
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 16 August 2023
Sec. Thoracic Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1230074
This article is part of the Research Topic New Progress in the Treatment of Bone and Soft Tissue Tumors View all 11 articles
 Shinji Miwa1*
Shinji Miwa1* Norio Yamamoto1
Norio Yamamoto1 Katsuhiro Hayashi1
Katsuhiro Hayashi1 Akihiko Takeuchi1
Akihiko Takeuchi1 Kentaro Igarashi1
Kentaro Igarashi1 Yuta Taniguchi1
Yuta Taniguchi1 Sei Morinaga1
Sei Morinaga1 Yohei Asano1
Yohei Asano1 Takayuki Nojima1,2
Takayuki Nojima1,2 Hiroyuki Tsuchiya1
Hiroyuki Tsuchiya1Giant cell tumors of bone (GCTB) sometimes metastasize to distant organs. In this case report, we present pulmonary metastases of GCTB mimicking malignancies. A 49-year-old man underwent two surgical treatments for a GCTB of the right proximal radius. At the time of the second surgery, no lesions were observed on chest radiography. Three years after surgery, the patient presented with cough and dyspnea, and chest radiography and computed tomography (CT) revealed multiple lung nodules. Positron emission tomography/CT revealed a high accumulation of 18F-fluoro-2-deoxy-D-glucose (18F-FDG) in multiple lesions. Based on the rapid growth and accumulation of 18F-FDG, a metastatic malignant tumor was suspected. CT-guided needle biopsy was performed, and the histology showed proliferation of spindle cells and multinuclear giant cells without malignant changes. Denosumab was administered because multiple lung lesions were unresectable. One month after denosumab treatment, CT showed marked shrinkage of the lesions, and the symptoms significantly improved. Eighteen months after the initial treatment with denosumab, the patient had no symptoms or tumor growth. Although its long-term efficacy and safety remain unclear, denosumab may be a treatment option for patients with unresectable pulmonary GCTB.
Giant cell tumor of bone (GCTB), a locally aggressive and rarely metastasizing tumor, is classified as intermediate malignancies according to the 2020 World Health Organization (WHO) classification (1–3). Histologically, GCTB consist of ovoid mononuclear cells and giant osteoclast-like cells (4, 5). Osteoclast-like multinuclear cells and their precursors express receptor activator of nuclear factor-kappa B (RANK), whereas mononuclear stromal cells express RANKL, which is necessary for the formation, function, and survival of the osteoclasts (6–9).
The standard treatment for GCTB is surgical excision, which consists of curettage and en bloc resection (10). In cases of unresectable GCTB, denosumab, a human monoclonal antibody targeting the nuclear factor-kappa B ligand (RANKL), is considered a treatment option (5, 11, 12). Denosumab binds to RANKL and blocks its binding to RANK on osteoclasts and osteoclast precursors, resulting in the inhibition of osteoclast differentiation and bone resorption by the osteoclasts (5, 12). Denosumab is widely used to prevent hypercalcemia, pathological fractures, and spinal cord compression in patients with metastatic bone diseases (13–15). Furthermore, high response rates have been reported in clinical studies on denosumab in patients with GCTB (5, 11). In a phase 2 study of denosumab in patients with GCTB, patients received 120 mg of subcutaneous denosumab (every 4 weeks with a loading dose on days 8 and 15 of the first cycle) (16). In this study, 163 of 169 (96%) patients were progression-free after a median follow-up of 13 months (16). In another study including 43 patients with resectable GCTB and 54 patients with unresectable GCTB, all tumors were controlled by denosumab treatment, whereas 40% of patients who discontinued denosumab showed tumor progression after a median of 8 months (12). Furthermore, it is reported that neoadjuvant treatment with denosumab can downstage the lesions by increasing the thickness of cortical bone and forming new cortical rim around the soft-tissue mass that facilitates joint salvage and decrease surgery invasiveness (1, 17–23). Although denosumab does not have direct cytotoxic effect on neoplastic stromal cells, it can inhibit pulmonary metastases. Denosumab prevents RANKL-mediated formation and activation of multinucleated giant cells from RANK-positive mononuclear preosteoclasts and macrophages, resulting in marked reduction in multinucleated giant cells (5, 24–26). Although denosumab is considered an effective treatment option for patients with unresectable GCTB, the indications, doses, and periods of denosumab use in metastatic GCTB remain unclear. Therefore, investigations into treatment strategies for unresectable metastatic GCTB are required.
In the management of malignant tumors, 18F-fluoro-2-deoxy-D-glucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) is one of the most useful diagnostic tools to assess grading, staging, therapeutic response, surgical planning, and expected prognosis (27). PET/CT can be used to differentiate between malignant and benign lesions (28). However, GCTB has high accumulation of 18F-FDG (27), and the high accumulation of 18F-FDG may mislead the diagnosis of the tumor (29–31). In this report, we present a case of pulmonary metastasis of GCTB that mimicked malignancies on radiographic examinations, which was successfully controlled by treatment with denosumab.
A 49-year-old man presented with right elbow pain. Radiography revealed osteolytic lesions and scalloping in the right proximal radius (Figure 1A). Magnetic resonance imaging showed a tumor lesion in the proximal radius with iso intensity on T1-weighted images and high intensity on T2-weighted images, and the lesion was enhanced by gadolinium (Figure 1B). 201Thallium (201Tl) scintigraphy and 99mTc-methylene diphosphonate (99mTc-MDP) scintigraphy showed an increased uptake of the 201Tl and 99mTc-MDP in the proximal radius. Open biopsy was performed, and the histology of the tumor showed proliferation of spindle cells and multinuclear osteoclast-like cells with collagen fibers and deposition of hemosiderin (Figure 1C). Immunohistological staining showed positivity for H3.3G34W in the tumor cells (Figure 1D). Based on the histological findings, the tumor was diagnosed as a GCTB. He underwent curettage with adjuvant treatment with ethanol and phenol, and the bone defect was augmented with α-tricalcium phosphate (αTCP) (Figure 1E). Two years after the initial surgery, radiography and CT revealed tumor recurrence. He underwent denosumab treatment (120 mg on days 1, 8, 15, 29, 56, and 84), curettage with adjuvant treatment with ethanol and phenol, and artificial bone grafting using α-TCP. Histological examination of the tumor specimen showed proliferation of spindle cells and multinuclear giant cells, consistent with GCTB recurrence. At the time of the second surgery, no pulmonary nodules were detected on chest radiography (Figure 2A).
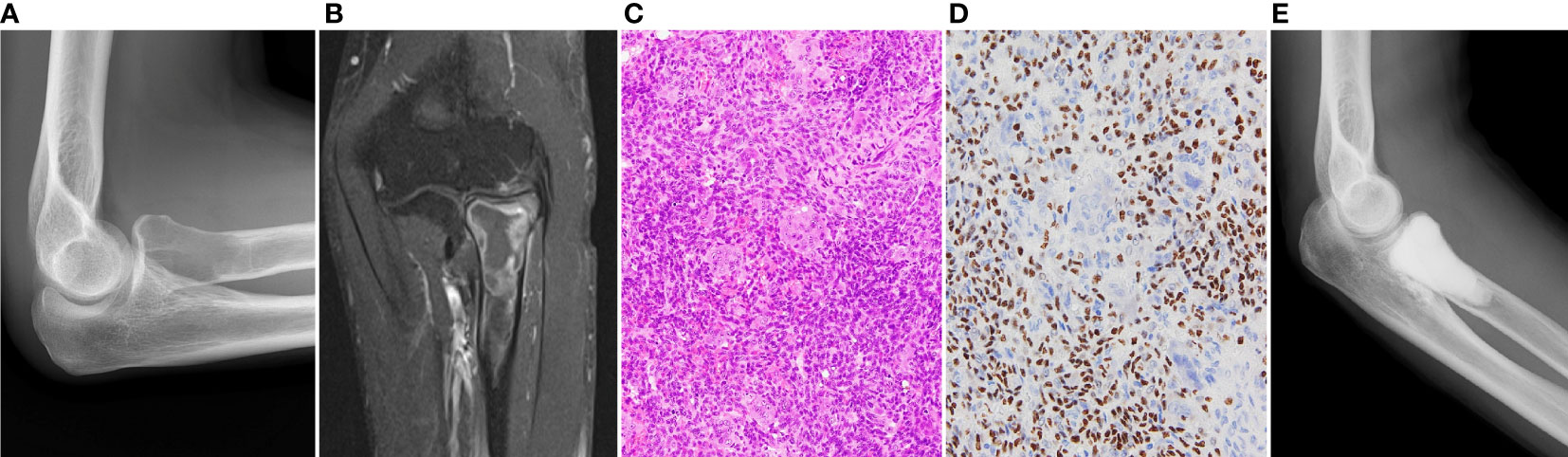
Figure 1 (A) Initial radiography shows an osteolytic lesion in the proximal radius. (B) In enhanced magnetic resonance imaging, the lesion is enhanced by gadolinium. (C) Histology of the specimen of the radius shows proliferation of spindle cells and multinucleated osteoclast-like cells, which is consistent with diagnosis of GCTB. (D) Immunohistological staining of H3.3G34W. (E) The lesion is curetted and augmented with α-tricalcium phosphate.
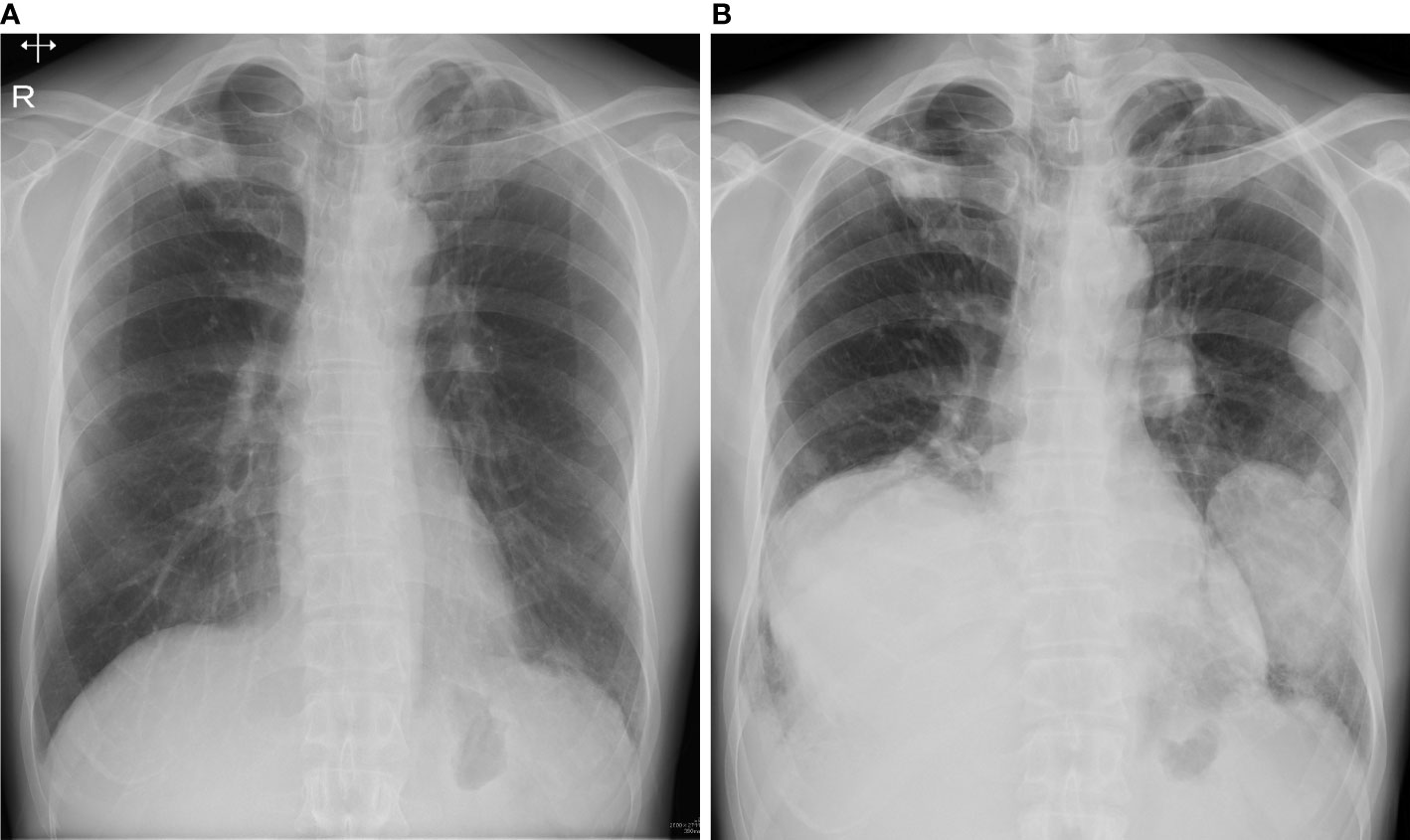
Figure 2 (A) At the time of second surgery, no pulmonary lesion was detected on radiography. (B) Three years after the second surgery, radiography showed multiple lung nodules.
Three years after the second surgery, the patient presented with cough and dyspnea. Radiography and CT revealed 31 nodules, including the largest lesion (15 × 13 cm) in the lungs (Figure 2B). PET/CT revealed a markedly increased uptake of 18F-FDG in the lesions (SUVmax = 11.8–12.2) (Figures 3A–C). Based on the clinical course and radiological findings, malignant metastasis was suspected. CT-guided needle biopsy was performed to confirm the diagnosis of multiple lung lesions. Histological examination of the specimen revealed proliferation of spindle cells and multinuclear osteoclast-like cells without malignant changes (Figure 3D), and the tumor cells were positive for H3.3G34W on immunohistological staining (Figure 3E). These findings were similar to the histology of the primary lesions of the proximal radius, and the lung lesions were diagnosed as multiple metastases from GCTB. Because the lung nodules were thought to be unresectable, he was treated with denosumab (120 mg subcutaneously on days 1, 8, 15, and 29 and every 4 weeks thereafter). One month after the initiation of denosumab treatment, shrinkages of the lung nodules and gradual improvement in pulmonary symptoms were observed (Figure 4). Then, the denosumab treatment continued every 4 weeks. During the denosumab treatment, the patient underwent chest X-ray every month, blood examination every 3 months, and chest CT every 6 months. After 3 months of denosumab treatment, the patient achieved a partial response according to the Response Evaluation Criteria in Solid Tumors criteria (32). During the treatment 7period, X-ray and CT showed no regrowth of the tumor, and there were no adverse events, such as hypocalcemia and osteoporosis of the jaw. Eighteen months after the initial treatment with denosumab, the patient showed no symptoms or disease progression and continued to undergo denosumab treatment (Figure 4).
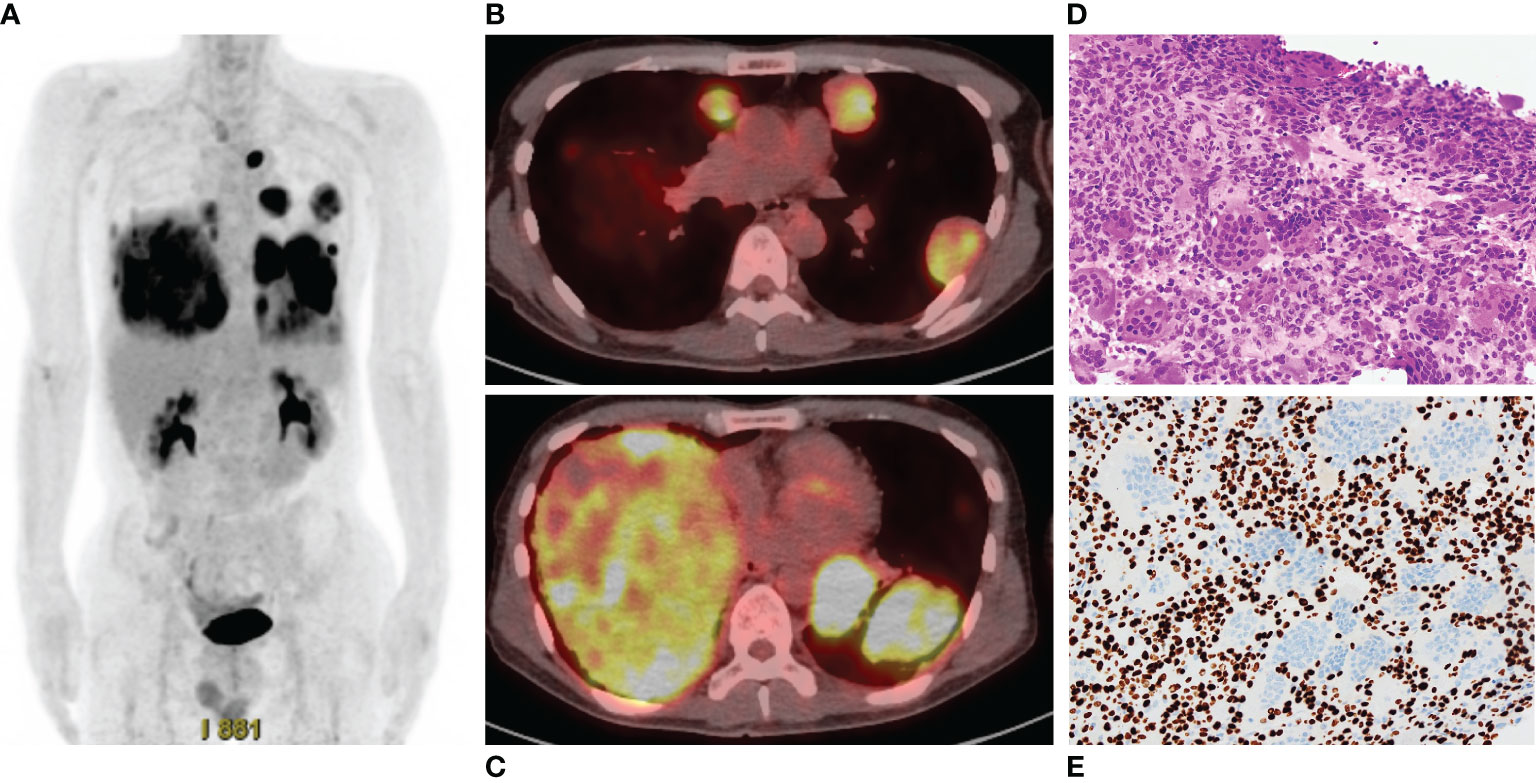
Figure 3 (A) Positron emission tomography/computed tomography shows high accumulation of 18F-fluoro-2-deoxy-D-glucose in the multiple lung lesions. (B, C). The maximum standardized uptake values are 11.8–12.2. (D) Histology of specimen of the lung nodule shows proliferation of spindle cells and multinuclear cells, which is similar to the findings of specimen of the radius. (E) Immunohistological staining of H3.3G34W.
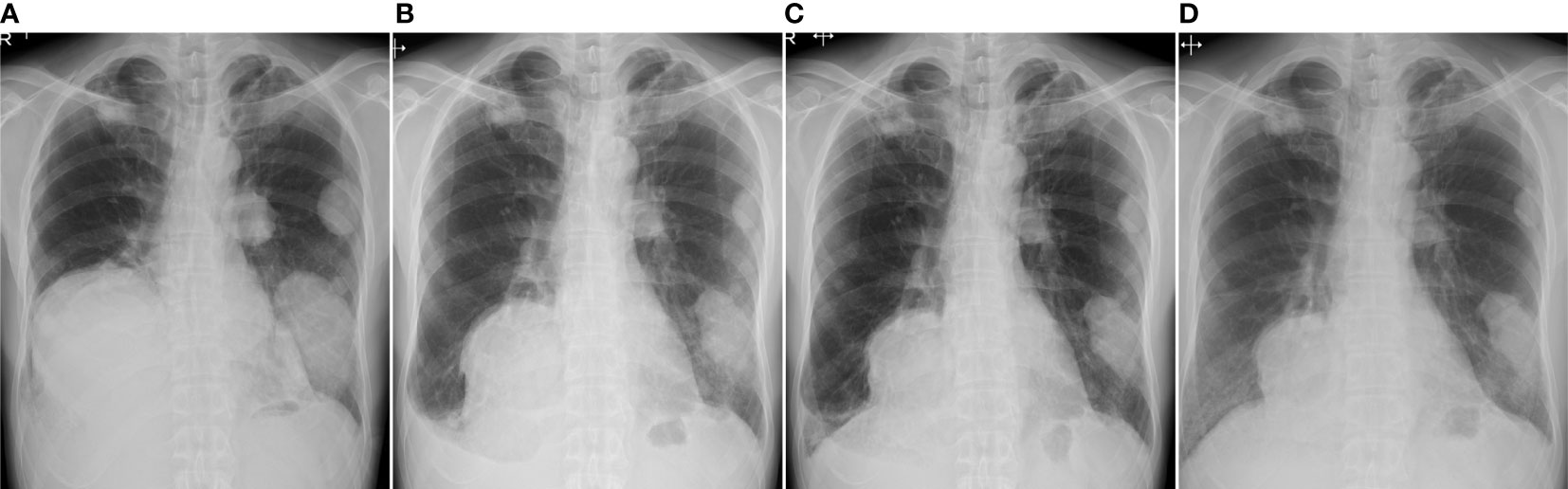
Figure 4 Radiography examination before treatment (A), 1 month (B), 3 months (C), and 18 months (D) after the initial treatment of denosumab. The pulmonary lesions show response to the treatment of denosumab.
In this report, we presented a case of multiple pulmonary metastases of GCTB. In this case, multiple pulmonary lesions were unresectable; however, these lesions were successfully controlled with denosumab treatment. The incidence of metastasis in GCTB has been reported to be 1–9% (33–37), and the lung is the most common site of metastasis, followed by the brain, kidney, bone, skin, and lymph nodes (34). Although most metastatic lung lesions of GCTB are slow-growing and can be controlled by tumor resection or observation (10, 33), some metastatic GCTB are aggressive and causes mortality (10, 38, 39). Although our follow-up protocol for patients with GCTB did not include chest X-ray, addition of chest X-ray or CT to the follow-up protocol is recommend to diagnose pulmonary metastases of GCTB earlier. Tsukamoto et al. reported that follow-up protocol after surgical treatment for GCTB included chest X-ray or CT every 4 months for the first 2 years, every 6 months for the next 3 years, and then annually (10). Chan et al. investigated the risk factors for pulmonary metastasis from GCTB in 291 patients with benign GCTB (39). In this study, only local recurrence was an independent risk factor for pulmonary metastases. Tsukamoto et al. investigated the outcomes and safety of initial observations in patients with pulmonary metastases of GCTB (10). In this study, 46% of patients with lung lesions ≤ 5 mm and all patients with lung lesions > 5 mm had disease progression. The patients with lung lesions ≤ 5 mm had significantly better progression-free survival than those with lung lesions > 5 mm (p = 0.022). Initial observation may be an option for patients with small pulmonary lesions. However, the present case required treatment because the pulmonary metastases were large and symptomatic.
In this case, clinical course and high accumulation of 18F-FDG in PET/CT mimicked malignancies. In previous reports, GCTB had higher accumulation of 18F-FDG (mean SUVmax = 8.4–16.8) than other benign lesions (28, 40–43). Uptake of FDG in tumor cells is associated with expression of glucose transporter protein (GLUT)-1, hexokinase II, and with gene upregulation for these proteins (44, 45). Ong et al. reported significantly greater GLUT-1 and hexokinase II in human cancer cell lines (46). On the other hand, other studies showed upregulation of GLUT-1 in monocyte-derived macrophages (47–49), and the high 18F-FDG uptake may be explained by high monocyte/macrophage content within GCTs (44, 50). Based on the reports, GCT and other lesions containing active macrophages should be considered in differential diagnoses of lesions with high accumulation of 18F-FDG.
There are several reports on systemic treatment for metastatic pulmonary GCTB (Table 1) (51–60). In a retrospective study of denosumab treatment in 7 patients with pulmonary GCTB, 3 patients showed partial response and 4 patients had stable disease (54). Based on the previous reports, denosumab can be one of the treatment options in patients with unresectable pulmonary GCTB (54, 56, 59, 60). However, denosumab can cause severe side effects, including hypocalcemia, hypophosphatemia, increased risk of atypical femoral fracture, and osteonecrosis of the jaw (5, 16). Therefore, long-term treatment with denosumab is not ideal therapeutic option, and discontinuation, dose reduction, or extension of the treatment interval, may be needed to avoid severe adverse effects of denosumab. Although denosumab can reduce tumor size by inhibiting osteoclastic differentiation and reducing giant cells, it is not cytocidal in the neoplastic stromal cells of GCTB, and discontinuation of the treatment may cause regrowth of the tumor (12). Tanikawa et al. reported a case treated with extended interval of the denosumab treatment for GCTB in the sphenoid bone (61). In their report, denosumab treatment (120 mg) for the first 2 years was performed every 4 weeks. Subsequently, the treatment interval was gradually extended, with 4 monthly dosing for the next 1 year, followed by a 6 monthly dosing for 2 years. During the extension of the treatment interval, slight growth of the lesion was observed, but it was thought to be acceptable range. They concluded that optimal extended dosing interval of denosumab treatment after achieving the stabilization of GCTB was 6 months. Thus, reducing the dose or extending the dosing interval as much as possible is desirable for patients who are unable to discontinue the medication.
In this case report, we present the pulmonary metastases from a GCTB mimicking malignancy. PET/CT revealed a high accumulation of 18F-FDG in the lesions. A metastatic malignant tumor was suspected in this case; however, histological examination revealed a metastatic GCTB without malignant changes. Although the pulmonary nodules were unresectable, they were controlled with denosumab. Denosumab may be a treatment option for patients with unresectable GCTB metastases. Further studies on dose reduction or extension of the treatment interval of denosumab treatment are demanded.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethical review and approval were not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided written informed consent to participate in this study. Written informed consent was obtained from the patients to publish any potentially identifiable images or data in this article.
The manuscript was drafted by ShM, NY, and HT. ShM, NY, KH, AT, KI, YT, SeM and YA examined and treated the patient. TN performed the histopathological assessment. NY, TN, and HT supervised this study. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Puri A, Gulia A, Hegde P, Verma V, Rekhi B. Neoadjuvant denosumab: its role and results in operable cases of giant cell tumour of bone. Bone Joint J (2019) 101-B:170–7. doi: 10.1302/0301-620X.101B2.BJJ-2018-0907.R2
2. Tsukamoto S, Mavrogenis AF, Kido A, Errani C. Current Concepts in the Treatment of Giant Cell Tumors of Bone. Cancers (Basel) (2021) 13:3647. doi: 10.3390/cancers13153647
3. WHO Classification of Tumours Editorial Board. WHO Classification of Tumours of Soft Tissue and Bone. 5th ed. Lyon, France: IARC Press (2020).
4. Bullough BM. Malignancy in giant cell tumour. World Health Organization classification of tumours. In: pathology and genetics of tumours of soft tissue and bone. Lyon: International Agency for Research on Cancer (IARC (2002).
5. Thomas D, Henshaw R, Skubitz K, Chawla S, Staddon A, Blay J-Y, et al. Denosumab in patients with giant-cell tumour of bone: an open-label, phase 2 study. Lancet Oncol (2010) 11:275–80. doi: 10.1016/S1470-2045(10)70010-3
6. Fuller K, Wong B, Fox S, Choi Y, Chambers TJ. TRANCE is necessary and sufficient for osteoblast-mediated activation of bone resorption in osteoclasts. J Exp Med (1998) 188:997–1001. doi: 10.1084/jem.188.5.997
7. Huang L, Xu J, Wood DJ, Zheng MH. Gene expression of osteoprotegerin ligand, osteoprotegerin, and receptor activator of NF-kappaB in giant cell tumor of bone: possible involvement in tumor cell-induced osteoclast-like cell formation. Am J Pathol (2000) 156:761–7. doi: 10.1016/S0002-9440(10)64942-5
8. Lacey DL, Tan HL, Lu J, Kaufman S, Van G, Qiu W, et al. Osteoprotegerin ligand modulates murine osteoclast survival in vitro and in vivo. Am J Pathol (2000) 157:435–48. doi: 10.1016/S0002-9440(10)64556-7
9. Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell (1998) 93:165–76. doi: 10.1016/S0092-8674(00)81569-X
10. Tsukamoto S, Ciani G, Mavrogenis AF, Ferrari C, Akahane M, Tanaka Y, et al. Outcome of lung metastases due to bone giant cell tumor initially managed with observation. J Orthop Surg Res (2020) 15:510. doi: 10.1186/s13018-020-02038-1
11. Chawla S, Blay J-Y, Rutkowski P, Le Cesne A, Reichardt P, Gelderblom H, et al. Denosumab in patients with giant-cell tumour of bone: a multicentre, open-label, phase 2 study. Lancet Oncol (2019) 20:1719–29. doi: 10.1016/S1470-2045(19)30663-1
12. Palmerini E, Chawla NS, Ferrari S, Sudan M, Picci P, Marchesi E, et al. Denosumab in advanced/unresectable giant-cell tumour of bone (GCTB): For how long? Eur J Cancer (2017) 76:118–24. doi: 10.1016/j.ejca.2017.01.028
13. Bekker PJ, Holloway DL, Rasmussen AS, Murphy R, Martin SW, Leese PT, et al. A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. J Bone Miner Res (2004) 19:1059–66. doi: 10.1359/JBMR.040305
14. McClung MR, Lewiecki EM, Cohen SB, Bolognese MA, Woodson GC, Moffett AH, et al. Denosumab in postmenopausal women with low bone mineral density. N Engl J Med (2006) 354:821–31. doi: 10.1056/NEJMoa044459
15. Lipton A, Steger GG, Figueroa J, Alvarado C, Solal-Celigny P, Body JJ, et al. Randomized active-controlled phase II study of denosumab efficacy and safety in patients with breast cancer-related bone metastases. J Clin Oncol (2007) 25:4431–7. doi: 10.1200/JCO.2007.11.8604
16. Chawla S, Henshaw R, Seeger L, Choy E, Blay J-Y, Ferrari S, et al. Safety and efficacy of denosumab for adults and skeletally mature adolescents with giant cell tumour of bone: interim analysis of an open-label, parallel-group, phase 2 study. Lancet Oncol (2013) 14:901–8. doi: 10.1016/S1470-2045(13)70277-8
17. Liang H, Liu X, Yang Y, Guo W, Yang R, Tang X, et al. Ultra-Short Course of Neo-Adjuvant Denosumab for Nerve-Sparing Surgery for Giant Cell Tumor of Bone in Sacrum. Spine (Phila Pa (2022) 1976) 47:691–701. doi: 10.1097/BRS.0000000000004318
18. Traub F, Singh J, Dickson BC, Leung S, Mohankumar R, Blackstein ME, et al. Efficacy of denosumab in joint preservation for patients with giant cell tumour of the bone. Eur J Cancer (2016) 59:1–12. doi: 10.1016/j.ejca.2016.01.006
19. Muller DA, Beltrami G, Scoccianti G, Campanacci DA, Franchi A, Capanna R. Risks and benefits of combining denosumab and surgery in giant cell tumor of bone-a case series. World J Surg Oncol (2016) 14:281. doi: 10.1186/s12957-016-1034-y
20. Errani C, Tsukamoto S, Leone G, Righi A, Akahane M, Tanaka Y, et al. Denosumab may increase the risk of local recurrence in patients with giant-cell tumor of bone treated with curettage. J Bone Joint Surg Am (2018) 100:496–504. doi: 10.2106/JBJS.17.00057
21. Chinder PS, Hindiskere S, Doddarangappa S, Pal U. Evaluation of local recurrence in giant-cell tumor of bone treated by neoadjuvant denosumab. Clin Orthop Surg (2019) 11:352–60. doi: 10.4055/cios.2019.11.3.352
22. Hindiskere S, Errani C, Doddarangappa S, Ramaswamy V, Rai M, Chinder PS. Is a short-course of preoperative denosumab as effective as prolonged therapy for giant cell tumor of bone? Clin Orthop Relat Res (2020) 478:2522–33. doi: 10.1097/CORR.0000000000001285
23. Treffel M, Lardenois E, Larousserie F, Karanian M, Gomez-Brouchet A, Bouvier C, et al. Denosumab-treated giant cell tumors of bone: a clinicopathologic analysis of 35 cases from the french group of bone pathology. Am J Surg Pathol (2020) 44:1–10. doi: 10.1097/PAS.0000000000001388
24. Lewiecki EM. Clinical use of denosumab for the treatment for postmenopausal osteoporosis. Curr Med Res Opin (2010) 26:2807–12. doi: 10.1185/03007995.2010.533651
25. Branstetter DG, Nelson SD, Manivel JC, Blay JY, Chawla S, Thomas DM, et al. Denosumab induces tumor reduction and bone formation in patients with giant-cell tumor of bone. Clin Cancer Res (2012) 18:4415–24. doi: 10.1158/1078-0432.CCR-12-0578
26. Rekhi B, Verma V, Gulia A, Jambhekar NA, Desai S, Juvekar SL, et al. Clinicopathological features of a series of 27 cases of post-denosumab treated giant cell tumors of bones: a single institutional experience at a tertiary cancer referral centre, india. Pathol Oncol Res (2017) 23:157–64. doi: 10.1007/s12253-016-0123-0
27. Hoshi M, Takada J, Oebisu N, Hata K, Ieguchi M, Nakamura H. Overexpression of hexokinase-2 in giant cell tumor of bone is associated with false positive in bone tumor on FDG-PET/CT. Arch Orthop Trauma Surg (2012) 132:1561–8. doi: 10.1007/s00402-012-1588-2
28. Miwa S, Mochizuki T, Yamamoto N, Shirai T, Hayashi K, Takeuchi A, et al. Efficacy and limitations of f-18-fluoro-2-deoxy-d-glucose positron emission tomography to differentiate between malignant and benign bone and soft tissue tumors. Anticancer Res (2018) 38:4065–72. doi: 10.21873/anticanres.12696
29. Aoki J, Watanabe H, Shinozaki T, Takagishi K, Ishijima H, Oya N, et al. FDG PET of primary benign and malignant bone tumors: standardized uptake value in 52 lesions. Radiology (2001) 219:774–7. doi: 10.1148/radiology.219.3.r01ma08774
30. Zhang Y, Reeve IP, Lewis DH. A case of giant cell tumor of sacrum with unusual pulmonary metastases: CT and FDG PET findings. Clin Nucl Med (2012) 37:920–1. doi: 10.1097/RLU.0b013e31825b2441
31. Makis W, Alabed YZ, Nahal A, Novales-Diaz JA, Hickeson M. Giant cell tumor pulmonary metastases mimic primary malignant pulmonary nodules on (18)F-FDG PET/CT. Nucl Med Mol Imag (2012) 46:134–7. doi: 10.1007/s13139-012-0134-z
32. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer (2009) 45:228–47. doi: 10.1016/j.ejca.2008.10.026
33. Dominkus M, Ruggieri P, Bertoni F, Briccoli A, Picci P, Rocca M, et al. Histologically verified lung metastases in benign giant cell tumours–14 cases from a single institution. Int Orthop (2006) 30:499–504. doi: 10.1007/s00264-006-0204-x
34. Gupta R, Seethalakshmi V, Jambhekar NA, Prabhudesai S, Merchant N, Puri A, et al. Clinicopathologic profile of 470 giant cell tumors of bone from a cancer hospital in western India. Ann Diagn Pathol (2008) 12:239–48. doi: 10.1016/j.anndiagpath.2007.09.002
35. Siebenrock KA, Unni KK, Rock MG. Giant-cell tumour of bone metastasising to the lungs. J Bone Joint Surg Br (1998) 80-B:43–7. doi: 10.1302/0301-620X.80B1.0800043
36. Tubbs WS, Brown LR, Beabout JW, Rock MG, Unni KK. Benign giant-cell tumor of bone with pulmonary metastases: clinical findings and radiologic appearance of metastases in 13 cases. AJR Am J Roentgenol (1992) 158:331–4. doi: 10.2214/ajr.158.2.1729794
37. Rosario M, Kim HS, Yun JY, Han I. Surveillance for lung metastasis from giant cell tumor of bone. J Surg Oncol (2017) 116:907–13. doi: 10.1002/jso.24739
38. Katz E, Nyska M, Okon E, Zajicek G, Robin G. Growth rate analysis of lung metastases from histologically benign giant cell tumor of bone. Cancer (1987) 59:1831–6. doi: 10.1002/1097-0142(19870515)59:10<1831::AID-CNCR2820591025>3.0.CO;2-A
39. Chan CM, Adler Z, Reith JD, Gibbs CP Jr. Risk factors for pulmonary metastases from giant cell tumor of bone. J Bone Joint Surg Am (2015) 97:420–8. doi: 10.2106/JBJS.N.00678
40. Hayashida K, Kawabata Y, Kato I, Kamiishi T, Matsuo K, Takeyama M, et al. Clinical and pathological analysis of giant cell tumor of bone with denosumab treatment and local recurrence. J Orthop Sci (2022) 27:215–21. doi: 10.1016/j.jos.2020.11.005
41. Hakozaki M, Tajino T, Yamada H, Hasegawa O, Tasaki K, Watanabe K, et al. Radiological and pathological characteristics of giant cell tumor of bone treated with denosumab. Diagn Pathol (2014) 9:111. doi: 10.1186/1746-1596-9-111
42. Engellau J, Seeger L, Grimer R, Henshaw R, Gelderblom H, Choy E, et al. Assessment of denosumab treatment effects and imaging response in patients with giant cell tumor of bone. World J Surg Oncol (2018) 16:191. doi: 10.1186/s12957-018-1478-3
43. Park HL, Yoo IR, Lee Y, Park SY, Jung CK. Giant cell tumor of the rib: two cases of F-18 FDG PET/CT findings. Nucl Med Mol Imaging (2017) 51:182–5. doi: 10.1007/s13139-016-0442-9
44. Selby L, Kukar M, Wang J, Beg M, Sullivan J. Pigmented villous nodular synovitis mimicking metastatic melanoma on PET-CT. Int J Surg Case Rep (2014) 5:231–3. doi: 10.1016/j.ijscr.2014.02.009
45. Hamada K, Tomita Y, Qiu Y, Zhang B, Ueda T, Myoui A, et al. 18F-FDG-PET of musculoskeletal tumors: a correlation with the expression of glucose transporter 1 and hexokinase II. Ann Nucl Med (2008) 22:699–705. doi: 10.1007/s12149-008-0173-9
46. Ong LC, Jin Y, Song IC, Yu S, Zhang K, Chow PK. 2-[18F]-2-deoxy-D-glucose (FDG) uptake in human tumor cells is related to the expression of GLUT-1 and hexokinase II. Acta Radiol (2008) 49:1145–53. doi: 10.1080/02841850802482486
47. Burke B, Giannoudis A, Corke KP, Gill D, Wells M, Ziegler-Heitbrock L, et al. Hypoxia-induced gene expression in human macrophages: implications for ischemic tissues and hypoxia-regulated gene therapy. Am J Pathol (2003) 163:1233–43. doi: 10.1016/S0002-9440(10)63483-9
48. Malide D, Davies-Hill TM, Levine M, Simpson IA. Distinct localization of GLUT-1, -3, and -5 in human monocyte-derived macrophages: effects of cell activation. Am J Physiol (1998) 274:E516–26. doi: 10.1152/ajpendo.1998.274.3.E516
49. Fu Y, Maianu L, Melbert BR, Garvey WT. Facilitative glucose transporter gene expression in human lymphocytes, monocytes, and macrophages: a role for GLUT isoforms 1, 3, and 5 in the immune response and foam cell formation. Blood Cells Mol Dis (2004) 32:182–90. doi: 10.1016/j.bcmd.2003.09.002
50. Pallas A, Hagge R, Borys D, Hunter J. Intense FDG uptake in an intra-articular localized giant-cell tumor of the tendon sheath (pigmented villonodular synovitis) mimics metastatic melanoma. Radiol Case Rep (2009) 4:343. doi: 10.2484/rcr.v4i4.343
51. Gong T, Luo Y, Wang Y, Zheng C, Fang J, Min L, et al. Multiple pulmonary metastases of recurrent giant cell tumor of bone with expression of VEGFR-2 successfully controlled by denosumab and apatinib: a case report and literature review. Cancer Manag Res (2021) 13:4447–54. doi: 10.2147/CMAR.S312846
52. Feng L, Ye T, Zhang J, Yuan S, Chen Y, Chen J. Stereotactic body radiotherapy for lung metastases in a patient with giant cell tumor of bone: a case report and literature review. Ann Transl Med (2022) 10:156. doi: 10.21037/atm-21-6575
53. Wang G, Jiang S, Li Z, Dong Y. Denosumab and Sunitinib in the treatment of giant-cell tumor of bone with pulmonary and bone metastases in an adolescent: A case report. Med (Baltimore) (2019) 98:e17778. doi: 10.1097/MD.0000000000017778
54. Luo Y, Tang F, Wang Y, Zhou Y, Min L, Zhang W, et al. Safety and efficacy of denosumab in the treatment of pulmonary metastatic giant cell tumor of bone. Cancer Manag Res (2018) 10:1901–6. doi: 10.2147/CMAR.S161871
55. Sachan DK, Bansal N, Gupta S, Kumar S. A rare case of giant cell tumour (GCT) of bone with lung metastases. BMJ Case Rep (2018) 2018:bcr2017221667. doi: 10.1136/bcr-2017-221667
56. Yamagishi T, Kawashima H, Ogose A, Sasaki T, Hotta T, Inagawa S, et al. Disappearance of giant cells and presence of newly formed bone in the pulmonary metastasis of a sacral giant-cell tumor following denosumab treatment: A case report. Oncol Lett (2016) 11:243–6. doi: 10.3892/ol.2015.3858
57. Wei F, Liu X, Liu Z, Jiang L, Dang G, Ma Q, et al. Interferon alfa-2b for recurrent and metastatic giant cell tumor of the spine: report of two cases. Spine (Phila Pa 1976) (2010) 35:E1418–22. doi: 10.1097/BRS.0b013e3181e7bf5a
58. Iwai T, Oebisu N, Hoshi M, Takada N, Nakamura H. Efficacy of Pazopanib in the Treatment of Metastatic Malignant Giant Cell Tumor of Soft Tissue: A Case Report. Curr Oncol (2022) 29:758–65. doi: 10.3390/curroncol29020064
59. Kudawara I, Kakunaga S, Takami K. Objective response of denosumab for multiple pulmonary metastases from giant cell tumor of bone: A case report and review of the literature. Curr Problems Cancer: Case Rep (2021) 3:100073. doi: 10.1016/j.cpccr.2021.100073
60. Egbert RC, Folsom R, Bell J, Rajani R. Denosumab Therapy for Giant Cell Tumor of Bone Pulmonary Metastasis. Case Rep Orthop (2017) 2017:2302597. doi: 10.1155/2017/2302597
Keywords: metastasis, giant cell tumor of bone, PET, denosumab, unresectable
Citation: Miwa S, Yamamoto N, Hayashi K, Takeuchi A, Igarashi K, Taniguchi Y, Morinaga S, Asano Y, Nojima T and Tsuchiya H (2023) Case Report: Unresectable pulmonary metastases of a giant cell tumor of bone treated with denosumab: a case report and review of literature. Front. Oncol. 13:1230074. doi: 10.3389/fonc.2023.1230074
Received: 08 June 2023; Accepted: 18 July 2023;
Published: 16 August 2023.
Edited by:
Duoyi Zhao, Fourth Affiliated Hospital of China Medical University, ChinaReviewed by:
Teruya Kawamoto, Kobe University, JapanCopyright © 2023 Miwa, Yamamoto, Hayashi, Takeuchi, Igarashi, Taniguchi, Morinaga, Asano, Nojima and Tsuchiya. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shinji Miwa, c21pd2EwMDFAeWFob28uY28uanA=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.