
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 25 July 2023
Sec. Gastrointestinal Cancers: Colorectal Cancer
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1222951
This article is part of the Research Topic Colorectal Cancer Awareness Month 2023: Diagnosis, Clinical Course, and Surgical Management of Metastatic Colorectal Cancer View all 12 articles
 Florian Huemer1
Florian Huemer1 Celine Dunkl1
Celine Dunkl1 Gabriel Rinnerthaler1,2
Gabriel Rinnerthaler1,2 Konstantin Schlick1
Konstantin Schlick1 Ronald Heregger1
Ronald Heregger1 Klaus Emmanuel3
Klaus Emmanuel3 Daniel Neureiter2,4
Daniel Neureiter2,4 Eckhard Klieser4
Eckhard Klieser4 Michael Deutschmann5
Michael Deutschmann5 Falk Roeder6
Falk Roeder6 Richard Greil1,2,7
Richard Greil1,2,7 Lukas Weiss1,2,7*
Lukas Weiss1,2,7*Background: Age-standardized mortality rates for metastatic colorectal cancer (mCRC) are highest among elderly patients. In current clinical guidelines, treatment recommendations for this patient population are based on a limited number of clinical trials.
Patients and methods: In this monocentric, retrospective analysis we characterized patients aged ≥70 years undergoing systemic therapy for mCRC and overall survival (OS) was investigated.
Results: We included 117 unselected, consecutive mCRC patients aged ≥70 years undergoing systemic therapy for mCRC between February 2009 and July 2022. Median OS was 25.6 months (95% CI: 21.8-29.4). The median age was 78 years (range: 70-90) and 21%, 48%, 26% and 5% had an ECOG performance score of 0, 1, 2, and 3, respectively. The median number of systemic therapy lines was 2 (range: 1-5). The choice of first-line chemotherapy backbone (doublet/triplet versus mono) did not impact OS (HR: 0.83, p=0.50) or the probability of receiving subsequent therapy (p=0.697). Metastasectomy and/or local ablative treatment in the liver, lung, peritoneum and/or other organs were applied in 26 patients (22%) with curative intent. First-line anti-EGFR-based therapy showed a trend towards longer OS compared to anti-VEGF-based therapy or chemotherapy alone in left-sided mCRC (anti-EGFR: 39.3 months versus anti-VEGF: 27.3 months versus chemotherapy alone: 13.8 months, p=0.105). In multivariable analysis, metastasectomy and/or local ablative treatment with curative intent (yes versus no, HR: 0.22, p<0.001), the ECOG performance score (2 versus 0, HR: 3.07, p=0.007; 3 versus 0, HR: 3.66, p=0.053) and the presence of liver metastases (yes versus no, HR: 1.79, p=0.049) were independently associated with OS.
Conclusions: Our findings corroborate front-line monochemotherapy in combination with targeted therapy as the treatment of choice for elderly mCRC patients with palliative treatment intent. Metastasectomy and/or local ablative treatment with curative intent are feasible and may improve OS in selected elderly mCRC patients.
Colorectal cancer (CRC) is the second most common cause of cancer-related death worldwide (1). The incidence rate of CRC considerably increases with age and age-standardized CRC mortality rates are highest among elderly patients (2, 3). Elderly metastatic CRC (mCRC) patients (≥70 years) are underrepresented in clinical trials and one out of four elderly mCRC patients does not receive chemotherapy-based palliative systemic therapy due to comorbidities, chronological age or poor performance status (4).
Therapeutic decision making and treatment recommendations by the European Society of Medical Oncology (ESMO) (5) and National Comprehensive Cancer Network (NCCN) (6) for elderly mCRC patients are mainly based on a limited number of clinical trials focusing on the elderly mCRC population (7–9). Fluorouracil-based monochemotherapy in combination with anti-VEGF-based therapy irrespective of sidedness (7, 9) or in combination with anti-EGFR-based therapy (8) as well as anti-EGFR monotherapy (10) in patients with RAS wild-type left-sided tumors represent recommended first-line protocols (5). A median overall survival of 14 and 21 months is achieved with the abovementioned first-line protocols among patients ≥75 years (10) and ≥70 years (7), respectively; however, data on the clinical outcome in the elderly mCRC population in the real-world setting are sparse.
While metastasectomy and/or local ablative treatment (+/- perioperative chemotherapy or previous conversion therapy) represent established approaches in eligible patients with oligometastatic CRC (5, 6), there is a paucity of evidence supporting this treatment concept with putative curative intent in the elderly oligometastatic CRC population.
The primary aim of this unicentric retrospective analysis was to evaluate the therapeutic management of mCRC patients ≥70 years of age and clinical outcome in a real-world setting. Furthermore, this analysis aimed at investigating the frequency, feasibility and efficacy of metastasectomy and/or local ablative treatment with putative curative intent in this elderly population.
This retrospective analysis was approved by the Ethics Committee of the provincial government of Salzburg, Austria (415-E/2343/5-2018). Patients with an age ≥70 years at the time point of histologically confirmed mCRC diagnosis and who received systemic therapy for mCRC at our tertiary cancer center (Department of Internal Medicine III, Paracelsus Medical University Salzburg, Austria) between February 2009 and July 2022 were included in this analysis. All included patients alive at the date of analysis signed an informed consent form. Early access within a named patient program was available for patients who had received regorafenib and/or TAS-102 before the respective approval by the European Medicines Agency (EMA). Data were extracted from medical records, including:
1. patient characteristics: mCRC diagnosis date, age, sex, Eastern Cooperative Oncology Group (ECOG) performance score
2. tumor characteristics: time point of metastases detection (synchronous versus metachronous), sidedness (right versus left), histological grade, metastatic distribution pattern at mCRC diagnosis, predictive tumor-tissue-based biomarkers (KRAS-, NRAS-, BRAF-, microsatellite-/mismatch-repair-status)
3. systemic therapy characteristics: number of systemic therapy lines, first-line chemotherapy backbone (mono- versus doublet or triplet chemotherapy), application of targeted therapy during first-line (no antibody versus anti-VEGF versus anti-EGFR), regorafenib and/or TAS-102 exposure and
4. local ablative treatment with curative intent: metastasectomy, microwave ablation (MWA), radiofrequency ablation (RFA), stereotactic body radiation therapy (SBRT), transarterial chemoembolization (TACE) and involved organ(s): liver, lung, peritoneum, other.
In order to draw a comparison in regard to age distribution and treatment intent between our unicentric elderly mCRC cohort and mCRC patients ≥70 years in the province of Salzburg (Austria), data from the Tumor Registry of the Province of Salzburg from 2013 to 2020 were used.
Baseline characteristics were compared using crosstabulation together with the chi-squared test, in case of categorical data. Continuous data were summarized using medians and ranges and compared between groups with the Mann-Whitney test. Uni- and multivariable analyses were based on Cox proportional hazard models. For multivariable analysis covariable selection, a backward stepwise procedure was performed using the Akaike information criterion (AIC) as selection criterion (11). OS was calculated from the date of mCRC diagnosis until death from any cause. Metastasectomy and/or local ablative treatment (yes versus no) as well as regorafenib and/or TAS-102 exposure were taken into account as time-dependent covariates, respectively. Patients alive at the last contact were censored. IBM SPSS Statistics version 27 (Armonk, NY, US) and the statistical software environment R (version 4.1.2, survival and MASS package) were used for statistical analyses. The complete data set is available from the corresponding author on reasonable request.
In this retrospective monocentric analysis, 117 mCRC patients aged ≥70 years, diagnosed between February 2009 and July 2022, and undergoing systemic therapy for mCRC were included. The baseline characteristics are depicted in Table 1.
The median age at mCRC diagnosis was 78 (range: 70-90). 21%, 48%, 26% and 5% had an ECOG PS of 0, 1, 2, and 3 with a median age of 75, 78, 78.5, and 82 years at mCRC diagnosis, respectively (p=0.087).
Eighty-seven patients (74%) were diagnosed with synchronous mCRC. The primary tumor location was left-sided in 76 patients (65%). Liver, lung and peritoneal metastases were detected in 80 (68%), 40 (34%) and 22 (19%) patients at the time point of mCRC diagnosis, respectively.
Among patients with available tumor-tissue-based biomarkers, KRAS-mutations, NRAS-mutations, BRAF V600E-mutations and MSI/MMRd were detected in 53%, 3%, 8% and 8%, respectively.
In first line, a monochemotherapy backbone was applied in 32 patients (28%), whereas 83 patients (72%) received a doublet or triplet chemotherapy backbone. The likelihood of applying a doublet or triplet chemotherapy backbone declined with increasing age (p<0.001, Table A.1) and with a worse ECOG PS (p=0.007, Table A.1). Two patients with MSI/MMRd received immune-checkpoint blockade as palliative first-line therapy.
Sixty-one patients (52%) were treated with anti-VEGF-based therapy in first line, whereas anti-EGFR based therapy was applied in 21 patients (18%). The remaining 35 patients (30%) did not receive targeted therapy in first-line. Anti-VEGF-based therapy, anti-EGFR-based therapy or no targeted therapy were documented in 38 (50%), 14 (18%), and 24 (32%) patients with left-sided and in 23 (56%), 7 (17%) and 11 (27%) patients with right-sided primary tumor localization (p=0.812).
The EMA approved third-line therapy options, regorafenib and TAS-102, were applied in 26 patients (22%) during the course of disease (only regorafenib: n=4 (3%), only TAS-102: n=13 (11%), regorafenib followed by TAS-102 or vice versa: n=9 (8%)).
The median number of systemic therapy lines in the study population was 2 (range: 1-5) and 52%, 27% 12% and 3% received a second-line, third-line, fourth-line and fifth-line therapy (Figure 1). The chemotherapy backbone in first line (mono versus doublet/triplet) did not statistically significantly impact the probability of receiving subsequent therapy (p=0.697, Figure 1).

Figure 1 Impact of first-line chemotherapy backbone on number of subsequent therapy lines. Relative number of systemic therapy lines among elderly mCRC patients undergoing first-line therapy with any systemic therapy (blue), a monochemotherapy backbone (green) or a doublet or triplet chemotherapy backbone (red).
Twenty-six patients (22%) underwent metastasectomy and/or local ablative treatment of metastases in the liver, lung, peritoneum or other organs with curative intent during their course of disease (Table A.2):
In twenty-three patients (20%) surgical metastasectomy was performed once, whereas nine (8%) and two patients (2%) underwent metastasectomy twice and three times during their course of disease, respectively. Stereotactic body radiation therapy (SBRT), radiofrequency ablation (RFA) or microwave ablation (MWA), and transarterial chemoembolization (TACE) were applied in six (5%), six (5%) and two (2%) cases, respectively.
Patients undergoing metastasectomy and/or local ablative treatment were more likely to receive a front-line doublet or triplet chemotherapy backbone (89% versus 67%, p=0.035) and showed a trend towards metachronous metastases (38% versus 22%, p=0.090) compared to patients without ablative measures (Table A.3).
According to the Tumor Registry of the Province of Salzburg (Austria), the following age distribution pattern was found between 2013 and 2020 in the province of Salzburg among mCRC patients ≥70 years: 70-74 years: 32%; 75-79 years: 35%; 80-84 years: 33%, ≥85 years: 0%.
Fifty-nine per cent of the abovementioned patients received palliative systemic therapy and the likelihood decreased with increasing age: 70-74 years: 74%, 75-79 years: 58%; 80-84 years: 44%.
After a median follow up of 38.4 months (95% CI: 29.3-47.5 months), the median OS in the entire monocentric cohort was 25.6 months (95% CI: 21.8-29.4 months).
A worse ECOG PS at diagnosis was associated with inferior OS (1 versus 0, HR: 1.45, p=0.24; 2 versus 0, HR: 1.58, p=0.22; 3 versus 0, HR: 4.97, p=0.01; Table 2). Chronological age at mCRC diagnosis did not impact survival (HR: 1.02, p=0.54; Table 2).
Neither sidedness (left-sided versus right-sided, HR: 1.10 p=0.71 log-rank, Figure 2A), nor KRAS mutational status (mutant versus wild-type, HR: 1.06 p=0.80; Table 2) proved as prognostic factors. The presence of liver metastases at the time point of mCRC diagnosis negatively influenced OS (present versus absent, HR: 1.82, p=0.03; Table 2).
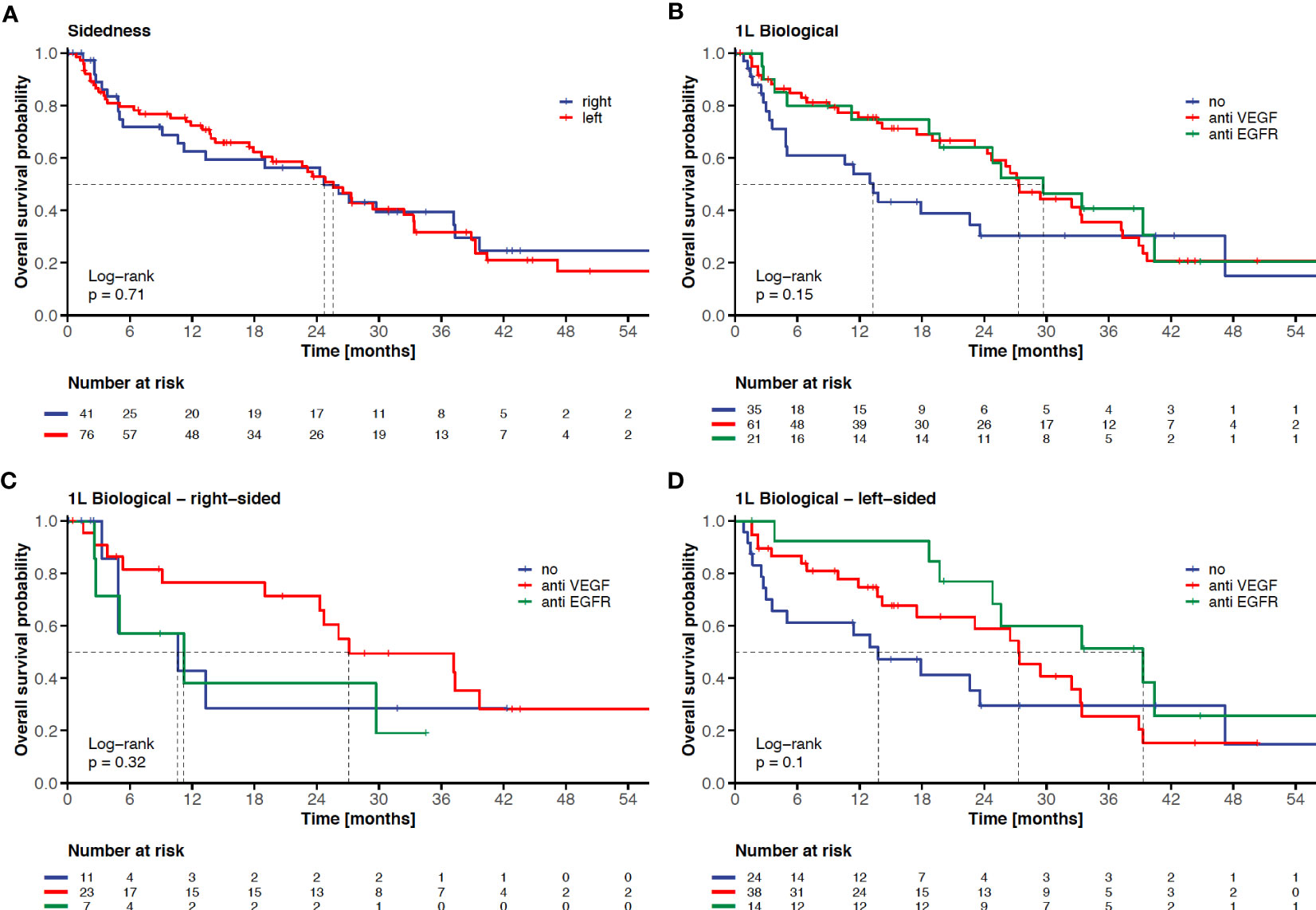
Figure 2 Impact of sidedness and 1L-targeted therapy on clinical outcome in elderly mCRC patients. KM-curves for overall survival according to sidedness (left-sided versus right-sided) (A), according to 1L-targeted therapy (no targeted therapy versus anti-VEGF-based therapy versus anti-EGFR-based therapy (B), according to 1L-targeted therapy in right-sided mCRC (C), and according to 1L-targeted therapy in left-sided mCRC (D). The tick marks on the curves represent censored patients.
The chemotherapy backbone of front-line therapy did neither affect OS in the entire cohort (doublet or triplet versus monochemotherapy, HR: 0.83, p=0.50; Table 2), nor among patients without metastasectomy and/or local ablative treatment (HR: 1.11, p=0.73). The addition of an anti-EGFR or anti-VEGF monoclonal antibody to chemotherapy in first line irrespective of the primary tumor localization resulted in a trend towards longer survival (anti-EGFR: 29.7 months versus anti-VEGF: 27.3 months versus no targeted therapy: 13.3 months, p=0.15 log-rank, Figure 2B). The choice of targeted therapy according to sidedness in first-line was associated with a trend towards superior survival with anti-EGFR-based therapy in left-sided disease (anti-EGFR: 39.3 months versus anti-VEGF: 27.3 months versus no targeted therapy: 13.8 months, p=0.105 log-rank; Figure 2D), while sidedness proved less predictive in right-sided disease (anti-VEGF: 27.1 months versus anti-EGFR: 11.2 months versus no targeted therapy: 10.6 months, p=0.325 log-rank; Figure 2C).
The application of more systemic therapy lines was associated with improved OS (≥2 versus 1, HR: 0.40, p<0.001; Table 2). Patients receiving regorafenib and/or TAS-102 during the course of disease did not show a survival benefit (yes versus no, HR: 1.14, p=0.67; Table 2) when considered as a time-dependent covariate. Seven patients were treated within clinical trials in first line and three patients in subsequent therapy lines.
Performing metastasectomy and/or applying local ablative treatment with curative intent statistically significantly improved OS (yes: 47.2 months versus no: 17.9 months, HR: 0.16, p<0.001, Table 2). The six-month survival rate was 100% after metastasectomy (liver, lung, peritoneum, other), SBRT (liver, lung), RFA/MWA (liver) and TACE (liver), respectively.
Based on a backward stepwise regression the following covariates were selected for multivariable analysis: sidedness (left-sided versus right-sided), liver metastases (present versus absent), ECOG PS (0 versus 1, 0 versus 2, 0 versus 3), regorafenib and/or TAS-102 exposure (yes versus no) and metastasectomy and/or local ablative treatment (yes versus no).
In multivariable analysis, metastasectomy and/or local ablative treatment (yes versus no, HR: 0.22, p<0.001), the ECOG performance score (2 versus 0, HR: 3.07, p=0.007; 3 versus 0, HR: 3.66, p=0.053) and the presence of liver metastases (yes versus no, HR: 1.79, p=0.049) remained statistically significantly and independently associated with survival (Figure 3).
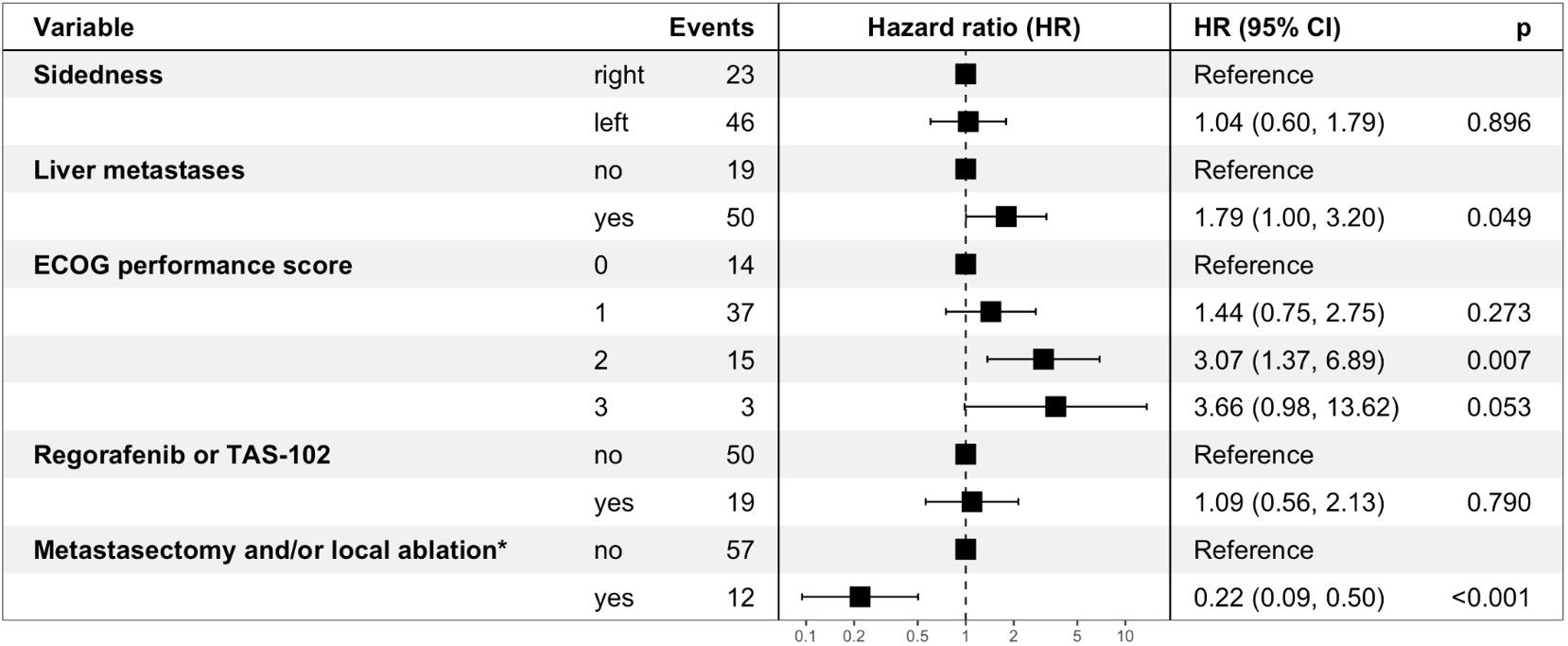
Figure 3 Multivariable analysis for overall survival – Forest Plot. ECOG performance score: Eastern Cooperative Oncology Group performance score. Regorafenib and/or TAS-102 exposure as well as metastasectomy and/or local ablative treatment were taken into consideration as time-dependent covariates. *involved organs: liver, lung, peritoneum, other.
As the aging population is highly represented among mCRC patients and due to the paucity of trial-based recommendations, therapeutic decision making in elderly mCRC patients remains challenging in clinical practice. In our unicentric, retrospective analysis we characterized patient and tumor characteristics and investigated clinical outcome in a representative elderly patient cohort undergoing systemic therapy for mCRC. The distribution of age categories within our elderly mCRC cohort was comparable to records of the Tumor Registry of the Province of Salzburg between 2013 and 2020: 70-74 years: 31% versus 32%; 75-79 years: 36% versus 35%; 80-84 years: 27% versus 33%, ≥85 years: 6% versus 0%. It is noteworthy, that only 59% of mCRC patients ≥70 years of age received palliative systemic therapy in the Province of Salzburg.
Based on the findings of our unicentric analysis we provide further evidence that OS of elderly mCRC patients undergoing systemic therapy in the real-world setting (mOS of 25.6 months) is comparable to landmark clinical trials (7, 12) (mOS of 19 to 21 months, Table A.4). Metastasectomy and/or local ablative treatment with curative intent proved feasible in selected elderly patients and resulted in a significant and clinically meaningful OS benefit (HR: 0.22, p<0.001, Figure 3). Furthermore, the observed trend towards superior OS with an anti-EGFR-based therapy in left-sided mCRC when compared to anti-VEGF-based therapy or chemotherapy alone (Figure 2D) sheds further light on the predictive value of sidedness and corroborates the preference of anti-EGFR-based therapy also in elderly patients with RAS/BRAF wild-type left-sided disease.
In a cross-trial comparison between our retrospective analysis and a pooled analysis (13) of the TRIBE (14) and TRIBE2 (15) study, fewer patients received subsequent therapy lines in our elderly mCRC cohort (2L: 77% versus 52%, 3L: 53% versus 27%, 4L: 27% versus 12%, 5L: 11% versus 3%, Figure 1). It is noteworthy that the median age at mCRC diagnosis in the aforementioned studies (TRIBE: 60.0 and 60.5 years; TRIBE2: 60.0 and 61.0 years) was considerably lower when compared to our cohort (78 years, range: 70-90 years). However, the probability to receive subsequent systemic therapy was higher in our cohort compared to mCRC patients in the AVEX trial (7) (52% versus 37%).
The chemotherapy backbone in first line (doublet or triplet versus mono) did neither impact the number of subsequent therapy lines (p=0.697, Figure 1), nor had an impact on clinical outcome in the entire cohort (HR: 0.83, p=0.50) or among patients not eligible for metastasectomy and/or local ablative treatment (HR: 1.11, p=0.73). The latter findings are in line with the MRC FOCUS2 (16) and FFCD 2001-02 (17) trials, where the addition of oxaliplatin (16) or irinotecan (17) to 5-FU or capecitabine did not improve OS in elderly and/or frail mCRC patients, but significantly increased the frequency of grade 3-4 toxicities (17). In this regard, it is noteworthy that the ECOG PS in our elderly cohort was comparable to the study population of the MRC FOCUS2 trial (16): ECOG 0: 21%/21%, ECOG 1: 48%/50%, ECOG 2: 26%/29%, ECOG 3: 5%/0%.
Higher treatment-related toxicity rates with a doublet chemotherapy backbone and a higher frequency of comorbidities have also been observed with increasing age in the CALGB 80405 study (18). Age demonstrated as a considerable prognostic factor in the FIRE-3 study (19) (≥65 years: 25.9 versus <65 years: 29.3 months, p=0.02) and CALGB 80405 study (18) (≥70 years versus <70 years: HR 1.32, p<0.001). Within our study population (range: 70-90 years), older patients showed a trend towards a worse ECOG PS (p=0.087), however, age as a continuous parameter did not show any additional prognostic value among mCRC patients ≥70 years (Table 2).
A worse ECOG PS at mCRC diagnosis showed a statistically significant and independent association with inferior OS (2 versus 0, HR: 3.07; 3 versus 0: HR; 3.66; Figure 3). While classification into the ECOG PS categories (from 0: fully active to 4: completely disabled) can be rapidly performed in daily clinical practice in younger patients, the latter performance score assessment can be challenging in elderly cancer patients due to physicians’ varying conception of the usual performance spectrum of elderly people. Considerable disparities between patient-reported and physician-reported ECOG PS ratings exist (20) and there is also a poor agreement in ECOG PS ratings between clinicians (21). Other scores such as the Charlson Comorbidity Index (22), which includes age and multiple comorbidities and classifies into four risk categories, proved as predictors of survival in (m)CRC (23–25). However, our findings confirm the ECOG PS as a time-saving prognosticator and helpful tool for therapeutic decision-making (e.g. chemotherapy intensity) in daily clinical practice in elderly mCRC patients. The International Society of Geriatric Oncology recommends geriatric assessment in older cancer patients aiming at influencing treatment choice, predicting treatment-related complications and predicting clinical outcome. Geriatric assessment should include functional status, comorbidities, cognition, mental health status, fatigue, social status and support, nutrition, and the presence of geriatric syndromes (26). Based on the retrospective nature of our analysis, only the functional status was extracted from medical records and geriatric assessment was not feasible.
Contrary to the literature (27), sidedness was not prognostic among elderly mCRC patients in our cohort (left-sided versus right-sided, HR: 1.10 p=0.71, Figure 2A), which may be explained by the application of front-line anti-VEGF-based therapy in the majority of cases with right-sided (58%) as well as left-sided (51%) primary tumor localization. Furthermore, a higher percentage of patients with right-sided primary tumors underwent metastasectomy and/or local ablative treatment (right-sided: 33% versus left-sided: 15%, Table A.3). This stands in contrast to the secondary metastasectomy rate among patients ≥65 years in the FIRE-3 study (19) (right-sided: 8%-13% versus left-sided: 15%-26%).
A post-hoc analysis of the FIRE-3 study in the subgroup of patients ≥ 65 years (n=199) could neither corroborate the survival benefit of cetuximab versus bevacizumab in left-sided mCRC (33.2 months versus 27.5 months, HR: 0.86, p=0.38), nor the disadvantage of first-line cetuximab-based therapy in right-sided disease (16.6 months versus 23.6 months, HR: 1.1, p=0.87) (19). Liver surgery for colorectal metastases with curative intent in elderly mCRC patients can yield a comparable OS benefit as in the young population (28, 29). For elderly mCRC patients undergoing CRC liver metastases resection an incidence of 60- to 90-day mortality ranging between 4% and 8% has been reported in population-based studies (29, 30).
According to the RAXO study, a nationwide Finnish prospective intervention study, up to 41% of mCRC patients can be classified as resectable with curative intent either upfront or after conversion therapy irrespective of chronological age (31). In our cohort, metastasectomy and/or local ablative treatment were performed in 22% of patients with technically resectable disease extent and adequate performance status and yielded a clinically meaningful and independent OS benefit (HR: 0.22, Figure 3). This is in line with the secondary metastasectomy rate (18%) and the OS advantage (HR: 0.44) of elderly patients in the FIRE-3 study (19). The latter findings should encourage us to identify eligible patients for metastasectomy and/or local ablative treatment with curative intent in the elderly mCRC population. The presence of liver metastases was a significant and independent negative prognostic factor (HR: 1.79, p=0.05) – presumably mainly driven by non-resectable and non-liver-limited disease.
However, we would like to emphasize that in the FIRE-3 and CALGB 80405 studies elderly patients were defined by ≥65 years and ≥70 years, respectively, and were all deemed fit for a doublet chemotherapy backbone (18, 19). Data from the Cardiovascular Health Study corroborate an increasing prevalence of frailty with higher chronological age (32). Therefore, the FIRE-3 and CALGB 80405 mCRC populations may not properly reflect the elderly and often frail mCRC population in the real-world setting.
Within the inclusion period of our retrospective analysis (2009-2022), regorafenib (33) as well as TAS-102 (34) have been established as EMA- and FDA-approved third-line therapy options based on a survival benefit versus placebo, respectively. In our cohort, one out of five patients received regorafenib and/or TAS-102 during the course of disease (Table 1). Since the availability of regorafenib and TAS-102 within named patient programs or based on the respective EMA approval, our treatment strategy has not favored one drug over the other in the time interval between 2014 and 2022 (Figure A.1). However, based on the toxicity profile of regorafenib (33), an increased skeletal muscle loss (35) and a higher frequency of hospitalizations with regorafenib compared to TAS-102 (36), regorafenib should be used with caution in elderly mCRC patients. Treatment with regorafenib and/or TAS-102 did not result in a survival advantage when taken into account as a time-dependent covariate (yes versus no, HR: 1.09, p=0.79, Figure 3). According to the ESMO Magnitude of Clinical Benefit Scale (ESMO-MCBS) (37), which is based on the extent of OS gain, QoL and toxicities, TAS-102 (MCBS: 3) proved superior to regorafenib (MCBS: 1) (5).
The SUNLIGHT study, a randomized phase 3 study comparing TAS-102 versus TAS-102 in combination with bevacizumab for third-line treatment of refractory mCRC, has met its primary endpoint, demonstrating an OS benefit with TAS-102 plus bevacizumab (10.8 months versus 7.5 months, HR: 0.61, p<0.001) (38). Due to the acceptable safety profile of TAS-102 combined with bevacizumab in previous studies (9, 38, 39) this combination may become a new third-line standard in the near future, particularly suitable for the elderly and frail mCRC population.
The availability of further new treatment options (40,) (41) within the inclusion period (2009-2022) may have also contributed to the encouraging clinical outcome (mOS of 25.6 months) compared to the experimental arm of the AVEX trial (7) (mOS of 20.7 months, Table A.4).
Potential limitations of our study include the retrospective nature and the length of the inclusion period (2009-2022). Within the latter time span, biomarker refinement for established therapies (42), numerous new therapies for all-comers (33, 34) and biomarker-defined targeted-therapies (40, 41) changed daily clinical practice resulting in heterogenous treatments strategies in our elderly mCRC cohort. As a consequence, the predictive biomarker status is incomplete in a relevant number of patients. Furthermore, the implementation of sidedness into first-line decision making took place after the Annual ASCO Meeting 2016 (27, 43, 44), therefore, sidedness as a predictive biomarker could only be applied in less than half of our elderly mCRC patients. It is noteworthy, that elderly patients undergoing only a best supportive care strategy were excluded from our analysis. Although the number of included patients in our analysis (n=117) was limited, the sample size was comparable to the experimental arms of the AVEX (n=140) and PANDA (n=93) landmark trials (Table A.4).
Clinical outcome among real-world elderly (≥70 years) mCRC patients is comparable to the results of first-line elderly mCRC landmark trials. First-line monochemotherapy plus targeted therapy based on sidedness and molecular status should be the treatment of choice. Based on proper patient selection, one out of five elderly mCRC patients qualifies for metastasectomy and/or local ablative treatment with curative intent. A doublet chemotherapy backbone +/- targeted therapy may be expedient in elderly mCRC patients who are candidates for metastasectomy and/or local ablative treatment. The latter ablative measures are feasible and yield a clinically meaningful survival benefit in selected elderly mCRC patients.
The raw data supporting the conclusions of this article will be made available by the authors on reasonable request.
The studies involving human participants were reviewed and approved by the Ethics Committee of the provincial government of Salzburg, Austria (415-E/2343/5-2018). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
FH: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Writing – original draft, Writing – review and editing. CD: Investigation, Writing – review and editing. GR: Formal analysis, Investigation, Methodology, Writing – review and editing. KS: Investigation, Writing – review and editing. RH: Investigation, Writing – review and editing. KE: Investigation, Writing – review and editing. DN: Investigation, Writing – review and editing. EK: Investigation, Writing – review and editing. MD: Investigation, Writing – review and editing. FR: Investigation, Writing – review and editing. RG: Funding Acquisition, Resources, Supervision, Writing – review and editing. LW: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review and editing. All authors contributed to the article and approved the submitted version.
This work was supported by Servier Austria GmbH without any role in the design of the analysis, interpretation of data or influence on the content of the manuscript.
FH received honoraria from Eli Lilly, Pierre Fabre, Amgen, Servier, Daiichi Sankyo, Merck, Sanofi and BMS; travel support from Servier, BMS, Roche, Merck, PharmaMar, Pfizer, Daiichi Sankyo, Sanofi and Pierre Fabre. GR received honoraria from Roche, Seagen, Daiichi Sankyo, Pfizer, Eli Lilly, Gilead, Novartis and Amgen; reports travel support from Amgen, Daiichi Sankyo, Eli Lilly, Gilead, Merck, Pfizer and Roche; reports a consulting or advisory role for Roche, AstraZeneca, Daiichi Sankyo, Gilead, Pfizer, Pierre Fabre, Eli Lilly, MSD, Novartis, Amgen and Merck. KS received honoraria and travel support from Servier, Amgen and Pfizer. Ronald Heregger received travel support from PharmaMar. DN received honoraria for advisory function from Boehringer Ingelheim Pharma GmbH & Co and Eli Lilly. MD received honoraria from Terumo Europe N.V. FR received travel grants and lecture honoraria from Intraop Medical and PharmaMar. RG reports a consulting or advisory role for Celgene, Novartis, Roche, BMS, Takeda, Abbvie, AstraZeneca, Janssen, MSD, Merck, Gilead, Daiichi Sankyo and Sanofi; honoraria from Celgene, Novartis, Amgen, Roche, BMS, Takeda, Abbvie, AstraZeneca, MSD, Merck, Sandoz, Gilead, Daiichi Sankyo, Sanofi; travel support from Celgene, Novartis, Roche, Amgen, BMS, Abbvie, AstraZeneca, Janssen, MSD, Gilead and Daiichi Sankyo; research funding from Celgene, Roche, Merck, Takeda, AstraZeneca, Novartis, Amgen, BMS, MSD, Sandoz, Abbvie, Gilead, Daiichi Sankyo. LW received honoraria from Amgen, Astellas, BMS, Daiichi Sankyo, GSK, Lilly, Merck, MSD, Novocure, PharmaMar, Pierre Fabre, Roche, Servier; consulting fees from Merck and MSD; research support from Novocure, Roche and Servier.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1222951/full#supplementary-material
Supplementary Table 1 | Association between 1L chemotherapy backbone and ECOG PS as well as age at mCRC diagnosis.
Supplementary Table 2 | Elderly mCRC patients undergoing metastasectomy and/or local ablative treatment with curative intent (N=26) MWA: microwave ablation, RFA: radiofrequency ablation, SBRT: stereotactic body radiation therapy, TACE: transarterial chemoembolization.
Supplementary Table 3 | Comparison of baseline characteristics between elderly mCRC patients undergoing metastasectomy and/or local ablative treatment versus not #Mann-Whitney-U-Test.
ECOG, Eastern Cooperative Oncology Group, mCRC, metastatic colorectal cancer.
Supplementary Table 4 | Comparison of baseline characteristics and clinical outcome between the Salzburg elderly mCRC real-world cohort and elderly mCRC landmark trials.
Supplementary Figure 1 | Regorafenib and TAS-102 exposure among elderly mCRC patients between 2014 and 2022 Cumulative cases of regorafenib (blue), TAS-102 (green) and total regorafenib and TAS-102 applications (red) between 2014 and 2022 among elderly mCRC patients.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
2. Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin (2020) 70:145–64. doi: 10.3322/caac.21601
3. Lieu CH, Renfro LA, de Gramont A, Meyers JP, Maughan TS, Seymour MT, et al. Association of age with survival in patients with metastatic colorectal cancer: analysis from the ARCAD clinical trials program. J Clin Oncol (2014) 32:2975–84. doi: 10.1200/JCO.2013.54.9329
4. Parakh S, Wong HL, Rai R, Ali S, Field K, Shapiro J, et al. Patterns of care and outcomes for elderly patients with metastatic colorectal cancer in Australia. J Geriatr Oncol (2015) 6:387–94. doi: 10.1016/j.jgo.2015.06.001
5. Cervantes A, Adam R, Rosello S, Arnold D, Normanno N, Taieb J, et al. Metastatic colorectal cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol (2022). doi: 10.1016/j.annonc.2022.10.003
6. National Comprehensive Cancer Network. Colon cancer (Version 2.2022). Available at: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf (Accessed December 24, 2022).
7. Cunningham D, Lang I, Marcuello E, Lorusso V, Ocvirk J, Shin DB, et al. Bevacizumab plus capecitabine versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer (AVEX): an open-label, randomised phase 3 trial. Lancet Oncol (2013) 14(11):1077–85. doi: 10.1016/S1470-2045(13)70154-2
8. Lonardi S, Schirripa M, Buggin F, Antonuzzo L, Merelli B, Boscolo G, et al. First-line FOLFOX plus panitumumab versus 5FU plus panitumumab in RAS-BRAF wild-type metastatic colorectal cancer elderly patients: the PANDA study. J Clin Oncol (2020) 38(15_suppl):4002–2. doi: 10.1200/JCO.2020.38.15_suppl.4002
9. Andre T, Falcone A, Shparyk Y, Moiseenko F, Polo-Marques E, Csoszi T, et al. Trifluridine-tipiracil plus bevacizumab versus capecitabine plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer ineligible for intensive therapy (SOLSTICE): a randomised, open-label phase 3 study. Lancet Gastroenterol Hepatol (2022). doi: 10.1016/S2468-1253(22)00334-X
10. Pietrantonio F, Cremolini C, Aprile G, Lonardi S, Orlandi A, Mennitto A, et al. Single-agent panitumumab in frail elderly patients with advanced RAS and BRAF wild-type colorectal cancer: challenging drug label to light up new hope. Oncologist (2015) 20:1261–5. doi: 10.1634/theoncologist.2015-0171
11. Heinze G, Wallisch C and Dunkler D. Variable selection - a review and recommendations for the practicing statistician. Biom J (2018) 60:431–49. doi: 10.1002/bimj.201700067
12. Kabbinavar FF, Hurwitz HI, Yi J, Sarkar S, Rosen O. Addition of bevacizumab to fluorouracil-based first-line treatment of metastatic colorectal cancer: pooled analysis of cohorts of older patients from two randomized clinical trials. J Clin Oncol (2009) 27:199–205. doi: 10.1200/JCO.2008.17.7931
13. Rossini D, Germani MM, Lonardi S, Pietrantonio F, Dell'Aquila E, Borelli B, et al. Treatments after second progression in metastatic colorectal cancer: a pooled analysis of the TRIBE and TRIBE2 studies. Eur J Cancer (2022) 170:64–72. doi: 10.1016/j.ejca.2022.04.019
14. Cremolini C, Loupakis F, Antoniotti C, Lupi C, Sensi E, Lonardi S, et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol (2015) 16:1306–15. doi: 10.1016/S1470-2045(15)00122-9
15. Cremolini C, Antoniotti C, Rossini D, Lonardi S, Loupakis F, Pietrantonio F, et al. Upfront FOLFOXIRI plus bevacizumab and reintroduction after progression versus mFOLFOX6 plus bevacizumab followed by FOLFIRI plus bevacizumab in the treatment of patients with metastatic colorectal cancer (TRIBE2): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol (2020) 21:497–507. doi: 10.1016/S1470-2045(19)30862-9
16. Seymour MT, Thompson LC, Wasan HS, Middleton G, Brewster AE, Shepherd SF, et al. Chemotherapy options in elderly and frail patients with metastatic colorectal cancer (MRC FOCUS2): an open-label, randomised factorial trial. Lancet (2011) 377:1749–59. doi: 10.1016/S0140-6736(11)60399-1
17. Aparicio T, Lavau-Denes S, Phelip JM, Maillard E, Jouve JL, Gargot D, et al. Randomized phase III trial in elderly patients comparing LV5FU2 with or without irinotecan for first-line treatment of metastatic colorectal cancer (FFCD 2001-02). Ann Oncol (2016) 27:121–7. doi: 10.1093/annonc/mdv491
18. McCleary NJ, Zhang S, Ma C, Ou FS, Bainter TM, Venook AP, et al. Age and comorbidity association with survival outcomes in metastatic colorectal cancer: CALGB 80405 analysis. J Geriatr Oncol (2022) 13:469–79. doi: 10.1016/j.jgo.2022.01.006
19. Fischer LE, Stintzing S, von Weikersthal LF, Modest DP, Decker T, Kiani A, et al. Efficacy of FOLFIRI plus cetuximab vs FOLFIRI plus bevacizumab in 1st-line treatment of older patients with RAS wild-type metastatic colorectal cancer: an analysis of the randomised trial FIRE-3. Br J Cancer (2022) 127:836–43. doi: 10.1038/s41416-022-01854-y
20. Popovic G, Harhara T, Pope A, Al-Awamer A, Banerjee S, Bryson J, et al. Patient-reported functional status in outpatients with advanced cancer: correlation with physician-reported scores and survival. J Pain Symptom Manage (2018) 55:1500–8. doi: 10.1016/j.jpainsymman.2018.02.015
21. Datta SS, Ghosal N, Daruvala R, Chakraborty S, Shrimali RK, van Zanten C, et al. How do clinicians rate patient's performance status using the ECOG performance scale? a mixed-methods exploration of variability in decision-making in oncology. Ecancermedicalscience (2019) 13:913. doi: 10.3332/ecancer.2019.913
22. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis (1987) 40:373–83. doi: 10.1016/0021-9681(87)90171-8
23. De Marco MF, Janssen-Heijnen ML, van der Heijden LH, Coebergh JW. Comorbidity and colorectal cancer according to subsite and stage: a population-based study. Eur J Cancer (2000) 36:95–9. doi: 10.1016/s0959-8049(99)00221-x
24. Ouellette JR, Small DG and Termuhlen PM. Evaluation of charlson-age comorbidity index as predictor of morbidity and mortality in patients with colorectal carcinoma. J Gastrointest Surg (2004) 8:1061–7. doi: 10.1016/j.gassur.2004.09.045
25. Baretti M, Rimassa L, Personeni N, Giordano L, Tronconi MC, Pressiani T, et al. Effect of comorbidities in stage II/III colorectal cancer patients treated with surgery and Neoadjuvant/Adjuvant chemotherapy: a single-center, observational study. Clin Colorectal Cancer (2018) 17:e489–98. doi: 10.1016/j.clcc.2018.03.010
26. Wildiers H, Heeren P, Puts M, Topinkova E, Janssen-Heijnen ML, Extermann M, et al. International society of geriatric oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol (2014) 32:2595–603. doi: 10.1200/JCO.2013.54.8347
27. Tejpar S, Stintzing S, Ciardiello F, Tabernero J, Van Cutsem E, Beier F, et al. Prognostic and predictive relevance of primary tumor location in patients with RAS wild-type metastatic colorectal cancer: retrospective analyses of the CRYSTAL and FIRE-3 trials. JAMA Oncol (2017) 3:194–201. doi: 10.1001/jamaoncol.2016.3797
28. Nassabein R, Mansour L, Richard C, Vandenbroucke-Menu F, Aubin F, Ayoub JP, et al. Outcomes of older patients with resectable colorectal liver metastases cancer (CRLM): single center experience. Curr Oncol (2021) 28:1899–908. doi: 10.3390/curroncol28030176
29. Adam R, Frilling A, Elias D, Laurent C, Ramos E, Capussotti L, et al. Liver resection of colorectal metastases in elderly patients. Br J Surg (2010) 97:366–76. doi: 10.1002/bjs.6889
30. Booth CM, Nanji S, Wei X, Mackillop WJ. Management and outcome of colorectal cancer liver metastases in elderly patients: a population-based study. JAMA Oncol (2015) 1:1111–9. doi: 10.1001/jamaoncol.2015.2943
31. Osterlund P, Salminen T, Soveri LM, Kallio R, Kellokumpu I, Lamminmaki A, et al. Repeated centralized multidisciplinary team assessment of resectability, clinical behavior, and outcomes in 1086 Finnish metastatic colorectal cancer patients (RAXO): a nationwide prospective intervention study. Lancet Reg Health Eur (2021) 3:100049. doi: 10.1016/j.lanepe.2021.100049
32. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci (2001) 56:M146–156. doi: 10.1093/gerona/56.3.m146
33. Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet (2013) 381:303–12. doi: 10.1016/S0140-6736(12)61900-X
34. Mayer RJ, Van Cutsem E, Falcone A, Yoshino T, Garcia-Carbonero R, Mizunuma N, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med (2015) 372:1909–19. doi: 10.1056/NEJMoa1414325
35. Huemer F, Schlintl V, Hecht S, Hackl H, Melchardt T, Rinnerthaler G, et al. Regorafenib is associated with increased skeletal muscle loss compared to TAS-102 in metastatic colorectal cancer. Clin Colorectal Cancer (2019) 18:159–166 e153. doi: 10.1016/j.clcc.2019.04.003
36. Huemer F, Piringer G, Schlintl V, Hackl H, Rinnerthaler G, Thaler J, et al. Hospitalizations and clinical outcome in metastatic colorectal cancer during regorafenib or TAS-102 therapy. Cancers (Basel) (2020) 12(10). doi: 10.3390/cancers12102812
37. Cherny NI, Dafni U, Bogaerts J, Latino NJ, Pentheroudakis G, Douillard JY, et al. ESMO-magnitude of clinical benefit scale version 1.1. Ann Oncol (2017) 28:2340–66. doi: 10.1093/annonc/mdx310
38. Prager GW, Taieb J, Fakih M, Ciardiello F, Van Cutsem E, Elez E, et al. Trifluridine-tipiracil and bevacizumab in refractory metastatic colorectal cancer. N Engl J Med (2023) 388:1657–67. doi: 10.1056/NEJMoa2214963
39. Pfeiffer P, Yilmaz M, Moller S, Zitnjak D, Krogh M, Petersen LN, et al. TAS-102 with or without bevacizumab in patients with chemorefractory metastatic colorectal cancer: an investigator-initiated, open-label, randomised, phase 2 trial. Lancet Oncol (2020) 21:412–20. doi: 10.1016/S1470-2045(19)30827-7
40. Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med (2015) 372:2509–20. doi: 10.1056/NEJMoa1500596
41. Siena S, Di Bartolomeo M, Raghav K, Masuishi T, Loupakis F, Kawakami H, et al. Trastuzumab deruxtecan (DS-8201) in patients with HER2-expressing metastatic colorectal cancer (DESTINY-CRC01): a multicentre, open-label, phase 2 trial. Lancet Oncol (2021) 22:779–89. doi: 10.1016/S1470-2045(21)00086-3
42. Misale S, Di Nicolantonio F, Sartore-Bianchi A, Siena S, Bardelli A. Resistance to anti-EGFR therapy in colorectal cancer: from heterogeneity to convergent evolution. Cancer Discov (2014) 4:1269–80. doi: 10.1158/2159-8290.CD-14-0462
43. Venook AP, Niedzwiecki D, Innocenti F, Fruth B, Greene C, O'Neil BH, et al. Impact of primary (1°) tumor location on overall survival (OS) and progression-free survival (PFS) in patients (pts) with metastatic colorectal cancer (mCRC): analysis of CALGB/SWOG 80405 (Alliance). J Clin Oncol (2016) 34:3504–4. doi: 10.1200/JCO.2016.34.15_suppl.3504
44. Arnold D, Lueza B, Douillard JY, Peeters M, Lenz HJ, Venook A, et al. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol (2017) 28:1713–29. doi: 10.1093/annonc/mdx175
Keywords: elderly, age, ECOG performance score, colorectal cancer, sidedness, local ablative treatment, metastasectomy
Citation: Huemer F, Dunkl C, Rinnerthaler G, Schlick K, Heregger R, Emmanuel K, Neureiter D, Klieser E, Deutschmann M, Roeder F, Greil R and Weiss L (2023) Management of metastatic colorectal cancer in patients ≥70 years - a single center experience. Front. Oncol. 13:1222951. doi: 10.3389/fonc.2023.1222951
Received: 22 May 2023; Accepted: 23 June 2023;
Published: 25 July 2023.
Edited by:
Samuel Aguiar Junior, A.C.Camargo Cancer Center, BrazilReviewed by:
Nuria Mulet Margalef, Catalan Institute of Oncology, SpainCopyright © 2023 Huemer, Dunkl, Rinnerthaler, Schlick, Heregger, Emmanuel, Neureiter, Klieser, Deutschmann, Roeder, Greil and Weiss. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lukas Weiss, bHUud2Vpc3NAc2Fsay5hdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.