
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol. , 10 January 2024
Sec. Neuro-Oncology and Neurosurgical Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1210873
This article is part of the Research Topic Improving Clinical Practice for the Diagnosis and Management of Patients with Leptomeningeal Metastasis View all 5 articles
The incidence of leptomeningeal metastases (LM) is increasing among breast cancer patients, but their prognosis remains dismal. Many therapeutic options are now available to treat HER2-positive (HER2+) metastatic breast cancer (MBC) involving the central nervous system (CNS). This case report illustrates a long-lasting response of more than 2 years in a patient with HER2+ MBC with LM after sequential administration of systemic and intrathecal (IT) anti-HER2 therapies and highlights that an appropriate treatment of HER2+ LM can result in durable survival.
Leptomeningeal metastasis (LM) corresponds to the spread of malignant cells in the subarachnoid space (leptomeninges). LM is a frequent complication of metastatic breast cancer (MBC), with high morbidity and mortality rates. Breast cancer (BC) is one of the most frequent primary tumor associated with LM (12-35%), with a median OS ranging from 3 to 4 months (1). LM is detected in approximately 10-12% of patients with BC at diagnosis (2) but is often underdiagnosed with a 20% rate of leptomeningeal involvement in autopsy series (3, 4). LM occurs in the presence of synchronous CNS metastases in 43%–52% of cases (5–7). The prevalence of HER2-low (IHC score of 1+ or 2+ with a negative FISH result) and HER2+ (IHC score of 2+ with a positive FISH result or IHC score of 3+) BM is approximately 15% and 50%, respectively (8). The HER2+ subtype seems to be more prone to developing LM, though only the triple-negative BC subtype has been consistently associated with an increased risk of LM in the literature (9, 10). Other risk factors of developing LM include a younger age, extracranial disease at diagnosis and/or a medical history of brain metastasis (BM) surgery, especially infratentorial BM (11, 12). Best supportive care remains the most appropriate strategy for patients with severe neurological impairment and when only weak therapeutic options are available. To guide treatment decision in patients with LM, clinicians can take into consideration the prognostic Curie score that integrates performance status, the number of chemotherapy (CT) regimens prior to LM diagnosis and negative hormone receptor status, and, in patients with LM from BC, the breast graded prognostic assessment (Breast-GPA) initially validated in patients with BC brain metastases (BCBM) (13). Other prognostic factors include initial response to treatment and protein levels in the cerebrospinal fluid (CSF) at diagnosis (1). However, though effective therapies are available in some cases, a treatment decision should be made as soon as possible.
The therapeutic strategy for patients with LM is based on expert opinions summarized in the European Association of Neuro-Oncology-European Society for Medical Oncology (EANO-ESMO) clinical practice guidelines published in 2017 (14). However, none of these therapeutic options increased overall survival (OS) in patients with LM. In HER2+ MBC patients, the improvement of systemic anti-HER2 targeted therapies has led to better extracranial response rates and prolonged OS. Small CNS metastases have an intact BBB which limits the penetration of drugs into the tumor, while larger CNS metastases show BBB disruption and inhomogeneity across the tumor. The incidence of LM is increasing as the CNS penetrance of systemic agents at therapeutic doses is limited by the meningeal-blood barrier.
Here, we present the case report of a patient with HER2+ BC with LM. Her therapeutic management included the sequential administration of both systemic and intra-CSF therapies with a prolonged OS over 2 years after the initial diagnostic of LM.
In 2016, a 38-year-old female patient underwent a right mastectomy with ipsilateral sentinel nodal dissection for a pT2N1mi(sn) non-specific infiltrating ductal carcinoma (SBR grade 3, Estrogen Receptor (ER) negative, Progesterone Receptor (PR) negative, HER2 3+, positive vascular emboli). The patient received adjuvant CT (3 cycles of fluorouracil-epirubicin-cyclophosphamide, 1 cycle of trastuzumab-docetaxel followed by 6 weekly cycles of trastuzumab-paclitaxel) and adjuvant local radiation therapy. Trastuzumab injections were continued to totalize 1 year of treatment.
After 2 years of follow-up, the patient was referred to an emergency department to explore headaches of rapid-onset associated with fatigue (KPS = 80%) and vomiting. Brain MRI revealed a left frontal meningeal lesion, isointense on T1-weighted imaging (T1-WI) and T2-weighted imaging (T2-WI), strongly enhanced after gadolinium injection, with multiple linear contrast-enhanced leptomeningeal lesions in the frontotemporal, peri-mesencephalic and peri-bulbar region (Figure 1A). There was no distant metastasis on the thoraco-abdominopelvic CT-scan. A stereotactic brain biopsy confirmed the diagnostic of LM with the same profile compared to the primary tumor (ER negative, PR negative, HER2 3+). The patient received stereotactic radiation therapy (5*6 Gy) for the left frontal lesion followed by systemic intravenous (IV) CT with paclitaxel, trastuzumab and pertuzumab. Despite a radiological partial response (PR) at 3 months on the left frontal lesion, other leptomeningeal lesions progressed, which was associated with a neurological decline (KPS = 50%, cognitive impairment, cerebellar syndrome) (Figures 1A, B). A lumbar puncture (LP) was performed and malignant cells were detected in the CSF. The patient was treated with dose-dense intra-CSF injections of methotrexate (MTX) (3 injections of 15 mg over a week) followed by weekly intra-CSF trastuzumab injections (150 mg) with concomitant IV pertuzumab. That therapeutic association led to a complete response (CR) in the CSF (no malignant cells found) and to a radiological PR. The patient experienced a durable neurological improvement (KPS = 80%) and could return home for 6 months (Figure 1B).
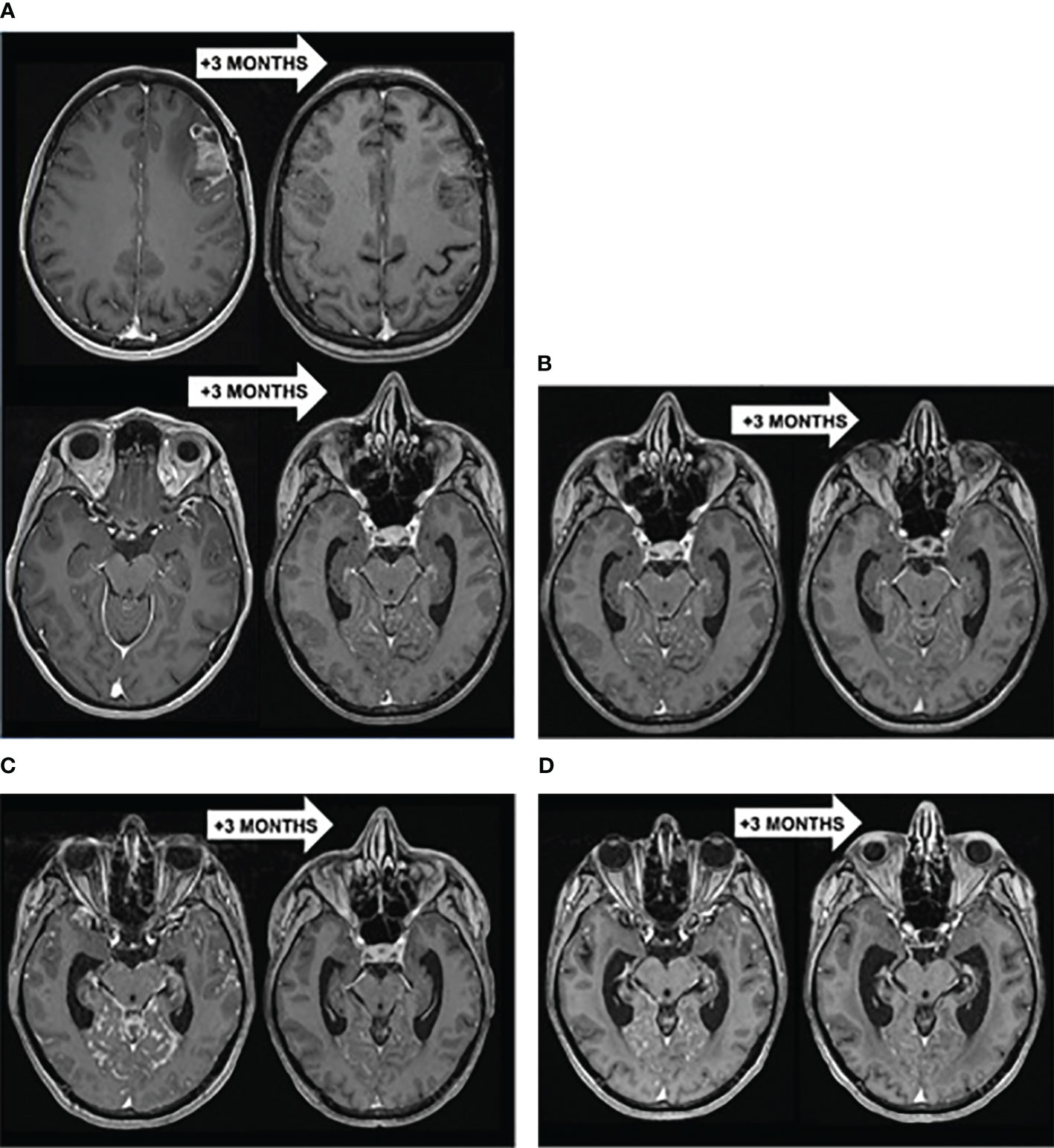
Figure 1 (A) Brain T1-WI MRI showing a PR of the left frontal lesion but progression of other LM lesions to stereotactic radiation therapy (5*6 Gy) followed by paclitaxel-trastuzumab-pertuzumab. (B) Brain T1-WI MRI showing a diffuse PR of LM to intra-CSF MTX (3*15 mg) followed by intra-CSF trastuzumab (150 mg) with concomitant IV pertuzumab. (C) Brain T1-WI MRI showing a diffuse PR of LM to WBRT (10*3 Gy) with concomitant IV trastuzumab-emtansine. (D) Brain T1-WI MRI showing a diffuse PR of LM to IV trastuzumab-deruxtecan.
LM recurred with a micronodular pattern in the cerebrum, brainstem and cerebellum. A new line of systemic trastuzumab-emtansine (T-DM1) in monotherapy was initiated but showed no clinico-radiological benefit after 3 cycles (Figure 1C). Consequently, the patient received a whole-brain radiotherapy (WBRT) (10*3 Gy) with concomitant T-DM1, resulting in a 3.5 months-PR when the patient could stay at home (Figure 1C). That improvement was followed by a neurological decline (KPS = 50%) and radiological progression of LM. A new systemic treatment combining tucatinib, trastuzumab and capecitabine was initiated with a transient clinical improvement for 1.5 months (KPS = 60%).
Eventually, the LM progressed (Figure 1D) and a last treatment of IV trastuzumab-deruxtecan (T-DXd) resulted in a clinico-radiological PR at 3 months with stable neurologic symptoms (KPS = 50%) (Figure 1D). After 5.4 months, the disease progressed again and the patient died 27 months after the diagnosis of LM, resulting in an OS of 6.8 months since the initiation of T-DXd.
LM is a diagnostic challenge often suspected based on the conjunction of clinical evaluation, radiological and CSF findings.
The clinical presentation consists in raised intracranial pressure, meningeal irritation and multifocal neurologic signs of rapid onset such as leg weakness, cauda equina syndrome or diplopia (15, 16).
Craniospinal MRI has a high sensitivity rate that can vary between 66% and 98%, depending on the series (17–20). Nevertheless, MRI sensitivity can greatly vary depending on the experience of the neuroradiologist and the use of appropriate sequences (contrast-enhanced T2 FLAIR, three-dimensional T1 black-blood fast spin-echo imaging) (21). Pathologic linear or nodular meningeal contrast-enhancements are classically visualized on gadolinium-injected T1-WI and localized on the cortical surface, gyri and sulci, cerebellar folia, the ventral surface of the brainstem and the spinal cord (22–24). Cranial nerves and spinal roots can also be pathologically enhanced.
Still, LM sometimes occurs without radiological evidence of LM. Given the very high sensitivity and specificity of the detection of malignant cells in the CSF compared to neuroimaging (17, 19, 25, 26), it is the gold standard for LM diagnosis and should be performed at diagnosis. Malignant cells are detected in approximately 66-90% of patients with LM from MBC (27). CSF profile might also include elevated protein rate (60-80%) and lymphocytic pleocytosis (50-60%). Although not specific to LM, increased lactate dehydrogenase (LDH) levels can be helpful in evaluating LM response follow-up (28). Increased Cancer antigen 15-3 (CA 15-3) CSF levels (> 3.0 IU/mL) can also be used to detect LM in MBC, with a sensitivity of 80% and a specificity of 70% (29). If the clinical suspicion of LM is high, LP should be repeated with a minimum CSF volume of 10.5 mL and be processed rapidly to minimize false-negative rates (26, 30). Still, CSF cytology can remain negative even in the presence of radiological features of LM (15, 30). A negative cytology should not prevent treatment initiation when symptoms and MRI are highly suggestive of LM. The diagnostic value of circulating tumor cell detection in the CSF is under investigation but is currently not routinely used (31, 32).
The EANO-ESMO clinical practice guidelines 2017 introduced a classification of LM based on CSF and clinico-radiological features to provide support for decision-making (14). Type I LM corresponds to a positive CSF cytology or biopsy, and type II to clinical findings and neuroimaging only. Each type is further classified into several subtypes according to clinico-radiological features: type A refers to linear disease only, type B to nodular disease only, type C to the combination of both and type D to a normal MRI. Other criteria such as the presence of synchronous or metachronous BM, pathology and molecular tumor profile (initial diagnosis/at relapse), prior treatments (focal and/or systemic) and the evolution of the extra-CNS disease are taken into consideration.
Patients with LM have seldom been included in large prospective BM trials, mainly due to their dismal prognosis. Consequently, data to guide therapeutic decision are lacking in that population. Therapeutic strategies are usually extrapolated from experience in patients with BCBM or even MBC. Treatment modalities include intrathecal (IT) chemotherapy (CT), WBRT and systemic CT (1) and their combination should be discussed in multidisciplinary tumor boards. The therapeutic options discussed hereafter are based on retrospective data and expert consensus, with a low level of evidence (Table 1).
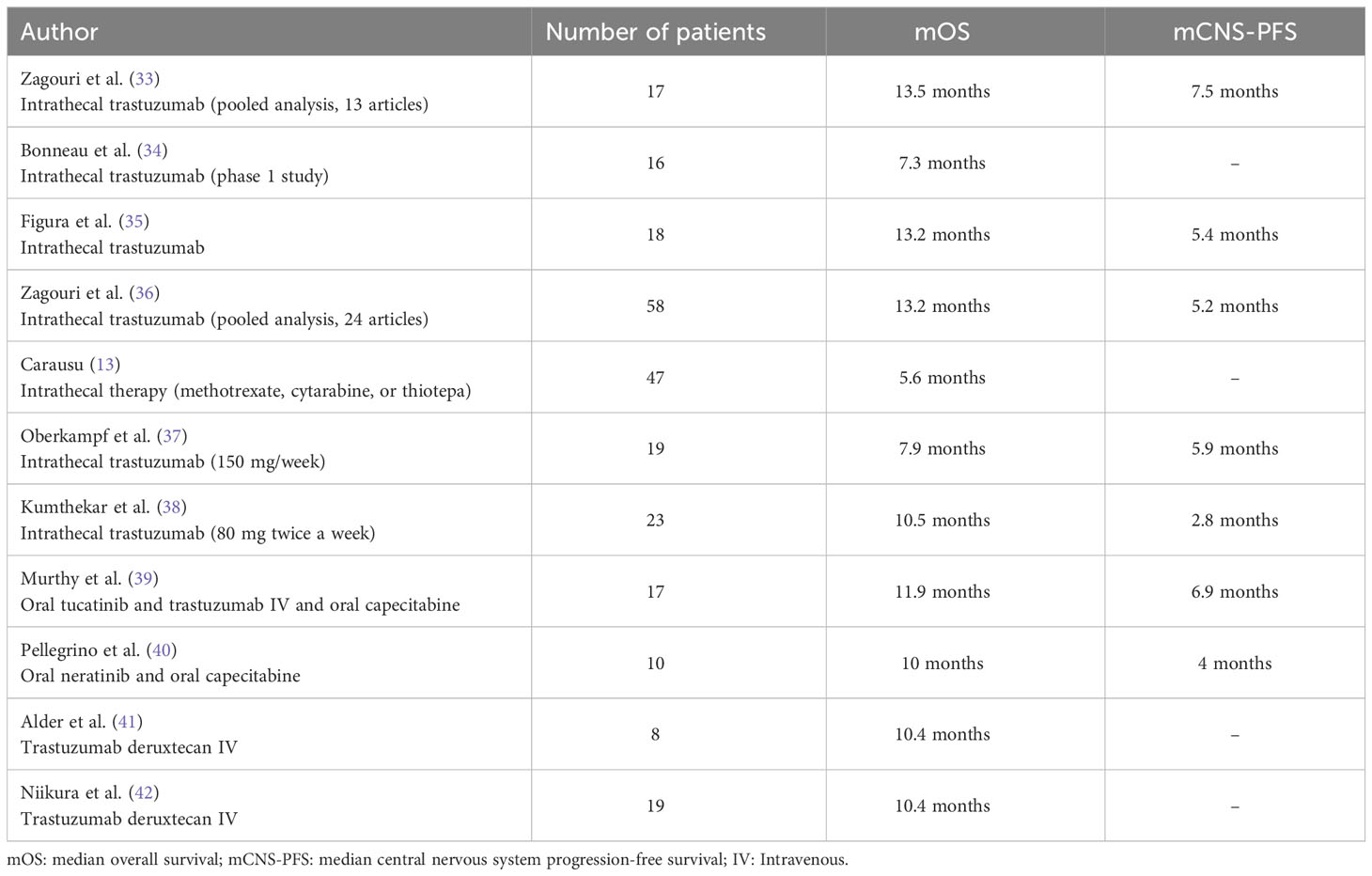
Table 1 Summary of published data on patients with leptomeningeal metastases from HER2-positive breast cancer with more than 5 patients and median overall survival available.
Intra-CSF pharmacotherapies are widely used in patients with LM across Europe, and should be restricted to patients with a life expectancy ≥ 1 month (14, 43). There are two routes of administration: repeated LP or intraventricular catheter, like Ommaya reservoirs. Four agents are classically used for intra-CSF injections: MTX, cytarabine (including liposomal cytarabine), topotecan and thiotepa (44, 45). There is no OS benefit of one agent over another and the combination of intra-CSF CT is not superior to monotherapy (46–48). The secondary analysis of a randomized trial showed that patients with LM (n = 100, 36 cases of primary BC) treated with intraventricular MTX had a longer progression-free survival (PFS) compared to lumbar administration (49). Ommaya reservoirs have a low complication rate (< 7.4% in the literature) with a uniform distribution in the CNS and avoid discomfort and post-procedural complications linked to repeated LP.
The EANO-ESMO 2017 classification provided a rationale for using intra-CSF pharmacotherapy in patients with linear or ependymal MRI contrast-enhancement and/or a positive cytology. Intra-CSF pharmacotherapy is recommended in patients with a positive CSF cytology (i.e. all type I LM), irrespective of their MRI presentation. In patients with a negative CSF cytology (type II LM), intra-CSF pharmacotherapy can also be considered in case of linear contrast enhancement (type IIA) or linear and nodular disease (type IIC). Conversely, a nodular meningeal disease alone (type IIB) predicts a poor CNS penetration of intra-CSF pharmacotherapy and can even increase the toxicity of chemotherapeutic agents due to CSF blocked flow. CSF flow studies can be done in this situation and radiation can be given to the site of the block to alleviate it.
Only 6 randomized trials prospectively explored the benefit of intra-CSF chemotherapy in LM, with contrasting results (46–48, 50–52). Two trials specifically assessed the efficacy of adding intra-CSF chemotherapy to systemic CT in patients with LM from BC. Boogerd et al. selectively enrolled patients with LM from BC and compared the combination of intra-CSF MTX with systemic CT (n = 17) to systemic CT alone (n = 18) (50). The study failed to demonstrate a clear benefit of adding intra-CSF MTX with similar response rates (59% versus 67%) and a shorter median OS (mOS) of 18.3 weeks in the experimental arm (versus 30.3 weeks, 95% CI: 5.5-34.3 weeks, p = 0.32). The poor prognosis observed in the experimental arm could partly be explained by a 18% Ommaya reservoirs revision rate and a higher rate of neurological complications (47% versus 6% in the control group, p = 0.0072). The DEPOSEIN open-label phase III trial evaluated the efficacy of adding intra-CSF liposomal cytarabine to systemic CT alone in 73 patients with LM from BC (52). The patients treated with the experimental treatment had a longer PFS related to LM (LM-PFS) of 3.8 months versus 2.2 months in the control arm (HR = 0.61, 95% CI: 0.38-0.98, p = 0.04) and a mOS of 7.3 months (95% CI: 3.9-9.6) versus 4.0 months (95% CI: 2.2-6.3) in the control group. The control group had a higher rate of HER2+ tumors (24% versus 6% in the experimental group) and most frequently received anti-HER2 therapies (n = 7 versus n = 2 in the experimental group).
Based on the poor CNS penetrance of systemic trastuzumab with a serum-to-CSF rate of 1/420 (53) and the concordant HER2 status between CSF cancer cells and primary tumor, case reports of long-responders (> 2 years) to intra-CSF trastuzumab were published (53, 54).
A first prospective multi-institutional phase I/II dose escalation trial was done to evaluate intra-CSF trastuzumab at a dose of 80 mg (2x/week for a month, then 1x/week for a month, then 1x/2 weeks) in 34 patients with LM from BC (55). The mOS was 12.1 months (95% CI: 4.3-19.6) with a clinical benefit in 69% of cases. Another phase I dose-escalation study published by Bonneau et al. assessed intra-CSF trastuzumab (150 mg weekly) in 19 patients with HER2+ LM from BC (34). At a dose of 150 mg, the mean trastuzumab CSF residual concentration was 27.88 mg/L, similar to the optimal inhibition concentration (30 mg/L). 3 patients had a clinical response, 7 were stable and 4 progressed with no reported dose-limiting toxicities. In a pooled analysis of 17 patients from 13 articles, the administration of intra-CSF trastuzumab was safe with a median PFS (mPFS) of 13.5 months and a median CNS-PFS of 7.5 months (33). A prospective trial compared the efficacy of intra-CSF trastuzumab (n = 18), single-agent intra-CSF CT (n = 15) or WBRT alone (n = 23) in 56 patients with HER2+ BC with LM (35). The CNS-PFS at 6 months was 44% with intra-CSF trastuzumab (versus 18% and 26% in the intra-CSF CT and WBRT groups) with a 1-year OS of 54% (versus 10%, and 19% in the intra-CSF CT and WBRT groups). More recently, a meta-analysis that included 58 patients with HER2+ LM treated with intra-CSF trastuzumab reported a shorter CNS-PFS of 5.2 months but a mOS of 13.2 months from intra-CSF trastuzumab initiation (36). Recently, a phase I/II prospective trial that enrolled 23 patients with HER2+ LM treated with IT trastuzumab was performed. At a dose of 80 mg twice weekly, the mOS was 10.5 months (95% CI 5.2-20.9) (38). A single-arm, non-randomized phase I/II trial (NCT04588545) will assess the efficacy of radiotherapy (RT) (WBRT or focal RT) followed by intra-CSF trastuzumab/pertuzumab (with pertuzumab dose-escalation) in HER2+ LM (56).
Case reports and retrospective series suggested some efficacy of systemic treatments in LM from MBC, but very few prospective trials are available to guide therapeutic decision. Consequently, guidelines for systemic treatments are extrapolated from the management of HER2+ BM (14, 57).
There is one case report of HER2+ LM response to T-DM1 published in the literature (58). The patient had BM and LM and received T-DM1 with concomitant WBRT after a first line of pertuzumab, trastuzumab and docetaxel. The patient experienced a CR after 3 cycles with a favorable safety profile and long-lasting CNS control > 13 months. In 2020, Higashiyamaa et al. published the case of a patient with heavily pretreated HER2+ BC with metastases to the CNS and liver (59). The diagnostic of LM was suspected based on clinical and MRI features, CSF cytology was not documented. The patient had an objective radiological improvement of LM lesions after 9 cycles of dose-adapted trastuzumab-deruxtecan (T-DXd). More recently, Alder et al. published a series of 8 patients with heavily pretreated HER2+ MBC and progressing LM treated with T-DXd. All the patients experienced a clinical benefit from T-DXd and 4 patients had a PR (41). We can cite the case of a patient with HER2-negative MBC (60) who experienced BM and LM treated with lapatinib (61). The patient had an objective neurological improvement during 9 months and experienced a rapid neurological decline when lapatinib was discontinued. One of the metastatic skin tumors was HER2 2+ (FISH -), which could suggest receptor conversion from HER2- to HER2+ in LM.
The first retrospective studies of patients with LM suggested some activity of systemic CT (50, 62, 63). In 2015, the case series of Chahal et al. evaluated IV thiotepa (40 mg/m2 every 21 days) in 13 patients with LM secondary to BM (64). 4 patients displayed a PR, 3 SD and 6 PD with a 69% 6-month survival rate and a 31% 1-year survival rate. The recent series of Pellegrino et al. evaluated the activity of Neratinib in association with Capecitabine in 10 patients with LM from heavily pretreated HER2+ BC (40). Patients experienced a 6-month OS of 60%, and a mOS of 10 months (95% CI: 2.00-17.0). 3-month intracranial PFS (IC-PFS) was 60% with a median IC-PFS of 4 months (95% CI: 2.00-6.0) and a median duration of neurological response of 6.5 months.
In 2015, Wu et al. assessed in a pilot study the benefit of bevacizumab combined with etoposide and cisplatin (BEEP) in 8 patients with LM from BC (65). The CNS-overall response rate (ORR) was 60% with a mOS of 4.7 months (95% CI: 0.3-9.0) and a CNS-PFS of 4.7 months (95% CI 0-10.5). The single-arm phase II trial published by Brastianos et al. assessed pembrolizumab efficacy in patients with solid tumor malignancies and LM (66). 15 patients had LM from BC in the cohort (83.3%), and 7 patients (35%) were HER2+ with a mOS of 4.4 months (90% CI: 1–6.8). Kumthekar et al. published an open-label phase II study in 28 patients with LM secondary to BM from BC treated with ANG1005 (600 mg/m2 every 3 weeks) (67). In the subset of patients with LM, 79% had intracranial disease control and mOS of 8 months (95% CI, 5.4-9.4), with a reasonable safety profile.
An ongoing phase II non-randomized study (NCT03501979) will test the safety and efficacy of the HER2CLIMB regimen (tucatinib, trastuzumab, capecitabine) in patients with HER2+ BC with LM, with encouraging tucatinib CSF concentration levels in the CSF pharmacokinetic analysis (68). A phase III randomized trial (NCT03613181) will compare ANG1005 to the physician’s best choice in patients with pretreated BCBM and newly diagnosed HER2-negative LM from BC.
In summary, the modification of systemic agents should be considered in case of LM diagnosis and take into account the primary tumor histology, molecular profile and previous systemic treatment lines.
Historically, RT was the treatment of choice for LM but no prospective trial assessed the efficacy and safety of WBRT or craniospinal irradiation (CSI) alone. Only one phase II trial tested the combination of intra-CSF MTX with concomitant involved-field radiotherapy (IF-RT) across LM from various cancer subtypes (69). Among the 59 patients included, 11 (19%) were treated for BC with a mOS of 5.4 months (unspecified HER2 status). The retrospectives series of WBRT alone in patients with LM showed no survival benefit (70–74). However, WBRT does provide symptomatic relief in case of hydrocephalus or seizures. WBRT alone (30 Gy in 10 fractions or 20 Gy in 5 fractions) is used in patients with synchronous BM, symptomatic linear disease or extensive nodular LM (14, 75) and is the treatment of choice in patients with a poor KPS without good systemic treatment options.
The neurotoxicity of concomitant CNS RT and intra-CSF CT was mostly evaluated in hematology studies. In the series of Kim et al., 80 patients with CNS lymphoma or leukemia received intra-CSF MTX in addition to WBRT and 63 patients received intra-CSF MTX alone. Leukoencephalopathy developed in 60 (75%) and 35 patients (55%), respectively (76). Even though the rate of leukoencephalopathy was higher in the combination group, it did not reach significance levels in univariate analysis; there was no comparison between treatment sequences (WBRT-intra-CSF MTX versus intra-CSF MTX-WBRT). A safety window should be observed between the end of WBRT and the beginning of intra-CSF injections to avoid radio-sensitization and high-grade MTX-induced neurotoxicity (77), as high as 20% when WBRT and intra-CSF MTX are combined (69).
Conventional photon craniospinal irradiation (CSI) should be avoided given the high risk of RT-induced bone marrow toxicity, enteritis and mucositis and the limited impact on clinical outcome. CSI could be an option in selected patients with limited extra-CNS disease and good KPS. Recently, a phase I clinical trial evaluated hypofractionated proton CSI (30 Gy in 10 fractions) alone using proton therapy in patients with LM (7/24 patients had BC, with 2 patients with HER2+ LM) with a better tolerability and some durable responses (4 patients had a CNS-PFS >12 months) (78).
Focal RT (single fractions or fractionated regimens) can be discussed in symptomatic type IIB LM prior to intra-CSF CT to restore a normal CSF flow pattern, improve delivery of intra-CSF treatment and limit the risk of toxicity. In the retrospective cohort of Wolf et al., 16 patients with focal LM (n = 5 (31%) from BC) received stereotactic radiosurgery (SRS) with a median dose of 16 Gy (79). 14 patients (87,5%) were actively treated with CT, targeted therapy or immunotherapy at the time of SRS. 5 patients experienced a SD and 8 patients a PR. The mOS from SRS was 10 months. 6 of the 7 patients with disease recurrence underwent a salvage WBRT (median time of 6 months), suggesting a delay of WBRT after SRS in some patients. Focal RT should also be considered in the presence of symptomatic cranial nerve impairment, cauda equina syndrome or skull base involvement.
Currently, there is no standard-of-care for HER2+ BC with LM. Large prospective trials excluded patients with LM and intracranial response rates of systemic anti-HER2 therapies and CT, such as capecitabine in HER2+ BC with LM, are extrapolated from the retrospective and prospective characterization of patients with BC with BM (57).
Trastuzumab is a systemic humanized anti-HER2 IgG1 monoclonal antibody widely prescribed in HER2+ MBC. Despite a lack of prospective trials assessing the specific impact of trastuzumab in HER2+ CNS disease, some studies suggest a clinical benefit. A retrospective analysis showed that patients that received trastuzumab for newly diagnosed HER2+ BM had a more favorable mOS of 11.9 months (versus 3 months without trastuzumab) (80). The prospective, observational registHER study confirmed a prolonged OS of 17.5 months in patients with newly diagnosed HER2+ CNS metastases treated with trastuzumab (versus 3.8 months without trastuzumab) (81). Trastuzumab seems to delay the onset of CNS metastases in HER2+ MBC. Intra-CSF cancer cells have a conserved HER2 status compared to the primary tumor in 94% of cases (54); consequently, the combination of systemic anti-HER2 therapies with CT should always be considered in this population.
Patients with CNS metastases were excluded from the phase III CLEOPATRA trial that assessed the addition of pertuzumab to docetaxel and trastuzumab for first-line treatment of HER2+ MBC (82). In an exploratory analysis, Swain et al. evaluated the incidence and time to development of CNS metastases in patients from CLEOPATRA (83). The incidence of CNS metastases was comparable between treatment arms (12.6% versus 13.7%) but the onset of CNS metastases was delayed in the pertuzumab group (15.0 versus 11.9 months, HR = 0.58, 95% CI: 0.39-0.85) and associated with a better mOS (34.4 versus 26.3 months). Patients with stable CNS metastases were included in the PERUSE trial, which confirmed paclitaxel as a more tolerable alternative to docetaxel in combination with trastuzumab and pertuzumab with similar mPFS (19.2-23.2 versus 18.7 months) and mOS (64.0-70.9 versus 57.1 months) compared to CLEOPATRA (84, 85). More recently, the PATRICIA phase II study assessed the efficacy of high-dose trastuzumab (6 mg/kg weekly) in combination with pertuzumab in 39 patients with progressive HER2+ CNS metastases after prior RT (86). Despite a low CNS ORR of 11% (95% CI: 3-25), a significant proportion of patients experienced a clinical benefit at 4 (68%) and 6 months (51%). Notably, 2 patients had an ongoing intracranial and extracranial disease stabilization for > 2 years. Patients with LM were excluded.
In the second-line setting, T-DM1 is approved for patients with HER2+ MBC. T-DM1 is an antibody drug conjugate (ADC) composed of trastuzumab covalently conjugated with the microtubule-inhibitory agent DM1 (derivative of maytansine) by means of a stable linker (87). ADCs allow targeted intracellular delivery of highly cytotoxic agents to cancer cells and decrease their overall toxicity profile. The efficacy of T-DM1 was compared to lapatinib in combination with capecitabine (L+C) in patients with HER2+ locally advanced breast cancer (LABC) or MBC previously treated with trastuzumab and a taxane in the phase III EMILIA trial (88). Patients with active BM were excluded from EMILIA but 19.8% of the patients included in the trial had stable CNS metastases. Krop et al. published in 2015 an exploratory analysis of patients with CNS metastasis included in EMILIA (89). The mOS of patients with CNS metastasis at baseline was improved in the T-DM1 arm compared to L+C (26.8 versus 12.9 months, HR = 0.38; p = 0.008) with similar PFS (5.9 vs 5.7 months). Patients previously treated with lapatinib were excluded from EMILIA. Consequently, the phase III TH3RESA trial assessed the efficacy of T-DM1 compared to the best physician’s choice in patients with HER2+ MBC who received trastuzumab- and lapatinib-based treatments in previous lines (90). 67 patients (11.1%) included in TH3RESA had asymptomatic or treated BM (N=40 in the T-DM1 arm, N=27 in the physician’s choice arm) with a mPFS of 6.2 months in the T-DM1 group (versus 3.3 months in the physician’s choice group). After the approval of T-DM1, multiple retrospective series reported an ORR ranging between 20% and 44% in patients with HER2+ CNS metastases (91–94).
After T-DM1, subsequent treatment lines are not uniformly codified. Due to the development of resistance and dose-limiting toxicities with T-DM1 or concomitant SRS (95, 96), other HER2-targeted therapies such as T-DXd were developed. T-DXd is an ADC composed of trastuzumab, a tetrapeptide-based cleavable linker and a topoisomerase I inhibitor, exatecan derivative. T-DXd is a more efficient and specific anti-HER2 ADC compared to T-DM1 due to its linker cleaved by tumor enzymes (cathepsins B and L), higher drug-to-antibody ratio (97) and a bystander killing effect that also targets surrounding cancer cells with heterogenic HER2 expression (98). Based on the results of the phase II DESTINY-Breast01 trial (99), T-DXd was approved in second line for HER2+ MBC after one line of anti-HER2-based regimens. Patients with untreated or symptomatic CNS metastases were excluded, but 24 patients with treated and/or asymptomatic BM were included. Jerusalem et al. reported a 58.3% ORR in that subgroup with a mPFS of 18.1 months (95% CI: 6.7-18.1) (100). A subgroup analysis of 82 patients with HER2+ BM from the DESTINY-Breast03 trial showed an improved mPFS of 15 months in the T-DXd group (versus 3 months in the T-DM1 group, HR = 0.25, 95% CI: 0.31-0.45) and a 63.9% IC-ORR (versus 33.4% in the T-DM1 group) (101). In a recently published update of the DESTINY-Breast03 trial, the mOS was not reached (95% CI: 40.5 months–N/A) in the T-DXd group and was not reached (34.0 months–N/A) in the T-DM1 group (HR = 0,64, 95% CI: 0,47-0,87, p = 0.0037) (102). That OS benefit was observed across all the subgroups analyzed, including those with baseline BM. The single-arm phase II DEBBRAH trial (NCT04420598) assessed the efficacy of T-DXd (5.4 mg/kg) in patients with pretreated HER2+ or HER2-low stable (n = 8, cohort 1), untreated (n = 4, cohort 2), or progressing BCBM after local therapy (n = 9, cohort 3) (103). The first results reported an overall IC-ORR of 46.2% (95% CI: 19.2-74.9) in patients with active BMs (cohorts 2 and 3). Additional results are expected in the population of patients with LM (103). The ongoing multicentric single-arm phase II TUXEDO-4 trial (NCT06048718) will analyze the efficacy of T-DXd in patients with HER2-low BC with active BM.
Finally, the recent prospective, open-label, single-arm, phase II TUXEDO-1 trial evaluated the efficacy of T-DXd in patients with newly diagnosed untreated HER2+ BM or progressive BM after previous local therapy or previous exposure to trastuzumab and pertuzumab (104). Among the 15 patients enrolled, 2 had a CR, 9 had a PR and 3 had a SD with a best IC-ORR rate of 73.3% (95% CI: 48.1-89.1%). The median PFS was 14 months (95% CI 11.0 months to not recorded) and median OS was not reached at a median follow-up of 12 months.
Data on response to T-DXd in patients with HER2+ BC with LM were lacking until very recently. The study populations of the DESTINY-Breast01 and DESTINY-Breast03 studies did not include patients with active BM and/or LM. The multicentric, retrospective ROSET-BM study assessed the benefit of T-DXd in 104 patients with HER2+ MBC with BM and/or LM (105). IC-ORR was 62.7% in that cohort with a median IC-PFS of 16.1 months (95% CI: 12.2–N/A). Among the 19 patients with LM, the 1-year PFS and OS rates were 60.7% (95% CI: 34,5–79,1) and 87.1% (95%CI, 57.3–96.6), respectively. Alder et al. published a series of 8 patients with heavily pretreated HER2+ MBC and progressing LM treated with T-DXd. All the patients experienced a clinical benefit from T-DXd and 4 patients had a PR (41).
In subsequent lines, anti-HER2 TKI can cross the BBB with an IC-ORR ranging between 47% and 66% (106–108). The HER2CLIMB study evaluated the combination of tucatinib, capecitabine and trastuzumab for heavily pretreated patients with HER2+ MBC. Supported by the preliminary efficacy data of the phase I trial, the HER2CLIMB study included 291 patients (47.5%) with untreated or previously treated progressing BM at baseline (109). The mPFS of patients with BM was 7.6 months (95% CI: 6.2-9.5) in the experimental arm (versus 5.4 months in the placebo group, 95% CI: 4.1-5.7). Interestingly, that benefit was similar in a subgroup analysis of patients with active BM (HR = 0.49, 95% CI: 0.30-0.80). At a median OS follow-up of 29.6 months, mOS was 24.7 months for the tucatinib combination group versus 19.2 months in the placebo group (HR = 0.73, 95% CI: 0.59-0.90, p = 0.004) (110). In the updated exploratory analysis of the HER2CLIMB trial published in 2023 with an additional follow-up of 15.6 months, the median CNS-PFS was 9.6 months in the tucatinib-combination group compared to 5.6 months in the placebo group for patients with active BMs (95% CI: 7.6-11.1 vs 2.9-5.6) and the risk of developing new brain lesions was reduced by 45.1% in the tucatinib-combination group (HR = 0.55, 95% CI: 0.36-0.85, p = 0.006) (111). Yan et al. published the case of a patient resistant to neratinib and capecitabine that demonstrated a significant response of LM to tucatinib (112). In the TBCR049 trial, tucatinib was detectable in CSF within 2 hours after drug administration, with concentrations ranging from 0.57 to 25 ng/mL and CSF-to-plasma ratios of 0.83 (68). The NALA trial compared the activity of lapatinib (L+C) and neratinib (N+C) in combination with capecitabine in HER2+ MBC (113). 101 patients (16%) had asymptomatic or stable BM. The N+C group displayed an improved mPFS of 8.8 months compared to L+C in the overall population (versus 6.6 months, HR = 0.76, 95% CI: 0.63-0.93). The incidence of intervention for CNS disease was lower in the N+C group versus L+C group, suggesting a delayed CNS metastasis onset. Hence, a combination of L+C or N+C is an interesting option to avoid infusion therapies in patients with HER2+ BM previously treated with at least two anti-HER2 regimens.
Despite the dismal prognosis classically associated with BC with LM, this case report highlights that long-term responses are possible with the sequential administration of anti-HER2 targeted therapies combined with local treatments. According to the recommendations of the ASCO Eligibility Criteria Working Group, future prospective trials should include patients with CNS metastases, including LM, to assess the CNS efficacy of new treatments and optimize treatment sequencing.
AdB and LL designed and edited the text, table and figure as well as their legends under the supervision of TB. All authors provided scientific advice and critically revised the manuscript. We confirm that all coauthors have contributed to the generation of the manuscript and have given final approval of the version to be published.
Dr. TB has received compensation for his advisory role for Astra Zeneca, Novartis, Pfzer, Roche and SeaGen. He also has received research support from Roche, Pfzer, SeaGen and Astra Zeneca.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Le Rhun E, Preusser M, van den Bent M, Andratschke N, Weller M. How we treat patients with leptomeningeal metastases. ESMO Open (2019) 4:e000507. doi: 10.1136/esmoopen-2019-000507
2. Lamba N, Wen PY, Aizer AA. Epidemiology of brain metastases and leptomeningeal disease. Neuro-Oncol (2021) 23(9):1447–56. doi: 10.1093/neuonc/noab101
3. Shapiro W, Posner J, Ushio Y, Chernik N, Young D. Treatment of meningeal neoplasms. Cancer Treat Rep (1977) 1977:733–43.
4. Posner J, Chernik N. Intracranial metastases from systemic cancer. Adv Neurology (1978) 1978:579–92.
5. Griguolo G, Pouderoux S, Dieci MV, Jacot W, Bourgier C, Miglietta F, et al. Clinicopathological and treatment-associated prognostic factors in patients with breast cancer leptomeningeal metastases in relation to tumor biology. Oncologist (2018) 23(11):1289–99. doi: 10.1634/theoncologist.2018-0200
6. Le Rhun E, Taillibert S, Zairi F, Kotecki N, Devos P, Mailliez A, et al. A retrospective case series of 103 consecutive patients with leptomeningeal metastasis and breast cancer. J Neurooncol (2013) 113(1):83–92. doi: 10.1007/s11060-013-1092-8
7. Morikawa A, Jordan L, Rozner R, Patil S, Boire A, Pentsova E, et al. Characteristics and outcomes of patients with breast cancer with leptomeningeal metastasis. Clin Breast Cancer (2017) 17(1):23–8. doi: 10.1016/j.clbc.2016.07.002
8. Gampenrieder SP, Rinnerthaler G, Tinchon C, Petzer A, Balic M, Heibl S, et al. Landscape of HER2-low metastatic breast cancer (MBC): results from the Austrian AGMT_MBC-Registry. Breast Cancer Res (2021) 23(1):112. doi: 10.1186/s13058-021-01492-x
9. Altundag K, Bondy ML, Mirza NQ, Kau S-W, Broglio K, Hortobagyi GN, et al. Clinicopathologic characteristics and prognostic factors in 420 metastatic breast cancer patients with central nervous system metastasis: CNS Metastases in Breast Cancer. Cancer (2007) 110(12):2640–7. doi: 10.1002/cncr.23088
10. Yust-Katz S, Garciarena P, Liu D, Yuan Y, Ibrahim N, Yerushalmi R, et al. Breast cancer and leptomeningeal disease (LMD): hormone receptor status influences time to development of LMD and survival from LMD diagnosis. J Neurooncol (2013) 114(2):229–35. doi: 10.1007/s11060-013-1175-6
11. Johnson MD, Avkshtol V, Baschnagel AM, Meyer K, Ye H, Grills IS, et al. Surgical resection of brain metastases and the risk of leptomeningeal recurrence in patients treated with stereotactic radiosurgery. Int J Radiat Oncol (2016) 94(3):537–43. doi: 10.1016/j.ijrobp.2015.11.022
12. Norris LK, Grossman SA, Olivi A. Neoplastic meningitis following surgical resection of isolated cerebellar metastasis: A potentially preventable complication. J Neurooncol (1997) 32:215–23. doi: 10.1023/a:1005723801479
13. Carausu M, Carton M, Darlix A, Pasquier D, Leheurteur M, Debled M, et al. Breast cancer patients treated with intrathecal therapy for leptomeningeal metastases in a large real-life database. ESMO Open (2021) 6(3):100150. doi: 10.1016/j.esmoop.2021.100150
14. Le Rhun E, Weller M, Brandsma D, Van den Bent M, de Azambuja E, Henriksson R, et al. EANO–ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up of patients with leptomeningeal metastasis from solid tumours. Ann Oncol (2017) 28:iv84–99. doi: 10.1093/annonc/mdx221
15. Clarke JL, Perez HR, Jacks LM, Panageas KS, DeAngelis LM. Leptomeningeal metastases in the MRI era. Neurology (2010) 74:1449–54. doi: 10.1212/WNL.0b013e3181dc1a69
16. Clarke JL. Leptomeningeal metastasis from systemic cancer. Contin Lifelong Learn Neurol (2012) 18:328–42. doi: 10.1212/01.CON.0000413661.58045.e7
17. Freilich RJ, Krol G, Deangelis LM. Neuroimaging and cerebrospinal fluid cytology in the diagnosis of leptomeningeal metastasis. Ann Neurol (1995) 38(1):51–7. doi: 10.1002/ana.410380111
18. Singh SK, Agris JM, Leeds NE, Ginsberg LE. Intracranial leptomeningeal metastases: comparison of depiction at FLAIR and contrast-enhanced MR imaging. Radiology (2000) 217(1):50–3. doi: 10.1148/radiology.217.1.r00oc3550
19. Straathof CSM, de Bruin HG, Dippel DWJ, Vecht C. The diagnostic accuracy of magnetic resonance imaging and cerebrospinal fluid cytology in leptomeningeal metastasis. J Neurol (1999) 246(9):810–4. doi: 10.1007/s004150050459
20. Zeiser R, Burger JA, Bley TA, Windfuhr-Blum M, Schulte-Mönting J, Behringer DM. Clinical follow-up indicates differential accuracy of magnetic resonance imaging and immunocytology of the cerebral spinal fluid for the diagnosis of neoplastic meningitis - a single centre experience: Differential Accuracy of MRI and Immunocytology of CSF. Br J Haematol (2004) 124(6):762–8. doi: 10.1111/j.1365-2141.2004.04853.x
21. Park YW, Ahn SJ. Comparison of contrast-enhanced T2 FLAIR and 3D T1 black-blood fast spin-echo for detection of leptomeningeal metastases. Investig Magn Reson Imaging (2018) 22(2):86. doi: 10.13104/imri.2018.22.2.86
22. Prabhu RS, Turner BE, Asher AL, Marcrom SR, Fiveash JB, Foreman PM, et al. A multi-institutional analysis of presentation and outcomes for leptomeningeal disease recurrence after surgical resection and radiosurgery for brain metastases. Neuro-Oncol (2019) 21(8):1049–59. doi: 10.1093/neuonc/noz049
23. Turner BE, Prabhu RS, Burri SH, Brown PD, Pollom EL, Milano MT, et al. Nodular leptomeningeal disease—A distinct pattern of recurrence after postresection stereotactic radiosurgery for brain metastases: A multi-institutional study of interobserver reliability. Int J Radiat Oncol (2020) 106(3):579–86. doi: 10.1016/j.ijrobp.2019.10.002
24. Kirkpatrick JP. Classifying leptomeningeal disease: an essential element in managing advanced metastatic disease in the central nervous system. Int J Radiat Oncol (2020) 106(3):587–8. doi: 10.1016/j.ijrobp.2019.12.016
25. Chamberlain MC. Comparative spine imaging in leptomeningeal metastases. J Neurooncol (1995) 23(3):233–8. doi: 10.1007/BF01059954
26. Chamberlain MC, Kormanik PA, Glantz MJ. A comparison between ventricular and lumbar cerebrospinal fluid cytology in adult patients with leptomeningeal metastases. Neuro-Oncol (2001) 3(1):42–5. doi: 10.1215/S1522851700000338
27. Gauthier H, Guilhaume MN, Bidard FC, Pierga JY, Girre V, Cottu PH, et al. Survival of breast cancer patients with meningeal carcinomatosis. Ann Oncol (2010) 21(11):2183–7. doi: 10.1093/annonc/mdq232
28. Angus L, Martens JWM, van den Bent MJ, Sillevis Smitt PAE, Sleijfer S, Jager A. Novel methods to diagnose leptomeningeal metastases in breast cancer. Neuro-Oncol (2019) 21(4):428–39. doi: 10.1093/neuonc/noy186
29. Le Rhun E, Kramar A, Salingue S, Girot M, Rodrigues I, Mailliez A, et al. CSF CA 15-3 in breast cancer-related leptomeningeal metastases. J Neurooncol (2014) 117(1):117–24. doi: 10.1007/s11060-014-1361-1
30. Glantz MJ, Cole BF, Glantz LK, Cobb J, Mills P, Lekos A, et al. Cerebrospinal fluid cytology in patients with cancer: Minimizing false-negative results. Cancer (1998) 82(4):733–9. doi: 10.1002/(SICI)1097-0142(19980215)82:4<733::AID-CNCR17>3.0.CO;2-Z
31. Milojkovic Kerklaan B, Pluim D, Bol M, Hofland I, Westerga J, van Tinteren H, et al. EpCAM-based flow cytometry in cerebrospinal fluid greatly improves diagnostic accuracy of leptomeningeal metastases from epithelial tumors. Neuro-Oncol (2016) 18(6):855–62. doi: 10.1093/neuonc/nov273
32. Lee JS, Melisko ME, Magbanua MJM, Kablanian AT, Scott JH, Rugo HS, et al. Detection of cerebrospinal fluid tumor cells and its clinical relevance in leptomeningeal metastasis of breast cancer. Breast Cancer Res Treat (2015) 154(2):339–49. doi: 10.1007/s10549-015-3610-1
33. Zagouri F, Sergentanis TN, Bartsch R, Berghoff AS, Chrysikos D, de Azambuja E, et al. Intrathecal administration of trastuzumab for the treatment of meningeal carcinomatosis in HER2-positive metastatic breast cancer: a systematic review and pooled analysis. Breast Cancer Res Treat (2013) 139(1):13–22. doi: 10.1007/s10549-013-2525-y
34. Bonneau C, Paintaud G, Trédan O, Dubot C, Desvignes C, Dieras V, et al. Phase I feasibility study for intrathecal administration of trastuzumab in patients with HER2 positive breast carcinomatous meningitis. Eur J Cancer (2018) 95:75–84. doi: 10.1016/j.ejca.2018.02.032
35. Figura NB, Rizk VT, Mohammadi H, Evernden B, Mokhtari S, Yu HM, et al. Clinical outcomes of breast leptomeningeal disease treated with intrathecal trastuzumab, intrathecal chemotherapy, or whole brain radiation therapy. Breast Cancer Res Treat (2019) 175(3):781–8. doi: 10.1007/s10549-019-05170-7
36. Zagouri F, Zoumpourlis P, Le Rhun E, Bartsch R, Zografos E, Apostolidou K, et al. Intrathecal administration of anti-HER2 treatment for the treatment of meningeal carcinomatosis in breast cancer: A metanalysis with meta-regression. Cancer Treat Rev (2020) 88:102046. doi: 10.1016/j.ctrv.2020.102046
37. Oberkampf F, Gutierrez M, Trabelsi Grati O, Le Rhun É, Trédan O, Turbiez I, et al. Phase II study of intrathecal administration of trastuzumab in patients with HER2-positive breast cancer with leptomeningeal metastasis. Neuro Oncol (2022), noac180. doi: 10.1093/neuonc/noac180
38. Kumthekar PU, Avram MJ, Lassman AB, Lin NU, Lee E, Grimm SA, et al. A phase I/II study of intrathecal trastuzumab in human epidermal growth factor receptor 2-positive (HER2-positive) cancer with leptomeningeal metastases: Safety, efficacy, and cerebrospinal fluid pharmacokinetics. Neuro-Oncol (2023) 25(3):557–65. doi: 10.1093/neuonc/noac195
39. Murthy RK, O Brien B, Berry DA, Singareeka-Raghavendra A, Monroe MG, Johnson J, et al. Abstract PD4-02: Safety and efficacy of a tucatinib-trastuzumab-capecitabine regimen for treatment of leptomeningeal metastasis (LM) in HER2-positive breast cancer: Results from TBCRC049, a phase 2 non-randomized study. Cancer Res (2022) 82:PD4–02–PD4–02. doi: 10.1158/1538-7445.SABCS21-PD4-02
40. Pellerino A, Soffietti R, Bruno F, Manna R, Muscolino E, Botta P, et al. Neratinib and capecitabine for the treatment of leptomeningeal metastases from HER2-positive breast cancer: A series in the setting of a compassionate program. Cancers (2022) 14(5):1192. doi: 10.3390/cancers14051192
41. Alder L, Trapani D, Bradbury C, Van Swearingen AED, Tolaney SM, Khasraw M, et al. Durable responses in patients with HER2+ breast cancer and leptomeningeal metastases treated with trastuzumab deruxtecan. NPJ Breast Cancer (2023) 9(1):19. doi: 10.1038/s41523-023-00519-0
42. Niikura N, Yamanaka T, Nomura H, Shiraishi K, Kusama H, Yamamoto M, et al. Treatment with trastuzumab deruxtecan in patients with HER2-positive breast cancer and brain metastases and/or leptomeningeal disease (ROSET-BM). npj Breast Cancer (2023) 9:1–8. doi: 10.1038/s41523-023-00584-5
43. Le Rhun E, Rudà R, Devos P, Hoang-Xuan K, Brandsma D, Pérez Segura P, et al. Diagnosis and treatment patterns for patients with leptomeningeal metastasis from solid tumors across Europe. J Neurooncol (2017) 133(2):419–27. doi: 10.1007/s11060-017-2452-6
44. Groves MD. New strategies in the management of leptomeningeal metastases. Arch Neurol (2010) 67(3):305–12. doi: 10.1001/archneurol.2010.18
45. Jaeckle KA, Dixon JG, Anderson SK, Moreno‐Aspitia A, Colon‐Otero G, Hebenstreit K, et al. Intra-CSF topotecan in treatment of breast cancer patients with leptomeningeal metastases. Cancer Med (2020) 9(21):7935–42. doi: 10.1002/cam4.3422
46. Glantz MJ, Jaeckle KA, Chamberlain MC, Phuphanich S, Recht L, Swinnen LJ, et al. A randomized controlled trial comparing intrathecal sustained-release cytarabine (DepoCyt) to intrathecal methotrexate in patients with neoplastic meningitis from solid tumors1. Clin Cancer Res (1999) 5(11):3394–402.
47. Grossman SA, Finkelstein DM, Ruckdeschel JC, Trump DL, Moynihan T, Ettinger DS. Randomized prospective comparison of intraventricular methotrexate and thiotepa in patients with previously untreated neoplastic meningitis. Eastern Cooperative Oncol Group J Clin Oncol (1993) 11(3):561–9. doi: 10.1200/JCO.1993.11.3.561
48. Hitchins RN, Bell DR, Woods RL, Levi JA. A prospective randomized trial of single-agent versus combination chemotherapy in meningeal carcinomatosis. J Clin Oncol (1987) 5(10):1655–62. doi: 10.1200/JCO.1987.5.10.1655
49. Glantz MJ, Van Horn A, Fisher R, Chamberlain MC. Route of intracerebrospinal fluid chemotherapy administration and efficacy of therapy in neoplastic meningitis. Cancer (2010) 116(8):1947–52. doi: 10.1002/cncr.24921
50. Boogerd W, van den Bent MJ, Koehler PJ, Heimans JJ, van der Sande JJ, Aaronson NK, et al. The relevance of intraventricular chemotherapy for leptomeningeal metastasis in breast cancer: a randomised study. Eur J Cancer (2004) 40(18):2726–33. doi: 10.1016/j.ejca.2004.08.012
51. Shapiro WR, Schmid M, Glantz M, Miller JJ. A randomized phase III/IV study to determine benefit and safety of cytarabine liposome injection for treatment of neoplastic meningitis. J Clin Oncol (2006) 24(18_suppl):1528–8. doi: 10.1200/jco.2006.24.18_suppl.1528
52. Le Rhun E, Wallet J, Mailliez A, Le Deley MC, Rodrigues I, Boulanger T, et al. Intrathecal liposomal cytarabine plus systemic therapy versus systemic chemotherapy alone for newly diagnosed leptomeningeal metastasis from breast cancer. Neuro-Oncol (2020) 22(4):524–38. doi: 10.1093/neuonc/noz201
53. Stemmler HJ, Schmitt M, Willems A, Bernhard H, Harbeck N, Heinemann V. Ratio of trastuzumab levels in serum and cerebrospinal fluid is altered in HER2-positive breast cancer patients with brain metastases and impairment of blood–brain barrier. Anticancer Drugs (2007) 18(1):23–8. doi: 10.1097/01.cad.0000236313.50833.ee
54. Park IH, Kwon Y, Ro JY, Lee KS, Ro J. Concordant HER2 status between metastatic breast cancer cells in CSF and primary breast cancer tissue. Breast Cancer Res Treat (2010) 123(1):125–8. doi: 10.1007/s10549-009-0627-3
55. Figura NB, Long W, Yu M, Robinson TJ, Mokhtari S, Etame AB, et al. Intrathecal trastuzumab in the management of HER2+ breast leptomeningeal disease: a single institution experience. Breast Cancer Res Treat (2018) 169(2):391–6. doi: 10.1007/s10549-018-4684-3
56. Ahmed KA, Kim Y, DeJesus M, Kumthekar P, Williams NO, Palmer JD, et al. Trial in progress: Phase I/II study of radiation therapy followed by intrathecal trastuzumab/pertuzumab in the management of HER2+ breast leptomeningeal disease. J Clin Oncol (2021) 39(15_suppl):TPS1099–TPS1099. doi: 10.1200/JCO.2021.39.15_suppl.TPS1099
57. Dudani S, Mazzarello S, Hilton J, Hutton B, Vandermeer L, Fernandes R, et al. Optimal management of leptomeningeal carcinomatosis in breast cancer patients—A systematic review. Clin Breast Cancer (2016) 16(6):456–70. doi: 10.1016/j.clbc.2016.07.014
58. Ricciardi GRR, Russo A, FranChina T, Schifano S, Mastroeni G, Santacaterina A, et al. Efficacy of T-DM1 for leptomeningeal and brain metastases in a HER2 positive metastatic breast cancer patient: new directions for systemic therapy - a case report and literature review. BMC Cancer (2018) 18(1):97. doi: 10.1186/s12885-018-3994-5
59. Higashiyama N, Nangia J, Shafaee MN, Chen N, Michael BL, Rimawi M, et al. Dose-reduced trastuzumab deruxtecan can be safely used in liver failure and active leptomeningeal metastases. Curr Probl Cancer Case Rep (2020) 2:100034. doi: 10.1016/j.cpccr.2020.100034
60. Toi M, Iwata H, Fujiwara Y, Ito Y, Nakamura S, Tokuda Y, et al. Lapatinib monotherapy in patients with relapsed, advanced, or metastatic breast cancer: efficacy, safety, and biomarker results from Japanese patients phase II studies. Br J Cancer (2009) 101(10):1676–82. doi: 10.1038/sj.bjc.6605343
61. Onishi H, Morisaki T, Nakafusa Y, Nakashima Y, Yokohata K, Katano M. Objective response with lapatinib in patients with meningitis carcinomatosa derived from HER2/HER1-negative breast cancer. Int J Clin Oncol (2011) 16(6):718–21. doi: 10.1007/s10147-011-0195-5
62. Glantz MJ, Cole BF, Recht L, Akerley W, Mills P, Saris S, et al. High-dose intravenous methotrexate for patients with nonleukemic leptomeningeal cancer: is intrathecal chemotherapy necessary? J Clin Oncol (1998) 16(4):1561–7. doi: 10.1200/JCO.1998.16.4.1561
63. Bokstein F, Lossos A, Siegal T. Leptomeningeal metastases from solid tumors: A comparison of two prospective series treated with and without intra-cerebrospinal fluid chemotherapy. Cancer (1998) 82(9):1756–63. doi: 10.1002/(SICI)1097-0142(19980501)82:9<1764::AID-CNCR24>3.0.CO;2-1
64. Chahal J, Stopeck A, Clarke K, Livingston RB, Chalasani P. Intravenous thiotepa for treatment of breast cancer-related leptomeningeal carcinomatosis: case series. Neurol Sci (2015) 36(9):1691–3. doi: 10.1007/s10072-015-2259-1
65. Wu PF, Lin CH, Kuo CH, Chen W-W, Yeh D-C, Liao H-W, et al. A pilot study of bevacizumab combined with etoposide and cisplatin in breast cancer patients with leptomeningeal carcinomatosis. BMC Cancer (2015) 15(1):299. doi: 10.1186/s12885-015-1290-1
66. Brastianos PK, Lee EQ, Cohen JV, Tolaney SM, Lin NU, Wang N, et al. Single-arm, open-label phase 2 trial of pembrolizumab in patients with leptomeningeal carcinomatosis. Nat Med (2020) 26(8):1280–4. doi: 10.1038/s41591-020-0918-0
67. Kumthekar P, Tang SC, Brenner AJ, Kesari S, Piccioni DE, Anders C, et al. ANG1005, a brain-penetrating peptide–drug conjugate, shows activity in patients with breast cancer with leptomeningeal carcinomatosis and recurrent brain metastases. Clin Cancer Res (2020) 26(12):2789–99. doi: 10.1158/1078-0432.CCR-19-3258
68. Stringer-Reasor EM, O’Brien BJ, Topletz-Erickson A, White JB, Lobbous M, Riley K, et al. Pharmacokinetic (PK) analyses in CSF and plasma from TBCRC049, an ongoing trial to assess the safety and efficacy of the combination of tucatinib, trastuzumab and capecitabine for the treatment of leptomeningeal metastasis (LM) in HER2 positive breast cancer. J Clin Oncol (2021) 39(15_suppl):1044–4. doi: 10.1200/JCO.2021.39.15_suppl.1044
69. Pan Z, Yang G, He H, Zhao G, Yuan T, Li Y, et al. Concurrent radiotherapy and intrathecal methotrexate for treating leptomeningeal metastasis from solid tumors with adverse prognostic factors: A prospective and single-arm study. Int J Cancer (2016) 139(8):1864–72. doi: 10.1002/ijc.30214
70. Morris PG, Reiner AS, Szenberg OR, Clarke JL, Panageas KS, Perez HR, et al. Leptomeningeal metastasis from non-small cell lung cancer: survival and the impact of whole brain radiotherapy. J Thorac Oncol (2012) 7(2):382–5. doi: 10.1097/JTO.0b013e3182398e4f
71. Park JH. Clinical outcomes of leptomeningeal metastasis in patients with non-small cell lung cancer in the modern chemotherapy era. Lung Cancer (2012) 76:387–92. doi: 10.1016/j.lungcan.2011.11.022
72. Gwak HS, Joo J, Kim S, Yoo H, Shin SH, Han J-Y, et al. Analysis of treatment outcomes of intraventricular chemotherapy in 105 patients for leptomeningeal carcinomatosis from non–small-cell lung cancer. J Thorac Oncol (2013) 8(5):599–605. doi: 10.1097/JTO.0b013e318287c943
73. Kuiper JL, Hendriks LE, van der Wekken AJ, de Langen AJ, Bahce I, Thunnissen E, et al. Treatment and survival of patients with EGFR -mutated non-small cell lung cancer and leptomeningeal metastasis: A retrospective cohort analysis. Lung Cancer (2015) 89(3):255–61. doi: 10.1016/j.lungcan.2015.05.023
74. Abouharb S, Ensor J, Loghin ME, Katz R, Moulder SL, Esteva FJ, et al. Leptomeningeal disease and breast cancer: the importance of tumor subtype. Breast Cancer Res Treat (2014) 146(3):477–86. doi: 10.1007/s10549-014-3054-z
75. Soffietti R, Cornu P, Delattre JY, Grant R, Graus F, Grisold W, et al. EFNS Guidelines on diagnosis and treatment of brain metastases: report of an EFNS Task Force. Eur J Neurol (2006) 13(7):674–81. doi: 10.1111/j.1468-1331.2006.01506.x
76. Kim JY, Kim ST, Nam DH, Lee JI, Park K, Kong DS. Leukoencephalopathy and disseminated necrotizing leukoencephalopathy following intrathecal methotrexate chemotherapy and radiation therapy for central nerve system lymphoma or leukemia. J Korean Neurosurg Soc (2011) 50(4):304. doi: 10.3340/jkns.2011.50.4.304
77. Bhojwani D, Sabin ND, Pei D, Yang JJ, Khan RB, Panetta JC, et al. Methotrexate-induced neurotoxicity and leukoencephalopathy in childhood acute lymphoblastic leukemia. J Clin Oncol (2014) 32(9):949–59. doi: 10.1200/JCO.2013.53.0808
78. Yang TJ, Wijetunga NA, Yamada J, Wolden S, Mehallow M, Goldman DA, et al. Clinical trial of proton craniospinal irradiation for leptomeningeal metastases. Neuro-Oncol (2021) 23(1):134–43. doi: 10.1093/neuonc/noaa152
79. Wolf A, Donahue B, Silverman JS, Chachoua A, Lee JK, Kondziolka D. Stereotactic radiosurgery for focal leptomeningeal disease in patients with brain metastases. J Neurooncol (2017) 134(1):139–43. doi: 10.1007/s11060-017-2497-6
80. Church DN, Modgil R, Guglani S, Bahl A, Hopkins K, Braybrooke JP, et al. Extended survival in women with brain metastases from HER2 overexpressing breast cancer. Am J Clin Oncol (2008) 31(3):250–4. doi: 10.1097/COC.0b013e31815a43c4
81. Brufsky AM, Mayer M, Rugo HS, Kaufman PA, Tan-Chiu E, Tripathy D, et al. Central nervous system metastases in patients with HER2-positive metastatic breast cancer: incidence, treatment, and survival in patients from registHER. Clin Cancer Res (2011) 17(14):4834–43. doi: 10.1158/1078-0432.CCR-10-2962
82. Swain SM, Baselga J, Kim SB, Ro J, Semiglazov V, Campone M, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med (2015) 372(8):724–34. doi: 10.1056/NEJMoa1413513
83. Swain SM, Baselga J, Miles D, Im Y-H, Quah C, Lee LF, et al. Incidence of central nervous system metastases in patients with HER2-positive metastatic breast cancer treated with pertuzumab, trastuzumab, and docetaxel: results from the randomized phase III study CLEOPATRA. Ann Oncol (2014) 25(6):1116–21. doi: 10.1093/annonc/mdu133
84. Bachelot T, Ciruelos E, Schneeweiss A, Puglisi F, Peretz-Yablonski T, Bondarenko I, et al. Preliminary safety and efficacy of first-line pertuzumab combined with trastuzumab and taxane therapy for HER2-positive locally recurrent or metastatic breast cancer (PERUSE). Ann Oncol (2019) 30(5):766–73. doi: 10.1093/annonc/mdz061
85. Miles D, Ciruelos E, Schneeweiss A, Puglisi F, Peretz-Yablonski T, Campone M, et al. Final results from the PERUSE study of first-line pertuzumab plus trastuzumab plus a taxane for HER2-positive locally recurrent or metastatic breast cancer, with a multivariable approach to guide prognostication. Ann Oncol (2021) 32(10):1245–55. doi: 10.1016/j.annonc.2021.06.024
86. Lin NU, Pegram M, Sahebjam S, Ibrahim N, Fung A, Cheng A, et al. Pertuzumab plus high-dose trastuzumab in patients with progressive brain metastases and HER2-positive metastatic breast cancer: primary analysis of a phase II study. J Clin Oncol (2021) 39(24):2667–75. doi: 10.1200/JCO.20.02822
87. Lewis Phillips GD, Li G, Dugger DL, Crocker LM, Parsons KL, Mai E, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody–cytotoxic drug conjugate. Cancer Res (2008) 68(22):9280–90. doi: 10.1158/0008-5472.CAN-08-1776
88. Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med (2012) 367(19):1783–91. doi: 10.1056/NEJMoa1209124
89. Krop IE, Lin NU, Blackwell K, Guardino E, Huober J, Lu M, et al. Trastuzumab emtansine (T-DM1) versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer and central nervous system metastases: a retrospective, exploratory analysis in EMILIA. Ann Oncol (2015) 26(1):113–9. doi: 10.1093/annonc/mdu486
90. Krop IE, Kim SB, González-Martín A, LoRusso PM, Ferrero J-M, Smitt M, et al. Trastuzumab emtansine versus treatment of physician’s choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol (2014) 15(7):689–99. doi: 10.1016/S1470-2045(14)70178-0
91. Montemurro F, Delaloge S, Barrios CH, Wuerstlein R, Anton A, Brain E, et al. Trastuzumab emtansine (T-DM1) in patients with HER2-positive metastatic breast cancer and brain metastases: exploratory final analysis of cohort 1 from KAMILLA, a single-arm phase IIIb clinical trial☆. Ann Oncol (2020) 31(10):1350–8. doi: 10.1016/j.annonc.2020.06.020
92. Fabi A, Alesini D, Valle E, Moscetti L, Caputo R, Caruso M, et al. T-DM1 and brain metastases: Clinical outcome in HER2-positive metastatic breast cancer. Breast (2018) 41:137–43. doi: 10.1016/j.breast.2018.07.004
93. Jacot W, Pons E, Frenel JS, Guiu S, Levy C, Heudel PE, et al. Efficacy and safety of trastuzumab emtansine (T-DM1) in patients with HER2-positive breast cancer with brain metastases. Breast Cancer Res Treat (2016) 157(2):307–18. doi: 10.1007/s10549-016-3828-6
94. Bartsch R, Berghoff AS, Preusser M. Breast cancer brain metastases responding to primary systemic therapy with T-DM1. J Neurooncol (2014) 116(1):205–6. doi: 10.1007/s11060-013-1257-5
95. Carlson JA, Nooruddin Z, Rusthoven C, Elias A, Borges VF, Diamond JR, et al. Trastuzumab emtansine and stereotactic radiosurgery: an unexpected increase in clinically significant brain edema. Neuro-Oncol (2014) 16(7):1006–9. doi: 10.1093/neuonc/not329
96. Donaghy H. Effects of antibody, drug and linker on the preclinical and clinical toxicities of antibody-drug conjugates. mAbs (2016) 8(4):659–71. doi: 10.1080/19420862.2016.1156829
97. Ogitani Y, Aida T, Hagihara K, Yamaguchi J, Ishii C, Harada N, et al. DS-8201a, A novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin Cancer Res (2016) 22(20):5097–108. doi: 10.1158/1078-0432.CCR-15-2822
98. Ogitani Y, Hagihara K, Oitate M, Naito H, Agatsuma T. Bystander killing effect of DS -8201a, a novel anti-human epidermal growth factor receptor 2 antibody–drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci (2016) 107(7):1039–46. doi: 10.1111/cas.12966
99. Cortés J, Kim SB, Chung WP, Im S-A, Park YH, Hegg R, et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N Engl J Med (2022) 386(12):1143–54. doi: 10.1056/NEJMoa2115022
100. Modi S, Saura C, Yamashita T, Park YH, Kim S-B, Tamura K, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med (2020) 382(7):610–21. doi: 10.1056/NEJMoa1914510
101. Hurvitz S, Kim SB, Chung WP, Im S-A, Park YH, Hegg R, et al. Abstract GS3-01: Trastuzumab deruxtecan (T-DXd; DS-8201a) vs. trastuzumab emtansine (T-DM1) in patients (pts) with HER2+ metastatic breast cancer (mBC): subgroup analyses from the randomized phase 3 study DESTINY-Breast03. Cancer Res (2022) 82(Suppl 4):GS3–01. doi: 10.1158/1538-7445.SABCS21-GS3-01
102. Hurvitz SA, Hegg R, Chung WP, Im S-A, Jacot W, Ganju V, et al. Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet (2023) 401(10371):105–17. doi: 10.1016/S0140-6736(22)02420-5
103. Pérez-García JM, Batista MV, Cortez P, Ruiz-Borrego M, Cejalvo JM, de la Haba-Rodriguez J, et al. Trastuzumab deruxtecan in patients with central nervous system involvement from HER2-positive breast cancer: the DEBBRAH trial. Neuro-Oncol (2023) 25:157–66. doi: 10.1093/neuonc/noac144
104. Bartsch R, Berghoff AS, Furtner J, Marhold M, Bergen ES, Roider-Schur S, et al. Trastuzumab deruxtecan in HER2-positive breast cancer with brain metastases: a single-arm, phase 2 trial. Nat Med (2022) 28(9):1840–7. doi: 10.1038/s41591-022-01935-8
105. Yamanaka T, Niikura N, Nomura H, Kusama H, Yamamoto M, Matsuura K, et al. Abstract PD7-01: Trastuzumab deruxtecan for the treatment of patients with HER2-positive breast cancer with brain and/or leptomeningeal metastases: A multicenter retrospective study (ROSET-BM study). Cancer Res (2023) 83(Suppl 5):PD7–01. doi: 10.1158/1538-7445.SABCS22-PD7-01
106. Bachelot T, Romieu G, Campone M, Diéras V, Cropet C, Dalenc F, et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol (2013) 14(1):64–71. doi: 10.1016/S1470-2045(12)70432-1
107. Freedman RA, Gelman RS, Anders CK, Melisko ME, Parsons HA, Cropp AM, et al. TBCRC 022: A phase II trial of neratinib and capecitabine for patients with human epidermal growth factor receptor 2–positive breast cancer and brain metastases. J Clin Oncol (2019) 37(13):1081–9. doi: 10.1200/JCO.18.01511
108. Lin NU, Borges V, Anders C, Murthy RK, Paplomata E, Hamilton E, et al. Intracranial efficacy and survival with tucatinib plus trastuzumab and capecitabine for previously treated HER2-positive breast cancer with brain metastases in the HER2CLIMB trial. J Clin Oncol (2020) 38(23):2610–9. doi: 10.1200/JCO.20.00775
109. Murthy RK, Loi S, Okines A, Paplomata E, Hamilton E, Hurvitz SA, et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N Engl J Med (2020) 382(7):597–609. doi: 10.1056/NEJMoa1914609
110. Curigliano G, Mueller V, Borges V, Hamilton E, Hurvitz S, Loi S, et al. Tucatinib versus placebo added to trastuzumab and capecitabine for patients with pretreated HER2+ metastatic breast cancer with and without brain metastases (HER2CLIMB): final overall survival analysis. Ann Oncol (2022) 33(3):321–9. doi: 10.1016/j.annonc.2021.12.005
111. Lin NU, Murthy RK, Abramson V, Anders C, Bachelot T, Bedard PL, et al. Tucatinib vs placebo, both in combination with trastuzumab and capecitabine, for previously treated ERBB2 (HER2)-positive metastatic breast cancer in patients with brain metastases: updated exploratory analysis of the HER2CLIMB randomized clinical trial. JAMA Oncol (2023) 9(2):197–205. doi: 10.1001/jamaoncol.2022.5610
112. Yan F, Rinn KJ, Kullnat JA, Wu AY, Ennett MD, Scott EL, et al. Response of leptomeningeal metastasis of breast cancer with a HER2/neu activating variant to tucatinib: A case report. J Natl Compr Canc Netw (2022) 20(7):745–52. doi: 10.6004/jnccn.2022.7006
113. Saura C, Oliveira M, Feng YH, Dai M-S, Chen S-W, Hurvitz SA, et al. Neratinib plus capecitabine versus lapatinib plus capecitabine in HER2-positive metastatic breast cancer previously treated with ≥ 2 HER2-directed regimens: phase III NALA trial. J Clin Oncol (2020) 38(27):3138–49. doi: 10.1200/JCO.20.00147
Keywords: leptomeningeal metastasis, breast cancer, HER2, trastuzumab-deruxtecan, tucatinib
Citation: de Bernardi A, Bachelot T and Larrouquère L (2024) Long-term response to sequential anti-HER2 therapies including trastuzumab-deruxtecan in a patient with HER2-positive metastatic breast cancer with leptomeningeal metastases: a case report and review of the literature. Front. Oncol. 13:1210873. doi: 10.3389/fonc.2023.1210873
Received: 23 April 2023; Accepted: 13 December 2023;
Published: 10 January 2024.
Edited by:
Giuseppe La Rocca, Orthopedic and Head and Neck Sciences, Agostino Gemelli University Polyclinic (IRCCS), ItalyReviewed by:
Henry Kaplan, Swedish Cancer Institute, United StatesCopyright © 2024 de Bernardi, Bachelot and Larrouquère. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Louis Larrouquère, bG91aXMubGFycm91cXVlcmVAbHlvbi51bmljYW5jZXIuZnI=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.