
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 10 August 2023
Sec. Gynecological Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1203127
 Soo Jin Park1
Soo Jin Park1 Kwon Joong Na2
Kwon Joong Na2 Maria Lee1,3
Maria Lee1,3 In Kyu Park2,4
In Kyu Park2,4 Hyun Hoon Chung1,3
Hyun Hoon Chung1,3 Chang Hyun Kang2,4
Chang Hyun Kang2,4 Jae-Weon Kim1,3
Jae-Weon Kim1,3 Noh Hyun Park1,3
Noh Hyun Park1,3 Young-Tae Kim2,4
Young-Tae Kim2,4 Yong Sang Song1,3
Yong Sang Song1,3 Samina Park2,4*
Samina Park2,4* Hee Seung Kim1,3*
Hee Seung Kim1,3*Introduction: To evaluate the survival impact of supradiaphragmatic lymphadenectomy as part of debulking surgery in stage IVB ovarian cancer with thoracic lymph node metastasis (LNM).
Methods: We retrospectively enrolled patients diagnosed with stage IVB ovarian, fallopian or primary peritoneal cancer between 2010 and 2020, carrying cardiophrenic, parasternal, anterior mediastinal or supraclavicular lymph nodes ≥5 mm on axial chest computed tomography. All tumors were classified into the abdominal (abdominal tumors and cardiophrenic lymph nodes) and supradiaphragmatic (parasternal, anterior mediastinal or supraclavicular lymph nodes) categories depending on the area involved. Residual tumors were classified into <5 vs ≥5 mm in the abdominal and supradiaphragmatic areas. Based on the site of recurrence, they were divided into abdominal, supradiaphragmatic and other areas.
Results: A total of 120 patients underwent primary debulking surgery (PDS, n=68) and interval debulking surgery after neoadjuvant chemotherapy (IDS/NAC, n=53). Residual tumors in the supradiaphragmatic area ≥5 mm adversely affected progression-free survival (PFS) and overall survival (OS) with marginal significance after PDS despite the lack of effect on survival after IDS/NAC (adjusted hazard ratios [HRs], 6.478 and 6.370; 95% confidence intervals [CIs], 2.224-18.864 and 0.953-42.598). Further, the size of residual tumors in the abdominal area measuring ≥5 mm diminished OS after IDS/NAC (adjusted HR, 9.330; 95% CIs, 1.386-62.800).
Conclusion: Supradiaphragmatic lymphadenectomy during PDS may improve survival in patients diagnosed with stage IVB ovarian cancer manifesting thoracic LNM. Further, suboptimal debulking surgery in the abdominal area may be associated with poor OS after IDS/NAC.
Trial registration: ClinicalTrials.gov (NCT05005650; https://clinicaltrials.gov/ct2/show/NCT05005650; first registration, 13/08/2021).
Research Registry (Research Registry UIN, researchregistry7366; https://www.researchregistry.com/browse-the-registry#home/?view_2_search=researchregistry7366&view_2_page=1).
Early diagnosis of ovarian cancer is known to be very important, with a five-year survival rate of over 80% expected (1, 2). However, screening using a combination of transvaginal ultrasound and serum CA-125 levels may not reduce death in the general population significantly, and most patients with ovarian cancer are still diagnosed with stage III-IV disease associated with a fatal prognosis (3, 4).
In particular, stage IVB disease with parenchymal metastasis involving liver and extra-abdominal lymph node metastasis (LNM) ranges from 12% to 15% (1). Since patients with stage IVB disease often carry unresectable tumors during primary debulking surgery (PDS), 25-30% of gynecologic oncologists prefer interval debulking surgery after neoadjuvant chemotherapy (IDS/NAC) due to similar survival benefit but lower rates of complication (5, 6).
However, thoracic LNM, involving cardiophrenic, mediastinal or supraclavicular LNM, is sometimes resectable in collaboration with thoracic surgeons. Thus, supradiaphragmatic lymphadenectomy can lead to optimal debulking surgery (ODS) in patients with stage IVB ovarian cancer and thoracic LNM. Despite this surgical feasibility, comparative evidence of survival benefit associated with supradiaphragmatic lymphadenectomy in these patients is unavailable. Thus, we investigated the effect of supradiaphragmatic lymphadenectomy as a component of ODS on survival of patients in stage IVB ovarian cancer along with thoracic LNM.
We searched an institutional database of patients with ovarian, fallopian, or primary peritoneal cancers between 2010 and 2020. The study was designed as a retrospective study, which included only patients with epithelial ovarian, fallopian, or primary peritoneal cancers; underwent PDS or IDS/NAC; International Federation of Obstetrics and Gynecology (FIGO) stage IVB disease; transthoracic LNM such as cardiophrenic, parasternal, anterior mediastinal, or supraclavicular LNM. However, we excluded stage IVB patients with posterior LNM, including middle or posterior mediastinal LNM, pleural seeding, and lung parenchymal metastasis. The Institutional Review Board of Seoul National University Hospital approved this study in advance (No. 1908-173-1059, September 3rd 2019), and we waived the patients’ consent because of a retrospective design. Moreover, this study has been registered in the ClinicalTrials.gov (NCT05005650; https://clinicaltrials.gov/ct2/show/NCT05005650) and the Research Registry (Research Registry UIN, researchregistry7366; https://www.researchregistry.com/browse-the-registry#home/?view_2_search=researchregistry7366&view_2_page=1).
In the earlier years of the study period, our institution followed a standardized protocol for the assessment of patients’ eligibility for complete cytoreduction based on CT and PET-CT scans to evaluate the disease extent and feasibility of ODS. In the later years of the study period, refinements were made to our assessment protocol by incorporating multidisciplinary team approaches to achieve maximal cytoreduction, especially for stage IVB patients presenting supradiaphragmatic metastasis.
Abdominal tumors were resected using debulking surgery as described in our previous report (7). First, we performed laparotomic staging operations, including hysterectomy, bilateral salpingo-oophorectomy, and pelvic or para-aortic lymphadenectomy. In this study, we removed enlarged lymph nodes selectively during pelvic or para-aortic lymphadenectomy. In addition, we conducted ultra-radical procedures including appendectomy, splenectomy, distal pancreatectomy, superficial liver mass excision or liver wedge resection, portal triad stripping, bowel resection, and anastomosis with or without prophylactic ileostomy in individual cases as needed. We performed en bloc pelvic resection or parietal peritonectomy to remove peritoneal metastasis.
Cardiophrenic lymph node dissection was performed trans-abdominally via diaphragmatic incision or trans-thoracically using video-assisted thoracic surgery (VATS) to remove thoracic LNM. Parasternal or anterior mediastinal lymph nodes were dissected via VATS. In particular, en bloc excision of internal mammary vessels near the metastatic lymph nodes was performed. Supraclavicular lymph nodes were dissected via 4 cm lateral supraclavicular incision. Bilateral approaches were adopted if indicated. ODS was defined as the size of residual tumors in the abdominal or supradiaphragmatic areas <5 mm after debulking surgery, whereas suboptimal debulking surgery (SDS) was defined as the size ≥5 mm.
We collected the following clinical and pathologic parameters: age, American Society of Anesthesiology (ASA) score, treatment types such as PDS and IDS/NAC, tumor origin, histology, use of bevacizumab adjuvant chemotherapy, the size of residual tumors and recurrent sites. We further evaluated the extent of surgical resection and surgical outcomes, including operation time, estimated blood loss, hospitalization, and acute grade 3 or 4 complications based on Memorial Sloan Kettering Cancer Center (MSKCC) grading criteria (8). Moreover, we used the modified Surgical Complexity Score (SCS) system by adding distal pancreatectomy, cholecystectomy, portal triad stripping, adrenalectomy, and lymphadenectomy in the cardiophrenic, internal mammary, and supraclavicular regions to evaluate the level of surgical complexity (9). In the modified SCS system, 18 procedures were scored from 1 to 3, and total scores divided all patients into the following three complexity score groups: low, ≤3; intermediate, 4–7; high, ≥8 (Supplementary Table 1).
In this study, we divided the surgical resection area into two compartments. The first compartment included the abdominal area, including abdominal tumors and cardiophrenic lymph nodes because cardiophrenic lymph node dissection was performed by either gynecologic oncologists or thoracic surgeons. The second compartment involved the supradiaphragmatic area, including parasternal, anterior mediastinal, or supraclavicular lymph nodes. Enlarged lymph nodes in the supradiaphragmatic area were resected if they were 5 mm or larger on axial chest computed tomography (CT) before surgery according to the criteria specified in previous studies (10, 11). Thus, based on size, the residual tumors in the adnominal and supradiaphragmatic areas were classified into two groups: <5 and ≥5 mm.
Survival outcomes included progression-free survival (PFS) and overall survival (OS). PFS was defined as the time interval from the treatment start date to the recurrence or last follow-up date. OS was defined as the duration from the treatment start date to cancer-related death or last follow-up date. Further, we investigated the recurrence pattern in the abdominal, supradiaphragmatic, and other areas.
The primary outcome of this study is survival outcomes of PFS in patients according to the size of residual disease in the supradiaphragmatic area. The secondary outcome include OS, subgroup analysis in high-grade serous type, surgical outcome, and recurrent sites. Chi-squared or Fisher’s exact test was used to analyze categorical variables and Student’s T-test for continuous variables. Survival outcomes were determined via Kaplan-Meier method with log-rank or Breslow test and identified independent factors affecting PFS and OS via Cox proportional-hazards regression analysis using hazard ratios (HRs) and 94% confidence intervals (CIs). SPSS software version 25.0 (SPSS Inc., Chicago IL, USA) was used for statistical analysis in this study.
A total of 120 eligible patients were included in our institutional database: 68 (56.2%) patients received PDS, and 53 (43.8%) patients underwent IDS/NAC. Supradiaphragmatic LNM <5 mm without resection, <5 mm with resection and 5 mm were identified in 41 (33.9%), 11 (9.1%) and 16 patients (13.2%) treated with PDS, and 29 (24%), 8 (6.6%) and 16 (13.2%) treated NAC/IDS, respectively. In 100 patients with HGSC of the ovary, supradiaphragmatic LNM <5 mm without resection, <5 mm with resection and 5 mm were identified in 32 (32%), 9 (9%) and 13 patients (13%) treated with PDS, and 26 (26%), 7 (7%) and 13 (13%) treated NAC/IDS, respectively (Figure 1).
Table 1 depicts the clinical and pathological characteristics of all patients. There was no difference in age, ASA score, tumor origin, histology, use of bevacizumab, the size of residual tumors in the abdominal area or the supradiaphragmatic area between patients treated with PDS and those treated with IDS/NAC. Among patients who underwent PDS, 82.4% had histologic type of HGSC, while among patients who underwent IDS, 90.6% had HGSC histologic type.
Supplementary Table 2 shows locations of enlarged and resected lymph nodes, and Supplementary Table 3 demonstrates the pathologic outcomes of resected lymph nodes in the cardiophrenic, parasternal, anterior mediastinal and supraclavicular regions. There was no difference in the distribution of enlarged and resected lymph nodes and the rate of metastatic lymph nodes among resected cases.
Table 2 shows the extent of surgical resection during both PDS and IDS/NAC. The two groups did not differ in terms of surgical procedures, including hysterectomy, bilateral salpingo-oophorectomy, pelvic or para-aortic lymphadenectomy, omentectomy, appendectomy, splenectomy, distal pancreatectomy, liver wedge resection, cholecystectomy, portal triad stripping, bowel resection and anastomosis, prophylactic ileostomy, parasternal, anterior mediastinal and supraclavicular lymphadenectomy. However, superficial liver mass excision, diaphragmatic peritonectomy, and cardiophrenic lymphadenectomy were more common in PDS than in IDS/NAC. When we compared the modified SCS between the two groups, high scores ≥8 was more frequent in PDS than in IDS/NAC (77.8% vs 49.1%).
In terms of surgical outcomes, operation time was longer, and the estimated blood loss was higher in PDS than in IDS/NAC. However, hospitalization and acute grade 3 or 4 complications did not differ between the two groups (Supplementary Table 4).
When we divided all patients according to the criteria for ODS in the abdominal and supradiaphragmatic areas, no use of bevacizumab after PDS and non-high-grade serous carcinoma (non-HGSC) after IDS/NAC were factors affecting poor PFS and OS (adjusted HRs, 4.214 and 11.445; 95% CIs, 1.648-10.772 and 2.498-52.437), whereas the size of residual tumors in the abdominal and supradiaphragmatic areas ≥5 mm after PDS was a factor affecting poor PFS and OS (adjusted HRs, 1.726 and 2.097; 95% CIs, 0.968-3.077 and 0.665-6.615; Supplementary Tables 5, 6).
For clarifying the effect of supradiaphragmatic lymphadenectomy in these patients, we divided the size of residual tumors in the supradiaphragmatic area into the three groups as follows: <5 mm without resection; <5 mm with resection; ≥5 mm. Supplementary Table 7 shows the comparison of clinicopathologic characteristics according to the size of residual tumors in the diaphragmatic area. There were no differences in age, ASA score and histology among the three groups, whereas bevacizumab was used more commonly in patients treated with PDS who had the size of residual tumors in the supradiaphragmatic area <5 mm after resection (54.5%), and the size of residual tumors in the abdominal area ≥5 mm was more common when the size of residual tumors in the supradiaphragmatic area was 5 mm or more after IDS/NAC (62.5%).
In terms of PFS, there were no differences based on the size of residual tumors in the supradiaphragmatic area in patients treated with IDS/NAC and those with HGSC of the ovary (median, 17.1 vs 10 vs 14.7 months; 17.1 vs 22.2 vs 18.2 months in < 5 mm without resection, <5 mm with resection, and ≥5 mm; P = 0.19 and 0.48). However, PFS was different according to the size of residual tumors in the supradiaphragmatic area in patients treated with PDS and those with HGSC of the ovary with marginal significance (mean, 26.6 vs 36.9 vs 28.4 months; 23.2 vs 43.8 vs 26.3 months in <5 mm without resection, <5 mm with resection, and ≥5 mm; P = 0.09 and 0.05; Figure 2).
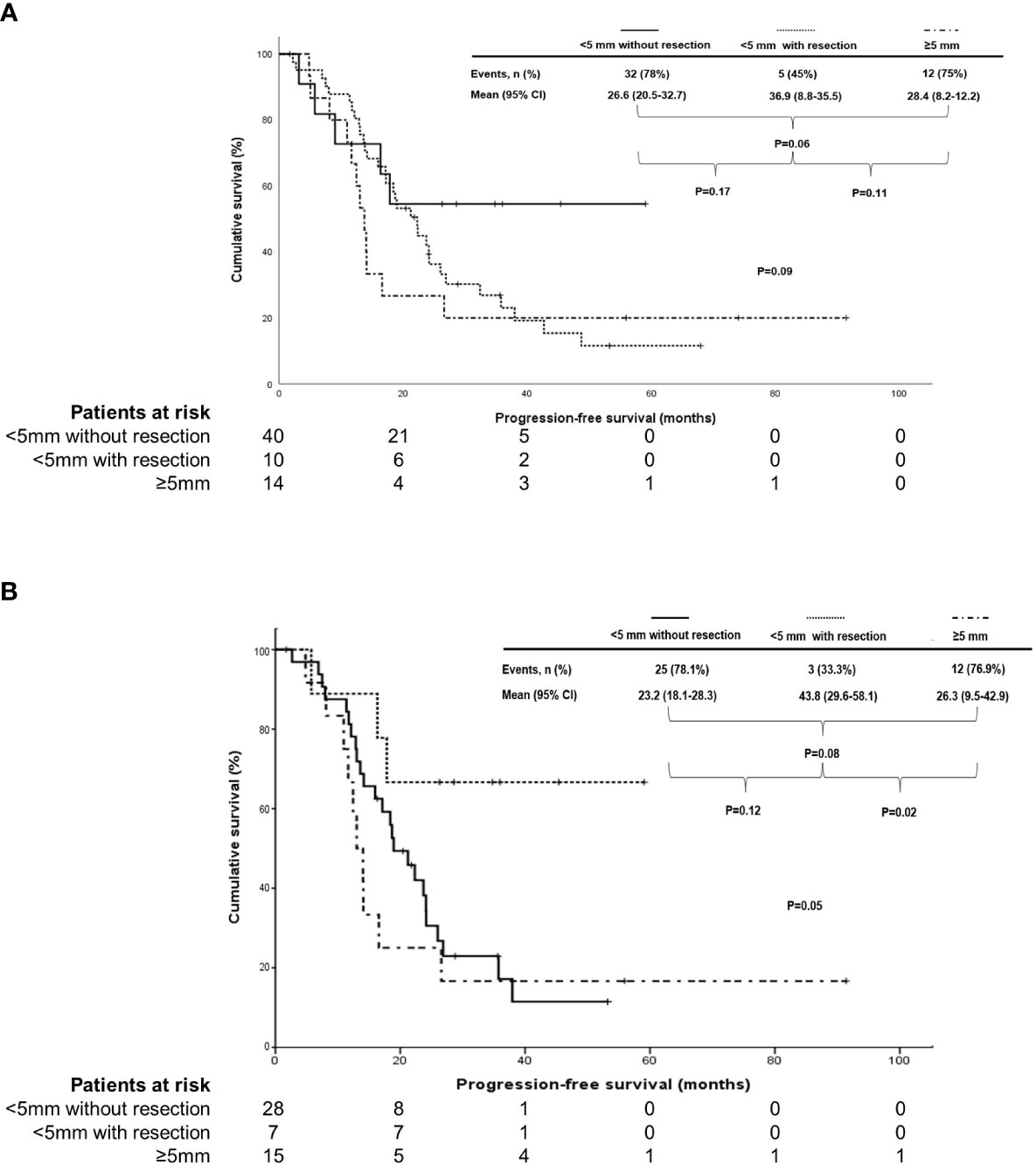
Figure 2 Subgroup analysis in comparison of progression-free survival based on the size of residual tumors in the supradiaphragmatic area (<5 mm without resection vs>5 mm with resection vs≥5 mm) in (A) stage IVB patients with thoracic lymph node metastasis who underwent primary debulking surgery (PDS) and in (B) those who underwent PDS with high-grade serous carcinoma of the ovary.
In terms of OS, there was no difference in OS based on the size of residual tumors in the supradiaphragmatic area in patients treated with PDS and those with HGSC of the ovary (mean, 64.8 vs 48.7 vs 103.4 months; 61 vs 47.8 vs 73.5 months in < 5 mm without resection, <5 mm with resection, and ≥5 mm; P=0.94 and 0.88). Moreover, OS was not different according to the size of residual tumors in the supradiaphragmatic area in patients treated with IDS/NAC and those with HGSC of the ovary (mean, 56.8 vs 48.3 vs 74.2 months; 57.1 vs 52 vs 86.8 months in <5 mm without resection, <5 mm with resection, and ≥ 5 mm; P=0.79 and 0.31).
In patients treated with PDS, no use of bevacizumab was an unfavorable factor for PFS (adjusted HR, 7.240; 95% CI, 2.249-23.304), and the size of residual tumors in the supradiaphragmatic area ≥5 mm was an adverse factor for PFS and OS (adjusted HRs, 6.478 and 6.370; 95% CIs, 2.249-23.304 and 2.224-18.864; Table 3). When we performed subgroup analyses for only patients with HGSC of the ovary, no use of bevacizumab was also a factor related with decreased PFS (adjusted HR, 7.408; 95% CI, 2.044-26.846), and the size of residual tumors in the supradiaphragmatic area ≥5 mm was also a factor associated with decreased PFS and OS (adjusted HRs, 5.945 and 19.685; 95% CIs, 1.805-19.579 and 1.756-220.660; Table 4). However, the size of residual tumors in the supradiaphragmatic area ≥5 mm was not related to survival in those treated with IDS/NAC, whereas the size of residual tumors in the abdominal area was related to decreased OS in patients treated with IDS/NAC and those with HGSC of the ovary (adjusted HRs, 9.330 and 6.209; 95% Cis, 1.386-62.800 and 1.110-34.738; Supplementary Tables 8, 9).
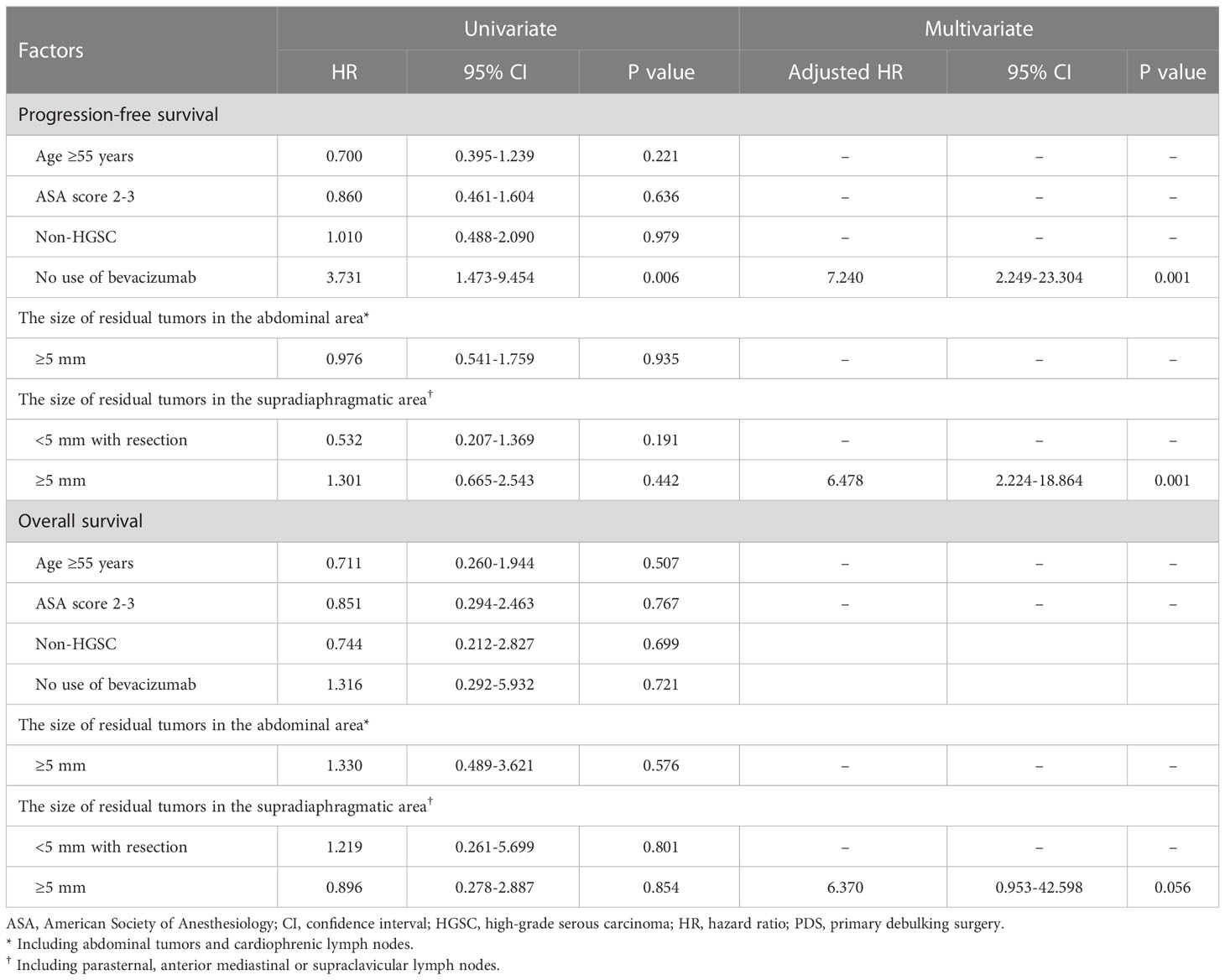
Table 3 Factors affecting progression-free and overall survivals in patients treated with primary debulking surgery.
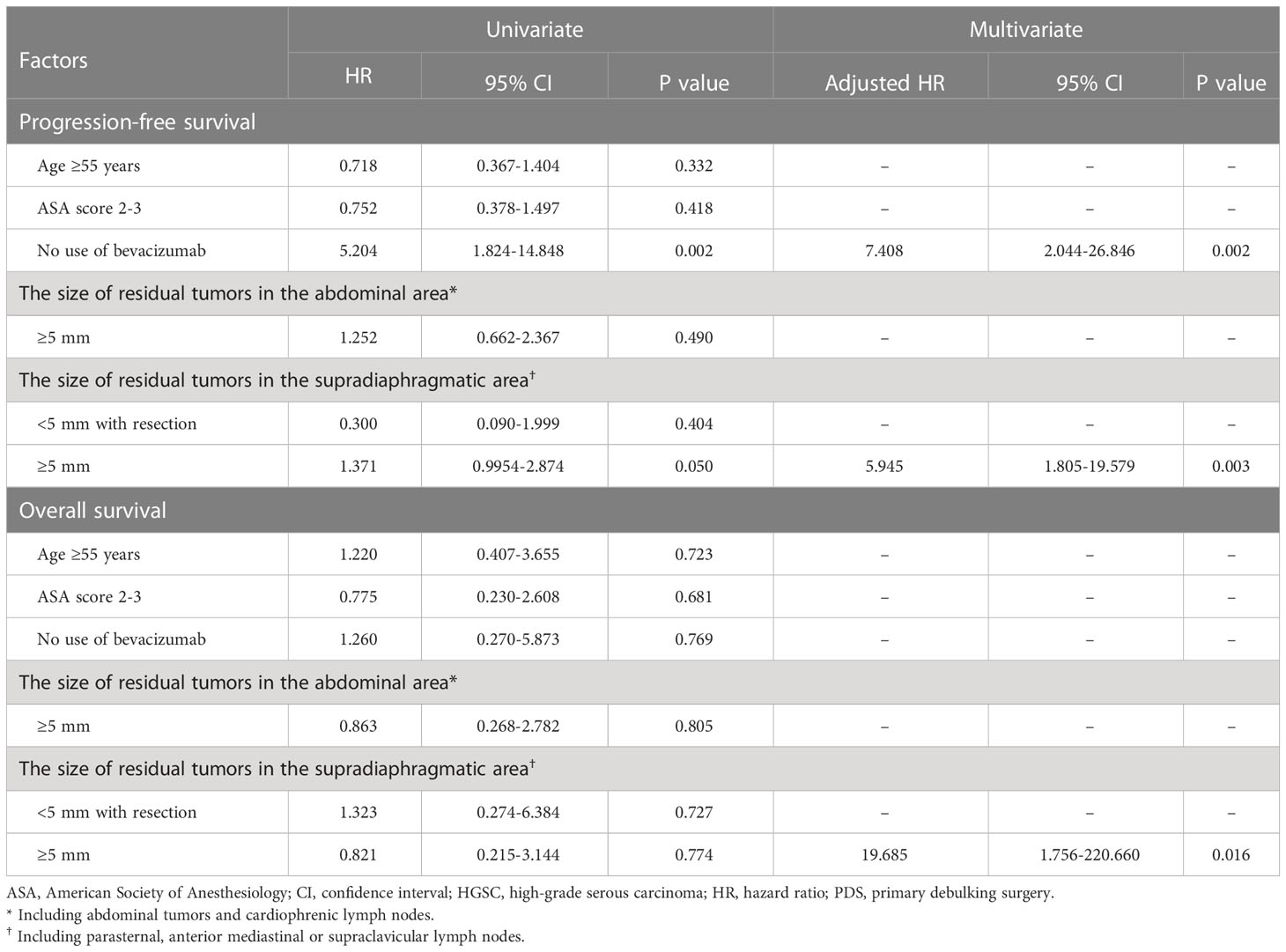
Table 4 Factors affecting progression-free survival in patients with high-grade serous carcinoma of the ovary who underwent primary debulking surgery.
Supplementary Table 10 depicts specific recurrence sites between PDS and IDS/NAC, which shows no difference in them between the two groups. Table 5 shows recurrence sites based on the size of residual tumors in the supradiaphragmatic area. As a result, there was no difference in recurrence sites according to the size of residual tumors in the supradiaphragmatic area after PDS and IDS/NAC.
VATS has been used to detect unexpected LNM or pulmonary metastasis with surgical feasibility and safety in various types of malignancies (12). Even though VATS has been shown to increase survival by facilitating resection of solitary metastases in some cancers, including colon and pancreatic cancers (13–15), the therapeutic role of resection of multiple metastatic tumors in the intrathoracic area has yet to be reported.
In ovarian cancer, the diagnostic and prognostic roles of VATS are also becoming increasingly important. Based on previous studies, VATS is expected to lead to cancer upstaging and change of management in 41% of patients with advanced ovarian cancer (16). Also, enlarged cardiophrenic lymph nodes may predict upper abdominal disease involvement but still benefit from complete resection of abdominal disease (17, 18). Further, macroscopic intrathoracic disease detected on VATS represents an unfavorable prognostic factor (18, 19). However, the therapeutic effect of tumour resection via VATS is also limited and controversial in ovarian cancer (20). Therefore, this study is the first study of its kind evaluating the therapeutic effect of supradiaphragmatic lymphadenectomy, and the well-known effect of ODS on improved survival may not be apparent in stage IVB ovarian cancer with transthoracic LNM.
In terms of lymphatic drainage, the transthoracic and posterior lymphatic pathways contribute to LNM from the abdomen to the supradiaphragmatic lymph nodes. In particular, lymphatic tumour cells of the abdominal surface of the diaphragm invade the parasternal or anterior mediastinal lymph nodes and further into the supraclavicular lymph nodes via the transthoracic pathway. However, lymphatic tumour cells can diffuse through the diaphragm into the aortic hiatus and the thoracic duct via the posterior lymphatic pathway (21). In this study, we selected only stage IVB patients with transthoracic LNM because LNM via the transthoracic pathway is dominant, and lymphadenectomy by VATS is feasible, compared with LNM via posterior lymphatic pathway, which can lead to ODS in these patients (21–23).
In this study, we defined ODS as the size of residual tumor <5 mm in the abdominal and supradiaphragmatic areas due to the two following reasons. First, lymph nodes <5 mm in the supradiaphragmatic area are not resected with VATS generally because it is difficult to localize them (24). Second, cardiophrenic, parasternal and anterior mediastinal lymph nodes are not palpable or visually notable during VATS because they are located in the extrapleural space (25). Third, thoracic procedures should be performed within a short time without serious complications not to interrupt subsequent abdominal debulking surgery and not to reduce the delivery rate of adjuvant chemotherapy (26). Thus, we conducted VATS as a minimally invasive procedure instead of thoracotomy.
As a result, the rate of ODS was similar between PDS and IDS/NAC in the abdominal and supradiaphragmatic areas, whereas operation time was longer, estimated blood loss was more, and the modified SCS was higher in PDS than in IDS/NAC. However, there was no difference in the rate of grade 3 or 4 complications between the two groups. It means that recovery speed after surgery was similar between the two treatments, which could be supported by the finding of no difference in hospitalization between the two groups. In particular, there was no serious complication related to VATS, requiring subsequent intervention in this study.
In terms of survival, ODS in the supradiaphragmatic area improved PFS and OS, especially in patients undergoing PDS. These findings were also observed in those with HGSC of the ovary after PDS. Even though the results of Lymphadenectomy in Ovarian Neoplasms (LION) included stage IIB-IV disease, and thereby did not reveal the effect of lymphadenectomy in stage IVB disease definitely, it emphasizes that the standard procedure may be to remove only suspicious lymph nodes in a case where complete resection may be reached (27). In this study, we also found that PFS could be improved if you could resect tumors ≥5 mm in the supradiaphragmatic area during PDS, suggesting that supradiaphragmatic lymphadenectomy may be important during ODS for complete resection in stage IVB disease with thoracic LNM.
However, the therapeutic effect of supradiaphragmatic lymphadenectomy was not apparent in patients who underwent IDS/NAC, suggesting that NAC has the potential to decrease the size of enlarged lymph nodes but does not eliminate hidden LNMs, which cannot be resected due to size less than 5 mm on axial chest CT before IDS. This hypothesis is supported by previous studies where radiologic lymph node status after NAC differed from pathologic lymph node status and did not affect survival in advanced ovarian cancer (28, 29).
As the other prognostic factors, no use of bevacizumab decreased PFS after PDS, suggesting that these patients should be included in a high-risk group requiring bevacizumab therapy to decrease the risk of disease recurrence (30). Furthermore, the study findings suggest that SDS in the abdominal area remained associated with poor OS after IDS/NAC. Interestingly, no significant impact on OS was observed after PDS. Although ODS is determined by the size of residual tumors, it is clearly distinct from maximal debulking surgery, defined as the removal of resectable lesions if possible. Previous studies have shown that SDS may reflect a lack of effort to perform maximal debulking surgery, leading to poor prognosis in stage IIIC to IVB disease (31–33). Conversely, the attempt to reduce tumour burden via maximal debulking surgery can be expected to increase OS in stage IVB patients with transthoracic LNM during IDS/NAC. Furthermore, tumors that did not respond well to the NAC and remained after surgery may have a more aggressive biology, leading to a greater negative impact on overall survival.
However, this study has some limitations. First, a retrospective study design is associated with an inherent bias for evaluating the effect of supradiaphragmatic lymphadenectomy. Second, the small number of stage IVB patients with only supradiaphragmatic LNM act as a bias to evaluate the surgical effect. When we consider that the number of stage IVB patients with only supradiaphragmatic LNM is relatively small, and the efficacy of thoracic surgery for these patients has not been reported yet, so there are various positions on surgical resection, which makes it difficult to conduct a multi-center retrospective study, large-scale prospective studies are essential to validate these findings. Third, we excluded patients with stage IVB harboring supradiaphragmatic LNM via the posterior lymphatic pathway because it is relatively rare, and the surgical resection is not facilitated by VATS. Fourth, only a small subset of patients received first-line PARP inhibitors, and a significant number of patients did not undergo genetic testing, conducting a meaningful subgroup analysis based on maintenance therapies and BRCA status becomes challenging. Fifth, modifications to our assessment protocol may have influenced the outcomes observed in our study. It is essential to recognize the evolving nature of advanced ovarian cancer surgical management during the specified time interval. Future research should aim to investigate the impact of these changes on patient outcomes. Sixth, it is important to note that including the cardiophrenic area within the abdominal region for analysis could introduce a potential bias, and therefore, the interpretation of our findings should be approached with caution.
In conclusion, the findings of this study highlight the potential therapeutic benefit of supradiaphragmatic lymphadenectomy during PDS in improving PFS and OS of stage IVB ovarian cancer patients with thoracic LNM. It is important to consider that the impact of supradiaphragmatic lymphadenectomy may diminish during IDS/NAC. Furthermore, SDS in the abdominal area remains associated with poor OS after IDS/NAC. These results emphasize the significance of PDS with no residual tumor as the optimal approach for these patients and highlight the importance of considering these factors in clinical practice and treatment decision-making. Further studies are warranted to validate these findings and optimize the management strategies for stage IVB ovarian cancer patients with thoracic LNM.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Seoul National University Hospital Institutional Review Board (1908-173-1059). Written informed consent was not provided because this study poses a low risk to the subjects and utilizes only retrospective medical records, and the Seoul National University Hospital Institutional Review Board has waived the required written consent from the subjects.
SP and HK conceived and designed this study. SJP, SP and HK collected and analyzed the data. SP, KN, ML, IP, HC, CK, J-WK, NP, Y-TK, YS, SP and HK interpreted the data. SJP, SP, and HK drafted this article. All the authors finally approved the submitted version.
This work was supported by Commercializations Promotion Agency for R&D Outcomes grant funded by the Korea government (the Ministry of Science and ICT) (Project No. 1711177795).
We thank to Dreampac Corp. (Wonju, Korea) and Precision Medicine for Peritoneal Metastasis Corp. (Wonju, Korea) for their support. Moreover, we deeply appreciate the investigators of the “Reduction of cycles of neoadjuvant chemotherapy for advanced epithelial ovarian, fallopian or primary peritoneal cancer (ROCOCO)” trial group for their advice in interpreting the results (NCT03693248).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1203127/full#supplementary-material
1. Heintz AP, Odicino F, Maisonneuve P, Quinn MA, Benedet JL, Creasman WT, et al. Carcinoma of the ovary. FIGO 26th annual report on the results of treatment in gynecological cancer. Int J Gynaecol Obstet (2006) 95(Suppl 1):S161–92. doi: 10.1016/S0020-7292(06)60033-7
2. Kim M, Suh DH, Lee KH, Eom KY, Lee JY, Lee YY, et al. Major clinical research advances in gynecologic cancer in 2019. J Gynecol Oncol (2020) 31(3):e48. doi: 10.3802/jgo.2020.31.e48
3. Buys SS, Partridge E, Black A, Johnson CC, Lamerato L, Isaacs C, et al. Effect of screening on ovarian cancer mortality: the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. JAMA (2011) 305(22):2295–303. doi: 10.1001/jama.2011.766
4. Menon U, Gentry-Maharaj A, Burnell M, Singh N, Ryan A, Karpinskyj C, et al. Ovarian cancer population screening and mortality after long-term follow-up in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet (2021) 397(10290):2182–93. doi: 10.1016/S0140-6736(21)00731-5
5. Park SJ, Lee EJ, Lee TS, Wang KL, Okamoto A, Ochiai K, et al. Asian perspective on debulking surgery for advanced ovarian cancer: An E-survey. Eur J Surg Oncol (2021) 47(5):1111–6. doi: 10.1016/j.ejso.2020.11.012
6. Park SJ, Kim J, Kim SN, Lee EJ, Oh S, Seol A, et al. Practice patterns of surgery for advanced ovarian cancer: analysis from international surveys. Jpn J Clin Oncol (2019) 49(2):137–45. doi: 10.1093/jjco/hyy175
7. Kim HS, Bristow RE, Chang SJ. Total parietal peritonectomy with en bloc pelvic resection for advanced ovarian cancer with peritoneal carcinomatosis. Gynecol Oncol (2016) 143(3):688–89. doi: 10.1016/j.ygyno.2016.10.014
8. Chi DS, Franklin CC, Levine DA, Akselrod F, Sabbatini P, Jarnagin WR, et al. Improved optimal cytoreduction rates for stages IIIC and IV epithelial ovarian, fallopian tube, and primary peritoneal cancer: a change in surgical approach. Gynecol Oncol (2004) 94(3):650–54. doi: 10.1016/j.ygyno.2004.01.029
9. Aletti GD, Eisenhauer EL, Santillan A, Axtell A, Aletti G, Holschneider C, et al. Identification of patient groups at highest risk from traditional approach to ovarian cancer treatment. Gynecol Oncol (2011) 120(1):23–8. doi: 10.1016/j.ygyno.2010.09.010
10. Lim MC, Lee HS, Jung DC, Choi JY, Seo SS, Park SY. Pathological diagnosis and cytoreduction of cardiophrenic lymph node and pleural metastasis in ovarian cancer patients using video-assisted thoracic surgery. Ann Surg Oncol (2009) 16(7):1990–6. doi: 10.1245/s10434-009-0486-5
11. Yoo HJ, Lim MC, Song YJ, Jung YS, Kim SH, Yoo CW, et al. Transabdominal cardiophrenic lymph node dissection (CPLND) via incised diaphragm replace conventional video-assisted thoracic surgery for cytoreductive surgery in advanced ovarian cancer. Gynecol Oncol (2013) 129(2):341–45. doi: 10.1016/j.ygyno.2012.12.023
12. Bölükbas S, Sponholz S, Kudelin N, Eberlein M, Schirren J. Risk factors for lymph node metastases and prognosticators of survival in patients undergoing pulmonary metastasectomy for colorectal cancer. Ann Thorac Surg (2014) 97(6):1926–32. doi: 10.1016/j.athoracsur.2014.02.026
13. Rotolo N, De Monte L, Imperatori A, Dominioni L. Pulmonary resections of single metastases from colorectal cancer. Surg Oncol (2007) 16(Suppl 1):S141–4. doi: 10.1016/j.suronc.2007.10.007
14. Robinson LA, Tanvetyanon T, Springett G, Fontaine J, Toloza E, Hodul P, et al. Pulmonary metastasectomy for suspected pancreaticobiliary cancer. J Thorac Cardiovasc Surg (2016) 152(1):75–82. doi: 10.1016/j.jtcvs.2016.02.066
15. Lumachi F, Mazza F, Del Conte A, Lo Re G, Ermani M, Chiara GB, et al. Short-term Survival of Patients with Lung Metastases from Colorectal and Non-colorectal Cancer Who Underwent Pulmonary Metastasectomy [published correction appears in Anticancer Res (2015) Jul;35(7):4371]. Anticancer Res (2015) 35(6):3563–6.
16. Di Guilmi J, Salvo G, Mehran R, Sood AK, Coleman RL, Lu KH, et al. Role of video-assisted thoracoscopy in advanced ovarian cancer: A literature review. Int J Gynecol Cancer (2016) 26(4):801–6. doi: 10.1097/IGC.0000000000000680
17. Boerner T, Filippova OT, Chi AJ, Iasonos A, Zhou QC, Long Roche K, et al. Video-assisted thoracic surgery in the primary management of advanced ovarian carcinoma with moderate to large pleural effusions: A Memorial Sloan Kettering Cancer Center Team Ovary Study. Gynecol Oncol (2020) 159(1):66–71. doi: 10.1016/j.ygyno.2020.07.101
18. Luger AK, Steinkohl F, Aigner F, Jaschke W, Marth C, Zeimet AG, et al. Enlarged cardiophrenic lymph nodes predict disease involvement of the upper abdomen and the outcome of primary surgical debulking in advanced ovarian cancer. Acta Obstet Gynecol Scand (2020) 99(8):1092–9. doi: 10.1111/aogs.13835
19. Kengsakul M, Nieuwenhuyzen-de Boer GM, Bijleveld AHJ, Udomkarnjananun S, Kerr SJ, Niehot CD, et al. Survival in advanced-stage epithelial ovarian cancer patients with cardiophrenic lymphadenopathy who underwent cytoreductive surgery: A systematic review and meta-analysis. Cancers (2021) 13(19):5017. doi: 10.1136/ijgc-2021-IGCS.280
20. Nasser S, Kyrgiou M, Krell J, Haidopoulos D, Bristow R, Fotopoulou C. A review of thoracic and mediastinal cytoreductive techniques in advanced ovarian cancer: extending the boundaries. Ann Surg Oncol (2017) 24(12):3700–5. doi: 10.1245/s10434-017-6051-8
21. Hynninen J, Auranen A, Carpén O, Dean K, Seppänen M, Kemppainen J, et al. FDG PET/CT in staging of advanced epithelial ovarian cancer: frequency of supradiaphragmatic lymph node metastasis challenges the traditional pattern of disease spread. Gynecol Oncol (2012) 126(1):64–8. doi: 10.1016/j.ygyno.2012.04.023
23. Abu-Hijleh MF, Habbal OA, Moqattash ST. The role of the diaphragm in lymphatic absorption from the peritoneal cavity. J Anat (1995) 186(Pt 3):453–67.
24. Prader S, Vollmar N, du Bois A, Heitz F, Schneider S, Ataseven B, et al. Pattern and impact of metastatic cardiophrenic lymph nodes in advanced epithelial ovarian cancer. Gynecol Oncol (2019) 152(1):76–81. doi: 10.1016/j.ygyno.2018.11.001
25. Holloway BJ, Gore ME, A’Hern RP, Parsons C. The significance of paracardiac lymph node enlargement in ovarian cancer. Clin Radiol (1997) 52(9):692–7. doi: 10.1016/S0009-9260(97)80034-7
26. Jiang G, Yang F, Li X, Liu J, Li J, Zhao H, et al. Video-assisted thoracoscopic surgery is more favorable than thoracotomy for administration of adjuvant chemotherapy after lobectomy for non-small cell lung cancer. World J Surg Oncol (2011) 9:170. doi: 10.1186/1477-7819-9-170
27. Harter P, Sehouli J, Lorusso D, Reuss A, Vergote I, Marth C, et al. A randomized trial of lymphadenectomy in patients with advanced ovarian neoplasms. N Engl J Med (2019) 380(9):822–2. doi: 10.1056/NEJMoa1808424
28. Komatsu H, Iida Y, Osaku D, Shimogai R, Chikumi J, Sato SB, et al. Effects of pretreatment radiological and pathological lymph node statuses on prognosis in patients with ovarian cancer who underwent interval debulking surgery with lymphadenectomy following neoadjuvant chemotherapy. J Obstet Gynaecol Res (2021) 47(1):152–8. doi: 10.1111/jog.14446
29. He M, Lai Y, Peng H, Tong C. Role of lymphadenectomy during interval debulking surgery performed after neoadjuvant chemotherapy in patients with advanced ovarian cancer. Front Oncol (2021) 11:646135. doi: 10.3389/fonc.2021.646135
30. Oza AM, Cook AD, Pfisterer J, Embleton A, Ledermann JA, Pujade-Lauraine E, et al. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): overall survival results of a phase 3 randomised trial. Lancet Oncol (2015) 16(8):928–36. doi: 10.1016/S1470-2045(15)00086-8
31. Ren Y, Jiang R, Yin S, You C, Liu D, Cheng X, et al. Radical surgery versus standard surgery for primary cytoreduction of bulky stage IIIC and IV ovarian cancer: an observational study. BMC Cancer (2015) 15:583. doi: 10.1186/s12885-015-1525-1
32. Park SJ, Shim SH, Ji YI, Kwon SH, Lee EJ, Lee M, et al. Reduction of cycles of neoadjuvant chemotherapy for advanced epithelial ovarian, fallopian or primary peritoneal cancer (ROCOCO): study protocol for a phase III randomized controlled trial. BMC Cancer (2020) 20(1):385. doi: 10.1186/s12885-020-06886-2
Keywords: supradiaphragmatic lymphadenectomy, stage IVB ovarian cancer, thoracic lymph node metastasis, residual tumors, overall survival
Citation: Park SJ, Na KJ, Lee M, Park IK, Chung HH, Kang CH, Kim J-W, Park NH, Kim Y-T, Song YS, Park S and Kim HS (2023) Impact of supradiaphragmatic lymphadenectomy on the survival of patients in stage IVB ovarian cancer with thoracic lymph node metastasis. Front. Oncol. 13:1203127. doi: 10.3389/fonc.2023.1203127
Received: 10 April 2023; Accepted: 11 July 2023;
Published: 10 August 2023.
Edited by:
Valerio Gallotta, Agostino Gemelli University Polyclinic (IRCCS), ItalyReviewed by:
Raffaella Ergasti, Agostino Gemelli University Polyclinic (IRCCS), ItalyCopyright © 2023 Park, Na, Lee, Park, Chung, Kang, Kim, Park, Kim, Song, Park and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Samina Park, c2FtaW5hcGFyazEyMDNAZ21haWwuY29t;c2FtODQxMjAzQHNudS5hYy5rcg==; Hee Seung Kim, YmJvZGRpMDMxMUBzbnUuYWMua3I=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.