- 1Department of Radiation Oncology, Capital Medical University Xuanwu Hospital, Beijing, China
- 2School of Basic Medical Sciences, Capital Medical University, Beijing, China
Immunotherapy has revolutionized the treatment of metastatic non-small cell lung cancer (NSCLC). Oligometastasis has been associated with better prognosis than widespread metastatic disease and may be curable by stereotactic body radiotherapy (SBRT). SBRT can stimulate immunogenic anti-tumor activity, which can be further augmented when combined with immunotherapy, such as immune checkpoint inhibitors (ICIs). Thus, its combination with immunotherapy was recognized as a promising treatment option, especially in the metastatic setting. However, the most optimal approach to combine SBRT with immunotherapy remains controversial with early clinical evidence emerging. Here, we review the current clinical evidence supporting the combination of SBRT with immunotherapy in the treatment of metastatic NSCLC. Also, we discuss the current controversies and areas for further exploration associated with this treatment strategy.
Introduction
Lung cancer is the top cause of cancer-related deaths in the US and more than half of patients are diagnosed with metastatic disease, resulting in high mortality rates (1). Non-small cell lung cancer (NSCLC), the most common type of lung cancer, accounts for approximately 84% of all lung cancers (2). Immunotherapy, particularly immune checkpoint inhibitors (ICIs), has improved the survival of patients with metastatic NSCLC. However, response rates to ICIs remain suboptimal (3). SBRT, a form of precise radiotherapy, delivers high doses of radiation to the tumor target(s) with minimal side effects, and has been reported to induce immunogenic cell death (4, 5). Combining ICIs with local therapies like stereotactic body radiotherapy (SBRT) has shown promise in enhancing treatment efficacy both locally and distantly in areas outside of the irradiated area. Such distant effect is called the which abscopal effect. Such synergy between SBRT and ICIs, especially the combined treatment approach’s association with increased frequency of an abscopal response warrants further exploration and validation in large randomized controlled trials (RCTs) (6). Here, we provide an overview of the latest developments and challenges in combining SBRT with immunotherapy for stage IV NSCLC.
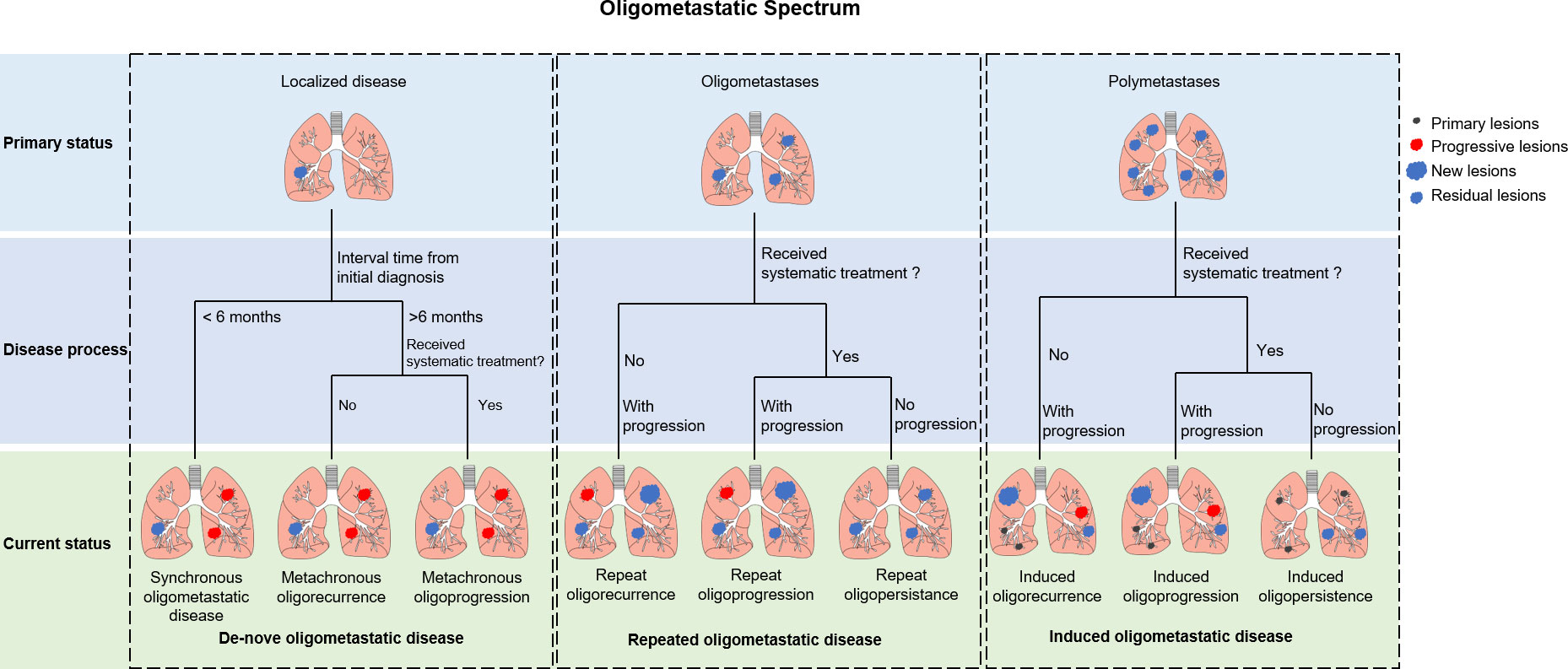
Oligometastases in advanced non-small cell lung cancer: a systematic classification
The term “oligometastases” refers to an intermediate state between local disease and widespread metastases (7). Oligometastatic disease (OMD) is a state where “cure” or “long-time disease control” can be achieved through radical treatment (7, 8). Radiotherapy, surgery, and radiofrequency ablation have made previously untreatable lesions safer and more effective to treat, which prompted changes the definition of OMD (8). In 2019, the International Association for the Study of Lung Cancer (IASLC) proposed a consensus report that defined synchronous oligometastatic NSCLC as the presence of 1-5 distant metastases in a maximum of 3 organs (8). In 2020, the European Society for Radiotherapy and Oncology (ESTRO) and American Society for Radiation Oncology (ASTRO) reached a consensus on the definition of OMD, which is now defined as 1-5 metastatic lesions that can be safely treated (9).
OMD’s 5-year overall survival (OS) ranges between 8.3% to 86% (10), suggesting that OMD with similar imaging features may possess substantially different biological characteristics. Hellman and Weichselbaum proposed that oligometastases may arise from the progression of primary tumor or the eradication of widespread metastases (11). The ESTRO and EORTC have established a classification system to accurately describe the status of OMD, which can be categorized into three subtypes based on the patient’s historical diagnosis: de-novo, repeated, and induced OMD (Figure 1) (12). De-novo OMD occurs when patients do not have a prior history of metastases. It can be categorized as synchronous (< 6 months) or metachronous (> 6 months) based on the interval between primary and current diagnosis. Metachronous OMD can occur during a treatment-free interval (metachronous oligorecurrence) or during active systemic therapy (metachronous oligoprogression). Whereas repeat OMD represents OMD in patients with de-novo OMD that response to local and systemic treatments poorly; a state of OMD may be induced by systemic therapy in patients with polymetastatic disease. Repeat and induced OMD can also be classified into oligorecurrence (no treatment and progression), oligoprogression (treatment and progression), and oligopersistence (treatment but no progression), depending on the patient’s clinical status upon presentation.
Immune checkpoint inhibitors improve the survival of stage IV NSCLC patients
In recent years, ICIs have significantly transformed the treatment of metastatic NSCLC. Monoclonal antibodies target specific inhibitory immune checkpoints, such as cytotoxic T-lymphocyte antigen-4 (CTLA-4), programmed death 1 (PD1), or programmed death ligand 1 (PD-L1), and have improved OS and progression-free survival (PFS) in patients with metastatic NSCLC in multiple large-scale phase III RCTs, particularly in patients with PD-L1 tumor proportion score (TPS) ≥ 50% (Supplementary Table 1). CheckMate-017 and CheckMate-057 evaluated Nivolumab as second-line treatment for advanced NSCLC, with improved PFS and OS compared to docetaxel (13, 14). In 2021, updated data showed that Nivolumab increased 5-year OS rate by over five-fold (13.4% vs. 2.6%) (15). Checkmate-078 further confirmed Nivolumab’s effectiveness in Chinese patients (16). However, Checkmate-026 did not demonstrate a survival benefit from Nivolumab over chemotherapy in the first-line setting (17). Alternatively, Checkmate-9LA and Checkmate-227 showed a benefit from first-line Nivolumab plus Ipilimumab in PD-L1 positive NSCLC patients (18, 19). Single-agent Pembrolizumab as a second-line treatment for metastatic NSCLC patients with PD-L1 TPS ≥ 1% provided a sustained and clinically meaningful improvement in PFS and OS, with more than doubled 5-year OS rate compared to docetaxel (20). For advanced NSCLC patients with a PD-L1 TPS >50%, the KEYNOTE-024 trial showed that single-agent Pembrolizumab provided long-term survival benefits, enabling patients to avoid chemotherapy (21). The KEYNOTE-042 trial further expanded the patient population benefiting from Pembrolizumab to include those with a PD-L1 TPS score ≥1% (22). For PD-L1 negative patients, Pembrolizumab plus chemotherapy has been established as a first-line treatment option based on the results from the KEYNOTE-189 and KEYNOTE-407 trials, which nearly doubled 5-year OS rate compared to chemotherapy alone (23, 24).
Atezolizumab, the first FDA-approved PD-L1 monoclonal antibody, is promising for advanced NSCLC. In the OAK trial, it improved OS compared to chemotherapy for previously treated NSCLC (median OS: 13.8 vs. 9.6 months, p = 0.0003) (25). In the first-line treatment of advanced NSCLC, the Impower110 trial showed that Atezolizumab monotherapy provided significant benefit over chemotherapy in patients with a high PD-L1 expression, offering another “chemotherapy waiver” treatment option (26). IMpower130 evaluated Atezolizumab plus chemotherapy for first-line treatment of non-squamous NSCLC patients with wild-type EGFR/ALK. PD-L1 expression was associated with better PFS (27). Impower150 added Atezolizumab to chemotherapy and Bevacizumab, significantly prolonging PFS and OS in metastatic non-squamous NSCLC patients, regardless of PD-L1 expression or EGFR/ALK status (28). Overall, Atezolizumab is promising for metastatic NSCLC patients, particularly those with high PD-L1 expression. Several other PD-1/PD-L1 inhibitors have also been safely applied in the first- and second-line treatment of advanced NSCLC (29–40). Despite the rapid clinical adaptation of ICI’s, most patients respond to ICI-containing regimens poorly. No consensus has been reached regarding to patient selection despite the exploration of over 40 potential predictive biomarkers for tumor response to ICIs (41). PD-L1 expression is the most accepted predictive biomarker for ICI response, but ≤ 26% of patients have PD-L1 levels above 50% (18). As a strategy to enhance treatment response in stage IV NSCLC, combining ICIs with local radiotherapy may be a promising approach to further enhance local and systemic tumor control (42).
Local ablative therapies for oligometastatic NSCLC in the pre-ICI era
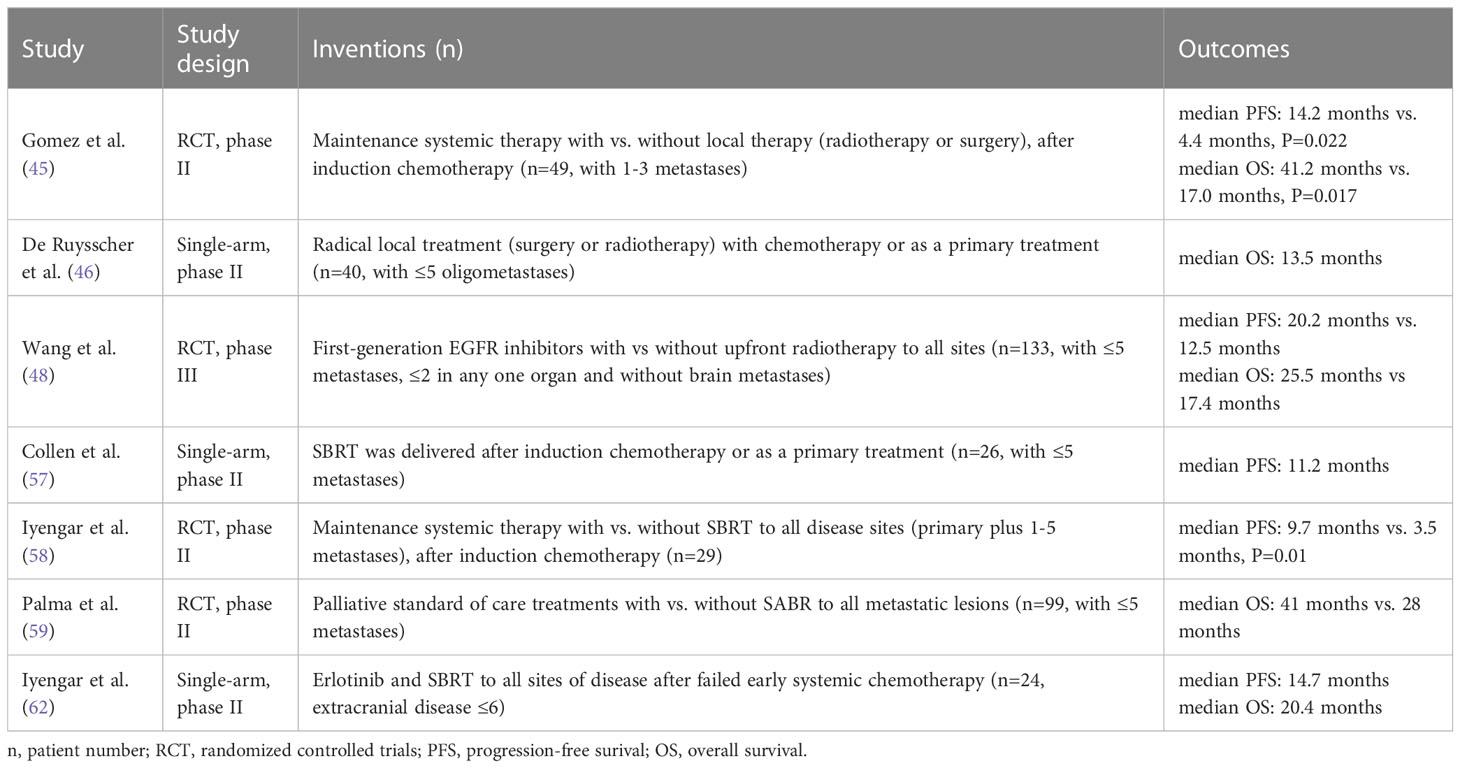
Local therapies like radiotherapy and surgery may cure some NSCLC patients with OMD (43). Comprehensive local therapy improved OS (27.1 vs. 13.1 months) and PFS (11.3 vs. 8.0 months) in NSCLC patients with ≤3 synchronous metastases (44). In a phase II study, local consolidative therapy (surgery, radiation therapy, or both) extended PFS from 4.4 to 14.2 months in 49 oligometastatic NSCLC patients (≤3 metastases) after first-line systemic therapy (45). In a single-arm phase II trial, a small number of 5-year survivors (7.7%) was observed after radical local treatment in oligometastatic NSCLC patients who also received systemic therapy (46). Furthermore, Xu et al. found that local ablative therapy may improve the survival of EGFR-mutant oligometastatic NSCLC patients treated with first-line EGFR-tyrosine kinase inhibitors (TKIs) (47). In the SINDAS trial, upfront radiotherapy added to first-line EGFR-TKI significantly improved PFS and OS in EGFR-mutated NSCLC patients with synchronous OMD (median PFS: 20.2 vs. 12.5 months; median OS: 25.5 vs. 17.4 months) (48).
SBRT is a safe and effective treatment for not only early stage, but also oligometastatic NSCLC, which represent a significant portion of patients with metastatic NSCLC (49–56). In several phase II trials, SBRT following chemotherapy or targeted therapy has been shown to improve survival outcomes in oligometastatic NSCLC patients (57–59). Ongoing RCTs are exploring whether SBRT improves survival in patients receiving maintenance therapy (60, 61). For second-line treatment of oligometastatic NSCLC, combining SBRT with targeted agents led to a median PFS of 14.7 months and a median OS of 20.4 months, which warrants further exploration (62). Overall, incorporating SBRT into the treatment regimen of oligometastatic NSCLC has been shown to be tolerable, and may lead to promising survival outcome. Table 1 summarizes the prospective studies on SBRT for oligometastatic NSCLC.
Clinical efficacy of SBRT combined with immunotherapy in metastatic NSCLC patients
Radiotherapy promotes the release of tumor-associated antigens and immune cell infiltration into the tumor, which can lead to an abscopal effect where unirradiated tumors respond (63). Combining SBRT with immunotherapy has gained great attention due to its ability to induce the abscopal effect (64). Compared with conventionally fractionated radiation therapy (CFRT), SBRT is associated with less lymphopenia and better clinical efficacy (65–67). Therefore, combining SBRT and immunotherapy appears promising as an emerging cancer treatment. The efficacy of combined radio-immunotherapy is affected by various factors, including radiation dose, fractionation schedule, treatment volume, and delivery sequence with an ICI. The most optimal dose fractionation schedule for maximum immune activation is not yet determined. In preclinical studies, either a single fraction of 15 Gy or 3 Gy×5 fractions elicited an immune response, but 15Gy x 1 fraction had a stronger immune-stimulatory effect (68). However, 7.5 Gy×2F and 5 Gy×3F were suggested to lead to superior anti-tumor immunity than a single ablative dose of 15 Gy. The medium-sized radiation dose of 7.5 Gy/F was recommended for best tumor control, due to its associated with strong anti-tumor immunity and low Treg numbers (69).
Although radiotherapy schedules varied in various trials, 8 Gy × 3 fractions has been very commonly used (70–72). Some studies found differences in efficacy to be associated with different dose fractionation regimens and treatment to different sites (73). Welsh et al. found that combining SBRT (50 Gy/4F) with immunotherapy yielded a 38% abscopal response rate (ARR), while the rate was only 10% with more protracted course of radiotherapy (45 Gy/15F) (74). Similar results were found in a pooled analysis of the MDACC and PEMBRO-RT trials favoring SBRT delivered in 3-4 fractions (67). Large-scale clinical studies are needed to explore the best SBRT dose and fractionation schedules to be combined with immunotherapy. Tumor regression in lesions exposed to low-dose irradiation during SBRT treatment has also been reported, suggesting the additional benefit of adding low-dose radiotherapy (LDRT) to selected metastatic lesions in patients receiving SBRT and immunotherapy (75, 76). Clinical trials investigating how to best combined LDRT, SBRT, and immunotherapy are warranted. Intra-tumoral heterogeneity may partially account for inconsistent efficacy of combining immunotherapy with SBRT (77).
Triggering of the abscopal effect is also by the tumor site(s) selected for local therapy, as not all metastatic sites are equally involved in systemic immune surveillance (78). For instance, irradiation of liver sites instead of lung sites may produce greater T-cell activation (79). However, since infiltration of activated T cells among multiple metastatic sites is complex and not intuitively predictable, SBRT to multiple or all tumor sites may be most effective for the generation of systemic antitumor immunity (67, 78, 80–82). This treatment approach is further supported by the following facts: acquired resistance to PD-1 axis inhibitors is often limited to no more than three metastatic sites (83); mixed progression is common in NSCLC (45%) and is associated with improved survival compared to those who experience widespread progression (84); SBRT to more than one lesion was shown to be associated with a trend toward improved overall tumor control (85).
Possible sequences of combing radiotherapy with immunotherapy include its administration before, concurrently with, or after radiotherapy. In preclinical studies, concurrent anti-PD-L1 agent and radiotherapy generated the most effective anti-tumor immune response (86). Buchwald et al. suggested that anti-PD-1/L1 and radiotherapy should be given simultaneously or, if not, radiotherapy should precede checkpoint blockers, as radiation to tumors after anti-PD-1/L1 therapy may abrogate the recently infiltrated and reactivated T-cell response (87). It is noteworthy that the above findings are primarily based on conventional fractionated radiotherapy, but for SBRT, intervention at any stage of immunotherapy may lead to survival benefits. Bestvina et al. presented the first randomized comparison of concurrent versus sequential dual checkpoint blockade and SBRT. They demonstrated no statistically significant difference in the median PFS between the concurrent and sequential groups (7.9 months vs. 4.7 months, p = 0.43) (73). Several clinical studies have also shown that the incidence of treatment-related toxicity following immunotherapy administered concurrently or sequentially with SBRT are not significantly different (70, 72, 73, 79). However, the current studies are largely limited by sample size, and the most optimal sequence of SBRT and immunotherapy needs further assessment in larger prospective trials.
Given the redundancy in the mechanisms of ICI- and radiation-induced toxic effects, radiation oncologists commonly avoid concurrent SBRT and ICI due to concerns about increased toxicity (88). However, current evidence only demonstrated a slightly elevated risk of certain toxic reactions in patients undergoing SBRT during immunotherapy (89). In the PEMBRO-RT trial, the addition of SBRT to Pembrolizumab increased the incidence of pneumonia from 8% to 26% and G3+ immune-related pneumonitis from 0% to 5%, but the total number of adverse events did not significantly differ from Pembrolizumab monotherapy (85 vs. 68 events, p = 0.076) (70). Multiple other studies have also demonstrated that the addition of SBRT to immunotherapy does not increase the incidence of severe adverse events (AEs) in patients with advanced NSCLC (71, 72, 74, 90). As shown in a large retrospective study, patients receiving radiotherapy within 90 days of starting ICI therapy may have a slightly higher incidence of adverse events (AEs), particularly low-grade AEs, compared to those receiving RT more than 90 days after starting ICI therapy (89). However, the clinical applicability of this conclusion to patients with advanced NSCLC still requires further verification given the varying AE spectra observed in different cancer types treated with PD-1 inhibitors (91). The sequence of SBRT and immunotherapy does not appear to induce significant difference in the incidence of severe AEs. Also shown by Bestvina et al. no statistical difference was present between patients receiving concurrent or sequential SBRT with the administration of Ipilimumab and Nivolumab (13 vs. 14 patients with G3+ immune-related AEs) (73). Other studies also confirmed the tolerability of concurrent SBRT and immunotherapy in NSCLC patients (72, 74, 79, 90). Welsh et al. found that the incidence of G3+ AEs was similar when either conventional fractionated radiotherapy or SBRT was combined with immunotherapy (74). Similarly, Schoenfeld et al. demonstrated no significant difference in the incidence of G3+AEs between the Durvalumab/Tremelimumab (D/T) plus LDRT group and the D/T plus SBRT group (15% and 12%, separately, P=0.27) (72). Reaching a consensus on the toxicity profile of the SBRT & ICI combination is difficult due to sample-size limitations and heterogeneities in patient population and treatment regimen. Meanwhile, the development of multisystem irAEs may also be associated with improved survival in patients with advanced NSCLC treated with ICIs (89, 92). Prospective trials are needed to investigate the most optimal sequence of combining radiotherapy and ICI to improve patient survival while maintaining a low incidence of treatment-related toxicities. It is also important to further explore how to best select patients for the combined treatment regimens. For example, some studies suggested that the combination of SBRT and immunotherapy may be more suitable for patients with low PD-L1 expression due to the better efficacy of immunotherapy alone in patients with high PD-L1 expression (70, 74). Thus, the selection of patients based on the presence and/or the level of specific biomarkers may help overcome the obstacle of hard-to-meet study endpoints in current clinical trials. Further investigations in this area are warranted. A summary of the clinical outcome and toxicity profile following SBRT combined with ICIs in prospective studies is provided in Table 2.
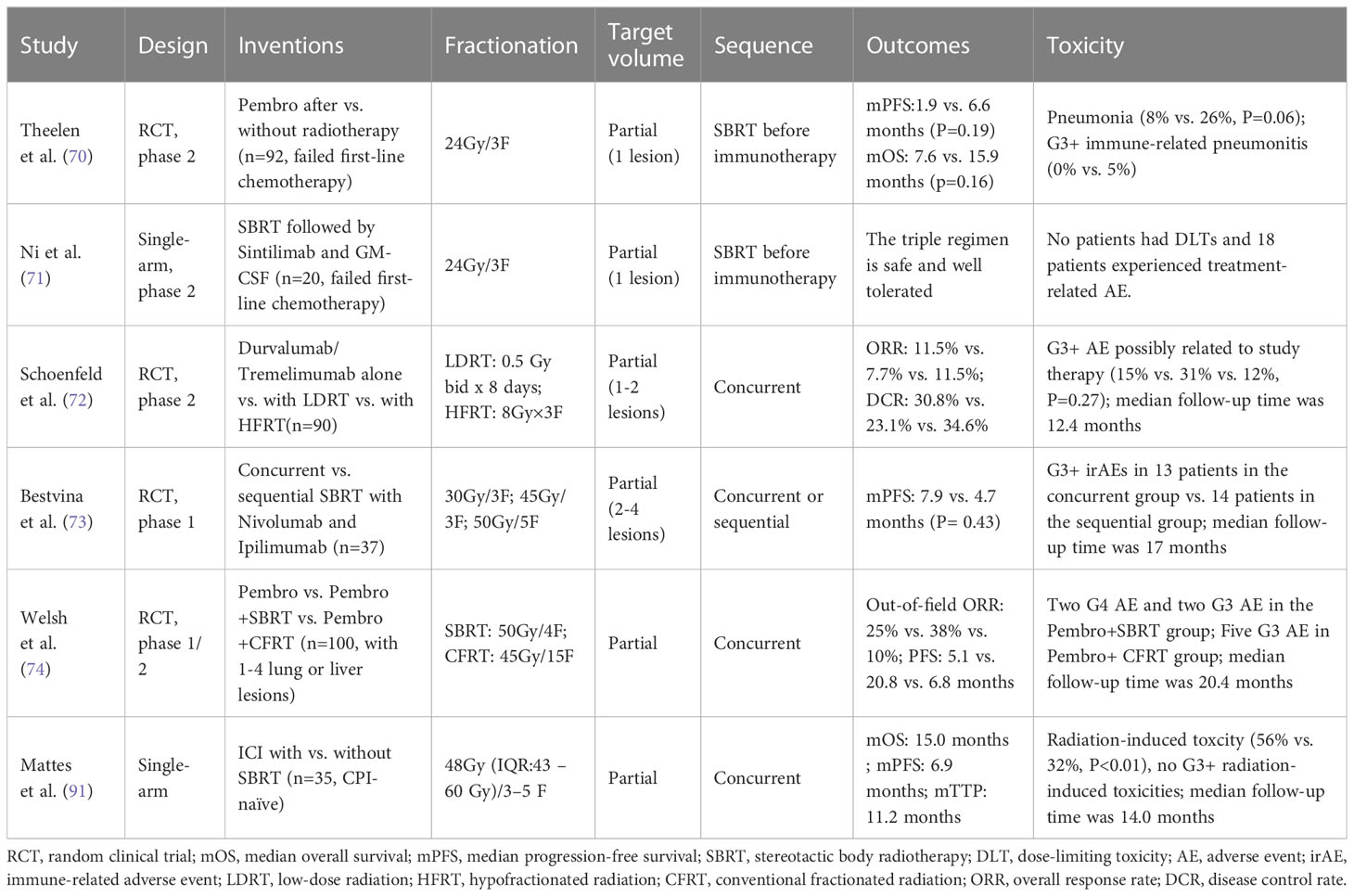
Table 2 Clinical trial summary of SBRT and immunotherapy combination therapy for advanced non-small-cell lung cancer.
Conclusion
Combining SBRT with immunotherapy is a promising treatment option for patients with metastatic NSCLC, especially those with OMD. Despite the emergence of early clinical evidence, the most optimal strategy for combining SBRT and immunotherapy remains to be further investigated to determine the most effective radiation dose/fractionation, target, and sequence with immunotherapy. For instance, the 8 Gy x 3 fractions regimen led to more prominent abscopal effect than higher single fractional dose of 20 Gy in vivo; likely due to DNA exonuclease Trex1 induction by high fractional doses above 12-18 Gy, leading to subsequent cytosolic DNA degradation and decreased immunogenicity (93, 94). Such finding led to the question of whether doses that are much lower than a biologically effective dose (BED) of 100 Gy10 should be considered when delivering “immunogenic” SBRT in combination with ICIs, in contrast to delivering ablative doses with a BED of ≥ 100 Gy10. Full dose SBRT remains to be recommended when it’s combined with ICIs for metastases based on limited evidence and expert opinion by the EORTC-ESTRO OligoCare consortium (95). In the evidence reviewed by this consortium, the risk of in-field severe toxicity was increased when thoracic SBRT was combined with an anti-CTLA-4 antibody (12%), or anti-PD-(L)1/anti-CTLA-4 combinations (26%). The majority of experts in the consortium recommended not to deliver an ICI and SBRT on the same day, and a time interval of at least one week between anti-CTLA-4 or anti-CTLA-4/anti-PD-(L)1 antibodies and SBRT administration (95). Given the increased utilization of local therapy with systemic therapy in the management of oligometastatic NSCLC, a clinical practice guideline was also released jointly by the ASTRO and ESTRO in 2023 (96). In this guideline, oligometastatic NSCLC’s definition, choice of local treatment and its sequencing with systemic therapy, radiotherapy dose/fractionation and techniques, as while as the indications for additional local therapy upon progression were reviewed. It further defined durable local control as a minimum of 85% at 2 years, while recognizing the likelihood of acceptable local control following SBRT delivering lower BED’s (50-75 Gy10) when it’s combined with systemic therapy. As implied by the European and European/American consensus recommendations, additional efforts should be directed toward conducting high quality clinical investigations into how to effectively combine SBRT and immunotherapy, which will provide insights and ultimately improve the care of patients with advanced stage NSCLC.
Author contributions
XL drafted the manuscript, created the figures and tables. AC generated the review’s concepts and structure. AC completed the final editing of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1211815/full#supplementary-material
References
1. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin (2023) 73:17–48. doi: 10.3322/caac.21763
2. Ganti AK, Klein AB, Cotarla I, Seal B, Chou E. Update of incidence, prevalence, survival, and initial treatment in patients with non-small cell lung cancer in the US. JAMA Oncol (2021) 7:1824–32. doi: 10.1001/jamaoncol.2021.4932
3. Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim S-W, Carcereny Costa E, et al. Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N Engl J Med (2019) 381:2020–31. doi: 10.1056/NEJMoa1910231
4. Nestle U, Adebahr S, Kaier K, Gkika E, Schimek-Jasch T, Hechtner M, et al. Quality of life after pulmonary stereotactic fractionated radiotherapy (SBRT): results of the phase II STRIPE trial. Radiother Oncol (2020) 148:82–8. doi: 10.1016/j.radonc.2020.03.018
5. Ye J, Mills BN, Zhao T, Han BJ, Murphy JD, Patel AP, et al. Assessing the magnitude of immunogenic cell death following chemotherapy and irradiation reveals a new strategy to treat pancreatic cancer. Cancer Immunol Res (2020) 8:94–107. doi: 10.1158/2326-6066.CIR-19-0373
6. Rodríguez Plá M, Dualde Beltrán D, Ferrer Albiach E. Immune checkpoints inhibitors and SRS/SBRT synergy in metastatic non-small-cell lung cancer and melanoma: A systematic review. IJMS (2021) 22:11621. doi: 10.3390/ijms222111621
8. Dingemans A-MC, Hendriks LEL, Berghmans T, Levy A, Hasan B, Faivre-Finn C, et al. Definition of synchronous oligometastatic non–small cell lung cancer—A consensus report. J Thorac Oncol (2019) 14:2109–19. doi: 10.1016/j.jtho.2019.07.025
9. Lievens Y, Guckenberger M, Gomez D, Hoyer M, Iyengar P, Kindts I, et al. Defining oligometastatic disease from a radiation oncology perspective: An ESTRO-ASTRO consensus document. Radiother Oncol (2020) 148:157–66. doi: 10.1016/j.radonc.2020.04.003
10. Ashworth A, Rodrigues G, Boldt G, Palma D. Is there an oligometastatic state in non-small cell lung cancer? A systematic review of the literature. Lung Cancer (2013) 82:197–203. doi: 10.1016/j.lungcan.2013.07.026
11. Weichselbaum RR, Hellman S. Oligometastases revisited. Nat Rev Clin Oncol (2011) 8:378–82. doi: 10.1038/nrclinonc.2011.44
12. Guckenberger M, Lievens Y, Bouma AB, Collette L, Dekker A, deSouza NM, et al. Characterisation and classification of oligometastatic disease: a European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus recommendation. Lancet Oncol (2020) 21:e18–28. doi: 10.1016/S1470-2045(19)30718-1
13. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med (2015) 373:1627–39. doi: 10.1056/NEJMoa1507643
14. Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WEE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med (2015) 373:123–35. doi: 10.1056/NEJMoa1504627
15. Borghaei H, Gettinger S, Vokes EE, Chow LQM, Burgio MA, de Castro Carpeno J, et al. Five-year outcomes from the randomized, phase III trials checkMate 017 and 057: nivolumab versus docetaxel in previously treated non–small-cell lung cancer. JCO (2021) 39:723–33. doi: 10.1200/JCO.20.01605
16. Wu Y-L, Lu S, Cheng Y, Zhou C, Wang J, Mok T, et al. Nivolumab versus docetaxel in a predominantly chinese patient population with previously treated advanced NSCLC: checkMate 078 randomized phase III clinical trial. J Thorac Oncol (2019) 14:867–75. doi: 10.1016/j.jtho.2019.01.006
17. Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M, et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med (2017) 376:2415–26. doi: 10.1056/NEJMoa1613493
18. Paz-Ares L, Ciuleanu T-E, Cobo M, Schenker M, Zurawski B, Menezes J, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol (2021) 22:198–211. doi: 10.1016/S1470-2045(20)30641-0
19. Brahmer JR, Lee J-S, Ciuleanu T-E, Bernabe Caro R, Nishio M, Urban L, et al. Five-year survival outcomes with nivolumab plus ipilimumab versus chemotherapy as first-line treatment for metastatic non-small-cell lung cancer in checkMate 227. J Clin Oncol (2023) 41:1200–12. doi: 10.1200/JCO.22.01503
20. Herbst RS, Garon EB, Kim D-W, Cho BC, Gervais R, Perez-Gracia JL, et al. Five year survival update from KEYNOTE-010: pembrolizumab versus docetaxel for previously treated, programmed death-ligand 1-positive advanced NSCLC. J Thorac Oncol (2021) 16:1718–32. doi: 10.1016/j.jtho.2021.05.001
21. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Updated analysis of KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol (2019) 37:537–46. doi: 10.1200/JCO.18.00149
22. Mok TSK, Wu Y-L, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet (2019) 393:1819–30. doi: 10.1016/S0140-6736(18)32409-7
23. Gadgeel S, Rodríguez-Abreu D, Speranza G, Esteban E, Felip E, Dómine M, et al. Updated analysis from KEYNOTE-189: pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non–small-cell lung cancer. JCO (2020) 38:1505–17. doi: 10.1200/JCO.19.03136
24. Paz-Ares L, Vicente D, Tafreshi A, Robinson A, Soto Parra H, Mazières J, et al. A randomized, placebo-controlled trial of pembrolizumab plus chemotherapy in patients with metastatic squamous NSCLC: protocol-specified final analysis of KEYNOTE-407. J Thorac Oncol (2020) 15:1657–69. doi: 10.1016/j.jtho.2020.06.015
25. Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet (2017) 389:255–65. doi: 10.1016/S0140-6736(16)32517-X
26. Jassem J, de Marinis F, Giaccone G, Vergnenegre A, Barrios CH, Morise M, et al. Updated overall survival analysis from IMpower110: atezolizumab versus platinum-based chemotherapy in treatment-naive programmed death-ligand 1-selected NSCLC. J Thorac Oncol (2021) 16:1872–82. doi: 10.1016/j.jtho.2021.06.019
27. West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol (2019) 20:924–37. doi: 10.1016/S1470-2045(19)30167-6
28. Socinski MA, Nishio M, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, et al. IMpower150 final overall survival analyses for atezolizumab plus bevacizumab and chemotherapy in first-line metastatic nonsquamous NSCLC. J Thorac Oncol (2021) 16:1909–24. doi: 10.1016/j.jtho.2021.07.009
29. Yang Y, Sun J, Wang Z, Fang J, Yu Q, Han B, et al. Updated overall survival data and predictive biomarkers of sintilimab plus pemetrexed and platinum as first-line treatment for locally advanced or metastatic nonsquamous NSCLC in the phase 3 ORIENT-11 study. J Thorac Oncol (2021) 16:2109–20. doi: 10.1016/j.jtho.2021.07.015
30. Zhou C, Wu L, Fan Y, Wang Z, Liu L, Chen G, et al. Sintilimab plus platinum and gemcitabine as first-line treatment for advanced or metastatic squamous NSCLC: results from a randomized, double-blind, phase 3 trial (ORIENT-12). J Thorac Oncol (2021) 16:1501–11. doi: 10.1016/j.jtho.2021.04.011
31. Lu S, Wu L, Jian H, Chen Y, Wang Q, Fang J, et al. Sintilimab plus bevacizumab biosimilar IBI305 and chemotherapy for patients with EGFR-mutated non-squamous non-small-cell lung cancer who progressed on EGFR tyrosine-kinase inhibitor therapy (ORIENT-31): first interim results from a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol (2022) 23:1167–79. doi: 10.1016/S1470-2045(22)00382-5
32. Zhou C, Huang D, Fan Y, Yu X, Liu Y, Shu Y, et al. Tislelizumab versus docetaxel in patients with previously treated advanced NSCLC (RATIONALE-303): A phase 3, open-label, randomized controlled trial. J Thorac Oncol (2023) 18:93–105. doi: 10.1016/j.jtho.2022.09.217
33. Lu S, Wang J, Yu Y, Yu X, Hu Y, Ai X, et al. Tislelizumab plus chemotherapy as first-line treatment for locally advanced or metastatic nonsquamous NSCLC (RATIONALE 304): A randomized phase 3 trial. J Thorac Oncol (2021) 16:1512–22. doi: 10.1016/j.jtho.2021.05.005
34. Wang J, Lu S, Yu X, Hu Y, Sun Y, Wang Z, et al. Tislelizumab plus chemotherapy vs chemotherapy alone as first-line treatment for advanced squamous non-small-cell lung cancer: A phase 3 randomized clinical trial. JAMA Oncol (2021) 7:709–17. doi: 10.1001/jamaoncol.2021.0366
35. Sezer A, Kilickap S, Gümüş M, Bondarenko I, Özgüroğlu M, Gogishvili M, et al. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: a multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet (2021) 397:592–604. doi: 10.1016/S0140-6736(21)00228-2
36. Gogishvili M, Melkadze T, Makharadze T, Giorgadze D, Dvorkin M, Penkov K, et al. Cemiplimab plus chemotherapy versus chemotherapy alone in non-small cell lung cancer: a randomized, controlled, double-blind phase 3 trial. Nat Med (2022) 28:2374–80. doi: 10.1038/s41591-022-01977-y
37. Zhou C, Chen G, Huang Y, Zhou J, Lin L, Feng J, et al. Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non-small-cell lung cancer (CameL): a randomised, open-label, multicentre, phase 3 trial. Lancet Respir Med (2021) 9:305–14. doi: 10.1016/S2213-2600(20)30365-9
38. Ren S, Chen J, Xu X, Jiang T, Cheng Y, Chen G, et al. Camrelizumab plus carboplatin and paclitaxel as first-line treatment for advanced squamous NSCLC (CameL-sq): A phase 3 trial. J Thorac Oncol (2022) 17:544–57. doi: 10.1016/j.jtho.2021.11.018
39. Zhou C, Wang Z, Sun Y, Cao L, Ma Z, Wu R, et al. SugeMalimab versus placebo, in combination with platinum-based chemotherapy, as first-line treatment of metastatic non-small-cell lung cancer (GEMSTONE-302): interim and final analyses of a double-blind, randomised, phase 3 clinical trial. Lancet Oncol (2022) 23:220–33. doi: 10.1016/S1470-2045(21)00650-1
40. Wang Z, Wu L, Li B, Cheng Y, Li X, Wang X, et al. Toripalimab plus chemotherapy for patients with treatment-naive advanced non-small-cell lung cancer: A multicenter randomized phase III trial (CHOICE-01). J Clin Oncol (2023) 41:651–63. doi: 10.1200/JCO.22.00727
41. Zhou F, Qiao M, Zhou C. The cutting-edge progress of immune-checkpoint blockade in lung cancer. Cell Mol Immunol (2021) 18:279–93. doi: 10.1038/s41423-020-00577-5
42. MacManus M, Hegi-Johnson F. Overcoming immunotherapy resistance in NSCLC. Lancet Oncol (2022) 23:191–3. doi: 10.1016/S1470-2045(21)00711-7
43. Ashworth AB, Senan S, Palma DA, Riquet M, Ahn YC, Ricardi U, et al. An individual patient data metaanalysis of outcomes and prognostic factors after treatment of oligometastatic non–small-cell lung cancer. Clin Lung Cancer (2014) 15:346–55. doi: 10.1016/j.cllc.2014.04.003
44. Sheu T, Heymach JV, Swisher SG, Rao G, Weinberg JS, Mehran R, et al. Propensity score–matched analysis of comprehensive local therapy for oligometastatic non-small cell lung cancer that did not progress after front-line chemotherapy. Int J Radiat OncologyBiologyPhysics (2014) 90:850–7. doi: 10.1016/j.ijrobp.2014.07.012
45. Gomez DR, Tang C, Zhang J, Blumenschein GR, Hernandez M, Lee JJ, et al. Local consolidative therapy vs. Maintenance therapy or observation for patients with oligometastatic non–small-cell lung cancer: long-term results of a multi-institutional, phase II, randomized study. JCO (2019) 37:1558–65. doi: 10.1200/JCO.19.00201
46. De Ruysscher D, Wanders R, Hendriks LE, van Baardwijk A, Reymen B, Houben R, et al. Progression-free survival and overall survival beyond 5 years of NSCLC patients with synchronous oligometastases treated in a prospective phase II trial (NCT 01282450). J Thorac Oncol (2018) 13:1958–61. doi: 10.1016/j.jtho.2018.07.098
47. Xu Q, Zhou F, Liu H, Jiang T, Li X, Xu Y, et al. Consolidative local ablative therapy improves the survival of patients with synchronous oligometastatic NSCLC harboring EGFR activating mutation treated with first-line EGFR-TKIs. J Thorac Oncol (2018) 13:1383–92. doi: 10.1016/j.jtho.2018.05.019
48. Wang X-S, Bai Y-F, Verma V, Yu R-L, Tian W, Ao R, et al. Randomized trial of first-line tyrosine kinase inhibitor with or without radiotherapy for synchronous oligometastatic EGFR -mutated non-small cell lung cancer. JNCI: J Natl Cancer Instit (2022) 115(6):742–8. doi: 10.1093/jnci/djac015
49. Narayanasamy G, Desai D, Morrill S, Zhang X, Galhardo E, Maraboyina S, et al. Technical Note: A planning technique to lower normal tissue toxicity in lung SBRT plans based on two likely dependent RTOG metrics. Med Phys (2018) 45:2325–8. doi: 10.1002/mp.12833
50. Fujimoto D, Yoshioka H, Kataoka Y, Morimoto T, Hata T, Kim YH, et al. Pseudoprogression in previously treated patients with non–small cell lung cancer who received nivolumab monotherapy. J Thorac Oncol (2019) 14:468–74. doi: 10.1016/j.jtho.2018.10.167
51. Baumann P, Nyman J, Hoyer M, Wennberg B, Gagliardi G, Lax I, et al. Outcome in a prospective phase II trial of medically inoperable stage I non–small-cell lung cancer patients treated with stereotactic body radiotherapy. JCO (2009) 27:3290–6. doi: 10.1200/JCO.2008.21.5681
52. No HJ, Raja N, Von Eyben R, Das M, Roy M, Myall N, et al. Characterization of metastatic non-small cell lung cancer and oligometastatic incidence in an era of changing treatment paradigms. Int J Radiat OncologyBiologyPhysics (2022) 114:603–10. doi: 10.1016/j.ijrobp.2022.04.050
53. Chmura S, Winter KA, Robinson C, Pisansky TM, Borges V, Al-Hallaq H, et al. Evaluation of safety of stereotactic body radiotherapy for the treatment of patients with multiple metastases: findings from the NRG-BR001 phase 1 trial. JAMA Oncol (2021) 7:845. doi: 10.1001/jamaoncol.2021.0687
54. Zeng Y, Ni J, Yu F, Zhou Y, Zhao Y, Li S, et al. The value of local consolidative therapy in Osimertinib-treated non-small cell lung cancer with oligo-residual disease. Radiat Oncol (2020) 15:207. doi: 10.1186/s13014-020-01651-y
55. Guo T, Ni J, Yang X, Li Y, Li Y, Zou L, et al. Pattern of recurrence analysis in metastatic EGFR-mutant NSCLC treated with osimertinib: implications for consolidative stereotactic body radiation therapy. Int J Radiat OncologyBiologyPhysics (2020) 107:62–71. doi: 10.1016/j.ijrobp.2019.12.042
56. Chan OSH, Lam KC, Li JYC, Choi FPT, Wong CYH, Chang ATY, et al. ATOM: A phase II study to assess efficacy of preemptive local ablative therapy to residual oligometastases of NSCLC after EGFR TKI. Lung Cancer (2020) 142:41–6. doi: 10.1016/j.lungcan.2020.02.002
57. Collen C, Christian N, Schallier D, Meysman M, Duchateau M, Storme G, et al. Phase II study of stereotactic body radiotherapy to primary tumor and metastatic locations in oligometastatic nonsmall-cell lung cancer patients. Ann Oncol (2014) 25:1954–9. doi: 10.1093/annonc/mdu370
58. Iyengar P, Wardak Z, Gerber DE, Tumati V, Ahn C, Hughes RS, et al. Consolidative radiotherapy for limited metastatic non–small-cell lung cancer: A phase 2 randomized clinical trial. JAMA Oncol (2018) 4:e173501. doi: 10.1001/jamaoncol.2017.3501
59. Palma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet (2019) 393:2051–8. doi: 10.1016/S0140-6736(18)32487-5
60. Tibdewal A, Agarwal JP, Srinivasan S, Mummudi N, Noronha V, Prabhash K, et al. Standard maintenance therapy versus local consolidative radiation therapy and standard maintenance therapy in 1–5 sites of oligometastatic non-small cell lung cancer: a study protocol of phase III randomised controlled trial. BMJ Open (2021) 11:e043628. doi: 10.1136/bmjopen-2020-043628
61. Belluomini L, Dionisi V, Palmerio S, Vincenzi S, Avancini A, Casali M, et al. Study design and rationale for espera trial: A multicentre, randomized, phase II clinical trial evaluating the potential efficacy of adding SBRT to pembrolizumab-pemetrexed maintenance in responsive or stable advanced non-squamous NSCLC after chemo-immunotherapy induction. Clin Lung Cancer (2022) 23:e269–72. doi: 10.1016/j.cllc.2021.07.004
62. Iyengar P, Kavanagh BD, Wardak Z, Smith I, Ahn C, Gerber DE, et al. Phase II trial of stereotactic body radiation therapy combined with erlotinib for patients with limited but progressive metastatic non–small-cell lung cancer. JCO (2014) 32:3824–30. doi: 10.1200/JCO.2014.56.7412
63. Herrera FG, Bourhis J, Coukos G. Radiotherapy combination opportunities leveraging immunity for the next oncology practice: Radiation-Immunotherapy Combinations. CA: A Cancer J Clin (2017) 67:65–85. doi: 10.3322/caac.21358
64. Ngwa W, Irabor OC, Schoenfeld JD, Hesser J, Demaria S, Formenti SC. Using immunotherapy to boost the abscopal effect. Nat Rev Cancer (2018) 18:313–22. doi: 10.1038/nrc.2018.6
65. Wu G, Baine MJ, Zhao N, Li S, Li X, Lin C. Lymphocyte-sparing effect of stereotactic body radiation therapy compared to conventional fractionated radiation therapy in patients with locally advanced pancreatic cancer. BMC Cancer (2019) 19:977. doi: 10.1186/s12885-019-6220-1
66. Chen D, Verma V, Patel RR, Barsoumian HB, Cortez MA, Welsh JW. Absolute lymphocyte count predicts abscopal responses and outcomes in patients receiving combined immunotherapy and radiation therapy: analysis of 3 phase 1/2 trials. Int J Radiat OncologyBiologyPhysics (2020) 108:196–203. doi: 10.1016/j.ijrobp.2020.01.032
67. Theelen WSME, Chen D, Verma V, Hobbs BP, Peulen HMU, Aerts JGJV, et al. Pembrolizumab with or without radiotherapy for metastatic non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Respir Med (2021) 9:467–75. doi: 10.1016/S2213-2600(20)30391-X
68. Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol (2005) 174:7516–23. doi: 10.4049/jimmunol.174.12.7516
69. Schaue D, Ratikan JA, Iwamoto KS, McBride WH. Maximizing tumor immunity with fractionated radiation. Int J Radiat OncologyBiologyPhysics (2012) 83:1306–10. doi: 10.1016/j.ijrobp.2011.09.049
70. Theelen WSME, Peulen HMU, Lalezari F, van der Noort V, de Vries JF, Aerts JGJV, et al. Effect of pembrolizumab after stereotactic body radiotherapy vs pembrolizumab alone on tumor response in patients with advanced non–small cell lung cancer: results of the PEMBRO-RT phase 2 randomized clinical trial. JAMA Oncol (2019) 5:1276. doi: 10.1001/jamaoncol.2019.1478
71. Ni J, Zhou Y, Wu L, Ai X, Dong X, Chu Q, et al. Sintilimab, stereotactic body radiotherapy and granulocyte–macrophage colony stimulating factor as second-line therapy for advanced non-small cell lung cancer: safety run-in results of a multicenter, single-arm, phase II trial. Radiat Oncol (2021) 16:177. doi: 10.1186/s13014-021-01905-3
72. Schoenfeld JD, Giobbie-Hurder A, Ranasinghe S, Kao KZ, Lako A, Tsuji J, et al. Durvalumab plus tremelimumab alone or in combination with low-dose or hypofractionated radiotherapy in metastatic non-small-cell lung cancer refractory to previous PD(L)-1 therapy: an open-label, multicentre, randomised, phase 2 trial. Lancet Oncol (2022) 23:279–91. doi: 10.1016/S1470-2045(21)00658-6
73. Bestvina CM, Pointer KB, Karrison T, Al-Hallaq H, Hoffman PC, Jelinek MJ, et al. A phase 1 trial of concurrent or sequential ipilimumab, nivolumab, and stereotactic body radiotherapy in patients with stage IV NSCLC study. J Thorac Oncol (2022) 17:130–40. doi: 10.1016/j.jtho.2021.08.019
74. Welsh J, Menon H, Chen D, Verma V, Tang C, Altan M, et al. Pembrolizumab with or without radiation therapy for metastatic non-small cell lung cancer: a randomized phase I/II trial. J Immunother Cancer (2020) 8:e001001. doi: 10.1136/jitc-2020-001001
75. Menon H, Chen D, Ramapriyan R, Verma V, Barsoumian HB, Cushman TR, et al. Influence of low-dose radiation on abscopal responses in patients receiving high-dose radiation and immunotherapy. J immunother Cancer (2019) 7:237. doi: 10.1186/s40425-019-0718-6
76. Yin L, Xue J, Li R, Zhou L, Deng L, Chen L, et al. Effect of low-dose radiation therapy on abscopal responses to hypofractionated radiation therapy and anti-PD1 in mice and patients with non-small cell lung cancer. Int J Radiat OncologyBiologyPhysics (2020) 108:212–24. doi: 10.1016/j.ijrobp.2020.05.002
77. Almendro V, Marusyk A, Polyak K. Cellular heterogeneity and molecular evolution in cancer. Annu Rev Pathol Mech Dis (2013) 8:277–302. doi: 10.1146/annurev-pathol-020712-163923
78. Poleszczuk JT, Luddy KA, Prokopiou S, Robertson-Tessi M, Moros EG, Fishman M, et al. Abscopal benefits of localized radiotherapy depend on activated T-cell trafficking and distribution between metastatic lesions. Cancer Res (2016) 76:1009–18. doi: 10.1158/0008-5472.CAN-15-1423
79. Tang C, Welsh JW, de Groot P, Massarelli E, Chang JY, Hess KR, et al. Ipilimumab with stereotactic ablative radiation therapy: phase I results and immunologic correlates from peripheral T cells. Clin Cancer Res (2017) 23:1388–96. doi: 10.1158/1078-0432.CCR-16-1432
80. Arina A, Gutiontov SI, Weichselbaum RR. Radiotherapy and immunotherapy for cancer: from “Systemic” to “Multisite. ” Clin Cancer Res (2020) 26:2777–82. doi: 10.1158/1078-0432.CCR-19-2034
81. Nguyen NP, Ali A, Vinh-Hung V, Gorobets O, Chi A, Mazibuko T, et al. Stereotactic body radiotherapy and immunotherapy for older patients with oligometastases: A proposed paradigm by the international geriatric radiotherapy group. Cancers (2022) 15:244. doi: 10.3390/cancers15010244
82. Bauml JM, Mick R, Ciunci C, Aggarwal C, Davis C, Evans T, et al. Pembrolizumab after completion of locally ablative therapy for oligometastatic non–small cell lung cancer: A phase 2 trial. JAMA Oncol (2019) 5:1283. doi: 10.1001/jamaoncol.2019.1449
83. Gettinger SN, Wurtz A, Goldberg SB, Rimm D, Schalper K, Kaech S, et al. Clinical features and management of acquired resistance to PD-1 axis inhibitors in 26 patients with advanced non–small cell lung cancer. J Thorac Oncol (2018) 13:831–9. doi: 10.1016/j.jtho.2018.03.008
84. Osorio JC, Arbour KC, Le DT, Durham JN, Plodkowski AJ, Halpenny DF, et al. Lesion-level response dynamics to programmed cell death protein (PD-1) blockade. JCO (2019) 37:3546–55. doi: 10.1200/JCO.19.00709
85. Chicas-Sett R, Zafra J, Rodriguez-Abreu D, Castilla-Martinez J, Benitez G, Salas B, et al. Combination of SABR with anti-PD-1 in oligoprogressive non-small cell lung cancer and melanoma: results of a prospective multicenter observational study. Int J Radiat OncologyBiologyPhysics (2022) 114:655–65. doi: 10.1016/j.ijrobp.2022.05.013
86. Dovedi SJ, Adlard AL, Lipowska-Bhalla G, McKenna C, Jones S, Cheadle EJ, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res (2014) 74:5458–68. doi: 10.1158/0008-5472.CAN-14-1258
87. Buchwald ZS, Wynne J, Nasti TH, Zhu S, Mourad WF, Yan W, et al. Radiation, immune checkpoint blockade and the abscopal effect: A critical review on timing, dose and fractionation. Front Oncol (2018) 8:612. doi: 10.3389/fonc.2018.00612
88. Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature (2015) 520:373–7. doi: 10.1038/nature14292
89. Anscher MS, Arora S, Weinstock C, Amatya A, Bandaru P, Tang C, et al. Association of radiation therapy with risk of adverse events in patients receiving immunotherapy: A pooled analysis of trials in the US food and drug administration database. JAMA Oncol (2022) 8:232. doi: 10.1001/jamaoncol.2021.6439
90. Mattes MD, Eubank TD, Almubarak M, Wen S, Marano GD, Jacobson GM, et al. A prospective trial evaluating the safety and systemic response from the concurrent use of radiation therapy with checkpoint inhibitor immunotherapy in metastatic non–small cell lung cancer. Clin Lung Cancer (2021) 22:268–73. doi: 10.1016/j.cllc.2021.01.012
91. Khoja L, Day D, Wei-Wu Chen T, Siu LL, Hansen AR. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol (2017) 28:2377–85. doi: 10.1093/annonc/mdx286
92. Maher VE, Fernandes LL, Weinstock C, Tang S, Agarwal S, Brave M, et al. Analysis of the association between adverse events and outcome in patients receiving a programmed death protein 1 or programmed death ligand 1 antibody. JCO (2019) 37:2730–7. doi: 10.1200/JCO.19.00318
93. Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, et al. Fractionated but Not Single-dose Radiotherapy Induces an Immune-mediated Abscopal Effect when Combined with Anti-CTLA-4 Antibody. Clin Cancer Res (2009) 15:5379–88. doi: 10.1158/1078-0432.CCR-09-0265
94. Vanpouille-Box C, Alard A, Aryankalayil MJ, Sarfraz Y, Diamond JM, Schneider RJ, et al. DNA enxonuclease trex1 regulates radiotherapy-induced tumor immunogenicity. Nat Comm (2017) 8:15618. doi: 10.1038/ncomms15618
95. Kroeze SGC, Pavic M, Stellamans K, Lievens Y, Becherini C, Scorsetti M, et al. Metastases-directed stereotactic body radiotherapy in combination with targeted therapy or immunotherapy: systemic review and consensus recommendations by the EORTC-ESTRO oligoCare consortium. Lancet Oncol (2023) 24:e121–e132.96. doi: 10.1016/S1470-2045(22)00752-5
Keywords: non-small cell lung cancer, metastasis, stereotactic body radiotherapy, immunotherapy, immune checkpoint inhibitors, abscopal effect
Citation: Liu X and Chi A (2023) Combining stereotactic body radiotherapy with immunotherapy in stage IV non-small cell lung cancer. Front. Oncol. 13:1211815. doi: 10.3389/fonc.2023.1211815
Received: 25 April 2023; Accepted: 28 July 2023;
Published: 06 September 2023.
Edited by:
Joaquim Bosch Barrera, Catalan Institute of Oncology, SpainReviewed by:
Arturo Navarro-Martin, Duran i Reynals Hospital, SpainCopyright © 2023 Liu and Chi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexander Chi, YWNoaWF6MjAxMEBnbWFpbC5jb20=
 Xiaoli Liu
Xiaoli Liu Alexander Chi
Alexander Chi