
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
METHODS article
Front. Oncol. , 30 June 2023
Sec. Radiation Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1216852
This article is part of the Research Topic Radiotherapy for Brain Metastases View all 10 articles
 Tian Chen1†
Tian Chen1† Mengqiu Tang1†
Mengqiu Tang1† Yang Zhou2
Yang Zhou2 Zhepei Wang3
Zhepei Wang3 Shiwei Li4
Shiwei Li4 Hongcai Wang4
Hongcai Wang4 Yangfang Lu1
Yangfang Lu1 Jinguo Wang1
Jinguo Wang1 Weiyu Shen5*
Weiyu Shen5*Background: Studies on the prognostic factors for patients with brain oligo-metastasis treated with fractionated stereotactic radiotherapy (FSRT) usually focus on the size of metastatic tumor and radiation dose. Some inflammatory indicators have predictive value in non-small cell lung cancer (NSCLC) with brain metastasis receiving stereotactic radiotherapy. However, the prognostic value of inflammatory indicators in NSCLC patients with brain oligo-metastasis treated with FSRT, and their effect on radiotherapy dose is unknown.
Methods: A total of 95 advanced NSCLC patients with brain oligo-metastasis who had undergone FSRT treatment at Ningbo Medical Center Lihuili Hospital between January 2015 and April 2022 were enrolled into the study. Neutrophil to lymphocyte ratio (NLR), platelet lymphocyte ratio (PLR), lymphocyte to monocyte ratio (LMR), tumor diameter and biologically effective dose (BED10) were analyzed using Chi-square test. Univariate and multivariate Cox regressions were used to identify predictors of survival.
Results: Tumor diameter (< 2 cm), BED10 (≥ 48Gy) and LMR (≥ 4) were found to be independently associated with good intracranial local control survival (i-LCS) through multivariate analysis. The median i-LCS was longer in patients with 2 independent risk factors (tumor diameter ≥ 2 and LMR < 4) administered with BED10 > 53.6Gy compared with patients administered with BED10 ≤ 53.6Gy (20.7 months vs 12.0 months, P = 0.042). LMR ≥ 4 (P = 0.019) and positivity for driver gene mutations (P = 0.011) were independently associated with better overall survival (OS).
Conclusions: LMR is an independent prognostic factor of i-LCS and OS in NSCLC patients with brain oligo-metastasis treated with FSRT. Patients with tumor diameter ≥ 2 and LMR < 4 should be treated with BED10 greater than 53.6Gy.
Brain metastases are the most common intracranial tumors in adult accounting for about 20-40 percent (1). Lung cancer is the most common primary malignant tumor that results in the brain metastases, with non-small cell lung cancer (NSCLC) accounting for more than 60 percent of the lung tumors (1, 2). The prognoses of patients with brain metastases arising from NSCLC varies greatly with the median survival time ranging from 6.9 to 46.8 months (3). Several high-technique models, such as diagnosis-specific graded prognostic assessment (DS-GPA), Graded Prognostic Assessment for Lung Cancer Using Molecular Markers (Lung-molGPA), score index for radiosurgery (SIR), and basic score for brain metastases (BSBM), are used to evaluate the prognosis of NSCLC patients (3–6), but the techniques are ineffective in evaluating the prognosis of NSCLC patients with brain oligo-metastasis treated with fractionated stereotactic radiotherapy (FSRT). FSRT has a higher local control rate and fewer side effects than stereotactic radiosurgery (SRS) therapy and has thus been widely used in the clinic. Compared with SRS therapy, FSRT showed a different biological effectiveness. For example, hypoxic tumor cells may survive after SRS, but FSRT, which is based on the principle of reoxidation, has better control rate in tumor (7), suggesting that the SIR model might not apply to the FSRT patients. There is need to identify prognostic factors for NSCLC patients with brain oligo-metastasis receiving FSRT therapy. Current studies on prognostic factors for oligo-metastasis patients treated with FSRT focus on the size of metastatic tumor and radiation dose. However, there is still no standard evaluation method for tumor size, radiation dose and fractionation scheme, with different studies suggesting different radiotherapy biologically effective dose (BED) (8–10). Therefore, there is need to develop and enhance predictive indexes indicating the efficacy and survival of NSCLC patients with brain oligo-metastasis receiving FSRT.
Since the discovery of the relationship between inflammation and cancer in 1863 by R Virchow (11), inflammatory indicators such as neutrophil-lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR), lymphocyte to monocyte ratio (LMR), regulatory T cells and peripheral memory CD4+ T cell, have been used in predicting efficacy and survival in different kinds of cancer (12–16). Inflammatory indicators have also been associated with prognosis of surgery, chemotherapy, targeted therapy, immunotherapy (17–20), and curative effect of radiotherapy (21, 22). In our previous study, we demonstrated that NLR, PLR and LMR constitute a simple and effective prediction index, in locally advanced esophageal cancer treated with surgery, and radiotherapy and in NSCLC treated with anti-vascular targeted therapy (23, 24). Several studies have also demonstrated the predictive value of some inflammatory indicators in NSCLC patients with brain metastasis receiving SRS therapy (25, 26). Although FSRT is similar to radiosurgery, the predictive value of inflammatory indicators in NSCLC patients with brain oligo-metastasis treated with FSRT and their effect on radiotherapy dose is unknown.
This study is a retrospective analysis of the association between inflammatory indicators (NLR, PLR and LMR) and the local control rate and survival of NSCLC patients with brain oligo-metastasis treated with FSRT, and their effect on radiotherapy dose.
This was a retrospective study involving NSCLC patients with oligometastatic brain metastases who had been treated with FSRT at Ningbo Medical Center Lihuili Hospital between January 2015 and January 2022. The inclusion criteria was as follows: (i) pathological findings of metastatic or recurrent NSCLC; (ii) 3 or less brain metastases; (iii) FSRT used to treat brain metastases; (iv) availability of results for routine blood tests carried out two weeks prior to treatment. The exclusion criteria was as follows: (i) co-administration of FSRT and targeted drugs (osimertnib, almonertinib, furmonertinib, alectinib) during the stable disease stage; (ii) assessable focus was treated with FSRT previously; (iii) lack of relevant hematological data within 2 weeks prior to FSRT treatment; (iv) in an acute infection state when obtaining blood inflammation indicators; (v) absence of efficacy evaluation and follow-up information. In the end, 95 patients were enrolled into the study.
The FSRT treatment plan was based on the preference of the attending physician because the tumors were located near or within a critical structure. The head was first immobilized with an aquaplast, and then a computed tomography (CT) scan with intravenous contrast was acquired to plan radiotherapy. Fusing magnetic resonance (MR) T1-weighted imaging with CT images within two weeks of treatment planning. In CT and MR images, the gross tumor volume (GTV) was defined as the contrast medium-enhancing tumor, the clinical target volume (CTV) represented the GTV, while the planning target volume (PTV) was considered as CTV plus a 2-4mm margin. A single split dose of FSRT was set from 3.5-7Gy. Approximately 90% of the maximum dose was applied to the peripheral area, and 95% of the PTV was covered by the peripheral dose. Radiation therapy was administered 5 times a week. We evaluated the dose response of various FSRT fractionation schedules according to a biologically effective dose using an alpha/beta ratio of 10 (BED10) as a measure of the biological effectiveness of the treatment.
The following hematology indexes were evaluated up to 2 weeks prior to FSRT: neutrophil count (× 109/L), platelet count (× 109/L), lymphocyte count (× 109/L) and monocytes count (× 109/L). NLR was defined as the neutrophil count divided by the lymphocyte count. Similarly, PLR was the ratio of the platelet count to the lymphocyte count, LMR was the ratio of the lymphocyte count to the monocytes count. The cutoff values were defined as 5, 180 and 4 for NLR, PLR, and LMR, respectively (25, 27–29). For tumor diameter, the cut off value was 2cm (median tumor diameter), and for BED 10, the cut off value was 48 Gy.
Taking brain enhanced MR re-examination after FSRT in 1-2 months, subsequently checking per 2-3 months, checking enhanced MR immediately at the appearance of intracranial hypertension or neuropsychiatric symptoms. Intracranial local control (i-LC) was defined as no significant increase in the size tumor lesion treated with FSRT on follow-up MR. Intracranial local control survival (i-LCS) was the primary end point of assessment. It was defined as the time from the start of radiation therapy to the time enlargement of the tumor treated with FSRT was observed. Overall survival (OS) was calculated from the date of initiation of FSRT to the time of death from any reason or last time of follow up. OS was the secondary end point of assessment.
The statistical analyses were performed using a social science statistical software package, version 26.0 (SPSS Inc., Chicago, IL, US). Chi-squared tests were used to analyze categorical variables. A Kaplan-Meier survival curve was plotted and compared with a log-rank-test curve. Factors for survival were identified using univariate and multivariate Cox regression analyses. Statistical significance was deemed to be a P-value < 0.05.
The median age of the study participants was 63 years (range from 37 to 79 years).Adenocarcinoma was the most common type of cancer (n = 79, 83.2%), with 13 patients having squamous cell carcinomas, 2 patients having poorly differentiated carcinoma, and 1 patient having large cell carcinoma. Only 11 patients had karnofsky performance status (KPS) scores less than 80, while the rest had scores greater than or equal to 80. The maximum diameter of intracranial metastases ranged from 0.6 to 6.4cm, with a median diameter of 2.0cm. A total of 38 patients (40.0%) had confirmed EGFR mutations, 3 patients (5.1%) had ALK rearrangement and one patient had MET-14 jumping mutation. Most of the patients had no history of brain radiotherapy (n = 84, 88.4%). The median BED10 was 53.6Gy (range from 37.5 to 85.1 Gy) and the number of splits was 5-18. The detailed information of patient characteristics and baseline data are shown in Table 1.
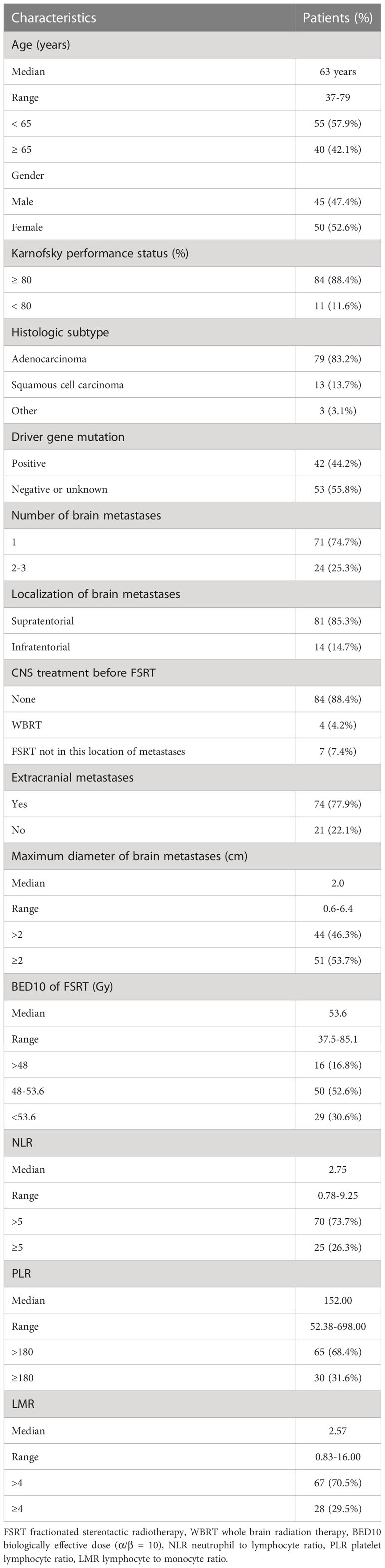
Table 1 Basic characteristics of non-small cell lung cancer patients with oligometastatic brain metastases.
The follow-up time ranged from 3.0 to 37.7 months, with a median of 20.6 months. The local control rates at 6 and 12 months were 82.9% and 66.5%, respectively. The median i-LCS and OS were 15.8 months and 19.4 months, respectively. (Figure S1A, B).
Univariate analyses found that tumor diameter, BED10, PLR and LMR were significant risk factors for i-LCS. Other factors including age, gender, number of brain metastases and presence of extracranial metastasis at diagnosis were not associated with i-LCS in univariate analyses (Table 2). Median i-LCS was significantly shorter in patients with tumor diameter ≥ 2 cm, compared to patients with smaller tumors (13.7 months vs. 31.5 months, HR: 2.595, 95% CI: 1.417-4.750, P = 0.002) (Figure 1A). Median i-LCS was significantly longer in patients with BED10 ≥ 48Gy, compared to patients with less BED10 (17.0 months vs 5.5 months, HR: 0.241, 95% CI: 0.124-0.468, P = 0.001) (Figure 1B). Median i-LCS was significantly longer among patients with PLR ≥ 180, compared to patients with lower values (10.7 months vs 16.5 months, HR: 2.023, 95% CI: 1.116-3.669, P = 0.020) (Figure 1C). Median i-LCS was significantly longer in patients with LMR ≥ 4, compared to patients with lower values (not reached vs 14.0 months, HR: 0.306, 95% CI: 0.147-0.636, P = 0.001) (Figure 1D). The results of multivariate analysis demonstrated that tumor diameter (< 2 cm), BED10 (≥ 48Gy) and LMR (≥ 4) were independently associated with good i-LCS (Table 3).
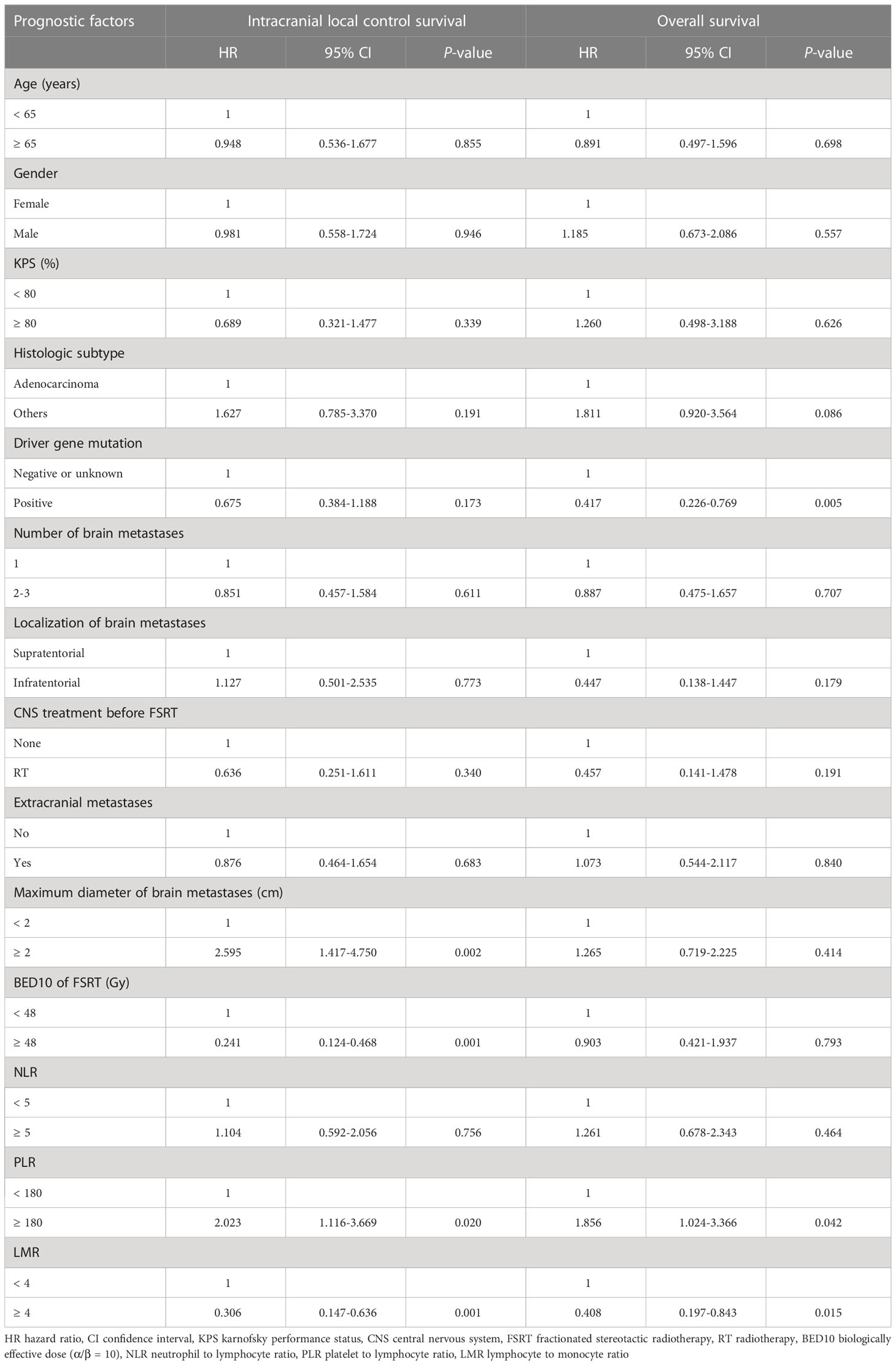
Table 2 Univariate analysis of factors associated with intracranial local control survival and overall survival.
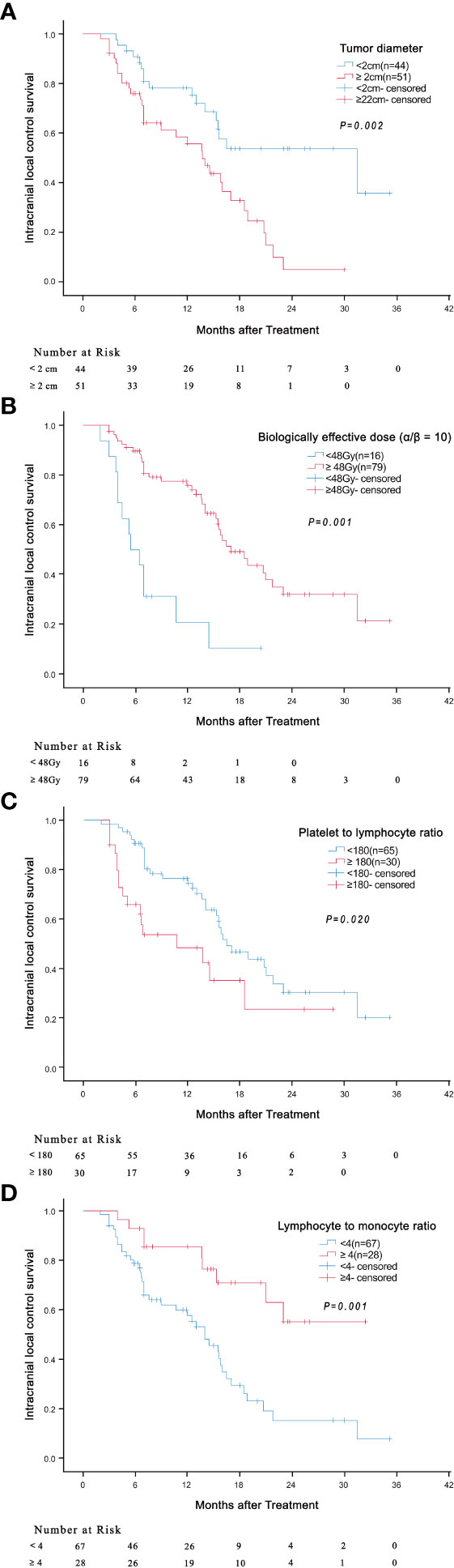
Figure 1 Association of tumor diameter (≥ 2cm versus < 2cm) ((A) P = 0.002), BED10 (≥ 48Gy versus < 48Gy) ((B) P = 0.001), PLR (≥ 180 versus < 180) ((C) P = 0.020) and LMR (≥ 4 versus < 4) ((D) P = 0.001) with intracranial local control survival.
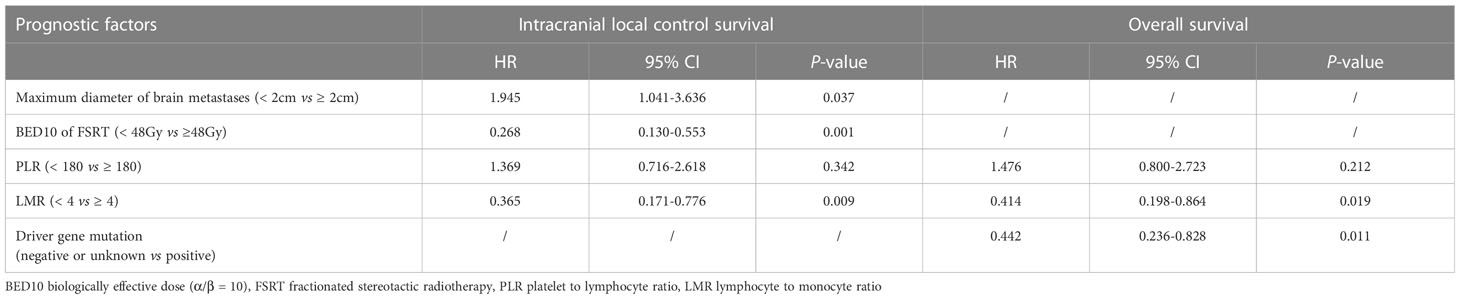
Table 3 Multivariate analysis of factors associated with intracranial local control survival and overall survival.
We then evaluated the effect of the independent prognostic factors on radiotherapy among the patients with BED10 ≥ 48Gy. In total, 49 patients had BED10 ≥ 48Gy, with the median BED10 being 53.6Gy. Surprisingly, in patients with 2 independent risk factors (tumor diameter ≥ 2 and LMR < 4), the i-LCS was longer in patients with BED10 greater than 53.6Gy, compared to patients with BED10 less than 53.6Gy (20.7 months vs 12.0 months, HR: 0.290, 95% CI: 0.082-1.030, P = 0.042) (Figure 2A). There was no significant difference in i-LCS among patients with different BED10 values and with only 1 independent risk factor (P = 0.101, Figure 2B)
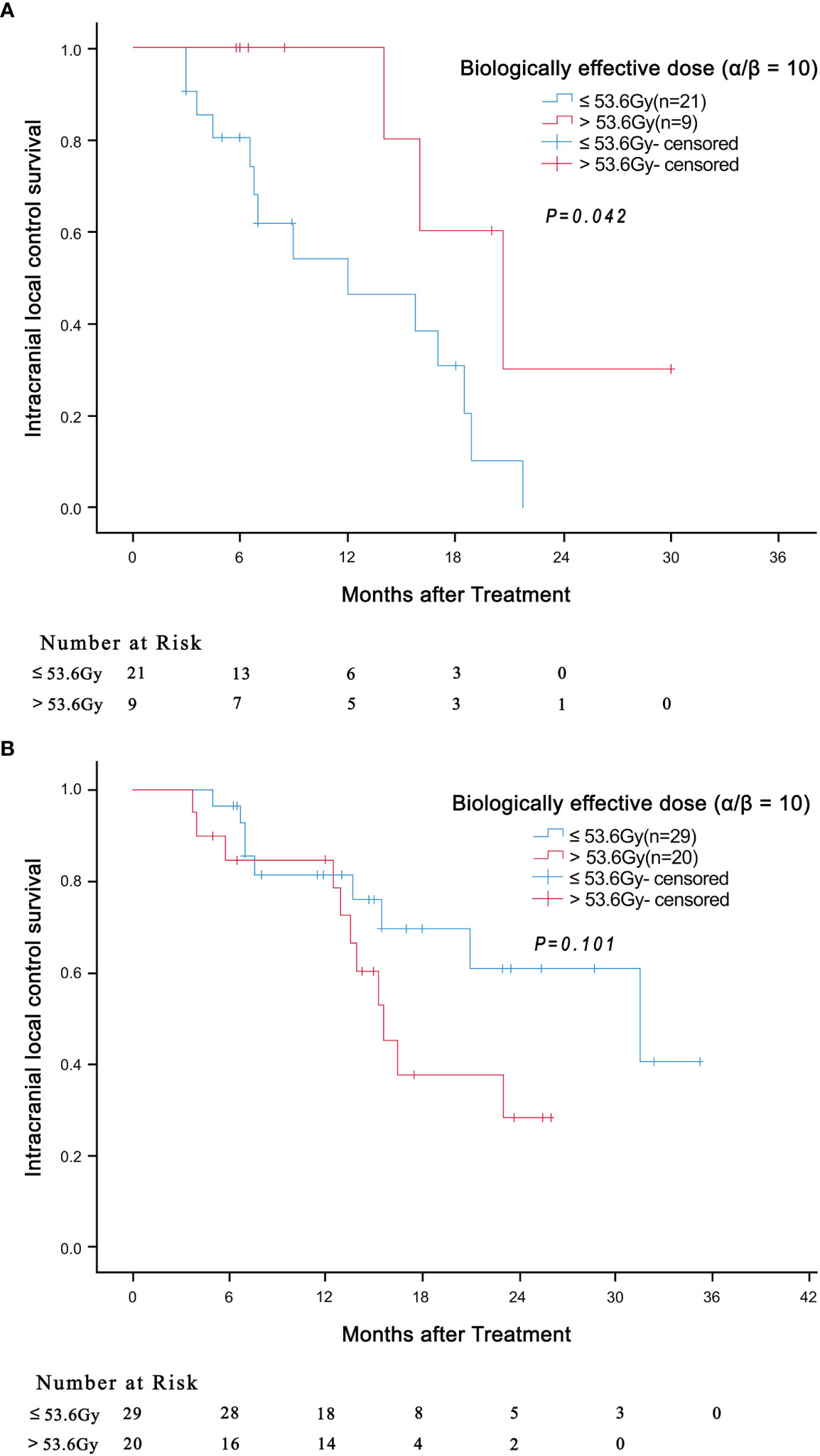
Figure 2 Association of BED10 (> 53.6Gy versus ≤ 53.6Gy) with intracranial local control survival in patients with 2 independent risk factors (tumor diameter ≥ 2 and LMR < 4) ((A) P = 0.042) and in patients with only 1 independent risk factor ((B) P = 0.101).
Results of univariate analysis revealed that driver gene mutations, PLR< 180 and LMR ≥ 4 were associated with better OS, but not BED10 (Table 2). Patients positive for driver gene mutations had longer median OS than patients negative for driver gene mutations or with unknown mutations (30.0 months vs 15.0 months, HR: 0.417, 95% CI: 0.226-0.769, P = 0.005) (Figure 3A). The median OS of patients with PLR <180 was significantly shorter compared to patients with higher values (14.8 months vs 20.5 months, HR: 1.856, 95% CI: 1.024-3.366, P = 0.042) (Figure 3B). The median OS of patients with LMR ≥ 4 was significantly longer compared to patients with lower values (32.4 months vs 16.8 months, HR: 0.408, 95% CI: 0.197-0.843, P = 0.015) (Figure 3C). Multivariate analysis demonstrated that LMR ≥ 4 and presence of driver gene mutations were independently associated with better OS (Table 3).
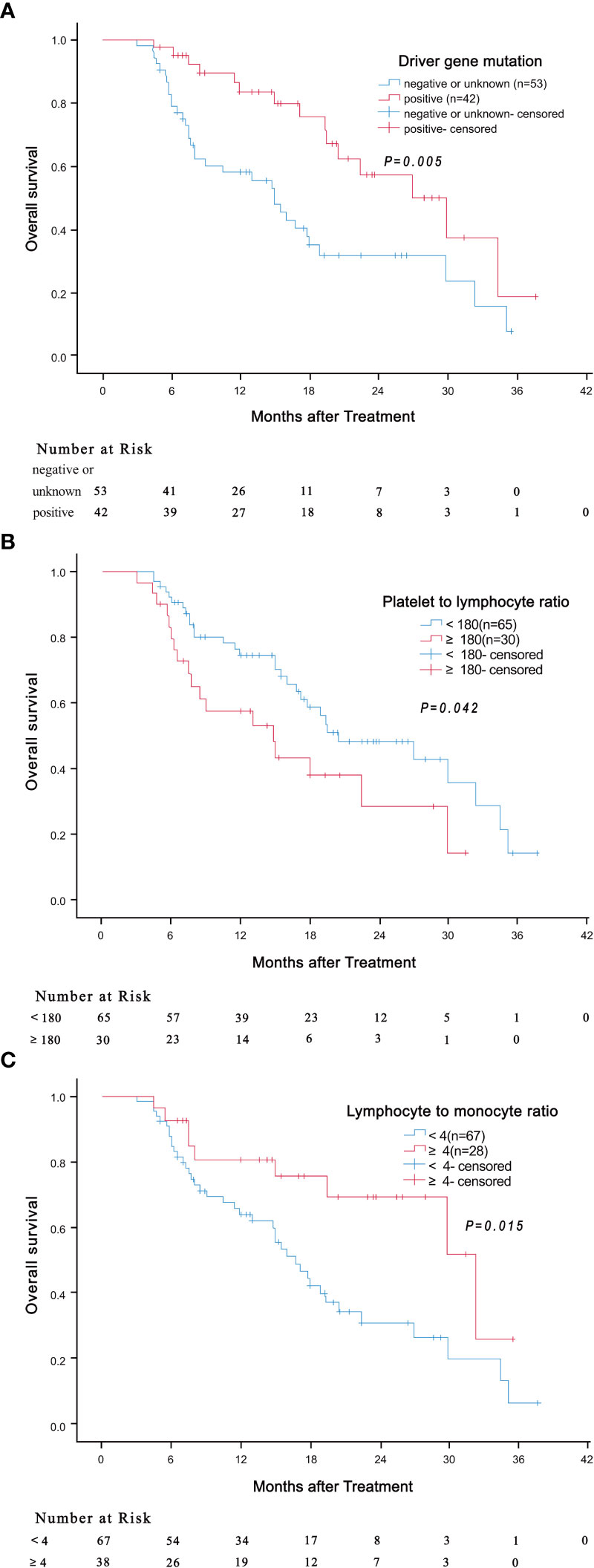
Figure 3 Association of driver gene mutation (A, P = 0.005), PLR (B, P = 0.042) and LMR (C, P = 0.015) with overall survival.
To our knowledge, this is the first study to evaluate the predictive value of inflammatory indicators in NSCLC patients with brain oligo-metastasis treated with FSRT. Our results showed that, in addition to tumor diameter and BED 10, LMR (an inflammatory indicator), was also an independent prognostic factor for i-LCS. Tumor diameter and BED10 had previously been identified as prognostic factors for i-LCS (9, 30, 31), but our study is the first to identify LMR value as an independent prognostic factor. Anna Cho et al. (25) reported that NLR, PLR and LMR were independent prognostic factors of overall survival in NSCLC patients with brain metastases after Gamma Knife Radiosurgery. However, Aijie Li et al. (32) proposed that LMR was the only independent prognostic factor of overall survival in NSCLC patients with brain metastases. The differences in these finding could be due to differences in the study population. The participants in our study were all NSCLC patients with oligometastatic brain metastases who had undergone FSRT treatment. We found that LMR as the only inflammatory indicator that acted as an independent prognostic factor of overall survival, although PLR was identified as prognostic factor during univariate analysis but was negative in multivariable analysis. Therefore LMR ≥ 4 is an excellent independent prognostic factor for i-LCS and overall survival in NSCLC patients with oligometastatic brain metastases treated with FSRT. We also found that positivity for driver gene mutations was an independent prognostic indicator of overall survival, which was consistent with the findings of Aijie Li et al. (32). The reason why driver gene mutations were independent prognostic indicators of overall survival but not i-LCS may be attributed to the overall poor extracranial control rate in patients negative for driver gene mutations, which leads to the death from extracranial lesions.
It is generally acknowledged that the cut-off value of LMR is 4 (25, 26), which was confirmed in our study. There is no consensus on the cut-off value of tumor diameter. Some articles indicate that the cut-off value of tumor diameter is 1cm in brain metastases receiving radiosurgery (33), and 2cm or 3cm in brain metastases receiving FSRT (34, 35). The i-LCS was significantly better in patients with lower cut-off value. Our results showed the cut-off value was 2cm demarcated by the median value. The median i-LCS was just 13.7 months in patients with tumors above 2cm but was as long as 31.5 months in patients with tumors below 2 treated with FSRT. The effective radiation dose for clinical application is still controversial. It is generally agreed that the radiation dose cut-off value for SRS treatment in brain metastases is 18Gy, with local control deteriorating significantly in patients receiving the dose below 18Gy (36). The use of BED is determining the curative effect of SRS is debatable (37), but is usually applied in determining the curative effect of FSRT treatment. Several divide-up radiotherapy plans have been suggested (8–10). One review summarized and compared the curative effect of different BED values, and concluded that BED12 values greater than 40Gy (which equals to BED10 greater than 48Gy) achieved a higher local control rate (38). Another study showed the 1 year local control rate was 100% for BED10 greater than 48Gy but was only 33% for BED10 less that 48Gy in treating postoperative metastasis tumor bed using FSRT (39). Thus it is generally believed the cut-off value of BED10 is 48Gy. In our study, we found that the median i-LCS of patients who received BED10 greater than 48Gy was significantly longer than for patient who received less BED10 (17.0 months vs 5.5 months). Results from multivariate analysis indicated the BED10 was an independent prognostic factor of i-LCS. Samuel R et al. (34) assessed if enhancement of BED value improved i-LCS, and found that enhancing BED10 value did not improve i-LCS in patients with a tumor diameter of more than 3cm. However, we found that administration of BED 10 greater than 53.6Gy improved the i-LCS of patients with two independent risk factors (tumor diameter ≥ 2 and LMR < 4), but had no benefit in the patients with 1 or no independent risk factor. The difference in findings may be because the study by Samuel R et al. enrolled patients with only 1 independent risk factor (tumor diameter ≥3cm) and did not screen the patients for obstinate resistance to radiotherapy. Unlike their results, this study analyzed the LMR value below 4 as the other independent risk factor. Thus got benefit by improving BED value in patients simultaneously possessed two independent risk factors (tumor diameter ≥ 2 and LMR < 4) equivalent to possessing obstinate resistance to radiotherapy.
Neutrophils inhibit immune functions and induce resistance to chemoradiotherapy by secreting cytokines and chemokines (13, 40, 41). Platelets are a critical source of cytokines, such as transforming growth factor-β, platelet-derived growth factor, and vascular endothelial growth factor (VEGF), which induce angiogenesis and cell invasion (42, 43). Moreover, lymphocytes can produce several cytokines, including IFN-γ and perforin, to prevent tumor development and induce apoptosis in cancer cells (36). Monocytes are innate immune cells that play important roles in tumor progression, invasion and metastasis and can be grouped into macrophages and myeloid-derived suppressor cells (44, 45). These findings from literature suggest that NLR, PLR and LMR have potential roles as prognostic factors in tumor development and treatment. In our study, we found that LMR but not NLR was a prognostic factor in the NSCLC patients with oligometastatic brain metastases receiving FSRT treatment. The difference between the two factors could be due to the effect of glucocorticoids. Glucocorticoids are usually administered to reduce the intracranial pressure before radiation therapy once the diagnosis of brain metastasis has been confirmed. The use of glucocorticoids can affect neutrophil counts which affects NLR value resulting in a negative result. It is also possible that NLR has no prognostic value in patients receiving FSRT treatment.
There are some limitations in our study. First, this was a retrospective study involving a small sample from a single center, which may have caused analytical bias. Second, the lack of follow-up data for different treatments before and after the radiotherapy may have influenced the analysis. Third, adverse reactions such as acute cerebral edema and radionecrosis are difficult to detect and were not reported during follow-up, thus adverse reactions analysis could not be carried out. Fourthly, selection bias may have been present despite the strict inclusion criteria, and thus the findings need to be validated in future prospective studies. Fifth, a part of patients from our study had been pronounced dead because of the extracranial lesions before the intracranial lesions, and this point was linked to the end of intracranial follow-up which resulted in attrition bias. Therefore, there is need for multi-center prospective randomized clinical trials with large sample size to validate the prognostic value of LMR in NSCLC patients with oligometastatic brain metastases treated with FSRT and its effect on radiotherapy dose.
LMR is a prognostic factor for i-LCS and OS in NSCLC patients with oligometastatic brain metastases treated with FSRT. The basic dose for BED10 was greater that 48Gy, but should be increased to greater than 53.6Gy in patients with tumor diameter ≥ 2 and LMR < 4.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ningbo Medical Center Lihuili Hospital ethics committee. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements. Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
TC and WS contributed to the conception and design of the study. TC and MT wrote the article together. SL, YL, and JW contributed to the acquisition and analysis of the data. YZ, ZW, and HW participated in revising of the article. All authors contributed to the article and approved the submitted version.
This work was supported by the Ningbo Clinical Research Center for thoracic and breast neoplasms (grant number 2021L002), the National Natural Science Foundation of China (grant number 21906014) and the Ningbo Medical and Health Leading Academic Discipline Project (grant number 2022-F04).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1216852/full#supplementary-material
Supplementary Figure 1 | Curves of intracranial local control survival (A) and overall survival (B).
1. Patchell RA. The management of brain metastases. Cancer Treat Rev (2003) 29(6):533–40. doi: 10.1016/s0305-7372(03)00105-1
2. Wu YL, Planchard D, Lu S, Sun H, Yamamoto N, Kim DW, et al. Pan-Asian adapted clinical practice guidelines for the management of patients with metastatic non-small-cell lung cancer: a CSCO-ESMO initiative endorsed by JSMO, KSMO, MOS, SSO and TOS. Ann Oncol (2019) 30(2):171–210. doi: 10.1093/annonc/mdy554
3. Sperduto PW, Yang TJ, Beal K, Pan H, Brown PD, Bangdiwala A, et al. Estimating survival in patients with lung cancer and brain metastases: an update of the graded prognostic assessment for lung cancer using molecular markers (Lung-molGPA). JAMA Oncol (2017) 3(6):827–31. doi: 10.1001/jamaoncol.2016.3834
4. Sperduto PW, Kased N, Roberge D, Xu Z, Shanley R, Luo X, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol (2012) 30(4):419–25. doi: 10.1200/JCO.2011.38.0527
5. Lorenzoni J, Devriendt D, Massager N, David P, Ruíz S, Vanderlinden B, et al. Radiosurgery for treatment of brain metastases: estimation of patient eligibility using three stratification systems. Int J Radiat Oncol Biol Physics (2004) 60(1):218–24. doi: 10.1016/j.ijrobp.2004.02.017
6. Franzin A, Snider S, Picozzi P, Bolognesi A, Serra C, Vimercati A, et al. Evaluation of different score index for predicting prognosis in gamma knife radiosurgical treatment for brain metastasis. Int J Radiat Oncol Biol Physics (2009) 74(3):707–13. doi: 10.1016/j.ijrobp.2008.08.062
7. Van De Voorde L, Vanneste B, Houben R, Damen P, van den Bogaard J, Lammering G, et al. Image-guided stereotactic ablative radiotherapy for the liver: a safe and effective treatment. Eur J Surg Oncol (2015) 41(2):249–56. doi: 10.1016/j.ejso.2014.10.053
8. Ishihara T, Yamada K, Harada A, Isogai K, Tonosaki Y, Demizu Y, et al. Hypofractionated stereotactic radiotherapy for brain metastases from lung cancer: evaluation of indications and predictors of local control. Strahlentherapie Und Onkol (2016) 192(6):386–93. doi: 10.1007/s00066-016-0963-2
9. Loo M, Clavier JB, Attal Khalifa J, Moyal E, Khalifa J. Dose-response effect and dose-toxicity in stereotactic radiotherapy for brain metastases: a review. Cancers (2021) 13(23):6086. doi: 10.3390/cancers13236086
10. Musunuru HB, Witt JS, Yadav P, Francis DM, Kuczmarska-Haas A, Labby ZE, et al. Impact of adjuvant fractionated stereotactic radiotherapy dose on local control of brain metastases. J Neuro-Oncol (2019) 145(2):385–90. doi: 10.1007/s11060-019-03308-7
11. Balkwill F, Mantovani A. Inflammation and cancer: back to virchow? Lancet (London England) (2001) 357(9255):539–45. doi: 10.1016/S0140-6736(00)04046-0
12. Zhang X, Hu D, Lin X, Zhang H, Xia Y, Lin J, et al. Prognostic value of an inflammation-related index in 6,865 Chinese patients with postoperative digestive tract cancers: the FIESTA study. Front Oncol (2019) 9:427. doi: 10.3389/fonc.2019.00427
13. Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol/Hematol (2013) 88(1):218–30. doi: 10.1016/j.critrevonc.2013.03.010
14. Templeton AJ, Ace O, McNamara MG, Al-Mubarak M, Vera-Badillo FE, Hermanns T, et al. Prognostic role of platelet to lymphocyte ratio in solid tumors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev (2014) 23(7):1204–12. doi: 10.1158/1055-9965.EPI-14-0146
15. Liu C, Sun B, Hu X, Zhang Y, Wang Q, Yue J, et al. Stereotactic ablative radiation therapy for pulmonary recurrence-based oligometastatic non-small cell lung cancer: survival and prognostic value of regulatory T cells. Int J Radiat Oncol Biol Physics (2019) 105(5):1055–64. doi: 10.1016/j.ijrobp.2019.08.012
16. Liu C, Hu Q, Xu B, Hu X, Su H, Li Q, et al. Peripheral memory and naïve T cells in non-small cell lung cancer patients with lung metastases undergoing stereotactic body radiotherapy: predictors of early tumor response. Cancer Cell Int (2019) 19:121. doi: 10.1186/s12935-019-0839-5
17. Lang C, Egger F, Alireza Hoda M, Saeed Querner A, Ferencz B, Lungu V, et al. Lymphocyte-to-monocyte ratio is an independent prognostic factor in surgically treated small cell lung cancer: an international multicenter analysis. Lung Cancer (Amsterdam Netherlands) (2022) 169:40–6. doi: 10.1016/j.lungcan.2022.05.010
18. Tada T, Kumada T, Hiraoka A, Michitaka K, Atsukawa M, Hirooka M, et al. Neutrophil-to-lymphocyte ratio is associated with survival in patients with unresectable hepatocellular carcinoma treated with lenvatinib. Liver Int (2020) 40(4):968–76. doi: 10.1111/liv.14405
19. Yao Y, Yuan D, Liu H, Gu X, Song Y. Pretreatment neutrophil to lymphocyte ratio is associated with response to therapy and prognosis of advanced non-small cell lung cancer patients treated with first-line platinum-based chemotherapy. Cancer Immunol Immunother (2013) 62(3):471–9. doi: 10.1007/s00262-012-1347-9
20. Peng L, Wang Y, Liu F, Qiu X, Zhang X, Fang C, et al. Peripheral blood markers predictive of outcome and immune-related adverse events in advanced non-small cell lung cancer treated with PD-1 inhibitors. Cancer Immunol Immunother (2020) 69(9):1813–22. doi: 10.1007/s00262-020-02585-w
21. Sebastian NT, Raj R, Prasad R, Barney C, Brownstein J, Grecula J, et al. Association of pre- and posttreatment neutrophil-lymphocyte ratio with recurrence and mortality in locally advanced non-small cell lung cancer. Front Oncol (2020) 10:598873. doi: 10.3389/fonc.2020.598873
22. Ng SP, Bahig H, Jethanandani A, Sturgis EM, Johnson FM, Elgohari B, et al. Prognostic significance of pre-treatment neutrophil-to-lymphocyte ratio (NLR) in patients with oropharyngeal cancer treated with radiotherapy. Br J Cancer (2021) 124(3):628–33. doi: 10.1038/s41416-020-01106-x
23. Wang C, Tong J, Tang M, Lu Y, Liang G, Zhang Z, et al. Pretreatment neutrophil-to-Lymphocyte ratio and platelet-to-Lymphocyte ratio as prognostic factors and reference markers of treatment options for locally advanced squamous cell carcinoma located in the middle and upper esophagus. Cancer Manage Res (2021) 13:1075–85. doi: 10.2147/CMAR.S294344
24. Chen T, Song C, Liang G, Xu X, Wang C, Zhang Z, et al. Neutrophil-to-Lymphocyte ratio, platelet-to-Lymphocyte ratio, and their variations as a basis for a prediction model in advanced NSCLC patients receiving anlotinib. Dis Markers (2022) 2022:5879137. doi: 10.1155/2022/5879137
25. Cho A, Untersteiner H, Hirschmann D, Fitschek F, Dorfer C, Rössler K, et al. Pre-radiosurgery leucocyte ratios and modified glasgow prognostic score predict survival in non-small cell lung cancer brain metastases patients. J Neuro-Oncol (2021) 151(2):257–65. doi: 10.1007/s11060-020-03660-z
26. Cho A, Kranawetter B, Untersteiner H, Khalaveh F, Dorfer C, Rössler K, et al. Neutrophil-to-Lymphocyte ratio is superior to other leukocyte-based ratios as a prognostic predictor in non-small cell lung cancer patients with radiosurgically treated brain metastases under immunotherapy or targeted therapy. World Neurosurg (2021) 151:e324–e31. doi: 10.1016/j.wneu.2021.04.033
27. Mandaliya H, Jones M, Oldmeadow C, Nordman II. Prognostic biomarkers in stage IV non-small cell lung cancer (NSCLC): neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), platelet to lymphocyte ratio (PLR) and advanced lung cancer inflammation index (ALI). Trans Lung Cancer Res (2019) 8(6):886–94. doi: 10.21037/tlcr.2019.11.16
28. Gu X, Sun S, Gao XS, Xiong W, Qin S, Qi X, et al. Prognostic value of platelet to lymphocyte ratio in non-small cell lung cancer: evidence from 3,430 patients. Sci Rep (2016) 6:23893. doi: 10.1038/srep23893
29. Cao D, Xu H, Xu X, Guo T, Ge W. A reliable and feasible way to predict the benefits of nivolumab in patients with non-small cell lung cancer: a pooled analysis of 14 retrospective studies. Oncoimmunology (2018) 7(11):e1507262. doi: 10.1080/2162402X.2018.1507262
30. Matsuyama T, Kogo K, Oya N. Clinical outcomes of biological effective dose-based fractionated stereotactic radiation therapy for metastatic brain tumors from non-small cell lung cancer. Int J Radiat Oncol Biol Physics (2013) 85(4):984–90. doi: 10.1016/j.ijrobp.2012.09.008
31. Redmond KJ, Gui C, Benedict S, Milano MT, Grimm J, Vargo JA, et al. Tumor control probability of radiosurgery and fractionated stereotactic radiosurgery for brain metastases. Int J Radiat Oncol Biol Physics (2021) 110(1):53–67. doi: 10.1016/j.ijrobp.2020.10.034
32. Li A, Mu X, He K, Wang P, Wang D, Liu C, et al. Prognostic value of lymphocyte-to-monocyte ratio and systemic immune-inflammation index in non-small-cell lung cancer patients with brain metastases. Future Oncol (London England) (2020) 16(30):2433–44. doi: 10.2217/fon-2020-0423
33. Chang EL, Hassenbusch SJ 3rd, Shiu AS, Lang FF, Allen PK, Sawaya R, et al. The role of tumor size in the radiosurgical management of patients with ambiguous brain metastases. Neurosurgery (2003) 53(2):272–80. doi: 10.1227/01.neu.0000073546.61154.9a
34. Marcrom SR, McDonald AM, Thompson JW, Popple RA, Riley KO, Markert JM, et al. Fractionated stereotactic radiation therapy for intact brain metastases. Adv Radiat Oncol (2017) 2(4):564–71. doi: 10.1016/j.adro.2017.07.006
35. Kwon AK, Dibiase SJ, Wang B, Hughes SL, Milcarek B, Zhu Y. Hypofractionated stereotactic radiotherapy for the treatment of brain metastases. Cancer (2009) 115(4):890–8. doi: 10.1002/cncr.24082
36. Shiau CY, Sneed PK, Shu HK, Lamborn KR, McDermott MW, Chang S, et al. Radiosurgery for brain metastases: relationship of dose and pattern of enhancement to local control. Int J Radiat Oncol Biol Physics (1997) 37(2):375–83. doi: 10.1016/s0360-3016(96)00497-x
37. Brown JM, Koong AC. High-dose single-fraction radiotherapy: exploiting a new biology? Int J Radiat Oncol Biol Phys (2008) 71(2):324–5. doi: 10.1016/j.ijrobp.2008.02.003
38. Wiggenraad R, Verbeek-de Kanter A, Kal HB, Taphoorn M, Vissers T, Struikmans H. Dose-effect relation in stereotactic radiotherapy for brain metastases. a systematic review. Radiother Oncol (2011) 98(3):292–7. doi: 10.1016/j.radonc.2011.01.011
39. Kumar AMS, Miller J, Hoffer SA, Mansur DB, Coffey M, Lo SS, et al. Postoperative hypofractionated stereotactic brain radiation (HSRT) for resected brain metastases: improved local control with higher BED(10). J Neuro-Oncol (2018) 139(2):449–54. doi: 10.1007/s11060-018-2885-6
40. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol (2014) 15(11):e493–503. doi: 10.1016/S1470-2045(14)70263-3
41. Powell DR, Huttenlocher A. Neutrophils in the tumor microenvironment. Trends Immunol (2016) 37(1):41–52. doi: 10.1016/j.it.2015.11.008
42. Schumacher D, Strilic B, Sivaraj KK, Wettschureck N, Offermanns S. Platelet-derived nucleotides promote tumor-cell transendothelial migration and metastasis via P2Y2 receptor. Cancer Cell (2013) 24(1):130–7. doi: 10.1016/j.ccr.2013.05.008
43. Yun SH, Sim EH, Goh RY, Park JI, Han JY. Platelet activation: the mechanisms and potential biomarkers. BioMed Res Int (2016) 2016:9060143. doi: 10.1155/2016/9060143
44. Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J Clin Invest (2015) 125(9):3356–64. doi: 10.1172/JCI80005
Keywords: lymphocyte to monocyte ratio (LMR), biologically effective dose (BED), brain oligo-metastasis, fractionated stereotactic radiotherapy (FSRT), intracranial local control survival (i-LCS)
Citation: Chen T, Tang M, Zhou Y, Wang Z, Li S, Wang H, Lu Y, Wang J and Shen W (2023) Pretreatment lymphocyte-to-monocyte ratio as a prognostic factor and influence on dose-effect in fractionated stereotactic radiotherapy for oligometastatic brain metastases in non-small cell lung cancer patients. Front. Oncol. 13:1216852. doi: 10.3389/fonc.2023.1216852
Received: 04 May 2023; Accepted: 14 June 2023;
Published: 30 June 2023.
Edited by:
Xiangpan Li, Renmin Hospital of Wuhan University, ChinaReviewed by:
Feifei Teng, Shandong University Cancer Center, ChinaCopyright © 2023 Chen, Tang, Zhou, Wang, Li, Wang, Lu, Wang and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weiyu Shen, bmJsaGxzd3lAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.