
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 21 July 2023
Sec. Cancer Molecular Targets and Therapeutics
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1220745
Background: The pulmonary sarcomatoid carcinoma (PSC) is a rare and aggressive subtype of NSCLC with rapid progression and poor prognosis, and is resistant to conventional chemotherapy. Most PSC cases have potential targetable genomic alterations. Approximately 7% of PSC patients have BRAF mutations, and the efficacy of dabrafenib and trametinib in BRAFV600E mutated PSC is unclear.
Case presentation: Our report describes a patient with mutated BRAFV600E PSC who underwent surgery and adjuvant chemotherapy early but quickly relapsed. Both chemotherapy and immunotherapy were ineffective for him, combined dabrafenib and trametinib produced a 6-month progression-free survival, and a partial response was observed in the tumor response evaluation. As a result of financial pressure, he stopped taking the targeted drugs, and his disease rapidly progressed.
Conclusion: Dabrafenib combined with trametinib provides partial remission in patients with advanced PSC with BRAFV600E mutations, and large-scale NGS panels could offer more options for PSC treatment.
In less than 1% of all lung cancers, pulmonary sarcomatoid carcinoma (PSC) is a rare subtype of non-small cell lung cancer (NSCLC), with high malignancy and poor prognosis (1). Despite early diagnosis, PSC patients have a worse prognosis compared to other types of NSCLC (2). According to the analyzes of the National Cancer Data Base, the 5-year survival rate for PSC was only 14.4% and the median overall survival (OS) was only 6.4 months (3). Because PSC is greatly resistant to conventional first-line chemotherapy drugs (4), there is an urgent need to actively explore the multimodal treatment of PSC. Previous studies have shown that most PSC cases have potential targetable genomic alterations and intermediate or high tumor mutation burden (TMB) (5). One study showed that patients with PSC treated with chemotherapy combined with immunotherapy as first-line therapy achieved an objective response rate (ORR) of 73.8% and a median progression-free survival (mPFS) of 10.3 months (6). There are also some case reports that the use of targeted drugs significantly alleviates disease and even survival benefits in patients with PSC (7–9). Thus, immunotherapy and targeted therapy may offer new opportunities for the treatment of PSC.
BRAF, a member of the Ras/mitogen-activated protein kinase signaling pathway, is downstream of KRAS, and directly phosphorylates MEK, which in turn phosphorylates ERK. The pathway culminates in the transcription of genes favoring proliferation and survival (10). The mutation frequency of BRAF in PSC is 7.2% (5). Moreover, the most common BRAF mutation, V600E (Val600Glu), was observed in 1–2% of lung adenocarcinomas (11). The combination of dabrafenib and trametinib is safe and effective in NSCLC patients with BRAFV600E mutations (12–14). However, existing studies have not reported the efficacy of this combination in patients with PSC. Here, we report a patient with BRAFV600E mutated-PSC who underwent surgery and postoperative adjuvant chemotherapy at an early stage but quickly relapsed. After relapsing, he received chemoradiotherapy in combination with immunotherapy and continued to progress rapidly, after which he achieved six months of PFS with dabrafenib in combination with trametinib. Despite his financial difficulties, he stopped using targeted drugs and switched to immunotherapy, after which his disease progressed rapidly.
A 67-year-old man, with a 30-year history of smoking and a five-year history of hypertension, came to Zhongnan Hospital of Wuhan University with a cough on February 1, 2021. Since the nodule of the left lower lobe did not change significantly (Figure S1), the attending doctor ignored it. The patient’s cough did not abate and he went to another hospital on April 8, 2021. A PET-CT examination revealed a nodule in the basal segment of the left inferior lobe with cavity and increased metabolism. Then, he underwent a lower left lobectomy and lymph node dissection on April 15. The pathological findings of the excised tissue suggested PSC (d=1.3cm) and no lymph node metastasis. In addition, the tumor stage was T1aN0M0. Considering the elevated risk of postoperative recurrence and poor prognosis of PSC, he then received four cycles of postoperative adjuvant chemotherapy (gemcitabine 2200 mg d1, d8 + cisplatin 40 mg d1~d3) from June 3 through August 6 in another hospital, during which severe myelosuppression and anemia occurred, and reexamination showed no tumor recurrence.
However, on October 21, 2021, he came to Zhongnan Hospital of Wuhan University again due to fatigue and chest pain, and out-patient CT showed enlarged nodules of left lung and subpleural, enlarged mediastinal lymph nodes and multiple low-density shadows on left ribs (Figure S2). To clarify the histology of the mass, a lung needle biopsy was performed on November 16 (Figure 1A) and an enhanced CT was reviewed (Figure 2A). Microscopically, two morphologies of cells, spindle cells, and epithelial cells were seen (Figure 1B). The immunohistochemical results were as follows: CK7 (+), NapsinA (focal +), TTF-1 (partial +), VIMENTIN (spindle cell +), CK (+), P40 (-), Ki-67 (50%) (Figures 1C–I), CK5/6 (-), SYN (-), CD56 (-), CgA (-). PD-L1 combined positive score (CPS) of the small amount of tissue taken for biopsy was 0%. The above results support the diagnosis of lung adenocarcinoma poorly differentiated with spindle cell components (15). Combined with the pathological diagnosis of the original surgical specimen (Figure S3), it was consistent with the involvement of PSC. Based on the clinical information, we considered it was a PSC recurrence. Upon next-generation sequencing (NGS) analysis of the biopsy tissue sample, the patient was identified with BRAFV600E mutation (exon 15, 22.7% abundance), KRASG12A mutation (exon 2, 5.71% abundance) (Figure 1J), PIK3CAE707K mutation (exon 14, 1.02% abundance) and TP53H179R mutation (exon 5, 29.65% abundance).
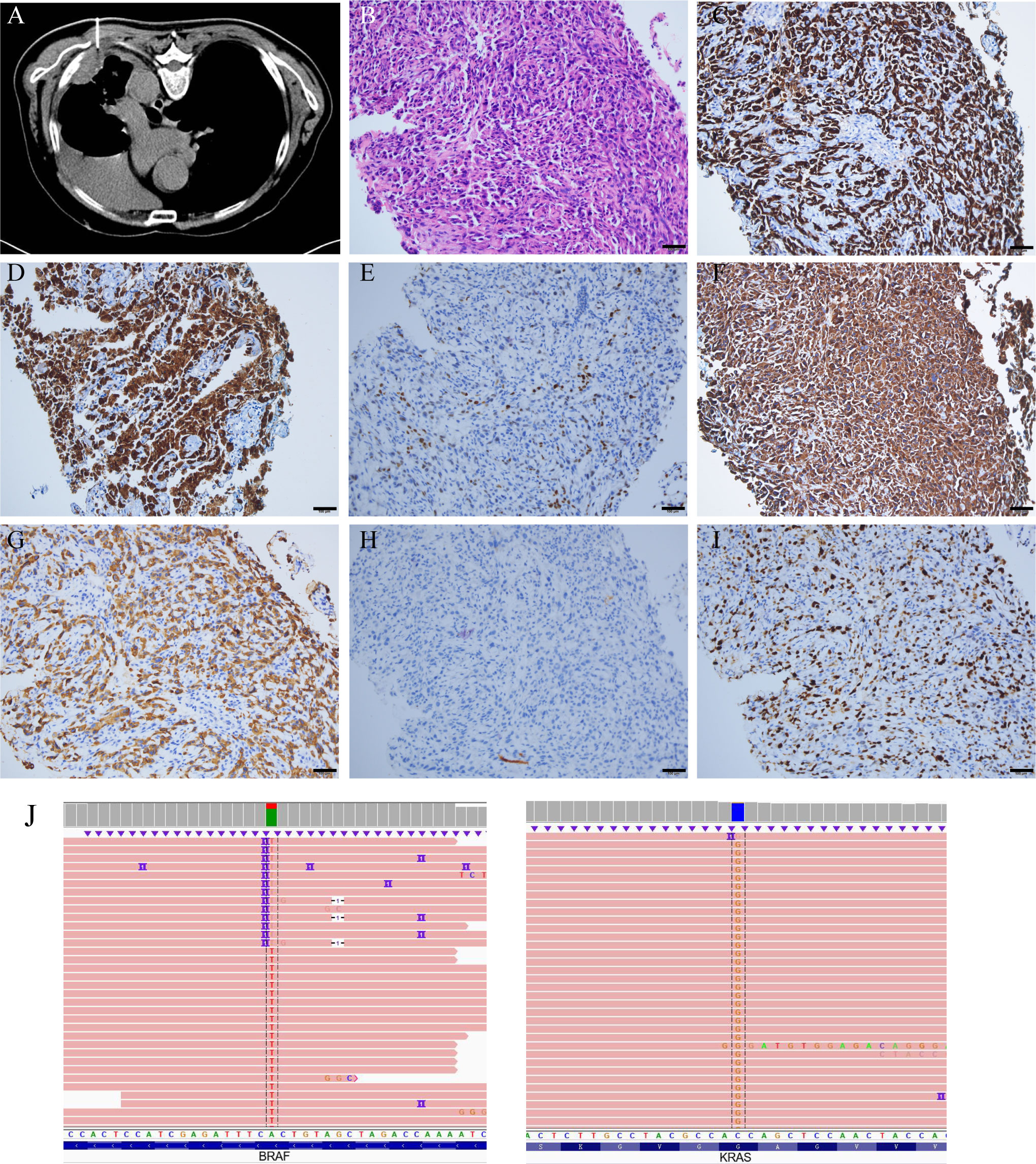
Figure 1 CT-guided needle biopsy, and its pathological staining and NGS analysis. (A) CT-guided needle biopsy. (B) HE staining, spindle cells, scale bars: 100μm. (C) CK7 (+). (D) NapsinA (focal +). (E) TTF-1 (partial +). (F) VIMENTIN (spindle cell +). (G) CK (+). (H) P40 (-). (I) Ki-67 (50%). (J) NGS revealed the presence of the BRAFV600E mutation (exon 15, 22.7% abundance) and KRASG12A mutation (exon 2, 5.71% abundance). Technical details are provided in the Supplementary Material S5.
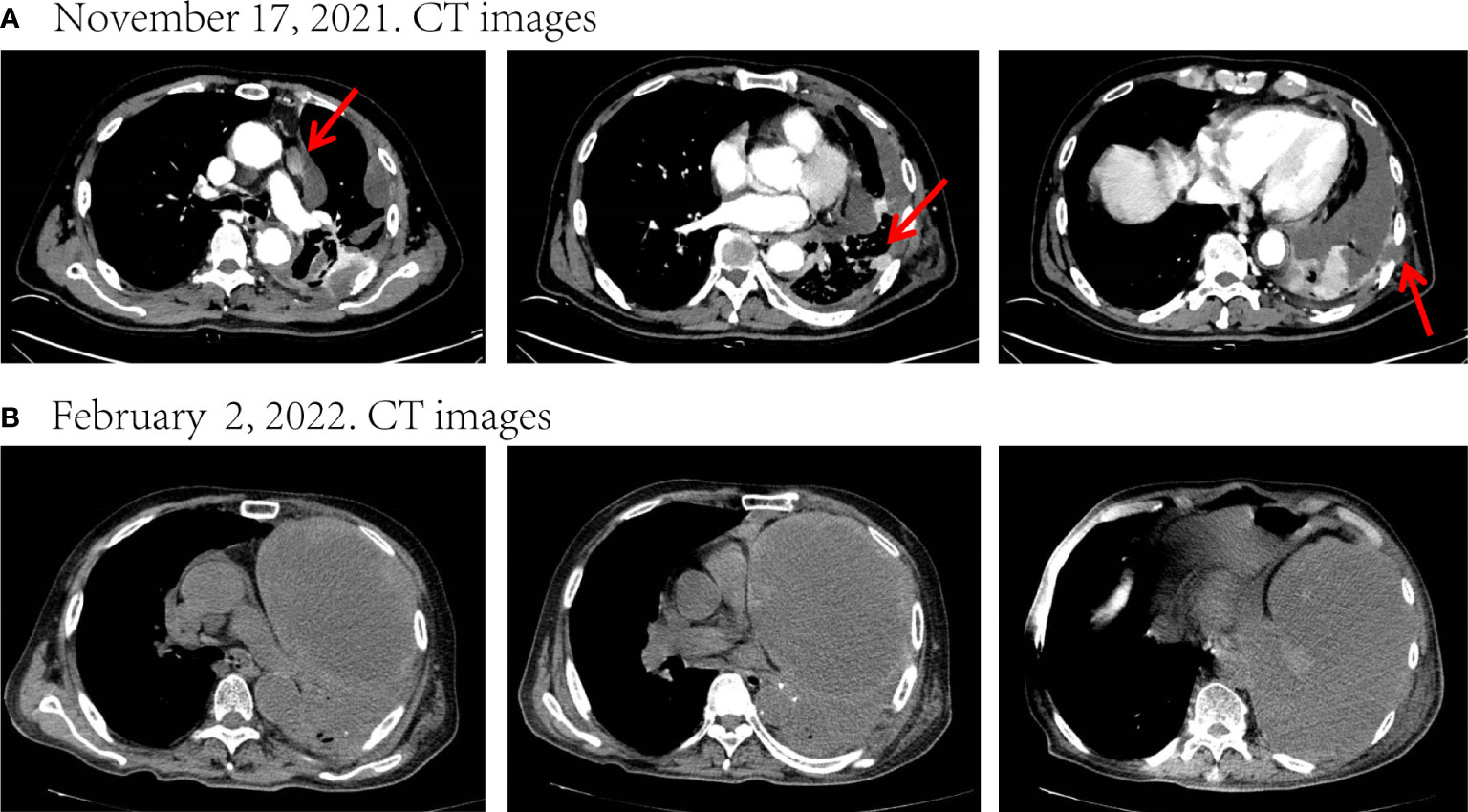
Figure 2 Disease status after chemoradiotherapy and two-cycle immunotherapy. (A) CT images before treatment. (B) After chemoradiotherapy and two-cycle immunotherapy, there was a marked increase in the size of the mass and a massive encapsulated malignant pleural effusion leading to left atelectasis. The tumor response evaluation is a progressive disease (PD). Red arrows indicate tumor or lymph node lesions.
To relieve the chest pain, he began to receive radiotherapy (Dt=45Gy/15F) on November 29 for the destruction of the ribs and the surrounding carcinoma tissue (Figure S4). After radiotherapy, his chest pain eased significantly. He refused targeted therapy for financial reasons. Later, he received one-cycle chemotherapy (albumin-bound paclitaxel 400mg d1 + carboplatin 400mg d1) in combination with tislelizumab (200mg d1) on December 18 and was pleural infused with cisplatin (40mg) on December 20. Unfortunately, he developed severe post-chemotherapy myelosuppression, malnutrition, and pulmonary infection, as well as a large pleural effusion, and the family refused to perform pleurocentesis. After symptomatic treatment, the patient continued to be unable to tolerate chemotherapy, so he was only treated with tislelizumab (200mg d1) on January 22, 2022. However, his condition continued to deteriorate. On February 2, he was re-admitted to the hospital due to fever and dyspnea. CT showed that the left lung was atelectatic due to a tumor and massive pleural effusion (Figure 2B), and the Eastern Cooperative Oncology Group (ECOG) score was up to 3. He could no longer tolerate chemotherapy and immunotherapy, and after adequate communication, he opted for targeted therapy with oral dabrafenib and trametinib.
Although he suffered adverse reactions such as pruritus and loss of appetite during the course of the targeted medication, he improved after treatment. Meanwhile, his reexamination results showed that malignant pleural effusion was significantly reduced and the mass significantly retreated (Figures 3A–C). The tumor response evaluation reached partial response (PR). Nonetheless, he stopped taking dabrafenib and trametinib in July 2022 because of financial pressures and began palliative antitumor therapy with tislelizumab (200mg d1) on August 12. His last imaging examination revealed strict left lung atelectasis, increased malignant pleural effusion, and mass enlargement (Figure 3D). Through follow-up, we learned that he passed away at home on November 7, 2022.
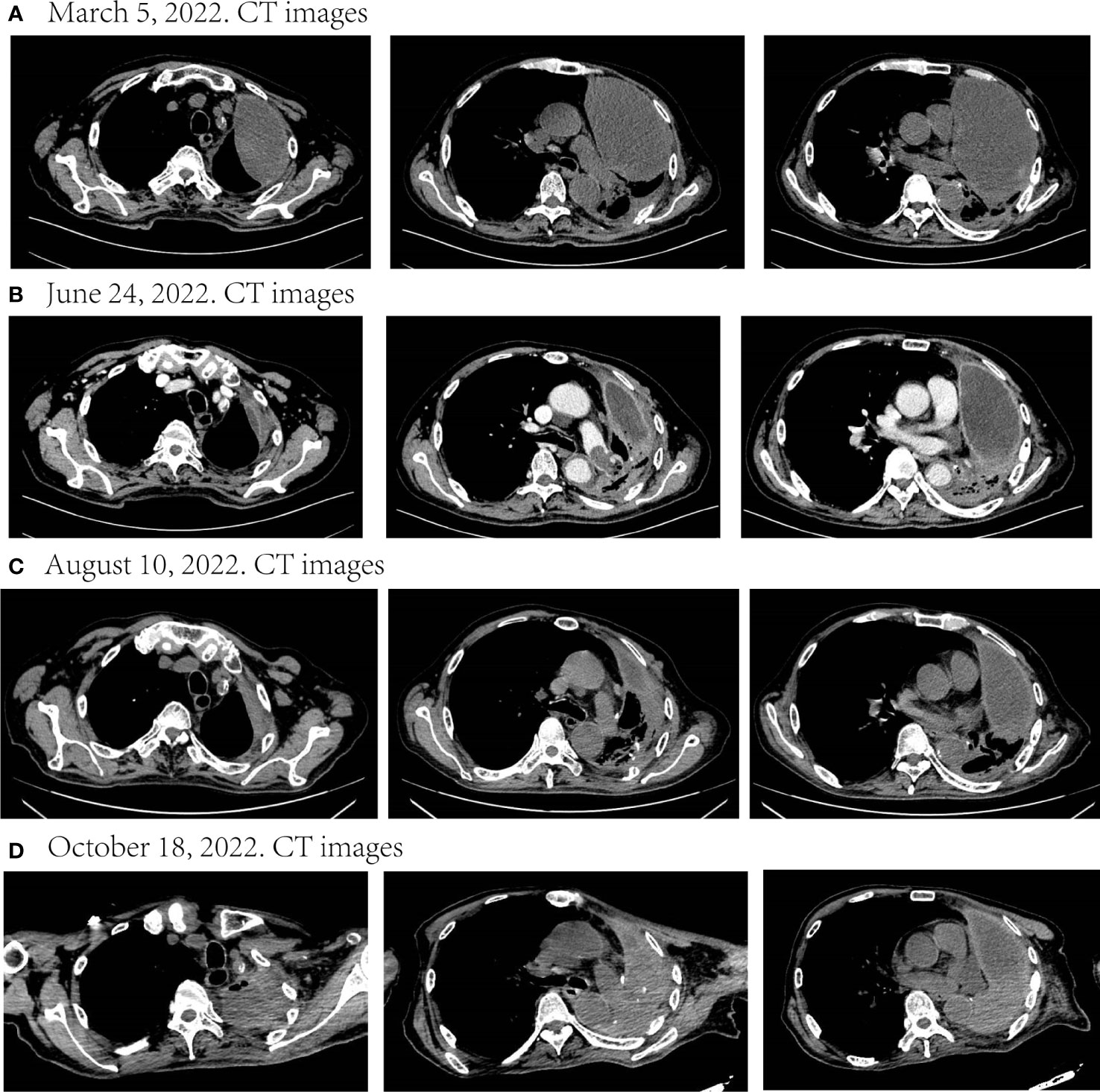
Figure 3 Disease status after targeted therapy and palliative immunotherapy. (A–C) During nearly six months of targeted therapy, the malignant pleural effusion continued to decrease and the mass continued to shrink. The tumor response evaluation of the three times respectively was SD, PR, and SD. (D) After discontinuation of targeted therapy and palliation with immunotherapy, the disease progressed. SD, stable disease; PR, partial response.
PSC is a rare and aggressive subtype of NSCLC that progresses rapidly, has a poor prognosis, and is resistant to conventional chemotherapy. The incidence of PSC is less than 1% of all primary lung tumors (16). Based on histological features, PSC can be classified as pleomorphic carcinoma, pulmonary blastoma, and carcinosarcoma. Pleomorphic carcinoma is the most common and can be divided into giant cell carcinoma and spindle cell carcinoma (15). The occurrence of PSC, especially pleomorphic carcinoma, may be related to epithelial-mesenchymal transition (EMT). Some case reports have confirmed the transformation of lung squamous cell carcinoma or lung adenocarcinoma into PSC during treatment, confirming the EMT process (17, 18). PSC has a poor prognosis due to its difficulty in early diagnosis, insensitivity to conventional treatment, and poor biological behavior (4). Chen et al. analyzed the cases in the SEER database and found that the OS of PSC was still much lower than other types of NSCLC after balancing different characteristics (19). In previous SEER-based studies, the median OS (mOS) was 6.0 months for PSC patients with all stages and 3.0 months for patients with advanced disease (20, 21). Currently, most people with PSC are still treated according to the standard treatment for NSCLC, and there is an urgent need to explore effective treatments for PSC.
For patients with PSC at an early stage, surgery is the best option (22, 23). However, surgeons are seldom asked to make management decisions regarding PSC (24), and there is still debate about whether adjuvant chemotherapy can benefit patients with PSC. A study based on the SEER database concluded that adjuvant chemotherapy did not significantly benefit OS in patients with PSC (25). While Abdallah et al. through the analysis of the National Cancer Database found that adjuvant chemotherapy appears to be associated with improved survival in stage II and stage III, but not stage I patients (26). In the study of Maneenil et al., 33 out of 45 patients who underwent complete resection had a definite recurrence (27). To reduce the likelihood of recurrence, the patient in our report received adjuvant chemotherapy despite being stage I at the time of surgery. In terms of the choice of chemotherapy regimen, previous studies have shown that PSC is insensitive to most chemotherapy drugs (28), but platinum-based combination chemotherapy can improve OS, with mOS of 3.95 months in patients receiving platinum-based chemotherapy, while only 0.95 months in patients who do not receive (29). Therefore, platinum-based combination chemotherapy remains a better option for PSC compared to alternative chemotherapy regimens.
Currently, immunotherapy has shown increasing potential for PSC treatment. Immune-checkpoint inhibitors (ICIs) achieve the purpose of anti-tumor by enhancing the activity of CD4+ and CD8+ T lymphocytes, which can improve the clinical prognosis of various tumors. The KEYNOTE-189 trial showed that for patients with metastatic nonsquamous NSCLC without sensitizing EGFR or ALK mutations, regardless of the patient’s programmed cell death-ligand 1 (PD-L1) expression, even <1%, the use of chemotherapy in combination with immunotherapy as first-line treatment can significantly improve the survival time of patients (30, 31). The RATIONALE-307 trial showed that tislelizumab combined with paclitaxel and carboplatin significantly improved PFS and ORR in patients with advanced squamous NSCLC, regardless of PD-L1 expression (32). ICIs have been used as adjuvant therapy for patients with advanced lung cancer (4). About 40.6% of Chinese patients with PSC were shown to have high TMB (33), and most PSC patients had significantly higher PD-L1 expression than NSCLC patients (34), suggesting that PSC patients may benefit from ICIs treatment. According to one case report, a patient of PSC with low expression of PD-L1 showed a remarkable response to pembrolizumab plus cytotoxic chemotherapy as the first-line treatment (35). In a cohort study of 42 PSC patients treated with ICIs, the ORR of the overall population was 73.8%, regardless of PD-L1 expression status, suggesting that chemotherapy combined with immunotherapy has a significant efficacy as the first-line treatment for PSC patients (6). Thus, while CPS=0 for PD-L1 in this patient, immunotherapy could theoretically benefit him.
Whereas neither chemotherapy nor immunotherapy resulted in remission in this patient we report on, only targeted therapy has benefited him. Previous studies have shown that most PSC cases have potential targetable genomic alterations (5). One of the most popular is the mesenchymal to epithelial transition (MET) proto-oncogene alterations, and METex14 skipping mutation is the most common and well-studied mutation type, occurring in about 22%~31.8% of PSC patients (36, 37). A phase II study showed savolitinib in Chinese patients with PSC and other NSCLCs harboring METex14 skipping mutation with an ORR of 49.2% (38). There are still numerous potential therapeutic targets in PSC that are worth exploring. Yang et al. performed whole exome sequencing on 56 PSC samples, of which TP53 mutations were the most common, accounting for 79% of the entire cohort. 57% of the patients harbored mutations in genes of the receptor tyrosine kinase/RAS pathway: 14% carried KRAS mutations, and 7% carried BRAF mutations. Two of the four patients with the BRAF mutation had the V600E mutation (39). In addition, a recent study demonstrated that BRAF mutation did not modulate the NSCLC response to ICIs (40). This evidence suggests that BRAF mutations are not a disadvantage to the efficacy of ICIs. V600E is the most common type of BRAF mutations, accounting for about 1-2% of lung adenocarcinomas (11). Dabrafenib and trametinib inhibits BRAF and MEK, and in its phase II trial, demonstrated a response of 67% and duration of response of 9.8 months in patients with previously treated BRAFV600E-mutant metastatic NSCLC (13, 14), a response of 64% and a median response duration of 10.4 months in patients with previously untreated NSCLC (12). The National Medical Products Administration (NMPA) approved dabrafenib in combination with trametinib for the treatment of BRAFV600E mutated NSCLC in China on March 24, 2022. However, no PSC patients were included in these cohorts, and the efficacy of dabrafenib in combination with trametinib in PSC patients with BRAFV600E mutations is unclear. As a result, the patient was not treated with targeted drugs as soon as the genetic test results were clear. Moreover, in China, treatment with dabrafenib combined with trametinib costs about 22,000 yuan (3,157 $) per month at that time, much higher than the average monthly income of Chinese people (3,074 yuan, 448 $), which causes a serious financial burden for an average family. Therefore, the patient eventually gave up on continuing to take targeted drugs. The rapid progression of the disease in this patient despite subsequent maintenance with tislelizumab is evidence from another perspective that dabrafenib combined with trametinib effectively controls tumor progression in this patient.
To our knowledge, this is the first case report describing a clinical response to dabrafenib combined with trametinib in a patient with PSC harboring BRAFV600E mutation, which demonstrates BRAF is one of the main drive oncogenes of PSC and is sensitive matched targeted therapy (Figure 4). Besides, this patient’s ECOG score had reached 3 when using targeted therapy. Targeted therapy controlled patient tumor progression without severe side effects, suggesting that targeted therapy is also appropriate for patients with high ECOG scores. Large-scale NGS panels may be able to provide more treatment options for patients with PSC. Moreover, this patient also had KRASG12A mutation, PIK3CAE707K mutation, and TP53H179R mutation. In resected NSCLC patients, KRAS transition mutations are significantly poor prognostic factors (41). According to the study of Lococo et al., PSC patients with mutations have a worse prognosis, especially the KRAS mutations alone or in combination with TP53, which is associated with decreased survival probability and occurrence of local metastases at recurrence (42). PIK3CA mutations are also associated with a poorer postoperative prognosis for PSC (43). However, in another clinical trial, NSCLC with KRAS co-mutated with TP53 had a better response to immunotherapy (44). We do not know how these mutations work together over the course of the disease, and whether they contribute to this patient’s insensitivity to chemotherapy and immunotherapy. The development of targeted drugs with multi-gene targets may be able to benefit such patients.
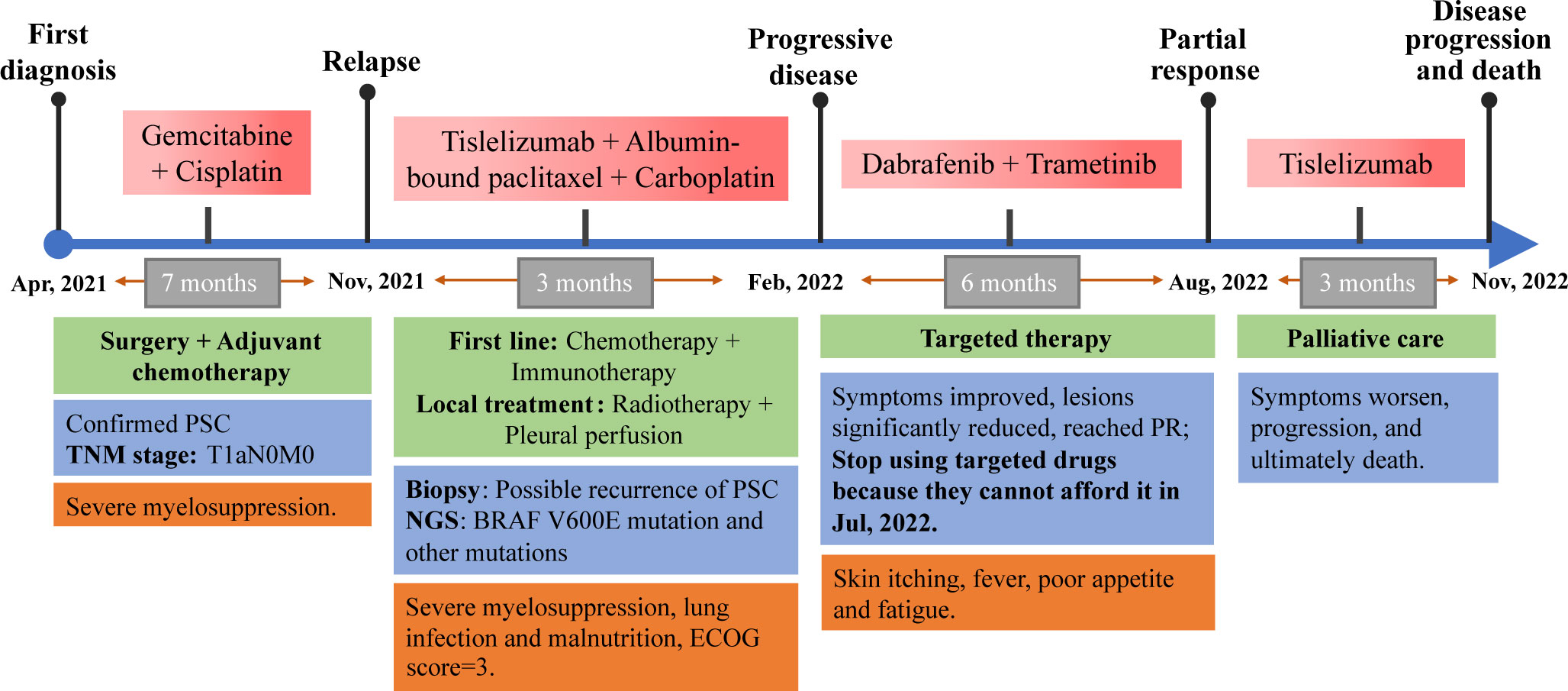
Figure 4 Complete diagnosis and treatment of this PSC patient. This patient with PSC had a rapid relapse following surgery and adjuvant chemotherapy in the early stages of the disease. NGS suggested that he had a BRAFV600E mutation, but he refused targeted therapy for financial reasons, so chemotherapy combined with immunotherapy was used. However, the disease progressed rapidly, and the patient’s general condition deteriorated sharply. Desperate, the patient tried targeted therapy, achieved remission and six months of PFS. But he stopped taking targeted drugs for financial reasons, the disease progressed and eventually died. Due to the need for text editing, the time axis in the image is not proportional to the length of time.
There also were limitations in this case. The patient underwent surgery and post-operative adjuvant chemotherapy at another hospital, the details of which are not available to us to rule out potential causes of recurrence. The patient had too few biopsies to accurately measure PD-L1 expression. In addition, larger prospective clinical trials are needed to confirm the efficacy of dabrafenib in combination with trametinib in BRAFV600E mutant PSC.
Dabrafenib in combination with trametinib provides partial remission in patients with advanced PSC with the BRAFV600E mutation, which is more effective than chemotherapy in combination with immunotherapy, and it is also applicable when the ECOG score is high. Large-scale NGS panels may be able to provide more treatment options for patients with PSC.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
RF: visualization and writing - original draft. JG: visualization and writing - review & editing. ZL: supervision and writing - review & editing. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1220745/full#supplementary-material
1. Zhang L, Lin W, Yang Z, Li R, Gao Y, He J. Multimodality treatment of pulmonary sarcomatoid carcinoma: a review of current state of art. J Oncol (2022) 2022:8541157. doi: 10.1155/2022/8541157
2. Roesel C, Terjung S, Weinreich G, Hager T, Chalvatzoulis E, Metzenmacher M, et al. Sarcomatoid carcinoma of the lung: a rare histological subtype of non-small cell lung cancer with a poor prognosis even at earlier tumour stages. Interact Cardiovasc Thorac Surg (2017) 24:407–13. doi: 10.1093/icvts/ivw392
3. Steuer CE, Behera M, Liu Y, Fu C, Gillespie TW, Saba NF, et al. Pulmonary sarcomatoid carcinoma: an analysis of the national cancer data base. Clin Lung Cancer (2017) 18:286–92. doi: 10.1016/j.cllc.2016.11.016
4. Li X, Wu D, Liu H, Chen J. Pulmonary sarcomatoid carcinoma: progress, treatment and expectations. Ther Adv Med Oncol (2020) 12:1758835920950207. doi: 10.1177/1758835920950207
5. Schrock AB, Li SD, Frampton GM, Suh J, Braun E, Mehra R, et al. Pulmonary sarcomatoid carcinomas commonly harbor either potentially targetable genomic alterations or high tumor mutational burden as observed by comprehensive genomic profiling. J Thorac Oncol (2017) 12:932–42. doi: 10.1016/j.jtho.2017.03.005
6. Zhou F, Guo H, Zhou X, Xie H, Tian T, Zhao W, et al. Immune checkpoint inhibitors plus chemotherapy in patients with locally advanced or metastatic pulmonary sarcomatoid carcinoma: a multicentric real-world study. Ther Adv Med Oncol (2022) 14:17588359221136759. doi: 10.1177/17588359221136759
7. Wu Y, Yan Z, Pan J, Chang X, Huang B, Luo D, et al. Partial response to pralsetinib in an advanced pulmonary sarcomatoid carcinoma patient harboring a KIF5B-RET rearrangement: a case report. World J Surg Oncol (2022) 20:386. doi: 10.1186/s12957-022-02848-z
8. Piao MN, Ma XT, Tankere P, Liam CK, Li JL, Wang JP. Anlotinib combined with chemotherapy and immunotherapy for advanced pulmonary sarcomatoid cancer: a case report and literature review. Ann Transl Med (2022) 10:1030. doi: 10.21037/atm-22-4312
9. Li YF, Zhao XF, Tian Y, Xiao XY, Yan CY, Shen H. Case report: pulmonary sarcomatoid carcinoma complicating TP53 mutation treated successfully with tislelizumab combined with anlotinib-a case report. Front Genet (2022) 13:949989. doi: 10.3389/fgene.2022.949989
10. Paik PK, Arcila ME, Fara M, Sima CS, Miller VA, Kris MG, et al. Clinical characteristics of patients with lung adenocarcinomas harboring BRAF mutations. J Clin Oncol (2011) 29:2046–51. doi: 10.1200/JCO.2010.33.1280
11. Barlesi F, Mazieres J, Merlio JP, Debieuvre D, Mosser J, Lena H, et al. Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French cooperative thoracic intergroup (IFCT). Lancet (2016) 387:1415–26. doi: 10.1016/S0140-6736(16)00004-0
12. Planchard D, Smit EF, Groen HJM, Mazieres J, Besse B, Helland ÅChecktae, et al. Dabrafenib plus trametinib in patients with previously untreated BRAF(V600E)-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol (2017) 18:1307–16. doi: 10.1016/S1470-2045(17)30679-4
13. Planchard D, Besse B, Groen HJM, Souquet PJ, Quoix E, Baik CS, et al. Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol (2016) 17:984–93. doi: 10.1016/S1470-2045(16)30146-2
14. Planchard D, Kim TM, Mazieres J, Quoix E, Riely G, Barlesi F, et al. Dabrafenib in patients with BRAF(V600E)-positive advanced non-small-cell lung cancer: a single-arm, multicentre, open-label, phase 2 trial. Lancet Oncol (2016) 17:642–50. doi: 10.1016/S1470-2045(16)00077-2
15. Nicholson AG, Tsao MS, Beasley MB, Borczuk AC, Brambilla E, Cooper WA, et al. The 2021 WHO classification of lung tumors: impact of advances since 2015. J Thorac Oncol (2022) 17:362–87. doi: 10.1016/j.jtho.2021.11.003
16. Weissferdt A. Pulmonary sarcomatoid carcinomas: a review. Adv Anat Pathol (2018) 25:304–13. doi: 10.1097/PAP.0000000000000202
17. Xie X, Chen X, Luo N, Qi Y, Li M, Feng X. Transformation of invasive lung adenocarcinoma with ALK rearrangement into pulmonary sarcomatoid carcinoma. J Cancer Res Clin Oncol (2022) 148:2165–8. doi: 10.1007/s00432-022-04022-0
18. Arshi J, Sauer M, Yin F. Rapid sarcomatoid transformation of lung squamous cell carcinoma after neoadjuvant therapy: a case report. Anticancer Res (2020) 40:1625–9. doi: 10.21873/anticanres.14111
19. Chen M, Yang Q, Xu Z, Luo B, Li F, Yu Y, et al. Survival analysis and prediction model for pulmonary sarcomatoid carcinoma based on SEER database. Front Oncol (2021) 11:630885. doi: 10.3389/fonc.2021.630885
20. Yendamuri S, Caty L, Pine M, Adem S, Bogner P, Miller A, et al. Outcomes of sarcomatoid carcinoma of the lung: a surveillance, epidemiology, and end results database analysis. Surgery (2012) 152:397–402. doi: 10.1016/j.surg.2012.05.007
21. Rahouma M, Kamel M, Narula N, Nasar A, Harrison S, Lee B, et al. Pulmonary sarcomatoid carcinoma: an analysis of a rare cancer from the surveillance, epidemiology, and end results database. Eur J Cardiothorac Surg (2018) 53:828–34. doi: 10.1093/ejcts/ezx417
22. Han S, Fang J, Lu S, Wang L, Li J, Cheng M, et al. Response and acquired resistance to savolitinib in a patient with pulmonary sarcomatoid carcinoma harboring MET exon 14 skipping mutation: a case report. Onco Targets Ther (2019) 12:7323–8. doi: 10.2147/OTT.S210365
23. Hou J, Xing L, Yuan Y. A clinical analysis of 114 cases of sarcomatoid carcinoma of the lung. Clin Exp Med (2018) 18:555–62. doi: 10.1007/s10238-018-0517-2
24. Batchelor TJP. Commentary: resected pulmonary sarcomatoid carcinoma-a defined treatment paradigm, or just the end of the beginning of the search? J Thorac Cardiovasc Surg (2022) 163:1683–4. doi: 10.1016/j.jtcvs.2021.02.060
25. Liang L, Liu Z, Wang C, Xie S. Adjuvant chemotherapy is not a decisive factor in improving the overall survival of pulmonary sarcoma: a population-based study. Front Oncol (2022) 12:940839. doi: 10.3389/fonc.2022.940839
26. Abdallah HM, Martinez-Meehan D, Lutfi W, Dhupar R, Grenda T, Schuchert MJ, et al. Adjuvant chemotherapy for pulmonary sarcomatoid carcinoma: a retrospective analysis of the national cancer database. J Thorac Cardiovasc Surg (2022) 163:1669–1681.e1663. doi: 10.1016/j.jtcvs.2021.01.081
27. Maneenil K, Xue Z, Liu M, Boland J, Wu F, Stoddard SM, et al. Sarcomatoid carcinoma of the lung: the Mayo clinic experience in 127 patients. Clin Lung Cancer (2018) 19:e323–33. doi: 10.1016/j.cllc.2017.12.008
28. Nian J, Zhu Y, Fu Q, Yang G, Wang X. Significant response of pulmonary sarcomatoid carcinoma with obstructive atelectasis to treatment with the PD-1 inhibitor camrelizumab combined with transbronchial cryoablation: a case report and literature review. Front Oncol (2022) 12:1013047. doi: 10.3389/fonc.2022.1013047
29. Vieira T, Girard N, Ung M, Monnet I, Cazes A, Bonnette P, et al. Efficacy of first-line chemotherapy in patients with advanced lung sarcomatoid carcinoma. J Thorac Oncol (2013) 8:1574–7. doi: 10.1097/01.JTO.0000437008.00554.90
30. Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-Small-Cell lung cancer. N Engl J Med (2018) 378:2078–92. doi: 10.1056/NEJMoa1801005
31. Garassino MC, Gadgeel S, Speranza G, Felip E, Esteban E, Dómine M, et al. Pembrolizumab plus pemetrexed and platinum in nonsquamous non-Small-Cell lung cancer: 5-year outcomes from the phase 3 KEYNOTE-189 study. J Clin Oncol (2023) 41(11):1992–8. doi: 10.1200/JCO.22.01989
32. Wang J, Lu S, Yu X, Hu Y, Sun Y, Wang Z, et al. Tislelizumab plus chemotherapy vs chemotherapy alone as first-line treatment for advanced squamous non-Small-Cell lung cancer: a phase 3 randomized clinical trial. JAMA Oncol (2021) 7:709–17. doi: 10.1001/jamaoncol.2021.0366
33. Liang X, Li Q, Xu B, Hu S, Wang Q, Li Y, et al. Mutation landscape and tumor mutation burden analysis of Chinese patients with pulmonary sarcomatoid carcinomas. Int J Clin Oncol (2019) 24:1061–8. doi: 10.1007/s10147-019-01454-6
34. Roesel C, Kambartel K, Kopeika U, Berzins A, Voshaar T, Krbek T. Lazarus-Type tumour response to therapy with nivolumab for sarcomatoid carcinomas of the lung. Curr Oncol (2019) 26:e270–3. doi: 10.3747/co.26.4377
35. Taniguchi H, Takemoto S, Ozasa M, Honda N, Suyama T, Umeyama Y, et al. Remarkable response to pembrolizumab with platinum-doublet in PD-L1-low pulmonary sarcomatoid carcinoma: a case report. Thorac Cancer (2021) 12:1126–30. doi: 10.1111/1759-7714.13890
36. Liu XW, Chen XR, Rong YM, Lyu N, Xu CW, Wang F, et al. MET exon 14 skipping mutation, amplification and overexpression in pulmonary sarcomatoid carcinoma: a multi-center study. Transl Oncol (2020) 13:100868. doi: 10.1016/j.tranon.2020.100868
37. Gong C, Xiong H, Qin K, Wang J, Cheng Y, Zhao J, et al. MET alterations in advanced pulmonary sarcomatoid carcinoma. Front Oncol (2022) 12:1017026. doi: 10.3389/fonc.2022.1017026
38. Lu S, Fang J, Li X, Cao L, Zhou J, Guo Q, et al. Once-daily savolitinib in Chinese patients with pulmonary sarcomatoid carcinomas and other non-small-cell lung cancers harbouring MET exon 14 skipping alterations: a multicentre, single-arm, open-label, phase 2 study. Lancet Respir Med (2021) 9:1154–64. doi: 10.1016/S2213-2600(21)00084-9
39. Yang Z, Xu J, Li L, Li R, Wang Y, Tian Y, et al. Integrated molecular characterization reveals potential therapeutic strategies for pulmonary sarcomatoid carcinoma. Nat Commun (2020) 11:4878. doi: 10.1038/s41467-020-18702-3
40. Li H, Zhang Y, Xu Y, Huang Z, Cheng G, Xie M, et al. Tumor immune microenvironment and immunotherapy efficacy in BRAF mutation non-small-cell lung cancer. Cell Death Dis (2022) 13:1064. doi: 10.1038/s41419-022-05510-4
41. Sato K, Akamatsu H, Koh Y, Ogawa K, Isa SI, Ando M, et al. Differential properties of KRAS transversion and transition mutations in non-small cell lung cancer: associations with environmental factors and clinical outcomes. BMC Cancer (2022) 22:1148. doi: 10.1186/s12885-022-10246-7
42. Lococo F, Gandolfi G, Rossi G, Pinto C, Rapicetta C, Cavazza A, et al. Deep sequencing analysis reveals that KRAS mutation is a marker of poor prognosis in patients with pulmonary sarcomatoid carcinoma. J Thorac Oncol (2016) 11:1282–92. doi: 10.1016/j.jtho.2016.04.020
43. Kojima K, Imai S, Samejima H, Fujiwara A, Tokunaga T, Yoon H, et al. PIK3CA mutations associated with a poor postoperative prognosis in patients with pulmonary pleomorphic carcinoma: a retrospective cohort study. BMC Cancer (2022) 22:1066. doi: 10.1186/s12885-022-10176-4
44. West HJ, McCleland M, Cappuzzo F, Reck M, Mok TS, Jotte RM, et al. Clinical efficacy of atezolizumab plus bevacizumab and chemotherapy in KRAS-mutated non-small cell lung cancer with STK11, KEAP1, or TP53 comutations: subgroup results from the phase III IMpower150 trial. J Immunother Cancer (2022) 10(2):e003027. doi: 10.1136/jitc-2021-003027
Keywords: pulmonary sarcomatoid carcinoma, BRAF V600E mutation, dabrafenib, trametinib, case report
Citation: Fang R, Gong J and Liao Z (2023) An advanced pulmonary sarcomatoid carcinoma patient harboring a BRAFV600E mutation responds to dabrafenib and trametinib: a case report and literature review. Front. Oncol. 13:1220745. doi: 10.3389/fonc.2023.1220745
Received: 11 May 2023; Accepted: 28 June 2023;
Published: 21 July 2023.
Edited by:
Gennaro Daniele, Agostino Gemelli University Polyclinic (IRCCS), ItalyReviewed by:
Lin Wu, Central South University, ChinaCopyright © 2023 Fang, Gong and Liao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhengkai Liao, emxpYW9Ad2h1LmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.