- 1Department of Biotechnology, Guru Ghasidas Vishwavidyalaya, Bilaspur, Chhattisgarh, India
- 2Department of Surgical Gastroenterology, All India Institute of Medical Sciences, Raipur, Chhattisgarh, India
- 3Centre for Excellence in Genomics, Trivitron Healthcare Pvt. Ltd., Chennai, India
- 4Department of Zoology, Dr. Bhawan Singh Porte Government College, Pendra, Chhattisgarh, India
- 5Department of Botany, Sri Satguru Jagjit Singh Namdhari College, Gharwa, Jharkhand, India
- 6Department of Forensic Sciences, Guru Ghasidas Vishwavidyalaya, Bilaspur, Chhattisgarh, India
T-cell malignancy is a broad term used for a diverse group of disease subtypes representing dysfunctional malignant T cells transformed at various stages of their clonal evolution. Despite having similar clinical manifestations, these disease groups have different disease progressions and diagnostic parameters. The effective diagnosis and prognosis of such a diverse disease group demands testing of molecular entities that capture footprints of the disease physiology in its entirety. MicroRNAs (miRNAs) are a group of noncoding RNA molecules that regulate the expression of genes and, while doing so, leave behind specific miRNA signatures corresponding to cellular expression status in an altered stage of a disease. Using miRNAs as a diagnostic tool is justified, as they can effectively distinguish expressional diversity between various tumors and within subtypes of T-cell malignancies. As global attention for cancer diagnosis shifts toward liquid biopsy, diagnosis using miRNAs is more relevant in blood cancers than in solid tumors. We also lay forward the diagnostic significance of miRNAs that are indicative of subtype, progression, severity, therapy response, and relapse. This review discusses the potential use and the role of miRNAs, miRNA signatures, or classifiers in the diagnosis of major groups of T-cell malignancies like T-cell acute lymphoblastic lymphoma (T-ALL), peripheral T-cell lymphoma (PTCL), extranodal NK/T-cell lymphoma (ENKTCL), and cutaneous T-cell lymphoma (CTCL). The review also briefly discusses major diagnostic miRNAs having prominent metabolic roles in these malignancies to highlight their importance among other dysregulated miRNAs.
1 Introduction
Cancer research has made remarkable progress in recent years, bringing many potent drugs to the fore for its treatment and management. However, the effectiveness of these cancer therapies largely depends on accurate and timely diagnosis. In most cases, delayed or inaccurate diagnosis leads to faulty clinical judgment, which incurs toxic responses in patients and renders therapeutic endeavors less effective (1). This disadvantage of diagnostic delays and inaccuracy can be overcome by investigating circulating fluids that may be home to disease-specific markers, especially in cases of lymphoma more than in solid tumors (2). Although the incidence of various T-cell lymphomas may be statistically rare, it is considered very aggressive and is often associated with poorer disease outcomes (3). Patients harboring such malignancies can also face treatment failures, as they develop resistance to established chemotherapeutic regimens faster than solid tumors (4). To diagnose such aggressive malignancies, conventional methods may not be sufficient to identify these diseases as early as possible. However, the growing prospects of liquid biopsy can be exploited to diagnose these lymphomas (5). One such biomarker is microRNAs (miRNAs), whose global expression can be investigated not only in patient tissues but also in their circulating fluids, providing scope for liquid biopsy (6). However, miRNA profiling, as a diagnostic option is still limited within the research domain; its applicability in routine clinical practice has not yet materialized as it requires high-throughput techniques. The present review describes the diagnostic and prognostic potential of miRNAs that are relevant for the efficient discriminatory diagnosis of a particular T-cell lymphoma from a wide spectrum of T-cell malignant lymphomas (TCLs).
miRNAs were first discovered in Caenorhabditis elegans as lin-4, a noncoding RNA reported to downregulate the expression of another gene, lin-14 (7). miRNAs are short (usually 21–23 nucleotides), noncoding (nc), ssRNA molecules produced endogenously in animal and plant cells that function posttranscriptionally, consequently regulating gene expression. Most often, miRNAs repress gene expression by binding to the 3′untranslated regions (3′UTRs) of their respective mRNA targets, thus cleaving them to suppress translation (8). These miRNAs can co-express and co-regulate one or more cellular pathways and result in a phenotypic consequence of oncogenic or onco-suppressive functions. miRNAs and their repressive roles have been widely studied using both in vitro and in silico models, in addition to their gene-inducing capabilities (7–9).
Cancers have overwhelming involvement in multiple pathways, for which miRNAs certainly create a miRNA signature while regulating those pathways and may indicate the cell’s physiological, metabolic, pathologic, and tumor-progression status (10). It is also noteworthy that T-cell malignant formations shed circulating miRNAs in circulating body fluids, which can be quantified to design promising diagnostic applications and monitor therapy response during treatment (10). In this review, we discuss different miRNAs with important diagnostic and prognostic applications in various types of T-cell malignancies. For this review, the diversity of T-cell malignancies is based on various pathological studies and the WHO classification (11). However, a clear classification is yet to be explored and is beyond the scope of this review (12). Hence, we discuss major T-cell malignancies and their dysregulated miRNAs, contributing to aspects of diagnostic or prognostic values in adult T-lymphoblastic leukemia/lymphoma (T-ALL), peripheral T-cell lymphoma (PTCL) and its types, extranodal T-cell lymphoma (ENKTCL), and cutaneous T-cell lymphoma (CTCL) (Figure 1). We also discuss how diagnostic miRNAs have a significant role in reprogramming cancer metabolism, as we underline emerging evidence suggesting a crucial role of miRNAs in regulating metabolic processes such as insulin sensitivity, glucose and lipid metabolism, and energy homeostasis. As metabolic miRNAs are being used for diagnostic and prognostic applications in metabolic disorders (13), they can have similar diagnostic and prognostic utilities in cancers as well, which undergo metabolic reprogramming as a key hallmark toward transformation. A recent computational biology study using machine learning has identified seven key miRNAs associated with mRNAs involved in lipid metabolism that are differentially expressed between prostate cancer and its benign counterpart. The differential expression of this lipid metabolism-related signature was also validated by PCR and in publicly available datasets (14). In addition to forming a molecular signature, miRNA expression profiles can identify distinct metabolic phenotypes within a disease spectrum. By identifying patients who may respond differently to a particular therapy or need specialized care, sub-phenotyping patients based on miRNA signatures can help precision medicine efforts (15, 16). Therefore, miRNAs along with their downstream metabolite signatures, can also potentially distinguish high-risk patient groups from others (17). The diagnostic and prognostic potential of miRNA profiling can be harnessed in clinical settings if it can be narrowed down to a few miRNAs defining a particular cancer subtype. The diagnostic focus required to distinguish a spectrum of disease manifestations can also be achieved by quantifying key miRNAs and their metabolic targets. Moreover, finding out how dysregulated miRNAs affect functional outcomes can reveal their role in metabolic processes verified through in vitro and in vivo research, which can also prove the miRNAs’ potential as therapeutic targets.
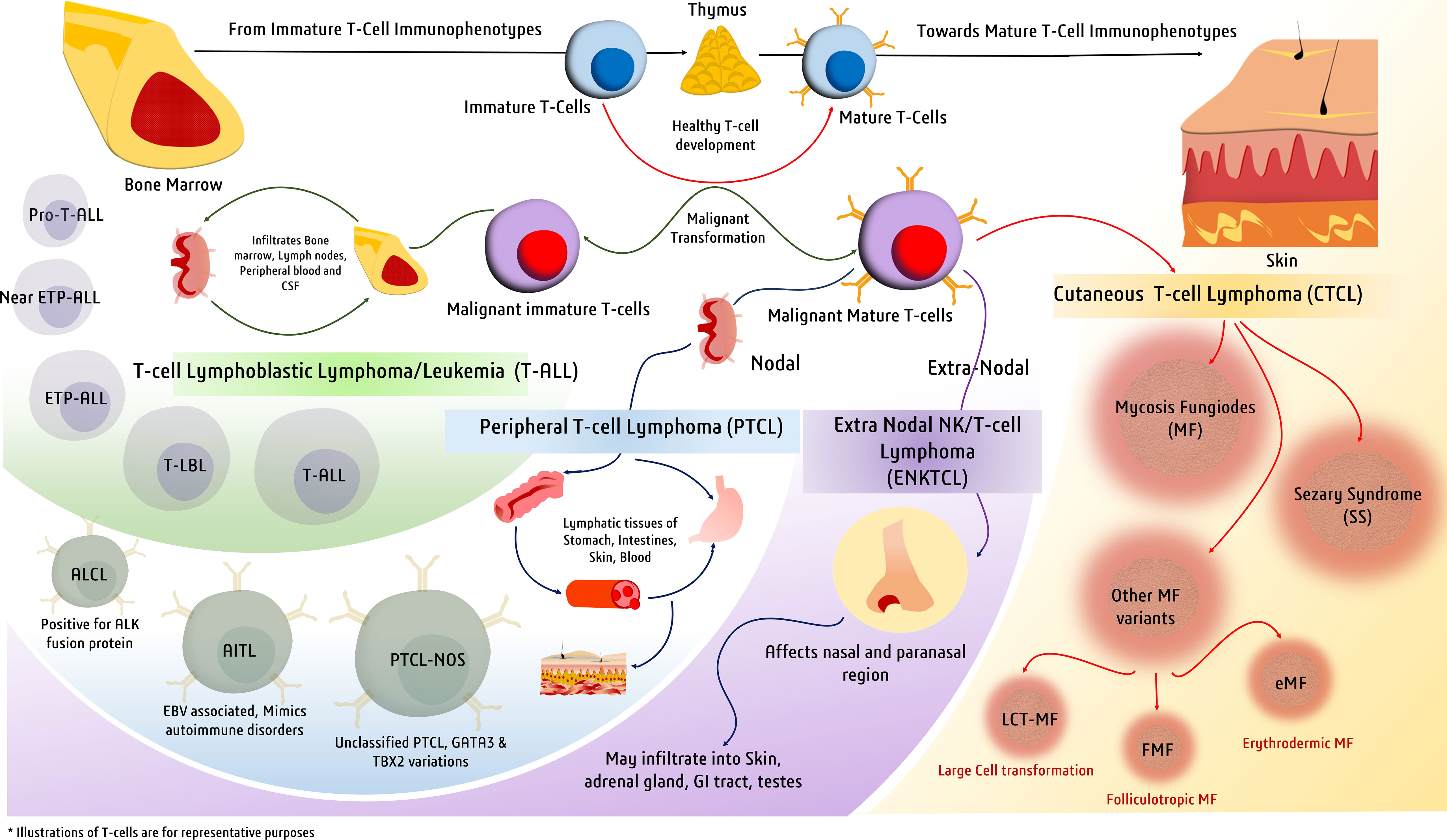
Figure 1 Major types of T-cell malignancies. Malignant transformation at various stages of T-cell development gives rise to a spectrum of malignancies defined by a variety of clinical manifestations.
2 T-lymphoblastic leukemia/lymphoma
According to the International Lymphoma study group and the WHO, T-lymphoplastic leukemia/lymphoma comprises two kinds of malignancies namely T-cell lymphoblastic lymphoma (T-LBL) and T-ALL (18, 19). Both are malignancies of immature thymocytes that share large similarities in morphology and immunophenotypes (20, 21). Both malignancies have considerable infiltration in the mediastinum, differing in their proportion of bone marrow infiltration. T-ALL has more (>25%) bone marrow infiltration, whereas T-LBL has considerably high infiltration in the lymph nodes along with low infiltration in the bone marrow (<25%). Malignant thymocytes in T-LBL and T-ALL also infiltrate peripheral blood, bone marrow, and cerebrospinal fluid (22). As far as genetic and epigenetic aberrations in both T-LBL and T-ALL are concerned, their molecular features largely overlap with some differential mutations in each. The early T-cell precursor LBL/ALL (ETP-LBL or ETP-ALL) is another aggressive subtype of immature T-cell malignancy whose mutational and transcriptional profiles closely resemble those of myeloid progenitors and hematopoietic stem cells (21, 22). However, there exist other immunophenotypic subgroups, such as Near-ETP-ALL and Pro-T-ALL, whose distinct phenotypic and clinical presentations are not clear enough (23).
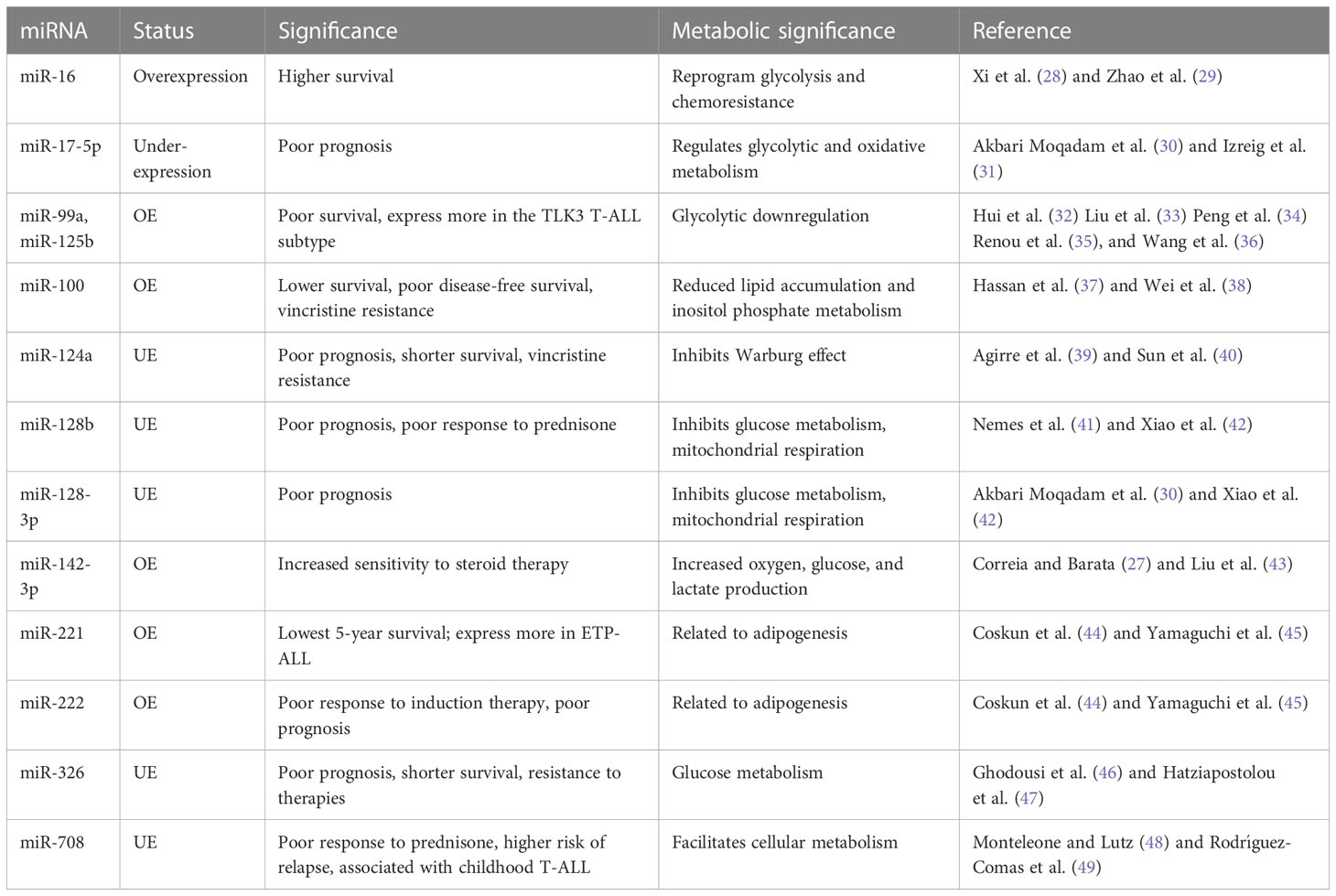
These T-ALL malignancies can be sub-grouped based on the distinct expression of various transcription factors, such as TAL1/TAL2, TLX1/TLX3, HOXA, and LMO1/LYL1, collectively known as type-A abnormalities (24). T-ALL is reported to have dysregulation of cell cycle regulation pathways such as the NOTCH-MYC-FBXW7 pathway, CDKN2A, CDK4/6 complex, kinase pathways such as JAK/STAT5 and PIK3/AKT/MTOR, and RNA metabolism pathways such as RPL5/10/11/22, CNOT3, and EIF4A (25). MicroRNAs involved in regulating these pathways are expected to have preferential diagnostic potential. In the case of T-ALL, miRNAs regulating the NOTCH1 pathway to achieve unchecked proliferation, apoptosis inhibition, and resistance to drug therapies may play a significant role in diagnosis (25). MicroRNAs, such as miR-128-3p, 148a-3p, miR-181a/b, miR-363-3p, and miR-20b-5p, are also involved in the pathogenesis of T-ALL (26). Other miRNAs suspected to regulate T-ALL pathobiology are miR-342, miR-223, miR-150, miR-142-3p, miR-93, miR-92, miR-26a, miR-20a, miR-19b, and miR-16 (27) (Table 1).
2.1 miRNAs having diagnostic significance in T-ALL
When T-ALL microRNA expression was compared with that of healthy T cells, higher expression of miR-128a and miR-181b was observed alongside significant underexpression of miR-100 and let-7e (50). A study noted a general downregulation of miR-708 among all T-cell malignant patient groups, whereas miR-196b was found to be upregulated significantly only in patients with T-ALL immunophenotypes (51). A separate study evaluated the increased expression of seven miRNAs (miR-92, miR-20a, miR-19a, miR-19b, miR-18a, miR-17-3p, and miR-17-5p) in the miRNA cluster (miR-17-92) and reported their involvement in chromosomal rearrangement activity, including translocation (27). Among these miRNAs, significant overexpression of miR-19b and miR-19a has been reported in patients with T-ALL. An miRNA panel that co-expresses miR-26a and miR-92, along with miR-19a and miR-19b, is strongly associated with T-ALL patients (52). Moreover, among miRNA species that are underexpressed, miR-150 has a significant role in increasing hematopoietic progenitor growth and cell proliferation in T-ALL (53). The underexpressing miRNAs, miR-30 and miR-451, are believed to be tumor suppressive in nature, inhibiting NOTCH1 and NOTCH2 expression. These aforementioned miRNAs play a significant role in regulating the signaling pathways involved in the progression of T-ALL (Figure 2) (54).
In a study, several miRNAs were reported to have stage- and subtype-specific expression statuses. For instance, upregulated miR-196b in T-ALL corresponds with a more immature immunophenotype of malignant T cells, whereas upregulated miR-363 and miR-19a correspond with a more mature malignant T-cell immunophenotype (44, 55). Another miRNA couple, miR-221 and miR-222, have high expression in T-ALL cases but more significantly in its ETP-ALL subtype, as high as eight- and fivefold, respectively (56). Moreover, an independent study used microRNA profiling and noted the under-expression of miR-124a, miR-146b-5p, miR-150, miR-193b-3p, miR-326, miR-451, miR-30, and miR-708 in T-ALL. Among them, lower expression of miR-146b-5p was correlated with the TAL1+ T-ALL patient group (35). Lower expression of a different miRNA, miR-193b-3p, was associated with the TAL-rearranged T-ALL subtype and the NOTCH-induced T-ALL subgroup. MicroRNAs can also differentially associate with adult and pediatric T-ALL disease biology; for instance, in children with T-ALL, miR-221 was found to overexpress significantly in their peripheral blood mononuclear cells (PBMNCs). Interestingly, miR-221 is also associated with the refractory stage of T-ALL, signifying its prognostic role in both adult and pediatric cases. Similarly, a different miRNA (miR-664) was found to be overexpressed in pediatric T-ALL, underlying a possible association with miR-221 (Figure 2). Additionally, miR-664 has been reported to confer malignant features such as cell growth promotion, cell migration, and apoptotic inhibition of cells in T-ALL (51) (Table 1).
2.2 miRNAs and prognosis of T-ALL disease
Dysregulated miRNAs can also indicate survival outcomes in patients; for instance, a miRNA signature of overexpressed miR-221 and miR-222 in T-ALL, more so in ETP-ALL, is reported to be associated with the lowest 5-year survival scores and is also known to induce chemoresistance (44). Overexpression of miR-221 was found to be associated with poorer response to induction therapy, risk typing, and blood cell counts, making it a reliable marker for the diagnosis and prognosis of T-ALL (57). A different pair of miR-99a and miR-125b is associated with the TLK3 T-ALL subtype that exhibits poorer patient survival (35). Patients diagnosed with T-ALL have lower expression of miR-30 and miR-100, and while the role of miR-30 is still under investigation, miR-100 underexpression correlates with poor overall and disease-free survival rates, making it a high-risk prognostic biomarker (27, 37). As high expression of miR-30 in breast cancer is associated with better survival and prognosis (58), its low expression in T-ALL may possibly resemble poor survival, which requires further investigation. Lower expression of miR-326, along with high expression of BAALC (brain and acute lymphoblastic leukemia cytoplasmic protein), is associated with poor survival, especially in children, along with leukemogenesis, shorter survival, resistance to therapy, and MRD (minimal residual disease) (46). There are other miRNAs, for instance, miR-16, which, despite having a nonsignificant overexpression in T-ALL, gave better 1-year survival rates as high as 50% in patients having comparatively higher levels of miR-16 (28). Another, miR-142-3p, is overexpressed in T-ALL cells and is involved in the downregulation of the cAMP/PKA pathway, causing enhanced cell migration. On the one hand, this miRNA causes increased sensitivity to steroid therapy; on the other hand, its inhibition causes increased sensitivity to dexamethasone in patients with prednisone resistance (27). A poorer response to prednisone is also associated with lower expression of miR-708, a miRNA associated with childhood T-ALL. Higher levels of miR-708 can be of diagnostic significance and can signify moderate survival, whereas lower expression of miR-708 is associated with higher risk and higher chances of relapse (59) (Figure 2) (Table 1).
2.3 Metabolic roles of dysregulated miRNAs in T-ALL
T-ALL finds significant overexpression and underexpression of many miRNAs, amongst which we discuss prominently dysregulated miRNAs having metabolic roles, either reported in T-cell malignancies or in other cancers. In T-ALL, an upregulated miR-16, positively influencing patient survival (28), is reported to reprogram glycolysis and chemoresistance (29) in cervical cancer by activating PKD4, and also increase the radiosensitivity of prostate cancer cells (60). A downregulated let-7e in T-ALL was reported to regulate metabolic rewiring by inhibiting oxidative phosphorylation and adipogenesis and increasing lactate accumulation in a breast cancer study (61). A negative prognostic factor of underexpressing miR-17-5p in T-ALL leads to tumor metabolic reprogramming via its cluster miR-17-92 in B-lymphoma cells (31). Similarly, miR-99a and miR-125b, resembling poor prognosis and patient survival, were reported to be involved in glycolytic downregulation (34, 36) by facilitating decreased glucose uptake and oxygen consumption (32, 33). Moreover, an upregulated miR-100 attributed to poor overall and disease-free survival in T-ALL is involved in reduced lipid accumulation in metabolic syndromes (62) and probably involved in inositol phosphate metabolism in multiple myeloma (38). Similarly, miR-124a and miR-128b were found to be downregulated in T-ALL, and are associated with poor prognosis, and have a role in the inhibition of the Warburg effect and suppression of glucose metabolism, respectively (40, 42). Furthermore, the enhanced miR-221 and miR-222 in T-ALL are not yet known to have any metabolic reprogramming function in cancer, but they are reported to have roles in adipogenesis in obese conditions (45). An underexpressed miR-326 is found to impact key glucose metabolism regulators (47). The significance of miR-708, which is found underexpressed in T-ALL and correlates with poor survival, has not yet been investigated for its metabolic role in cancer, but it has been reported to affect glucose metabolism by influencing insulin secretion (49) and also influence prostaglandin E2 production, affecting tumorigenesis (48). Thus, these miRNAs with diagnostic and prognostic significance expectedly mold the metabolic status of cancer cells by prominently affecting glucose metabolism to support malignant growth (Figure 2).
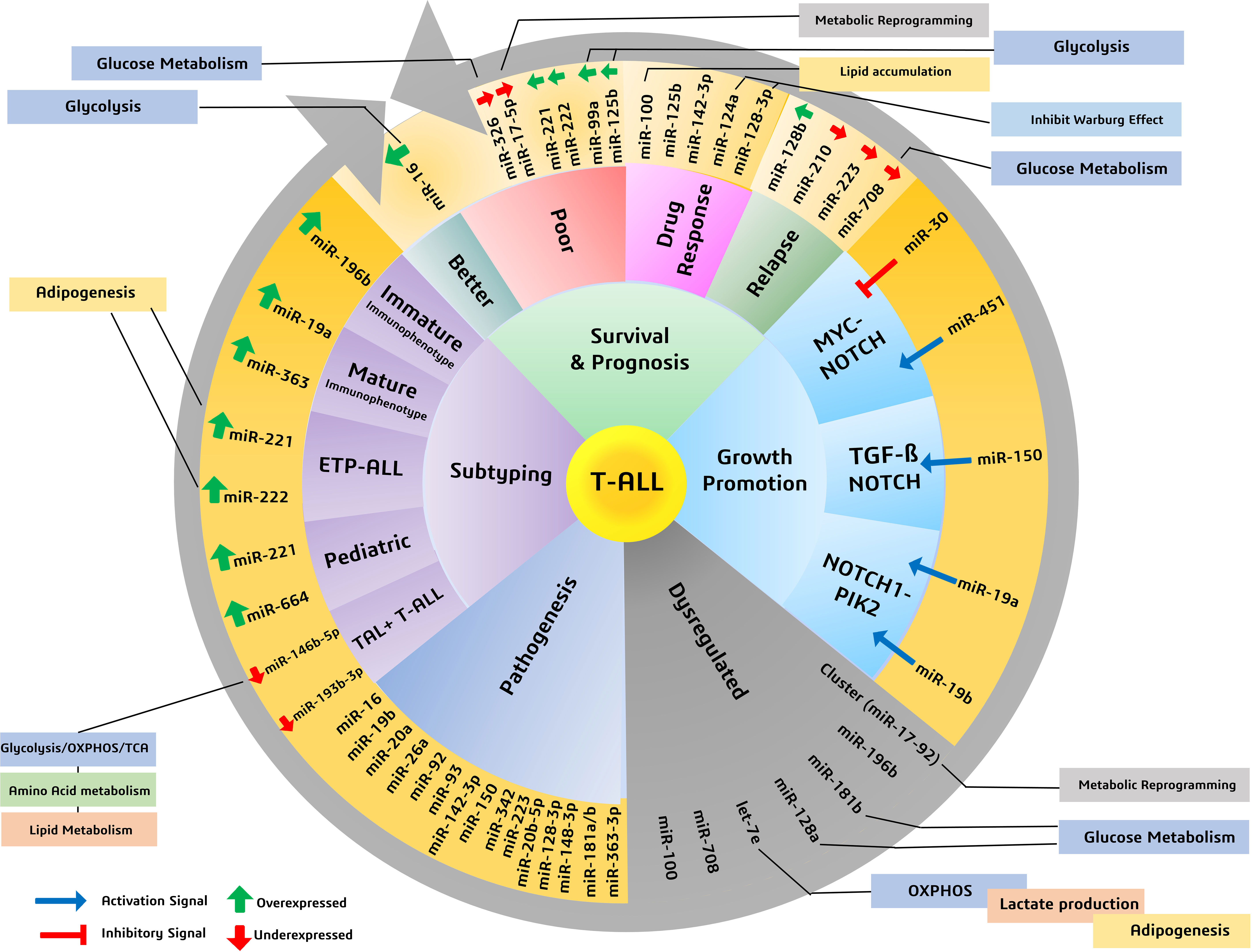
Figure 2 Various miRNAs and their significance in patient survival outcomes, prognosis, and subtyping, highlighting miRNAs involved in metabolic reprogramming of cancers.
3 Peripheral T-cell lymphomas
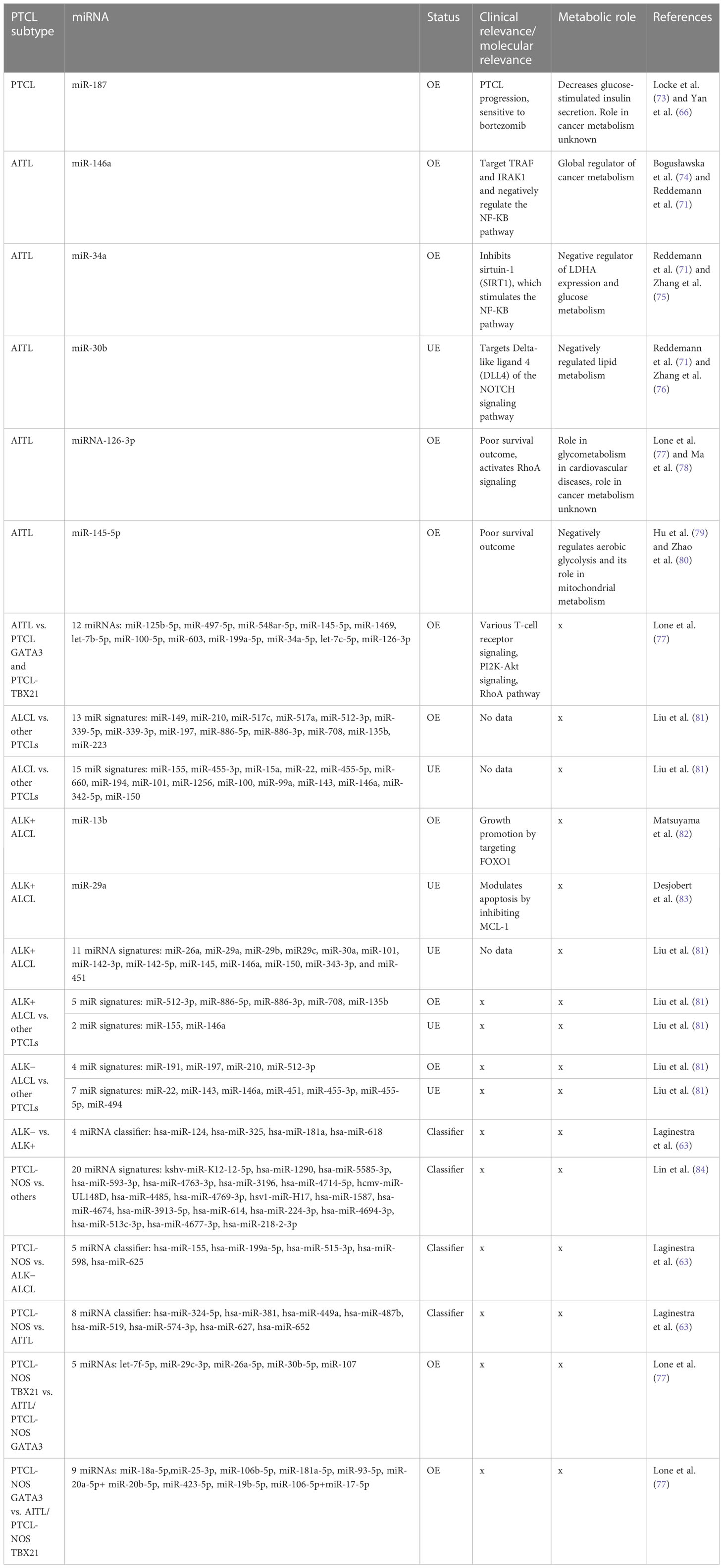
Unlike T-ALL, which originates from immature thymocytes, PTCL is a malignancy of mature T cells. It is a heterogeneous class of nodal and some extranodal tumors, corresponding to approximately 10%–15% of all lymphoid malignancies. PTCL can be subdivided into three major types: angioimmunoblastic T-cell lymphoma (AITL), anaplastic large-cell lymphoma (ALCL), and peripheral T-cell lymphoma-not otherwise specified (PTCL-NOS) (63). As far as the diagnosis of PTCLs is concerned, conventional diagnostic methodologies have not performed efficiently, and therefore, more than 30% of all PTCL cases remain unclassified and remain at the intersection of various subtypes. Although at the molecular level, these subgroups, despite sharing an overwhelming number of commonly altered pathways and transcriptional signatures, have distinct expression levels revealed by gene expression profiling of patients (64). MicroRNA profiling of PTCL cases revealed specific miRNA signatures based on differential expression of various miRNAs when compared to normal cells. Many miRNA profiling studies, however, have reported differentially expressed miRNAs within various subtypes (65), but their diagnostic accuracies will depend on their practical ability to discriminate the subtypes at the molecular level. A notable miRNA, miR-187 overexpression, is associated with peripheral T-cell lymphoma progression, which is also related to sensitivity to bortezomib (66) and is associated with better survival in other cancers (67). However, its clinical role in PTCL patient outcomes and prognosis remains unclear.
3.1 Angioimmunoblastic T-cell lymphoma
AITL is a rare but the second most frequently reported malignant lymphoma of peripheral T cells that originates in mature T lymphocytes. It also accounts for around 1%–2% of all non-Hodgkin lymphoma (NHL) cases. Clinically, AITL cases manifest symptoms such as lymphadenopathy, hepatosplenomegaly, hypergammaglobulinemia, and anemia. Less common symptoms include arthritis, ascites, and some autoimmune characteristics, such as circulating rheumatoid antibodies, antismooth muscle antibodies, and hemolytic anemia (68). Generally, death in AITL patients occurs due to overwhelming immunodeficiency and not due to a serious tumor load (69). AITL pathogenesis and progression are also associated with EBV; in some cases, its onset closely mimics an EBV infection confirmed by EBV-specific antibodies in AITL-positive patient serology (70). miRNA profiling of 30 patients with AITL revealed suppressed expression of four miRNAs, miR-140-3p, let-7g, miR-30b, and miR-664, whereas three miRNAs, miR-146a, miR-193b, and miR-34a, were significantly upregulated (71). Among these, miR-146a and miR-34a influence the expression of the EBV-associated protein LMP1 in an NF-κB-dependent manner, thus helping in tumorigenesis; however, these miRNAs are also overexpressed in B-cell lymphomas (72), indicating their lower specificity for AITLs.
Genome-wide miRNA expression profiling of AITL biopsy samples when compared with their polarized CD4+ T-cell counterparts, produced by polarizing them and grouping them based on their cytokine signatures, revealed many overlapping and distinct miRNA signatures. In general, AITL was associated with many differentially expressed miRNAs compared with their normal counterparts or other subtypes of PTCL-NOS, such as the PTCL-GATA3 and PTCL-TBX21 subtypes. These 13 differentially expressed miRNAs (Table 2) signify a distinct miRNA signature that distinguishes AITL from other T-cell malignancies. Among these miRNAs, miR-126-3p is important as it regulates angiogenesis (85), maintains endothelial vasculature (86), and has roles in T-cell activation (87) and differentiation (88), all of which are interesting roles in the context of AITL pathobiology. Moreover, two miRNAs, miR-126-3p and miR-145-5p, are important because they show marginal and significant associations with AITL overall survival (OS) (Figure 3). Ectopic expression of miR-126-3p in cell line models showed repression of sphingosine-1 phosphate receptor-2 (S1PR2), which has been reported to activate RhoA signaling and is associated with poor prognosis, whereas ectopic expression of miR-145-5p represses ROCK1, which is a downstream target of RhoA. Hence, these miRNAs, along with their association with poor prognosis, are also responsible for regulating Rho-GTPase and inhibiting T-cell migration, as observed in AITL pathobiology (77). These enriched miRNA signatures, when fed into an in silico computational annotation tool (89) for pathway analysis, showed the involvement of two major pathways. These enriched miRNAs activated T-cell receptor signaling along with the activation of PI3K-Akt signaling (77). Therefore, these overexpressing miRNAs and their involvement in peculiar AITL pathobiology hold promising potential and call for more focused investigations.
3.2 Anaplastic large cell lymphoma
ALCL is a malignancy of mature T lymphocytes characterized by anaplastic characteristics, such as horseshoe-shaped nuclei with large pleomorphic lymphoid cells. Molecularly, ALCL is further subdivided into two groups, ALK + ALCL and ALK− ALCL, based on rearrangement (translocation) of the anaplastic lymphoma kinase (ALK) gene (90). Diseases in which this gene is rearranged give rise to the expression of chimeric nucleophosmin (NPM) protein, which causes constitutive ALK expression (91). This translocation and eventual formation of the fusion protein NPM-ALK is the definitive molecular feature of ALK+ ALCL, whereas the other subgroup, that is, ALK− ALCL, is coarsely defined as cases devoid of expressing the fusion protein NPM-ALK. This NPM-ALK fusion protein activates many growth-promoting and anti-apoptotic pathways, such as cJun, cMyc, Jak/STAT, and PI3K-Akt/mTOR (92). In a major study in which miRNA profiling of ALCL cases was performed and compared to miRNA profiles of other PTCL cases, such as AITL and PTCL-NOS, diagnostic miRNA signatures were identified after nullifying miRNAs that were also expressed in stromal cells. This diagnostic miRNA signature differentiates ALCL from other PTCL cases and comprises 13 upregulated miRNAs (Table 2), led by the highest expression of miR-149 along with others, and 15 downregulated miRNAs (Table 2), led by miR-155. These miRNA signatures do hold promising diagnostic potential owing to their discriminatory nature regarding various molecular and clinical manifestations of PTCLs (81).
3.2.1 ALK+ and ALK− ALCL
ALK+ ALCL has positive expression of the oncogene fusion protein NPM-ALK, which promotes miR-135b expression by activating signal transducer and activator of transcription 3 (STAT3). This miR-135b also targeted FOXO1 and inhibited its growth suppression activities (82). As STAT3 activation is essential for ALK-mediated transformation, its knockdown in ALK cells and forced expression of a certain miRNA cluster (miR-17-92) result in STAT3 rescue, suggesting a positive role of this cluster in STAT3 and ALK-mediated transformation (93). Similar to most ALCL phenotypes that overexpress anti-apoptotic genes like myeloid cell leukemia-1 (MCL1), it has been reported that this overexpression is done by the NPM-ALK fusion oncoprotein by downmodulating another miRNA called miR-29a (83). However, the role of miR-29a in determining clinical outcomes and patient survival remains unclear.
ALK+ cell lines overexpressed six miRNAs (miR-17, miR-20a, miR-20b, miR-93, miR-106a, and miR-886-3p), where miR-886-3p had very significant overexpression (≥16-fold) when compared with ALK− cell lines (90). Studies on ALK+ ALCL cell lines and ALK+ ALCL clinical samples have revealed many deregulated miRNAs in comparison to their healthy counterparts. Of these deregulated miRNAs, the repressed expression of 11 miRNAs has been reported in ALK+ ALCL (Table 2) (81).
To differentiate ALCL subtypes from other PTCL subtypes, research suggests unique miRNA signatures associated with both ALK+ and ALK− subtypes. ALK+ ALCL has a significant association with seven miRNAs, of which the expression of five miRNAs was elevated (miR-512-3p, miR-886-5p, miR-886-3p, miR-708, and miR-135b) and the expression of two miRNAs (miR-146a and miR-155) was repressed (Table 2) (81). In contrast, ALK− ALCL was found to be associated with 11 miRNAs, of which four were elevated (led by miR-191) and seven had lower expression (led by miR-22) (Figure 3) (81). Therefore, these miRNAs can be used as classifiers to diagnose and differentiate ALK subtypes of ALCL from other PTCL subtypes.
3.3 PTCL-NOS (not otherwise specified)
PTCL-NOS consists of a group of malignancies that are not similar to the other main PTCL subtypes. Generally, PTCL-NOS is molecularly characterized by an aberrant T-cell immunophenotype, usually with the loss of CD5 and CD7 (94). Similar to many other PTCLs, PTCL-NOS shows molecular expression similarity to activated CD4+ and CD8+ T lymphocytes in in vitro studies. In PTCL-NOS, overexpression of miR-187 has been reported to be associated with tumor progression and a poor prognosis (66). In this regard, many studies have attempted to classify PTCL-NOS cases based on their miRNA expression patterns. A study by Laginestra et al. found 158 miRNAs that significantly affect the PTCL-NOS transcriptome compared to normal activated CD8+ and CD4+ T lymphocytes. Of these miRNAs, there was a significant underexpression of miR-132-3p, a miRNA found to be downregulated in many other solid tumors, but in PTCL-NOS cases, it showed differential expression compared to normally activated CD4+ and CD8+ T cells (63). In addition to the above study, another miRNA profiling study reported a miRNA signature of 20 miRNAs (led by kshv-miR-K12-12-5p) in PTCL-NOS that can be used for diagnostic purposes while narrowing down five miRNAs, namely hsa-miR-224-3p, hsa-miR-614, hsa-miR-3196, hsa-miR-1290, and hsa-miR-4485, (Table 2), which related to common cancer progression pathways (84). They reported a significant increase in expression of miR-1290 and miR-4485 in PTCL-NOS among all other subtypes (84).
Although miRNA expression analysis has shown a large number of miRNAs expressed in various subtypes of PTCLs, their diagnostic efficacy has not been assessed in previous studies. Laginestra et al. (63) not only studied differentially expressed miRNAs in PTCL subtypes but also narrowed down the huge number of miRNAs to a set of few miRNA classifiers and devised a practical tool for their diagnostic efficiency. According to this study, PTCL-NOS can be differentiated from ALCL/ALK− using a miRNA classifier comprising five miRNAs (led by has-miR-155) (Table 2). Similarly, PTCL-NOS can be distinguished from AITL using another miRNA classifier with eight miRNAs (led by has-miR-324-5p) and a four-miRNA classifier (led by hsa-miR-124) (Table 2) to differentiate ALK+ and ALK− ALCLs (63). However, a few other studies have subdivided PTCL-NOS into two groups, namely PTCL-NOS-GATA3 and PTCL-NOS-TBX21, based on patient gene expression profiling (GEP). The PTCL-NOS-GATA3 subtype is associated with PI3KP and mTOR upregulation, whereas PTCL-NOS-TBX21 activates the NF-κB pathway. As far as the PTCL-NOS-GATA3 subtype is concerned, it showed nine distinct miRNAs (led by miR-18a-5p), like a signature peculiar to PTCL-NOS-GATA3 (77). PTCL-NOS-TBX21 also showed five distinct miRNA expressions (led by let-7f-5p) when compared against AITL or other PTCL-NOS-GATA3 cases (Figure 3) (77). As the incidence of PTCL-NOS is the highest among all PTCLs, an increasing number of clinical studies validating these miRNA classifiers will help to understand and validate their diagnostic accuracies and their effect on survival outcomes.
3.4 Metabolic roles of dysregulated miRNAs in PTCLs
As PTCL has several subtypes, upregulation of microRNA miR-187 reported across PTCL subtypes is known to decrease glucose-stimulated insulin secretion (GSIS) in metabolic syndromes; however, its role in cancer metabolic reprogramming is unknown (73). In AITL, the overexpression of miR-146a has a global implication on cancer metabolic reprogramming, starting from glycolysis, oxidative phosphorylation, and TCA, along with amino acid and lipid metabolism (74). The miR-34a, which is also overexpressed in AITL, has a negative role in regulating glucose metabolism by lowering the expression of lactate dehydrogenase A (LDHA) (95), whereas the downregulated miR-30b has a negative role in regulating lipid metabolism as reported in hepatocellular carcinoma (76). Similarly, an overexpressed miR-126-3p, suggesting poor prognosis in AITL, expectedly is a global regulator of angiogenesis, having a role in neovascularization and glycometabolism in cardiovascular diseases (78). This miR-126 is also suggested to have been enriched in microvesicles (96), indicating its prospective role as a noninvasive circulating biomarker potentially useful for clinical management. Another miRNA, miR-145, associated with poor survival in AITL, finds significance in negatively regulating aerobic glycolysis and has also been found to inhibit mitochondrial metabolism in cervical cancer and ovarian cancer (79, 80).
In ALCL, few miRNAs among the 13 found to have diagnostic specificity against other PTCLs have metabolic roles. Among them are the highly upregulated hypoxia-responsive miR-210 and miR-517, which are implicated in facilitating aerobic glycolysis (75, 97), whereas miR-708 may have a role in facilitating cellular metabolism (48). For ALK+ ALCL, the upregulated diagnostic miRNA signature, including miR-512 and miR-135b, is reported to have an inhibitory effect on glycolysis (98, 99), whereas a repressed miR-155 may signify reduced glucose metabolism (100). Another downregulated miR-146a in ALK+ ALCL may signify an increase in the Warburg effect and lactate export (101) to support cancer growth. Like ALK+ ALCL, miR-210 is also upregulated in ALK− ALCL, which has a well-known role in facilitating the Warburg effect by decreasing tri-carboxylic acid (TCA) cycle activity and increasing lactate production (102). Furthermore, in PTCL-NOS-TBX21, miRNAs let-7f and miR-26a were found to be most upregulated and have well-documented roles in modulating metabolism in cancers (103, 104), whereas an upregulated miR-30b is known to negatively regulate lipid metabolism (76). In PTCL-NOS-TBX21, an upregulated miR-106b is one of the global regulators of cancer metabolism (74). In this TBX21 subtype, an upregulated miR-181a is found to reprogram metabolism to influence glucose metabolism (105), lactate production (106), and lipid metabolism (107), and can inhibit IDH1/2 and TCA cycle enzymes (108). Moreover, an upregulated miR-20 is reported to have been involved in increasing fatty acid synthesis in separate studies (109) on metastatic cancers; therefore, its levels in aggressive forms of PTCL-NOS can be of clinical and diagnostic importance (Figure 3).
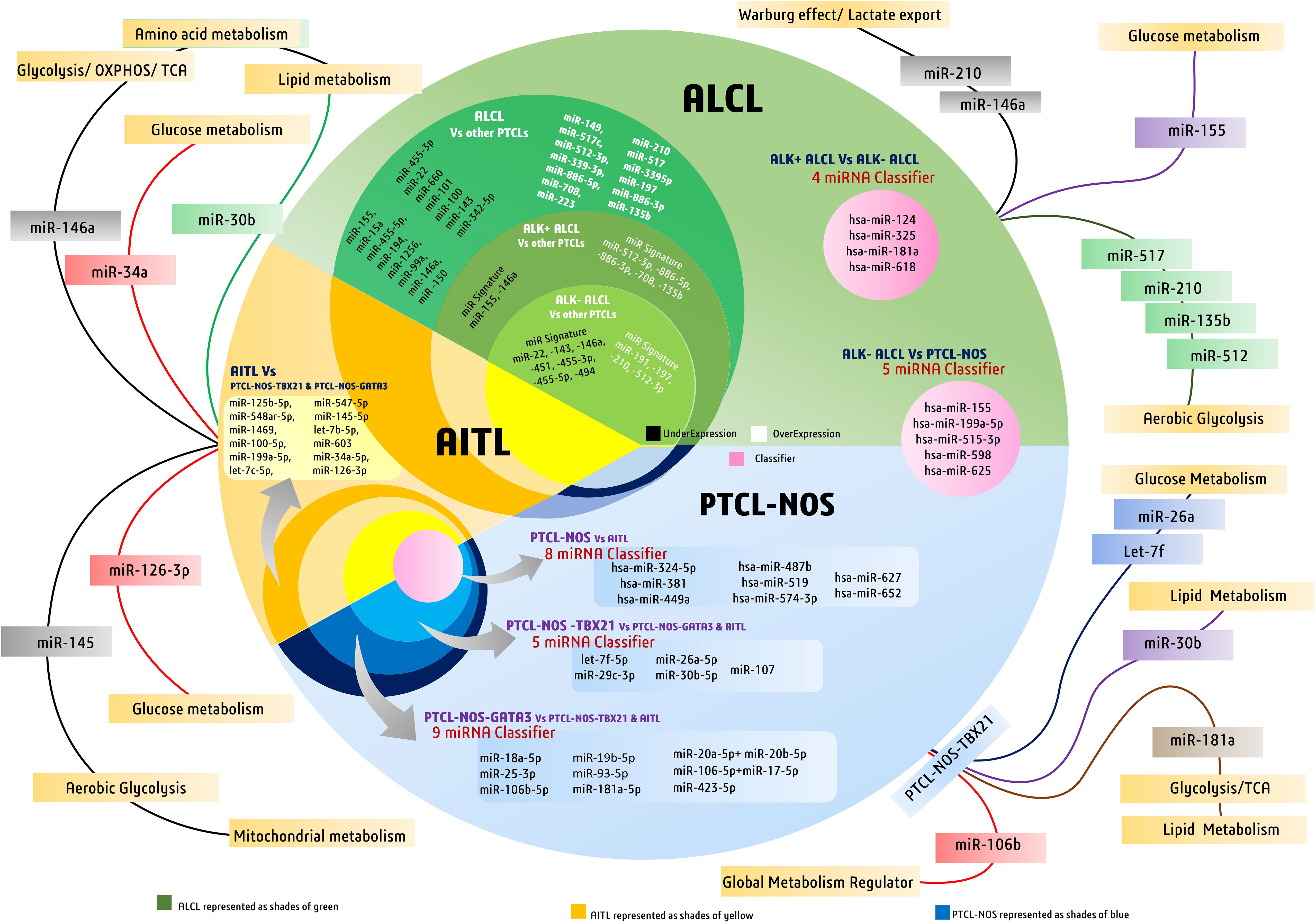
Figure 3 Discriminatory miRNAs and miRNA signatures in major peripheral T-cell lymphoma (PTCL) subtypes like PTCL-NOS, AITL, and ALCL. The figure also highlights major diagnostic miRNAs of PTCLs involved in metabolic regulation in cancers.
4 Extranodal NK/T-cell lymphomas
Another subtype of PTCL, extranodal NK/T-cell lymphomas, should be discussed separately because of their peculiar nature of affecting non-nodal regions. Almost 80% of the reported cases occur around the nose and the region associated with the nasopharynx and oropharynx (110). As far as the incidence of ENKTCL is concerned, it is more commonly diagnosed in men than in women, along with an ethnic bias where it is reported more in Asian, South American, and Central American populations than in Western countries (111). Apart from its apparent clinical course associated with extensive necrosis, ENKTCL is also associated with Epstein–Barr virus (EBV) infection, a peculiar and defining feature of this malignancy. This malignancy is sustained by upregulation of pro-proliferation pathways like JAK/STAT3-related pathways, NF-κB pathways, RUNX3, platelet-derived growth factor (PDGF) pathway, NOTCH1, and aurora kinase pathway, among others (112).
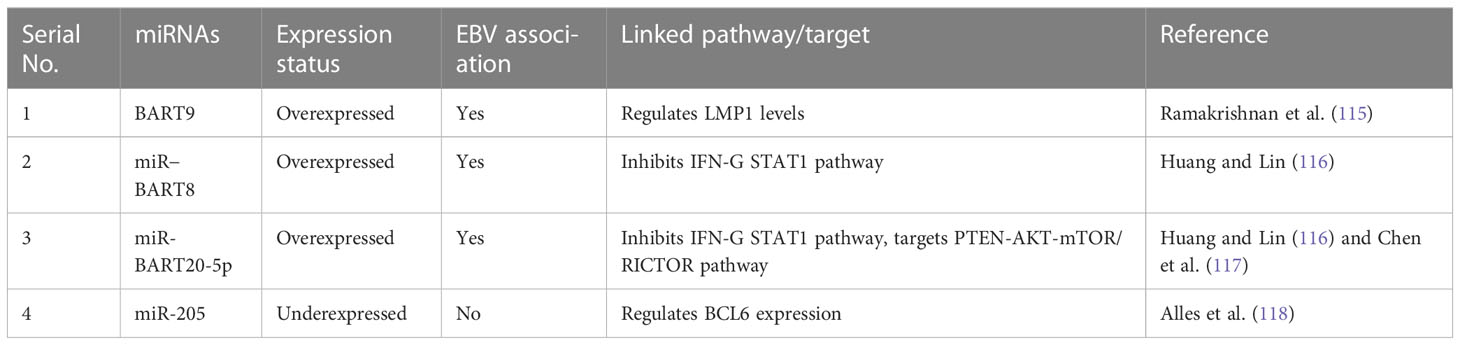
When considering the role of miRNAs in ENKTCL malignancies, earlier studies have shown the diagnostic significance of circulating miR-221, whose upregulation in plasma is so enriched that it can be quantified without total mRNA extraction (113). miR-221 is also upregulated in T-ALL and associated with shorter survival (56). Earlier studies also found downregulation of miR-26a, miR-26b, miR-28-5p, miR-101, and miR-363 in nasal-type ENKTCL when compared to normal NK cells (114). As more than 90% of ENKTCL cases have EBV infection, EBV-encoded miRNAs also play a significant role in driving the transformation of NK/T cells and evading scrutiny by cytotoxic T cells. Among these EBV-encoded miRNAs, BART9 is directly involved in modulating latent membrane protein 1 (LMP1) expressed in almost all EBV-associated lymphomas, providing ENKTCL and its relationship with BART9 with a discriminatory edge compared to other non-EBV-associated lymphomas (115). Other EBV-encoded miRNAs, such as miR-BART20-5p and miR-BART8, are upregulated and involved in inhibiting the IFN-γ STAT1 pathway (116) and evading immune scrutiny, whereas miR-BART9 was found to directly inhibit transcription factor T-bet, resulting in repressed expression of IFN-γ (117). In non-EBV-associated lymphomas, two miRNAs (miR-205 and miR-142-3p) were found to support tumor progression by upregulating the expression of BCL6 and interleukin-A. Interestingly, these two miRNAs were found to be repressed in EBV-associated lymphomas (Table 3) (118), indicating their diagnostic role in discriminating between EBV and non-EBV-linked lymphomas. Various other miRNAs such as miR-21, miR-155, miR-221, miR-223, and miR-4943p are overexpressed, and several others such as miR-15a, miR-16, miR-143, miR-146a, miR-150, and miR-205 are found to be underexpressed in independent studies but have less diagnostic potential because of their association with other malignancies (119).
5 Cutaneous T-cell lymphomas
Cutaneous T-cell lymphoma is a heterogeneous group of malignancies with different clinical, histological, and prognostic features that are grouped according to their apparent effects on skin and cutaneous tissues. A general feature of all CTCLs is the presence of malignant T cells proliferating in a chronic inflammatory environment that expands to the skin, like the sezary syndrome (SS) and mycosis fungicides (MF), which are aggressive variants of CTCLs and constitute the majority of the cases (120). Past research has successfully identified miRNA signatures that differentiate CTCLs from other similar diseases/malignancies, for example, benign inflammatory dermatosis (121). These specific miRNA signatures may be potent in not only identifying CTCLs from other tumors/lymphomas but can also differentiate between various subtypes of CTCLs such as MF, erythrodermic mycosis fungoides (eMF), SS and others (122, 123).
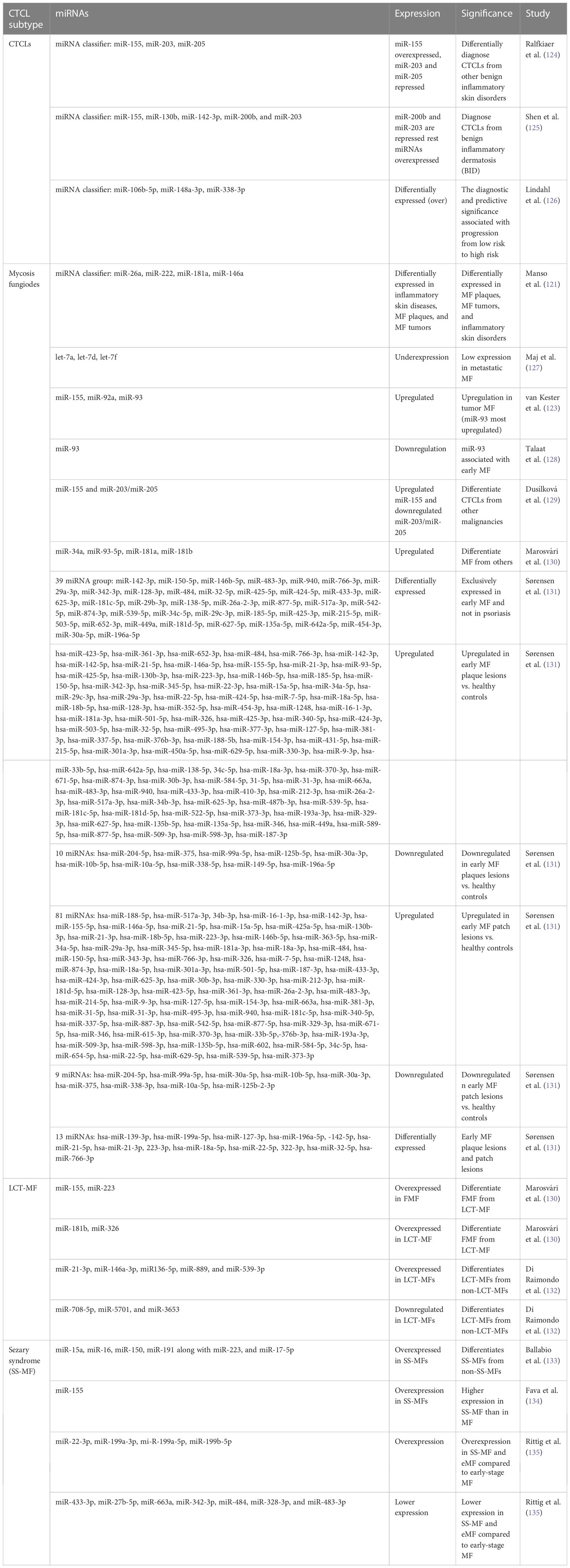
In CTCLs, miR-155 has been reported to be upregulated and act as an oncogene across studies. A three-miRNA classifier consisting of upregulated miR-155 along with downregulated miR-203 and miR-205 (tumor suppressors) can diagnose CTCLs from other benign skin diseases with more than 90% confidence interval (Table 4) (124). MicroRNA profiling revealed significant upregulation of miR-326, miR-663, and miR-711 and downregulation of miR-203, miR-205, and miR-718 in CTCLs. This study identified miR-326, miR-663, and miR-711 as the most induced miRNAs in CTCL cases. Using miR-103 and miR-425-5p expression as a qPCR reference, three miRNAs, including downregulated miR-203 and miR-205 and upregulated miR-326, were used for PCR validation of CTCL cases with enhanced diagnostic capabilities (124) (Table 4).
5.1 Mycosis fungoides
One of the most prevalent types of CTCL is the indolent form known as MF, which accounts for almost 50% of all CTCL cases (120). An early-stage MF resembles small patches and plaques on the skin that advance to form large multifocal itchy lesions and cutaneous tumors in later stages, which may start disseminating malignant T cells to the surrounding nodes, peripheral blood, and vital organs (136). MFs can also be characterized in many groups based on their different clinical and pathological presentations (for example, folliculotropic MF, erythrodermic MF, unilesional MF, hyperpigmented MF, pagetoid MF, granulomatous MF, granulomatous slack skin MF, and vasculature atropicans MF) (137, 138).
In MF, several miRNAs have been implicated to not only have diagnostic potential but also act as oncogenes and drive the pathogenesis of MF. One of them is miR-155, whose upregulation has been consistently observed in various studies concerning MFs. Considering MF’s resemblance to inflammatory dermatoses, differential expression of microRNAs in both disease types is reportedly apparent (121, 139). A study observed significantly higher upregulation of miR-155, miR-92a, miR-92b, and miR-93 in MFs than in inflammatory dermatoses, among which miR-93 showed the highest upregulation. Many miRNAs, including miR-93, miR-146a, miR-181, miR-203, miR-205, miR-16, miR-342-3p, and miR-146b-5p, can diagnose MFs early and efficiently (121) (140). These miRNAs can also be used for differential diagnosis of MFs. Among these, miR-93 is important, as its levels are suggestive of specific stages of MFs. In advanced MFs, miR-93 is upregulated when compared with inflammatory dermatosis, whereas another study suggested its downregulation in early MFs when compared with normal and eczema cases (128).
Although using single miRNAs, such as miR-155, may not be very specific for MFs, its high expression is also seen in other benign forms, such as inflammatory dermatoses. In another study, a four-miRNA classifier with miR-181a, miR-146a, miR-222, and miR-26a could differentiate MF plaques from inflammatory dermatoses. The same study reported that miR-26a and miR-222 were upregulated in early MFs, whereas miR-146a and miR-181a were upregulated in tumoral MFs (121). A study on miRNA profiling in various stages of MFs, in contrast to atopic dermatoses, identified various deregulated miRNAs. Upregulated miR-155, downregulated miR-203, and miR-205 have been reported to distinguish early MFs against atopic dermatoses. A miRNA classifier consisting of upregulated miR-302, miR-155, miR-150, miR-940, and miR-1913 and downregulated miR-149, miR-141, miR-205, miR-221, miR-203, miR-27b, miR-23b, and miR-let7b is deregulated between MF stage 1 and atopic dermatoses. On the other hand, a miRNA classifier with upregulated miR-181a, miR-93, miR-92a-1, miR-107, and miR-15b and downregulated miR-302c, miR-10a, and miR-31 has been found to distinguish MF stages 1 and 2. Another group of deregulated miRNAs consisting of overexpressed miR-93, miR-451, miR-425, miR-142-5p, and miR-421 and downregulated miR-34b, miR-193b, miR-205, miR-1247, and others can discriminate between MF stages 1 and 3, stage 4, and PTCL-not otherwise specified cases (141).
Early MF lesions manifest as plaques and patches which may appear similar to skin inflammation. Therefore, a more recent observation factored out commonly expressed miRNAs in early MF lesions and skin inflammation (psoriasis) to find 39 miRNAs differentially expressing in early MF (Table 4) and 12 miRNAs differentially and exclusively expressing in psoriasis cases, while 70 differentially expressed miRNAs remain common in early MF and psoriasis (131). When healthy controls were compared with early plaque and patch lesions, respectively, many miRNAs were differentially expressed (> 2-fold changes) in these lesions (Table 4). Interestingly, upregulation of miR-142-5p, miR-21-3p, and miR-155-5p was recorded in both plaque and patch lesions of early MF. When plaque and patch lesions are considered, they differentially expressed 13 miRNAs (Table 4), of which 11 miRNAs led by miR-142, miR-21, and miR-22 were found highly expressed in infiltrated plaque lesions and not in patch lesions. Levels of miR-155 and miR-146 remain similar in both MF lesion types (131).
Interestingly, few studies detecting miRNAs in plasma found upregulated miR-155 and downregulated miR-203/miR-205 in MF patients with 100% specificity (129). When it comes to predicting clinical outcomes of MF patients, deregulated miR-21, miR-155, miR-17, miR-25, and miR-106b are related to poor outcome and are associated with progressive MF disease (Figure 4) (141).
5.2 Large cell transformation of mycosis fungoides diagnosis
Another advanced form of MF, called large cell transformation of mycosis fungoides (LCT-MF), is associated with rapid progression of the disease and poor survival outcomes (142). LCT-MFs are characterized by the presence of large T cells that are almost four times bigger than normal T cells and have been reported as an independent prognostic factor for poor survival in MF and SS (143, 144). These malignant transformed T cells (LCT) are often found in advanced stages of MF or SS and very rarely in early stages (145). A study showed significant upregulation of miR-34a, miR-93-5p, miR-181a, miR-181b, and miR-326 in LCT-MF when compared to control cases (130). Another study focusing on the miRNA profile of patients with LCT-MF found the highest upregulation of miRNAs like miR-21-3p, miR-146a-3p, miR136-5p, miR-889, and miR-539-3p, suggesting its role in tumor progression. They also found significant downregulation of miR-708-5p, miR-5701, and miR-3653, suggesting their tumor-suppressive roles (132). These miRNAs in combination could be used to differentially diagnose LCT-MF from non-LCT-MF cases.
5.3 Folliculotropic mycosis fungoides
An aggressive subtype of MF known as folliculotropic MF (FMF), which involves deep infiltration of malignant T cells in and around hair follicles of the patient’s body, has a quite similar miRNA profile to LCT-MF with upregulation of miR-34a, miR-93-5p, and miR-181a (130). Both LCT-MF and FMF cases can be distinguished by upregulated miR-181b and miR-326 in LCT-MF and FMF (130). There have been no discriminatory miRNAs discovered to date to diagnose FMF cases positively.
5.4 Sezary syndrome
SS is a rare form of CTCL that can be distinguished from other types of lymphomas molecularly by the expression of CD4+ and morphologically by the cerebriform appearance of the nucleus, commonly known as sezary cells. These sezary cells can be found in the peripheral blood, skin, and lymph nodes of the suffering patient. Among all the miRNAs, miR-21 specifically regulates the oncogenic progression in SS by activating the STAT3 signaling pathway. This activation of the signaling pathway is also strongly regulated by IL-21 cytokine. Hence, targeting the miR-21 can provide a new therapeutic approach to SS management (146).
On the other hand, while miR-155 is implicated in several malignancies of T cells and in discriminating various subtypes of CTCLs, miR-155 expression was reported to be higher in SS-MF in comparison to MFs in general (134). Various miRNA expression studies showed elevated levels of miR-15a, miR-16, miR-150, and miR-191 along with miR-223 and miR-17-5p in SS-MF. Although in validation experiments all the above miRNAs failed to predict SS-MF, a distinct level of miR-223 had correctly predicted SS-MF from nonerythrodermic MFs with 90% accuracy (133). Another study focused on early-stage MF, eMF (advanced stage MF), and sezary syndrome, where they found 27 deregulated miRNAs, out of which 14 miRNAs showed lower expression and 13 miRNAs showed enhanced expression in eMF when compared to SS-MF. When compared with early-stage MF, both SS-MF and eMF had overexpressed four miRNAs (miR-22-3p, miR-199a-3p, miR-199a-5p, and miR-199b-5p) and less expression of seven miRNAs (miR-433-3p, miR-27b-5p, miR-663a, miR-342-3p, miR-484, miR-328-3p, and miR-483-3p) (Figure 4) (147).
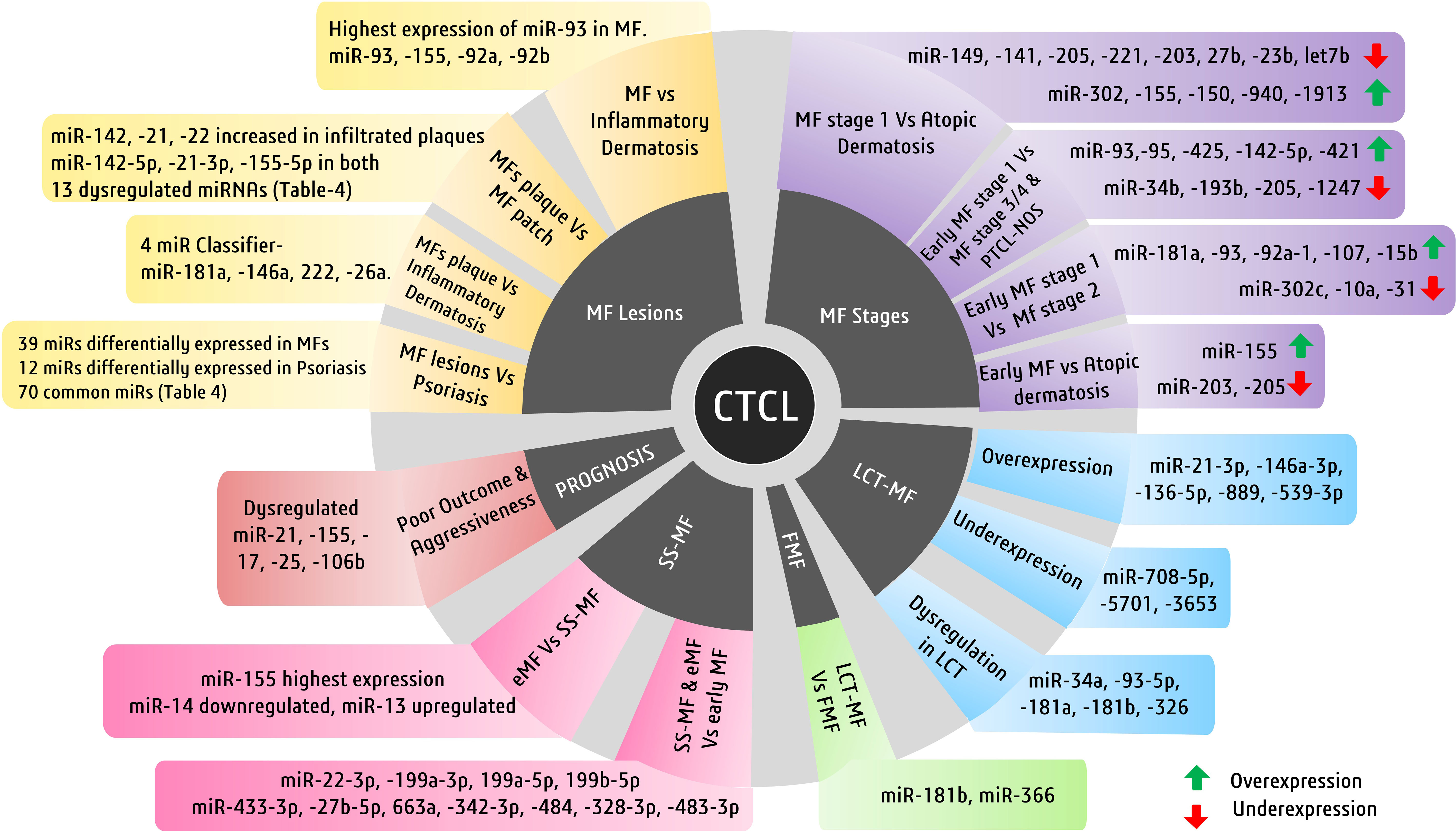
Figure 4 Major cutaneous T-cell lymphoma (CTCL) subtypes and miRNAs of diagnostic and prognostic significance. Illustrated here are the miRNAs involved in mycosis fungiode (MF) diagnosis in a stage- and lesion-specific diagnosis.
5.5 Diagnostic miRNAs of CTCLs and their metabolic significance
In CTCLs, miR-155 is found upregulated irrespective of its subtype but is interestingly found downregulated in PTCLs, where it has a positive role in regulating and driving glucose metabolism (100, 148, 149). Its increased expression in CTCL subtypes may signify a significant role of glucose metabolism in the pathogenesis of CTCLs. The miR-326, which is upregulated in CTCLs in general more so in MFs, along with its FMF variant, is underexpressed in T-ALL. It is known to modulate glucose metabolism by influencing many key regulators (47). Moreover, upregulated miR-146a in all CTCL subtypes and AITL are well known as global regulators of cancer metabolism, influencing glycolysis and TCA cycle activity (74). Similarly, miR-181 is found to regulate glucose and lipid metabolism is upregulated in PTCL-NOS-TBX21 and CTCL subtypes more prominently in mycosis fungiodes and also in T-ALL (77, 105–107, 121). This indicates a similar contribution of miRNA regulators of glucose metabolism to the metabolic landscape of these T-cell malignancies and, hence, can suggest interesting future studies in the discovery of more accurate diagnostic miRNAs.
6 Applicability of miRNA-based diagnosis and new avenues
miRNAs for diagnosis have been of research interest in cancers for a long time now. Its application in the diagnosis of T-cell malignancies is, however, of more importance given the wide range of pathobiological, clinical, and cytogenetic variations in its malignant subtypes (150). To avoid inaccurate clinical judgment (151), it is essential to devise new methods of diagnosis that cannot only capture specific tumor markers but also quantify molecular variations among overlapping clinical features. miRNA profiling can be employed to look for miRNA classifiers for specific patterns in T-cell malignancy subtypes (152). For instance, in AITL biology, miR-146a and miR-34a are associated with EBV-linked AITL cases; therefore, their upregulation can be used to establish EBV-associated AITL cases and rule out non-EBV cases. Similarly, miR-30b is significantly associated with angiogenesis and can be used for the differential diagnosis of AITL from others. Moreover, subtypes like extranodal NK/T-cell lymphomas (ENKTCL) overexpresses EBV-associated miRNAs like miR-BART8, miR-BART9, and miR-BART20-5p, along with non-EBV-associated miRNAs found to be over/underexpressed (116). These miRNAs, in combination, can be effectively used to differentially diagnose and discriminate ENKTCL lymphomas from other subtypes.
Circulating miRNAs have attracted a great deal of interest as potential diagnostic biomarkers because of their stability and detectability. miRNAs derived from extracellular vesicles (EVs) (54) and exosomes (153) may reflect a miRNA signature that may resemble a very early disease condition (154). For instance, miR-126 overexpressed in AITL signifies poor prognosis, and its enrichment in the exosome is found to distinguish early and advanced non-small cell lung cancer (NSCLC), while it is downregulated for advanced NSCLC (155). This miR-126 can be investigated in AITL for its possible correlation with the stage and aggressiveness of AITL and whether its exosomal enrichment in body fluids can be used as a noninvasive tool to monitor disease and suggest better therapies. Similarly, in ALCL, an upregulated miR-223 can have a potential role in inducing chemoresistance, whose expression levels can be quantified in exosomes to potentially diagnose and use for disease surveillance (156). Studies also indicate that an upregulated miR-197 in ALK− ALCL can be investigated for its enrichment in exosomes as it has been implicated in metastatic promotion in lung cancer (157). The above insights project miRNAs and their profiling as powerful tools to be potentially used for differential diagnosis of malignancies with overlapping clinical manifestations.
More recently, liquid biopsies, which include examining circulating miRNAs in bodily fluids, have presented a simple, noninvasive method for early and efficient diagnosis along with disease tracking and surveillance capacities (158). However, assessing tumor-specific miRNA expression by liquid biopsy or miRNA enrichment in EVs or exosomes requires many clinical-based studies to establish their clinical applicability. Currently, the applicability of liquid biopsies in clinical settings is debatable (159). On the one hand, the method provides a noninvasive and easily accessible option that incurs less discomfort and risk to the patient than standard tissue biopsies, evaluation of biomarkers derived from it, remains unstandardized (160). The task of standardizing miRNA profiling data analysis is ongoing as platforms and procedures used by various laboratories differ, causing results to fluctuate and making it difficult to reproduce and compare findings (160). A liquid biopsy also gives clinicians and scientists an upper hand in the early detection of tumors, along with its promising potential to record the molecular heterogeneity of tumors, thus providing scope for precise diagnosis and personalized treatment strategies. However, molecular and genetic complexities of T-cell malignancies can influence miRNA profiles depending on the tumor microenvironment, disease stage, individual patient characteristics, or the presence of any additional disease (161). Moreover, circulating miRNA levels can be affected by a number of preanalytical factors, including sample type, storage conditions, and RNA separation techniques. The processing of samples and the selection of the sample type (serum, plasma, or other biofluids) can affect the stability and production of miRNA (162). For trustworthy diagnostic outcomes and to reduce technical variability, these preanalytical factors must be standardized. The clinical relevance, sensitivity, specificity, and positive predictive value of diagnostic tests must be demonstrated through thorough validation and clinical investigations, just like with any other diagnostic test (163). Additionally, in order to authorize miRNA-based diagnostic tests for clinical use, regulatory authorities demand strong proof to avoid faulty clinical judgments while in use (164).
Tumor-associated miRNAs can be detected and quantified in body fluids such as serum (165) or plasma (166) using techniques such as reverse transcriptase PCR (167), microarray (124), and next-generation sequencing (NGS) that were traditionally used for miRNA profiling studies. Quantitative PCR and microarray analysis are two miRNA detection methods that have limitations in terms of sensitivity and specificity. Results that are falsely positive or falsely negative can be recorded, which can reduce the precision of miRNA-based diagnosis. More reliable and powerful detection techniques such as droplet digital PCR (ddPCR) promise increased sensitivity, absolute quantification and precision, multiplexing capabilities, and resistance to PCR inhibitors, and their ability to detect rare variants, which can lead to better-standardized results than traditionally used methods (168).
In malignancies or otherwise, miRNAs were never singularly used for diagnosis, as there are overlapping miRNAs in various malignant disease phenotypes. Therefore, miRNA classifiers and panels encompassing a wide range of over- and underexpressing miRNAs are fit to be used for diagnostic applications. In this regard, many researchers have already devised miRNA classifiers whose diagnostic applicability has been validated in clinical settings (140, 169, 170). However, research is still underway to identify and validate particular miRNAs linked to better prognosis and accurate diagnosis. In this regard, miRNAs having a metabolic impact on tumors may be investigated for their promising diagnostic potential. Many studies have identified miRNA species that have regulatory roles in reprogramming cell metabolism to support or inhibit cancer progression. Such studies often develop an overexpression system using many strategies, such as transfecting cells with miRNA-viral construct or miRNA mimics to transiently overexpress or inhibit a particular miRNA in order to study their functional roles. A brief summary of the methodological strategies and outcomes of such studies is featured in Supplementary Table 1. Future investigations exploring such metabolic miRNAs for their diagnostic potential along with the metabolic status of patients could be potentially utilized for more accurate subtyping and subgrouping of diseases and leading precision medicine efforts.
7 Conclusion
Various malignancies of the T-cell display overlapping physical symptoms despite each one having different molecular and disease progression profiles. Even though imaging techniques such as PET and CT scans can efficiently diagnose the location, stage, and spread of cancer, they cannot suggest valuable information regarding the best therapeutic choice with minimum toxicity and maximum survival. However, investigating disease-specific molecular biomarkers in tumor tissues or in circulating fluids is a promising avenue of molecular diagnostics and can reduce cancer mortality by suggesting the best therapeutic choices for better survival outcomes. Studies capturing miRNA signatures and unique miRNAs in miRNAome need more variety in terms of patient diversity in addition to the range of miRNAs used for screening. miRNAs involved in regulating diverse signaling pathways can be included in the panel of miRNAs for better diagnosis and categorization of malignant T-cell subtypes. Therefore, more such studies identifying and evaluating tissue or serum miRNAs derived from EVs or exosomes will help identify specific signature miRNAs for various other clinical outcomes and may lead to early and efficient diagnosis and therapy of this diverse disease group. Future studies can explore miRNAs along with their metabolic impact to devise more precise miRNA panels for differential diagnostic purposes.
Author contributions
Corresponding and lead authors from Guru Ghasidas Vishwavidyalaya wrote and edited the manuscript. All authors contributed intellectual contributions to designing, editing, and proofreading the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors acknowledge institutional and administrative support of Department of Biotechnology, Guru Ghasidas Vishwavidyalaya (GGV), Bilaspur and UGC-SAP program. ICMR-Senior Research Fellowship support to co-author Sapnita Shinde (SS) is acknowledged. Collaborative and research support of clinicians of All India Institute of Medical Sciences (AIIMS), Raipur is appreciated. The authors also appreciate research support of our respective collaborators.
Conflict of interest
Authors ST and GV were employed by the company Trivitron Healthcare Pvt. Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1230273/full#supplementary-material
References
1. Li Y, Gao X, Kong L-Z, Li J. Misdiagnosis of angioimmunoblastic T−cell lymphoma: A case report. Oncol Lett (2023) 25(6):250. doi: 10.3892/ol.2023.13836
2. Poynton E, Okosun J. Liquid biopsy in lymphoma: Is it primed for clinical translation? EJHaem (2021) 2(3):616–27. doi: 10.1002/jha2.212
3. Tang T, Khoo LP, Lim C, Ham JS, Kim SJ, Hong H, et al. Outcomes of patients with peripheral T-cell lymphoma in first complete remission: data from three tertiary Asian cancer centers. Blood Cancer J (2017) 7(12):653. doi: 10.1038/s41408-017-0030-y
4. Düzova A, Kutluk T, Kanra G, Büyükpamukçu M, Akyüz C, Seçmeer G, et al. Monotherapy with meropenem versus combination therapy with piperacillin plus amikacin as empiric therapy for neutropenic fever in children with lymphoma and solid tumors. Turkish J Pediatr (2001) 43(2):105–9. Available at: https://pubmed.ncbi.nlm.nih.gov/11432485/.
5. Lv L, Liu Y. Clinical application of liquid biopsy in non-hodgkin lymphoma. Front Oncol (2021) 11:658234. doi: 10.3389/fonc.2021.658234
6. Izzotti A, Carozzo S, Pulliero A, Zhabayeva D, Ravetti JL, Bersimbaev R. Extracellular MicroRNA in liquid biopsy: applicability in cancer diagnosis and prevention. Am J Cancer Res (2016) 6(7):1461–93. Available at: https://pubmed.ncbi.nlm.nih.gov/27508091/.
7. Vella MC, Choi E-Y, Lin S-Y, Reinert K, Slack FJ. The C. elegans microRNA let-7 binds to imperfect let-7 complementary sites from the lin-41 3′ UTR. Genes Dev (2004) 18(2):132–7. doi: 10.1101/gad.1165404
8. O’Brien J, Hayder H, Zayed Y, Peng C. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol (2018) 9:402. doi: 10.3389/fendo.2018.00402
9. Li Z, Xu R, Li N. MicroRNAs from plants to animals, do they define a new messenger for communication? Nutr Metab (2018) 15(1):68. doi: 10.1186/s12986-018-0305-8
10. Mendell JT, Olson EN. MicroRNAs in stress signaling and human disease. Cell (2012) 148(6):1172–87. doi: 10.1016/j.cell.2012.02.005
11. Alaggio R, Amador C, Anagnostopoulos I, Attygalle AD, Araujo IB, de O, et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: lymphoid neoplasms. Leukemia (2022) 36(7):1720–48. doi: 10.1038/s41375-022-01620-2
12. Sehn LH, Soulier J. Introduction to the review series on T-cell Malignancies. Blood (2017) 129(9):1059–60. doi: 10.1182/blood-2017-01-741389
13. Refeat MM, Hassan NA-M, Ahmad IH, Mostafa ERM, Amr KS. Correlation of circulating miRNA-33a and miRNA-122 with lipid metabolism among Egyptian patients with metabolic syndrome. J Genet Eng Biotechnol (2021) 19(1):147. doi: 10.1186/s43141-021-00246-8
14. Zhai T, Dou M, Ma Y, Wang H, Liu F, Zhang L, et al. Lipid metabolism-related miRNAs with potential diagnostic roles in prostate cancer. Lipids Health Dis (2023) 22(1):39. doi: 10.1186/s12944-023-01804-4
15. Li G, Wu Z, Gu J, Zhu Y, Zhang T, Wang F, et al. Metabolic signature-based subtypes may pave novel ways for low-grade glioma prognosis and therapy. Front Cell Dev Biol (2021) 9:755776. doi: 10.3389/fcell.2021.755776
16. Srivastava A, Vinod PK. Identification and characterization of metabolic subtypes of endometrial cancer using a systems-level approach. Metabolites (2023) 13(3):409. doi: 10.3390/metabo13030409
17. Tan G, Wang H, Yuan J, Qin W, Dong X, Wu H, et al. Three serum metabolite signatures for diagnosing low-grade and high-grade bladder cancer. Sci Rep (2017) 7(1):46176. doi: 10.1038/srep46176
18. Harris NL, Jaffe ES, Stein H, Banks PM, Chan JKC, Cleary ML, et al. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood (1994) 84(5):1361–92. doi: 10.1182/blood.V84.5.1361.1361
19. Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood J Am Soc Hematol (2016) 127(20):2391–405. doi: 10.1182/blood-2016-03-643544
20. Hoelzer D, Gökbuget N. T-cell lymphoblastic lymphoma and T-cell acute lymphoblastic leukemia: a separate entity? Clin Lymphoma Myeloma (2009) 9(Suppl 3):S214–21. doi: 10.3816/CLM.2009.s.015
21. Kroeze E, Loeffen JLC, Poort VM, Meijerink JPP. T-cell lymphoblastic lymphoma and leukemia: different diseases from a common preMalignant progenitor? Blood Adv (2020) 4(14):3466–73. doi: 10.1182/bloodadvances.2020001822
22. Wang P, Peng X, Deng X, Gao L, Zhang X, Feng Y. Diagnostic challenges in T-lymphoblastic lymphoma, early T-cell precursor acute lymphoblastic leukemia or mixed phenotype acute leukemia: A case report. Medicine (2018) 97(41):e12743.. doi: 10.1097/MD.0000000000012743
23. Genescà E, la Starza R. Early T-cell precursor ALL and beyond: immature and ambiguous lineage T-ALL subsets. Cancers (2022) 14(8):1873. doi: 10.3390/cancers14081873
24. Tan TK, Zhang C, Sanda T. Oncogenic transcriptional program driven by TAL1 in T-cell acute lymphoblastic leukemia. Int J Hematol (2019) 109(1):5–17. doi: 10.1007/s12185-018-2518-z
25. Follini E, Marchesini M, Roti G. Strategies to overcome resistance mechanisms in T-cell acute lymphoblastic leukemia. Int J Mol Sci (2019) 20(12):3021. doi: 10.3390/ijms20123021
26. Dawidowska M, Jaksik R, Drobna M, Szarzyńska-Zawadzka B, Kosmalska M, Sędek Ł., et al. Comprehensive Investigation of miRNome Identifies Novel Candidate miRNA-mRNA Interactions Implicated in T-Cell Acute Lymphoblastic Leukemia. Neoplasia (New York N.Y.) (2019) 21(3):294–310. doi: 10.1016/j.neo.2019.01.004
27. Correia NC, Barata JT. MicroRNAs and their involvement in T-ALL: A brief overview. Adv Biol Regul (2019) 74:100650. doi: 10.1016/j.jbior.2019.100650
28. Xi Y, Li J, Zan L, Wang J, Wang G, Ning Y. Micro-RNA-16 expression in paraffin-embedded specimen correlates with overall survival of T-lymphoblastic lymphoma/leukemia. Hum Pathol (2013) 44(6):1011–6. doi: 10.1016/j.humpath.2012.08.023
29. Zhao Z, Ji M, Wang Q, He N, Li Y. miR-16-5p/PDK4-mediated metabolic reprogramming is involved in chemoresistance of cervical cancer. Mol Ther Oncolytics (2020) 17:509–17. doi: 10.1016/j.omto.2020.05.008
30. Akbari Moqadam F, Lange-Turenhout EAM, Ariës IM, Pieters R, den Boer ML. MiR-125b, miR-100 and miR-99a co-regulate vincristine resistance in childhood acute lymphoblastic leukemia. Leukemia Res (2013) 37(10):1315–-1321. doi: 10.1016/j.leukres.2013.06.027
31. Izreig S, Samborska B, Johnson RM, Sergushichev A, Ma EH, Lussier C, et al. The miR-17∼92 microRNA Cluster Is a Global Regulator of Tumor Metabolism. Cell Rep (2016) 16(7):1915–28. doi: 10.1016/j.celrep.2016.07.036
32. Hui L, Zhang J, Guo X. MiR-125b-5p suppressed the glycolysis of laryngeal squamous cell carcinoma by down-regulating hexokinase-2. Biomedicine Pharmacotherapy (2018) 103:1194–201. doi: 10.1016/j.biopha.2018.04.098
33. Liu Z, Smith KR, Khong HT, Huang J, Ahn E-YE, Zhou M, et al. miR-125b regulates differentiation and metabolic reprogramming of T cell acute lymphoblastic leukemia by directly targeting A20. Oncotarget (2016) 7(48):78667–79. doi: 10.18632/oncotarget.12018
34. Peng M, Yin N, Chhangawala S, Xu K, Leslie CS, Li MO. Aerobic glycolysis promotes T helper 1 cell differentiation through an epigenetic mechanism. Sci (New York N.Y.) (2016) 354(6311):481–4. doi: 10.1126/science.aaf6284
35. Renou L, Boelle P-Y, Deswarte C, Spicuglia S, Benyoucef A, Calvo J, et al. Homeobox protein TLX3 activates miR-125b expression to promote T-cell acute lymphoblastic leukemia. Blood Adv (2017) 1(12):733–47. doi: 10.1182/bloodadvances.2017005538
36. Wang G, Lu Y, Di S, Xie M, Jing F, Dai X. miR−99a−5p inhibits glycolysis and induces cell apoptosis in cervical cancer by targeting RRAGD. Oncol Lett (2022) 24(1):228. doi: 10.3892/ol.2022.13349
37. Hassan NM, Refaat LA, Ismail GN, Abdellateif M, Fadel SA, AbdelAziz RS. Diagnostic, prognostic and predictive values of miR-100 and miR-210 in pediatric acute lymphoblastic Leukemia. Hematol (Amsterdam Netherlands) (2020) 25(1):405–13. doi: 10.1080/16078454.2020.1843753
38. Wei X, Feng Y, Fu Y, Liu F, Chen Q, Zhang W, et al. miR-100-5p is upregulated in multiple myeloma and involves in the pathogenesis of multiple myeloma through targeting MTMR3. Hematology (2023) 28(1):2196857. doi: 10.1080/16078454.2023.2196857
39. Agirre X, Vilas-Zornoza A, Jiménez-Velasco A, Martin-Subero JI, Cordeu L, Gárate L, et al. Epigenetic silencing of the tumor suppressor microRNA hsa-miR-124a regulates CDK6 expression and confers a poor prognosis in acute lymphoblastic leukemia. Cancer Res (2009) 69(10):4443–-4453. doi: 10.1158/0008-5472.CAN-08-4025
40. Sun Y, Zhao X, Zhou Y, Hu Y. miR-124, miR-137 and miR-340 regulate colorectal cancer growth via inhibition of the Warburg effect. Oncol Rep (2012) 28(4):1346–52. doi: 10.3892/or.2012.1958
41. Nemes K, Csóka M, Nagy N, Márk Á, Váradi Z, Dankó T, et al. Expression of certain leukemia/lymphoma related microRNAs and its correlation with prognosis in childhood acute lymphoblastic leukemia. Pathol Oncol Research : POR (2015) 21(3):597–-604. doi: 10.1007/s12253-014-9861-z
42. Xiao M, Lou C, Xiao H, Yang Y, Cai X, Li C, et al. MiR-128 regulation of glucose metabolism and cell proliferation in triple-negative breast cancer. Br J Surg (2018) 105(1):75–85. doi: 10.1002/bjs.10646
43. Liu S, Xiao Z, Ai F, Liu F, Chen X, Cao K, et al. miR-142-5p promotes development of colorectal cancer through targeting SDHB and facilitating generation of aerobic glycolysis. Biomedicine Pharmacotherapy (2017) 92:1119–-1127. doi: 10.1016/j.biopha.2017.05.134
44. Coskun E, Neumann M, Schlee C, Liebertz F, Heesch S, Goekbuget N, et al. MicroRNA profiling reveals aberrant microRNA expression in adult ETP-ALL and functional studies implicate a role for miR-222 in acute leukemia. Leukemia Res (2013) 37(6):647–56. doi: 10.1016/j.leukres.2013.02.019
45. Yamaguchi S, Zhang D, Katayama A, Kurooka N, Sugawara R, Albuayjan HHH, et al. Adipocyte-specific inhibition of mir221/222 ameliorates diet-induced obesity through targeting ddit4. Front Endocrinol (2022) 12:750261. doi: 10.3389/fendo.2021.750261
46. Ghodousi ES, Aberuyi N, Rahgozar S. Simultaneous changes in expression levels of BAALC and miR-326: a novel prognostic biomarker for childhood ALL. Japanese J Clin Oncol (2020) 50(6):671–8. doi: 10.1093/jjco/hyaa025
47. Hatziapostolou M, Polytarchou C, Iliopoulos D. miRNAs link metabolic reprogramming to oncogenesis. Trends Endocrinol Metab (2013) 24(7):361–73. doi: 10.1016/j.tem.2013.03.002
48. Monteleone NJ, Lutz CS. miR-708-5p targets oncogenic prostaglandin E2 production to suppress a pro-tumorigenic phenotype in lung cancer cells. Oncotarget (2020) 11(26):2464–83. doi: 10.18632/oncotarget.27614
49. Rodríguez-Comas J, Moreno-Asso A, Moreno-Vedia J, Martín M, Castaño C, Marzà-Florensa A, et al. Stress-induced microRNA-708 impairs β-cell function and growth. Diabetes (2017) 66(12):3029–40. doi: 10.2337/db16-1569
50. Wallaert A, Van Loocke W, Hernandez L, Taghon T, Speleman F, Van Vlierberghe P. Comprehensive miRNA expression profiling in human T-cell acute lymphoblastic leukemia by small RNA-sequencing. Sci Rep (2017) 7(1):7901. doi: 10.1038/s41598-017-08148-x
51. Drobna M, Szarzyńska-Zawadzka B, Dawidowska M. T-cell acute lymphoblastic leukemia from miRNA perspective: Basic concepts, experimental approaches, and potential biomarkers. Blood Rev (2018) 32(6):457–72. doi: 10.1016/j.blre.2018.04.003
52. Mavrakis KJ, van der Meulen J, Wolfe AL, Liu X, Mets E, Taghon T, et al. A cooperative microRNA-tumor suppressor gene network in acute T-cell lymphoblastic leukemia (T-ALL). Nat Genet (2011) 43(7):673–8. doi: 10.1038/ng.858
53. Rawoof A, Swaminathan G, Tiwari S, Nair RA, Dinesh Kumar L. LeukmiR: a database for miRNAs and their targets in acute lymphoblastic leukemia. Database: J Biol Database Curation 2020 (2020). doi: 10.1093/database/baz151
54. Colangelo T, Panelli P, Mazzarelli F, Tamiro F, Melocchi V, De Santis E, et al. Extracellular vesicle microRNAs contribute to Notch signaling pathway in T-cell acute lymphoblastic leukemia. Mol Cancer (2022) 21(1):226. doi: 10.1186/s12943-022-01698-3
55. Coskun E, von der Heide EK, Schlee C, Kühnl A, Gökbuget N, Hoelzer D, et al. The role of microRNA-196a and microRNA-196b as ERG regulators in acute myeloid leukemia and acute T-lymphoblastic leukemia. Leukemia Res (2011) 35(2):208–13. doi: 10.1016/j.leukres.2010.05.007
56. Gimenes-Teixeira HL, Lucena-Araujo AR, dos Santos GA, Zanette DL, Scheucher PS, Oliveira LC, et al. Increased expression of miR-221 is associated with shorter overall survival in T-cell acute lymphoid leukemia. Exp Hematol Oncol (2013) 2(1):10. doi: 10.1186/2162-3619-2-10
57. Li S-W, Li H, Zhang Z-P, Zhuo F, Li Z-X. [Expression and clinical significance of miR-146a and miR-221 in childhood acute T lymphoblastic leukemia]. Zhongguo shi yan xue ye xue za zhi (2020) 28(2):436–41. doi: 10.19746/j.cnki.issn.1009-2137.2020.02.013
58. Jamshidi M, Fagerholm R, Muranen TA, Kaur S, Potdar S, Khan S, et al. High miR-30 expression associates with improved breast cancer patient survival and treatment outcome. Cancers (2021) 13(12):2907. doi: 10.3390/cancers13122907
59. Huang W, Wang W-T, Fang K, Chen Z-H, Sun Y-M, Han C, et al. MIR-708 promotes phagocytosis to eradicate T-ALL cells by targeting CD47. Mol Cancer (2018) 17(1):12. doi: 10.1186/s12943-018-0768-2
60. Wang F, Mao A, Tang J, Zhang Q, Yan J, Wang Y, et al. microRNA-16-5p enhances radiosensitivity through modulating Cyclin D1/E1-pRb-E2F1 pathway in prostate cancer cells. J Cell Physiol (2019) 234(8):13182–90. doi: 10.1002/jcp.27989
61. Sharma P, Sharma V, Ahluwalia TS, Dogra N, Kumar S, Singh S. Let-7a induces metabolic reprogramming in breast cancer cells via targeting mitochondrial encoded ND4. Cancer Cell Int (2021) 21(1):629. doi: 10.1186/s12935-021-02339-3
62. Smolka C, Schlösser D, Hohnloser C, Bemtgen X, Jänich C, Schneider L, et al. MiR-100 overexpression attenuates high fat diet induced weight gain, liver steatosis, hypertriglyceridemia and development of metabolic syndrome in mice. Mol Med (2021) 27(1):101. doi: 10.1186/s10020-021-00364-6
63. Laginestra MA, Piccaluga PP, Fuligni F, Rossi M, Agostinelli C, Righi S, et al. Pathogenetic and diagnostic significance of microRNA deregulation in peripheral T-cell lymphoma not otherwise specified. Blood Cancer J (2014) 4(11):e259–9. doi: 10.1038/bcj.2014.78
64. Iqbal J, Wright G, Wang C, Rosenwald A, Gascoyne RD, Weisenburger DD, et al. Gene expression signatures delineate biological and prognostic subgroups in peripheral T-cell lymphoma. Blood (2014) 123(19):2915–23. doi: 10.1182/blood-2013-11-536359
65. Piccaluga PP, Laginestra MA, Rossi M, De Leo A, Gibellini D, Gazzola A, et al. Identification of Differentially Expressed miRNAs in Peripheral t-Cell Lymphomas. Blood (2011) 118(21):773. doi: 10.1182/blood.V118.21.773.773
66. Yan Z-X, Wu L-L, Xue K, Zhang Q-L, Guo Y, Romero M, et al. MicroRNA187 overexpression is related to tumor progression and determines sensitivity to bortezomib in peripheral T-cell lymphoma. Leukemia (2014) 28(4):880–7. doi: 10.1038/leu.2013.291
67. Peng W, Sha H, Sun X, Zou R, Zhu Y, Zhou G, et al. Role and mechanism of miR-187 in human cancer. Am J Trans Res (2020) 12(9):4873–84. Available at: https://pubmed.ncbi.nlm.nih.gov/33042395/.
68. Xu B, Liu P. No survival improvement for patients with angioimmunoblastic T-cell lymphoma over the past two decades: a population-based study of 1207 cases. PloS One (2014) 9(3):e92585. doi: 10.1371/journal.pone.0092585
69. Dogan A, Attygalle AD, Kyriakou C. Angioimmunoblastic T-cell lymphoma. Br J Haematology (2003) 121(5):681–91. doi: 10.1046/j.1365-2141.2003.04335.x
70. Facchinelli D, Polino A, Dima F, Parisi A, Ambrosetti A, Veneri D. Two cases of angioimmunoblastic T-cell lymphoma with concomitant positive serology for acute Epstein-Barr virus infection. Hematol Rep (2017) 9(3):7088. doi: 10.4081/hr.2017.7088
71. Reddemann K, Gola D, Schillert A, Knief J, Kuempers C, Ribbat-Idel J, et al. Dysregulation of microRNAs in angioimmunoblastic T-cell lymphoma. Anticancer Res (2015) 35(4):2055 LP – 2061. Available at: https://pubmed.ncbi.nlm.nih.gov/25862860/.
72. Forte E, Salinas RE, Chang C, Zhou T, Linnstaedt SD, Gottwein E, et al. The Epstein-Barr virus (EBV)-induced tumor suppressor microRNA MiR-34a is growth promoting in EBV-infected B cells. J Virol (2012) 86(12):6889–98. doi: 10.1128/JVI.07056-11
73. Locke JM, da Silva Xavier G, Dawe HR, Rutter GA, Harries LW. Increased expression of miR-187 in human islets from individuals with type 2 diabetes is associated with reduced glucose-stimulated insulin secretion. Diabetologia (2014) 57(1):122–8. doi: 10.1007/s00125-013-3089-4
74. Bogusławska J, Popławski P, Alseekh S, Koblowska M, Iwanicka-Nowicka R, Rybicka B, et al. MicroRNA-mediated metabolic reprograming in renal cancer. Cancers (2019) 11(12):1825. doi: 10.3390/cancers11121825
75. Zhang D, Li Z, Li T, Luo D, Feng X, Liu Y, et al. miR-517a promotes Warburg effect in HCC by directly targeting FBP1. OncoTargets Ther (2018) 11:8025–32. doi: 10.2147/OTT.S172084
76. Zhang Q, Ma X-F, Dong M-Z, Tan J, Zhang J, Zhuang L-K, et al. MiR-30b-5p regulates the lipid metabolism by targeting PPARGC1A in Huh-7 cell line. Lipids Health Dis (2020) 19(1):76. doi: 10.1186/s12944-020-01261-3
77. Lone W, Bouska A, Sharma S, Amador C, Saumyaranjan M, Herek TA, et al. Genome-wide miRNA expression profiling of molecular subgroups of peripheral T-cell lymphoma. Clin Cancer Res (2021) 27(21):6039–53. doi: 10.1158/1078-0432.CCR-21-0573
78. Ma Y, Liu H, Wang Y, Xuan J, Gao X, Ding H, et al. Roles of physical exercise-induced MiR-126 in cardiovascular health of type 2 diabetes. Diabetol Metab Syndrome (2022) 14(1):169. doi: 10.1186/s13098-022-00942-6
79. Hu C, Liu T, Zhang W, Sun Y, Jiang D, Zhang X, et al. miR-145 inhibits aerobic glycolysis and cell proliferation of cervical cancer by acting on MYC. FASEB J (2023) 37(4):e22839. doi: 10.1096/fj.202201189RR
80. Zhao S, Zhang Y, Pei M, Wu L, Li J. miR-145 inhibits mitochondrial function of ovarian cancer by targeting ARL5B. J Ovarian Res (2021) 14(1):8. doi: 10.1186/s13048-020-00762-0
81. Liu C, Iqbal J, Teruya-Feldstein J, Shen Y, Dabrowska MJ, Dybkaer K, et al. MicroRNA expression profiling identifies molecular signatures associated with anaplastic large cell lymphoma. Blood (2013) 122(12):2083–92. doi: 10.1182/blood-2012-08-447375
82. Matsuyama H, Suzuki HI, Nishimori H, Noguchi M, Yao T, Komatsu N, et al. miR-135b mediates NPM-ALK-driven oncogenicity and renders IL-17-producing immunophenotype to anaplastic large cell lymphoma. Blood (2011) 118(26):6881–92. doi: 10.1182/blood-2011-05-354654
83. Desjobert C, Renalier M-H, Bergalet J, Dejean E, Joseph N, Kruczynski A, et al. MiR-29a down-regulation in ALK-positive anaplastic large cell lymphomas contributes to apoptosis blockade through MCL-1 overexpression. Blood (2011) 117(24):6627–37. doi: 10.1182/blood-2010-09-301994
84. Lin Y, Chen W-M, Wang C, Chen X-Y. MicroRNA profiling in peripheral T-cell lymphoma, not otherwise specified. Cancer Biomarkers (2017) 18:339–47. doi: 10.3233/CBM-160126
85. Alique M, Bodega G, Giannarelli C, Carracedo J, Ramírez R. MicroRNA-126 regulates Hypoxia-Inducible Factor-1α which inhibited migration, proliferation, and angiogenesis in replicative endothelial senescence. Sci Rep (2019) 9(1):7381. doi: 10.1038/s41598-019-43689-3
86. Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, et al. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell (2008) 15(2):261–71. doi: 10.1016/j.devcel.2008.07.002
87. Chu F, Hu Y, Zhou Y, Guo M, Lu J, Zheng W, et al. MicroRNA-126 deficiency enhanced the activation and function of CD4(+) T cells by elevating IRS-1 pathway. Clin Exp Immunol (2018) 191(2):166–79. doi: 10.1111/cei.13067
88. Hu L, Xu H, Lu J, Zhou Y, Chu F, Zheng W, et al. MicroRNA-126 deficiency affects the development of thymus CD4+ Single-positive cells through elevating IRS-1. Int Arch Allergy Immunol (2018) 177(3):207–18. doi: 10.1159/000490710
89. Kern F, Fehlmann T, Solomon J, Schwed L, Grammes N, Backes C, et al. miEAA 2.0: integrating multi-species microRNA enrichment analysis and workflow management systems. Nucleic Acids Res (2020) 48(W1):W521–8. doi: 10.1093/nar/gkaa309
90. Merkel O, Hamacher F, Laimer D, Sifft E, Trajanoski Z, Scheideler M, et al. Identification of differential and functionally active miRNAs in both anaplastic lymphoma kinase (ALK)+ and ALK- anaplastic large-cell lymphoma. Proc Natl Acad Sci United States America (2010) 107(37):16228–33. doi: 10.1073/pnas.1009719107
91. Morris SW, Kirstein MN, Valentine MB, Dittmer KG, Shapiro DN, Saltman DL, et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Sci (New York N.Y.) (1994) 263(5151):1281–4. doi: 10.1126/science.8122112
92. Lim MS, Carlson ML, Crockett DK, Fillmore GC, Abbott DR, Elenitoba-Johnson OF, et al. The proteomic signature of NPM/ALK reveals deregulation of multiple cellular pathways. Blood (2009) 114(8):1585–95. doi: 10.1182/blood-2009-02-204735
93. Spaccarotella E, Pellegrino E, Ferracin M, Ferreri C, Cuccuru G, Liu C, et al. STAT3-mediated activation of microRNA cluster 17~92 promotes proliferation and survival of ALK-positive anaplastic large cell lymphoma. Haematologica (2014) 99(1):116–24. doi: 10.3324/haematol.2013.088286
94. Oluwasanjo A, Kartan S, Johnson W, Alpdogan O, Gru A, Mishra A, et al. Peripheral T-cell lymphoma, not otherwise specified (PTCL-NOS). Cancer Treat Res (2019) 176:83–98. doi: 10.1007/978-3-319-99716-2_4
95. Zhang R, Su J, Xue S-L, Yang H, Ju L-L, Ji Y, et al. HPV E6/p53 mediated down-regulation of miR-34a inhibits the Warburg effect through targeting LDHA in cervical cancer. Am J Cancer Res (2016) 6(2):312–20. Available at: https://pubmed.ncbi.nlm.nih.gov/27186405/.
96. Wu K, Yang Y, Zhong Y, Ammar HM, Zhang P, Guo R, et al. The effects of microvesicles on endothelial progenitor cells are compromised in type 2 diabetic patients via downregulation of the miR-126/VEGFR2 pathway. Am J Physiol Endocrinol Metab (2016) 310(10):E828–37. doi: 10.1152/ajpendo.00056.2016
97. Du Y, Wei N, Ma R, Jiang S, Song D. A miR-210-3p regulon that controls the Warburg effect by modulating HIF-1α and p53 activity in triple-negative breast cancer. Cell Death Dis (2020) 11(9):731. doi: 10.1038/s41419-020-02952-6
98. Chu K, Gao G, Yang X, Ren S, Li Y, Wu H, et al. miR-512-5p induces apoptosis and inhibits glycolysis by targeting p21 in non-small cell lung cancer cells. Int J Oncol (2016) 48(2):577–86. doi: 10.3892/ijo.2015.3279
99. Yang Y, Ishak Gabra MB, Hanse EA, Lowman XH, Tran TQ, Li H, et al. MiR-135 suppresses glycolysis and promotes pancreatic cancer cell adaptation to metabolic stress by targeting phosphofructokinase-1. Nat Commun (2019) 10(1):809. doi: 10.1038/s41467-019-08759-0
100. Kim S, Lee E, Jung J, Lee JW, Kim HJ, Kim J, et al. microRNA-155 positively regulates glucose metabolism via PIK3R1-FOXO3a-cMYC axis in breast cancer. Oncogene (2018) 37(22):2982–91. doi: 10.1038/s41388-018-0124-4
101. Montes-Mojarro I-A, Steinhilber J, Griessinger CM, Rau A, Gersmann A-K, Kohlhofer U, et al. CD147 a direct target of miR-146a supports energy metabolism and promotes tumor growth in ALK+ ALCL. Leukemia (2022) 36(8):2050–63. doi: 10.1038/s41375-022-01617-x
102. Ullmann P, Qureshi-Baig K, Rodriguez F, Ginolhac A, Nonnenmacher Y, Ternes D, et al. Hypoxia-responsive miR-210 promotes self-renewal capacity of colon tumor-initiating cells by repressing ISCU and by inducing lactate production. Oncotarget (2016) 7(40):65454–70. doi: 10.18632/oncotarget.11772
103. Chen B, Liu Y, Jin X, Lu W, Liu J, Xia Z, et al. MicroRNA-26a regulates glucose metabolism by direct targeting PDHX in colorectal cancer cells. BMC Cancer (2014) 14:443. doi: 10.1186/1471-2407-14-443
104. Nguyen LH, Zhu H. Lin28 and let-7 in cell metabolism and cancer. Trans Pediatr (2015) 4(1):4–11. doi: 10.3978/j.issn.2224-4336.2015.01.05
105. Zhuang X, Chen Y, Wu Z, Xu Q, Chen M, Shao M, et al. Mitochondrial miR-181a-5p promotes glucose metabolism reprogramming in liver cancer by regulating the electron transport chain. Carcinogenesis (2020) 41(7):972–83. doi: 10.1093/carcin/bgz174
106. Wei Z, Cui L, Mei Z, Liu M, Zhang D. miR-181a mediates metabolic shift in colon cancer cells via the PTEN/AKT pathway. FEBS Lett (2014) 588(9):1773–9. doi: 10.1016/j.febslet.2014.03.037
107. Chu B, Wu T, Miao L, Mei Y, Wu M. MiR-181a regulates lipid metabolism via IDH1. Sci Rep (2015) 5:8801. doi: 10.1038/srep08801
108. Tanaka H, Sasayama T, Tanaka K, Nakamizo S, Nishihara M, Mizukawa K, et al. MicroRNA-183 upregulates HIF-1α by targeting isocitrate dehydrogenase 2 (IDH2) in glioma cells. J Neuro-Oncology (2013) 111(3):273–83. doi: 10.1007/s11060-012-1027-9
109. Song K, Liu C, Zhang J, Yao Y, Xiao H, Yuan R, et al. Integrated multi-omics analysis reveals miR-20a as a regulator for metabolic colorectal cancer. Heliyon (2022) 8(3):e09068. doi: 10.1016/j.heliyon.2022.e09068
110. Vose J, Armitage J, Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol (2008) 26(25):4124–30. doi: 10.1200/JCO.2008.16.4558
111. Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood (2016) 127(20):2375–90. doi: 10.1182/blood-2016-01-643569
112. de Mel S, Hue SS-S, Jeyasekharan AD, Chng W-J, Ng S-B. Molecular pathogenic pathways in extranodal NK/T cell lymphoma. J Hematol Oncol (2019) 12(1):33. doi: 10.1186/s13045-019-0716-7
113. Guo H-Q, Huang G-L, Guo C-C, Pu X-X, Lin T-Y. Diagnostic and prognostic value of circulating miR-221 for extranodal natural killer/T-cell lymphoma. Dis Markers (2010) 29(5):251–8. doi: 10.3233/DMA-2010-0755
114. Ng S-B, Yan J, Huang G, Selvarajan V, Tay JL-S, Lin B, et al. Dysregulated microRNAs affect pathways and targets of biologic relevance in nasal-type natural killer/T-cell lymphoma. Blood (2011) 118(18):4919–29. doi: 10.1182/blood-2011-07-364224
115. Ramakrishnan R, Donahue H, Garcia D, Tan J, Shimizu N, Rice AP, et al. Epstein-barr virus BART9 miRNA modulates LMP1 levels and affects growth rate of nasal NK T cell lymphomas. PloS One (2011) 6(11):e27271. doi: 10.1371/journal.pone.0027271
116. Huang W-T, Lin C-W. EBV-Encoded miR-BART20-5p and miR-BART8 Inhibit the IFN-γ–STAT1 Pathway Associated with Disease Progression in Nasal NK-Cell Lymphoma. Am J Pathol (2014) 184(4):1185–97. doi: 10.1016/j.ajpath.2013.12.024
117. Chen H-H, Huang W-T, Yang L-W, Lin C-W. The PTEN-AKT-mTOR/RICTOR Pathway in Nasal Natural Killer Cell Lymphoma Is Activated by miR-494-3p via PTEN But Inhibited by miR-142-3p via RICTOR. Am J Pathol (2015) 185(5):1487–99. doi: 10.1016/j.ajpath.2015.01.025
118. Alles J, Menegatti J, Motsch N, Hart M, Eichner N, Reinhardt R, et al. miRNA expression profiling of Epstein–Barr virus-associated NKTL cell lines by Illumina deep sequencing. FEBS Open Bio (2016) 6(4):251–63. doi: 10.1002/2211-5463.12027
119. Gao F, He S, Jin A. MiRNAs and lncRNAs in NK cell biology and NK/T-cell lymphoma. Genes Dis (2021) 8(5):590–602. doi: 10.1016/j.gendis.2020.08.006
120. Samimi S, Rook AH, Kim EJ. Update on epidemiology of cutaneous T-cell lymphoma. Curr Dermatol Rep (2013) 2(1):35–41. doi: 10.1007/s13671-012-0038-2
121. Manso R, Martínez-Magunacelaya N, Eraña-Tomás I, Monsalvez V, Rodríguez-Peralto JL, Ortiz-Romero P-L, et al. Mycosis fungoides progression could be regulated by microRNAs. PloS One (2018) 13(6):e0198477. doi: 10.1371/journal.pone.0198477
122. Narducci MG, Arcelli D, Picchio MC, Lazzeri C, Pagani E, Sampogna F, et al. MicroRNA profiling reveals that miR-21, miR486 and miR-214 are upregulated and involved in cell survival in Sézary syndrome. Cell Death Dis (2011) 2(4):e151. doi: 10.1038/cddis.2011.32
123. van Kester MS, Ballabio E, Benner MF, Chen XH, Saunders NJ, van der Fits L, et al. miRNA expression profiling of mycosis fungoides. Mol Oncol (2011) 5(3):273–80. doi: 10.1016/j.molonc.2011.02.003
124. Ralfkiaer U, Hagedorn PH, Bangsgaard N, Løvendorf MB, Ahler CB, Svensson L, et al. Diagnostic microRNA profiling in cutaneous T-cell lymphoma (CTCL). Blood (2011) 118(22):5891–900. doi: 10.1182/blood-2011-06-358382
125. Shen X, Wang B, Li K, Wang L, Zhao X, Xue F, et al. MicroRNA signatures in diagnosis and prognosis of cutaneous t-cell lymphoma. J Invest Dermatol (2018) 138(9):2024–-2032. doi: 10.1016/j.jid.2018.03.1500
126. Lindahl LM, Besenbacher S, Rittig AH, Celis P, Willerslev-Olsen A, Gjerdrum LMR, et al. Prognostic miRNA classifier in early-stage mycosis fungoides: development and validation in a Danish nationwide study. Blood (2018) 131(7):759–-770. doi: 10.1182/blood-2017-06-788950
127. Maj J, Jankowska-Konsur A, Sadakierska-Chudy A, Noga L, Reich A. Altered microRNA expression in mycosis fungoides. Br J Dermatol (2012) 166(2):331–-336. doi: 10.1111/j.1365-2133.2011.10669.x
128. Talaat IM, Abdelmaksoud RE, Guimei M, Agamia NF, Nugud A, El-Serafi AT. Potential role for microRNA-16 (miR-16) and microRNA-93 (miR-93) in diagnosis and prediction of disease progression in mycosis fungoides in Egyptian patients. PloS One (2019) 14(10):e0224305. doi: 10.1371/journal.pone.0224305
129. Dusílková N, Bašová P, Polívka J, Kodet O, Kulvait V, Pešta M, et al. Plasma miR-155, miR-203, and miR-205 are Biomarkers for Monitoring of Primary Cutaneous T-Cell Lymphomas. Int J Mol Sci (2017) 18(10):2136. doi: 10.3390/ijms18102136
130. Marosvári D, Téglási V, Csala I, Marschalko M, Bödör C, Timár B, et al. Altered microRNA expression in folliculotropic and transformed mycosis fungoides. Pathol Oncol Research: POR (2015) 21:821–5. doi: 10.1007/s12253-015-9897-8
131. Sørensen ST, Litman T, Gluud M, Celis P, Torres-Rusillo S, Willerslev-Olsen A, et al. miRNA signature in early-stage mycosis fungoides. Acta Dermato-Venereologica (2022) 102(SE-Articles):adv00785. doi: 10.2340/actadv.v102.628
132. Di Raimondo C, Han Z, Su C, Wu X, Qin H, Sanchez JF, et al. Identification of a distinct miRNA regulatory network in the tumor microenvironment of transformed mycosis fungoides. Cancers (2021) 13(22):5854. doi: 10.3390/cancers13225854
133. Ballabio E, Mitchell T, van Kester MS, Taylor S, Dunlop HM, Chi J, et al. MicroRNA expression in Sezary syndrome: identification, function, and diagnostic potential. Blood (2010) 116(7):1105–13. doi: 10.1182/blood-2009-12-256719
134. Fava P, Bergallo M, Astrua C, Brizio M, Galliano I, Montanari P, et al. miR-155 expression in primary cutaneous T-cell lymphomas (CTCL). J Eur Acad Dermatol Venereology: JEADV (2017) 31(1):e27–9. doi: 10.1111/jdv.13597
135. Rittig AH, Lindahl LM, Johansen C, Celis P, Ødum N, Iversen L, et al. The microRNA expression profile differs between erythrodermic mycosis fungoides and sézary syndrome. Acta Dermato-Venereologica (2019) 99(12):1148–-1153. doi: 10.2340/00015555-3306
136. Miyashiro D, Sanches JA. Mycosis fungoides and Sézary syndrome: clinical presentation, diagnosis, staging, and therapeutic management. Front Oncol (2023) 13:1141108. doi: 10.3389/fonc.2023.1141108
137. Cather JC, Vance EA, Menter MA. Diverse cutaneous manifestations associated with a single disease. Proc (Baylor University. Med Center) (2002) 15(4):433–6. doi: 10.1080/08998280.2002.11927876
138. Virmani P, Myskowski PL, Pulitzer M. Unusual variants of mycosis fungoides. Diagn Histopathology (Oxford England) (2016) 22(4):142–51. doi: 10.1016/j.mpdhp.2016.04.004
139. Gug G, Solovan C. From benign inflammatory dermatosis to cutaneous lymphoma. DNA copy number imbalances in mycosis fungoides versus large plaque parapsoriasis. Medicina (Kaunas Lithuania) (2021) 57(5):502. doi: 10.3390/medicina57050502
140. Marstrand T, Ahler CB, Ralfkiaer U, Clemmensen A, Kopp KL, Sibbesen NA, et al. Validation of a diagnostic microRNA classifier in cutaneous T-cell lymphomas. Leukemia lymphoma (2014) 55(4):957–8. doi: 10.3109/10428194.2013.815352
141. Ralfkiaer U, Lindahl LM, Litman T, Gjerdrum L-M, Ahler CB, Gniadecki R, et al. MicroRNA expression in early mycosis fungoides is distinctly different from atopic dermatitis and advanced cutaneous T-cell lymphoma. Anticancer Res (2014) 34(12):7207–17. Available at: https://pubmed.ncbi.nlm.nih.gov/25503151/.
142. Kang X, Kong B, Chen Q, Zhao S. Low expression of miR-138 inhibit the proliferation, migration and invasion of colorectal cancer and affect patient survival by targeting SIRT1. Trans Cancer Res (2021) 10(7):3548–59. doi: 10.21037/tcr-21-559
143. Salhany KE, Cousar JB, Greer JP, Casey TT, Fields JP, Collins RD. Transformation of cutaneous T cell lymphoma to large cell lymphoma. A clinicopathologic and immunologic study. Am J Pathol (1988) 132(2):265–77. Available at: https://pubmed.ncbi.nlm.nih.gov/3261136/.
144. Bontoux C, de Masson A, Thonnart N, Ram-Wolff C, Caraguel F, Batista L, et al. Large-cell transformation is an independent poor prognostic factor in Sézary syndrome: analysis of 117 cases. Br J Dermatol (2022) 187(5):815–7. doi: 10.1111/bjd.21738
145. Diamandidou E, Colome-Grimmer M, Fayad L, Duvic M, Kurzrock R. Transformation of mycosis fungoides/sezary syndrome: clinical characteristics and prognosis. Blood (1998) 92(4):1150–9. doi: 10.1182/blood.V92.4.1150
146. van der Fits L, van Kester MS, Qin Y, Out-Luiting JJ, Smit F, Zoutman WH, et al. MicroRNA-21 expression in CD4+ T cells is regulated by STAT3 and is pathologically involved in Sézary syndrome. J Invest Dermatol (2011) 131(3):762–8. doi: 10.1038/jid.2010.349
147. Gluud M, Willerslev-Olsen A, Gjerdrum LM, Lindahl LM, Buus TB, Andersen MH, et al. MicroRNAs in the pathogenesis, diagnosis, prognosis and targeted treatment of cutaneous T-cell lymphomas. Cancers (2020) 12(5):1229. doi: 10.3390/cancers12051229
148. Bacci M, Giannoni E, Fearns A, Ribas R, Gao Q, Taddei ML, et al. miR-155 drives metabolic reprogramming of ER+ Breast cancer cells following long-term estrogen deprivation and predicts clinical response to aromatase inhibitors. Cancer Res (2016) 76(6):1615–26. doi: 10.1158/0008-5472.CAN-15-2038
149. Zanoaga O, Braicu C, Chiroi P, Andreea N, Hajjar N, Mărgărit S, et al. The role of miR-155 in nutrition: modulating cancer-associated inflammation. Nutrients (2021) 13(7):1229. doi: 10.3390/nu13072245
150. Samad MA, Mahboob E, Shafiq A, Ur Rehman MH, Sheikh A, Tharwani ZH. Types of T-cell lymphoma-a cytogenetic perspective. Ann Med Surg (2012) (2022) 84:104844. doi: 10.1016/j.amsu.2022.104844
151. Herrera AF, Crosby-Thompson A, Friedberg JW, Abel GA, Czuczman MS, Gordon LI, et al. Comparison of referring and final pathology for patients with T-cell lymphoma in the National Comprehensive Cancer Network. Cancer (2014) 120(13):1993–9. doi: 10.1002/cncr.28676
152. Cirillo M, Craig AFM, Borchmann S, Kurtz DM. Liquid biopsy in lymphoma: Molecular methods and clinical applications. Cancer Treat Rev (2020) 91:102106. doi: 10.1016/j.ctrv.2020.102106
153. Ryu KJ, Lee JY, Choi ME, Yoon SE, Cho J, Ko YH, et al. Serum-derived exosomal microRNA profiles can predict poor survival outcomes in patients with extranodal natural killer/T-cell lymphoma. Cancers (2020) 12(12):3548. doi: 10.3390/cancers12123548
154. Casalone E, Birolo G, Pardini B, Allione A, Russo A, Catalano C, et al. Serum extracellular vesicle-derived microRNAs as potential biomarkers for pleural mesothelioma in a European prospective study. Cancers (2023) 15(1):125. doi: 10.3390/cancers15010125
155. Grimolizzi F, Monaco F, Leoni F, Bracci M, Staffolani S, Bersaglieri C, et al. Exosomal miR-126 as a circulating biomarker in non-small-cell lung cancer regulating cancer progression. Sci Rep (2017) 7(1):15277. doi: 10.1038/s41598-017-15475-6
156. Zhu X, Shen H, Yin X, Yang M, Wei H, Chen Q, et al. Macrophages derived exosomes deliver miR-223 to epithelial ovarian cancer cells to elicit a chemoresistant phenotype. J Exp Clin Cancer Res (2019) 38(1):81. doi: 10.1186/s13046-019-1095-1
157. Chang R-M, Fu Y, Zeng J, Zhu X-Y, Gao Y. Cancer-derived exosomal miR-197-3p confers angiogenesis via targeting TIMP2/3 in lung adenocarcinoma metastasis. Cell Death Dis (2022) 13(12):1032. doi: 10.1038/s41419-022-05420-5
158. Palmirotta R, Lovero D, Cafforio P, Felici C, Mannavola F, Pellè E, et al. Liquid biopsy of cancer: a multimodal diagnostic tool in clinical oncology. Ther Adv Med Oncol (2018) 10:1758835918794630. doi: 10.1177/1758835918794630
159. Alba-Bernal A, Lavado-Valenzuela R, Domínguez-Recio ME, Jiménez-Rodriguez B, Queipo-Ortuño MI, Alba E, et al. Challenges and achievements of liquid biopsy technologies employed in early breast cancer. EBioMedicine (2020) 62. doi: 10.1016/j.ebiom.2020.103100
160. Esagian SM, Grigoriadou G, Nikas IP, Boikou V, Sadow PM, Won J-K, et al. Comparison of liquid-based to tissue-based biopsy analysis by targeted next generation sequencing in advanced non-small cell lung cancer: a comprehensive systematic review. J Cancer Res Clin Oncol (2020) 146(8):2051–66. doi: 10.1007/s00432-020-03267-x
161. Nikanjam M, Kato S, Kurzrock R. Liquid biopsy: current technology and clinical applications. J Hematol Oncol (2022) 15(1):131. doi: 10.1186/s13045-022-01351-y
162. MacLellan SA, MacAulay C, Lam S, Garnis C. Pre-profiling factors influencing serum microRNA levels. BMC Clin Pathol (2014) 14:27. doi: 10.1186/1472-6890-14-27
163. Finkle JD, Boulos H, Driessen TM, Lo C, Blidner RA, Hafez A, et al. Validation of a liquid biopsy assay with molecular and clinical profiling of circulating tumor DNA. NPJ Precis Oncol (2021) 5(1):63. doi: 10.1038/s41698-021-00202-2
164. Tomasik B, Skrzypski M, Bieńkowski M, Dziadziuszko R, Jassem J. Current and future applications of liquid biopsy in non-small-cell lung cancer-a narrative review. Trans Lung Cancer Res (2023) 12(3):594–614. doi: 10.21037/tlcr-22-742
165. Weber JA, Baxter DH, Zhang S, Huang DY, Huang KH, Lee MJ, et al. The microRNA spectrum in 12 body fluids. Clin Chem (2010) 56(11):1733–41. doi: 10.1373/clinchem.2010.147405
166. Khare D, Goldschmidt N, Bardugo A, Gur-Wahnon D, Ben-Dov IZ, Avni B. Plasma microRNA profiling: Exploring better biomarkers for lymphoma surveillance. PloS One (2017) 12(11):e0187722. doi: 10.1371/journal.pone.0187722
167. Drobna M, Szarzyńska-Zawadzka B, Daca-Roszak P, Kosmalska M, Jaksik R, Witt M, et al. Identification of endogenous control miRNAs for RT-qPCR in T-cell acute lymphoblastic leukemia. Int J Mol Sci (2018) 19(10):2858. doi: 10.3390/ijms19102858
168. Ouyang T, Liu Z, Han Z, Ge Q. MicroRNA detection specificity: recent advances and future perspective. Analytical Chem (2019) 91(5):3179–86. doi: 10.1021/acs.analchem.8b05909
169. Borel F, Konstantinova P, Jansen PLM. Diagnostic and therapeutic potential of miRNA signatures in patients with hepatocellular carcinoma. J Hepatol (2012) 56(6):1371–83. doi: 10.1016/j.jhep.2011.11.026
Keywords: T-cell malignancies, MicroRNAs, diagnosis, lymphomas, prognosis, metabolism
Citation: Mondal D, Shinde S, Paul S, Thakur S, Velu GSK, Tiwari AK, Dixit V, Amit A, Vishvakarma NK and Shukla D (2023) Diagnostic significance of dysregulated miRNAs in T-cell malignancies and their metabolic roles. Front. Oncol. 13:1230273. doi: 10.3389/fonc.2023.1230273
Received: 28 May 2023; Accepted: 17 July 2023;
Published: 10 August 2023.
Edited by:
Nelida Ines Noguera, University of Rome Tor Vergata, ItalyReviewed by:
Zi-jun Xu, Jiangsu University Affiliated People’s Hospital, ChinaElisabetta De Marinis, Sapienza University of Rome, Italy
Copyright © 2023 Mondal, Shinde, Paul, Thakur, Velu, Tiwari, Dixit, Amit, Vishvakarma and Shukla. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dhananjay Shukla, c2RoYW5udUBnbWFpbC5jb20=
 Deepankar Mondal
Deepankar Mondal Sapnita Shinde
Sapnita Shinde Souvik Paul
Souvik Paul Suresh Thakur
Suresh Thakur GSK Velu3
GSK Velu3 Atul Kumar Tiwari
Atul Kumar Tiwari Vineeta Dixit
Vineeta Dixit Ajay Amit
Ajay Amit Naveen Kumar Vishvakarma
Naveen Kumar Vishvakarma Dhananjay Shukla
Dhananjay Shukla