- 1Department of Obstetrics and Gynecology, Yongin Severance Hospital, Yonsei University College of Medicine, Yongin, Republic of Korea
- 2Department of Obstetrics and Gynecology, Samsung Medical Center, Seoul, Republic of Korea
- 3Department of Obstetrics and Gynecology, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Republic of Korea
- 4Department of Obstetrics and Gynecology, Seoul National University College of Medicine, Seoul, Republic of Korea
- 5Departments of Obstetrics and Gynecology, Ajou University School of Medicine, Suwon, Republic of Korea
- 6Department of Obstetrics and Gynecology, Institute of Women’s Medical Life Science, Yonsei Cancer Center, Severance Hospital, Yonsei University College of Medicine, Seoul, Republic of Korea
Objective: There is a lack of multi-institutional large-volume and long-term follow-up data on comparisons between robot-assisted surgery and conventional laparoscopic surgery. This study compared the surgical and long-term survival outcomes between patients who underwent robot-assisted or conventional laparoscopic surgery for endometrial cancer.
Methods: We retrospectively reviewed the data of patients from five large academic institutions who underwent either robot-assisted or conventional laparoscopic surgery for the treatment of endometrial cancer between 2012 and 2017, ensuring at least 5 years of potential follow-up. Intra- and postoperative outcomes, long-term disease-free survival, and overall survival were compared.
Results: The study cohort included 1,003 unselected patients: 551 and 452 patients received conventional laparoscopic and robot-assisted surgery, respectively. The median follow-up duration was 57 months. Postoperative complications were significantly less likely to occur in the robot-assisted surgery group compared to the laparoscopic surgery group (7.74% vs. 13.79%, P = 0.002), primarily limited to minor complications. There were no significant differences in survival: 5-year disease-free survival was 91.2% versus 90.0% (P = 0.628) and overall survival was 97.9% versus 96.8% (P = 0.285) in the robot-assisted and laparoscopic surgery cohorts, respectively. Cox proportional hazard regression models demonstrated that the mode of surgery was not associated with disease-free survival (hazard ratio, 0.897; confidence interval, 0.563–1.429) or overall survival (hazard ratio, 0.791; confidence interval, 0.330–1.895) after adjusting for confounding factors.
Conclusion: Robot-assisted surgery for endometrial cancer demonstrates comparable long-term survival outcomes and a reduced incidence of postoperative minor complications when compared to conventional laparoscopic surgery.
1 Introduction
Endometrial cancer is the most common malignancy of the female reproductive tract in developed countries (1). Surgery is an essential step in the management of the disease. The need for postoperative adjuvant treatment to minimize recurrence can be determined from the pathological analysis of the surgical specimen (2).
Minimally invasive surgery (MIS) for early stage endometrial cancer offers equivalent survival outcomes with reduced intra- and postoperative morbidity, compared to laparotomic surgery (3, 4). Specifically, the introduction of robot-assisted laparoscopic surgery (RS) has encouraged more gynecologic oncologists to adopt MIS when treating endometrial cancer (5). RS provides potential benefits over conventional laparoscopic surgery (LS), including binocular view, additional degrees of rotational freedom, decreased reliance on skilled assistance, and relatively shallower learning curve. Moreover, RS has been reported as a feasible, safe, and reproducible alternative to LS, even in the cases of obese or elderly patients with endometrial cancer (6, 7). As a result, the proportion of endometrial cancers treated through MIS has gradually increased, approaching 90% at high-volume hospitals, which reflects the increasing use of RS (8, 9). However, there is a lack of large-volume, long-term follow-up data on the direct comparison of oncologic and surgical outcomes between RS and LS for the treatment of endometrial cancer. Currently, most reports suggest that short-term complication rates and survival outcomes for patients with endometrial cancer are similar between RS and LS (10, 11).
This study compared the long-term survival outcomes and surgical outcomes, including intra- and postoperative complications, between patients who underwent RS or LS for endometrial cancer in five high-volume hospitals in Korea.
2 Materials and methods
2.1 Study population and data collection
This retrospective multicenter study was approved by the institutional review board of Yonsei university of Korea (Approval No. 4-2021-0988). The requirement of informed consent was waived due to the retrospective nature of the study. The study was conducted in accordance with the Declaration of Helsinki. We reviewed the medical records of each institution and identified patients who met the following inclusion criteria: (1) age ≥18 years, (2) pathologically confirmed endometrial cancer, (3) diagnosis made between January 2012 and June 2017, and (4) having undergone laparoscopic or robot-assisted MIS, including total hysterectomy and peritoneal lavage, with or without lymph node biopsy for the treatment of endometrial cancer. Patients were excluded if they met any of the following criteria: (1) immunocompromised or pregnant, (2) synchronous double primary cancers, (3) treated medically or with radiation alone, and (4) lost to follow-up without evidence of disease recurrence or death.
2.2 Data analysis
Patients who underwent robot-assisted surgery for endometrial cancer were assigned to the RS group, whereas those who underwent conventional laparoscopic surgery were assigned to the LS group. Differences in the demographic characteristics, comorbidities, and detailed intra- and postoperative outcomes, including complications, details of surgical procedures, results of pathological analysis, and survival outcomes were compared between the RS and LS groups. Disease-free survival (DFS) was defined as the duration from the date of surgery to the recurrence or end of follow-up. Overall survival (OS) was defined as the interval between the date of surgery and death or the end of follow-up, whichever came first.
Patients were classified into subgroups based on the number of ports utilized during the procedure. An analysis of subgroups was conducted to evaluate and compare the surgical outcomes and survival rates between patients who underwent multiport laparoscopy (mLS), single port laparoscopy (sLS), multiport robot-assisted surgery (mRS), and single port robot-assisted surgery (sRS).
2.3 Statistical analysis
Demographic, surgical, and pathological characteristics were compared using paired t-tests and chi-square tests. Kaplan–Meier methods with log-rank tests were used for survival analysis. Multivariable Cox proportional hazards models were used to determine the hazard ratio (HR) to predict recurrence or death after adjusting for confounding variables. All statistical analyses were conducted using the IBM SPSS Statistics software (version 26.0; SPSS Inc., Chicago, IL, USA).
3 Results
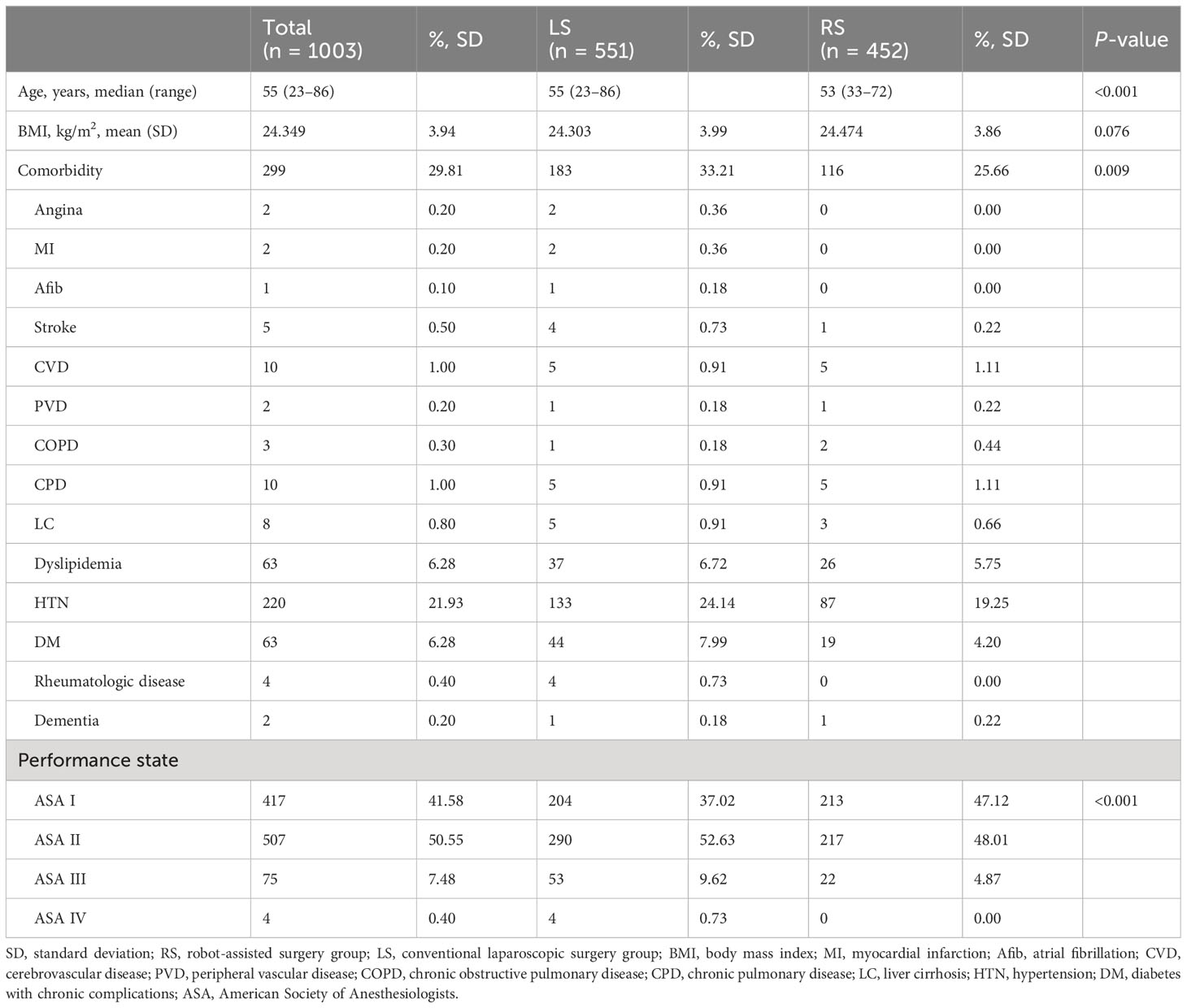
A total of 1,003 patients who underwent surgical treatment for endometrial cancer during the study window were identified from five participating centers: 551 (54.9%) and 452 (45.1%) patients underwent LS and RS, respectively. Based on the number of ports, 456 (45.5%), 95 (9.5%), 419 (41.8%), and 33 (3.3%) patients were classified into the mLS, sLS, mRS, and sRS groups, respectively. The average age of the patients was 55 years at the time of surgery, and the RS group was significantly younger, with fewer overall comorbidities and a more favorable performance status as compared with the LS group. There were no significant differences in body mass index. Patient characteristics, including comorbidities and performance status, are presented in Table 1. Patient characteristics classified according to the number of ports utilized are demonstrated in Supplementary Table 1.
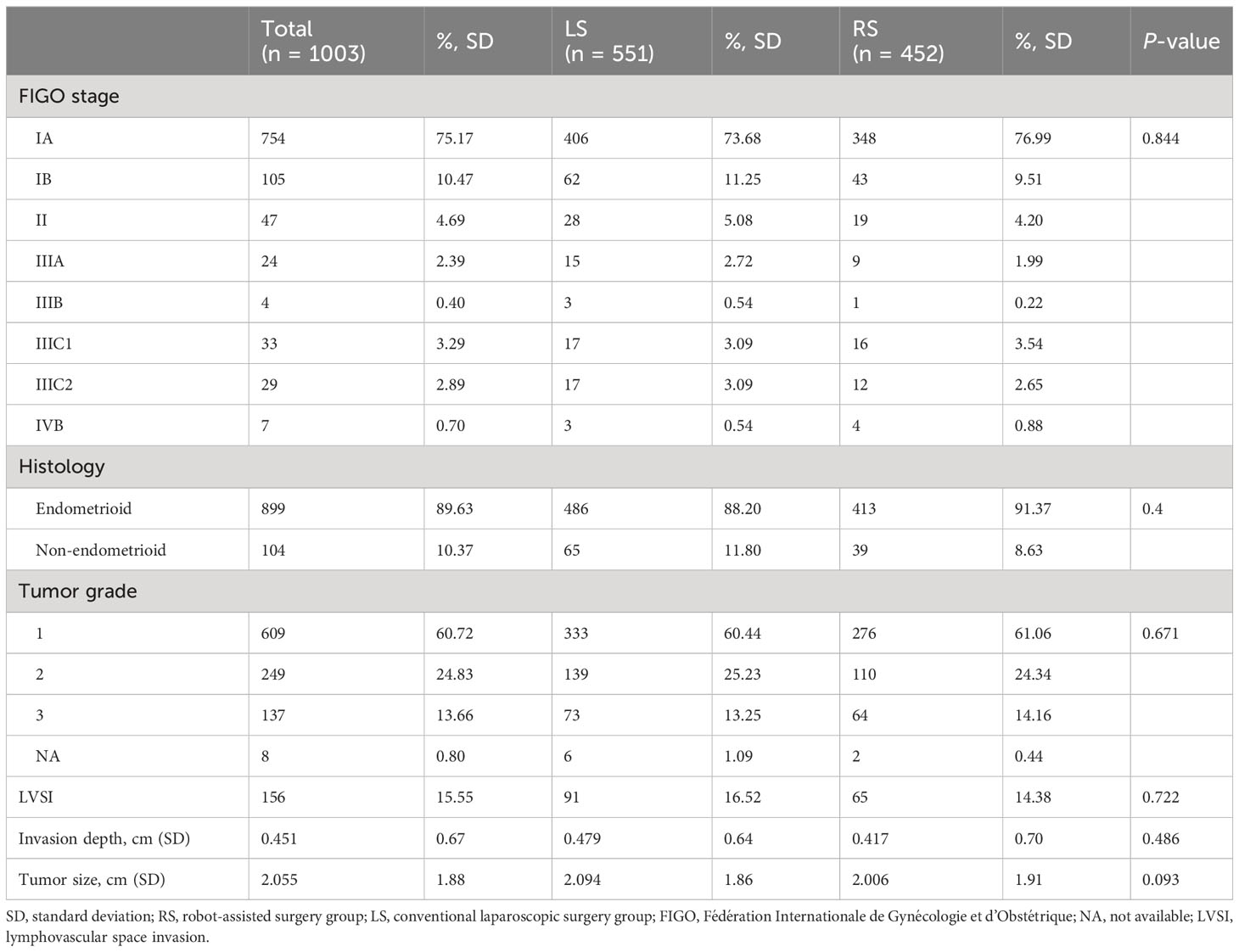
Surgical staging, including total hysterectomy and peritoneal lavage with or without lymph node biopsy, was performed for all the included patients. There were no significant differences in the FIGO stage, histologic type, tumor grade, lympho-vascular space invasion (LVSI), invasion depth, and tumor size between the two cohorts (Table 2). No significant variations in the pathologic outcomes were observed in relation to the number of ports utilized, as per the data presented in Supplementary Table 2.
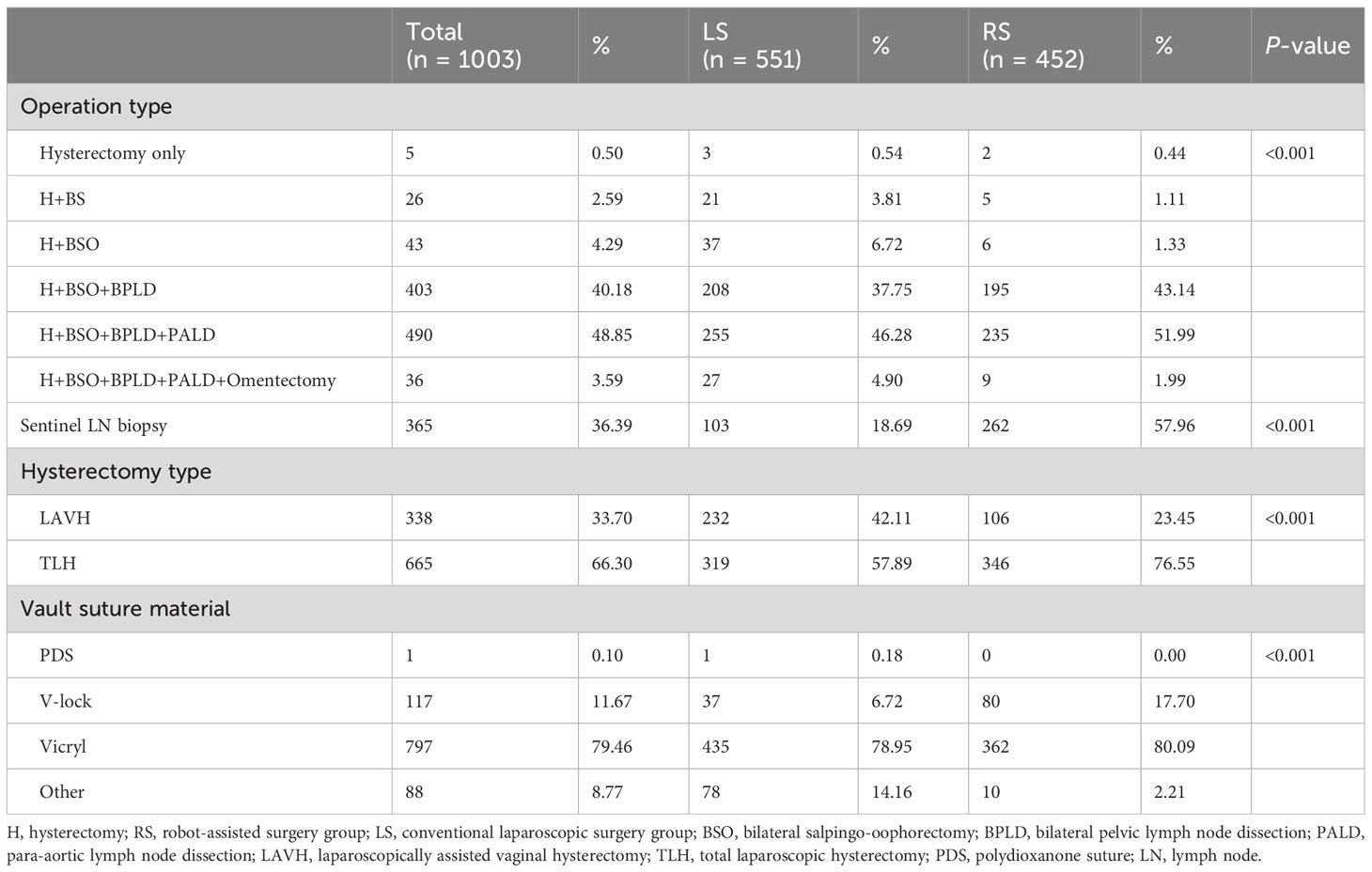
Compared with the LS group, the RS group underwent more complicated procedures, including para-aortic lymph node dissection (54.0% vs. 51.2%). Sentinel lymph node sampling was performed more frequently in the RS group as compared with the LS group (58.0% vs. 18.7%; P < 0.001). Total laparoscopic hysterectomy was performed more frequently in the RS group (76.6% vs. 57.9; P < 0.001). Most vault sutures were performed using vicryl; however, V-lock sutures were more common in the RS group than in the LS group (17.7% vs. 6.7%) (Table 3). The operative description in correlation to the number of ports utilized during the procedure is presented in the Supplementary Table 3.
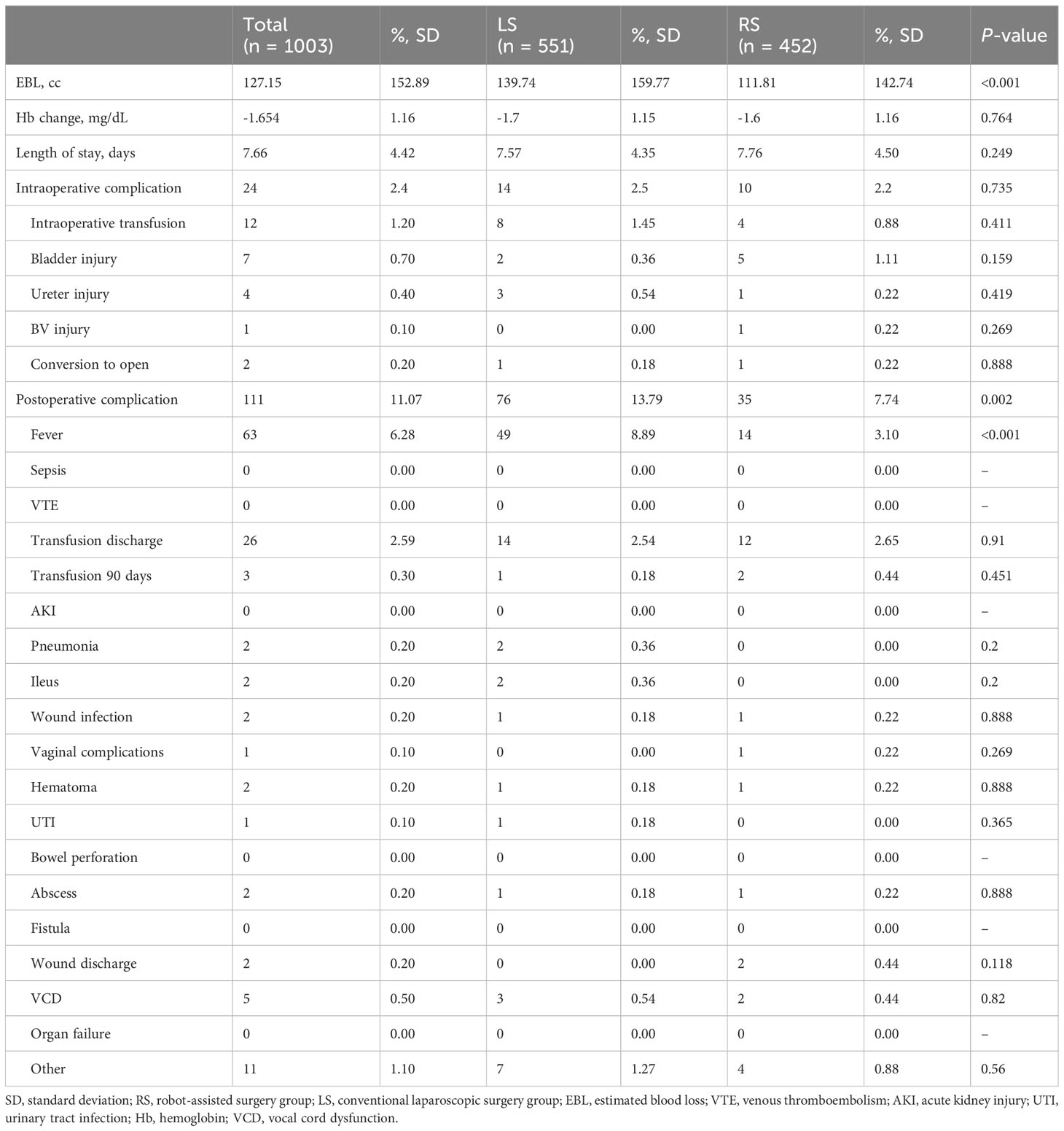
Significantly less blood loss was observed during surgery in the RS group compared to the LS group (111.8 cc vs. 139.4 cc; P < 0.001). However, this reduction in blood loss did not result in a significant change in hemoglobin levels, likely due to minimal bleeding in both groups. There was no difference in the incidence of intraoperative complications between the two groups. Compared with the LS group, postoperative complications were significantly less in the RS group compared with the LS group (7.7% vs. 13.8%; P = 0.002), especially postoperative fever (3.1% vs. 8.9% in the LS and RS groups, respectively; P < 0.001) (Table 4). Supplementary Table 4 presents the surgical results according to the number of ports utilized. Detailed Grade 3 postoperative complications are presented in Supplementary Table 5.
The median follow-up periods were 57 and 49 months in the RS cohort and 60 months in the LS cohort. Ninety patients (9.0%) experienced recurrence during the study period. These included 53 (9.6%) and 37 (8.2%) patients in the LS and RS groups, respectively. The median time to first recurrence was 15 and 12 months in the RS and LS groups, respectively (P = 0.368). Overall, 66.0% of recurrences in the LS and 70.2% in the RS cohort occurred less than 24 months after surgery (P = 0.608). There was no significant difference in the 5-year DFS in the RS and LS cohorts (91.2% vs. 90.0%, respectively; P = 0.628) (Figure 1). A total of 26 patients died during the study period, including 17 (3.1%) and 9 (2.0%) patients in the LS and RS groups, respectively. There was no significant difference in the 5-year overall rate of 97.9% vs. 96.8% for the RS and the LS groups, respectively (P = 0.285) (Figure 1). No significant variations were observed in the DFS and OS between the mLS, sLS, mRS, and sRS groups, as per the data presented in the Supplementary Figure 1. The 5-year DFS rates in the mLS, sLS, mRS, and sRS groups were 89.2%, 93.6%, 90.6%, and 100.0%, respectively. Similarly, the 5-year OS rates in the mLS, sLS, mRS, and sRS groups were 96.6%, 97.9%, 97.7%, and 100.0%, respectively (Supplementary Figure 1).
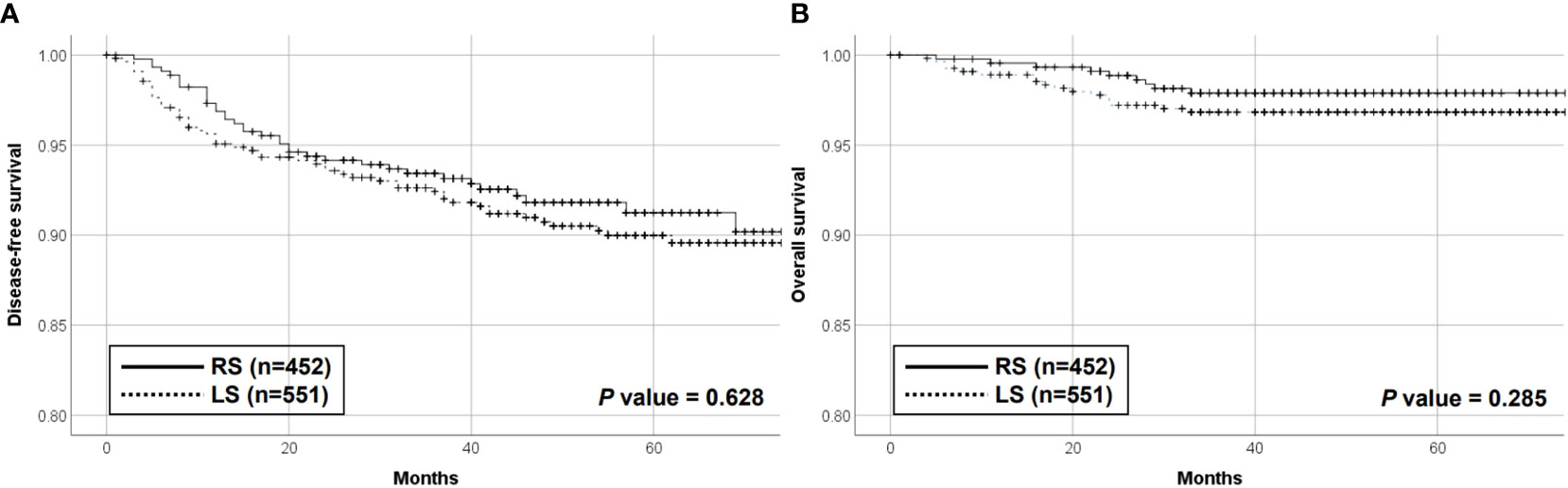
Figure 1 Disease-free survival (A) and overall survival (B) of the RS and LS groups. RS, robot-assisted surgery group; LS, conventional laparoscopic surgery group.
Cox proportional hazards regression models demonstrated that the mode of surgery was not associated with DFS (HR, 0.897; confidence interval [CI], 0.563–1.429; P = 0.648) or OS (HR, 0.791; CI, 0.330–1.895; P = 0.598) after adjusting for confounding factors including age, comorbidity, FIGO stage, grade, and LVSI (Table 5).
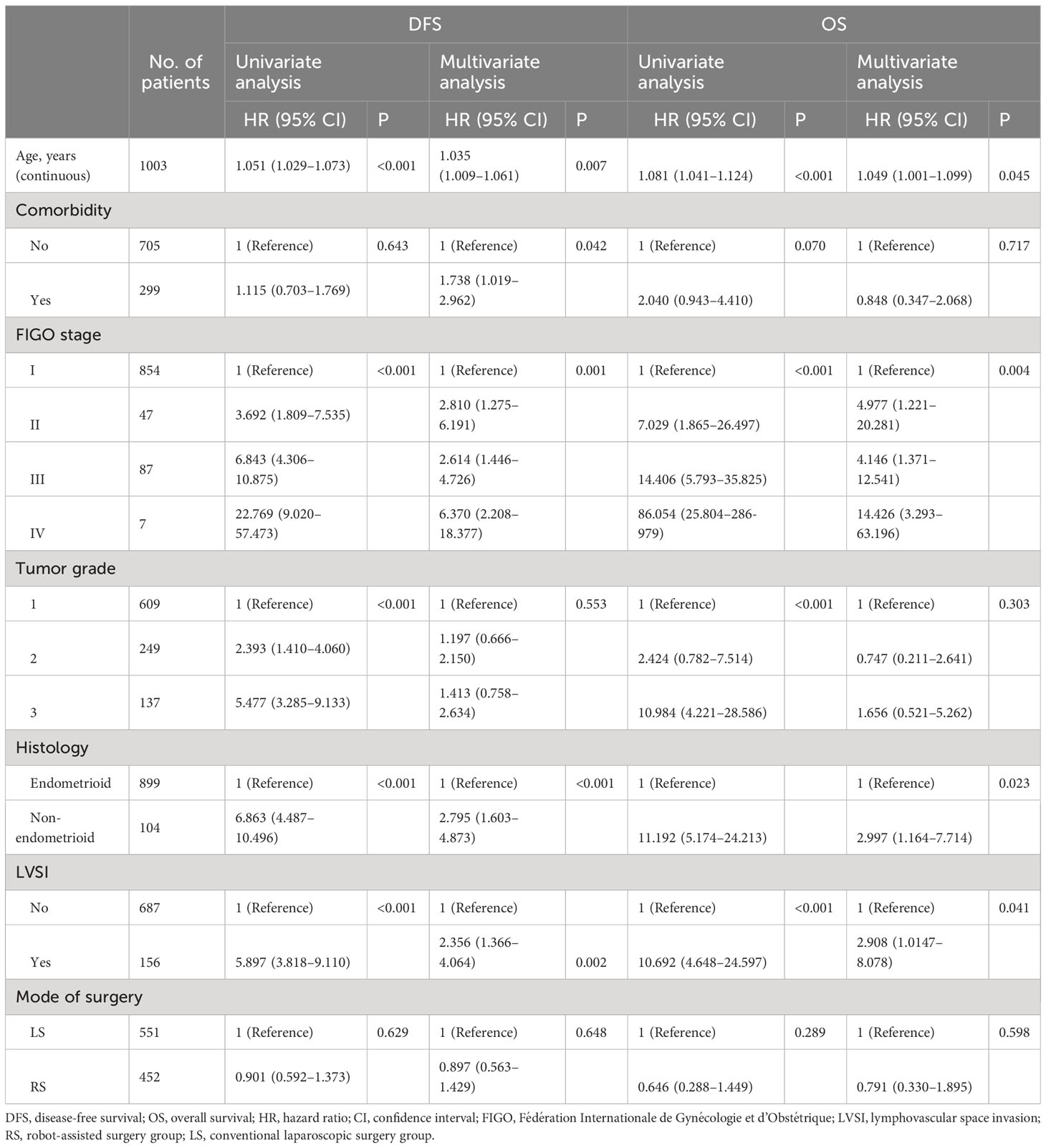
Table 5 Univariate and multivariate analysis of various factors for progression-free survival and overall survival.
4 Discussion
4.1 Principle findings
This study compared the long-term oncologic outcomes between RS and conventional LS in the era of a shift in the standard of care in endometrial cancer management toward MIS, after the introduction of robotic surgery in the field of gynecologic oncology (12). We observed that RS did not compromise survival outcomes when compared with conventional LS for endometrial cancer. In addition, RS was associated with significantly fewer postoperative complications when compared with LS. To the best of our knowledge, this is the largest multicenter study to compare oncologic and operative outcomes according to the modes of surgery used for the treatment of endometrial cancer in this shifting era of surgical procedures.
4.2 Results in the context of what is known
MIS, which includes laparoscopy and robotic surgical approaches, has significantly improved the management of endometrial cancer, and has largely replaced open surgery. MIS offers various advantages, such as reducing intra- and postoperative complications, improving patient satisfaction, and demonstrating cost efficacy (13). The introduction of robotic surgery has further enhanced surgical precision, visualization, and maneuverability, resulting in lower blood loss, reduced postoperative complications, and comparable oncological outcomes. This advancement has led to notable changes in real-world practice, particularly in high-volume hospitals. While two randomized controlled trials have reported reduced surgical complications with MIS in endometrial cancer, there is limited data available for a direct comparison between RS and LS for this condition (14, 15).
Our study encompassing 1,003 Korean patients demonstrated that patients who underwent RS were significantly less likely to develop postoperative complications as compared with those who underwent LS, which is in accordance with the results of previous studies (8, 16). This could be due to the advantages of RS, including binocular three-dimensional view, additional degrees of rotational freedom, and decreased reliance on skilled assistance. Thus, RS is gentler, causes only minor damage to the internal organs, produces less postoperative pain, and aids in faster return to a normal diet and ambulation (17, 18). Moreover, in the RS group, para-aortic lymph node dissection or sentinel lymph node sampling, which is important for staging, was performed more frequently than that in the LS group. This could be due to the Firefly technology of the robotic system (19, 20).
4.3 Clinical implications
Strict evaluation is necessary for any changes in the surgical approach for cancer patients to ensure that the long-term survival outcomes are not compromised. Regarding the survival outcomes of MIS for endometrial cancer, most of the available studies compared laparotomic surgery with LS rather than with RS and demonstrated that laparoscopy could be a non-inferior alternative to the traditional laparotomic approach (3, 21). Several previous studies have reported comparative survival outcomes between RS and LS for endometrial cancer (22–28). Cardenas-Goicoechea et al. observed that there were no significant differences in survival between the RS and LS cohorts (3-year PFS was 88.4% and 83.3% and 3-year OS was 93.6% and 93.3% for the LS and RS groups, respectively) (27). Similarly, Corrado et al. demonstrated that the 3-year OS was 88.4% and 91.5% and the 3-year PFS was 91.7% and 91.5% for the LS and RS groups, respectively (26). Additionally, Brudie et al. noted a 3-year PFS of 89.3% and 3-year OS of 89.1% (28), and Kilgore et al. reported a 5-year OS of 89.1% (29) in patients who underwent RS for endometrial cancer. However, the previous studies had follow-up periods ranging from 17.7 months to 47 months. These durations may have been insufficient to detect significant proportions of the DFS and OS events, unlike our study, which had a longer follow-up period of 57 months.
In Korea, the first Ministry of Food and Drug Safety approval of the da Vinci Surgical system for hysterectomy was granted in 2005, and these devices have been utilized in clinical practice since 2006 (30).
4.4 Research implications
A previous Korean nationwide cohort study encompassing 5,065 patients from 2012 to 2016 provided evidence that RS is a safe surgical alternative to LS, demonstrating comparable survival outcomes (5-year PFS, 93.1%; 5-year OS, 94.8%) in the RS group (5). Nevertheless, variables such as surgical stage or cell types, which may influence the oncologic outcomes of endometrial cancer, could not be considered in population-based research.
Our study included all the detailed clinical and pathologic variables from high-volume hospitals where robotic surgery procedures are actively performed, indicating that the adoption of RS did not compromise the long-term survival outcomes compared with conventional MIS after adjusting for factors including age, comorbidity, FIGO stage, grade, and LVSI.
4.5 Strengths and limitations
The strengths of our study are its long-term follow-up and large sample size. Although randomized controlled trials have excellent internal validity, they do not necessarily determine the impact of a specific surgical method in real-world patients as frail subgroups tend to be excluded owing to the strict inclusion criteria for enrollment (31). We believe that our data are reliable for comparing the operative and oncologic outcomes in the real clinical practice environment. This is because we analyzed well-collected clinicopathological data of unselected patients from the five largest hospitals in Korea, where both RS and LS were actively performed for endometrial cancer. Meanwhile, the retrospective nature of our study and unmeasured confounding variables are the major limitations of this study. Due to the collection of data from five different hospitals, there were substantial differences in patient characteristics, such as age, comorbidity, and performance state, making it challenging to directly accept the survival rate comparison. Consequently, it was necessary to rely on Cox regression multivariate analysis to make reasonable estimations. Potential selection bias, especially owing to the selection of patients who can afford to undergo RS, may also exist. The results of the current study should be interpreted in the context of these limitations.
Robot-assisted surgery for endometrial cancer exhibits comparable long-term survival outcomes but a lower occurrence of postoperative minor complications compared to conventional laparoscopic surgery.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Yonsei university of Korea (Approval No. 4-2021-0988). Written informed consent was not provided because the requirement of informed consent was waived due to the retrospective nature of the study.
Author contributions
KE: writing original draft, review, and editing. T-JK: conceptualization, data curation, resources, and editing. J-YP: data curation, and resources. HK: data curation, resources, and editing. JP: data curation, resources, and editing. YK: conceptualization, data curation, resources, supervision, writing, and editing. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that this study received funding from Intuitive Surgical Inc. The funder was not involved in the study design, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1219371/full#supplementary-material
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin (2019) 69(1):7–34. doi: 10.3322/caac.21551
2. Wright JD, Barrena Medel NI, Sehouli J, Fujiwara K, Herzog TJ. Contemporary management of endometrial cancer. Lancet (2012) 379(9823):1352–60. doi: 10.1016/s0140-6736(12)60442-5
3. Walker JL, Piedmonte MR, Spirtos NM, Eisenkop SM, Schlaerth JB, Mannel RS, et al. Recurrence and survival after random assignment to laparoscopy versus laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group LAP2 Study. J Clin Oncol (2012) 30(7):695–700. doi: 10.1200/jco.2011.38.8645
4. Walker JL, Piedmonte MR, Spirtos NM, Eisenkop SM, Schlaerth JB, Mannel RS, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol (2009) 27(32):5331. doi: 10.1200/JCO.2009.22.3248
5. Eoh KJ, Nam EJ, Kim SW, Shin M, Kim SJ, Kim JA, et al. Nationwide comparison of surgical and oncologic outcomes in endometrial cancer patients undergoing robotic, laparoscopic, and open surgery: a population-based cohort study. Cancer Res Treat (2021) 53(2):549–57. doi: 10.4143/crt.2020.802
6. Corrado G, Vizza E, Cela V, Mereu L, Bogliolo S, Legge F, et al. Laparoscopic versus robotic hysterectomy in obese and extremely obese patients with endometrial cancer: A multi-institutional analysis. Eur J Surg Oncol (2018) 44(12):1935–41. doi: 10.1016/j.ejso.2018.08.021
7. Corrado G, Vizza E, Perrone AM, Mereu L, Cela V, Legge F, et al. Comparison between laparoscopic and robotic surgery in elderly patients with endometrial cancer: a retrospective multicentric study. Front Oncol (2021) 11:724886. doi: 10.3389/fonc.2021.724886
8. Bergstrom J, Aloisi A, Armbruster S, Yen T-T, Casarin J, Leitao MM Jr., et al. Minimally invasive hysterectomy surgery rates for endometrial cancer performed at National Comprehensive Cancer Network (NCCN) Centers. Gynecol Oncol (2018) 148(3):480–4. doi: 10.1016/j.ygyno.2018.01.002
9. Casarin J, Song C, Multinu F, Cappuccio S, Liu E, Butler KA, et al. Implementing robotic surgery for uterine cancer in the United States: better outcomes without increased costs. Gynecol Oncol (2020) 156(2):451–8. doi: 10.1016/j.ygyno.2019.11.016
10. Vuorinen RK, Maenpaa MM, Nieminen K, Tomas EI, Luukkaala TH, Auvinen A, et al. Costs of robotic-assisted versus traditional laparoscopy in endometrial cancer. Int J Gynecol Cancer (2017) 27(8):1788–93. doi: 10.1097/igc.0000000000001073
11. Ran L, Jin J, Xu Y, Bu Y, Song F. Comparison of robotic surgery with laparoscopy and laparotomy for treatment of endometrial cancer: a meta-analysis. PloS One (2014) 9(9):e108361. doi: 10.1371/journal.pone.0108361
12. Lau S, Vaknin Z, Ramana-Kumar AV, Halliday D, Franco EL, Gotlieb WH. Outcomes and cost comparisons after introducing a robotics program for endometrial cancer surgery. Obstet Gynecol (2012) 119(4):717–24. doi: 10.1097/AOG.0b013e31824c0956
13. Kornblith AB, Huang HQ, Walker JL, Spirtos NM, Rotmensch J, Cella D. Quality of life of patients with endometrial cancer undergoing laparoscopic international federation of gynecology and obstetrics staging compared with laparotomy: a Gynecologic Oncology Group study. J Clin Oncol (2009) 27(32):5337. doi: 10.1200/JCO.2009.22.3529
14. Walker JL, Piedmonte MR, Spirtos NM, Eisenkop SM, Schlaerth JB, Mannel RS, et al. Recurrence and survival after random assignment to laparoscopy versus laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group LAP2 Study. J Clin Oncol (2012) 30(7):695. doi: 10.1200/JCO.2011.38.8645
15. Janda M, Gebski V, Davies LC, Forder P, Brand A, Hogg R, et al. Effect of total laparoscopic hysterectomy vs total abdominal hysterectomy on disease-free survival among women with stage I endometrial cancer: a randomized clinical trial. Jama (2017) 317(12):1224–33. doi: 10.1001/jama.2017.2068
16. Salehi S, Åvall-Lundqvist E, Legerstam B, Carlson JW, Falconer H. Robot-assisted laparoscopy versus laparotomy for infrarenal paraaortic lymphadenectomy in women with high-risk endometrial cancer: a randomised controlled trial. Eur J Cancer (2017) 79:81–9. doi: 10.1016/j.ejca.2017.03.038
17. Jorgensen SL, Mogensen O, Wu C, Lund K, IaChina M, Korsholm M, et al. Nationwide introduction of minimally invasive robotic surgery for early-stage endometrial cancer and its association with severe complications. JAMA Surg (2019) 154(6):530–8. doi: 10.1001/jamasurg.2018.5840
18. Galaal K, Donkers H, Bryant A, Lopes AD. Laparoscopy versus laparotomy for the management of early stage endometrial cancer. Cochrane Database Syst Rev (2018) 10:Cd006655. doi: 10.1002/14651858.CD006655.pub3
19. Saotome K, Yamagami W, Machida H, Ebina Y, Kobayashi Y, Tabata T, et al. Impact of lymphadenectomy on the treatment of endometrial cancer using data from the JSOG cancer registry. Obstet Gynecol Sci (2021) 64(1):80–9. doi: 10.5468/ogs.20186
20. Kim YN, Kim YT. Sentinel lymph node biopsy in high-risk endometrial cancer: performance, outcomes, and future avenues. Obstet Gynecol Sci (2022) 65(5):395–405. doi: 10.5468/ogs.22146
21. Siesto G, Uccella S, Ghezzi F, Cromi A, Zefiro F, Serati M, et al. Surgical and survival outcomes in older women with endometrial cancer treated by laparoscopy. Menopause (2010) 17(3):539–44. doi: 10.1097/gme.0b013e3181c4e9f5
22. Argenta PA, Mattson J, Rivard CL, Luther E, Schefter A, Vogel RI. Robot-assisted versus laparoscopic minimally invasive surgery for the treatment of stage I endometrial cancer. Gynecol Oncol (2022) 165(2):347–52. doi: 10.1016/j.ygyno.2022.03.007
23. Chambers LM, Carr C, Freeman L, Jernigan AM, Michener CM. Does surgical platform impact recurrence and survival? A study of utilization of multiport, single-port, and robotic-assisted laparoscopy in endometrial cancer surgery. Am J Obstet Gynecol (2019) 221(3):243. doi: 10.1016/j.ajog.2019.04.038
24. Perrone E, Capasso I, Pasciuto T, Gioè A, Alletti SG, Restaino S, et al. Laparoscopic vs. robotic-assisted laparoscopy in endometrial cancer staging: large retrospective single-institution study. J Gynecol Oncol (2021) 32(3):e45. doi: 10.3802/jgo.2021.32.e45
25. Cardenas-Goicoechea J, Shepherd A, Momeni M, Mandeli J, Chuang L, Gretz H, et al. Survival analysis of robotic versus traditional laparoscopic surgical staging for endometrial cancer. Am J Obstet Gynecol (2014) 210(2):160. e1–. e11. doi: 10.1016/j.ajog.2013.10.871
26. Corrado G, Cutillo G, Pomati G, Mancini E, Sperduti I, Patrizi L, et al. Surgical and oncological outcome of robotic surgery compared to laparoscopic and abdominal surgery in the management of endometrial cancer. Eur J Surg Oncol (2015) 41(8):1074–81. doi: 10.1016/j.ejso.2015.04.020
27. Cardenas-Goicoechea J, Shepherd A, Momeni M, Mandeli J, Chuang L, Gretz H, et al. Survival analysis of robotic versus traditional laparoscopic surgical staging for endometrial cancer. Am J Obstet Gynecol (2014) 210(2):160.e1–.e11. doi: 10.1016/j.ajog.2013.10.871
28. Brudie LA, Backes FJ, Ahmad S, Zhu X, Finkler NJ, GEt B, et al. Analysis of disease recurrence and survival for women with uterine Malignancies undergoing robotic surgery. Gynecol Oncol (2013) 128(2):309–15. doi: 10.1016/j.ygyno.2012.11.005
29. Kilgore JE, Jackson AL, Ko EM, Soper JT, Van Le L, Gehrig PA, et al. Recurrence-free and 5-year survival following robotic-assisted surgical staging for endometrial carcinoma. Gynecol Oncol (2013) 129(1):49–53. doi: 10.1016/j.ygyno.2012.12.020
30. Kim YT, Kim SW, Hyung WJ, Lee SJ, Nam EJ, Lee WJ. Robotic radical hysterectomy with pelvic lymphadenectomy for cervical carcinoma: a pilot study. Gynecol Oncol (2008) 108(2):312–6. doi: 10.1016/j.ygyno.2007.10.015
Keywords: endometrial neoplasms, robotic surgical procedures, laparoscopy, mortality, postoperative complications
Citation: Eoh KJ, Kim T-J, Park J-Y, Kim HS, Paek J and Kim YT (2023) Robot-assisted versus conventional laparoscopic surgery for endometrial cancer: long-term comparison of outcomes. Front. Oncol. 13:1219371. doi: 10.3389/fonc.2023.1219371
Received: 09 May 2023; Accepted: 17 August 2023;
Published: 15 September 2023.
Edited by:
Petra Zusterzeel, Radboud University Medical Centre, NetherlandsReviewed by:
Carlo De Cicco Nardone, Campus Bio-Medico University Hospital, ItalyGiacomo Corrado, Department of Woman and Child Health and Public Health, Agostino Gemelli University Polyclinic (IRCCS), Italy
Copyright © 2023 Eoh, Kim, Park, Kim, Paek and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Young Tae Kim, eXRrY2hvaUB5dWhzLmFj
 Kyung Jin Eoh
Kyung Jin Eoh Tae-Joong Kim
Tae-Joong Kim Jeong-Yeol Park
Jeong-Yeol Park Hee Seung Kim
Hee Seung Kim Jiheum Paek
Jiheum Paek Young Tae Kim6*
Young Tae Kim6*