
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 18 August 2023
Sec. Surgical Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1230021
 Xunjian Zhou1
Xunjian Zhou1 Zhi Duan1
Zhi Duan1 Ting Tao1
Ting Tao1 Zhen Li1
Zhen Li1 Ning Wang2
Ning Wang2 Qimei Xu1
Qimei Xu1 Meiyan Wei1
Meiyan Wei1 Zheng Zhong3
Zheng Zhong3 Ran Liu1
Ran Liu1 Qinghua Yin4
Qinghua Yin4 Lixin Xiong4
Lixin Xiong4 Hui Chen1*
Hui Chen1*Malignant rhabdoid tumors (MRTs) are rare tumors with high mortality rates and poor prognoses. MRTs occur mainly in the central nervous system, kidneys, and soft tissues, but rarely in the omentum. MRTs occur more commonly in infants and children and less frequently in adults. Here, we report the first observed case of MRT in an adult omentum. A 35-year-old man with abdominal distension and pain was admitted to the emergency department. Previously, several hospitals considered patients with cirrhosis who had not received active treatment. Computed tomography and magnetic resonance imaging revealed diffuse omental thickening and massive ascites. The surgery was performed at our hospital, and the pathological diagnosis was MRT with a SMARCB1(INI-1) deletion. Postoperatively, his symptoms improved, and he underwent five cycles of chemotherapy. However, 6 months after surgery, the tumor developed liver metastases, and the patient subsequently died. Primary MRT of the greater omentum is rare, and its pathological diagnosis usually requires extensive clinicopathological evaluation of various differential diagnoses and an appropriate work-up to exclude other malignancies associated with SMARCB1 deletion. At the same time, the lack of specific signs of omental MRT and its rapid progression should alert clinicians.
Malignant rhabdoid tumors (MRTs) are rare and aggressive tumors that are more common in infants and young children than those in adults (1). In children, two-thirds of MRTs arise in the central nervous system, a disease known as atypical teratoid rhabdoid tumor (AT/RT). The rest occur in the kidneys, called MRTs, or in the soft tissues of other anatomical sites, sometimes called extrarenal or extracranial rhabdoid tumors (2). The absence of SMARCB1(INI-1) expression is an important feature of MRT. However, the pathological diagnosis of primary greater omentum MRT is difficult to distinguish from metastases of SMARCB1 deletion malignancies at other sites. Simultaneously, due to the lack of characteristic clinical manifestations, MRT can be easily missed by clinicians, thus delaying patient treatment. To date, no adult cases of omental MRT have been reported (3). Here, we report the first case of MRT in an adult omentum.
The patient was a 35-year-old man who developed abdominal distension 1 month before presentation, and no significant abnormalities were found during a rectoscopy at a primary hospital. More than 10 d prior, he had visited two other hospitals for treatment because of bloating and abdominal pain. One hospital doctor considered the patient to have a fatty liver, while the other hospital doctors believed it to be cirrhosis; they did not further examine or treat the patient. Ten days prior, the patient visited our hospital with a gradual worsening of abdominal pain. Physical examination revealed mild tenderness in the lower left abdomen, no rebound tenderness, and positive abdominal mobility dullness. Abdominal computed tomography (CT) enhancement at our hospital showed severe ascites and diffuse nodular thickening of the greater omentum, the nature of which was not determined, and the possibility of cirrhosis was considered (Figures 1A, B). Abdominal magnetic resonance imaging (MRI) revealed irregular thickening of the peritoneum with massive ascites of unknown etiology (Figures 1C–F). No noticeable abnormalities were observed in the liver, gallbladder, bile duct, spleen, pancreas, kidney, bladder, prostate, or intestinal walls (Supplementary Figure 1). In addition, the father of the patient had a history of an ascending colon tumor, and the carbohydrate antigen 125 level was elevated to 200 U/mL (normal < 35 U/mL). A gastrointestinal endoscopy revealed polyps in the colon and several small duodenal ulcers. No malignant lesions were observed upon pathological examination. Viral hepatitis markers were negative, serum AFP and CEA were normal, and head and chest CT showed no abnormalities. Upon admission, the patient’s condition deteriorated rapidly, with incomplete intestinal obstruction, moderate iron deficiency anemia (hemoglobin, 81 g/L), and various severe electrolyte and metabolic disturbances. The patient’s basic condition was poor. After full communication with the patient’s family, the surgeon opted for emergency surgery. A large area of ascites was observed in the abdominal cavity during surgery. The entire omentum was enlarged like fish meat, which had partly invaded the bladder, abdominal wall, and mesentery.
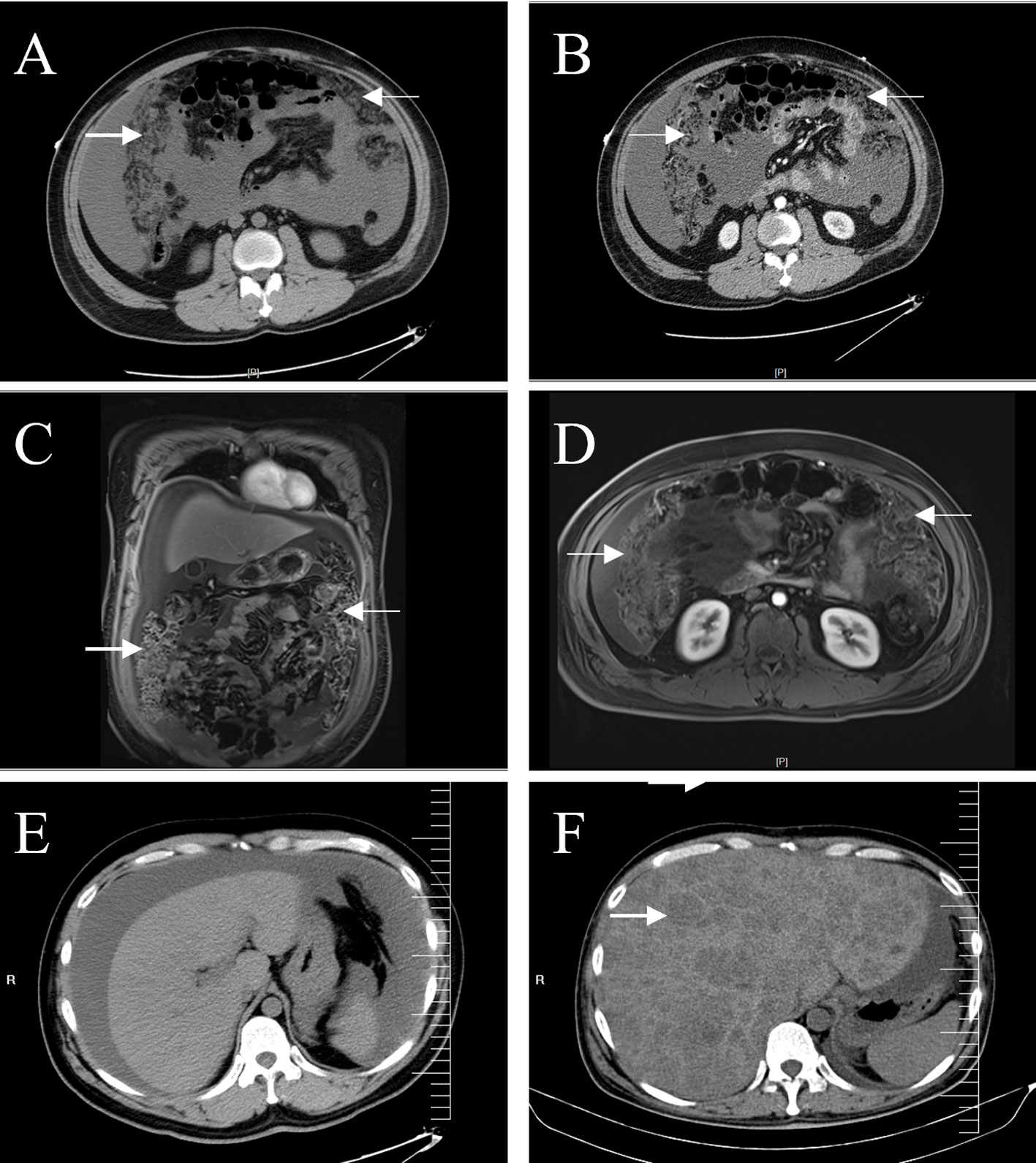
Figure 1 Abdominal CT and MRI. A plain (A) and enhanced (B) abdominal CT scans show a dense, blurred, and thickened omentum (arrows), with large amounts of fluid visible in the abdominal cavity. Coronal (C) and axial (D) enhanced T1-weighted images showed more clearly the irregularly thickened omentum (arrows). CT images of the liver showed no space-occupying lesions on admission (E), and multiple low-density lesions (arrow) were observed in the liver six months later (F).
In general, the surface of the tumor was gray-red, and the cut surface was gray-red, gray-yellow, solid, and hard (Figure 2A). Microscopically, most of the tumor cells were distributed in solid sheets, and a small portion showed a glandular growth pattern. The tumor diffusely infiltrated the omental adipose tissue. The tumor cells were large in volume, rich in the cytoplasm, eosinophilic, and had large nuclei. The nucleus was located on one side of the cell, showing “rhabdoid” morphology, some of the nuclei were vacuolar, the nucleolus was obvious, and nuclear division was common (Figures 2B–D).
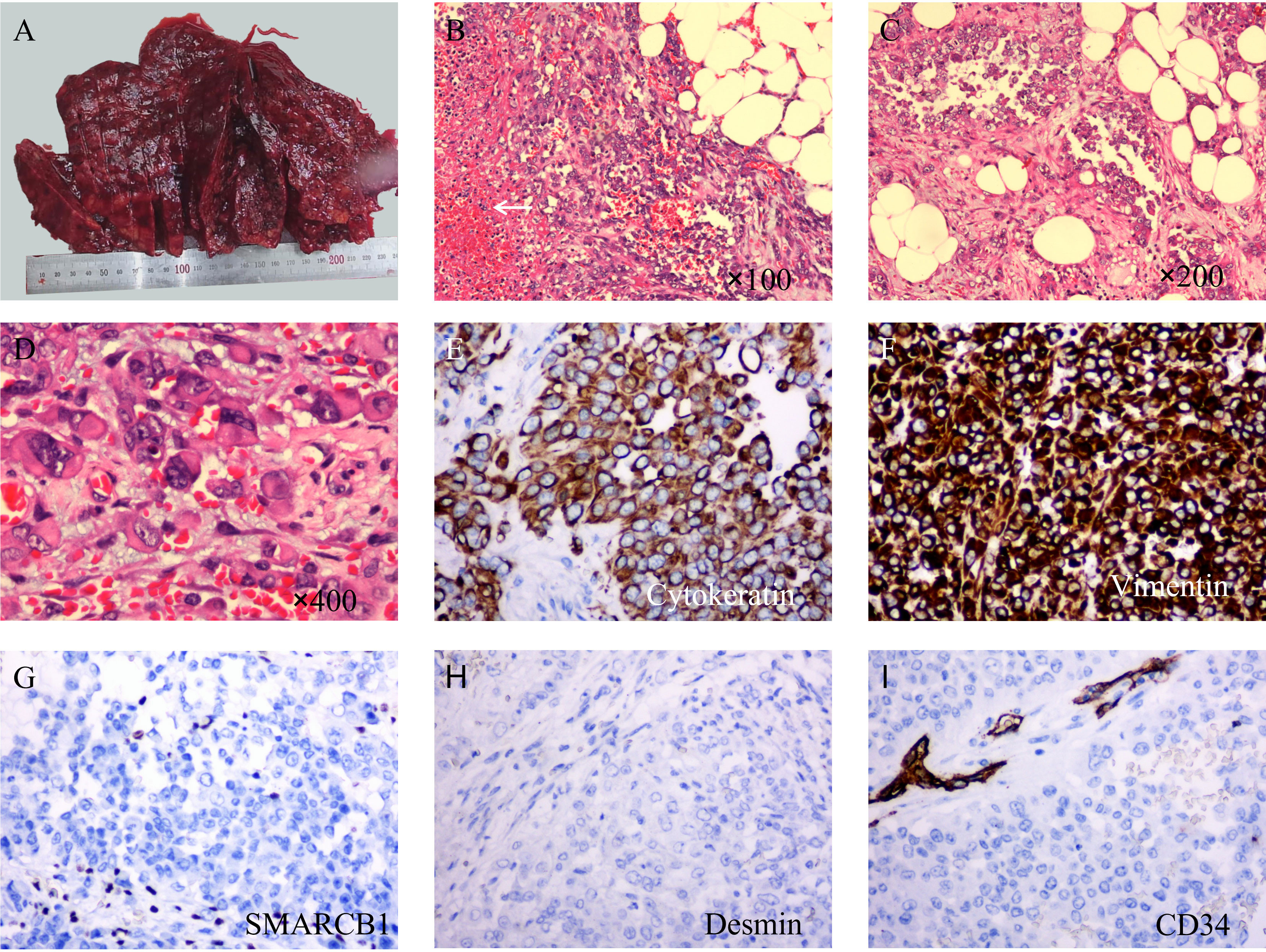
Figure 2 Macroscopic examination, hematoxylin & eosin staining, and immunohistochemical staining of tumors. (A) The surface of the tumor is gray-red and not very smooth. (B) At low magnification, the tumor cells showed a diffuse sheet-like growth pattern with poor intercellular adhesion, and necrosis (arrow). (C) In some areas, the tumor cells showed an adenoid growth pattern. (D) Under high magnification, the tumor cells are large in size, with abundant cytoplasm, an eosinophilic red color, large nuclei, deviated, vacuolated, and obvious nucleoli. (E) Immunohistochemical staining showed diffuse expression of cytokeratin (×200). (F) Vimentin is also diffusely positive and stains more deeply (×200). (G) SMARCB1(INI-1) was completely absent in the tumor cells while being positive in the surrounding cells. (H) Desmin is negative (×200). (I) CD34 was completely negative in tumor cells; however, peripheral vessels were positive (×200).
Immunohistochemical and molecular detection: Immunohistochemical expression in the solid and glandular growth regions was similar, and the expression levels of cytokeratin, EMA, and vimentin were diffusely positive, but those of SMARCA4, desmin, and CD34 were negative (Figures 2F–I). Approximately 50% of the tumor cells were Ki67 positive. Importantly, SMARCB1 expression was lost. Similarly, a fluorescence in situ hybridization (FISH) assay revealed a SMARCB1 deletion (Figure 3). In addition, immunohistochemistry of the tumor cells was positive for CK19, MUC5AC, MDM2, CDK4, BCL2, E-cadherin, and WT1. The tumors did not express CD31, ERG, D2-40, calretinin, actin, S100, SOX10, melana-A, HMB45, chromogranin A, synaptophysin, CD56, CD45, Sall-4, CD117, CD68, CK5/6, CK7, CK20, CDX2, SATB2, TTF-1, hepatocyte-1, glypican-3, Pax8, MUC2, or MUC6. Simultaneously, 507 fusion or rearrangement genes in common soft tissue tumors, including Ewing’s sarcoma and synovial sarcoma, were examined; however, no significant genes were found. Ultimately, the patient was diagnosed with MRT based on the results of auxiliary examinations.
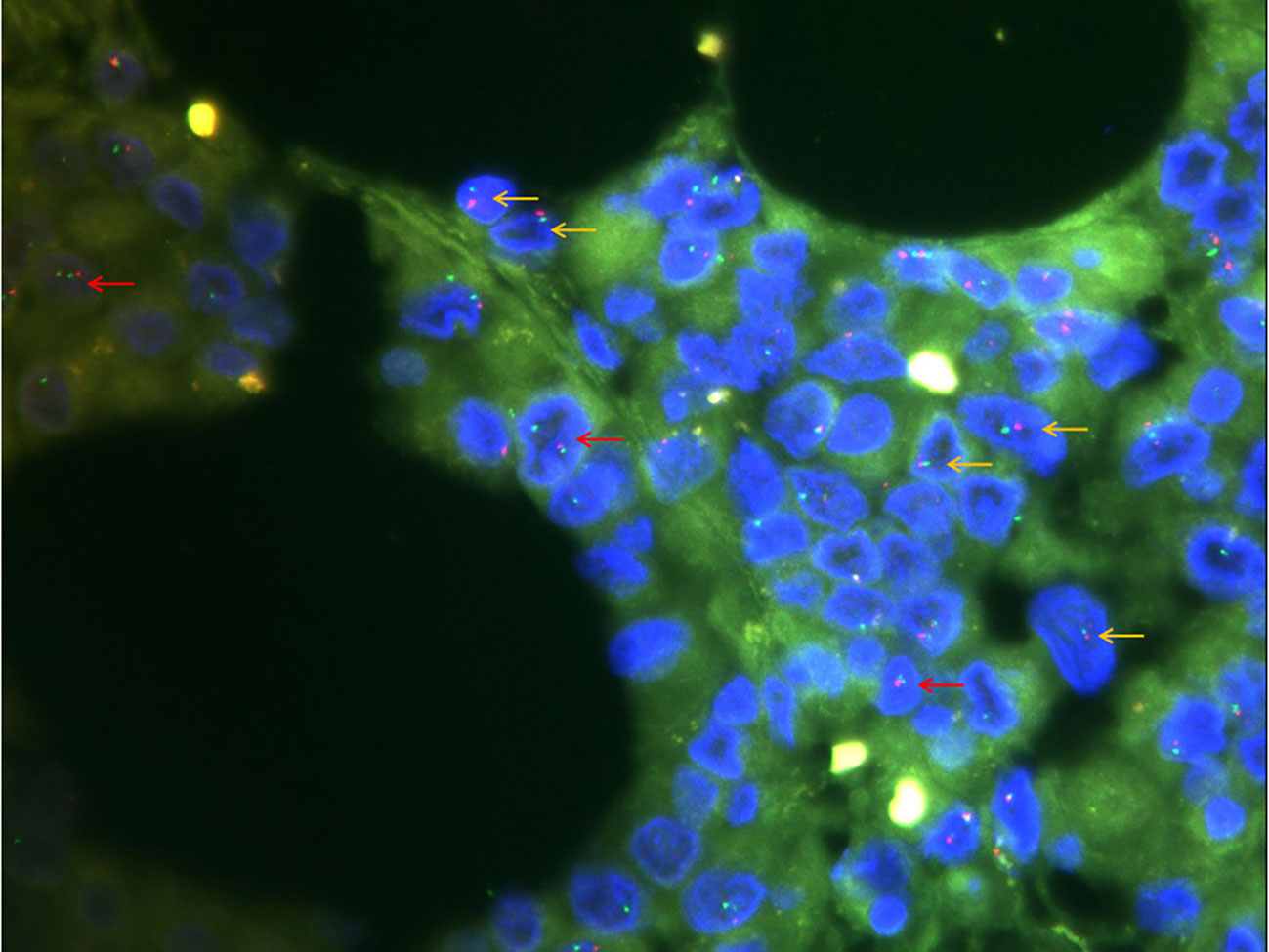
Figure 3 Fluorescence in situ hybridization detection of SMARCB1 (×1000). Of the 100 cells counted, 20% had a red-green monomer (orange arrow) and 41% had 1R2G (red arrow), indicating SMARCB1 heterozygous deletion.
After two surgical debulking procedures, the patient was administered five cycles of ifosfamide + etoposide + mesna chemotherapy, and the ascites decreased. Unfortunately, 6 months after surgery, an MRI revealed metastases to the patient’s liver, and the patient died within a few days (Figure 4).
In 1998, MRT sequencing identified mutations, deletions, and other somatic alterations in SMARCB1 (4). SMARCB1, located at 22q11.2, is one of the core subunit proteins of the ATP-dependent SWI/SNF chromatin remodeling complex (2, 5). SMARCB1, also known as INI1/BAF47, is ubiquitously expressed in the nucleus of normal cells and is an important tumor suppressor (4). Animal studies have shown that the disruption of SMARCB1 expression leads to early embryonic death in mice (6). Although the immunohistochemical deletion of SMARCB1 is believed to contribute to the histological diagnosis of MRT, aberrant SMARCB1 expression has also been found in a variety of tumors, including epithelioid and synovial sarcomas (7). The abnormal protein expression of SMARCB1 can be divided into three modes: complete loss, mosaic expression, and reduced expression. Complete loss of SMARCB1 is observed in MRTs, epithelioid sarcomas, renal medullary carcinomas, epithelioid malignant peripheral nerve sheath tumors, myoepithelial tumors, extraosseous myxoid chondrosarcomas, pediatric chordomas, pancreatic undifferentiated rhabdoid carcinomas, sinus basaloid tumor carcinomas, and gastrointestinal rhabdoid carcinomas. Mosaic expression of SMARCB1 is observed in schwannomatosis, gastrointestinal stromal tumors, and fibromyxoma ossification. Decreased SMARCB1 expression is observed in synovial sarcoma (4).
In this case, immunohistochemistry of the tumor cells showed SMARCB1 deletion, which should be differentiated from poorly differentiated carcinomas with SMARCB1 deletion. Ber-EP4 and MOC31 are believed to be present in a wide range of normal human and tumor epithelia and mature squamous cells and hepatocytes, playing a role in differentiated carcinomas and sarcomas (8). In this case, Ber-EP4 and MOC31 were only positive in small clusters of cells. More importantly, the clinician performed gastrointestinal endoscopy, chest and abdominal ultrasound, CT, MRI, and other examinations, but no space-occupying lesions were detected in the other organs; therefore, the above diagnosis can be excluded. Claudin-4 is a marker of epithelial differentiation that is expressed in almost all cancer types. Immunohistochemistry for claudin-4 was performed on 130 SWI/SNF complex-deficient tumors. Of these, 90% of MRTs (9/10) were claudin-4 negative, while 80% (16/20) of SWI/SNF complex-deficient undifferentiated carcinomas were claudin-4 membrane-positive (≥ 5% of cells), suggesting that claudin- 4 may play a role in differentiating MRT from SMARCB1-deficient differentiated carcinomas (9).
Second, the presented case must be differentiated from proximal epithelioid sarcoma. Proximal epithelioid sarcoma generally occurs in the body midline, is characterized by biphasic differentiation and SMARCB1 gene deletion, and often has a morphology similar to that of MRT (10). Recent studies have shown that SALL4 and glypican-3 are expressed in some MRT cases and rarely in epithelioid sarcomas, but both were negative in the present case (10, 11). Compared with MRT, epithelioid sarcoma usually occurs in adults; therefore, epithelioid sarcoma is the most important differential diagnosis of the disease presented in this case. Morphologically, MRT usually presents as large, poorly adherent pleomorphic cells, whereas epithelioid sarcomas are often round or occasionally spindle-shaped with little pleomorphism (7). It is generally believed that at least 50% of epithelioid sarcomas express CD34. In addition, 38–68% (41/109, 19/28) of MRT cases were recently reported to be ERG-positive, while FLI1 and D2-40 are expressed in a higher proportion in epithelioid sarcoma (12–14). More importantly, Kohashi et al. analyzed 53 epithelioid angiosarcomas, of which 45 (84.9%) showed loss of SMARCB1 protein expression using immunohistochemical staining. Molecular testing of 39 of these patients revealed that only four (10.3%) had alterations at the DNA level sufficient to suppress gene expression, and the frequency of SMARCB1 gene alterations was significantly lower than that in patients with MRT. In addition, MRT has a worse prognosis than epithelioid sarcoma (3, 15). Although the patient received active treatment, he still developed liver metastases and died after 6 months. Combined with the FISH detection of SMARCB1 deletion and negative immunohistochemistry results for CD34 and ERG, the presented case is more in line with MRT.
Finally, the reported case must be differentiated from other tumors with biphasic differentiation, such as synovial sarcoma and malignant mesothelioma, which are features of MRTs. Synovial sarcomas originate from unknown pluripotent stem cells that differentiate bidirectionally into mesenchymal and epithelial cells. Synovial sarcomas tend to grow in the extremities, usually near large joints (7). Poorly differentiated synovial sarcomas can also exhibit a “rhabdoid” morphology, and reduced SMARCB1 expression can be observed in synovial sarcomas (16). Although the tumor cells of poorly differentiated synovial sarcomas are large, round, epithelioid, and frequently mitotic, they lack the significant pleomorphism of MRT tumor cells. In addition, immunohistochemical staining showed complete deletion of SMARCB1 expression rather than reduced expression, and no SYT-SSX fusion gene was detected, which is a relatively specific molecular change in synovial sarcoma; therefore, synovial sarcoma was excluded (17). Biphasic malignant mesothelioma usually has both epithelioid and sarcomatoid cells and usually does not have a SMARCB1 deletion. However, a rare case of malignant mesothelioma has recently been reported, with morphological features very similar to those of malignant rhabdomyoma and immunohistochemical staining showing a SMARCB1 deletion (18). Importantly, the rare malignant mesothelioma case expressed various mesothelial cell markers. However, all mesothelioma markers were negative except for the focal immunohistochemical expression of WT1 in the case presented at our hospital, allowing biphasic malignant mesotheliomas to be excluded.
Mutations in genes encoding the subunits of the Switch/Sucrose non-fermentable (SWI/SNF) chromatin remodeling complex are common and occur in nearly 25% of tumors (2). Approximately 95% of MRTs have SMARCB1 mutations, and another 5% have SMARCA4 mutations (2, 5).
Likewise, an inactivating mutation of SMARCA4 is an important molecular driver (19). SMARCA4-deficient tumors, similar to SMARCB1-deficient tumors, often exhibit a “rhabdoid” morphology, and both tumor types encode subunits of the SWI/SNF chromatin remodeling complex. Nearly 90% of small cell carcinomas of the ovary and hypercalcemic types have biallelic inactivating mutations in SMARCA4 but rarely in SMARCB1 (20). Thoracic SMARCA4-deficient undifferentiated tumors are newly identified rare tumor entities that present as large compressive and infiltrating mediastinal, pulmonary, or pleural masses and are often associated with smoking. They are undifferentiated tumors composed of epithelioid or rhabdoid tumor cells with a high mutational burden. Similarly, an inactivating mutation in SMARCA4 is an important molecular driver (19).
In addition to SMARCB1 and SMARCA4, at least seven genes encoding SWI/SNF complex subunits have been identified to be repeatedly mutated in various tumor types, including ARID1A, ARID1B, ARID2, SMARCA2, PBRM1, SMARCC1, and SMARCC2 (21). Among these, ARID1A is the SWI/SNF composite gene with the highest mutation frequency and was found to be mutated in nearly 50% of ovarian clear cell carcinomas and endometrioid carcinomas. The next most frequent SWI/SNF composite genes with mutations are SMARCA4 and PBRM1, with PBRM1 mutations found in 41% of patients with clear-cell renal cell carcinoma (2). Studies have shown that SMARCB1 plays a role in various signaling pathways, such as the p16-RB, canonical WNT, sonic hedgehog, and polycomb pathways (4). Molecular inhibitors of related pathways, such as EZH2, HDAC, CDK4/6, and mTOR, have entered clinical trials for MRT. In particular, EZH2 inhibitors, which directly target the negative consequences of SMARCB1 loss prevalent in MRT, may improve MRT prognosis (22). Significant research progress has recently been made in immunosuppressive therapy for treating many solid tumors. Some studies have shown that the tumor cell proportion score of PD-L1 is higher in some MRT cases than that in other malignancies, indicating that these patients may benefit from immune checkpoint inhibitors (23). However, in the current case, we neither tested for PD-L1 nor attempted PD-L1-targeted therapy.
We report a rare case of MRT in the omentum of an adult male, which is the first reported case of MRT in an adult omentum. The patient had relatively complete examination data, treatment interventions, and prognostic information. However, whole-exon sequencing, which may provide information for studying the pathogenesis and pathological diagnosis of MRT, was not performed in this case.
The case reported in this study can provide useful insights for clinicians and pathologists. MRT of the omentum is rare; owing to the lack of characteristic signs and symptoms, it is easily missed and misdiagnosed. For patients with unexplained ascites, clinicians should consider the possibility of malignant tumors and actively improve the relevant auxiliary examinations. Although the morphology of MRT overlaps with that of other SWI/SNF complex-deficient tumors, it is necessary to identify MRT because of its poor prognosis. This requires a pathologist to conduct an extensive clinicopathological assessment of the differential diagnosis and perform appropriate examinations. The prognosis of MRT is poor, and conventional surgical treatment and chemotherapy have little effect on the patient’s prognosis. Therefore, the pathogenesis and treatment of MRT require further study.
In summary, we report the first case of MRT in an adult omentum. MRTs of the omentum are rare, and as they lack specific signs and symptoms, they can be easily overlooked or missed. As such, we believe this finding deserves the attention of clinicians. At the same time, the morphology of MRT overlaps with that of other tumors, especially those with SWI/SNF complex deficiency. Pathologists must conduct extensive clinicopathological assessments of the differential diagnosis and appropriate examinations to make an accurate pathological diagnosis. Most MRTs are accompanied by SMARCB1 deletions, which may provide new research directions for the treatment of this tumor.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Written informed consent was obtained for the publication of this case report.
XZ completed the data collection and the writing of the first draft. HC came up with the initial idea and completed the molecular testing. ZD, TT, and QX assisted in data interpretation. ZL and NW revised the manuscript. MW and RL assisted in completing part of the immunohistochemical experiments. ZZ provided the images. QY and LX provides surgical and patient information. All authors contributed to the article and approved the submitted version.
This study was supported by the Research Project of the Health Commission of Hunan Province (202201042974, B202303088127), Changsha Natural Science Foundation (kq2202004), and the Research Project of the First Hospital of Changsha (Y2022-17, Y2022-19).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1230021/full#supplementary-material
1. Schaefer IM, Hornick JL. SWI/SNF complex-deficient soft tissue neoplasms: An update. Semin Diagn Pathol (2021) 383:222–31. doi: 10.1053/j.semdp.2020.05.005
2. Mittal P, Roberts CWM. The SWI/SNF complex in cancer - biology, biomarkers and therapy. Nat Rev Clin Oncol (2020) 177:435–48. doi: 10.1038/s41571-020-0357-3
3. Nam SH, Park JA, Kim YM. Primary Malignant rhabdoid tumor of greater omentum in 10-year-old girl. Ann Surg Treat Res (2014) 861:50–3. doi: 10.4174/astr.2014.86.1.50
4. Kohashi K, Oda Y. Oncogenic roles of SMARCB1/INI1 and its deficient tumors. Cancer Sci (2017) 1084:547–52. doi: 10.1111/cas.13173
5. Pawel BR. SMARCB1-deficient tumors of childhood: A practical guide. Pediatr Dev Pathol (2018) 211:6–28. doi: 10.1177/1093526617749671
6. Guidi CJ, Sands AT, Zambrowicz BP, Turner TK, Demers DA, Webster W, et al. Disruption of Ini1 leads to peri-implantation lethality and tumorigenesis in mice. Mol Cell Biol (2001) 2110:3598–603. doi: 10.1128/MCB.21.10.3598-3603.2001
7. Hollmann TJ, Hornick JL. INI1-deficient tumors: diagnostic features and molecular genetics. Am J Surg Pathol (2011) 3510:e47–63. doi: 10.1097/PAS.0b013e31822b325b
8. Vojtek M, Walsh MD, Papadimos DJ, Shield PW. Claudin-4 immunohistochemistry is a useful pan-carcinoma marker for serous effusion specimens. Cytopathol (2019) 306:614–9. doi: 10.1111/cyt.12765
9. Naso JR, Churg A. Claudin-4 shows superior specificity for mesothelioma vs non-small-cell lung carcinoma compared with MOC-31 and Ber-EP4. Hum Pathol (2020) 100:10–4. doi: 10.1016/j.humpath.2020.04.005
10. Kohashi K, Yamada Y, Hotokebuchi Y, Yamamoto H, Taguchi T, Iwamoto Y, et al. ERG and SALL4 expressions in SMARCB1/INI1-deficient tumors: a useful tool for distinguishing epithelioid sarcoma from Malignant rhabdoid tumor. Hum Pathol (2015) 462:225–30. doi: 10.1016/j.humpath.2014.10.010
11. Chan ES, Pawel BR, Corao DA, Venneti S, Russo P, Santi M, et al. Immunohistochemical expression of glypican-3 in pediatric tumors: an analysis of 414 cases. Pediatr Dev Pathol (2013) 164:272–7. doi: 10.2350/12-06-1216-OA.1
12. Armah HB, Parwani AV. Epithelioid sarcoma. Arch Pathol Lab Med (2009) 1335:814–9. doi: 10.1043/1543-2165-133.5.814
13. Miettinen M, Wang Z, Sarlomo-Rikala M, Abdullaev Z, Pack SD, Fetsch JF. ERG expression in epithelioid sarcoma: a diagnostic pitfall. Am J Surg Pathol (2013) 3710:1580–5. doi: 10.1097/PAS.0b013e31828de23a
14. Stockman DL, Hornick JL, Deavers MT, Lev DC, Lazar AJ, Wang WL. ERG and FLI1 protein expression in epithelioid sarcoma. Mod Pathol (2014) 274:496–501. doi: 10.1038/modpathol.2013.161
15. Kohashi K, Izumi T, Oda Y, Yamamoto H, Tamiya S, Taguchi T, et al. Infrequent SMARCB1/INI1 gene alteration in epithelioid sarcoma: a useful tool in distinguishing epithelioid sarcoma from Malignant rhabdoid tumor. Hum Pathol (2009) 403:349–55. doi: 10.1016/j.humpath.2008.08.007
16. Rekhi B, Vogel U. Utility of characteristic 'Weak to Absent' INI1/SMARCB1/BAF47 expression in diagnosis of synovial sarcomas. APMIS (2015) 1237:618–28. doi: 10.1111/apm.12395
17. Feng X, Huang YL, Zhang Z, Wang N, Yao Q, Pang LJ, et al. The role of SYT-SSX fusion gene in tumorigenesis of synovial sarcoma. Pathol Res Pract (2021) 222:153416. doi: 10.1016/j.prp.2021.153416
18. Kimura N, Hasegawa M, Hiroshima K. SMARCB1/INI1/BAF47- deficient pleural Malignant mesothelioma with rhabdoid features. Pathol Int (2018) 682:128–32. doi: 10.1111/pin.12623
19. Mardinian K, Adashek JJ, Botta GP, Kato S, Kurzrock R. SMARCA4: implications of an altered chromatin-remodeling gene for cancer development and therapy. Mol Cancer Ther (2021) 2012:2341–51. doi: 10.1158/1535-7163.MCT-21-0433
20. Perret R, Chalabreysse L, Watson S, Serre I, Garcia S, Forest F, et al. SMARCA4-deficient thoracic sarcomas: clinicopathologic study of 30 cases with an emphasis on their nosology and differential diagnoses. Am J Surg Pathol (2019) 434:455–65. doi: 10.1097/PAS.0000000000001188
21. Savas S, Skardasi G. The SWI/SNF complex subunit genes: Their functions, variations, and links to risk and survival outcomes in human cancers. Crit Rev Oncol Hematol (2018) 123:114–31. doi: 10.1016/j.critrevonc.2018.01.009
22. Nemes K, Fruhwald MC. Emerging therapeutic targets for the treatment of Malignant rhabdoid tumors. Expert Opin Ther Targets (2018) 224:365–79. doi: 10.1080/14728222.2018.1451839
Keywords: malignant rhabdoid tumor, SMARCB1, omentum, pathological diagnosis, case report
Citation: Zhou X, Duan Z, Tao T, Li Z, Wang N, Xu Q, Wei M, Zhong Z, Liu R, Yin Q, Xiong L and Chen H (2023) Malignant rhabdoid tumor of the omentum in an adult male: a case report and literature review. Front. Oncol. 13:1230021. doi: 10.3389/fonc.2023.1230021
Received: 27 May 2023; Accepted: 25 July 2023;
Published: 18 August 2023.
Edited by:
Satvinder Singh Mudan, The London Clinic, United KingdomReviewed by:
Ravi Hari Phulware, All India Institute of Medical Sciences, Rishikesh, IndiaCopyright © 2023 Zhou, Duan, Tao, Li, Wang, Xu, Wei, Zhong, Liu, Yin, Xiong and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Chen, Mzc5MDMwNTUwQHFxLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.