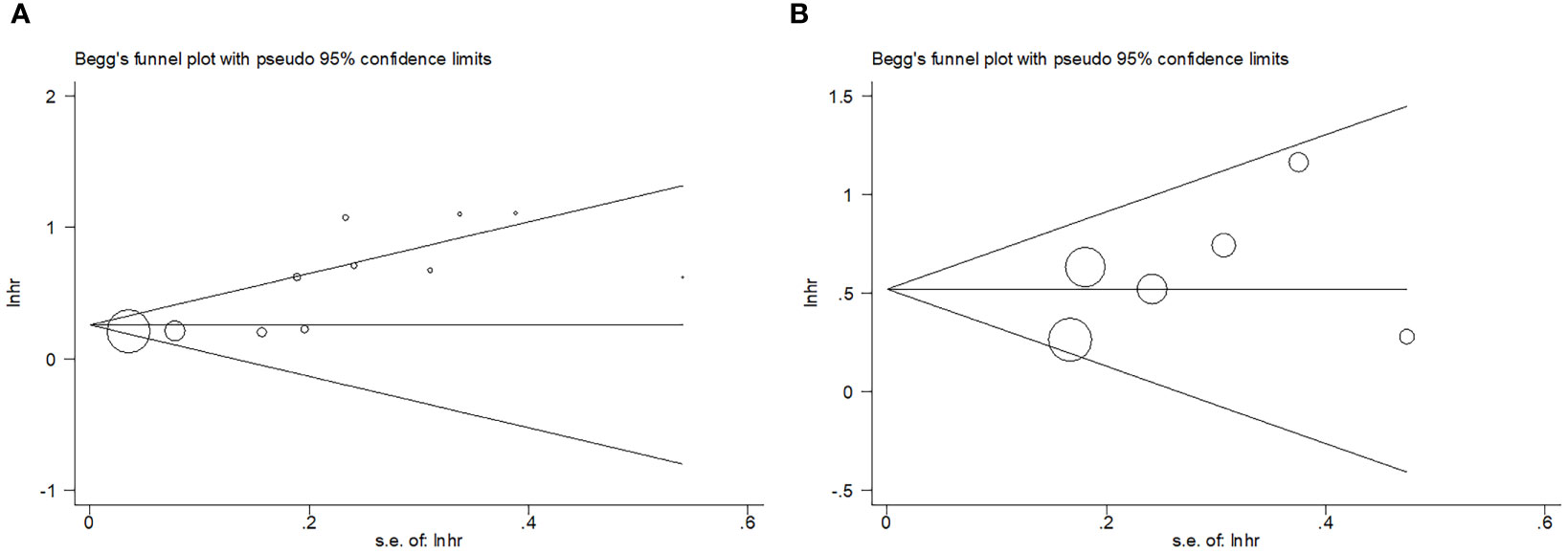
Figure 5 Publication test by Begg’ test. (A) Begg’s test for OS, p=0.082; (B) Begg’s test for PFS, p=0.452.
Although previous studies have evaluated the significance of CRP levels in determining the prognoses of patients with CC, the results were inconsistent. The present meta-analysis, which included 12 articles encompassing 2,204 cases, aimed to definitively identify the relationship of CRP levels with CC prognoses. Based on the findings of the present meta-analysis, increased serum CRP levels are significantly predictive of OS and PFS in patients with CC, and as demonstrated by the subgroup and sensitivity analyses, this relationship was very reliable. Taken together, these results suggest that serum CRP level may be a useful prognostic biomarker for patients with CC. To the best of our knowledge, this is the first meta-analysis to explore the prognostic significance of serum CRP levels in patients with CC.
CRP is a well-established biomarker for inflammation and can be easily assayed in clinical practice (42). Additionally, as an acute-phase protein generated by the liver, CRP is indicative of whether inflammation exists, as well as its level in the body, while maintaining certain advantages, such as a stable half-life, cost-effectiveness, simple measurement, and standardization (43). There are multiple mechanisms underlying the prognostic value of CRP in patients with CC. First, the presence of tumor tissue causes inflammation, which, in turn, increases serum CRP levels (44); therefore, increased CRP levels indicate tumor necrosis or local tissue injury. Second, IL-6 can induce hepatocytes to produce CRP, as can other factors, such as IL-1, TNF-α, and transforming growth factor β (TGFβ) (45), while via vascular endothelial growth factor (VEGF), IL-6 may promote angiogenesis in the presence of inflammation (46). Third, it has been shown that CRP induces an inflammatory tumor microenvironment that activates certain signaling pathways important for tumor proliferation, angiogenesis, and metastasis (47). It is likely, therefore, that patients with CC and a systemic inflammatory response, as indicated by elevated serum CRP levels, will be given a poor prognosis.
The present meta-analysis has several strengths. To the best of the authors’ knowledge, this is the first meta-analysis to explore the relationship between CRP levels and survival outcomes in patients with CC. Second, all included studies were of high quality (NOS score ≥ 6), which contributes to the overall statistical power of this meta-analysis. Third, sensitivity analyses and publication bias tests confirmed the reliability of the results of the present meta-analysis.
Numerous recent meta-analyses have reported the use of CRP in determining the prognoses of a variety of solid tumors. In a meta-analysis of 12 articles, Liu et al. (48) reported that serum CRP levels predicted poor OS in patients with prostate cancer. Li et al. (49) demonstrated that an increased CRP level indicated poor OS in patients with bone tumors, based on a meta-analysis of 816 participants. The results of a meta-analysis of 44 studies indicated that CRP levels could be used to predict OS and cancer-specific survival (CSS) in urological cancer cases (50). Jin et al. (51) carried out a meta-analysis encompassing 1,649 patients, the results of which showed that elevated CRP levels were indicative of poor OS in patients with non-small cell lung cancer, while Zheng et al. (52) reported that increased serum CRP levels indicated poor OS and recurrence-free survival (RFS) in patients with hepatocellular carcinoma, based on a meta-analysis of 10 studies. Notably, due to the fact that CRP is not specific for patients with CC. The level of CRP, as discussed above, could also offer insight into the prognosis of various types of cancer. In this case, the high level of CRP could serve as a prognostic marker only for those patients with CC without other cancers and treatment histories.
The present meta-analysis does have some limitations. First, the included studies were all of a retrospective nature, which may have introduced selection bias. Second, the cut-off values of CRP levels ranged from 2.6–10, but we were unable to determine an optimal cut-off value for determining the prognosis of CC based on CRP levels. Third, our sample size was relatively small, as many of the included studies had relatively small sample sizes. Therefore, larger prospective studies are required to verify our findings.
In conclusion, the results of the present meta-analysis indicated that increased CRP levels are markedly associated with poor OS and PFS in patients with CC. Therefore, serum CRP level could be an inexpensive and independent factor for determining the prognoses of patients with CC in a clinical setting.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
SY conceived and designed the study, interpreted the data, and drafted the manuscript. ZZ and LS designed and revised the manuscript. SY and ZZ selected the articles. ZZ and LS retrieved the data. All authors contributed to the article and approved the submitted version.
This study was supported by Huzhou Science and Technology Plan Project (Grant No. 2022GY45).
We would like to thank Editage (www.editage.com) for English language editing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
CRP, C-reactive protein; CC, cervical cancer; HR, hazard ratio; CI, confidence interval; OS, overall survival; PFS, progression-free survival; HPV, human papillomavirus; FIGO, International Federation of Gynecology and Obstetrics; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; NOS, Newcastle-Ottawa Scale; REM, random-effects model; FEM, fixed-effects model.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
3. Cohen PA, Jhingran A, Oaknin A, Denny L. Cervical cancer. Lancet (2019) 393(10167):169–82. doi: 10.1016/s0140-6736(18)32470-x
4. Kjær SK, Frederiksen K, Munk C, Iftner T. Long-term absolute risk of cervical intraepithelial neoplasia grade 3 or worse following human papillomavirus infection: role of persistence. J Natl Cancer Institute (2010) 102(19):1478–88. doi: 10.1093/jnci/djq356
5. Ramirez PT, Frumovitz M, Pareja R, Lopez A, Vieira M, Ribeiro R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. New Engl J Med (2018) 379(20):1895–904. doi: 10.1056/NEJMoa1806395
6. Quinn MA, Benedet JL, Odicino F, Maisonneuve P, Beller U, Creasman WT, et al. Carcinoma of the cervix uteri. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J gynaecology obstetrics: Off Organ Int Fed Gynaecol Obs (2006) 95 Suppl 1:S43–103. doi: 10.1016/s0020-7292(06)60030-1
7. van Harten-Gerritsen AS, Balvers MG, Witkamp RF, Kampman E, van Duijnhoven FJ. Vitamin D, inflammation, and colorectal cancer progression: A review of mechanistic studies and future directions for epidemiological studies. Cancer epidemiology Biomarkers Prev Publ Am Assoc Cancer Research cosponsored by Am Soc Prev Oncol (2015) 24(12):1820–8. doi: 10.1158/1055-9965.Epi-15-0601
8. Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature (2017) 541(7637):321–30. doi: 10.1038/nature21349
9. Gewurz H, Mold C, Siegel J, Fiedel B. C-reactive protein and the acute phase response. Adv Intern Med (1982) 27:345–72. doi: 10.1080/21548331.1982.11702332
10. Liang-Fonseca J, Geissler K. Significance of C-reactive protein in patients with chronic myelomonocytic leukemia. Wiener medizinische Wochenschrift (1946) 173(1-2):15–20. doi: 10.1007/s10354-022-00981-8
11. O'Brian D, Prunty M, Hill A, Shoag J. The role of C-reactive protein in kidney, bladder, and prostate cancers. Front Immunol (2021) 12:721989. doi: 10.3389/fimmu.2021.721989
12. Potempa LA, Rajab IM, Olson ME, Hart PC. C-reactive protein and cancer: interpreting the differential bioactivities of its pentameric and monomeric, modified isoforms. Front Immunol (2021) 12:744129. doi: 10.3389/fimmu.2021.744129
13. Li HY, Wang J, Wu YX, Zhang L, Liu ZP, Filep JG, et al. Topological localization of monomeric C-reactive protein determines proinflammatory endothelial cell responses. J Biol Chem (2014) 289(20):14283–90. doi: 10.1074/jbc.M114.555318
14. Wang CY, Hsieh MJ, Chiu YC, Li SH, Huang HW, Fang FM, et al. Higher serum C-reactive protein concentration and hypoalbuminemia are poor prognostic indicators in patients with esophageal cancer undergoing radiotherapy. Radiotherapy Oncol J Eur Soc Ther Radiol Oncol (2009) 92(2):270–5. doi: 10.1016/j.radonc.2009.01.002
15. Fujita T, Iwamura M, Ishii D, Tabata K, Matsumoto K, Yoshida K, et al. C-reactive protein as a prognostic marker for advanced renal cell carcinoma treated with sunitinib. Int J Urol Off J Japanese Urological Assoc (2012) 19(10):908–13. doi: 10.1111/j.1442-2042.2012.03071.x
16. Beer TM, Lalani AS, Lee S, Mori M, Eilers KM, Curd JG, et al. C-reactive protein as a prognostic marker for men with androgen-independent prostate cancer: results from the ASCENT trial. Cancer (2008) 112(11):2377–83. doi: 10.1002/cncr.23461
17. Hwang JE, Kim HN, Kim DE, Choi HJ, Jung SH, Shim HJ, et al. Prognostic significance of a systemic inflammatory response in patients receiving first-line palliative chemotherapy for recurred or metastatic gastric cancer. BMC Cancer (2011) 11:489. doi: 10.1186/1471-2407-11-489
18. Polterauer S, Grimm C, Tempfer C, Sliutz G, Speiser P, Reinthaller A, et al. C-reactive protein is a prognostic parameter in patients with cervical cancer. Gynecol Oncol (2007) 107(1):114–7. doi: 10.1016/j.ygyno.2007.06.001
19. Nakamura K, Nishida T, Haruma T, Haraga J, Omichi C, Ogawa C, et al. Pretreatment platelet-lymphocyte ratio is an independent predictor of cervical cancer recurrence following concurrent chemoradiation therapy. Mol Clin Oncol (2015) 3(5):1001–6. doi: 10.3892/mco.2015.595
20. Xiao Y, Ren YK, Cheng HJ, Wang L, Luo SX. Modified Glasgow prognostic score is an independent prognostic factor in patients with cervical cancer undergoing chemoradiotherapy. Int J Clin Exp Pathol (2015) 8(5):5273–81.
21. Bodner-Adler B, Kimberger O, Schneidinger C, Kölbl H, Bodner K. Prognostic significance of pre-treatment serum C-reactive protein level in patients with adenocarcinoma of the uterine cervix. Anticancer Res (2016) 36(9):4691–6. doi: 10.21873/anticanres.11022
22. He X, Li JP, Liu XH, Zhang JP, Zeng QY, Chen H, et al. Prognostic value of C-reactive protein/albumin ratio in predicting overall survival of Chinese cervical cancer patients overall survival: comparison among various inflammation based factors. J Cancer (2018) 9(10):1877–84. doi: 10.7150/jca.23320
23. Wang WJ, Li Y, Zhu J, Gao MJ, Shi JP, Huang YQ. Prognostic values of systemic inflammation response (SIR) parameters in resectable cervical cancer. Dose-response Publ Int Hormesis Soc (2019) 17(1):1559325819829543. doi: 10.1177/1559325819829543
24. An Q, Liu W, Yang Y, Yang B. Preoperative fibrinogen-to-albumin ratio, a potential prognostic factor for patients with stage IB-IIA cervical cancer. BMC Cancer (2020) 20(1):691. doi: 10.1186/s12885-020-07191-8
25. Wang H, Wang MS, Zhou YH, Shi JP, Wang WJ. Prognostic values of LDH and CRP in cervical cancer. Onco Targets Ther (2020) 13:1255–63. doi: 10.2147/ott.S235027
26. Bakir MS, Birge O, Tuncer HA, Dogan S, Simsek T. Prognostic value of combined glucose and C-reactive protein (CRP) in cervical cancer. Eur J Gynaecological Oncol (2021) 42(6):1242–51. doi: 10.31083/j.ejgo4206180
27. Taguchi A, Nakajima Y, Furusawa A, Yoshino Y, Takao M, Kashiyama T, et al. High neutrophil-to-lymphocyte ratio is a predictor of short-term survival for patients with recurrent cervical cancer after radiation-based therapy. J obstetrics gynaecology Res (2021) 47(5):1862–70. doi: 10.1111/jog.14712
28. Li Y, Li Z, Zhang G. Clinical utility of red blood cell distribution width for the diagnosis and prognosis of cervical cancer. Int J Gen Med (2022) 15:2597–606. doi: 10.2147/ijgm.S354569
29. Zheng X, Gu H, Cao X, Pan B, Xiang H, Ju M, et al. Tislelizumab for cervical cancer: A retrospective study and analysis of correlative blood biomarkers. Front Immunol (2023) 14:1113369. doi: 10.3389/fimmu.2023.1113369
30. Moher D, Liberati A, Tetzlaff J, Altman DG, Grp P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Internal Med (2009) 151(4):264–W64. doi: 10.7326/0003-4819-151-4-200908180-00135
31. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
33. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med (2002) 21(11):1539–58. doi: 10.1002/sim.1186
34. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical Res ed) (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
35. Jiang KY, Qi LL, Liu XB, Wang Y, Wang L. Prognostic value of Siglec-15 expression in patients with solid tumors: A meta-analysis. Front Oncol (2022) 12:1073932. doi: 10.3389/fonc.2022.1073932
36. Li Z, Xu S, Chen L, Huang S, Kuerban X, Li T. Prognostic significance of ING3 expression in patients with cancer: A systematic review and meta-analysis. Front Oncol (2023) 13:1090860. doi: 10.3389/fonc.2023.1090860
37. Wang L, Qin X, Zhang Y, Xue S, Song X. The prognostic predictive value of systemic immune index and systemic inflammatory response index in nasopharyngeal carcinoma: A systematic review and meta-analysis. Front Oncol (2023) 13:1006233. doi: 10.3389/fonc.2023.1006233
38. Mao H, Yang F. Prognostic significance of systemic immune-inflammation index in patients with ovarian cancer: a meta-analysis. Front Oncol (2023) 13:1193962. doi: 10.3389/fonc.2023.1193962
39. DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clin trials (1986) 7(3):177–88. doi: 10.1016/0197-2456(86)90046-2
40. Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Institute (1959) 22(4):719–48.
41. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics (1994) 50(4):1088–101. doi: 10.2307/2533446
42. Kretschmer A, Tilki D. Biomarkers in prostate cancer - Current clinical utility and future perspectives. Crit Rev oncology/hematology (2017) 120:180–93. doi: 10.1016/j.critrevonc.2017.11.007
43. Lee SH, Kim KH, Choi CW, Kim SJ, Kim DH, Choi CI, et al. Reduction rate of C-reactive protein as an early predictor of postoperative complications and a reliable discharge indicator after gastrectomy for gastric cancer. Ann Surg Treat Res (2019) 97(2):65–73. doi: 10.4174/astr.2019.97.2.65
44. Allin KH, Bojesen SE, Nordestgaard BG. Baseline C-reactive protein is associated with incident cancer and survival in patients with cancer. J Clin Oncol Off J Am Soc Clin Oncol (2009) 27(13):2217–24. doi: 10.1200/jco.2008.19.8440
45. Kuta AE, Baum LL. C-reactive protein is produced by a small number of normal human peripheral blood lymphocytes. J Exp Med (1986) 164(1):321–6. doi: 10.1084/jem.164.1.321
46. Huang SP, Wu MS, Wang HP, Yang CS, Kuo ML, Lin JT. Correlation between serum levels of interleukin-6 and vascular endothelial growth factor in gastric carcinoma. J Gastroenterol Hepatol (2002) 17(11):1165–9. doi: 10.1046/j.1440-1746.2002.02873.x
47. Gueron G, De Siervi A, Vazquez E. Advanced prostate cancer: reinforcing the strings between inflammation and the metastatic behavior. Prostate Cancer prostatic Dis (2012) 15(3):213–21. doi: 10.1038/pcan.2011.64
48. Liu T, Zhuo L. The role of C-reactive protein in the prognosis of prostate cancer: A meta-analysis. J Environ Public Health (2023) 2023:6222324. doi: 10.1155/2023/6222324
49. Li W, Luo X, Liu Z, Chen Y, Li Z. Prognostic value of C-reactive protein levels in patients with bone neoplasms: A meta-analysis. PloS One (2018) 13(4):e0195769. doi: 10.1371/journal.pone.0195769
50. Zhou L, Cai X, Liu Q, Jian ZY, Li H, Wang KJ. Prognostic role of C-reactive protein in urological cancers: A meta-analysis. Sci Rep (2015) 5:12733. doi: 10.1038/srep12733
51. Jin Y, Sun Y, Shi X, Zhao J, Shi L, Yu X. Prognostic value of circulating C-reactive protein levels in patients with non-small cell lung cancer: a systematic review with meta-analysis. J Cancer Res Ther (2014) 10 Suppl:C160–6. doi: 10.4103/0973-1482.145854
Keywords: C-reactive protein, cervical cancer, meta-analysis, prognosis, evidence-based medicine
Citation: Yang S, Zhang Z and Shen L (2023) Prognostic significance of C-reactive protein in patients with cervical cancer: a meta-analysis. Front. Oncol. 13:1232409. doi: 10.3389/fonc.2023.1232409
Received: 31 May 2023; Accepted: 08 August 2023;
Published: 01 September 2023.
Edited by:
Francesco Fanfani, Agostino Gemelli University Polyclinic (IRCCS), ItalyReviewed by:
Francesco Ricchetti, Sacro Cuore Don Calabria Hospital (IRCCS), ItalyCopyright © 2023 Yang, Zhang and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Linglong Shen, Y2hhb3JlbjUxMjExMzIwMjNAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.