- 1Department of General Surgery, West China Hospital, Sichuan University, Chengdu, China
- 2Gastric Cancer Center, West China Hospital, Sichuan University, Chengdu, China
- 3Division of Gastrointestinal Surgery, Department of General Surgery, West China Hospital, Sichuan University, Chengdu, China
- 4Department of General Surgery, West China Shangjin Hospital, Sichuan University, Chengdu, China
- 5Colorectal Cancer Center, West China Hospital, Sichuan University, Chengdu, China
- 6Department of Pathology, West China Shangjin Hospital, Sichuan University, Chengdu, China
- 7Department of Anesthesiology, West China Shangjin Hospital, Sichuan University, Chengdu, China
Background: Breast and vulvar metastases from rectal signet ring cell carcinoma (SRCC) represent a rare and obscure clinical entity associated with poor survival. Managing patients with metastatic rectal SRCC is extremely challenging due to the absence of high-quality evidence.
Case presentation: A 26-year-old woman presented with progressively worsening anal pain, constipation, and hematochezia for approximately two years. Following the diagnosis of locally advanced rectal cancer (cT3N0-1M0), she received neoadjuvant chemotherapy with modified FOLFOX6 regimen and underwent laparoscopic abdominoperineal resection. Metastases to the breast and vulva developed during postoperative chemotherapy. Genetic testing revealed RAS/BRAF wild-type and microsatellite instability (MSI)-low status. Though sequential administration of irinotecan plus tegafur and tegafur plus raltitrexed-based chemotherapy in combination with bevacizumab, the disease progressed rapidly. Sadly, the patient passed away 15 months after initial diagnosis due to rapidly progressive disease.
Conclusion: Rectal SRCC is associated with younger on-set, aggressive behaviors, and worse survival outcomes. Due to poor cohesiveness, SRCC tends to develop metastases. A patient’s medical history and immunohistochemical staining (such as CK20, CK7, and CDX-2) can aid in identifying the tumor origin of breast and vulvar metastases. Mutations and signaling pathways predominant in the tumorigenesis of SRCC remains unveiled. There is poor effect of conventional chemotherapies, targeted and immunotherapies for colorectal adenocarcinoma on SRCC, so novel therapies are needed to treat this patient population.
Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed malignancy and the second leading cause of cancer-related deaths worldwide (1). Signet ring cell carcinoma (SRCC), characterized by the presence of over 50% signet ring cells, represents one of the rarest histopathologic subtypes of CRC (1-2.4%) and is associated with poorer survival outcomes (2, 3). Signet ring cells are featured by an eccentrically displaced nucleus due to excess intracellular mucin, imparting a signet ring appearance under an optical microscope (4). Although the median age at CRC diagnosis is 63-69 years (5), colorectal SRCC is associated with a younger on-set (6). Due to poor cohesiveness, SRCC tends to develop metastases (7). Distant metastases from colorectal SRCC are most frequently found in the liver, followed by the distant lymph node, bone, lung, and brain (8).
Most breast metastases originate from the contralateral breast cancer (9). Breast metastases from extramammary malignancies are exactly uncommon, with incidence rates ranging between 0.2% and 2.7% in previous studies (10, 11). Except CRC, hematological malignancies as well as several primary solid tumors (including melanoma, rhabdomyosarcoma, and lung cancer) usually disseminate to the breast (12). Similarly, accounting for only 4% of gynecological malignancies, primary vulvar cancer is uncommon and mainly affects postmenopausal women (13). Vulvar metastasis is even rarer, representing only 5-8% of all vulvar cancers (14, 15).
In accordance with principles of the CAse REport (CARE) guidelines (16), we present a case report of a young female patient with breast and vulvar metastases from resected rectal SRCC. This case report aims to enhance the clinicians’ recognition and awareness of the invasiveness and occult metastasis of colorectal SRCC.
Case presentation
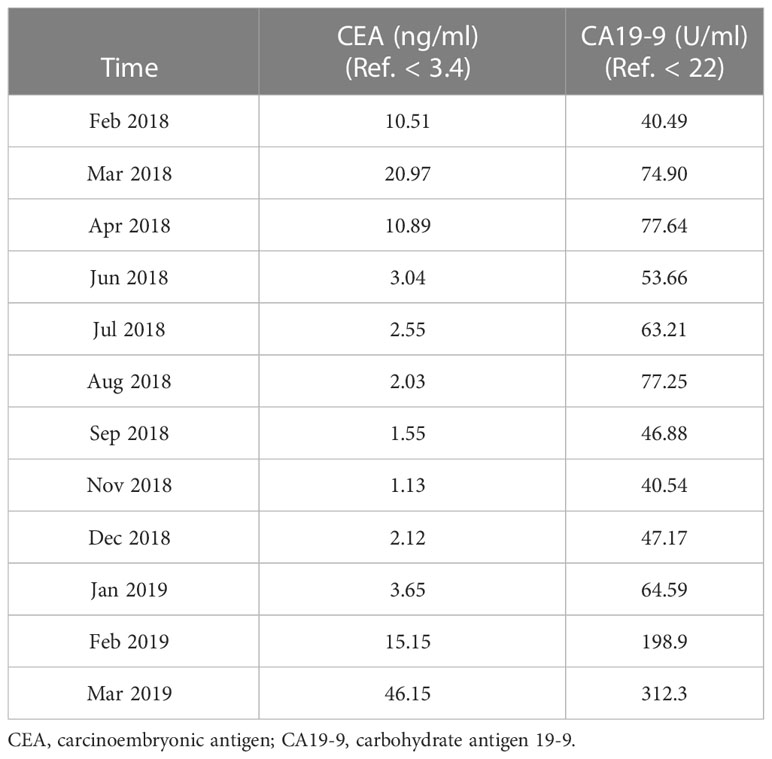
In February 2018, a 26-year-old female patient presented to our hospital with progressively worsening anal pain, constipation, and hematochezia for approximately two years. She had given birth right two and a half years previously. She had no prior medical history and family history of gastrointestinal malignancies. On admission, her vital signs were stable. Digital rectal examination revealed an ulcerated 5-cm indurated lesion located at the nine o’clock position and 2 cm from the anal verge, with bleeding upon palpation. The blood tests showed elevated carcinoembryonic antigen (CEA) (10.51 ng/ml; reference range, < 3.4 ng/ml) and carbohydrate antigen 19-9 (CA19-9) (40.49 U/ml; reference range, < 22 U/ml). The diagnosis of poorly differentiated adenocarcinoma was established by flexible sigmoidoscopy with biopsies and histological examination. Computed tomography (CT) of the chest and abdomen and magnetic resonance imaging (MRI) of the pelvic confirmed the tumor stage of cT3N1M0 (Figure 1A). Radiologic examinations did not detect metastasis to the liver, bone, lung, or breast.
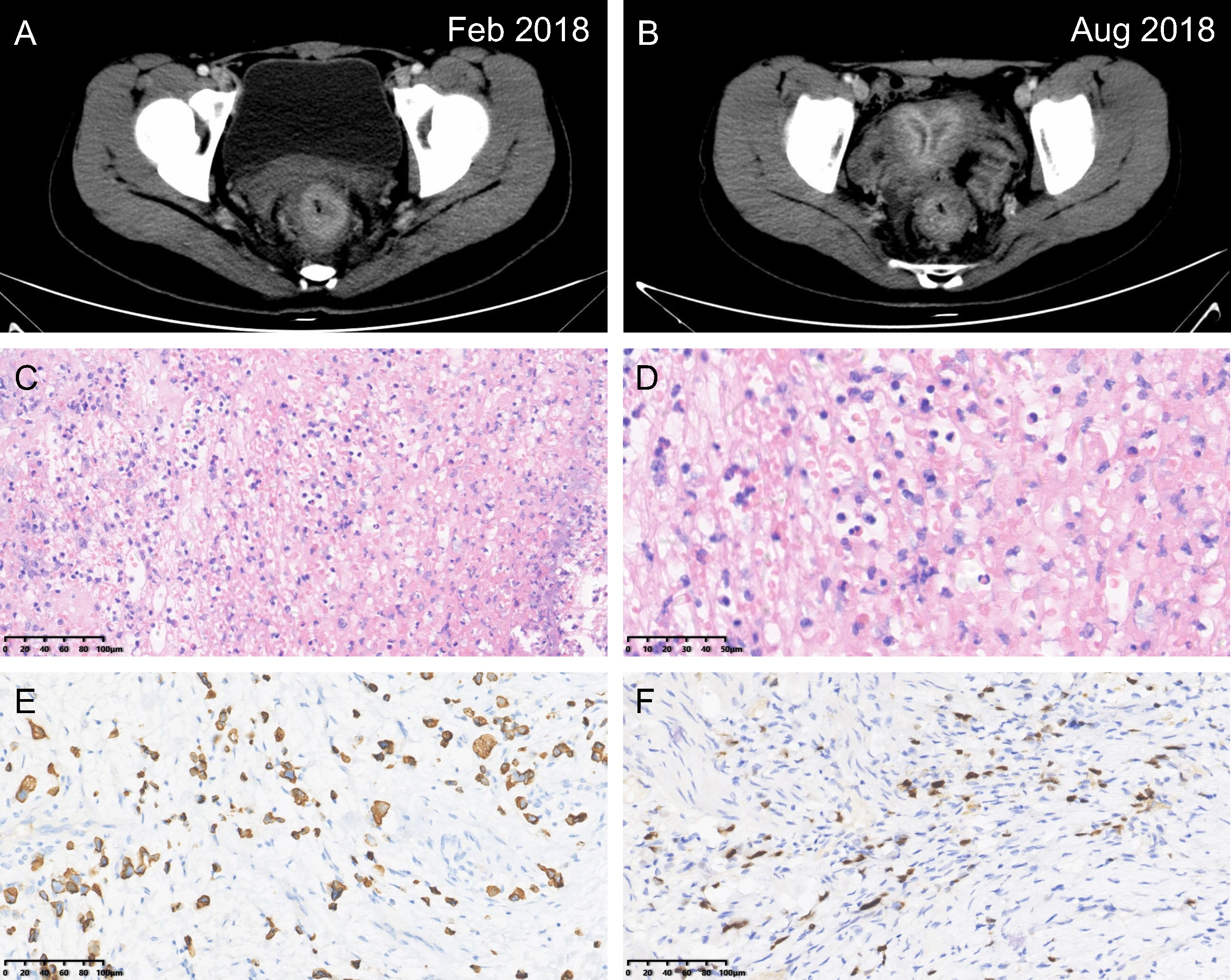
Figure 1 Rectal signet ring cell carcinoma. Computed tomography images at the initial diagnosis (A) and after the implementation of neoadjuvant therapy (B). Hematoxylin & eosin staining imparting a signet ring appearance (C, magnification power: 20×; D, magnification power: 40×). Immunohistochemical staining revealing (E) CK20 (+), and (F) CDX-2 (+) (magnification power: 20×).
Following consultancy with the multidisciplinary team, the patient underwent prophylactic transverse colostomy to avoid upcoming obstruction and then received 6 cycles of modified FOLFOX-6 neoadjuvant chemotherapy. The scheduled long-course radiotherapy (50Gy/25f) was ceased after the first time due to severe anal incontinence and myelosuppression. Laparoscopic abdominoperineal resection (R0) with a permanent colostomy was performed for her 6 weeks after the termination of neoadjuvant therapy (Figure 1B). Guided by the fast-track surgery pathway, the patient’s recovery was uneventful, with discharge on postoperative day 5. The final diagnosis of rectal SRCC (ypT3N1bM0, Tumor Regression Grade 2) was determined via postoperative pathologic findings (Figures 1C–F). Two out of eighteen mesorectal lymph nodes were identified with tumor involvement. Meanwhile, the proficient mismatch repair (MMR) was detected. The postoperative chemotherapy was consistent with the neoadjuvant regimen and initiated 4 weeks after surgery.
After the second cycle of adjuvant modified FOLFOX-6, a painless, firm mass was palpable in the right breast and the area of vulva, respectively. The follow-up CT examination found the right breast mass (Figure 2A). Ultrasound-guided core needle biopsies were performed for her. Hematoxylin & eosin and immunohistochemical (IHC) staining indicated the metastases to the breast (positive: CK20, CDX-2, E-cadherin; negative: CK7, PR, ER, Her-2, GATA3, GCDFP15) (Figures 2B–E) and vulva (positive: CDX-2, PCK, CEA, Alcian Blue) (Figures 2F–I) from rectal SRCC. In addition, genetic testing demonstrated RAS/BRAF wild-type and microsatellite instability-low (MSI-L).
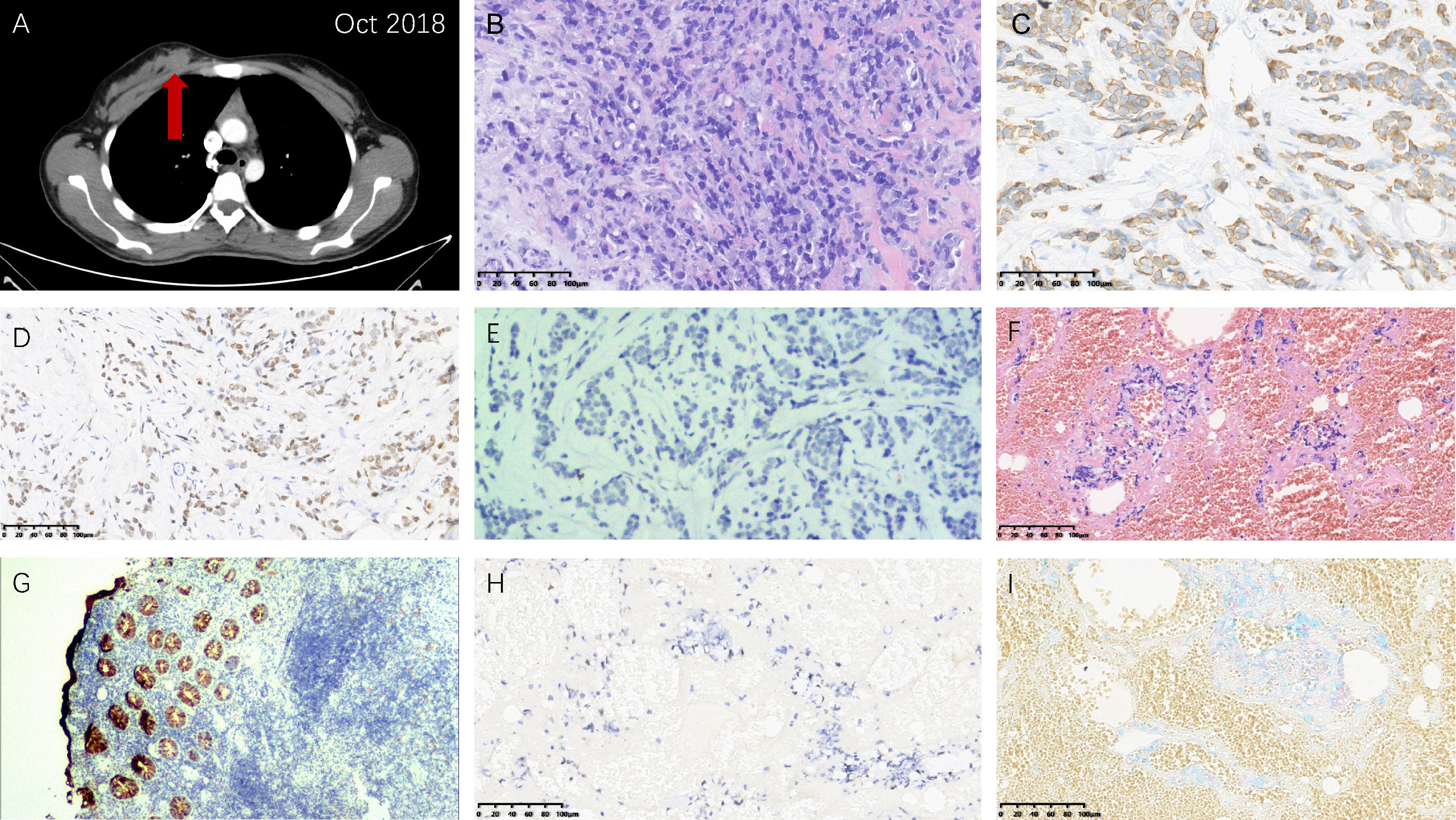
Figure 2 Right breast and vulvar metastases from rectal signet ring cell carcinoma. (A) Computed tomography image showing the right breast metastasis. Hematoxylin & eosin staining demonstrating morphological characteristics of (B) the right breast and (F) vulvar metastases similar with the primary rectal carcinoma (magnification power: 20×). Immunohistochemical staining: (C) CK20 (+) (magnification power: 20×), (D) CDX-2 (+) (magnification power: 20×), and (E) GATA3 (-) (magnification power: 100×) of the right breast metastasis tissue; (G) CK20 (+) (magnification power: 40×), (H) CDX-2 (+) (magnification power: 20×), and (I) Alcian Blue (+) (magnification power: 20×) of the vulvar metastasis tissue.
In December 2018, metastases to bilateral lung have been developed and the evaluation of efficacy was identified as progressive disease. Then the chemotherapy regimen was changed to irinotecan (290mg, day1, q3w) plus tegafur (50mg bid, day1-14, q3w). However, multiple metastases throughout the body (including the left breast) were found 3 months later. Tegafur (50mg, bid, day1-14, q3w) plus raltitrexed (4mg, day1, q3w)-based chemotherapy in combination with bevacizumab (400mg, day1, q3w) as the third-line treatment did not provide favorable efficacy. Unfortunately, the patient passed away 15 months after initial diagnosis due to rapidly progressive disease. The results of serum tumor markers (CEA and CA19-9) are listed in Table 1. Though limited sensitivity, the reduction of tumor markers at the early phase represented favorable response to neoadjuvant therapy, while the elevation of tumor markers at the late phase indicated rapidly progressive disease. The timeline with clinical data from the episode of care is shown in Figure 3.
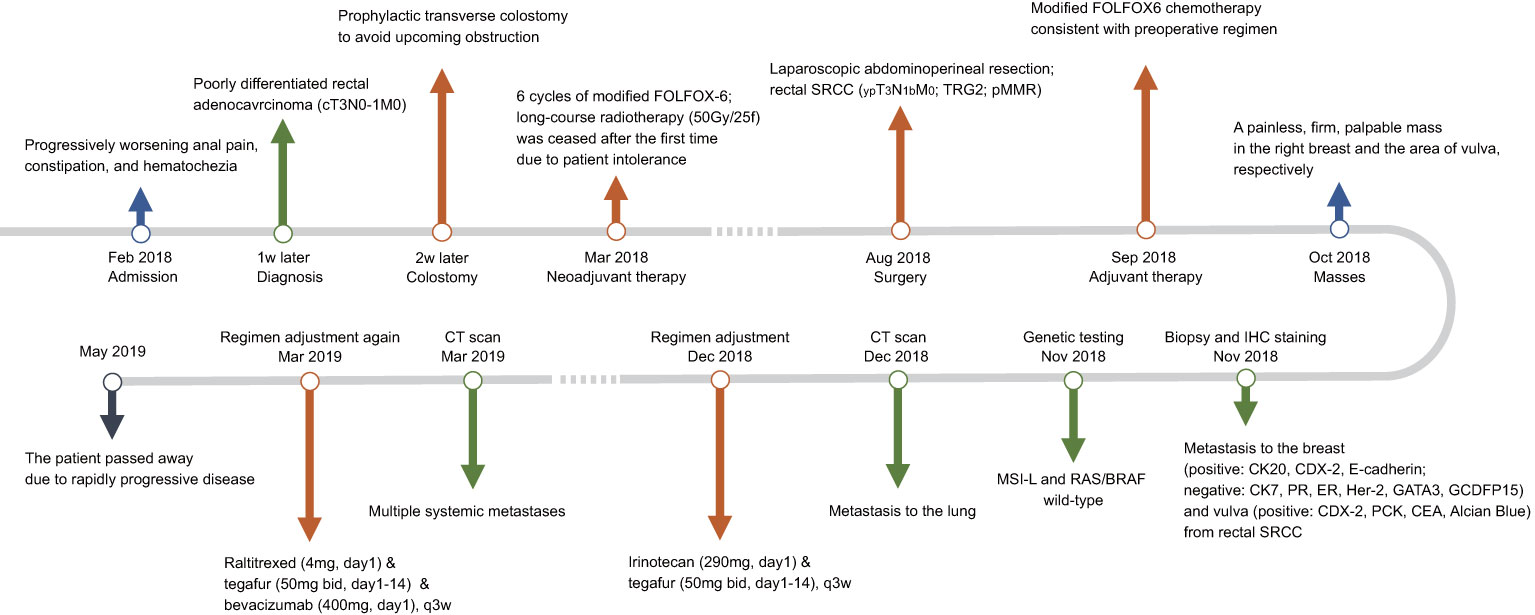
Figure 3 Timeline with clinical data from the episode of care. SRCC, signet ring cell carcinoma; TRG2, tumor regression grade 2; pMMR, proficient mismatch repair; IHC, immunohistochemical; MSI-L, microsatellite instability-low; CT, computed tomography.
Discussion
Compared to the liver or lung metastases, rectal cancer breast metastasis (RCBM), let alone vulvar metastasis, is rather uncommon in existing literature (17–19). Except 2 male patients, RCBM mainly involved the female in previous reports (20, 21). The rareness of this entity might be ascribed to its hormone status and the relatively poor blood supply of a large amount of fibrous tissue, especially in Eastern female (22, 23). Besides hematogenous spread, lymphatic dissemination of cancer cells to thoracic duct might play a critical role in RCBM (24). RCBM presents at a younger age of 47.7 years (17) than primary rectal cancer (63 years) (5). The mean interval between the initial diagnosis of rectal cancer and the occurrence of breast metastasis was reported to be 28.4 months (range, 5 months to 18 years) (17). Williams et al. (25) suggested that breast metastasis usually served as a part of systemic metastases and was associated with poor expected survival, with a median survival of 10 months post diagnosis. Therefore, a thorough examination to evaluate metastases to other sites is required.
The most essential evidence to help establish the diagnosis of RCBM are the past medical history and morphological assessment of hematoxylin and eosin-stained sections in comparison to the primary tumor (10). Furthermore, IHC using specific primary antibodies remains the golden standard for the diagnosis of RCBM. The combination of cytokeratin 20 (CK20) and cytokeratin 7 (CK7) is of great benefit to the categorization of carcinomas and discrimination of primary adenocarcinomas from metastatic ones (26, 27). Meanwhile, caudal type homeobox 2 (CDX-2) is only expressed by intestine cells in adults and can be also employed to determine the colorectal adenocarcinoma (28). Conversely, GATA binding protein 3 (GATA3) is highly expressed in well-differentiated primary breast cancer (29). When conducting IHC assay, panels of antibodies, rather than any individuals, should be relied on (10).
There is a high incidence of MSI in CRC and about 15% of sporadic CRCs progress via an MSI-dependent pathway (30). The testing of MMR/MSI status has been recommended by current guidelines for Lynch syndrome screening in all newly diagnosed CRC and for treatment selection (including chemotherapy and immunotherapy) in metastatic CRC (31, 32). The methods of MMR/MSI detection mainly include IHC, polymerase chain reaction-capillary electrophoresis fragment analysis (PCR-CE), and next-generation sequencing (NGS) panel (33). The CRC patients with dMMR/MSI-high (MSI-H) cannot benefit from fluorouracil-based chemotherapy (34). However, immune checkpoint inhibitor (ICI) therapy, such as programmed cell death protein-1 (PD-1) and programmed cell death ligand-1 (PD-L1) inhibitors, has reversed the treatment landscape and improved survival of these patients in recent studies (35–37). Notably, increasing combination approaches are underway to transform MSS CRC (which accounting for 90-95% of CRC) from the immune desert into immunologically ‘hot’ cancer (38, 39).
Compare to common subtypes of CRC, such as adenocarcinoma and mucinous adenocarcinoma, SRCC is associated with younger on-set (6), aggressive behaviors (7), and a worse overall and disease-free survival (40). Limited whole-exome sequencing and gene panel sequencing profiles demonstrate that general driver mutations associated with CRC (e.g. APC, KRAS, and PIK3CA) are not frequent in SRCC (41, 42). Mutations and signaling pathways predominant in the tumorigenesis of SRCC remains unveiled. Through a Surveillance, Epidemiology, and End Results (SEER) database-based study, Wu et al. concluded that only cancer-specific resection improved the cancer-specific survival in primary colorectal SRCC (8). However, whether surgical removal of breast metastases in patients with limited metastatic disease may confer a survival benefit remains controversial, particularly under the condition of SRCC (25). Prior to metastasectomy, a thorough assessment and systemic treatment should be taken into consideration.
In conclusion, the current case showed that rectal SRCC is associated with younger on-set, aggressive behaviors, and worse survival outcomes. Due to poor cohesiveness, SRCC tends to develop metastases. A patient’s medical history and immunohistochemical staining (such as CK20, CK7, and CDX-2) can aid in identifying the tumor origin of breast and vulvar metastases. Mutations and signaling pathways predominant in the tumorigenesis of SRCC remains unveiled. There is poor effect of conventional chemotherapies, targeted and immunotherapies for colorectal adenocarcinoma on SRCC, so novel therapies are needed to treat this patient population.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by Ethical Committee on Biomedical Research, West China Hospital, Sichuan University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
MZ, BZ, YH, and WY conceived and designed the present study. YH and WY drafted the manuscript. QM, ZC, and YY were primarily responsible for literature search and review. JG and TY collected and collated the clinical data. All authors contributed substantially to revising and polishing the manuscript critically for important intellectual content and approved of the final version to be published.
Funding
This study was funded by the 1·3·5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (Grant No. ZYJC18034 and ZYJC21021) and Sichuan Provincial Administration of Traditional Chinese Medicine (Grant No. 2023MS173).
Acknowledgments
We sincerely appreciate the professional and precise interpretation of the pathologic images from Dr. Da-Gang Zhou (Department of Pathology, Chengdu Pidu District Hospital of Traditional Chinese Medicine).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Anthony T, George R, Rodriguez-Bigas M, Petrelli NJ. Primary signet-ring cell carcinoma of the colon and rectum. Ann Surg Oncol (1996) 3(4):344–8. doi: 10.1007/BF02305663
3. Kakar S, Smyrk TC. Signet ring cell carcinoma of the colorectum: correlations between microsatellite instability, clinicopathologic features and survival. Mod Pathol (2005) 18(2):244–9. doi: 10.1038/modpathol.3800298
4. Barresi V, Bonetti LR, Domati F, Baron L. Prognostic relevance of histopathological features in signet ring cell carcinoma of the colorectum. Virchows Arch (2016) 469(3):267–75. doi: 10.1007/s00428-016-1983-0
5. Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, et al. SEER cancer statistics review, 1975-2017. Natl Cancer Institute (2020).
6. Nitsche U, Zimmermann A, Späth C, Müller T, Maak M, Schuster T, et al. Mucinous and signet-ring cell colorectal cancers differ from classical adenocarcinomas in tumor biology and prognosis. Ann Surg (2013) 258(5):775–82. doi: 10.1097/SLA.0b013e3182a69f7e
7. Chen J, Cai R, Ren G, Zhao J, Li H, Guo C, et al. Differences in clinicopathological characteristics and computed tomography findings between signet ring cell carcinoma and nonsignet ring cell carcinoma in early and advanced gastric cancer. Cancer Med (2018) 7(4):1160–9. doi: 10.1002/cam4.1417
8. Wu J, Fang D, Man D, Wu W, Wang Q, Li Y, et al. Clinical correlates and prognostic value of different metastatic sites in gastric and colorectal signet ring cell arcinoma. Engineering (2020) 6(9):1028–34. doi: 10.1016/j.eng.2020.06.007
9. Begg CB, Ostrovnaya I, Geyer FC, Papanastasiou AD, Ng CKY, Sakr RA, et al. Contralateral breast cancers: independent cancers or metastases? Int J Cancer (2018) 142(2):347–56. doi: 10.1002/ijc.31051
10. Lee AH. The histological diagnosis of metastases to the breast from extramammary malignancies. J Clin Pathol (2007) 60(12):1333–41. doi: 10.1136/jcp.2006.046078
11. Lee SK, Kim WW, Kim SH, Hur SM, Kim S, Choi JH, et al. Characteristics of metastasis in the breast from extramammary malignancies. J Surg Oncol (2010) 101(2):137–40. doi: 10.1002/jso.21453
12. Lee AHS, Hodi Z, Soomro I, Sovani V, Abbas A, Rakha E, et al. Histological clues to the diagnosis of metastasis to the breast from extramammary malignancies. Histopathology (2020) 77(2):303–13. doi: 10.1111/his.14141
13. Olawaiye AB, Cuello MA, Rogers LJ. Cancer of the vulva: 2021 update. Int J Gynaecol Obstet (2021) 155 Suppl 1(Suppl 1):7–18. doi: 10.1002/ijgo.13881
14. Akpak YK, Dandin Ö, Gün İ, Atay V, Haholu A. A rare case of vulvar skin metastasis of rectal cancer after surgery. Int J Dermatol (2014) 53(6):e337–338. doi: 10.1111/ijd.12230
15. Krathen RA, Orengo IF, Rosen T. Cutaneous metastasis: a meta-analysis of data. South Med J (2003) 96(2):164–7. doi: 10.1097/01.SMJ.0000053676.73249.E5
16. Riley DS, Barber MS, Kienle GS, Aronson JK, von Schoen-Angerer T, Tugwell P, et al. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol (2017) 89:218–35. doi: 10.1016/j.jclinepi.2017.04.026
17. Dai Y, Jin Y, Lan A, Ding N, Jiang L, Liu S. Breast metastasis from rectal signet-ring cell carcinoma: a case report and review of literature. Front Oncol (2022) 12:873354. doi: 10.3389/fonc.2022.873354
18. Wang DD, Yang SJ, Chen WX. Breast metastasis from rectal carcinoma: a case report and review of the literature. Malawi Med J (2021) 33(3):226–8. doi: 10.4314/mmj.v33i3.11
19. Hasegawa H, Nagata Y, Sakakibara Y, Miyake M, Mori K, Masuda N, et al. Breast metastasis from rectal cancer with BRAF V600E mutation: a case report with a review of the literature. Clin J Gastroenterol (2020) 13(2):153–7. doi: 10.1007/s12328-019-01035-0
20. Wang T, Lv YG, Yan QG, Yuan SF, Ling R, Chen JH, et al. Rectal carcinoma metastatic to the male breast after 7 years: case report. Onkologie (2011) 34(10):544–6. doi: 10.1159/000332225
21. Gur EO, Onak C, Bayar EE, Yazıcı A, Kokulu İ, Hacıyanlı S, et al. Rectum mucinous adenocarcinoma metastasis to bilateral breast in a male patient: a case report. Breast J (2020) 26(3):517–9. doi: 10.1111/tbj.13580
22. Jochimsen PR, Brown RC. Metastatic melanoma in the breast masquerading as fibroadenoma. JAMA (1976) 236(24):2779–80.
23. Lee SH, Park JM, Kook SH, Han BK, Moon WK. Metastatic tumors to the breast: mammographic and ultrasonographic findings. J Ultrasound Med (2000) 19(4):257–62. doi: 10.7863/jum.2000.19.4.257
24. Burn JI, Watne AL, Moore GE. The role of the thoracic duct lymph in cancer dissemination. Br J Cancer (1962) 16(4):608–15. doi: 10.1038/bjc.1962.71
25. Williams SA, Ehlers RA 2nd, Hunt KK, Yi M, Kuerer HM, Singletary SE, et al. Metastases to the breast from nonbreast solid neoplasms: presentation and determinants of survival. Cancer (2007) 110(4):731–7. doi: 10.1002/cncr.22835
26. Chu PG, Weiss LM. Keratin expression in human tissues and neoplasms. Histopathology (2002) 40(5):403–39. doi: 10.1046/j.1365-2559.2002.01387.x
27. Tot T. Cytokeratins 20 and 7 as biomarkers: usefulness in discriminating primary from metastatic adenocarcinoma. Eur J Cancer (2002) 38(6):758–63. doi: 10.1016/s0959-8049(02)00008-4
28. Kaimaktchiev V, Terracciano L, Tornillo L, Spichtin H, Stoios D, Bundi M, et al. The homeobox intestinal differentiation factor CDX2 is selectively expressed in gastrointestinal adenocarcinomas. Mod Pathol (2004) 17(11):1392–13999. doi: 10.1038/modpathol.3800205
29. Kouros-Mehr H, Slorach EM, Sternlicht MD, Werb Z. GATA-3 maintains the differentiation of the luminal cell fate in the mammary gland. Cell (2006) 127(5):1041–55. doi: 10.1016/j.cell.2006.09.048
30. Hause RJ, Pritchard CC, Shendure J, Salipante SJ. Classification and characterization of microsatellite instability across 18 cancer types. Nat Med (2016) 22(11):1342–50. doi: 10.1038/nm.4191
31. National Comprehensive Cancer Network. NCCN guidelines: treatment by cancer type [rectal cancer version 3] (2022). Available at: https://www.nccn.org/guidelines/.
32. Luchini C, Bibeau F, Ligtenberg MJL, Singh N, Nottegar A, Bosse T, et al. ESMO recommendations on microsatellite instability testing for immunotherapy in cancer, and its relationship with PD-1/PD-L1 expression and tumour mutational burden: a systematic review-based approach. Ann Oncol (2019) 30(8):1232–43. doi: 10.1093/annonc/mdz116
33. Amato M, Franco R, Facchini G, Addeo R, Ciardiello F, Berretta M, et al. Microsatellite instability: from the implementation of the detection to a prognostic and predictive role in cancers. Int J Mol Sci (2022) 23(15):8726. doi: 10.3390/ijms23158726
34. de Rosa N, Rodriguez-Bigas MA, Chang GJ, Veerapong J, Borras E, Krishnan S, et al. DNA Mismatch repair deficiency in rectal cancer: benchmarking its impact on prognosis, neoadjuvant response prediction, and clinical cancer genetics. J Clin Oncol (2016) 34(25):3039–46. doi: 10.1200/JCO.2016.66.6826
35. Cercek A, Lumish M, Sinopoli J, Weiss J, Shia J, Lamendola-Essel M, et al. PD-1 blockade in mismatch repair-deficient, locally advanced rectal cancer. N Engl J Med (2022) 386(25):2363–76. doi: 10.1056/NEJMoa2201445
36. Xiao BY, Zhang X, Cao TY, Li DD, Jiang W, Kong LH, et al. Neoadjuvant immunotherapy leads to major response and low recurrence in localized mismatch repair-deficient colorectal cancer. J Natl Compr Canc Netw (2023) 21(1):60–66.e5. doi: 10.6004/jnccn.2022.7060
37. Chen G, Jin Y, Guan WL, Zhang RX, Xiao WW, Cai PQ, et al. Neoadjuvant PD-1 blockade with sintilimab in mismatch-repair deficient, locally advanced rectal cancer: an open-label, single-centre phase 2 study. Lancet Gastroenterol Hepatol (2023) 8(5):422–31. doi: 10.1016/S2468-1253(22)00439-3
38. Saunders MP, Graham J, Cunningham D, Plummer R, Church D, Kerr R, et al. CXD101 and nivolumab in patients with metastatic microsatellite-stable colorectal cancer (CAROSELL): a multicentre, open-label, single-arm, phase II trial. ESMO Open (2022) 7(6):100594. doi: 10.1016/j.esmoop.2022.100594
39. Lemech C, Dredge K, Bampton D, Hammond E, Clouston A, Waterhouse NJ, et al. Phase ib open-label, multicenter study of pixatimod, an activator of TLR9, in combination with nivolumab in subjects with microsatellite-stable metastatic colorectal cancer, metastatic pancreatic ductal adenocarcinoma and other solid tumors. J Immunother Cancer (2023) 11(1):e006136. doi: 10.1136/jitc-2022-006136
40. Li Y, Li J, Wang R, Zhang L, Fu G, Wang X, et al. Frequent RNF43 mutation contributes to moderate activation of wnt signaling in colorectal signet-ring cell carcinoma. Protein Cell (2020) 11(4):292–8. doi: 10.1007/s13238-020-00691-0
41. Nam JY, Oh BY, Hong HK, Bae JS, Kim TW, Ha SY, et al. Molecular characterization of colorectal signet-ring cell carcinoma using whole-exome and RNA sequencing. Transl Oncol (2018) 11(4):836–44. doi: 10.1016/j.tranon.2018.04.007
Keywords: rectal signet ring cell carcinoma, breast metastasis, vulvar metastasis, systemic treatment, immunohistochemical staining, mismatch repair, microsatellite stability
Citation: Han Y, Yang W, Ma Q, Cai Z, Yang Y, Gou J, Yuan T, Zhang M and Zhang B (2023) Case Report: Systemic treatment for breast and vulvar metastases from resected rectal signet ring cell carcinoma. Front. Oncol. 13:1213888. doi: 10.3389/fonc.2023.1213888
Received: 28 April 2023; Accepted: 22 June 2023;
Published: 06 July 2023.
Edited by:
Airazat M. Kazaryan, Østfold Hospital, NorwayReviewed by:
Alexander Petrovsky, Russian Cancer Research Center NN Blokhin, RussiaSamvel Bardakhchyan, Professor R.H. Yolyan Blood Center, Armenia
Copyright © 2023 Han, Yang, Ma, Cai, Yang, Gou, Yuan, Zhang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingming Zhang, bW16aGFuZ21kQDE2My5jb20=; Bo Zhang, emhhbmdib19zY3VAc2N1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Yihui Han
Yihui Han Wenming Yang
Wenming Yang Qin Ma
Qin Ma Zhaolun Cai
Zhaolun Cai Yun Yang1,4,5
Yun Yang1,4,5 Bo Zhang
Bo Zhang