
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Oncol., 08 August 2023
Sec. Radiation Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1213122
Biologically younger, fully independent octogenarians are able to tolerate most oncological treatments. Increasing frailty results in decreasing eligibility for certain treatments, e.g., chemotherapy and surgery. Most brain metastases are not an isolated problem, but part of widespread cancer dissemination, often in combination with compromised performance status. Multidisciplinary assessment is key in this vulnerable patient population where age, frailty, comorbidity and even moderate additional deficits from brain metastases or their treatment may result in immobilization, hospitalization, need for nursing home care, termination of systemic anticancer treatment etc. Here, we provide examples of successful treatment (surgery, radiosurgery, systemic therapy) and best supportive care, and comment on the limitations of prognostic scores, which often were developed in all-comers rather than octogenarians. Despite selection bias in retrospective studies, survival after radiosurgery was more encouraging than after whole-brain radiotherapy. Prospective research with focus on octogenarians is warranted to optimize outcomes.
The negative impact of increasing age on prognosis has already been confirmed in the recursive partitioning analysis (RPA) of historical brain metastases trials (accrual 1979-1993), the cut-off being 65 years (1). Only 13% of patients were 70 years of age or older and the mainstay of treatment was whole-brain radiotherapy (WBRT). Since then, many countries have witnessed an increase in people older than 80 years, with heterogeneous patterns of cancer incidence, comorbidity and frailty (2, 3). However, biologically younger, fully independent octogenarians are not uncommon. Prospective clinical trials are no longer inaccessible for these patients, e.g., after geriatric assessment (4, 5). As in all age groups, oncological treatment is most commonly administered outside of clinical trials, i.e. according to standard clinical practice. Regarding brain metastases, a relatively common type of distant dissemination in patients with lung or breast cancer or malignant melanoma (6, 7), special consideration must be given to cognitive function, especially in patients with well-preserved baseline function (8). A subset of octogenarians maintains normal cognitive function despite high prevalence and incidence of cognitive decline attributed to neurodegeneration. Brain metastases treatment that prolongs survival, but compromises functional independence might not be in line with octogenarians’ goals of care. Given that sophisticated and personalized management approaches exist, while age group-specific prospective trials are lacking (9), multidisciplinary assessment of pros and cons of different options is encouraged (10–12).
Rades et al. reported a retrospective analysis of WBRT, the historical standard approach that is less commonly employed now, in 94 octogenarian patients (13). Their median survival was 2.0 months and the authors proposed a survival score featuring three prognostic groups based on Eastern Cooperative Oncology Group (ECOG) performance status (PS), number of lesions (single versus multiple), and extracranial metastases (present versus absent). Nieder et al. validated these results in an independent cohort of 50 patients (14). Median survival was 2.1 months. In their study, other factors like cancer type (better survival for breast cancer and malignant melanoma) and lack of steroid treatment were significantly associated with survival too. However, the Rades et al. score resulted in useful stratification. WBRT does not result in guaranteed symptom palliation and neither is it complication-free, as recently reviewed by our group (15). Thus, consideration should be given to two alternative options: best supportive care (BSC) (16, 17), if active brain metastases treatment is unable to extend survival beyond the median observed by Rades et al. and Nieder et al., or stereotactic radiosurgery (SRS) if the prognostic tools and the clinicians’ multidisciplinary assessment predict survival clearly beyond 2 months. Regardless of combination of prognostic features, ECOG PS 0-2 is required to become part of a subgroup with longer survival.
Encouraging results were achieved with SRS, as suggested by a case-matched study comparing treatment results for patients 80 years of age or older versus patients 65-79 years of age (18). Overall, 165 patients were 80+ years old. Median survival time was shorter in these patients (5.3 months) than in the younger, matched group (6.9 months). However, this difference was not statistically significant (HR 1.1, 95% CI 0.9-1.4, p=0.2). A different study included 106 patients age 80 years and older who received SRS (19). The median survival was 7.1 months. Six-month and 12-month rates of local tumor control (per lesion) were 94% and 89%, respectively. Repeat SRS, salvage WBRT and surgical resection were subsequently required in 25, 4 and 1 patient, respectively. Karnofsky PS ≥ 70, controlled primary disease/no extracranial metastases and female sex were independent factors predicting better survival. Tumor volume >2 mL was the only factor predicting a higher rate of local failure. Chen et al. reported a retrospective study suggesting that WBRT was associated with increased toxicity compared with SRS in elderly and very elderly (80+) patients with brain metastases (20). Other authors have also confirmed that SRS is efficacious and safe in this population (21), albeit in absence of prospective longitudinal cognitive and quality of life analyses. The fact that additional salvage treatment might be needed after SRS is well known from the literature and not age-dependent (22). Previous limitations regarding maximum number of lesions eligible for SRS (often 3-4) are not stringently applied anymore (6, 7, 10). Figure 1 shows case-based recommendations for the common scenarios of SRS and BSC.
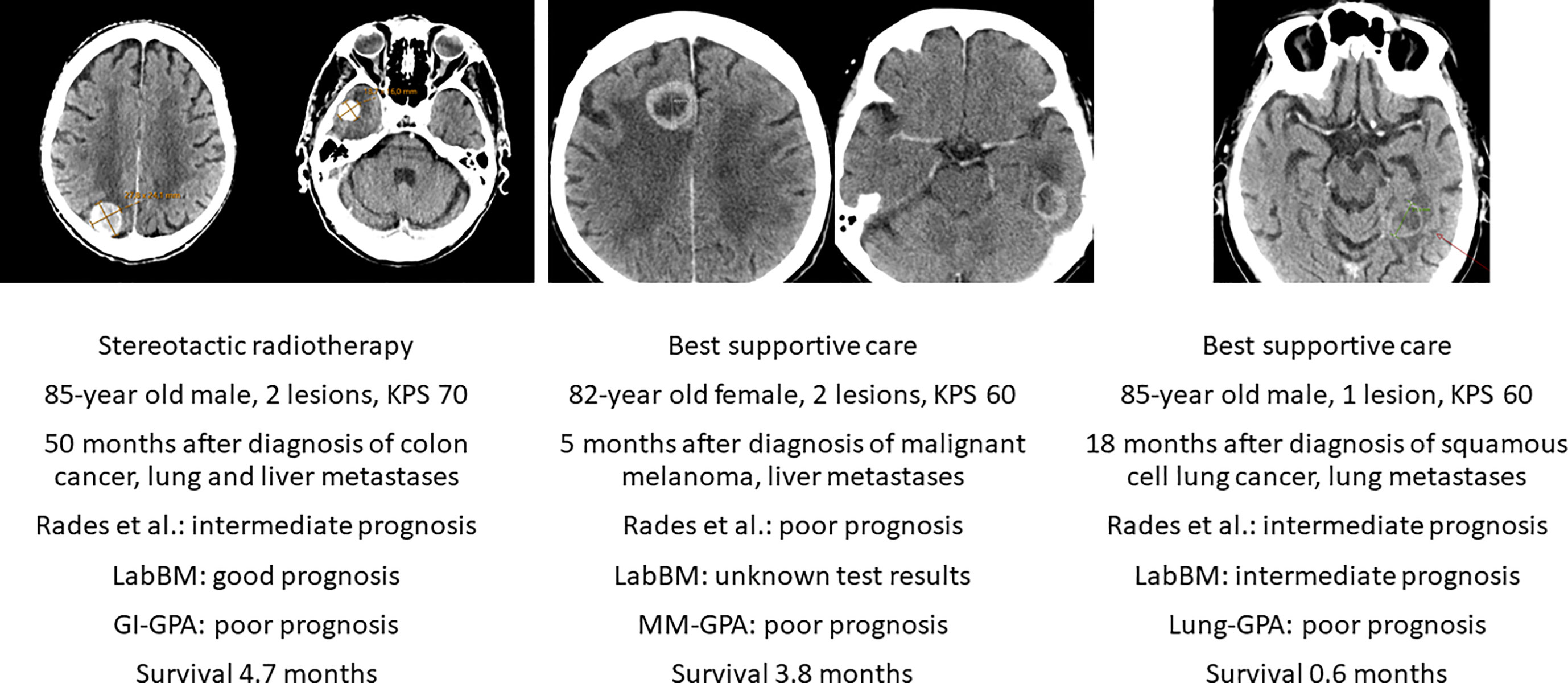
Figure 1 Axial computed tomography scans of three deceased patients with known survival outcome managed with stereotactic radiotherapy or best supportive care without systemic therapy after diagnosis of brain metastases. KPS, Karnofsky performance status. LabBM (23) and graded prognostic assessment (GPA) were calculated as described in the original studies (24).
Neurosurgical resection should be considered in medically operable patients whose survival can be extended by surgery, if radiotherapy is less likely to result in equivalent outcome. The combination of a single, large and accessible brain metastasis and absent/well controlled extracranial disease might prompt the multidisciplinary team to recommend surgery, as illustrated in Figure 2. Surgery was evaluated in a retrospective analysis of the Nationwide Inpatient Sample (1998–2005) published in 2011 (25). Age older than 80 years and higher Charlson comorbidity scores were found to be important prognostic factors for inpatient outcome. Therefore, thorough pre-operative assessment is necessary to confirm the appropriateness and safety of this approach (26). Post-operative irradiation of the cavity/tumor bed (27, 28) can be offered also in octogenarians.
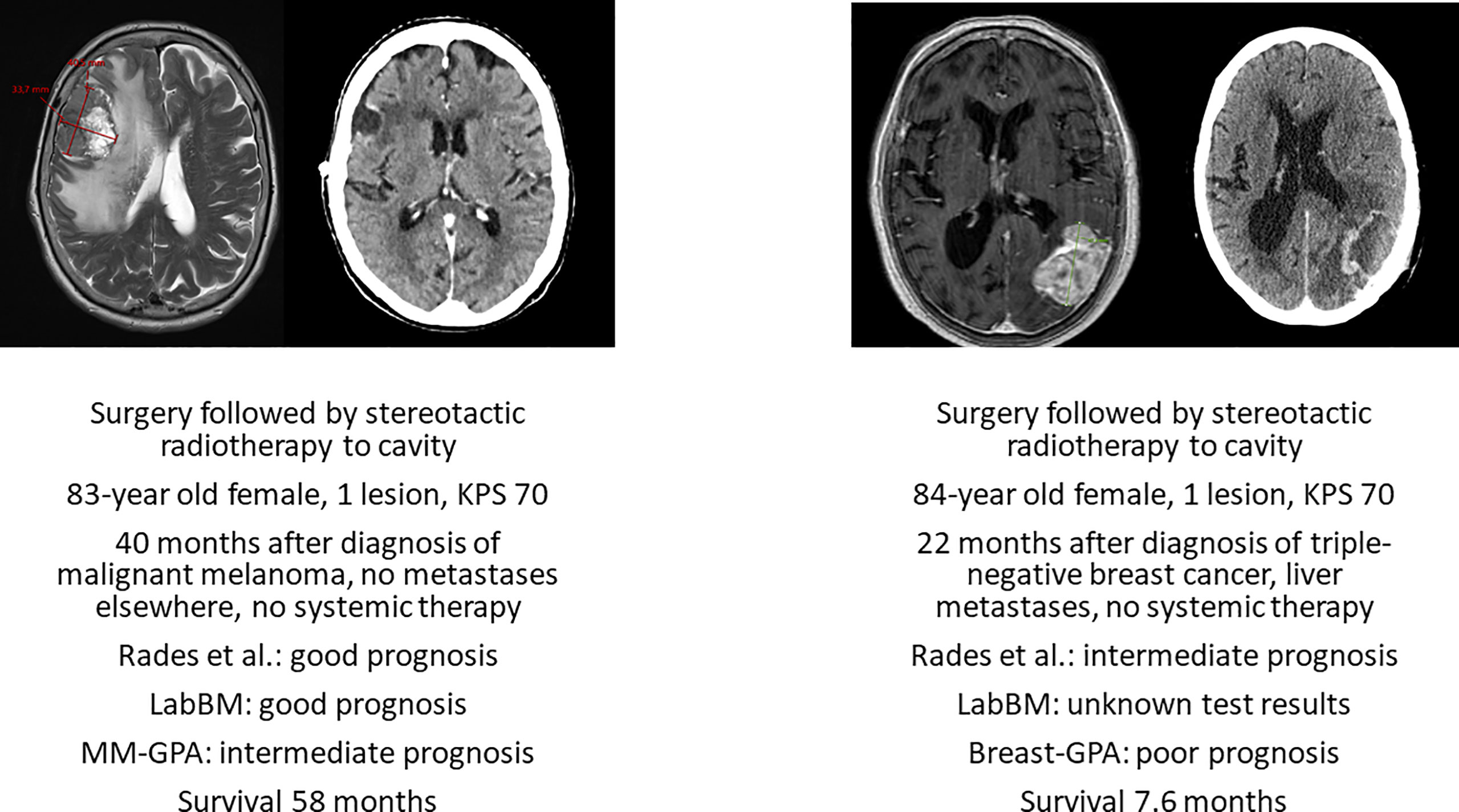
Figure 2 Axial pre-operative magnetic resonance imaging scans and post-operative radiation treatment planning scans of two deceased patients with known survival outcome managed with surgery and cavity-confined stereotactic radiotherapy without systemic therapy after diagnosis of brain metastases. KPS, Karnofsky performance status. LabBM (23) and graded prognostic assessment (GPA) were calculated as described in the original studies (24).
Deferring local treatment and tailoring it to patterns of extra- and intracranial response and availability of further lines of systemic treatment might be an option for octogenarians eligible for upfront systemic therapy (29). The phase II OCEAN study of osimertinib for radiotherapy-naive brain metastases from NSCLC (sensitizing EGFR mutation-positive) included patients with an age range of 41 to 84 years (30). The ALEX trial in patients with a different target (treatment-naive advanced anaplastic lymphoma kinase mutation-positive (ALK+) NSCLC) reported an age range of 18-81 years (31). The upper limit was identical in the phase 2 study of patients with metastatic melanoma and at least one measurable, non-irradiated brain metastasis (tumor diameter, 0.5 to 3 cm) and no neurologic symptoms who received nivolumab plus ipilimumab for up to four doses, followed by nivolumab (32). Overall, most patients in these trials were considerably younger, resulting in sparse, if any, evidence for octogenarians. Such patients were not included at all in several studies of human epidermal growth factor receptor 2-positive breast cancer and brain metastases (33–35). Even if dedicated studies for octogenarians are needed to provide firm conclusions, individual decisions for primary systemic therapy are justified (Figure 3), as also reflected in one of the authors’ single-institution patterns of care analysis (Figure 4). Six percent of these Norwegian patients were managed with primary systemic therapy.
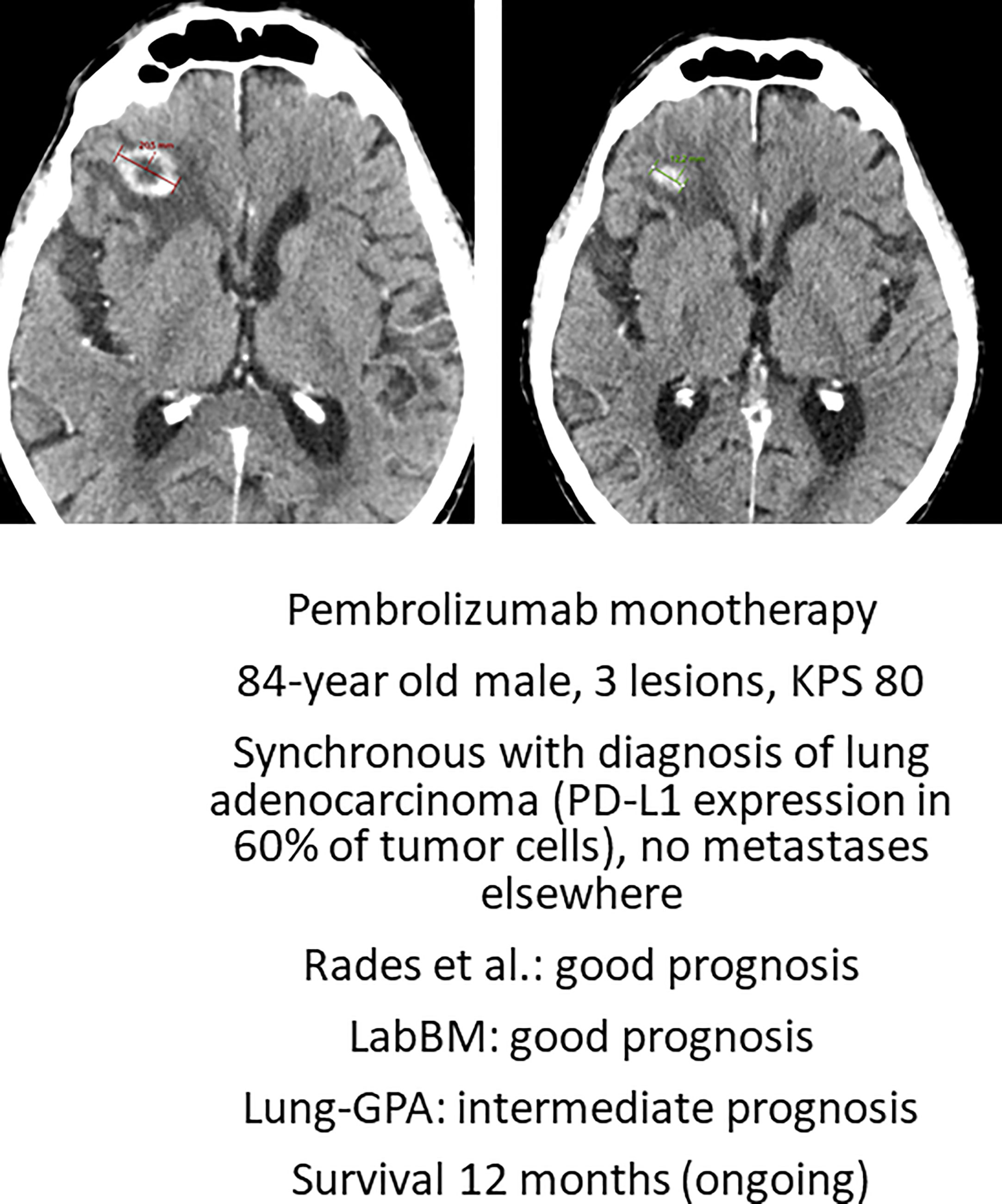
Figure 3 Axial computed tomography scan before Pembrolizumab and after 2.5 months of treatment. This patient is still alive. KPS, Karnofsky performance status; PD-L1, programmed death ligand 1. LabBM (23) and graded prognostic assessment (GPA) were calculated as described in the original studies (24).
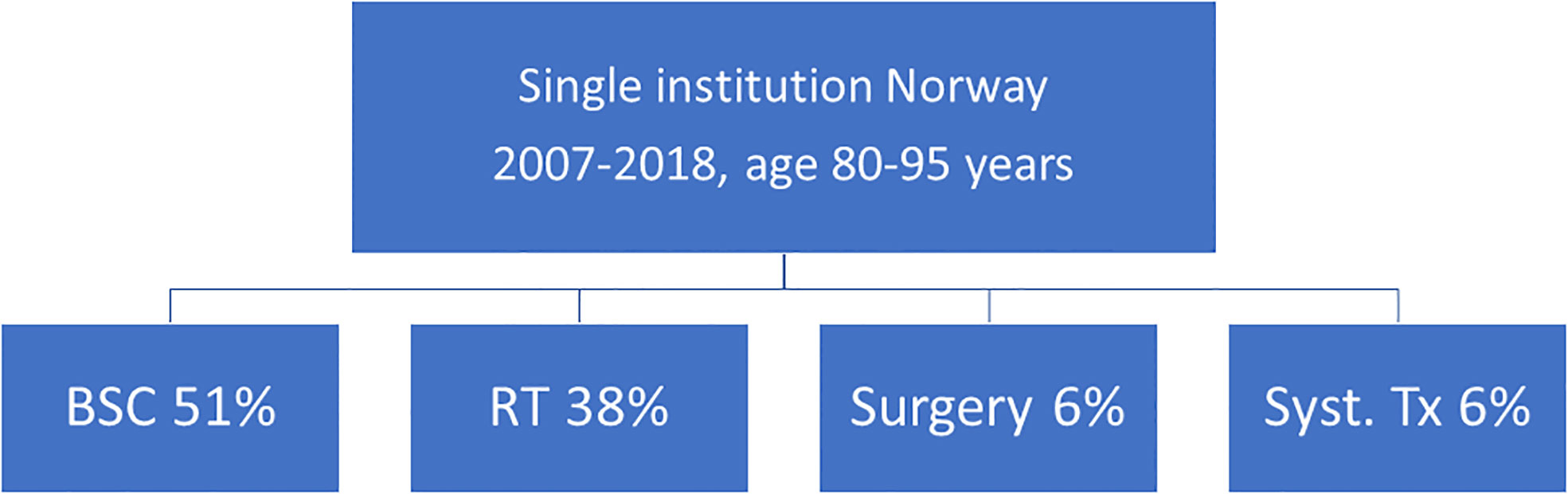
Figure 4 Approaches selected in one of the authors’ institutions. All patients with brain metastases were monitored in a continuously updated database (14, 17). BSC, best supportive care; RT, radiotherapy; Tx, treatment.
BSC was the preferred strategy in a large proportion of patients at Nordland Hospital. The longest observed survival was 6.1 months in octogenarians managed with BSC. Given that survival after WBRT was disappointing (13, 14), more efficacious, yet function-preserving SRS (or fractionated variants) should be considered, if KPS is ≥70 and active treatment is needed. Median survival in the literature was around 6 months. Selected patients with good KPS might benefit from surgical resection (large, symptomatic metastasis) or primary systemic therapy tailored to specific targets (small, asymptomatic metastases; simultaneous extracranial activity needed). As illustrated in the Figures, prognostic assessment is still imperfect and inconsistent between different scores (often developed in all-comers). Scores alone are not sufficient for decision-making, in part because frailty and comorbidity are not included in commonly used scores, despite their important impact on oncological treatment choices in the elderly and oldest old. Multidisciplinary assessment is key in such a vulnerable patient population where age, frailty, and even moderate additional deficits from brain metastases or their treatment may result in immobilization, hospitalization, need for nursing home care, termination of systemic anticancer treatment etc. Often, patient caregivers can supplement important information during decision-making and definition of the goals of treatment. If the oncologist in charge lacks confidence in a patient’s ability to tolerate treatment or provide appropriate consent, geriatric assessment should be incorporated during preparation of attempted treatment (36, 37).
The data analyzed in this study is subject to the following licenses/restrictions: Our institutional brain metastases dataset (Nordland Hospital) is available for external analyses on reasonable request from the corresponding author. Requests to access these datasets should be directed to CN, Y2Fyc3Rlbi5uaWVkZXJAbmxzaC5ubw==.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study.
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by CN. The first draft of the manuscript was written by CN. and all authors commented on previous versions of the manuscript. All authors contributed to the article and approved the submitted version.
Open access publication funded by UiT - The Arctic University of Norway.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys (1997) 37(4):745–51. doi: 10.1016/s0360-3016(96)00619-0
2. Habbous S, Alibhai SMH, Menjak IB, Forster K, Holloway CMB, Darling G. The effect of age on the opportunity to receive cancer treatment. Cancer Epidemiol (2022) 81:102271. doi: 10.1016/j.canep.2022.102271
3. de Ruiter JC, Heineman DJ, Daniels JM, van Diessen JN, Damhuis RA, Hartemink KJ. The role of surgery for stage I non-small cell lung cancer in octogenarians in the era of stereotactic body radiotherapy in the Netherlands. Lung Cancer (2020) 144:64–70. doi: 10.1016/j.lungcan.2020.04.005
4. Nguyen D, Mika G, Ninh A. Age-based exclusions in clinical trials: A review and new perspectives. Contemp Clin Trials (2022) 114:106683. doi: 10.1016/j.cct.2022.106683
5. Lee W, Cheng SJ, Grant SJ, Marcum ZA, Devine B. Use of geriatric assessment in cancer clinical trials: A systematic review. J Geriatr Oncol (2022) 13(7):907–13. doi: 10.1016/j.jgo.2022.04.014
6. Aizer AA, Lamba N, Ahluwalia MS, Aldape K, Boire A, Brastianos PK, et al. Brain metastases: A Society for Neuro-Oncology (SNO) consensus review on current management and future directions. Neuro Oncol (2022) 24(10):1613–46. doi: 10.1093/neuonc/noac118
7. Kraft J, Mayinger M, Willmann J, Brown M, Tanadini-Lang S, Wilke L, et al. Management of multiple brain metastases: a patterns of care survey within the German Society for Radiation Oncology. J Neurooncol (2021) 152(2):395–404. doi: 10.1007/s11060-021-03714-w
8. Lehrer EJ, Jones BM, Dickstein DR, Green S, Germano IM, Palmer JD, et al. The cognitive effects of radiotherapy for brain metastases. Front Oncol (2022) 12:893264. doi: 10.3389/fonc.2022.893264
9. VanderWalde N, Hurria A, Jagsi R. Improving consistency and quality of care for older adults with cancer: The challenges of developing consensus guidelines for radiation therapy. Int J Radiat Oncol Biol Phys (2017) 98(4):721–5. doi: 10.1016/j.ijrobp.2016.11.042
10. Tsui DCC, Camidge DR, Rusthoven CG. Managing central nervous system spread of lung cancer: The state of the art. J Clin Oncol (2022) 40(6):642–60. doi: 10.1200/JCO.21.01715
11. Karschnia P, Le Rhun E, Vogelbaum MA, van den Bent M, Grau SJ, Preusser M, et al. The evolving role of neurosurgery for central nervous system metastases in the era of personalized cancer therapy. Eur J Cancer (2021) 156:93–108. doi: 10.1016/j.ejca.2021.07.032
12. Nieder C, Guckenberger M, Gaspar LE, Rusthoven CG, De Ruysscher D, Sahgal A, et al. Management of patients with brain metastases from non-small cell lung cancer and adverse prognostic features: multi-national radiation treatment recommendations are heterogeneous. Radiat Oncol (2019) 14(1):33. doi: 10.1186/s13014-019-1237-9
13. Rades D, Delikanli C, Schild SE, Kristiansen C, Tvilsted S, Janssen S. The first survival score for patients aged ≥80 years irradiated for brain metastases. Biol (Basel) (2022) 11(10):1434. doi: 10.3390/biology11101434
14. Nieder C, Dalhaug A. Prognostic factors and independent validation of a risk stratification model in octogenarian patients irradiated for brain metastases. Anticancer Res (2023) 43(2):749–53. doi: 10.21873/anticanres.16214
15. Nieder C, Andratschke NH, Grosu AL. Brain metastases: Is there still a role for whole-brain radiation therapy? Semin Radiat Oncol (2023) 33(2):129–38. doi: 10.1016/j.semradonc.2023.01.005
16. Mulvenna P, Nankivell M, Barton R, Faivre-Finn C, Wilson P, McColl E, et al. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): results from a phase 3, non-inferiority, randomised trial. Lancet (2016) 388(10055):2004–14. doi: 10.1016/S0140-6736(16)30825-X
17. Nieder C, Norum J, Dalhaug A, Aandahl G, Pawinski A. Radiotherapy versus best supportive care in patients with brain metastases and adverse prognostic factors. Clin Exp Metastasis (2013) 30(6):723–9. doi: 10.1007/s10585-013-9573-x
18. Watanabe S, Yamamoto M, Sato Y, Kawabe T, Higuchi Y, Kasuya H, et al. Stereotactic radiosurgery for brain metastases: a case-matched study comparing treatment results for patients 80 years of age or older versus patients 65-79 years of age. J Neurosurg (2014) 121(5):1148–57. doi: 10.3171/2014.6.JNS132790
19. Yomo S, Hayashi M. Is upfront stereotactic radiosurgery a rational treatment option for very elderly patients with brain metastases? A retrospective analysis of 106 consecutive patients age 80 years and older. BMC Cancer (2016) 16(1):948. doi: 10.1186/s12885-016-2983-9
20. Chen L, Shen C, Redmond KJ, Page BR, Kummerlowe M, Mcnutt T, et al. Use of stereotactic radiosurgery in elderly and very elderly patients with brain metastases to limit toxicity associated with whole brain radiation therapy. Int J Radiat Oncol Biol Phys (2017) 98(4):939–47. doi: 10.1016/j.ijrobp.2017.02.031
21. Acker G, Hashemi SM, Fuellhase J, Kluge A, Conti A, Kufeld M, et al. Efficacy and safety of CyberKnife radiosurgery in elderly patients with brain metastases: a retrospective clinical evaluation. Radiat Oncol (2020) 15(1):225. doi: 10.1186/s13014-020-01655-8
22. Fritz C, Borsky K, Stark LS, Tanadini-Lang S, Kroeze SGC, Krayenbühl J, et al. Repeated courses of radiosurgery for new brain metastases to defer whole brain radiotherapy: Feasibility and outcome with validation of the new prognostic metric brain metastasis velocity. Front Oncol (2018) 8:551. doi: 10.3389/fonc.2018.00551
23. Berghoff AS, Wolpert F, Holland-Letz T, Koller R, Widhalm G, Gatterbauer B, et al. Combining standard clinical blood values for improving survival prediction in patients with newly diagnosed brain metastases-development and validation of the LabBM score. Neuro Oncol (2017) 19(9):1255–62. doi: 10.1093/neuonc/now290
24. Sperduto PW, Mesko S, Li J, Cagney D, Aizer A, Lin NU, et al. Survival in patients with brain metastases: Summary report on the updated diagnosis-specific graded prognostic assessment and definition of the eligibility quotient. J Clin Oncol (2020) 38(32):3773–84. doi: 10.1200/JCO.20.01255
25. Grossman R, Mukherjee D, Chang DC, Purtell M, Lim M, Brem H, et al. Predictors of inpatient death and complications among postoperative elderly patients with metastatic brain tumors. Ann Surg Oncol (2011) 18(2):521–8. doi: 10.1245/s10434-010-1299-2
26. Kerschbaumer J, Krigers A, Demetz M, Pinggera D, Klingenschmid J, Pichler N, et al. The Clinical Frailty Scale as useful tool in patients with brain metastases. J Neurooncol (2022) 158(1):51–7. doi: 10.1007/s11060-022-04008-5
27. Redmond KJ, De Salles AAF, Fariselli L, Levivier M, Ma L, Paddick I, et al. Stereotactic radiosurgery for postoperative metastatic surgical cavities: A critical review and International Stereotactic Radiosurgery Society (ISRS) practice guidelines. Int J Radiat Oncol Biol Phys (2021) 111(1):68–80. doi: 10.1016/j.ijrobp.2021.04.016
28. Minniti G, Niyazi M, Andratschke N, Guckenberger M, Palmer JD, Shih HA, et al. Current status and recent advances in resection cavity irradiation of brain metastases. Radiat Oncol (2021) 16(1):73. doi: 10.1186/s13014-021-01802-9
29. Andratschke N, Kraft J, Nieder C, Tay R, Califano R, Soffietti R, et al. Optimal management of brain metastases in oncogenic-driven non-small cell lung cancer (NSCLC). Lung Cancer (2019) 129:63–71. doi: 10.1016/j.lungcan.2018.12.009
30. Yamaguchi H, Wakuda K, Fukuda M, Kenmotsu H, Mukae H, Ito K, et al. A phase II study of Osimertinib for radiotherapy-naive central nervous system metastasis from NSCLC: Results for the T790M cohort of the OCEAN study (LOGIK1603/WJOG9116L). J Thorac Oncol (2021) 16(12):2121–32. doi: 10.1016/j.jtho.2021.07.026
31. Gadgeel S, Peters S, Mok T, Shaw AT, Kim DW, Ou SI, et al. Alectinib versus crizotinib in treatment-naive anaplastic lymphoma kinase-positive (ALK+) non-small-cell lung cancer: CNS efficacy results from the ALEX study. Ann Oncol (2018) 29(11):2214–22. doi: 10.1093/annonc/mdy405
32. Tawbi HA, Forsyth PA, Algazi A, Hamid O, Hodi FS, Moschos SJ, et al. Combined Nivolumab and Ipilimumab in melanoma metastatic to the brain. N Engl J Med (2018) 379(8):722–30. doi: 10.1056/NEJMoa1805453
33. Bartsch R, Berghoff AS, Furtner J, Marhold M, Bergen ES, Roider-Schur S, et al. Trastuzumab deruxtecan in HER2-positive breast cancer with brain metastases: a single-arm, phase 2 trial. Nat Med (2022) 28(9):1840–7. doi: 10.1038/s41591-022-01935-8
34. Lin NU, Pegram M, Sahebjam S, Ibrahim N, Fung A, Cheng A, et al. Pertuzumab plus high-dose trastuzumab in patients with progressive brain metastases and HER2-positive metastatic breast cancer: Primary analysis of a phase II study. J Clin Oncol (2021) 39(24):2667–75. doi: 10.1200/JCO.20.02822
35. Freedman RA, Gelman RS, Anders CK, Melisko ME, Parsons HA, Cropp AM, et al. Translational Breast Cancer Research Consortium. TBCRC 022: A phase II trial of Neratinib and Capecitabine for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases. J Clin Oncol (2019) 37(13):1081–9. doi: 10.1200/JCO.18.01511
36. Cree A, O'Donovan A, O'Hanlon S. New horizons in radiotherapy for older people. Age Ageing (2019) 48(5):605–12. doi: 10.1093/ageing/afz089
37. Eriksen GF, Šaltytė Benth J, Grønberg BH, Rostoft S, Kirkhus L, Kirkevold Ø, et al. Geriatric impairments are prevalent and predictive of survival in older patients with cancer receiving radiotherapy: a prospective observational study. Acta Oncol (2022) 61(4):393–402. doi: 10.1080/0284186X.2021.2009561
Keywords: brain metastases, radiotherapy, best supportive care (BSC), surgery, prognosis
Citation: Nieder C, Andratschke NH and Grosu AL (2023) How we treat octogenarians with brain metastases. Front. Oncol. 13:1213122. doi: 10.3389/fonc.2023.1213122
Received: 27 April 2023; Accepted: 25 July 2023;
Published: 08 August 2023.
Edited by:
Raphael Pfeffer, Assuta Medical Center, IsraelReviewed by:
Fabio Trippa, Santa Maria Hospital, ItalyCopyright © 2023 Nieder, Andratschke and Grosu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carsten Nieder, Y2Fyc3Rlbi5uaWVkZXJAbmxzaC5ubw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.