- 1The First College of Clinical Medicine, Guizhou University of Traditional Chinese Medicine, Guiyang, Guizhou, China
- 2Department of Medical Affairs, The First Affiliated Hospital of Guizhou University of Traditional Chinese Medicine, Guiyang, Guizhou, China
- 3Research Laboratory, The First Affiliated Hospital of Guizhou University of Traditional Chinese Medicine, Guiyang, Guizhou, China
- 4Development Planning Division, Guizhou University of Traditional Chinese Medicine, Guiyang, Guizhou, China
- 5Student Management Office, The First Affiliated Hospital of Guizhou University of Traditional Chinese Medicine, Guiyang, Guizhou, China
- 6Dean’s Office, The First Affiliated Hospital of Guizhou University of Traditional Chinese Medicine, Guiyang, Guizhou, China
Purpose: To investigate the efficacy of the application of microecological agents in patients with perioperative colorectal cancer.
Methods: The seven electronic databases including PubMed, Cochrane Library, Excerpt Medica Database (Embase), Web of Science (WOS), Chinese Biomedical Literature Database (CBM), China National Knowledge Infrastructure (CNKI), and Wan-fang Database were systematically searched for eligible studies from 2000 to February 2023.
Results: A total of 38 randomized controlled clinical trials were included in this study, with a total of 1765 patients in the microecological preparation group and 1769 patients in the control group. All data were analyzed using Review Manager 5.4 and R 4.2.2 software. Meta-analysis showed that in the perioperative period of colorectal cancer, the microecological agents group reduced patients’ adverse drug reactions, improved intestinal flora with Lactobacillus (SMD, 3.0858, [2.0197; 4.1520], p< 0. 0001), Bifidobacterium (SMD, 2.1551, [1.6145; 2.6956], p< 0.0001) and Escherichia coli (SMD, -1.1393, [-1.6247; -0.6538], p< 0.0001); protection of intestinal mucosal barrier function, endotoxin (SMD, -2.6850 [-4.1399; -1.2301], p=0.0003), DAO (SMD, -2.5916, [-3.4694; -1.7137], p<0.0001) and plasma D-lactate (SMD, -5.4726, [-9.8901; -1.0551], p= 0.0152), reduced inflammatory response, IL-6 (SMD, -3.1279 [-5.7706; -0.4852], p=0.0204) and CRP (SMD, -3.9698 [-7.6296; -0.3100], p=0.0335); improved the immune function of the organism, CD4+ (SMD, 1.5817 [1.0818; 2.0817], p< 0.0001), CD4+/CD8+ (SMD, 1.2938 [0.9693; 1.6183] p< 0.0001) and IgG (SMD, 1.1376 [0.2993; 1.9759] p=0.0078), improved short-term clinical efficacy, ORR (RR, 1.5105 [1.2306; 1.8541], p< 0.0001) and DCR (RR, 0.3896 [0.2620; 0.5795], p< 0.0001).
Conclusion: By increasing the number of beneficial flora such as Lactobacillus and Bifidobacterium and decreasing the number of harmful flora such as Escherichia coli, the micro-ecological preparation group is beneficial in improving the ecological dysregulation in colorectal cancer patients receiving different treatments in the perioperative period. The microecological preparation group was able to reduce many types of adverse drug reactions, such as infections and gastrointestinal discomfort, compared to the control group. The microecological agents also reduced inflammatory responses, decreased the increase in harmful metabolites, enhanced patients’ immune function, protected intestinal mucosal barrier function, and improved short-term clinical outcomes.
Systematic review registration: https://inplasy.com/inplasy-2023-4-0051/, identifier INPLASY202340051.
1 Introduction
Colorectal cancer(CRC) is one of the top three causes of cancer deaths worldwide, and the number of cases and deaths are on the rise, and the incidence rate among young people (20-49 years old) has increased significantly, with CRC ranking third in incidence rate and second in mortality rate in 2020 (1, 2).
Microorganisms play a crucial role in human health and disease development, colonizing various parts of our body (3–5), and having different types of crosstalk with various organs, but the highest numbers are found in the intestine (6). Gut microbes interact with the immune system, providing signals to promote the maturation of immune cells and the normal development of immune function (7, 8), which in turn is a major force in the regulation of cancer. Studies have shown that the occurrence of CRC is closely related to disorders of the intestinal microbiota (9).
CRC patients have significant ecological dysbiosis in their intestinal flora, and the various treatments that CRC patients receive during the perioperative period can cause changes in intestinal flora, and intestinal flora disorders can cause a series of adverse effects including increased intestinal inflammatory responses and harmful metabolites. In addition to preoperative mechanical bowel preparation, chemotherapy, radiotherapy, antibiotics and acid suppressants, CRC surgery itself and the stress response to surgery may also affect the intestinal flora and cause significant changes in the intestinal flora structure, which may affect postoperative recovery, short-term complications and long-term oncologic outcomes (10). In recent years, microecological preparations have been successfully used to improve the intestinal microbiota for the treatment of CRC and to mitigate treatment-mediated side effects (11). A large number of probiotic bacteria, their metabolites and other prebiotic components have been shown to influence CRC incidence and mediate intestinal immunity, while they also exhibit anti-inflammatory properties (12, 13). Gut microbial metabolites, which are very important regulators of the interaction between the gut microbiota and the host immune system (14), are abundant and include short chain fatty acids (SCFAs), tryptophan metabolites, vitamins and bile acids. These metabolites have different functions, e.g. Clostridium difficile bacteria, Bifidobacterium bifidum, Streptococcus and Lactobacillus in the gut produce SCFAs that can modulate intestinal immune function by binding to G protein-coupled receptors (GPCRs), and inhibiting the activity of histone deacetylases (HDACs) (15). However, bound bile acids, such as glycochenodeoxycholic acid and glycodeoxycholic acid, promotes tumorigenesis by stimulating cancer cell growth and increasing IL-6 expression (16, 17). And oral microecological agents, not only targeting systemic immunity, are also adept at managing mucosal immunity, thus addressing the inability of systemic immunity to affect the mucosal layer in the colon (18, 19). Microecological preparation is a general term for a class of cultures (live bacteria, dead bacteria or their metabolites) that can effectively participate in the establishment of intestinal micro-ecological balance, promote the growth of normal flora and inhibit the proliferation of pathogens after ingestion by animals, which can improve the health status and growth performance of the organism (20). According to their material composition and mode of action, micro-ecological agents can be divided into three categories: probiotics, prebiotics and synbiotics (21).
In this study, we conducted a systematic evaluation and meta-analysis of intestinal flora alterations, intestinal mucosal barrier-related factors, immune function-related indices, inflammatory factors, clinical efficacy and adverse effects produced after intervention with microecological agents in the perioperative period of CRC to provide a basis for the involvement of microecological agents in the perioperative treatment of CRC.
2 Methods
The protocol for this systematic review was registered on INPLASY (unique ID number) and is available in full at inpla sy.com (https://inplasy.com/inplasy-2023-4-0051/).
2.1 Eligibility criteria and outcome measures
According to the PICOS acronym (22), the inclusion criteria were as follows:
Participants (P): ① All cases included in the study must have pathologically confirmed CRC, and no metastases to the liver or other sites ② No microecological agents, antibiotics or laxatives within 1 month prior to surgery, have an indication for surgery and undergo radical CRC surgery ③ Approved by the hospital ethics committee, the patient and family understand and are informed, voluntarily participate in this study and sign the informed consent form ④ No restrictions by gender, race or country were found.
Intervention(I): Randomized controlled clinical trial of oral microecological preparations in the perioperative period for colorectal cancer and the content of the microecological preparations is not limited.
Comparison(C): On the basis of the control group, patients in the test group received oral microecological preparations.
Outcomes(O): Clinical efficacy and safety of microecological agents.
Study design(S): Randomized controlled clinical trials.
Exclusion criteria (1): non-randomized controlled trials (2) unclear dose and periodicity of microecological agents (3) incomplete test results (4) lack of sufficient data.
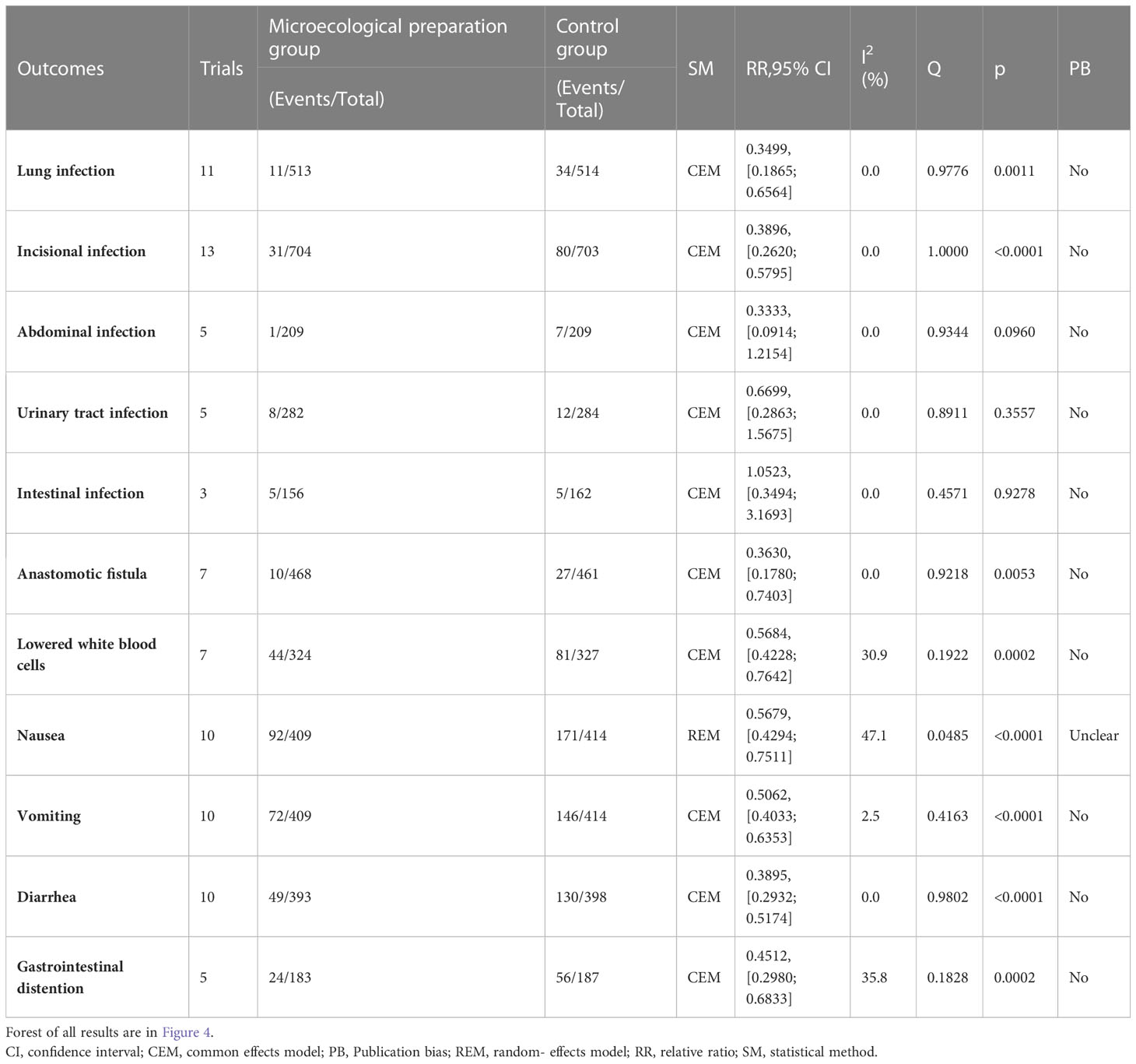
The primary outcome included two efficacy measures: (I) changes in intestinal flora: mainly involving changes in the numbers of Lactobacillus, Bifidobacterium, Escherichia coli, and Enterococcus faecalis; and (II) adverse drug reactions, assessed by detecting hematologic toxicity (leukopenia), gastrointestinal reactions (nausea, vomiting, diarrhea, flatulence), infections (pulmonary, abdominal, urinary, intestinal, incisional), and anastomotic fistulas.
Secondary outcome indicators included four efficacy measures: (i) short-term clinical efficacy ORR, DCR, short-term clinical efficacy according to the World Health Organization (WHO) criteria and Response Evaluation Criteria in Solid Tumors (RECIST), short-term tumor remission including complete remission (CR), partial remission (PR), stable disease remission (SD), progressive disease remission (PD), ORR, disease control rate ORR was defined as the sum of CR and PR, and DCR was the sum of CR, PR, and SD; (ii) immune function indicators CD4+,CD8+,CD4+/CD8+, and IgA, IgG; (iii) intestinal mucosal barrier detection indicators endotoxin, Diamine oxidase (DAO), plasma D-lactate; (iv) inflammatory factors IL-6, TNF-α, CRP.
2.2 Search strategy and study selection
Literature search in both international (Cochrane Library, PubMed, EMBASE, and Web of Science) and Chinese (CBM, CNKI, and Wan-fang Database) databases will be systematically searched for eligible studies from 2000 to February 2023, were independently conducted by two researchers. First, the MeSH database was searched by entering Colorectal Cancer、Intestinal flora、Randomized controlled clinical trials in turn, and then by searching for the terms ((Colorectal Neoplasm or Colorectal Tumor or Colorectal Cancer or Colorectal Carcinoma) AND (Intestinal flora or Gastrointestinal Microbiome or Gut Microbiota or Gastrointestinal Microbial Community or Intestinal Microbiome)) AND (Controlled Clinical Trials, Randomized or Randomized controlled trials or Clinical Studies) or (((Colorectal Neoplasm or Colorectal Tumor or Colorectal Cancer or Colorectal Carcinoma) AND (Intestinal flora or Gastrointestinal Microbiome or Gut Microbiota or Gastrointestinal Microbial Community or Intestinal Microbiome)) AND (Controlled Clinical Trials, Randomized or Randomized controlled trials or Clinical Studies)) AND (Probiotic or Probiotics) for screening. Two investigators independently screened titles and abstracts and then read the full text of the relevant literature to confirm inclusion, and any discrepancies were discussed with a third investigator.
2.3 Data extraction
The following study and participant characteristics were extracted for this study, including first author, year of publication, study type, sample size, mean age of participants, drug type, drug intervention dose and duration, and outcome indicators. Any disagreements were resolved by consensus.
2.4 Quality assessment and evidence level
The quality of the studies was assessed by the Cochrane risk of bias tool Review Manager 5.4. Included studies were assessed at three levels, including low, unclear, and high risk of bias. The review criteria covered seven areas, including random sequence generation, allocation concealment, blinding of investigators sequence generation, allocation concealment, blinding of participants and staff, blinding of participants and staff for outcome assessment, blinding for outcome assessment, incomplete outcome data, selective reporting, and other sources of bias. Sources of bias.
2.5 Statistical analyses
Statistical analyses were performed using Review Manager 5.4 and R 4.2.2 software. The outcomes were mainly represented by risk ratio (RR) and standardized mean difference (SMD) with its 95% CIs. Two- tailed p< 0.05 was considered to be statistically significant. Cochrane’s Q test and I2 statistics were used to assess heterogeneity between studies; p ≤ 0.1 or I2 > 50% indicated the presence of statistical heterogeneity, and a random-effects model was used to calculate the results when statistical heterogeneity was not present, and a fixed-effects model (common effects model) was used when statistical heterogeneity was not present. Publication bias was tested using funnel plot tests when more than 10 studies reported the same results. Sensitivity analyses were performed by removing one study at a time from the pooled analysis to explore the effect of individual studies on the pooled results. Subgroup analysis was performed according to whether or not combined chemotherapy was administered.
3 Results
3.1 Literature search and study characteristics
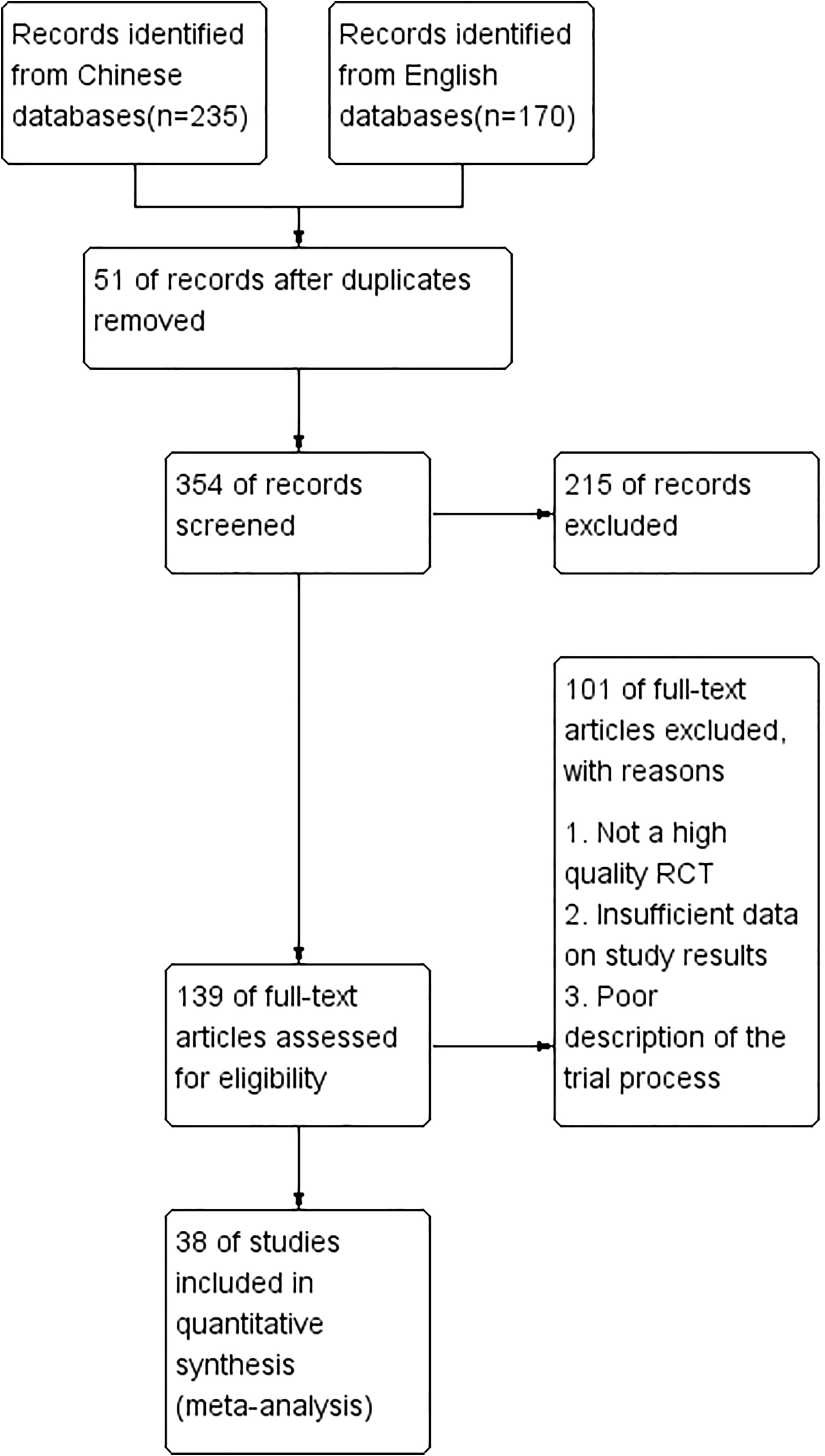
A total of 405 papers were initially retrieved, and after screening titles and abstracts, 139 papers were entered for full-text reading, and 38 studies with a total of 1765 patients in the microecological preparation group and 1769 in the control group were finally included for meta-analysis (Figure 1). 38 studies were randomized controlled clinical studies, and their characteristics are shown in Table S1.
3.2 Methodological bias of the included studies
The method of random assignment was clearly described in all 38 studies, suggesting that there was no selection bias in all included samples. Blinding of investigators and subjects was explicitly mentioned l in some studies and not specifically described in others, suggesting possible implementation bias and measurement bias. All data were complete and did not appear to be selectively reported. Other biases are unclear, and the characteristics and quality of all included studies are shown in Figure S1.
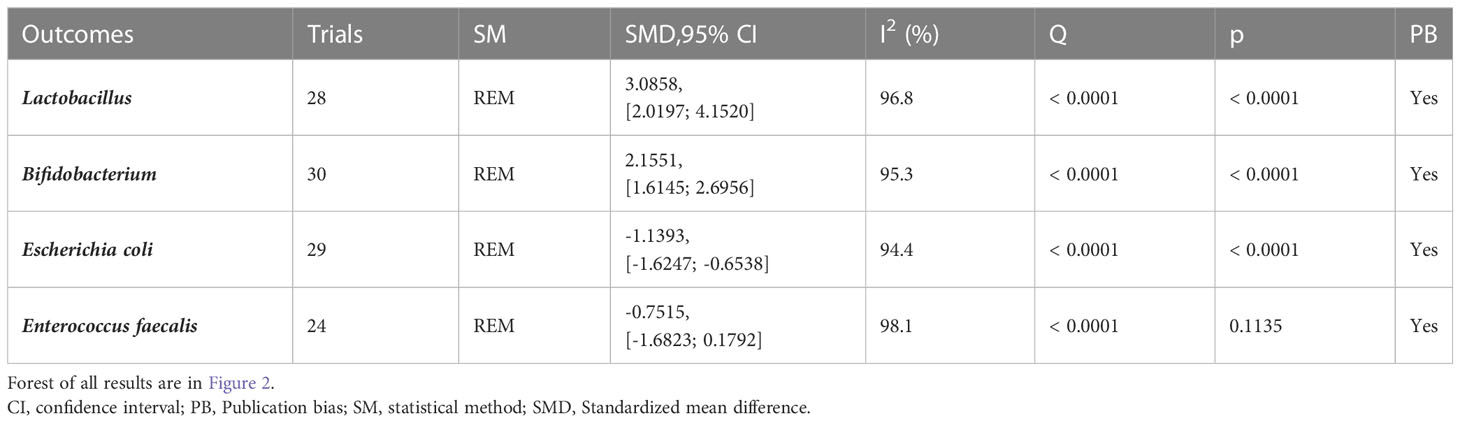
3.3 Intestinal flora
30 (23–52) reported alterations in intestinal flora (Table 1; Figures 3, S2). Lactobacillus (I2 = 96.8), Bifidobacterium (I2 = 95.3), Escherichia coli (I2 = 94.4) and Enterococcus faecalis (I2 = 98.1) were statistically heterogeneous, so the random- effects model was used for all four data. Meta-analysis showed that the microecological agents in the Lactobacillus (SMD, 3.0858, [2.0197; 4.1520], p< 0.0001) in the microecological preparation group, both in combination with chemotherapy (SMD, 3.09, [2.02; 4.15], p< 0.0001) and without chemotherapy (SMD, 3.75, [1.78; 5.73], p< 0.0001), improved better than the control group. The same conclusion was found for Bifidobacterium (SMD, 2.1551, [1.6145; 2.6956], p< 0.0001) and E. coli (SMD, -1.1393, [-1.6247; -0.6538], p< 0.0001). No statistically significant results were found for both groups in Enterococcus faecalis (SMD, -0.7515, [-1.6823; 0.1792], p=0.1135>0.05).
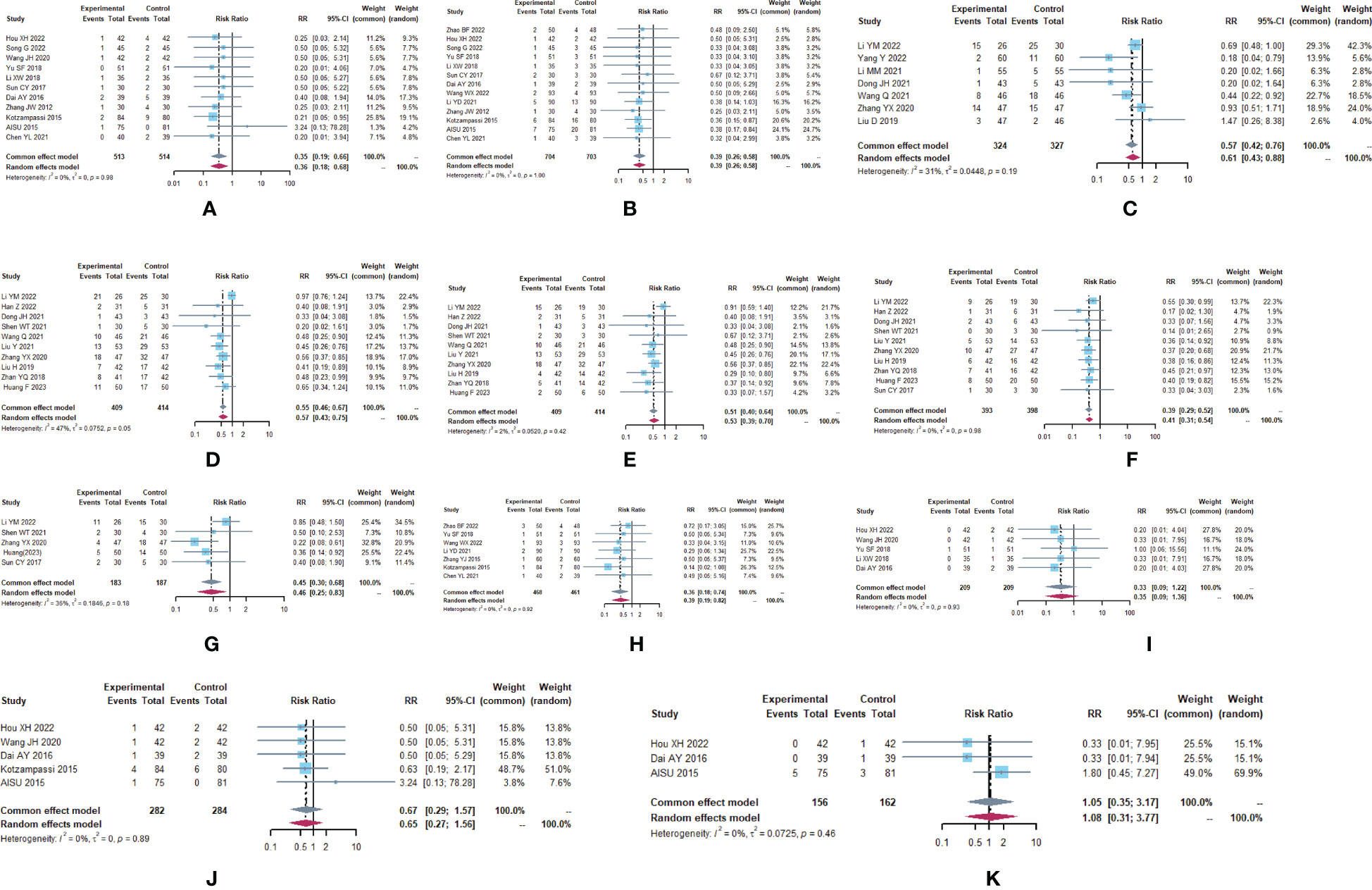
Figure 2 Adverse drug reactions (A) Forest plot of lung infection analysis results. (B) Forest plot of incision infection analysis results. (C) Forest plot of leukopenia analysis results. (D) Forest plot of nausea analysis results. (E) Forest plot of vomiting analysis results. (F) Forest plot of diarrhea analysis results. (G) Forest plot of the results of the analysis of gastrointestinal distension. (H) Forest plot of the results of anastomotic fistula analysis. (I) Forest plot of the results of the analysis of abdominal infections. (J) Forest plot of urinary tract infection analysis results. (K) Forest plot of the results of the analysis of intestinal infections.
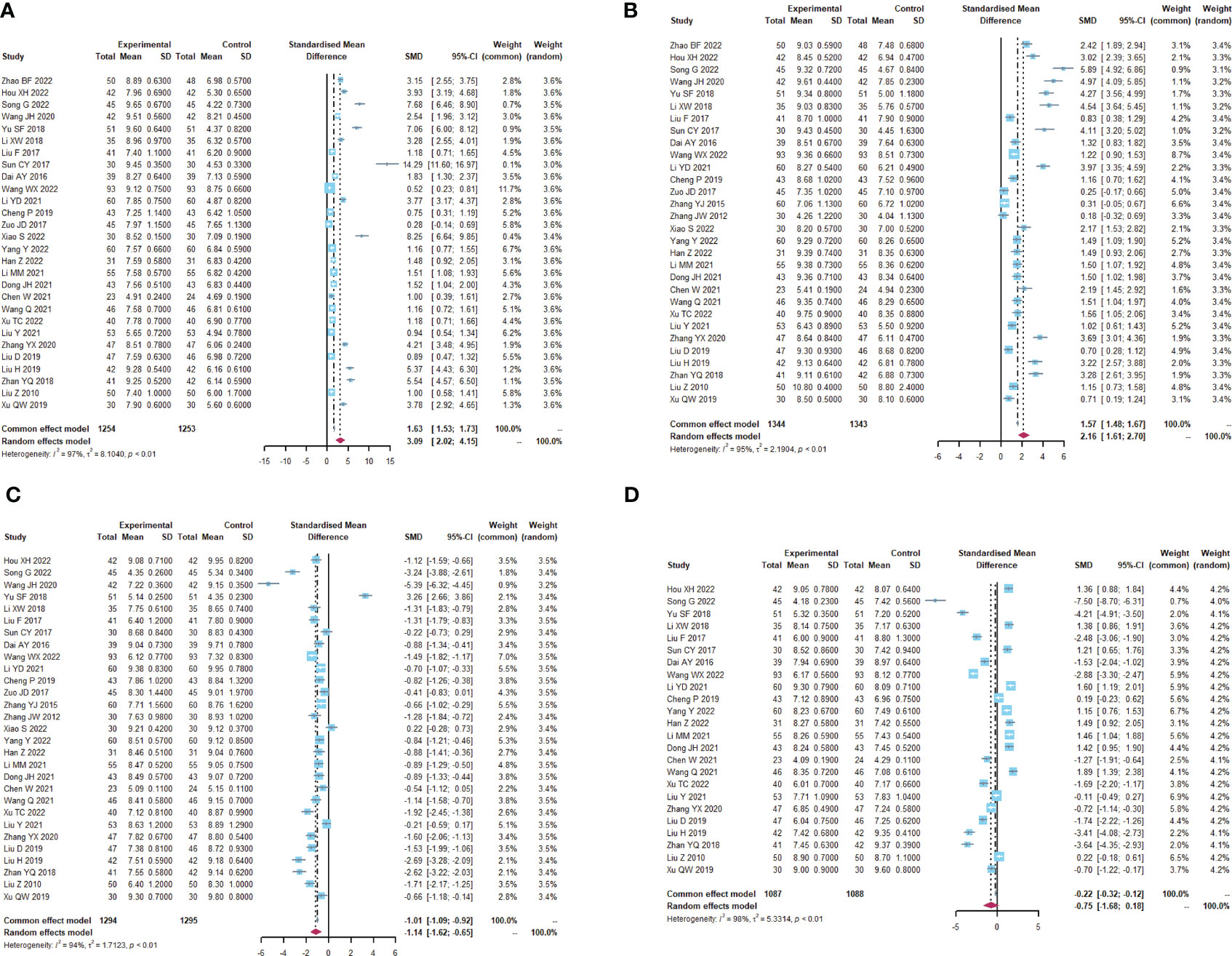
Figure 3 Altered intestinal flora. (A) Forest plot of the results of Lactobacillus analysis. (B) Forest plot of the results of Bifidobacterium analysis. (C) Forest plot of the results of E. coli analysis. (D) Forest plot of the results of the analysis of Enterococcus faecalis.
3.4 Adverse drug reactions
A total of 29 trials (24–28, 30–32, 34–39, 41–45, 47, 49–51, 53–58) reported adverse drug reactions (Table 2; Figure 2), with moderate heterogeneity in nausea (I2 = 47.1). The results showed that compared to the control group, the microecological agent group showed pulmonary infection (RR, 0.3499, [0.1865; 0.6564], p=0.0011), incisional infection (RR, 0.3896, [0.2620; 0.5795], p<0.0001), leukocytopenia (RR, 0.5684, [0.4228; 0.7642], p=0.0002), nausea (RR, 0.5679, [0.4294; 0.7511], p<0.0001), vomiting (RR, 0.5679, [0.4294; 0.7511], p<0.0001), diarrhea (RR, 0.3895, [0.2932; 0.5174], p< 0.0001), gastrointestinal distention (RR, 0.4512, [0.2980; 0.6833], p=0.0002), and anastomotic fistula (RR, 0.3630, [0.1780; 0.7403], p=0.0053) were at low risk. The results between the two groups for abdominal infection (RR, 0.3333, [0.0914; 1.2154], p=0.0960), urinary tract infection (RR, 0.6699, [0.2863; 1.5675], p=0.3557), and intestinal infection (RR, 1.0523, [0.3494; 3.1693], p=0.9278) were not statistically significant.
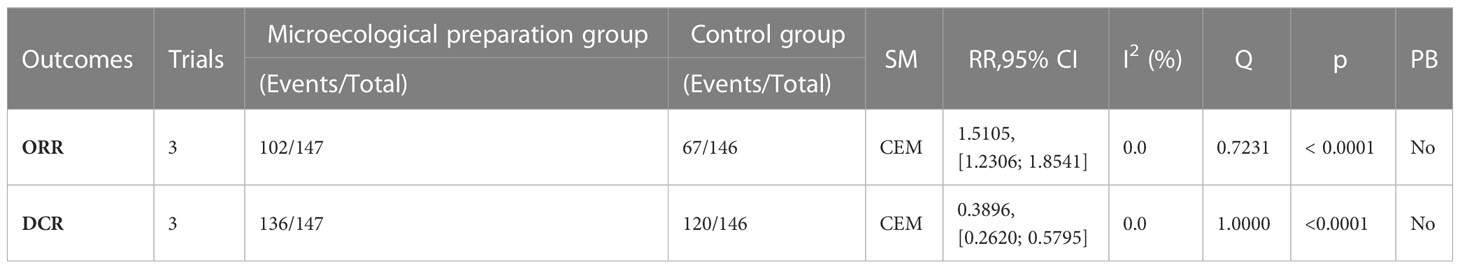
3.5 Short-term clinical efficacy
A total of 293 subjects from 3 trials (26, 29, 39)reported short-term clinical efficacy (Table 3; Figure 4). meta-analysis showed no heterogeneity in ORR and DCR results (I2 = 0). Compared with the control group, the microecological preparation group had better ORR (RR, 1.5105 [1.2306; 1.8541], p< 0.0001) and DCR (RR, 0.3896 [0.2620; 0.5795], p< 0.0001).
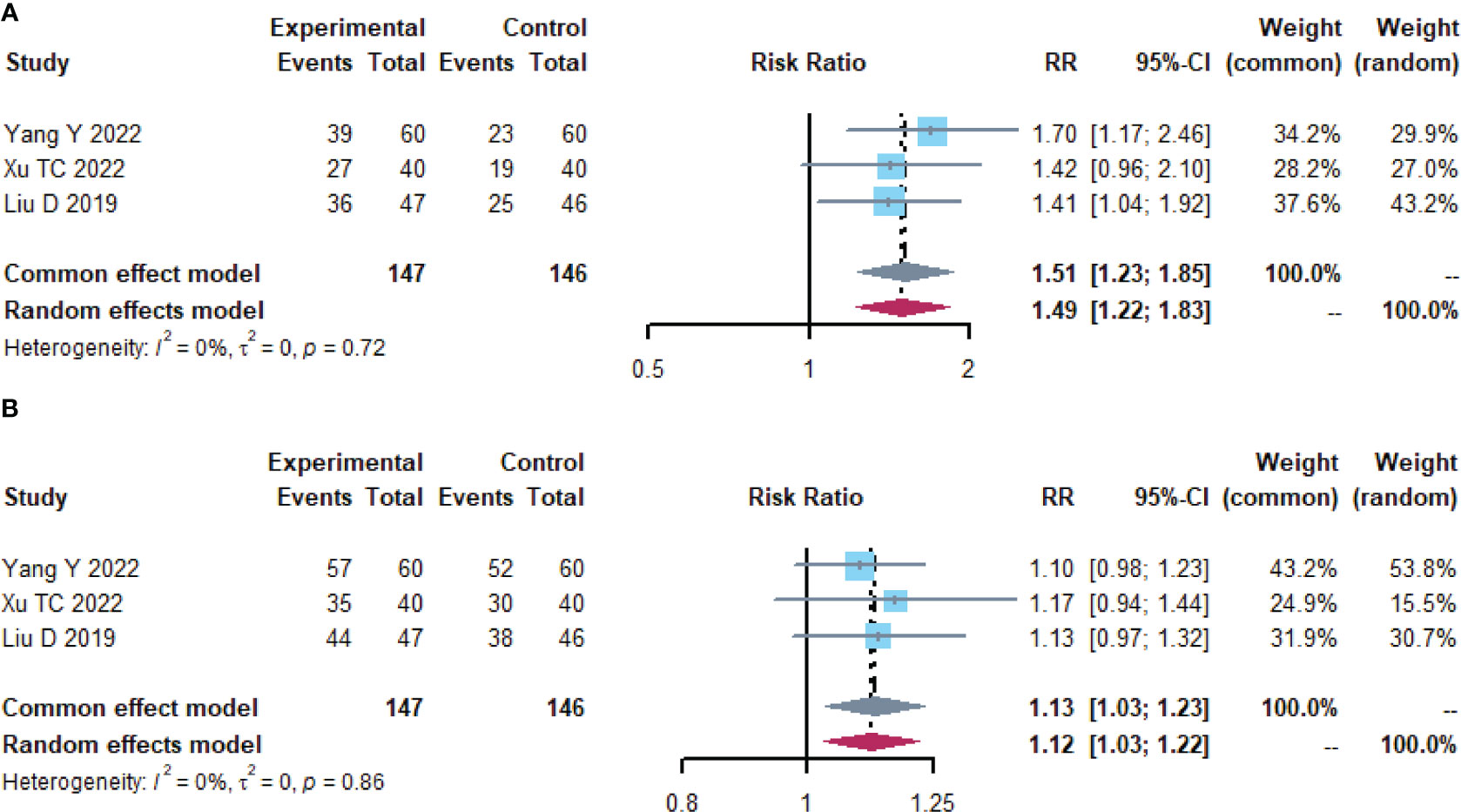
Figure 4 Short-term clinical efficacy. (A) Forest plot of ORR analysis results. (B) Forest plot of DCR analysis results.
3.6 Intestinal mucosal barrier function
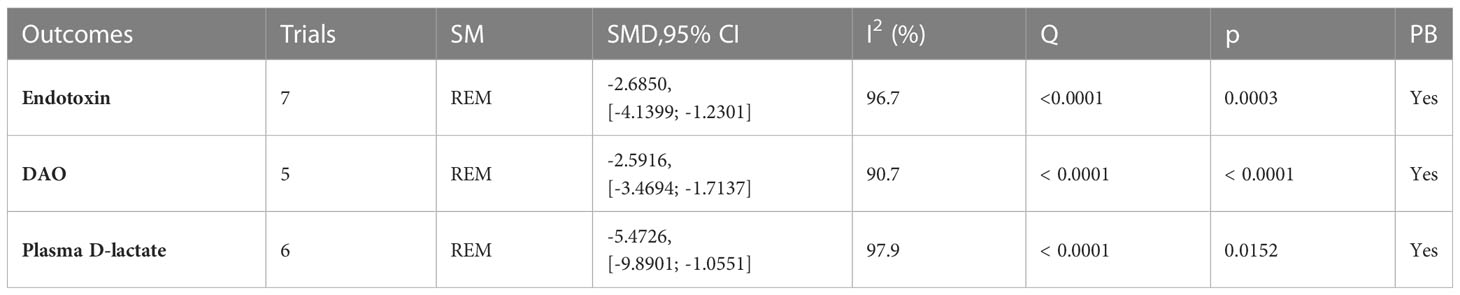
A total of 12 trials (25, 32–34, 36, 37, 42, 46, 49–51, 59) reported the detection of intestinal mucosal barrier-related factors (Table 4; Figures 5, S3), endotoxin (I2 = 96.7), DAO (I2 = 90.7) and plasma D-lactate (I2 = 97.9) were statistically heterogeneous and therefore all were calculated using a random effects model. Meta-analysis results showed that compared to the control group, the microecological preparation group improved endotoxin (SMD, -2.6850[-4.1399; -1.2301], p=0.0003), DAO (SMD, -2.5916, [-3.4694; -1.7137], p<0.0001) and plasma D-lactate (SMD, -5.4726, [-9.8901; -1.0551], p= 0.0152) better.

Figure 5 Intestinal mucosal barrier function. (A) Forest plot of endotoxin analysis results. (B) Forest plot of DAO analysis results. (C) Forest plot of plasma D-lactate analysis results.
3.7 Immune function
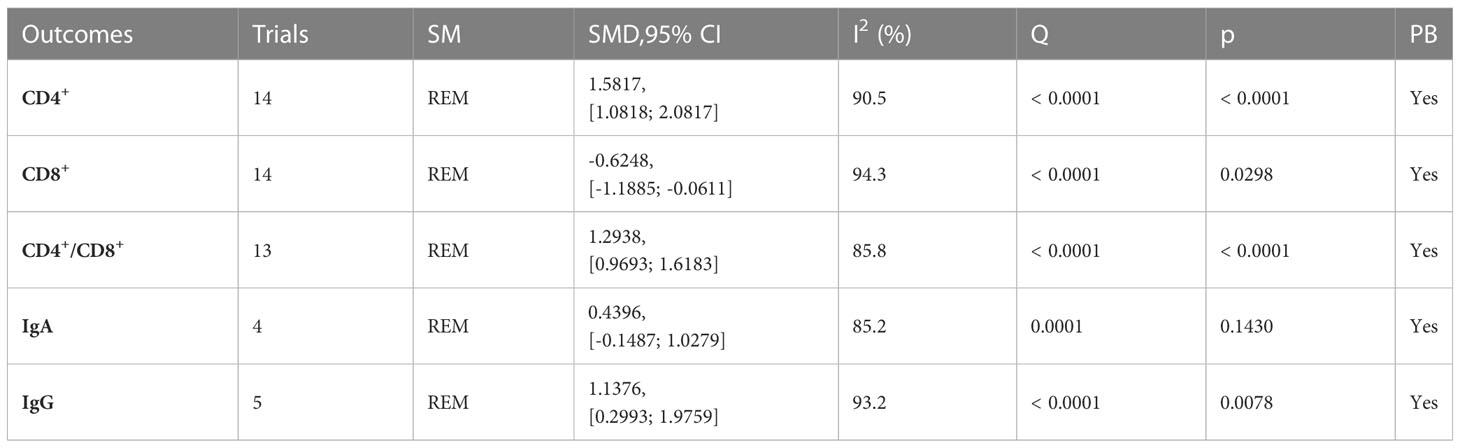
A total of 19 trials (23, 24, 26–34, 38–40, 43, 48, 55, 59, 60) reported immune function-related indices (Table 5; Figures 6, S4), CD4+(I2 = 90.5), CD8+ (I2 = 94.3), CD4+/CD8+ (I2 = 85.8), IgA (I2 = 85.2), IgG (I2 = 93.2) were statistically heterogeneous, so a random-effects model was used. Meta-analysis results showed that the microecological preparation group improved CD4+ (SMD, 1.5817 [1.0818; 2.0817], p< 0.0001) CD4+/CD8+ (SMD, 1.2938[0.9693; 1.6183] p< 0.0001) and IgG (SMD, 1.1376[0.2993; 1.9759] p=0.0078) compared to the control group. The difference between the two groups for CD8+ (SMD, -0.6248[-1.1885; -0.0611] p=0.0298) and IgA (SMD, 0.4396 [-0.1487; 1.0279], p= 0.1430) were not statistically significant.
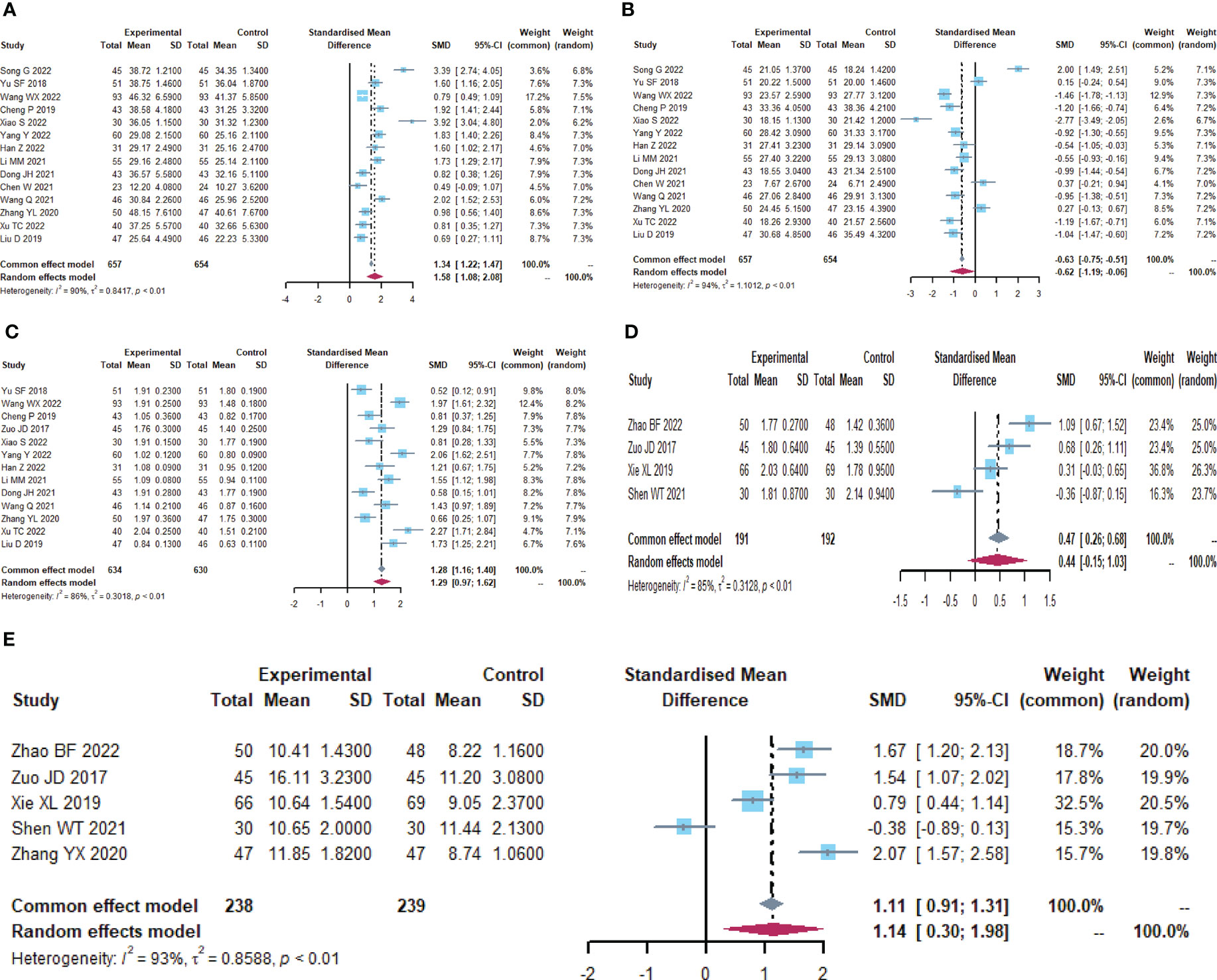
Figure 6 Immune function (A) Forest plot of CD4+ analysis results. (B) Forest plot of CD8+ analysis results. (C) Forest plot of CD4+/CD8+ analysis results. (D) Forest plot of IgA analysis results. (E) Forest plot of IgG analysis results.
3.8 Inflammatory factors
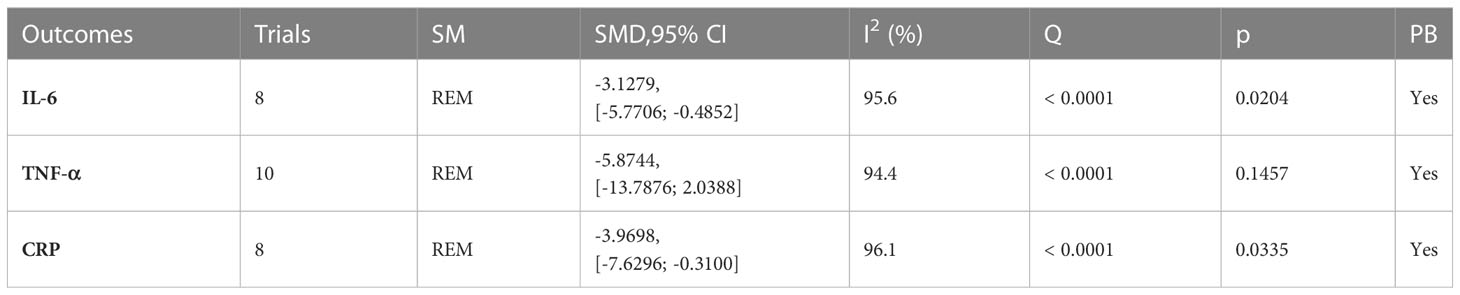
A total of 12 trials (23, 24, 26, 28, 30, 32, 34, 38, 42, 44, 55, 56) reported on inflammatory factor-related indices (Table 6; Figures 7, S5). IL-6 (I2 = 95.6), TNF-α (I2 = 94.4), and CRP (I2 = 96.1) were statistically heterogeneous, so a random-effects model was chosen. IL-6 (SMD, -3.1279[-5.7706; -0.4852], p=0.0204) and CRP (SMD, -3.9698[-7.6296; -0.3100], p=0.0335) were improved in the microecological preparation group compared to the control group. no statistical difference was found in TNF-α (SMD, -5.8744[-13.7876; 2.0388], p=0.1457) between the two groups.

Figure 7 Inflammatory factors. (A) Forest plot of IL-6 analysis results. (B) Forest plot of TNF-α analysis results. (C) Forest plot of CRP analysis results.
3.9 Publication bias analysis
Funnel plots were used to examine Lactobacillus, Bifidobacterium, Escherichia coli, Enterococcus faecalis, Lung infection, Incisional infections, Nausea, Vomiting. Diarrhea, CD4+, CD8+, CD4+/CD8+, publication bias of TNF-α (Figure 8).
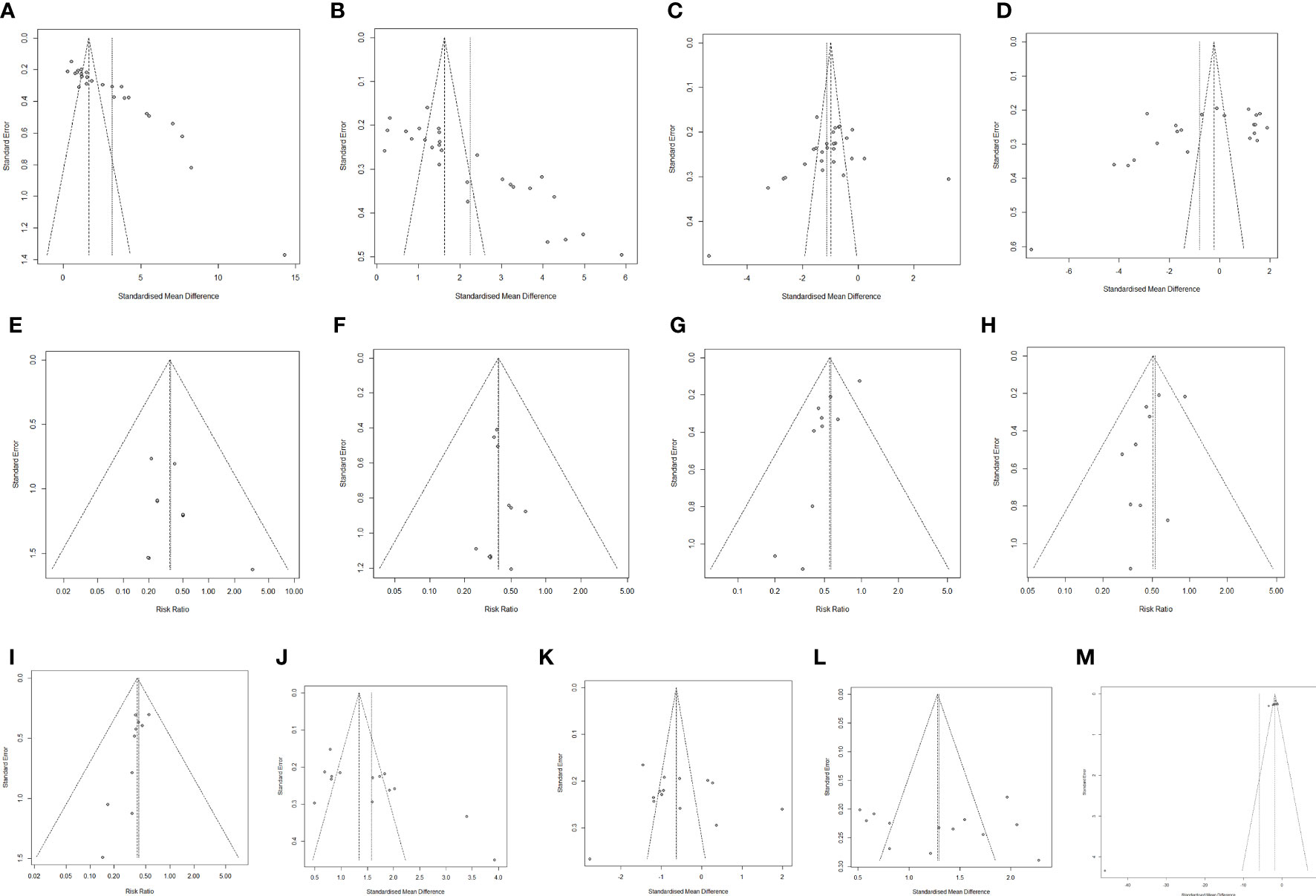
Figure 8 Publication bias analysis. (A) Lactobacillus. (B) Bifidobacterium. (C) Escherichia coli. (D) Enterococcus faecalis. (E) Lung infection (F) Incisional infections. (G) Nausea. (H) Vomiting. (I) Diarrhea. (J) CD4+. (K) CD8+. (L) CD4+/CD8+. (M) TNF-α.
3.10 Sensitivity analysis
To assess the stability of the results. The meta-analysis of the remaining literature was combined after sequentially excluding one literature, and the changes in the combined results were observed to assess whether the results of the original meta-analysis were significantly changed by certain studies (Figure S6).
4 Discussion
In recent years, a growing number of studies have shown that microecological agents can be used to treat CRC and alleviate side effects due to treatment. Meta-analysis included 38 trials containing 3,234 patients to assess whether the addition of microecological agents is beneficial in improving outcome indicators in the perioperative period of CRC.
The development of CRC is strongly associated with disturbances in the gut microbiota. The data showed that the microecological preparation group was beneficial in improving the ecological dysbiosis brought about by various treatments received by CRC patients in the perioperative period, increasing the number of beneficial flora such as Lactobacillus and Bifidobacterium, while reducing harmful flora such as E. coli. However, under certain conditions, it may have the opposite effect. Bifidobacteria may play an important role in altering host metabolism during parasitic infections, thereby promoting the development of cholangiocarcinoma (CC) (61). In intrahepatic cholangiocarcinoma (ICC), Lactobacillus and Alloscardovia were positively correlated with taurocholanol deoxycholic acid. Plasma tauroarsodeoxycholic acid was negatively correlated with Pseudomonas spp. and survival time, but positively correlated with vascular invasion (17, 62).
CRC surgery itself and the stress response to surgery can affect patients’ postoperative recovery as well as short-term complications. Compared with the control group, the microecological preparation group was able to reduce many types of adverse drug reactions such as infections and gastrointestinal discomfort. The intestinal microflora can influence the efficacy and adverse effects of chemotherapeutic drugs by regulating the body’s immune response (63, 64), regulating the body’s hormone levels (65, 66), regulating the body’s metabolic levels (67, 68), and regulating the metabolism and transport of chemotherapeutic drugs (69–72).Therefore, appropriate supplementation of probiotics, prebiotics or synbiotics by micro-ecological means is beneficial to regulate the homeostasis of the intestinal microflora and thus reduce the adverse effects of chemotherapeutic drugs.
At the same time, the microecological agents also reduced the inflammatory response, decreased the increase of harmful metabolites, enhanced the immune function of patients, and improved short-term clinical outcomes. The harmful metabolites of the gut flora, such as ammonia, phenols and p-cresol, are involved in the development and progression of cancer through chronic inflammation and DNA damage (73, 74). For example, high levels of lipopolysaccharide (LPS), entering the bloodstream can cause a number of severe pathophysiological responses, including fever, coagulation and shock, by disrupting the host’s immune, complement and coagulation systems (75). Primary bile acids enter the large intestine and are converted by intestinal bacteria into secondary bile acids, a class of metabolites with pro-cancer effects that can promote tumour development by stimulating oxidative stress (e.g. reactive oxygen species and reactive nitrogen species), inducing cellular DNA damage and activating EGFR and NF-κB (76–78).
5 Conclusion
The subtle interactions between the intestinal flora and human physiology can influence multiple aspects of health. Microbial-epithelial interactions can maintain intestinal barrier function, modulate resistance to infection and intestinal immune function, and maintain host metabolism.
CRC is one of the top three causes of cancer deaths worldwide, and surgery is the primary treatment for colorectal cancer. However, trauma, disturbance of normal intestinal flora, decreased intestinal mucosal barrier function, increased systemic inflammation, decreased immune function, and also the risk of postoperative infection may occur after surgery (79).
Probiotics have antitumor activity by a variety of mechanisms. The most common probiotic flora are two genera of Lactobacilli and Bifidobacteria, which are naturally present in the human digestive system. For example, antioxidants produced by Lactobacilli are able to fight against angiogenic factors, reduce DNA damage, reduce inflammation and tumor size, and inhibit the expression of tumor-specific proteins and polyamine components (80). In addition, prebiotics are fermentable components present in foods that alter the composition and activity of the intestinal microbiota and promote host health. One of the most commonly used prebiotics is resistant starch, which increases the biological activity of a wide range of probiotic bacteria, especially bifidobacteria, and modifies the immune response (81). Prebiotics are organic substances that are not digested or absorbed by the host, but can selectively promote the metabolism and proliferation of beneficial bacteria in the body, thereby improving the health of the host. Commonly used prebiotics include Fructo oligosaccharide, xylo-oligosaccharides and inulin. Studies have shown that Fructo oligosaccharide can reduce the number and activity of carcinogenic enzymes and regulate the body’s immune capacity, and the short-chain fatty acids and lactic acid it ferments to produce in the colon can reduce intestinal pH and ammonia concentration, which is conducive to the reduction and inhibition of intestinal spoilage substances (82) Xylo-oligosaccharides can inhibit the invasion of exogenous pathogenic bacteria, improve the body’s immune response and protect the barrier function of the intestinal mucosa (83).
Our study found that in the perioperative period of CRC, a more effective treatment regimen in the microecological agent group was accompanied by reduced adverse drug reactions in patients, improved intestinal flora, improved short-term clinical outcomes, enhanced body immune function, and reduced inflammatory responses. According to the World Health Organization, appropriate doses of probiotics are beneficial to human health. Proper consumption of microecological agents, such as probiotics or prebiotics, may be a promising way to prevent and treat CRC.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
Conceptualization: XW. Methodology: XW. Formal analysis and investigation: XW and LP. Writing—original draft preparation: XW. Writing—review and editing: XW, FW. Funding acquisition: DT and BY. Resources: XW. Supervision: DT and FL. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This work was financially supported by National Natural Science Foundation of China (nos. 82260957, 82274610, 81860819, 81960818),the Key Research on “TCM Modernization” of National Key Research and Development Plan(2019YFC171250401), Guizhou Traditional Chinese Medicine Tumor Inheritance and Science and Technology Innovation Talent Base (No. Deaf leader-[2018] No. 3), Guizhou high-level innovative talent training plan (100 levels) (no. Yankehe Talents [2016] no. 4032), TCM graduate school workstation (No. Teaching and research JYSZ-[2014]018), The Big Health Science and Technology Cooperation Project of the First Affiliated Hospital of Guizhou University of Traditional Chinese Medicine (No. Building branch [2019] 9–2, No. Building branch [2019] 9-2-35, No. Building branch [2019] 9–2-30), Guizhou Province Traditional Chinese Medicine Oncology Inheritance and Scientific and Technological Innovation Talent Team (Qian Kehe Platform Talents [2020]5013), Guizhou Provincial Science and Technology Plan Project (Qianke Foundation ZK[2022] 487, Qianke Foundation ZK[2022] 498,Qianke Foundation [2021] 095).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1229177/full#supplementary-material
References
1. Sharma R, Abbasi-Kangevari M, Abd-Rabu R, Abidi H, Abu-Gharbieh E, Acuna JM, et al. Global, regional, and national burden of colorectal cancer and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol Hepatol (2022) 7(7):627–47. doi: 10.1016/S2468-1253(22)00044-9
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
3. Neugent ML, Hulyalkar NV, Nguyen VH, Zimmern PE, De Nisco NJ. Advances in understanding the human urinary microbiome and its potential role in urinary tract infection. mBio (2020) 11(2):e00218-20. doi: 10.1128/mBio.00218-20
4. Sender R, Fuchs S, Milo R. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell (2016) 164(3):337–40. doi: 10.1016/j.cell.2016.01.013
5. de Vos WM, Tilg H, Van Hul M, Cani PD. Gut microbiome and health: mechanistic insights. Gut (2022) 71(5):1020–32. doi: 10.1136/gutjnl-2021-326789
6. Lloyd-Price J, Mahurkar A, Rahnavard G, Crabtree J, Orvis J, Hall AB, et al. Strains, functions and dynamics in the expanded Human Microbiome Project. Nature (2017) 550(7674):61–6. doi: 10.1038/nature23889
7. de Groot P, Nikolic T, Pellegrini S, Sordi V, Imangaliyev S, Rampanelli E, et al. Faecal microbiota transplantation halts progression of human new-onset type 1 diabetes in a randomised controlled trial. Gut (2021) 70(1):92–105. doi: 10.1136/gutjnl-2020-322630
8. Kootte RS, Levin E, Salojärvi J, Smits LP, Hartstra AV, Udayappan SD, et al. Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell Metab (2017) 26(4):611–9.e6. doi: 10.1016/j.cmet.2017.09.008
9. Tilg H, Adolph TE, Gerner RR, Moschen AR. The intestinal microbiota in colorectal cancer. Cancer Cell (2018) 33(6):954–64. doi: 10.1016/j.ccell.2018.03.004
10. Wu Y, Wang T. Progress of research on the effect of perioperative treatment of colorectal cancer on intestinal flora. Shandong Med J (2022) 62(10):5. doi: 10.3969/j.issn.1002-266X.2022.10.023
11. Bhandari A, Woodhouse M, Gupta S. Colorectal cancer is a leading cause of cancer incidence and mortality among adults younger than 50 years in the USA: a SEER-based analysis with comparison to other young-onset cancers. J Invest Medicine: Off Publ Am Fed Clin Res (2017) 65(2):311–5. doi: 10.1136/jim-2016-000229
12. Singh S, Singh M, Gaur S. Probiotics as multifaceted oral vaccines against colon cancer: A review. Front Immunol (2022) 13:1002674. doi: 10.3389/fimmu.2022.1002674
13. Khani S, Hosseini HM, Taheri M, Nourani MR, Imani Fooladi AA. Probiotics as an alternative strategy for prevention and treatment of human diseases: a review. Inflammation Allergy Drug Targets (2012) 11(2):79–89. doi: 10.2174/187152812800392832
14. Levy M, Thaiss CA, Elinav E. Metabolites: messengers between the microbiota and the immune system. Genes Dev (2016) 30(14):1589–97. doi: 10.1101/gad.284091.116
15. Hao X, Lv H, Zhang Q, Liu H, Yi F. Progress in the study of gut microbial metabolites mediating intestinal immunity. Feed Res (2023) 1–16.
16. Özdirik B, Müller T, Wree A, Tacke F, Sigal M. The role of microbiota in primary sclerosing cholangitis and related biliary Malignancies. Int J Mol Sci (2021) 22(13):6975. doi: 10.3390/ijms22136975
17. Jia X, Lu S, Zeng Z, Liu Q, Dong Z, Chen Y, et al. Characterization of gut microbiota, bile acid metabolism, and cytokines in intrahepatic cholangiocarcinoma. Hepatol (Baltimore Md) (2020) 71(3):893–906. doi: 10.1002/hep.30852
18. Shah T, Baloch Z, Shah Z, Cui X, Xia X. The intestinal microbiota: impacts of antibiotics therapy, colonization resistance, and diseases. Int J Mol Sci (2021) 22(12):6597. doi: 10.3390/ijms22126597
19. Durán-Lobato M, Niu Z, Alonso MJ. Oral delivery of biologics for precision medicine. Advanced materials (Deerfield Beach Fla) (2020) 32(13):e1901935. doi: 10.1002/adma.201901935
20. Guarner F, Schaafsma GJ. Probiotics. Int J Food Microbiol (1998) 39(3):237–8. doi: 10.1016/S0168-1605(97)00136-0
21. Yu L, Ma L, Du Y, Liu S, Ping Y, Hu Y, et al. Advances in microecological preparations. Chin J Microecolog (2012) 24(01):84–6.
22. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open medicine: peer-reviewed independent open-access J (2009) 3(3):e123–30. doi: 14306/renhyd.18.3.114
23. Xiao S. Analysis of the significance of oxaliplatin in combination with Bifidobacterium trisporium capsules on immune function in postoperative colorectal cancer patients. Modern Drug Appl China (2022) 16(13):3. doi: 10.14164/j.cnki.cn11-5581/r.2022.13.039
24. Zhao B. A randomized controlled study of compound probiotics on postoperative rehabilitation of patients with colorectal cancer. [master’s thesis]. Guilin Medical College (2022) 9–19. doi: 10.27806/d.cnki.gglyx.2022.000026
25. Hou X. Study on the effect of Bifidobacterium bifidum triptans capsules on postoperative intestinal mucosal function in colorectal cancer patients. Sci Tech Inf Gansu (2022) 003):051. doi: 10.3969/j.issn.1672-6375.2022.03.025
26. Yang Y, He Y, Jia F, Li E, Zhi J. Effects of Bifidobacterium triple viable capsule combined with FOLFOX regimen on intestinal micro-ecology and anti-tumor immune response after colorectal cancer surgery. J Hunan Normal University(Medical Sciences) (2022) 19(01):95–8.
27. Han Z, Wang J, Li H, Wang J, Sun Y. Effect of bifidobacterium combined with adjuvant chemotherapy on intestinal flora and immune function in patients with colorectal cancer. Med Innovation China (2022) 19(01):29–33.
28. Song G. Influence of hypodermic long-standing vaccum drainage combined with microecological preparation on the incision infected rate and enteric flora change of colorectal cancer patients underwent surgery. Chin J Coloproctology (2022) 42(1):3.
29. Xu T. Effect of bifidobacterium triptans combined with rapid rehabilitation care on intestinal flora and CD4 CD8 CD4 _CD8 levels in postoperative patients with colorectal cancer. Primary Care Forum (2022) 026):026. doi: 10.19435/j.1672-1721.2022.26.042
30. Wang W, Xie J, Wang Y. Bifidobacterium tetrakis tablets in combination with enteral nutrition emulsion Rexin in postoperative colon cancer. Pract Clin J Integrated Traditional Chin Western Med (2022) 22(14):4. doi: 10.13638/j.issn.1671-4040.2022.14.007
31. Li M. Influence of bifibacterium tripletvaccine combined with XELOX scheme of chemotherapy on postoperative T-cell subpopulation and intestinal flora of colorectal cancer patients. Chin J Coloproctology (2021) 41(3):3.
32. Dong J, Cheng X, Yang Z, Li M, Yu K. Curative efficacy of bifidobacterium triple viable capsule combined with oxaliplatin in treatment of postoperative colorectal cancer and its effects on intestinal flora and immune function. Med J West China (2019) 31(3):5.
33. Chen W. Effect of probiotics combind with enteral nutrition on postoperative chemotherapy in patients with colorectal cancer. [master’s thesis]. Hubei Medical College (2022) 10–22. doi: 10.27913/d.cnki.ghyby.2021.000038
34. Wang Q, Gong L, Zheng H. Effects of live Clostridium butyricum tablets on intestinal flora balance, toxic and side effects, and immune inflammatory indexes in colorectal cancer patients on postoperative FOLFOX4 chemotherapy. World Chin J Digestology (2021) 29(08):435–42. doi: 10.11569/wcjd.v29.i8.435
35. Liu Y. Relationship between intestinal microbial community and prognosis in patients with advanced colorectal cancer chemotherapy. Chin J Microecology (2021) 33(08):958–61 + 66. doi: 10.13381/j.cnki.cjm.202108019
36. Li Y, Wu C, Li H. The status of intestinal flora and the treatment of Bifidobacterium after laparoscopic operation in patients with colon cancer. J Hunan Normal University(Medical Sciences) (2021) 18(2):4.
37. Wang J, Wu L. Effect of laparoscopic surgery combined with microecological agents on intestinal flora and intestinal barrier function in patients with colorectal cancer. Chin J Microecology (2020) 32(03):298–301 + 5. doi: 10.13381/j.cnki.cjm.202003012
38. Zhang Y, Zhou X, Gao X. Effect of probiotic intervention on postoperative chemotherapy complications, prognosis and intestinal flora in patients with colon cancer. Modern Interventional Diagnosis Treat Gastroenterology (2020) 25(06):768–71.
39. Liu D, Su W, Wu Z. Effect of Bifidobacterium trivium combined with XELOX chemotherapy on intestinal flora and immune function after colorectal cancer surgery. China Med Herald (2019) 16(32):119–22.
40. Cheng P. Effect of oral probiotic preparations on intestinal flora in patients after radical surgery for colon cancer. Health Prot Promotion (2019) 345(08):31–2. doi: CNKI:SUN:YSXD.0.2019-08-026
41. Liu H, Liu Y, Peng R, Zuo W, Gou J, Li Y, et al. Analysis of the effect of probiotics on intestinal flora in patients undergoing chemotherapy for colon cancer. World Latest Med Inf (2019) 19(45):56. doi: 10.19613/j.cnki.1671-3141.2019.45.027
42. Xu Q, Xu P, Cen Y, Li W. Effects of preoperative oral administration of glucose solution combined with postoperative probiotics on inflammation and intestinal barrier function in patients after colorectal cancer surgery. Oncol Lett (2019) 18(1):694–8. doi: 10.3892/ol.2019.10336
43. Yu S. Effects of preoperative nutritional support combined with intestinal probiotic intervention on patients with colorectal cancer. Med J Natl Defending Forces Southwest China (2018) 28(09):837–9.
44. Li X. Influence of perioperative supplement of Bifid Triple Viable Capsules on inflammatory reactions and intestinal microecology in patients with colorectal cancer. Chin J Microecolog (2018) 30(01):75–8. doi: 10.13381/j.cnki.cjm.201801019
45. Zhan Y. Effect of probiotics on intestinal flora in patients with colon cancer undergoing chemotherapy. China Pract Med (2018) 13(32):105–7. doi: 10.14163/j.cnki.11-5547/r.2018.32.057
46. Liu F. Influence of Bifid Triple Viable Capsules on intestinal flora and intestinal mucosal barrier function of patients with post-operation diarrhea after colostomy. Chin J Microecolog (2017) 29(07):814–7. doi: 10.13381/j.cnki.cjm.201707016
47. Sun C, He Y, He R. Study on the clinical effect of preoperative application of microecological agents for colorectal cance. Electronic J Clin Med Literature (2017) 4(01):34–5. doi: 10.16281/j.cnki.jocml.2017.01.020
48. Zuo J, Liu G, Yuan K. Effect of Bifidobacterium bifidum supplementation with Lactobacillus rhamnosus triplex on intestinal flora after intestinal surgery. China Modern Med (2017) 24(05):25–7.
49. Dai A. Influence of Bifid Triple Viable Capsules on intestinal flora and intestinal permeability after surgery for colorectal cancer. Chin J Microecolog (2016) 28(04):425–8. doi: 10.13381/j.cnki.cjm.201604013
50. Zhang Y, Zhou Z, Huang R. Analysis of the effect of preoperative intestinal flora supplementation with Bifidobacterium trisporus after colorectal cancer surgery. Strait Pharm J (2015) 27(10):155–6.
51. Zhang JW, Du P, Gao J, Yang BR, Fang WJ, Ying CM. Preoperative probiotics decrease postoperative infectious complications of colorectal cancer. Am J Med Sci (2012) 343(3):199–205. doi: 10.1097/MAJ.0b013e31823aace6
52. Liu Z, Qin H, Yang Z, Xia Y, Liu W, Yang J, et al. Randomised clinical trial: the effects of perioperative probiotic treatment on barrier function and post-operative infectious complications in colorectal cancer surgery - a double-blind study. Alimentary Pharmacol Ther (2011) 33(1):50–63. doi: 10.1111/j.1365-2036.2010.04492.x
53. Huang F, Li S, Chen W, Han Y, Yao Y, Yang L, et al. Postoperative probiotics administration attenuates gastrointestinal complications and gut microbiota dysbiosis caused by chemotherapy in colorectal cancer patients. Nutrients (2023) 15(2):356. doi: 10.3390/nu15020356
54. Li Y. Effects of xylooligosaccharide on improving quality of life in colorectal cancer patients receiving chemotherapy by regulating gut microbiota. [master's thesis]. Jiangnan University (2022). 10–16 doi: 10.27169/d.cnki.gwqgu.2022.001569
55. Shen W, Cai G, Luo Y, Li Z, Lin K, Gu H, et al. Effect of microecological agents on gastrointestinal and inflammatory responses in patients undergoing chemotherapy for colorectal cancer. J Shantou Univ Med College (2021) 34(4):4. doi: 10.13401/j.cnki.jsumc.2021.04.005
56. Chen Y, Zhang Y, Che Z. Effect of intestinal microecological intervention on complications after operation and during chemotherapy in patients with colon cancer. J Clin Med Pract (2021) 25(20):73–7.
57. Kotzampassi K, Stavrou G, Damoraki G, Georgitsi M, Basdanis G, Tsaousi G, et al. A four-probiotics regimen reduces postoperative complications after colorectal surgery: A randomized, double-blind, placebo-controlled study. World J Surg (2015) 39(11):2776–83. doi: 10.1007/s00268-015-3071-z
58. Aisu N, Tanimura S, Yamashita Y, Yamashita K, Maki K, Yoshida Y, et al. Impact of perioperative probiotic treatment for surgical site infections in patients with colorectal cancer. Exp Ther Med (2015) 10(3):966–72. doi: 10.3892/etm.2015.2640
59. Zhang Y, Chen Q, Liu Y. Effect of microecological agents on early colorectal cancer after radical resection. Chin J Microecolog (2020) 32(10):1167–72. doi: 10.13381/j.cnki.cjm.202010012
60. Xie X, He Y, Li H, Yu D, Na L, Sun T, et al. Effects of prebiotics on immunologic indicators and intestinal microbiota structure in perioperative colorectal cancer patients. Nutr (Burbank Los Angeles County Calif) (2019) 61:132–42. doi: 10.1016/j.nut.2018.10.038
61. Ketpueak T, Thiennimitr P, Apaijai N, Chattipakorn SC, Chattipakorn N. Association of chronic opisthorchis infestation and microbiota alteration on tumorigenesis in cholangiocarcinoma. Clin Trans gastroenterology. (2020) 12(1):e00292. doi: 10.14309/ctg.0000000000000292
62. Rao B, Ren T, Wang X, Wang H, Zou Y, Sun Y, et al. Dysbiosis in the human microbiome of cholangiocarcinoma. Front Physiol (2021) 12:715536. doi: 10.3389/fphys.2021.715536
63. Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillère R, Hannani D, et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Sci (New York NY) (2013) 342(6161):971–6. doi: 10.1126/science.1240537
64. Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Sci (New York NY) (2013) 342(6161):967–70. doi: 10.1126/science.1240527
65. Sfanos KS, Markowski MC, Peiffer LB, Ernst SE, White JR, Pienta KJ, et al. Compositional differences in gastrointestinal microbiota in prostate cancer patients treated with androgen axis-targeted therapies. Prostate Cancer Prostatic Dis (2018) 21(4):539–48. doi: 10.1038/s41391-018-0061-x
66. Pernigoni N, Zagato E, Calcinotto A, Troiani M, Mestre RP, Calì B, et al. Commensal bacteria promote endocrine resistance in prostate cancer through androgen biosynthesis. Sci (New York NY) (2021) 374(6564):216–24. doi: 10.1126/science.abf8403
67. He Y, Fu L, Li Y, Wang W, Gong M, Zhang J, et al. Gut microbial metabolites facilitate anticancer therapy efficacy by modulating cytotoxic CD8(+) T cell immunity. Cell Metab (2021) 33(5):988–1000.e7. doi: 10.1016/j.cmet.2021.03.002
68. Geng HW, Yin FY, Zhang ZF, Gong X, Yang Y. Butyrate suppresses glucose metabolism of colorectal cancer cells via GPR109a-AKT signaling pathway and enhances chemotherapy. Front Mol Biosci (2021) 8:634874. doi: 10.3389/fmolb.2021.634874
69. Yan A, Culp E, Perry J, Lau JT, MacNeil LT, Surette MG, et al. Transformation of the anticancer drug doxorubicin in the human gut microbiome. ACS Infect diseases (2018) 4(1):68–76. doi: 10.1021/acsinfecdis.7b00166
70. Okuda H, Nishiyama T, Ogura K, Nagayama S, Ikeda K, Yamaguchi S, et al. Lethal drug interactions of sorivudine, a new antiviral drug, with oral 5-fluorouracil prodrugs. Drug Metab Dispos (1997) 25(2):270–3.
71. Scott TA, Quintaneiro LM, Norvaisas P, Lui PP, Wilson MP, Leung KY, et al. Host-microbe co-metabolism dictates cancer drug efficacy in C. elegans. Cell (2017) 169(3):442–56.e18. doi: 10.1016/j.cell.2017.03.040
72. García-González AP, Ritter AD, Shrestha S, Andersen EC, Yilmaz LS, Walhout AJM. Bacterial metabolism affects the C. elegans response to cancer chemotherapeutics. Cell (2017) 169(3):431–41.e8. doi: 10.1016/j.cell.2017.03.046
73. Le Gall G, Guttula K, Kellingray L, Tett AJ, Ten Hoopen R, Kemsley EK, et al. Metabolite quantification of faecal extracts from colorectal cancer patients and healthy controls. Oncotarget (2018) 9(70):33278–89. doi: 10.18632/oncotarget.26022
74. Marchesi JR, Adams DH, Fava F, Hermes GD, Hirschfield GM, Hold G, et al. The gut microbiota and host health: a new clinical frontier. Gut (2016) 65(2):330–9. doi: 10.1136/gutjnl-2015-309990
75. Raetz CR, Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem (2002) 71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414
76. Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature (2013) 499(7456):97–101. doi: 10.1038/nature12347
77. Goodwin AC, Destefano Shields CE, Wu S, Huso DL, Wu X, Murray-Stewart TR, et al. Polyamine catabolism contributes to enterotoxigenic Bacteroides fragilis-induced colon tumorigenesis. Proc Natl Acad Sci USA(2011) 108(37):15354–9. doi: 10.1073/pnas.1010203108
78. Azcárate-Peril MA, Sikes M, Bruno-Bárcena JM. The intestinal microbiota, gastrointestinal environment and colorectal cancer: a putative role for probiotics in prevention of colorectal cancer? Am J Physiol Gastrointestinal Liver Physiol (2011) 301(3):G401–24. doi: 10.1152/ajpgi.00110.2011
79. McMillan DC, Canna K, McArdle CS. Systemic inflammatory response predicts survival following curative resection of colorectal cancer. Br J Surg (2003) 90(2):215–9. doi: 10.1002/bjs.4038
80. Eslami M, Yousefi B, Kokhaei P, Hemati M, Nejad ZR, Arabkari V, et al. Importance of probiotics in the prevention and treatment of colorectal cancer. J Cell Physiol (2019) 234(10):17127–43. doi: 10.1002/jcp.28473
81. Ranadheera R, Baines S, Adams M. Importance of food in probiotic efficacy. Food Res Int (2010) 43(1):1–7. doi: 10.1016/j.foodres.2009.09.009
82. Zhong Y, Chen H. Oligofructose: A new approach to bowel cancer prevention and treatment. Sci Technol Food Industry (2014) 35(17):28–31.
Keywords: colorectal cancer, perioperative period, microecological agents, meta-analysis, intestinal flora
Citation: Wang X, Pan L, Wang F, Long F, Yang B and Tang D (2023) Interventional effects of oral microecological agents on perioperative indicators of colorectal cancer: a meta-analysis. Front. Oncol. 13:1229177. doi: 10.3389/fonc.2023.1229177
Received: 26 May 2023; Accepted: 07 August 2023;
Published: 23 August 2023.
Edited by:
Huang He, Tianjin University, ChinaReviewed by:
Hongwei Yao, Beijing Friendship Hospital, ChinaLi Zhang, University of Minnesota Twin Cities, United States
Copyright © 2023 Wang, Pan, Wang, Long, Yang and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dongxin Tang, dGFuZ2Rvbmd4aW4xMjA0QHNpbmEuY29t
 Xueyan Wang
Xueyan Wang Lijun Pan2
Lijun Pan2 Feiqing Wang
Feiqing Wang Dongxin Tang
Dongxin Tang