- 1Department of Gynecological Surgery and Gynecological Oncology, Medical University of Lodz, Lodz, Poland
- 2Department of Gynecological, Endoscopic and Oncological Surgery, Polish Mother’s Health Center—Research Institute, Lodz, Poland
- 3Department of Surgical and Endoscopic Gynecology, Medical University of Lodz, Lodz, Poland
- 4Laboratory of Virology, Institute of Medical Biology of the Polish Academy of Sciences, Lodz, Poland
Ovarian cancer, especially high-grade serous type, is the most lethal gynecological malignancy. The lack of screening programs and the scarcity of symptomatology result in the late diagnosis in about 75% of affected women. Despite very demanding and aggressive surgical treatment, multiple-line chemotherapy regimens and both approved and clinically tested targeted therapies, the overall survival of patients is still unsatisfactory and disappointing. Research studies have recently brought some more understanding of the molecular diversity of the ovarian cancer, its unique intraperitoneal biology, the role of cancer stem cells, and the complexity of tumor microenvironment. There is a growing body of evidence that individualization of the treatment adjusted to the molecular and biochemical signature of the tumor as well as to the medical status of the patient should replace or supplement the foregoing therapy. In this review, we have proposed the principles of the novel regimen of the therapy that we called the “DEPHENCE” system, and we have extensively discussed the results of the studies focused on the ovarian cancer stem cells, other components of cancer metastatic niche, and, finally, clinical trials targeting these two environments. Through this, we have tried to present the evolving landscape of treatment options and put flesh on the experimental approach to attack the high-grade serous ovarian cancer multidirectionally, corresponding to the “DEPHENCE” system postulates.
Introduction
Ovarian cancer, especially its type II according to the dualistic model proposed by Kurman and Shih (1), represented mostly by the high-grade serous ovarian cancer (HGSOC) is the most lethal tumor of the female genital tract. The cumulative 5-year survival in the population of patients with all clinical stages does not exceed 48% (2). Despite the fact that some cases of the HGSOC are primarily chemo-refractory, the most of the cancers belonging to this group are chemosensitive to first-line chemotherapy; however, they quickly acquire the secondary chemoresistance that constitutes the main problem in effective management. Moreover, the HGSOC possesses unique behavior that allows spreading of the tumor cells, mostly in the form of cellular spheroids, from the primary tumor into the distant localizations in the peritoneal cavity. Therefore, the HGSOC is a highly malignant, rapidly progressive tumor characterized by poor prognosis and mortality reaching 90% of all ovarian cancer cases (3).
Ovarian cancer stem cells
One of the main problems in the treatment of HGSOC is the existence of ovarian cancer stem cells (OCSCs) that reside inside tumor niches and cooperate with surrounding cells that compose tumor microenvironment (TME). The character of this cooperation shapes tumor behavior and influences several processes including dormancy, proliferation, metastasis, and, most of all, chemoresistance (4). Cancer stem cells are a population of cells capable of self-renewal and reproduction of the original phenotype of the tumor and are enriched especially in the advanced, disseminated, and recurrent tumors (5). There are two functionally distinct populations of CSCs, proliferating and quiescent, which occupy different niches inside the tumor. The proliferative CSCs are chemoresistant but vulnerable to overdoses of the chemotherapeutics; however, quiescent CSCs are in the autophagic state and could survive even high doses of anti-cancer drugs, thus enabling tumor relapse (6). One of the key phenomena responsible for regulation of stemness is epithelial–mesenchymal transition (EMT) viewed as a continuum of phenotype cellular states from complete epithelial and proliferative state, through several intermediate hybrid states to complete mesenchymal and invasive phenotype. Cancer stem cells could represent any of these steps due to the outstanding plasticity (7). This plasticity of CSCs is highly dependent on the patient’s immunosurveillance as well as on epigenetic and environmental signals from the TME (6). The most recognized stressors that could influence both phenotype and function of CSCs are hypoxia, acidity, mechanical stress, immunological response, epigenetic changes like DNA methylation, histone and non-coding RNA modifications, and, finally, activation of stemness signaling pathways (8–11).
The problem of the stemness is directly connected to the cancer dormancy that is dependent on the presence of circulating tumor cells (CTCs) and disseminated tumor cells (DTCs) that have partially overlapping functions and are enriched by the population of quiescent CSCs (12). CTCs, DTCs, and CSCs are able to produce micrometastases that migrate and home inside the target organs in the pre-metastatic niches composed from tumor cells and recruited local stromal and immune cells from the environment. Quiescent CSCs and dormant DTCs inside pre-metastatic niche show overexpression of signaling pathways, enabling them to survive in stressful conditions, including chemotherapeutics (13–15).
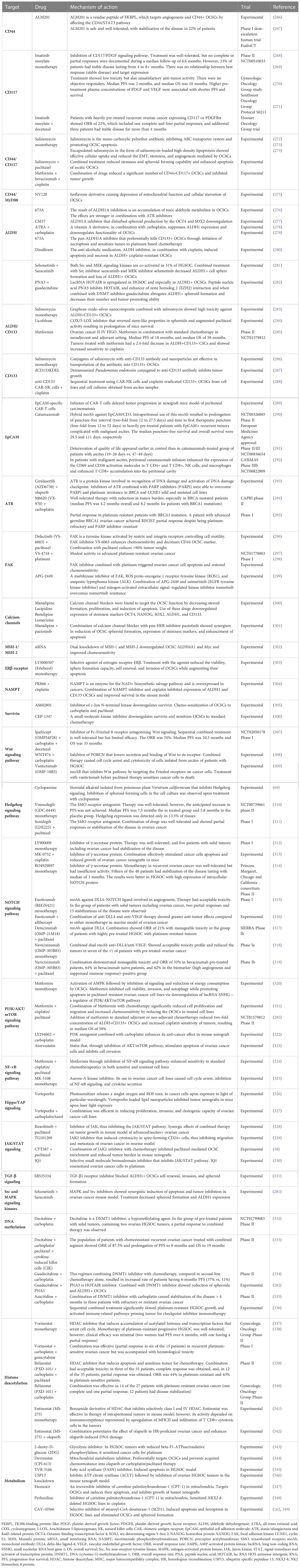
Ovarian CSCs are characterized by cell surface CD44, CD117, CD133, CD24, MyD88, epithelial cell adhesion molecule (EpCAM), leucine-rich repeat containing G protein–coupled receptor-5 (LGR5), and LGR6] and intracellular [aldehyde dehydrogenase (ALDH), sex determining region Y-box 2 (SOX2), octamer-binding transcription factor-4 (OCT4), homeobox protein NANOG transcription factor (NANOG), and forkhead box protein M1 (FOXM1)] markers, as well as by their specific behavior (“side-population” cells). The markers for characterization of OCSCs, their function, and clinical significance are presented in Table 1. The OCSC markers unfortunately are not cancer stem cell specific, as they are also present on normal stem cells. Another feature of OCSCs is activation of signaling pathways upregulating their stemness, cancer proliferative capability, and chemoresistance. The most important and studied pathways for preservation of OCSC function are Wnt/β-catenin, Hedgehog, Hippo/yes-associated protein (YAP), neurogenic locus notch homolog (NOTCH), nuclear factor-κ-light chain enhancer of activated B cells (NF-κB), hypoxia-induced factor-1α (HIF-1α), PI3K/protein kinase B (AKT), Janus kinase (JAK)/signal transducer and activator of transcription protein (STAT), transforming growth factor–β (TGF-β), and Rho/Rho-associated protein kinase (Rho/ROCK) pathways. The functional and clinical characterization of these pathways is included in Table 2.
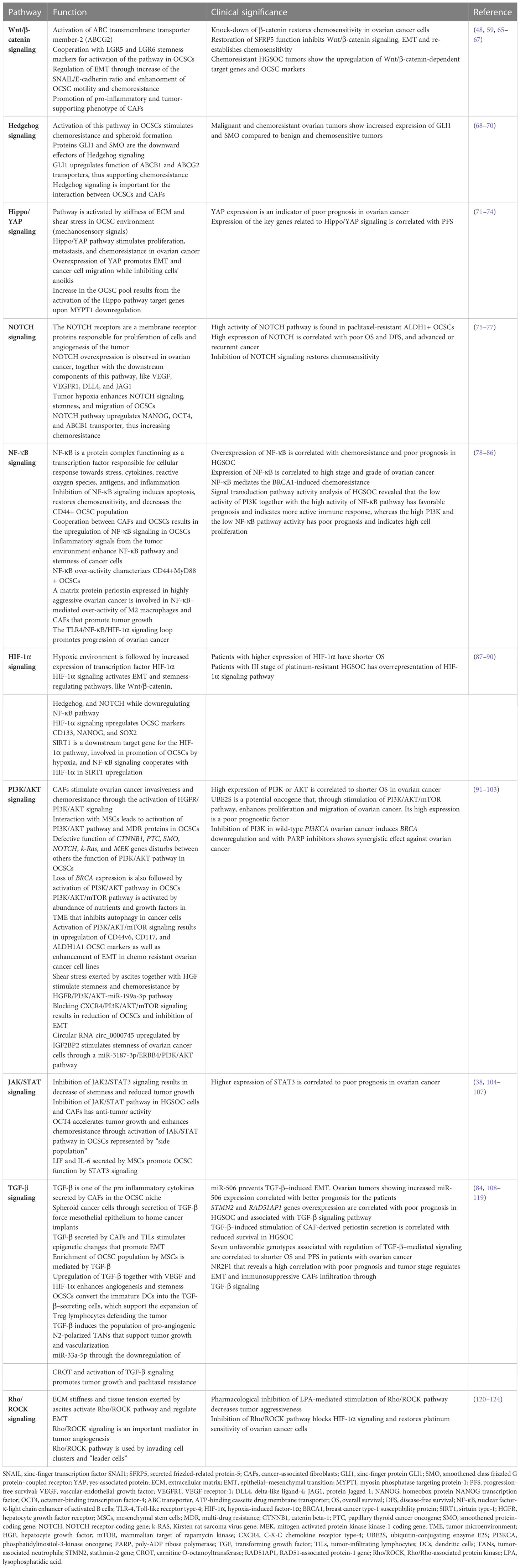
Table 2 The functional and clinical characterization of ovarian cancer stem cell signaling pathways.
Tumor microenvironment in ovarian cancer
Ascites is a unique microenvironment for OCSCs and is responsible for exceptional biology of ovarian cancer, shaped by the transcoelomic spread of peritoneal implants. The EMT process enables the tumor cells from primary localization to seed in the form of multiform cellular conglomerates, mostly adopting the form of spheroids enriched in OCSCs. They are transported in fluid into distant places of peritoneal cavity, with the predisposition to home into the adipose tissue collections inside peritoneum, like “milky spots”, omental fat, mesentery, or bowel appendices (125). Sphere-forming cells express OCSC markers CD44v6, CD117, ALDH1, and NANOG and are resistant to anoikis despite lack of anchorage to the surface (16, 126). The presence of cytokines [interleukin-6 (IL-6), IL-8, IL-10, and vascular endothelial growth factor (VEGF)], osteoprotegerin, and exosomes containing micro RNAs (miRNAs), cytokines, and growth factors further enhances stemness in the spheroids (38, 68, 127–129). Spheroids adhere to and destroy the mesothelium, go through the mesenchymal/epithelial transition, and start to proliferate (130, 131). TGF-β, CD133, and CD44 from spheroids stimulate mesothelium to produce fibronectin for cancer cells adhesion, enhance attachment of floating cells to the epithelial surface, and stimulate secretion of metalloproteinase-9 (MMP-9) that supports mesothelial invasion (108, 132). The initial opinions on random transportation of cellular conglomerates have been replaced by the theory of collective invasion, according to that clusters of cancer cells migrate in a directed and coordinated way (133). Collective invasion is described by some characteristic features, mainly preservation of cell-cell junctions, interaction with surface cells and ECM on their way, cooperative cytoskeleton dynamics enabling migration of clusters as a single unit, and multicellular polarity (120, 133–135). Despite a collective behavior, not all cells in the cluster are invasion-competent, and the population of cells that rule invasion is called “leader cells”. These cells delineate the way, change cellular contractility, and grow invadopodia, as well as respond to environmental signals (120, 136–138). Their presence at the front of the cluster results in its polarization. The coordinated movement requires rearrangement of the cytoskeleton, actinomyosin contraction, and activation of PI3K and Rho/ROCK pathways (120, 135, 139). After adhesion to the mesothelial surface, “leader cells” express proteolytic enzymes and penetrate the basement membrane (120, 140). The phenotype of “leader cells” is characterized by the keratin-14 (KRT14) expression. Their functional phenotype resembles the OCSC phenotype but does not correlate to EMT. The KRT14+ cells are able to re-establish the epithelial cells, show clonogenicity, are abundant in metastases, are enriched in response to chemotherapy, and promote the chemoresistance (120, 140–143). Cancer-associated fibroblasts (CAFs) present in TME play important role in collective invasion by regulation of TME remodeling to “pave” the routs for migrating cell clusters (120, 144). After exposition to chemotherapy, the population of apoptosis-resistant “leader cells” increases and shows expression of ALDH1 and CD44v6 stemness markers together with chemoresistance. Functional impairment of the “leader cells” restores chemosensitivity in vitro (145). After homing into peritoneal environment, OCSCs reside inside the “metastatic niche” composed of several cell populations, ECM components, lipids, exosomes, regulatory RNAs, and hypoxia that are orchestrated to support the OCSCs. Table 3 presents the function and clinical significance of the main components of the TME inside the “metastatic niche”.
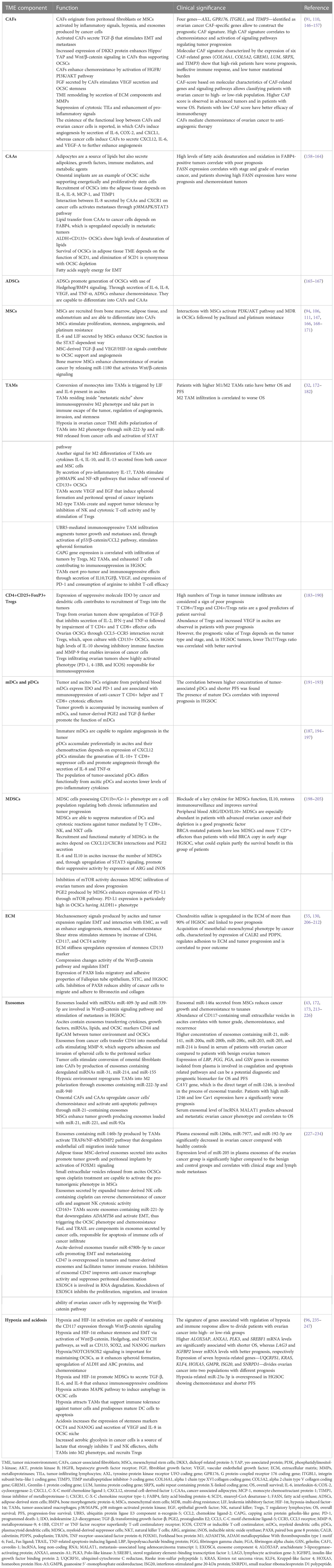
Table 3 The function and clinical significance of the main components of the tumor microenvironment inside the “metastatic niche”.
Obstacles in the treatment of the HGSOC
The treatment of ovarian cancer is based on debulking cytoreductive surgery, platinum-taxane–based first line chemotherapy, second-line chemotherapy, and targeted therapy approved by the FDA (Food and Drug Administration) and EMA (European Medicines Agency) using bevacizumab and poly-ADP-ribose polymerase (PARP) inhibitors. Several others drugs are being tested in clinical trials including programmed death-1 (PD-1)/ programmed death ligand-1 (PD-L1) inhibitors. HGSOC is initially a chemosensitive tumor, especially in the cases of positive BRCA germinal or somatic mutations. However, recurrent tumors are mostly chemoresistant due to activation of many mechanisms associated with the exceptional function and proliferative activity of OCSCs or reverse BRCA mutations occurring during the treatment. Moreover, the unique pattern of cancer spread inside peritoneal cavity that utilizes both collective invasion and sanguiferous route is relatively early phenomena in the course of the disease. The important obstacle in the effective treatment of HGSOC is also tumor heterogeneity comprehended as spatial heterogeneity in the different areas of the tumor, the inter-patient heterogeneity, and temporal heterogeneity between primary tumors, metastases, and recurrent disease. Even OCSCs themselves exhibit unexpected phenotypic plasticity and may differ in the same patient or among different patients depending on the cancer molecular type, advancement of the disease, patient health, and treatment scheme. The conclusion from those observations is that the use of the uniform treatment for all patients or for all temporal stages of the tumor is an oversimplification that results in observed unsatisfactory results in the context of both OS and PFS. The complexity of interaction between tumor cells, OCSCs, and TME in metastatic niche is another factor of great importance for supporting tumor growth, enhancing chemoresistance and the immune attack defiance. Therefore, tumor environment with all its components should also be treated as a target for anti-cancer therapy.
Remarks on the targeting of the OCSCs
Taking the abovementioned reflections into consideration, the interesting targets for multidirectional treatment are OCSCs themselves and the components of OCSC microenvironment, particularly metastatic niche. One of the most explored areas of anti-OCSCs therapy is drugs directed against OCSC markers, signaling pathways, and epigenetic regulators. Targeting OCSC markers is important as chemotherapy, whereas decreasing tumor burden simultaneously increases the number of OCSCs. After exposition to chemotherapy, increased numbers of ascitic EpCAM+, CD44+, and OCT4+ cells were noted (248). Similarly, recurrent tumors contain more ALDHA1+, CD133+, and CD44+ OCSCs than primary tumors (49). These phenomena are observed not only in standard platinum/taxol-based chemotherapy but also in the tumors treated with PARP inhibitors (PARPis), where increased numbers of CD133+ and CD117+ OCSCs precede the acquired PARP resistance (249). However, targeting OCSC markers has to overcome two problems. The first one is that OCSC markers are not able to distinguish cancer stem cells exclusively, as about 75% of known cancer stem cell markers are also present on the surface of embryonic and adult stem cells (250). For instance, CD44 is present on hematopoietic cells, MSCs, and adipose-derived stem cells (251–253). CD117 is positive on 25% of embryonic stem cells (254), whereas CD166 is also found on epithelial cells, MSCs, and intestinal stem cells (255, 256). Intracellular cancer stem cell markers, like NANOG, OCT4, and SOX2, are also present in normal stem cells (257, 258). The second problem is associated with the fact that there is no universal cancer stem cell marker known. Tumor heterogeneity, differentiation status, and environment are reasons for OCSC different types. Therefore, the more effective strategy of elimination of OCSCs relies on targeting of at least two OCSC markers simultaneously. Targeting the signaling pathways used by OCSCs is also reasoned by the fact that many of them are likewise OCSC markers, activated after exposition to chemotherapy (259). Epigenetic regulation in ovarian cancer is associated with both hypermethylation and hypomethylation of DNA, as well as with histone methylation and acethylation. Hypermethylation of DNA contributes to formation of OCSCs (260). The CpG islands of many onco-suppressor genes were shown to be hypermethylated in ovarian cancer, leading to the loss of DNA-repair function and cell cycle control desynchronization (261). Upon chemotherapy, hypermethylation of genes responsible for cell resistance to apoptosis was detected (262). Gene hypomethylation is frequently observed in advanced HGSOC and correlates with worse survival (263). Histone methylation is engaged in upregulation of ATP-binding cassette drug membrane (ABC) transporters in chemoresistant OCSCs (264). Disturbed function of histone deacetylases promotes tumor progression (265). Table 4 contains data on both the experimental and clinical trials of targeting OCSCs.
Remarks on the targeting of the tumor microenvironment
One of the most important targets in TME is CAFs. However, the past experience with anti-CAFs therapy has indicated that the aim in this approach should be to revert CAFs functionally back to normal fibroblasts, rather than eradicating them completely from the TME. Eradication of CAFs has proved to change the tumor into more aggressive phenotype, instead of eliminating tumor cells (350). It is even more important taking into consideration that CAF populations of different tumor-promoting abilities and phenotype (CD49e+, fibroblast activation protein FAP-high or FAP-low) have been identified (351). The reprogramming of M2 tumor-associated macrophages (TAMs), another key population of tumor-supporting cells, into M1 phenotype could be similarly to CAFs, which is a better option than eliminating them completely (352). Another, recently identified population of cells in TME is cancer-associated mesothelial cells (CAMs) that originate from peritoneal normal mesothelial cells activated by cancer-derived promoting factors that induce mesothelial–mesenchymal transition and secretion of factors, enhancing peritoneal metastases and chemoresistance (353). Hepatocyte growth factor (HGF) released from ovarian cancer cells in hypoxic conditions induces the senescence of mesothelial cells and downregulates the expression of junctional proteins that results in disintegration of mesothelial integrity and enables cancer invasion through the mesothelial barrier (354, 355). Phenotypic changes of mesothelial cells to CAMs are mediated by TGF-β and CD44 and annexin A2 secreted inside exosomes from cancer cells (356–358). In response to those changes, CAMs secrete VEGF and upregulate fibronectin expression in ECM, thus promoting tumor vascularization and binding of tumor cells’ integrins to ECM to support metastases (108, 359). Moreover, CAMs increase secretion of IL-8 and CCL2 that stimulate pyruvate dehydrogenase kinase-1 in cancer cells followed by increased expression of integrins to enhance adhesion and migration (360, 361). Interaction between intelectin-1 on CAMs and lipoprotein receptor–related protein-1 on cancer cells also contributes to invasion by upregulation of MMP-1 (362). CAMs pre-stimulated by cancer cell–derived TGF-β secret osteopontin, which, in turn, activates CD44/PI3K/AKT pathway in OCSCs, leading to ABC transporters’ overexpression and chemoresistance (363). M2-shifted TAMs also support CAMs activity by macrophage inflammatory protein-1β that activates P-selectin secretion by CAMs, followed by stimulation of CD24 on the cancer cells’ surface and increased adhesion (364). CAMs are, in turn, able to polarize the TAM phenotype into M2 type (365). CAMs are also capable to regulate the expression of glucose transporter type 4, resulting in increased glucose intake by cancer cells and growth promotion (362). Because of all above functions, CAMs are an interesting target for anti-TME therapy in ovarian cancer.
The next promising target for the therapy is metabolism of cancer cells. Cancer cells use both aerobic glycolysis (the Warburg effect) and oxidative phosphorylation (OXPHOS). Aerobic glycolysis protects cells from oxydative stress and fuels proliferation. However, OXPHOS and resistance to glucose deprivation in tumor environment are a metabolic adaptation enabling chemoresistance. Both ways of glucose metabolism are therefore used by cancer cells, including OCSCs and are another sign of their plasticity (366–368). The metabolic interactions between omental adipocytes and OCSCs are another reason for cancer progression and chemoresistance. Fatty acids could be very efficient source of energy that fuels the spread and growth of peritoneal implants (369). Adipocytes are stimulated by cancer cells to release fatty acids into metastatic niche, and, in turn, adipocytes induce expression of fatty acid receptor CD36 on cancer cells, thus enhancing uptake of fatty acids by cancer (370). Colonization of omental tissue depends on expression of salt-inducible kinase 2 (SIK2) in cancer cells. SIK2 kinase stimulates cell proliferation in PI3K/AKT-mediated manner and enhances paclitaxel resistance in HGSOC cells (371). Moreover, fatty acid oxidase and fatty acid synthase (FASN) have been shown to sustain survival of cancer cells in TME and increase resistance to anoikis and chemotherapy and spheroid formation in HGSOC lines (347, 372). Ovarian cancer CSCs indicate increased concentration of unsaturated lipids and what enhances cell membrane fluidity and facilitates OCSC plasticity and self-renewal. Inhibition of desaturases inhibits spheroid formation and abrogates tumor growth and metastases (373).
Another potential target for anti-TME therapy in HGSOC is exosomes. The identification of their origin inside TME and the recognition of their cargo have the key role in exosome-directed therapy. Exosomes could be also used as potential vehicles for the transportation of drugs into the tumor. It was also found that exosomes secreted from untreated tumors have a significant influence on the expression of many genes involved in functional change of fibroblasts into CAFs and in stimulation of tumor metastases. Such ability was less evident in exosomes secreted by pre-treated tumors (374). The situation is, therefore, complicated, as it seems that exosomes differ depending not only on the type of secreting cell but also on its functional status and temporal changes during therapy. Exosomes are able to influence several mechanisms of tumor growth. Their cargo, including proteins, neoantigens, cytokines, growth factors, and miRNAs, is responsible for cancer progression, metastases, and chemoresistance. Exosomes contain also modulators of immune response capable of inhibition of macrophages: natural killer (NK) cells, dendritic cells (DCs), and B and T lymphocytes (375, 376). Exosomes negatively regulate immunosurveillance of the host against tumor, through inhibition of T lymphocytes, NK cells, DCs, and monocytes in tumor environment and ascites (227, 377–379). Exosomes stimulate tumor angiogenesis affecting the VEGF and HIF-1α expression and by activation of Wnt/β-catenin and NF-κB signaling pathways (380, 381). Exosomes influence also stroma remodeling by cooperation with CAFs and adipocyte-derived stem cells (165). Recently, tumor-derived exosomal miR-141 was identified as a regulator of stromal-tumor interactions and inducer of tumor-promoting stromal niche by activation of YAP/chemokine (C-X-C motif) ligand 1 (GROα)/CXCR signaling pathway (382). One of the most interesting vectors of information between cancer cells and TME is non-coding miRNAs and long non-coding RNAs (lncRNAs). They were found in the serum and ascites of patients with ovarian cancer (227, 228); however, their presence in tumor-derived exosomes ensures safe and undisturbed transportation to the target cells. Non-coding RNAs play extremely important functions. Exosomes loaded with miR-1246 are able to enhance pro-tumorigenic effects of M2-shifted TAMs and to facilitate paclitaxel resistance (383). Cancer cell–derived miR-21-3p, miR-222, miR-125b-5p, miR-181d-5p, and miR-940 target TAMs and polarize them into M2 phenotype (172, 384). miR-99a-5p affects human peritoneal mesothelial cells and enhances cancer cell invasion (385). The Let-7a and miR-200a regulate tumor invasiveness (386). Exosomes containing lncRNAs ENST00000444164 and ENST0000043768 are responsible for activation of NF-κB signaling in cancer cells (387). Table 5 presents data on targeting the components of TME and OCSC niche.
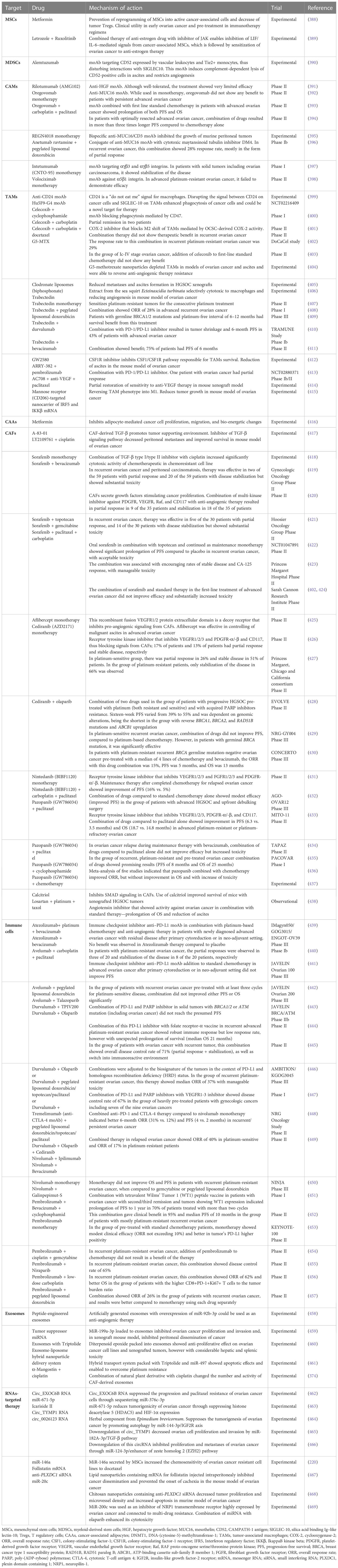
Table 5 Data on the experimental and clinical trials of drugs targeting the tumor microenvironment and OCSC metastatic niche.
A novel regimen of therapy
The urgent need for improvement of efficacy in the HGSOC treatment is obvious, and many researchers have called attention to the novel approaches in diagnosis, monitoring, and management of patients with ovarian cancer. We have learned from the experience from therapy of hematologic cancers and several solid tumors that the individual approach to the treatment based on genetic, molecular, or metabolic signatures of the patients and the cancer itself usually results in better treatment efficacy and improved outcome. However, such individualization of therapy is much more difficult to be used in solid tumors, compared to hematologic malignancies, and ovarian cancer due to its unique biology is even more demanding and challenging target.
In the recent article devoted to OCSCs and OCSC-targeted treatment (470), we proposed that the novel complex standard of ovarian cancer therapy called the “DEPHENCE” system (“Dynamic PHarmacologic survEillaNCE”) should be worked out. In our opinion, it ought to be based on the following rules:
1. avoidance of monotherapy, as usually combination of several drugs directed against different targets, is more efficient and, if properly orchestrated, could be less toxic;
2. identification of the markers for pharmacologic compliance or resistance of the tumor and stratification of the patients according to the prognosis of treatment efficacy;
3. performing the sampling of the tumor (primary, metastatic, and recurrent) repetitively for characterization of genetic signature and TME features, which could change in the course of the disease and in the response to the treatment;
4. using the repeated biopsy of the tumor, but preferentially liquid biopsy, which enables to obtain more complex picture of growing tumor, as compared to standard biopsy the results of liquid biopsy do not depend on the site of the harvest of the sample;
5. such approach and individualization of the therapy could enable to restore the pharmacologic surveillance over the tumor that fits the actual status of both tumor and the patient;
6. every line of treatment should simultaneously target cancer cells, OCSCs, and elements of TME, as well as should generate potentialization of the patient’s immune status;
7. HGSOC molecular types and different phases of the disease need different approach to the therapy;
8. at the beginning, such therapy could allow for stabilization of the disease, hopefully enabling prolongation of PFS and OS; however, in a distant future the goal of this approach should be complete curation.
We think that the necessary components incorporated into the DEPHENCE system should also be
1. identification of the high-risk population of women (gene mutations, single-nucleotide polymorphisms, metabolic syndromes, and environmental factors);
2. searching for the techniques of early detection or even for the screening tools both in the high-risk and general populations;
3. searching for the infection factors responsible potentially for ovarian cancer origin (viruses, microbiome disturbances);
4. looking for prognosis biomarkers of ovarian cancer.
The practical implementation of the “DEPHENCE” system in the diagnosis and therapy of ovarian cancer is still awaiting, although the first signs of its use can be seen in the attempts to classify the molecular signatures of the tumors and TME components (158, 458, 471–476), to personalize therapy according to the tumor origin, histology, and most of all to genomic and epigenomic disturbances. The first such studies grouped HGSOC tumors T into four subtypes: C1, high stromal response; C2, high immune signature; C4, low stromal response; and C5, mesenchymal, with low immune signature. These subtypes differed in the extent of immune infiltration, desmoplasia, and EMT predisposition, and what could suggest different approach to the treatment, including immunotherapy, and patients from the C1 and C5 subtypes showed poor survival compared with other subtypes (3). Another genomic classification was proposed by The Cancer Genome Atlas Research Network, which, based on the genomic pattern, divided the ovarian cancer into four subtypes: mesenchymal, immunoreactive, proliferative, and differentiated. Mesenchymal and proliferative subtypes showed profound desmoplasia and invasive gene expression pattern, with limited immune infiltration and activation of stemness markers. Both were characterized by unfavorable prognosis. Immunoreactive subtype showed extensive immune infiltration and, similar to differentiated more mature tumors, had better prognosis (477–479). The next analysis of tumor genome identified three novel ovarian cancer subtypes named tumor-enriched, immune-enriched, and mixed. The meaning of these subtypes for therapy implies that tumor-enriched tumors should be treated with tumor killing therapy, whereas immune-enriched tumors with immunotherapy or mixture of both approaches (480). Molecular characterization of platinum-refractory and platinum-resistant ovarian tumors identified three tumor clusters: cluster 1 with overrepresentation of growth factor signaling pathways, cluster 2 with pathways regulating cell survival in hypoxic conditions and senescence, and cluster 3 related to cellular senescence. A possible treatment of choice for cluster 1 could be tyrosine kinase or angiokinase inhibitors, cluster 2 could theoretically response to mTOR inhibitors, whereas cluster 3 could be treated with the deacetylase inhibitors (87, 481, 482). Another single-cell transcriptome study revealed the heterogeneity of HGSOC, which was found to be composed of several cell clusters. The first one called EC1 showed gene enrichment for glycolysis/gluconeogenesis and ECM-receptor interactions. The EC2 subtype expressed genes, suggesting their origin from tube epithelium. The EC3 subtype showed overexpression of genes associated with function of ABC transporters, suggesting a potential to be a drug-resistant subtype. EC4 subtype was characterized by the immune response-related pathways indicating the activity of EC4 cells in immune response. The chemoresistance responsible genes were strongly represented in EC5 cell population (483).
Epigenomic analysis of immune-related lncRNAs revealed RNAs having the potential to divide the population of patients with ovarian cancer into high-risk and low-risk groups characterized by a shorter or longer overall survival (OS), respectively. High-risk score tumors were positively correlated with abundant representation of checkpoint and immunosuppressive molecules, indicating the group of patients with compromised anti-tumor immune response (484). The DNA methylation signatures represent another epigenetic point of interest in ovarian tumors. The hypomethylated upregulated tumor necrosis factor (TNF), estrogen receptor 1 (ESR1), mucin 1 (MUC1) genes, and hypermethylated downregulated forkhead box O1 (FOXO1) gene could serve as targets for epigenetic therapy and were correlated with patients’ prognosis (485).
According to the TME components, the four different CAF subsets (S1 to S4) were identified in ovarian tumors. The HGSOC of mesenchymal subtype, defined by stromal gene signatures and poor survival, had high numbers of CAF-S1 cells, which attracted and sustained immunosuppressive infiltration of Treg CD25+FoxP3+ T lymphocytes (475). The study of immunological profile of HGSOC showed the presence of activated‐immune and CAF‐immune subtypes. Activated-immune subtype showed anti-tumor features exemplified by active immune response and better prognosis. The CAF‐immune subtype was characterized by tumor‐promoting signals like, activated stroma, M2 macrophages, and a poor prognosis. The activated‐immune subtype was more likely than the CAF‐immune subtype to respond to checkpoint blockade immunotherapy (486).
The most painful problem in ovarian cancer therapy is the acquired chemoresistance following the initial good response to the first-line chemotherapy. Therefore, identification of the biomarkers of chemoresistance is one of the most important activities in ovarian cancer surveillance. The classic biomarkers of platinum and PARP chemosensitivity are the germinal and somatic mutations of BRCA1/2 genes (487). However, the reversion mutations in BRCA genes and in other homologous recombination repair (HR) genes were found to be responsible for secondary resistance to platinum- and PARPi–based therapy (488, 489). On the basis of the homologous recombination deficiency, insertions and deletions, copy number changes, and mutational signatures, a combined predictor of platinum resistance, named DRDscore, was established, and, when validated in a cohort of patients with HGSOC, it reached sensitivity of 91% (490). Four miRNA biomarkers (miR-454-3p, miR-98-5p, miR-183-5p, and miR-22-3p) identified in ovarian cancer tissues were able to discriminate between platinum-sensitive and platinum-resistant patients with HGSOC (491). Treatment using PARPis results in acquired PARPi resistance. The reason for this is a promotion of STAT3 activity both in tumor cells and populations of immune and CAF cells, followed by creation of an immunosuppressive environment. Treatment of olaparib-resistant ovarian cancer cell line with napabucasin, the STAT3 inhibitor, improved PARPi sensitivity (492). Hypoxia and therapy-induced senescence are the key drivers of primary chemo-refractoriness and secondary chemoresistance of HGSOC (493). Hypoxic TME induces the M2-phenotype in TAMs, which, in turn, secrete exosomes containing miR-223 that, when transported into ovarian cancer cells, makes them chemoresistant (494). To overcome chemoresistance, there are plenty of different drug combinations tested in both experimental and clinical settings (Tables 4, 5). Simultaneously, identification of potentially resistant tumors is of the utmost importance for successful therapy. Identification of ovarian cancer cells with high-stress signature and disturbed drug responsiveness could optimize the subsequent therapy to attenuate their function or eliminate them from the tumor (493, 495, 496). Moreover, as HGSOC tumors are characterized by temporal heterogeneity, the repetitive circulating tumor DNA (ctDNA)/CTCs testing should be performed to have the most actual picture of the disease.
The exploration of the infection factors in the origin or predisposition to ovarian cancer is also being realized in the analysis of microbiome and viral infections (497–499). Another field of intensive investigation is searching for prognostic biomarkers (500–503). It is a lot of work to do to safely and effectively combine different drugs, but the practical use of the “DEPHENCE” system philosophy could, in our opinion, lead doctors and researchers in proper direction.
Author contributions
All authors contributed to the conception of the review. Data collection and analysis were performed by JW, MW, and EP. The first version of manuscript was written by JW and MW, and all authors commented on previous versions of the paper. EP and MW read and approved the final version of the manuscript, made linguistic corrections, and revised the text. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by the National Science Centre of Poland, grant number 2019/33/B/NZ7/02872.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kurman RJ, Shih I-M. The dualistic model of ovarian carcinogenesis. Am J Pathol (2016) 186:733–47. doi: 10.1016/j.ajpath.2015.11.011
2. Survival rates for ovarian cancer (2022). Available at: https://www.cancer.org/cancer/ovarian-cancer/detection-diagnosis-staging/survival-rates.html.
3. Tothill RW, Tinker AV, George J, Brown R, Fox SB, Lade S, et al. Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome. Clin Cancer Res (2008) 14:5198–208. doi: 10.1158/1078-0432.CCR-08-0196
4. Muñoz-Galván S, Carnero A. Targeting cancer stem cells to overcome therapy resistance in ovarian cancer. Cells (2020) 9:1402. doi: 10.3390/cells9061402
5. Wang X. Stem cells in tissues, organoids, and cancers. Cell Mol Life Sci (2019) 76:4043–70. doi: 10.1007/s00018-019-03199-x
6. Batlle E, Clevers H. Cancer stem cells revisited. Nat Med (2017) 23:1124–34. doi: 10.1038/nm.4409
7. Tam WL, Weinberg RA. The epigenetics of epithelial-mesenchymal plasticity in cancer. Nat Med (2013) 19:1438–49. doi: 10.1038/nm.3336
8. Berabez N, Durand S, Gabut M. Post-transcriptional regulations of cancer stem cell homeostasis. Curr Opin Oncol (2019) 31:100–7. doi: 10.1097/CCO.0000000000000503
9. Marcucci F, Bellone M, Caserta CA, Corti A. Pushing tumor cells towards a malignant phenotype: stimuli from the microenvironment, intercellular communications and alternative roads: stimuli promoting a malignant phenotype. Int J Cancer (2014) 135:1265–76. doi: 10.1002/ijc.28572
10. Takebe N, Miele L, Harris PJ, Jeong W, Bando H, Kahn M, et al. Targeting notch, hedgehog, and wnt pathways in cancer stem cells: clinical update. Nat Rev Clin Oncol (2015) 12:445–64. doi: 10.1038/nrclinonc.2015.61
11. Taniguchi H, Suzuki Y, Natori Y. The evolving landscape of cancer stem cells and ways to overcome cancer heterogeneity. Cancers (2019) 11:532. doi: 10.3390/cancers11040532
12. Risson E, Nobre AR, Maguer-Satta V, Aguirre-Ghiso JA. The current paradigm and challenges ahead for the dormancy of disseminated tumor cells. Nat Cancer (2020) 1:672–80. doi: 10.1038/s43018-020-0088-5
13. Hen O, Barkan D. Dormant disseminated tumor cells and cancer stem/progenitor-like cells: similarities and opportunities. Semin Cancer Biol (2020) 60:157–65. doi: 10.1016/j.semcancer.2019.09.002
14. Schewe DM, Aguirre-Ghiso JA. ATF6α-Rheb-mTOR signaling promotes survival of dormant tumor cells in vivo. Proc Natl Acad Sci (2008) 105:10519–24. doi: 10.1073/pnas.0800939105
15. De Angelis M, Francescangeli F, Zeuner A. Breast cancer stem cells as drivers of tumor chemoresistance, dormancy and relapse: new challenges and therapeutic opportunities. Cancers (2019) 11:1569. doi: 10.3390/cancers11101569
16. Tjhay F, Motohara T, Tayama S, Narantuya D, Fujimoto K, Guo J, et al. CD 44 variant 6 is correlated with peritoneal dissemination and poor prognosis in patients with advanced epithelial ovarian cancer. Cancer Sci (2015) 106:1421–8. doi: 10.1111/cas.12765
17. Bourguignon LYW, Peyrollier K, Xia W, Gilad E. Hyaluronan-CD44 interaction activates stem cell marker nanog, stat-3-mediated MDR1 gene expression, and ankyrin-regulated multidrug efflux in breast and ovarian tumor cells. J Biol Chem (2008) 283:17635–51. doi: 10.1074/jbc.M800109200
18. Zhang S, Balch C, Chan MW, Lai H-C, Matei D, Schilder JM, et al. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res (2008) 68:4311–20. doi: 10.1158/0008-5472.CAN-08-0364
19. Grass GD, Tolliver LB, Bratoeva M, Toole BP. CD147, CD44, and the epidermal growth factor receptor (EGFR) signaling pathway cooperate to regulate breast epithelial cell invasiveness. J Biol Chem (2013) 288:26089–104. doi: 10.1074/jbc.M113.497685
20. Motohara T, Fujimoto K, Tayama S, Narantuya D, Sakaguchi I, Tashiro H, et al. CD44 variant 6 as a predictive biomarker for distant metastasis in patients with epithelial ovarian cancer. Obstet Gynecol (2016) 127:1003–11. doi: 10.1097/AOG.0000000000001420
21. Liu M, Mor G, Cheng H, Xiang X, Hui P, Rutherford T, et al. High frequency of putative ovarian cancer stem cells with CD44/CK19 coexpression is associated with decreased progression-free intervals in patients with recurrent epithelial ovarian cancer. Reprod Sci (2013) 20:605–15. doi: 10.1177/1933719112461183
22. Zhang J, Chang B, Liu J. CD44 standard form expression is correlated with high-grade and advanced-stage ovarian carcinoma but not prognosis. Hum Pathol (2013) 44:1882–9. doi: 10.1016/j.humpath.2013.02.016
23. Kar K, Ghosh S, Roy AK. A study of CD44 positive cancer cells in epithelial ovarian cancer and their correlation with P53 and Ki67. J Lab Physicians (2021) 13:050–7. doi: 10.1055/s-0041-1724235
24. Wu Y, Wang T, Xia L, Zhang M. LncRNA WDFY3-AS2 promotes cisplatin resistance and the cancer stem cell in ovarian cancer by regulating hsa-miR-139-5p/SDC4 axis. Cancer Cell Int (2021) 21:284. doi: 10.1186/s12935-021-01993-x
25. Golebiewska A, Brons NHC, Bjerkvig R, Niclou SP. Critical appraisal of the side population assay in stem cell and cancer stem cell research. Cell Stem Cell (2011) 8:136–47. doi: 10.1016/j.stem.2011.01.007
26. Luo L, Zeng J, Liang B, Zhao Z, Sun L, Cao D, et al. Ovarian cancer cells with the CD117 phenotype are highly tumorigenic and are related to chemotherapy outcome. Exp Mol Pathol (2011) 91:596–602. doi: 10.1016/j.yexmp.2011.06.005
27. Raspollini MR, Amunni G, Villanucci A, Baroni G, Taddei A, Taddei GL. C-KIT expression and correlation with chemotherapy resistance in ovarian carcinoma: an immunocytochemical study. Ann Oncol (2004) 15:594–7. doi: 10.1093/annonc/mdh139
28. Chen M, Su J, Feng C, Liu Y, Zhao L, Tian Y. Chemokine CCL20 promotes the paclitaxel resistance of CD44 + CD117 + cells via the Notch1 signaling pathway in ovarian cancer. Mol Med Rep (2021) 24:635. doi: 10.3892/mmr.2021.12274
29. Stemberger-Papić S, Vrdoljak-Mozetic D, Ostojić DV, Rubesa-Mihaljević R, Krigtofić I, Brncić-Fisher A, et al. Expression of CD133 and CD117 in 64 serous ovarian cancer cases. Coll Antropol (2015) 39:745–53.
30. Mazzoldi EL, Pavan S, Pilotto G, Leone K, Pagotto A, Frezzini S, et al. A juxtacrine/paracrine loop between c-kit and stem cell factor promotes cancer stem cell survival in epithelial ovarian cancer. Cell Death Dis (2019) 10:412. doi: 10.1038/s41419-019-1656-4
31. Roy L, Bobbs A, Sattler R, Kurkewich JL, Dausinas PB, Nallathamby P, et al. CD133 promotes adhesion to the ovarian cancer metastatic niche. Cancer Growth Metastasis (2018) 11:117906441876788. doi: 10.1177/1179064418767882
32. Xiang T, Long H, He L, Han X, Lin K, Liang Z, et al. Interleukin-17 produced by tumor microenvironment promotes self-renewal of CD133+ cancer stem-like cells in ovarian cancer. Oncogene (2015) 34:165–76. doi: 10.1038/onc.2013.537
33. Baba T, Convery PA, Matsumura N, Whitaker RS, Kondoh E, Perry T, et al. Epigenetic regulation of CD133 and tumorigenicity of CD133+ ovarian cancer cells. Oncogene (2009) 28:209–18. doi: 10.1038/onc.2008.374
34. Zhang J, Guo X, Chang DY, Rosen DG, Mercado-Uribe I, Liu J. CD133 expression associated with poor prognosis in ovarian cancer. Mod Pathol (2012) 25:456–64. doi: 10.1038/modpathol.2011.170
35. Kusumbe AP, Mali AM, Bapat SA. CD133-expressing stem cells associated with ovarian metastases establish an endothelial hierarchy and contribute to tumor vasculature. Stem Cells (2009) 27:498–508. doi: 10.1634/stemcells.2008-0868
36. Ferrandina G, Martinelli E, Petrillo M, Prisco MG, Zannoni G, Sioletic S, et al. CD133 antigen expression in ovarian cancer. BMC Cancer (2009) 9:221. doi: 10.1186/1471-2407-9-221
37. Qin Q, Sun Y, Fei M, Zhang J, Jia Y, Gu M, et al. Expression of putative stem marker nestin and CD133 in advanced serous ovarian cancer. Neoplasma (2012) 59:310–6. doi: 10.4149/neo_2012_040
38. Abubaker K, Luwor RB, Zhu H, McNally O, Quinn MA, Burns CJ, et al. Inhibition of the JAK2/STAT3 pathway in ovarian cancer results in the loss of cancer stem cell-like characteristics and a reduced tumor burden. BMC Cancer (2014) 14:317. doi: 10.1186/1471-2407-14-317
39. Davidson B, Holth A, Hellesylt E, Tan TZ, Huang RY-J, Tropé C, et al. The clinical role of epithelial-mesenchymal transition and stem cell markers in advanced-stage ovarian serous carcinoma effusions. Hum Pathol (2015) 46:1–8. doi: 10.1016/j.humpath.2014.10.004
40. Davidson B. CD24 is highly useful in differentiating high-grade serous carcinoma from benign and malignant mesothelial cells. Hum Pathol (2016) 58:123–7. doi: 10.1016/j.humpath.2016.08.005
41. Gao M-Q, Choi Y-P, Kang S, Youn JH, Cho N-H. CD24+ cells from hierarchically organized ovarian cancer are enriched in cancer stem cells. Oncogene (2010) 29:2672–80. doi: 10.1038/onc.2010.35
42. Nakamura K, Terai Y, Tanabe A, Ono YJ, Hayashi M, Maeda K, et al. CD24 expression is a marker for predicting clinical outcome and regulates the epithelial-mesenchymal transition in ovarian cancer via both the akt and ERK pathways. Oncol Rep (2017) 37:3189–200. doi: 10.3892/or.2017.5583
43. Runz S, Keller S, Rupp C, Stoeck A, Issa Y, Koensgen D, et al. Malignant ascites-derived exosomes of ovarian carcinoma patients contain CD24 and EpCAM. Gynecol Oncol (2007) 107:563–71. doi: 10.1016/j.ygyno.2007.08.064
44. Fu J, Shang Y, Qian Z, Hou J, Yan F, Liu G, et al. Chimeric antigen receptor-T (CAR-T) cells targeting epithelial cell adhesion molecule (EpCAM) can inhibit tumor growth in ovarian cancer mouse model. J Vet Med Sci (2021) 83:241–7. doi: 10.1292/jvms.20-0455
45. Akhter MZ, Sharawat SK, Kumar V, Kochat V, Equbal Z, Ramakrishnan M, et al. Aggressive serous epithelial ovarian cancer is potentially propagated by EpCAM+CD45+ phenotype. Oncogene (2018) 37:2089–103. doi: 10.1038/s41388-017-0106-y
46. The PLOS ONE Staff. Correction: the MyD88+ phenotype is an adverse prognostic factor in epithelial ovarian cancer. PloS One (2014) 9:e108833. doi: 10.1371/journal.pone.0108833
47. Liu W, Zhang J, Gan X, Shen F, Yang X, Du N, et al. LGR 5 promotes epithelial ovarian cancer proliferation, metastasis, and epithelial–mesenchymal transition through the Notch1 signaling pathway. Cancer Med (2018) 7:3132–42. doi: 10.1002/cam4.1485
48. Schindler AJ, Watanabe A, Howell SB. LGR5 and LGR6 in stem cell biology and ovarian cancer. Oncotarget (2018) 9:1346–55. doi: 10.18632/oncotarget.20178
49. Steg AD, Bevis KS, Katre AA, Ziebarth A, Dobbin ZC, Alvarez RD, et al. Stem cell pathways contribute to clinical chemoresistance in ovarian cancer. Clin Cancer Res (2012) 18:869–81. doi: 10.1158/1078-0432.CCR-11-2188
50. Silva IA, Bai S, McLean K, Yang K, Griffith K, Thomas D, et al. Aldehyde dehydrogenase in combination with CD133 defines angiogenic ovarian cancer stem cells that portend poor patient survival. Cancer Res (2011) 71:3991–4001. doi: 10.1158/0008-5472.CAN-10-3175
51. House CD, Jordan E, Hernandez L, Ozaki M, James JM, Kim M, et al. NFκB promotes ovarian tumorigenesis via classical pathways that support proliferative cancer cells and alternative pathways that support ALDH+ cancer stem–like cells. Cancer Res (2017) 77:6927–40. doi: 10.1158/0008-5472.CAN-17-0366
52. Liebscher CA, Prinzler J, Sinn BV, Budczies J, Denkert C, Noske A, et al. Aldehyde dehydrogenase 1/epidermal growth factor receptor coexpression is characteristic of a highly aggressive, poor-prognosis subgroup of high-grade serous ovarian carcinoma. Hum Pathol (2013) 44:1465–71. doi: 10.1016/j.humpath.2012.12.016
53. Li Y, Chen K, Li L, Li R, Zhang J, Ren W. Overexpression of SOX2 is involved in paclitaxel resistance of ovarian cancer via the PI3K/Akt pathway. Tumor Biol (2015) 36:9823–8. doi: 10.1007/s13277-015-3561-5
54. Wen Y, Hou Y, Huang Z, Cai J, Wang Z. SOX2 is required to maintain cancer stem cells in ovarian cancer. Cancer Sci (2017) 108:719–31. doi: 10.1111/cas.13186
55. Ip CKM, Li S-S, Tang MYH, Sy SKH, Ren Y, Shum HC, et al. Stemness and chemoresistance in epithelial ovarian carcinoma cells under shear stress. Sci Rep (2016) 6:26788. doi: 10.1038/srep26788
56. Levasseur DN, Wang J, Dorschner MO, Stamatoyannopoulos JA, Orkin SH. Oct4 dependence of chromatin structure within the extended nanog locus in ES cells. Genes Dev (2008) 22:575–80. doi: 10.1101/gad.1606308
57. Peng S, Maihle NJ, Huang Y. Pluripotency factors Lin28 and Oct4 identify a sub-population of stem cell-like cells in ovarian cancer. Oncogene (2010) 29:2153–9. doi: 10.1038/onc.2009.500
58. Fan Q, Cai Q, Xu Y. FOXM1 is a downstream target of LPA and YAP oncogenic signaling pathways in high grade serous ovarian cancer. Oncotarget (2015) 6:27688–99. doi: 10.18632/oncotarget.4280
59. Nagaraj AB, Joseph P, Kovalenko O, Singh S, Armstrong A, Redline R, et al. Critical role of wnt/β-catenin signaling in driving epithelial ovarian cancer platinum resistance. Oncotarget (2015) 6:23720–34. doi: 10.18632/oncotarget.4690
60. Zhou J, Wang Y, Wang Y, Yin X, He Y, Chen L, et al. FOXM1 modulates cisplatin sensitivity by regulating EXO1 in ovarian cancer. PloS One (2014) 9:e96989. doi: 10.1371/journal.pone.0096989
61. Fox RG, Park FD, Koechlein CS, Kritzik M, Reya T. Musashi signaling in stem cells and cancer. Annu Rev Cell Dev Biol (2015) 31:249–67. doi: 10.1146/annurev-cellbio-100814-125446
62. Kudinov AE, Karanicolas J, Golemis EA, Boumber Y. Musashi RNA-binding proteins as cancer drivers and novel therapeutic targets. Clin Cancer Res (2017) 23:2143–53. doi: 10.1158/1078-0432.CCR-16-2728
63. Chen P, Li Q, Yang Z. Musashi-1 expression is a prognostic factor in ovarian adenocarcinoma and correlates with ALDH-1 expression. Pathol Oncol Res (2015) 21:1133–40. doi: 10.1007/s12253-015-9943-6
64. Chen H, Liu J, Wang H, Cheng Q, Zhou C, Chen X, et al. Inhibition of RNA-binding protein musashi-1 suppresses malignant properties and reverses paclitaxel resistance in ovarian carcinoma. J Cancer (2019) 10:1580–92. doi: 10.7150/jca.27352
65. Teeuwssen, Fodde. Wnt signaling in ovarian cancer stemness, EMT, and therapy resistance. J Clin Med (2019) 8:1658. doi: 10.3390/jcm8101658
66. Su H-Y, Lai H-C, Lin Y-W, Liu C-Y, Chen C-K, Chou Y-C, et al. Epigenetic silencing of SFRP5 is related to malignant phenotype and chemoresistance of ovarian cancer through wnt signaling pathway. Int J Cancer (2010) 127:555–67. doi: 10.1002/ijc.25083
67. Raghavan S, Mehta P, Xie Y, Lei YL, Mehta G. Ovarian cancer stem cells and macrophages reciprocally interact through the WNT pathway to promote pro-tumoral and malignant phenotypes in 3D engineered microenvironments. J Immunother Cancer (2019) 7:190. doi: 10.1186/s40425-019-0666-1
68. Chen Y, Bieber MM, Teng NNH. Hedgehog signaling regulates drug sensitivity by targeting ABC transporters ABCB1 and ABCG2 in epithelial ovarian cancer. Mol Carcinog (2014) 53:625–34. doi: 10.1002/mc.22015
69. Rocconi R. Hedgehog signaling pathway regulates the growth of ovarian cancer spheroid forming cells. Int J Oncol (2011) 39:797–804. doi: 10.3892/ijo.2011.1093
70. Song X, Yan L, Lu C, Zhang C, Zhu F, Yang J, et al. Activation of hedgehog signaling and its association with cisplatin resistance in ovarian epithelial tumors. Oncol Lett (2018) 15:5569–76. doi: 10.3892/ol.2018.8008
71. Kim S, Kim B, Song YS. Ascites modulates cancer cell behavior, contributing to tumor heterogeneity in ovarian cancer. Cancer Sci (2016) 107:1173–8. doi: 10.1111/cas.12987
72. Overholtzer M, Zhang J, Smolen GA, Muir B, Li W, Sgroi DC, et al. Transforming properties of YAP , a candidate oncogene on the chromosome 11q22 amplicon. Proc Natl Acad Sci (2006) 103:12405–10. doi: 10.1073/pnas.0605579103
73. Xia Y, Chang T, Wang Y, Liu Y, Li W, Li M, et al. YAP promotes ovarian cancer cell tumorigenesis and is indicative of a poor prognosis for ovarian cancer patients. PloS One (2014) 9:e91770. doi: 10.1371/journal.pone.0091770
74. Kubelac P, Braicu C, Raduly L, Chiroi P, Nutu A, Cojocneanu R, et al. Comprehensive analysis of the expression of key genes related to hippo signaling and their prognosis impact in ovarian cancer. Diagnostics (2021) 11:344. doi: 10.3390/diagnostics11020344
75. Park JT, Chen X, Tropè CG, Davidson B, Shih I-M, Wang T-L. Notch3 overexpression is related to the recurrence of ovarian cancer and confers resistance to carboplatin. Am J Pathol (2010) 177:1087–94. doi: 10.2353/ajpath.2010.100316
76. McAuliffe SM, Morgan SL, Wyant GA, Tran LT, Muto KW, Chen YS, et al. Targeting notch, a key pathway for ovarian cancer stem cells, sensitizes tumors to platinum therapy. Proc Natl Acad Sci (2012) 109:E2939-48. doi: 10.1073/pnas.1206400109
77. Perez-Fidalgo JA, Ortega B, Simon S, Samartzis EP, Boussios S. NOTCH signalling in ovarian cancer angiogenesis. Ann Transl Med (2020) 8:1705–5. doi: 10.21037/atm-20-4497
78. Gonzalez-Torres C, Gaytan-Cervantes J, Vazquez-Santillan K, Mandujano-Tinoco EA, Ceballos-Cancino G, Garcia-Venzor A, et al. NF-κB participates in the stem cell phenotype of ovarian cancer cells. Arch Med Res (2017) 48:343–51. doi: 10.1016/j.arcmed.2017.08.001
79. Harte MT, Gorski JJ, Savage KI, Purcell JW, Barros EM, Burn PM, et al. NF-κB is a critical mediator of BRCA1-induced chemoresistance. Oncogene (2014) 33:713–23. doi: 10.1038/onc.2013.10
80. Kaltschmidt C, Banz-Jansen C, Benhidjeb T, Beshay M, Förster C, Greiner J, et al. A role for NF-κB in organ specific cancer and cancer stem cells. Cancers (2019) 11:655. doi: 10.3390/cancers11050655
81. Lin S-C, Liao Y-C, Chen P-M, Yang Y-Y, Wang Y-H, Tung S-L, et al. Periostin promotes ovarian cancer metastasis by enhancing M2 macrophages and cancer-associated fibroblasts via integrin-mediated NF-κB and TGF-β2 signaling. J BioMed Sci (2022) 29:109. doi: 10.1186/s12929-022-00888-x
82. Zhao B, Niu X, Huang S, Yang J, Wei Y, Wang X, et al. TLR4 agonist and hypoxia synergistically promote the formation of TLR4/NF-κB/HIF-1α loop in human epithelial ovarian cancer. Anal Cell Pathol (2022) 2022:1–19. doi: 10.1155/2022/4201262
83. Shuang T, Wang M, Zhou Y, Shi C. Over-expression of nuclear NF-κB1 and c-rel correlates with chemoresistance and prognosis of serous epithelial ovarian cancer. Exp Mol Pathol (2016) 100:139–44. doi: 10.1016/j.yexmp.2015.11.030
84. Guo R-X, Qiao Y-H, Zhou Y, Li L-X, Shi H-R, Chen K-S. Increased staining for phosphorylated AKT and nuclear factor-κB p65 and their relationship with prognosis in epithelial ovarian cancer. Pathol Int (2008) 58:749–56. doi: 10.1111/j.1440-1827.2008.02306.x
85. Wang L, Wang C, Jin S, Qu D, Ying H. Expression of NF-κB and PTEN in primary epithelial ovarian carcinoma and the correlation with chemoresistance. Int J Clin Exp Pathol (2015) 8:10953–63.
86. van Lieshout L, van de Stolpe A, van der Ploeg P, Bowtell D, de Hullu J, Piek J. Signal transduction pathway activity in high-grade, serous ovarian carcinoma reveals a more favorable prognosis in tumors with low PI3K and high NF-κB pathway activity: a novel approach to a long-standing enigma. Cancers (2020) 12:2660. doi: 10.3390/cancers12092660
87. McDonald M, Salinas E, Devor E, Newtson A, Thiel K, Goodheart M, et al. Molecular characterization of non-responders to chemotherapy in serous ovarian cancer. Int J Mol Sci (2019) 20:1175. doi: 10.3390/ijms20051175
88. Liu L, Salnikov AV, Bauer N, Aleksandrowicz E, Labsch S, Nwaeburu C, et al. Triptolide reverses hypoxia-induced epithelial–mesenchymal transition and stem-like features in pancreatic cancer by NF-κB downregulation. Int J Cancer (2014) 134:2489–503. doi: 10.1002/ijc.28583
89. Qin J, Liu Y, Lu Y, Liu M, Li M, Li J, et al. Hypoxia-inducible factor 1 alpha promotes cancer stem cells-like properties in human ovarian cancer cells by upregulating SIRT1 expression. Sci Rep (2017) 7:10592. doi: 10.1038/s41598-017-09244-8
90. Daponte A, Ioannou M, Mylonis I, Simos G, Minas M, Messinis IE, et al. Prognostic significance of hypoxia-inducible factor 1 alpha(HIF-1alpha) expression in serous ovarian cancer: an immunohistochemical study. BMC Cancer (2008) 8:335. doi: 10.1186/1471-2407-8-335
91. Deying W, Feng G, Shumei L, Hui Z, Ming L, Hongqing W. CAF-derived HGF promotes cell proliferation and drug resistance by up-regulating the c-Met/PI3K/Akt and GRP78 signalling in ovarian cancer cells. Biosci Rep (2017) 37:BSR20160470. doi: 10.1042/BSR20160470
92. Kwon Y, Smith BD, Zhou Y, Kaufman MD, Godwin AK. Effective inhibition of c-MET-mediated signaling, growth and migration of ovarian cancer cells is influenced by the ovarian tissue microenvironment. Oncogene (2015) 34:144–53. doi: 10.1038/onc.2013.539
93. Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer (2006) 6:392–401. doi: 10.1038/nrc1877
94. Park CW, Kim K-S, Bae S, Son HK, Myung P-K, Hong HJ, et al. Cytokine secretion profiling of human mesenchymal stem cells by antibody array. Int J Stem Cells (2009) 2:59–68. doi: 10.15283/ijsc.2009.2.1.59
95. Gorodetska I, Kozeretska I, Dubrovska A. BRCA genes: the role in genome stability, cancer stemness and therapy resistance. J Cancer (2019) 10:2109–27. doi: 10.7150/jca.30410
96. Parzych KR, Klionsky DJ. An overview of autophagy: morphology, mechanism, and regulation. Antioxid Redox Signal (2014) 20:460–73. doi: 10.1089/ars.2013.5371
97. Deng J, Bai X, Feng X, Ni J, Beretov J, Graham P, et al. Inhibition of PI3K/Akt/mTOR signaling pathway alleviates ovarian cancer chemoresistance through reversing epithelial-mesenchymal transition and decreasing cancer stem cell marker expression. BMC Cancer (2019) 19:618. doi: 10.1186/s12885-019-5824-9
98. Hassan AA, Artemenko M, Tang MKS, Shi Z, Chen L-Y, Lai H-C, et al. Ascitic fluid shear stress in concert with hepatocyte growth factor drive stemness and chemoresistance of ovarian cancer cells via the c-Met-PI3K/Akt-miR-199a-3p signaling pathway. Cell Death Dis (2022) 13:537. doi: 10.1038/s41419-022-04976-6
99. Zi D, Li Q, Xu C, Zhou Z-W, Song G-B, Hu C-B, et al. CXCR4 knockdown enhances sensitivity of paclitaxel via the PI3K/Akt/mTOR pathway in ovarian carcinoma. Aging (2022) 14:4673–98. doi: 10.18632/aging.203241
100. Wang S, Li Z, Zhu G, Hong L, Hu C, Wang K, et al. RNA-Binding protein IGF2BP2 enhances circ_0000745 abundancy and promotes aggressiveness and stemness of ovarian cancer cells via the microRNA-3187-3p/ERBB4/PI3K/AKT axis. J Ovarian Res (2021) 14:154. doi: 10.1186/s13048-021-00917-7
101. Cai J, Xu L, Tang H, Yang Q, Yi X, Fang Y, et al. The role of the PTEN/PI3K/Akt pathway on prognosis in epithelial ovarian cancer: a meta-analysis. Oncol (2014) 19:528–35. doi: 10.1634/theoncologist.2013-0333
102. Zhang M, Liu Y, Yin Y, Sun Z, Wang Y, Zhang Z, et al. UBE2S promotes the development of ovarian cancer by promoting PI3K/AKT/mTOR signaling pathway to regulate cell cycle and apoptosis. Mol Med (2022) 28:62. doi: 10.1186/s10020-022-00489-2
103. Wang D, Li C, Zhang Y, Wang M, Jiang N, Xiang L, et al. Combined inhibition of PI3K and PARP is effective in the treatment of ovarian cancer cells with wild-type PIK3CA genes. Gynecol Oncol (2016) 142:548–56. doi: 10.1016/j.ygyno.2016.07.092
104. Izar B, Tirosh I, Stover EH, Wakiro I, Cuoco MS, Alter I, et al. A single-cell landscape of high-grade serous ovarian cancer. Nat Med (2020) 26:1271–9. doi: 10.1038/s41591-020-0926-0
105. Ruan Z, Yang X, Cheng W. OCT4 accelerates tumorigenesis through activating JAK/STAT signaling in ovarian cancer side population cells. Cancer Manag Res (2018) 11:389–99. doi: 10.2147/CMAR.S180418
106. McLean K, Tan L, Bolland DE, Coffman LG, Peterson LF, Talpaz M, et al. Leukemia inhibitory factor functions in parallel with interleukin-6 to promote ovarian cancer growth. Oncogene (2019) 38:1576–84. doi: 10.1038/s41388-018-0523-6
107. Shang A-Q, Wu J, Bi F, Zhang Y-J, Xu L-R, Li L-L, et al. Relationship between HER2 and JAK/STAT-SOCS3 signaling pathway and clinicopathological features and prognosis of ovarian cancer. Cancer Biol Ther (2017) 18:314–22. doi: 10.1080/15384047.2017.1310343
108. Kenny HA, Chiang C-Y, White EA, Schryver EM, Habis M, Romero IL, et al. Mesothelial cells promote early ovarian cancer metastasis through fibronectin secretion. J Clin Invest (2014) 124:4614–28. doi: 10.1172/JCI74778
109. Zhang Q, Peng C. Cancer−associated fibroblasts regulate the biological behavior of cancer cells and stroma in gastric cancer (Review). Oncol Lett (2017) 15:691–98. doi: 10.3892/ol.2017.7385
110. Cardenas H, Vieth E, Lee J, Segar M, Liu Y, Nephew KP, et al. TGF-β induces global changes in DNA methylation during the epithelial-to-mesenchymal transition in ovarian cancer cells. Epigenetics (2014) 9:1461–72. doi: 10.4161/15592294.2014.971608
111. McLean K, Gong Y, Choi Y, Deng N, Yang K, Bai S, et al. Human ovarian carcinoma–associated mesenchymal stem cells regulate cancer stem cells and tumorigenesis via altered BMP production. J Clin Invest (2011) 121:3206–19. doi: 10.1172/JCI45273
112. Pasquet M, Golzio M, Mery E, Rafii A, Benabbou N, Mirshahi P, et al. Hospicells (ascites-derived stromal cells) promote tumorigenicity and angiogenesis. Int J Cancer (2009) 126:2090–101. doi: 10.1002/ijc.24886
113. Li X, Gao X, Yuan J, Wang F, Xu X, Wang C, et al. The miR-33a-5p/CROT axis mediates ovarian cancer cell behaviors and chemoresistance via the regulation of the TGF-β signal pathway. Front Endocrinol (2022) 13:950345. doi: 10.3389/fendo.2022.950345
114. Yang D, Sun Y, Hu L, Zheng H, Ji P, Pecot CV, et al. Integrated analyses identify a master MicroRNA regulatory network for the mesenchymal subtype in serous ovarian cancer. Cancer Cell (2013) 23:186–99. doi: 10.1016/j.ccr.2012.12.020
115. Shang B, Liu Y, Jiang S, Liu Y. Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: a systematic review and meta-analysis. Sci Rep (2015) 5:15179. doi: 10.1038/srep15179
116. Yue H, Li W, Chen R, Wang J, Lu X, Li J. Stromal POSTN induced by TGF-β1 facilitates the migration and invasion of ovarian cancer. Gynecol Oncol (2021) 160:530–8. doi: 10.1016/j.ygyno.2020.11.026
117. Zhao H, Gao Y, Chen Q, Li J, Ren M, Zhao X, et al. RAD51AP1 promotes progression of ovarian cancer via TGF-β/Smad signalling pathway. J Cell Mol Med (2021) 25:1927–38. doi: 10.1111/jcmm.15877
118. Gagno S, Poletto E, Bartoletti M, Quartuccio L, Romualdi C, Garziera M, et al. A TGF-β associated genetic score to define prognosis and platinum sensitivity in advanced epithelial ovarian cancer. Gynecol Oncol (2020) 156:233–42. doi: 10.1016/j.ygyno.2019.10.019
119. Liang Q, Xu Z, Liu Y, Peng B, Cai Y, Liu W, et al. NR2F1 regulates TGF-β1-Mediated epithelial-mesenchymal transition affecting platinum sensitivity and immune response in ovarian cancer. Cancers (2022) 14:4639. doi: 10.3390/cancers14194639
120. Moffitt L, Karimnia N, Stephens A, Bilandzic M. Therapeutic targeting of collective invasion in ovarian cancer. Int J Mol Sci (2019) 20:1466. doi: 10.3390/ijms20061466
121. Bryan BA, Dennstedt E, Mitchell DC, Walshe TE, Noma K, Loureiro R, et al. RhoA/ROCK signaling is essential for multiple aspects of VEGF-mediated angiogenesis. FASEB J (2010) 24:3186–95. doi: 10.1096/fj.09-145102
122. McGrail DJ, Kieu QMN, Dawson MR. Metastatic ovarian cancer cell malignancy is increased on soft matrices through a mechanosensitive Rho/ROCK pathway. J Cell Sci (2014) jcs:144378. doi: 10.1242/jcs.144378
123. Ogata S, Morishige K-I, Sawada K, Hashimoto K, Mabuchi S, Kawase C, et al. Fasudil inhibits lysophosphatidic acid-induced invasiveness of human ovarian cancer cells. Int J Gynecol Cancer (2009) 19:1473–80. doi: 10.1111/IGC.0b013e3181c03909
124. Ohta T, Takahashi T, Shibuya T, Amita M, Henmi N, Takahashi K, et al. Inhibition of the Rho/ROCK pathway enhances the efficacy of cisplatin through the blockage of hypoxia-inducible factor-1α in human ovarian cancer cells. Cancer Biol Ther (2012) 13:25–33. doi: 10.4161/cbt.13.1.18440
125. Meza-Perez S, Randall TD. Immunological functions of the omentum. Trends Immunol (2017) 38:526–36. doi: 10.1016/j.it.2017.03.002
126. Liao J, Qian F, Tchabo N, Mhawech-Fauceglia P, Beck A, Qian Z, et al. Ovarian cancer spheroid cells with stem cell-like properties contribute to tumor generation, metastasis and chemotherapy resistance through hypoxia-resistant metabolism. PloS One (2014) 9:e84941. doi: 10.1371/journal.pone.0084941
127. Graves LE, Ariztia EV, Navari JR, Matzel HJ, Stack MS, Fishman DA. Proinvasive properties of ovarian cancer ascites-derived membrane vesicles. Cancer Res (2004) 64:7045–9. doi: 10.1158/0008-5472.CAN-04-1800
128. Milliken D, Scotton C, Raju S, Balkwill F, Wilson J. Analysis of chemokines and chemokine receptor expression in ovarian cancer ascites. Clin Cancer Res Off J Am Assoc Cancer Res (2002) 8:1108–14.
129. Yamamoto CM, Oakes ML, Murakami T, Muto MG, Berkowitz RS, Ng S-W. Comparison of benign peritoneal fluid- and ovarian cancer ascites-derived extracellular vesicle RNA biomarkers. J Ovarian Res (2018) 11:20. doi: 10.1186/s13048-018-0391-2
130. Bregenzer, Horst, Mehta, Novak, Repetto, Mehta. The role of cancer stem cells and mechanical forces in ovarian cancer metastasis. Cancers (2019) 11:1008. doi: 10.3390/cancers11071008
131. Yeung T-L, Leung CS, Yip K-P, Au Yeung CL, Wong STC, Mok SC. Cellular and molecular processes in ovarian cancer metastasis. a review in the theme: cell and molecular processes in cancer metastasis. Am J Physiol-Cell Physiol (2015) 309:C444–56. doi: 10.1152/ajpcell.00188.2015
132. Shishido A, Mori S, Yokoyama Y, Hamada Y, Minami K, Qian Y, et al. Mesothelial cells facilitate cancer stem−like properties in spheroids of ovarian cancer cells. Oncol Rep (2018) 40:2105–14. doi: 10.3892/or.2018.6605
133. Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol (2009) 10:445–57. doi: 10.1038/nrm2720
134. Montell DJ. Morphogenetic cell movements: diversity from modular mechanical properties. Science (2008) 322:1502–5. doi: 10.1126/science.1164073
135. Mayor R, Etienne-Manneville S. The front and rear of collective cell migration. Nat Rev Mol Cell Biol (2016) 17:97–109. doi: 10.1038/nrm.2015.14
136. Cheung KJ, Gabrielson E, Werb Z, Ewald AJ. Collective invasion in breast cancer requires a conserved basal epithelial program. Cell (2013) 155:1639–51. doi: 10.1016/j.cell.2013.11.029
137. Khalil AA, Friedl P. Determinants of leader cells in collective cell migration. Integr Biol (2010) 2:568. doi: 10.1039/c0ib00052c
138. Volkmer J-P, Sahoo D, Chin RK, Ho PL, Tang C, Kurtova AV, et al. Three differentiation states risk-stratify bladder cancer into distinct subtypes. Proc Natl Acad Sci (2012) 109:2078–83. doi: 10.1073/pnas.1120605109
139. Pandya P, Orgaz JL, Sanz-Moreno V. Actomyosin contractility and collective migration: may the force be with you. Curr Opin Cell Biol (2017) 48:87–96. doi: 10.1016/j.ceb.2017.06.006
140. Cheung KJ, Padmanaban V, Silvestri V, Schipper K, Cohen JD, Fairchild AN, et al. Polyclonal breast cancer metastases arise from collective dissemination of keratin 14-expressing tumor cell clusters. Proc Natl Acad Sci (2016) 113:E854-63. doi: 10.1073/pnas.1508541113
141. Zhu F, Qian W, Zhang H, Liang Y, Wu M, Zhang Y, et al. SOX2 is a marker for stem-like tumor cells in bladder cancer. Stem Cell Rep (2017) 9:429–37. doi: 10.1016/j.stemcr.2017.07.004
142. Papafotiou G, Paraskevopoulou V, Vasilaki E, Kanaki Z, Paschalidis N, Klinakis A. KRT14 marks a subpopulation of bladder basal cells with pivotal role in regeneration and tumorigenesis. Nat Commun (2016) 7:11914. doi: 10.1038/ncomms11914
143. Kurtova AV, Xiao J, Mo Q, Pazhanisamy S, Krasnow R, Lerner SP, et al. Blocking PGE2-induced tumour repopulation abrogates bladder cancer chemoresistance. Nature (2015) 517:209–13. doi: 10.1038/nature14034
144. Neri S, Ishii G, Hashimoto H, Kuwata T, Nagai K, Date H, et al. Podoplanin-expressing cancer-associated fibroblasts lead and enhance the local invasion of cancer cells in lung adenocarcinoma: PDPN-CAFs lead and enhance cancer cell invasion. Int J Cancer (2015) 137:784–96. doi: 10.1002/ijc.29464
145. Karimnia N, Wilson AL, Green E, Matthews A, Jobling TW, Plebanski M, et al. Chemoresistance is mediated by ovarian cancer leader cells in vitro. J Exp Clin Cancer Res (2021) 40:276. doi: 10.1186/s13046-021-02086-3
146. Cai J, Tang H, Xu L, Wang X, Yang C, Ruan S, et al. Fibroblasts in omentum activated by tumor cells promote ovarian cancer growth, adhesion and invasiveness. Carcinogenesis (2012) 33:20–9. doi: 10.1093/carcin/bgr230
147. Rynne-Vidal A, Jiménez-Heffernan J, Fernández-Chacón C, López-Cabrera M, Sandoval P. The mesothelial origin of carcinoma associated-fibroblasts in peritoneal metastasis. Cancers (2015) 7:1994–2011. doi: 10.3390/cancers7040872
148. Zhang Y, Tang H, Cai J, Zhang T, Guo J, Feng D, et al. Ovarian cancer-associated fibroblasts contribute to epithelial ovarian carcinoma metastasis by promoting angiogenesis, lymphangiogenesis and tumor cell invasion. Cancer Lett (2011) 303:47–55. doi: 10.1016/j.canlet.2011.01.011
149. Jang K, Kim M, Gilbert CA, Simpkins F, Ince TA, Slingerland JM. VEGFA activates an epigenetic pathway upregulating ovarian cancer-initiating cells. EMBO Mol Med (2017) 9:304–18. doi: 10.15252/emmm.201606840
150. Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer (2016) 16:582–98. doi: 10.1038/nrc.2016.73
151. Sun W, Fu S. Role of cancer-associated fibroblasts in tumor structure, composition and the microenvironment in ovarian cancer (Review). Oncol Lett (2019) 18:2173–78. doi: 10.3892/ol.2019.10587
152. Zeng L, Wang X, Wang F, Zhao X, Ding Y. Identification of a gene signature of cancer-associated fibroblasts to predict prognosis in ovarian cancer. Front Genet (2022) 13:925231. doi: 10.3389/fgene.2022.925231
153. Feng S, Xu Y, Dai Z, Yin H, Zhang K, Shen Y. Integrative analysis from multicenter studies identifies a WGCNA-derived cancer-associated fibroblast signature for ovarian cancer. Front Immunol (2022) 13:951582. doi: 10.3389/fimmu.2022.951582
154. Zou R, Jiang Q, Jin T, Chen M, Yao L, Ding H. Pan-cancer analyses and molecular subtypes based on the cancer-associated fibroblast landscape and tumor microenvironment infiltration characterization reveal clinical outcome and immunotherapy response in epithelial ovarian cancer. Front Immunol (2022) 13:956224. doi: 10.3389/fimmu.2022.956224
155. Sulaiman R, De P, Aske JC, Lin X, Dale A, Koirala N, et al. Patient-derived primary cancer-associated fibroblasts mediate resistance to anti-angiogenic drug in ovarian cancers. Biomedicines (2023) 11:112. doi: 10.3390/biomedicines11010112
156. Erez N, Glanz S, Raz Y, Avivi C, Barshack I. Cancer associated fibroblasts express pro-inflammatory factors in human breast and ovarian tumors. Biochem Biophys Res Commun (2013) 437:397–402. doi: 10.1016/j.bbrc.2013.06.089
157. Ko SY, Barengo N, Ladanyi A, Lee J-S, Marini F, Lengyel E, et al. HOXA9 promotes ovarian cancer growth by stimulating cancer-associated fibroblasts. J Clin Invest (2012) 122:3603–17. doi: 10.1172/JCI62229
158. Ahmed N, Escalona R, Leung D, Chan E, Kannourakis G. Tumour microenvironment and metabolic plasticity in cancer and cancer stem cells: perspectives on metabolic and immune regulatory signatures in chemoresistant ovarian cancer stem cells. Semin Cancer Biol (2018) 53:265–81. doi: 10.1016/j.semcancer.2018.10.002
159. Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab (2004) 89:2548–56. doi: 10.1210/jc.2004-0395
160. Nieman KM, Kenny HA, Penicka CV, Ladanyi A, Buell-Gutbrod R, Zillhardt MR, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med (2011) 17:1498–503. doi: 10.1038/nm.2492
161. Mukherjee A, Chiang C-Y, Daifotis HA, Nieman KM, Fahrmann JF, Lastra RR, et al. Adipocyte-induced FABP4 expression in ovarian cancer cells promotes metastasis and mediates carboplatin resistance. Cancer Res (2020) 80:1748–61. doi: 10.1158/0008-5472.CAN-19-1999
162. Li J, Condello S, Thomes-Pepin J, Ma X, Xia Y, Hurley TD, et al. Lipid desaturation is a metabolic marker and therapeutic target of ovarian cancer stem cells. Cell Stem Cell (2017) 20:303–314.e5. doi: 10.1016/j.stem.2016.11.004
163. Zhou Z, Lu Y, Wang Y, Du L, Zhang Y, Tao J. Let-7c regulates proliferation and osteodifferentiation of human adipose-derived mesenchymal stem cells under oxidative stress by targeting SCD-1. Am J Physiol-Cell Physiol (2019) 316:C57–69. doi: 10.1152/ajpcell.00211.2018
164. Cai Y, Wang J, Zhang L, Wu D, Yu D, Tian X, et al. Expressions of fatty acid synthase and HER2 are correlated with poor prognosis of ovarian cancer. Med Oncol (2015) 32:391. doi: 10.1007/s12032-014-0391-z
165. Cho JA, Park H, Lim EH, Kim KH, Choi JS, Lee JH, et al. Exosomes from ovarian cancer cells induce adipose tissue-derived mesenchymal stem cells to acquire the physical and functional characteristics of tumor-supporting myofibroblasts. Gynecol Oncol (2011) 123:379–86. doi: 10.1016/j.ygyno.2011.08.005
166. Coffman LG, Choi Y-J, McLean K, Allen BL, di Magliano MP, Buckanovich RJ. Human carcinoma-associated mesenchymal stem cells promote ovarian cancer chemotherapy resistance via a BMP4/HH signaling loop. Oncotarget (2016) 7:6916–32. doi: 10.18632/oncotarget.6870
167. Wen Y, Guo Y, Huang Z, Cai J, Wang Z. Adipose-derived mesenchymal stem cells attenuate cisplatin-induced apoptosis in epithelial ovarian cancer cells. Mol Med Rep (2017) 16:9587–92. doi: 10.3892/mmr.2017.7783
168. Spaeth EL, Dembinski JL, Sasser AK, Watson K, Klopp A, Hall B, et al. Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PloS One (2009) 4:e4992. doi: 10.1371/journal.pone.0004992
169. Touboul C, Lis R, Al Farsi H, Raynaud CM, Warfa M, Althawadi H, et al. Mesenchymal stem cells enhance ovarian cancer cell infiltration through IL6 secretion in an amniochorionic membrane based 3D model. J Transl Med (2013) 11:28. doi: 10.1186/1479-5876-11-28
170. Bu S, Wang Q, Zhang Q, Sun J, He B, Xiang C, et al. Human endometrial mesenchymal stem cells exhibit intrinsic anti-tumor properties on human epithelial ovarian cancer cells. Sci Rep (2016) 6:37019. doi: 10.1038/srep37019
171. Gu Z-W, He Y-F, Wang W-J, Tian Q, Di W. MiR-1180 from bone marrow-derived mesenchymal stem cells inducesglycolysis and chemoresistance in ovarian cancer cells by upregulating the wnt signalingpathway. J Zhejiang Univ-Sci B (2019) 20:219–37. doi: 10.1631/jzus.B1800190
172. Chen X, Ying X, Wang X, Wu X, Zhu Q, Wang X. Exosomes derived from hypoxic epithelial ovarian cancer deliver microRNA-940 to induce macrophage M2 polarization. Oncol Rep (2017) 38:522–8. doi: 10.3892/or.2017.5697
173. Yin M, Li X, Tan S, Zhou HJ, Ji W, Bellone S, et al. Tumor-associated macrophages drive spheroid formation during early transcoelomic metastasis of ovarian cancer. J Clin Invest (2016) 126:4157–73. doi: 10.1172/JCI87252
174. Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol (2002) 23:549–55. doi: 10.1016/S1471-4906(02)02302-5
175. Duluc D, Delneste Y, Tan F, Moles M-P, Grimaud L, Lenoir J, et al. Tumor-associated leukemia inhibitory factor and IL-6 skew monocyte differentiation into tumor-associated macrophage-like cells. Blood (2007) 110:4319–30. doi: 10.1182/blood-2007-02-072587
176. Masoumi Moghaddam S, Amini A, Morris DL, Pourgholami MH. Significance of vascular endothelial growth factor in growth and peritoneal dissemination of ovarian cancer. Cancer Metastasis Rev (2012) 31:143–62. doi: 10.1007/s10555-011-9337-5
177. Zeng X-Y, Xie H, Yuan J, Jiang X-Y, Yong J-H, Zeng D, et al. M2-like tumor-associated macrophages-secreted EGF promotes epithelial ovarian cancer metastasis via activating EGFR-ERK signaling and suppressing lncRNA LIMT expression. Cancer Biol Ther (2019) 20:956–66. doi: 10.1080/15384047.2018.1564567
178. Song M, Yeku OO, Rafiq S, Purdon T, Dong X, Zhu L, et al. Tumor derived UBR5 promotes ovarian cancer growth and metastasis through inducing immunosuppressive macrophages. Nat Commun (2020) 11:6298. doi: 10.1038/s41467-020-20140-0
179. Macciò A, Gramignano G, Cherchi MC, Tanca L, Melis L, Madeddu C. Role of M1-polarized tumor-associated macrophages in the prognosis of advanced ovarian cancer patients. Sci Rep (2020) 10:6096. doi: 10.1038/s41598-020-63276-1
180. Tan Q, Liu H, Xu J, Mo Y, Dai F. Integrated analysis of tumor-associated macrophage infiltration and prognosis in ovarian cancer. Aging (2021) 13:23210–32. doi: 10.18632/aging.203613
181. Jiang S, Yang Y, Zhang Y, Ye Q, Song J, Zheng M, et al. Overexpression of CAPG is associated with poor prognosis and immunosuppressive cell infiltration in ovarian cancer. Dis Markers (2022) 2022:1–18. doi: 10.1155/2022/9719671
182. Wang H, Yung MMH, Ngan HYS, Chan KKL, Chan DW. The impact of the tumor microenvironment on macrophage polarization in cancer metastatic progression. Int J Mol Sci (2021) 22:6560. doi: 10.3390/ijms22126560
183. You Y, Li Y, Li M, Lei M, Wu M, Qu Y, et al. Ovarian cancer stem cells promote tumour immune privilege and invasion via CCL5 and regulatory T cells. Clin Exp Immunol (2017) 191:60–73. doi: 10.1111/cei.13044
184. Knutson KL, Maurer MJ, Preston CC, Moysich KB, Goergen K, Hawthorne KM, et al. Regulatory T cells, inherited variation, and clinical outcome in epithelial ovarian cancer. Cancer Immunol Immunother (2015) 64:1495–504. doi: 10.1007/s00262-015-1753-x
185. Toker A, Nguyen LT, Stone SC, Yang SYC, Katz SR, Shaw PA, et al. Regulatory T cells in ovarian cancer are characterized by a highly activated phenotype distinct from that in melanoma. Clin Cancer Res (2018) 24:5685–96. doi: 10.1158/1078-0432.CCR-18-0554
186. Marchenko S, Piwonski I, Hoffmann I, Sinn BV, Kunze CA, Monjé N, et al. Prognostic value of regulatory T cells and T helper 17 cells in high grade serous ovarian carcinoma. J Cancer Res Clin Oncol (2022) 149:2523–36. doi: 10.1007/s00432-022-04101-2
187. Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med (2004) 10:942–9. doi: 10.1038/nm1093
188. Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, et al. Intraepithelial CD8 + tumor-infiltrating lymphocytes and a high CD8 + /regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci (2005) 102:18538–43. doi: 10.1073/pnas.0509182102
189. Bamias A, Koutsoukou V, Terpos E, Tsiatas ML, Liakos C, Tsitsilonis O, et al. Correlation of NK T-like CD3+CD56+ cells and CD4+CD25+(hi) regulatory T cells with VEGF and TNFα in ascites from advanced ovarian cancer: association with platinum resistance and prognosis in patients receiving first-line, platinum-based chemotherapy. Gynecol Oncol (2008) 108:421–7. doi: 10.1016/j.ygyno.2007.10.018
190. Woo EY, Chu CS, Goletz TJ, Schlienger K, Yeh H, Coukos G, et al. Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res (2001) 61:4766–72.
191. Labidi-Galy SI, Sisirak V, Meeus P, Gobert M, Treilleux I, Bajard A, et al. Quantitative and functional alterations of plasmacytoid dendritic cells contribute to immune tolerance in ovarian cancer. Cancer Res (2011) 71:5423–34. doi: 10.1158/0008-5472.CAN-11-0367
192. Huarte E, Cubillos-Ruiz JR, Nesbeth YC, Scarlett UK, Martinez DG, Buckanovich RJ, et al. Depletion of dendritic cells delays ovarian cancer progression by boosting antitumor immunity. Cancer Res (2008) 68:7684–91. doi: 10.1158/0008-5472.CAN-08-1167
193. Truxova I, Kasikova L, Hensler M, Skapa P, Laco J, Pecen L, et al. Mature dendritic cells correlate with favorable immune infiltrate and improved prognosis in ovarian carcinoma patients. J Immunother Cancer (2018) 6:139. doi: 10.1186/s40425-018-0446-3
194. Zou W, Machelon V, Coulomb-L’Hermin A, Borvak J, Nome F, Isaeva T, et al. Stromal-derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells. Nat Med (2001) 7:1339–46. doi: 10.1038/nm1201-1339
195. Krempski J, Karyampudi L, Behrens MD, Erskine CL, Hartmann L, Dong H, et al. Tumor-infiltrating programmed death receptor-1+ dendritic cells mediate immune suppression in ovarian cancer. J Immunol (2011) 186:6905–13. doi: 10.4049/jimmunol.1100274
196. Wertel I, Polak G, Bednarek W, Barczyński B, Roliński J, Kotarski J. Dendritic cell subsets in the peritoneal fluid and peripheral blood of women suffering from ovarian cancer. Cytometry B Clin Cytom (2008) 74B:251–8. doi: 10.1002/cyto.b.20410
197. Wei S, Kryczek I, Zou L, Daniel B, Cheng P, Mottram P, et al. Plasmacytoid dendritic cells induce CD8+ regulatory T cells in human ovarian carcinoma. Cancer Res (2005) 65:5020–6. doi: 10.1158/0008-5472.CAN-04-4043
198. Yanagisawa K, Exley MA, Jiang X, Ohkochi N, Taniguchi M, Seino K. Hyporesponsiveness to natural killer T-cell ligand α-galactosylceramide in cancer-bearing state mediated by CD11b+ gr-1+ cells producing nitric oxide. Cancer Res (2006) 66:11441–6. doi: 10.1158/0008-5472.CAN-06-0944
199. Hart KM, Byrne KT, Molloy MJ, Usherwood EM, Berwin B. IL-10 immunomodulation of myeloid cells regulates a murine model of ovarian cancer. Front Immunol (2011) 2:29. doi: 10.3389/fimmu.2011.00029
200. Obermajer N, Muthuswamy R, Odunsi K, Edwards RP, Kalinski P. PGE2-induced CXCL12 production and CXCR4 expression controls the accumulation of human MDSCs in ovarian cancer environment. Cancer Res (2011) 71:7463–70. doi: 10.1158/0008-5472.CAN-11-2449
201. Pi R, Yang Y, Hu X, Li H, Shi H, Liu Y, et al. Dual mTORC1/2 inhibitor AZD2014 diminishes myeloid-derived suppressor cells accumulation in ovarian cancer and delays tumor growth. Cancer Lett (2021) 523:72–81. doi: 10.1016/j.canlet.2021.09.017
202. Komura N, Mabuchi S, Shimura K, Yokoi E, Kozasa K, Kuroda H, et al. The role of myeloid-derived suppressor cells in increasing cancer stem-like cells and promoting PD-L1 expression in epithelial ovarian cancer. Cancer Immunol Immunother (2020) 69:2477–99. doi: 10.1007/s00262-020-02628-2
203. Okła K, Czerwonka A, Wawruszak A, Bobiński M, Bilska M, Tarkowski R, et al. Clinical relevance and immunosuppressive pattern of circulating and infiltrating subsets of myeloid-derived suppressor cells (MDSCs) in epithelial ovarian cancer. Front Immunol (2019) 10:691. doi: 10.3389/fimmu.2019.00691
204. Wu L, Deng Z, Peng Y, Han L, Liu J, Wang L, et al. Ascites-derived IL-6 and IL-10 synergistically expand CD14+HLA-DR-/low myeloid-derived suppressor cells in ovarian cancer patients. Oncotarget (2017) 8:76843–56. doi: 10.18632/oncotarget.20164
205. Lee J, Botesteanu D, Tomita Y, Yuno A, Lee M, Kohn E, et al. Patients with BRCA mutated ovarian cancer may have fewer circulating MDSC and more peripheral CD8+ T cells compared with women with BRCA wild−type disease during the early disease course. Oncol Lett (2019) 18:3914–24. doi: 10.3892/ol.2019.10731
206. Kumar SM, Liu S, Lu H, Zhang H, Zhang PJ, Gimotty PA, et al. Acquired cancer stem cell phenotypes through Oct4-mediated dedifferentiation. Oncogene (2012) 31:4898–911. doi: 10.1038/onc.2011.656
207. Roy Choudhury A, Gupta S, Chaturvedi PK, Kumar N, Pandey D. Mechanobiology of cancer stem cells and their niche. Cancer Microenviron (2019) 12:17–27. doi: 10.1007/s12307-019-00222-4
208. Klymenko Y, Kim O, Stack M. Complex determinants of epithelial: mesenchymal phenotypic plasticity in ovarian cancer. Cancers (2017) 9:104. doi: 10.3390/cancers9080104
209. Soriano AA, de Cristofaro T, Di Palma T, Dotolo S, Gokulnath P, Izzo A, et al. PAX8 expression in high-grade serous ovarian cancer positively regulates attachment to ECM via integrin β3. Cancer Cell Int (2019) 19:303. doi: 10.1186/s12935-019-1022-8
210. van der Steen SCHA, Bulten J, Van de Vijver KK, van Kuppevelt TH, Massuger LFAG. Changes in the extracellular matrix are associated with the development of serous tubal intraepithelial carcinoma into high-grade serous carcinoma. Int J Gynecol Cancer (2017) 27:1072–81. doi: 10.1097/IGC.0000000000000933
211. Vallen MJE, Massuger LFAG, ten Dam GB, Bulten J, van Kuppevelt TH. Highly sulfated chondroitin sulfates, a novel class of prognostic biomarkers in ovarian cancer tissue. Gynecol Oncol (2012) 127:202–9. doi: 10.1016/j.ygyno.2012.06.022
212. Ojasalu K, Brehm C, Hartung K, Nischak M, Finkernagel F, Rexin P, et al. Upregulation of mesothelial genes in ovarian carcinoma cells is associated with an unfavorable clinical outcome and the promotion of cancer cell adhesion. Mol Oncol (2020) 14:2142–62. doi: 10.1002/1878-0261.12749
213. Gutwein P, Stoeck A, Riedle S, Gast D, Runz S, Condon TP, et al. Cleavage of L1 in exosomes and apoptotic membrane vesicles released from ovarian carcinoma cells. Clin Cancer Res (2005) 11:2492–501. doi: 10.1158/1078-0432.CCR-04-1688
214. Mitra AK, Zillhardt M, Hua Y, Tiwari P, Murmann AE, Peter ME, et al. MicroRNAs reprogram normal fibroblasts into cancer-associated fibroblasts in ovarian cancer. Cancer Discovery (2012) 2:1100–8. doi: 10.1158/2159-8290.CD-12-0206
215. Au Yeung CL, Co N-N, Tsuruga T, Yeung T-L, Kwan S-Y, Leung CS, et al. Exosomal transfer of stroma-derived miR21 confers paclitaxel resistance in ovarian cancer cells through targeting APAF1. Nat Commun (2016) 7:11150. doi: 10.1038/ncomms11150
216. Wu Q, Wu X, Ying X, Zhu Q, Wang X, Jiang L, et al. Suppression of endothelial cell migration by tumor associated macrophage-derived exosomes is reversed by epithelial ovarian cancer exosomal lncRNA. Cancer Cell Int (2017) 17:62. doi: 10.1186/s12935-017-0430-x
217. Lopatina T, Gai C, Deregibus MC, Kholia S, Camussi G. Cross talk between cancer and mesenchymal stem cells through extracellular vesicles carrying nucleic acids. Front Oncol (2016) 6:125. doi: 10.3389/fonc.2016.00125
218. Alharbi M, Lai A, Guanzon D, Palma C, Zuñiga F, Perrin L, et al. Ovarian cancer-derived exosomes promote tumour metastasis in vivo: an effect modulated by the invasiveness capacity of their originating cells. Clin Sci (2019) 133:1401–19. doi: 10.1042/CS20190082
219. Qu Q, Liu L, Cui Y, Chen Y, Wang Y, Wang Y. Exosomes from human omental adipose-derived mesenchymal stem cells secreted into ascites promote peritoneal metastasis of epithelial ovarian cancer. Cells (2022) 11:3392. doi: 10.3390/cells11213392
220. Qiu L, Wang J, Chen M, Chen F, Tu W. Exosomal microRNA−146a derived from mesenchymal stem cells increases the sensitivity of ovarian cancer cells to docetaxel and taxane via a LAMC2−mediated PI3K/Akt axis. Int J Mol Med (2020) 46:609–20. doi: 10.3892/ijmm.2020.4634
221. Vera N, Acuña-Gallardo S, Grünenwald F, Caceres-Verschae A, Realini O, Acuña R, et al. Small extracellular vesicles released from ovarian cancer spheroids in response to cisplatin promote the pro-tumorigenic activity of mesenchymal stem cells. Int J Mol Sci (2019) 20:4972. doi: 10.3390/ijms20204972
222. Luo H, Zhou Y, Zhang J, Zhang Y, Long S, Lin X, et al. NK cell-derived exosomes enhance the anti-tumor effects against ovarian cancer by delivering cisplatin and reactivating NK cell functions. Front Immunol (2023) 13:1087689. doi: 10.3389/fimmu.2022.1087689
223. Zhang X, Wang J, Liu N, Wu W, Li H, Chen J, et al. Molecular mechanism of CD163+ tumor-associated macrophage (TAM)-derived exosome-induced cisplatin resistance in ovarian cancer ascites. Ann Transl Med (2022) 10:1014–4. doi: 10.21037/atm-22-4267
224. Shnaider PV, Petrushanko IYU, Aleshikova OI, Babaeva NA, Ashrafyan LA, Borovkova EI, et al. Expression level of CD117 (KIT) on ovarian cancer extracellular vesicles correlates with tumor aggressiveness. Front Cell Dev Biol (2023) 11:1057484. doi: 10.3389/fcell.2023.1057484
225. Zhang W, Ou X, Wu X. Proteomics profiling of plasma exosomes in epithelial ovarian cancer: a potential role in the coagulation cascade, diagnosis and prognosis. Int J Oncol (2019) 54:1719–33. doi: 10.3892/ijo.2019.4742
226. Cai J, Gong L, Li G, Guo J, Yi X, Wang Z. Exosomes in ovarian cancer ascites promote epithelial–mesenchymal transition of ovarian cancer cells by delivery of miR-6780b-5p. Cell Death Dis (2021) 12:210. doi: 10.1038/s41419-021-03490-5
227. Keng. Exosomes in the ascites of ovarian cancer patients: origin and effects on anti-tumor immunity. Oncol Rep (2011) 25:749–62. doi: 10.3892/or.2010.1119
228. Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol (2008) 110:13–21. doi: 10.1016/j.ygyno.2008.04.033
229. Shimizu A, Sawada K, Kobayashi M, Yamamoto M, Yagi T, Kinose Y, et al. Exosomal CD47 plays an essential role in immune evasion in ovarian cancer. Mol Cancer Res (2021) 19:1583–95. doi: 10.1158/1541-7786.MCR-20-0956
230. Kanlikilicer P, Bayraktar R, Denizli M, Rashed MH, Ivan C, Aslan B, et al. Corrigendum to ‘Exosomal miRNA confers chemo resistance via targeting Cav1/p-gp/M2-type macrophage axis in ovarian cancer’ [EBioMedicine 38 (2018) 100–112]. EBioMedicine (2020) 52:102630. doi: 10.1016/j.ebiom.2020.102630
231. Qiu J-J, Lin X-J, Tang X-Y, Zheng T-T, Lin Y-Y, Hua K-Q. Exosomal Metastasis−Associated lung adenocarcinoma transcript 1 promotes angiogenesis and predicts poor prognosis in epithelial ovarian cancer. Int J Biol Sci (2018) 14:1960–73. doi: 10.7150/ijbs.28048
232. Chen L, Wang K, Li L, Zheng B, Zhang Q, Zhang F, et al. Plasma exosomal miR-1260a, miR-7977 and miR-192-5p as diagnostic biomarkers in epithelial ovarian cancer. Future Oncol (2022) 18:2919–31. doi: 10.2217/fon-2022-0321
233. Xiong C, Sun Z, Yu J, Lin Y. Exosome component 4 promotes epithelial ovarian cancer cell proliferation, migration, and invasion via the wnt pathway. Front Oncol (2021) 11:797968. doi: 10.3389/fonc.2021.797968
234. Zhu Z, Chen Z, Wang M, Zhang M, Chen Y, Yang X, et al. Detection of plasma exosomal miRNA-205 as a biomarker for early diagnosis and an adjuvant indicator of ovarian cancer staging. J Ovarian Res (2022) 15:27. doi: 10.1186/s13048-022-00961-x
235. Li S-S, Ma J, Wong AST. Chemoresistance in ovarian cancer: exploiting cancer stem cell metabolism. J Gynecol Oncol (2018) 29:e32. doi: 10.3802/jgo.2018.29.e32
236. Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell (2010) 40:294–309. doi: 10.1016/j.molcel.2010.09.022
237. Vander Linden C, Corbet C. Therapeutic targeting of cancer stem cells: integrating and exploiting the acidic niche. Front Oncol (2019) 9:159. doi: 10.3389/fonc.2019.00159
238. Corbet C, Feron O. Tumour acidosis: from the passenger to the driver’s seat. Nat Rev Cancer (2017) 17:577–93. doi: 10.1038/nrc.2017.77
239. Toutirais O, Chartier P, Dubois D, Bouet F, Lévêque J, Catros-Quemener V, et al. Constitutive expression of TGF-bêta1, interleukin-6 and interleukin-8 by tumor cells as a major component of immune escape in human ovarian carcinoma. Eur Cytokine Netw (2003) 14:246–55.
240. Mills C. M1 and M2 macrophages: oracles of health and disease. Crit Rev Immunol (2012) 32:463–88. doi: 10.1615/CritRevImmunol.v32.i6.10
241. Naldini A, Morena E, Pucci A, Miglietta D, Riboldi E, Sozzani S, et al. Hypoxia affects dendritic cell survival: role of the hypoxia-inducible factor-1α and lipopolysaccharide. J Cell Physiol (2012) 227:587–95. doi: 10.1002/jcp.22761
242. Seo EJ, Kim DK, Jang IH, Choi EJ, Shin SH, Lee SI, et al. Hypoxia-NOTCH1-SOX2 signaling is important for maintaining cancer stem cells in ovarian cancer. Oncotarget (2016) 7:55624–38. doi: 10.18632/oncotarget.10954
243. Chen X, Lan H, He D, Xu R, Zhang Y, Cheng Y, et al. Multi-omics profiling identifies risk hypoxia-related signatures for ovarian cancer prognosis. Front Immunol (2021) 12:645839. doi: 10.3389/fimmu.2021.645839
244. Huang Y, Zhou Y, Zhang M. Identification of seven hypoxia-related genes signature and risk score models for predicting prognosis for ovarian cancer. Funct Integr Genomics (2023) 23:39. doi: 10.1007/s10142-022-00956-3
245. Todeschini P, Salviato E, Romani C, Raimondi V, Ciccarese F, Ferrari F, et al. Comprehensive profiling of hypoxia-related miRNAs identifies miR-23a-3p overexpression as a marker of platinum resistance and poor prognosis in high-grade serous ovarian cancer. Cancers (2021) 13:3358. doi: 10.3390/cancers13133358
246. Kouidhi S, Ben Ayed F, Benammar Elgaaied A. Targeting tumor metabolism: a new challenge to improve immunotherapy. Front Immunol (2018) 9:353. doi: 10.3389/fimmu.2018.00353
247. Renner K, Singer K, Koehl GE, Geissler EK, Peter K, Siska PJ, et al. Metabolic hallmarks of tumor and immune cells in the tumor microenvironment. Front Immunol (2017) 8:248. doi: 10.3389/fimmu.2017.00248
248. Latifi A, Luwor RB, Bilandzic M, Nazaretian S, Stenvers K, Pyman J, et al. Isolation and characterization of tumor cells from the ascites of ovarian cancer patients: molecular phenotype of chemoresistant ovarian tumors. PloS One (2012) 7:e46858. doi: 10.1371/journal.pone.0046858
249. Bellio C, DiGloria C, Foster R, James K, Konstantinopoulos PA, Growdon WB, et al. PARP inhibition induces enrichment of DNA repair–proficient CD133 and CD117 positive ovarian cancer stem cells. Mol Cancer Res (2019) 17:431–45. doi: 10.1158/1541-7786.MCR-18-0594
250. Kim W-T, Ryu CJ. Cancer stem cell surface markers on normal stem cells. BMB Rep (2017) 50:285–98. doi: 10.5483/BMBRep.2017.50.6.039
251. Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood (2005) 106:1901–10. doi: 10.1182/blood-2005-04-1417
252. Maleki M, Ghanbarvand F, Behvarz MR, Ejtemaei M, Ghadirkhomi E. Comparison of mesenchymal stem cell markers in multiple human adult stem cells. Int J Stem Cells (2014) 7:118–26. doi: 10.15283/ijsc.2014.7.2.118
253. Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell (2002) 13:4279–95. doi: 10.1091/mbc.e02-02-0105
254. Sundberg M, Jansson L, Ketolainen J, Pihlajamäki H, Suuronen R, Skottman H, et al. CD Marker expression profiles of human embryonic stem cells and their neural derivatives, determined using flow-cytometric analysis, reveal a novel CD marker for exclusion of pluripotent stem cells. Stem Cell Res (2009) 2:113–24. doi: 10.1016/j.scr.2008.08.001
255. Zannettino ACW, Paton S, Arthur A, Khor F, Itescu S, Gimble JM, et al. Multipotential human adipose-derived stromal stem cells exhibit a perivascular phenotype in vitro and in vivo. J Cell Physiol (2008) 214:413–21. doi: 10.1002/jcp.21210
256. Wang F, Scoville D, He XC, Mahe MM, Box A, Perry JM, et al. Isolation and characterization of intestinal stem cells based on surface marker combinations and colony-formation assay. Gastroenterology (2013) 145:383–395.e21. doi: 10.1053/j.gastro.2013.04.050
257. Clarke MF, Fuller M. Stem cells and cancer: two faces of eve. Cell (2006) 124:1111–5. doi: 10.1016/j.cell.2006.03.011
258. Dvorak P, Dvorakova D, Hampl A. Fibroblast growth factor signaling in embryonic and cancer stem cells. FEBS Lett (2006) 580:2869–74. doi: 10.1016/j.febslet.2006.01.095
259. Takahashi A, Hong L, Chefetz I. How to win the ovarian cancer stem cell battle: destroying the roots. Cancer Drug Resist (2021) 3:1021–33. doi: 10.20517/cdr.2020.93
260. Esteller M. Epigenetic gene silencing in cancer: the DNA hypermethylome. Hum Mol Genet (2007) 16:R50–9. doi: 10.1093/hmg/ddm018
261. Teodoridis JM, Hall J, Marsh S, Kannall HD, Smyth C, Curto J, et al. CpG island methylation of DNA damage response genes in advanced ovarian cancer. Cancer Res (2005) 65:8961–7. doi: 10.1158/0008-5472.CAN-05-1187
262. Ahmed N, Kadife E, Raza A, Short M, Jubinsky PT, Kannourakis G. Ovarian cancer, cancer stem cells and current treatment strategies: a potential role of magmas in the current treatment methods. Cells (2020) 9:719. doi: 10.3390/cells9030719
263. Zhang W, Klinkebiel D, Barger CJ, Pandey S, Guda C, Miller A, et al. Global DNA hypomethylation in epithelial ovarian cancer: passive demethylation and association with genomic instability. Cancers (2020) 12:764. doi: 10.3390/cancers12030764
264. To SKY, Mak ASC, Eva Fung YM, Che C-M, Li S-S, Deng W, et al. β-catenin downregulates dicer to promote ovarian cancer metastasis. Oncogene (2017) 36:5927–38. doi: 10.1038/onc.2017.185
265. Roca, Di Gennaro, Budillon. Implication for cancer stem cells in solid cancer chemo-resistance: promising therapeutic strategies based on the use of HDAC inhibitors. J Clin Med (2019) 8:912. doi: 10.3390/jcm8070912
266. Annett S, Moore G, Short A, Marshall A, McCrudden C, Yakkundi A, et al. FKBPL-based peptide, ALM201, targets angiogenesis and cancer stem cells in ovarian cancer. Br J Cancer (2020) 122:361–71. doi: 10.1038/s41416-019-0649-5
267. El Helali A, Plummer R, Jayson GC, Coyle VM, Drew Y, Mescallado N, et al. A first-in-human phase I dose-escalation trial of the novel therapeutic peptide, ALM201, demonstrates a favourable safety profile in unselected patients with ovarian cancer and other advanced solid tumours. Br J Cancer (2022) 127:92–101. doi: 10.1038/s41416-022-01780-z
268. Coleman RL, Broaddus RR, Bodurka DC, Wolf JK, Burke TW, Kavanagh JJ, et al. Phase II trial of imatinib mesylate in patients with recurrent platinum- and taxane-resistant epithelial ovarian and primary peritoneal cancers. Gynecol Oncol (2006) 101:126–31. doi: 10.1016/j.ygyno.2005.09.041
269. Schilder RJ, Sill MW, Lee RB, Shaw TJ, Senterman MK, Klein-Szanto AJ, et al. Phase II evaluation of imatinib mesylate in the treatment of recurrent or persistent epithelial ovarian or primary peritoneal carcinoma: a gynecologic oncology group study. J Clin Oncol (2008) 26:3418–25. doi: 10.1200/JCO.2007.14.3420
270. Alberts DS, Liu PY, Wilczynski SP, Jang A, Moon J, Ward JH, et al. Phase II trial of imatinib mesylate in recurrent, biomarker positive, ovarian cancer (Southwest oncology group protocol S0211). Int J Gynecol Cancer (2007) 17:784–8. doi: 10.1111/j.1525-1438.2007.00882.x
271. Matei D, Emerson RE, Schilder J, Menning N, Baldridge LA, Johnson CS, et al. Imatinib mesylate in combination with docetaxel for the treatment of patients with advanced, platinum-resistant ovarian cancer and primary peritoneal carcinomatosis: a Hoosier oncology group trial. Cancer (2008) 113:723–32. doi: 10.1002/cncr.23605
272. Lee H-G, Shin S-J, Chung H-W, Kwon S-H, Cha S-D, Lee J-E, et al. Salinomycin reduces stemness and induces apoptosis on human ovarian cancer stem cell. J Gynecol Oncol (2017) 28:e14. doi: 10.3802/jgo.2017.28.e14
273. Zou M, Yin X, Zhou X, Niu X, Wang Y, Su M. Salinomycin-loaded high-density lipoprotein exerts promising anti-ovarian cancer effects by inhibiting epithelial–mesenchymal transition. Int J Nanomed (2022) Volume 17:4059–71. doi: 10.2147/IJN.S380598
274. Fan Y, Cheng H, Liu Y, Liu S, Lowe S, Li Y, et al. Metformin anticancer: reverses tumor hypoxia induced by bevacizumab and reduces the expression of cancer stem cell markers CD44/CD117 in human ovarian cancer SKOV3 cells. Front Pharmacol (2022) 13:955984. doi: 10.3389/fphar.2022.955984
275. Alvero AB, Montagna MK, Holmberg JC, Craveiro V, Brown D, Mor G. Targeting the mitochondria activates two independent cell death pathways in ovarian cancer stem cells. Mol Cancer Ther (2011) 10:1385–93. doi: 10.1158/1535-7163.MCT-11-0023
276. Grimley E, Cole AJ, Luong TT, McGonigal SC, Sinno S, Yang D, et al. Aldehyde dehydrogenase inhibitors promote DNA damage in ovarian cancer and synergize with ATM/ATR inhibitors. Theranostics (2021) 11:3540–51. doi: 10.7150/thno.51885
277. Nwani N, Condello S, Wang Y, Swetzig W, Barber E, Hurley T, et al. A novel ALDH1A1 inhibitor targets cells with stem cell characteristics in ovarian cancer. Cancers (2019) 11:502. doi: 10.3390/cancers11040502
278. Whitworth JM, Londoño-Joshi AI, Sellers JC, Oliver PJ, Muccio DD, Atigadda VR, et al. The impact of novel retinoids in combination with platinum chemotherapy on ovarian cancer stem cells. Gynecol Oncol (2012) 125:226–30. doi: 10.1016/j.ygyno.2011.12.425
279. Chefetz I, Grimley E, Yang K, Hong L, Vinogradova EV, Suciu R, et al. A pan-ALDH1A inhibitor induces necroptosis in ovarian cancer stem-like cells. Cell Rep (2019) 26:3061–3075.e6. doi: 10.1016/j.celrep.2019.02.032
280. Guo F, Yang Z, Sehouli J, Kaufmann AM. Blockade of ALDH in cisplatin-resistant ovarian cancer stem cells In vitro synergistically enhances chemotherapy-induced cell death. Curr Oncol (2022) 29:2808–22. doi: 10.3390/curroncol29040229
281. Simpkins F, Jang K, Yoon H, Hew KE, Kim M, Azzam DJ, et al. Dual src and MEK inhibition decreases ovarian cancer growth and targets tumor initiating stem-like cells. Clin Cancer Res (2018) 24:4874–86. doi: 10.1158/1078-0432.CCR-17-3697
282. Wang W, Fang F, Ozes A, Nephew KP. Targeting ovarian cancer stem cells by dual inhibition of HOTAIR and DNA methylation. Mol Cancer Ther (2021) 20:1092–101. doi: 10.1158/1535-7163.MCT-20-0826
283. Choi Y-J, Gurunathan S, Kim J-H. Graphene oxide–silver nanocomposite enhances cytotoxic and apoptotic potential of salinomycin in human ovarian cancer stem cells (OvCSCs): a novel approach for cancer therapy. Int J Mol Sci (2018) 19:710. doi: 10.3390/ijms19030710
284. Hirst J, Pathak HB, Hyter S, Pessetto ZY, Ly T, Graw S, et al. Licofelone enhances the efficacy of paclitaxel in ovarian cancer by reversing drug resistance and tumor stem-like properties. Cancer Res (2018) 78:4370–85. doi: 10.1158/0008-5472.CAN-17-3993
285. Brown JR, Chan DK, Shank JJ, Griffith KA, Fan H, Szulawski R, et al. Phase II clinical trial of metformin as a cancer stem cell-targeting agent in ovarian cancer. JCI Insight (2020) 5:e133247. doi: 10.1172/jci.insight.133247
286. Mi Y, Huang Y, Deng J. The enhanced delivery of salinomycin to CD133+ ovarian cancer stem cells through CD133 antibody conjugation with poly(lactic-co-glycolic acid)-poly(ethylene glycol) nanoparticles. Oncol Lett (2018) 15:6611–21. doi: 10.3892/ol.2018.8140
287. Skubitz APN, Taras EP, Boylan KLM, Waldron NN, Oh S, Panoskaltsis-Mortari A, et al. Targeting CD133 in an in vivo ovarian cancer model reduces ovarian cancer progression. Gynecol Oncol (2013) 130:579–87. doi: 10.1016/j.ygyno.2013.05.027
288. Klapdor R, Wang S, Hacker U, Büning H, Morgan M, Dörk T, et al. Improved killing of ovarian cancer stem cells by combining a novel chimeric antigen receptor–based immunotherapy and chemotherapy. Hum Gene Ther (2017) 28:886–96. doi: 10.1089/hum.2017.168
289. Ang WX, Li Z, Chi Z, Du S-H, Chen C, Tay JCK, et al. Intraperitoneal immunotherapy with T cells stably and transiently expressing anti-EpCAM CAR in xenograft models of peritoneal carcinomatosis. Oncotarget (2017) 8:13545–59. doi: 10.18632/oncotarget.14592
290. Berek JS, Edwards RP, Parker LP, DeMars LR, Herzog TJ, Lentz SS, et al. Catumaxomab for the treatment of malignant ascites in patients with chemotherapy-refractory ovarian cancer: a phase II study. Int J Gynecol Cancer (2014) 24:1583–9. doi: 10.1097/IGC.0000000000000286
291. Wimberger P, Gilet H, Gonschior A-K, Heiss MM, Moehler M, Oskay-Oezcelik G, et al. Deterioration in quality of life (QoL) in patients with malignant ascites: results from a phase II/III study comparing paracentesis plus catumaxomab with paracentesis alone. Ann Oncol (2012) 23:1979–85. doi: 10.1093/annonc/mds178
292. Fossati M, Buzzonetti A, Monego G, Catzola V, Scambia G, Fattorossi A, et al. Immunological changes in the ascites of cancer patients after intraperitoneal administration of the bispecific antibody catumaxomab (anti-EpCAM×anti-CD3). Gynecol Oncol (2015) 138:343–51. doi: 10.1016/j.ygyno.2015.06.003
293. Kim H, Xu H, George E, Hallberg D, Kumar S, Jagannathan V, et al. Combining PARP with ATR inhibition overcomes PARP inhibitor and platinum resistance in ovarian cancer models. Nat Commun (2020) 11:3726. doi: 10.1038/s41467-020-17127-2
294. Shah PD, Wethington SL, Pagan C, Latif N, Tanyi J, Martin LP, et al. Combination ATR and PARP inhibitor (CAPRI): a phase 2 study of ceralasertib plus olaparib in patients with recurrent, platinum-resistant epithelial ovarian cancer. Gynecol Oncol (2021) 163:246–53. doi: 10.1016/j.ygyno.2021.08.024
295. Yap TA, O’Carrigan B, Penney MS, Lim JS, Brown JS, de Miguel Luken MJ, et al. Phase I trial of first-in-Class ATR inhibitor M6620 (VX-970) as monotherapy or in combination with carboplatin in patients with advanced solid tumors. J Clin Oncol (2020) 38:3195–204. doi: 10.1200/JCO.19.02404
296. Kang Y, Hu W, Ivan C, Dalton HJ, Miyake T, Pecot CV, et al. Role of focal adhesion kinase in regulating YB–1–Mediated paclitaxel resistance in ovarian cancer. JNCI J Natl Cancer Inst (2013) 105:1485–95. doi: 10.1093/jnci/djt210
297. Patel MR, Infante JR, Moore KN, Keegan M, Poli A, Padval M, et al. Phase 1/1b study of the FAK inhibitor defactinib (VS-6063) in combination with weekly paclitaxel for advanced ovarian cancer. J Clin Oncol (2014) 32:5521–1. doi: 10.1200/jco.2014.32.15_suppl.5521
298. Diaz Osterman CJ, Ozmadenci D, Kleinschmidt EG, Taylor KN, Barrie AM, Jiang S, et al. FAK activity sustains intrinsic and acquired ovarian cancer resistance to platinum chemotherapy. eLife (2019) 8:e47327. doi: 10.7554/eLife.47327
299. Fang DD, Tao R, Wang G, Li Y, Zhang K, Xu C, et al. Discovery of a novel ALK/ROS1/FAK inhibitor, APG-2449, in preclinical non-small cell lung cancer and ovarian cancer models. BMC Cancer (2022) 22:752. doi: 10.1186/s12885-022-09799-4
300. Lee H, Kim JW, Kim DK, Choi DK, Lee S, Yu JH, et al. Calcium channels as novel therapeutic targets for ovarian cancer stem cells. Int J Mol Sci (2020) 21:2327. doi: 10.3390/ijms21072327
301. Lee H, Kim JW, Lee D-S, Min S-H. Combined poziotinib with manidipine treatment suppresses ovarian cancer stem-cell proliferation and stemness. Int J Mol Sci (2020) 21:7379. doi: 10.3390/ijms21197379
302. Löblein MT, Falke I, Eich HT, Greve B, Götte M, Troschel FM. Dual knockdown of musashi RNA-binding proteins MSI-1 and MSI-2 attenuates putative cancer stem cell characteristics and therapy resistance in ovarian cancer cells. Int J Mol Sci (2021) 22:11502. doi: 10.3390/ijms222111502
303. He Y, Alejo S, Venkata PP, Johnson JD, Loeffel I, Pratap UP, et al. Therapeutic targeting of ovarian cancer stem cells using estrogen receptor beta agonist. Int J Mol Sci (2022) 23:7159. doi: 10.3390/ijms23137159
304. Nacarelli T, Fukumoto T, Zundell JA, Fatkhutdinov N, Jean S, Cadungog MG, et al. NAMPT inhibition suppresses cancer stem-like cells associated with therapy-induced senescence in ovarian cancer. Cancer Res (2020) 80:890–900. doi: 10.1158/0008-5472.CAN-19-2830
305. Yamamoto M, Suzuki S, Togashi K, Sanomachi T, Seino S, Kitanaka C, et al. AS602801, an anticancer stem cell candidate drug, reduces survivin expression and sensitizes A2780 ovarian cancer stem cells to carboplatin and paclitaxel. Anticancer Res (2018) 38:6699–706. doi: 10.21873/anticanres.13038
306. Togashi K, Okada M, Yamamoto M, Suzuki S, Sanomachi T, Seino S, et al. A small-molecule kinase inhibitor, CEP-1347, inhibits survivin expression and sensitizes ovarian cancer stem cells to paclitaxel. Anticancer Res (2018) 38:4535–42. doi: 10.21873/anticanres.12757
307. Moore KN, Gunderson CC, Sabbatini P, McMeekin DS, Mantia-Smaldone G, Burger RA, et al. A phase 1b dose escalation study of ipafricept (OMP 54F28) in combination with paclitaxel and carboplatin in patients with recurrent platinum-sensitive ovarian cancer. Gynecol Oncol (2019) 154:294–301. doi: 10.1016/j.ygyno.2019.04.001
308. Boone JD, Arend RC, Johnston BE, Cooper SJ, Gilchrist SA, Oelschlager DK, et al. Targeting the wnt/β-catenin pathway in primary ovarian cancer with the porcupine inhibitor WNT974. Lab Invest (2016) 96:249–59. doi: 10.1038/labinvest.2015.150
309. Fischer MM, Cancilla B, Yeung VP, Cattaruzza F, Chartier C, Murriel CL, et al. WNT antagonists exhibit unique combinatorial antitumor activity with taxanes by potentiating mitotic cell death. Sci Adv (2017) 3:e1700090. doi: 10.1126/sciadv.1700090
310. Kaye SB, Fehrenbacher L, Holloway R, Amit A, Karlan B, Slomovitz B, et al. Randomized, placebo-controlled study of vismodegib as maintenance therapy in patients with ovarian cancer in second or third complete remission. Clin Cancer Res (2012) 18:6509–18. doi: 10.1158/1078-0432.CCR-12-1796
311. Stathis A, Hess D, von Moos R, Homicsko K, Griguolo G, Joerger M, et al. Phase I trial of the oral smoothened inhibitor sonidegib in combination with paclitaxel in patients with advanced solid tumors. Invest New Drugs (2017) 35:766–72. doi: 10.1007/s10637-017-0454-z
312. Pant S, Jones SF, Kurkjian CD, Infante JR, Moore KN, Burris HA, et al. A first-in-human phase I study of the oral notch inhibitor, LY900009, in patients with advanced cancer. Eur J Cancer (2016) 56:1–9. doi: 10.1016/j.ejca.2015.11.021
313. Chen X, Gong L, Ou R, Zheng Z, Chen J, Xie F, et al. Sequential combination therapy of ovarian cancer with cisplatin and γ-secretase inhibitor MK-0752. Gynecol Oncol (2016) 140:537–44. doi: 10.1016/j.ygyno.2015.12.011
314. Diaz-Padilla I, Wilson MK, Clarke BA, Hirte HW, Welch SA, Mackay HJ, et al. A phase II study of single-agent RO4929097, a gamma-secretase inhibitor of notch signaling, in patients with recurrent platinum-resistant epithelial ovarian cancer: a study of the princess Margaret, Chicago and California phase II consortia. Gynecol Oncol (2015) 137:216–22. doi: 10.1016/j.ygyno.2015.03.005
315. Chiorean EG, LoRusso P, Strother RM, Diamond JR, Younger A, Messersmith WA, et al. A phase I first-in-Human study of enoticumab (REGN421), a fully human delta-like ligand 4 (Dll4) monoclonal antibody in patients with advanced solid tumors. Clin Cancer Res (2015) 21:2695–703. doi: 10.1158/1078-0432.CCR-14-2797
316. Huang J, Hu W, Hu L, Previs RA, Dalton HJ, Yang X-Y, et al. Dll4 inhibition plus aflibercept markedly reduces ovarian tumor growth. Mol Cancer Ther (2016) 15:1344–52. doi: 10.1158/1535-7163.MCT-15-0144
317. Coleman RL, Handley KF, Burger R, Molin GZD, Stagg R, Sood AK, et al. Demcizumab combined with paclitaxel for platinum-resistant ovarian, primary peritoneal, and fallopian tube cancer: the SIERRA open-label phase ib trial. Gynecol Oncol (2020) 157:386–91. doi: 10.1016/j.ygyno.2020.01.042
318. Jimeno A, Moore KN, Gordon M, Chugh R, Diamond JR, Aljumaily R, et al. A first-in-human phase 1a study of the bispecific anti-DLL4/anti-VEGF antibody navicixizumab (OMP-305B83) in patients with previously treated solid tumors. Invest New Drugs (2019) 37:461–72. doi: 10.1007/s10637-018-0665-y
319. Fu S, Corr BR, Culm-Merdek K, Mockbee C, Youssoufian H, Stagg R, et al. Phase ib study of navicixizumab plus paclitaxel in patients with platinum-resistant ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol (2022) 40:2568–77. doi: 10.1200/JCO.21.01801
320. Yu Z, Wang Y, Wang B, Zhai J. Metformin affects paclitaxel sensitivity of ovarian cancer cells through autophagy mediated by long noncoding RNASNHG7/miR-3127-5p axis. Cancer Biother Radiopharm (2022) 37:792–801. doi: 10.1089/cbr.2019.3390
321. Bishnu A, Sakpal A, Ghosh N, Choudhury P, Chaudhury K, Ray P. Long term treatment of metformin impedes development of chemoresistance by regulating cancer stem cell differentiation through taurine generation in ovarian cancer cells. Int J Biochem Cell Biol (2019) 107:116–27. doi: 10.1016/j.biocel.2018.12.016
322. Westfall SD, Skinner MK. Inhibition of phosphatidylinositol 3-kinase sensitizes ovarian cancer cells to carboplatin and allows adjunct chemotherapy treatment. Mol Cancer Ther (2005) 4:1764–71. doi: 10.1158/1535-7163.MCT-05-0192
323. Jones HM, Fang Z, Sun W, Clark LH, Stine JE, Tran A-Q, et al. Atorvastatin exhibits anti-tumorigenic and anti-metastatic effects in ovarian cancer in vitro. Am J Cancer Res (2017) 7:2478–90.
324. dos Santos Guimarães I, Ladislau-Magescky T, Tessarollo NG, dos Santos DZ, Gimba ERP, Sternberg C, et al. Chemosensitizing effects of metformin on cisplatin- and paclitaxel-resistant ovarian cancer cell lines. Pharmacol Rep (2018) 70:409–17. doi: 10.1016/j.pharep.2017.11.007
325. Chefetz I, Holmberg JC, Alvero AB, Visintin I, Mor G. Inhibition of aurora-a kinase induces cell cycle arrest in epithelial ovarian cancer stem cells by affecting NFĸB pathway. Cell Cycle (2011) 10:2206–14. doi: 10.4161/cc.10.13.16348
326. Michy, Massias, Bernard, Vanwonterghem, Henry, Guidetti, et al. Verteporfin-loaded lipid nanoparticles improve ovarian cancer photodynamic therapy In vitro and In vivo. Cancers (2019) 11:1760. doi: 10.3390/cancers11111760
327. Dasari VR, Carey DJ, Gogoi R. Synergistic enhancement of efficacy of platinum drugs with verteporfin in ovarian cancer cells. BMC Cancer (2020) 20:273. doi: 10.1186/s12885-020-06752-1
328. Han ES, Wen W, Dellinger TH, Wu J, Lu SA, Jove R, et al. Ruxolitinib synergistically enhances the anti-tumor activity of paclitaxel in human ovarian cancer. Oncotarget (2018) 9:24304–19. doi: 10.18632/oncotarget.24368
329. Burgos-Ojeda D, Wu R, McLean K, Chen Y-C, Talpaz M, Yoon E, et al. CD24+ ovarian cancer cells are enriched for cancer-initiating cells and dependent on JAK2 signaling for growth and metastasis. Mol Cancer Ther (2015) 14:1717–27. doi: 10.1158/1535-7163.MCT-14-0607
330. Bagratuni T, Mavrianou N, Gavalas NG, Tzannis K, Arapinis C, Liontos M, et al. JQ1 inhibits tumour growth in combination with cisplatin and suppresses JAK/STAT signalling pathway in ovarian cancer. Eur J Cancer (2020) 126:125–35. doi: 10.1016/j.ejca.2019.11.017
331. Wen H, Qian M, He J, Li M, Yu Q, Leng Z. Inhibiting of self-renewal, migration and invasion of ovarian cancer stem cells by blocking TGF-β pathway. PloS One (2020) 15:e0230230. doi: 10.1371/journal.pone.0230230
332. Fan H, Lu X, Wang X, Liu Y, Guo B, Zhang Y, et al. Low-dose decitabine-based chemoimmunotherapy for patients with refractory advanced solid tumors: a phase I/II report. J Immunol Res (2014) 2014:1–14. doi: 10.1155/2014/371087
333. Zhang Y, Mei Q, Liu Y, Li X, Brock MV, Chen M, et al. The safety, efficacy, and treatment outcomes of a combination of low-dose decitabine treatment in patients with recurrent ovarian cancer. OncoImmunology (2017) 6:e1323619. doi: 10.1080/2162402X.2017.1323619
334. Oza AM, Matulonis UA, Alvarez Secord A, Nemunaitis J, Roman LD, Blagden SP, et al. A randomized phase II trial of epigenetic priming with guadecitabine and carboplatin in platinum-resistant, recurrent ovarian cancer. Clin Cancer Res (2020) 26:1009–16. doi: 10.1158/1078-0432.CCR-19-1638
335. Falchook GS, Fu S, Naing A, Hong DS, Hu W, Moulder S, et al. Methylation and histone deacetylase inhibition in combination with platinum treatment in patients with advanced malignancies. Invest New Drugs (2013) 31:1192–200. doi: 10.1007/s10637-013-0003-3
336. Wong-Brown MW, van der Westhuizen A, Bowden NA. Sequential azacitidine and carboplatin induces immune activation in platinum-resistant high-grade serous ovarian cancer cell lines and primes for checkpoint inhibitor immunotherapy. BMC Cancer (2022) 22:100. doi: 10.1186/s12885-022-09197-w
337. Modesitt SC, Sill M, Hoffman JS, Bender DP. A phase II study of vorinostat in the treatment of persistent or recurrent epithelial ovarian or primary peritoneal carcinoma: a gynecologic oncology group study. Gynecol Oncol (2008) 109:182–6. doi: 10.1016/j.ygyno.2008.01.009
338. Matulonis U, Berlin S, Lee H, Whalen C, Obermayer E, Penson R, et al. Phase I study of combination of vorinostat, carboplatin, and gemcitabine in women with recurrent, platinum-sensitive epithelial ovarian, fallopian tube, or peritoneal cancer. Cancer Chemother Pharmacol (2015) 76:417–23. doi: 10.1007/s00280-015-2813-9
339. Dizon DS, Damstrup L, Finkler NJ, Lassen U, Celano P, Glasspool R, et al. Phase II activity of belinostat (PXD-101), carboplatin, and paclitaxel in women with previously treated ovarian cancer. Int J Gynecol Cancer (2012) 22:979–86. doi: 10.1097/IGC.0b013e31825736fd
340. Dizon DS, Blessing JA, Penson RT, Drake RD, Walker JL, Johnston CM, et al. A phase II evaluation of belinostat and carboplatin in the treatment of recurrent or persistent platinum-resistant ovarian, fallopian tube, or primary peritoneal carcinoma: a gynecologic oncology group study. Gynecol Oncol (2012) 125:367–71. doi: 10.1016/j.ygyno.2012.02.019
341. Smith HJ, McCaw TR, Londono AI, Katre AA, Meza-Perez S, Yang ES, et al. The antitumor effects of entinostat in ovarian cancer require adaptive immunity. Cancer (2018) 124:4657–66. doi: 10.1002/cncr.31761
342. Gupta VG, Hirst J, Petersen S, Roby KF, Kusch M, Zhou H, et al. Entinostat, a selective HDAC1/2 inhibitor, potentiates the effects of olaparib in homologous recombination proficient ovarian cancer. Gynecol Oncol (2021) 162:163–72. doi: 10.1016/j.ygyno.2021.04.015
343. Hernlund E, Hjerpe E, Åvall-Lundqvist E, Shoshan M. Ovarian carcinoma cells with low levels of β-F1-ATPase are sensitive to combined platinum and 2-deoxy- d -glucose treatment. Mol Cancer Ther (2009) 8:1916–23. doi: 10.1158/1535-7163.MCT-09-0179
344. Bellio C, DiGloria C, Spriggs DR, Foster R, Growdon WB, Rueda BR. The metabolic inhibitor CPI-613 negates treatment enrichment of ovarian cancer stem cells. Cancers (2019) 11:1678. doi: 10.3390/cancers11111678
345. Ventura R, Mordec K, Waszczuk J, Wang Z, Lai J, Fridlib M, et al. Inhibition of de novo palmitate synthesis by fatty acid synthase induces apoptosis in tumor cells by remodeling cell membranes, inhibiting signaling pathways, and reprogramming gene expression. EBioMedicine (2015) 2:808–24. doi: 10.1016/j.ebiom.2015.06.020
346. Han C, Yang L, Choi HH, Baddour J, Achreja A, Liu Y, et al. Amplification of USP13 drives ovarian cancer metabolism. Nat Commun (2016) 7:13525. doi: 10.1038/ncomms13525
347. Sawyer BT, Qamar L, Yamamoto TM, McMellen A, Watson ZL, Richer JK, et al. Targeting fatty acid oxidation to promote anoikis and inhibit ovarian cancer progression. Mol Cancer Res (2020) 18:1088–98. doi: 10.1158/1541-7786.MCR-19-1057
348. Zhu J, Wu G, Song L, Cao L, Tan Z, Tang M, et al. NKX2-8 deletion-induced reprogramming of fatty acid metabolism confers chemoresistance in epithelial ovarian cancer. EBioMedicine (2019) 43:238–52. doi: 10.1016/j.ebiom.2019.04.041
349. Tesfay L, Paul BT, Konstorum A, Deng Z, Cox AO, Lee J, et al. Stearoyl-CoA desaturase 1 protects ovarian cancer cells from ferroptotic cell death. Cancer Res (2019) 79:5355–66. doi: 10.1158/0008-5472.CAN-19-0369
350. Dasari S, Fang Y, Mitra AK. Cancer associated fibroblasts: naughty neighbors that drive ovarian cancer progression. Cancers (2018) 10:406. doi: 10.3390/cancers10110406
351. Hussain A, Voisin V, Poon S, Karamboulas C, Bui NHB, Meens J, et al. Distinct fibroblast functional states drive clinical outcomes in ovarian cancer and are regulated by TCF21. J Exp Med (2020) 217:e20191094. doi: 10.1084/jem.20191094
352. Cummings M, Freer C, Orsi NM. Targeting the tumour microenvironment in platinum-resistant ovarian cancer. Semin Cancer Biol (2021) 77:3–28. doi: 10.1016/j.semcancer.2021.02.007
353. Sandoval P, Jiménez-Heffernan JA, Rynne-Vidal Á, Pérez-Lozano ML, Gilsanz Á, Ruiz-Carpio V, et al. Carcinoma-associated fibroblasts derive from mesothelial cells via mesothelial-to-mesenchymal transition in peritoneal metastasis. J Pathol (2013) 231:517–31. doi: 10.1002/path.4281
354. Mikuła-Pietrasik J, Uruski P, Pakuła M, Maksin K, Szubert S, Woźniak A, et al. Oxidative stress contributes to hepatocyte growth factor-dependent pro-senescence activity of ovarian cancer cells. Free Radic Biol Med (2017) 110:270–9. doi: 10.1016/j.freeradbiomed.2017.06.015
355. Pakuła M, Witucka A, Uruski P, Radziemski A, Moszyński R, Szpurek D, et al. Senescence-related deterioration of intercellular junctions in the peritoneal mesothelium promotes the transmesothelial invasion of ovarian cancer cells. Sci Rep (2019) 9:7587. doi: 10.1038/s41598-019-44123-4
356. Rynne-Vidal A, Au-Yeung CL, Jiménez-Heffernan JA, Pérez-Lozano ML, Cremades-Jimeno L, Bárcena C, et al. Mesothelial-to-mesenchymal transition as a possible therapeutic target in peritoneal metastasis of ovarian cancer: mesothelial-to-mesenchymal transition and peritoneal metastatic niche. J Pathol (2017) 242:140–51. doi: 10.1002/path.4889
357. Nakamura K, Sawada K, Kinose Y, Yoshimura A, Toda A, Nakatsuka E, et al. Exosomes promote ovarian cancer cell invasion through transfer of CD44 to peritoneal mesothelial cells. Mol Cancer Res (2017) 15:78–92. doi: 10.1158/1541-7786.MCR-16-0191
358. Gao L, Nie X, Gou R, Hu Y, Dong H, Li X, et al. Exosomal ANXA2 derived from ovarian cancer cells regulates epithelial-mesenchymal plasticity of human peritoneal mesothelial cells. J Cell Mol Med (2021) 25:10916–29. doi: 10.1111/jcmm.16983
359. Fujikake K, Kajiyama H, Yoshihara M, Nishino K, Yoshikawa N, Utsumi F, et al. A novel mechanism of neovascularization in peritoneal dissemination via cancer-associated mesothelial cells affected by TGF-β derived from ovarian cancer. Oncol Rep (2017) 39:193–200. doi: 10.3892/or.2017.6104
360. Siu MKY, Jiang Y, Wang J, Leung THY, Ngu SF, Cheung ANY, et al. PDK1 promotes ovarian cancer metastasis by modulating tumor-mesothelial adhesion, invasion, and angiogenesis via α5β1 integrin and JNK/IL-8 signaling. Oncogenesis (2020) 9:24. doi: 10.1038/s41389-020-0209-0
361. Yasui H, Kajiyama H, Tamauchi S, Suzuki S, Peng Y, Yoshikawa N, et al. CCL2 secreted from cancer-associated mesothelial cells promotes peritoneal metastasis of ovarian cancer cells through the P38-MAPK pathway. Clin Exp Metastasis (2020) 37:145–58. doi: 10.1007/s10585-019-09993-y
362. Au-Yeung C-L, Yeung T-L, Achreja A, Zhao H, Yip K-P, Kwan S-Y, et al. ITLN1 modulates invasive potential and metabolic reprogramming of ovarian cancer cells in omental microenvironment. Nat Commun (2020) 11:3546. doi: 10.1038/s41467-020-17383-2
363. Qian J, LeSavage BL, Hubka KM, Ma C, Natarajan S, Eggold JT, et al. Cancer-associated mesothelial cells promote ovarian cancer chemoresistance through paracrine osteopontin signaling. J Clin Invest (2021) 131:e146186. doi: 10.1172/JCI146186
364. Carroll MJ, Fogg KC, Patel HA, Krause HB, Mancha A-S, Patankar MS, et al. Alternatively-activated macrophages upregulate mesothelial expression of p-selectin to enhance adhesion of ovarian cancer cells. Cancer Res (2018) 78:3560–73. doi: 10.1158/0008-5472.CAN-17-3341
365. Asem M, Young AM, Oyama C, Claure de la Zerda A, Liu Y, Yang J, et al. Host Wnt5a potentiates microenvironmental regulation of ovarian cancer metastasis. Cancer Res (2020) 80:1156–70. doi: 10.1158/0008-5472.CAN-19-1601
366. Jia D, Lu M, Jung KH, Park JH, Yu L, Onuchic JN, et al. Elucidating cancer metabolic plasticity by coupling gene regulation with metabolic pathways. Proc Natl Acad Sci (2019) 116:3909–18. doi: 10.1073/pnas.1816391116
367. Desbats MA, Giacomini I, Prayer-Galetti T, Montopoli M. Metabolic plasticity in chemotherapy resistance. Front Oncol (2020) 10:281. doi: 10.3389/fonc.2020.00281
368. Pastò A, Pagotto A, Pilotto G, De Paoli A, De Salvo GL, Baldoni A, et al. Resistance to glucose starvation as metabolic trait of platinum-resistant human epithelial ovarian cancer cells. Oncotarget (2017) 8:6433–45. doi: 10.18632/oncotarget.14118
369. Zhao G, Cardenas H, Matei D. Ovarian cancer–why lipids matter. Cancers (2019) 11:1870. doi: 10.3390/cancers11121870
370. Ladanyi A, Mukherjee A, Kenny HA, Johnson A, Mitra AK, Sundaresan S, et al. Adipocyte-induced CD36 expression drives ovarian cancer progression and metastasis. Oncogene (2018) 37:2285–301. doi: 10.1038/s41388-017-0093-z
371. Ahmed AA, Lu Z, Jennings NB, Etemadmoghadam D, Capalbo L, Jacamo RO, et al. SIK2 is a centrosome kinase required for bipolar mitotic spindle formation that provides a potential target for therapy in ovarian cancer. Cancer Cell (2010) 18:109–21. doi: 10.1016/j.ccr.2010.06.018
372. Bauerschlag DO, Maass N, Leonhardt P, Verburg FA, Pecks U, Zeppernick F, et al. Fatty acid synthase overexpression: target for therapy and reversal of chemoresistance in ovarian cancer. J Transl Med (2015) 13:146. doi: 10.1186/s12967-015-0511-3
373. Yi M, Li J, Chen S, Cai J, Ban Y, Peng Q, et al. Emerging role of lipid metabolism alterations in cancer stem cells. J Exp Clin Cancer Res (2018) 37:118. doi: 10.1186/s13046-018-0784-5
374. Borzdziłowska P, Bednarek I. Alpha mangostin and cisplatin as modulators of exosomal interaction of ovarian cancer cell with fibroblasts. Int J Mol Sci (2022) 23:8913. doi: 10.3390/ijms23168913
375. Cheng L, Wu S, Zhang K, Qing Y, Xu T. A comprehensive overview of exosomes in ovarian cancer: emerging biomarkers and therapeutic strategies. J Ovarian Res (2017) 10:73. doi: 10.1186/s13048-017-0368-6
376. Keller S, König A-K, Marmé F, Runz S, Wolterink S, Koensgen D, et al. Systemic presence and tumor-growth promoting effect of ovarian carcinoma released exosomes. Cancer Lett (2009) 278:73–81. doi: 10.1016/j.canlet.2008.12.028
377. Shenoy GN, Loyall J, Berenson CS, Kelleher RJ, Iyer V, Balu-Iyer SV, et al. Sialic acid–dependent inhibition of T cells by exosomal ganglioside GD3 in ovarian tumor microenvironments. J Immunol (2018) 201:3750–8. doi: 10.4049/jimmunol.1801041
378. Bretz NP, Ridinger J, Rupp A-K, Rimbach K, Keller S, Rupp C, et al. Body fluid exosomes promote secretion of inflammatory cytokines in monocytic cells via toll-like receptor signaling. J Biol Chem (2013) 288:36691–702. doi: 10.1074/jbc.M113.512806
379. Labani-Motlagh A, Israelsson P, Ottander U, Lundin E, Nagaev I, Nagaeva O, et al. Differential expression of ligands for NKG2D and DNAM-1 receptors by epithelial ovarian cancer-derived exosomes and its influence on NK cell cytotoxicity. Tumor Biol (2016) 37:5455–66. doi: 10.1007/s13277-015-4313-2
380. Yi H, Ye J, Yang X-M, Zhang L-W, Zhang Z-G, Chen Y-P. High-grade ovarian cancer secreting effective exosomes in tumor angiogenesis. Int J Clin Exp Pathol (2015) 8:5062–70.
381. Tang MKS, Yue PYK, Ip PP, Huang R-L, Lai H-C, Cheung ANY, et al. Soluble e-cadherin promotes tumor angiogenesis and localizes to exosome surface. Nat Commun (2018) 9:2270. doi: 10.1038/s41467-018-04695-7
382. Mo Y, Leung LL, Mak CSL, Wang X, Chan W-S, Hui LMN, et al. Tumor-secreted exosomal miR-141 activates tumor-stroma interactions and controls premetastatic niche formation in ovarian cancer metastasis. Mol Cancer (2023) 22:4. doi: 10.1186/s12943-022-01703-9
383. Kanlikilicer P, Bayraktar R, Denizli M, Rashed MH, Ivan C, Aslan B, et al. Exosomal miRNA confers chemo resistance via targeting Cav1/p-gp/M2-type macrophage axis in ovarian cancer. eBioMedicine (2018) 38:100–12. doi: 10.1016/j.ebiom.2018.11.004
384. Ying X, Wu Q, Wu X, Zhu Q, Wang X, Jiang L, et al. Epithelial ovarian cancer-secreted exosomal miR-222-3p induces polarization of tumor-associated macrophages. Oncotarget (2016) 7:43076–87. doi: 10.18632/oncotarget.9246
385. Yoshimura A, Sawada K, Nakamura K, Kinose Y, Nakatsuka E, Kobayashi M, et al. Exosomal miR-99a-5p is elevated in sera of ovarian cancer patients and promotes cancer cell invasion by increasing fibronectin and vitronectin expression in neighboring peritoneal mesothelial cells. BMC Cancer (2018) 18:1065. doi: 10.1186/s12885-018-4974-5
386. Kobayashi M, Salomon C, Tapia J, Illanes SE, Mitchell MD, Rice GE. Ovarian cancer cell invasiveness is associated with discordant exosomal sequestration of let-7 miRNA and miR-200. J Transl Med (2014) 12:4. doi: 10.1186/1479-5876-12-4
387. Zamaraev AV, Volik PI, Sukhikh GT, Kopeina GS, Zhivotovsky B. Long non-coding RNAs: a view to kill ovarian cancer. Biochim Biophys Acta BBA Rev Cancer (2021) 1876:188584. doi: 10.1016/j.bbcan.2021.188584
388. Taylor SE, Chan DK, Yang D, Bruno T, Lieberman R, Siddiqui J, et al. Shifting the soil: metformin treatment decreases the protumorigenic tumor microenvironment in epithelial ovarian cancer. Cancers (2022) 14:2298. doi: 10.3390/cancers14092298
389. Tan L, Tondo-Steele K, Foster C, McIlwain C, Bolland DE, Crawford HC, et al. Inhibition of tumor microenvironment cytokine signaling sensitizes ovarian cancer cells to antiestrogen therapy. Cancers (2022) 14:4675. doi: 10.3390/cancers14194675
390. Pulaski HL, Spahlinger G, Silva IA, McLean K, Kueck AS, Reynolds RK, et al. Identifying alemtuzumab as an anti-myeloid cell antiangiogenic therapy for the treatment of ovarian cancer. J Transl Med (2009) 7:49. doi: 10.1186/1479-5876-7-49
391. Martin LP, Sill M, Shahin MS, Powell M, DiSilvestro P, Landrum LM, et al. A phase II evaluation of AMG 102 (rilotumumab) in the treatment of persistent or recurrent epithelial ovarian, fallopian tube or primary peritoneal carcinoma: a gynecologic oncology group study. Gynecol Oncol (2014) 132:526–30. doi: 10.1016/j.ygyno.2013.12.018
392. Berek J, Taylor P, McGuire W, Smith LM, Schultes B, Nicodemus CF. Oregovomab maintenance monoimmunotherapy does not improve outcomes in advanced ovarian cancer. J Clin Oncol (2009) 27:418–25. doi: 10.1200/JCO.2008.17.8400
393. Battaglia A, Buzzonetti A, Fossati M, Scambia G, Fattorossi A, Madiyalakan MR, et al. Translational immune correlates of indirect antibody immunization in a randomized phase II study using scheduled combination therapy with carboplatin/paclitaxel plus oregovomab in ovarian cancer patients. Cancer Immunol Immunother (2020) 69:383–97. doi: 10.1007/s00262-019-02456-z
394. Brewer M, Angioli R, Scambia G, Lorusso D, Terranova C, Panici PB, et al. Front-line chemo-immunotherapy with carboplatin-paclitaxel using oregovomab indirect immunization in advanced ovarian cancer: a randomized phase II study. Gynecol Oncol (2020) 156:523–9. doi: 10.1016/j.ygyno.2019.12.024
395. Crawford A, Haber L, Kelly MP, Vazzana K, Canova L, Ram P, et al. A mucin 16 bispecific T cell–engaging antibody for the treatment of ovarian cancer. Sci Transl Med (2019) 11:eaau7534. doi: 10.1126/scitranslmed.aau7534
396. Santin AD, Vergote I, González-Martín A, Moore K, Oaknin A, Romero I, et al. Safety and activity of anti-mesothelin antibody–drug conjugate anetumab ravtansine in combination with pegylated-liposomal doxorubicin in platinum-resistant ovarian cancer: multicenter, phase ib dose escalation and expansion study. Int J Gynecol Cancer (2023) 33:562–70. doi: 10.1136/ijgc-2022-003927
397. Mullamitha SA, Ton NC, Parker GJM, Jackson A, Julyan PJ, Roberts C, et al. Phase I evaluation of a fully human anti–αv integrin monoclonal antibody (CNTO 95) in patients with advanced solid tumors. Clin Cancer Res (2007) 13:2128–35. doi: 10.1158/1078-0432.CCR-06-2779
398. Bell-McGuinn KM, Matthews CM, Ho SN, Barve M, Gilbert L, Penson RT, et al. Single-arm study of the anti-α5β1 integrin antibody volociximab as monotherapy in patients with platinum-resistant advanced epithelial ovarian or primary peritoneal cancer. Gynecol Oncol (2011) 121:273–9. doi: 10.1016/j.ygyno.2010.12.362
399. Barkal AA, Brewer RE, Markovic M, Kowarsky M, Barkal SA, Zaro BW, et al. CD24 signalling through macrophage siglec-10 is a target for cancer immunotherapy. Nature (2019) 572:392–6. doi: 10.1038/s41586-019-1456-0
400. Sikic BI, Lakhani N, Patnaik A, Shah SA, Chandana SR, Rasco D, et al. First-in-Human, first-in-Class phase I trial of the anti-CD47 antibody Hu5F9-G4 in patients with advanced cancers. J Clin Oncol (2019) 37:946–53. doi: 10.1200/JCO.18.02018
401. Gupta R, Cristea M, Frankel P, Ruel C, Chen C, Wang Y, et al. Randomized trial of oral cyclophosphamide versus oral cyclophosphamide with celecoxib for recurrent epithelial ovarian, fallopian tube, and primary peritoneal cancer. Cancer Treat Res Commun (2019) 21:100155. doi: 10.1016/j.ctarc.2019.100155
402. Legge F, Paglia A, D’Asta M, Fuoco G, Scambia G, Ferrandina G. Phase II study of the combination carboplatin plus celecoxib in heavily pre-treated recurrent ovarian cancer patients. BMC Cancer (2011) 11:214. doi: 10.1186/1471-2407-11-214
403. Reyners AKL, de Munck L, Erdkamp FLG, Smit WM, Hoekman K, Lalisang RI, et al. A randomized phase II study investigating the addition of the specific COX-2 inhibitor celecoxib to docetaxel plus carboplatin as first-line chemotherapy for stage IC to IV epithelial ovarian cancer, fallopian tube or primary peritoneal carcinomas: the DoCaCel study. Ann Oncol (2012) 23:2896–902. doi: 10.1093/annonc/mds107
404. Penn CA, Yang K, Zong H, Lim J-Y, Cole A, Yang D, et al. Therapeutic impact of nanoparticle therapy targeting tumor-associated macrophages. Mol Cancer Ther (2018) 17:96–106. doi: 10.1158/1535-7163.MCT-17-0688
405. Robinson-Smith TM, Isaacsohn I, Mercer CA, Zhou M, Van Rooijen N, Husseinzadeh N, et al. Macrophages mediate inflammation-enhanced metastasis of ovarian tumors in mice. Cancer Res (2007) 67:5708–16. doi: 10.1158/0008-5472.CAN-06-4375
406. Germano G, Frapolli R, Belgiovine C, Anselmo A, Pesce S, Liguori M, et al. Role of macrophage targeting in the antitumor activity of trabectedin. Cancer Cell (2013) 23:249–62. doi: 10.1016/j.ccr.2013.01.008
407. Casado A, Callata HR, Manzano A, Marquina G, Alonso T, Gajate P, et al. Trabectedin for reversing platinum resistance and resensitization to platinum in patients with recurrent ovarian cancer. Future Oncol (2019) 15:271–80. doi: 10.2217/fon-2018-0554
408. Takahashi S, Takekuma M, Tamura K, Takehara K, Nomura H, Ono M, et al. A phase I study of combined trabectedin and pegylated liposomal doxorubicin therapy for advanced relapsed ovarian cancer. Int J Clin Oncol (2021) 26:1977–85. doi: 10.1007/s10147-021-01973-1
409. Monk BJ, Herzog TJ, Wang G, Triantos S, Maul S, Knoblauch R, et al. A phase 3 randomized, open-label, multicenter trial for safety and efficacy of combined trabectedin and pegylated liposomal doxorubicin therapy for recurrent ovarian cancer. Gynecol Oncol (2020) 156:535–44. doi: 10.1016/j.ygyno.2019.12.043
410. Toulmonde M, Brahmi M, Giraud A, Chakiba C, Bessede A, Kind M, et al. Trabectedin plus durvalumab in patients with advanced pretreated soft tissue sarcoma and ovarian carcinoma (TRAMUNE): an open-label, multicenter phase ib study. Clin Cancer Res (2022) 28:1765–72. doi: 10.1158/1078-0432.CCR-21-2258
411. Colombo N, Zaccarelli E, Baldoni A, Frezzini S, Scambia G, Palluzzi E, et al. Multicenter, randomised, open-label, non-comparative phase 2 trial on the efficacy and safety of the combination of bevacizumab and trabectedin with or without carboplatin in women with partially platinum-sensitive recurrent ovarian cancer. Br J Cancer (2019) 121:744–50. doi: 10.1038/s41416-019-0584-5
412. Moughon DL, He H, Schokrpur S, Jiang ZK, Yaqoob M, David J, et al. Macrophage blockade using CSF1R inhibitors reverses the vascular leakage underlying malignant ascites in late-stage epithelial ovarian cancer. Cancer Res (2015) 75:4742–52. doi: 10.1158/0008-5472.CAN-14-3373
413. Johnson M, Dudek AZ, Sukari A, Call J, Kunk PR, Lewis K, et al. ARRY-382 in combination with pembrolizumab in patients with advanced solid tumors: results from a phase 1b/2 study. Clin Cancer Res (2022) 28:2517–26. doi: 10.1158/1078-0432.CCR-21-3009
414. Lyons YA, Pradeep S, Wu SY, Haemmerle M, Hansen JM, Wagner MJ, et al. Macrophage depletion through colony stimulating factor 1 receptor pathway blockade overcomes adaptive resistance to anti-VEGF therapy. Oncotarget (2017) 8:96496–505. doi: 10.18632/oncotarget.20410
415. Zhang F, Parayath NN, Ene CI, Stephan SB, Koehne AL, Coon ME, et al. Genetic programming of macrophages to perform anti-tumor functions using targeted mRNA nanocarriers. Nat Commun (2019) 10:3974. doi: 10.1038/s41467-019-11911-5
416. Tebbe C, Chhina J, Dar SA, Sarigiannis K, Giri S, Munkarah AR, et al. Metformin limits the adipocyte tumor-promoting effect on ovarian cancer. Oncotarget (2014) 5:4746–64. doi: 10.18632/oncotarget.2012
417. Yamamura S, Matsumura N, Mandai M, Huang Z, Oura T, Baba T, et al. The activated transforming growth factor-beta signaling pathway in peritoneal metastases is a potential therapeutic target in ovarian cancer. Int J Cancer (2012) 130:20–8. doi: 10.1002/ijc.25961
418. Gao Y, Shan N, Zhao C, Wang Y, Xu F, Li J, et al. LY2109761 enhances cisplatin antitumor activity in ovarian cancer cells. Int J Clin Exp Pathol (2015) 8:4923–32.
419. Matei D, Sill MW, Lankes HA, DeGeest K, Bristow RE, Mutch D, et al. Activity of sorafenib in recurrent ovarian cancer and primary peritoneal carcinomatosis: a gynecologic oncology group trial. J Clin Oncol (2011) 29:69–75. doi: 10.1200/JCO.2009.26.7856
420. Lee J-M, Annunziata CM, Hays JL, Cao L, Choyke P, Yu M, et al. Phase II trial of bevacizumab and sorafenib in recurrent ovarian cancer patients with or without prior-bevacizumab treatment. Gynecol Oncol (2020) 159:88–94. doi: 10.1016/j.ygyno.2020.07.031
421. Ramasubbaiah R, Perkins SM, Schilder J, Whalen C, Johnson CS, Callahan M, et al. Sorafenib in combination with weekly topotecan in recurrent ovarian cancer, a phase I/II study of the Hoosier oncology group. Gynecol Oncol (2011) 123:499–504. doi: 10.1016/j.ygyno.2011.08.033
422. Chekerov R, Hilpert F, Mahner S, El-Balat A, Harter P, De Gregorio N, et al. Sorafenib plus topotecan versus placebo plus topotecan for platinum-resistant ovarian cancer (TRIAS): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol (2018) 19:1247–58. doi: 10.1016/S1470-2045(18)30372-3
423. Welch SA, Hirte HW, Elit L, Schilder RJ, Wang L, MacAlpine K, et al. Sorafenib in combination with gemcitabine in recurrent epithelial ovarian cancer: a study of the princess Margaret hospital phase II consortium. Int J Gynecol Cancer (2010) 20:787–93. doi: 10.1111/IGC.0b013e3181e273a8
424. Hainsworth JD, Thompson DS, Bismayer JA, Gian VG, Merritt WM, Whorf RC, et al. Paclitaxel/carboplatin with or without sorafenib in the first-line treatment of patients with stage III/IV epithelial ovarian cancer: a randomized phase II study of the Sarah cannon research institute. Cancer Med (2015) 4:673–81. doi: 10.1002/cam4.376
425. Colombo N, Mangili G, Mammoliti S, Kalling M, Tholander B, Sternas L, et al. A phase II study of aflibercept in patients with advanced epithelial ovarian cancer and symptomatic malignant ascites. Gynecol Oncol (2012) 125:42–7. doi: 10.1016/j.ygyno.2011.11.021
426. Matulonis UA, Berlin S, Ivy P, Tyburski K, Krasner C, Zarwan C, et al. Cediranib, an oral inhibitor of vascular endothelial growth factor receptor kinases, is an active drug in recurrent epithelial ovarian, fallopian tube, and peritoneal cancer. J Clin Oncol (2009) 27:5601–6. doi: 10.1200/JCO.2009.23.2777
427. Hirte H, Lheureux S, Fleming GF, Sugimoto A, Morgan R, Biagi J, et al. A phase 2 study of cediranib in recurrent or persistent ovarian, peritoneal or fallopian tube cancer: a trial of the princess Margaret, Chicago and California phase II consortia. Gynecol Oncol (2015) 138:55–61. doi: 10.1016/j.ygyno.2015.04.009
428. Lheureux S, Oaknin A, Garg S, Bruce JP, Madariaga A, Dhani NC, et al. EVOLVE: a multicenter open-label single-arm clinical and translational phase II trial of cediranib plus olaparib for ovarian cancer after PARP inhibition progression. Clin Cancer Res (2020) 26:4206–15. doi: 10.1158/1078-0432.CCR-19-4121
429. Liu JF, Brady MF, Matulonis UA, Miller A, Kohn EC, Swisher EM, et al. Olaparib with or without cediranib versus platinum-based chemotherapy in recurrent platinum-sensitive ovarian cancer (NRG-GY004): a randomized, open-label, phase III trial. J Clin Oncol (2022) 40:2138–47. doi: 10.1200/JCO.21.02011
430. Lee J-M, Moore RG, Ghamande S, Park MS, Diaz JP, Chapman J, et al. Cediranib in combination with olaparib in patients without a germline BRCA1/2 mutation and with recurrent platinum-resistant ovarian cancer: phase IIb CONCERTO trial. Clin Cancer Res (2022) 28:4186–93. doi: 10.1158/1078-0432.CCR-21-1733
431. Ledermann JA, Hackshaw A, Kaye S, Jayson G, Gabra H, McNeish I, et al. Randomized phase II placebo-controlled trial of maintenance therapy using the oral triple angiokinase inhibitor BIBF 1120 after chemotherapy for relapsed ovarian cancer. J Clin Oncol (2011) 29:3798–804. doi: 10.1200/JCO.2010.33.5208
432. du Bois A, Kristensen G, Ray-Coquard I, Reuss A, Pignata S, Colombo N, et al. Standard first-line chemotherapy with or without nintedanib for advanced ovarian cancer (AGO-OVAR 12): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol (2016) 17:78–89. doi: 10.1016/S1470-2045(15)00366-6
433. Pignata S, Lorusso D, Scambia G, Sambataro D, Tamberi S, Cinieri S, et al. MITO-11: a randomized multicenter phase II trial testing the addition of pazopanib to weekly paclitaxel in platinum-resistant or -refractory advanced ovarian cancer (AOC). J Clin Oncol (2014) 32:5503–3. doi: 10.1200/jco.2014.32.15_suppl.5503
434. Joly F, Fabbro M, Berton D, Lequesne J, Anota A, Puszkiel A, et al. Paclitaxel with or without pazopanib for ovarian cancer relapsing during bevacizumab maintenance therapy: the GINECO randomized phase II TAPAZ study. Gynecol Oncol (2022) 166:389–96. doi: 10.1016/j.ygyno.2022.06.022
435. Dinkic C, Eichbaum M, Schmidt M, Grischke EM, Gebauer G, Fricke HC, et al. Pazopanib (GW786034) and cyclophosphamide in patients with platinum-resistant, recurrent, pre-treated ovarian cancer - results of the PACOVAR-trial. Gynecol Oncol (2017) 146:279–84. doi: 10.1016/j.ygyno.2017.05.013
436. Zhang Y, Fang H, Wang X, Wang H, Pan G, Chen J. The efficacy and safety of pazopanib plus chemotherapy in treating recurrent or persistent ovarian cancer:: a systematic review and meta-analysis. Am J Clin Oncol (2023) 46:254–62. doi: 10.1097/COC.0000000000000999
437. Yeung T-L, Sheng J, Leung CS, Li F, Kim J, Ho SY, et al. Systematic identification of druggable epithelial–stromal crosstalk signaling networks in ovarian cancer. JNCI J Natl Cancer Inst (2019) 111:272–82. doi: 10.1093/jnci/djy097
438. Zhao Y, Cao J, Melamed A, Worley M, Gockley A, Jones D, et al. Losartan treatment enhances chemotherapy efficacy and reduces ascites in ovarian cancer models by normalizing the tumor stroma. Proc Natl Acad Sci (2019) 116:2210–9. doi: 10.1073/pnas.1818357116
439. Moore KN, Bookman M, Sehouli J, Miller A, Anderson C, Scambia G, et al. Atezolizumab, bevacizumab, and chemotherapy for newly diagnosed stage III or IV ovarian cancer: placebo-controlled randomized phase III trial (IMagyn050/GOG 3015/ENGOT-OV39). J Clin Oncol (2021) 39:1842–55. doi: 10.1200/JCO.21.00306
440. Moroney JW, Powderly J, Lieu CH, Bendell JC, Eckhardt SG, Chang C-W, et al. Safety and clinical activity of atezolizumab plus bevacizumab in patients with ovarian cancer: a phase ib study. Clin Cancer Res (2020) 26:5631–7. doi: 10.1158/1078-0432.CCR-20-0477
441. Monk BJ, Colombo N, Oza AM, Fujiwara K, Birrer MJ, Randall L, et al. Chemotherapy with or without avelumab followed by avelumab maintenance versus chemotherapy alone in patients with previously untreated epithelial ovarian cancer (JAVELIN ovarian 100): an open-label, randomised, phase 3 trial. Lancet Oncol (2021) 22:1275–89. doi: 10.1016/S1470-2045(21)00342-9
442. Pujade-Lauraine E, Fujiwara K, Ledermann JA, Oza AM, Kristeleit R, Ray-Coquard I-L, et al. Avelumab alone or in combination with chemotherapy versus chemotherapy alone in platinum-resistant or platinum-refractory ovarian cancer (JAVELIN ovarian 200): an open-label, three-arm, randomised, phase 3 study. Lancet Oncol (2021) 22:1034–46. doi: 10.1016/S1470-2045(21)00216-3
443. Schram AM, Colombo N, Arrowsmith E, Narayan V, Yonemori K, Scambia G, et al. Avelumab plus talazoparib in patients with BRCA1/2 - or ATM -altered advanced solid tumors: results from JAVELIN BRCA/ATM, an open-label, multicenter, phase 2b, tumor-agnostic trial. JAMA Oncol (2023) 9:29. doi: 10.1001/jamaoncol.2022.5218
444. Zamarin D, Walderich S, Holland A, Zhou Q, Iasonos AE, Torrisi JM, et al. Safety, immunogenicity, and clinical efficacy of durvalumab in combination with folate receptor alpha vaccine TPIV200 in patients with advanced ovarian cancer: a phase II trial. J Immunother Cancer (2020) 8:e000829. doi: 10.1136/jitc-2020-000829
445. Lampert EJ, Zimmer A, Padget M, Cimino-Mathews A, Nair JR, Liu Y, et al. Combination of PARP inhibitor olaparib, and PD-L1 inhibitor durvalumab, in recurrent ovarian cancer: a proof-of-Concept phase II study. Clin Cancer Res (2020) 26:4268–79. doi: 10.1158/1078-0432.CCR-20-0056
446. Lee J-Y, Kim B-G, Kim J-W, Lee JB, Park E, Joung J-G, et al. On behalf of Korean gynecologic oncology group (KGOG) investigators. biomarker-guided targeted therapy in platinum-resistant ovarian cancer (AMBITION; KGOG 3045): a multicentre, open-label, five-arm, uncontrolled, umbrella trial. J Gynecol Oncol (2022) 33:e45. doi: 10.3802/jgo.2022.33.e45
447. Zimmer AS, Nichols E, Cimino-Mathews A, Peer C, Cao L, Lee M-J, et al. A phase I study of the PD-L1 inhibitor, durvalumab, in combination with a PARP inhibitor, olaparib, and a VEGFR1–3 inhibitor, cediranib, in recurrent women’s cancers with biomarker analyses. J Immunother Cancer (2019) 7:197. doi: 10.1186/s40425-019-0680-3
448. Zamarin D, Burger RA, Sill MW, Powell DJ, Lankes HA, Feldman MD, et al. Randomized phase II trial of nivolumab versus nivolumab and ipilimumab for recurrent or persistent ovarian cancer: an NRG oncology study. J Clin Oncol (2020) 38:1814–23. doi: 10.1200/JCO.19.02059
449. Liu JF, Herold C, Gray KP, Penson RT, Horowitz N, Konstantinopoulos PA, et al. Assessment of combined nivolumab and bevacizumab in relapsed ovarian cancer: a phase 2 clinical trial. JAMA Oncol (2019) 5:1731. doi: 10.1001/jamaoncol.2019.3343
450. Hamanishi J, Takeshima N, Katsumata N, Ushijima K, Kimura T, Takeuchi S, et al. Nivolumab versus gemcitabine or pegylated liposomal doxorubicin for patients with platinum-resistant ovarian cancer: open-label, randomized trial in Japan (NINJA). J Clin Oncol (2021) 39:3671–81. doi: 10.1200/JCO.21.00334
451. Manning-Geist BL, Gnjatic S, Aghajanian C, Konner J, Kim SH, Sarasohn D, et al. Phase I study of a multivalent WT1 peptide vaccine (Galinpepimut-s) in combination with nivolumab in patients with WT1-expressing ovarian cancer in second or third remission. Cancers (2023) 15:1458. doi: 10.3390/cancers15051458
452. Zsiros E, Lynam S, Attwood KM, Wang C, Chilakapati S, Gomez EC, et al. Efficacy and safety of pembrolizumab in combination with bevacizumab and oral metronomic cyclophosphamide in the treatment of recurrent ovarian cancer: a phase 2 nonrandomized clinical trial. JAMA Oncol (2021) 7:78. doi: 10.1001/jamaoncol.2020.5945
453. Matulonis UA, Shapira-Frommer R, Santin AD, Lisyanskaya AS, Pignata S, Vergote I, et al. Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: results from the phase II KEYNOTE-100 study. Ann Oncol (2019) 30:1080–7. doi: 10.1093/annonc/mdz135
454. Walsh CS, Kamrava M, Rogatko A, Kim S, Li A, Cass I, et al. Phase II trial of cisplatin, gemcitabine and pembrolizumab for platinum-resistant ovarian cancer. PloS One (2021) 16:e0252665. doi: 10.1371/journal.pone.0252665
455. Konstantinopoulos PA, Waggoner S, Vidal GA, Mita M, Moroney JW, Holloway R, et al. Single-arm phases 1 and 2 trial of niraparib in combination with pembrolizumab in patients with recurrent platinum-resistant ovarian carcinoma. JAMA Oncol (2019) 5:1141. doi: 10.1001/jamaoncol.2019.1048
456. Liao JB, Gwin WR, Urban RR, Hitchcock-Bernhardt KM, Coveler AL, Higgins DM, et al. Pembrolizumab with low-dose carboplatin for recurrent platinum-resistant ovarian, fallopian tube, and primary peritoneal cancer: survival and immune correlates. J Immunother Cancer (2021) 9:e003122. doi: 10.1136/jitc-2021-003122
457. Lee EK, Xiong N, Cheng S-C, Barry WT, Penson RT, Konstantinopoulos PA, et al. Combined pembrolizumab and pegylated liposomal doxorubicin in platinum resistant ovarian cancer: a phase 2 clinical trial. Gynecol Oncol (2020) 159:72–8. doi: 10.1016/j.ygyno.2020.07.028
458. Wang J, Wang C, Li Y, Li M, Zhu T, Shen Z, et al. Potential of peptide-engineered exosomes with overexpressed miR-92b-3p in anti-angiogenic therapy of ovarian cancer. Clin Transl Med (2021) 11:e425. doi: 10.1002/ctm2.425
459. Kobayashi M, Sawada K, Miyamoto M, Shimizu A, Yamamoto M, Kinose Y, et al. Exploring the potential of engineered exosomes as delivery systems for tumor-suppressor microRNA replacement therapy in ovarian cancer. Biochem Biophys Res Commun (2020) 527:153–61. doi: 10.1016/j.bbrc.2020.04.076
460. Liu H, Shen M, Zhao D, Ru D, Duan Y, Ding C, et al. The effect of triptolide-loaded exosomes on the proliferation and apoptosis of human ovarian cancer SKOV3 cells. BioMed Res Int (2019) 2019:1–14. doi: 10.1155/2019/2595801
461. Li L, He D, Guo Q, Zhang Z, Ru D, Wang L, et al. Exosome-liposome hybrid nanoparticle codelivery of TP and miR497 conspicuously overcomes chemoresistant ovarian cancer. J Nanobiotechnol (2022) 20:50. doi: 10.1186/s12951-022-01264-5
462. Zheng Y, Li Z, Yang S, Wang Y, Luan Z. CircEXOC6B suppresses the proliferation and motility and sensitizes ovarian cancer cells to paclitaxel through miR-376c-3p/FOXO3 axis. Cancer Biother Radiopharm (2022) 37:802–14. doi: 10.1089/cbr.2020.3739
463. Peng D, Wu T, Wang J, Huang J, Zheng L, Wang P, et al. microRNA-671-5p reduces tumorigenicity of ovarian cancer via suppressing HDAC5 and HIF-1α expression. Chem Biol Interact (2022) 355:109780. doi: 10.1016/j.cbi.2021.109780
464. Yuan D, Guo T, Qian H, Ge H, Zhao Y, Huang A, et al. Icariside II suppresses the tumorigenesis and development of ovarian cancer by regulating miR-144-3p/IGF2R axis. Drug Dev Res (2022) 83:1383–93. doi: 10.1002/ddr.21967
465. Rao Y, Zhang W, Li D, Li X, Ma Y, Qu P. Circ TYMP1 inhibits carcinogenesis and cisplatin resistance in ovarian cancer by reducing Smad2/3 phosphorylation via a MicroRNA-182A-3p/TGF1B axis. Contrast Media Mol Imaging (2022) 2022:1–9. doi: 10.1155/2022/1032557
466. Yang X, Wang J, Li H, Sun Y, Tong X. Downregulation of hsa_circ_0026123 suppresses ovarian cancer cell metastasis and proliferation through the miR−124−3p/EZH2 signaling pathway. Int J Mol Med (2020) 47:668–76. doi: 10.3892/ijmm.2020.4804
467. Korzun T, Moses AS, Kim J, Patel S, Schumann C, Levasseur PR, et al. Nanoparticle-based follistatin messenger RNA therapy for reprogramming metastatic ovarian cancer and ameliorating cancer-associated cachexia. Small (2022) 18:2204436. doi: 10.1002/smll.202204436
468. Kim GH, Won JE, Byeon Y, Kim MG, Wi TI, Lee JM, et al. Selective delivery of PLXDC1 small interfering RNA to endothelial cells for anti-angiogenesis tumor therapy using CD44-targeted chitosan nanoparticles for epithelial ovarian cancer. Drug Delivery (2018) 25:1394–402. doi: 10.1080/10717544.2018.1480672
469. Vescarelli E, Gerini G, Megiorni F, Anastasiadou E, Pontecorvi P, Solito L, et al. MiR-200c sensitizes olaparib-resistant ovarian cancer cells by targeting neuropilin 1. J Exp Clin Cancer Res (2020) 39:3. doi: 10.1186/s13046-019-1490-7
470. Wilczyński JR, Wilczyński M, Paradowska E. Cancer stem cells in ovarian cancer–a source of tumor success and a challenging target for novel therapies. Int J Mol Sci (2022) 23:2496. doi: 10.3390/ijms23052496
471. Köbel M, Kang EY. The evolution of ovarian carcinoma subclassification. Cancers (2022) 14:416. doi: 10.3390/cancers14020416
472. Lupia M, Melocchi V, Bizzaro F, Lo Riso P, Dama E, Baronio M, et al. Integrated molecular profiling of patient-derived ovarian cancer models identifies clinically relevant signatures and tumor vulnerabilities. Int J Cancer (2022) 151:240–54. doi: 10.1002/ijc.33983
473. Liu X, Wu A, Wang X, Liu Y, Xu Y, Liu G, et al. Identification of metabolism-associated molecular subtype in ovarian cancer. J Cell Mol Med (2021) 25:9617–26. doi: 10.1111/jcmm.16907
474. Fang F, Cardenas H, Huang H, Jiang G, Perkins SM, Zhang C, et al. Genomic and epigenomic signatures in ovarian cancer associated with resensitization to platinum drugs. Cancer Res (2018) 78:631–44. doi: 10.1158/0008-5472.CAN-17-1492
475. Givel A-M, Kieffer Y, Scholer-Dahirel A, Sirven P, Cardon M, Pelon F, et al. miR200-regulated CXCL12β promotes fibroblast heterogeneity and immunosuppression in ovarian cancers. Nat Commun (2018) 9:1056. doi: 10.1038/s41467-018-03348-z
476. Li Y, Tian R, Liu J, Li J, Tan H, Wu Q, et al. Deciphering the immune landscape dominated by cancer-associated fibroblasts to investigate their potential in indicating prognosis and guiding therapeutic regimens in high grade serous ovarian carcinoma. Front Immunol (2022) 13:940801. doi: 10.3389/fimmu.2022.940801
477. The Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature (2011) 474:609–15. doi: 10.1038/nature10166
478. Irani S. Emerging insights into the biology of metastasis: a review article. Iran J Basic Med Sci (2019) 22:833–47. doi: 10.22038/ijbms.2019.32786.7839
479. Konecny GE, Wang C, Hamidi H, Winterhoff B, Kalli KR, Dering J, et al. Prognostic and therapeutic relevance of molecular subtypes in high-grade serous ovarian cancer. JNCI J Natl Cancer Inst (2014) 106:dju249. doi: 10.1093/jnci/dju249
480. Liu B, Ji X, Li J, Zhu N, Long J, Zhuang X, et al. Integrative analysis identifies three molecular subsets in ovarian cancer. Clin Transl Med (2022) 12:e1029. doi: 10.1002/ctm2.1029
481. Ledermann JA, Embleton AC, Raja F, Perren TJ, Jayson GC, Rustin GJS, et al. Cediranib in patients with relapsed platinum-sensitive ovarian cancer (ICON6): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet (2016) 387:1066–74. doi: 10.1016/S0140-6736(15)01167-8
482. Li D, Marchenko ND, Moll UM. SAHA shows preferential cytotoxicity in mutant p53 cancer cells by destabilizing mutant p53 through inhibition of the HDAC6-Hsp90 chaperone axis. Cell Death Differ (2011) 18:1904–13. doi: 10.1038/cdd.2011.71
483. Hao Q, Li J, Zhang Q, Xu F, Xie B, Lu H, et al. Single-cell transcriptomes reveal heterogeneity of high-grade serous ovarian carcinoma. Clin Transl Med (2021) 11:e500. doi: 10.1002/ctm2.500
484. Zhang X, Yang Q. An immune-related lncRNA pairing model for predicting the prognosis and immune-infiltrating cell condition in human ovarian cancer. BioMed Res Int (2022) 2022:1–17. doi: 10.1155/2022/3168408
485. Gong G, Lin T, Yuan Y. Integrated analysis of gene expression and DNA methylation profiles in ovarian cancer. J Ovarian Res (2020) 13:30. doi: 10.1186/s13048-020-00632-9
486. Lu X, Ji C, Jiang L, Zhu Y, Zhou Y, Meng J, et al. Tumour microenvironment-based molecular profiling reveals ideal candidates for high-grade serous ovarian cancer immunotherapy. Cell Prolif (2021) 54:e12979. doi: 10.1111/cpr.12979
487. Patch A-M, Christie EL, Etemadmoghadam D, Garsed DW, George J, Fereday S, et al. Whole–genome characterization of chemoresistant ovarian cancer. Nature (2015) 521:489–94. doi: 10.1038/nature14410
488. Lin KK, Harrell MI, Oza AM, Oaknin A, Ray-Coquard I, Tinker AV, et al. BRCA reversion mutations in circulating tumor DNA predict primary and acquired resistance to the PARP inhibitor rucaparib in high-grade ovarian carcinoma. Cancer Discovery (2019) 9:210–9. doi: 10.1158/2159-8290.CD-18-0715
489. Kondrashova O, Nguyen M, Shield-Artin K, Tinker AV, Teng NNH, Harrell MI, et al. Secondary somatic mutations restoring RAD51C and RAD51D associated with acquired resistance to the PARP inhibitor rucaparib in high-grade ovarian carcinoma. Cancer Discovery (2017) 7:984–98. doi: 10.1158/2159-8290.CD-17-0419
490. Li Y, Zhang X, Gao Y, Shang C, Yu B, Wang T, et al. Development of a genomic signatures-based predictor of initial platinum-resistance in advanced high-grade serous ovarian cancer patients. Front Oncol (2021) 10:625866. doi: 10.3389/fonc.2020.625866
491. Qi X, Yu C, Wang Y, Lin Y, Shen B. Network vulnerability-based and knowledge-guided identification of microRNA biomarkers indicating platinum resistance in high-grade serous ovarian cancer. Clin Transl Med (2019) 8:28. doi: 10.1186/s40169-019-0245-6
492. Martincuks A, Song J, Kohut A, Zhang C, Li Y-J, Zhao Q, et al. PARP inhibition activates STAT3 in both tumor and immune cells underlying therapy resistance and immunosuppression in ovarian cancer. Front Oncol (2021) 11:724104. doi: 10.3389/fonc.2021.724104
493. Lee AH, Mejia Peña C, Dawson MR. Comparing the secretomes of chemorefractory and chemoresistant ovarian cancer cell populations. Cancers (2022) 14:1418. doi: 10.3390/cancers14061418
494. Zhu X, Shen H, Yin X, Yang M, Wei H, Chen Q, et al. Macrophages derived exosomes deliver miR-223 to epithelial ovarian cancer cells to elicit a chemoresistant phenotype. J Exp Clin Cancer Res (2019) 38:81. doi: 10.1186/s13046-019-1095-1
495. Zhang K, Erkan EP, Jamalzadeh S, Dai J, Andersson N, Kaipio K, et al. Longitudinal single-cell RNA-seq analysis reveals stress-promoted chemoresistance in metastatic ovarian cancer. Sci Adv (2022) 8:eabm1831. doi: 10.1126/sciadv.abm1831
496. Kan T, Zhang S, Zhou S, Zhang Y, Zhao Y, Gao Y, et al. Single-cell RNA-seq recognized the initiator of epithelial ovarian cancer recurrence. Oncogene (2022) 41:895–906. doi: 10.1038/s41388-021-02139-z
497. Banerjee S, Tian T, Wei Z, Shih N, Feldman MD, Alwine JC, et al. The ovarian cancer oncobiome. Oncotarget (2017) 8:36225–45. doi: 10.18632/oncotarget.16717
498. Kania KD, Haręża D, Wilczyński JR, Wilczyński M, Jarych D, Malinowski A, et al. The toll-like receptor 4 polymorphism Asp299Gly is associated with an increased risk of ovarian cancer. Cells (2022) 11:3137. doi: 10.3390/cells11193137
499. Haręża DA, Wilczyński JR, Paradowska E. Human papillomaviruses as infectious agents in gynecological cancers. oncogenic properties of viral proteins. Int J Mol Sci (2022) 23:1818. doi: 10.3390/ijms23031818
500. Morand S, Devanaboyina M, Staats H, Stanbery L, Nemunaitis J. Ovarian cancer immunotherapy and personalized medicine. Int J Mol Sci (2021) 22:6532. doi: 10.3390/ijms22126532
501. Bonifácio VDB. Ovarian cancer biomarkers: moving forward in early detection. In: Serpa J, editor. Tumor microenvironment. advances in experimental medicine and biology. Cham: Springer International Publishing (2020). p. 355–63. doi: 10.1007/978-3-030-34025-4_18
502. Zhu JW, Charkhchi P, Akbari MR. Potential clinical utility of liquid biopsies in ovarian cancer. Mol Cancer (2022) 21:114. doi: 10.1186/s12943-022-01588-8
Keywords: high-grade serous ovarian cancer, ovarian cancer stem cells, metastatic niche, tumor microenvironment, chemo-resistance, experimental therapy, clinical trial
Citation: Wilczyński JR, Wilczyński M and Paradowska E (2023) “DEPHENCE” system—a novel regimen of therapy that is urgently needed in the high-grade serous ovarian cancer—a focus on anti-cancer stem cell and anti-tumor microenvironment targeted therapies. Front. Oncol. 13:1201497. doi: 10.3389/fonc.2023.1201497
Received: 06 April 2023; Accepted: 07 June 2023;
Published: 28 June 2023.
Edited by:
Livia Elena Sima, Institute of Biochemistry of the Romanian Academy, RomaniaReviewed by:
Salvatore Condello, Indiana University Bloomington, United StatesXiaoyan Zhang, Fudan University, China
Copyright © 2023 Wilczyński, Wilczyński and Paradowska. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jacek R. Wilczyński, anJ3aWxAcG9zdC5wbA==
 Jacek R. Wilczyński
Jacek R. Wilczyński Miłosz Wilczyński2,3
Miłosz Wilczyński2,3