- 1Department of Neonatology, Children’s Hospital, Zhejiang University School of Medicine, National Clinical Research Center for Child Health, Hangzhou, China
- 2Laboratory for Diagnosis and Therapy of Neonatal Diseases of Zhejiang Province, Hangzhou, China
Infantile fibrosarcoma (IFS) is a rare tumor in childhood characterized by a single, localized, painless mass that grows rapidly but has a relatively indolent biological behavior and a favorable prognosis. Eighty-five percent of infantile fibrosarcomas are associated with t (12;15) (p13;25) chromosomal translocation resulting in ETV6-NTRK3 gene fusion, which provides the target for targeted therapy. Here, we report a case of IFS in a newborn with a mass in the left lower extremity confirmed by imaging, histopathological examination, tissue FISH testing, and high-throughput sequencing to detect gene rearrangement. Based on gene fusion targeted drug testing results, the patient was treated with standard doses of larotrectinib, resulting in significant mass shrinkage with no adverse effects, demonstrating the treatment effect of targeted therapy. This case provides a reference for using larotrectinib in newborns with IFS.
1 Introduction
Infantile fibrosarcoma (IFS) is a malignant tumor originating from fibroblasts in fibrous tissue and is the most common soft tissue tumor in children under one year of age, with 40% of cases present at birth (1). No standard treatment for IFS; surgical resection combined with chemotherapy is recommended. Recent studies revealed that over 85% of children with IFS exhibit a t (12,15)(p13;25) chromosomal translocation (2). This translocation leads to the fusion of the ETV6-NTRK3 genes, synthesizing fusion proteins that drive tumor development. In recent years, treatment with larotrectinib has shown good responsiveness and safety in treating IFS (3–5), but its use in newborns is rare. This article reports a case of larotrectinib treatment for IFS in a newborn.
2 Case presentation
A male newborn, born at a gestational age of 39 weeks and four days, was found to have swelling in the left thigh, with limited mobility at birth, and was admitted to Children’s Hospital, Zhejiang University School of Medicine. Physical examination revealed swelling in the left lower extremity, with a circumference of 29 cm (Figure 1A), local skin temperature, and limited mobility, while the right lower extremity had a circumference of 20 cm with normal mobility. After admission, magnetic resonance imaging (MRI) and enhanced CT scans of the lower limb were complete, and the results revealed a soft tissue mass in the thigh, measuring approximately 8.25 cm × 8.72 cm × 7.86 cm, with possible hemorrhage (Figures 2A, B. Subsequently, an ultrasound-guided fine-needle biopsy of the left thigh mass was performed under general anesthesia, and the histopathological results (Figure 3) suggested a soft tissue tumor with spindle cells. Immunohistochemical staining with monoclonal antibodies showed Desmin (weakly positive), CD34 (partially positive), EMA (focally positive), Pan-Trk (weakly positive), Vimentin (positive), INI1 (present), BRG1 (present), CD56 (partially positive), MyoD1 (negative), CD99 (positive), CK (AE1/AE3) (focally positive), Myogenin (negative), Ki-67 (approximately 30% positive), and SMA (positive) (Figure 4). Fluorescence in situ hybridization (FISH) revealed ETV6 gene breakage (Figure 5). High-throughput sequencing for gene rearrangement detection and targeted drug testing showed ETV6-NTRK3 rearrangement, and larotrectinib, entrectinib, and crizotinib were sensitive. The final diagnosis was infantile fibrosarcoma. At 20 days of age, oral larotrectinib (Vitrakvi, 50 ml/1.0 g) was started at a dose of 22 mg/dose twice a day (3.64 kg body weight, 100 mg/m2, twice a day). After ten days of treatment, the circumference of the left thigh decreased to 24.5 cm (Figure 1B). One month of follow-up showed a further reduction in circumference to 20 cm (Figure 1C), and ultrasound B revealed a mass size of 6.0 cm × 5.0 cm × 3.0 cm.
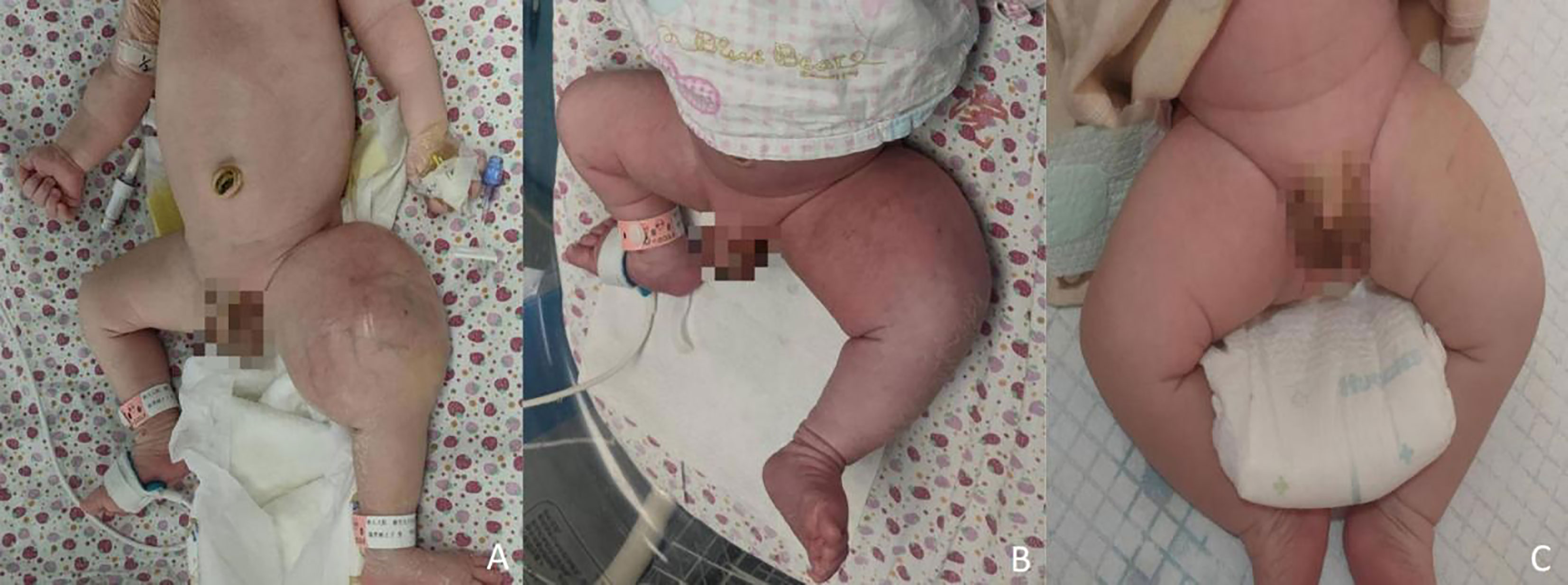
Figure 1 Changes in the left lower limb mass. (A) before treatment; (B) after ten days; (C) after one month.

Figure 2 Imaging results. (A) MRI examination of the lower limb: a circular soft tissue mass in the left lower limb, measuring 8.25cm x 8.72cm x 7.86cm, closely adjacent to the femur and surrounding it, with unclear demarcation from the surrounding muscle tissue, and mixed-signal intensity in the mass. (B) enhanced CT of the lower limb: the mass is mainly supplied by the left femoral artery, with flux into the femoral vein, and many tortuous vessels are visible within the lesion. (C, D) MRI of the lower extremity revealed a significant mass reduction after four months of treatment.
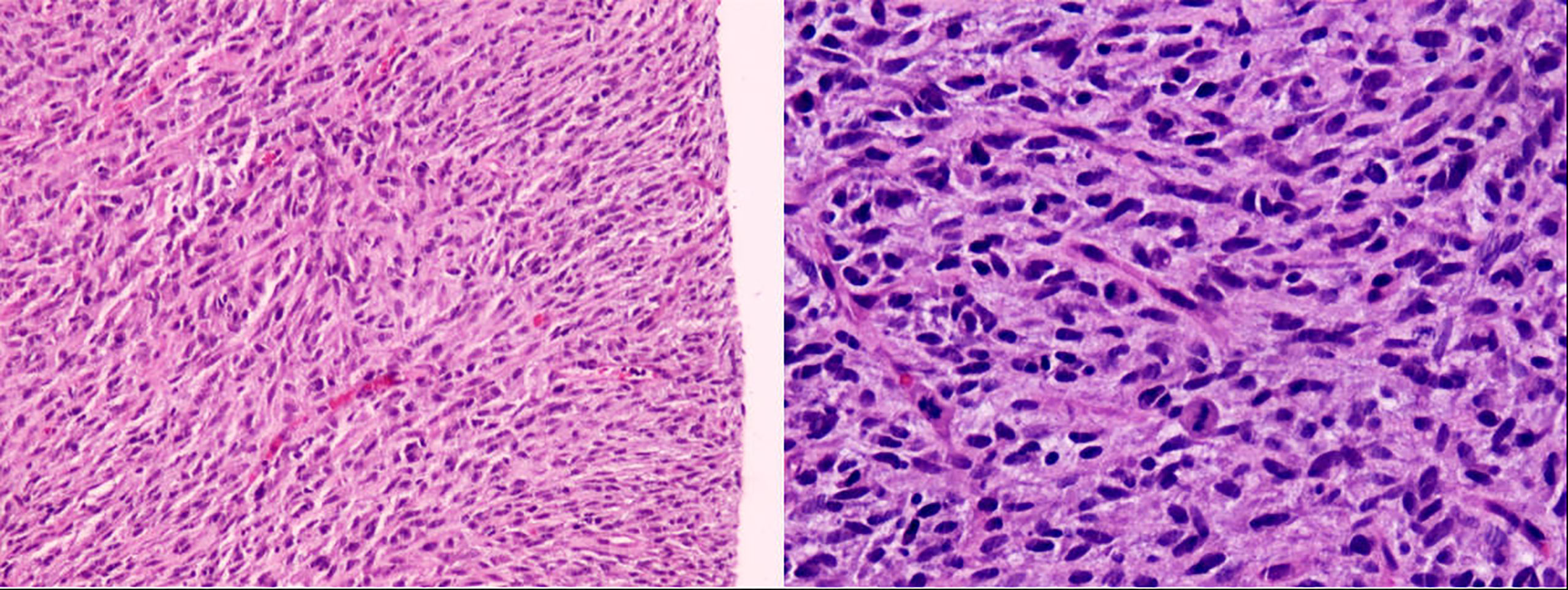
Figure 3 Pathology (microscopic examination shows spindle-shaped tumor cells with eosinophilic cytoplasm and frequent mitotic figures).
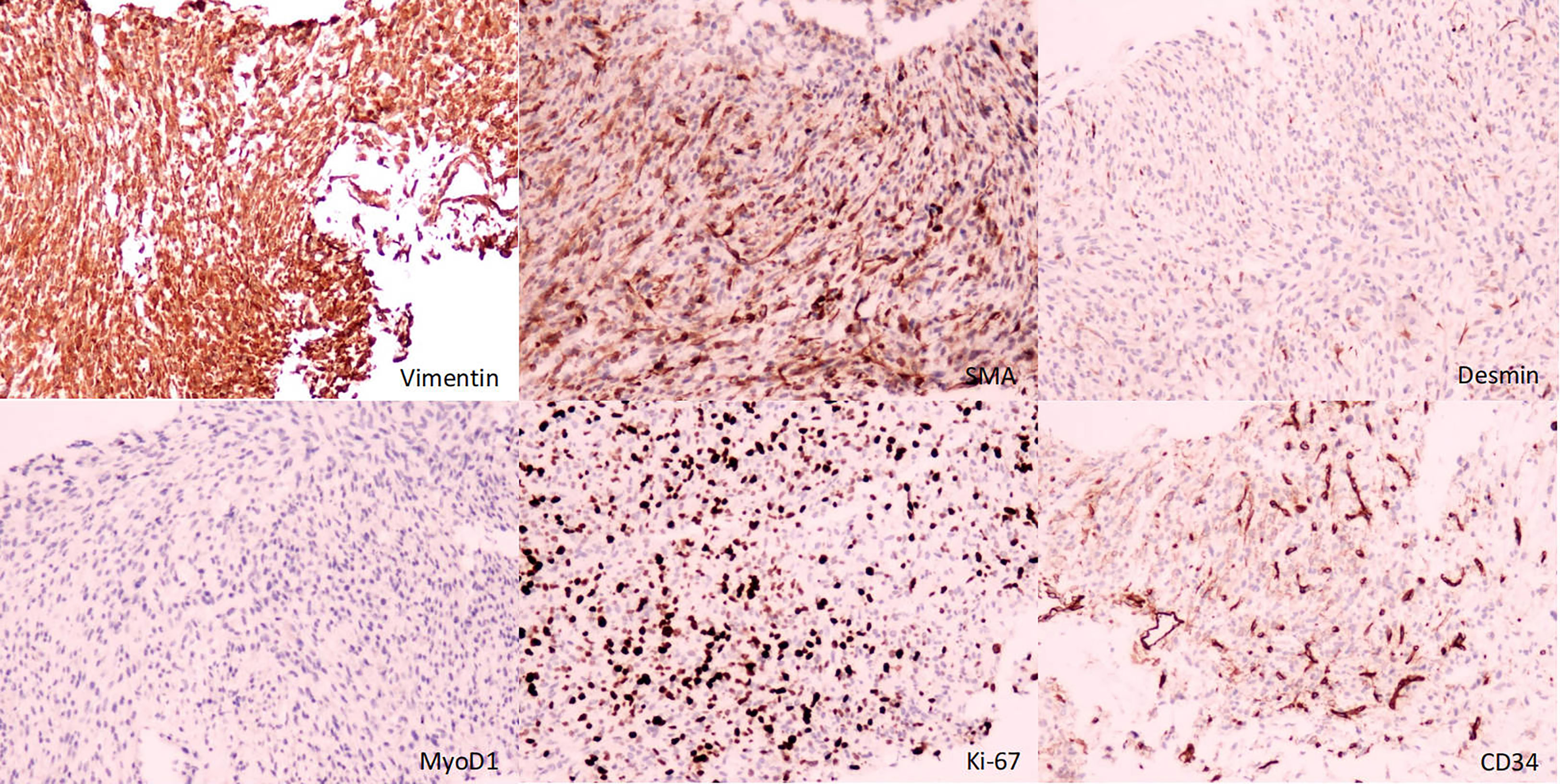
Figure 4 Immunohistochemistry (immunohistochemical monoclonal antibodies to Vimentin, SMA, Desmin, MyoD1, Ki-67, and CD34, respectively).
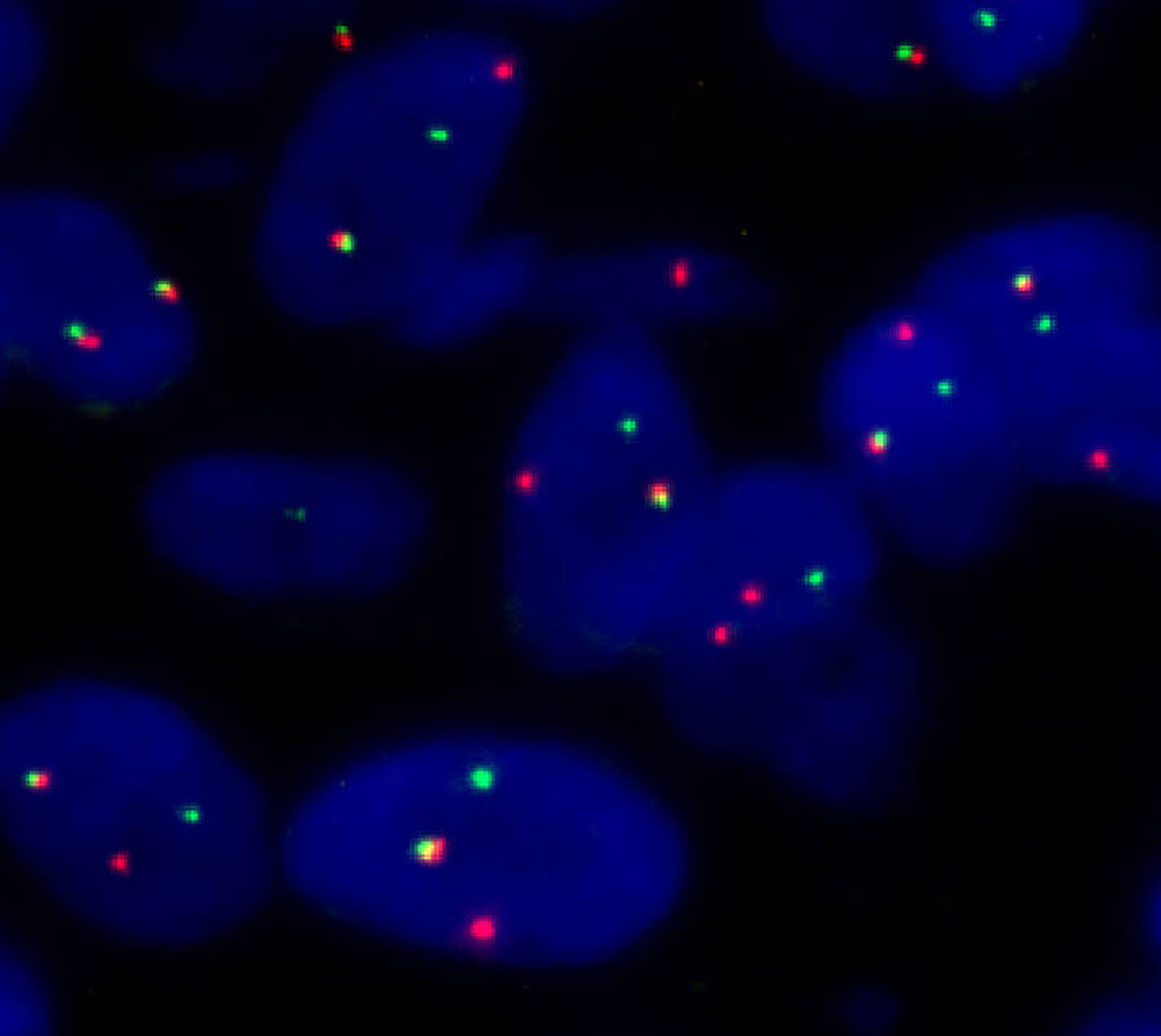
Figure 5 Fluorescence in situ hybridization (FISH) (separate red and green signals revealed ETV6 breakage).
The patient has been followed for four months, and the tumor has shrunk significantly. But it still wrapped around the femur, and the boundary with the surrounding muscles is unclear(Figures 2C, D). The efficacy of larotinib therapy and the intricate nature of the tumor have prompted us to abandon any surgical approach at this time and maintain medical care until the tumor vanishes. We only considered surgery when larotinib resistance surfaced during treatment.
3 Discussion
IFS is a rare tumor that often presents as a single localized painless mass with rapid growth, most commonly found in the extremities and trunk and less commonly in the oral cavity, head and neck, colon, jejunum, periorbital, abdomen, and other sites (6–9). In this case, the mass of the newborn is located in the left lower limb, consistent with previous literature reports (10).
IFS must be differentiated from fibromatosis, hemangioendothelioma, rhabdomyosarcoma, lymphangioma, and neuroblastoma, but it isn’t easy to differentiate them based only on imaging examinations. We improved an aspiration biopsy of the lesion and histopathological examination to confirm the diagnosis, which showed spindle cells arranged in a fishbone-like pattern, with a high mitotic rate and focal necrosis visible under the microscope. Immunohistochemistry showed diffuse expression of Vimentin, minimal expression, or almost no expression of Desmin, CD34, and MyoD1. Tissue FISH examination confirmed the presence of ETV6 breakage, and high-throughput sequencing for gene rearrangement detection showed positive results for the ETV6-NTRK3 fusion gene. The detections mentioned above confirmed the diagnosis of IFS. However, it should be noted that the ETV6-NTRK3 fusion gene is not a completely specific marker for IFS, as this fusion gene has also been reported in patients with congenital mesoblastic nephroma, secretory carcinoma of salivary glands, secretory breast carcinoma, thyroid carcinoma, glioma, and some hematological disorders (11–16).
The current first-line infantile fibrosarcoma (IFS) treatment combines chemotherapy and surgery. However, chemotherapy can induce bone marrow suppression (17). Alkylating agents can cause renal and gonadal damage, while anthracycline drugs can lead to cardiac toxicity (10). Moreover, there are unique differences in the absorption, distribution, metabolism, and excretion of chemotherapy drugs in newborns, making the use of chemotherapy complex (18). Furthermore, IFS tumors are closely associated with adjacent blood vessels, nerves, or bones, posing a high risk of disability if complete surgical resection is attempted. Considering these factors, targeted therapy was used for a newborn with the ETV6-NTRK3 fusion gene. This fusion gene drives the expression of a fusion protein with sustained tyrosine kinase activity, acting as a driving factor in continuous downstream signal transduction, oncogenic transformation, and tumor growth (19). Larotrectinib, a tyrosine kinase inhibitor that works by blocking the altered fusion protein, is applicable for treating all types of solid tumors with NTRK gene fusion. The study demonstrated an overall response rate of 94% in paediatric patients with solid tumors containing fusions of the NTRK gene who received larotrectinib (20). Increasing evidence also supports the clinical benefits associated with larotrectinib (3, 4, 21, 22). However, caution should be exercised due to the potential presence of publication bias in the available reports. Treatment with standard doses of larotrectinib (100 mg/m2, twice a day) was started at 20 days of age, and after one month of treatment, the tumor size decreased from 8.25 cm × 8.72 cm × 7.86 cm to 6.0 cm × 5.0 cm × 3.0 cm. By the fourth month of treatment, the tumor had significantly decreased in size; however, an accurate measurement could not be obtained due to unclear demarcation between the tumor and surrounding muscle tissue. In summary, larotrectinib demonstrated favorable therapeutic efficacy in this patient, providing further evidence for its application in neonatal infantile fibrosarcoma.
Larotrectinib has been demonstrated to tolerate in pediatric patients, with most experiencing grade 1 or 2 adverse events. The most commonly observed adverse reactions include elevated alanine aminotransferase levels, leukopenia, neutropenia, vomiting, and other adverse events such as anemia, constipation, hypoalbuminemia, cough, and weight gain (5, 23). In this particular case, the patient has been followed up to date, and no adverse reactions have been observed. However, due to the relatively short duration of its current use, the long-term effects of larotrectinib on the growth and development of children have not been definitively determined.
A literature search using the keywords “infantile fibrosarcoma” and “larotrectinib” in the PubMed, Web of Science, and Embase databases revealed that this case is the second reported case of the use of larotrectinib for IFS in newborns since Caldwell KJ’s report (24). However, unlike Caldwell KJ’s case, larotrectinib was started at standard doses in this case rather than starting with a lower dose (1 mg/kg, twice a day) before escalating to standard doses. In our case, the visible deformity was no longer observed after one month, whereas Caldwell KJ’s reported case still had small deformities visible after six cycles. It is unknown whether the potential difference in dosage of Laroctinib is related to factors other than the size of the tumor itself. Our report suggests that standard doses of larotrectinib are safe for newborns with IFS.
Several literature reports on the issue of resistance to larotrectinib (5, 25, 26). The follow-up period for this case is relatively short, and the development of drug resistance is currently unclear. A longer follow-up period is necessary to gain more insight, highlighting a limitation of this particular case.
4 Conclusions
Pathological diagnosis is the gold standard for diagnosing IFS, and the ETV6-NTRK3 fusion gene helps confirm the diagnosis. Treatment with standard doses of larotrectinib is safe for newborns with IFS.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
DW, FZ and WF reviewed the literature and contributed to manuscript drafting. TY and JP were responsible for revising the manuscript for important intellectual content. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chung EB, Enzinger FM. Infantile fibrosarcoma. Cancer (1976) 38(2):729–39. doi: 10.1002/1097-0142(197608)38:2<729::AID-CNCR2820380216>3.0.CO;2-Z
2. Demetri GD, Antonescu CR, Bjerkehagen B, Bovée JVMG, Boye K, Chacón M, et al. Diagnosis and management of tropomyosin receptor kinase (TRK) fusion sarcomas: expert recommendations from the World Sarcoma Network. Ann Oncol (2020) 31(11):1506–17. doi: 10.1016/j.annonc.2020.08.2232
3. Corral Sánchez MD, Jiménez Carrascoso R, Rubio Aparicio P, Plaza López de Sabando D, Sastre Urgelles A, Pozo-Kreilinger JJ, et al. Therapeutic strategies and clinical evolution of patients with infantile fibrosarcoma: a unique paediatric case series. Clin Transl Oncol (2023). doi: 10.1007/s12094-023-03175-9
4. Bielack SS, Cox MC, Nathrath M, Apel K, Blattmann C, Holl T, et al. Rapid, complete and sustained tumour response to the TRK inhibitor larotrectinib in an infant with recurrent, chemotherapy-refractory infantile fibrosarcoma carrying the characteristic ETV6-NTRK3 gene fusion. Ann Oncol (2019) 30(Suppl_8):viii31–viii5. doi: 10.1093/annonc/mdz382
5. Laetsch TW, DuBois SG, Mascarenhas L, Turpin B, Federman N, Albert CM, et al. Larotrectinib for paediatric solid tumours harbouring NTRK gene fusions: phase 1 results from a multicentre, open-label, phase 1/2 study. Lancet Oncol (2018) 19(5):705–14. doi: 10.1016/S1470-2045(18)30119-0
6. Kotb M, Aboelela A, Eshiba A, Sheta E, Abdallah D. Jejunal infantile fibrosarcoma: An unusual cause of neonatal intestinal obstruction. Congenit Anom (Kyoto). (2021) 61(5):199–201. doi: 10.1111/cga.12433
7. Manta AI, Vittorio A, Sullivan TJ. Long term follow-up of congenital infantile fibrosarcoma of the orbital region. Orbit (2022), :1–4. doi: 10.1080/01676830.2022.2067566
8. Sıvrıkoz TS, Uygu LS, Kunt İşgüder Ç, Aygun E, Kalelioglu IH, Has R. The giant infantile fibrosarcoma of fetal oropharynx and anterior neck. Fetal Pediatr Pathol (2022) 41(3):451–6. doi: 10.1080/15513815.2020.1809038
9. Parmar V, Peters RT, Cheesman E, Edi-Osagie N, Craigie RJ. Congenital infantile fibrosarcoma of the colon: a case series and literature review. Pediatr Surg Int (2014) 30(10):1079–85. doi: 10.1007/s00383-014-3589-4
10. Orbach D, Rey A, Cecchetto G, Oberlin O, Casanova M, Thebaud E, et al. Infantile fibrosarcoma: management based on the European experience. J Clin Oncol (2010) 28(2):318–23. doi: 10.1200/JCO.2009.21.9972
11. Church AJ, Calicchio ML, Nardi V, Skalova A, Pinto A, Dillon DA, et al. Recurrent EML4-NTRK3 fusions in infantile fibrosarcoma and congenital mesoblastic nephroma suggest a revised testing strategy. Mod Pathol (2018) 31(3):463–73. doi: 10.1038/modpathol.2017.127
12. Horowitz DP, Sharma CS, Connolly E, Gidea-Addeo D, Deutsch I. Secretory carcinoma of the breast: results from the survival, epidemiology and end results database. Breast (2012) 21(3):350–3. doi: 10.1016/j.breast.2012.02.013
13. Sun J, Li J, Tian Z, Zhang C, Xia R, He Y. Atypical/unbalanced ETV6/NTRK3 rearrangement in salivary secretory carcinoma with a focus on the incidence, the patterns, and the clinical implications. J Oral Pathol Med (2022) 51(8):721–9. doi: 10.1111/jop.13341
14. Leeman-Neill RJ, Kelly LM, Liu P, Brenner AV, Little MP, Bogdanova TI, et al. ETV6-NTRK3 is a common chromosomal rearrangement in radiation-associated thyroid cancer. Cancer (2014) 120(6):799–807. doi: 10.1002/cncr.28484
15. Biswas A, Rajesh Y, Das S, Banerjee I, Kapoor N, Mitra P, et al. Therapeutic targeting of RBPJ, an upstream regulator of ETV6 gene, abrogates ETV6-NTRK3 fusion gene transformations in glioblastoma. Cancer Lett (2022) 544:215811. doi: 10.1016/j.canlet.2022.215811
16. Kralik JM, Kranewitter W, Boesmueller H, Marschon R, Tschurtschenthaler G, Rumpold H, et al. Characterization of a newly identified ETV6-NTRK3 fusion transcript in acute myeloid leukemia. Diagn Pathol (2011) 6:19. doi: 10.1186/1746-1596-6-19
17. Othieno-Abinya NA, Waweru A, Nyabola LO. Chemotherapy induced myelosuppression. East Afr Med J (2007) 84(1):8–15. doi: 10.4314/eamj.v84i1.9485
18. Siegel SE, Moran RG. Problems in the chemotherapy of cancer in the neonate. Am J Pediatr Hematol Oncol (1981) 3(3):287–96.
19. Vaishnavi A, Le AT, Doebele RC. TRKing down an old oncogene in a new era of targeted therapy. Cancer Discovery (2015) 5(1):25–34. doi: 10.1158/2159-8290.CD-14-0765
20. van Tilburg CM, DuBois SG, Albert CM, Federman N, Nagasubramanian R, Geoerger B, et al. Larotrectinib efficacy and safety in pediatric TRK fusion cancer patients. Am Soc Clin Oncol (2019). doi: 10.1200/JCO.2019.37.15_suppl.10010
21. Lapeña LM, Caldas MCS, Ramírez C, Basilio MS, Junco PT, Rodríguez-Laguna L, et al. Larotrectinib as an effective therapy in congenital infantile fibrosarcoma: report of two cases. Eur J Pediatr Surg Rep (2022) 10(1):e76–e9. doi: 10.1055/s-0042-1748866
22. Corral Sánchez MD, Galán Gómez V, Sastre Urgelles A, Plaza López de Sabando D, Rubio Aparicio P, Martínez Martínez L, et al. Treatment of infantile fibrosarcoma associated to an abdominal aortic aneurysm with larotrectinib: a case report. Pediatr Hematol Oncol (2021) 38(5):504–9. doi: 10.1080/08880018.2021.1889730
23. Drilon A, Laetsch TW, Kummar S, DuBois SG, Lassen UN, Demetri GD, et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med (2018) 378(8):731–9. doi: 10.1056/NEJMoa1714448
24. Caldwell KJ, de la Cuesta E, Morin C, Pappo A, Helmig S. A newborn with a large NTRK fusion positive infantile fibrosarcoma successfully treated with larotrectinib. Pediatr Blood Cancer. (2020) 67(9):e28330. doi: 10.1002/pbc.28330
25. Thorwarth A, Haase K, Röefzaad C, Pajtler KW, Schramm K, Hauptmann K, et al. Genomic evolution and personalized therapy of an infantile fibrosarcoma harboring an NTRK oncogenic fusion. JCO Precis Oncol (2022) 6:e2100283. doi: 10.1200/PO.21.00283
Keywords: infantile fibrosarcoma, newborn, ETV6-NTRK3, larotrectinib, treatment
Citation: Wang D, Zhang F, Feng W, Pan J and Yuan T (2023) Larotrectinib treatment for infantile fibrosarcoma in newborns: a case report and literature review. Front. Oncol. 13:1206833. doi: 10.3389/fonc.2023.1206833
Received: 16 April 2023; Accepted: 11 July 2023;
Published: 27 July 2023.
Edited by:
Eleanor Chen, University of Washington, United StatesReviewed by:
Anderson Collier III, Nemours Foundation, United StatesErica Kao, Brooke Army Medical Center, United States
Copyright © 2023 Wang, Zhang, Feng, Pan and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tianming Yuan, eXVhbnRpYW5taW5nQHpqdS5lZHUuY24=
 Dandan Wang
Dandan Wang Fanhui Zhang
Fanhui Zhang Wanli Feng1,2
Wanli Feng1,2 Tianming Yuan
Tianming Yuan