- 1Department of Gastrointestinal Surgery, Clinical Medical College and The First Affiliated Hospital of Chengdu Medical College, Chengdu, Sichuan, China
- 2Department of Gastrointestinal Surgery, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu, China
- 3Department of Urology, People’s Hospital Affiliated to Chongqing Three Gorges Medical College, Chongqing, China
- 4Department of Pharmacy, Three Gorges Hospital Affiliated to Chongqing University, Chongqing, China
Background and objective: Lateral pelvic lymph node (LPLN) metastasis is one of the prominent reasons for local recurrence (LR) in patients with rectal cancer (RC). The evaluation criteria of lateral lymph node dissection (LLND) for patients in eastern (mainly in Japan) and western countries have been controversial. The aim of this study was to analyse the risk factors for LPLN metastasis in order to guide surgical methods.
Methods: We searched relevant databases (Embase (Ovid), Medline (Ovid), PubMed, Cochrane Library, and Web of Science) for articles published between 1 January 2000 and 05 October 2022 to evaluate the risk factors for LPLN metastasis in patients with RC in this meta-analysis.
Results: A total of 24 articles with 5843 patients were included in this study. The overall results showed that female sex, age <60 years, pretherapeutic CEA level >5 ng/ml, clinical T4 stage (cT4), clinical M1 stage (cM1), distance of the tumour from the anal verge (AV) <50 mm, tumour centre located below the peritoneal reflection (Rb), short axis (SA) of LPLN ≥8 mm before nCRT, short axis (SA) of LPLN ≥5 mm after nCRT, border irregularity of LPLN, tumour size ≥50 mm, pathological T3-4 stage (pT3-4), pathological N2 stage (pN2), mesorectal lymph node metastasis (MLNM), lymphatic invasion (LI), venous invasion (VI), CRM (+) and poor differentiation were significant risk factors for LPLN metastasis (P <0.05).
Conclusion: This study summarized almost all potential risk factors of LPLN metastasis and expected to provide effective treatment strategies for patients with LRC. According to the risk factors of lateral lymph node metastasis, we can adopt different comprehensive treatment strategies. High-risk patients can perform lateral lymph node dissection to effectively reduce local recurrence; In low-risk patients, we can avoid overtreatment, reduce complications and trauma caused by lateral lymph node dissection, and maximize patient survival and quality of life.
1 Introduction
Colorectal cancer (CRC) is the third most common malignant tumour in the world. In 2020, it was ranked as the fourth leading cause of cancer death, second to lung cancer (1), and its burden is estimated to increase by 60% to more than 2.2 million new cases and 1.1 million cancer deaths by 2030 (2).
Local recurrence (LR) of RC is still a serious clinical problem that is related to low survival and high incidence rates. It diffuses through the superior lymphatic drainage of the inferior mesenteric artery as well as the lateral lymphatic drainage of the internal iliac artery outside the rectum (3, 4). Lateral pelvic lymph node (LPLN) metastasis is considered the main cause of LR in patients with low rectal cancer (LRC) (5–7). Several studies verified that the incidence of LPLN metastasis in patients with LRC was approximately 15% (8), while the incidence of stages T3 and T4 exceeded 20% (9, 10). Klusters M et al. assumed that lymph and tumour cells wound flow into the LPLN system when the tumour is crushed during surgical resection. In addition, the LPLN system was left untouched during standard TME, and partial damage during rapid dissection of the lateral ligament led to lateral positive lymphatic residue. Finally, lymph converged in the presacral area and flowed into serum, which might have led to local tumour recurrence (11). It is urgent to find the relevant risk factors for LPLN metastasis. However, recent studies have shown that lateral recurrence has become the most common recurrence mode, accounting for up to 50%~82.7%. Lateral recurrence after rectal cancer surgery has been heavily discussed and is a barrier to prevention and treatment of colorectal surgery (6).
However, there has been no meta-analysis to clarify the risk factors for LPLN metastasis in patients with LRC to date. We included all significantly relevant articles to compile this meta-analysis to further guide the treatment of rectal cancer patients with suspected LPLN metastasis. It can guide us to identify which patients with rectal cancer need lateral lymph node dissection to reduce the risk of local recurrence.
2 Materials and methods
2.1 Literature search
Studies published up to 05 October 2022 were identified by searching Embase (Ovid), Medline (Ovid), PubMed, Cochrane Library, and Web of Science. No regional restriction was imposed. Articles were confined to human studies published in English. The search algorithms consisted of Medical Subject Headings (MeSH) and free text terms, including the following: “Rectal cancer”, “Lateral pelvic lymph node metastasis”, and “risk factor”. Eligible literature was identified by reading the included relevant articles.
2.2 Article selection
Inclusion criteria: (1) participants: rectal cancer patients with clinically suspected LPLN metastasis; (2) intervention: pathological examination confirmed positive metastasis of LPLN; (3) comparison: pathological examination confirmed negative metastasis of LPLN; (4) outcome measures: report at least one of the endpoints listed in Table 1; (5) study design: randomized controlled trials, prospective or retrospective cohort and case-control studies. Studies were excluded if: (1) they were reviews, case reports, conference articles or unrelated studies (the article did not contain rectal cancer, lymphatic metastasis, or risk factor analysis); (2) the metastatic lymph node was not LPLN; and (3) no outcome measures of interest were reported.
2.3 Outcomes of interest
We tried to screen all comparable data of the included articles as fully as possible. When a certain indicator contains data with more than 2 articles, it is considered as “Outcomes of Interest”. The indicators were as follows: Sex, age, pretherapeutic CEA level (ng/ml), border irregularity of LPLN, mixed signal intensity of LPLN, short axis of LPLN before CRT (mm), short axis of LPLN after CRT (mm), distance of the tumour from the AV (50 mm or 40 mm), tumour location, tumour size (mm), cT, cN, cM, pT, pN, LI, MLNM, VI, PI, CRM and differentiation.
2.4 Data extraction and outcome measures
Two authors (ZDX and TL) independently screened all the included studies and extracted the relevant data. Divergence of views was resolved through discussion between the authors. When consensus could not be reached, the third author (RMN) was consulted, and a discussion ensued until a consensus was reached. The following relevant information was extracted from all the included studies: reference, journal, country, number of patients, LPLN (+) rate, age, operation method and endpoints.
2.5 Study quality assessment
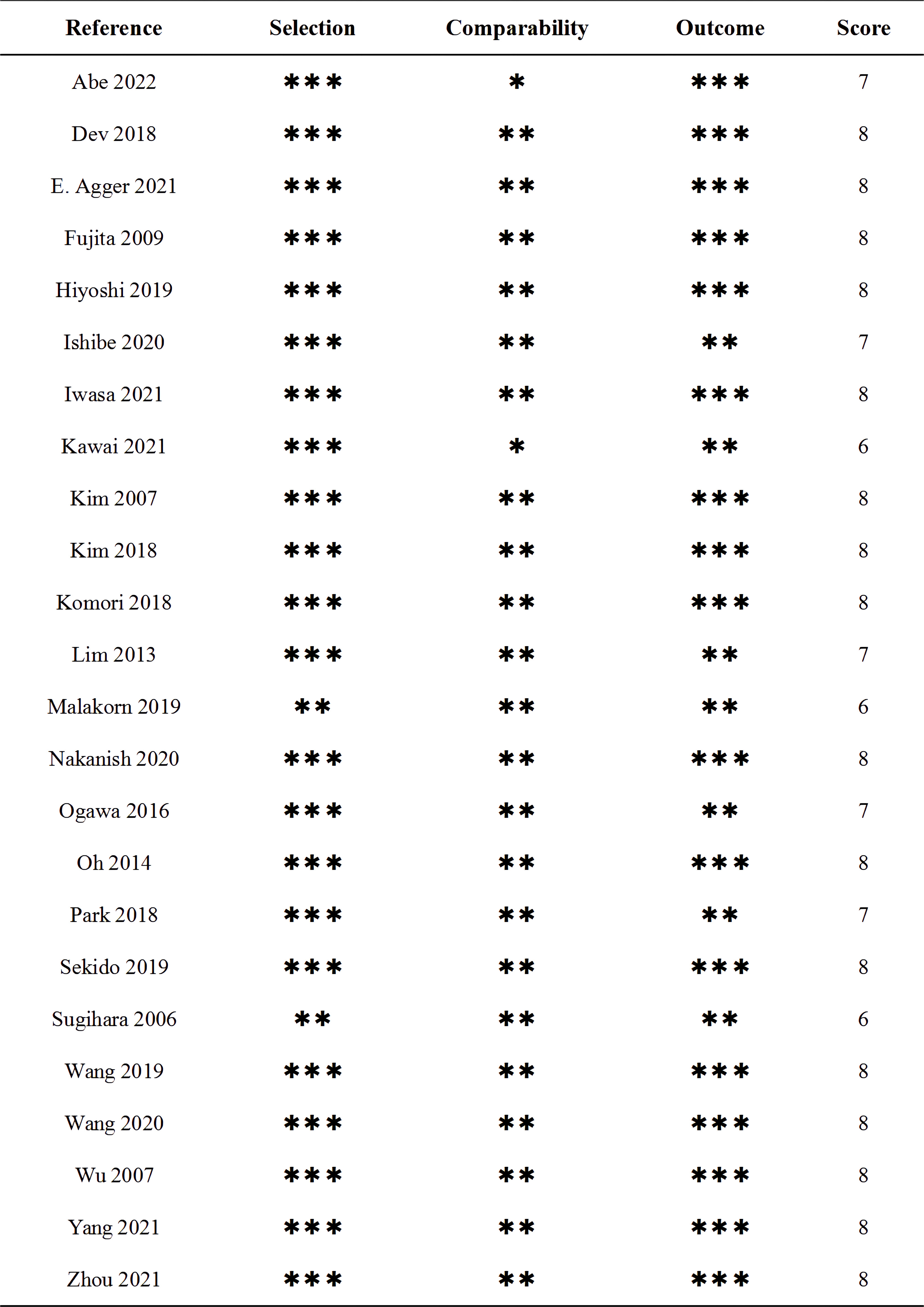
The quality of the enrolled studies was evaluated by two authors independently using the Newcastle Ottawa Scale (NOS), with a maximum of nine points per study (34). Studies with a score <6 were considered low-quality studies and excluded. For this systematic review, we adhered to the Meta-analysis of Observational Studies guidelines and the Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) statement (35).
2.6 Statistical analysis
We used RevMan 5.4 software from the Cochrane Collaboration for all statistical analyses. Odds ratios (ORs) with 95% confidence intervals (CIs) were assessed to analyse dichotomous variables. p of Q test >0.1 and I2 < 50% illustrated a lack of heterogeneity, and in this case, the pooled estimate was calculated by a fixed effects model. Otherwise, when p of Q test <0.1 or I2 >50%, a random effects model was adopted. A leave-one-out sensitivity analysis was performed by excluding one study back and forth to confirm that our results were not driven by any single trial. Publication bias was assessed by visual inspection of the symmetry of a funnel plot. The level of significance was defined as p <0.05 (test for heterogeneity was set at p <0.1).
3 Results
3.1 Study selection and characteristics
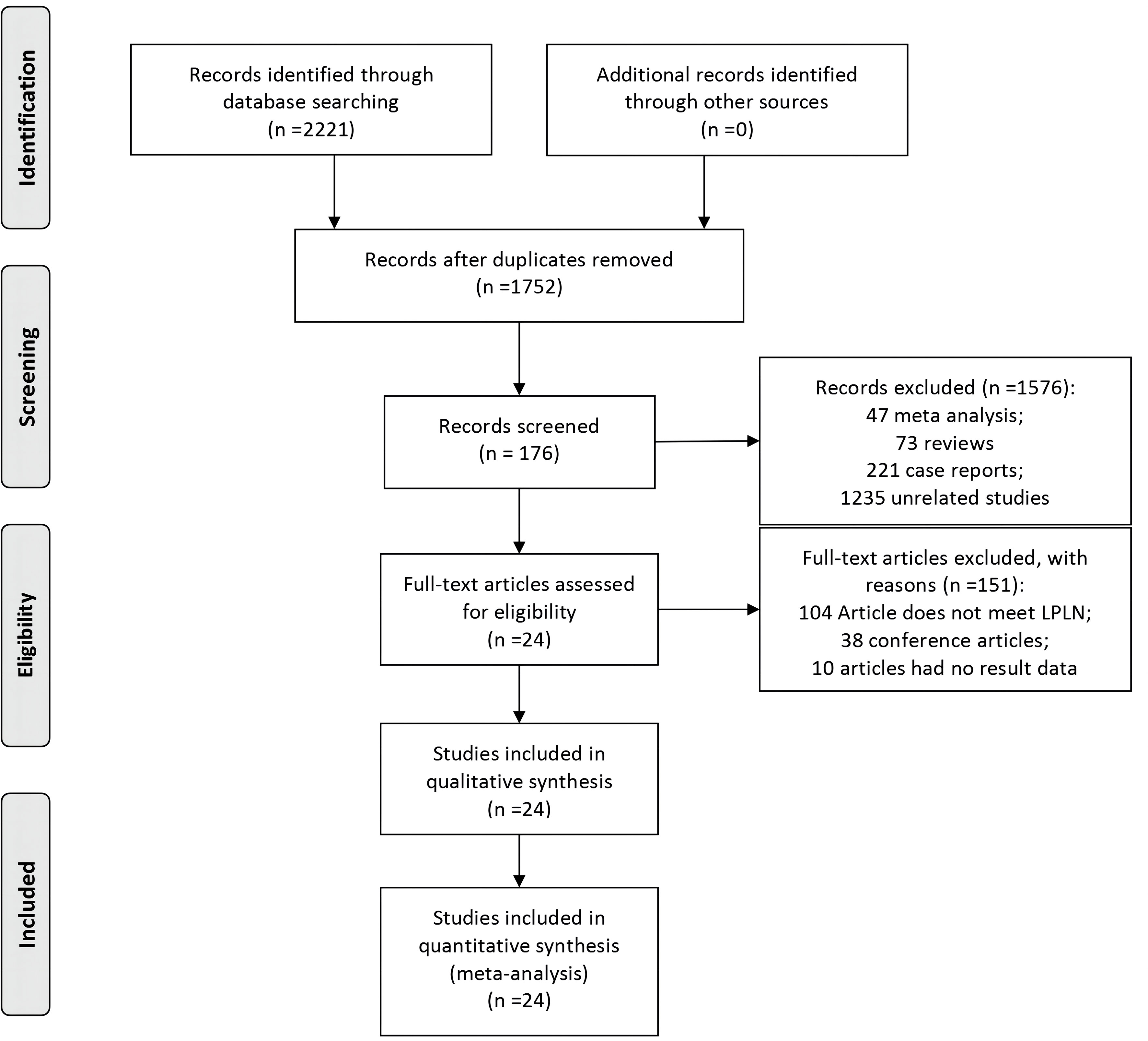
The flow chart for the inclusion of articles is shown in Figure 1. A total of 24 studies were eventually included in the quantitative synthesis by screening databases through search strategies in advance (6, 7, 12–33). The baseline characteristics and lymph details of the studies are displayed in Table 1. A total of 24 retrospective articles with 5843 patients were included in this study, of which the LPLN-positive rate was between 6.5% and 51.6%. Most articles were reported in East Asia (12 in Japan, 5 in Korea, 4 in China and 1 in India), but 2 were reported in Western countries (1 in Sweden and the other in America). The NOS scores of the studies are displayed in Figure 2, and all studies scored 6 points or higher.
3.2 Outcomes of baseline characteristics
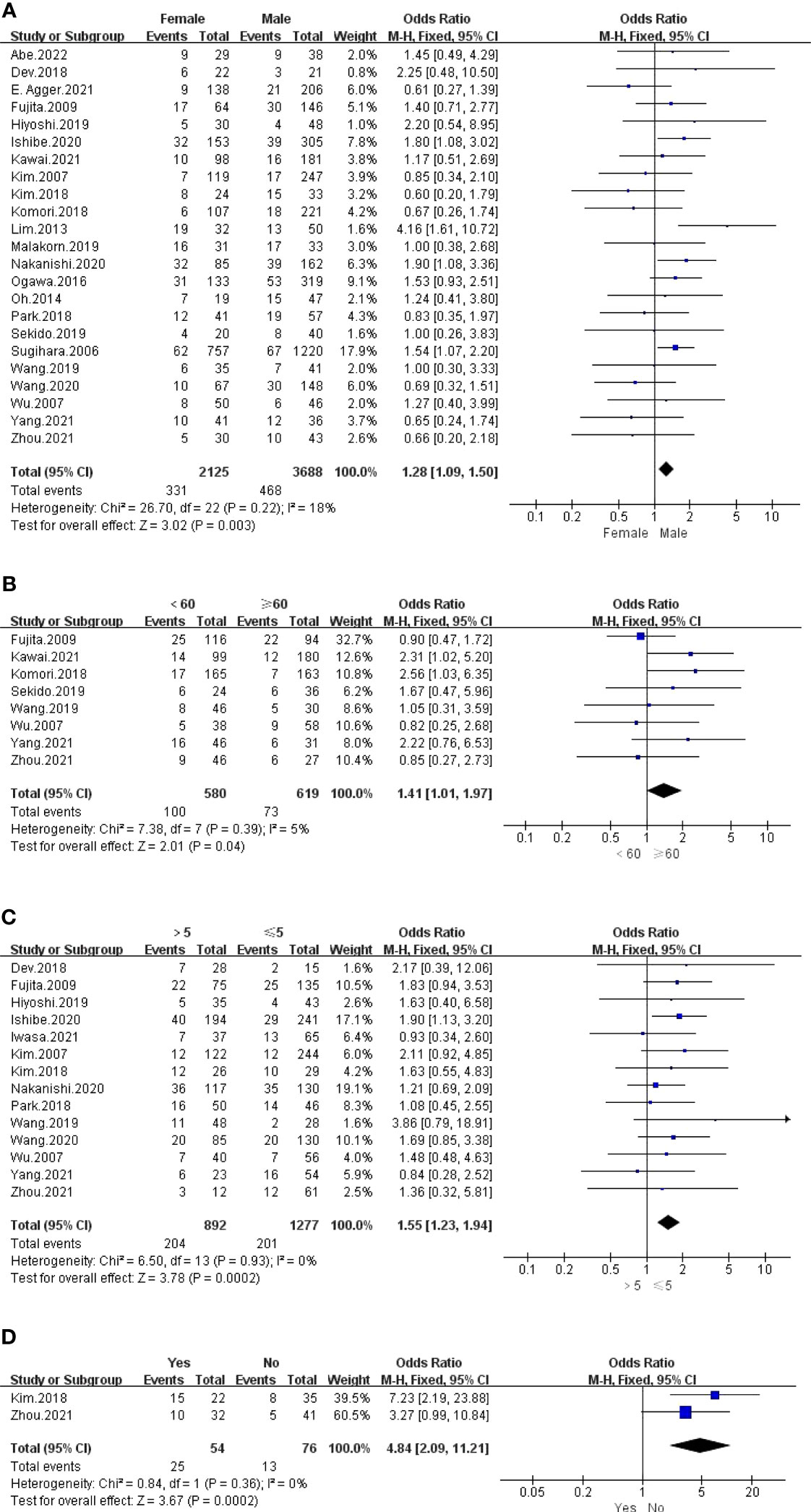
The outcomes are summarized in Figures 3A, B. For all outcomes, low statistical heterogeneity existed between the studies, and the fixed effects model was used. The pooled results showed a significantly higher risk of LPLN metastasis in females (OR: 1.28, 95% CI: 1.09-1.50, I2 = 18%, P =0.003) and age <60 years (OR: 1.41, 95% CI: 1.01-1.97, I2 = 5%, P =0.04).
3.3 Preoperative examination results
3.3.1 Pretherapy CEA level (ng/ml)
The outcome is listed in Figure 3C. No statistical heterogeneity existed between the studies; thus, the fixed effects model was used. Graphics demonstrated that a pretherapeutic CEA level >5 ng/ml was strongly associated with LPLN metastasis (OR: 1.55, 95% CI: 1.23-1.94, I2 = 0%, P =0.0002).
3.3.2 Tumour border and signal characteristics on MRI
The outcomes are listed in Figures 3D and S1. Pooled results revealed a significantly higher risk of LPLN metastasis with border irregularity on MRI (OR: 4.84, 95% CI: 2.09-11.21, I2 = 0%, P =0.0002). Regarding tumour signal characteristics, the random effects model was used due to obvious statistical heterogeneity, photographs seemed to not affect LPLN metastasis (OR: 3.98, 95% CI: 0.77-20.56, I2 = 76%, P =0.10).
3.3.3 SA of LPLN on MRI/CT (mm)
The outcomes are summarized in Figures 4A and S2. The fixed effects model was used because no statistical heterogeneity existed in the SA of LPLN ≥5 mm after nCRT, while the random effects model was used because obvious statistical heterogeneity existed in the SA of LPLN ≥8 mm before nCRT. Overall, the results showed that both SA of LPLN ≥5 mm after nCRT (OR: 17.93, 95% CI: 10.02-32.07, I2 = 0%, P <0.00001) and SA of LPLN ≥8 mm before nCRT (OR: 9.33, 95% CI: 3.51-24.83, I2 = 68%, P <0.00001) proved to be hazard factors for LPLN metastasis.
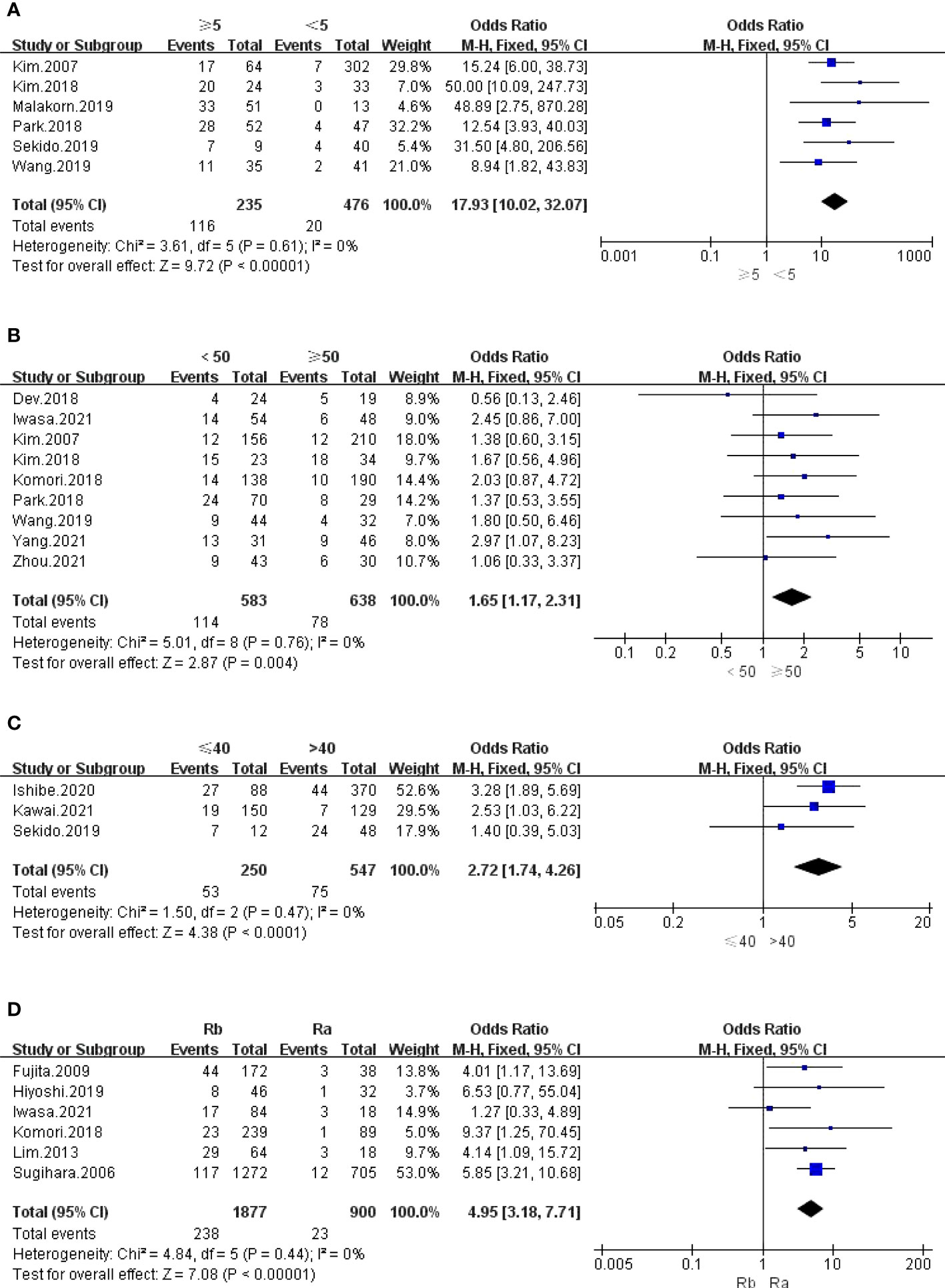
Figure 4 (A) LPLN ≥5mm after nCRT; (B) Distance of the tumor from the AV <50mm; (C) Distance of the tumor from the AV ≤40mm; (D) Tumor center located Rb.
3.3.4 Tumour location and size
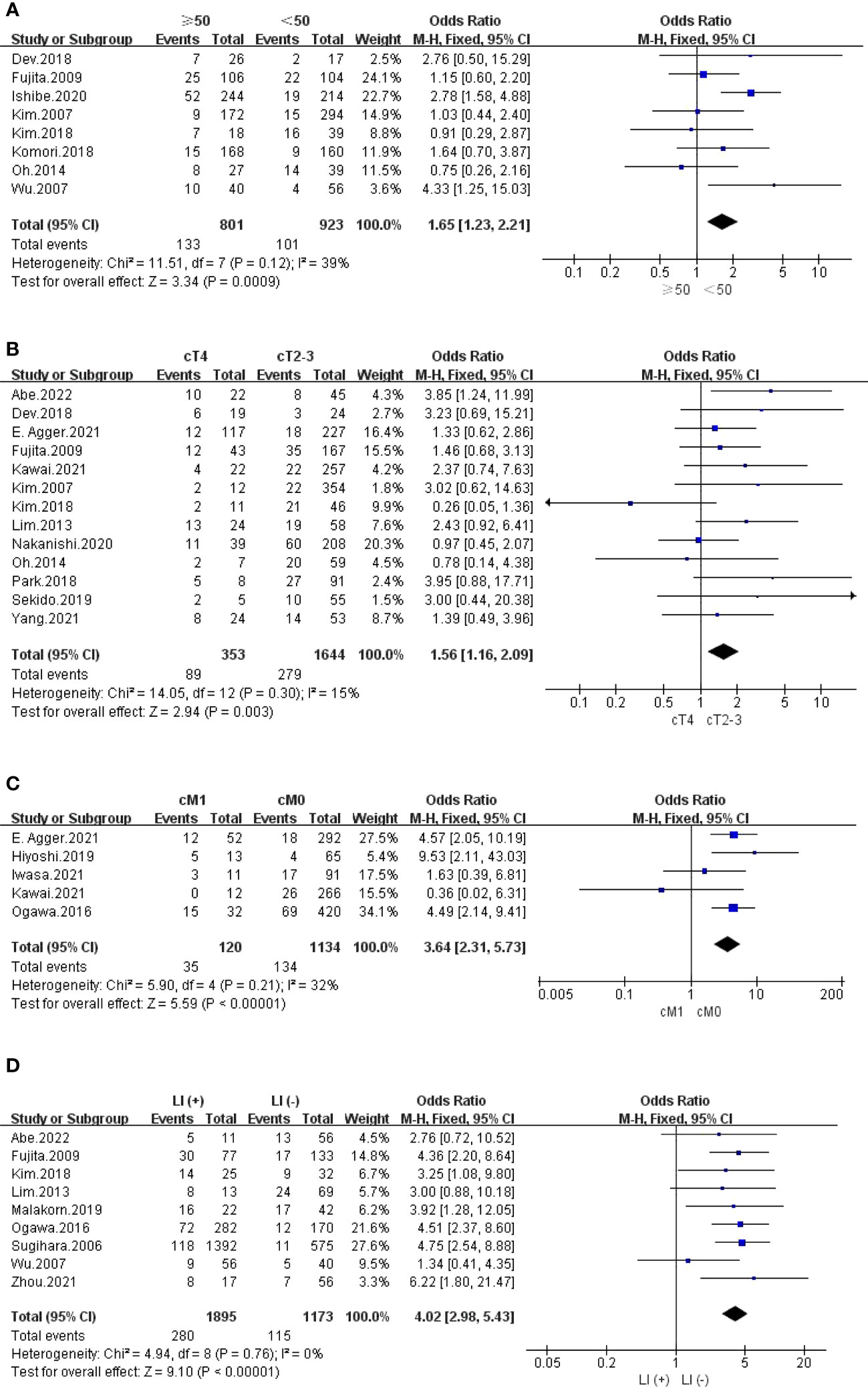
The outcomes are summarized in Figures 4B–D and 5A. The fixed effects model was used owing to low statistical heterogeneity. Overall, the results showed a significantly higher risk of LPLN metastasis in the distance of the tumour from the AV <50 mm (OR: 1.65, 95% CI: 1.17-2.31, I2 = 0%, P =0.004) or ≤40 mm (OR: 2.72, 95% CI: 1.74-4.26, I2 = 0%, P <0.0001), tumour centre located Rb (OR: 4.95, 95% CI: 3.18-7.71, I2 = 0%, P <0.00001), and tumour size ≥50 mm (OR: 1.65, 95% CI: 1.23-2.21, I2 = 39%, P =0.0009).
3.3.5 cTNM stage
The outcomes are summarized in Figures 5B, C and S3. The fixed effects model was used owing to low statistical heterogeneity. Pooled results revealed that both cT4 (OR: 1.56, 95% CI: 1.16-2.09, I2 = 15%, P =0.003) and cM1 (OR: 3.64, 95% CI: 2.31-5.73, I2 = 32%, P <0.00001) were hazard factors for LPLN metastasis. However, cN2-3 did not seem to affect LPLN metastasis (OR: 1.09, 95% CI: 0.61-1.93, I2 = 0%, P =0.77).
3.4 Postoperative examination results
3.4.1 pTN stage
The outcomes are summarized in Figures S4, 5. The random effects model was used because obvious statistical heterogeneity existed in pT stage, while the fixed effects model was used because no statistical heterogeneity existed in pN stage. Overall, the results showed that both pT3-4 (OR: 2.81, 95% CI: 1.83-4.30, I2 = 59%, P <0.00001) and pN2 (OR: 7.61, 95% CI: 4.88-11.85, I2 = 0%, P <0.00001) were conspicuous hazard factors for LPLN metastasis.
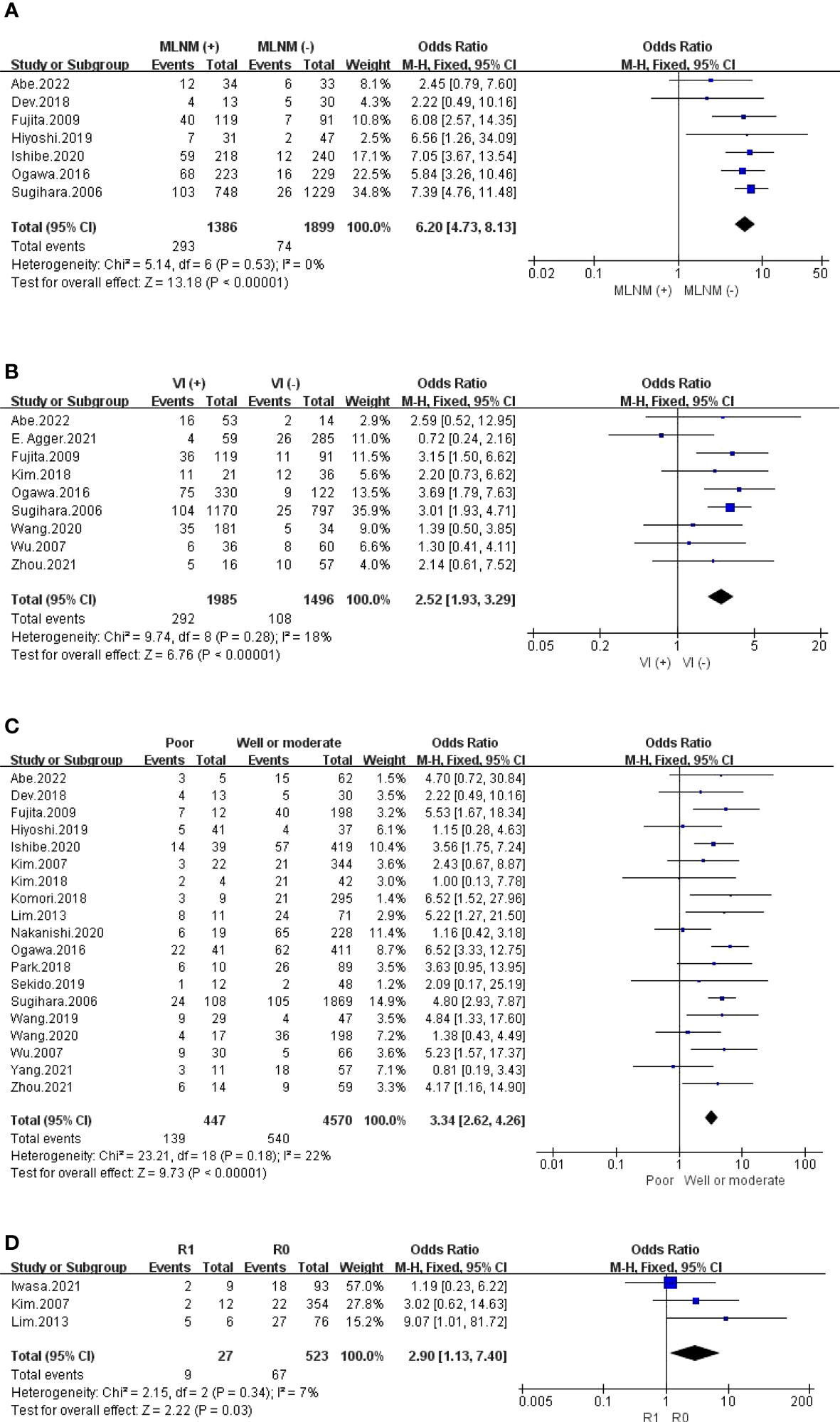
3.4.2 Invasion
The outcomes are summarized in Figures 5D, 6A, B and S6. Regarding LI, MLNM and VI, the fixed effects model was used owing to low statistical heterogeneity. Overall, the results showed a significantly higher risk of LPLN metastasis in LI (OR: 4.02, 95% CI: 2.98-5.43, I2 = 0%, P <0.00001), MLNM (OR: 6.20, 95% CI: 4.73-8.13, I2 = 0%, P <0.00001) and VI (OR: 2.52, 95% CI: 1.93-3.29, I2 = 18%, P <0.00001). While the random effects model was used because obvious statistical heterogeneity existed in the PI, photographs seemed to not affect LPLN metastasis (OR: 1.45, 95% CI: 0.86-2.45, I2 = 56%, P =0.17).
3.4.3 Differentiation and CRM
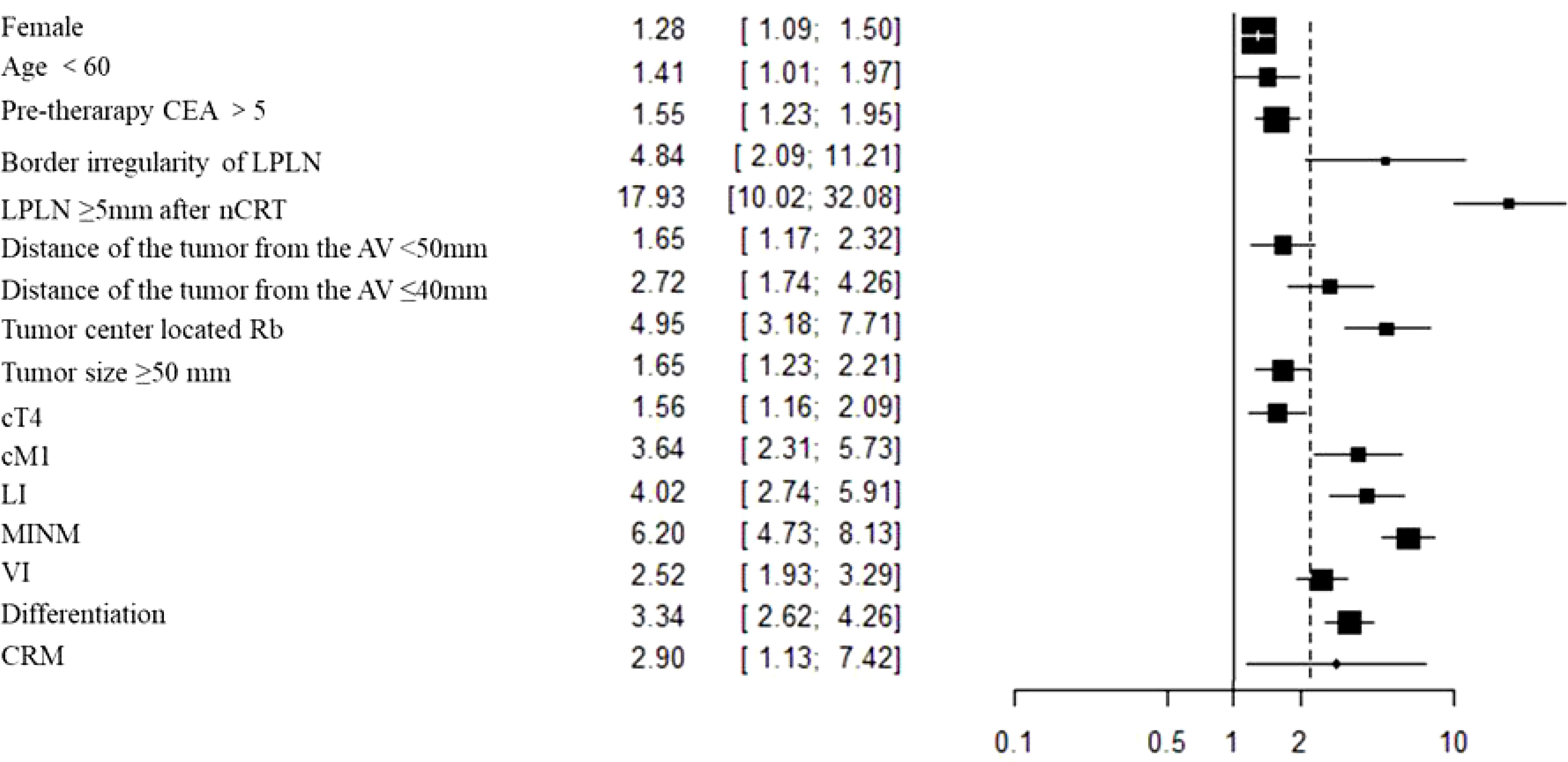
The outcomes are summarized in Figures 6C, D. The fixed effects model was used owing to low statistical heterogeneity. Overall, the results showed a significantly higher risk of LPLN metastasis in poor differentiation (OR: 3.34, 95% CI: 2.62-4.26, I2 = 22%, P <0.00001) and R1 (OR: 2.90, 95% CI: 1.13-7.40, I2 = 7%, P =0.03). The total valuable variables as possible risk factors for LPLN metastasis were summarized in Figure 7. The funnel plot of publication bias which included various indicators were listed in Figure 8.
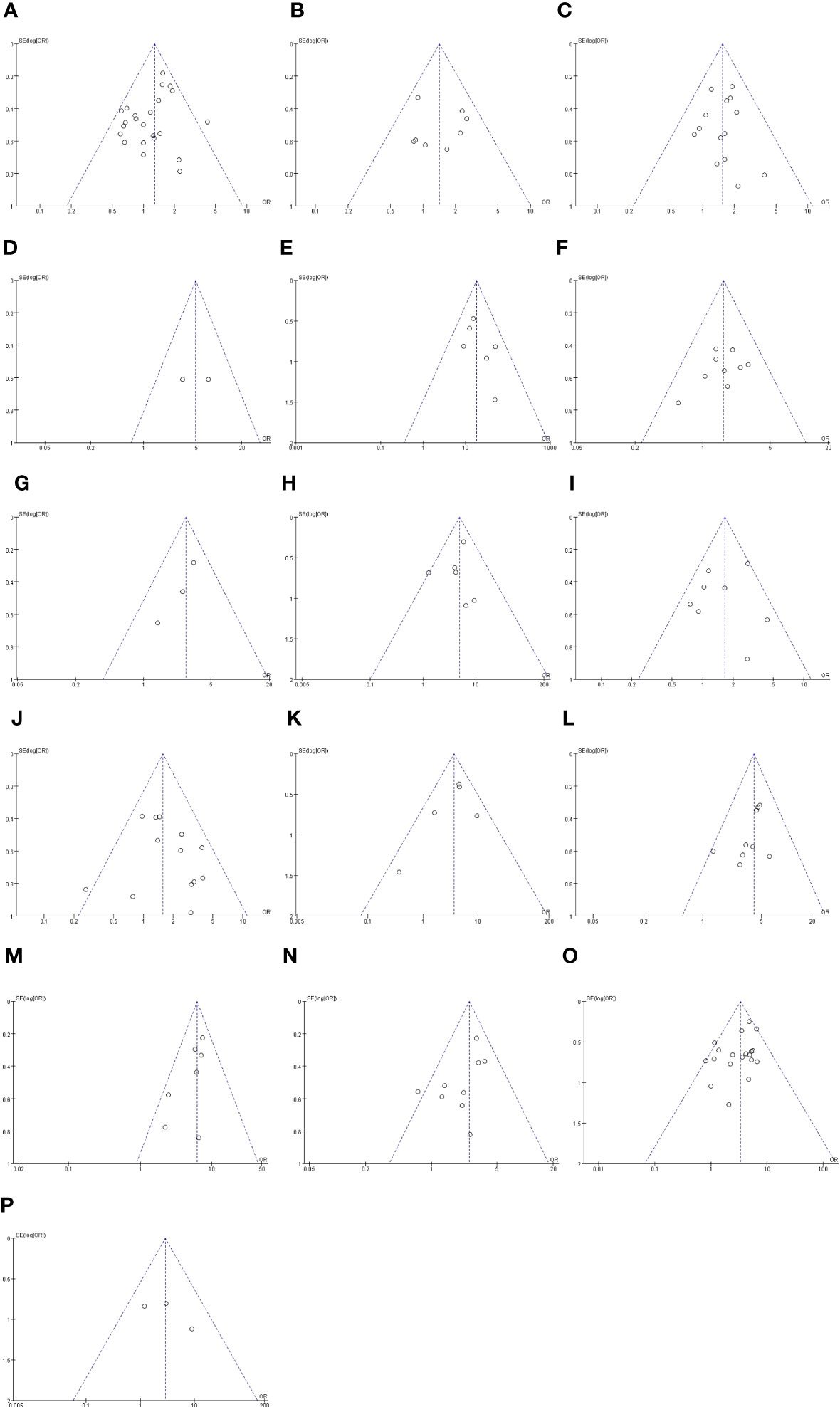
Figure 8 Funnel plot of publication bias in the meta-analysis. (A) Gender. (B) Age (C) Pre-therapy CEA level. (D) Border irregularity of LPLN. (E) SA of LPLN ≥5mm after nCRT. (F) The distance of the tumor from the AV <50mm. (G) The distance of the tumor from the AV ≤40mm. (H) Tumor center located Rb. (I) Tumor size ≥50 mm. (J) cT. (K) cM. (L) LI. (M) MLNM. (N) VI. (O) Differentiation. (P) CRM.
4 Discussion
Surgical treatment is the main treatment for rectal cancer, in which radical resection and regional lymph node dissection are the key to success. The special drainage characteristics of rectal cancer lymph nodes determine the extent of lymph node dissection. The rectal lymphatic drainage area is distributed along the medial space of the obturator foramen of the internal iliac artery, and once metastasis occurs, it will spread upwards, laterally and downwards. It is worth noting that lateral lymph node metastasis is a metastatic pathway of low rectal cancer. Chemoradiotherapy has a poor effect and affects the prognosis of patients with rectal cancer. Previous literature has reported that LPLN metastasis is the main cause of LR in patients with LRC. Postoperative LR is a serious complication in patients with LRC that leads to pain, ureteral and intestinal obstruction, fistula and inflammation and significantly reduces the quality of life of patients. The prevention of LR is crucial because of the poor treatment effect when LR develops (36). Lateral lymph node metastasis is a common problem in the diagnosis and treatment of low rectal cancer, but there is still controversy between Eastern and Western scholars on whether TME should be combined with lateral lymph node dissection for middle and low rectal cancer (37). The studies and literature of Japanese scholars have confirmed that the effect of lateral lymph node dissection is affirmative, which can significantly reduce the local recurrence rate and significantly improve the 5-year survival rate. At present, lateral lymph node dissection has become a standard procedure in Japan. However, this procedure is not widely accepted in Western countries. The treatment for advanced rectal cancer in Europe and America is preoperative neoadjuvant chemoradiotherapy (nCRT)+TME as a standard treatment strategy based on the explanation that LPLN is considered to be a systemic disease as well as unresectable by the TME procedure alone (38). In addition, several studies have authenticated that nCRT can reduce the rate of local recurrence (39, 40). However, Ogura et al. refuted that it is still a problem with the treatment of nCRT before TME and cleared the lower proportion of local recurrence when TME was combined with LLND (41).
In recent years, on account of recognition of local disease rather than a systemic disease about LPLN (8, 42, 43), the Japanese Society for Cancer of the Colon and Rectum recommends the LLND procedure for advanced LRC, especially located below the peritoneal reflection (Rb), which is able to reduce the rate of LPLN metastasis (44), but complications such as longer operation time, higher blood loss and sexual dysfunction occur sequentially (8, 45–47). Consequently, the TME+LLND group was compared with the TME+nCRT group, and Kusters et al. found that the local recurrence rate in both groups was lower than that of the TME alone group, although there was no significant difference (48). In contrast, J.S. Williamson et al. supported the point of views that LLND, especially internal iliac lymph node metastasis, should be considered a resectable local disease and that enlarged lymph nodes that do not respond to nCRT should be surgically dissected (49).
We performed a meta-analysis to identify the risk factors for LPLN metastasis and to provide a more scientific and accurate evaluation index for lateral lymph node dissection. Our results showed that female sex, age <60 years, pretherapeutic CEA level >5 ng/ml, cT4, cM1, distance of the tumour from the AV <50 mm, tumour centre located Rb, SA of LPLN ≥8 mm before nCRT, SA of LPLN ≥5 mm after nCRT, border irregularity of LPLN, tumour size ≥50 mm, pT3-4, pN2, MLNM, LI, VI, CRM (+) and poor differentiation were risk factors for LPLN metastasis.
Similar to previous studies (42, 50, 51), our results showed that female sex was independently associated with LPLN metastasis, potentially owing to the anatomical difference between the male and female pelvis (52). Age <60 years was risk factor as well, because younger patients had the higher basal metabolic rate, and the faster the tumor progression, the higher rate of LPLN metastasis. In addition, a pretherapeutic CEA level >5 ng/ml was associated with LPLN metastasis as well.Elevated CEA levels often indicate a later tumour stage and a greater risk of lateral lymph node metastasis. Similarly, tumour size ≥50 mm was related to LPLN metastasis because the larger the tumour diameter, the greater the depth of invasion, and the higher the probability of LPLN metastasis. Additionally, lower tumour location was also related to LPLN metastasis; however, no accurate standard was determined. Nine studies used AV =50 mm, while three studies used 40 mm as the critical level. The other 6 studies used peritoneal reentry as the cut-off. Anatomically, compared with the tumour centre located at the Ra, the lymphatic drainage of the tumour centre located at the Rb was more complex (53), In addition, the internal iliac and obturator lymph nodes were the most common LPLN metastasis pathways, which were located at the Rb (54, 55). The lower the tumour location, the more drainage to the lateral lymph node region, and thus the higher the probability of LPLN metastasis.
Several studies showed that there was no significant difference between the sensitivity and specificity of CT and MRI in the diagnosis of LPLN metastasis (21, 23). A SA of the LPLN ≥8 mm before CRT was a significant risk factor for LPLN metastasis; however, there was no standard for the selection of cut-off for lymph node diameter. Some studies increased the cut-off to 7 mm (25, 31, 41), which could fully delaminate transverse local recurrence (41). Fujita et al. even advanced the cut-off to 5 mm (23, 29). More researchers predicted the risk of LPLN metastasis through the ROC curve area, and the size of the lymph node corresponding to the largest area was selected as the cut-off (26, 27, 32, 52). Although the standard is not unified, most studies set the critical value of lymph node size before nCRT as 8 mm. The results of our data analysis support that a pre-CRT SA of LPLN ≥8 mm is a significant risk factor for LPLN metastasis. Similarly, our results also showed that the SA of LPLN ≥5 mm after nCRT was significantly related to LPLN metastasis. Most of the included articles suggested 5 mm as the cut-off, except that Kawai et al. suggested 8 mm (17) and Zhou et al. suggested 7 mm (15), because 100% sensitivity was observed for a size ≥ 5 mm after nCRT to predict LPLN metastasis (18). Therefore, a SA ≥5 mm in the remaining LPLN after nCRT should be one of the clear signals of LPLN metastasis. In general, both before and after nCRT, our results showed that LPLN enlargement was significantly related to LPLN metastasis. In addition to lymph node enlargement, the specific imaging features of lymph node metastasis are also very important. Notably, the morphology of lymph nodes on MRI showing irregular boundaries and mixed signal intensity is often suggestive of LPLN metastasis, and our analysis of results also demonstrates that the irregular boundaries of LPLN is risk factor for lateral lymph node metastasis, which is consistent with previous research results (56). Some studies even found that it could improve the prediction ability of MRI for LPLN metastasis in place of lymph node size (57). Regarding the depth of cancer invasion, Wang et al. considered it an important indicator for LPLN metastasis assessed by preoperative diagnostic imaging (28). Our results showed that patients with cT4 stage were more likely to have LPLN metastasis than those with cT2-3 stage; equally, patients with cM1 stage were more prone to have LPLN metastasis than patients with cM0. Rectal lymphatic vessels arise from the lamina propria of the rectal mucosa in anatomy; thus, the percentage of lymph node metastasis is approximately 8-15% in patients with early RC (58, 59). When the tumour invades the submucosa, cancer cells have more opportunity to spread through the lymphatic vessels, leading to LPLN metastasis. In addition, the deeper the infiltration, the higher the probability of LPLN metastasis. The overall study shows that the risk of LPLN metastasis is closely related to the clinical stage of the tumour, and the later the clinical stage of the tumour, the higher the risk of LPLN metastasis.
Current studies have shown that lymphatic, venous and perineural invasions are risk factors for LR of RC, with 1 recurrence confirmed by histopathological examination in every 4 to 5 patients (60, 61). Our results showed that LI (including MLNM) and VI were strong predictors of LPLN metastasis. It is clear that LI (including MLNM) and VI indicate a later stage of the tumor and an increased risk of lateral lymph node metastasis. In addition, compared with well or moderate differentiation, our results showed that poor differentiation was a risk factor for LPLN metastasis. It is obvious that poorly differentiated carcinoma has stronger invasive and metastatic abilities and is more likely to have distant metastasis, so poorly differentiated RC is more likely to have lateral lymph node metastasis. Echoing previous reports, compared with tubular and papillary adenocarcinoma, mucinous and signet ring adenocarcinoma that did not respond to radiotherapy had a higher probability of LPLN metastasis (3, 7, 62). In addition, we also analysed whether a positive circumferential margin was a risk factor for LPLN metastasis. The results showed that a positive circumferential margin (R1) was also a risk factor for LPLN metastasis. It was obvious that RC patients with R1 were often in a later clinical stage and more likely to develop lateral lymph node metastasis.
Currently, more methods of predicting LPLN metastasis are being developed. However, it is necessary to explore better techniques to help surgeons make more accurate judgements. Dev et al. proposed a risk stratification nomogram based on important predictors of LPLN metastasis to comprehensively evaluate and guide treatment (20). Miyake et al. used a novel one-step nucleic acid amplification (OSNA) assay to calculate LPLN metastasis targeting lymph node micrometastasis with 100% sensitivity and 86% specificity, which was significantly higher than that of CT and MRI (63). Iwasa et al. proved that the presence of the middle rectal artery (MRA) assessed by ceMRI could accurately predict bilateral LPLN metastasis (including micrometastasis) (25). Abe et al. proved that extramural venous invasion on MRI (MRI-EMVI) was independently related to LPLN metastasis and proposed that it could more accurately predict LPLN metastasis combined with lymph node size (12). As we mentioned above, treatments for LPLN metastasis of LRC differ between eastern and western countries. We supported that combining the advantages of both treatments, developing strengths and avoiding weaknesses, may achieve an unprecedented effect. We recommend patients with the following risk factors: Age <60 years, female, elevated CEA level, large tumor volume, low distance from anal margin, enlarged lymph nodes with irregular enhancement (especially SA of LPLN ≥8 mm before nCRT, SA of LPLN ≥5 mm after nCRT), pT 3 - 4, pN 2 can be given priority to comprehensive treatment including lateral lymph node dissection. Other low-risk factors can be carefully performed lateral lymph node dissection to avoid complications and trauma caused by lateral lymph node dissection. There were some limitations in our study. First, most of the articles included were retrospective studies. Second, except for two articles from Western countries (America and Switzerland), the rest were from Eastern countries, which might have caused our research to be slightly biased towards the Eastern perspective.
5 Conclusion
Our studies proved that female sex, age <60 years, pretherapeutic CEA level >5 ng/ml, cT4, cM1, distance of the tumour from the AV <50 mm, tumour centre located Rb, SA of LPLN ≥8 mm before nCRT, SA of LPLN ≥5 mm after nCRT, border irregularity of LPLN, tumour size ≥50 mm, pT3-4, pN2, MLNM, LI, VI, CRM (+) and poor differentiation were risk factors for LPLN metastasis. In conclusion, although lateral lymph node dissection can reduce the local recurrence rate, increase the number of lymph nodes harvested, and achieve more accurate assessment of rectal cancer, it also has the risk of increasing surgery-related complications. Whether to perform lateral lymph node dissection in clinical practice can be judged in combination with the above risk factors so that patients with rectal cancer who need lateral lymph node dissection can be accurately screened out to reduce the risk of unnecessary surgical trauma. According to the risk factors of lateral lymph node metastasis, we can adopt different comprehensive treatment strategies. High-risk patients can perform lateral lymph node dissection to effectively reduce local recurrence; In low-risk patients, we can avoid overtreatment, reduce complications and trauma caused by lateral lymph node dissection, and maximize patient survival and quality of life.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
D-xZ, ZY, and LT acquisition of data, analysis and interpretation of data, and drafting the article; M-nR and Z-lL collect and organize data, and revising the article; J-wX conception and design of the study, and critical revision. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by National Natural Science Foundation of China, NO.30700773, NO.81070378 and NO.81270561; Special Research Fund for the First Affiliated Hospital of Chengdu Medical College, NO.CYFY2019YB08; and High-level Talents Introduction Fund for the First Affiliated Hospital of Chengdu Medical College, NO.CYFY2018GQ17. the Key Project of Sichuan Provincial Department of Science and Technology Applied Basic Research Program, China (NO. 2022NSFSC0050).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1219608/full#supplementary-material
Abbreviations
AV, anal verge; CIs, confidence intervals; cM1, clinical M1 stage; cN, clinical N stage; CRC, Colorectal cancer; ESGAR, the European Society of Gastrointestinal and Abdominal Radiology; LI, lymphatic invasion; LLND, lateral lymph node dissection; LPLN, Lateral pelvic lymph node; LR, local recurrence; LRC, low rectal cancer; MeSH, Medical Subject Headings; MLNM, mesorectal lymph node metastasis; MRA, middle rectal artery; MRI-EMVI, extramural venous invasion on MRI; NOS, Newcastle Ottawa Scale; ORs, Odds ratios; OSNA, one-step nucleic acid amplification; pN, pN stage; pN2, pathological N2 stage; pT3-4, pathological T3-4 stage; R1, positive circumferential margin; Rb, below the peritoneal reflection; RC, rectal cancer; SA, short axis; VI, venous invasion.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut (2017) 66(4):683–91. doi: 10.1136/gutjnl-2015-310912
3. Takahashi T, Ueno M, Azekura K, Ohta H. Lateral node dissection and total mesorectal excision for rectal cancer. Dis Colon Rectum (2000) 43(10 Suppl):S59–68. doi: 10.1007/BF02237228
4. Ueno H, Yamauchi C, Hase K, Ichikura T, Mochizuki H. Clinicopathological study of intrapelvic cancer spread to the iliac area in lower rectal adenocarcinoma by serial sectioning. Br J Surg (1999) 86(12):1532–7. doi: 10.1046/j.1365-2168.1999.01271.x
5. Haanappel A, Kroon HM, Schaap DP, Bedrikovetski S, Dudi-Venkata NN, Lee HX, et al. Lateral lymph node metastases in locally advanced low rectal cancers may not be treated effectively with neoadjuvant (Chemo)Radiotherapy only. Front Oncol (2019) 9:1355. doi: 10.3389/fonc.2019.01355
6. Kim TH, Jeong SY, Choi DH, Kim DY, Jung KH, Moon SH, et al. Lateral lymph node metastasis is a major cause of locoregional recurrence in rectal cancer treated with preoperative chemoradiotherapy and curative resection. Ann Surg Oncol (2008) 15(3):729–37. doi: 10.1245/s10434-007-9696-x
7. Sugihara K, Kobayashi H, Kato T, Mori T, Mochizuki H, Kameoka S, et al. Indication and benefit of pelvic sidewall dissection for rectal cancer. Dis Colon Rectum (2006) 49(11):1663–72. doi: 10.1007/s10350-006-0714-z
8. Fujita S, Akasu T, Mizusawa J, Saito N, Kinugasa Y, Kanemitsu Y, et al. Postoperative morbidity and mortality after mesorectal excision with and without lateral lymph node dissection for clinical stage II or stage III lower rectal cancer (JCOG0212): results from a multicentre, randomised controlled, non-inferiority trial. Lancet Oncol (2012) 13(6):616–21. doi: 10.1016/S1470-2045(12)70158-4
9. Yano H, Moran BJ. The incidence of lateral pelvic side-wall nodal involvement in low rectal cancer may be similar in Japan and the West. Br J Surg (2008) 95(1):33–49. doi: 10.1002/bjs.6061
10. Ueno M, Oya M, Azekura K, Yamaguchi T, Muto T. Incidence and prognostic significance of lateral lymph node metastasis in patients with advanced low rectal cancer. Br J Surg (2005) 92(6):756–63. doi: 10.1002/bjs.4975
11. Kusters M, Wallner C, Lange MM, DeRuiter MC, van de Velde CJ, Moriya Y, et al. Origin of presacral local recurrence after rectal cancer treatment. Br J Surg (2010) 97(10):1582–7. doi: 10.1002/bjs.7180
12. Abe T, Yasui M, Imamura H, Matsuda C, Nishimura J, Haraguchi N, et al. Combination of extramural venous invasion and lateral lymph node size detected with magnetic resonance imaging is a reliable biomarker for lateral lymph node metastasis in patients with rectal cancer. World J Surg Oncol (2022) 20(1):5. doi: 10.1186/s12957-021-02464-3
13. Dev K, Veerenderkumar KV, Krishnamurthy S. Incidence and predictive model for lateral pelvic lymph node metastasis in lower rectal cancer. Indian J Surg Oncol (2018) 9(2):150–6. doi: 10.1007/s13193-017-0719-1
14. Agger E, Akerlund V, Ekberg O, Jorgren F, Lydrup ML, Buchwald P. Management, treatment and prognostic significance of lateral lymph node metastases in rectal cancer-a regional cohort study. Int J Colorectal Dis (2021) 36(12):2707–14. doi: 10.1007/s00384-021-04018-1
15. Fujita S, Yamamoto S, Akasu T, Moriya Y. Risk factors of lateral pelvic lymph node metastasis in advanced rectal cancer. Int J Colorectal Dis (2009) 24(9):1085–90. doi: 10.1007/s00384-009-0704-4
16. Hiyoshi Y, Miyamoto Y, Kiyozumi Y, Eto K, Nagai Y, Iwatsuki M, et al. Risk factors and prognostic significance of lateral pelvic lymph node metastasis in advanced rectal cancer. Int J Clin Oncol (2020) 25(1):110–7. doi: 10.1007/s10147-019-01523-w
17. Ishibe A, Watanabe J, Suwa Y, Suzuki S, Nakagawa K, Suwa H, et al. Oncological outcomes of lateral lymph node dissection (LLND) for locally advanced rectal cancer: is LLND alone sufficient? Int J Colorectal Dis (2021) 36(2):293–301. doi: 10.1007/s00384-020-03760-2
18. Iwasa Y, Koyama F, Marugami N, Kuge H, Nakamoto T, Obara S, et al. The middle rectal artery detected by contrast-enhanced magnetic resonance imaging predicts lateral lymph node metastasis in lower rectal cancer. Int J Colorectal Dis (2021) 36(8):1677–84. doi: 10.1007/s00384-021-03887-w
19. Kawai K, Shiratori H, Hata K, Nozawa H, Tanaka T, Nishikawa T, et al. Optimal size criteria for lateral lymph node dissection after neoadjuvant chemoradiotherapy for rectal cancer. Dis Colon Rectum (2021) 64(3):274–83. doi: 10.1097/DCR.0000000000001866
20. Kim MJ, Hur BY, Lee ES, Park B, Joo J, Kim MJ, et al. Prediction of lateral pelvic lymph node metastasis in patients with locally advanced rectal cancer with preoperative chemoradiotherapy: Focus on MR imaging findings. PloS One (2018) 13(4):e0195815. doi: 10.1371/journal.pone.0195815
21. Komori K, Fujita S, Mizusawa J, Kanemitsu Y, Ito M, Shiomi A, et al. Predictive factors of pathological lateral pelvic lymph node metastasis in patients without clinical lateral pelvic lymph node metastasis (clinical stage II/III): The analysis of data from the clinical trial (JCOG0212). Eur J Surg Oncol (2019) 45(3):336–40. doi: 10.1016/j.ejso.2018.11.016
22. Lim SB, Yu CS, Kim CW, Yoon YS, Park SH, Kim TW, et al. Clinical implication of additional selective lateral lymph node excision in patients with locally advanced rectal cancer who underwent preoperative chemoradiotherapy. Int J Colorectal Dis (2013) 28(12):1667–74. doi: 10.1007/s00384-013-1761-2
23. Malakorn S, Yang Y, Bednarski BK, Kaur H, You YN, Holliday EB, et al. Who should get lateral pelvic lymph node dissection after neoadjuvant chemoradiation? Dis Colon Rectum (2019) 62(10):1158–66. doi: 10.1097/DCR.0000000000001465
24. Nakanishi R, Akiyoshi T, Toda S, Murakami Y, Taguchi S, Oba K, et al. Radiomics approach outperforms diameter criteria for predicting pathological lateral lymph node metastasis after neoadjuvant (Chemo)Radiotherapy in advanced low rectal cancer. Ann Surg Oncol (2020) 27(11):4273–83. doi: 10.1245/s10434-020-08974-w
25. Ogawa S, Hida J, Ike H, Kinugasa T, Ota M, Shinto E, et al. The important risk factor for lateral pelvic lymph node metastasis of lower rectal cancer is node-positive status on magnetic resonance imaging: study of the Lymph Node Committee of Japanese Society for Cancer of the Colon and Rectum. Int J Colorectal Dis (2016) 31(10):1719–28. doi: 10.1007/s00384-016-2641-3
26. Oh HK, Kang SB, Lee SM, Lee SY, Ihn MH, Kim DW, et al. Neoadjuvant chemoradiotherapy affects the indications for lateral pelvic node dissection in mid/low rectal cancer with clinically suspected lateral node involvement: a multicenter retrospective cohort study. Ann Surg Oncol (2014) 21(7):2280–7. doi: 10.1245/s10434-014-3559-z
27. Park BK, Lee SJ, Hur BY, Kim MJ, Chan Park S, Chang HJ, et al. Feasibility of selective lateral node dissection based on magnetic resonance imaging in rectal cancer after preoperative chemoradiotherapy. J Surg Res (2018) 232:227–33. doi: 10.1016/j.jss.2018.05.047
28. Sekido Y, Nishimura J, Fujino S, Ogino T, Miyoshi N, Takahashi H, et al. Predicting lateral pelvic lymph node metastasis based on magnetic resonance imaging before and after neoadjuvant chemotherapy for patients with locally advanced lower rectal cancer. Surg Today (2020) 50(3):292–7. doi: 10.1007/s00595-019-01886-7
29. Wang P, Zhou S, Zhou H, Liang J, Zhou Z. Evaluating predictive factors for determining the presence of lateral pelvic node metastasis in rectal cancer patients following neoadjuvant chemoradiotherapy. Colorectal Dis (2019) 21(7):791–6. doi: 10.1111/codi.14595
30. Wang L, HIrano Y, Heng G, Ishii T, Kondo H, Hara K, et al. The significance of lateral lymph node metastasis in low rectal cancer: a propensity score matching study. J Gastrointest Surg (2021) 25(7):1866–74. doi: 10.1007/s11605-020-04825-x
31. Wu ZY, Wan J, Li JH, Zhao G, Yao Y, Du JL, et al. Prognostic value of lateral lymph node metastasis for advanced low rectal cancer. World J Gastroenterol (2007) 13(45):6048–52. doi: 10.3748/wjg.v13.45.6048
32. Yang X, Gu C, Hu T, Wei M, Meng W, Wang Z, et al. Indications and oncological outcomes of selective dissection for clinically suspected lateral lymph node metastasis in patients with rectal cancer based on pretreatment imaging. Tech Coloproctol (2021) 25(4):425–37. doi: 10.1007/s10151-020-02386-4
33. Zhou S, Jiang Y, Pei W, Liang J, Zhou Z. Risk factors and prognostic significance of lateral pelvic lymph node dissection after neoadjuvant chemoradiotherapy for rectal patients with clinically suspected lateral lymph node metastasis. BMC Surg (2021) 21(1):441. doi: 10.1186/s12893-021-01443-5
34. Lo CK, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers' to authors' assessments. BMC Med Res Methodol (2014) 14:45. doi: 10.1186/1471-2288-14-45
35. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ (2009) 339:b2535. doi: 10.1136/bmj.b2535
36. Garcia-Aguilar J, Cromwell JW, Marra C, Lee SH, Madoff RD, Rothenberger DA. Treatment of locally recurrent rectal cancer. Dis Colon Rectum (2001) 44(12):1743–8. doi: 10.1007/BF02234449
37. Peacock O, Chang GJ. The landmark series: Management of lateral lymph nodes in locally advanced rectal cancer. Ann Surg Oncol (2020) 27(8):2723–31. doi: 10.1245/s10434-020-08639-8
38. Ishihara S, Kanemitsu Y, Murono K, Otani K, Yasuda K, Nishikawa T, et al. Oncological benefit of lateral pelvic lymph node dissection for rectal cancer treated without preoperative chemoradiotherapy: a multicenter retrospective study using propensity score analysis. Int J Colorectal Dis (2016) 31(7):1315–21. doi: 10.1007/s00384-016-2607-5
39. Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med (2004) 351(17):1731–40. doi: 10.1056/NEJMoa040694
40. Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med (2001) 345(9):638–46. doi: 10.1056/NEJMoa010580
41. Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med (2006) 355(11):1114–23. doi: 10.1056/NEJMoa060829
42. Ogura A, Konishi T, Cunningham C, Garcia-Aguilar J, Iversen H, Toda S, et al. Neoadjuvant (Chemo)radiotherapy with total mesorectal excision only is not sufficient to prevent lateral local recurrence in enlarged nodes: Results of the multicenter lateral node study of patients with low cT3/4 rectal cancer. J Clin Oncol (2019) 37(1):33–43. doi: 10.1200/JCO.18.00032
43. Akiyoshi T, Watanabe T, Miyata S, Kotake K, Muto T, Sugihara K. Japanese Society for Cancer of the C, Rectum: Results of a Japanese nationwide multi-institutional study on lateral pelvic lymph node metastasis in low rectal cancer: is it regional or distant disease? Ann Surg (2012) 255(6):1129–34. doi: 10.1097/SLA.0b013e3182565d9d
44. Watanabe T, Itabashi M, Shimada Y, Tanaka S, Ito Y, Ajioka Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) Guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol (2015) 20(2):207–39. doi: 10.1007/s10147-015-0801-z
45. Watanabe T, Itabashi M, Shimada Y, Tanaka S, Ito Y, Ajioka Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2010 for the treatment of colorectal cancer. Int J Clin Oncol (2012) 17(1):1–29. doi: 10.1007/s10147-011-0315-2
46. Akasu T, Sugihara K, Moriya Y. Male urinary and sexual functions after mesorectal excision alone or in combination with extended lateral pelvic lymph node dissection for rectal cancer. Ann Surg Oncol (2009) 16(10):2779–86. doi: 10.1245/s10434-009-0546-x
47. Georgiou P, Tan E, Gouvas N, Antoniou A, Brown G, Nicholls RJ, et al. Extended lymphadenectomy versus conventional surgery for rectal cancer: a meta-analysis. Lancet Oncol (2009) 10(11):1053–62. doi: 10.1016/S1470-2045(09)70224-4
48. Tsukamoto S, Fujita S, Ota M, Mizusawa J, Shida D, Kanemitsu Y, et al. Long-term follow-up of the randomized trial of mesorectal excision with or without lateral lymph node dissection in rectal cancer (JCOG0212). Br J Surg (2020) 107(5):586–94. doi: 10.1002/bjs.11513
49. Kusters M, Beets GL, van de Velde CJ, Beets-Tan RG, Marijnen CA, Rutten HJ, et al. A comparison between the treatment of low rectal cancer in Japan and the Netherlands, focusing on the patterns of local recurrence. Ann Surg (2009) 249(2):229–35. doi: 10.1097/SLA.0b013e318190a664
50. Williamson JS, Quyn AJ, Sagar PM. Rectal cancer lateral pelvic sidewall lymph nodes: a review of controversies and management. Br J Surg (2020) 107(12):1562–9. doi: 10.1002/bjs.11925
51. Kobayashi H, Mochizuki H, Kato T, Mori T, Kameoka S, Shirouzu K, et al. Outcomes of surgery alone for lower rectal cancer with and without pelvic sidewall dissection. Dis Colon Rectum (2009) 52(4):567–76. doi: 10.1007/DCR.0b013e3181a1d994
52. Akiyoshi T, Matsueda K, Hiratsuka M, Unno T, Nagata J, Nagasaki T, et al. Indications for lateral pelvic lymph node dissection based on magnetic resonance imaging before and after preoperative chemoradiotherapy in patients with advanced low-Rectal cancer. Ann Surg Oncol (2015) 22 Suppl 3:S614–620. doi: 10.1245/s10434-015-4565-5
53. Ishibe A, Ota M, Watanabe J, Suwa Y, Suzuki S, Kanazawa A, et al. Prediction of lateral pelvic lymph-node metastasis in low rectal cancer by magnetic resonance imaging. World J Surg (2016) 40(4):995–1001. doi: 10.1007/s00268-015-3299-7
54. Kim JH, Beets GL, Kim MJ, Kessels AG, Beets-Tan RG. High-resolution MR imaging for nodal staging in rectal cancer: are there any criteria in addition to the size? Eur J Radiol (2004) 52(1):78–83. doi: 10.1016/j.ejrad.2003.12.005
55. Kim SH, Chang HJ, Kim DY, Park JW, Baek JY, Kim SY, et al. What is the ideal tumor regression grading system in rectal cancer patients after preoperative chemoradiotherapy? Cancer Res Treat (2016) 48(3):998–1009. doi: 10.4143/crt.2015.254
56. Kusters M, Slater A, Muirhead R, Hompes R, Guy RJ, Jones OM, et al. What to do with lateral nodal disease in low locally advanced rectal cancer? A call for further reflection and research. Dis Colon Rectum (2017) 60(6):577–85. doi: 10.1097/DCR.0000000000000834
57. Amano K, Fukuchi M, Kumamoto K, Hatano S, Ohno H, Osada H, et al. Pre-operative evaluation of lateral pelvic lymph node metastasis in lower rectal cancer: Comparison of three different imaging modalities. J Anus Rectum Colon (2020) 4(1):34–40. doi: 10.23922/jarc.2019-022
58. Yu SK, Chand M, Tait DM, Brown G. Magnetic resonance imaging defined mucinous rectal carcinoma is an independent imaging biomarker for poor prognosis and poor response to preoperative chemoradiotherapy. Eur J Cancer (2014) 50(5):920–7. doi: 10.1016/j.ejca.2013.12.007
59. Miyake Y, Mizushima T, Hata T, Takahashi H, Hanada H, Shoji H, et al. Inspection of perirectal lymph nodes by one-Step nucleic acid amplification predicts lateral lymph node metastasis in advanced rectal cancer. Ann Surg Oncol (2017) 24(13):3850–6. doi: 10.1245/s10434-017-6069-y
60. Huang L, Wang X, Wen C, Yang X, Song M, Chen J, et al. Hsa-miR-19a is associated with lymph metastasis and mediates the TNF-alpha induced epithelial-to-mesenchymal transition in colorectal cancer. Sci Rep (2015) 5:13350. doi: 10.1038/srep13350
61. Kyo K, Sameshima S, Takahashi M, Furugori T, Sawada T. Impact of autonomic nerve preservation and lateral node dissection on male urogenital function after total mesorectal excision for lower rectal cancer. World J Surg (2006) 30(6):1014–9. doi: 10.1007/s00268-005-0050-9
62. Uyama I, Sugioka A, Matsui H, Fujita J, Komori Y, Hanai T, et al. Laparoscopic lateral node dissection with autonomic nerve preservation for advanced lower rectal cancer. J Am Coll Surg (2001) 193(5):579–84. doi: 10.1016/S1072-7515(01)01042-0
Keywords: rectal cancer, lateral pelvic lymph node metastasis, risk factors, meta-analysis, LPLN
Citation: Zeng D-x, Yang Z, Tan L, Ran M-n, Liu Z-l and Xiao J-w (2023) Risk factors for lateral pelvic lymph node metastasis in patients with lower rectal cancer: a systematic review and meta-analysis. Front. Oncol. 13:1219608. doi: 10.3389/fonc.2023.1219608
Received: 01 June 2023; Accepted: 31 July 2023;
Published: 06 September 2023.
Edited by:
Aldo Rocca, University of Molise, ItalyReviewed by:
Paulo Roberto Stevanato Filho, AC Camargo Cancer Center, BrazilDaniel Neureiter, Salzburger Landeskliniken, Austria
Copyright © 2023 Zeng, Yang, Tan, Ran, Liu and Xiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiang-wei Xiao, eGlhb2ppYW5nd2VpMjAxOEAxNjMuY29t
†These authors share first authorship
 De-xing Zeng
De-xing Zeng Zhou Yang2†
Zhou Yang2† Ling Tan
Ling Tan Jiang-wei Xiao
Jiang-wei Xiao