- Department of Rehabilitation Medicine, General Hospital of Northern Theater Command, Shenyang, China
Osteosarcoma (OS) is a primary malignant bone tumor that occurs in children and adolescents, and the PI3K/AKT pathway is overactivated in most OS patients. MicroRNAs (miRNAs) are highly conserved endogenous non-protein-coding RNAs that can regulate gene expression by repressing mRNA translation or degrading mRNA. MiRNAs are enriched in the PI3K/AKT pathway, and aberrant PI3K/AKT pathway activation is involved in the development of osteosarcoma. There is increasing evidence that miRNAs can regulate the biological functions of cells by regulating the PI3K/AKT pathway. MiRNA/PI3K/AKT axis can regulate the expression of osteosarcoma-related genes and then regulate cancer progression. MiRNA expression associated with PI3K/AKT pathway is also clearly associated with many clinical features. In addition, PI3K/AKT pathway-associated miRNAs are potential biomarkers for osteosarcoma diagnosis, treatment and prognostic assessment. This article reviews recent research advances on the role and clinical application of PI3K/AKT pathway and miRNA/PI3K/AKT axis in the development of osteosarcoma.
1 Introduction
Osteosarcoma (OS) is a primary malignant bone tumor derived from bone forming mesenchymal stem cells. It is highly malignant and can be locally aggressive and often leading to pulmonary or even systemic metastases. Children and adolescents are the most common patients with osteosarcoma, second only to lymphoma and brain tumors in the childhood and adolescent population (1–3). The distal end of the femur is the most common site for osteosarcoma, followed by the proximal end of the tibia and humerus (2, 4–6). Local invasion is observed in more than 85% of osteosarcoma patients, with lung metastases being the most common in 74% of patients with metastases, followed by bone metastases in 9% of patients, and both bone and lung metastases in 8% of patients with metastases (6). In recent decades, surgery combined with new chemotherapy has been recognized as the standard treatment for osteosarcoma, significantly improving overall survival and quality of life (7). Emerging chemotherapy regimens include cisplatin (DDP), adriamycin (DOX), methotrexate (MTX), and isocyclophosphamide (IFO) (7). However, the therapeutic effect of chemotherapeutic agents is limited by various reasons, such as escape of apoptosis, reduced drug uptake, and increased drug metabolism. Systemic metastasis limits the effectiveness of surgical resection, so metastatic and drug resistance often result in unsatisfactory outcomes and prognosis for patients with osteosarcoma (8). The problems described above involve changes in multiple biological processes, including changes in genetic and epigenetic characteristics. Understanding and studying the molecular changes of genes associated with the formation of osteosarcoma and associated signaling pathways will help uncover the mechanisms underlying its occurrence and development, providing new directions for the diagnosis, targeted therapy, and prognosis of osteosarcoma.
More than 98% of the genes in the human genome are composed of noncoding genes (9–11). Since they lack the ability to encode proteins, their transcripts are considered non-coding Rna (ncRNA) (12, 13). With the development of high-throughput sequencing technology, the characteristics of ncRNAs have gradually emerged, and microRNA (miRNA, miR), long ncrna (lncRNA) and circular RNAs (circRNA) are considered as classical ncRNA (14–16). Among them, miRNA is a conserved endogenous non-coding RNA of approximately 22 nucleotides in length (17, 18). MiRNA can bind to the untranslated region (UTR) of mRNA and regulate the expression of target genes by inhibiting the translation or degradation of mRNA, thereby affecting a variety of intracellular signaling pathways and playing an important role in the formation of tumors (19). The abnormal expression of miRNA in tumor cells can affect the malignant biological behavior of tumor cells (20–23), and osteosarcoma is no exception. MiRNA have a very important role in the development of osteosarcoma and are clearly associated with many clinical features (24–27). MiRNAs are expected to become effective biomarkers for diagnosis, targeted therapy and prognosis of osteosarcoma patients.
It is well known that the PI3K/AKT pathway plays an extremely important role in the life activities of cells (28), It can be activated by insulin growth factors and cytokines under physiological conditions and participates in the regulation of a variety of intracellular signal transduction and cell biological processes (29), such as cell growth, differentiation, transcriptional regulation, protein synthesis, metabolism, autophagy, cell proliferation, apoptosis, angiogenesis, migration, and cytoskeletal reorganization (30–36). Aberrant activation of the PI3K/AKT pathway has been observed in almost all types of tumor cells (37–39), such as ovarian cancer (40, 41), lung cancer (42, 43), gastric cancer (44, 45), pancreatic cancer (46, 47), breast cancer (48, 49), hepatocellular carcinoma (50, 51), lymphoma (52, 53), osteosarcoma (54) and so on. Therefore, an in-depth study of the function of PI3K/AKT pathway in carcinogenesis is of great importance.
Recently, there is increasing evidence that the interaction between miRNA and PI3K/AKT pathway has an important role in the biological process of osteosarcoma (55–58). Moreover, this interaction has been found to be significantly associated with many clinical features (59, 60), and these studies provide a new perspective for the diagnosis, targeted therapy and prognosis of osteosarcoma patients. In recent years, the study of miRNA associated with the PI3K/AKT pathway has also been a hot spot for investigating mechanisms related to osteosarcoma development. In this review, we review the molecular mechanisms and functional roles of the miRNA/PI3K/AKT axis in the pathogenesis and progression of osteosarcoma.
2 PI3K/AKT pathway in osteosarcoma
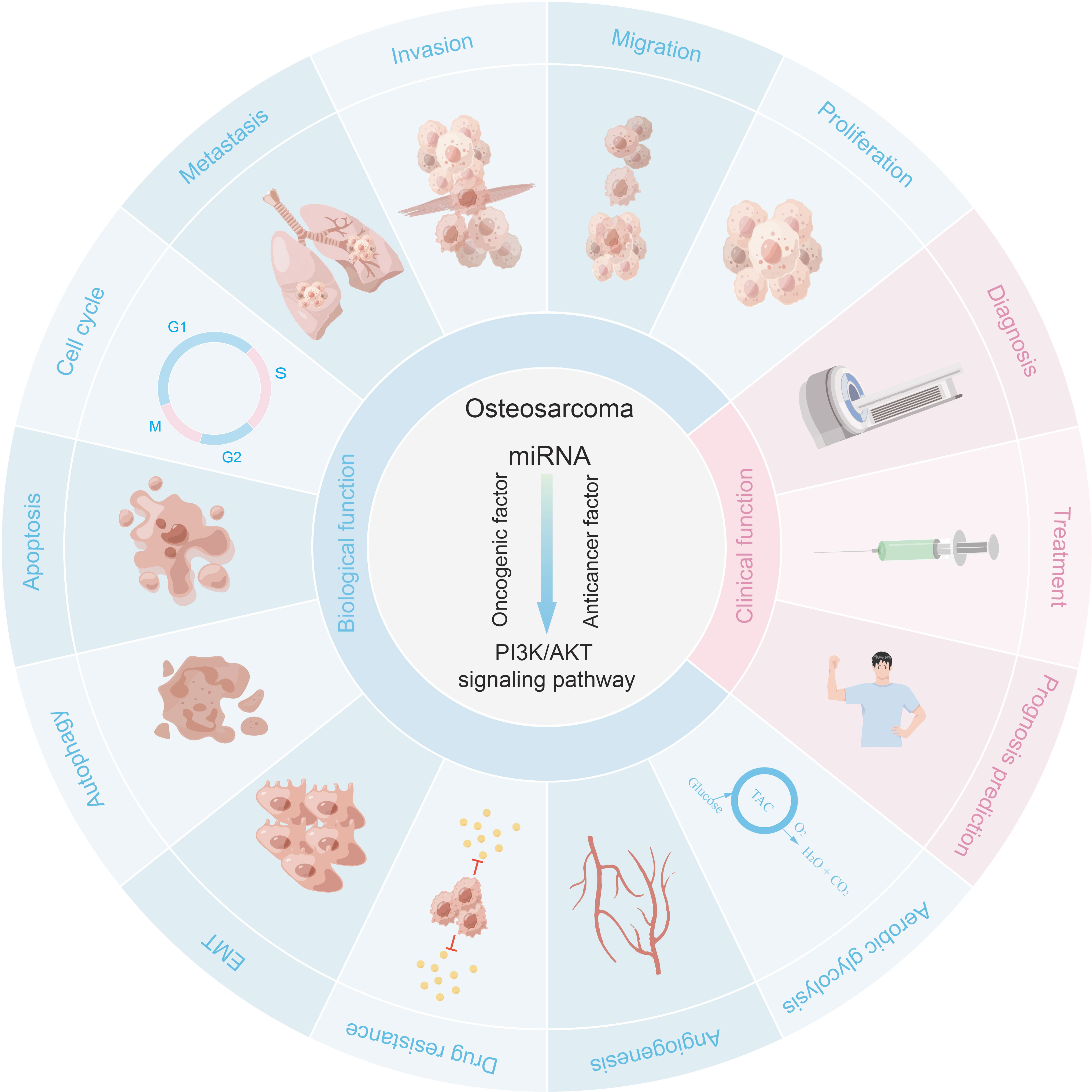
It is well known that aberrant activation of the PI3K/AKT pathway may lead to tumorigenesis. In recent years, a large amount of evidence has shown that dysregulation of PI3K/AKT pathway is involved in a variety of pathological processes of OS, including OS occurrence, proliferation, metastasis, migration, invasion, cell cycle progression, apoptosis, autophagy, angiogenesis, chemoresistance, Epithelial-Mesenchymal Transition (EMT), aerobic glycolysis, etc. This section introduces the PI3K/AKT pathway and outlines the mechanisms involved in the role of PI3K/AKT pathway in the development of osteosarcoma.
2.1 Overview of the PI3K/AKT pathway
Abnormalities in the PI3K/AKT pathway are common in osteosarcoma, and the PI3K/AKT pathway plays a critical role in regulating the growth, proliferation, differentiation, migration, metastasis, infiltration, apoptosis, and drug resistance of osteosarcoma (61–65). It has been demonstrated that the dysregulation of major factors in this signaling pathway in osteosarcoma cells is closely related to the activation and inhibition of other downstream signaling pathways. PI3K, a member of the large family of lipid kinases, is a downstream effector of receptor tyrosine kinases (RTKs) and G protein-coupled receptors (GPCRs) (66). Based on the differences in structure and function of PI3K, it has been classified into three subclasses, class I, II and III (36, 67). Among them, class I PI3K is the most relevant to tumors, and the most intensive research has been conducted on class I. Class I is further divided into class IA and class IB, and they are composed of the p110α, β, γ and δ catalytic subunits encoded by the PIK3CA, PIK3CB, PIK3CG and PIK3CD genes, respectively, and the PIK3R1, PIK3R2 and PIK3R3 genes encoding p85α, p85β and p55γ regulatory subunits of the PIK3R1, PIK3R2 and PIK3R3 genes (68, 69). The binding between subunits not only stabilizes the structure of PI3K but also provides sites for activation of PI3K by RTKs, GPCRs, and oncogenes (e.g. Ras) (36, 67, 70). Various molecules including insulin, epithelial growth factor (EGF), glucose, fibroblast growth factor (FGF) and vascular endothelial growth factor (VEGF) can activate PI3K via RTKs and GPCRs (71, 72). Activated PI3K can convert phosphatidylinositol 3,4-bisphosphate (PIP2) to 3,4,5-trisphosphate (PIP3), and PIP3 can bind to phosphatidylinositol-dependent kinase 1 (PDK1) to phosphorylate AKT (73). During this process, the negative regulator phosphatase and tensin homologue (PTEN) can invert PIP3 to PIP2 to limit the intensity of this activation process (74).
AKT is a serine/threonine kinase encoded by the PKB gene. AKT can lead to the activation of the downstream PI3K/AKT pathway through the phosphorylation of various substrates, including AKT1, AKT2 and AKT3, which is an extremely important protein molecule in the PI3K/AKT pathway (75). Activation of AKT is mainly the result of PDK-1 and mTORC2 phosphorylation at threonine 308 and serine 473, respectively (76, 77).AKT can also be inhibited by dephosphorylation of CTMP, PP2A, and tcl1 (78–80), after which activated AKT is transferred to the cytoplasm and nucleus, where it can activate or inhibit matrix metalloproteinases (MMPs) through phosphorylation and dephosphorylation, cyclic-dependent kinase (CDKs), MDM2, GSK3β, FOXO1, and other downstream substrates (71, 81–83), thus affecting various cellular signaling pathways and metabolic pathways and leading to abnormal life activities in normal cells.
2.2 Role of PI3K/AKT pathway in OS development
2.2.1 Malignant phenotype of osteosarcoma
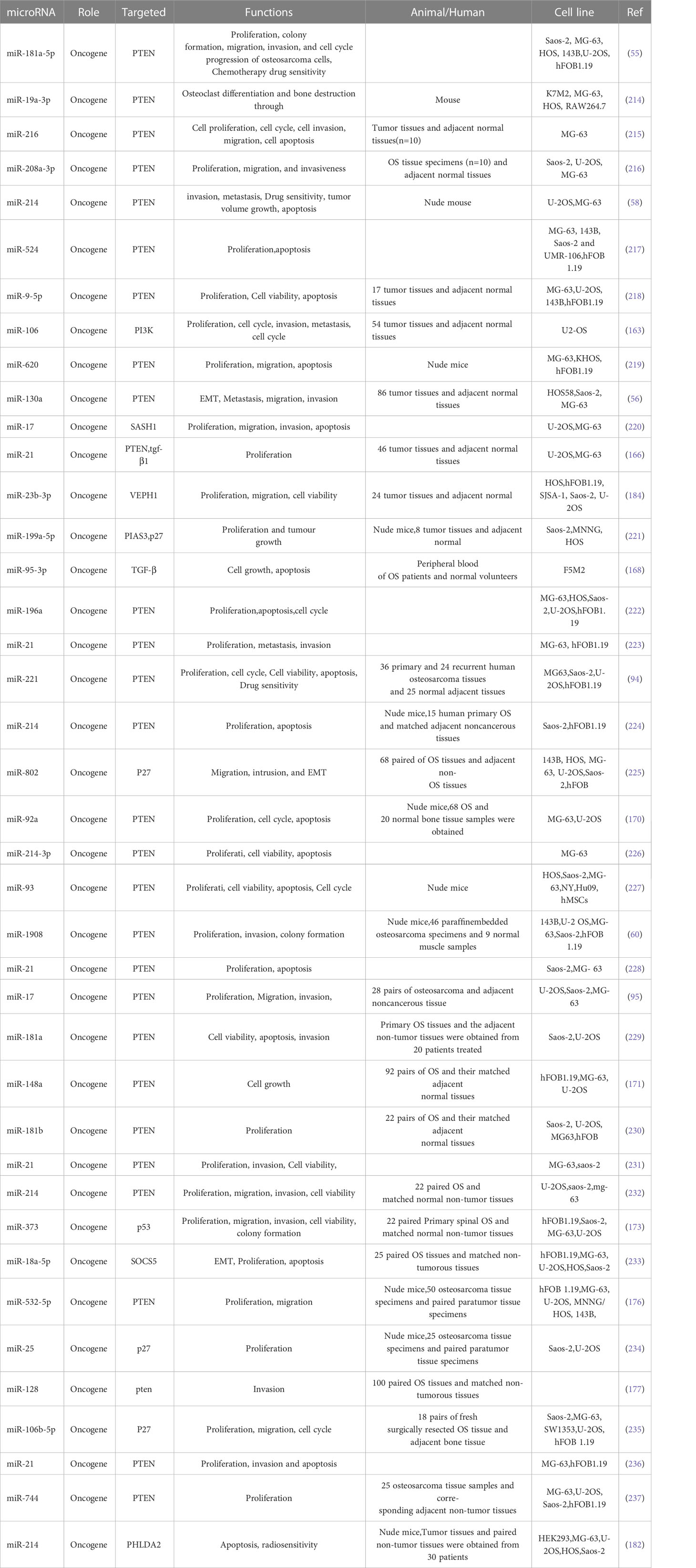
PI3K/AKT pathway and its upstream and downstream related molecules can have a significant impact on the formation of osteosarcoma and the associated malignant phenotype. This evidence strongly suggests the importance of the PI3K/AKT pathway for osteosarcoma formation, as evidenced by whole-genome sequencing analysis of OS cell lines that revealed significant upregulation of AKT expression, followed by significant inhibition of proliferation in all cell lines by the addition of the metamorphic AKT inhibitor MK-2206 (84). The levels of phosphorylated PI3K (p-PI3K) and phosphorylated AKT (p-AKT) are closely related to the activation of PI3K/AKT pathway, downregulation of fatty acid synthase (FAS) significantly reduces the expression levels of p-PI3K and p-AKT, which in turn reduced the proliferation and invasion of U-2OS cells (Figure 1A) (84). Chordin-like 2 (CHRDL2) is one of the Bone morphogenic proteins (BMPs) antagonist that prevents the interaction between BMPs and their cognate cell surface receptors (89). CHRDL2 is highly expressed in osteosarcoma tissues, and it was confirmed experimentally that CHRDL2 promotes the metastasis and proliferation of osteosarcoma cells through the BMP-9/PI3K/AKT pathway (Figure 1B) (86). Cyclooxygenase-2 (COX-2) is a membrane-bound protein closely related to inflammatory diseases. It is an inducible cyclooxygenase and rate-limiting enzyme for prostaglandin synthesis. Most tissue cells do not express COX-2 under physiological conditions, but it shows an increasing trend under pathological conditions such as inflammation and tumor (90). In osteosarcoma, COX-2 affects the expression levels of vimentin, E-cadherin, MMP-9 and MMP-2 by activating the PI3K/AKT/NF-κ b signaling pathway, leading to a significant increase in the migratory ability of osteosarcoma cells (Figure 1C) (87). Studies have reported that glycoprotein non-metastatic melanoma protein B (GPNMB) affects the metastasis of tumor cells in addition to being associated with tissue regeneration, inflammation, and cell proliferation (91). GPNMB may also be a potential target for targeted therapy of osteosarcoma, as it has been found that GPNMB can regulate the metastasis and proliferation of osteosarcoma cells by affecting the PI3K/AKT/mTOR pathway (92). PTEN, the first tumor suppressor gene identified with tyrosine phosphatase activity (93), is a potent negative regulator of AKT and plays a crucial role in controlling PI3K/AKT signaling activation (94). MiR-221, miR-17, and miR-128 overexpression leads to the 3-UTR of PTEN by directly binding diminished inhibition of PTEN and activation of the PI3K/AKT pathway, which significantly promotes OS cell proliferation, migration and invasion (94, 95). In a similar vein, HER4 is a member of the ErbB family, and it has been demonstrated that HER4 can promote osteosarcoma progression in part by affecting the PTEN/PI3K/AKT pathway (Figure 1D) (88). On the other hand, if we block the PI3K/AKT signaling cascade by various means can prevent the development of OS-related malignant phenotypes. A recent study showed that OS cell proliferation was significantly inhibited after Ski knockdown, and in-depth studies revealed a significant decrease in the protein levels of p-PI3K and p-AKT in the cells, thus the mechanism was hypothesized to be the knockdown of Ski blocking the PI3K/AKT pathway (Figure 2A) (62). Melanoma deficiency factor 2 (AIM2) is lowly expressed in osteosarcoma cells, and overexpression of AIM2 inhibits the levels of p-PI3K, p-AKT and p-mTOR thereby suppressing the proliferation, invasion and migration of osteosarcoma cells, a process that can be reversed by LY294002, suggesting that AIM2 is a tumor suppressor (61). However, this study lacks research on the upstream mechanism of AIM2, and no animal experiments have been conducted to further confirm this conclusion. Therefore, it has certain limitations. Molecules such as Schisandrin B (Sch B), Budding uninhibited by benzimidazoles 1 (BUB1), can affect the malignant phenotype of osteosarcoma by activating or inhibiting the PI3K/AKT pathway (100, 101).
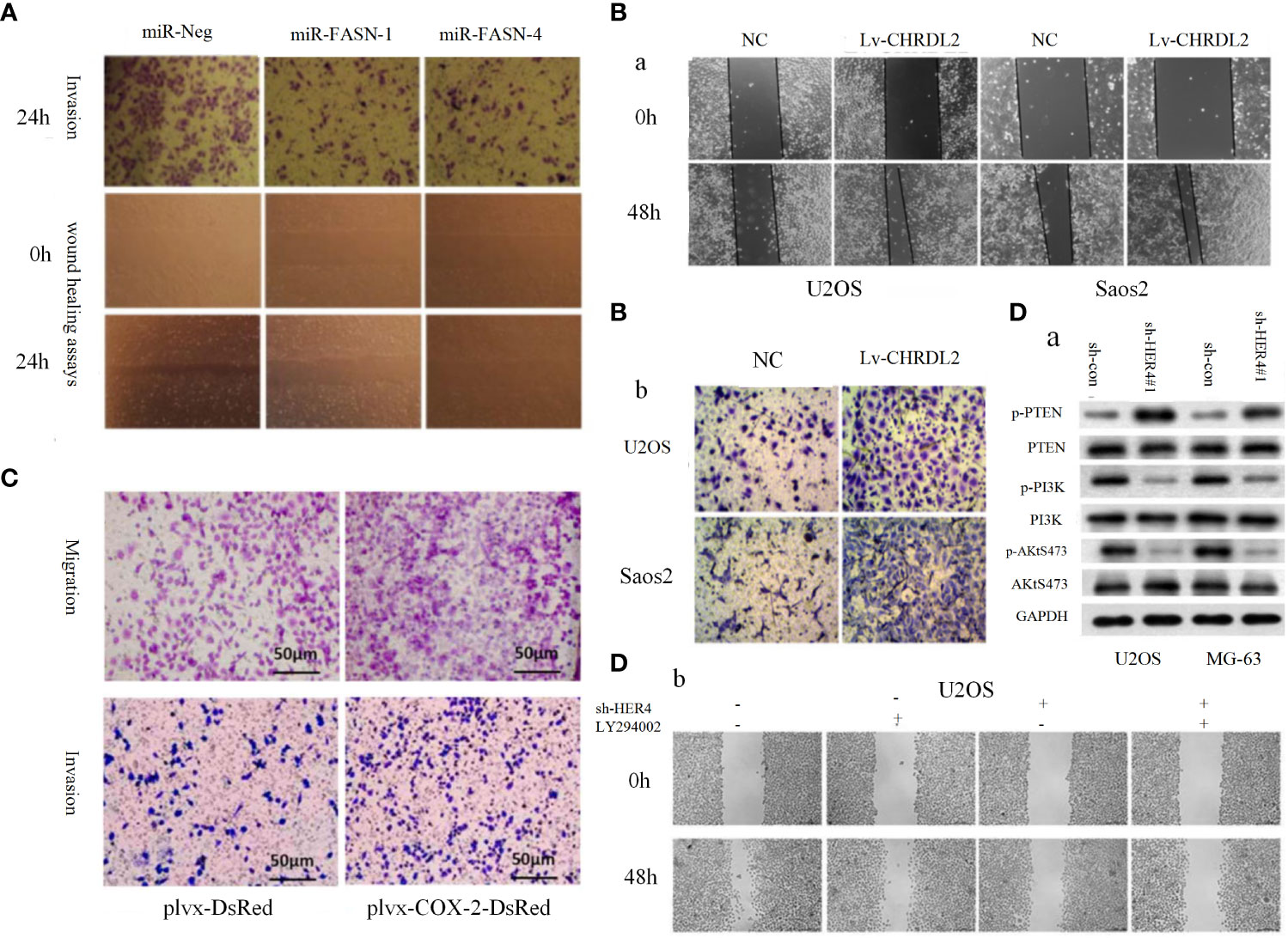
Figure 1 (A) Representative images of the transwell invasion assays and wound healing assays are shown for each group (85). (B) Overexpression of CHRDL2 promoted osteosarcoma cell proliferation and mobility. (a) Wound healing assay. (b) Transwell assay (86). (C) COX-2 overexpression increases migration and invasion in MG-63 cells (87). (D) The role of HER4 in the PI3K/mTOR signaling pathway. (D-a) The western blotting was used to measure the protein expression of p-PI3K, p-AKT, and p-PTEN. (D-b) Wound healing assay was performed to measure the migration ability of cells (88).
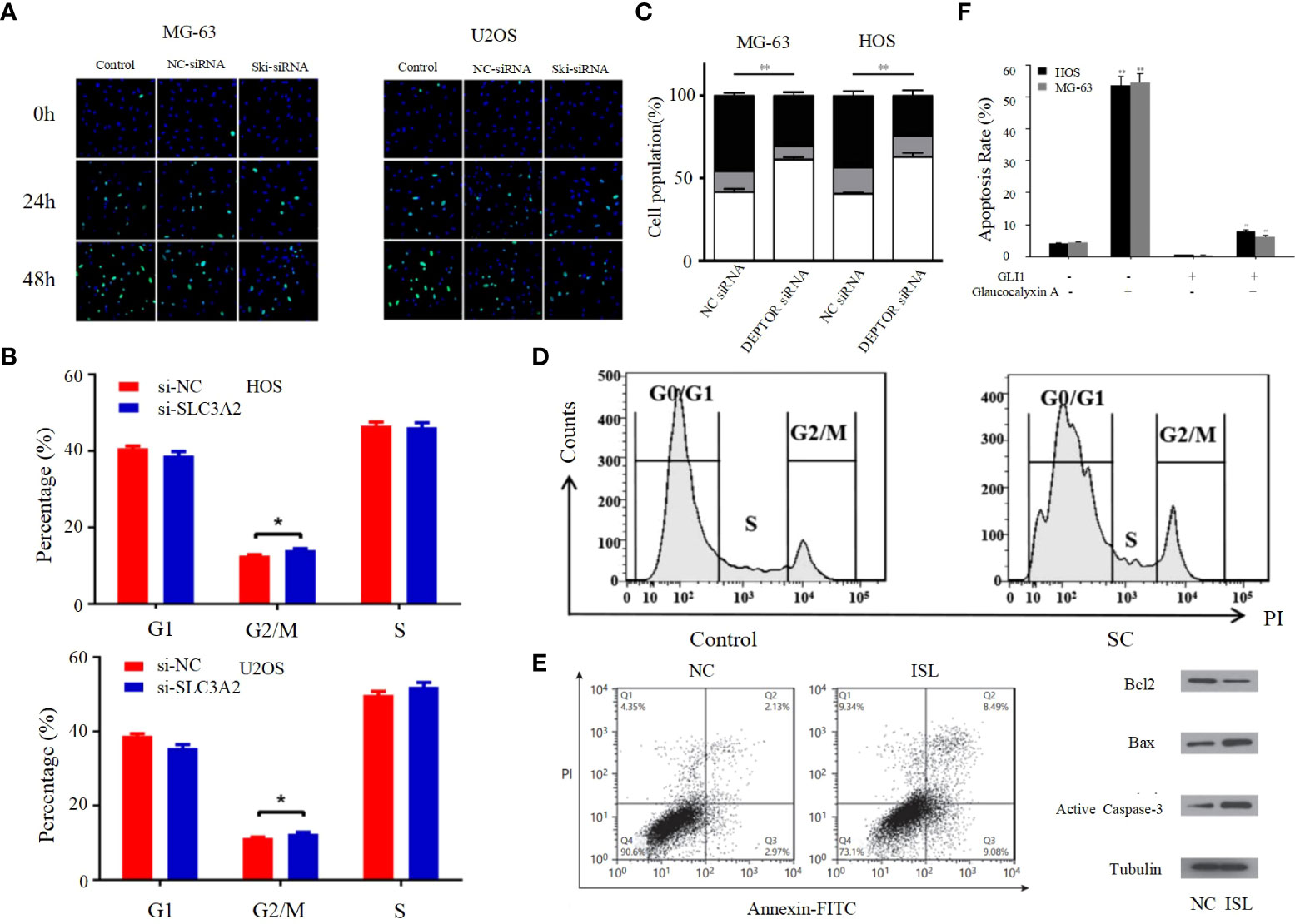
Figure 2 (A) Proliferation rates of MG63/U2OS cells among the control, NC-siRNA and Ski-siRNA groups at 24 and 48 h following transfection (62). (B) Cell cycle profiles determined by propidium iodide (PI) staining and flow cytometry assays of (a) MNNG/HOS and (b) U2OS cells transfected with si-SLC3A2 or si-NC (96). (C) Flow cytometric analysis of the percentage of cells in different phases of the cell cycle with three independent experiments (97). (D) Following SC treatment, cell cycle distribution was determined by flow cytometry at 24 h (98). (E) ISL treatment induces apoptosis in U2OS cells (63). (F) The apoptotic rates of HOS and MG-63 cells were detected by Annexin V/PI double-staining assay (99). *p < 0.05, **p < 0.01.
2.2.2 Cell cycle
The entire process of cell division from the completion of one division to the end of the next is called the cell cycle, and the strictly conserved cell cycle control mechanism is the main regulatory mechanism of cell proliferation; cancer is the result of continuous overdivision of cells, and therefore dysregulation of the cell cycle is closely related to the biological behavior of osteosarcoma (102). The cell cycle consists of interphase and mitotic phase consisting of G1, S and G2 phases. The transition from G1 to S phase and G2 to M phase are two very important phases of the cell cycle, which are very complex and active, and are particularly susceptible to abnormal environmental conditions thus appearing to lead to abnormal cell cycle emergence (64). Kexiang Zhang et al. recently found that Notch1 inhibited PI3K/AKT signaling, leading to S-phase block and effectively inhibiting the proliferation of osteosarcoma cells (103). Yong Cui et al. also demonstrated that S-phase block could be induced in osteosarcoma cells by altering the PI3K/AKT pathway (Figure 2B) (104). In addition, G2/M phase block can also be caused through the PI3K/AKT pathway, thereby inhibiting the proliferation of OS cells, as confirmed by Bin Zhu et al. (Figure 2C) (96). PI3K/AKT pathway can also be inhibited by activating mTOR thereby inducing G0/G1 phase cell cycle arrest in OS cell lines (97). Cyclin family members can interact with CDK proteins to form an active heterodimer complex, which is necessary for the formation of specific phases of the cell cycle. Therefore, cyclin and CDK proteins play an important role in the progression of the cell cycle (105). It has been demonstrated that the PI3K/AKT signaling pathway can influence the cell cycle progression of osteosarcoma cells by affecting the expression of cell cycle proteins and CDK. For example, DA-LIANG KONG et al. significantly inhibited the phosphorylation of AKT by sodium cantharidininate (SC) without inhibiting mTOR, JNK or p38, and inhibition of AKT phosphorylation decreased the expression of CDK4, CDK6 and cyclin D1, which induced G0/G1 phase block in MG-63 cells that further inhibited the proliferation of osteosarcoma cells (Figure 2D) (104). This shows that the PI3K/AKT signaling pathway has an extremely important role in influencing the cell cycle progression of osteosarcoma cells, and its in-depth study is of great significance.
2.2.3 Apoptosis
Apoptosis, a self-destructive mechanism present in cells, has the main role of removing senescent and abnormal cells and maintaining a normal physiological state of internal environmental homeostasis. In pathological states, the homeostasis of apoptosis can be dysregulated, which can negatively affect the organism and may lead to the development of a range of tumors, including osteosarcoma (106, 107). Apoptosis is mainly initiated by the death receptor pathway, which is mediated by death receptors including tumor necrosis factor (TNF) receptors, TNF-related apoptosis-inducing ligand (TRAIL) receptors and Fas, and the mitochondrial apoptosis pathway, also known as the Bcl-2 regulatory pathway, as it is mainly regulated by the Bcl-2 family (108). Several recent studies have suggested that in osteosarcoma, abnormalities in the PI3K/AKT pathway may affect the apoptotic program of osteosarcoma cells (100, 101, 109–117). For example, Jing Chen et al. used Isoliquiritigenin (ISL) to inhibit the PI3K/AKT pathway and found that the protein expression levels of Bax and active Caspase-3 were elevated, while Bcl-2 levels were significantly decreased, and further studies revealed that apoptosis was accelerated and the invasion, proliferation and migration of osteosarcoma cells were inhibited (Figure 2E) (63), thus suggesting that the mitochondrial apoptotic pathway would be partially affected by the PI3K/AKT pathway. This conclusion was further supported by the use of agonists of the PI3K/AKT pathway to reverse the regulation of apoptosis and proliferation in a study by Songjia Ni et al. (118).Sineocolis homolog box homolog 1 (SIX1), an evolutionarily conserved transcription factor (119), is a key regulator of embryonic development and is associated with tumorigenesis and development (120). In osteosarcoma cells, overexpression of SIX1 inhibits apoptosis, promotes cell migration, invasion and proliferation, and in-depth studies have revealed that this function is closely related to reduced PTEN expression and activation of the PI3K/AKT pathway (121). Glaucocalyxin A has properties including inhibition of platelet aggregation (122), immunosuppressive, antioxidant and DNA damage protective activity, and cytotoxic activity (123). Recently, it was found that Glaucocalyxin A can induce apoptosis in osteosarcoma cells by increasing the ratio of Bax to Bcl-2, triggering reactive oxygen species (ROS) generation, decreasing mitochondrial membrane potential and inducing caspase-3 and caspase-9 cleavage, as found by using PI3K activators and inhibitors This function of Glaucocalyxin A is mainly achieved by inhibiting the nuclear translocation of GLI1 through the regulation of PI3K/AKT pathway (Figure 2F) (99). Furthermore, it has been found that inhibition of the PI3K/AKT/mTOR signaling pathway promotes apoptosis in osteosarcoma cells induced by chemotherapeutic agents, including DOX and methotrexate (MTX) (Figure 3A) (124). This finding has positive implications for contributing to the current challenge of chemoresistance.
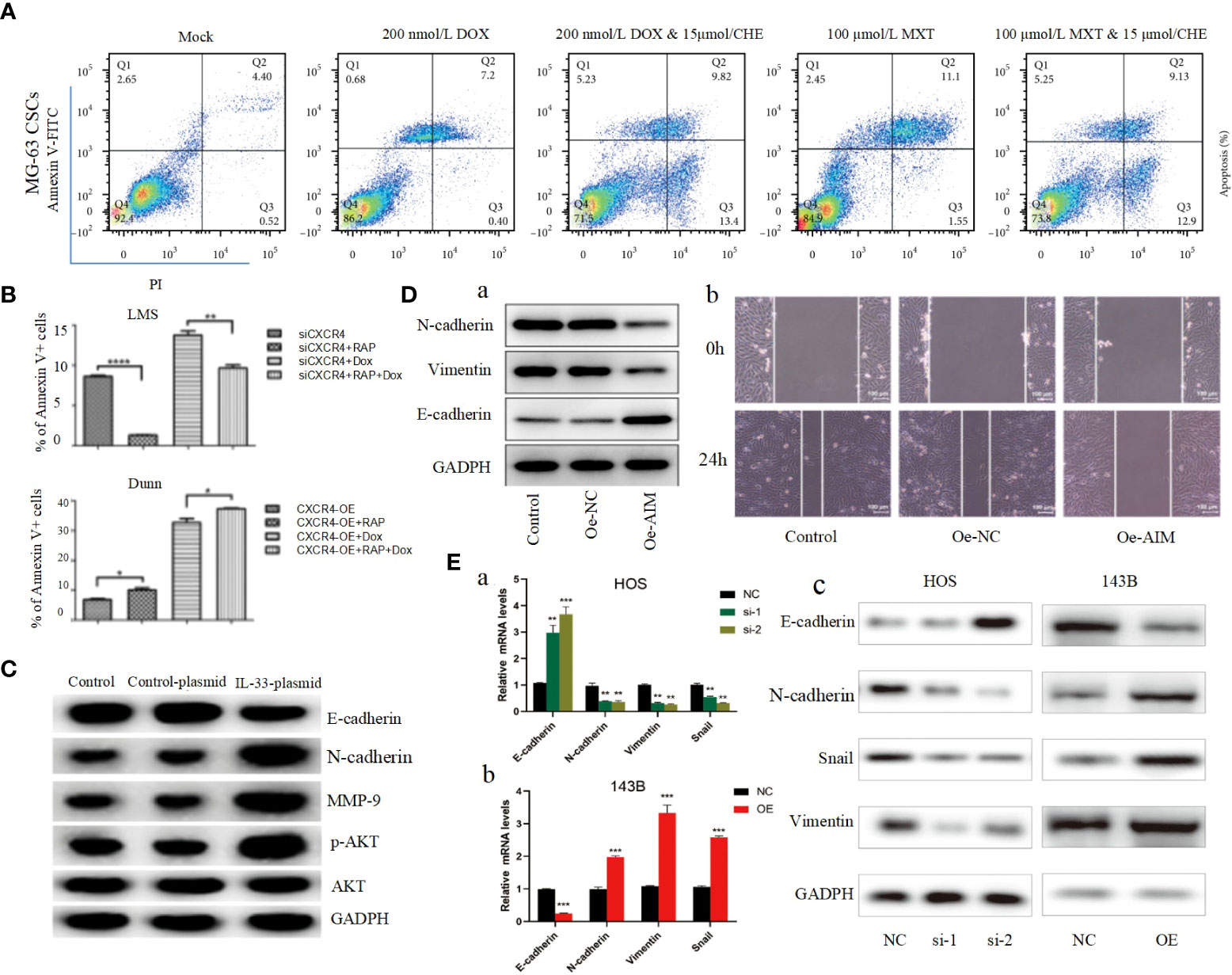
Figure 3 (A) After being cocultured using CHE with DOX or MTX, apoptosis was measured by performing Annexin V-FITC/PI double staining followed by flow cytometry assay (124). (B) The apoptosis ratios for each group (percentage of Annexin V+ cells) were determined by flow cytometry (125). (C) The protein levels of the EMT markers, E-cadherin, N-cadherin, MMP-9, p-AKT, and AKT in transfected cells were detected by Western blot (126). (D) Overexpression of AIM2 inhibits osteosarcoma cell invasion, migration and EMT. (A) Western blotting was used to assess the levels of EMT-related proteins, including N-cadherin, Vimentin and E-cadherin. (B) Wound healing assay was utilized to detect cell migration (61). (E) ZCCHC12 promoted OS cell EMT progression, qRT-PCR (A, B) and western blot analysis (C) were performed to examine EMT-related markers in OS cells after ZCCHC12 knockdown or overexpression (104). *p < 0.05; **p < 0. 01; ***p < 0.001; ****p < 0.0001.
2.2.4 Autophagy
Autophagy is an intracellular degradation process with highly conserved characteristics. Damaged organelles and cytoplasmic proteins are encapsulated into double-layer vesicles, which then interact with lysosomes to form autolysosomes for degradation, thereby renewing cytoplasmic proteins or organelles (127, 128). Autophagy is particularly important for cells to maintain homeostasis and adapt to nutrient deficiencies in vivo. Three different types of autophagy include microautophagy, macroautophagy and molecular chaperone-mediated autophagy (129). There are multiple regulatory mechanisms of autophagy and the most studied mechanism, PI3K/AKT/mTOR pathway, is activated under normal nutritional conditions leading to autophagy inhibition, however, during nutritional deficiency, PI3K/AKT/mTOR pathway is inhibited leading to autophagy occurrence (129, 130). It is well known that LC3-II, ATG5 and p62 protein levels are closely related to autophagy levels, and several studies have shown that inhibition of the PI3K/AKT/mTOR pathway in osteosarcoma cells can increase the expression levels of LC3-II, ATG5 and p62, which increases autophagy and decreases the proliferative and invasive potential of osteosarcoma cells (64, 131, 132). Jinfeng Zhou et al. also found that activation of autophagic flux induced by inhibition of PI3K/AKT/mTOR signaling pathway sensitized OS to DOX (Figure 3B). Therefore, targeting the CXCR4/PI3K/AKT/mTOR autophagy axis may be an effective therapeutic strategy to overcome OS chemoresistance (125). Unfortunately, the above studies are relatively basic, if we can use electron microscopy and other experimental means to observe the autophagy behavior of osteosarcoma cells wound greatly increase the significance of the experimental results. Since the effect of autophagy on tumors is bidirectional, the different activation degree of PI3K/AKT pathway will lead to different degrees of autophagy activation, which may eventually produce tumor suppressor or carcinogenic effects. Therefore, it is not only a challenge but also an opportunity to study the relationship between PI3K/AKT and tumor autophagy.
2.2.5 EMT
EMT is a process of cell morphological characteristics change, which is characterized by the loss of epithelial cell phenotype and the transition to mesenchymal cell phenotype. The main manifestations include the down-regulation of epithelial cell markers, unstable cell-cell junctions, loss of basement membrane and apical polarity, and reorganization of the cytoskeleton (133). Although more attention has been paid to epithelial cancers, EMT also plays an important role in the formation of non-epithelial cancers, such as OS. EMT in OS is highly complex and regulated by multiple signaling pathways, including the PI3K/AKT pathway. E-calmodulin is a marker of primary epithelial tumors, and Shenyu Wang et al. found that by affecting the PI3K/AKT pathway can affect E-calmodulin levels and N-calmodulin levels in osteosarcoma cells, further altering the cell viability and EMT of osteosarcoma cells (Figure 3C) (126). Absent in melanoma 2 (AIM2) and Fer-1-like protein 4 (FER1L4) are two osteosarcoma inhibitors, and several studies have found that their overexpression inhibited the PI3K/AKT/mTOR signaling pathway, with an increase in E-calmodulin and a decrease in wave proteins and fibronectin, inhibiting EMT (Figure 3D) (61, 134). In contrast, STEAP2 (sixtransmembrane epithelial antigen of prostate 2), zinc finger CCHC domain containing 12 gene (ZCCHC12) (Figure 3E), and fibulin-4 (Figure 4A) are promoters of osteosarcoma, which can be induced by the PI3K/AKT/mTOR pathway to induce EMT and promote tumor growth, invasion and metastasis of osteosarcoma cells (104, 135, 139).
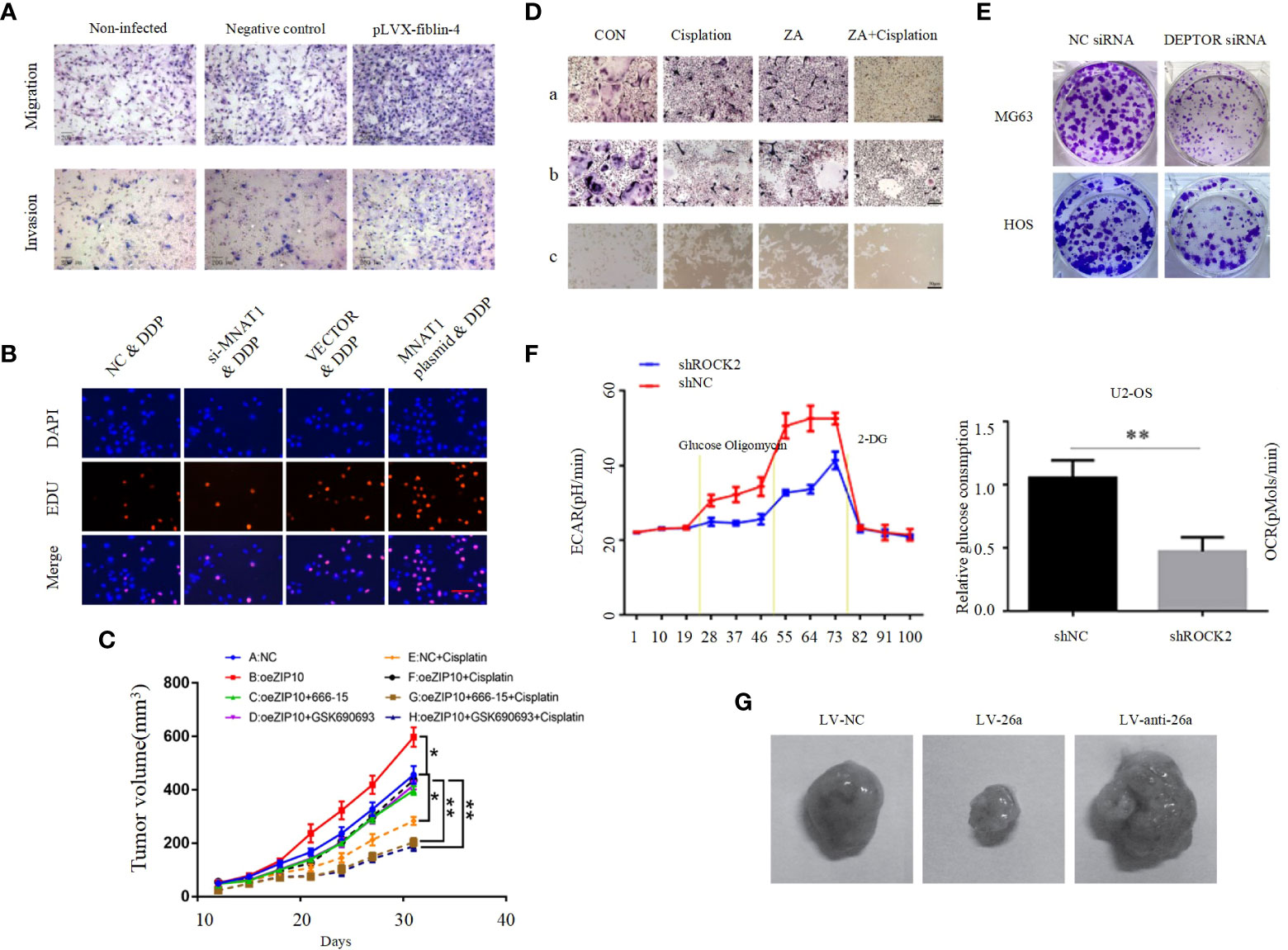
Figure 4 (A) Effect of fibulin-4 knockdown and overexpression on the migration and invasion of the differently invasive osteosarcoma cell subclones (135). (B) MNAT1 regulated OS chemo-sensitivity to DDP-based therapy (136). (C) Tumor weight in each group (54). (D) Cisplatin in synergy with ZA inhibited osteoclast formation, survival, and activation. (a) The TRAP staining of BMMs treated with M-CSF and RANKL for 4 days in the presence of cisplatin/ZA+cisplatin. (b) The mature osteoclasts were treated by cisplatin/ZA+cisplatin. (c) The bone resorption on Corning Osteo Assay 24-well plates of osteoclasts treated by cisplatin/ZA+cisplatin (65). (E) Colony formation capacity of osteosarcoma cells (97). (F) ROCK2 affects the level of glycolysis in OS cells. Extracellular acidification rate data revealed the glycolytic rate and capacity (137). (G) Tumor growth in mouse xenograft models. MG-63 cells infected with NC, miR-26a, or anti-miR-26a lentivirus were injected subcutaneously into nude mice (138). *p < 0.05, **p < 0.01.
2.2.6 Resistance to chemotherapy drugs
It is self-evident that chemotherapy plays an important role in the treatment of osteosarcoma. The application of chemotherapy has greatly improved the overall survival rate and quality of life of OS patients (140). However, the problem of chemoresistance has become increasingly prominent in recent years. The problem of chemoresistance has become a major obstacle to further improve the treatment effect of osteosarcoma patients. Chemotherapy resistance in OS can be mediated by a variety of mechanisms, mainly including significantly reduced intracellular drug accumulation, accelerated drug inactivation, increased DNA repair rate, disturbance of intracellular signal transduction pathways, and abnormal changes in apoptosis, autophagy and CSCs (124, 125, 141). The PI3K/AKT pathway affects chemoresistance in osteosarcoma has been demonstrated in several studies. Menage a trois 1 (MAT1) is a subunit in the cell cycle protein-dependent kinase-activated kinase (CAK) complex, and Chensheng Qiu et al. demonstrated that MAT1 is required to regulate OS chemosensitivity to DDP and achieves its action through the PI3K/AKT/mTOR pathway (Figure 4B) (136). A recent study found that knockdown of Zrt and Irt-related protein 10 (ZIP10) inhibited OS cell proliferation and chemoresistance, and that ZIP10 promoted Zn content-induced phosphorylation and activation of cAMP response element binding protein (CREB), which is a key component of integrin α10 (ITGA10) transcription and ITGA10 activation PI3K/AKT pathway, and does not stimulate the classical FAK or SRC pathways. It was further confirmed by in vivo experiments that ZIP10 mediates chemotherapy resistance in OS cells via the ITGA10-PI3K/AKT axis (Figure 4C) (54). Zoledronic acid (ZA) is a diphosphate compound used to treat bone diseases. It inhibits bone destruction caused by increased osteoclast activity (142). Previous studies found ZA to inhibit a variety of tumors including osteosarcoma, cervical and breast cancers (143–145). A recent study by Liang Liu et al. found that ZA combined with cisplatin significantly inhibited the malignant biological behavior of 143B cells, and that agonists of the PI3K/AKT pathway could reverse this result. This shows that ZA enhances the antitumor effects of cisplatin in osteosarcoma through the PI3K/AKT pathway and reduces chemoresistance and osteoclast activation (Figure 4D), and this study raises the possibility of using ZA in combination with cisplatin as a new strategy representing the fight against osteosarcoma (65). However, these studies related to drug resistance were supported only by cell and animal experiments without clinical trials. For example, YU et al. found that AMD 3100 could enhance the antitumor effect of adriamycin in an in situ OS mouse model. Decreased expression of p-PI3K, p-AKT, and p-mTOR were also observed. These results suggest that AMD 3100 promotes the antitumor effect of adriamycin on tumor growth in vivo (125).
2.2.7 Angiogenesis
Angiogenesis is not only essential for normal life activities, but is also crucial for tumors, as tumor growth also requires blood vessels to provide them with adequate nutrients. One study reported that Nude mice injected with human OS 3AB-OS pluripotent CSC showed high AKT levels along with a significant increase in tumor vascular density, and the significant increase in vascular density was suppressed after inhibition of the PI3K/AKT pathway (146). This experiment suggests that activation of the PI3K/AKT pathway is important for angiogenesis in OS. Some studies found that TGF, VEGF, PDGF and basic fibroblast growth factor (bFGF) play a pro-angiogenic role in OS progression (147, 148). As mentioned above, as activators, they can effectively activate the PI3K/AKT pathway. Thus, activated PI3K/AKT pathway plays a key role in TGF, PDGF and bFGF-induced OS angiogenesis, however, its clear mechanism still needs further in-depth study. Recently, DEP domain-containing mTOR interacting protein (DEPTOR) has been identified as an endogenous mTOR inhibitor. Considering the close relationship between DEPTOR and mTOR, DEPTOR is thought to play an important role in the pathogenesis of many cancers, and it was found that DEPTOR overexpression significantly inhibits mTOR and activates the PI3K/AKT pathway, which is required for OS cell proliferation, migration, invasion, angiogenic mimetic formation and survival (Figure 4E) (97).
2.2.8 Aerobic glycolysis
Increased aerobic glycolysis (Warburg effect) has become a hallmark of becoming cancerogenesis that can provide more intermediates for certain biosynthetic pathways and adaptation to hypoxic environments, a metabolic shift that leads to cancer cell proliferation and survival (149, 150). Rho-associated coiled-coiled-coil containing protein kinase 2 (ROCK2) is a serine-threonine kinase. As a downstream effector of the Rho subfamily of small gtpase, ROCK2 regulates cell morphology and migration. In osteosarcoma cells, ROCK2 can promote OS cell growth by inducing aerobic glycolysis. ROCK2 induces aerobic glycolysis mainly by activating p-PI3K/AKT pathway to promote the expression of mitochondrial hexokinase II (HKII). (Figure 4F) (137). Glucose metabolism assays demonstrated that PDGF/PDGFR-β effectively promoted aerobic glycolysis in osteosarcoma cells. PI3K/AKT pathway inhibitor LY294002 was used to perform WB assays and glucose metabolism assays. The results showed that PDGF/PDGFR-β promoted aerobic glycolysis in osteosarcoma cells mainly through the activation of PI3K/AKT/mTOR/c-Myc pathway (151).
3 MicroRNAs in the PI3K/AKT pathway
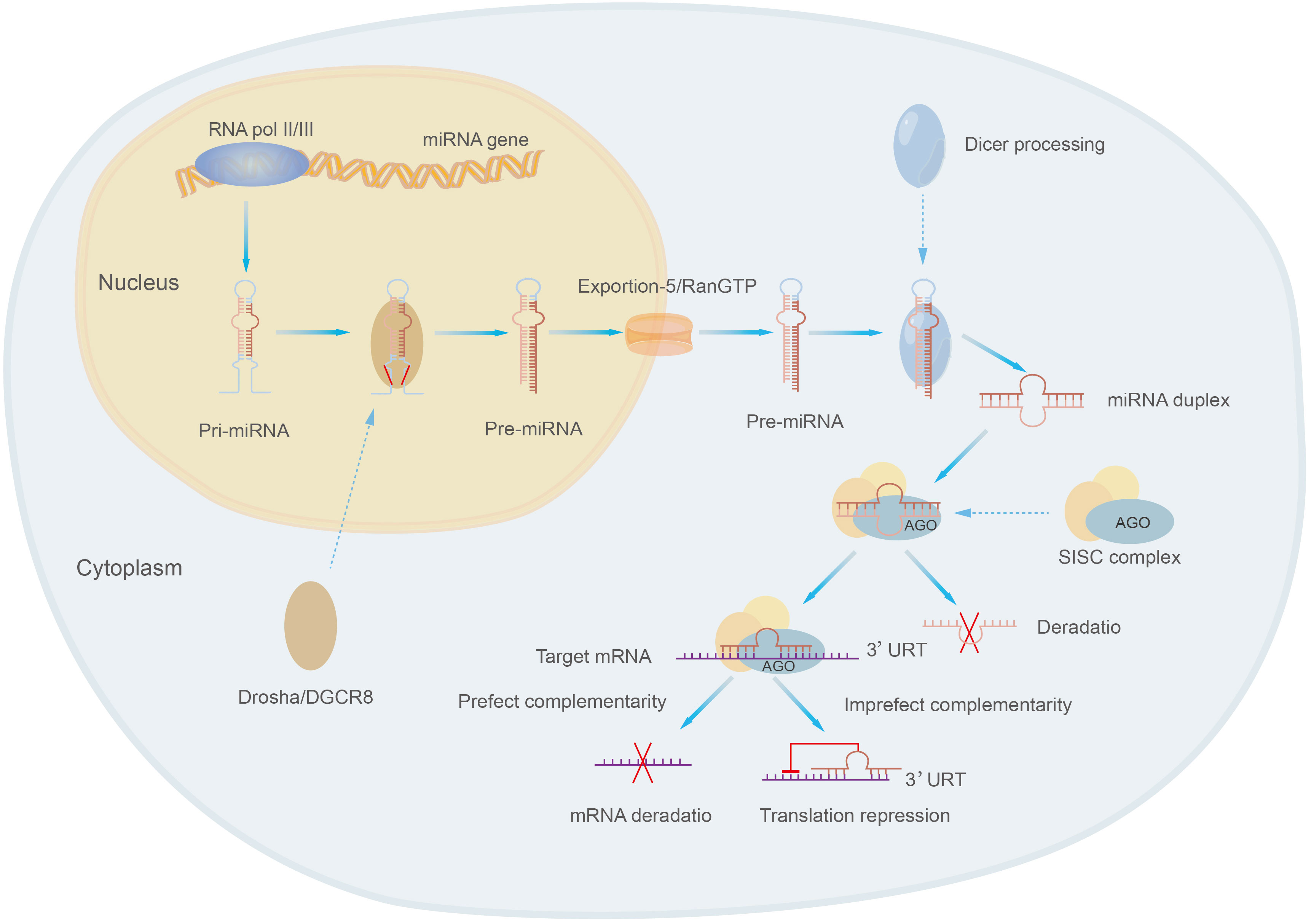
MicroRNAs are class of small molecular RNAs of 21-25 nucleotides in length, they are widely found in animals, plants and eukaryotic microorganisms and are a novel and important factor in regulating gene expression (19, 152). MicroRNAs were originally identified in Cryptobacterium hidradenum (153). MicroRNAs exist in several forms, including pri-miRNA, pre-miRNA and miRNA. Pri-miRNA is derived from the genome of eukaryotes and is transcribed and spliced to form pre-miRNA, then pre-miRNA is sheared into mature double-stranded microRNA by the nucleic acid endonuclease Dicer in the extracellular nucleus, and the mature double-stranded microRNA is associated with Argonaute (AGO) and forms a RNA-induced silencing complex (RISC) called the rna-induced silencing complex, which selects one strand of the double-stranded body to become a mature miRNA and discards the other strand (152, 154) (Figure 5). MiRNA present a single-stranded form structurally and, most characteristically, contain a structurally stable base pair between the RNA and the RNA within the precursor RNA structure. The expression of miRNA tends to be higher due to the wide intracellular distribution of small RNAs (155).
MicroRNAs mainly regulate gene expression by targeting with specific proteins in cooperation with specific mechanisms: miRNAs select appropriate mRNA targets through the interaction of the initiating RNA-mediated silencing complex (RISC) with the mRNA 3’-UTR complementary sequences of target genes, target recognition, physical hindrance of target sequences, translation inhibition, degradation and other steps to affect the expression of target genes and their functions, thus participating in the regulation of various cell biological processes, such as cell proliferation, apoptosis, differentiation and metabolism (106, 156, 157). For example, the interaction of miR-181a-5p with the 3’-UTR complementary sequence of PTEN leads to a decrease in PTEN, which results in the activation of PI3K/AKT pathway further affecting the development of osteosarcoma (55).
PI3K/AKT pathway is the main signaling pathway that affects the biological behavior of tumor cells. There is a close relationship between miRNA and PI3K/AKT pathway. On the one hand, miRNA can affect the activation and inhibition of the signaling pathway by targeting and regulating the expression of PI3K/AKT pathway-related genes. For example, in osteosarcoma cells miR-181a-5p (55) and miR-133a (158) can target PTEN genes and inhibit their expression, thus promoting the activation of PI3K/AKT pathway. In contrast, microRNAs such as miR-506-3p (159) and miR-126 (160) can target genes of the PI3K/AKT pathway and suppress their expression, thus inhibiting the activation of the signaling pathway. On the other hand, the PI3K/AKT pathway can also function by regulating the expression of miRNA. For example, the activation and inhibition of AKT can also affect the expression of miR-21, which further affects the activation of PI3K/AKT pathway (161).
In summary, miRNA and PI3K/AKT pathway are interacting with each other, they regulate each other, and for the occurrence and development of some diseases, such as tumorigenesis and metastasis, they are also related to the abnormalities of miRNA and PI3K/AKT pathway. Therefore, studying the interaction between miRNA and PI3K/AKT pathway in osteosarcoma is of great significance for further systematic and in-depth understanding of the pathogenesis of part of osteosarcoma and the development of new and effective diagnostic, therapeutic and prognostic strategies.
4 Role of microRNA/PI3K/AKT axis in osteosarcoma
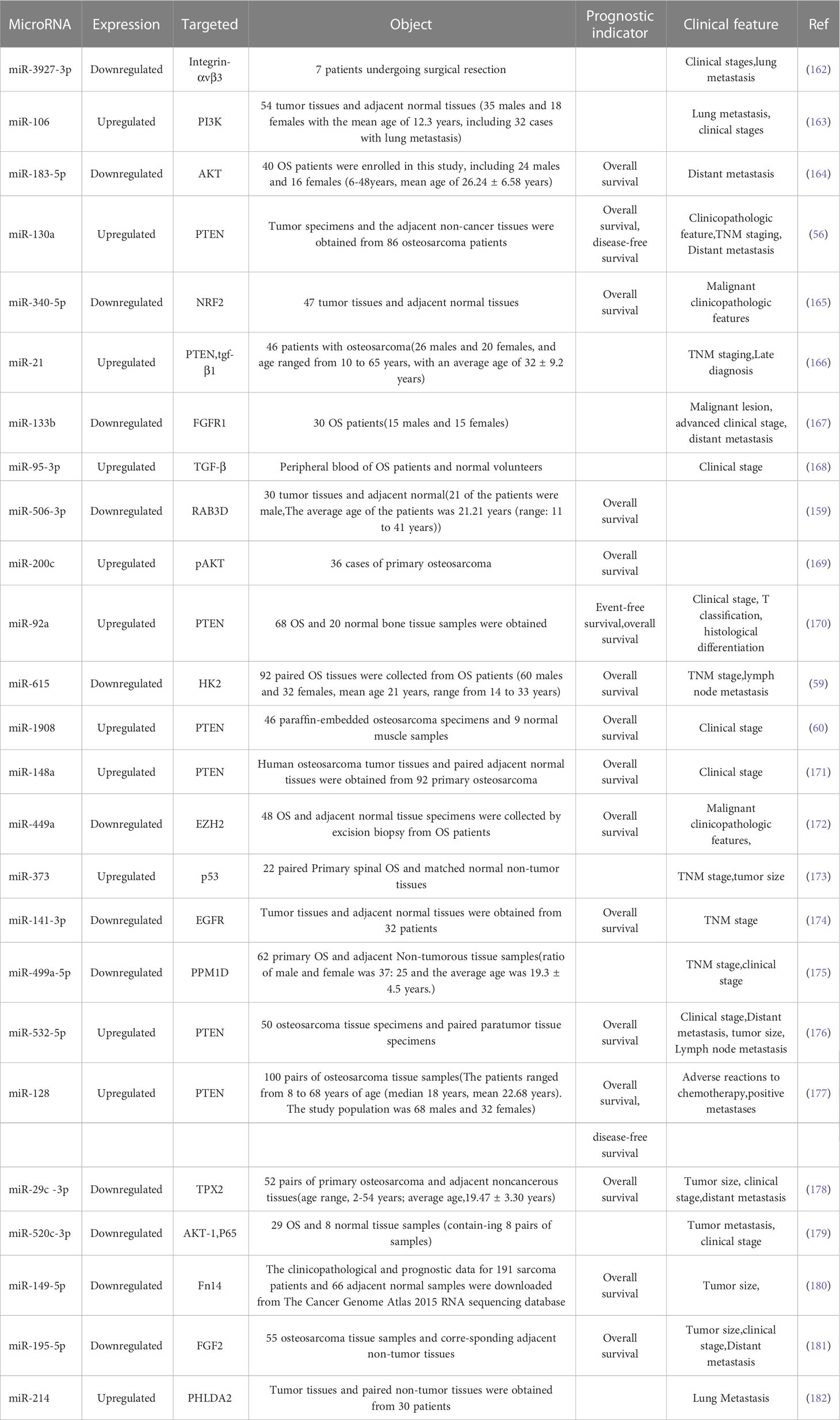
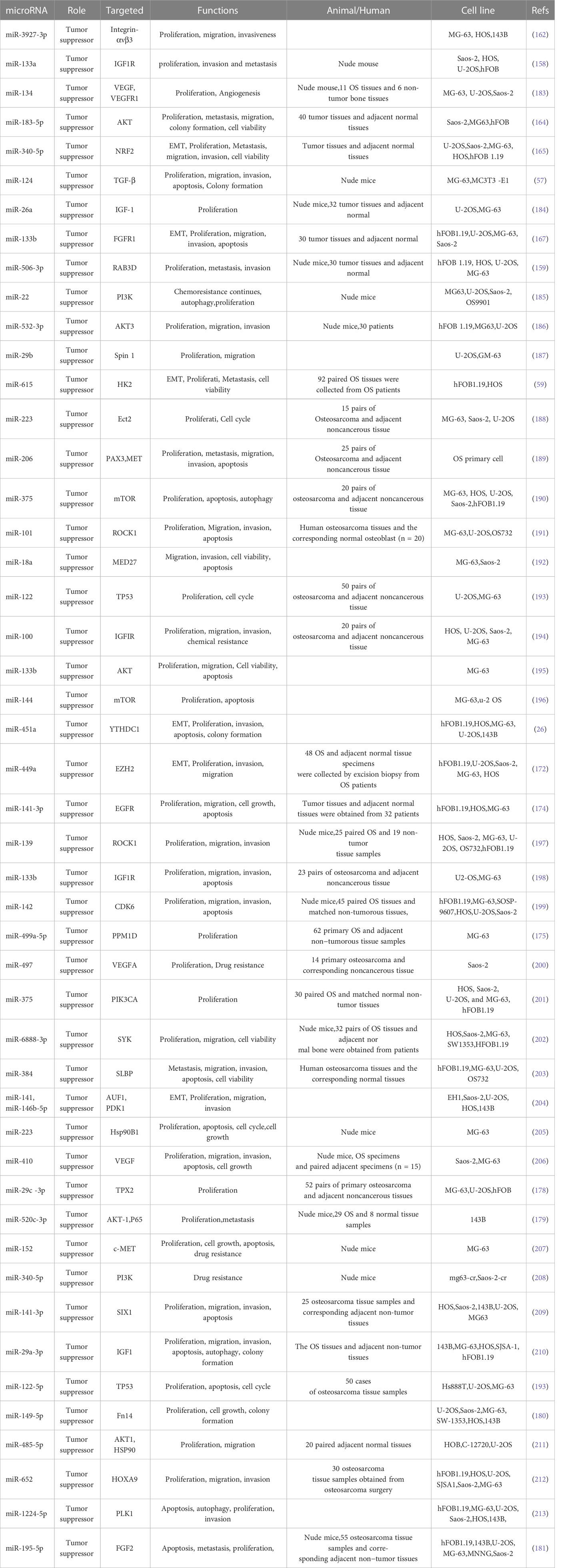
Many microRNAs associated with the PI3K/AKT pathway are aberrantly expressed in osteosarcoma. A new series of studies shows that a range of clinical features in patients with osteosarcoma are associated with the PI3K/AKT pathway and microRNAs (Table 1). In addition, the microRNA/PI3K/AKT axis promotes cancer progression by altering various biological properties of osteosarcoma cells. In this article, we will introduce in detail the Status of expression, clinical features, basic functions, and mechanism of microRNA/PI3K/AKT axis from two parts: cancer suppressor factors (Table 2) and cancer promoting factors (Table 3) that act on the PI3K/AKT pathway. It provides certain directions and ideas for further insight into the mechanisms associated with microRNA/PI3K/AKT axis-related osteosarcoma and the development of new therapeutic strategies.
4.1 Cancer suppressor acting on the PI3K/AKT pathway
Insulin-like growth factor-1 (IGF-1) is composed of 70 amino acids. IGF-1 can affect the proliferation, invasion, metastasis and drug resistance of tumor cells (238, 239). Recent studies found that miR-26a (Figure 4G) and miR-29a-3p were lowly expressed in osteosarcoma and negatively correlated with IGF-1 expression, and further studies revealed that overexpression of miR-26a and miR-29a-3p could target IGF-1 to inhibit the IGF-1R/PI3K/AKT pathway to affect apoptosis and autophagy in OS cells, as evidenced by a significant attenuation of invasion (138, 210). IGF-1 can act by activating the PI3K/AKT pathway through activation of the IGF-1R located on the cell surface, and therefore by affecting IGF-1R can also affect activation of the PI3K/AKT pathway.It was found that miR-133a, miR-133b (Figure 6A), and miR-100 can partially block this pathway, and miR-133a, miR-133b, and miR-100 can then target IGF-1R in human osteosarcoma cells to inhibit the activation of PI3K/AKT pathway and thus inhibit apoptosis, proliferation, invasion, metastasis, migration, and chemoresistance of osteosarcoma (158, 194, 198). VEGF can bind to VEGFR1 to activate PI3K/AKT pathway thereby promoting tumor proliferation and angiogenesis (240). MiR-134, miR-497 and miR-410 were found to be lowly expressed in osteosarcoma cells, and functionally microRNA-134, miR-497 and miR-410 could lead to reduced expression of VEGF and VEGFR1, thus inhibiting the PI3K/AKT pathway leading to osteosarcoma angiogenesis, proliferation, migration, invasion, cell growth, and drug resistance (Figure 6B) (183, 200, 206). MiR-124 and miR-21 are lowly expressed in osteosarcoma, and overexpression can act on TGF-β to affect the activation of PI3K/AKT pathway. As a result, the malignant degree of osteosarcoma cells decreased significantly. in addition miRNA -21 is also strongly associated with TNM staging in osteosarcoma patients (Figure 6C) (57, 166). Bingsheng Yang et al. found that miR-195-5p expression was significantly reduced in OS and negatively correlated with fibroblast growth factor-2 (FGF-2) expression. MiR-195-5p/FGF2/PI3K/AKT axis can affect the occurrence and metastasis of osteosarcoma (181). Fibroblast growth factor receptor-1 (FGFR-1) can mediate the activation of PI3K/AKT pathway. MiR-133b can target FGFR-1 in OS to inhibit PI3K/AKT pathway and thus inhibit the progression of osteosarcoma. MiR-195-5p and miR-133b were found by studying clinical samples 5p and miRNA-133b were also found to be closely associated with tumor size, distant metastasis and the clinical stage of the patients in OS patients, thus it is important to continue in-depth studies on miR-195-5p and miR-133b (167).
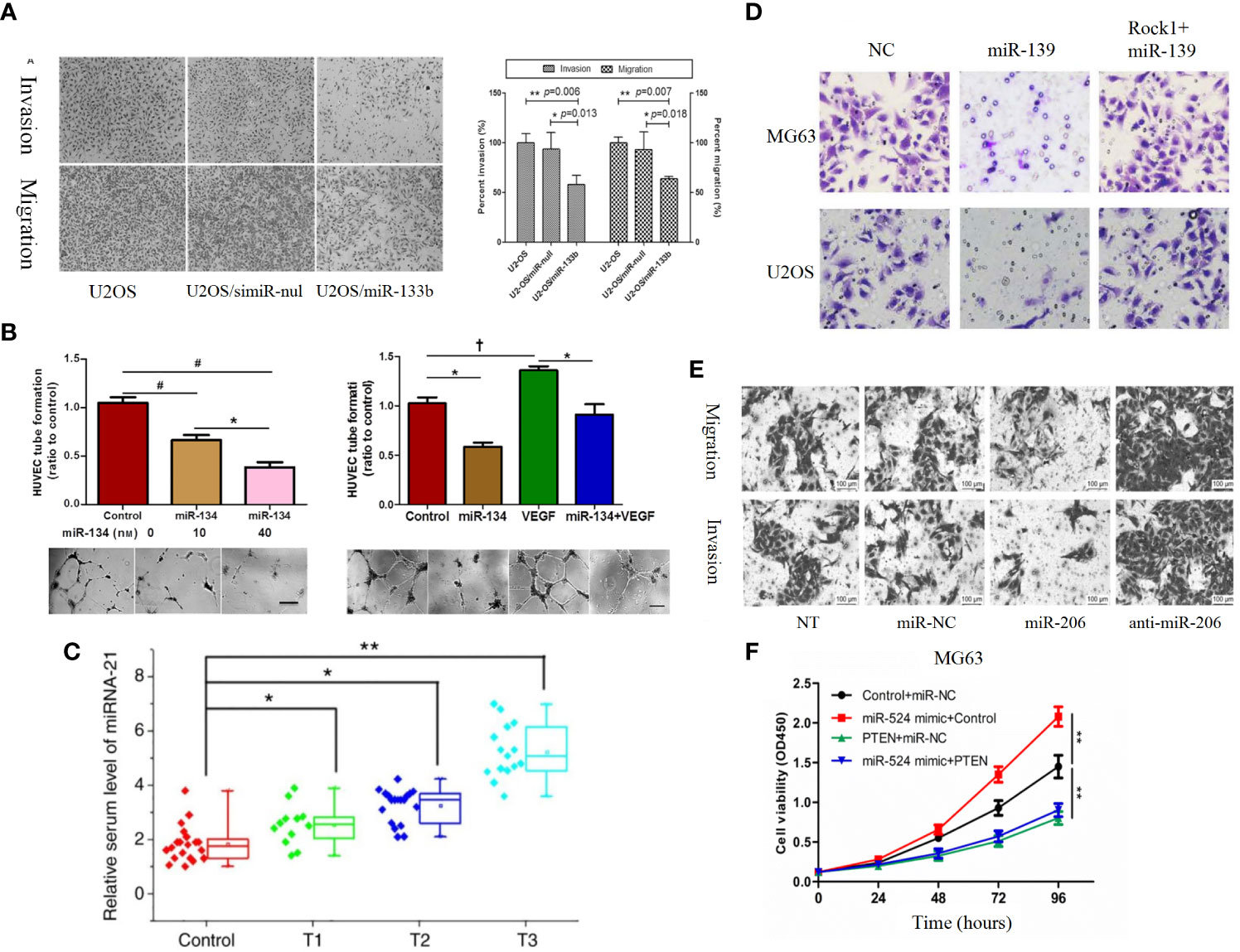
Figure 6 (A) Roles of miR-133b in invasion and migration of osteosarcoma cell lines. Over-expression of miR-133b suppressed cell invasion and migration in U2-OS cells. Cell invasion and migration were visualized and examined by inverted phase contrast microscope (198). (B) MiR-134 overexpression inhibits the proliferation of Saos-2 cells and their secretion of angiogenesis factors in vitro, but promotes Saos-2 cell apoptosis (183). (C) Serum levels of miR-21 increase as the T stage of osteosarcoma increases (166). (D) ROCK1 rescues cell proliferation and invasion in miR-139 overexpression OS cells. Representative images of transwell invasion assay (197). (E) Representative results of cell migration and invasion assays (189). (F) MG-63 (a) and 143 (b) cells are transfected with Control + miR-NC, miR-524 mimic + Control, PTEN + miR-NC or miR-524 mimic + PTEN, and cell proliferation in each group is detected by CCK-8 (217). †P < 0.05; *P < 0.01; **P < 0.001, #P < 0.0001.
There are also many microRNAs whose effects on the PI3K/AKT pathway are directly on PI3K and AKT molecules, for example, miR-340-5p is under-expressed in osteosarcoma as an oncogenic factor, and thus as a direct target gene of miR-340-5p, PI3K receives significantly less direct inhibition, leading to abnormal PI3K/AKT pathway and a OS development with a significant contribution (208). MiR-22 significantly inhibits the proliferation of MG63 cell line and MG63/CDDP cells, and enhanced the anti-proliferation ability of CDDP in vitro and in vivo. In addition, miR-22 can also reduce CDDP-induced autophagy and mediate the resistance of osteosarcoma cell lines to CDDP (185). AKT has an extremely important position in the PI3K/AKT pathway (241).Several studies found that miR-183-5p, miR-532-3p, miR-133b, miR-520c-3p and miR-485-5p were overexpressed in osteosarcoma, and overexpression would directly affect the formation of AKT leading to abnormal PI3K/AKT pathway, and the proliferation, migration, cell viability, and apoptosis of osteosarcoma cells would be affected (164, 179, 186, 195, 211). In addition, miR-183-5p and miR-520c-3p are also associated with clinical characteristics and prognosis of OS patients, including distant metastasis of tumors and overall survival of patients at clinical stage. Therefore the interaction mechanism of miR-183-5p and miR-520c-3p with PI3K/AKT pathway should be investigated in depth (164, 179). PI3K and Akt molecules are the core of PI3K/Akt pathway and are the most important signaling molecules. Therefore, mirnas directly acting on PI3K and Akt molecules can more directly affect the abnormal activation of PI3K/Akt pathway, which may have a more significant effect on osteosarcoma cells, and reduce the influence of the whole criss-crossing signal network, which is worthy of our study.
Luciferase assay and bioinformatic analysis confirmed that mTOR is a direct target gene of miR-375-3p and miR-144. mTOR is a key regulator of various life activities of cells and performs its various functions mainly by participating in the PI3K/AKT pathway. Reduced expression of miR-144 miR-375-3p in osteosarcoma cells leads to PI3K/AKT/mTOR pathway aberrant activation, which affects OS cell proliferation, apoptosis and autophagy (190, 196). ROCK1 is a serine/threonine kinase belonging to the Rho family that promotes the reorganization of the actin cytoskeleton (242). It has been found that microRNA interaction with ROCK1 may be related to the formation and progression of osteosarcoma, where miR-101 and miR-139 have been demonstrated as osteosarcoma suppressors by targeting rock1. It was found that by overexpressing miR-101 and miR-139 (Figure 6D) can downregulate rock1 while causing PI3K/AKT and JAK/STAT signaling pathway inactivation, ultimately leading to the malignant behavior of osteosarcoma cells can be partially suppressed, including biological behaviors such as abnormal proliferation, migration and invasion (191, 197). MiR-340-5p (165), miR-506-3p (159), miR-615 (59), miR-18a (192), miR-122-5p (193), miR-451a (26), miR-449a (172), miR-142 (199), miR-499a-5p (175), miR-6888-3p (202), miR-384 (203), and miR-223 (205), miR-29c-3p (178), miR-152 (207), miR-149-5p (180), miR-652 (212), miR-1224-5p (213), miR-146b-5p (204), miR-141-3p (209), miR-141 [198], miR-29b (187), and miR-223 (188) are all lowly expressed in osteosarcoma cells as tumor suppressors, and they can act directly on NRF2, RAB3D, HK2, MED27, TP53, YTHDC1, EZH2, CDK6, PPM1D, SYK, SLBP, Hsp90B1, TPX2, c-MET, Fn14, HOXA9, PLK1, PDK1, SIX1, AUF1, Spin 1, Ect2 and thus inhibit the PI3K/AKT pathway, which ultimately leads to the impact of osteosarcoma cells in proliferation, migration, invasion, apoptosis, autophagy, cell cycle, cell growth, etc., and inhibits the development of osteosarcoma and progression of osteosarcoma. In addition, miR-206 can also act on both PAX3 and MET target genes to achieve the inhibition of PI3K/AKT pathway through two pathways, so the inhibitory effect of miR-206 on osteosarcoma may be more obvious (Figure 6E) (189). Systematic in-depth analysis of clinical data from multiple osteosarcoma patients found a significant correlation between the expression of miR-506-3p in osteosarcoma tissues and patient prognosis. Specifically, lower expression levels of miR-506-3p were associated with worse prognoses (159). And the expression of miR-499a-5p was significantly correlated with TNM staging, showing that the lower the expression of miR-499a-5p, the higher the grade of TNM staging (175). In contrast, low expression of miR-340-5p (165), miR-615 (59), miR-449a (172), miR-29c-3p (178), and miR-149-5p (180) not only predicted a poorer prognosis for patients, but also correlated with osteosarcoma TNM stage, clinical stage, tumor size, distant metastasis and other clinical features are significantly correlated. Therefore, this part of MicroRNA that has been shown to correlate with patient prognosis and clinical characteristics deserves further in-depth study and hopefully can be applied to clinical practice.
4.2 Oncogenic factors acting on PI3K/AKT pathway
PTEN is a member of the protein tyrosine phosphatase gene family and represents the first identified tumor suppressor with bispecific phosphatase activity to date. In the PI3K/AKT pathway, PTEN plays a crucial role in the regulation of cellular signaling pathways by dephosphorylating phosphatidylinositol-3,4,5-trisphosphate (PIP3), the second messenger produced by PI3K, thereby negatively modulating the activity of serine/threonine protein kinase AKT (243, 244). Thus PTEN has been shown to act as a tumor suppressor gene by inactivating the PI3K/AKT pathway (198), ultimately participating in the regulation of proliferation, cell cycle, apoptosis, migration, invasion and metastasis during cancer development (245). In osteosarcoma, a number of studies have shown that abnormalities in the PI3K/AKT pathway can be caused by the interaction of microRNA with PTEN, which is basically in a high expression state in osteosarcoma and is generally considered as a pro-oncogenic factor. For example, Chen Sun et al. found that miR-181a-5p, which is highly expressed in osteosarcoma cells, could bind to the 3-URT of PTEN and reduce its protein expression, thus activating the PI3K/AKT pathway. Overexpression of PTEN or inhibition of AKT significantly inhibited the tumor-promoting effect of miR-181a-5p (55). Ming Zhuang et al. found that miR-524 was significantly upregulated in osteosarcoma tissues and osteosarcoma cell lines, miR-524 knockdown inhibited proliferation and promoted apoptosis in osteosarcoma cells, and bioinformatics analysis and luciferase analysis confirmed that PTEN was a direct target gene of miR-524. miR-524 activated PPI3K/AKT signaling by inhibiting PTEN pathway to induce osteosarcoma cell proliferation (Figure 6F) (217). miR-196a transfection also decreased PTEN expression in osteosarcoma cells and led to enhanced phosphorylation of PI3K and AKT. miR-196a should therefore be an oncogene in osteosarcoma. miR-196a overexpression affected MG63 and U-2OS by regulating the PTEN/PI3K/AKT pathway cell apoptosis, cell cycle, and proliferation (222). In osteosarcoma, there are many other microRNAs with similar mechanisms of action to the above microRNAs, including miR-19a-3p (214), miR-216 (215), miR-208a-3p (216), miR-214 (58), miR-9-5p (218), miR-620 (219), miR-130a (56), miR-21 (166), miR-21 (223), miR-221 (94), miR-214 (224), miR-92a (170), miR-214-3p (226), miR-93 (227), miR-1908 (60), miR-21 (228), miR-17 (95), miR-148a (171), miR-181b (230), miR-21 (231), miR-214 (232), miR-532-5p (176), miR-21 (236), miR-744 (237), which were found to be highly expressed in osteosarcoma tissues and osteosarcoma cell lines, all of which can target PTEN, bind to the 3,URT of PTEN and reduce its protein expression. Therefore, the aberrant activation of the PI3K/AKT pathway ultimately results in the facilitation of malignant biological processes such as proliferation, migration, invasion, cell cycle regulation, autophagy and apoptosis in osteosarcoma. In addition, overexpression of miR-181a-5p (55), miR-214 (58) and miR-221 (94) also significantly increased the sensitivity of osteosarcoma cells to some chemotherapeutic drugs, such as ADR and CDDP, which provides new hope to address the current problem of drug resistance in osteosarcoma. In terms of clinical characteristics and patient prognosis, miR-130a (56), miRNA-21 (166), miR-92a (170), miR-148a (171) and miR-532-5p (176) were significantly correlated with the clinical characteristics of patients, and the analysis of clinical and pathological data of patients with osteosarcoma revealed that The high expression of miR-148a (171) correlated with the clinical stage of patients, and the high expression of miR-130a (56)and miR-532-5p (176) correlated with the clinicopathological characteristics, TNM stage, and distant metastasis of patients. The high expression of miR-21 (166) correlated with the TNM stage and late diagnosis of patients, and miR-92a (170) high expression was not only correlated with clinical staging but also with T staging and histological differentiation. In terms of prognosis, high expression of miR-130a (56), miR-21 (166), miR-148a (171) and miR-532-5p (176) was found to lead to significantly lower overall and disease-free survival of patients, and these findings are sufficient to suggest that miR and PTEN/PI3K/AKT signaling pathway interactions lead to patient prognosis. The overall point is that higher microRNA expression of these oncogenic factors that directly target PTEN in osteosarcoma has a greater impact on the PI3K/AKT pathway, which ultimately leads to higher malignancy and worse prognosis for patients with osteosarcoma.
P27 (also known as CDKN1B) is a cyclin-dependent kinase inhibitor. As a downstream molecule of PI3K/AKT pathway, P27 is regulated by PI3K/AKT pathway, and the abnormal change of p27 significantly affects cell proliferation and cell cycle, making it a target to be considered in cancer therapy (246, 247). Several studies have confirmed that microRNAs can affect the function of PI3K/AKT pathway by influencing P27 and thus, for example, miR-199a-5p (Figure 7A) (221), miR-802 (225) and miR-25 (234) can act on P27 to affect its expression by the mechanism of miR-199a-5p, miR-802 and miR-25 can directly P27 the 3-UTR binding of mRNA and mediate a decrease in P27 protein levels, thus stimulating OS cell cycle progression. MiR-106b-5p was found by Chuan He et al. to cause a significant increase in the percentage of G0/G1 phase cells and a decrease in S and G2/M phase cells The number decreased, suggesting that miR-106b-5p blocks cell cycle progression by blocking osteosarcoma cells in G0/G1 phase.
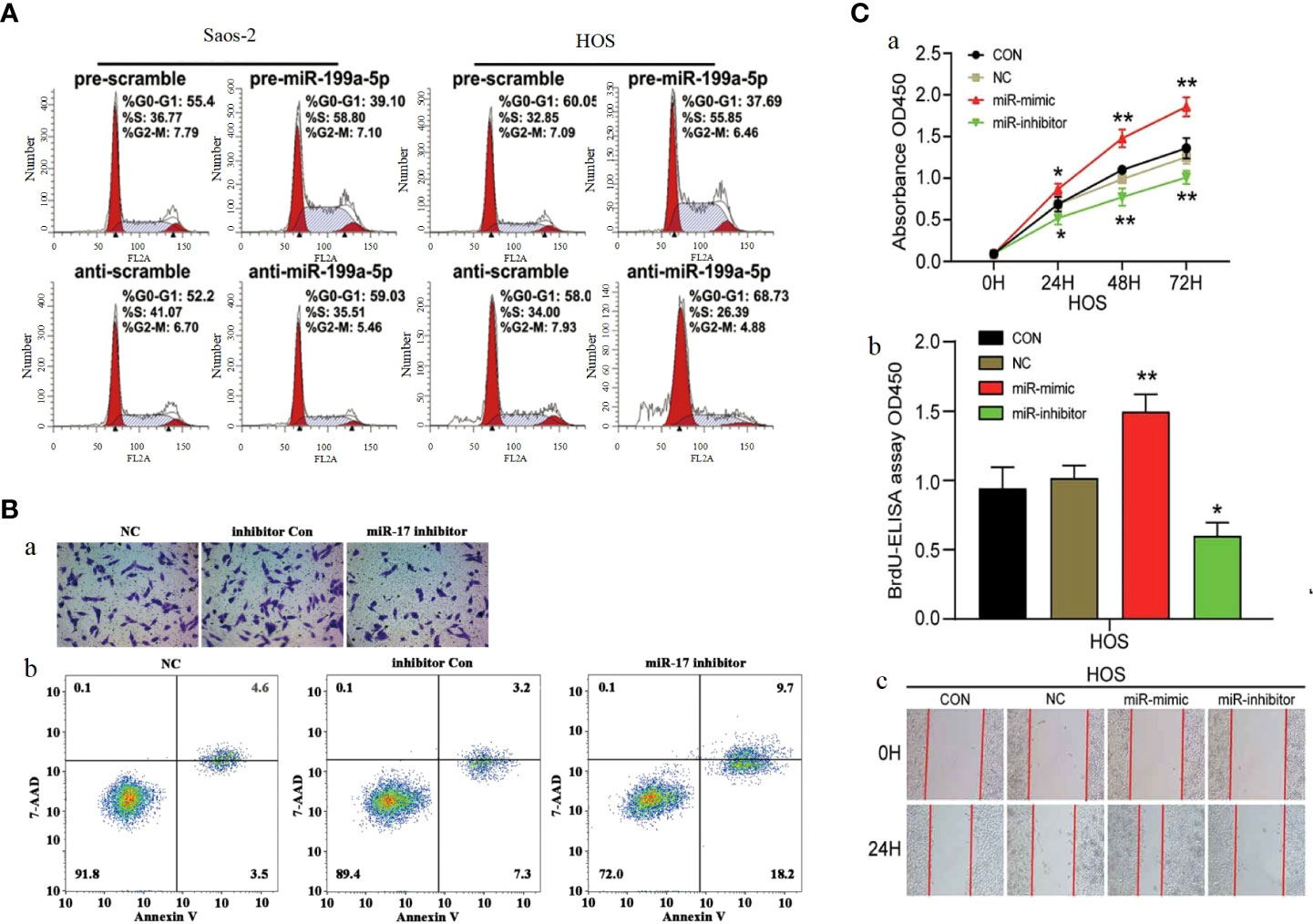
Figure 7 (A) Cell cycle analysis of Saos-2 and MNNG/HOS cells after transfection with pre/anti-miR-199a-5p or the corresponding control (221). (B) Knockdown of miR-17 inhibited cell proliferation, migration and invasion in OS cells.(a) Cell migration was detected using Transwell assay. (b) Flow cytometry demonstrated that knockdown of miR-17 induced cell apoptosis (220). (C) MiR-23b-3p promoted the cell viability, proliferation and migration in OS. (A) Cell viability was detected by CCK-8 assay. (B) The effect of miR-23b-3p on cell proliferation was detected by BrdU-ELISA. (C) Wound healing assays (184). *p < 0.05, **p < 0.01.
CDKN1A is a direct target gene of miR-106b-5p, and the expression of miR-106b-5p exhibits a negative correlation with that of CDKN1A. CDKN1A is a key protein that can interact with CDK and is involved in the regulation of cell cycle progression, proliferation, survival, motility and senescence by binding to CDK and/or its subunits (248) (249).. In addition, the oncogenic effect of miR-95-3p can also be achieved by acting on CDKN1A. In terms of clinical features, the high expression of miR-95-3p is positively correlated with the clinical stage of patients (168). MiR-17 is highly expressed in osteosarcoma cells, and Dajiang Wu et al. have demonstrated miR-17 was involved in the development of osteosarcoma by targeting the SASH1/PI3K/AKT pathway leading to OS cells proliferation, migration and inhibition of apoptosis (Figure 7B) (220). As an oncogenic factor in osteosarcoma, miR-23b-3p is highly expressed in osteosarcoma tissues and OS cell lines. MiR-23b-3p directly targets VEPH1 to inhibit the activation of PI3K/AKT pathway and significantly promoted the viability, proliferation and migration of OS cells (Figure 7C) (184). miR-18a-5p overexpression expression inhibits SOCS5 attenuated the effects of FER1L4 overexpression on OS cell apoptosis and the expression levels of PI3K, AKT, Twist1, N-cadherin and Vimentin. Downregulation of miR-18a-5p promotes SOCS5 can PI3K/AKT pathway activation (233). MiR-214 was shown to downregulate PHLDA2 expression by targeting 3’-UTR, by Yi Li et al. High levels of miR-214 were found in osteosarcoma tissues by qPCR analysis and positively correlated with lung metastasis. Knockdown of miR-214 significantly augmented the radiosensitivity of osteosarcoma cells both in vitro and in vivo. Further investigation revealed that PHLDA2 expression was markedly suppressed, which was significantly associated with the experimental outcomes. MiR-214 modulates PHLDA2 expression to activate the PI3K/AKT pathway. Collectively, our findings suggest that the miR-214/PHLDA2/AKT axis (182). Yufeng Liu et al. found that miR-373 was significantly overexpressed in spinal cord OS tissues and OS cell lines. Overexpression of miR-373 can enhance the malignant degree of osteosarcoma cell lines, which is due to its ability to affect the expression of p53 and its downstream target genes, leading to abnormal activation of PI3K/AKT-Rac1-JNK signaling pathway. In addition, miR-373 expression was also significantly correlated with TNM stage and size of osteosarcoma. The cancer-promoting mechanism and clinical characteristics of miR-373 are expected to provide new ideas for the treatment of spinal osteosarcoma (173).
5 Potential biomarkers in the microRNA/PI3K/AKT axis
Despite recent advances in medical technology, early diagnosis, further effective treatment and prognosis of OS remain a serious challenge, and finding new OS biomarkers may be a feasible approach. There is increasing evidence that PI3K/AKT pathway-associated microRNAs are closely associated with OS progression. Micrornas related to PI3K/AKT pathway may be of great significance in the early diagnosis, effective treatment and prognosis of osteosarcoma, and are expected to become potential biomarkers (Table 4). In this section, we will discuss in detail the possible potential applications of microRNAs related to PI3K/AKT pathway in clinical practice.
5.1 Diagnosis
It is well known that timely and accurate diagnosis of osteosarcoma is the key to the treatment of osteosarcoma, and also an important guarantee to improve the prognosis of patients with osteosarcoma. Identifying appropriate biomarkers has been a challenge in OS research. MicroRNAs related to the PI3K/AKT pathway have been reported to be used to aid in the diagnosis of many cancers. A comparison of tumor tissues from patients with osteosarcoma and the corresponding paracancerous tissues revealed that miR-106 (163), miR-130a (56), miR-21 (166), miR-95-3p (168), miR-92a (170), miR-1908 (60), and miR-148a (171), miR-373 (173), miR-532-5p (176), miR-128 (177) and other PI3K/AKT pathway-related microRNAs all showed high expression in osteosarcoma, and then the clinical and pathological data of these patients were analyzed and studied in depth, and their expression was correlated with clinical stage and TNM stage were positively correlated. While miR-183-5p (164), miR-133b (167), miR-615 (59), miR-499a-5p (175), miR-29c -3p (178), miR-520c-3p (179), miR-195-5p (181), miR 141-3p (174) and other PI3K/AKT pathway-related microRNAs all showed low expression in osteosarcoma, and their expression was negatively correlated with clinical stage and TNM stage. Therefore, these microRNAs can be used as valuable biomarkers for diagnosing the clinical stage and TNM stage of OS patients. In addition, miR-615 (59), miR-532-5p (176), also correlated with lymph node metastasis, and the low expression of miR-615 in osteosarcoma or high expression of miR-532-5p suggested the possibility of lymph node metastasis, so miR-615 and miR-532-5p are useful for diagnosing lymph node metastasis Therefore, miR-615 and miR-532-5p are meaningful for diagnosing lymph node metastasis. In terms of pathological diagnosis, it was found that low expression of miR-130a (56) and miR-449a (172) or high expression of miR-340-5p (165) suggested the possibility of malignant pathological features, so this kind of PI3K/AKT pathway-related miRNA has a certain supporting role for pathological diagnosis. However, we found that due to the insufficient stability of micrornas related to PI3K/AKT pathway, poor correlation between micrornas and the early stage of osteosarcoma, few clinical studies and other reasons, their use as biomarkers for the early diagnosis of osteosarcoma is limited. Therefore, more in-depth studies are needed to solve these problems.
5.2 Treatment
Currently, the treatment of OS mainly includes aggressive surgical resection, systemic chemotherapy and targeted radiation therapy. Although such a comprehensive treatment approach has achieved certain results, the resistance to chemotherapy and the insensitivity to targeted radiation therapy also lead to unsatisfactory treatment results and prognosis for many patients. microRNA-based targeted therapeutic strategies may provide new ideas for the development of OS therapy. Several studies have shown that microRNAs can modulate the chemosensitivity and radiosensitivity of OS by directly or indirectly interacting with PI3K/AKT pathway. Cisplatin is the traditional first-line chemotherapeutic agent for OS (250). Therefore, increasing the sensitivity of osteosarcoma cells to cisplatin is very meaningful for the treatment of osteosarcoma, and it was found that miR-181a-5p (55), miR-214 (58), miR-221 (94), miR-128 (177) and other microRNAs related to the PI3K/AKT pathway in osteosarcoma cells They are pro-oncogenic factors, and by inhibiting their expression, the sensitivity of osteosarcoma cells to CDDP can be improved. In contrast, miR-22 (185), miR-29b (187), miR-100 (194), miR-142 (199), miR-497 (200), miR-340-5p (208) and other microRNAs related to PI3K/AKT pathway showed bottom expression, and by promoting their expression, the sensitivity of osteosarcoma cells to CDDP could be improved. In addition, Ze-Yu Sun found that promoting miR-152 overexpression also improved OS sensitivity to gemcitabine (207). These findings are very important guidelines for our in-depth understanding and study of chemotherapeutic drug resistance in microRNA/PI3K/AKT pathway osteosarcoma cells, and we can target these microRNAs to find possible solutions to the resistance of osteosarcoma cells to pharmacotherapeutic drugs. In radiation therapy, Yi Li et al. demonstrated that upregulation of miR-214 significantly reduced the radioresistance of osteosarcoma cells, while upregulation of miR-214 increased its targeted binding to the 3’-UTR region of PHLDA2, resulting in decreased PHLDA2 expression and enhanced the radiosensitivity of osteosarcoma cells and a mouse xenograft model. This study suggests that it is feasible to enhance the radiosensitivity of sarcomas via the microRNA/PI3K/AKT axis (182). The treatment of osteosarcoma with mirnas associated with PI3K/Akt signaling pathway can be conducted from two aspects. One is to design drugs for new targets. For example, Formononetin (FN) can induce apoptosis of osteosarcoma cells by targeting miR-214-3 p and inhibit proliferation of osteosarcoma cells (226). The second is to conduct research on existing anti-osteosarcoma drugs to find miRNAs that can promote the effect of existing anti-osteosarcoma drugs, improve the sensitivity of existing drugs and toxicity against tumor cells.
5.3 Prognosis prediction
Early prognostic information is important in making treatment decisions for patients with osteosarcoma. In recent years, there has been increasing evidence that microRNAs associated with the PI3K/AKT pathway may have important prognostic value. miR-130a (56), miR-200c (169), miR-92a (251), miR-148a (171), miR-532-5p (176) and miR-128 (177) are promoters of osteosarcoma, and their high expression was found to be negatively correlated with the overall survival of patients. While miR-183-5p (164), miR-340-5p (165), miR-506-3p (159), miR-615 (59), miR-449a (172), miR-141-3p (174), miR-29c-3p (178), miR-149-5p (180) and miR-195-5p (181) low expression was negatively correlated with overall survival of Patients with Osteosarcoma. Among them, high expression of miR-130a (56), miR-92a (170) and miR-128 (177) also showed a negative correlation with the disease-free survival of patients. The expression profiles of these microRNAs associated with the PI3K/AKT pathway are of great interest as early prognostic information.Therefore, efforts need to be made to identify PI3K/AKT related miRNAs that are prognostic early in the development of osteosarcoma, which would be of great benefit to patients.
6 Conclusions and future perspectives
The PI3K/AKT pathway is heavily involved in the development of osteosarcoma and involved in various cellular functions of osteosarcoma cells, including the regulation of proliferation, migration, invasion, apoptosis, autophagy, angiogenesis, EMT, chemotherapy resistance and aerobic glycolysis during OS progression. MiRNAs are also involved in osteosarcoma caused by abnormal PI3K/AKT pathway. Identification and utilization of aberrant expression patterns of miRNAs associated with the PI3K/AKT pathway will facilitate clinical applications, including diagnosis, treatment, and prognosis of patients with osteosarcoma.
However, the current understanding of miRNA and PI3K/AKT pathway in the academic community is limited. The research on the mechanism of miRNA in osteosarcoma is mostly limited to a single or a few miRNA, and most of the related experiments are carried out in animals, human tissues and osteosarcoma cell lines, and the clinical practice research is relatively rare. This has limited our understanding of the miRNA/PI3K/AKT pathway. It also limits our knowledge and understanding of the interrelationship between miRNA/PI3K/AKT pathway and osteosarcoma development. In addition, MiRNA related to PI3K/AKT pathway are also potential therapeutic biomarkers for osteosarcoma. However, there are still many problems with miRNA as biomarkers for diagnosis, treatment and prognosis of osteosarcoma patients. For example, there are no drugs related to miRNA used for large-scale clinical treatment of osteosarcoma. As diagnostic biomarkers for osteosarcoma, most mirnas cannot be used as diagnostic markers for early osteosarcoma, such as miR-130a (56), miR-133b (167), miR-499a-5p (175) etc., which can only show diagnostic role in the late stage of osteosarcoma. Therefore, the significance of miRNA as biomarkers is limited at present.
MiRNA negatively or positively regulate the progression of osteosarcoma by directly or indirectly interacting with PI3K/AKT pathway. We can enhance the expression of repressor microRNA in osteosarcoma cells or inhibit the expression of pro-cancer microRNA in osteosarcoma cells to control cancer progression. For example, overexpression of MicroRNA-26a and miR-29a-3p can significantly reduce the proliferation, migration and invasion ability of OS (138, 210). MiR-524 knockdown can significantly inhibit the proliferation of osteosarcoma cells (245). MiRNA also affect the resistance of osteosarcoma cells to chemotherapeutic drugs. By inhibiting the expression of miR-181a-5p (55), miR-214 (58), miR-221 (94) and miR-128 (163), the sensitivity of osteosarcoma cells to cisplatin (CDDP) can be improved. Promoting the expression of mirnas such as miR-22 (208) can improve the sensitivity of osteosarcoma cells to cisplatin (CDDP) and enhance the therapeutic effect of chemotherapy drugs. Therefore, strengthening the research and investigation of the mechanism of miRNA involved in anti-tumor drug resistance will provide clinical support for strategies to overcome drug resistance, and should provide more effective new insights into the development of therapeutic methods involving miRNA. Further understanding of the structure and function of miRNA associated with PI3K/AKT signaling is also needed. In addition, further studies are needed to confirm the interaction between miRNA involved in the PI3K/AKT pathway and their related mechanisms. In the clinical application and dissemination of new therapies targeting miRNA/PI3K/AKT pathway, there are problems such as unclear indications and contraindications, unclear side effects, and imperfect coping strategies. For diagnosis and prognosis, we wanted to find a stable, easy to detect, sensitive, and specific expression of PI3K/AKT pathway related miRNA in the early stage of osteosarcoma. The ultimate goal is to translate the research results of miRNA/PI3K/AKT pathway in osteosarcoma into clinical practice, and to use these targets to develop various effective antitumor drugs.
Author contributions
YX and YY: These authors contributed equally to this work and share first authorship. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Luetke A, Meyers PA, Lewis I, Juergens H. Osteosarcoma treatment - where do we stand? a state of the art review. Cancer Treat Rev (2014) 40:523–32. doi: 10.1016/j.ctrv.2013.11.006
2. Rothzerg E, Ho XD, Xu J, Wood D, Martson A, Maasalu K, et al. Alternative splicing of leptin receptor overlapping transcript in osteosarcoma. Exp Biol Med (Maywood) (2020) 245:1437–43. doi: 10.1177/1535370220949139
3. Xu R, Feng F, Yu X, Liu Z, Lao L. LncRNA SNHG4 promotes tumour growth by sponging miR-224-3p and predicts poor survival and recurrence in human osteosarcoma. Cell proliferation (2018) 51:e12515. doi: 10.1111/cpr.12515
4. Nagarajan R, Kamruzzaman A, Ness KK, Marchese VG, Sklar C, Mertens A, et al. Twenty years of follow-up of survivors of childhood osteosarcoma: a report from the childhood cancer survivor study. Cancer (2011) 117:625–34. doi: 10.1002/cncr.25446
5. Zhang P, Li J. Down-regulation of circular RNA hsa_circ_0007534 suppresses cell growth by regulating miR-219a-5p/SOX5 axis in osteosarcoma. J Bone Oncol (2021) 27:100349. doi: 10.1016/j.jbo.2021.100349
6. Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Helmke K, Flege S, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol (2002) 20:776–90. doi: 10.1200/JCO.2002.20.3.776
7. Isakoff MS, Bielack SS, Meltzer P, Gorlick R. Osteosarcoma: current treatment and a collaborative pathway to success. J Clin Oncol (2015) 33:3029–35. doi: 10.1200/JCO.2014.59.4895
8. Fujiwara T, Ozaki T. Overcoming therapeutic resistance of bone sarcomas: overview of the molecular mechanisms and therapeutic targets for bone sarcoma stem cells. Stem Cells Int (2016) 2016:2603092. doi: 10.1155/2016/2603092
9. Engreitz JM, Haines JE, Perez EM, Munson G, Chen J, Kane M, et al. Local regulation of gene expression by lncRNA promoters, transcription and splicing. Nature (2016) 539:452–5. doi: 10.1038/nature20149
10. Lu S, Zhang J, Lian X, Sun L, Meng K, Chen Y, et al. A hidden human proteome encoded by 'non-coding' genes. Nucleic Acids Res (2019) 47:8111–25. doi: 10.1093/nar/gkz646
11. Saw PE, Xu X, Chen J, Song EW. Non-coding RNAs: the new central dogma of cancer biology. Sci China Life Sci (2021) 64:22–50. doi: 10.1007/s11427-020-1700-9
12. Matsui M, Corey DR. Non-coding RNAs as drug targets. Nat Rev Drug Discovery (2017) 16:167–79. doi: 10.1038/nrd.2016.117
13. Li G, Zhang T, Huang K, Zhu Y, Xu K, Gu J, et al. Long noncoding RNA GAS8-AS1: a novel biomarker in human diseases. BioMed Pharmacother (2021) 139:111572. doi: 10.1016/j.biopha.2021.111572
14. Beermann J, Piccoli MT, Viereck J, Thum T. Non-coding RNAs in development and disease: background, mechanisms, and therapeutic approaches. Physiol Rev (2016) 96:1297–325. doi: 10.1152/physrev.00041.2015
15. Chan JJ, Tay Y. Noncoding RNA:RNA regulatory networks in cancer. Int J Mol Sci (2018) 19. doi: 10.3390/ijms19051310
16. Zhu KP, Zhang CL, Ma XL, Hu JP, Cai T, Zhang L. Analyzing the interactions of mRNAs and ncRNAs to predict competing endogenous RNA networks in osteosarcoma chemo-resistance. Mol Ther (2019) 27:518–30. doi: 10.1016/j.ymthe.2019.01.001
17. Lu TX, Rothenberg ME. MicroRNA. J Allergy Clin Immunol (2018) 141:1202–7. doi: 10.1016/j.jaci.2017.08.034
18. Correia de Sousa M, Gjorgjieva M, Dolicka D, Sobolewski C, Foti M. Deciphering miRNAs' action through miRNA editing. Int J Mol Sci (2019) 20. doi: 10.3390/ijms20246249
19. Ali Syeda Z, Langden SSS, Munkhzul C, Lee M, Song SJ. Regulatory mechanism of MicroRNA expression in cancer. Int J Mol Sci (2020) 21. doi: 10.3390/ijms21051723
20. Shen Y, Zhao N, Zhao N, Hu X, He X, Xu Y, et al. Tumor-suppressive and oncogenic roles of microRNA-149-5p in human cancers. Int J Mol Sci (2022) 23. doi: 10.3390/ijms231810823
21. Mirzaei S, Zarrabi A, Asnaf SE, Hashemi F, Zabolian A, Hushmandi K, et al. The role of microRNA-338-3p in cancer: growth, invasion, chemoresistance, and mediators. Life Sci (2021) 268:119005. doi: 10.1016/j.lfs.2020.119005
22. Li X, Zeng Z, Wang J, Wu Y, Chen W, Zheng L, et al. MicroRNA-9 and breast cancer. BioMed Pharmacother (2020) 122:109687. doi: 10.1016/j.biopha.2019.109687
23. Dai YC, Pan Y, Quan MM, Chen Q, Pan Y, Ruan YY, et al. MicroRNA-1246 mediates drug resistance and metastasis in breast cancer by targeting NFE2L3. Front Oncol (2021) 11:677168. doi: 10.3389/fonc.2021.677168
24. Zu D, Dong Q, Chen S, Chen Y, Yao J, Zou Y, et al. miRNA-331-3p affects the proliferation, metastasis, and invasion of osteosarcoma through SOCS1/JAK2/STAT3. J Oncol (2022) 2022:6459029. doi: 10.1155/2022/6459029
25. Dai W, Liu H. MicroRNA-886 suppresses osteosarcoma cell proliferation and its maturation is suppressed by long non-coding RNA OXCT1-AS1. Bioengineered (2022) 13:5769–78. doi: 10.1080/21655979.2022.2031669
26. Cao D, Ge S, Li M. MiR-451a promotes cell growth, migration and EMT in osteosarcoma by regulating YTHDC1-mediated m6A methylation to activate the AKT/mTOR signaling pathway. J Bone Oncol (2022) 33:100412. doi: 10.1016/j.jbo.2022.100412
27. Soghli N, Ferns GA, Sadeghsoltani F, Qujeq D, Yousefi T, Vaghari-Tabari M. MicroRNAs and osteosarcoma: potential targets for inhibiting metastasis and increasing chemosensitivity. Biochem Pharmacol (2022) 201:115094. doi: 10.1016/j.bcp.2022.115094
28. Lawrence MS, Stojanov P, Mermel CH, Robinson JT, Garraway LA, Golub TR, et al. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature (2014) 505:495–501. doi: 10.1038/nature12912
29. Hoxhaj G, Manning BD. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat Rev Cancer (2020) 20:74–88. doi: 10.1038/s41568-019-0216-7
30. Edlind MP, Hsieh AC. PI3K-AKT-mTOR signaling in prostate cancer progression and androgen deprivation therapy resistance. Asian J Androl (2014) 16:378–86. doi: 10.4103/1008-682X.122876
31. Kim SG, Buel GR, Blenis J. Nutrient regulation of the mTOR complex 1 signaling pathway. Mol Cells (2013) 35:463–73. doi: 10.1007/s10059-013-0138-2
32. Mori S, Nada S, Kimura H, Tajima S, Takahashi Y, Kitamura A, et al. The mTOR pathway controls cell proliferation by regulating the FoxO3a transcription factor via SGK1 kinase. PloS One (2014) 9:e88891. doi: 10.1371/journal.pone.0088891
33. Holroyd AK, Michie AM. The role of mTOR-mediated signaling in the regulation of cellular migration. Immunol Lett (2018) 196:74–9. doi: 10.1016/j.imlet.2018.01.015
34. Jillson LK, Yette GA, Laajala TD, Tilley WD, Costello JC, Cramer SD. Androgen receptor signaling in prostate cancer genomic subtypes. Cancers (Basel) (2021) 13. doi: 10.3390/cancers13133272
35. Gasparri ML, Bardhi E, Ruscito I, Papadia A, Farooqi AA, Marchetti C, et al. PI3K/AKT/mTOR pathway in ovarian cancer treatment: are we on the right track? Geburtshilfe Frauenheilkd (2017) 77:1095–103. doi: 10.1055/s-0043-118907
36. Mabuchi S, Kuroda H, Takahashi R, Sasano T. The PI3K/AKT/mTOR pathway as a therapeutic target in ovarian cancer. Gynecol Oncol (2015) 137:173–9. doi: 10.1016/j.ygyno.2015.02.003
37. Papadimitrakopoulou V. Development of PI3K/AKT/mTOR pathway inhibitors and their application in personalized therapy for non-small-cell lung cancer. J Thorac Oncol (2012) 7:1315–26. doi: 10.1097/JTO.0b013e31825493eb
38. He L, Wu Y, Lin L, Wang J, Wu Y, Chen Y, et al. Hispidulin, a small flavonoid molecule, suppresses the angiogenesis and growth of human pancreatic cancer by targeting vascular endothelial growth factor receptor 2-mediated PI3K/Akt/mTOR signaling pathway. Cancer Sci (2011) 102:219–25. doi: 10.1111/j.1349-7006.2010.01778.x
39. Mahajan K, Mahajan NP. PI3K-independent AKT activation in cancers: a treasure trove for novel therapeutics. J Cell Physiol (2012) 227:3178–84. doi: 10.1002/jcp.24065
40. Zhang M, Liu Y, Yin Y, Sun Z, Wang Y, Zhang Z, et al. UBE2S promotes the development of ovarian cancer by promoting PI3K/AKT/mTOR signaling pathway to regulate cell cycle and apoptosis. Mol Med (2022) 28:62. doi: 10.1186/s10020-022-00489-2
41. Leng J, Li H, Niu Y, Chen K, Yuan X, Chen H, et al. Low-dose mono(2-ethylhexyl) phthalate promotes ovarian cancer development through PPARalpha-dependent PI3K/Akt/NF-kappaB pathway. Sci Total Environ (2021) 790:147990. doi: 10.1016/j.scitotenv.2021.147990
42. Shi L, Zhu W, Huang Y, Zhuo L, Wang S, Chen S, et al. Cancer-associated fibroblast-derived exosomal microRNA-20a suppresses the PTEN/PI3K-AKT pathway to promote the progression and chemoresistance of non-small cell lung cancer. Clin Trans Med (2022) 12. doi: 10.1002/ctm2.989
43. Yu J, Zhang L, Peng J, Ward R, Hao P, Wang J, et al. Dictamnine, a novel c-met inhibitor, suppresses the proliferation of lung cancer cells by downregulating the PI3K/AKT/mTOR and MAPK signaling pathways. Biochem Pharmacol (2022) 195:114864. doi: 10.1016/j.bcp.2021.114864
44. Wu S, Chen M, Huang J, Zhang F, Lv Z, Jia Y, et al. ORAI2 promotes gastric cancer tumorigenicity and metastasis through PI3K/Akt signaling and MAPK-dependent focal adhesion disassembly. Cancer Res (2021) 81:986–1000. doi: 10.1158/0008-5472.Can-20-0049
45. Shu X, Zhan PP, Sun LX, Yu L, Liu J, Sun LC, et al. BCAT1 activates PI3K/AKT/mTOR pathway and contributes to the angiogenesis and tumorigenicity of gastric cancer. Front Cell Dev Biol (2021) 9:659260. doi: 10.3389/fcell.2021.659260
46. Stanciu S, Ionita-Radu F, Stefani C, Miricescu D, Stanescu S II, Greabu M, et al. Targeting PI3K/AKT/mTOR signaling pathway in pancreatic cancer: from molecular to clinical aspects. Int J Mol Sci (2022) 23. doi: 10.3390/ijms231710132
47. Wu W, Li Q, Zhu Z, Li C, Lu P, Zhou X, et al. HTR1D functions as a key target of HOXA10-AS/miR-340-3p axis to promote the malignant outcome of pancreatic cancer via PI3K-AKT signaling pathway. Int J Biol Sci (2022) 18:3777–94. doi: 10.7150/ijbs.70546
48. Wang L, Zhou Y, Jiang L, Lu L, Dai T, Li A, et al. CircWAC induces chemotherapeutic resistance in triple-negative breast cancer by targeting miR-142, upregulating WWP1 and activating the PI3K/AKT pathway. Mol Cancer (2021) 20:43. doi: 10.1186/s12943-021-01332-8
49. Qu J, Li J, Zhang Y, He R, Liu X, Gong K, et al. AKR1B10 promotes breast cancer cell proliferation and migration via the PI3K/AKT/NF-kappaB signaling pathway. Cell Biosci (2021) 11:163. doi: 10.1186/s13578-021-00677-3
50. Pu Z, Duda DG, Zhu Y, Pei S, Wang X, Huang Y, et al. VCP interaction with HMGB1 promotes hepatocellular carcinoma progression by activating the PI3K/AKT/mTOR pathway. J Transl Med (2022) 20:212. doi: 10.1186/s12967-022-03416-5
51. Han W, Wang N, Kong R, Bao W, Lu J. Ligand-activated PPARdelta expression promotes hepatocellular carcinoma progression by regulating the PI3K-AKT signaling pathway. J Transl Med (2022) 20:86. doi: 10.1186/s12967-022-03288-9
52. Feng M, Yang K, Wang J, Li G, Zhang H. First report of FARSA in the regulation of cell cycle and survival in mantle cell lymphoma cells via PI3K-AKT and FOXO1-RAG1 axes. Int J Mol Sci (2023) 24. doi: 10.3390/ijms24021608
53. Zhang R, Huang T, Li J, Zhou H, Wang X. Effect of miR-27b on the proliferation and apoptosis of diffuse large b-cell lymphoma cells by targeting the regulation of MET/PI3K/AKT pathway. Discovery Oncol (2022) 13:137. doi: 10.1007/s12672-022-00589-9
54. Li H, Shen X, Ma M, Liu W, Yang W, Wang P, et al. ZIP10 drives osteosarcoma proliferation and chemoresistance through ITGA10-mediated activation of the PI3K/AKT pathway. J Exp Clin Cancer Res (2021) 40:340. doi: 10.1186/s13046-021-02146-8
55. Sun C, Chen C, Chen Z, Guo J, Yu ZH, Qian W, et al. MicroRNA-181a-5p promotes osteosarcoma progression via PTEN/AKT pathway. Anal Cell Pathol (Amst) (2022) 2022:3421600. doi: 10.1155/2022/3421600
56. Chen J, Yan D, Wu W, Zhu J, Ye W, Shu Q. MicroRNA-130a promotes the metastasis and epithelial-mesenchymal transition of osteosarcoma by targeting PTEN. Oncol Rep (2016) 35:3285–92. doi: 10.3892/or.2016.4719
57. Yu B, Jiang K, Zhang J. MicroRNA-124 suppresses growth and aggressiveness of osteosarcoma and inhibits TGF-beta-mediated AKT/GSK-3beta/SNAIL-1 signaling. Mol Med Rep (2018) 17:6736–44. doi: 10.3892/mmr.2018.8637
58. Ou L, Lin H, Song Y, Tan G, Gui X, Li J, et al. Efficient miRNA inhibitor with GO-PEI nanosheets for osteosarcoma suppression by targeting PTEN. Int J Nanomedicine (2020) 15:5131–46. doi: 10.2147/IJN.S257084
59. Sun L, Wang P, Zhang Z, Zhang K, Xu Z, Li S, et al. MicroRNA-615 functions as a tumor suppressor in osteosarcoma through the suppression of HK2. Oncol Lett (2020) 20:226. doi: 10.3892/ol.2020.12089
60. Yuan H, Gao Y. MicroRNA-1908 is upregulated in human osteosarcoma and regulates cell proliferation and migration by repressing PTEN expression. Oncol Rep (2015) 34:2706–14. doi: 10.3892/or.2015.4242
61. Zheng J, Liu C, Shi J, Wen K, Wang X. AIM2 inhibits the proliferation, invasion and migration, and promotes the apoptosis of osteosarcoma cells by inactivating the PI3K/AKT/mTOR signaling pathway. Mol Med Rep (2022) 25. doi: 10.3892/mmr.2021.12569
62. Zhao X, Fang Y, Wang X, Yang Z, Li D, Tian M, et al. Knockdown of ski decreases osteosarcoma cell proliferation and migration by suppressing the PI3K/Akt signaling pathway. Int J Oncol (2020) 56:206–18. doi: 10.3892/ijo.2019.4914
63. Chen J, Liu C, Yang QQ, Ma RB, Ke Y, Dong F, et al. Isoliquiritigenin suppresses osteosarcoma U2OS cell proliferation and invasion by regulating the PI3K/Akt signalling pathway. Chemotherapy (2018) 63:155–61. doi: 10.1159/000490151
64. Yue Z, Guan X, Chao R, Huang C, Li D, Yang P, et al. Diallyl disulfide induces apoptosis and autophagy in human osteosarcoma MG-63 cells through the PI3K/Akt/mTOR pathway. Molecules (Basel Switzerland) (2019) 24. doi: 10.3390/molecules24142665
65. Liu L, Geng H, Mei C, Chen L. Zoledronic acid enhanced the antitumor effect of cisplatin on orthotopic osteosarcoma by ROS-PI3K/AKT signaling and attenuated osteolysis. Oxid Med Cell Longev (2021) 2021:6661534. doi: 10.1155/2021/6661534
66. Osaki M, Oshimura M, Ito H. PI3K-akt pathway: its functions and alterations in human cancer. Apoptosis (2004) 9:667–76. doi: 10.1023/B:APPT.0000045801.15585.dd
67. Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nat Rev Drug Discovery (2014) 13:140–56. doi: 10.1038/nrd4204
68. Ahmad A, Biersack B, Li Y, Kong D, Bao B, Schobert R, et al. Targeted regulation of PI3K/Akt/mTOR/NF-kappaB signaling by indole compounds and their derivatives: mechanistic details and biological implications for cancer therapy. Anticancer Agents Med Chem (2013) 13:1002–13. doi: 10.2174/18715206113139990078
69. Dornan GL, Burke JE. Molecular mechanisms of human disease mediated by oncogenic and primary immunodeficiency mutations in class IA phosphoinositide 3-kinases. Front Immunol (2018) 9:575. doi: 10.3389/fimmu.2018.00575
70. Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol (2010) 11:329–41. doi: 10.1038/nrm2882
71. De Santis MC, Gulluni F, Campa CC, Martini M, Hirsch E. Targeting PI3K signaling in cancer: challenges and advances. Biochim Biophys Acta Rev Cancer (2019) 1871:361–6. doi: 10.1016/j.bbcan.2019.03.003
72. Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet (2006) 7:606–19. doi: 10.1038/nrg1879
73. Liu L, Meng T, Zheng X, Liu Y, Hao R, Yan Y, et al. Transgelin 2 promotes paclitaxel resistance, migration, and invasion of breast cancer by directly interacting with PTEN and activating PI3K/Akt/GSK-3beta pathway. Mol Cancer Ther (2019) 18:2457–68. doi: 10.1158/1535-7163.MCT-19-0261
74. Lee YR, Chen M, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor: new modes and prospects. Nat Rev Mol Cell Biol (2018) 19:547–62. doi: 10.1038/s41580-018-0015-0
75. Scheid MP, Woodgett JR. PKB/AKT: functional insights from genetic models. Nat Rev Mol Cell Biol (2001) 2:760–8. doi: 10.1038/35096067
76. Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, et al. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase balpha. Curr Biol (1997) 7:261–9. doi: 10.1016/s0960-9822(06)00122-9
77. Majchrzak A, Witkowska M, Smolewski P. Inhibition of the PI3K/Akt/mTOR signaling pathway in diffuse large b-cell lymphoma: current knowledge and clinical significance. Molecules (Basel Switzerland) (2014) 19:14304–15. doi: 10.3390/molecules190914304
78. Maira SM, Galetic I, Brazil DP, Kaech S, Ingley E, Thelen M, et al. Carboxyl-terminal modulator protein (CTMP), a negative regulator of PKB/Akt and v-akt at the plasma membrane. Science (2001) 294:374–80. doi: 10.1126/science.1062030
79. Scarpa M, Singh P, Bailey CM, Lee JK, Kapoor S, Lapidus RG, et al. PP2A-activating drugs enhance FLT3 inhibitor efficacy through AKT inhibition-dependent GSK-3beta-Mediated c-myc and pim-1 proteasomal degradation. Mol Cancer Ther (2021) 20:676–90. doi: 10.1158/1535-7163.MCT-20-0663
80. Pekarsky Y, Koval A, Hallas C, Bichi R, Tresini M, Malstrom S, et al. Tcl1 enhances akt kinase activity and mediates its nuclear translocation. Proc Natl Acad Sci United States America (2000) 97:3028–33. doi: 10.1073/pnas.97.7.3028
81. Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discovery (2009) 8:627–44. doi: 10.1038/nrd2926
82. Fu K, Robbins SR, McDougall JJ. Osteoarthritis: the genesis of pain. Rheumatol (Oxford) (2018) 57:iv43–50. doi: 10.1093/rheumatology/kex419
83. Manning BD, Toker A. AKT/PKB signaling: navigating the network. Cell (2017) 169:381–405. doi: 10.1016/j.cell.2017.04.001
84. Kuijjer ML, van den Akker BE, Hilhorst R, Mommersteeg M, Buddingh EP, Serra M, et al. Kinome and mRNA expression profiling of high-grade osteosarcoma cell lines implies akt signaling as possible target for therapy. BMC Med Genomics (2014) 7:4. doi: 10.1186/1755-8794-7-4
85. Wang TF, Wang H, Peng AF, Luo QF, Liu ZL, Zhou RP, et al. Inhibition of fatty acid synthase suppresses U-2 OS cell invasion and migration via downregulating the activity of HER2/PI3K/AKT signaling pathway in vitro. Biochem Biophys Res Commun (2013) 440:229–34. doi: 10.1016/j.bbrc.2013.09.024
86. Chen H, Pan R, Li H, Zhang W, Ren C, Lu Q, et al. CHRDL2 promotes osteosarcoma cell proliferation and metastasis through the BMP-9/PI3K/AKT pathway. Cell Biol Int (2021) 45:623–32. doi: 10.1002/cbin.11507
87. Zhang X, Qu P, Zhao H, Zhao T, Cao N. COX−2 promotes epithelial−mesenchymal transition and migration in osteosarcoma MG−63 cells via PI3K/AKT/NF−kappaB signaling. Mol Med Rep (2019) 20:3811–9. doi: 10.3892/mmr.2019.10598
88. Ma K, Zhang C. HER4 promotes osteosarcoma progression and predicts poor prognosis through the PTEN-PI3K/AKT pathway. J Cancer (2022) 13:290–303. doi: 10.7150/jca.62787
89. Fujisawa T, Huang Y, Sebald W, Zhang JL. The binding of von willebrand factor type c domains of chordin family proteins to BMP-2 and tsg is mediated by their SD1 subdomain. Biochem Biophys Res Commun (2009) 385:215–9. doi: 10.1016/j.bbrc.2009.05.041
90. Lee KS, Lee DH, Chun SY, Nam KS. Metastatic potential in MDA-MB-231 human breast cancer cells is inhibited by proton beam irradiation via the akt/nuclear factor-kappaB signaling pathway. Mol Med Rep (2014) 10:1007–12. doi: 10.3892/mmr.2014.2259
91. Maric G, Annis MG, Dong Z, Rose AA, Ng S, Perkins D, et al. GPNMB cooperates with neuropilin-1 to promote mammary tumor growth and engages integrin alpha5beta1 for efficient breast cancer metastasis. Oncogene (2015) 34:5494–504. doi: 10.1038/onc.2015.8
92. Jin R, Jin YY, Tang YL, Yang HJ, Zhou XQ, Lei Z. GPNMB silencing suppresses the proliferation and metastasis of osteosarcoma cells by blocking the PI3K/Akt/mTOR signaling pathway. Oncol Rep (2018) 39:3034–40. doi: 10.3892/or.2018.6346
93. Nussinov R, Zhang M, Tsai CJ, Jang H. Phosphorylation and driver mutations in PI3Kalpha and PTEN autoinhibition. Mol Cancer Res (2021) 19:543–8. doi: 10.1158/1541-7786.MCR-20-0818
94. Zhao G, Cai C, Yang T, Qiu X, Liao B, Li W, et al. MicroRNA-221 induces cell survival and cisplatin resistance through PI3K/Akt pathway in human osteosarcoma. PloS One (2013) 8:e53906. doi: 10.1371/journal.pone.0053906
95. Gao Y, Luo LH, Li S, Yang C. miR-17 inhibitor suppressed osteosarcoma tumor growth and metastasis via increasing PTEN expression. Biochem Biophys Res Commun (2014) 444:230–4. doi: 10.1016/j.bbrc.2014.01.061
96. Zhu B, Cheng D, Hou L, Zhou S, Ying T, Yang Q. SLC3A2 is upregulated in human osteosarcoma and promotes tumor growth through the PI3K/Akt signaling pathway. Oncol Rep (2017) 37:2575–82. doi: 10.3892/or.2017.5530
97. Hu B, Lv X, Gao F, Chen S, Wang S, Qing X, et al. Downregulation of DEPTOR inhibits the proliferation, migration, and survival of osteosarcoma through PI3K/Akt/mTOR pathway. Onco Targets Ther (2017) 10:4379–91. doi: 10.2147/OTT.S143518
98. Kong DL, Liu Y, Wang JY, Liu G, Zhang ML. Sodium cantharidinate suppresses human osteosarcoma MG−63 cell proliferation and induces cell cycle arrest by inhibition of PI3K/AKT activation. Oncol Rep (2019) 41:1351–8. doi: 10.3892/or.2018.6906
99. Zhu J, Sun Y, Lu Y, Jiang X, Ma B, Yu L, et al. Glaucocalyxin a exerts anticancer effect on osteosarcoma by inhibiting GLI1 nuclear translocation via regulating PI3K/Akt pathway. Cell Death Dis (2018) 9:708. doi: 10.1038/s41419-018-0684-9
100. Wang Y, Chen J, Huang Y, Yang S, Tan T, Wang N, et al. Schisandrin b suppresses osteosarcoma lung metastasis in vivo by inhibiting the activation of the wnt/beta−catenin and PI3K/Akt signaling pathways. Oncol Rep (2022) 47. doi: 10.3892/or.2022.8261
101. Huang Z, Wang S, Wei H, Chen H, Shen R, Lin R, et al. Inhibition of BUB1 suppresses tumorigenesis of osteosarcoma via blocking of PI3K/Akt and ERK pathways. J Cell Mol Med (2021) 25:8442–53. doi: 10.1111/jcmm.16805
102. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell (2011) 144:646–74. doi: 10.1016/j.cell.2011.02.013
103. Zhang K, Wu S, Wu H, Liu L, Zhou J. Effect of the Notch1-mediated PI3K-Akt-mTOR pathway in human osteosarcoma. Aging (Albany NY) (2021) 13:21090–101. doi: 10.18632/aging.203261
104. Cui Y, Dong YY. ZCCHC12 promotes the progression of osteosarcoma via PI3K/AKT pathway. Aging (Albany NY) (2022) 14:7505–16. doi: 10.18632/aging.204296
105. Ramakrishna A, Shreedhar B, Narayan T, Mohanty L, Shenoy S, Jamadar S. Cyclin D1 an early biomarker in oral carcinogenesis. J Oral Maxillofac Pathol (2013) 17:351–7. doi: 10.4103/0973-029X.125189
106. Lee YS, Dutta A. MicroRNAs in cancer. Annu Rev Pathol (2009) 4:199–227. doi: 10.1146/annurev.pathol.4.110807.092222
107. Hensley AP, McAlinden A. The role of microRNAs in bone development. Bone (2021) 143:115760. doi: 10.1016/j.bone.2020.115760
108. Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol (2008) 9:47–59. doi: 10.1038/nrm2308
109. Zhang S, Ren H, Sun H, Cao S. Dieckol exerts anticancer activity in human osteosarcoma (MG-63) cells through the inhibition of PI3K/AKT/mTOR signaling pathway. Saudi J Biol Sci (2021) 28:4908–15. doi: 10.1016/j.sjbs.2021.07.019
110. Zhang H, Jiang H, Zhang H, Liu J, Hu X, Chen L. Anti-tumor efficacy of phellamurin in osteosarcoma cells: involvement of the PI3K/AKT/mTOR pathway. Eur J Pharmacol (2019) 858:172477. doi: 10.1016/j.ejphar.2019.172477
111. Liu MY, Liu F, Li YJ, Yin JN, Gao YL, Wang XY, et al. Ginsenoside Rg5 inhibits human osteosarcoma cell proliferation and induces cell apoptosis through PI3K/Akt/mTORC1-related LC3 autophagy pathway. Oxid Med Cell Longev (2021) 2021:5040326. doi: 10.1155/2021/5040326
112. Gao S, Sun H, Cheng C, Wang G. BRCA1-associated protein-1 suppresses osteosarcoma cell proliferation and migration through regulation PI3K/Akt pathway. DNA Cell Biol (2017) 36:386–93. doi: 10.1089/dna.2016.3579
113. Da W, Tao L, Zhu Y. The inhibitory effect of CTAB on human osteosarcoma through the PI3K/AKT signaling pathway. Int J Oncol (2021) 59. doi: 10.3892/ijo.2021.5222
114. Wu X, Yu H, Zhou H, Li Z, Huang H, Xiao F, et al. Proanthocyanidin B2 inhibits proliferation and induces apoptosis of osteosarcoma cells by suppressing the PI3K/AKT pathway. J Cell Mol Med (2020) 24:11960–71. doi: 10.1111/jcmm.15818
115. Mickymaray S, Alfaiz FA, Paramasivam A, Veeraraghavan VP, Periadurai ND, Surapaneni KM, et al. Rhaponticin suppresses osteosarcoma through the inhibition of PI3K-Akt-mTOR pathway. Saudi J Biol Sci (2021) 28:3641–9. doi: 10.1016/j.sjbs.2021.05.006
116. Huang F, Liao F, Ma G, Hu Y, Zhang C, Xu P, et al. TBRG4 knockdown suppresses proliferation and growth of human osteosarcoma cell lines MG63 through PI3K/Akt pathway. Onco Targets Ther (2020) 13:7271–81. doi: 10.2147/OTT.S249477
117. Huang Y, Chen J, Yang S, Tan T, Wang N, Wang Y, et al. Cinnamaldehyde inhibits the function of osteosarcoma by suppressing the wnt/beta-catenin and PI3K/Akt signaling pathways. Drug design Dev Ther (2020) 14:4625–37. doi: 10.2147/DDDT.S277160
118. Ni S, Li J, Qiu S, Xie Y, Gong K, Duan Y. KIF21B expression in osteosarcoma and its regulatory effect on osteosarcoma cell proliferation and apoptosis through the PI3K/AKT pathway. Front Oncol (2020) 10:606765. doi: 10.3389/fonc.2020.606765
119. Wu W, Ren Z, Liu H, Wang L, Huang R, Chen J, et al. Core promoter analysis of porcine Six1 gene and its regulation of the promoter activity by CpG methylation. Gene (2013) 529:238–44. doi: 10.1016/j.gene.2013.07.102
120. Micalizzi DS, Wang CA, Farabaugh SM, Schiemann WP, Ford HL. Homeoprotein Six1 increases TGF-beta type I receptor and converts TGF-beta signaling from suppressive to supportive for tumor growth. Cancer Res (2010) 70:10371–80. doi: 10.1158/0008-5472.CAN-10-1354
121. Yu C, Zhang B, Li YL, Yu XR. SIX1 reduces the expression of PTEN via activating PI3K/AKT signal to promote cell proliferation and tumorigenesis in osteosarcoma. BioMed Pharmacother (2018) 105:10–7. doi: 10.1016/j.biopha.2018.04.028
122. Gao LW, Zhang J, Yang WH, Wang B, Wang JW. Glaucocalyxin a induces apoptosis in human leukemia HL-60 cells through mitochondria-mediated death pathway. Toxicol In Vitro (2011) 25:51–63. doi: 10.1016/j.tiv.2010.09.006
123. Xiao X, Cao W, Jiang X, Zhang W, Zhang Y, Liu B, et al. A negative akt regulator, specifically induces apoptosis in human brain glioblastoma U87MG cells. Acta Biochim Biophys Sin (2013) 45:946–52. doi: 10.1093/abbs/gmt097
124. Chen Z, Yang H, Zhang Q, Hu Q, Zhao Z. Chelerythrine inhibits stemness of cancer stem-like cells of osteosarcoma and PI3K/AKT/mTOR signal. J Oncol (2022) 2022:6435431. doi: 10.1155/2022/6435431
125. Liao YX, Lv JY, Zhou ZF, Xu TY, Yang D, Gao QM, et al. CXCR4 blockade sensitizes osteosarcoma to doxorubicin by inducing autophagic cell death via PI3K−Akt−mTOR pathway inhibition. Int J Oncol (2021) 59. doi: 10.3892/ijo.2021.5229
126. Wang S, Zhao G, Zhao S, Qiao Y, Yang H. The effects of interleukin-33 (IL-33) on osteosarcoma cell viability, apoptosis, and epithelial-mesenchymal transition are mediated through the PI3K/AKT pathway. Med Sci Monit (2020) 26:e920766. doi: 10.12659/MSM.920766
127. Yang Z, Klionsky DJ. Eaten alive: a history of macroautophagy. Nat Cell Biol (2010) 12:814–22. doi: 10.1038/ncb0910-814
128. Yang Z, Klionsky DJ. An overview of the molecular mechanism of autophagy. Curr Top Microbiol Immunol (2009) 335:1–32. doi: 10.1007/978-3-642-00302-8_1
129. Niu J, Yan T, Guo W, Wang W, Zhao Z. Insight into the role of autophagy in osteosarcoma and its therapeutic implication. Front Oncol (2019) 9:1232. doi: 10.3389/fonc.2019.01232
130. Xu Z, Han X, Ou D, Liu T, Li Z, Jiang G, et al. Targeting PI3K/AKT/mTOR-mediated autophagy for tumor therapy. Appl Microbiol Biotechnol (2020) 104:575–87. doi: 10.1007/s00253-019-10257-8
131. Pang H, Wu T, Peng Z, Tan Q, Peng X, Zhan Z, et al. Baicalin induces apoptosis and autophagy in human osteosarcoma cells by increasing ROS to inhibit PI3K/Akt/mTOR, ERK1/2 and beta-catenin signaling pathways. J Bone Oncol (2022) 33:100415. doi: 10.1016/j.jbo.2022.100415
132. Zhao GS, Gao ZR, Zhang Q, Tang XF, Lv YF, Zhang ZS, et al. TSSC3 promotes autophagy via inactivating the src-mediated PI3K/Akt/mTOR pathway to suppress tumorigenesis and metastasis in osteosarcoma, and predicts a favorable prognosis. J Exp Clin Cancer Res (2018) 37:188. doi: 10.1186/s13046-018-0856-6
133. Hinton K, Kirk A, Paul P, Persad S. Regulation of the epithelial to mesenchymal transition in osteosarcoma. Biomolecules (2023) 13. doi: 10.3390/biom13020398
134. Ma L, Zhang L, Guo A, Liu LC, Yu F, Diao N, et al. Overexpression of FER1L4 promotes the apoptosis and suppresses epithelial-mesenchymal transition and stemness markers via activating PI3K/AKT signaling pathway in osteosarcoma cells. Pathol Res Pract (2019) 215:152412. doi: 10.1016/j.prp.2019.04.004
135. Zhang D, Wang S, Chen J, Liu H, Lu J, Jiang H, et al. Fibulin-4 promotes osteosarcoma invasion and metastasis by inducing epithelial to mesenchymal transition via the PI3K/Akt/mTOR pathway. Int J Oncol (2017) 50:1513–30. doi: 10.3892/ijo.2017.3921
136. Qiu C, Su W, Shen N, Qi X, Wu X, Wang K, et al. MNAT1 promotes proliferation and the chemo-resistance of osteosarcoma cell to cisplatin through regulating PI3K/Akt/mTOR pathway. BMC Cancer (2020) 20:1187. doi: 10.1186/s12885-020-07687-3
137. Deng B, Deng J, Yi X, Zou Y, Li C. ROCK2 promotes osteosarcoma growth and glycolysis by up-regulating HKII via phospho-PI3K/AKT signalling. Cancer Manag Res (2021) 13:449–62. doi: 10.2147/CMAR.S279496
138. Tan X, Fan S, Wu W, Zhang Y. MicroRNA-26a inhibits osteosarcoma cell proliferation by targeting IGF-1. Bone Res (2015) 3:15033. doi: 10.1038/boneres.2015.33
139. Zhang D, Liu H, Wang W, Xu G, Yin C, Wang S. STEAP2 promotes osteosarcoma progression by inducing epithelial-mesenchymal transition via the PI3K/AKT/mTOR signaling pathway and is regulated by EFEMP2. Cancer Biol Ther (2022) 23:1–16. doi: 10.1080/15384047.2022.2136465
140. D'Adamo DR. Appraising the current role of chemotherapy for the treatment of sarcoma. Semin Oncol (2011) 38 Suppl 3:S19–29. doi: 10.1053/j.seminoncol.2011.09.004
141. He H, Ni J, Huang J. Molecular mechanisms of chemoresistance in osteosarcoma (Review). Oncol Lett (2014) 7:1352–62. doi: 10.3892/ol.2014.1935
142. Zhang S, Gangal G, Uludag H. 'Magic bullets' for bone diseases: progress in rational design of bone-seeking medicinal agents. Chem Soc Rev (2007) 36:507–31. doi: 10.1039/b512310k
143. Ferrari S, Palmerini E. Adjuvant and neoadjuvant combination chemotherapy for osteogenic sarcoma. Curr Opin Oncol (2007) 19:341–6. doi: 10.1097/CCO.0b013e328122d73f
144. Liu H, Wang SH, Chen SC, Chen CY, Lin TM. Zoledronic acid blocks the interaction between breast cancer cells and regulatory T-cells. BMC Cancer (2019) 19:176. doi: 10.1186/s12885-019-5379-9
145. Wang L, Liu Y, Zhou Y, Wang J, Tu L, Sun Z, et al. Zoledronic acid inhibits the growth of cancer stem cell derived from cervical cancer cell by attenuating their stemness phenotype and inducing apoptosis and cell cycle arrest through the Erk1/2 and akt pathways. J Exp Clin Cancer Res (2019) 38:93. doi: 10.1186/s13046-019-1109-z
146. Di Fiore R, Guercio A, Puleio R, Di Marco P, Drago-Ferrante R, D'Anneo A, et al. Modeling human osteosarcoma in mice through 3AB-OS cancer stem cell xenografts. J Cell Biochem (2012) 113:3380–92. doi: 10.1002/jcb.24214
147. Kuzmanov A, Wielockx B, Rezaei M, Kettelhake A, Breier G. Overexpression of factor inhibiting HIF-1 enhances vessel maturation and tumor growth via platelet-derived growth factor-c. Int J Cancer (2012) 131:E603–613. doi: 10.1002/ijc.27360
148. Klotzsche-von Ameln A, Prade I, Grosser M, Kettelhake A, Rezaei M, Chavakis T, et al. PHD4 stimulates tumor angiogenesis in osteosarcoma cells via TGF-alpha. Mol Cancer Res (2013) 11:1337–48. doi: 10.1158/1541-7786.MCR-13-0201
149. Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol (2011) 27:441–64. doi: 10.1146/annurev-cellbio-092910-154237
150. Lu J, Tan M, Cai Q. The warburg effect in tumor progression: mitochondrial oxidative metabolism as an anti-metastasis mechanism. Cancer Lett (2015) 356:156–64. doi: 10.1016/j.canlet.2014.04.001
151. Tang HY, Guo JQ, Sang BT, Cheng JN, Wu XM. PDGFRbeta modulates aerobic glycolysis in osteosarcoma HOS cells via the PI3K/AKT/mTOR/c-myc pathway. Biochem Cell Biol (2022) 100:75–84. doi: 10.1139/bcb-2021-0305
152. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell (2004) 116:281–97. doi: 10.1016/s0092-8674(04)00045-5
153. Lee RC, Feinbaum RL, Ambros V. The c. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell (1993) 75:843–54. doi: 10.1016/0092-8674(93)90529-y
154. Denli AM, Tops BB, Plasterk RH, Ketting RF, Hannon GJ. Processing of primary microRNAs by the microprocessor complex. Nature (2004) 432:231–5. doi: 10.1038/nature03049
155. Kim VN, Nam JW. Genomics of microRNA. Trends Genet (2006) 22:165–73. doi: 10.1016/j.tig.2006.01.003
156. Akgul B, Erdogan I. Intracytoplasmic re-localization of miRISC complexes. Front Genet (2018) 9:403. doi: 10.3389/fgene.2018.00403
157. Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell (2009) 136:215–33. doi: 10.1016/j.cell.2009.01.002
158. Chen G, Fang T, Huang Z, Qi Y, Du S, Di T, et al. MicroRNA-133a inhibits osteosarcoma cells proliferation and invasion via targeting IGF-1R. Cell Physiol Biochem (2016) 38:598–608. doi: 10.1159/000438653
159. Jiashi W, Chuang Q, Zhenjun Z, Guangbin W, Bin L, Ming H. MicroRNA-506-3p inhibits osteosarcoma cell proliferation and metastasis by suppressing RAB3D expression. Aging (Albany NY) (2018) 10:1294–305. doi: 10.18632/aging.101468
160. Chen L, Wang J, Wang B, Yang J, Gong Z, Zhao X, et al. MiR-126 inhibits vascular endothelial cell apoptosis through targeting PI3K/Akt signaling. Ann Hematol (2016) 95:365–74. doi: 10.1007/s00277-015-2567-9
161. Ni SJ, Zhao LQ, Wang XF, Wu ZH, Hua RX, Wan CH, et al. CBX7 regulates stem cell-like properties of gastric cancer cells via p16 and AKT-NF-kappaB-miR-21 pathways. J Hematol Oncol (2018) 11:17. doi: 10.1186/s13045-018-0562-z
162. Tsai HC, Lai YY, Hsu HC, Fong YC, Lien MY, Tang CH. CCL4 stimulates cell migration in human osteosarcoma via the mir-3927-3p/Integrin alphavbeta3 axis. Int J Mol Sci (2021) 22. doi: 10.3390/ijms222312737
163. Xu M, Zhang YY, Wang HF, Yang GS. The expression and function of miRNA-106 in pediatric osteosarcoma. Eur Rev Med Pharmacol Sci (2017) 21:715–22.
164. Shao JH, Yuan S, Qian QR, Zhu J. MicroRNA-183-5p suppresses the malignant progression of osteosarcoma via binding to AKT. Eur Rev Med Pharmacol Sci (2019) 23:8203–10. doi: 10.26355/eurrev_201910_19127
165. Fan HP, Wang SY, Shi YY, Sun J. MicroRNA-340-5p inhibits the malignant phenotypes of osteosarcoma by directly targeting NRF2 and deactivating the PI3K/AKT pathway. Eur Rev Med Pharmacol Sci (2021) 25:3661–9. doi: 10.26355/eurrev_202105_25932
166. Hu X, Li L, Lu Y, Yu X, Chen H, Yin Q, et al. miRNA-21 inhibition inhibits osteosarcoma cell proliferation by targeting PTEN and regulating the TGF-beta1 signaling pathway. Oncol Lett (2018) 16:4337–42. doi: 10.3892/ol.2018.9177
167. Gao G, Tian Z, Zhu HY, Ouyang XY. miRNA-133b targets FGFR1 and presents multiple tumor suppressor activities in osteosarcoma. Cancer Cell Int (2018) 18:210. doi: 10.1186/s12935-018-0696-7
168. Zhao X, Yang Y, Xu J, Luo Y, Xin Y, Wang Y. Downregulation of microRNA-95-3p suppresses cell growth of osteosarcoma via CDKN1A/p21 expression. Oncol Rep (2018) 39:289–97. doi: 10.3892/or.2017.6065
169. Berlanga P, Munoz L, Piqueras M, Sirerol JA, Sanchez-Izquierdo MD, Hervas D, et al. miR-200c and phospho-AKT as prognostic factors and mediators of osteosarcoma progression and lung metastasis. Mol Oncol (2016) 10:1043–53. doi: 10.1016/j.molonc.2016.04.004
170. Xiao J, Yu W, Hu K, Li M, Chen J, Li Z. miR-92a promotes tumor growth of osteosarcoma by targeting PTEN/AKT signaling pathway. Oncol Rep (2017) 37:2513–21. doi: 10.3892/or.2017.5484
171. Zhang H, Wang Y, Xu T, Li C, Wu J, He Q, et al. Increased expression of microRNA-148a in osteosarcoma promotes cancer cell growth by targeting PTEN. Oncol Lett (2016) 12:3208–14. doi: 10.3892/ol.2016.5050
172. Lu DG, Tang QL, Wei JH, He FY, Lu L, Tang YJ. Targeting EZH2 by microRNA-449a inhibits osteosarcoma cell proliferation, invasion and migration via regulation of PI3K/AKT signaling pathway and epithelial-mesenchymal transition. Eur Rev Med Pharmacol Sci (2020) 24:1656–65. doi: 10.26355/eurrev_202002_20339
173. Liu Y, Cheng Z, Pan F, Yan W. MicroRNA-373 promotes growth and cellular invasion in osteosarcoma cells by activation of the PI3K/AKT-Rac1-JNK pathway: the potential role in spinal osteosarcoma. Oncol Res (2017) 25:989–99. doi: 10.3727/096504016X14813867762123
174. Wang J, Wang G, Li B, Qiu C, He M. miR-141-3p is a key negative regulator of the EGFR pathway in osteosarcoma. Onco Targets Ther (2018) 11:4461–78. doi: 10.2147/OTT.S171304
175. Liu J, Huang L, Su P, Song T, Zhang W, Fan J, et al. MicroRNA-499a-5p inhibits osteosarcoma cell proliferation and differentiation by targeting protein phosphatase 1D through protein kinase b/glycogen synthase kinase 3beta signaling. Oncol Lett (2018) 15:4113–20. doi: 10.3892/ol.2018.7814
176. Liu Y, Qiu G, Luo Y, Li S, Xu Y, Zhang Y, et al. Circular RNA ROCK1, a novel circRNA, suppresses osteosarcoma proliferation and migration via altering the miR-532-5p/PTEN axis. Exp Mol Med (2022) 54:1024–37. doi: 10.1038/s12276-022-00806-z
177. Tian Z, Guo B, Yu M, Wang C, Zhang H, Liang Q, et al. Upregulation of micro-ribonucleic acid-128 cooperating with downregulation of PTEN confers metastatic potential and unfavorable prognosis in patients with primary osteosarcoma. Onco Targets Ther (2014) 7:1601–8. doi: 10.2147/OTT.S67217
178. Zhu D, Xu X, Zhang M, Wang T. TPX2 regulated by miR-29c-3p induces cell proliferation in osteosarcoma via the AKT signaling pathway. Oncol Lett (2022) 23:143. doi: 10.3892/ol.2022.13262
179. Liu Y, Li G, Zhang Y, Li L, Zhang Y, Huang X, et al. Nectin-4 promotes osteosarcoma progression and metastasis through activating PI3K/AKT/NF-kappaB signaling by down-regulation of miR-520c-3p. Cancer Cell Int (2022) 22:252. doi: 10.1186/s12935-022-02669-w
180. Xu RD, Feng F, Yu XS, Liu ZD, Lao LF. miR-149-5p inhibits cell growth by regulating TWEAK/Fn14/PI3K/AKT pathway and predicts favorable survival in human osteosarcoma. Int J Immunopathol Pharmacol (2018) 32:2058738418786656. doi: 10.1177/2058738418786656
181. Yang B, Li L, Tong G, Zeng Z, Tan J, Su Z, et al. Circular RNA circ_001422 promotes the progression and metastasis of osteosarcoma via the miR-195-5p/FGF2/PI3K/Akt axis. J Exp Clin Cancer Res (2021) 40:235. doi: 10.1186/s13046-021-02027-0
182. Li Y, Song X, Liu Z, Li Q, Huang M, Su B, et al. Upregulation of miR-214 induced radioresistance of osteosarcoma by targeting PHLDA2 via PI3K/Akt signaling. Front Oncol (2019) 9:298. doi: 10.3389/fonc.2019.00298
183. Zhang L, Lv Z, Xu J, Chen C, Ge Q, Li P, et al. MicroRNA-134 inhibits osteosarcoma angiogenesis and proliferation by targeting the VEGFA/VEGFR1 pathway. FEBS J (2018) 285:1359–71. doi: 10.1111/febs.14416
184. Fan L, Cao X, Lei Y. MicroRNA miR-23b-3p promotes osteosarcoma by targeting ventricular zone expressed PH domain-containing 1 (VEPH1)/phosphatidylinositol 3-kinase/protein kinase b (PI3K/AKT) pathway. Bioengineered (2021) 12:12568–82. doi: 10.1080/21655979.2021.2010383
185. Meng CY, Zhao ZQ, Bai R, Zhao W, Wang YX, Xue HQ, et al. MicroRNA−22 mediates the cisplatin resistance of osteosarcoma cells by inhibiting autophagy via the PI3K/Akt/mTOR pathway. Oncol Rep (2020) 43:1169–86. doi: 10.3892/or.2020.7492
186. Shi Z, Wang K, Xing Y, Yang X. CircNRIP1 encapsulated by bone marrow mesenchymal stem cell–derived extracellular vesicles aggravates osteosarcoma by modulating the miR-532-3p/AKT3/PI3K/AKT axis. Front Oncol (2021) 11:658139. doi: 10.3389/fonc.2021.658139
187. Li S, Bai H, Chen X, Gong S, Xiao J, Li D, et al. Soft substrate promotes osteosarcoma cell self-renewal, differentiation, and drug resistance through miR-29b and its target protein spin 1. ACS Biomater Sci Eng (2020) 6:5588–98. doi: 10.1021/acsbiomaterials.0c00816
188. Xu J, Yao Q, Hou Y, Xu M, Liu S, Yang L, et al. MiR-223/Ect2/p21 signaling regulates osteosarcoma cell cycle progression and proliferation. BioMed Pharmacother (2013) 67:381–6. doi: 10.1016/j.biopha.2013.03.013
189. Zhan FB, Zhang XW, Feng SL, Cheng J, Zhang Y, Li B, et al. MicroRNA-206 reduces osteosarcoma cell malignancy In vitro by targeting the PAX3-MET axis. Yonsei Med J (2019) 60:163–73. doi: 10.3349/ymj.2019.60.2.163
190. Sun X, Wei B, Peng ZH, Fu QL, Wang CJ, Zheng JC, et al. Knockdown of lncRNA XIST suppresses osteosarcoma progression by inactivating AKT/mTOR signaling pathway by sponging miR-375-3p. Int J Clin Exp Pathol (2019) 12:1507–17.
191. Jiang R, Zhang C, Liu G, Gu R, Wu H. MicroRNA-101 inhibits proliferation, migration and invasion in osteosarcoma cells by targeting ROCK1. Am J Cancer Res (2017) 7:88–97.
192. Ding J, Sha L, Shen P, Huang M, Cai Q, Li J. MicroRNA-18a inhibits cell growth and induces apoptosis in osteosarcoma by targeting MED27. Int J Oncol (2018) 53:329–38. doi: 10.3892/ijo.2018.4374
193. Li KW, Wang SH, Wei X, Hou YZ, Li ZH. Mechanism of miR-122-5p regulating the activation of PI3K-Akt-mTOR signaling pathway on the cell proliferation and apoptosis of osteosarcoma cells through targeting TP53 gene. Eur Rev Med Pharmacol Sci (2020) 24:12655–66. doi: 10.26355/eurrev_202012_24163
194. Liu Y, Zhu ST, Wang X, Deng J, Li WH, Zhang P, et al. MiR-100 inhibits osteosarcoma cell proliferation, migration, and invasion and enhances chemosensitivity by targeting IGFIR. Technol Cancer Res Treat (2016) 15:NP40–48. doi: 10.1177/1533034615601281
195. Ying B, Huang H, Li H, Song M, Wu S, Ying H. Procaine inhibits proliferation and migration and promotes cell apoptosis in osteosarcoma cells by upregulation of MicroRNA-133b. Oncol Res (2017) 25:1463–70. doi: 10.3727/096504017X14878518291077
196. Ren YF, Zhang TH, Zhong S, Zhao YT, Lv YN. miR-144 suppresses proliferation and induces apoptosis of osteosarcoma cells via direct regulation of mTOR expression. Oncol Lett (2018) 15:1163–9. doi: 10.3892/ol.2017.7364
197. Fan G, He Z, Cao L, Shi X, Wu S, Zhou G. miR-139 inhibits osteosarcoma cell proliferation and invasion by targeting ROCK1. Front Biosci (Landmark Ed) (2019) 24:1167–77. doi: 10.2741/4773
198. Zhao H, Li M, Li L, Yang X, Lan G, Zhang Y. MiR-133b is down-regulated in human osteosarcoma and inhibits osteosarcoma cells proliferation, migration and invasion, and promotes apoptosis. PloS One (2013) 8:e83571. doi: 10.1371/journal.pone.0083571
199. Zhang L, Zhao G, Ji S, Yuan Q, Zhou H. Downregulated long non-coding RNA MSC-AS1 inhibits osteosarcoma progression and increases sensitivity to cisplatin by binding to MicroRNA-142. Med Sci Monit (2020) 26:e921594. doi: 10.12659/MSM.921594
200. Shao XJ, Miao MH, Xue J, Xue J, Ji XQ, Zhu H. The down-regulation of MicroRNA-497 contributes to cell growth and cisplatin resistance through PI3K/Akt pathway in osteosarcoma. Cell Physiol Biochem (2015) 36:2051–62. doi: 10.1159/000430172
201. Shi ZC, Chu XR, Wu YG, Wu JH, Lu CW, Lu RX, et al. MicroRNA-375 functions as a tumor suppressor in osteosarcoma by targeting PIK3CA. Tumour Biol (2015) 36:8579–84. doi: 10.1007/s13277-015-3614-9
202. Wu W, Wang L, Li S. Hox transcript antisense RNA knockdown inhibits osteosarcoma progression by regulating the phosphoinositide 3-kinase/AKT pathway through the microRNA miR-6888-3p/spleen tyrosine kinase axis. Bioengineered (2022) 13:9397–410. doi: 10.1080/21655979.2022.2059614
203. Wang Y, Huang H, Li Y. Knocking down miR-384 promotes growth and metastasis of osteosarcoma MG63 cells by targeting SLBP. Artif Cells Nanomed Biotechnol (2019) 47:1458–65. doi: 10.1080/21691401.2019.1601099
204. Al-Khalaf HH, Aboussekhra A. MicroRNA-141 and microRNA-146b-5p inhibit the prometastatic mesenchymal characteristics through the RNA-binding protein AUF1 targeting the transcription factor ZEB1 and the protein kinase AKT. J Biol Chem (2014) 289:31433–47. doi: 10.1074/jbc.M114.593004
205. Li G, Cai M, Fu D, Chen K, Sun M, Cai Z, et al. Heat shock protein 90B1 plays an oncogenic role and is a target of microRNA-223 in human osteosarcoma. Cell Physiol Biochem (2012) 30:1481–90. doi: 10.1159/000343336
206. Zhao D, Jia P, Wang W, Zhang G. VEGF-mediated suppression of cell proliferation and invasion by miR-410 in osteosarcoma. Mol Cell Biochem (2015) 400:87–95. doi: 10.1007/s11010-014-2265-2
207. Sun ZY, Jian YK, Zhu HY, Li B. lncRNAPVT1 targets miR-152 to enhance chemoresistance of osteosarcoma to gemcitabine through activating c-MET/PI3K/AKT pathway. Pathol Res Pract (2019) 215:555–63. doi: 10.1016/j.prp.2018.12.013
208. Song L, Zhou Z, Gan Y, Li P, Xu Y, Zhang Z, et al. Long noncoding RNA OIP5-AS1 causes cisplatin resistance in osteosarcoma through inducing the LPAATbeta/PI3K/AKT/mTOR signaling pathway by sponging the miR-340-5p. J Cell Biochem (2019) 120:9656–66. doi: 10.1002/jcb.28244
209. Ji Q, Zhu J, Fang CL, Jin H, Zhan DP, Huang J. Down-regulation of MIAT suppresses osteosarcoma progression by acting as a ceRNA for miR-141-3p to regulate SIX1-mediated PI3K/AKT pathway. Eur Rev Med Pharmacol Sci (2020) 24:2218–28. doi: 10.26355/eurrev_202003_20487
210. Qi S, Xu L, Han Y, Chen H, Cheng A. miR-29a-3p mitigates the development of osteosarcoma through modulating IGF1 mediated PI3k/Akt/FOXO3 pathway by activating autophagy. Cell Cycle (2022) 21:1980–95. doi: 10.1080/15384101.2022.2078614
211. Liu Q, Wang Z, Zhou X, Tang M, Tan W, Sun T, et al. miR-485-5p/HSP90 axis blocks Akt1 phosphorylation to suppress osteosarcoma cell proliferation and migration via PI3K/AKT pathway. J Physiol Biochem (2020) 76:279–90. doi: 10.1007/s13105-020-00730-8
212. Yang C, Chen Y, Xiong W, Xu K. miR-652 inhibits the proliferation, migration, and invasion of osteosarcoma cells by targeting HOXA9 and regulating the PI3K/Akt signaling pathway. J Oncol (2022) 2022:4809312. doi: 10.1155/2022/4809312
213. Jin B, Jin D, Zhuo Z, Zhang B, Chen K. MiR-1224-5p activates autophagy, cell invasion and inhibits epithelial-to-Mesenchymal transition in osteosarcoma cells by directly targeting PLK1 through PI3K/AKT/mTOR signaling pathway. Onco Targets Ther (2020) 13:11807–18. doi: 10.2147/OTT.S274451
214. Luo T, Zhou X, Jiang E, Wang L, Ji Y, Shang Z. Osteosarcoma cell-derived small extracellular vesicles enhance osteoclastogenesis and bone resorption through transferring MicroRNA-19a-3p. Front Oncol (2021) 11:618662. doi: 10.3389/fonc.2021.618662
215. Jiang P, Yang X, Li Y, Chen J. miRNA-216 knockdown has effects to suppress osteosarcoma via stimulating PTEN. Food Sci Nutr (2020) 8:4708–16. doi: 10.1002/fsn3.1587
216. Fu Y, Wang Y, Bi K, Yang L, Sun Y, Li B, et al. MicroRNA-208a-3p promotes osteosarcoma progression via targeting PTEN. Exp Ther Med (2020) 20:255. doi: 10.3892/etm.2020.9385
217. Zhuang M, Qiu X, Cheng D, Zhu C, Chen L. MicroRNA-524 promotes cell proliferation by down-regulating PTEN expression in osteosarcoma. Cancer Cell Int (2018) 18:114. doi: 10.1186/s12935-018-0612-1
218. Yao P, Ni Y, Liu C. Long non-coding RNA 691 regulated PTEN/PI3K/AKT signaling pathway in osteosarcoma through miRNA-9-5p. Onco Targets Ther (2020) 13:4597–606. doi: 10.2147/OTT.S249827
219. Han G, Guo Q, Ma N, Bi W, Xu M, Jia J. Apatinib inhibits cell proliferation and migration of osteosarcoma via activating LINC00261/miR-620/PTEN axis. Cell Cycle (2021) 20:1785–98. doi: 10.1080/15384101.2021.1949132
220. Wu D, Zhang H, Ji F, Ding W. MicroRNA-17 promotes osteosarcoma cells proliferation and migration and inhibits apoptosis by regulating SASH1 expression. Pathol Res Pract (2019) 215:115–20. doi: 10.1016/j.prp.2018.10.012
221. Wang C, Ba X, Guo Y, Sun D, Jiang H, Li W, et al. MicroRNA-199a-5p promotes tumour growth by dual-targeting PIAS3 and p27 in human osteosarcoma. Sci Rep (2017) 7:41456. doi: 10.1038/srep41456
222. Shang Y, Wang LQ, Guo QY, Shi TL. MicroRNA-196a overexpression promotes cell proliferation and inhibits cell apoptosis through PTEN/Akt/FOXO1 pathway. Int J Clin Exp Pathol (2015) 8:2461–72.
223. Li C, Xu B, Miu X, Deng Z, Liao H, Hao L. Inhibition of miRNA-21 attenuates the proliferation and metastasis of human osteosarcoma by upregulating PTEN. Exp Ther Med (2018) 15:1036–40. doi: 10.3892/etm.2017.5477
224. Wang X, Sun J, Fu C, Wang D, Bi Z. MicroRNA-214 regulates osteosarcoma survival and growth by directly targeting phosphatase and tensin homolog. Mol Med Rep (2014) 10:3073–9. doi: 10.3892/mmr.2014.2616
225. Gao LF, Jia S, Zhang QM, Xia YF, Li CJ, Li YH. MicroRNA-802 promotes the progression of osteosarcoma through targeting p27 and activating PI3K/AKT pathway. Clin Transl Oncol (2022) 24:266–75. doi: 10.1007/s12094-021-02683-w
226. Li K, Shen H, Lu M, Chen J, Yin Q, Li P. Formononetin inhibits osteosarcoma cell proliferation and promotes apoptosis by regulating the miR-214-3p/phosphatase and tensin homolog pathway. Transl Cancer Res (2020) 9:4914–21. doi: 10.21037/tcr-20-2296
227. Kawano M, Tanaka K, Itonaga I, Ikeda S, Iwasaki T, Tsumura H. microRNA-93 promotes cell proliferation via targeting of PTEN in osteosarcoma cells. J Exp Clin Cancer Res (2015) 34:76. doi: 10.1186/s13046-015-0192-z
228. Yang J, Zou Y, Jiang D. Honokiol suppresses proliferation and induces apoptosis via regulation of the miR−21/PTEN/PI3K/AKT signaling pathway in human osteosarcoma cells. Int J Mol Med (2018) 41:1845–54. doi: 10.3892/ijmm.2018.3433
229. Jiang C, Fang X, Zhang H, Wang X, Li M, Jiang W, et al. Triptolide inhibits the growth of osteosarcoma by regulating microRNA-181a via targeting PTEN gene in vivo and vitro. Tumour Biol (2017) 39:1010428317697556. doi: 10.1177/1010428317697556
230. Wang W, Wang Z, Chen S, Zang X, Miao J. Interleukin-1beta/nuclear factor-kappaB signaling promotes osteosarcoma cell growth through the microRNA-181b/phosphatase and tensin homolog axis. J Cell Biochem (2019) 120:1763–72. doi: 10.1002/jcb.27477
231. Zheng M, Wu Y. Piceatannol suppresses proliferation and induces apoptosis by regulation of the microRNA−21/phosphatase and tensin homolog/protein kinase b signaling pathway in osteosarcoma cells. Mol Med Rep (2020) 22:3985–93. doi: 10.3892/mmr.2020.11484
232. Liu CJ, Yu KL, Liu GL, Tian DH. MiR−214 promotes osteosarcoma tumor growth and metastasis by decreasing the expression of PTEN. Mol Med Rep (2015) 12:6261–6. doi: 10.3892/mmr.2015.4197
233. Ye F, Tian L, Zhou Q, Feng D. LncRNA FER1L4 induces apoptosis and suppresses EMT and the activation of PI3K/AKT pathway in osteosarcoma cells via inhibiting miR-18a-5p to promote SOCS5. Gene (2019) 721:144093. doi: 10.1016/j.gene.2019.144093
234. Wang XH, Cai P, Wang MH, Wang Z. microRNA−25 promotes osteosarcoma cell proliferation by targeting the cell−cycle inhibitor p27. Mol Med Rep (2014) 10:855–9. doi: 10.3892/mmr.2014.2260
235. He C, Chen H, Liu Y, Li X, Zhang C, Qin Q, et al. miR-106b-5p promotes cell proliferation and cell cycle progression by directly targeting CDKN1A in osteosarcoma. Exp Ther Med (2020) 19:3203–10. doi: 10.3892/etm.2020.8574
236. Lv C, Hao Y, Tu G. MicroRNA-21 promotes proliferation, invasion and suppresses apoptosis in human osteosarcoma line MG63 through PTEN/Akt pathway. Tumour Biol (2016) 37:9333–42. doi: 10.1007/s13277-016-4807-6
237. Yu W, Chen PB, Chen FC, Ding SL, Pan XY. MicroRNA-744 promotes proliferation of osteosarcoma cells by targeting PTEN. Mol Med Rep (2020) 21:2276–82. doi: 10.3892/mmr.2020.11030
238. Li ZJ, Ying XJ, Chen HL, Ye PJ, Chen ZL, Li G, et al. Insulin-like growth factor-1 induces lymphangiogenesis and facilitates lymphatic metastasis in colorectal cancer. World J Gastroenterol (2013) 19:7788–94. doi: 10.3748/wjg.v19.i43.7788
239. Djiogue S, Nwabo Kamdje AH, Vecchio L, Kipanyula MJ, Farahna M, Aldebasi Y, et al. Insulin resistance and cancer: the role of insulin and IGFs. Endocr Relat Cancer (2013) 20:R1–R17. doi: 10.1530/ERC-12-0324
240. Lichtenberger BM, Tan PK, Niederleithner H, Ferrara N, Petzelbauer P, Sibilia M. Autocrine VEGF signaling synergizes with EGFR in tumor cells to promote epithelial cancer development. Cell (2010) 140:268–79. doi: 10.1016/j.cell.2009.12.046
241. Toker A, Dibble CC. PI 3-kinase signaling: AKTing up inside the cell. Mol Cell (2018) 71:875–6. doi: 10.1016/j.molcel.2018.09.006
242. Guo F, Cogdell D, Hu L, Yang D, Sood AK, Xue F, et al. MiR-101 suppresses the epithelial-to-mesenchymal transition by targeting ZEB1 and ZEB2 in ovarian carcinoma. Oncol Rep (2014) 31:2021–8. doi: 10.3892/or.2014.3106
243. Sun H, Lesche R, Li DM, Liliental J, Zhang H, Gao J, et al. PTEN modulates cell cycle progression and cell survival by regulating phosphatidylinositol 3,4,5,-trisphosphate and akt/protein kinase b signaling pathway. Proc Natl Acad Sci United States America (1999) 96:6199–204. doi: 10.1073/pnas.96.11.6199
244. Yamada KM, Araki M. Tumor suppressor PTEN: modulator of cell signaling, growth, migration and apoptosis. J Cell Sci (2001) 114:2375–82. doi: 10.1242/jcs.114.13.2375
245. Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem (1998) 273:13375–8. doi: 10.1074/jbc.273.22.13375
246. Chu IM, Hengst L, Slingerland JM. The cdk inhibitor p27 in human cancer: prognostic potential and relevance to anticancer therapy. Nat Rev Cancer (2008) 8:253–67. doi: 10.1038/nrc2347
247. Blain SW, Scher HI, Cordon-Cardo C, Koff A. p27 as a target for cancer therapeutics. Cancer Cell (2003) 3:111–5. doi: 10.1016/s1535-6108(03)00026-6
248. Kreis NN, Louwen F, Yuan J. Less understood issues: p21(Cip1) in mitosis and its therapeutic potential. Oncogene (2015) 34:1758–67. doi: 10.1038/onc.2014.133
249. Abbas T, Dutta A. p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer (2009) 9:400–14. doi: 10.1038/nrc2657
250. Kager L, Tamamyan G, Bielack S. Novel insights and therapeutic interventions for pediatric osteosarcoma. Future Oncol (2017) 13:357–68. doi: 10.2217/fon-2016-0261
Keywords: MicroRNA, PI3K/AKT pathway, osteosarcoma, mechanism, biomarker
Citation: Xiang Y, Yang Y, Liu J and Yang X (2023) Functional role of MicroRNA/PI3K/AKT axis in osteosarcoma. Front. Oncol. 13:1219211. doi: 10.3389/fonc.2023.1219211
Received: 08 May 2023; Accepted: 01 June 2023;
Published: 19 June 2023.
Edited by:
Zhiyu Zhang, Fourth Affiliated Hospital of China Medical University, ChinaReviewed by:
Jiyu Han, Tongji University, ChinaWeilin Zhang, Affiliated Hospital of Guangdong Medical University, China
Copyright © 2023 Xiang, Yang, Liu and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yingxin Yang, eGlhbmd5dWJvMjAyMEAxMjYuY29t
†These authors have contributed equally to this work and share first authorship
 Yubo Xiang
Yubo Xiang Yingxin Yang
Yingxin Yang Jia Liu
Jia Liu