- 1Department of Physiology, National Cheng Kung University Medical College, Tainan, Taiwan
- 2School of Medicine, National Sun Yat-sen University College of Medicine, Kaohsiung, Taiwan
- 3Department of Education and Research, An Nan Hospital, China Medical University, Tainan, Taiwan
- 4Department of Anesthesia, An Nan Hospital, China Medical University, Tainan, Taiwan
Recently, we have read a very interesting paper entitled “The association between tyrosine kinase inhibitors and fatal arrhythmia in patients with non-small lung cancer in Taiwan” by Chang et al., published in your journal, Frontiers in Oncology. In their study conducted in Taiwan, it was observed that there is a potential correlation between the use of tyrosine kinase inhibitors and fatal cardiac arrhythmias in patients with non-small cell lung cancer. Therefore, we would like to express some related opinions about the paper to provide reference to the readers.
Indeed, many compounds known to suppress the activity of tyrosine kinases in various types of neoplastic cells have been increasing used in clinical practices to treat various types of advance cancers. However, these drugs are recognized to exert inhibitory effects on ion channels in the cell membrane, such as erg-mediated K+ channels, which can potentially lead to cardiac arrhythmias. Therefore, regarding the use of tyrosine kinase inhibitors, we may discuss this from two perspectives: clinical and basic research.
First, on the level of clinical research
1. There have been many different tyrosine kinase inhibitors (such as lapatinib and sorafenib) approved for the treatment of various serious cancers, including non-small cell lung carcinoma (1, 2). With appropriate administration, there is evidence that many good treatment outcomes can occur, bringing many positive implications for disease treatment and prognosis. However, the use of these drugs can sometimes cause a lengthening in cardiac action potential or prolongation of the QTc interval on the electrocardiogram, leading to severe Torsade Pointe arrhythmia (1). As a result, there may be an increased risk of sudden death due to cardiac causes. This is an area where healthcare professionals and even family members need to be more aware and understanding. On the other hand, encouraging patients to carry portable electrocardiogram monitors with them may be an important tool for early detection.
2. In addition to tyrosine kinase inhibitors, many cyclin-dependent kinase (CDK) inhibitors (such as ribociclib) have also shown good treatment outcomes for patients with various severe cancers. However, there are also frequent occurrences of QTc interval prolongation issues (3). It is therefore important to note that if these different anti-cancer drugs are used in combination, there is a high likelihood of significantly increasing the occurrence of cardiac arrhythmias.
3. Of note, in patients with concomitant atrial fibrillation, the administration of tyrosine kinase inhibitors can block potassium ion currents, similar to the effects of quinidine or procainamide, causing a shortening of the atrioventricular node refractoriness and leading to rapid ventricular response. This aspect should also be given special attention in clinical practice to prevent unexpected events.
4. Many cancer patients receiving tyrosine kinase inhibitors may experience changes in their blood electrolyte levels. For example, changes in potassium ion concentration can affect the QTc interval on electrocardiogram, leading to arrhythmias (4, 5). Therefore, the concentration of potassium ions in the blood can also affect the effect of tyrosine kinase inhibitors on QTc intervals.
Second, on the level of basic academic research
1. Although the blockade of hERG K+ currents by tyrosine kinase inhibitors may be one of the reasons for QTc interval prolongation induced by these drugs (6, 7), inhibition of other potassium currents can also lead to lengthening of the cardiac action potential and concurrent prolongation of the QTc interval (7). Therefore, in drug development, in addition to studying the degree of inhibition of tyrosine kinase activity by different drugs, it is necessary to conduct more in-depth research on the mechanisms of action of these new anti-neoplastic drugs on various ion currents on the cell membrane, such as current intensity or kinetics of opening or closing, to reduce the risk of QTc interval prolongation after their administration (8).
2. In addition to hERG K+ currents, blocking slowly activating delayed-rectifier K+ currents (also known as KCNQ1 or KV7.1 current) can also lead to QTc interval prolongation. Moreover, the current inactivation of certain delayed rectifier K+ currents (especially KV2.1 or KV3.1 current) can cause QTc interval prolongation, and even polymorphic ventricular tachycardia (9). In addition, some drugs that activate voltage-gated sodium currents can also cause QTc interval prolongation (10, 11).
3. Tyrosine kinase inhibitors have IC50 values in the nM range for tyrosine kinase inhibition (11), while the doses that cause inhibition of K+ currents by tyrosine kinase inhibitors are in the µM range (7, 8). The difference between the two is about 100-1000 times. Moreover, for drugs like zanubrutinib, the pharmacokinetics show a maximum blood concentration of about 400 ng/ml (approximately 0.85 µM) (12). Therefore, the impact on membrane K+ currents seems to be minimal.
4. Finally, whether the inhibition of these membrane ion currents directly or indirectly participate in the suppression of tyrosine kinase activity is also an important subject for further research.
In summary, as shown in Figure 1, apart from its inhibition of tyrosine kinases, the compounds known to suppress the activity of tyrosine kinases may affect the activity of erg-mediated K+ channels. However, how the inhibition of these ionic channels can indirectly alter the activity of tyrosine kinases remains to be further investigated. It is hoped that the understanding and research in this area can be further enhanced, while providing better assistance for the care and treatment of patients.
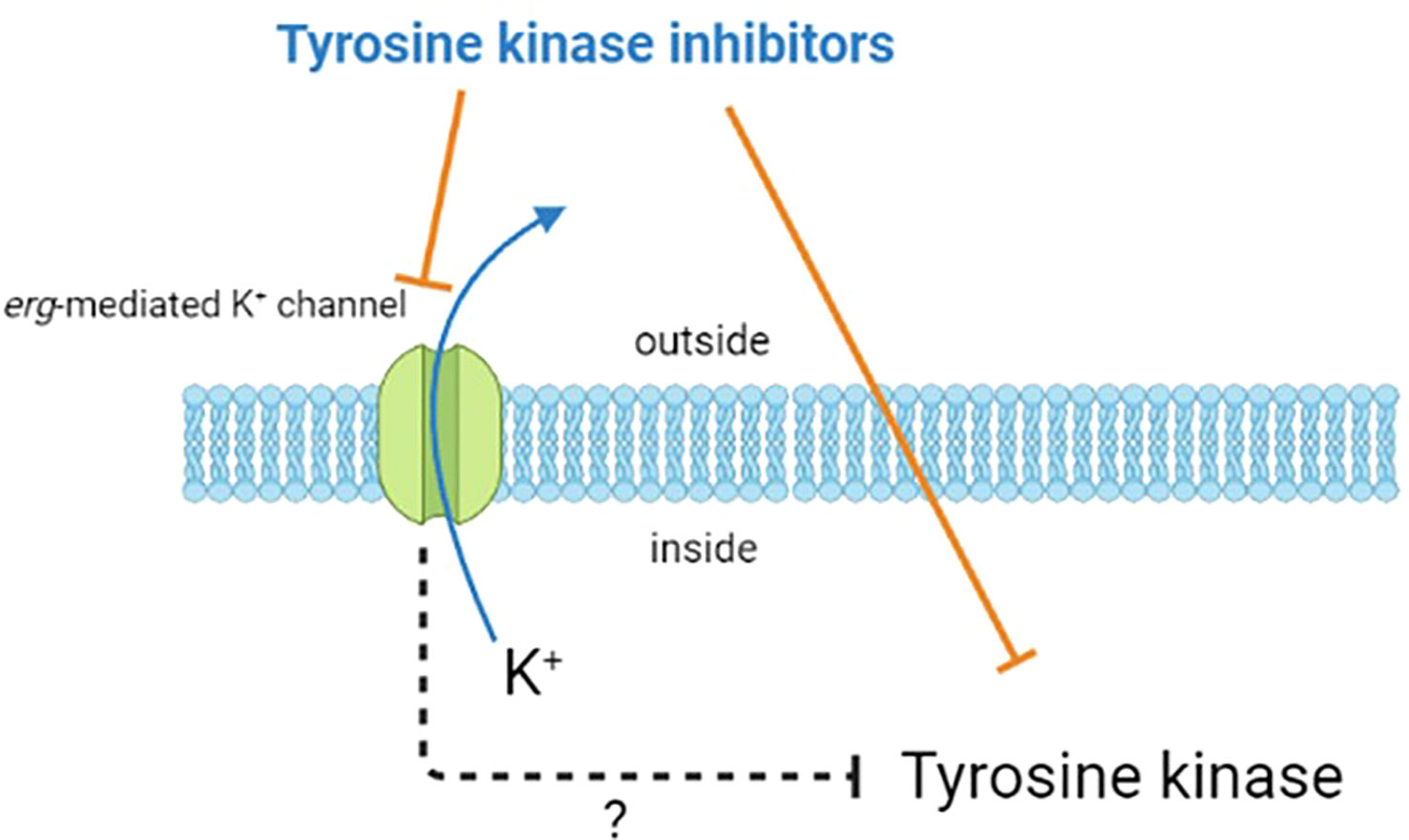
Figure 1 Diagram illustrating the potential effects of tyrosine kinase inhibitors. Besides inhibiting the activity of tyrosine kinases in cells, they may also block erg-mediated K+ channels. However, the indirect impact of this channel blockade on the activity of tyrosine kinases is current unclear and require further research.
Author contributions
S-NW and ES drafted the manuscript. C-HH prepared the format for submission and help to review and polish the English wordings. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chang WT, Lin HW, Chang TC, Lin SH, Li YH. The association between tyrosine kinase inhibitors and fatal arrhythmia in patients with non-small lung cancer in Taiwan. Front Oncol (2023) 13:1172036. doi: 10.3389/fonc.2023.1172036
2. Hartmann JT, Haap M, Kopp HG, Lipp HP. Tyrosine kinase inhibitors – a review on pharmacology, metabolism and side effects. Curr Drug Metab (2009) 10(5):470–81. doi: 10.2174/138920009788897975
3. Liu PY, Chang WT, Wu SN. Characterization of the synergistic inhibition of IK(erg) and IK(DR) by ribociclib, a cyclin-dependent kinase 4/6 inhibitor. Int J Mol Sci (2020) 21:8078. doi: 10.3390/ijms21218078
4. Wu SN. Simulation of the cardiac action potential based on the Hodgkin-Huxley kinetics with the use of Microsoft excel spreadsheets. Chin J Physiol (2004) 47(1):15–22.
5. Sung RJ, Wu SN, Wu JS, Chang HD, Luo CH. Electrophysiological mechanism of ventricular arrhythmias in relation to Andersen-tawil syndrome under conditions of reduced IK1 simulation study. Am J Physiol Heart Circ Physiol (2006) 291(6):H2597–607. doi: 10.1152/ajpheart.00393.2006
6. So EC, Hsing CH, Liang CH, Wu SN. The action of mdiv-1, an inhibitor of mitochondrial fission, on rapidly activating delayed rectifier k+ current and membrane potential in HL-1 murine atrial cardiomyocytes. Eur J Pharmacol (2012) 683(1-3):1–9. doi: 10.1016/j.ejphar.2012.02.012
7. Chang WT, Liu PY, Lee K, Feng YH, Wu SN. Differential inhibitory actions of multitarget tyrosine kinase inhibitors on different ionic current types in cardiomyocytes. Int J Mol Sci (2020) 21(5):1672. doi: 10.3390/ijms21051672
8. Chen YT, Masbuchin AN, Fang YH, Hsu LW, Wu SN, Yen CJ, et al. Pentraxin 3 regulates tyrosine-kinase inhibitor-associated cardiomyocyte contraction and mitochondrial dysfunction via ERK/JNK signalling pathwarys. BioMed Pharmacother (2023) 157:113962. doi: 10.1016/j.biopha.2022.113962
9. Chang WT, Wu SN. Activation of voltage-gated sodium current and inhibition of erg-mediated potassium current caused by telmisartan, an antagonist of angiotensin II type-1 receptor, in HL-1 atrial cardiomyocytes. Clin Exp Pharmacol Physiol (2018) 45(8):797–807. doi: 10.1111/1440-1681.12943
10. So EC, Wu SN, Lo YC, Su K. Differential regulation of tefluthrin and telmisartan on the gating charges of INa activation and inactivation as well as on resurgent and persistent INa in a pituitary cell line (GH3). Toxicol Lett (2018) 285:104–12. doi: 10.1016/j.toxlet.2018.01.002
11. Traxler P, Furet P. Strategies toward the design of novel and selective protein tyrosine kinase inhibitors. Pharmacol Ther (1999) 82(2-3):195–206. doi: 10.1016/S0163-7258(98)00044-8
Keywords: tyrosine kinase inhibitor, ion channels, erg-mediated K+ channel, QTc interval, cardiac arrhythmia
Citation: Hu C-H, Wu S-N and So EC (2023) Tyrosine kinase inhibitors, ionic currents, and cardiac arrhythmia. Front. Oncol. 13:1218821. doi: 10.3389/fonc.2023.1218821
Received: 08 May 2023; Accepted: 19 June 2023;
Published: 24 July 2023.
Edited by:
Annarosa Arcangeli, University of Florence, ItalyReviewed by:
Claudia Duranti, University of Florence, ItalyCopyright © 2023 Hu, Wu and So. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sheng-Nan Wu, c253dUBtYWlsLm5ja3UuZWR1LnR3; Edmund Cheung So, ZWRtdW5kc290d0BnbWFpbC5jb20=
 Chi-Hao Hu
Chi-Hao Hu Sheng-Nan Wu
Sheng-Nan Wu Edmund Cheung So
Edmund Cheung So