- 1Department of Radiology, Suining Central Hospital, Suining, China
- 2Department of Radiology, The Third Hospital of Mianyang, Sichuan Mental Health Center, Mianyang, China
- 3Department of Radiology and Imaging, Institute of Rehabilitation and Development of Brain Function, The Second Clinical Medical College of North Sichuan Medical College, Nanchong Central Hospital, Nanchong, China
- 4Sichuan Key Laboratory of Medical Imaging, Department of Radiology, Affiliated Hospital of North Sichuan Medical College, Nanchong, China
- 5Department of Pathology, Suining Central Hospital, Suining, China
- 6Department of Radiology, Lixian People’s Hospital, Aba, China
Background: Urachal tumors are exceedingly rare, and adenocarcinoma is the most common malignant urachal neoplasm. Here, an especially rare patient of primary urachal leiomyosarcoma from our hospital was reported, and only five patients have been reported thus far since 1981.
Case description: A 24-year-old man was admitted due to urinary tract symptoms. Both urogenital ultrasonography and contrast-enhanced computed tomography showed a mass at the dome of the urinary bladder. Laparoscopic surgical resection was performed, and histopathologic examination of the mass confirmed the diagnosis of urachal leiomyosarcoma. No recurrence was noted after one and a half years.
Conclusions: Because the leiomyosarcoma located in the extraperitoneal space of Retzius and may manifest with nonspecific abdominal or urinary symptoms, early and definitive preoperative diagnosis is challenging. Partial cystectomy with complete excision of the urachus is recommended. Because only a few patients have been recorded, clinical outcomes and recurrence risks are difficult to assess.
Introduction
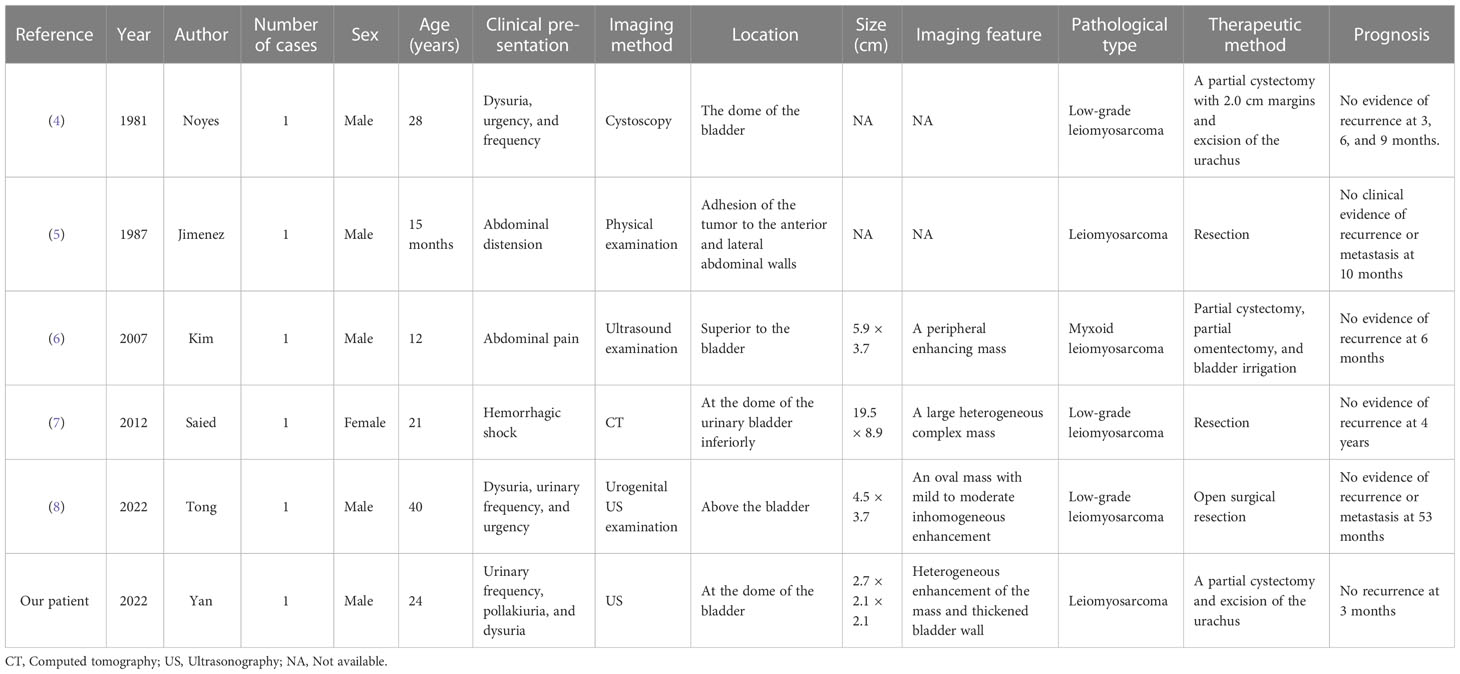
The urachus is a ductal remnant that arises embryologically, originating from the involution of the allantois and cloaca and extending between the bladder dome and the umbilicus (1). The tubular urachus normally involutes before birth remaining as a fibrous band with no known function (2). It is located in the loose connective tissue between the transversalis fascia and parietal peritoneum. Patients with urachal disease are usually asymptomatic because of its anatomical characteristics. Urachal diseases are usually found in conjunction with an infection or in urinary or other past abdominal and pelvic symptoms. The detection rate of urachal leiomyosarcomas is very low. Furthermore, urachal carcinoma accounts for < 1% of all bladder tumors, and approximately 80% of urachal carcinomas (UrC) are adenocarcinomas (3). We report a particularly rare patient of urachal leiomyosarcoma with a literature review conducted to shed more light on this disease (Table 1) (4–8).
Case presentation
A 24-year-old man manifested the symptoms of dysuria, urinary frequency, and urgency for more than a week. Urogenital ultrasonography (US) revealed a hypoechoic mass at the dome of the bladder. The patient was treated with anti-inflammatory therapy and discharged from the local hospital after improvement. Thereafter, the patient sought consultation at our hospital for further evaluation. Contrast-enhanced abdominal computed tomography (CT) showed a heterogeneous 2.7 cm × 2.1 cm × 2.1 cm mass at the dome of the bladder (Figure 1). The boundary of the mass was obscure, and the adjacent bladder wall was irregularly thickened. Inhomogeneous enhancement of the mass revealed a thickened bladder wall in the arterial and venous phases. Putative clinical diagnosis included urachal adenocarcinoma, squamous cell carcinoma, urachal cystic mass, or abscess. Cystoscopy showed that the tumor had invaded the dome and right-side bladder wall, and the biopsy revealed some normal urothelium. Chest CT and whole-body positron emission tomography/computed tomography (PET/CT) showed no metastasis of tumor. After sufficient preoperative preparation, a partial cystectomy and excision of the urachus were scheduled for both definitive diagnosis and therapeutic purposes. The intraoperative findings are: the tumor had infiltrated the whole urachus and adhered to the peritoneum; the tumor was located at the dome of the bladder, infiltrating the bladder wall, with a size of 4.2 cm × 3.5 cm × 3.5 cm; the tumor was fish-meat like appearance; no necrosis or bleeding was noted. Pathological and immunohistochemical examinations (desmin (+), SMA (+), Bcl-2 (–), MyoD1 (-), myogenin (-), CD117 (-), CD34 (-), DOG1 (-), S100 (-), PHH3 (+, with mitotic activity), Ki-67 (+, 20%)) of the mass confirmed the diagnosis of a leiomyosarcoma originating from the urachus (Figure 2). Our diagnostic criteria for interpreting a carcinoma as of urachal origin are (9, 10): (a) tumor located in the dome or anterior bladder wall; (b) tumor growth in the bladder wall; (c) absence of atypical intestinal metaplasia and cystitis/glandularis beyond the dome/anterior wall; (d) absence of a urothelial bladder neoplasia; (e) exclusion of a primary adenocarcinoma of a different origin. Some urachal remnants were identified within the tumor tissue that near the abdominal wall side, covered with columnar and transitional epithelium cells. The result is in accordance with the consensus statement by the Canadian Urological Association and Genitourinary Medical Oncologists of Canada (10). In the consensus, urachal remnants were reported in only 15%-62.5% of patients; their absence should not be considered exclusionary for a urachal primary. The patient was discharged after a week (Figure 3), and no recurrence was noted at one and a half years (Figure 4).
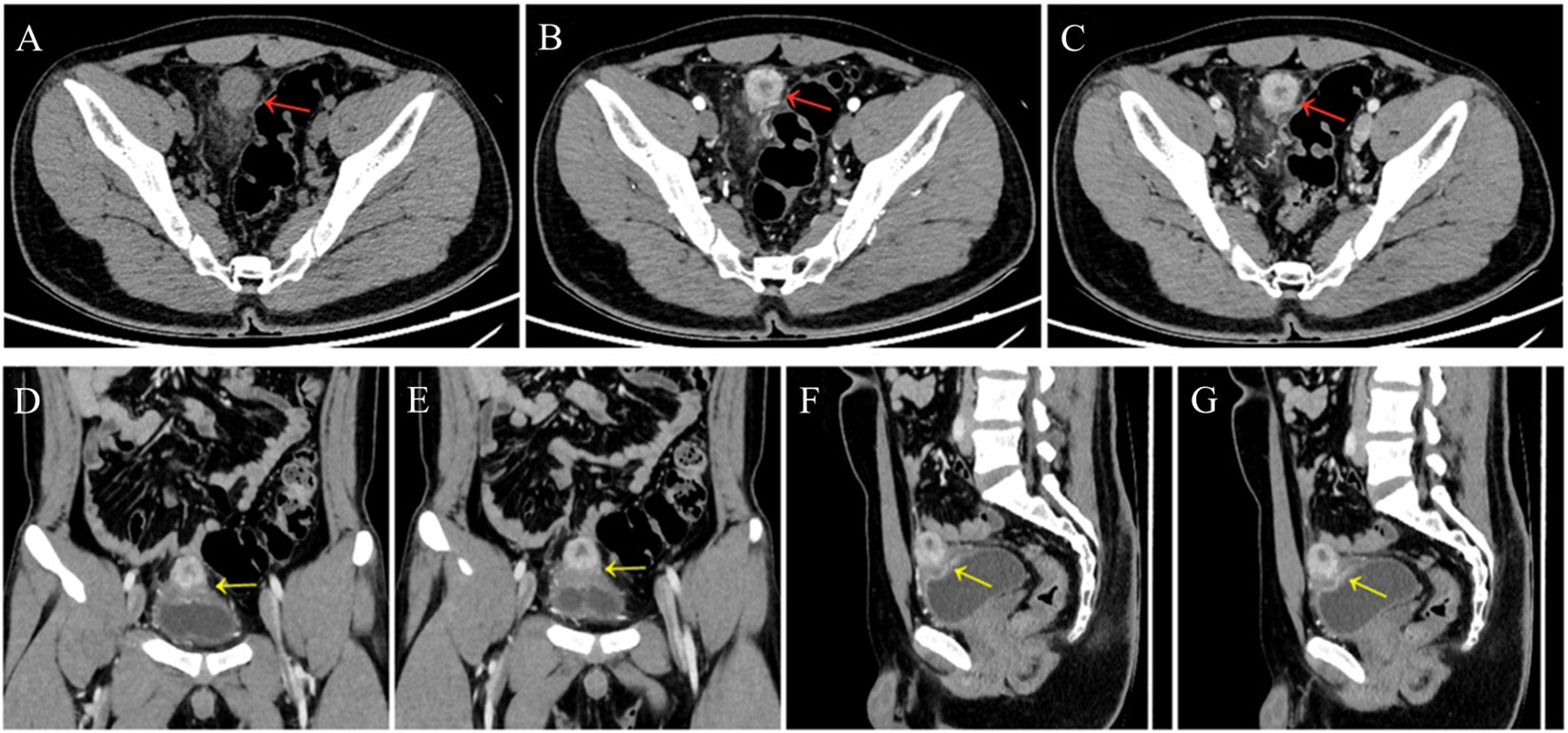
Figure 1 A 24-year-old man presented a slightly hypodense mass (red arrows) at the dome of the bladder (A), at arterial phase (B, D, F) and venous phase (C, E, G); contrast-enhanced CT scan showed peripheral enhancement of the mass and no enhancement of necrosis. Coronal (D, E) and sagittal (F, G) images showed the thickened bladder wall (yellow arrows).
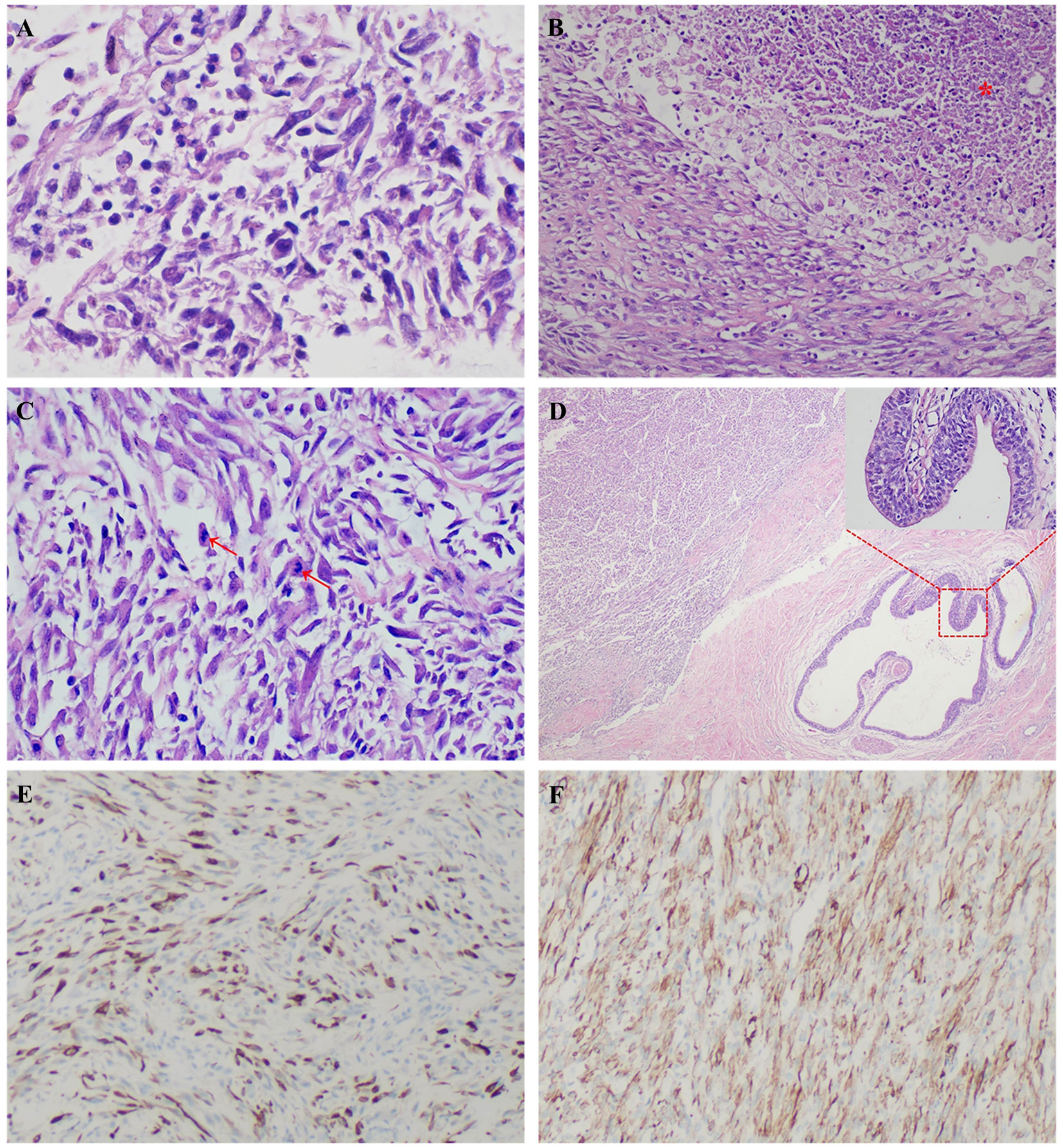
Figure 2 A 24-year-old man was diagnosed with a primary urachal leiomyosarcoma. Microscopic view showed irregular intersecting bundles of spindle cells with mild cell pleomorphism (A), HE × 600), which indicating a well differentiated leiomyosarcoma. There was necrosis observed in this tumor (B), red star, HE × 200). Some of the nuclei showed atypia (C), red arrows, HE × 400). Some urachal remnants were identified within the tumor tissue that near the abdominal wall side (D), HE × 40), covered with columnar and transitional epithelium cells (D), red square, HE × 400). Immunohistochemical stains showed positivity for desmin (E), × 100) and smooth muscle actin (SMA, (F), ×100), compatible with leiomyosarcoma.
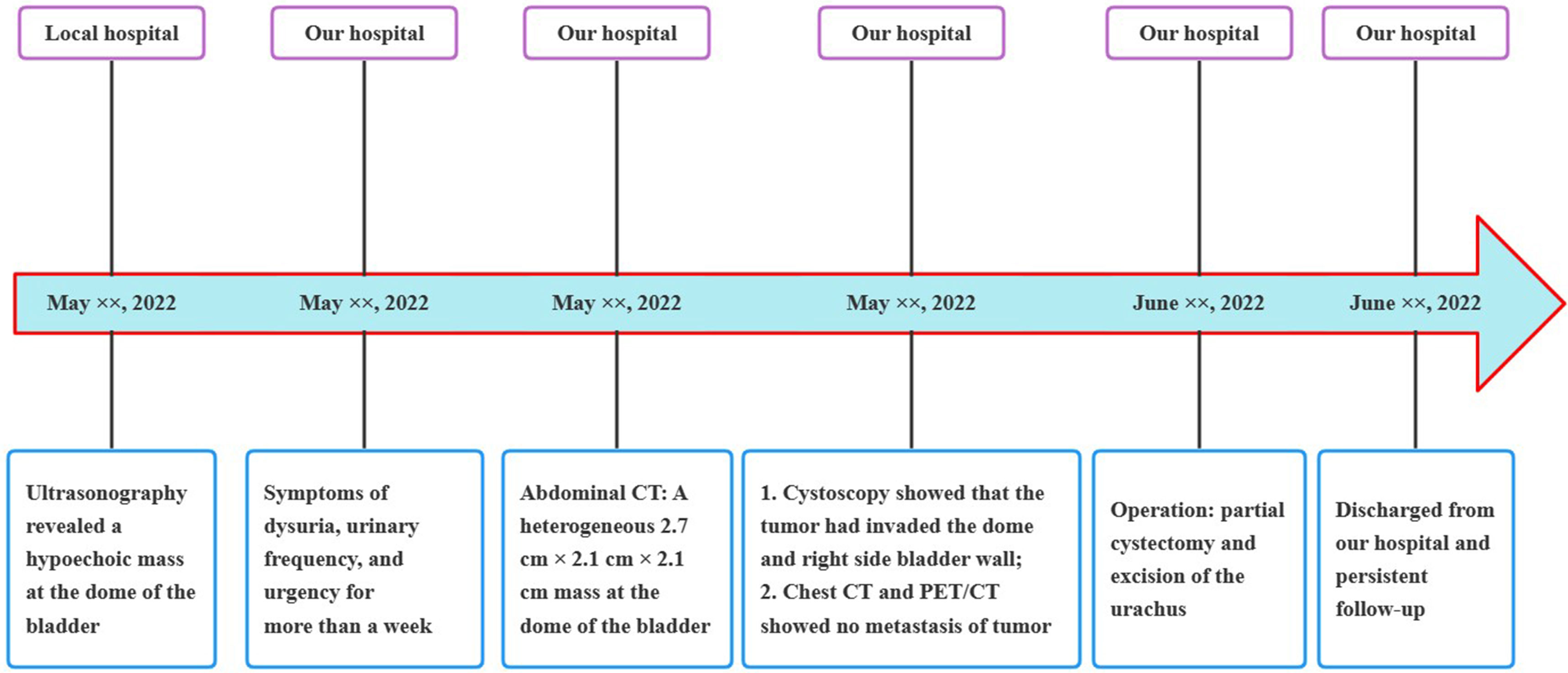
Figure 3 Medical timeline of the patient with primary urachal leiomyosarcoma. CT, computed tomography; PET/CT, positron emission tomography/computed tomography.
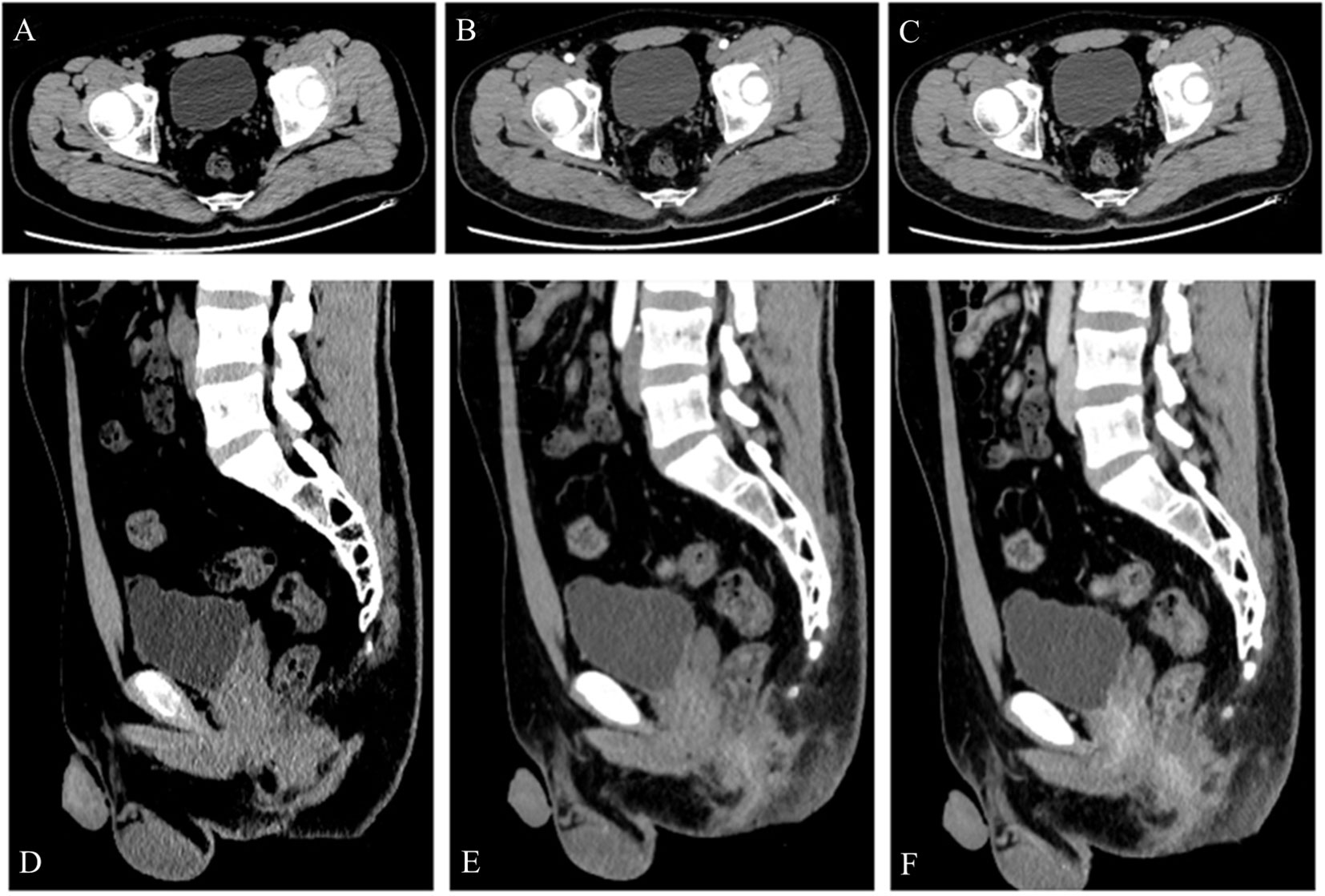
Figure 4 A 24-year-old man was diagnosed with a urachal leiomyosarcoma. One and a half years after surgery, contrast-enhanced CT examination (A–F) showed no recurrence of the tumor.
Discussion
Clinical features
The urachus is a 3.0 - 10.0 cm tubular structure extending from the allantois to the early fetal bladder. During embryonic development, the upper part of the allantois will be reduced gradually. After birth, it is replaced by fibromuscular tissue, also known as the median umbilical ligament. In patient of partial obliteration of the urachus, various diseases including fistula, diverticulum, or tumor may occur. The tumor primarily occurs in males and is usually localized in the bladder dome or anterior wall. Urachal adenocarcinoma is the most common pathological type (up to 80%), with an occasional occurrence of squamous cell carcinoma (1). The patient of urachal leiomyosarcoma is extremely rare. Urachal leiomyosarcomas are typically silent because of their extraperitoneal location, but initial symptoms of infection, local invasion, or metastatic disease may include hematuria, irritative voiding symptoms, and suprapubic pain (2, 11). Prognosis is generally poor due to the anatomic location, late onset of symptoms, and high risk of metastasis. Tumor grade and surgical margin are the most important prognostic factors.
Histologic and pathologic features
Leiomyosarcoma is a malignant neoplasm characterized by smooth muscle differentiation. The World Health Organization (WHO) proposed a histological classification of leiomyosarcoma which is divided into myxoid and epithelioid leiomyosarcoma (12). Leiomyosarcomas of the retroperitoneal region, digestive tract, and uterus are more commonly observed. Urachal leiomyosarcoma derived from the smooth muscle tissue of the urachus is considered extremely rare. Similar to leiomyosarcoma in other parts of the body, urachal leiomyosarcomas usually demonstrate noticeable interlacing bundles of muscle fascicles with spindle cells that have cigar-shaped nuclei and abundant eosinophilic cytoplasm. Most tumors have pleomorphism, necrosis, and a high frequency of mitoses. Immunohistochemical studies showed that tumor cells are immunopositive to smooth muscle actin and desmin, but are immunonegative to keratin, S-100, HMB-45, MyoD1 and myogenin. Myogenic markers such as actin and desmin are also expressed in rhabdomyosarcoma and non-myogenic sarcoma. MyoD1 and myogenin are useful in deafferenting leiomyosarcoma from rhabdomyosarcoma, as they are regulatory transcriptional factors expressed in the early stages of skeletal muscle differentiation. Most studies have confirmed that they are sensitive and specific immunohistochemical markers for rhabdomyosarcoma, and an overexpression of myogenin particularly facilitates the diagnosis (13). Therefore, when myogenin is negatively expressed, it is a very useful marker to help identify rhabdomyosarcoma. Expressions of MyoD1 and myogenin in the tumor of our patient both are negative. Leiomyosarcoma is usually graded according to the three-level classification standard of the French Federation of Cancer Centers Sarcoma Group. The local recurrence rate or metastasis is related to the tumor grade. Primary urachal leiomyosarcoma has no specific imaging findings. Therefore, the diagnosis of urachal leiomyosarcoma is often difficult and delayed. Moreover, the final diagnosis is confirmed through pathologic examination.
Imaging diagnostic modalities
US is the preferred method in the detection of urachal leiomyosarcoma. US can show the urachal tract between the bladder dome and the umbilicus allowing localization of the tumor. The CT appearance of a leiomyosarcoma is not specific; necrotic or cystic change is usually observed and reveals no enhancement; therefore, it shows an uneven and avid enhancement on contrast-enhanced CT. On MRI, the urachal leiomyosarcoma is reported to be isointense on T1-weighted imaging (T1WI) and heterogeneously hyperintense on T2-weighted imaging (T2WI). A high signal intensity on T2WI is associated with fluid, necrosis, or mucin. The contrast-enhanced MRI findings are similar to that of the contrast-enhanced CT.
Recent studies demonstrate that 18F-fluorodeoxyglucose (FDG) PET/CT may be helpful for diagnosing primary and recurrent urachal adenocarcinoma (14). On 18F-FDG PET/CT, the primary tumor and metastasis are classified as 18F-FDG-avid (above the local background). In identifying the primary focus, FDG PET/CT does not seem to yield additional information compared with CT or MR. However, it may detect metastatic lesions not seen on other imaging modalities, causing changes in tumor stage and patient management (15).
Staging
Currently, there are some different staging approaches (e.g., Sheldon, Mayo, and modified TNM staging systems) for urachal cancer, but none of them have been fully validated. The Sheldon staging system is complex and has eight categories, while the Mayo system is simpler and more proportional for patient distribution. Based on the Mayo system, urachal cancer is classified into four stages: Stage I, tumor confined to urachus and/or bladder; Stage II, tumor extending beyond the muscular layer of urachus and/or bladder; Stage III, tumor infiltrating the regional lymph node; and Stage IV, tumor infiltrating non-regional lymph nodes or distant sites (16). Therefore, our patient can be classified as the stage II of the Mayo system.
Compared with other modalities for staging of urachal cancer, MRI has more advantages in soft tissue resolution and multi-plane imaging capabilities. For primary UrC, MRI can provide the tumors’ location, size, morphology, extent of peritoneal tissue, and/or local structures beyond bladder invasion. For recurrent UrC, it can accurately evaluate recurrence and metastatic patterns. Studies have shown Mayo stage at MRI is highly consistent with pathology (17).
Notably, UrC are often mucinous in histology, and this can lead to low or absent 18F-FDG uptake due to hypocellularity of tumors which may limit the sensitivity of 18F-FDG PET/CT (18). Physicians and PET readers should realize the potential pitfall in trying to stage urachal cancer either in the context of primary or recurrent urachal tumors and consider combining with other imaging modalities such as MRI, to further evaluate mucinous malignancy (19).
Differential diagnosis
Urachal carcinoma commonly occurs in the middle-aged and elderly people and primarily in men (20). Despite the fact that the urachus is lined by urothelium, more than 80% of UrC are adenocarcinomas (1). On US, Urachal adenocarcinomas usually appear as a midline soft-tissue mass over the apex of the bladder with complex echogenicity. On CT, the mass can be cystic, solid, or mixed. It usually appears as a midline mass near the bladder dome with areas of low attenuation representing mucin content. Approximately 50% - 70% of tumors showed calcification (21). On MRI, sagittal images may be the best modality for the evaluation of urachal neoplasms. The solid part of the lesion appeared as isointense on T1WI and T2WI compared to soft tissue. There is also a high signal intensity area on T2WI images that is associated with mucin content. In gadolinium diethylenetriamine pentaacetic acid (Gd-DTPA) enhancement scan, the solid part is enhanced, and the area of T2WI hyperintensity will demonstrate enhancement of glandular tissue which can be differentiated from fluid or necrosis (1). In recent years, MRI has become one of the most used modalities in bladder cancer evaluation (22). Most of the literature, though, on the MR imaging of UrC are case reports and reviews. The research on clinical applications of MRI is limited. A recent study (17) showed that for primary UrC, MRI is able to accurately identify the tumor location (in relation to the bladder), morphology, maximum diameters, invasion of adjacent structures, Mayo stage on MRI, and multiparametric MRI features of urachal tumor; for recurrent UrC, MRI can effectively evaluate the local tumor recurrence (location and MRI features), detect lymph nodes, and distant metastasis (23). Therefore, MRI can offer effective assessment to preoperative staging, detection of local recurrence, and aid in the evaluation of the tumor response in the treatment (24).
A detailed description of some urachal neoplasms is summarized in Table 2.
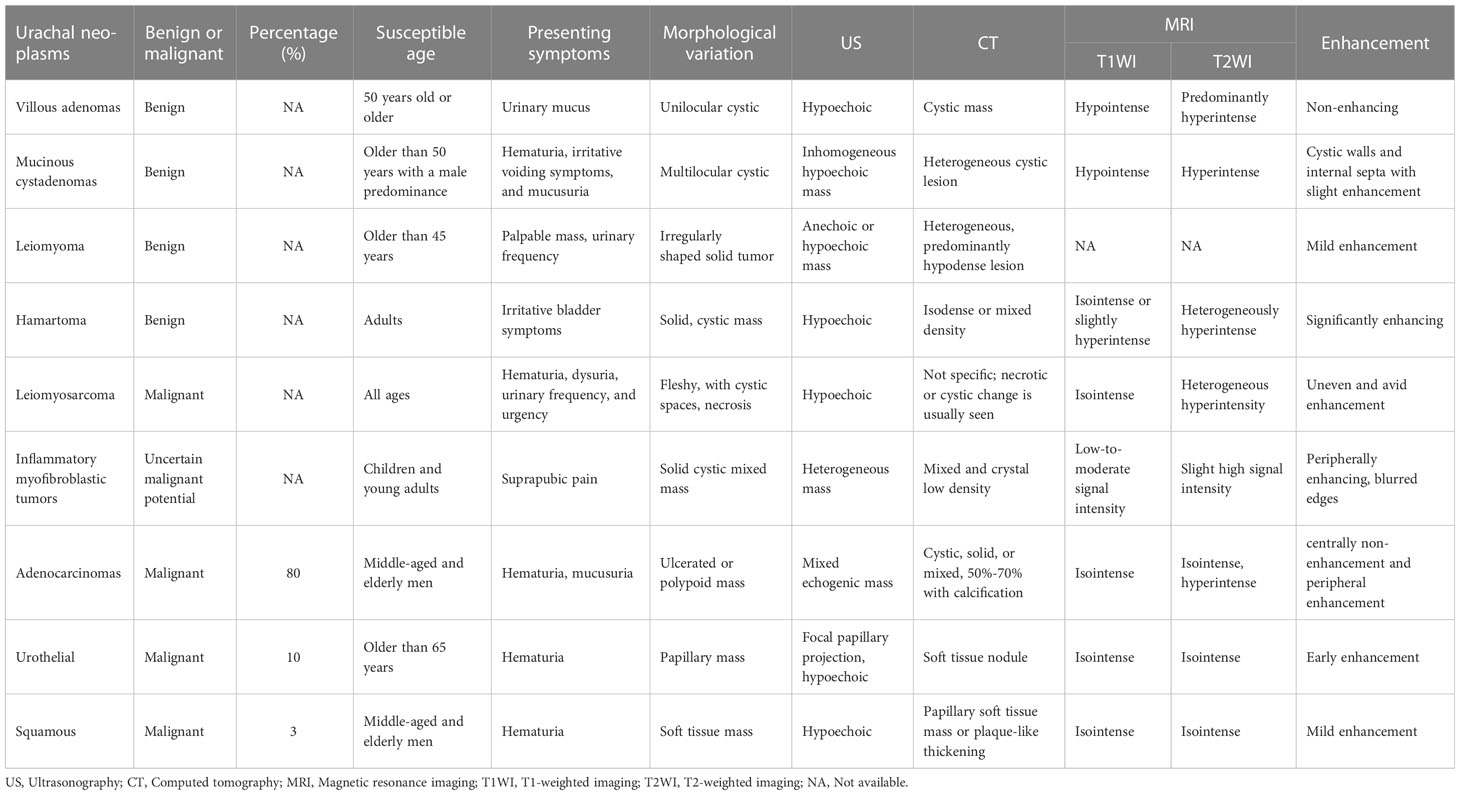
Table 2 Clinical and imaging features of urachal leiomyosarcoma, common benign or malignant urachal neoplasms.
Treatment and prognosis
The recommended treatment of urachal carcinoma is partial cystectomy and excision of the urachus including a peritoneal cuff which is the first treatment intervention for most patients of urachal leiomyosarcoma that have been reported (4). As a result of the lack of data on urachal leiomyosarcomas, adjuvant chemotherapy and radiotherapy for urachal leiomyosarcoma are controversial and the clinical outcomes and prognosis are difficult to assess (25). A previous study reported a spontaneously ruptured urachal myxoid leiomyosarcoma in an adolescent patient who was treated with radiation therapy and alternating adjuvant chemotherapy (vincristine, doxorubicin and cyclophosphamide regimen, vincristine, dactinomycin and cyclophosphamide regimen) (6). However, no long-term follow-up data were reported in that study. In addition, neoadjuvant chemotherapy for bladder leiomyosarcoma may provide reference for neoadjuvant regimens are doxorubicin, ifosfamide, cisplatin, adriamycin, and vincristine (26).
The patient in our report was not treated with adjuvant chemotherapy or radiotherapy, and no recurrence was noted at one and a half years. We think that urachal tumors are not particularly sensitive to radiotherapy. As a result, radiotherapy can be considered for patients unfit for surgery, postoperative positive margins, and incurable disease (palliative radiation). For chemotherapy, we suggest that it should not be recommended on a routine basis, as types of treatment used are heterogeneous and the benefits are unclear. Postoperative chemotherapy may be considered due to positive margins, lymph node or peritoneal involvement, or unresected umbilicus and a high likelihood of relapse (10). Finally, cystoscopic evaluation has the tendency to cause local recurrence. Thus, imaging examination can be used to evaluate whether there are new or recurrent lesions after surgery.
Follow-up
As a result of the lack of data on urachal leiomyosarcomas, follow-up scheme of this entity is not clear in the literature (10). For the patient in our study, we referred to the follow-up scheme for urachal carcinoma and bladder cancer (9, 27–30). Specifically, we recommended that the patient a regular follow-up after the operation including visits to an outpatient clinic every 3 months for the first 2 years and every 6 months for the following years. We also advised the patient to undergo routine blood and biochemical analyses, physical examinations, chest radiographies, and US/CT/MRI examinations of the abdomen and pelvis, as appropriate. When there was any evidence of suspected recurrence, chest, abdominal, and pelvic CT scans; brain MRI; bone scintigraphy; and PET/CT might be performed, as appropriate.
Conclusions
Leiomyosarcoma is a type of soft-tissue sarcoma usually occurring in the soft tissues of the extremities and trunk. It is aggressive and spreads hematogenously which are characteristics of a malignant neoplasm. Primary leiomyosarcoma of the urachus is extremely rare, and it tends to be easily misdiagnosed. Understanding the embryological basis of these urachal disorders and their imaging features coupled with histopathological, examination is crucial for the correct diagnosis and management.
Patient perspective
Our patient reported the following about his disease: “Throughout my clinical care, I have been concerned that my disease would come back. I now have an abdominal ultrasonography every three months to keep track of my disease. Fortunately, I am now in good health. Thanks are given to the doctors and nurses who have taken care of me and helped me.”
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional Ethics Committee of the Suining Central Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
JY, QD, and GY participated in the study design and writing of the manuscript. JY, QD, GY, CT, HL, and YL participated in clinical data collection and analysis, and carried out the interpretation of the CT images. LY performed the pathological and immunohistochemical examinations. GY, MM, AB, RL, GT and BF all revised the paper critically for intellectual content. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by grants from the Sichuan Provincial Commission of Health (No. 19PJ284), the Science and Technology Association of Suining City (No. 6), and the Suining Central Hospital (Nos. 2021y09 and 2022ypj01).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Parada Villavicencio C, Adam SZ, Nikolaidis P, Yaghmai V, Miller FH. Imaging of the urachus: AnoMalies, complications, and mimics. Radiographics. (2016) 36(7):2049–63. doi: 10.1148/rg.2016160062
2. Yu JS, Kim KW, Lee HJ, Lee YJ, Yoon CS, Kim MJ. Urachal remnant diseases: spectrum of CT and US findings. Radiographics. (2001) 21(2):451–61. doi: 10.1148/radiographics.21.2.g01mr02451
3. Herr HW, Bochner BH, Sharp D, Dalbagni G, Reuter VE. Urachal carcinoma: contemporary surgical outcomes. J Urol (2007) 178(1):74–8.
4. Noyes D, Vinson RK. Urachal leiomyosarcoma. Urology. (1981) 17(3):279–80. doi: 10.1016/0090-4295(81)90053-4
5. Jimenez CL, Carpenter BF, Robb I. Leiomyosarcoma. Ultrastruct Pathol (1987) 11(5-6):765–9. doi: 10.3109/01913128709048465
6. Kim SO, Kwon D, Choi C, Baek HJ, Park K, Ryu SB. A case of spontaneous rupture perforation of leiomyosarcoma in the urachus of a young boy. Int J Urol (2007) 14(10):960–2. doi: 10.1111/j.1442-2042.2007.01826.x
7. Saied A, Salzman S, Hochwald SN, Trevino JG. Traumatic intraperitoneal rupture of a urachal leiomyosarcoma: unique presentation as hemorrhagic shock. Am Surg (2012) 78(9):E409–11.
8. Tong S, Jia Z. Primary urachal leiomyosarcoma: A rare case report and literature review. Urol Case Rep (2022) 44:102143. doi: 10.1016/j.eucr.2022.102143
9. Loizzo D, Pandolfo SD, Crocerossa F, Guruli G, Ferro M, Paul AK, et al. Current management of urachal carcinoma: An evidence-based guide for clinical practice. Eur Urol Open Sci (2022) 39:1–6. doi: 10.1016/j.euros.2022.02.009
10. Hamilou Z, North S, Canil C, Wood L, Hotte S, Sridhar SS, et al. Management of urachal cancer: A consensus statement by the Canadian Urological Association and Genitourinary Medical Oncologists of Canada. Can Urol Assoc J (2020) 14(3):E57–64. doi: 10.5489/cuaj.5946
11. Johansen TE, Jebsen PW. Carcinoma of the urachus. A Case Rep Rev literature Int Urol Nephrol (1993) 25(1):59–63. doi: 10.1007/BF02552255
12. WHO Classification of Tumors Editorial Board Eds. In: World Health Organization Classification of Soft Tissue and Bone Tumours, 5th ed. Lyon: IARC Press.
13. Cessna MH, Zhou H, Perkins SL, Tripp SR, Layfield L, Daines C, et al. Are myogenin and myoD1 expression specific for rhabdomyosarcoma? A study of 150 cases, with emphasis on spindle cell mimics. Am J Surg Pathol (2001) 25(9):1150–7. doi: 10.1097/00000478-200109000-00005
14. Yang G, Wang Z, Liu S, Wu F, Li D. A rare case of urachal carcinoma metastatic to thoracic vertebra detected by FDG PET/CT. Clin Nucl Med (2017) 42(7):544–6. doi: 10.1097/RLU.0000000000001680
15. Das JP, Vargas HA, Ghafoor S, Goh AC, Ulaner GA. Clinical utility of 18F-FDG PET/CT for staging and treatment planning in urachal adenocarcinoma. J Nucl Med (2021) 62(5):643–7. doi: 10.2967/jnumed.120.251561
16. Ashley RA, Inman BA, Sebo TJ, Leibovich BC, Blute ML, Kwon ED, et al. Urachal carcinoma: clinicopathologic features and long-term outcomes of an aggressive Malignancy. Cancer. (2006) 107(4):712–20. doi: 10.1002/cncr.22060
17. Das JP, Woo S, Ghafoor S, Andrieu PIC, Ulaner GA, Donahue TF, et al. Value of MRI in evaluating urachal carcinoma: A single center retrospective study. Urol Oncol (2022) 40(7):345.e9–345.e17. doi: 10.1016/j.urolonc.2022.02.017
18. Das JP, Vargas HA, Ulaner GA. Mucinous urachal adenocarcinoma: a potential non fluorodeoxyglucose-avid pitfall on 18fluorine-fluorodeoxyglucose positron emission tomography/computed tomography. World J Nucl Med (2020) 19(4):432–4. doi: 10.4103/wjnm.WJNM_60_19
19. Das JP, Vargas HA, Lee A, Hutchinson B, O'Connor E, Kok HK, et al. The urachus revisited: multimodal imaging of benign & Malignant urachal pathology. Br J Radiol (2020) 93(1110):20190118. doi: 10.1259/bjr.20190118
20. Machida H, Ueno E, Nakazawa H, Fujimura M, Kihara T. Computed tomographic appearance of urachal carcinoma associated with urachal diverticulum misdiagnosed by cystoscopy. Abdom Imaging (2008) 33(3):363–6. doi: 10.1007/s00261-007-9256-7
21. Thali-Schwab CM, Woodward PJ, Wagner BJ. Computed tomographic appearance of urachal adenocarcinomas: review of 25 cases. Eur Radiol (2005) 15(1):79–84. doi: 10.1007/s00330-004-2408-z
22. Woo S, Ghafoor S, Das JP, Gangai N, Goh AC, Vargas HA. Plasmacytoid urothelial carcinoma of the bladder: MRI features and their association with survival. Urol Oncol (2022) 40(3):108.e1–108.e10. doi: 10.1016/j.urolonc.2021.09.017
23. Caglic I, Panebianco V, Vargas HA, Bura V, Woo S, Pecoraro M, et al. MRI of bladder cancer: local and nodal Staging. J Magn Reson Imaging (2020) 52(3):649–67. doi: 10.1002/jmri.27090
24. Gandhi N, Krishna S, Booth CM, Breau RH, Flood TA, Morgan SC, et al. Diagnostic accuracy of magnetic resonance imaging for tumour staging of bladder cancer: systematic review and meta-analysis. BJU Int (2018) 122(5):744–53. doi: 10.1111/bju.14366
25. Szarvas T, Módos O, Niedworok C, Reis H, Szendröi A, Szász MA, et al. Clinical, prognostic, and therapeutic aspects of urachal carcinoma-A comprehensive review with meta-analysis of 1,010 cases. Urol Oncol (2016) 34(9):388–98. doi: 10.1016/j.urolonc.2016.04.012
26. Fakhoury M, Hwang RR, Silletti J, Bjurlin MA. Bladder leiomyosarcoma: A rare, but aggressive diagnosis. Curr Urol (2016) 9(3):166–8. doi: 10.1159/000447135
27. Duan F, Zhai W, Zhang B, Guo S. Urachal carcinoma: Impact of recurrence pattern and lymphadenectomy on long-term outcomes. Cancer Med (2020) 9(12):4166–74.
28. Yu YD, Ko YH, Kim JW, Jung SI, Kang SH, Park J, et al. The prognosis and oncological predictor of urachal carcinoma of the bladder: A large scale multicenter cohort study analyzed 203 patients with long term follow-up. Front Oncol (2021) 11:683190.
29. Guerin M, MIran C, Colomba E, Cabart M, Herrmann T, Pericart S, et al. Urachal carcinoma: a large retrospective multicentric study from the French Genito-Urinary Tumor Group. Front Oncol (2023) 13:1110003.
30. National Health Commission of the People’s Republic of China. Guidelines for the diagnosis and treatment of bladder cancer (version 2022) . Available at: http://www.nhc.gov.cn/yzygj/s2911/202204/a0e67177df1f439898683e1333957c74/files/7224e506d4a24b90a9df0424888ba38a.pdf.
Keywords: urachus, urachal tumor, leiomyosarcoma, computed tomography, case report
Citation: Yan J, Li H, Yan G, Duan Q, Tang C, McClure MA, Bhetuwal A, Li Y, Yang L, Li R, Tan G and Feng B (2023) Primary urachal leiomyosarcoma: a case report and literature review of clinical, pathological, and medical imaging features. Front. Oncol. 13:1228178. doi: 10.3389/fonc.2023.1228178
Received: 24 May 2023; Accepted: 25 July 2023;
Published: 17 August 2023.
Edited by:
Cicero Matthew R. Habito, Harvard Medical School, United StatesReviewed by:
Caterina Gaudiano, Sant’Orsola-Malpighi Polyclinic, ItalySean R. Williamson, Cleveland Clinic, United States
Copyright © 2023 Yan, Li, Yan, Duan, Tang, McClure, Bhetuwal, Li, Yang, Li, Tan and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gaowu Yan, eWFuZ2Fvd3UxOTg5QDE2My5jb20=
†These authors have contributed equally to this work
 Jing Yan1†
Jing Yan1† Gaowu Yan
Gaowu Yan Morgan A. McClure
Morgan A. McClure Anup Bhetuwal
Anup Bhetuwal