- Department of Gastroenterology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, China
Background: Patients with early gastric cancer have increased risk of developing multiple primary malignancies (MPM) due to improved survival rates. The purpose of this study was to evaluate the clinicopathological features of MPM and to generate a useful tool for predicting the development of MPM after early gastric cancer.
Methods: We selected 1025 early gastric cancer patients with complete medical records for a retrospective analysis. The Cox proportional risk regression model was used to analyze the independent risk factors for the development of MPM in early gastric cancer. RStudio software was used to compare the OS of early gastric cancer patients with and without MPM, and a nomogram was established to predict the probability of MPM 1-, 2-, 3-year after early gastric cancer. The predictive effectiveness of the nomogram was evaluated by the C-index and calibration curve. Decision curve analysis (DCA) measured the applicability of the nomogram to clinical practice.
Results: Of the 1025 patients with early gastric cancer, 66 patients (6.4%) had 69 primary cancers other than early gastric cancer. They had a median follow-up of 41 months, and their cumulative incidence of MPM was 4.9%, 5.4% and 5.9% after 1-, 2-, and 3- year, respectively. Oesophageal cancer was the most frequently detected MPM, followed by lung and colorectal cancers. Male (p=0.038), age ≥65 years (p=0.003), smoking history (p=0.036), and lymph node metastasis (p=0.013) were independent risk factors for MPM in patients with early gastric cancer. Patients with early gastric cancer with MPM had a worse OS prognosis than patients with early gastric cancer without MPM (p<0.001). The internally validated nomogram predicted the probability of developing MPM after early gastric cancer (C index= 0.697). The calibration chart showed that the predicted probability of MPM in early gastric cancer was similar to the observed result, and the DCA showed strong clinical practicability.
Conclusion: After the diagnosis and treatment of early gastric cancer, we should be alert to the possibility of MPM and perform regular and careful monitoring.
1 Introduction
Gastric cancer is the fifth most common malignant tumour in the world and ranks fourth in global cancer mortality rate after lung cancer, colorectal cancer and liver cancer (1, 2). There are approximately 1.2 million new cases of gastric cancer each year worldwide, of which China accounts for approximately 40%. Early gastric cancer accounts for approximately 20% of cases at diagnosis, and the 5-year survival rate after endoscopic resection can reach 92.6% (3). Multiple primary malignancies (MPM) are two or more primary malignancies occurring simultaneously or consecutively in the same individual, which can originate from the same organ, paired organs, different parts of the same system, or different organs of different systems (4–7), although they can display similar histologic subclassifications, each with different clinical features.
In the last two decades, the detection and survival rates of patients with gastric cancer have increased significantly due to the widespread availability of endoscopic screening programs and the development of minimally invasive endoscopic techniques, and the improved prognosis of gastric cancer combined with the continued influence of genetic and behavioural risk factors has led to an increase in the incidence of associated MPM (8, 9). The incidence of gastric cancer with a second primary malignancy (SPM) is 0.7-11% (10–18), and SPM associated with gastric cancer is usually colorectal, lung and liver cancers (8, 9). Studies on early gastric cancer-associated MPM are scarce and not recent, particularly lacking in minimally invasive endoscopic techniques. The prognosis of early gastric cancer is improving, but clinicians may pay attention to late tumour recurrence and metastasis while ignoring the occurrence of early tumour MPM. When symptomatic MPM is diagnosed, it is usually more advanced than asymptomatic MPM, and the patient’s performance status may not allow for the best cancer treatment, resulting in poor prognosis and short survival time for patients. Therefore, it is important to recognize the clinical and pathological features of patients with MPM associated with early gastric cancer in order to detect MPM early enough for treatment to increase patient survival and improve patient quality of life.
The purpose of this study was to determine the clinicopathological features and outcomes of MPM and to analyse the risk factors for the development of MPM associated with early gastric cancer, with the aim of generating a useful predictive tool for the risk of developing MPM in patients with early gastric cancer. Such a tool would help clinicians identify the clinical course and prognostic factors of early gastric cancer patients with concurrent and metachronous primary cancers, which would facilitate to provide early prevention and diagnosis of MPM.
2 Methods
2.1 Patient and case data
From 1 January 2010 to 31 March 2022, among 1355 patients with early gastric cancer confirmed by gastroscopy and pathological biopsy at the Provincial Hospital of Shandong First Medical University, we retrospectively analysed the medical records of 1025 patients with clinicopathological features of early gastric cancer who had adequate medical records and were available for postoperative follow-up. Early gastric cancer refers to cancerous tissue limited to the gastric mucosal layer or submucosal layer, regardless of its extent or whether there is lymph node metastasis. Exclusion criteria: (1) endoscopic and pathological confirmation of submucosal tumours, including smooth muscle tumours, mesenchymal tumours, fibrous nerve sheath tumours, neuroendocrine tumours, odoriferous pancreas, lipomas, cysts, capillary haemangiomas, etc.; (2) pathological confirmation of low-grade intraepithelial neoplasia, mid- to late-stage gastric cancer and residual gastric cancer. Following Warren and Gates (7), the diagnostic criteria for MPM were as follows: (1) each tumour had definite malignant histopathological changes; (2) each tumour had an independent pathological type; and (3) the possibility of invasion and metastasis of the second cancer as the first primary cancer was excluded. A second tumour was defined as synchronous MPM if it was found concurrently with the first tumour or within 6 months; if it was found >6 months later, it was defined as metachronous MPM. The diagnosis of each malignancy in patients with MPM is identified by a histopathologist. Haematologic malignancies were excluded from the study, and only solid malignancies were included.
2.2 Observed indicators
The observation indices were data on patients’ age, sex, history of smoking and drinking, lesion location, maximum lesion diameter, gross appearance, degree of differentiation, depth of invasion, degree of surrounding mucosal atrophy, degree of intestinal metaplasia, whether the lesion site was multifocal, and whether there was lymph node metastasis at the time of diagnosis of early gastric cancer, and the type of the MPM.
Occasional smokers were considered non-smokers, and social drinkers were considered non-drinkers. The lesions were divided into the upper 1/3 of the stomach (cardia, fundus), the middle 1/3 (body of the stomach) and the lower 1/3 (sinus, including the pylorus). The size of the lesion was determined based on pathological measurements and is expressed as the maximum diameter of the lesion. Superficial lesions (Type 0) were classified as elevated (0-I), flat (0-II) and depressed (0-III) according to the Paris classification update criteria (19). Type 0-II was divided into three subtypes, 0-IIa, 0-IIb and 0-IIc, which had slight elevation, flatness and slight depression, respectively. Lesions with both slight elevation and slight depression were 0-IIa+IIc. According to the degree of histological differentiation, WHO staging, and Lauren staging, highly and moderately differentiated ductal adenocarcinoma and papillary adenocarcinoma are differentiated types, while hypofractionated adenocarcinoma, indolent cell carcinoma and mucinous adenocarcinoma are undifferentiated types, and the presence of both types makes for a mixed type. The deepest cancer tissue located above the mucosal muscle layer was called intramucosal carcinoma, and a 500 μm vertical distance from the deepest lesion through the mucosal muscle layer was used as the threshold value to distinguish superficial (SM1) and deep (≥SM2) submucosal infiltration. The excised specimens were fixed in 10% formalin and then examined histopathologically. Histological mapping was performed after serial sectioning of endoscopic specimens at 2-mm intervals and surgical specimens at 5-mm intervals. Gastrointestinal pathologists assessed the degree of differentiation, gross appearance, maximum lesion diameter, depth of invasion, degree of surrounding mucosal atrophy, degree of intestinal metaplasia, whether the lesion site was multifocal and lymph node metastasis according to the Japanese Classification of Gastric Cancer.
2.3 Follow-up
After treatment, patients with early gastric cancer visited the outpatient clinic for follow-up every 3 months in the 1st year and then at intervals of 6-12 months. Follow-up examinations included physical examination, blood analysis, ultrasonography, computed tomography (CT) scan and endoscopy. MPM was evaluated by ultrasonography, computed tomography (CT), positron emission tomography (PET-CT), endoscopy, and histological biopsy. Vital status (all-cause mortality) was assessed by case analysis and telephone follow-up. The follow-up period was defined as the number of years from the diagnosis of early gastric cancer to the date of all-cause death, the date of MPM diagnosis, or the date of the last follow-up visit, whichever occurred first. When we considered the risk of MPM, death was a competing event. Unlike deletion, competing events could not occur at the same time as the event of concern. Overall survival (OS) was defined as the time from diagnosis to death or to the last follow-up visit for patients with early gastric cancer without MPM and the time from MPM diagnosis to death or to the last follow-up visit for patients with early gastric cancer with MPM.
2.4 Statistical analysis
In this study, continuous variables are described as means with standard deviations, and categorical variables are expressed as numbers with percentages. Age and sex conformed to a normal distribution and were compared by the one-sample t test, comparisons between categorical variables were made using the chi-square test or Fisher’s exact test, and rank data and non-normally distributed variables were compared using the Wilcoxon signed rank sum test. Risk factors for the development of MPM in patients with early gastric cancer were analysed using Cox regression models, and differences were considered statistically significant when P<0.05. RStudio software was used to see whether there was a statistically significant difference in the OS of early gastric cancer patients with vs. without MPM. Nomogram plots were constructed based on the prediction models established using the results of the Cox multifactorial regression model analysis. The predictive power of the models was evaluated using the consistency index (C-index), and calibration plots were drawn to validate the nomogram. Decision curve analysis (DCA) was done to quantify the applicability of the nomogram to clinical practice. All statistical analyses were analysed using SPSS 26 (IBM Corporation, Armonk, NY, USA) and RStudio (2023.03.0).
3 Results
3.1 MPM and overall survival
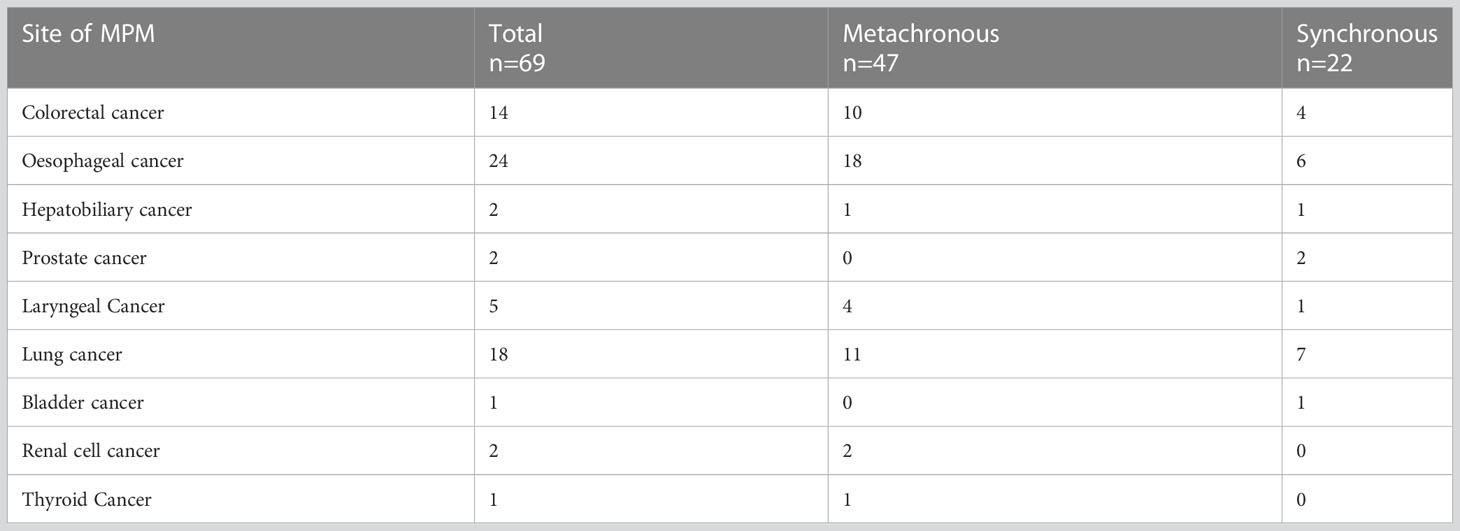
A total of 1025 patients with early gastric cancer were included in this study, 66 (6.4%) of whom had 69 primary cancers other than early gastric cancer (Table 1), 47 (68.1%) had metachronous MPM, and 22 (31.9%) had concurrent MPM. There were 24 cases of oesophageal cancer, 18 cases of lung cancer, 14 cases of colorectal cancer, 5 cases of pharyngeal cancer, 2 cases of hepatobiliary cancer, and 2 cases of prostate cancer. The mean time interval between the diagnosis of early gastric cancer and the diagnosis of MPM was 10.06 months. The cumulative incidence of MPM after treatment of early gastric cancer was 4.9% at 1 year, 5.4% at 2 years, and 5.9% at 3 years.
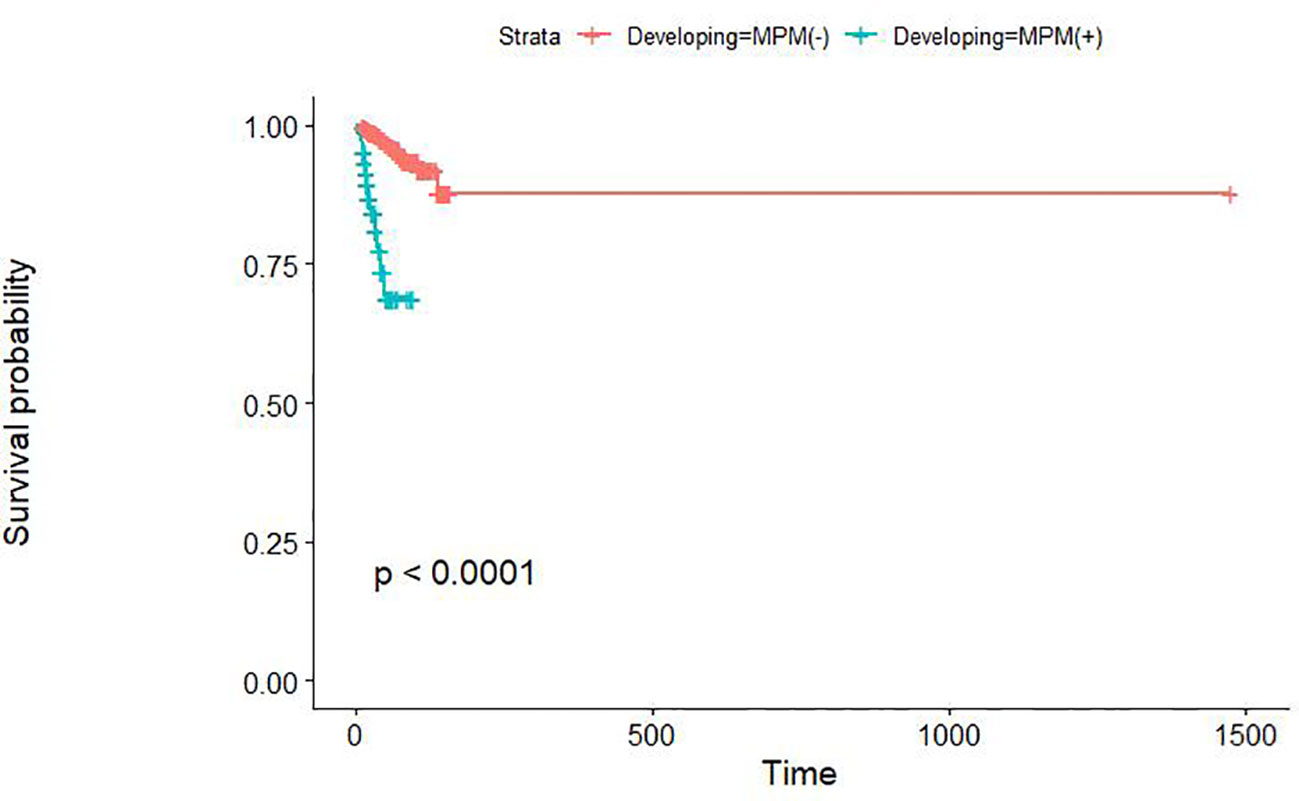
Early gastric cancer patients without MPM had a median survival of 43 months and a 3-year OS rate of 98.3%, while the early gastric cancer patients with MPM had values of 23.50 months and 77.7%, respectively. The overall survival rate of patients with MPM was significantly lower than that of early gastric cancer patients without MPM (p<0.01) (Figure 1). The cause-of-death patterns of early gastric cancer patients with and without MPM were different: 29 of the 960 early gastric cancer patients without MPM died during follow-up, whose cause of death was mainly other chronic diseases or natural death, while 12 of the 66 early gastric cancer patients with MPM died during follow-up, in whom the cause of death was mainly the progression of MPM.
3.2 Predictive factors for MPM
Table 2 compares the clinicopathologic data between the patients with and without MPM. The MPM group had more male (93.9% vs. 76.1%, p=0.001) and older (65.44 ± 7.946 years vs. 62.00 ± 9.525, p=0.004) patients than the group without MPM. The MPM group more often had a history of smoking (68.2% vs. 45.5%, p=0.000) and lymph node metastasis (13.6% vs. 5.3%, p=0.005). In contrast, patients with early gastric cancer without other primary cancers and patients with early gastric cancer with other primary cancers were similar in terms of history of drinking, lesion location, maximum lesion diameter, degree of differentiation, depth of invasion, degree of surrounding mucosal atrophy and intestinal metaplasia, and whether the lesion site was multifocal.
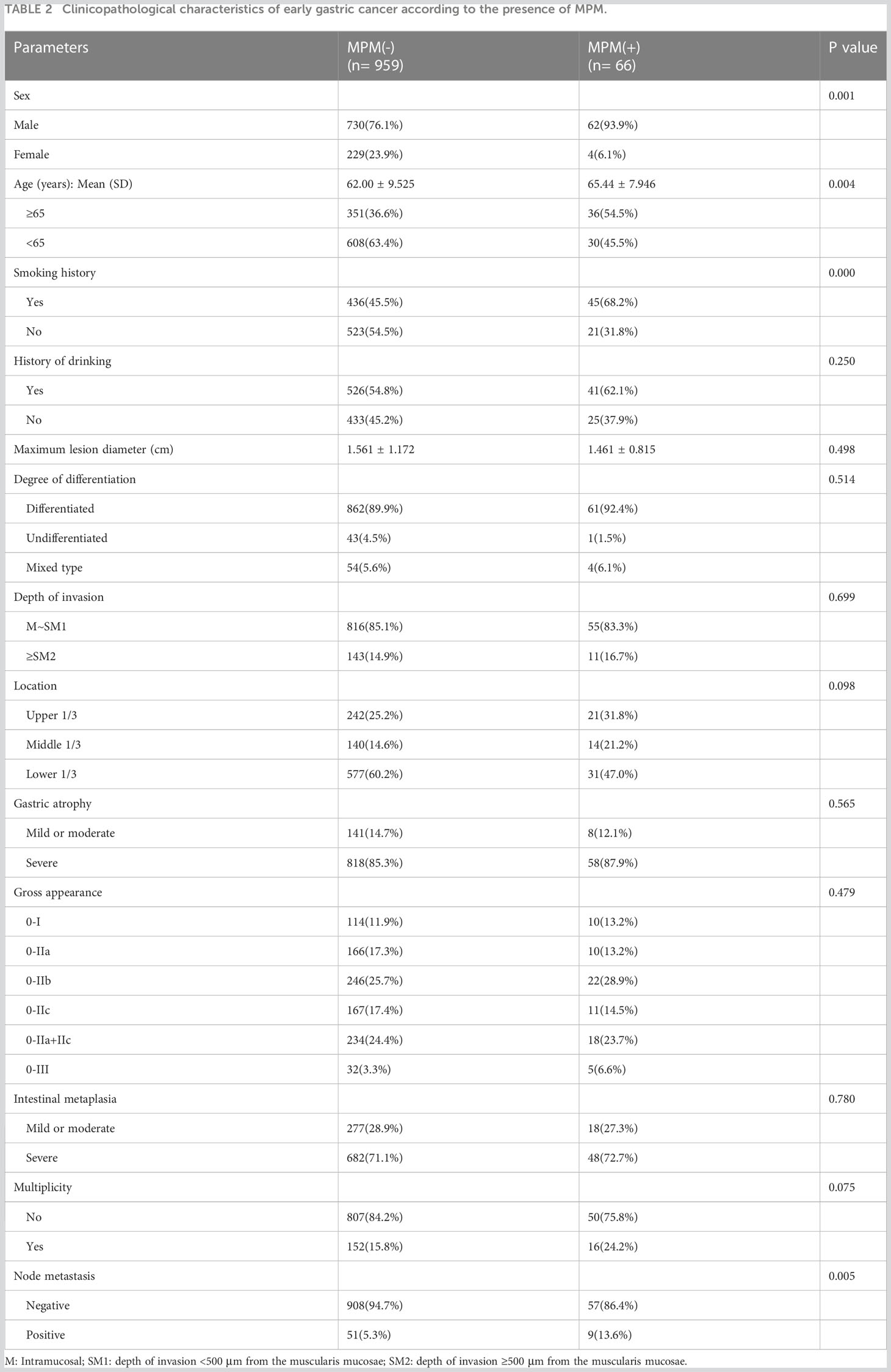
Table 2 Clinicopathological characteristics of early gastric cancer according to the presence of MPM.
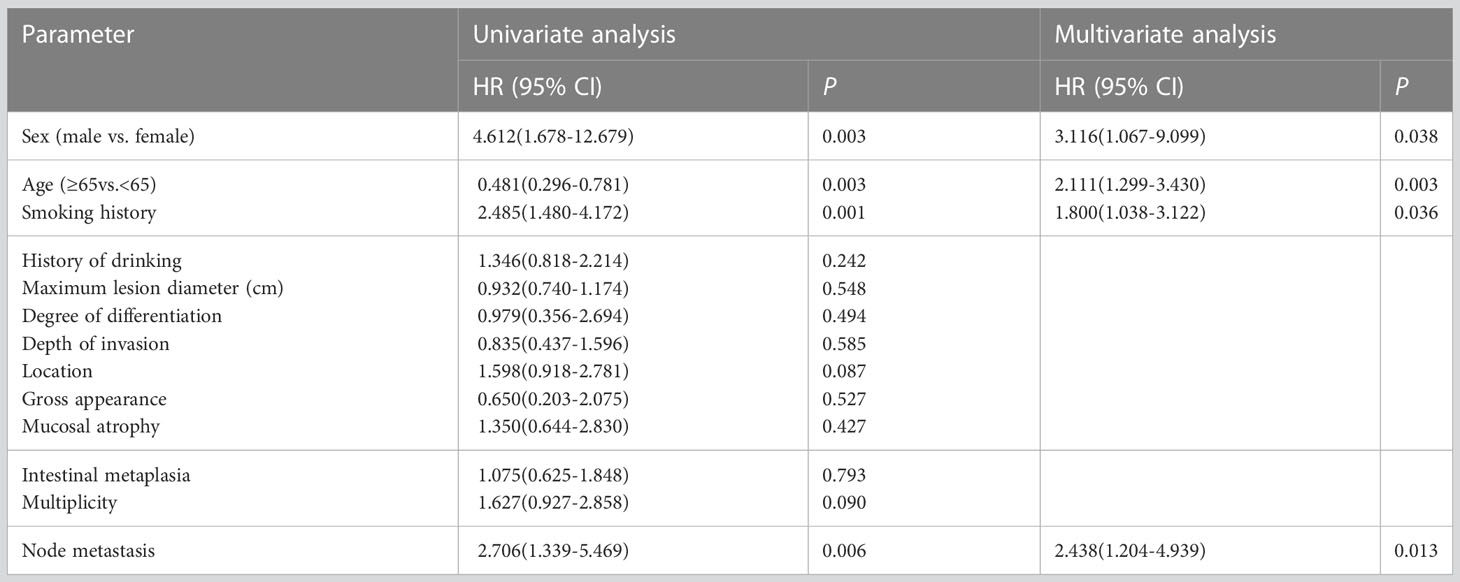
Cox univariate regression analysis was performed on all variables, and variables with p<0.05 were then subjected to Cox multivariate regression analysis, which showed that male sex, age older than 65 years at the time of GC diagnosis, history of smoking and lymph node metastasis were independent predictors of MPM among early gastric cancer patients (Table 3).
3.3 Nomogram
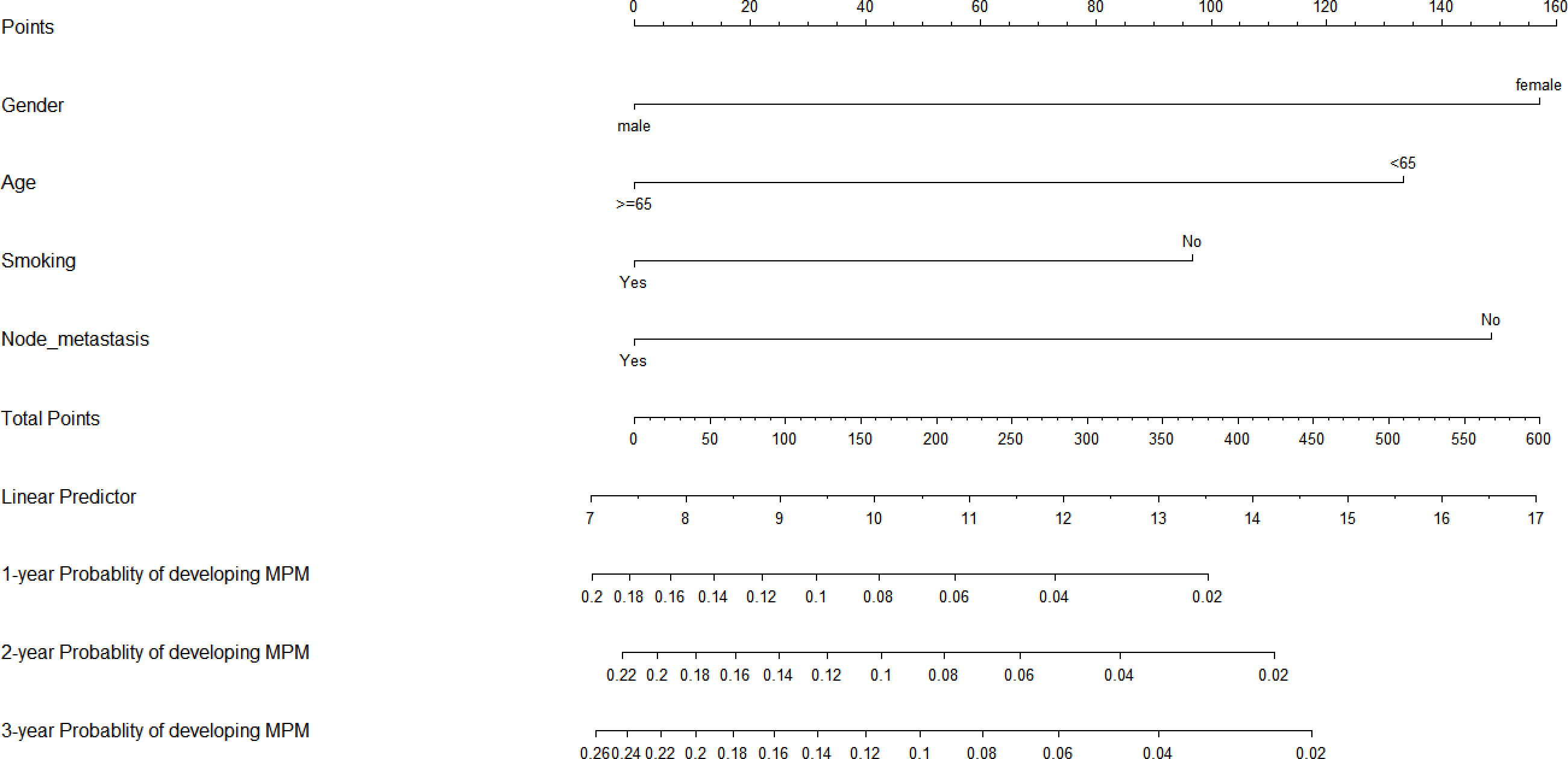
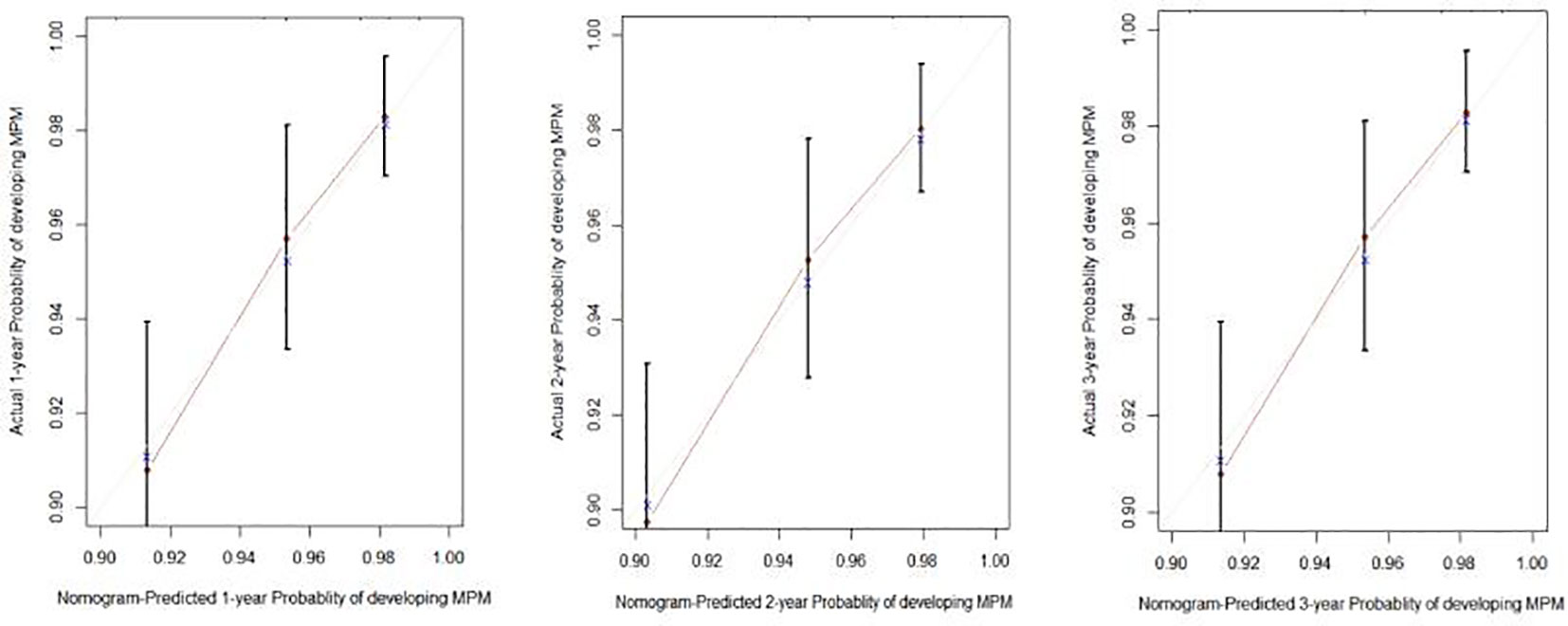
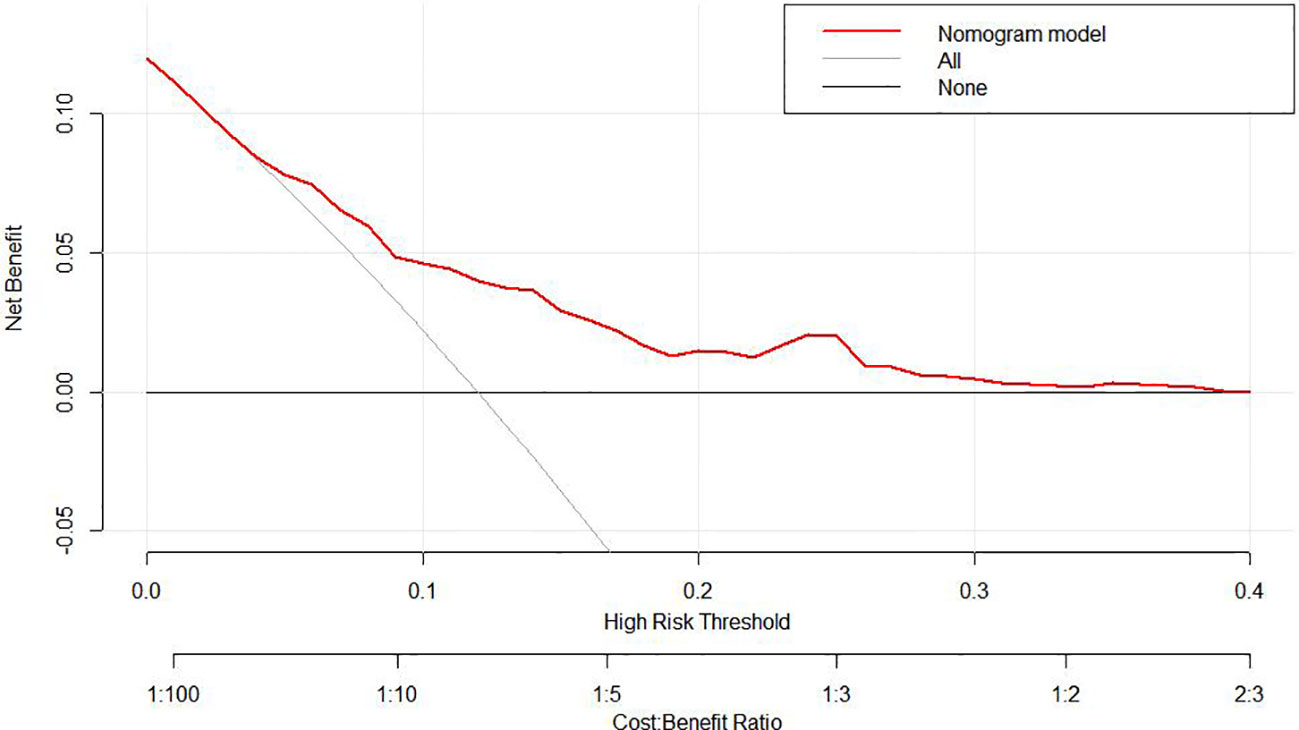
To predict the development of MPM, we generated a nomogram (Figure 2) based on the results of the Cox regression. From the Cox regression model, male sex (p = 0.038), age over 65 years (p = 0.003), history of smoking (p = 0.036) and lymph node metastasis (p = 0.013) were associated with the development of metachronous MPC. Each variable category in the nomogram corresponds to its respective score evaluation. All the category scores are summed to obtain the total score. By drawing a line vertically downwards from the total score scale point to the horizontal axis, the intersection point is the predicted probability of developing MPM in early gastric cancer patients at 1 year, 2 years, and 3 years. The consistency index of the model was 0.697, and the calibration curves of the nomogram were all similar to or basically fit the 45° diagonal line (Figure 3). In addition, the DCA showed strong clinical practicability (Figure 4).
4 Discussion
With economic and social progress, people have gradually become more aware of matters of health, and advances in active medical examination behaviours and minimally invasive endoscopic techniques have led to a higher detection rate and cure rate of early gastric cancer, with good prognosis. In general, the survival rate has improved, the risk period has grown longer, and the prevalence of MPM in early gastric cancer patients has increased. Domestic and international data indicate that the risk for the development of SPM in the cancer population is twice that of the general population (20, 21). The incidence of multiple primary malignancies associated with early gastric cancer in this study was 6.4%, similar to the results of previous studies (10–18). However, the most common MPM site for gastric cancer in previous studies was colorectal cancer, followed by lung and liver cancer (16, 18, 22–24), while oesophageal cancer was the most common MPM associated with early gastric cancer in the present study, followed by lung and colorectal cancer. In addition to the possible similarity of external risk factors (environment, smoking and drinking history, and diet) (20, 25, 26), we speculate that the predilection for these MPM sites may be because the respiratory tract epithelium and digestive tract epithelium, as well as various digestive glands that are specialized forms of digestive tract epithelium, all have endodermal development and are more likely to be influenced by the same genes and environmental signals during embryonic organ development (27). This conjecture needs to be tested by stem-cell-to-organoid modelling.
Our study showed that there was a significant difference in OS between patients with early gastric cancer with MPM and those without MPM. However, the prognosis of MPM patients itself is worse than that of patients without MPM, which may be due not only to their higher tumour load and comorbid conditions but also to higher psychological burden (28, 29). Therefore, most MPM patients still have a poor prognosis even under regular follow-up. Our nomogram suggests that patients at high risk may need more frequent follow-up or more accurate means of follow-up, which would help to detect MPM at an early stage, thus helping patients achieve good treatment results at an early stage and avoiding late detection of MPM, which would lead them to miss the best time for treatment, worsening their prognosis and the quality and duration of survival. The optimal surveillance interval for MPM should be determined in future studies.
Patients with early gastric cancer, especially males, those aged ≥65 years, and those with lymphovascular involvement, tend to have a greater risk of second primary cancers and of recurrence (23, 30–33). Smoking is an independent risk factor for second primary cancer and increases the incidence of second primary cancer in patients with gastric cancer (34–36), in line with our results. Although our nomogram may be biased due to not enough MPM events, this nomogram can provide a more personalized prediction of MPM for early gastric cancer patients than the independent risk factor model. External validation in independent patient groups is needed to improve the accuracy of the nomogram.
There are several limitations of this study. Since it was retrospective, patient information was obtained mainly through case records and telephone follow-up, so recall bias may have occurred because lifestyle changes may come about as the cancer progresses and symptoms appear. We included only the history of smoking and drinking at the time of detection of early gastric cancer and ignored changes in habits after treatment began, but cancer patients may adopt a healthier lifestyle after diagnosis or treatment (37, 38). We also only analysed the presence or absence of a history of smoking and did not analyse individual smoking status. Since this study was a single-centre study, its results cannot be easily extrapolated or generalized. We also did not gather any information on H. pylori infection or eradication. The association between H. pylori and gastric cancer has been confirmed in several studies, H. pylori infection being associated with gastric cancer incidence worldwide (39–45), and one study showed that H. pylori eradication significantly reduced the incidence of gastric cancer (by 46%) and reduced gastric cancer mortality (by 39%) (46). Therefore, once H. pylori is detected, early eradication therapy is recommended to maximize the prevention of gastric cancer.
In conclusion, the risk of MPM after early gastric cancer treatment is still high. Age ≥65 years, male sex, smoking and the presence of lymph node metastasis are risk factors for MPM in early gastric cancer patients. Since oesophageal and lung cancers are common MPMs and patients with early gastric cancer with MPM have a poor OS prognosis, it is recommended that after the early gastric cancer is cured, in addition to regular monitoring for gastric cancer recurrence, follow-up oesophageal and chest examinations should also be given particular attention. We generated and validated a nomogram to predict the probability of MPM in patients with early gastric cancer. If this tool proves useful to screen for MPM, we can improve the clinical prognosis of patients with early gastric cancer.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
NC contributed to the study design and wrote the article. LS and JG did the data analysis. NC and RJ generated and improved the figures and tables. LS proofread the manuscript. JJ reviewed the article. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Youth Program of Natural Science Foundation of Shandong Province (grant number ZR2021QH327).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Fock KM. Review article: the epidemiology and prevention of gastric cancer. Aliment Pharmacol Ther (2014) 40(3):250–60. doi: 10.1111/apt.12814
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
3. Waddingham W, Nieuwenburg SAV, Carlson S, Rodriguez-Justo M, Spaander M, Kuipers EJ, et al. Recent advances in the detection and management of early gastric cancer and its precursors. Frontline Gastroenterol (2021) 12(4):322–31. doi: 10.1136/flgastro-2018-101089
4. Amer MH. Multiple neoplasms, single primaries, and patient survival. Cancer Manag Res (2014) 6:119–34. doi: 10.2147/CMAR.S57378
5. Demandante CG, Troyer DA, Miles TP. Multiple primary malignant neoplasms: case report and a comprehensive review of the literature. Am J Clin Oncol (2003) 26(1):79–83. doi: 10.1097/00000421-200302000-00015
6. Vogt A, Schmid S, Heinimann K, Frick H, Herrmann C, Cerny T, et al. Multiple primary tumours: challenges and approaches, a review. ESMO Open (2017) 2(2):e000172. doi: 10.1136/esmoopen-2017-000172
7. Warren S, Cancer AJ. Multiple primary malignant tumors. A survey of the literature and a statistical study. Gastroenterology (1932) 93(4):779. doi: 10.1016/0016-5085(87)90440-9
8. Elia I, Haigis MC. Metabolites and the tumour microenvironment: from cellular mechanisms to systemic metabolism. Nat Metab (2021) 3(1):21–32. doi: 10.1038/s42255-020-00317-z
9. Yang XB, Zhang LH, Xue JN, Wang YC, Yang X, Zhang N, et al. High incidence combination of multiple primary malignant tumors of the digestive system. World J Gastroenterol (2022) 28(41):5982–92. doi: 10.3748/wjg.v28.i41.5982
10. Aslam SS, Sridhar H, Rao MY. A case of synchronous malignancy of stomach and kidney. Kathmandu Univ Med J (KUMJ) (2013) 11(41):94–5. doi: 10.3126/kumj.v11i1.11053
11. Oh SJ, Bae DS, Suh BJ. Synchronous triple primary cancers occurring in the stomach, kidney, and thyroid. Ann Surg Treat Res (2015) 88(6):345–8. doi: 10.4174/astr.2015.88.6.345
12. Ha TK, An JY, Youn HG, Noh JH, Sohn TS, Kim S. Surgical outcome of synchronous second primary cancer in patients with gastric cancer. Yonsei Med J (2007) 48(6):981–7. doi: 10.3349/ymj.2007.48.6.981
13. Lawniczak M, Gawin A, Jaroszewicz-Heigelmann H, Rogoza-Mateja W, Raszeja-Wyszomirska J, Bialek A, et al. Synchronous and metachronous neoplasms in gastric cancer patients: a 23-year study. World J Gastroenterol (2014) 20(23):7480–7. doi: 10.3748/wjg.v20.i23.7480
14. Hu KN, Lai WH, Tseng PT, Wang WC, Shen KH. Synchronous primary gastric cancer and renal cell carcinoma: A case report and literatures review. Sci U (2012) 23(1):28–30. doi: 10.1016/j.urols.2011.12.007
15. Cosme Jimenez A, Bujanda Fernandez de Pierola L, Ojeda Perez E, Gil Lasa I, Barrio Andres J, Torrado Cadena J, et al. Gastric carcinoma associated with other primary malignant neoplasms. A retrospective study of 25 cases. Rev Clin Esp (2000) 200(1):7–11. doi: 10.1016/s0014-2565(00)70543-1
16. Ikeda Y, Saku M, Kawanaka H, Nonaka M, Yoshida K. Features of second primary cancer in patients with gastric cancer. Oncology (2003) 65(2):113–7. doi: 10.1159/000072335
17. Lee JH, Park JG, Bae JM, Bae JS, Ryu KW, Lee JS, et al. Gastric cancer patients at high-risk of having synchronous cancer. World J Gastroenterol (2006) 12(16):2588–2592. doi: 10.3748/wjg.v12.i16.2588
18. Eom BW, Lee HJ, Yoo MW, Cho JJ, Kim WH, Yang HK, et al. Synchronous and metachronous cancers in patients with gastric cancer. J Surg Oncol (2008) 98(2):106–10. doi: 10.1002/jso.21027
19. Miyaoka M, Yao K, Tanabe H, Kanemitsu T, Otsu K, Imamura K, et al. Diagnosis of early gastric cancer using image enhanced endoscopy: a systematic approach. Transl Gastroenterol Hepatol (2020) 5:50. doi: 10.21037/tgh.2019.12.16
20. Utada M, Ohno Y, Hori M, Soda M. Incidence of multiple primary cancers and interval between first and second primary cancers. Sci C (2014) 105(7):890–6. doi: 10.1111/cas.12433
21. Lunet BN. The contribution of second primary cancers to the mortality of patients with a gastric first primary cancer. Eur J Gastroenterol Hepatol (2019) 31(4):471–7. doi: 10.1097/MEG.0000000000001348
22. Lundegardh G, Hansson LE, Nyren O, Adami HO, Krusemo UB. The risk of gastrointestinal and other primary malignant diseases following gastric cancer. Acta Oncol (1991) 30(1):1–6. doi: 10.3109/02841869109091804
23. Kim C, Chon H, Kang B, Kim K, Jeung HC, Chung H, et al. Prediction of metachronous multiple primary cancers following the curative resection of gastric cancer. BMC Cancer (2013) 13:394. doi: 10.1186/1471-2407-13-394
24. Aoyama T, Ju M, Komori K, Tamagawa H, Tamagawa A, Maezawa Y, et al. The clinical impact of synchronous and metachronous other primary cancer in gastric cancer patients who receive curative treatment. In Vivo (2022) 36(5):2514–20. doi: 10.21873/invivo.12987
25. Pena A. Carcinogenic food contaminants. Cancer Invest (2007) 25(3):189–96. doi: 10.1080/07357900701208733
26. Mysuru Shivanna L. A review on dietary and non-dietary risk factors associated with gastrointestinal cancer. J Gastrointest Cancer Urooj A (2016) 47(3):247–54. doi: 10.1007/s12029-016-9845-1
27. Zorn AM, Wells JM. Vertebrate endoderm development and organ formation. Annu Rev Cell Dev Biol (2009) 25:221–51. doi: 10.1146/annurev.cellbio.042308.113344
28. Thong M, Mols F, Verhoeven R, Liu L, Andrykowski MA, Roukema JA, et al. Multiple primary cancer survivors have poorer health status and well-being than single primary cancer survivors: a study from the population-based PROFILES registry. Psychooncology (2013) 22(8):1834–42. doi: 10.1002/pon.3227
29. Belcher SM, Hausmann EA, Cohen SM, Donovan HS, Schlenk EA. Examining the relationship between multiple primary cancers and psychological distress: A review of current literature. Psychooncology (2017) 26(12):2030–9. doi: 10.1002/pon.4299
30. Yamamoto M, Yamanaka T, Baba H, Kakeji Y, Maehara Y. The postoperative recurrence and the occurrence of second primary carcinomas in patients with early gastric carcinoma. J Surg Oncol (2008) 97(3):231–5. doi: 10.1002/jso.20946
31. Ikeda Y, Saku M, Kishihara F, Maehara Y. Effective follow-up for recurrence or a second primary cancer in patients with early gastric cancer. Br J Surg (2005) 92(2):235–9. doi: 10.1002/bjs.4758
32. Chen S-C, Liu C-J, Hu Y-W, Yeh C-M, Hu LY, Wang YP, et al. Second primary malignancy risk among patients with gastric cancer: a nationwide population-based study in Taiwan. Gastric Cancer (2016) 19(2):490–7. doi: 10.1007/s10120-015-0482-3
33. Morais S, Antunes L, Bento MJ, Lunet N. Risk of second primary cancers among patients with a first primary gastric cancer: A population-based study in North Portugal. Epidemiol C (2017) 50:85. doi: 10.1016/j.canep.2017.08.007
34. Kinoshita Y, Tsukuma H, Ajiki W, Kinoshita N, Oshima A, Hiratsuka M, et al. The risk for second primaries in gastric cancer patients: adjuvant therapy and habitual smoking and drinking. J Epidemiol (2000) 10(5):300. doi: 10.2188/jea.10.300
35. Hiyama T, Sato T, Yoshino K, Tsukuma H, Hanai A, Fujimoto I, et al. Second primary cancer following laryngeal cancer with special reference to smoking habits. Jpn J Cancer Res (1992) 83(4):334–9. doi: 10.1111/j.1349-7006.1992.tb00111.x
36. Tabuchi T, Ozaki K, Ioka A, Miyashiro I. Joint and independent effect of alcohol and tobacco use on the risk of subsequent cancer incidence among cancer survivors: A cohort study using cancer registries. Int J Cancer (2015) 137(9):2114–23. doi: 10.1002/ijc.29575
37. Blanchard CM, Denniston MM, Baker F, Ainsworth SR, Courneya KS, Hann DM, et al. Do adults change their lifestyle behaviors after a cancer diagnosis? Am J Health Behav (2003) 27(3):246–56. doi: 10.5993/AJHB.27.3.6
38. Pacheco-Figueiredo L, Antunes L, Bento MJ, Lunet N. Health-related behaviours in the EpiPorto study: cancer survivors versus participants with no cancer history. EJC Prev (2011) 20(4):348–54. doi: 10.1097/CEJ.0b013e328345f923
39. González CA, Megraud F, Buissonniere A, Lujan Barroso L, Agudo A, Duell EJ, et al. Helicobacter pylori infection assessed by ELISA and by immunoblot and noncardia gastric cancer risk in a prospective study: the Eurgast-EPIC project. Ann Oncol (2012) 23(5):1320–4. doi: 10.1093/annonc/mdr384
40. Lochhead P, El-Omar EM. Helicobacter pylori infection and gastric cancer. Best Pract Res Clin Gastroenterol (2007) 21(2):281–97. doi: 10.1016/j.bpg.2007.02.002
41. Plummer M, Franceschi S, Vignat J, Forman D, Martel CD. Global burden of gastric cancer attributable to Helicobacter pylori. Int J Cancer (2015) 136(2):487–90. doi: 10.1002/ijc.28999
42. Parsonnet J, Vandersteen D, Goates J, Sibley RK, Pritikin J, Chang Y. Helicobacter pylori infection in intestinal- and diffuse-type gastric adenocarcinomas. J Natl Cancer Inst (1991) 83(9):640–3. doi: 10.1093/jnci/83.9.640
43. Hansson L-E, Engstrand L, Nyrén O, Lindgren A. Prevalence of Helicobacter pylori infection in subtypes of gastric cancer. Gastroenterology (1995) 109(3):885–8. doi: 10.1097/00042737-199510000-00030
44. Peleteiro B, Bastos A, Ferro A, Lunet N, Diseases D. Prevalence of Helicobacter pylori infection worldwide: a systematic review of studies with national coverage. Sciences (2015) 60(9):2849–9. doi: 10.1007/s10620-015-3779-5
45. Lu Y, Xiao F, Wang Y, Wang Z, Liu D, Hong F. Prevalence of helicobacter pylori in non-cardia gastric cancer in china: a systematic review and meta-analysis. Front Oncol (2022) 12:850389. doi: 10.3389/fonc.2022.850389
Keywords: early gastric cancer, multiple primary malignancies, nomogram, predictive factor, predictive tool
Citation: Chen N, Shi L, Ge J, Jia R and Jiang J (2023) Risk and prediction of multiple primary malignancies after early gastric cancer. Front. Oncol. 13:1205358. doi: 10.3389/fonc.2023.1205358
Received: 13 April 2023; Accepted: 10 July 2023;
Published: 25 July 2023.
Edited by:
Jun Lu, Fujian Medical University, ChinaReviewed by:
Natale Calomino, University of Siena, ItalyYosuke Tsuji, The University of Tokyo, Japan
Michela Giulii Capponi, Santo Spirito in Sassia Hospital, Italy
Copyright © 2023 Chen, Shi, Ge, Jia and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junmei Jiang, MTM2MjY0MDM5ODFAMTYzLmNvbQ==
 Na Chen
Na Chen Junmei Jiang
Junmei Jiang