
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol., 22 August 2023
Sec. Genitourinary Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1207536
This article is part of the Research TopicThe Mechanism of Tumor Evolution and Microenvironmental Changes of Genitourinary Oncology in Clinical Diagnosis and Treatment,volume IIView all 10 articles
 Jun Zhang1†
Jun Zhang1† Wen-Juan Wang1†
Wen-Juan Wang1† Li-Hong Chen1
Li-Hong Chen1 Ning Wang2
Ning Wang2 Ming-Wen Wang2
Ming-Wen Wang2 Hao Liu2
Hao Liu2 Li-Juan Pang1
Li-Juan Pang1 Han-Guo Jiang1
Han-Guo Jiang1 Yan Qi1*
Yan Qi1*Epithelioid angiomyolipoma (EAML) is a rare type of mesenchymal angiomyolipoma with potential malignancy in the kidney that can cause lymph node metastases, local recurrence, and distant metastases. Herein, we describe a case of EAML in the right kidney of a 51-year-old man who was admitted to the hospital with a right abdominal mass. Computed tomography revealed a heterogeneously enhanced mass with blurred margins, which was considered a malignant tumor. A radical nephrectomy was then performed. Two years later, the patient developed liver metastases from EAML and was administered sintilimab combined with bevacizumab. The patient survived after 6 months of follow-up. Histologically, the tumors showed clear boundaries and no obvious capsules. The tumor tissue mainly consisted of epithelioid tumor cells, thick-walled blood vessels, and a small amount of adipose tissue. Tumor cells with lipid vacuoles and acinar areas were large, round, polygonal, eosinophilic, or transparent in the cytoplasm. The enlarged and hyperchromatic nuclei were accompanied by distinct nucleoli and pathological mitosis. These histopathological findings resembled those of renal cell carcinoma, and immunohistochemical analysis was performed. The tumor cells were diffusely positive for HMB45, Melan-A, CK20, vimentin antibodies, and TFE3, suggesting that the tumor originated from perivascular epithelioid cells, excluding renal cell carcinoma. The Ki-67 index was 10%. These histopathological features were observed in liver mass puncture tissues. We also summarized 46 cases of EAML with distant metastasis and explored the clinicopathological features of EAML to improve the treatment of the disease. EAML is often ignored in the clinical setting, leading to metastasis and recurrence. Therefore, EAMLs require long-term follow-up, and timely detection of recurrent disease can improve the prognosis.
Renal angiomyolipoma (AML) belongs to the perivascular epithelioid tumor (PEComas) family of lesions, which originate in the mesenchymal tissue and are characterized by the coexpression of melanocytes (Melan) and muscle markers by perivascular clear and epithelioid cells (1). Epithelioid angiomyolipoma (EAML) is a rare and special subtype of AML. EAMLs have a major epithelioid component and potentially malignant behavior; they mainly consist of epithelioid cells with diverse morphology and abundant proliferation arranged in sheets, and the proportion of mature adipocytes tends to be <5% (2, 3). It can occur in the kidney, liver, bone, ileum, pelvic retroperitoneum, and most commonly in the kidney, with local invasion or metastasis. About one-third of patients may have lymph nodes, liver, lung, or spinal metastasis (4).
Recently, several cases of malignant EAML have been reported. Edmund et al. first reported a malignant EAML with liver metastasis in 2001 (5). However, only 17 studies have reported malignant EAML with liver metastasis at present (Table 1) (5–8, 11, 12, 16, 20–25, 27, 29, 32, 34). Additionally, there are no established criteria for predicting malignancy. The diagnosis of EAML may be challenging. In particular, the definite diagnosis of malignant EAML is established and confirmed by histological immunohistochemical findings owing to the similarity of its epithelioid morphology with that of renal cell carcinoma (RCC). Therefore, insights into the morphological characteristics and immunophenotype of this disease entity can aid in an accurate diagnosis.
A 51-year-old man with a history of a painless mass in the right upper abdomen for 20 days presented to the Department of Urology at Zhanjiang Central Hospital, Guangdong Medical University (Guangdong, China). A physical examination revealed a painless mass in the upper right abdomen. A computed tomography (CT) examination revealed a 13 cm × 9.7 cm × 13.8 cm heterogeneously enhanced mass with a blurred boundary in the right renal parenchyma. A high-density calcification shadow is observed. Radical right nephrectomy was performed for renal malignancy. Subsequent histological examination showed that the right kidney was 11 cm × 7 cm × 4 cm in size, with a fat capsule on the surface; the cut surface was gray-red and dark-red. No definite mass was observed in the renal parenchyma. A 14 cm × 13 cm × 8 cm mass with no capsule was linked to the kidney capsule. The tumor showed expansive growth and did not invade the perirenal fat. The section of the mass was reddish-gray yellow and soft in texture, and most necrotic changes were observed.
Resected tumor specimens were fixed in 10% neutral-buffered formalin and processed for immunohistochemistry using a standard protocol. Paraffin-embedded blocks were sectioned at a thickness of 5 μm and stained with hematoxylin–eosin and various antibodies. The antibody clones, working dilutions, and commercial sources are listed in Supplementary Table S1.
Microscopically, there was no fibrous membrane around the tumor tissue, which was clearly demarcated from the surrounding normal renal tissue at low power. The tumor tissue consisted of numerous epithelioid cells, smooth muscle cells, twisted thick-walled blood vessels (hyalinized vascular walls), and small amounts of adipose tissue. The staining of tumor cells was shallower than that of normal renal cells. They were unevenly distributed. Thick-walled blood vessels, adipose tissue, and fiberglass lesions were observed in the cell-sparse areas. A large number of epithelioid cells were observed in the cell-rich areas. The epithelioid tumor cells were arranged in tight sheets (Figures 1A–C). They were large, round, or polygonal with some adipose vacuolar and acinar areas and had abundant cytoplasm, which was stained eosinophilic or transparent (Figure 1D). The enlarged nuclei were deeply stained with distinct nucleoli. Necrotic and pathological mitosis were also observed (Figure 1E). Vacuolated and weird-type nuclei were also observed (Figure 1F). These morphologies were easily confused with the histological features of RCC. Therefore, we performed an immunohistochemical examination.
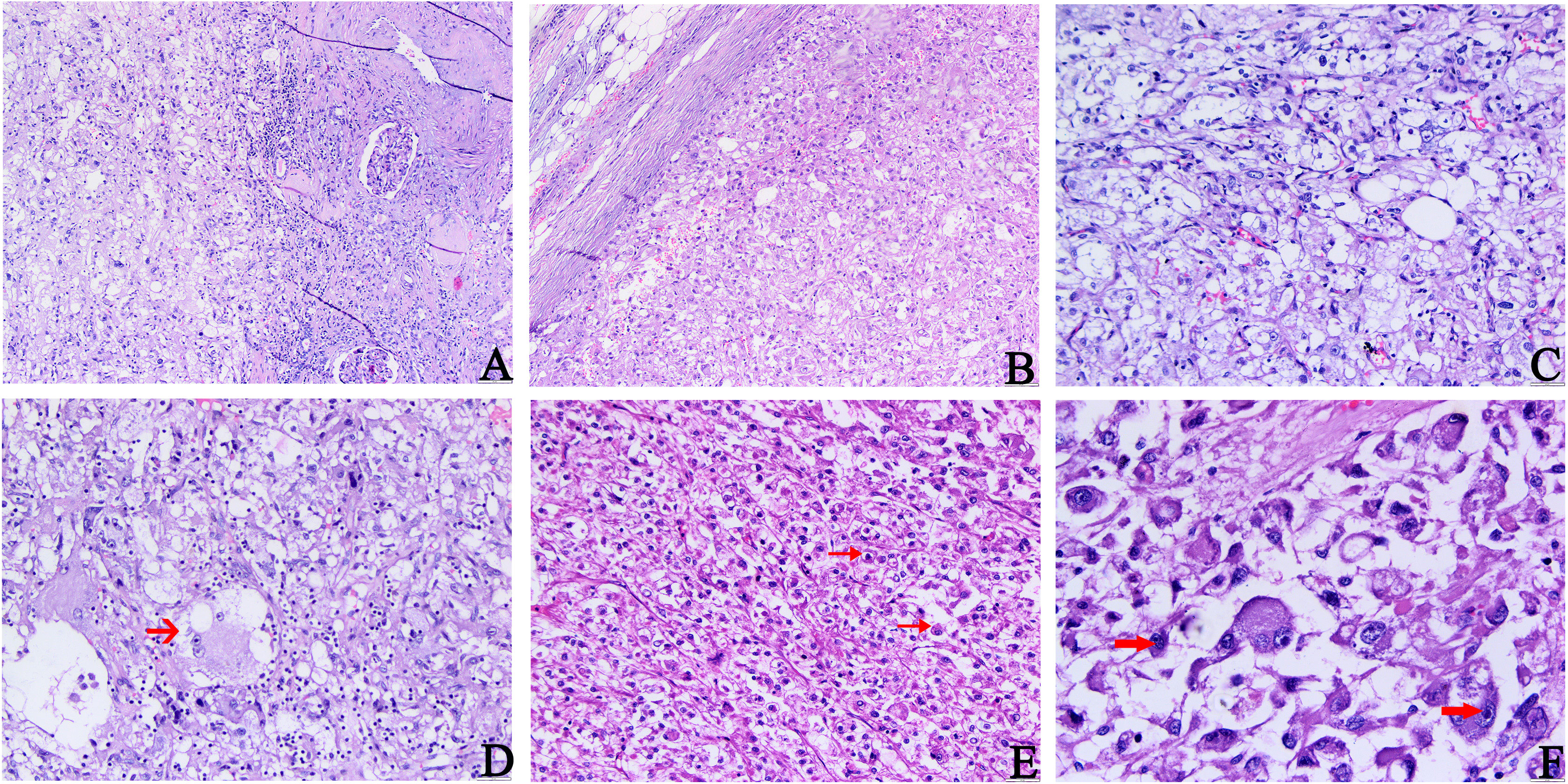
Figure 1 Histopathology features of primary tumors. (A) The boundary between tumor tissue and surrounding normal kidney tissue is not clear. (B) Epithelioid tumor cells were arranged in tight sheets. (C) Abundant epithelioid cells, a small amount of adipose tissue, and thick-walled blood vessels were seen in the tumor. (D–F) Epithelioid tumor cells are large and diverse, with some adipose vacuolar and acinar areas (indicated by the arrow in (D)), which are rich in eosinophilic or translucent cytoplasm. The nuclei were enlarged and deeply stained, partially vacuolated, and accompanied by obvious nucleoli (indicated by the arrow in (F)). The scattered megakaryocytes and occasionally pathological mitosis (indicated by the arrow in (E)) and necrosis could be observed (A, B H&E, ×100; C–E H&E, ×200; F, H&E, ×400).
Immunohistochemical staining revealed that the tumor cells were diffusively positive for human melanoma black 45 (HMB45), melan antigen (Melan-A), cytokeratin (CK) 20, and transcription factor enhancer 3 (TFE3), and partly positive for smooth muscle actin (SMA) (Figures 2A–E), which indicated that the tumor originated from peripheral vascular epithelioid cells, ruling out RCC. The tumor cells were diffusely vimentin-positive, and a Ki-67 index (Figure 2F) of 10% suggested that the tumor was malignant. The negative response of tumor cells to epithelial membrane antigen (EMA), PAX-8, CK, and CK7 excludes tumors of epithelial origin. The absence of melanocytes in the tumor tissue and the negative response of tumor cells to S100 and cluster of differentiation (CD) 117 precluded melanoma. Based on these pathological findings, the mass was confirmed as an EAML (Supplementary Table S1).
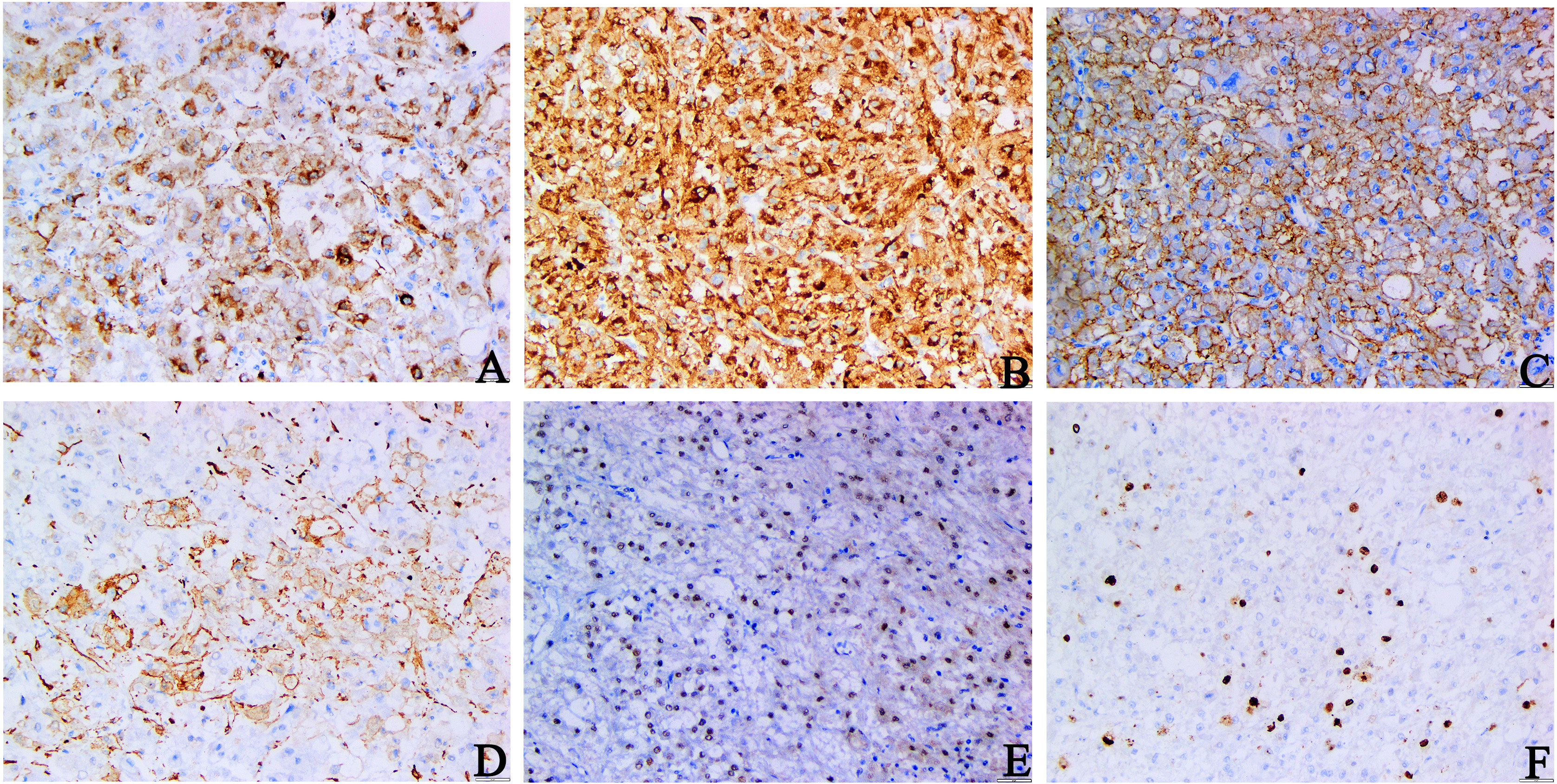
Figure 2 Immunohistochemical staining of primary tumors. (A–C) HMB45, Melan-A, CK20 staining: diffuse and strong positive cytoplasm of tumor cells. (D) SMA staining: tumor cells were positive. (E) TFE3 staining: tumor cell nuclear was positive. (F) Ki-67 staining: The Ki-67 index hit 10% (A–D, F original magnification is ×200; E original magnification is ×100).
The patient was not treated or followed up after surgery. Two years later, the patient was admitted to the hospital with right lumbago pain, chills, and a fever for more than 1 week. A physical examination revealed tenderness in the right lumbar region without pain. CT examination showed multiple nodules with unequal density in the right nephrectomy area and a mass of 13.9 cm × 12 cm × 12.6 cm mixed with a slightly low-density shadow in the right lobe of the liver with unclear boundary and heterogeneous enhancement, which was considered a metastatic tumor. A biopsy of a liver mass was carried out for a pathological examination. Microscopically, only a small number of tumor cells were observed in the liver biopsy samples. Significant atypical epithelioid tumor cells with hyperchromatic nuclei were observed. The morphological characteristics were similar to those of the primary lesion (Figures 3A–C). Combined with the history of malignant EAML and imaging findings, we suspected that the patient had developed liver metastases. To confirm this hypothesis, we performed an immunohistochemical examination, which suggested diffuse positivity of liver tumor cells for Melan-A (Figure 3D) and SMA (Figure 3E). Its proliferation index hit 5% (Figure 3F). These findings confirmed the diagnosis of EAML metastasis. The patient or his family members had no history of tuberous sclerosis (TSC) or other renal tumors. The patient received sintilimab combined with bevacizumab. The patient survived after 6 months of follow-up.
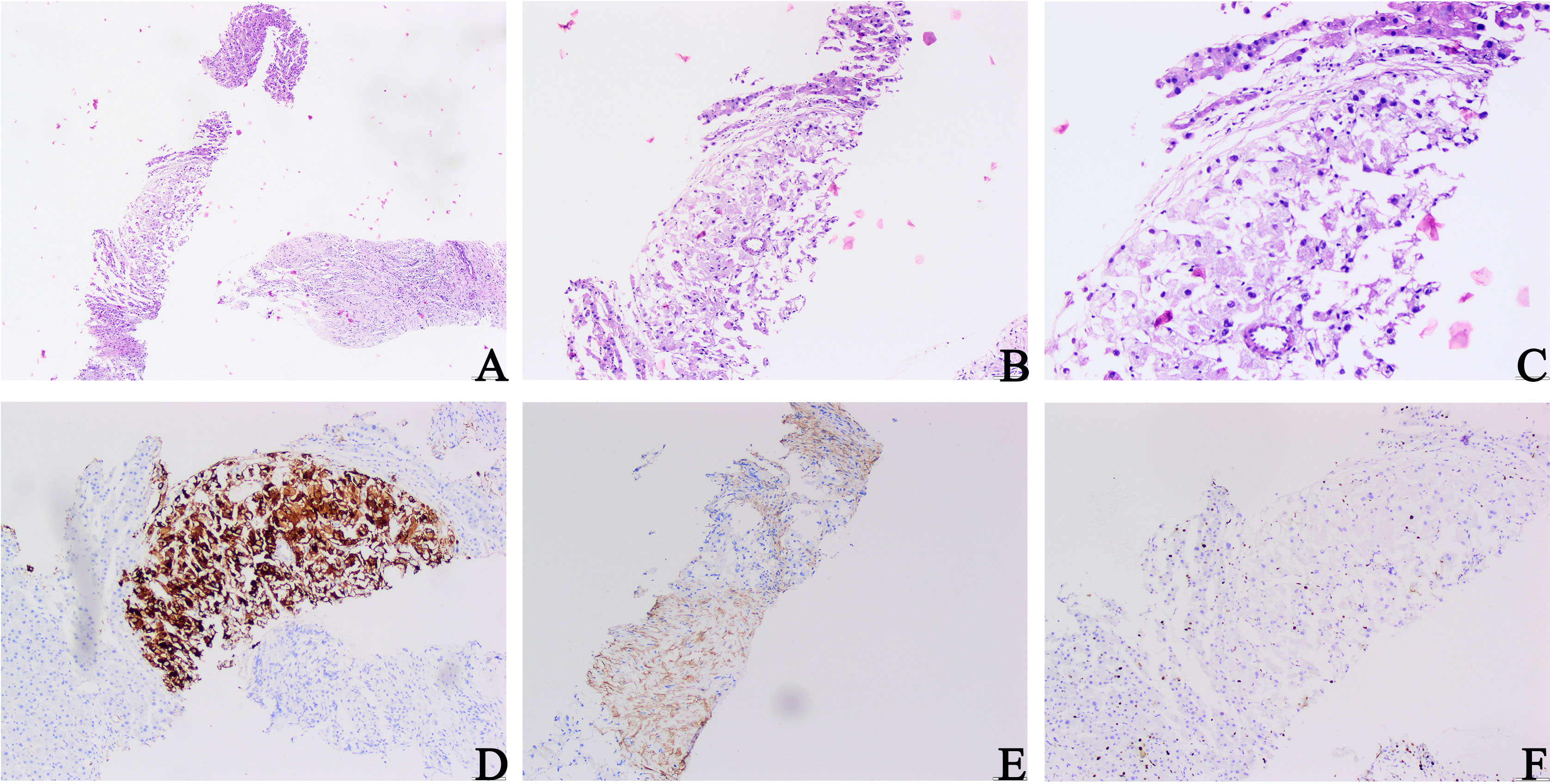
Figure 3 Microscopic architectural features of a liver mass biopsy. (A–C) Proliferating fibrous tissue, eosinophilic cytoplasm, and significantly hyperchromatic and enlarged nuclei of epithelioid tumor cells could be observed (H&E, A ×40; B ×100; C ×200). (D) Melan-A staining: diffuse and strong positive cytoplasm of tumor cells. (E) SMA staining: tumor cells were positive. (F) Ki-67 staining: the Ki-67 index hit 5% (D, F original magnification is ×200; E original magnification is ×100).
AML is a renal tumor that accounts for 2%–6.4% of all renal tumors (2, 3). Renal EAML, a subtype of AML, is a rare renal mesenchymal tumor with malignant potential that was first reported by Mai et al. (35). The development of renal AMLs may be associated with TSC. It is a systemic autosomal-dominant disease characterized by hamartomas of the lungs, skin, heart, brain, and kidney (36). It is usually caused by reduced or missing expression of the TSC1 (hamartin) or TSC2 (tuberin) genes (37). Renal AMLs are found in 80% of patients with TSC (38). Similarly, analysis of sporadic AMLs and EAMLs showed an association with TSC2 (9, 38, 39).
To date, only 46 cases of EAML with distant metastasis have been reported in the literature (Table 1) (5–34). Through a review of the literature, we found that these tumors occurred in people over 40 years of age (26/43, 60%, ranging from 7 to 78 years old; median, 49 years; mean, 44 years), and there was no significant difference in sex. The patients were mainly affected by unilateral kidney disease (21/28, 75%), with the left side being the most affected (12/19, 63%). The size of the EAML tumors excised from the kidneys ranged from 1 cm to 37 cm, with an average size of 14.89 cm. A total of 12 patients (12/46, 26%) had TSC. The most distant metastatic sites were the liver (28/46, 60%), lungs (19/46, 41%), and lymph nodes (10/46, 21%). Metastases to other sites, such as the pelvis, peritoneum, and rectus abdominis, are less common (Table 2). Some patients had signs of distant metastasis before the primary tumor was found, and the time range of distant metastasis ranged from 1 month to 12 years after primary tumor resection in most patients, with an average of 2.7 years. The longest postoperative survival time was 20 years (12), and the shortest was 2.5 months (32). We found that the efficacy of surgery or chemotherapy after EAML metastasis was unsatisfactory, with a poor prognosis and a low 5-year survival rate.
Most patients present with abdominal pain, hematuria, palpable masses, or clinical symptoms of EAML that metastasize to the lungs and cause fever, cough, and chest pain. A CT examination is of great significance in planning surgery and predicting patient prognosis, as it may help discover the location of tumors and determine whether they have metastasized. EAML tumors were large (usually >7 cm), and CT showed irregular mixed-density solid or multilocular mass shadows (usually >45 HU) with uneven enhancement and “fast in and slow out.” This phenomenon may be associated with higher cell density, reduced tumor stroma, abnormal hyperangiogenesis, the presence of intact tumor capsules, and the absence of tissue structures with reflux vessels (3). The imaging findings of the mass in our case showed an inhomogeneously enhanced mass, which was considered to be a malignant renal tumor, consistent with the above summary of imaging findings. However, the above results may be misdiagnosed as RCC or retroperitoneal sarcoma (40, 41). Therefore, further pathological examinations are required to confirm the diagnosis.
In addition to mature adipocytes, smooth muscle-like spindle cells, and clear, thick-walled blood vessels, EAML contains a variety of clear-to-eosinophilic and cytoplasmic epithelioid cells. There is no uniform standard for determining the number of epithelioid cells required for a final diagnosis of EAML. Current inclusion criteria range from 10% to 95%, whereas the new edition of the World Health Organization in 2016 recommends greater than 80% epithelioid cells as the diagnostic criteria for EAML (42). A precise diagnostic criterion is beneficial for identifying the characteristics of EAML as it can be used as a guide for clinical treatment. Additionally, the diagnosis of malignant renal EAML is controversial, and there are no unified malignant diagnostic criteria. With the accumulation of clinical cases in recent years, researchers have found that recurrence and metastasis rates of the disease are as high as 17% and 49%, respectively, and the mortality rate can reach 33% (43). Therefore, highly invasive biological behaviors and histological features should initially be considered malignant.
Lei (44) concluded that three or more of the following characteristics predicted an increased likelihood of malignancy: (1) necrosis, (2) tumor size >9 cm, (3) tumor thrombus formation in the vein, and (4) epithelioid cells >70% or atypical cells >60%. These criteria greatly aid in the accurate diagnosis of malignant EAML. The proportion of tumor epithelioid cells in our case was 80%, which was consistent with the diagnosis of EAML. The tumor cells showed obvious atypia and hyperchromatic nuclei, accompanied by evident nucleoli, pathological mitosis, and necrosis, consistent with the diagnosis of malignant EAML. Two years later, the patient developed EAML liver metastases, which further confirmed the diagnosis of a malignant tumor. However, when tumor nuclear atypia is evident and the adipose tissue content is significantly reduced, these tumors are most likely to be misdiagnosed as RCC or sarcoma. Therefore, the final diagnosis depends on immunohistochemical staining of the tumor. EAMLs exhibit specific immunohistochemical characteristics. The tumor cells are positive for melanoma-related markers such as HMB45, HMB50, Melan-A, SOX10, and myogenic markers such as SMA but negative for epithelial markers such as AE1/AE3 and EMA (45, 46). Compared with EAML, epithelial markers for RCC were positive, and melanocyte markers were negative. While EAML epithelial cell marker staining was negative, HMB45 and Melan-A staining were generally positive. These cells also expressed SMA. Staining for S-100 protein is usually negative. In this case, the tumor cells were diffuse, strongly positive for HMB45 and Melan-A, and negative for EMA, CK, CK7, and PAX-8, excluding the diagnosis of RCC (Supplementary Table S2) (47–50). Moreover, melanocytes in the tumor tissue and their negative responses to S100 and CD117 excluded the diagnosis of melanoma. To our surprise, our case was strongly positive for CK20 staining, which had not been seen in previous case reports. We reviewed relevant reports on the expression of CK20 in epithelioid tumors and found that epithelioid malignant mesothelioma (51) and malignant hepatic epithelioid hemangioendothelioma (52) could abnormally express CK20. Perhaps our case could serve as the first report of an anomalous EAML expression of CK20. Interestingly, the positive expression of TFE3 in our case can help clinicians adjust the follow-up visit treatment strategy.
Currently, the treatment options for EAML include surgery, chemotherapy, targeted therapy, endocrine therapy, and immunotherapy. Surgical excision is the primary treatment of choice for renal EAML (53). However, some patients experience recurrence or even distant metastasis after surgery. Malignant EAML is prone to tumor thrombus formation, which may cause distant metastasis. A study found that mutations in TSC1/TSC2 and translocations in TFE3 lead to overactivation of the mTOR complex (54). MTOR inhibitors inhibit mTOR activity to control tumors. However, there have been cases of limited efficacy of mTOR inhibitors in TFE3-rearranged malignant PEComas, and targeting VEGF/VEGFR signaling is probably a new effective treatment strategy for TFE3-associated malignant PEComas (54, 55). Therefore, it is important to determine whether TFE3 is positive to guide subsequent treatments. Lattanzi et al. first reported a case of malignant EAML with a TSC mutation and resistance to mTOR inhibitor therapy. After switching to PD-1 antibody therapy, the patient’s disease was effectively controlled, suggesting that PD-1 antibody therapy is a breakthrough in the treatment of anti-malignant EAML. Therefore, the standard treatment for EAML with distant metastases is mTOR inhibitor-targeted therapy (everolimus) combined with PD1 immunotherapy (bevacizumab), which is the most effective treatment strategy in the current study (56). However, its therapeutic effects on EAML relapse and distant metastasis remain unsatisfactory. As in our case, the clinicians and patients did not pay attention to long-term return visits and active treatment, which resulted in liver metastases. Therefore, long-term follow-up visits and active treatment are of great significance for detecting recurrence or metastasis as early as possible in EAML. Since there was no mTOR inhibitor available in our hospital, our patient was treated with bevacizumab (200 mg dL−1) combined with sintilimab (200 mg dL−1), and the patient and his family agreed to this treatment strategy. The treatment was effective, and the patient was stable.
EAML is a tumor of interstitial origin that expresses both myogenic and melanin markers. There is increasing evidence that it has malignant potential. Therefore, it is necessary to consider EAML other than RCC when dealing with intravascular thrombosis in renal tumors. The diagnosis is usually made based on histopathological examination; however, it is easily confused with other tumors, especially RCC. Immunohistochemical markers can be used for efficient differentiation to obtain an accurate diagnosis. Because of the risk of disease recurrence, which may occur very late, renal EAMLs require long-term follow-up to detect recurrence and metastasis as early as possible. In such cases, more efficient active treatment can be performed.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Zhanjiang Central Hospital, Guangdong Medical University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
JZ, M-WW and HL performed the experiments and analyzed the data; YQ designed and supervised the study. L-JP, H-GJ, NW and L-HC provided crucial input for the project; JZ, W-JW and YQ wrote the manuscript. All authors read and approved the final version of the manuscript.
This work was supported by grants from the National Natural Science Foundation of China (Grant No. 81860471), the Zhanjiang Science and Technology Development Special Fund Competitive Allocation Project—key projects of disease prevention and control (2021A05145), and the Provincial Science and Technology Special Fund (“college items + task list”) project—special topic of basic and applied research (2021A05236).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1207536/full#supplementary-material
1. Humphrey P, Moch H, Cubilla A, Ulbright T, Reuter V. The 2016 who classification of tumours of the urinary system and male genital organs-part B: prostate and bladder tumours. Eur Urol (2016) 70(1):106–19. doi: 10.1016/j.eururo.2016.02.028
2. Roberts S, Ladi-Seyedian S, Daneshmand S. Vena cava tumor thrombus associated with renal angiomyolipoma in a Jehovah's witness patient. Uro (2021) 156:e86–7. doi: 10.1016/j.urology.2021.08.005
3. Yang J, Liang C, Yang L. Advancements in the diagnosis and treatment of renal epithelioid angiomyolipoma: A narrative review. Kaohsiung J Med Sci (2022) 38(10):925–32. doi: 10.1002/kjm2.12586
4. Lee S, Lee J, Kim J, Ko S, Lee K, Hwang I, et al. Renal epithelioid angiomyolipoma with epithelial cysts mimicking cystic renal cell carcinoma: A case report of combination of two rare entities. J Korean Soc Radiol (2022) 83(5):1109–15. doi: 10.3348/jksr.2021.0172
5. Cibas E, Goss G, Kulke M, Demetri G. Fletcher CJTAjosp. Malignant epithelioid angiomyolipoma ('Sarcoma ex angiomyolipoma') of the kidney: A case report and review of the literature. Am J Surg Pathol (2001) 25(1):121–6. doi: 10.1097/00000478-200101000-00014
6. Radin R, Ma Y. Malignant epithelioid renal angiomyolipoma in a patient with tuberous sclerosis. %J journal of computer assisted tomography. J Comput Assist Tomogr (2001) 25(6). doi: 10.1159/000111739
7. Yamamoto T, Ito K, Suzuki K, Yamanaka H, Ebihara K, Sasaki A. Rapidly progressive Malignant epithelioid angiomyolipoma of the kidney. J Urol (2002) 168(1):190–1. doi: 10.1097/00005392-200207000-00046
8. Warakaulle D, Phillips R, Turner G, Davies D, Protheroe A. Malignant monotypic epithelioid angiomyolipoma of the kidney. J Clin Radiol (2004) 59(9):849–52. doi: 10.1016/j.crad.2004.02.009
9. Chuang C, Lin H, Tasi H, Lee K, Kao Y, Chuang F, et al. Clinical presentations and molecular studies of invasive renal epithelioid angiomyolipoma. Int Urol Nephrol (2017) 49(9):1527–36. doi: 10.1007/s11255-017-1629-4
10. Huang K, Huang C, Chung S, Pu Y, Shun C, Chen J. Malignant epithelioid angiomyolipoma of the kidney. J Formos Med Assoc (2007) 106:S51–4. doi: 10.1016/s0929-6646(09)60353-3
11. Sato K, Ueda Y, Tachibana H, Miyazawa K, Chikazawa I, Kaji S, et al. Malignant epithelioid angiomyolipoma of the kidney in a patient with tuberous sclerosis: an autopsy case report with P53 gene mutation analysis %J pathology - research and practice. PATHOL RES PRACT (2008) 204(10). doi: 10.1016/j.prp.2008.04.008
12. Nese N, Martignoni G, Fletcher C, Gupta R, Pan C, Kim H, et al. Pure epithelioid pecomas (So-called epithelioid angiomyolipoma) of the kidney: A clinicopathologic study of 41 cases: detailed assessment of morphology and risk stratification. Am J Surg Pathol (2011) 35(2):161–76. doi: 10.1097/PAS.0b013e318206f2a9
13. Moudouni S, Tligui M, Sibony M, Doublet J, Haab F, Gattegno B, et al. Malignant epithelioid renal angiomyolipoma involving the inferior vena cava in a patient with tuberous sclerosis. J Uro Int (2008) 80(1):102–4. doi: 10.1159/000111739
14. Lee H-Y, Huang C-W, Li C-C, Wu W-C, Liu T-C, Wu C-C, et al. Malignant renal epithelioid angiomyolipoma with an inferior vena cava and right atrium thrombus %J urological science. Urol Sci (2012) 23(4). doi: 10.1016/j.urols.2012.10.009
15. Li J, Zhu M, Wang Y. Malignant epithelioid angiomyolipoma of the kidney with pulmonary metastases and P53 gene mutation. World J Surg Oncol (2012) 10:213. doi: 10.1186/1477-7819-10-213
16. Xi S, Chen H, Wu X, Jiang H, Liu J, Wu Q, et al. Malignant renal angiomyolipoma with metastases in a child. Int J Surg Pathol (2014) 22(2):160–6. doi: 10.1177/1066896913497395
17. Yang S-L, Zhao J-L, Yan Z-J, liu J. Pulmonary metastatic renal epithelioid angiomyolipoma: A case report %J journal of practical cancer. J Pract Cancer (2013) 28(01):94.
18. Shi H, Cao Q, Li H, Zhen T, Lai Y, AJIjoc H, et al. Malignant perivascular epithelioid cell tumor of the kidney with rare pulmonary and ileum metastases. Int J Clin Exp Pathol (2014) 7(9):6357–63.
19. Wang M-H, Ling Y, Gao Q-Q, Huang Z-S, Wan M-Z, Chen Y-F, et al. Lung metastasis of renal epithelioid angiomyolipoma: A case report and literature review %J journal of clinical and pathological studies. J Clin Pathological Stud (2014) 34(03):326–9.
20. Konosu-Fukaya S, Nakamura Y, Fujishima F, Kasajima A, McNamara K, Takahashi Y, et al. Renal epithelioid angiomyolipoma with Malignant features: histological evaluation and novel immunohistochemical findings. Pathol Int (2014) 64(3):133–41. doi: 10.1111/pin.12142
21. Guo B, Song H, Yue J, Li G. Malignant renal epithelioid angiomyolipoma: A case report and review of the literature. Oncol Lett (2016) 11(1):95–8. doi: 10.3892/ol.2015.3846
22. Xiao Y-M, Liao H, Tan Z, Mao D, Wu Y, Li C. Clinical features and prognosis of renal epithelioid angiomyolipoma %J journal of oncology. J Oncol (2016) 22(07):579–82.
23. Shen B-H, Cheng G, Xie L-P eds. Malignant Epithelioid Renal Angiomyolipoma: A Case Report and Literature Review. In: 2009 Zhejiang Andrology and Urology Annual Conference. Jinyun, Zhejiang, China.
24. Park J, Lee C, Suh J, Kim G, Song B, Moon K. Renal epithelioid angiomyolipoma: histopathologic review, immunohistochemical evaluation and prognostic significance. Pathol Int (2016) 66(10):571–7. doi: 10.1111/pin.12458
25. Cho S, Choi H, Lee S. Rapidly progressing Malignant epithelioid renal angiomyolipoma: A case report. J Urol (2016) 13(2):2653–5.
26. Zhao R-R, Su Y-J. Lung metastasis of renal epithelioid angiomyolipoma: case report %J journal of clinical pulmonary science. J Clin Pulmonary Sci (2014) 19(07):1357–8.
27. Zhan R, Li Y, Chen C, Hu H, Zhang CJM. Primary kidney Malignant epithelioid angiomyolipoma: two cases report and review of literature. Med (Baltim) (2018) 97(32):e11805. doi: 10.1097/md.0000000000011805
28. Wang H, Zhan H, Yao Z, Liu Q. Malignant renal epithelioid angiomyolipoma with tfe3 gene amplification mimicking renal carcinoma. J Clin Nephrol Case Stud (2018) 6:11–5. doi: 10.5414/cncs109443
29. De Bree E, Stamatiou D, Chryssou E, Michelakis D, Tzardi MJM. Late local, peritoneal and systemic recurrence of renal angiomyolipoma: A case report. Mol Clin Oncol (2019) 10(1):43–8. doi: 10.3892/mco.2018.1755
30. PE J, Arden P, Ryan P, Patricia V, Sarah P. Epithelioid angiomyolipoma metastasis to the rectus abdominis. Can J Uro (2018) 25(5).
31. Erickson L. Malignant epithelioid angiomyolipoma of the kidney (Malignant perivascular epithelioid cell neoplasm). Mayo Clin Proc (2020) 95(1):205–6. doi: 10.1016/j.mayocp.2019.11.015
32. Gupta C, Malani A, Gupta V, Singh J, Ammar H. Metastatic retroperitoneal epithelioid angiomyolipoma. Autops Case Rep (2007) 60(4):428–31. doi: 10.1136/jcp.2006.039503
33. Al Umairi R, Al Shamsi R, Kamona A, Al Lawati F, Baqi S, Kurian G, et al. Renal epithelioid angiomyolipoma: A case report and review of literature. Oman Med J (2020) 35(5):e178. doi: 10.5001/omj.2020
34. Tessone I, Lichtbroun B, Srivastava A, Tabakin A, Polotti C, Groisberg R, et al. Massive Malignant epithelioid angiomyolipoma of the kidney. J Kidney Cancer VHL (2022) 9(2):13–8. doi: 10.15586/jkcvhl.v9i2.210
35. Martignoni G, Pea M, Rigaud G, Manfrin E, Colato C, Zamboni G, et al. Renal angiomyolipoma with epithelioid sarcomatous transformation and metastases: demonstration of the same genetic defects in the primary and metastatic lesions. Am J Surg Pathol (2000) 24(6):889–94. doi: 10.1097/00000478-200006000-00017
36. Lam H, Siroky B, Henske E. Renal disease in tuberous sclerosis complex: pathogenesis and therapy. Nat Rev Nephrol (2018) 14(11):704–16. doi: 10.1038/s41581-018-0059-6
37. Cai Y, Guo H, Wang W, Li H, Sun H, Shi B, et al. Assessing the outcomes of everolimus on renal angiomyolipoma associated with tuberous sclerosis complex in China: A two years trial. Orphanet J Rare Dis (2018) 13(1):43. doi: 10.1186/s13023-018-0781-y
38. Sauter M, Belousova E, Benedik M, Carter T, Cottin V, Curatolo P, et al. Rare manifestations and Malignancies in tuberous sclerosis complex: findings from the tuberous sclerosis registry to increase disease awareness (Tosca). Orphanet J Rare Dis (2021) 16(1):301. doi: 10.1186/s13023-021-01917-y
39. Henske E, Neumann H, Scheithauer B, Herbst E, Short M, Kwiatkowski D. Loss of heterozygosity in the tuberous sclerosis (Tsc2) region of chromosome band 16p13 occurs in sporadic as well as tsc-associated renal angiomyolipomas. Genes Chromosomes Cancer (1995) 13(4):295–8. doi: 10.1002/gcc.2870130411
40. Luo C, Liu Z, Gao M, Hu Q, He X, Xi Y, et al. Renal epithelioid angiomyolipoma: computed tomography manifestation and radiologic-pathologic correlation depending on different epithelioid component percentages. Abdom Radiol (NY) (2022) 47(1):310–9. doi: 10.1007/s00261-021-03313-3
41. Caliò A, Brunelli M, Segala D, Pedron S, Tardanico R, Remo A, et al. T(6;11) renal cell carcinoma: A study of seven cases including two with aggressive behavior, and utility of cd68 (Pg-M1) in the differential diagnosis with pure epithelioid pecoma/epithelioid angiomyolipoma. Mod Pathol (2018) 31(3):474–87. doi: 10.1038/modpathol.2017.144
42. Bansal A, Goyal S, Goyal A, Jana M. Who classification of soft tissue tumours 2020: an update and simplified approach for radiologists. Eur J Radiol (2021) 143:109937. doi: 10.1016/j.ejrad.2021.109937
43. Stock K, Slotta-Huspenina J, Kübler H, Autenrieth M. [Innovative ultrasound-based diagnosis of renal tumors]. Der Urologe Ausg A (2019) 58(12):1418–28. doi: 10.1007/s00120-019-01066-y
44. Lei J, Liu L, Wei Q, Song T, Yang L, Yuan H, et al. A four-year follow-up study of renal epithelioid angiomyolipoma: A multi-center experience and literature review. Sci Rep (2015) 5:10030. doi: 10.1038/srep10030
45. Kobayashi Y, Shimizu S, Arai H, Yoshida K, Honda M. Fat-poor leiomyomatous angiomyolipoma arising from renal parenchyma negative for hmb-45: A case report. Clin Case Rep (2022) 10(12):e6771. doi: 10.1002/ccr3.6771
46. Stone C, Lee M, Amin M, Yaziji H, Gown A, Ro J, et al. Renal angiomyolipoma: further immunophenotypic characterization of an expanding morphologic spectrum. Arch Pathol Lab Med (2001) 125(6):751–8. doi: 10.5858/2001-125-0751-ra
47. Zheng S, Bi X, Song Q, Yuan Z, Guo L, Zhang H, et al. A suggestion for pathological grossing and reporting based on prognostic indicators of Malignancies from a pooled analysis of renal epithelioid angiomyolipoma. Int Urol Nephrol (2015) 47(10):1643–51. doi: 10.1007/s11255-015-1079-9
48. Liu L, Feng Y, Guo C, Weng S, Xu H, Xing Z, et al. Multi-center validation of an immune-related lncrna signature for predicting survival and immune status of patients with renal cell carcinoma: an integrating machine learning-derived study. J Cancer Res Clin Oncol (2023). doi: 10.1007/s00432-023-05107-0
49. Liu C, Sunday H, Jing Z. Imaging diagnosis of renal epithelioid angiomyolipoma. J Med Imaging (Chinese) (2017) 27(09):1800–2. doi: 10.1007/s00432-023-05107-0
50. Jia Z, Tang L, Liu K, Guo A, Huang Q, Peng C, et al. Venous tumor thrombus consistency: is it a prognostic factor of survival for patients with renal cell carcinoma? Updates Surg (2023). doi: 10.1007/s13304-023-01544-1
51. Manur R, Lamzabi I. Aberrant cytokeratin 20 reactivity in epithelioid Malignant mesothelioma: A case report. Appl immunohistochem Mol morphology AIMM (2019) 27(10):e93–e6. doi: 10.1097/pai.0000000000000504
52. Boukhris I, Azzabi S, Kéchaou I, Chérif E, Kooli C, Romdhane K, et al. [Hypercalcemia related to pth-rp revealing Malignant hepatic epithelioid hemangioendothelioma]. Annales biologie clinique (2016) 74(1):98–102. doi: 10.1684/abc.2015.1118
53. De la Sancha C, Khan S, Alruwaii F, Cramer H, Saxena R. Hepatic angiomyolipoma with predominant epithelioid component: diagnostic clues on aspiration and core needle biopsies. Diagn Cytopathol (2021) 49(7):E238–E41. doi: 10.1002/dc.24688
54. Sanfilippo R, Jones R, Blay J, Le Cesne A, Provenzano S, Antoniou G, et al. Role of chemotherapy, vegfr inhibitors, and mtor inhibitors in advanced perivascular epithelioid cell tumors (Pecomas). Clin Cancer Res (2019) 25(17):5295–300. doi: 10.1158/1078-0432.Ccr-19-0288
55. Liapi A, Mathevet P, Herrera F, Hastir D, Sarivalasis A. Vegfr inhibitors for uterine metastatic perivascular epithelioid tumors (Pecoma) resistant to mtor inhibitors. A case report and review of literature. Front Oncol (2021) 11:641376. doi: 10.3389/fonc.2021.641376
Keywords: renal epithelioid angiomyolipoma, liver metastasis, histopathology, immunohistochemistry, differential diagnosis
Citation: Zhang J, Wang W-J, Chen L-H, Wang N, Wang M-W, Liu H, Pang L-J, Jiang H-G and Qi Y (2023) Primary renal malignant epithelioid angiomyolipoma with distant metastasis: a case report and literature review. Front. Oncol. 13:1207536. doi: 10.3389/fonc.2023.1207536
Received: 17 April 2023; Accepted: 28 July 2023;
Published: 22 August 2023.
Edited by:
Wen-Hao Xu, Fudan University, ChinaCopyright © 2023 Zhang, Wang, Chen, Wang, Wang, Liu, Pang, Jiang and Qi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Qi, cWl5YW55YW4tMTk5OEAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.