
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 29 June 2023
Sec. Cancer Immunity and Immunotherapy
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1218808
This article is part of the Research Topic Novel Immune Markers and Predictive Models for Immunotherapy and Prognosis in Breast and Gynecological Cancers View all 16 articles
Objective: The aim of this study was to evaluate the prognostic value of Butyrophilin-like protein 8 (BTNL8) expression in high-risk HPV (hrHPV) infection treated with photodynamic therapy.
Methods: A total of 93 patients with hrHPV infection were enrolled as research study subjects, along with 69 healthy women who served as controls. Serum samples were obtained from each participant, and BTNL8 levels were quantified. The patients were divided into high- and low-expression groups (n = 45 and n = 48, respectively), and both groups underwent photodynamic therapy. We recorded the following data: BTNL8 expression pre-treatment and at 3/6 months post-treatment, HPV negative conversion ratio, regression rate of low-grade squamous intraepithelial lesions (LSIL), incidence of adverse reactions, complication rate, serum inflammatory factors, persistence of HPV positivity, LSIL residue or recurrence, and incidence of high-grade cervical intraepithelial lesions (HCIL).
Results: Patients with HPV infection exhibited higher BTNL8 expression than healthy individuals. Compared to the low-expression group, the high-expression group showed increased HPV negative conversion ratios, LSIL regression rates, and levels of IL-17 and IL-23. This group also demonstrated decreased total complication rate, HPV positivity persistence, LSIL residue or recurrence, and IL-10 levels. Additionally, there was no significant difference between the two groups in terms of the number of adverse reactions and cases with LSIL residue/recurrence.
Conclusion: Serum BTNL8 expression may serve as a valuable tool for early screening and prognosis monitoring of patients with hrHPV infection.
Cervical cancer (CC) is the fourth most commonly diagnosed cancer in women. In 2020, approximately 604,127 new cases were reported globally, accounting for 3.1% of all cancer diagnoses, making it the ninth most common type of cancer (1). China, in particular, faces a significant challenge due to its high incidence rate. As per recent statistics, 106,000 confirmed cases of CC and 48,000 related deaths were reported in China in 2018 (2). Notably, high-risk human papillomavirus (hrHPV) infection is associated with up to 90% of all CC cases.
HPV, a small double-stranded DNA virus, circumvents the host’s innate immunity and integrates its genome into the host cell (3). When hrHPV infection persists, it causes its structural oncoproteins E6 and E7 to contribute to the inactivation of the tumor suppressor proteins p53 and pRB, thereby disrupting the normal functioning of DNA repair and apoptosis and triggering abnormal proliferation of normal cervical epithelial squamous cells, known as cervical intraepithelial neoplasia (CIN) (4–6). If left untreated, unresolved CIN1 will continue to progress into high-grade squamous intraepithelial lesions (HSIL), with massive genomic alterations that induce CC occurrence (7).
The treatment options for hrHPV infection include circumcision, cryotherapy, conization, therapeutic vaccine, and photodynamic therapy (8). Traditional treatment methods, which mainly encompass circumcision, cryotherapy, and conization, present some shortcomings, such as high recurrence rate, serious tissue damage, and potential structural destruction of cervical if improperly applied (9). Photodynamic therapy, a novel cancer treatment modality, involves the activation of photosensitive molecules by lasers of a specific wavelength. The activated photosensitive molecules prompt the production of reactive oxygen species (ROS) leading to irreversible cell apoptosis and necrosis, and consequently inhibiting the proliferation and invasion of cancer cells (10, 11). Photodynamic therapy offers advantages in fostering good tissue healing and the convenient utilization of light (12). Cang et al. (13) pointed out the potential of photodynamic therapy to effectively eliminate hrHPV infection, particularly HPV16 and HPV18. Gu et al. (14) propose that 5-aminolevulinic acid-mediated photodynamic therapy, an effective treatment for hrHPV infection co-occurring with cervical squamous intraepithelial lesions, is less likely to cause cervical injury. Therefore, photodynamic therapy emerges as a promising, effective therapy for hrHPV infection.
During the course of hrHPV infection, genes such as p16 and galectin-3 may demonstrate aberrant expression, which could serve as biomarkers of HPV infection for early screening for cervical lesions (15, 16). Thus, incorporating gene expression into the prognostic evaluation of hrHPV infections can facilitate dynamic detection post-therapy and expedite the investigation and treatment of recurrent cases. Butyrophilin-like protein 8 (BTNL8) is implicated in adaptive immune process and thus serves as a crucial regulatory factor of tumor immunity (17–19). Yang et al. (20) observed a significant correlation of BTNL8 expression with patients’ survival rate according to the relatively low survival rate in CC patients with high BTNL8 expression (<40%) and the over 60% of survival rate in CC patients with low expression. The results suggest BTNL8 as one possible biomarker for the prognosis of CC and its potential for postoperative evaluation of hrHPV infection.
As of now, the potential evaluative significance of BTNL8 in HPV infection has not been reported. Therefore, this study aims to assess the clinical efficacy and postoperative recurrence of photodynamic therapy in hrHPV infection through the lens of BTNL8, hoping to provide a reliable basis for the clinical dynamic monitoring of hrHPV infection.
A total of 93 women diagnosed with hrHPV infection at Jinhua Central Hospital from January 2020 to March 2022 were included in this study. The inclusion criteria were as follows: patients confirmed with hrHPV infection according to the PCR method (21) and cervical cytology (22), patients with detailed clinical data, patients not in pregnancy and lactation period, and those who were willing to participate in the research. The exclusion criteria were as follows: patients who had received HPV infection therapy; patients who had received immunosuppressive agents or glucocorticoids recently; patients with comorbid CC or other malignant tumors of the reproductive system; patients with comorbid dysfunction of the heart, lung, liver, or kidney; patients with comorbid mental disorder; and those allergic to photodynamic therapy. A total of 69 healthy women were enrolled as controls. All participants of the study offered signed informed consent forms after understanding the whole process of the study, and approval was obtained for the study from the ethics committee of our hospital.
5-Aminolevulinic acid-mediated photodynamic therapy was adopted for treating patients with hrHPV infection in this study. Specifically, each patient was given photodynamic therapy at 7 days after the menstrual period. Before therapy, the patient was required to empty his/her bladder to avoid interference. Start with a surface shave, the lesions were cleaned via normal saline and then given ultra-expensive disinfection, followed by even application of 20% 5-aminolevulinic acid (Shanghai Fudan-Zhangjiang Bio-Pharmaceutical Co., Ltd, 118 mg/piece) with cotton swabs (23). After 3 h, the lesions were irradiated with a Y-HN300 He-Ne laser therapy machine (Wuhan Yage Laser Equipment Co., Ltd.) (output wavelength: 632.8 nm; rated power: 300 mW) at 20 min each time, once every 7 days, for four consecutive treatments, and the observation course was 6 months.
(1) Clinical efficacy: Patients are asked to visit the hospital for regular follow-up at 3 months and 6 months after surgery. The negative convention of HPV and inversion of low-grade squamous intraepithelial lesions (LSIL) in patients at 3 months and 6 months after surgery were recorded. The LSIL residue/recurrence of LSIL lesions (the cervicovaginal part and the cervicovaginal part + cervical canal) at 6 months after surgery was evaluated and recorded.
(2) Serum BTNL8 expression: Serum was sampled from each patient with hrHPV infection before admission, and serum was also acquired from healthy women as controls. Serum was sampled from every patient before therapy and at 3 and 6 months after therapy. All blood specimens were acquired on an empty stomach. Total RNA of sampled serum was acquired via a plasma/serum RNA extraction kit (NORGEN, Canada), followed by fluorescent quantitation via a qPCR instrument. The design and synthesis of BTNL8 primers were completed by Shanghai Sangon Biotech Co., Ltd. The 2−ΔΔCt method was adopted for standardizing the gene expression.
(3) Serum inflammatory factor levels: Serum inflammatory factors were quantified via ELISA kits of IL-17 (ab100556), IL-23 (ab221837), and IL-10 (ab185986) (Abcam, Shanghai) at 3 months after surgery.
(4) Adverse reactions and complications: The adverse reactions and complications of patients within 3 months after surgery were recorded. Adverse reactions included colporrhagia, menstrual abdominal pain, and colpitis. Postoperative complications included bleeding, infection, menstrual disorder, prolonged menstrual period, and cervical duct adhesion.
(5) Prognostic indexes: Prognostic indexes at 6 months after surgery were recorded, including persistence in positive HPV, LSIL residue/recurrence, and progression to high-grade cervical intraepithelial lesions (HCIL).
This study adopted SPSS 20.0 for statistical analyses of data. Counting data were presented by n (%), and measurement data were presented by mean ± SD, all of which were analyzed via the normality test. The independent-samples t-test was utilized for inter-group comparison and the chi-square test was used for inter-group comparison of counting data, and the repeated measures analysis of variance was used for intra-group comparison. 95% CI was adopted. p < 0.05 implies a notable difference.
A total of 93 patients with hrHPV infection and 69 healthy women were enrolled, and compared in serum BTNL8 expression (Figure 1). The patients showed notably downregulated serum BTNL8 than the healthy women (p < 0.05). In contract to serum BTNL8 expression before therapy, the HPV infection group showed notably up-regulated BTNL8 expression at 3 and 6 months after photodynamic therapy (p < 0.05). ROC curves revealed the potential value of serum BTNL8 in screening hrHPV infection (AUC = 0.835, p < 0.05).
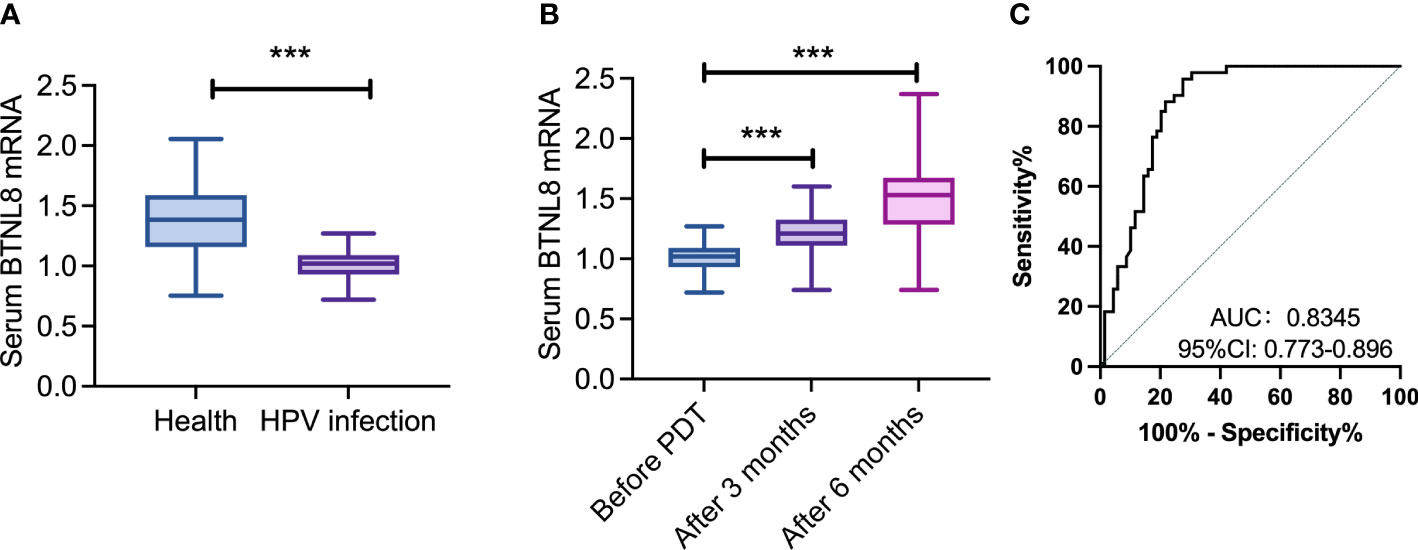
Figure 1 Aberrant serum BTNL8 expression in cases with hrHPV infection. (A) Comparison of serum BTNL8 expression levels between patients and healthy women. (B) Comparison of serum BTNL8 expression between patients after photodynamic therapy 3 months and before. (C) Comparison of serum BTNL8 expression levels between patients before and after photodynamic therapy for 6 months. *** vs. p < 0.001.
Patients were assigned to the high- and low-expression groups (n = 45 and 48, respectively) based on the median relative expression level of serum BTNL8 obtained prior to admission (1.02). The relative expression levels of BTNL8 in the two groups were 1.11 ± 0.06 and 0.93 ± 0.08, respectively. The former group consisted of patients at 35.69 ± 6.73 years old, with a course of disease of 15.76 ± 5.20 months and body mass index (BMI) of 20.72 ± 1.76 kg/m2, including 33 cases of cervicovaginal attacks and 12 cases of attacks in the cervicovaginal part + cervical canal. The latter group consisted of patients at 36.24 ± 6.20 years old, with a course of disease of 13.84 ± 4.54 months and a body mass index (BMI) of 20.99 ± 1.70 kg/m2, including 37 cases of cervicovaginal attacks and 11 cases of attacks in the cervicovaginal part + cervical canal. The two groups were similar in general data (Table 1).
The clinical efficacy at 3 and 6 months after photodynamic therapy were counted (Tables 2, 3). At 3 and 6 months after therapy, the high-expression group presented notably higher negative conversion ratio of HPV and inversion rate of LISL than the other (both p < 0.05). The quantification results of serum inflammatory factors in postoperative patients revealed notably higher IL-17 and IL-23 levels and a notably lower IL-10 level in the high-expression group than in the other group (all p < 0.04, Figure 2).
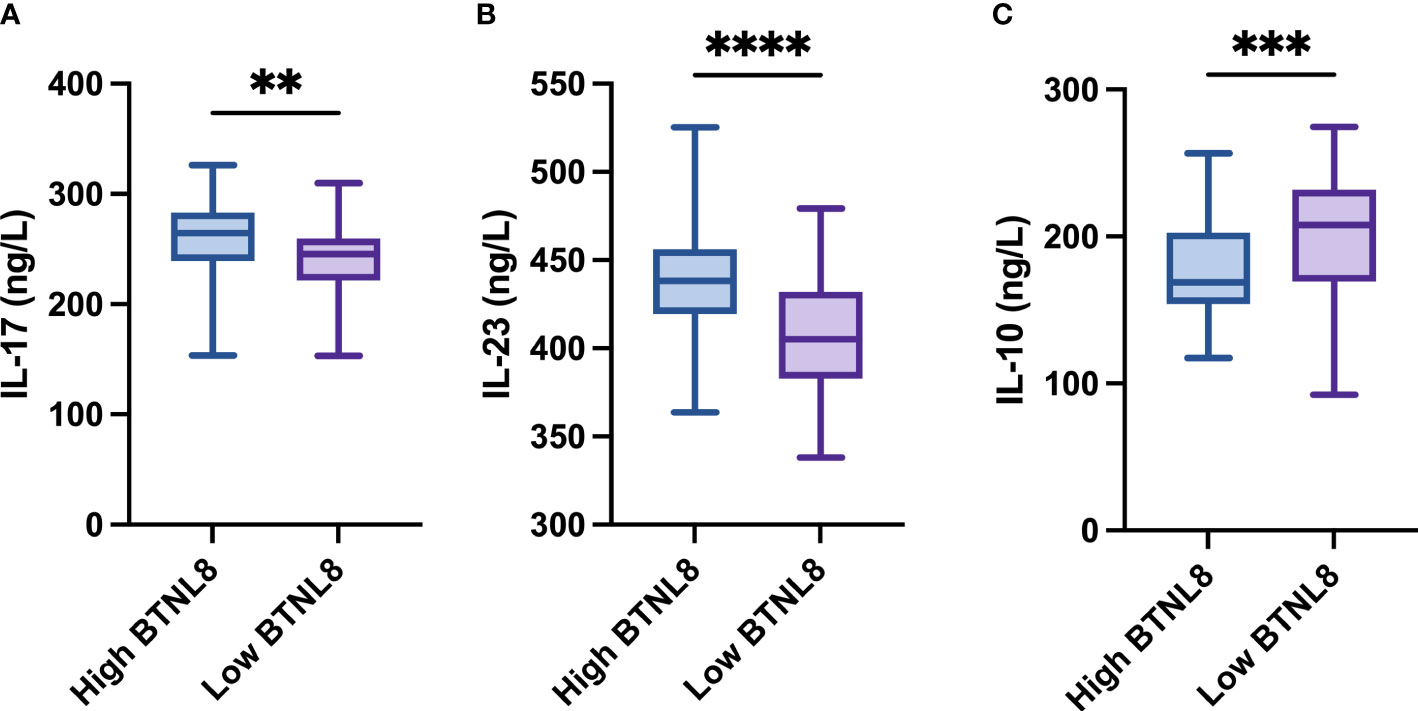
Figure 2 Comparison of BTNL8 expression with postoperative serum inflammatory factors. Comparison of BTNL8 expression with postoperative serum (A) IL-17 levels; (B) IL-23; (C) IL-10 levels. ** vs. p < 0.01; *** vs. p < 0.001; **** vs. p < 0.0001.
The postoperative adverse reactions (colporrhagia, menstrual abdominal pain, and colpitis) and complications in patients were counted. The low-expression group presented a total incidence of adverse reactions of 10.42%, with two cases of colporrhagia, two cases of menstrual abdominal pain, and one case of colpitis, while the other group presented a total incidence of adverse reactions of 9.76%, with two cases of colporrhagia, one case of menstrual abdominal pain, and one case of colpitis (Figure 3). The two groups were similar in the incidence of adverse reactions (p > 0.05).
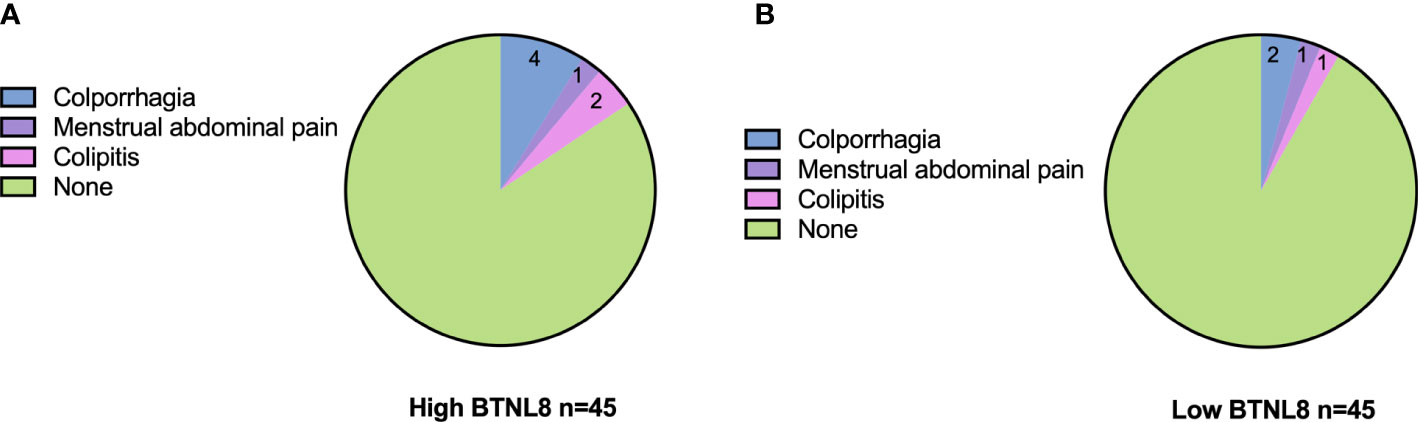
Figure 3 Associations of serum BTNL8 expression with postoperative adverse reactions. Distribution of postoperative adverse effects in patients in the (A) high BTNL8 expression group versus those in the (B) low BTNL8 expression group.
The associations of serum BTNL8 with postoperative complications (bleeding, infection, menstrual disorder, prolonged menstrual period, and cervical duct adhesion) were analyzed (Table 4). The low-expression group presented a total incidence of complications of 29.17%, with one case of bleeding, two cases of infection, four cases of menstrual disorder, five cases of prolonged menstruation, and two cases of cervical duct adhesion, while the other group presented a total incidence of complications of 11.11%, with one case of bleeding, one case of infection, two cases of menstrual disorder, no case of prolonged menstruation, and one case of cervical duct adhesion. The latter group presented a notably lower incidence than the former group.
The associations of BTNL8 expression with persistence in positive HPV, LSIL residue/recurrence, and HCIL were analyzed, and the results revealed 4 cases of persistent positive HPV (8.89%), 6 cases of LSIL residue/recurrence (12.50%), and 0 cases of progression to HCIL in the high-expression group and 12 cases of persistent positive HPV (25.00%), 15 cases of LSIL residue/recurrence (31.25%), and 0 cases of progression to HCIL in the other group (Table 5). The two groups were notably different in persistence in positive HPV and LSIL residue/recurrence (both p < 0.05).
The associations of BTNL8 expression with residual/recurrent sites of LSIL were also analyzed, and the results revealed two cases in the cervicovaginal part (4.44%) and four cases in the cervical part (8.89%) in the high-expression group, and six cases in the cervicovaginal part (12.50%) and nine cases in the cervicovaginal part + cervical canal (18.75%) in the other group (Table 6). The two groups were similar in the incidence rate in the cervicovaginal part and cervicovaginal part + cervical canal (p > 0.05).
Screening for hrHPV infection is a crucial link to prevent CC. At the current stage, hrHPV infection is mainly detected via membrane liquid-based ultrathin cytology, HPV-DNA detection, and colposcopy biopsy. With features of convenient and simple operation, serum marker-based detection can be adopted as one auxiliary means of the above methods, which is helpful for postoperative dynamic monitoring of patients. Troja et al. (24) believe a strong association of serum vitamin D with hrHPV infection. BTNL8 has been verified to be bound up with the survival of CC (20). Thus, we adopted it for evaluating clinical significance of photodynamic therapy for hrHPV infection. Our results imply the positive role of serum BTNL8 for the evaluation.
Firstly, we found downregulated BTNL8 in serum samples of patients with hrHPV infection and the induction of photodynamic therapy to BTNL8 expression, which may be attributed to the regulatory influence of BTNL8 on the immune system. In principle, photodynamic therapy triggers DNA damage, apoptosis, and necrosis through the production of ROS, during which it also stimulates a series of immune responses to clear cells (25, 26). BTNL8 happens to be one crucial factor associated with immune system, which mediates the dependent selection of T-cell receptors such as Vγ4+/Vδ1+ intraepithelial lymphocytes and γδT cells (27, 28). Thus, under the influence of photodynamic therapy on immune response in the tumor microenvironment, BTNL8 expression changes and acts as a key factor to regulate the proliferation of associated immune cells, which is probably the reason for the increase of BTNL8 under photodynamic forces. Furthermore, the high efficacy accompanied by high BTNL8 expression and the association of the high expression with serum inflammatory factors also verify the possible involvement of BTNL8 in the immune mechanism under photodynamic therapy for hrHPV infection. The specific function of BTNL8 will be discussed in future research. According to ROC-based analysis, BTNL8 facilitates distinguishing hrHPV infection. The results imply that serum BTNL8 is promising in the triage and management of HPV-positive patients to lower unnecessary colposcopy referrals and other related injuries (29).
In our investigation, we did not discern a significant correlation between BTNL8 expression and the occurrence of postoperative adverse events. However, the limited sample size of our study might obscure more nuanced impacts, potentially introducing selection bias and limiting the validity of these findings. In future investigations, we plan to enlarge our sample size, which will enable a more comprehensive and reliable analysis of the potential association between BTNL8 expression and postoperative adverse reactions in the context of hrHPV infection. Interestingly, our data pointed towards an inverse relationship between BTNL8 expression and complication incidence. Recurrence symptoms following photodynamic therapy in hrHPV-infected patients can manifest as persistent HPV positivity, residual or recurrent LSIL, or progression to HCIL (30–33).
Within our study cohort, no patients advanced to HCIL post-photodynamic therapy. Nonetheless, a higher BTNL8 expression was associated with significantly reduced instances of LSIL recurrence and persistent HPV positivity. It should be noted that we have not accounted for potential confounding factors that could impact BTNL8 expression within this study. Furthermore, we did not identify a correlation between serum BTNL8 expression and the location of LSIL residue or recurrence. This suggests that while serum BTNL8 levels may serve as an indicator of residual or recurrent LSIL, it does not appear to facilitate identification of the specific site of these conditions. This aspect warrants further exploration in future research.
To sum up, this study suggests the associations of serum BTNL8 expression with the clinical efficacy, complications, persistent positive HPV, and LSIL residue/recurrence in the therapy of HPV infection by photodynamic therapy, which is helpful to dynamically monitor the prognosis of patients, timely screen patients with recurrence risk, and potentially facilitate biomarker-based triage and management of HPV-positive patients.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by Ethics Committee of Jinhua Central Hospital. The patients/participants provided their written informed consent to participate in this study.
HL, SL, and LZ contributed to conception and design of the study. HL organized the data. DC performed the statistical analysis. YL wrote the first draft of the manuscript. YY, MC, and PC wrote sections of the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by Jinhua Municipal Science and Technology Project, approval number: No. 2020-4-019.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Arbyn M, Weiderpass E, Bruni L, de Sanjosé S, Saraiya M, Ferlay J, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Global Health (2020) 8(2):e191–203. doi: 10.1016/S2214-109X(19)30482-6
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
3. de Sanjose S, Brotons M, Pavon MA. The natural history of human papillomavirus infection. Best Pract Res Clin Obstetr Gynaecol (2018) 47:2–13. doi: 10.1016/j.bpobgyn.2017.08.015
4. Balasubramaniam SD, Balakrishnan V, Oon CE, Kaur G. Key molecular events in cervical cancer development. Med (Kaunas) (2019) 55(7):384. doi: 10.3390/medicina55070384
5. Liu C, Zhang M, Yan X, Ni Y, Gong Y, Wang C, et al. Single-cell dissection of cellular and molecular features underlying human cervical squamous cell carcinoma initiation and progression. Sci Adv (2023) 9(4):eadd8977. doi: 10.1126/sciadv.add8977
6. Moscicki A-B, Schiffman M, Burchell A, Albero G, Giuliano AR, Goodman MT, et al. Updating the natural history of human papillomavirus and anogenital cancers. Vaccine (2012) 30:F24–33. doi: 10.1016/j.vaccine.2012.05.089
7. Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet (2007) 370(9590):890–907. doi: 10.1016/S0140-6736(07)61416-0
8. Hoffman SR, Le T, Lockhart A, Sanusi A, Dal Santo L, Davis M, et al. Patterns of persistent HPV infection after treatment for cervical intraepithelial neoplasia (CIN): a systematic review. Int J Cancer (2017) 141(1):8–23. doi: 10.1002/ijc.30623
9. Wittmaack A, Dudley D, Boyle A. Maternal history of cervical surgery and preterm delivery: a retrospective cohort study. J Women's Health (2019) 28(11):1538–42. doi: 10.1089/jwh.2018.7457
10. Raizada K, Naik M. Photodynamic therapy for the eye. In StatPearls (StatPearls Publishing) (2020).
11. Donohoe C, Senge MO, Arnaut LG, Gomes-da-Silva LC. Cell death in photodynamic therapy: from oxidative stress to anti-tumor immunity. Biochimica et biophysica Acta. Reviews on cancer (2019) 1872(2):188308. doi: 10.1016/j.bbcan.2019.07.003
12. Dobson J, de Queiroz GF, Golding JP. Photodynamic therapy and diagnosis: principles and comparative aspects. Vet J (2018) 233:8–18. doi: 10.1016/j.tvjl.2017.11.012
13. Cang W, Gu L, Hong Z, Wu A, Di W, Qiu L. Effectiveness of photodynamic therapy with 5-aminolevulinic acid on HPV clearance in women without cervical lesions. Photodiagnosis Photodyn Ther (2021) 34:102293. doi: 10.1016/j.pdpdt.2021.102293
14. Gu L, Cheng M, Hong Z, Di W, Qiu L. The effect of local photodynamic therapy with 5-aminolevulinic acid for the treatment of cervical low-grade squamous intraepithelial lesions with high-risk HPV infection: a retrospective study. Photodiagnosis Photodyn Ther (2021) 33:102172. doi: 10.1016/j.pdpdt.2020.102172
15. Kumar R, Mandal S, Arora P, Mala Y, Khurana N. The expression of p16 and galectin-3 in cervical intraepithelial neoplasia (CIN) and squamous cell carcinoma (SCC) uterine cervix. J Obstetr Gynaecol (2021) 41(5):785–90. doi: 10.1080/01443615.2020.1803235
16. Curty G, Menezes AN, Brant AC, de Mulder Rougvie M, Moreira MÂM, Soares MA. Expression of retroelements in cervical cancer and their interplay with HPV infection and host gene expression. Cancers (2021) 13(14):3513. doi: 10.3390/cancers13143513
17. Riquelme P, Hutchinson JA. Novel molecules mediate specialized functions of human regulatory macrophages. Curr Opin Organ Transplant (2018) 23(5):533–7. doi: 10.1097/MOT.0000000000000560
18. Blazquez J-L, Benyamine A, Pasero C, Olive D. New insights into the regulation of γδ T cells by BTN3A and other BTN/BTNL in tumor immunity. Front Immunol (2018) 9:1601. doi: 10.3389/fimmu.2018.01601
19. Chapoval AI, Smithson G, Brunick L, Mesri M, Boldog FL, Andrew D, et al. BTNL8, a butyrophilin-like molecule that costimulates the primary immune response. Mol Immunol (2013) 56(4):819–28. doi: 10.1016/j.molimm.2013.08.003
20. Yang L, Yang Y, Meng M, Wang W, He S, Zhao Y, et al. Identification of prognosis-related genes in the cervical cancer immune microenvironment. Gene (2021) 766:145119. doi: 10.1016/j.gene.2020.145119
21. Zeng Z, Yang H, Li Z, He X, Griffith CC, Chen X, et al. Prevalence and genotype distribution of HPV infection in China: analysis of 51,345 HPV genotyping results from china's largest CAP certified laboratory. J Cancer (2016) 7(9):1037–43. doi: 10.7150/jca.14971
22. Wang J, Tang D, Wang J, Zhang Z, Chen Y, Wang K, et al. Genotype distribution and prevalence of human papillomavirus among women with cervical cytological abnormalities in xinjiang, China. Hum Vaccines Immunotherapeutics (2019) 15(7-8):1889–96. doi: 10.1080/21645515.2019.1578598
23. Zhao S, Liu D, Shi W, Kang Y, Li Q, Liu Q, et al. Efficacy of a new therapeutic option for vulvar intraepithelial neoplasia: superficial shaving combined with photodynamic therapy. Lasers Surg Med (2020) 52(6):488–95. doi: 10.1002/lsm.23185
24. Troja C, Hoofnagle AN, Szpiro A, Stern JE, Lin J, Winer RL. Serum concentrations of emerging vitamin d biomarkers and detection of prevalent high-risk HPV infection in mid-adult women. Cancer Epidemiol Biomarkers Prev (2020) 29(7):1468–74. doi: 10.1158/1055-9965.EPI-20-0126
25. Doix B, Trempolec N, Riant O, Feron O. Low photosensitizer dose and early radiotherapy enhance antitumor immune response of photodynamic therapy-based dendritic cell vaccination. Front Oncol (2019) 9:811. doi: 10.3389/fonc.2019.00811
26. Hou Y-j, Yang X-x, Liu R-q, Zhao D, Guo C-x, Zhu A-c, et al. Pathological mechanism of photodynamic therapy and photothermal therapy based on nanoparticles. Int J Nanomed (2020) 15:6827–38. doi: 10.2147/IJN.S269321
27. Vantourout P, Laing A, Woodward MJ, Zlatareva I, Apolonia L, Jones AW, et al. Heteromeric interactions regulate butyrophilin (BTN) and BTN-like molecules governing γδ T cell biology. Proc Natl Acad Sci (2018) 115(5):1039–44. doi: 10.1073/pnas.1701237115
28. Mayassi T, Ladell K, Gudjonson H, McLaren JE, Shaw DG, Tran MT, et al. Chronic inflammation permanently reshapes tissue-resident immunity in celiac disease. Cell (2019) 176(5):967–81. e19. doi: 10.1016/j.cell.2018.12.039
29. Clarke MA, Cheung LC, Castle PE, Schiffman M, Tokugawa D, Poitras N, et al. Five-year risk of cervical precancer following p16/Ki-67 dual-stain triage of HPV-positive women. JAMA Oncol (2019) 5(2):181–6. doi: 10.1001/jamaoncol.2018.4270
30. Pandey D, Solleti V, Jain G, Das A, Shama Prasada K, Acharya S, et al. Human papillomavirus (HPV) infection in early pregnancy: prevalence and implications. Infect Dis Obstetr Gynecol (2019) 2019:4376902. doi: 10.1155/2019/4376902
31. Abdulaziz AM, You X, Liu L, Sun Y, Zhang J, Sun S, et al. Management of high-grade squamous intraepithelial lesion patients with positive margin after LEEP conization: a retrospective study. Medicine (2021) 100(20):e26030. doi: 10.1097/MD.0000000000026030
32. Chen J-y, Wang Z-l, Wang Z-y, Yang X-s. The risk factors of residual lesions and recurrence of the high-grade cervical intraepithelial lesions (HSIL) patients with positive-margin after conization. Medicine (2018) 97(41):e12792. doi: 10.1097/MD.0000000000012792
Keywords: high-risk HPV infection, photodynamic therapy, BTNL8, prognosis evaluation, HPV
Citation: Lv H, Lou S, Zhang L, Cui D, Li Y, Yang Y, Chen M and Chen P (2023) Evaluation of the impacts of photodynamic therapy on the prognosis of patients with hrHPV infection based on BTNL8 expression. Front. Oncol. 13:1218808. doi: 10.3389/fonc.2023.1218808
Received: 08 May 2023; Accepted: 09 June 2023;
Published: 29 June 2023.
Edited by:
Chao Liu, Shandong Cancer Hospital, ChinaReviewed by:
Qinyong Hu, Renmin Hospital of Wuhan University, ChinaCopyright © 2023 Lv, Lou, Zhang, Cui, Li, Yang, Chen and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Yang, Z2Z5YW5neWluZ0BzaW5hLmNvbQ==; Meilan Chen, MTgyNTc5NjgzNDNAMTM5LmNvbQ==; Pan Chen, bHljaGVucGFuQDEyNi5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.