- 1Gatroenterology Associates of Central Georgia, Macon, GA, United States
- 2Mercer University School of Medicine, Macon, GA, United States
- 3Advanced Pathology Solutions, Department of Gastroenterology, Little Rock, AR, United States
- 4Department of Oral and Craniofacial Sciences, University of California San Francisco School of Dentistry, San Francisco, CA, United States
Introduction: Human Papillomavirus (HPV) is the primary risk factor for the development of anal intraepithelial neoplasia (AIN) and is a leading risk factor for anogenital squamous cell carcinoma (ASCC). Despite common shared risk factors for both HPV and syphilis, co-infection is not well documented, and the role of syphilitic infection in HPV-associated AIN and ASCC potentiation is not defined.
Case description/methods: A 72-year-old single male presented with complaints of mild rectal pain and intermittent rectal bleeding. A flexible sigmoidoscopy was performed, and a firm 4.5cm x 3cm perianal mass was detected and superficially biopsied. Pathology findings demonstrated evidence of a high grade squamous intraepithelial lesion (HGSIL, AIN II/III/AIS) with viral cytopathic effect, consistent with HPV infection. Much of the biopsied lesion showed acanthotic squamous mucosa with intraepithelial neutrophils and abundant submucosal plasma cells, suggesting possible syphilitic involvement. Subsequent immunohistochemical staining for p16 as a surrogate marker for HPV was positive, as was an immunohistochemical stain for spirochetes, supportive of co-infection with Treponema pallidum pallidum (T. pallidum), the causative agent in venereal syphilis. The patient was referred to an infectious disease specialist for syphilitic infection and was treated with penicillin with surprisingly complete resolution of the lesion. EUAs were performed 2- and 3-months following treatment without lesion recurrence. However, one year following diagnosis, a flexible sigmoidoscopy revealed a 5 mm recurrent HPV-related low-grade AIN 1 lesion at the dentate line.
Discussion: Resolution of the lesion by antibiotic treatment for syphilitic infection suggested that co-infection by T. pallidum may potentiate HPV-associated squamous cell carcinoma based on histological findings. Findings from this case, as well as a review of bacterial involvement and potentiation in various cancers, are reviewed here. Such findings offer new insight regarding the role of STI-associated bacteria and HPV co-infection in the establishment of AIN and may additionally propose new treatment modalities for ASCC.
Introduction
Anal squamous cell carcinoma (ASCC) is the most common cancer type of the anal region. ASCC cancer originates in the squamous cells, which make up both the lining of the anal canal and the anal margins (1). Anal cancer is a common disease that is a public health concern worldwide, with an increased incidence by two- to four-fold in Europe, Australia, and the United States among both men and women within the past three decades (2, 3). Anal cancer accounts for less than 1% of all new cancer diagnoses and for about 4% of all gastrointestinal tract cancers (4, 5). In 2020, anal cancer accounted for approximately 50,685 new cases and 19,293 deaths worldwide (6). In the United States alone, the American Cancer Society recorded 8,580 new adult cases (2,960 male and 5,620 female) and 1,160 deaths (480 male and 680 female) due to anal cancer in 2018 (7). In 2022, anal cancer was projected to account for 9,440 new adult cases (3,150 male and 6,290 female) and an estimated 1,670 deaths (740 male and 930 female) in the United States (6). These numbers represent a 10% increase in new cases and a 44% increase in anal cancer deaths from 2018 to 2022 (7). The 5-year survival rates for anal cancer has improved in the last couple of decades and demonstrates a favorable prognosis, that being ~75% (8). However, increased rates for human papillomavirus (HPV) associated anal cancer showcases the need for the discovery of novel therapeutic approaches (9).
HPV, one of the most common sexually transmitted infections (STI) in the United States, is the primary risk factor for the development of ASCC and its precursor lesion, anal intraepithelial neoplasia (AIN), especially with regard to the HPV16 and HPV18 subtypes (4, 10). Factors that increase the risk of HPV infection leading to malignancy include anal-receptive intercourse, high lifetime number of sexual partners, human immunodeficiency virus (HIV) infection, prior history of anogenital warts, lower genital tract malignancies, a history of other HPV-related cancers, autoimmune disorders, smoking, and history of transplantation/chronic immunosuppression (4, 5, 10–13). Results obtained from published studies from the 1990s showed that combined chemoradiotherapy was the backbone for the initial treatment (8, 14–16). Today, three-quarters of patients with ASCC receive chemoradiotherapy as their primary treatment option. Some of the morbidities associated with chemoradiotherapy include increased long-term cardiovascular toxicity (17), increased risk of infection, bruising and bleeding, nausea and vomiting, fatigue, and hair loss (18). Surgical treatment options are also available and remain the standard of care for recurrent and residual disease (19). Significant morbidity of anal stenosis, wound healing, and incontinence are common outcomes on the surgical route (20). GI adverse events, especially fecal incontinence, and sphincter insufficiency are some of the more common morbidities associated with anal cancer in general (21). Efficacy of treatment via chemoradiotherapy compared to surgical intervention has demonstrated that complete chemoradiotherapy has favorable long-term results (22). Early diagnosis and treatment are the best way to improve quality of life for patients with ASCC.
Treatment for anal intraepithelial lesions includes topical, ablative, and surgical approaches, where topical is generally the best option. All these treatment options are followed by active surveillance due to the high recurrence rate of AIN. Topicals, such as 5-fluorouracil, are applied either to the entire anal canal or the specific lesion and usually require 1-2 treatments. Topical treatment has demonstrated efficacy for complete resolution (or regression of high-grade lesions to low grade lesions) in 71%-79% of cases. Ablative therapy is effective only in patients with HIV (79% disease regression), while surgical excision of lesions is rarely used today due to its high morbidity and recurrence rate (23). Regular surveillance of patients with AIN, including digital rectal exam, anoscope, and application of Lugol’s solution, every 3 to 6 months is recommended by the American Society of Colon and Rectal Surgeons (24).
Despite common shared risk factors for both HPV and syphilis, co-infection by HPV and T. pallidum, the causative pathogen in syphilis, is not well documented. As such, the role of syphilitic infection in HPV-associated AIN and ASCC potentiation is not well-documented. However, co-infection by HPV and syphilis has been observed previously (25, 26). For example, a study conducted by the Sexually Transmitted Infection Ambulatory Clinic at the Santa Casa de Misericordia Hospital in Rio de Janeiro, Brazil demonstrated that 15.9% of patients were co-infected with HPV and syphilis (27). Here, we report a similar case of co-infection by HPV and T. pallidum as a fortuitous pathological finding in a biopsied AIN.
Case description
A 72-year-old single male with a history of esophageal resection for Barrett’s esophagus with high-grade dysplasia and stage 1A esophageal cancer (without chemotherapy, with apparent cure, and no recurrence to date), presented with complaints of mild rectal pain and intermittent rectal bleeding. Three weeks later, a flexible sigmoidoscopy was performed, and a firm perianal mass was detected and superficially biopsied (Figure 1A). The specimen—multiple fragments of tan tissue that measured from less than 1mm to 2mm—was stored in a formalin-filled container prior to processing and was then submitted into a single cassette for processing. Technical portions of these services (grossing, processing, embedding, microtomy, and staining) were performed at Gastroenterology Associates of Central Georgia, LLC, CLIA #11D2015828. Professional portions of these services (all staining and immunohistochemistry) was performed at Advanced Pathology Solutions, Dept. of Gastroenterology CLIA # 04D2037153 in North Little Rock, Arkansas.
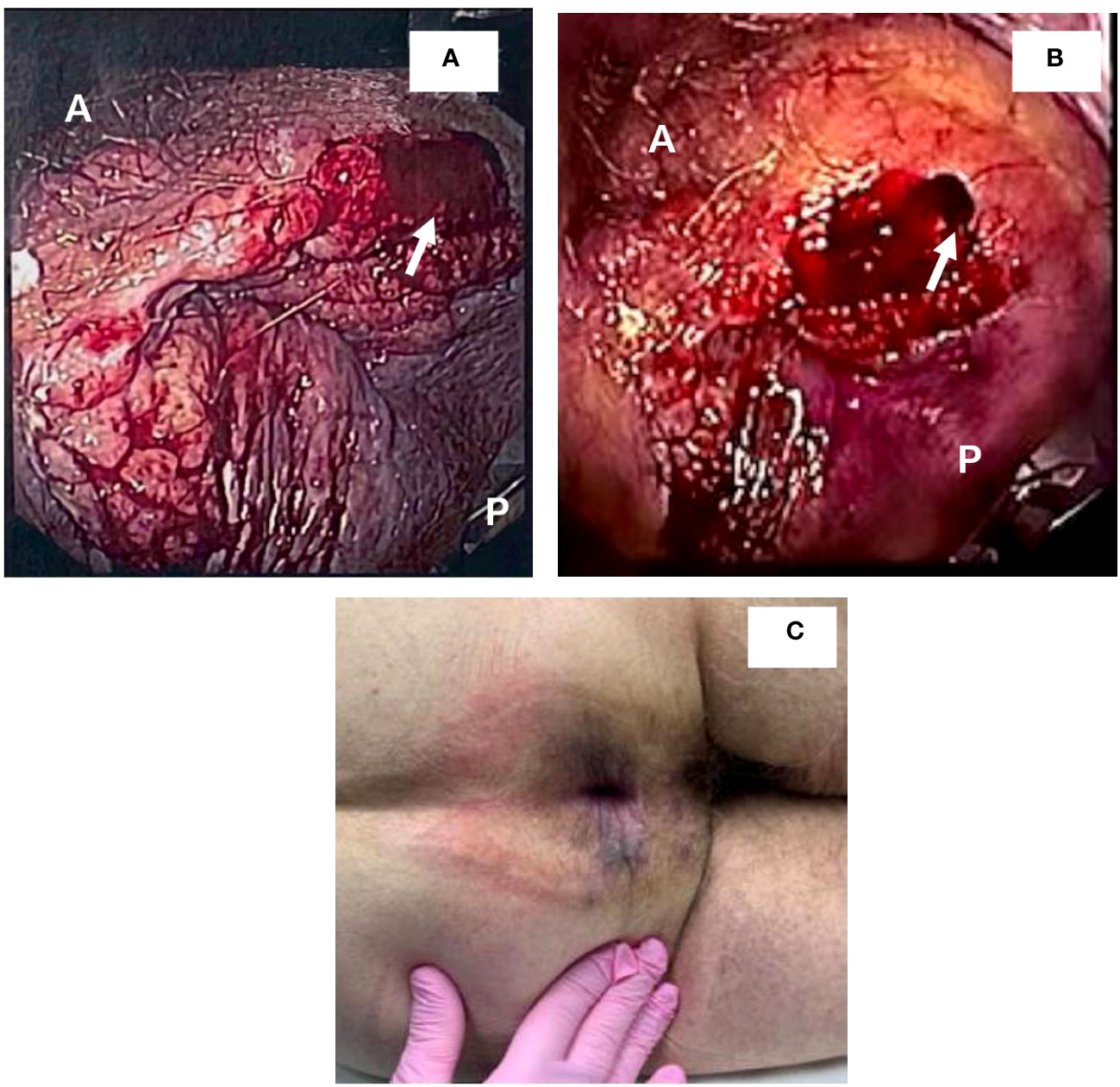
Figure 1 Detection and resolution of anal mass. (A, B) demonstrate views detected during sigmoidoscopy. A and P denote anterior and posterior positions, respectively. Arrows demonstrate areas that were biopsied. (C) demonstrates lesion resolution upon anal exam following treatment with penicillin.
The mass measured 4.5cm x 3cm, and pathological findings revealed the lesion to be high grade squamous intraepithelial lesion (HGSIL, AIN II/II/AIS) with viral cytopathic effect, consistent with HPV infection (Figures 2A, B). HPV diagnosis was confirmed using a p16 immunohistochemical stain (Figures 2A, B); the efficacy of this technique has proven comparable to the historically used 16S rRNA sequencing (28–30). Due to the firmness of the lesion and the superficiality of the biopsy, it cannot be discounted that the patient may have had squamous cell carcinoma at deeper levels. Much of the biopsied lesion demonstrated acanthotic squamous mucosa with intraepithelial neutrophils and abundant submucosal plasma cells, consistent with pathological indicators of syphilitic involvement. An RPR/FTA-ABS serology test was performed; the results were 1:128 and showed positive reactivity, respectively. Additionally, immunohistochemical stain (rabbit polyclonal antibody, BioCare Medical) for spirochetes (Figure 2C) was positive. These findings—including presence of an anal lesion, histological confirmation of spirochete infection, and additional serological confirmation—is consistent with primary syphilitic infection. No systemic manifestations of secondary or latent syphilis were observed.
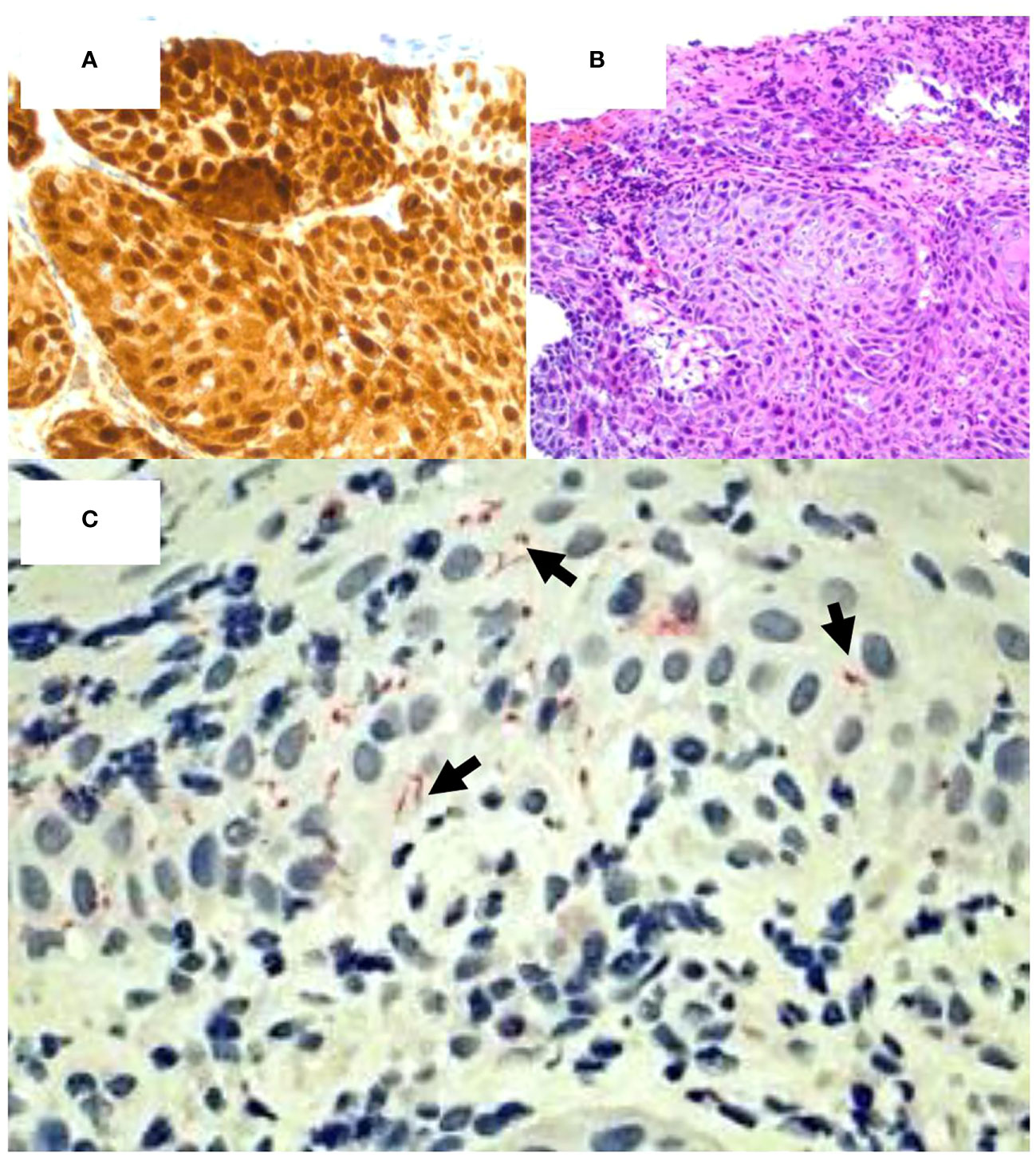
Figure 2 Coinfection of HPV and T. pallidum in high- grade squamous intraepithelial lesion. (A) p16 staining demonstrates HPV positivity. (Mag=20X). (B) H&E staining of p16 positive tissue fragment confirms HPV infection. (Mag=20X). (C) Spirochete stain (red) confirms the presence of syphilitic coinfection with HPV. (Mag=40X).
Of note, a PET and CT scan 1 year prior to diagnosis as well as a CT scan 7 months following diagnosis were negative for relevant findings to AIN. Furthermore, the patient denied any sexual history for the past 20 years; however, a colonoscopy performed one year prior to the diagnosis revealed a normal morphology, suggesting an acquired primary syphilis infection during the time between the colonoscopy and diagnostic sigmoidoscopy.
Following the procedure, the patient was checked for CMV, HSV1/2, HIV, and hepatitis panels. immunohistochemical stains for CMV and HSV1/2 were negative. Labs demonstrated hepatitis A antibody negative, hepatitis B surface antigen negative, hepatitis B core antibody IgM negative, hepatitis C virus antibody negative, and HIV non-reactive. The patient was referred to an infectious disease specialist and syphilitic infection was treated with 3X doses of 2.4 MU Bicillin LA administered intramuscularly for three consecutive weeks at 7-day intervals. A total of 4 mL of Bicillin LA was injected with 2 mL on the left buttock and 2 mL on the right buttock each week. The RPR titer came down from 1:128 to 1:16 following treatment. Upon follow up with gastroenterology, remarkable and surprising resolution of the suspected HPV actuated dysplastic lesion was observed (Figure 1B). Two subsequent EUAs were performed, 2- and 3- months following treatment, and were evaluated as normal with complete resolution of the lesion (Figure 1C). However, a sigmoidoscopy performed one year later revealed a recurrent 5mm AIN 1 lesion at the dentate line which was promptly removed. The lesion was evaluated using Hematoxylin and Eosin stain (Figures 3A, B) as well as p16 immunohistochemical stain, which showed focal p16 positivity indicating low grade dysplasia/AIN 1 (Figures 3C, D). No treatment was required for the recurrent lesion. A follow-up flexible sigmoidoscopy was scheduled one year following lesion resolution; however, the appointment was cancelled. Currently, the patient is being contacted to reschedule the flexible sigmoidoscopy.
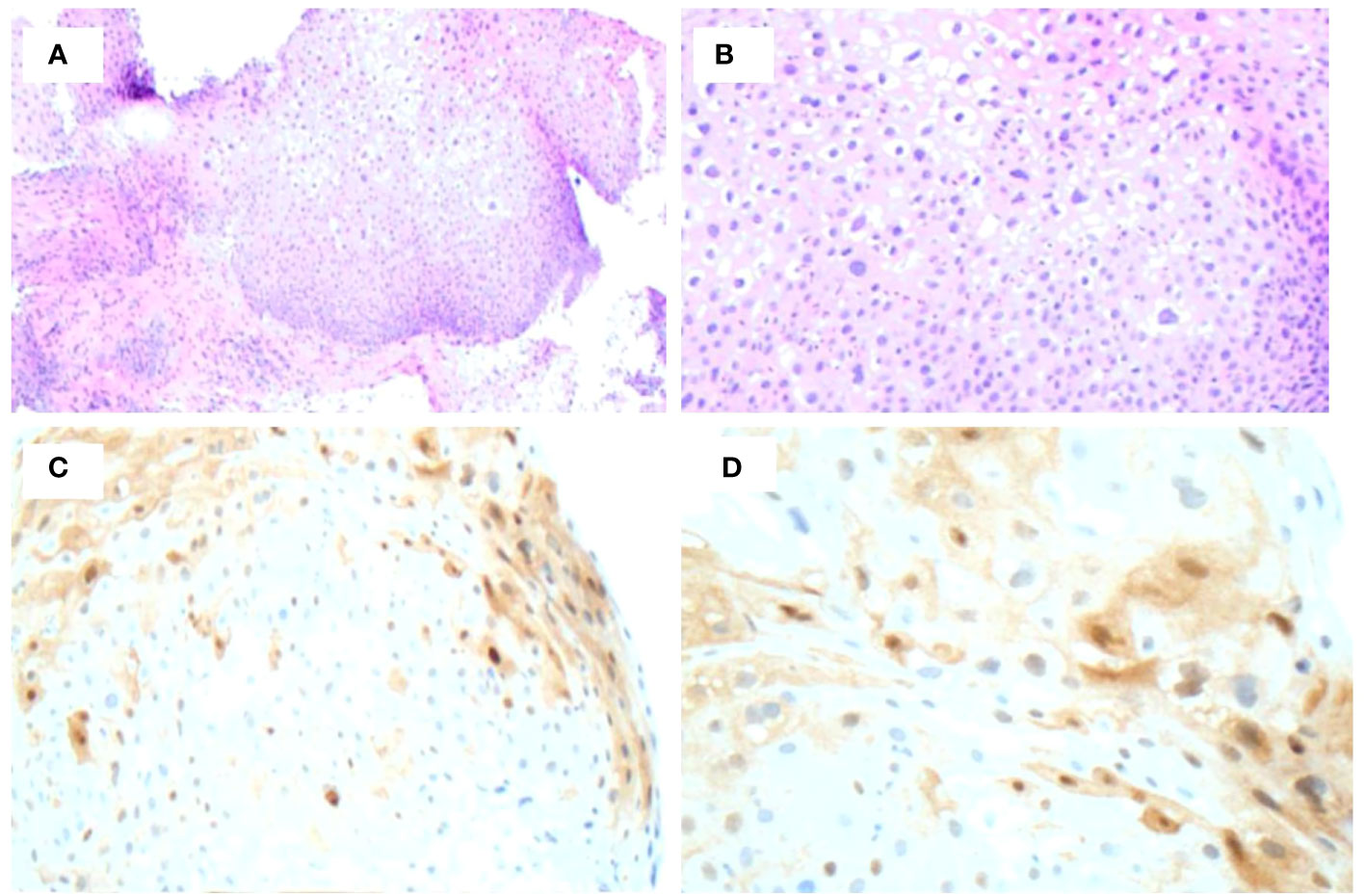
Figure 3 Biopsy of recurrent lesion one year following initial detection. (A, B) H&E staining of lesion demonstrates low-grade dysplasia. (C, D) p16 immunohistochemical stain demonstrates focal p16 positivity indicative or AIN 1.
Discussion
Bacterial potentiation of cancer
The resolution of the lesion by antibiotic treatment for syphilitic infection suggests that co-infection by T. pallidum may potentiate HPV-associated squamous cell carcinoma, as based on histological findings. Findings of co-infection by HPV and T. pallidum with specific regard to ASCC are limited. As such, a review of bacterial involvement and potentiation in various cancer subtypes, along with associated treatment modalities, is presented here considering the findings from this case.
Bacterial infections may contribute to the development of cancer through the production of toxins, interrupting cellular signaling, altering DNA, and interfering with the normal functioning of the immune system resulting in inflammation (31, 32). Numerous bacterial strains are related to cancers, such as Citrobacter rodentium in colorectal cancer, Helicobacter pylori in gastric cancer, Campylobacter jejuni in small intestinal lymphomas, Salmonella typhi in hepatobiliary carcinomas, Mycobacterium tuberculosis in lung cancer, and Chlamydia psittaci in ocular lymphomas (33–38). The idea of bacterial involvement in the potentiation of cancer was largely neglected until 1890, when Dr. William Russell suggested the causal relationship between bacteria and cancer (39), and supporting discoveries were made throughout the twentieth century (40, 41). In fact, it has been shown that specific tumors have distinct bacterial compositions, and these specific compositional differences can be seen in benign versus metastatic disease of the same tissue (42, 43). The microbial DNA of these bacteria can be sampled, analyzed, and then used to diagnose whether an individual has cancer, and even what specific type (44). It has even been shown that there are different microbiome compositions in underlying versus overlying breast tissue (42).
Potentiation of oral squamous cell carcinoma (OSCC) by bacteria has also been documented. OSCC has been identified as a consequential outcome to infection by Fusobacterium nucleatum and Porphyromonas gingivalis, both putative pathogens in periodontal disease (45). This association was demonstrated via utilization of a mouse model, in which administration of F. nucleatum and P. gingivalis increased carcinogenesis (46). In addition to OSCC, P. gingivalis has been shown to play a role in various digestive tract tumors, and F. nucleatum is associated with colorectal carcinoma (47, 48). Colorectal carcinoma, also known as colorectal cancer (CRC), is influenced by bacteria residing within the oral cavity, or the oral microbiota (49). Most notably, disruption of the oral microbiota may induce dysbiosis among the intestinal microbiota, which may subsequently lead to CRC (49, 50). The oral microbiota in CRC is distinct, and therefore, profiling the oral microbiome could be an effective screening method for CRC (51). Cervical squamous cell carcinoma (CSCC) is another squamous cell carcinoma, and the risk of developing CSCC is increased by Chlamydia trachomatis infection, a bacterial infection (52). Although HPV is one of the top causes of CSCC, multiple studies have shown that Chlamydia trachomatis infection is a significant risk factor that can further potentiate the development of CSCC (52–54).
Treatment of bacterial infection and improvement of cancer
Treatments for squamous cell carcinoma associated with HPV are typically unimodal or multimodal, depending on how early detection is made. HPV-associated head, neck, and oral squamous cell carcinomas are typically treated with surgery and aggressive radiotherapy or chemotherapy, and commonly accompanied by antibiotics for presumptive infection by pathogens associated with sexually transmitted infection (55–57). When antibiotics are administered, screening for sexually transmitted infection is not typically performed due to high efficacy. Antibiotics administration can range, but most administrations include quinolones or cotrimoxazole (58). Due to lack of screening, possible co-infection by STI-associated bacteria is typically undocumented. As a result, any synergistic benefits to STI-associated SCCs via treatment with antibiotics typically goes undocumented. With regard to CRC, the microbiome plays a pathologically unclear role in the recurrence and metastasis after surgical treatment (59) due to the wide variety of contributing bacteria (60, 61). Thus, specific bacterial identification is needed to mechanistically understand the improvement or worsening of squamous cell-associated cancers with presence of bacterial species.
Limitations in STI reporting in elderly populations. Cooperation in sexual reporting is often hindered among elderly populations due to (1) cultural and societal bias, (2) perceived stigma, embarrassment, and discrimination, (3) educational and training limitations among healthcare professionals, and the (4) quality of relationships shared among patients and healthcare professionals (62). In the described case, the patient denied having any sexual activity for 20 years. However, a colonoscopy performed one year prior to syphilitic and HIV-associated AIN demonstrated normal morphology. This observation possibly signifies that acquisition of syphilitic infection may have occurred during the one-year interval. HPV infection may have been acquired during this time also, or alternatively may have been latent and subsequently reactivated (63). There is increased evidence of acute STIs in the elderly (64); this may be due to multiple factors including loss of spouse (65), high risk sex (66), immunosuppression (67), and erectile dysfunction drugs (68). The CDC reports that STI incidence more than doubled in U.S. adults 65 years and older between 2007-2017. More specifically, rates of syphilitic infection increased nearly four-fold within this ten-year period, from 91 cases in 2007 to 349 cases in 2017 (69). This denial of recent sexual history may also be explained by susceptibility of self-reported sexual activity being under-reporting bias due to perception of social desirability. As such, self-identified sexual orientation may not serve as a reliable record of actual sexual preferences (63, 70).
Conclusion
Co-infection of T. pallidum and HPV-associated cancers may lead to synergistic effects that potentiate symptoms and/or impact treatment in ASCC. Here, the identification of T. pallidum allowed for targeted antibiotic intervention by penicillin that correlated with the improvement of the high grade squamous intra-epithelial lesion (HGSIL). Literature review indicates these synergistic interactions may be more common than initially thought but are typically left uncharacterized and unstudied due to generalized antibiotic administration. This case study indicates a clinical need for STI testing and follow up care in patients afflicted by HPV-associated cancers and other metastases.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This research was conducted with the informed consent of all participants. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
SS treated the patient and collected the sample. SL worked on the case description and figures. SS, HN, PB, FE, DJ, and RA wrote the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
The content of this manuscript has been presented in part at the The American Journal of Gastroenterology: Colorectal Cancer Prevention – Clinical Vignettes/Case Reports, Sedghi, Lea M. MSc1; Do, Jennifer S. MD2; Yoshida, Kenji BSc3; Cafro, Carolyn BSc3; Kapila, Yvonne L. DDS, PhD4; Sedghi, Shahriar MD5. S1980 Resolution of High-Grade Anal Squamous Intraepithelial Lesion With Antibiotics Proposes a New Role for Syphilitic Infection in Potentiation of HPV-Associated ASCC. The American Journal of Gastroenterology 116():p S866, October 2021. | DOI: 10.14309/01.ajg.0000781452.92695.31
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. What Is Anal Cancer? | Types of Anal Cancer. Available at: https://www.cancer.org/cancer/types/anal-cancer/about/what-is-anal-cancer.html.
2. Islami F, Ferlay J, Lortet-Tieulent J, Bray F, Jemal A. International trends in anal cancer incidence rates. Int J Epidemiol (2017) 46:924–38. doi: 10.1093/ije/dyw276
3. Wilkinson JR, Morris EJ, Downing A, Finan PJ, Aravani A, Thomas JD, et al. The rising incidence of anal cancer in England 1990-2010: a population-based study. Colorectal Dis Off J Assoc Coloproctol G. B Irel (2014) 16:O234–239. doi: 10.1111/codi.12553
4. Rao S, Guren MG, Khan K, Brown G, Renehan AG, Steigen SE, et al. Anal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up☆. Ann Oncol Off J Eur Soc Med Oncol (2021) 32:1087–100. doi: 10.1016/j.annonc.2021.06.015
5. Morton M, Melnitchouk N, Bleday R. Squamous cell carcinoma of the anal canal. Curr Probl Cancer (2018) 42:486–92. doi: 10.1016/j.currproblcancer.2018.11.001
6. Henry NL, Magnuson A, Mulrooney D, Hlubocky F, Patel J. Anal Cancer - Statistics. American Society of Clinical Oncology (ASCO): Cancer.Net (2012). Available at: https://www.cancer.net/cancer-types/anal-cancer/statistics.
7. Cancer Facts & Figures 2018 . Available at: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2018.html.
8. Sekhar H, Zwahlen M, Trelle S, Malcomson L, Kochhar R, Saunders MP, et al. Nodal stage migration and prognosis in anal cancer: a systematic review, meta-regression, and simulation study. Lancet Oncol (2017) 18:1348–59. doi: 10.1016/S1470-2045(17)30456-4
9. Lin C, Franceschi S, Clifford GM. Human papillomavirus types from infection to cancer in the anus, according to sex and HIV status: a systematic review and meta-analysis. Lancet Infect Dis (2018) 18:198–206. doi: 10.1016/S1473-3099(17)30653-9
10. Daling JR, Madeleine MM, Johnson LG, Schwartz SM, Shera KA, Wurscher MA, et al. Human papillomavirus, smoking, and sexual practices in the etiology of anal cancer. Cancer (2004) 101:270–80. doi: 10.1002/cncr.20365
11. de Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer (2017) 141:664–70. doi: 10.1002/ijc.30716
12. Bower M, Powles T, Newsom-Davis T, Thirlwell C, Stebbing J, Mandalia S, et al. HIV-associated anal cancer: has highly active antiretroviral therapy reduced the incidence or improved the outcome? J Acquir Immune Defic Syndr 1999 (2004) 37:1563–5. doi: 10.1097/00126334-200412150-00004
13. Edgren G, Sparén P. Risk of anogenital cancer after diagnosis of cervical intraepithelial neoplasia: a prospective population-based study. Lancet Oncol (2007) 8:311–6. doi: 10.1016/S1470-2045(07)70043-8
14. Epidermoid anal cancer: results from the UKCCCR randomised trial of radiotherapy alone versus radiotherapy, 5-fluorouracil, and mitomycin. UKCCCR Anal Cancer Trial Working Party. UK Co-ordinating Committee on Cancer Research. Lancet Lond Engl (1996) 348:1049–54. doi: 10.1016/S0140-6736(96)03409-5
15. Flam M, John M, Pajak TF, Petrelli N, Myerson R, Doggett S, et al. Role of mitomycin in combination with fluorouracil and radiotherapy, and of salvage chemoradiation in the definitive nonsurgical treatment of epidermoid carcinoma of the anal canal: results of a phase III randomized intergroup study. J Clin Oncol Off J Am Soc Clin Oncol (1996) 14:2527–39. doi: 10.1200/JCO.1996.14.9.2527
16. Bartelink H, Roelofsen F, Eschwege F, Rougier P, Bosset JF, Gonzalez DG, et al. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. J Clin Oncol Off J Am Soc Clin Oncol (1997) 15:2040–9. doi: 10.1200/JCO.1997.15.5.2040
17. Meinardi MT, Gietema JA, van Veldhuisen DJ, van der Graaf WT, de Vries EG, Sleijfer DT. Long-term chemotherapy-related cardiovascular morbidity. Cancer Treat Rev (2000) 26:429–47. doi: 10.1053/ctrv.2000.0175
18. Chemotherapy Side Effects. Available at: https://www.cancer.org/cancer/managing-cancer/treatment-types/chemotherapy/chemotherapy-side-effects.html.
19. Ghosn M, Kourie HR, Abdayem P, Antoun J, Nasr D. Anal cancer treatment: current status and future perspectives. World J Gastroenterol (2015) 21:2294–302. doi: 10.3748/wjg.v21.i8.2294
20. Osborne MC, Maykel J, Johnson EK, Steele SR. Anal squamous cell carcinoma: an evolution in disease and management. World J Gastroenterol (2014) 20:13052–9. doi: 10.3748/wjg.v20.i36.13052
21. Kouzy R, Abi Jaoude J, Lin D, El Alam MB, Minsky BD, Koay EJ, et al. Patient-reported GI outcomes in patients with anal cancer receiving modern chemoradiation. JCO Oncol Pract (2020) 16:e1524–31. doi: 10.1200/OP.20.00122
22. O’Neill BDP, Brown G, Heald RJ, Cunningham D, Tait DM. Non-operative treatment after neoadjuvant chemoradiotherapy for rectal cancer. Lancet Oncol (2007) 8:625–33. doi: 10.1016/S1470-2045(07)70202-4
23. Roberts JR, Siekas LL, Kaz AM. Anal intraepithelial neoplasia: A review of diagnosis and management. World J Gastrointest Oncol (2017) 9:50–61. doi: 10.4251/wjgo.v9.i2.50
24. Long KC, Menon R, Bastawrous A, Billingham R. Screening, surveillance, and treatment of anal intraepithelial neoplasia. Clin Colon Rectal Surg (2016) 29:57–64. doi: 10.1055/s-0035-1570394
25. Tayal S, Shaban F, Dasgupta K, Tabaqchali MA. A case of syphilitic anal condylomata lata mimicking Malignancy. Int J Surg Case Rep (2015) 17:69–71. doi: 10.1016/j.ijscr.2015.10.035
26. McCloskey JC, Kast WM, Flexman JP, McCallum D, French MA, Phillips M. Syndemic synergy of HPV and other sexually transmitted pathogens in the development of high-grade anal squamous intraepithelial lesions. Papillomavirus Res Amst Neth (2017) 4:90–8. doi: 10.1016/j.pvr.2017.10.004
27. de Sena Souza LM, Miller WM, da Costa Nery JA, de Andrade AFB, Asensi MD. A syphilis co-infection study in human papilloma virus patients attended in the sexually transmitted infection ambulatory clinic, Santa Casa de Misericórdia Hospital, Rio de Janeiro, Brazil. Braz J Infect Dis Off Publ Braz Soc Infect Dis (2009) 13:207–9. doi: 10.1590/S1413-86702009000300010
28. Rödel F, Wieland U, Fraunholz I, Kitz J, Rave-Fränk M, Wolff HA. Human papillomavirus DNA load and p16INK4a expression predict for local control in patients with anal squamous cell carcinoma treated with chemoradiotherapy. Int J Cancer (2015) 136:278–88. doi: 10.1002/ijc.28979
29. Urgoiti GBR, Gustafson K, Klimowicz AC, Petrillo SK, Magliocco AM, Doll CM. The prognostic value of HPV status and p16 expression in patients with carcinoma of the anal canal. PloS One (2014) 9:e108790. doi: 10.1371/journal.pone.0108790
30. Lu DW, El-Mofty SK, Wang HL. Expression of p16, Rb, and p53 proteins in squamous cell carcinomas of the anorectal region harboring human papillomavirus DNA. Mod Pathol Off J U S Can Acad Pathol Inc (2003) 16:692–9. doi: 10.1097/01.MP.0000077417.08371.CE
31. Lax AJ. Opinion: Bacterial toxins and cancer–a case to answer? Nat Rev Microbiol (2005) 3:343–9. doi: 10.1038/nrmicro1130
32. Baik SC, Youn HS, Chung MH, Lee WK, Cho MJ, Ko GH, et al. Increased oxidative DNA damage in Helicobacter pylori-infected human gastric mucosa. Cancer Res (1996) 56:1279–82.
33. De Spiegeleer B, Verbeke F, D'Hondt M, Hendrix A, Van De Wiele C, Burvenich C, et al. The quorum sensing peptides PhrG, CSP and EDF promote angiogenesis and invasion of breast cancer cells in vitro. PloS One (2015) 10:e0119471. doi: 10.1371/journal.pone.0119471
34. Correa P, Fox J, Fontham E, Ruiz B, Lin YP, Zavala D, et al. Helicobacter pylori and gastric carcinoma. Serum antibody prevalence in populations with contrasting cancer risks. Cancer (1990) 66:2569–74. doi: 10.1002/1097-0142(19901215)66:12<2569::AID-CNCR2820661220>3.0.CO;2-I
35. Mesnard B, De Vroey B, Maunoury V, Lecuit M. Immunoproliferative small intestinal disease associated with Campylobacter jejuni. Dig Liver Dis Off J Ital Soc Gastroenterol Ital Assoc Study Liver (2012) 44:799–800. doi: 10.1016/j.dld.2012.03.020
36. Caygill CP, Braddick M, Hill MJ, Knowles RL, Sharp JC. The association between typhoid carriage, typhoid infection and subsequent cancer at a number of sites. Eur J Cancer Prev Off J Eur Cancer Prev Organ ECP (1995) 4:187–93. doi: 10.1097/00008469-199504000-00010
37. Gupta PK, Tripathi D, Kulkarni S, Rajan MGR. Mycobacterium tuberculosis H37Rv infected THP-1 cells induce epithelial mesenchymal transition (EMT) in lung adenocarcinoma epithelial cell line (A549). Cell Immunol (2016) 300:33–40. doi: 10.1016/j.cellimm.2015.11.007
38. Ferreri AJM, Guidoboni M, Ponzoni M, De Conciliis C, Dell'Oro S, Fleischhauer K, et al. Evidence for an association between Chlamydia psittaci and ocular adnexal lymphomas. J Natl Cancer Inst (2004) 96:586–94. doi: 10.1093/jnci/djh102
39. Russell W. An address on a characteristic organism of cancer. Br Med J (1890) 2:1356–60. doi: 10.1136/bmj.2.1563.1356
40. L’esperance ES. Studies in hodgkin’s disease. Ann Surg (1931) 93:162–8. doi: 10.1097/00000658-193101000-00023
41. Diller IC. Tumor incidence in ICR/albino and C57/B16JNIcr male mice injected with organisms cultured from mouse Malignant tissue. Growth (1974) 38:507–17.
42. Hieken TJ, Chen J, Hoskin TL, Walther-Antonio M, Johnson S, Ramaker S, et al. The microbiome of aseptically collected human breast tissue in benign and Malignant disease. Sci Rep (2016) 6:30751. doi: 10.1038/srep30751
43. Nejman D, Livyatan I, Fuks G, Gavert N, Zwang Y, Geller LT, et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science (2020) 368:973–80. doi: 10.1126/science.aay9189
44. Poore GD, Kopylova E, Zhu Q, Carpenter C, Fraraccio S, Wandro S, et al. Microbiome analyses of blood and tissues suggest cancer diagnostic approach. Nature (2020) 579:567–74. doi: 10.1038/s41586-020-2095-1
45. Sami A, Elimairi I, Stanton C, Ross RP, Ryan CA. The role of the microbiome in oral squamous cell carcinoma with insight into the microbiome-treatment axis. Int J Mol Sci (2020) 21:8061. doi: 10.3390/ijms21218061
46. Binder Gallimidi A, Fischman S, Revach B, Bulvik R, Maliutina A, Rubinstein AM, et al. Periodontal pathogens Porphyromonas gingivalis and Fusobacterium nucleatum promote tumor progression in an oral-specific chemical carcinogenesis model. Oncotarget (2015) 6:22613–23. doi: 10.18632/oncotarget.4209
47. Liu X-B, Gao Z, Sun C, Wen H, Gao B, Li S, et al. The potential role of P.gingivalis in gastrointestinal cancer: a mini review. Infect Agent Cancer (2019) 14:23. doi: 10.1186/s13027-019-0239-4
48. Castellarin M, Warren RL, Freeman JD, Dreolini L, Krzywinski M, Strauss J, et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res (2012) 22:299–306. doi: 10.1101/gr.126516.111
49. Koliarakis I, Messaritakis I, Nikolouzakis TK, Hamilos G, Souglakos J, Tsiaoussis J, et al. Oral bacteria and intestinal dysbiosis in colorectal cancer. Int J Mol Sci (2019) 20:4146. doi: 10.3390/ijms20174146
50. Mo S, Ru H, Huang M, Cheng L, Mo X, Yan L. Oral-intestinal microbiota in colorectal cancer: inflammation and immunosuppression. J Inflamm Res (2022) 15:747–59. doi: 10.2147/JIR.S344321
51. Flemer B, Warren RD, Barrett MP, Cisek K, Das A, Jeffery IB, et al. The oral microbiota in colorectal cancer is distinctive and predictive. Gut (2018) 67:1454–63. doi: 10.1136/gutjnl-2017-314814
52. Madeleine MM, Anttila T, Schwartz SM, Saikku P, Leinonen M, Carter JJ, et al. Risk of cervical cancer associated with Chlamydia trachomatis antibodies by histology, HPV type and HPV cofactors. Int J Cancer (2007) 120:650–5. doi: 10.1002/ijc.22325
53. Koskela P, Anttila T, Bjørge T, Brunsvig A, Dillner J, Hakama M, et al. Chlamydia trachomatis infection as a risk factor for invasive cervical cancer. Int J Cancer (2000) 85:35–9. doi: 10.1002/(SICI)1097-0215(20000101)85:1<35::AID-IJC6>3.0.CO;2-A
54. Anttila T, Saikku P, Koskela P, Bloigu A, Dillner J, Ikäheimo I, et al. Serotypes of Chlamydia trachomatis and risk for development of cervical squamous cell carcinoma. JAMA (2001) 285:47–51. doi: 10.1001/jama.285.1.47
55. Marur S, Forastiere AA. Head and neck squamous cell carcinoma: update on epidemiology, diagnosis, and treatment. Mayo Clin Proc (2016) 91:386–96. doi: 10.1016/j.mayocp.2015.12.017
56. Feller L, Lemmer J. Oral squamous cell carcinoma: epidemiology, clinical presentation and treatment. J Cancer Ther (2012) 3:263–8. doi: 10.4236/jct.2012.34037
57. Penel N, Fournier C, Lefebvre D, Lefebvre J-L. Multivariate analysis of risk factors for wound infection in head and neck squamous cell carcinoma surgery with opening of mucosa. Study of 260 surgical procedures. Oral Oncol (2005) 41:294–303. doi: 10.1016/j.oraloncology.2004.08.011
58. Herbst C, Naumann F, Kruse EB, Monsef I, Bohlius J, Schulz H, et al. Prophylactic antibiotics or G-CSF for the prevention of infections and improvement of survival in cancer patients undergoing chemotherapy. Cochrane Database Syst Rev (2009) 1:CD007107. doi: 10.1002/14651858.CD007107.pub2
59. Gaines S, van Praagh JB, Williamson AJ, Jacobson RA, Hyoju S, Zaborin A, et al. Western diet promotes intestinal colonization by collagenolytic microbes and promotes tumor formation after colorectal surgery. Gastroenterology (2020) 158:958–970.e2. doi: 10.1053/j.gastro.2019.10.020
60. Shogan BD, Belogortseva N, Luong PM, Zaborin A, Lax S, Bethel C, et al. Collagen degradation and MMP9 activation by Enterococcus faecalis contribute to intestinal anastomotic leak. Sci Transl Med (2015) 7:286ra68. doi: 10.1126/scitranslmed.3010658
61. Shogan BD, Smith DP, Christley S, Gilbert JA, Zaborina O, Alverdy JC. Intestinal anastomotic injury alters spatially defined microbiome composition and function. Microbiome (2014) 2:35. doi: 10.1186/2049-2618-2-35
62. Ezhova I, Savidge L, Bonnett C, Cassidy J, Okwuokei A, Dickinson T. Barriers to older adults seeking sexual health advice and treatment: A scoping review. Int J Nurs Stud (2020) 107:103566. doi: 10.1016/j.ijnurstu.2020.103566
63. Poynten IM, Jin F, Molano M, Roberts JM, Hillman RJ, Templeton DJ, et al. Possible reactivation of latent anal human papillomavirus associated with markers of immune dysfunction in gay and bisexual men. Cancer Epidemiol Biomarkers Prev (2022) 31:1052–7. doi: 10.1158/1055-9965.EPI-21-1346
64. Wang C, Zhao P, Xiong M, Tucker JD, Ong JJ, Hall BJ, et al. New syphilis cases in older adults, 2004-2019: an analysis of surveillance data from south China. Front Med (2021) 8:781759. doi: 10.3389/fmed.2021.781759
65. Smith ML, Bergeron CD, Goltz HH, Coffey T, Boolani A. Sexually transmitted infection knowledge among older adults: psychometrics and test-retest reliability. Int J Environ Res Public Health (2020) 17:2462. doi: 10.3390/ijerph17072462
66. Tao X, Ghanem KG, Page KR, Gilliams E, Tuddenham S. Risk factors predictive of sexually transmitted infection diagnosis in young compared to older patients attending sexually transmitted diseases clinics. Int J STD AIDS (2020) 31:142–9. doi: 10.1177/0956462419886772
67. Relhan V, Bansal A, Hegde P, Sahoo B. Sexually transmitted infections in the elderly: A 6-year retrospective study in a tertiary care hospital in New Delhi. Indian J Sex Transm Dis AIDS (2021) 42:144–9. doi: 10.4103/ijstd.IJSTD_60_20
68. Jena AB, Goldman DP, Kamdar A, Lakdawalla DN, Lu Y. Sexually transmitted diseases among users of erectile dysfunction drugs: analysis of claims data. Ann Intern Med (2010) 153:1–7. doi: 10.7326/0003-4819-153-1-201007060-00003
69. AtlasPlus - Charts. Available at: https://gis.cdc.gov/grasp/nchhstpatlas/charts.html.
Keywords: coinfection, syphilis, human papillomavirus (HPV), anogenital squamous cell carcinoma (ASCC), anal intraepithelial lesions
Citation: Ranabhotu A, Habibian N, Patel B, Farrell E, Do J, Sedghi S and Sedghi L (2023) Case Report: Resolution of high grade anal squamous intraepithelial lesion with antibiotics proposes a new role for syphilitic infection in potentiation of HPV-associated ASCC. Front. Oncol. 13:1226202. doi: 10.3389/fonc.2023.1226202
Received: 28 June 2023; Accepted: 04 September 2023;
Published: 03 October 2023.
Edited by:
Marcelo A. Soares, National Cancer Institute (INCA), BrazilReviewed by:
Mark M. Huycke, College of Medicine, University of Oklahoma, United StatesMircea Ioan Popa, Carol Davila University of Medicine and Pharmacy, Romania
Copyright © 2023 Ranabhotu, Habibian, Patel, Farrell, Do, Sedghi and Sedghi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: L. Sedghi, bGVhLnNlZGdoaUBnbWFpbC5jb20=; S. Sedghi, R2lzZWRnaGlAYW9sLmNvbQ==; A. Ranabhotu, YXJhbmFiaG90dUBnYW9jZy5jb20=
 A. Ranabhotu
A. Ranabhotu N. Habibian
N. Habibian B. Patel1
B. Patel1 E. Farrell
E. Farrell S. Sedghi
S. Sedghi L. Sedghi
L. Sedghi