
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 25 September 2023
Sec. Cancer Molecular Targets and Therapeutics
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1197782
This article is part of the Research Topic Therapeutic Advances in Immune Checkpoint Inhibitors for Cancer Treatment View all 9 articles
 Ping Li1†
Ping Li1† Ming Hu1†
Ming Hu1† Mei Liu1†
Mei Liu1† Xiangyu Ren1
Xiangyu Ren1 Donghong Liu2
Donghong Liu2 Jiluo Liu1
Jiluo Liu1 Jianhua Yin1
Jianhua Yin1 Xiaojie Tan1
Xiaojie Tan1 Guangwen Cao1*
Guangwen Cao1*Background and aims: Systemic combinations have recently brought significant therapeutic benefits for advanced hepatocellular carcinoma (aHCC). To design the most effective combination regimens, a systematic review (PROSPERO ID: CRD42022321949) was conducted to evaluate the efficacy and safety of systemic combinations on aHCC.
Methods: We retrieved all the studies from PubMed, Embase, the Cochrane Central Register of Controlled Trials (CENTRAL), and China National Knowledge Infrastructure (CNKI) using the Medical Subject Headings (MeSH) terms until December 21, 2022. The effect indicators (hazard ratio [HR], relative risk [RR], and median) were pooled by a fixed- or random-effects model. A subgroup analysis was conducted according to types and specific therapies.
Results: In total, 88 eligible studies were selected from 7249 potential records. Each kind of combination treatment (chemotherapy plus chemotherapy, targeted plus immune checkpoint inhibitor (ICI) therapy, targeted plus chemotherapy, and targeted plus targeted therapy) had a better objective response rate (ORR) in patients with aHCC, compared to the monotherapy mostly with sorafenib (RR: 1.57 [1.44–1.71]; I2 = 30%). Of those, targeted plus ICI therapy showed better therapeutic efficiency in overall survival (median: 15.02 [12.67–17.38]), progression-free survival (median: 7.08 [6.42–7.74]), and ORR (RR: 1.81 [1.55–2.13]), compared to the monotherapy. Specifically, Atezo plus Beva showed all those benefits. Our pooled result showed all the combinations had increased ≥3 Grade treatment-related adverse events (TrAEs), with an RR of 1.25 [95% CI: 1.15–1.36], compared to the monotherapy.
Conclusion: The systemic combinations, especially targeted plus ICI therapy, including Atezo plus Beva, significantly improve clinical outcomes but increase side effects in patients with aHCC. Future trials should concentrate on improvement in therapeutic efficiency and reduction of toxicity of targeted plus ICI therapy.
Systematic review registration: https://www.crd.york.ac.uk/prospero, identifier CRD42022321949.
• All systemic combinations (chemotherapy plus chemotherapy, targeted therapy plus ICI therapy, targeted therapy plus chemotherapy, and targeted plus targeted therapies) significantly improve the objective response rate in patients with aHCC.
• The targeted therapy plus ICI therapy showed better therapeutic efficiency in overall survival, progression-free survival, and objective response rate, compared to the monotherapy.
• In particular, Atezo plus Beva in targeted therapy plus ICI therapy shows superiority in multiple clinical outcomes over other therapies.
• Increased treatment-related toxicity is evident in combination therapies except for targeted plus chemotherapy.
Hepatocellular carcinoma (HCC) is one of the most common cancers worldwide, with high incidence and comparable mortality (1). HCC at an early stage can be cured by topical treatments, like resection, liver transplantation, interventional embolization, radiofrequency ablation, and microwave ablation. For moderate-stage HCC, transcatheter arterial chemoembolization (TACE) shows therapeutic efficiency. More recently, some novel embolic materials and technologies have been employed, especially the superstable homogeneous iodinated formulation technology (SHIFT), showing long-term stability and favorable pharmaceutical value (2–5). However, over 50% of patients with HCC are diagnosed at advanced stages and therefore not suitable for surgical or locoregional therapies (6). Patients with advanced HCC, which is regarded as incurable, have limited treatment options and poor prognosis, until the advent of tyrosine kinase inhibitor (TKI) sorafenib for systemic therapy (7). According to the National Comprehensive Cancer Network (NCCN) and American Society of Clinical Oncology (ASCO) guidelines, systemic treatments were recommended for patients with advanced HCC (aHCC) (8, 9). Over the past decades, sorafenib was the leading systemic agent for those patients, followed by lenvatinib as well as other monotherapies (7, 10, 11). Recently, systemic combinations, like immunotherapy and targeted therapy, have brought significant benefits for those patients, which bring great changes to the treatment of advanced HCC (12). For instance, a randomized, phase III trial (EACH) in Asian patients with aHCC, showed progression-free survival (PFS; hazard ratio [HR]: 0.62, 95% confidence interval [CI]: 0.49–0.79), and response rate (8.15% vs. 2.67%, p = 0.02) benefits for FOLFOX4 (infusional fluorouracil, leucovorin, and oxaliplatin) over doxorubicin (13). Another two phase II clinical trials showed sorafenib–oxaliplatin–gemcitabine/capecitabine increased overall survival (OS), objective response rate (ORR), and PFS (14, 15). In a global, phase III trial (IMbrave150), atezolizumab (Atezo) combined with bevacizumab (Beva) resulted in better OS (HR: 0.58 [95% CI: 0.42–0.79]) and PFS (median: 6.8 [95% CI: 5.7–8.3] vs. 4.3 [95% CI: 4.0–5.6] months) than did sorafenib in patients with unresectable HCC (16). More importantly, Atezo plus Beva was listed as a preferred regimen, while sorafenib was listed as another recommended regimen in NCCN guidelines (8). Although systemic combination treatments had great potential to improve the prognosis of aHCC, some phase III trials failed in evaluating systemic combinations for those patients (17, 18). Furthermore, the most critical concern is whether the combination strategy would be a trend in anticancer therapy development and what kind of combinations would be the most optimal one. To facilitate the design of future combination regimens, we performed the systematic review by making an expanded comparison between any two combinations of chemotherapy, targeted therapy, immune checkpoint inhibitor (ICI) therapy, and single certain interventions.
We registered the protocol for this systematic review and meta-analysis on PROSPERO as recommended (https://www.crd.york.ac.uk/PROSPERO, ID: CRD42022321949).
A systematic literature search was performed using PubMed, Embase, the Cochrane Central Register of Controlled Trials (CENTRAL), and China National Knowledge Infrastructure (CNKI) using the Medical Subject Headings (MeSH) terms. The search covered the period from the inception date of each database until December 21, 2022. There was no restriction on publication status or language, and all the included non-English studies were translated into English. The keywords were as follows: (atezolizumab or bevacizumab or sorafenib or oxaliplatin or lenvatinib or pembrolizumab or nivolumab or camrelizumab or apatinib or tyrosine kinase inhibitor* or TKI or PD-1 or PD-L1 or immune checkpoint inhibitor* or ICI) and (hepatocellular cancer or liver cancer or hepatocellular carcinoma or HCC or liver neoplasms*) and (advanced or unresectable or inoperable).
The inclusion criteria were as follows: 1) studies that evaluated the effects of systemic combination therapies on aHCC, with or without controls; 2) studies that included research subjects with advanced/unresectable HCC; 3) studies that incorporated at least one of available endpoints (overall survival, progression-free survival, or objective response rate). The exclusion criteria were as follows: 1) studies that enrolled patients with cancers that metastasized to the liver, 2) studies with the combination of systemic and topical treatments, and 3) studies that lacked necessary information for data extraction.
Patients with aHCC were eligible, defined as advanced metastatic or unresectable hepatocellular carcinoma (i.e., patients with characteristics such as multifocal and/or infiltrative disease within the liver, vascular invasion, or extrahepatic spread), with the diagnosis confirmed by histologic or cytologic analysis or clinical features. Systemic combination therapies are defined as those systemic combination regimens for aHCC, recommended in the NCCN and ASCO guidelines (8, 9), or the actual combinations used clinically, including “atezolizumab+bevacizumab”, “bevacizumab+erlotinib”, “nivolumab+ipilimumab”, “capecitabine+oxaliplatin”, and “sorafenib+GEMOX”. Comparators were the systemic monotherapies including sorafenib, gemcitabine, or oxaliplatin. There was no restriction on the types of control treatment. The primary endpoints were OS at 6 months or longer, PFS at 6 months or longer, and ORR. OS was defined as the interval between the date of random assignment and the date of death from any cause; PFS was defined as the interval between random assignment and progression or death from any cause; ORR was defined as the percentage of patients who had a confirmed complete or partial response. Those endpoints were assessed according to Response Evaluation Criteria in Solid Tumors (RECIST) 1.1. The second endpoints were treatment-related adverse events (TrAEs) and ≥3 Grade TrAEs, according to the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) (version 3.0).
Our systematic searching was conducted according to the search terms we set before and followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (PRISMA 2020, http://www.prisma-statement.org). The headings and abstracts identified in those databases were reviewed and cross-checked by four investigators (LM, HM, RX, and LD) for the identification of studies that fulfill the eligible criteria. Moreover, reference lists of eligible studies, conference abstracts, and systematic reviews were reviewed to acquire relevant papers as well. For studies using the same data source, the most recent study or the study with the largest sample size was included. Some complicated papers were judged by panel discussion and further arbitrated by the third experienced reviewer (LP).
Prespecified data were independently extracted and double-checked by reviewers (LM, HM, RX, and LD). A standardized data extraction form was designed to manage the necessary items. The following information was extracted from all included publications (papers, abstracts, and reported data of registered trials): title, author, study design, country, sample size, experimental arms, control arms, other demographic characteristics, description of outcomes and adverse events, and their definitions. In particular, a study involving multiple systemic combination therapies was divided into a series of comparisons, each of which contained a group of combination and control regimens.
The characteristics of the included studies were synthesized and presented in a tabular form. Categorical variables were presented as counts (%), and continuous variables were described using means or medians. The HR, relative risk (RR), and median with corresponding 95% CI were pooled by the Mantel–Haenszel fixed-effects model if no evidence of significant heterogeneity existed; otherwise, the DerSimonian–Laird random-effects model was applied. The heterogeneity was assessed by I2 statistics, classified as low (I2 < 25), moderate (25 ≤ I2 < 75), and high heterogeneity (I2 ≥ 75). Generally, I2 statistic >50% was considered significant heterogeneity. The subgroup analysis was conducted by types and specific therapies of systemic combination therapy to detect the effects and heterogeneity of the treatments on aHCC across different combinations. The sensitivity analysis was performed by study design. The publication bias of the included studies was evaluated qualitatively by funnel plot and quantitatively by Egger’s test. These statistical analyses and plots were performed using “meta” and “metamedian” packages from R software, version 3.6.2 (R Foundation for Statistical Computing, Canberra, Austria).
The Cochrane Collaboration Tools were applied to assess the risk of bias in the included studies. In the tools, the Newcastle–Ottawa Scale (NOS) and version 2 of the Cochrane tool (RoB 2) were used to assess the risk of bias in cohort studies and randomized trials, respectively, as recommended by the Agency for Healthcare Research (19, 20). An Excel tool developed by the agency was applied to implement RoB 2 (https://www.riskofbias.info/welcome/rob-2-0-tool/current-version-of-rob-2). A cohort study was awarded a maximum of nine stars in three sections, and study quality was judged as follows: high risk of bias = 0–3; moderate risk of bias = 4–6; low risk of bias ≥ 7. RoB 2 contains five domains, and each of them was assessed as low risk of bias, some concerns (moderate risk of bias), or a high risk of bias. The judgment principles of the overall bias were as follows: low risk of bias if all domains were labeled as low risk, and high risk of bias if any of the domains were labeled as high risk; otherwise, the assessment result was some concerns. Two reviewers (LM and HM) assessed the risk of bias for each study independently.
In total, 7,249 potential records (papers, abstracts, and registered trials) were screened from databases and other relevant sources. Ultimately, 88 eligible studies (13, 15, 21–107) fulfilling the criteria were included after removing duplicates and reviewing the papers in detail (Figure 1). Of those studies, Dhooge 2012 was conducted in both Child-Pugh A and B cohorts; Hitron 2014 and Jiang 2019 contained two kinds of combination regimens, and IMbrave150 included global and China cohorts. Thus, 92 records were incorporated into the analyses. The baseline characteristics of the studies are described and summarized in Table 1. In total, 9,748 patients with aHCC from all around the world were included in our systematic review. There were 47 double-arm studies with 7,431 patients and 45 single-arm studies with 2,317 patients. The mean or median age of the total population was 60 years. Most studies were conducted in China (48, 52.2%), and the USA (18, 19.6%). The systemic regimens were the pairwise combinations of targeted therapy, ICI therapy, chemotherapy, and other therapies. Of those, the number and proportions of chemotherapy plus chemotherapy, targeted therapy plus chemotherapy, targeted therapy plus ICI therapy, and targeted plus targeted therapies were 25 (27.2%), 23 (25.0%), 20 (21.7%), and 16 (17.4%), respectively. Subsequently, four specific therapies of those combinations with study numbers greater than 3 were analyzed to probe heterogeneity: Atezo plus Beva, gemcitabine (Gemc) plus oxaliplatin (Oxal), erlotinib (Erlo) plus Beva, and sorafenib (Sora) plus gemcitabine and oxaliplatin (GEMOX). The majority of monotherapies was sorafenib (18, 38.3%), followed by gemcitabine (7, 14.9%). All studies included were trials (73, 79.3%) and cohorts (19, 20.7%).
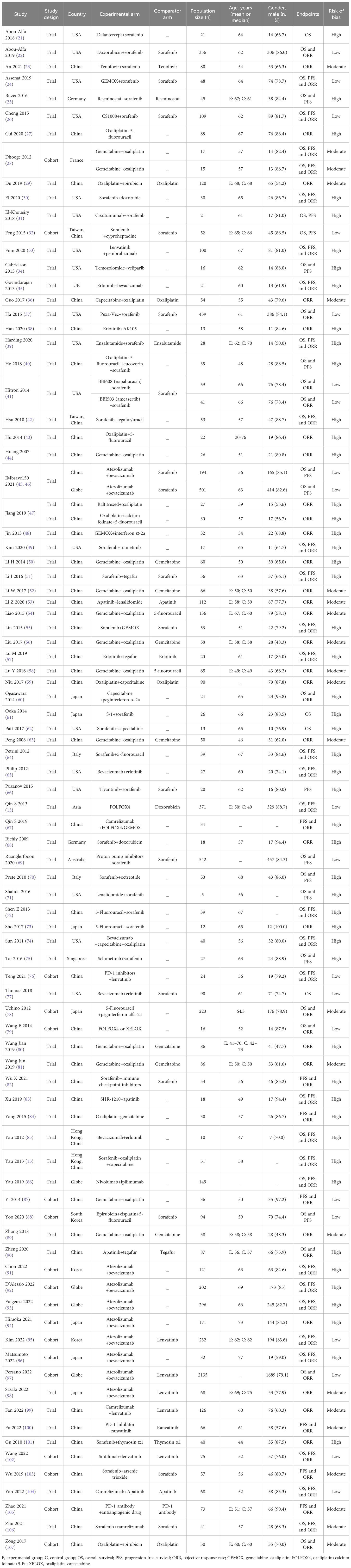
Table 1 The characteristics of studies and participants with advanced hepatocellular carcinoma included in the meta-analysis.
The effects of systemic combination interventions on OS were assessed in a total of 57 studies. In patients with aHCC, targeted therapy plus ICI therapy significantly increased OS (HR: 0.80 [95% CI: 0.68–0.94]; I2 = 0%) and prolonged median OS (15.02 [12.67–17.38] months vs. 8.55 [6.91–10.19] months), compared to the monotherapy. Further, the subgroup analysis of specific therapies indicated this effect was largely due to Atezo plus Beva (HR: 0.81 [0.69–0.96); median: 14.85 [9.87–19.83]). However, the OS benefits were not observed in other types of combinations (Figures 2, 3, S1, S2).
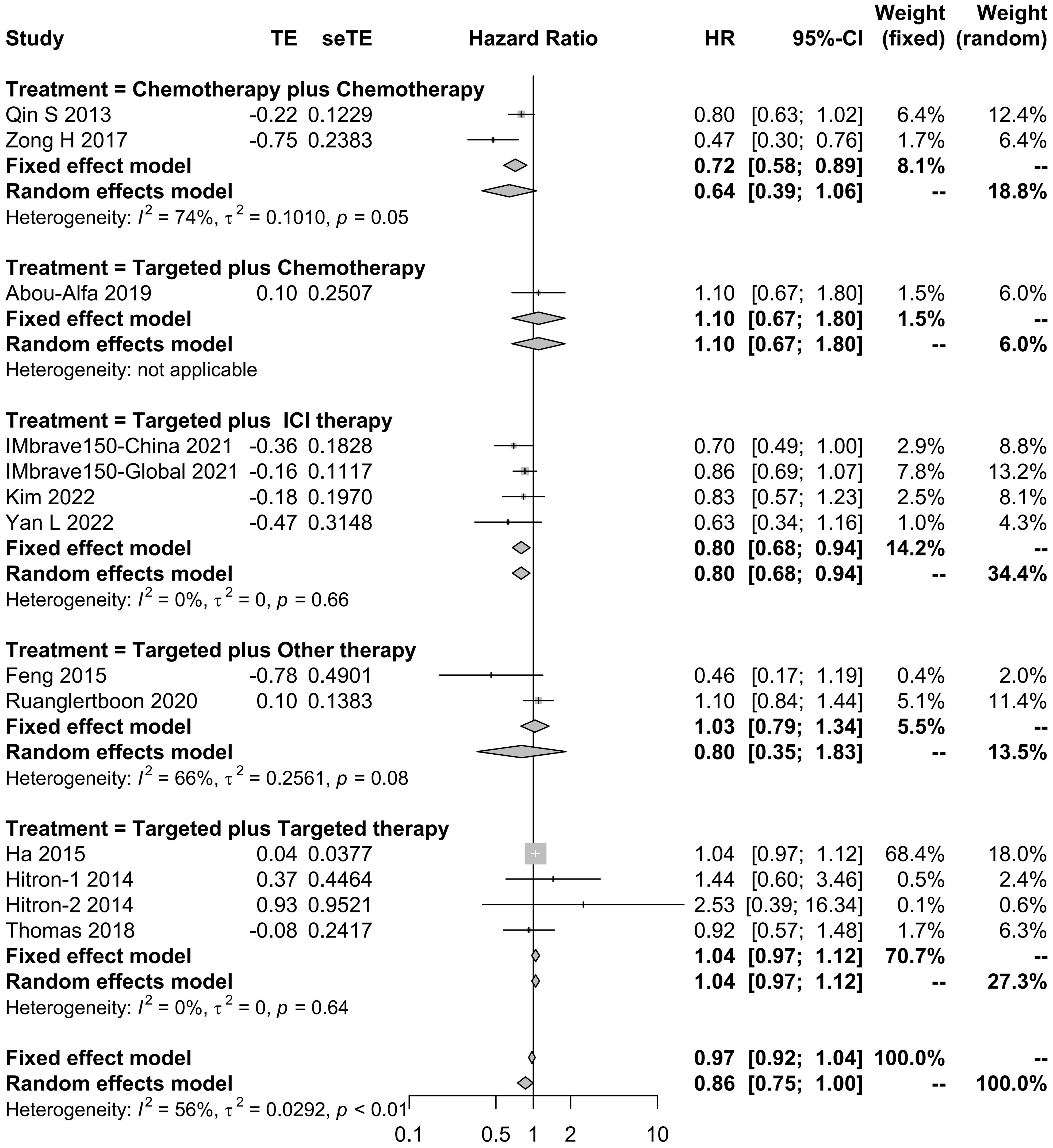
Figure 2 Forest plot for HR of overall survival for the systemic combination therapies, compared to the monotherapy in patients with aHCC. aHCC, advanced hepatocellular carcinoma; HR, hazard ratio; CI, confidence interval.
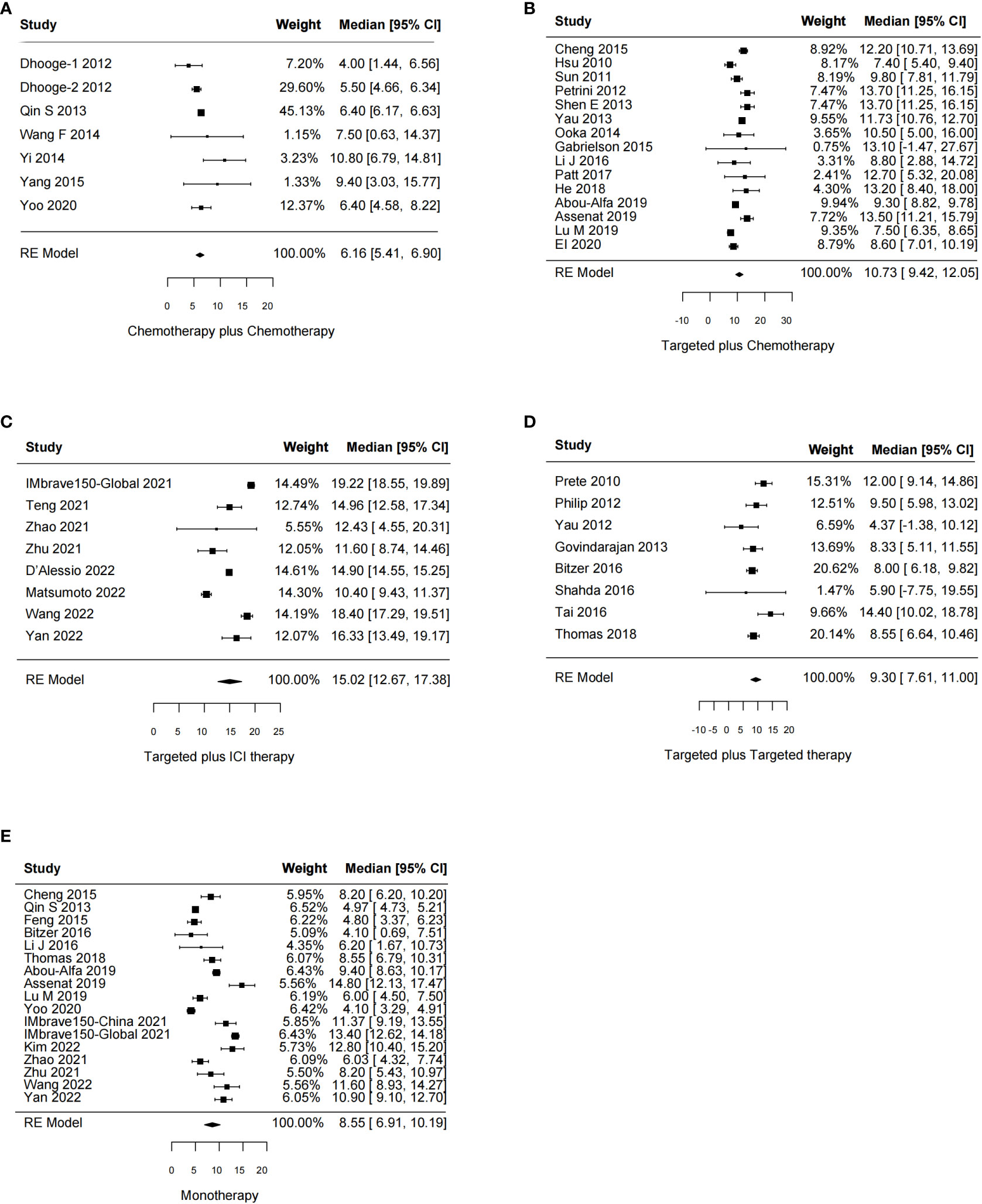
Figure 3 Forest plot for median overall survival of the systemic combination therapies, compared to the monotherapy (E) in patients with aHCC. (A) Chemotherapy plus chemotherapy. (B) Targeted plus chemotherapy. (C) Targeted plus ICI therapy. (D) Targeted plus targeted therapy. aHCC, advanced hepatocellular carcinoma; CI, confidence interval.
In total, 51 studies reported the effect of systemic combination interventions on PFS. The random-effects model indicated that targeted therapy plus ICI therapy had an estimated HR of 0.62 [95% CI: 0.46–0.84], which showed significant PFS benefits over monotherapy. Moreover, the estimated pooled results showed that median PFS was significantly improved if treated with targeted therapy plus chemotherapy (5.08 months [95% CI: 4.13–6.03]) or targeted therapy plus ICI therapy (7.08 months [95% CI: 6.42–7.74]), compared to the monotherapy (3.52 months [95% CI: 2.82–4.22]). Specifically, the median PFS was 5.91 [5.07-6.75] in Sora plus GEMOX and 6.47 [6.06–6.88] in Atezo plus Beva. However, the PFS and median PFS were not improved in the other types of systemic combinations, compared to the monotherapy (Figures 4, 5, S3, S4).
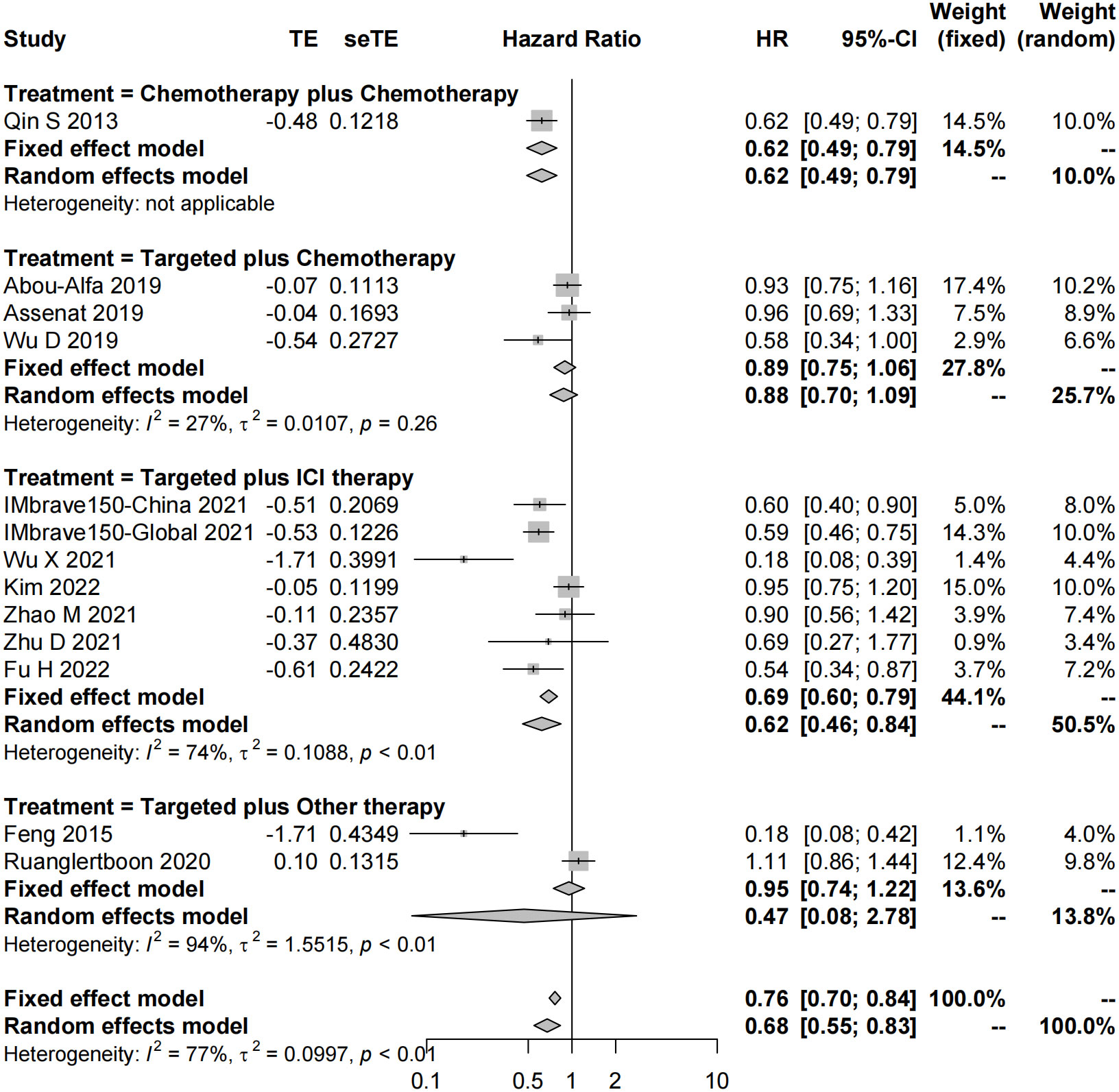
Figure 4 Forest plot for HR of progression-free survival of the systemic combination therapies, compared to the monotherapy in patients with aHCC. aHCC, advanced hepatocellular carcinoma; HR, hazard ratio; CI, confidence interval.
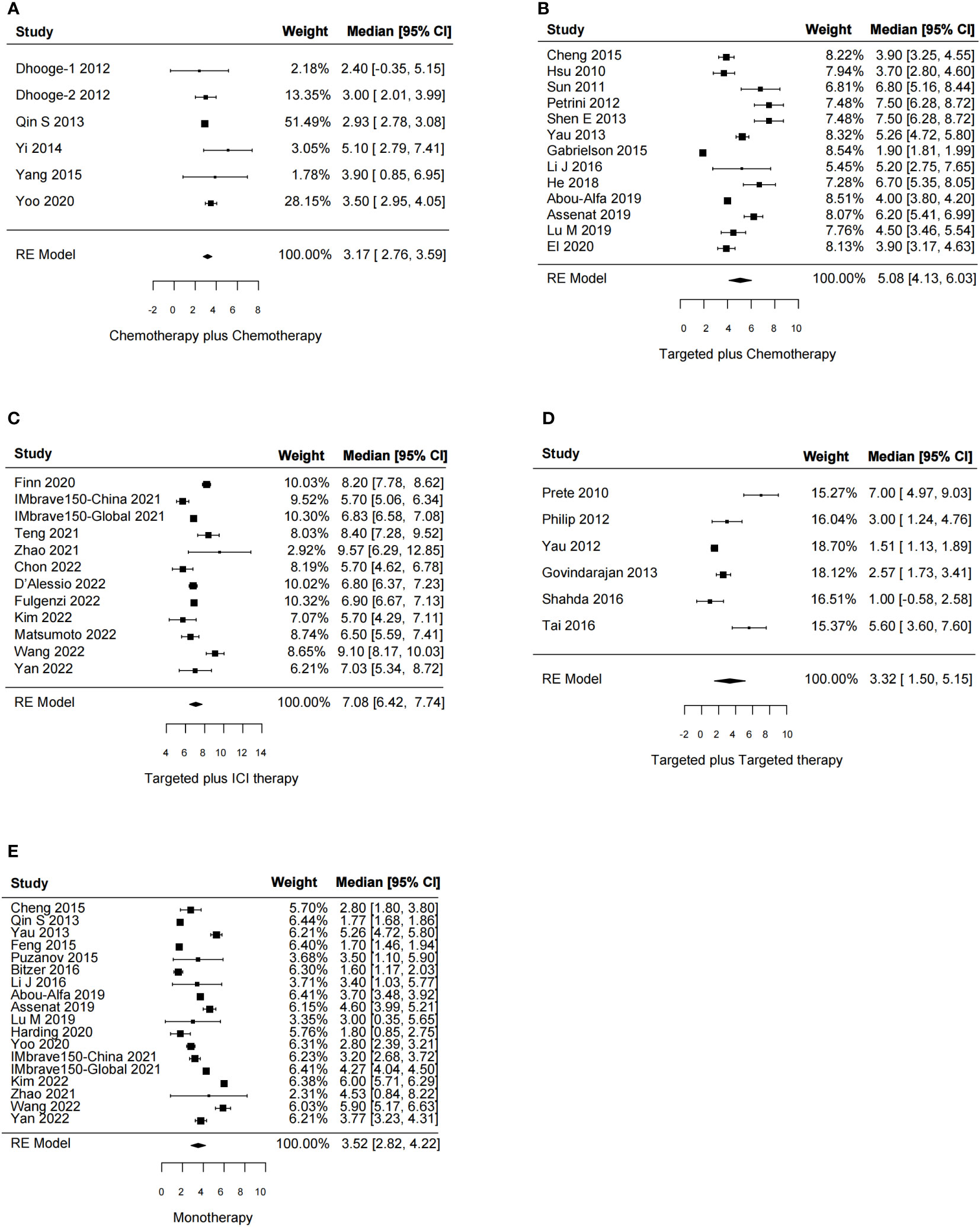
Figure 5 Forest plot for median progression-free survival of the systemic combination therapies, compared to the monotherapy (E) in patients with aHCC. (A) Chemotherapy plus chemotherapy. (B) Targeted plus chemotherapy. (C) Targeted plus ICI therapy. (D) Targeted plus targeted therapy. aHCC, advanced hepatocellular carcinoma; CI, confidence interval.
In total, 75 studies reported the effect of systemic combination interventions on ORR. Of those, 39 studies included comparisons. The pooled results of combination regimens indicated that the effects across those interventions were consistent, and overall heterogeneity was moderate (RR: 1.57 [95% CI: 1.44–1.71]; I2 = 30%). All systemic combination interventions had an improved ORR in patients with aHCC (chemotherapy plus chemotherapy: 1.53 [1.37–1.71], I2 = 14%; targeted therapy plus chemotherapy: 1.77 [1.22–2.55], I2 = 0%; targeted therapy plus ICI therapy: 1.81 [1.55–2.13], I2 = 49%; targeted plus targeted therapy: 1.23 [0.85–1.79], I2 = 56%), compared to the monotherapy. In the subgroup analysis of specific therapies, Atezo plus Beva, Gemc plus Oxal gained ORR benefits as well (Figures 6, S5).
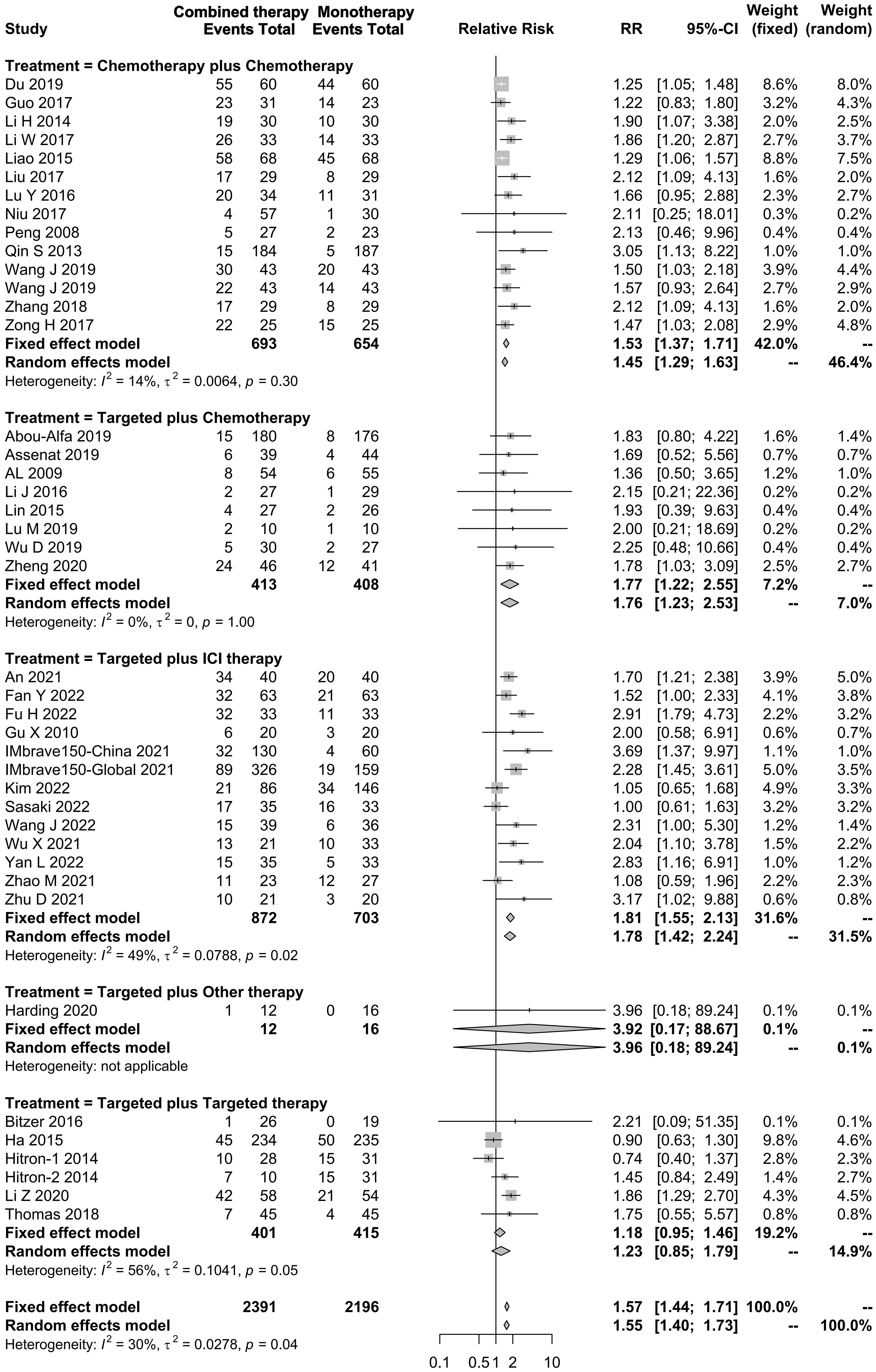
Figure 6 Forest plot for RR of objective response rate of the systemic combination therapies compared to the monotherapy in patients with aHCC. aHCC, advanced hepatocellular carcinoma; RR, relative risk; CI, confidence interval.
The safety profile of systematic combination therapy was also evaluated in this meta-analysis, including the overall TrAEs in 31 two-arm studies and ≥3 Grade TrAEs in 20 two-arm studies. The incidence rate of TrAEs among those combination interventions was comparable (RR: 1.00 [95% CI: 0.98–1.02]; I2 = 73%; Figure S6). For ≥3 Grade TrAEs, the pooled result estimated by the fixed-effects model indicated that the combinations had an increased RR of 1.25 [1.15–1.36], compared to the monotherapy (Figure S7). Moderate heterogeneity was detected across those interventions (I2 = 25%). In the subgroup analysis, the incidence rates of ≥3 Grade TrAEs in the chemotherapy plus chemotherapy, targeted therapy plus ICI therapy, and targeted plus targeted were significantly higher, compared to the monotherapy, with RR values of 1.19 [95% CI: 1.01–1.39], 1.26 [0.90–1.75], and 1.38 [1.16–1.64], respectively. However, it was not significant in targeted therapy plus chemotherapy (1.08 [0.93–1.25]).
For quality assessment, the risk of bias in most studies was high or moderate, which should be attributed to nearly half of the studies with a single arm. However, the quality of double-arm studies was generally acceptable, of which the proportion with low or moderate risk of bias was 89.4%. Funnel plots for the effects of systematic combination therapies on OS, PFS, and ORR were asymmetrical. Moreover, the results of Egger’s test indicated that publication bias was detected (OS, p = 0.084; PFS, p = 0.04; ORR, p = 0.002; ≥3 Grade TrAEs, p = 0.092; Figure S8).
In this systemic review, we evaluated the efficacy and safety of different systemic combination treatments on the prognosis of aHCC. All kinds of combination treatments (chemotherapy plus chemotherapy, targeted therapy plus ICI therapy, targeted therapy plus chemotherapy, and targeted plus targeted therapies) had better ORRs in patients with aHCC, compared to the monotherapy (Figure 6). Importantly, targeted therapy plus ICI therapy, especially Atezo plus Beva, showed superiority in multiple clinical outcomes (OS, PFS, and ORR) over other combinations. Except for targeted therapy plus chemotherapy, all the other combinations had an increased RR for ≥3 Grade TrAEs, compared to the monotherapy. Our findings indicated that the systemic combination regimens had a prominent advantage in treating advanced HCC, although adverse events should be taken into consideration. The pooled results were also calculated separately by study design, and the subgroups with the number of studies greater than 3 were presented. The results of trials and cohorts were generally consistent with studies combined together, indicating the robustness of the pooled results in this study (Figures S9–S11). In particular, targeted therapy plus ICI therapy should be given priority on further drug design and development in aHCC.
Previously, several systematic reviews investigated the effects of different systemic treatments on aHCC across lines of therapy (108–110). For instance, a systematic review provided evidence that the combination of PD-1/PD-L1 inhibitors with anti-VEGF agents improved clinical outcomes in patients with aHCC (ORR, p = 0.016; PFS, p < 0.001) but also increased immune-related toxicity (108). The other two network systemic reviews made a comparison between the specific systemic combination therapies and monotherapy (109, 110). It was demonstrated that the Atezo plus Beva combination prolonged OS, PFS, and ORR in patients with unresectable HCC in both the experimental setting and the real world (Figures S1–S4). Notably, systemic treatment should be selected based on the goals of individualized treatment. The outcomes of those studies were generally consistent with our findings. However, more clinical trials are needed to update long-term clinical outcomes. Moreover, safety is also an important factor affecting clinical decision-making. Our pooled analysis showed the combinations of chemotherapy plus chemotherapy, targeted therapy plus ICI therapy, and targeted plus targeted therapies had increased and comparable risk of suffering ≥3 Grade TrAEs, which were partly reported in another study (108). The treatment-related toxicity is critical for patients with aHCC.
The mechanisms by which the combination of targeted therapy plus ICI therapy improved the prognosis in aHCC remain largely unknown. Anti-angiogenesis therapy using multikinase inhibitors not only prunes blood vessels essential for cancer progression and metastasis but also has immune modulatory effects by increasing M1 polarization of macrophages and stimulating CD8+ T-cell function (111–113). Hence, immune checkpoint blockade and anti-angiogenesis synergistically increase anti-tumor activity in aHCC. However, high dosages of the kinase inhibitors may contribute to immune suppression in the tumor microenvironment (113), indicating that the immune modulatory dosage should be optimized to facilitate the design of future combination regimens. In the precise medicine era, identifying a universal therapy covering a large group is important but not enough. To further improve therapeutic effect, it is of great significance to find out the target patients of those combination treatments. It is reported that investigating treatment-related biomarkers, like immunotherapy, is a promising therapeutic strategy (114–116).
Our study had limitations. First, the heterogeneity existed in total systematic combinations, although types and specific therapies partly accounted for it. Second, some single-arm trials included in this meta-analysis could lead to potential bias. Despite this, the single-arm studies did not cause significant bias in major conclusions since they were only used to estimate the pooled median of OS and PFS. During the process of the study searching, we found an increasing number of studies evaluating the efficacy of new therapy (i.e., Atezo and Beva) in the clinic since 2022. We would exclude single-arm studies when the number of double-arm studies are large enough. Despite those disadvantages, this study provides the most convincing evidence indicating that combinations of systemic therapies especially targeted therapy plus ICI therapy have more advantages compared with monotherapy in treating aHCC.
Our systematic review and meta-analysis showed that the combinations of chemotherapy plus chemotherapy, targeted therapy plus ICI therapy, targeted therapy plus chemotherapy, and targeted plus targeted therapies significantly improve ORR in patients with aHCC. Furthermore, targeted therapy plus ICI therapy, especially Atezo plus Beva, shows superiority in multiple clinical outcomes over other combinations. Moreover, increased toxicity is evident in combination therapies except for targeted plus chemotherapy. Future trials should concentrate on improvement in the therapeutic efficiency and reduction of the treatment-related toxicity of targeted therapy plus ICI therapy.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Conception and design: PL and GC. Administrative support: GC. Provision of study materials or patients: none. Collection and assembly of data: ML, MH, XR, and DL. Data analysis and interpretation: PL and GC. Manuscript writing: all authors. Final approval of manuscript: all authors.
This work was supported by grant 2015CB554006 from the National Key Basic Research Program of China (GC); grants 91529305 (GC), 81520108021 (GC), 81673250 (GC), and 81521091 (GC) from the National Natural Science Foundation of China; grants GWV-10.1-XK17 from the “3-year public health promotion” program of Shanghai Municipal Health Commission (GC); and grant 2022QN021 (PL) from the Youth Fund of Naval Military Medical University.
We acknowledge all workers involved in study searching, data extraction, statistical analysis, and writing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1197782/full#supplementary-material
aHCC, advanced hepatocellular carcinoma; OS, overall survival; PFS, progression-free survival; ORR, objective response rate; TrAEs, treatment-related adverse events; ICI, immune checkpoint inhibitor; Atezo, atezolizumab; Beva, bevacizumab; Gemc, gemcitabine; Oxal, oxaliplatin; Erl, erlotinib; Sora, sorafenib; GEMOX, gemcitabine and oxaliplatin.
1. Liu Z, Suo C, Mao X, Jiang Y, Jin L, Zhang T, et al. Global incidence trends in primary liver cancer by age at diagnosis, sex, region, and etiology, 1990-2017. Cancer (2020) 126:2267–78. doi: 10.1002/cncr.32789
2. Cheng H, Yang X, Liu G. Superstable homogeneous iodinated formulation technology: revolutionizing transcatheter arterial chemoembolization. Sci Bull (Beijing) (2020) 65:1685–7. doi: 10.1016/j.scib.2020.06.029
3. Chen H, Cheng H, Dai Q, Cheng Y, Zhang Y, Li D, et al. A superstable homogeneous lipiodol-ICG formulation for locoregional hepatocellular carcinoma treatment. J Control Release (2020) 323:635–43. doi: 10.1016/j.jconrel.2020.04.021
4. Li Z, Cheng H, Mao J, Liu G. Conversion therapy of intermediate and advanced hepatocellular carcinoma using superstable homogeneous iodinated formulation technology. Sci China Life Sci (2022) 65:2114–7. doi: 10.1007/s11427-022-2142-3
5. Peng Y, Cheng H, Liu H, Zhang Y, Liu G. Super-stable homogeneous embolic agents advance the treatment of hepatocellular carcinoma. Iradiology (2023) 1:1–5. doi: 10.1002/ird3.22
6. Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int (2015) 35:2155–66. doi: 10.1111/liv.12818
7. Bruix J, Raoul JL, Sherman M, Mazzaferro V, Bolondi L, Craxi A, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol (2012) 57:821–9. doi: 10.1016/j.jhep.2012.06.014
8. Benson AB, D’Angelica MI, Abbott DE, Anaya DA, Anders R, Are C, et al. Hepatobiliary cancers, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw (2021) 19:541–65. doi: 10.6004/jnccn.2021.0022
9. Gordan JD, Kennedy EB, Abou-Alfa GK, Beg MS, Brower ST, Gade TP, et al. Systemic therapy for advanced hepatocellular carcinoma: ASCO guideline. J Clin Oncol (2020) 38:4317–45. doi: 10.1200/JCO.20.02672
10. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib vs sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet (2018) 391:1163–73. doi: 10.1016/S0140-6736(18)30207-1
11. Bruix J, da Fonseca LG, Reig M. Insights into the success and failure of systemic therapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol (2019) 16:617–30. doi: 10.1038/s41575-019-0179-x
12. Foerster F, Galle PR. The current landscape of clinical trials for systemic treatment of HCC. Cancers (2021) 13:1962. doi: 10.3390/cancers13081962
13. Qin S, Bai Y, Lim HY, Thongprasert S, Chao Y, Yang TS, et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. Clin Oncol (2013) 31:3501–8. doi: 10.1200/JCO.2012.44.5643
14. Assenat E, Pageaux GP, Thézenas S, Peron JM, Bécouarn Y, Seitz JF, et al. Sorafenib alone vs. sorafenib plus GEMOX as 1-line treatment for advanced HCC: the phase II randomised PRODIGE 10 trial. Br J Cancer (2019) 120:896–902. doi: 10.1038/s41416-019-0443-4
15. Yau TC, Cheung FY, Lee F, Choo SP, Wong H, TohA. Leung K. HC, et al. A multicenter phase II study of sorafenib, capecitabine, and oxaliplatin (SECOX) in patients with advanced hepatocellular carcinoma: Final results of Hong Kong-Singapore Hepatocellular Carcinoma Research Collaborative Group study. J Clin Oncol (2013) 31:15_suppl 4117-4117. doi: 10.1200/jco.2013.31.15_suppl.4117
16. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim T, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med (2020) 382:1894–905. doi: 10.1056/NEJMoa1915745
17. Zhu AX, Rosmorduc O, Evans TR, Ross PJ, Santoro A, Carrilho FJ, et al. SEARCH: a phase III, randomized, doubleblind, placebo-controlled trial of sorafenib plus erlotinib in patients with advanced hepatocellular carcinoma. J Clin Oncol (2015) 33:559–66. doi: 10.1200/JCO.2013.53.7746
18. Abou-Alfa GK, Niedzwieski D, Knox JJ, Kaubisch A, Posey J, Tan BR, et al. Phase III randomized study of sorafenib plus doxorubicin versus sorafenib in patients with advanced hepatocellular carcinoma (HCC): CALGB 80802 (Alliance). J Clin Oncol (2016) 34:15_suppl 4003–4003. doi: 10.1200/jco.2016.34.4_suppl.192
19. Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ontario K1J 8M5, Canada: Professor GA Wells, Department of Epidemiology and Commuunity Medicine, University of Ottawa (2020). Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
20. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ (2019) 366:l4898. doi: 10.1136/bmj.l4898
21. Abou-Alfa GK, Miksad RA, Tejani MA, Williamson S, Gutierrez ME, Olowokure OO, et al. Open-label study of dalantercept, an activin receptor-like kinase 1 ligand trap, plus sorafenib in advanced hepatocellular carcinoma. Oncologist (2019) 24:161–70. doi: 10.1634/theoncologist.2018-0654
22. Abou-Alfa GK, Shi Q, Knox JJ, Kaubisch A, Niedzwiecki D, Posey J, et al. Assessment of treatment with sorafenib plus doxorubicin vs sorafenib alone in patients with advanced hepatocellular carcinoma: phase 3 CALGB 80802 randomized clinical trial. JAMA Oncol (2019) 5:1582–8. doi: 10.1001/jamaoncol.2019.2792
23. An X, Li Y, Menggen C. Clinical observation of tenofovir and sorafenib in the treatment of patients with hepatitis B associated advanced primary liver cancer. J Prac Hepatol (2021) 24:600–2. doi: 10.3969/j.issn.1672-5069.2021.04.037
24. Assenat E, Pageaux GP, Thézenas S, Peron JM, Bécouarn Y, Seitz JF, et al. Sorafenib alone vs. sorafenib plus GEMOX as 1st-line treatment for advanced HCC: the phase II randomised PRODIGE 10 trial. Br J Cancer (2019) 120:896–902. doi: 10.1038/s41416-019-0443-4
25. Bitzer M, Horger M, Giannini EG, Ganten TM, Wörns MA, Siveke JT, et al. Resminostat plus sorafenib as second-line therapy of advanced hepatocellular carcinoma - The SHELTER study. J Hepatol (2016) 65:280–8. doi: 10.1016/j.jhep.2016.02.043
26. CS1008- in combination with sorafenib compared to sorafenib alone in subjects with advanced liver cancer (2009). Available at: https://clinicaltrials.gov/ct2/show/NCT01033240.
27. Cui J. Clinical analysis of oxaliplatin combined with 5-Fu in the treatment of advanced primary liver cancer. Guide Chin Med (2020) 18:138. doi: 10.15912/j.cnki.gocm.2020.06.112
28. Dhooge M, Coriat R, Mir O, Perkins G, Brezault C, Boudou-Rouquette P, et al. Feasibility of gemcitabine plus oxaliplatin in advanced hepatocellular carcinoma patients with Child-Pugh B cirrhosis. Oncology (2013) 84:32–8. doi: 10.1159/000342763
29. Du G, Wang H. Therapeutic effect of oxaliplatin combined with epirubicin in the interventional treatment of advanced primary liver cancer. Syst Med (2019) 4:121–3. doi: 10.19368/j.cnki.2096-1782.2019.14.121
30. El DI, Capanu M, Chou JF, Harding JJ, Ly M, Hrabovsky AD, et al. Phase II trial of sorafenib and doxorubicin in patients with advanced hepatocellular carcinoma after disease progression on sorafenib. Cancer Med (2020) 9:7453–9. doi: 10.1002/cam4.3389
31. El-Khoueiry AB, O’Donnell R, Semrad TJ, Mack P, Blanchard S, Bahary N, et al. A phase I trial of escalating doses of cixutumumab (IMC-A12) and sorafenib in the treatment of advanced hepatocellular carcinoma. Cancer Chemother Pharmacol (2018) 81:957–63. doi: 10.1007/s00280-018-3553-4
32. Feng YM, Feng CW, Lu CL, Lee MY, Chen CY, Chen SC. Cyproheptadine significantly improves the overall and progression-free survival of sorafenib-treated advanced HCC patients. Jpn J Clin Oncol (2015) 45:336–42. doi: 10.1093/jjco/hyv007
33. Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, et al. Phase ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol (2020) 38:2960–70. doi: 10.1200/JCO.20.00808
34. Gabrielson A, Tesfaye AA, Marshall JL, Pishvaian MJ, Smaglo B, Jha R, et al. Phase II study of temozolomide and veliparib combination therapy for sorafenib-refractory advanced hepatocellular carcinoma. Cancer Chemother Pharmacol (2015) 76:1073–9. doi: 10.1007/s00280-015-2852-2
35. Govindarajan R, Siegel E, Makhoul I, Williamson S. Bevacizumab and erlotinib in previously untreated inoperable and metastatic hepatocellular carcinoma. Am J Clin Oncol (2013) 36:254–7. doi: 10.1097/COC.0b013e318248d83f
36. Guo N, Wu Y, Zhao F. Clinical efficacy and safety analysis of capecitabine combined with oxaliplatin in patients with advanced liver cancer. Guizhou Med J (2017) 41:930–3. doi: 10.3969/j.issn.1000-744X.2017.09.013
37. Hepatocellular Carcinoma Study Comparing Vaccinia Virus Based immunotherapy Plus Sorafenib vs Sorafenib Alone (PHOCUS) (2015). Available at: https://clinicaltrials.gov/show/NCT02562755.
38. Han C, Ye S, Li J, Zhang P, Dong Y, Bai L. A preliminary study on effect and safety of anlotinib combined with anti-PD-1 antibody (AK105) in treatment of advanced hepatocellular carcinoma. Acad J Chin PLA Med Sch (2020) 41:868–72. doi: 10.3969/j.issn.2095-5227.2020.09.005+
39. Harding JJ, Kelley RK, Tan B, Capanu M, Do GK, Shia J, et al. Phase ib study of enzalutamide with or without sorafenib in patients with advanced hepatocellular carcinoma. Oncologist (2020) 25:e1825–36. doi: 10.1634/theoncologist.2020-0521
40. He MK, Zou RH, Li QJ, Zhou ZG, Shen JX, Zhang YF, et al. Phase II study of sorafenib combined with concurrent hepatic arterial infusion of oxaliplatin, 5-fluorouracil and leucovorin for unresectable hepatocellular carcinoma with major portal vein thrombosis. Cardiovasc Intervent Radiol (2018) 41:734–43. doi: 10.1007/s00270-017-1874-z
41. A study of BBI608 in combination with sorafenib, or BBI503 in combination with sorafenib in adult patients with hepatocellular carcinoma (2014). Available at: https://clinicaltrials.gov/show/NCT02279719.
42. Hsu CH, Shen YC, Lin ZZ, Chen PJ, Shao YY, Ding YH, et al. Phase II study of combining sorafenib with metronomic tegafur/uracil for advanced hepatocellular carcinoma. J Hepatol (2010) 53:126–31. doi: 10.1016/j.jhep.2010.01.035
43. Hu Q, Wei Y, Wang Y, Wan X, Hu J, Yuan L. Clinical observation on oxaliplatin in combination with 5-fluorouracil for advanced primary hepatocarcinoma. J Wannan Med Coll (2014) 33:328–31. doi: 10.3969/j.issn.1002-0217.2014.04.014
44. Huang H, Zheng Z, Li G. Clinical study of double-way chemotherapy with gemcitabine and oxaliplatin in the treatment of advanced primary hepatocarcinoma. Chongqing Med (2007) 36:2315–7. doi: 10.3969/j.issn.1671-8348.2007.22.029
45. Qin S, Ren Z, Feng YH, Yau T, Wang B, Zhao H, et al. Atezolizumab plus Bevacizumab versus Sorafenib in the Chinese Subpopulation with Unresectable Hepatocellular Carcinoma: Phase 3 Randomized, Open-Label IMbrave150 Study. Liver Cancer (2021) 10:296–308. doi: 10.1159/000513486
46. A study of atezolizumab in combination with bevacizumab compared with sorafenib in patients with untreated locally advanced or metastatic hepatocellular carcinoma (IMbrave150) (2018). Available at: https://clinicaltrials.gov/ct2/show/NCT03434379.
47. Jiang Z. Clinical study of combined chemotherapy in the treatment of advanced primary liver cancer. China Prar Med (2019) 14:124–7. doi: 10.14163/j.cnki.11-5547/r.2019.36.067
48. Jin Y, Lu J, Chen P, Li X, Mei K. Clinical analysis of 32 cases of gem and L-OHP with interferon regimen for the treatment of patients with advanced primary liver cancer. Prog Mod BioMed (2013) 13:4295–9. doi: CNKI:SUN:SWCX.0.2013-22-026
49. A phase I trial of trametinib in combination with sorafenib in patients with advanced hepatocellular cancer (2014). Available at: https://clinicaltrials.gov/ct2/show/NCT002292173.
50. Li H, Lei Y, Huang J, He Q, Sun Y. Study of the efficacy and safety of GEMOX regimen for the patients with advanced primary liver cancer. J Clin Exp Med (2014) 13:271–7. doi: 10.3969/j.issn.1671-4695.2014.04.010
51. Li J, Huang Y, Yuan J, Chen Q, Liu Q, Meng J, et al. Clinical study of Sorafenib combined with S-1 in advanced hepatocellular carcinoma. China J Mod Med (2016) 26:77–83. doi: 10.3969/j.issn.1005-8982.2016.24.017
52. Li W, Cao X. Short-term efficacy and adverse reactions of gemcitabine combined with oxaliplatin in the treatment of advanced liver cancer. Strait Pharm J (2017) 29:119–22. doi: 10.3969/j.issn.1006-3765.2017.06.054
53. Li Z, Chen R. Clinical efficacy of apatinib and lenalidomide in advanced primary liver cancer. J Guangdong Med Univ (2020) 38:325–8. doi: 10.3969/j.issn.1005-4057.2020.03.020
54. Liao H. Analysis of the effect of gemcitabine combined with oxaliplatin in the treatment of advanced liver cancer. Contemp Med Forum (2015) 13:233–5. doi: CNKI:SUN:QYWA.0.2015-17-198
55. Lin S, Liu C, Jiang G, Zhang R. Therapeutic effect and prognosis of sorafenib combined with GEMOX regimen in the treatment of advanced hepatocellular carcinoma. Chin Clin Oncol (2015) 20:517–21. doi: CNKI:SUN:LCZL.0.2015-06-010
56. Liu H, Zhou F, Yi S. Efficacy of gemcitabine combined with oxaliplatin in the treatment of advanced primary liver cancer. World Latest Med Info (2017) 17:96–7. doi: CNKI:SUN:WMIA.0.2017-41-066
57. Lu M, Kang M, Li B, Xu S, Luo M. Observation on Effect of single anlotinib or combined with tegafur in treating sorafenib resistant advanced hepatocellular carcinoma. J Mod Med Health (2020) 36:985–9. doi: CNKI:SUN:XYWS.0.2020-07-008
58. Lu Y. Clinical observation of GEMOX regimen in the treatment of advanced primary liver cancer. Guide Chin Med (2016) 14:96–8. doi: CNKI:SUN:YYXK.0.2016-28-075
59. Niu H, Guo J, Zhu F, Zhao H, Hou Z. Observation of effects of oxaliplatin combined with capecitabine in the treatment of primary hepatic carcinoma. Anti-tumor Pharm (2017) 7:360–4. doi: 10.3969/j.issn.2095-1264.2017.03.22
60. Ogasawara S, Chiba T, Ooka Y, Kanogawa N, Motoyama T, Suzuki E, et al. A phase I/II trial of capecitabine combined with peginterferon α-2a in Patients with sorafenib-refractory advanced hepatocellular carcinoma. Invest New Drugs (2014) 32:762–8. doi: 10.1007/s10637-014-0097-2
61. Ooka Y, Chiba T, Ogasawara S, Arai K, Suzuki E, Tawada A, et al. A phase I/II study of S-1 with sorafenib in patients with advanced hepatocellular carcinoma. Invest New Drugs (2014) 32:723–8. doi: 10.1007/s10637-014-0077-6
62. Patt Y, Rojas-Hernandez C, Fekrazad HM, Bansal P, Lee FC. Phase II trial of sorafenib in combination with capecitabine in patients with hepatocellular carcinoma: INST 08-20. Oncologist (2017) 22:1158–16. doi: 10.1634/theoncologist.2017-0168
63. Peng C, Xu A, Su X. An initial study of GEMOX regimen for the patients with advanced hepatocellular carcinoma. J Nantong Univ (Med Sci) (2008) 28:281–3. doi: 10.3969/j.issn.1674-7887.2008.04.017
64. Petrini I, Lencioni M, Ricasoli M, Iannopollo M, Orlandini C, Oliveri F, et al. Phase II trial of sorafenib in combination with 5-fluorouracil infusion in advanced hepatocellular carcinoma. Cancer Chemother Pharmacol (2012) 69:773–80. doi: 10.1007/s00280-011-1753-2
65. Philip PA, Mahoney MR, Holen KD, Northfelt DW, Pitot HC, Picus J, et al. Phase 2 study of bevacizumab plus erlotinib in patients with advanced hepatocellular cancer. Cancer (2012) 118:2424–30. doi: 10.1002/cncr.26556
66. Puzanov I, Sosman J, Santoro A, Saif MW, Goff L, Dy GK, et al. Phase 1 trial of tivantinib in combination with sorafenib in adult patients with advanced solid tumors. Invest New Drugs (2015) 33:159–68. doi: 10.1007/s10637-014-0167-5
67. Qin S, Chen Z, Liu Y, Xiong J, Ren Z, Meng Z, et al. A phase II study of anti–PD-1 antibody camrelizumab plus FOLFOX4 or GEMOX systemic chemotherapy as first-line therapy for advanced hepatocellular carcinoma or biliary tract cancer. Journal of Clinical Oncology (2019) 37:15_suppl 4074-4074. doi: 10.1200/JCO.2019.37.15_suppl.4074.
68. Richly H, Schultheis B, Adamietz IA, Kupsch P, Grubert M, Hilger RA, et al. Combination of sorafenib and doxorubicin in patients with advanced hepatocellular carcinoma: results from a phase I extension trial. Eur J Cancer (2009) 45:579–87. doi: 10.1016/j.ejca.2008.10.039
69. Ruanglertboon W, Sorich MJ, Logan JM, Rowland A, Hopkins AM. The effect of proton pump inhibitors on survival outcomes in advanced hepatocellular carcinoma treated with sorafenib. J Cancer Res Clin Oncol (2020) 146:2693–7. doi: 10.1007/s00432-020-03261-3
70. Prete SD, Montella L, Caraglia M, Maiorino L, Cennamo G, Montesarchio V, et al. Sorafenib plus octreotide is an effective and safe treatment in advanced hepatocellular carcinoma: multicenter phase II So. LAR. study. Cancer Chemother Pharmacol (2010) 66:837–44. doi: 10.1007/s00280-009-1226-z
71. Shahda S, Loehrer PJ, Clark RS, Spittler AJ, Althouse SK, Chiorean EG. Phase I study of lenalidomide and sorafenib in patients with advanced hepatocellular carcinoma. Oncologist (2016) 21:664–5. doi: 10.1634/theoncologist.2016-0071
72. Shen E, Hu J, Weng J. Clinical study of sorafenib combined with 5-fluorouracil in the treatment of advanced liver cancer. J Clin Exp Med (2013) 12:1573–5. doi: 10.3969/j.issn.1671-4695.2013.19.023
73. Sho T, Nakanishi M, Morikawa K, Ohara M, Kawagishi N, Izumi T, et al. A phase I study of combination therapy with sorafenib and 5-fluorouracil in patients with advanced hepatocellular carcinoma. Drugs R D (2017) 17:381–8. doi: 10.1007/s40268-017-0187-7
74. Sun W, Sohal D, Haller DG, Mykulowycz K, Rosen M, Soulen MC, et al. Phase 2 trial of bevacizumab, capecitabine, and oxaliplatin in treatment of advanced hepatocellular carcinoma. Cancer (2011) 117:3187–92. doi: 10.1002/cncr.25889
75. Tai WM, Yong WP, Lim C, Low LS, Tham CK, Koh TS, et al. A phase Ib study of selumetinib (AZD6244, ARRY-142886) in combination with sorafenib in advanced hepatocellular carcinoma (HCC). Ann Oncol (2016) 27:2210–5. doi: 10.1093/annonc/mdw415
76. Teng Y, Ding X, Li W, Chen J. Clinical effect of programmed cell death-1 inhibitor combined with lenvatinib in treatment of advanced primary liver cancer and related adverse events. J Clin Hepatol (2021) 37:606–11. doi: 10.3969/j.issn.1001-5256.2021.03.020
77. Bevacizumab and erlotinib or sorafenib as first-line therapy in treating patients with advanced liver cancer (2009). Available at: https://clinicaltrials.gov/ct2/show/results/NCT00881751.
78. Uchino K, Obi S, Tateishi R, Sato S, Kanda M, Sato T, et al. Systemic combination therapy of intravenous continuous 5-fluorouracil and subcutaneous pegylated interferon alfa-2a for advanced hepatocellular carcinoma. J Gastroenterol (2012) 47:1152–9. doi: 10.1007/s00535-012-0574-3
79. Wang F, Qin S, Hua H, Liu X, Yang L, Qu W, et al. Clinical observation of oxaliplatin-based regimen for sorafenib-resistant advanced primary liver carcinoma. Chin Clin Oncol (2014) 19:226–30. doi: CNKI:SUN:LCZL.0.2014-03-010
80. Wang J, Zhang J, Wu Z, Zhang Y. Clinical analysis of gemcitabine combined with oxaliplatin in the treatment of stage IV primary hepatocellular carcinoma. Elec J Clin Med Lit (2019) 6:151–2. doi: CNKI:SUN:LCWX.0.2019-94-129
81. Wang J. Feasibility and safety of gemcitabine combined with oxaliplatin in the treatment of advanced primary hepatocellular carcinoma. Clin Res (2019) 27:130–2. doi: CNKI:SUN:LCYN.0.2019-10-073
82. Wu X, Chen W, Zheng L, Fang S, Wu F, Zhao Z, et al. Efficacy and safety of sorafenib combined with immune checkpoint inhibitors in TACE refractory liver cancer. J HPB Surg (2021) 33:585–91. doi: 10.11952/j.issn.1007-1954.2021.10.003
83. Xu J, Zhang Y, Jia R, Yue C, Chang L, Liu R, et al. Anti-PD-1 antibody SHR-1210 combined with apatinib for advanced hepatocellular carcinoma, gastric, or esophagogastric junction cancer: an open-label, dose escalation and expansion study. Clin Cancer Res (2019) 25:515–23. doi: 10.1158/1078-0432.CCR-18-2484
84. Yang Y, Wang M, Wang Z, Wang J, Zheng R. Gemcitabine plus oxaliplatin for treatment of advanced primary liver cancer. J Bengbu Med Coll (2015) 40:1158–62. doi: 10.13898/j.cnki.issn.1000-2200.2015.09.005
85. Yau T, Wong H, Chan P, Yao TJ, Pang R, Cheung TT, et al. Phase II study of bevacizumab and erlotinib in the treatment of advanced hepatocellular carcinoma patients with sorafenib-refractory disease. Invest New Drugs (2012) 30:2384–90. doi: 10.1007/s10637-012-9808-8
86. Yau T, Kang YK, Kim TY, El-Khoueiry B. A, Santoro A, Sangro B, et al. Nivolumab (NIVO) + ipilimumab (IPI) combination therapy in patients (pts) with advanced hepatocellular carcinoma (aHCC): Results from CheckMate 040. J Clin Oncol (2019) 37:15_suppl.4012. doi: 10.1200/JCO.2019.37.15_suppl.4012
87. Yi W, Fang L, Liao X, Qin F, Zhou W, Li Y, et al. A Clinical study of Gemcitabine plus Oxaliplatin in the treatment of Advanced Primary hepatocellular carcinoma. J Guangxi Med Univ (2014) 31:756–60. doi: CNKI:SUN:GXYD.0.2014-05-012
88. Yoo SH, Kwon JH, Nam SW, Lee JY, Kim YW, Shim DJ, et al. Transarterial infusion of epirubicin and cisplatin combined with systemic infusion of 5-flurouracil versus sorafenib for hepatocellular carcinoma with refractoriness of transarterial chemoembolization using doxorubicin. Cancer Control (2020) 27:1–9. doi: 10.1177/1073274820935843
89. Zhang H. Efficacy of gemcitabine combined with oxaliplatin in the treatment of advanced primary hepatocellular carcinoma. Guide Chin Med (2018) 16:168–70. doi: CNKI:SUN:YYXK.0.2018-16-123
90. Zheng Z, Pan M. Efficacy and safety of apatinib combined with tegafur in the treatment of liver cancer after the failure of initial treatment. J Bengbu Med Coll (2020) 45:917–21. doi: 10.13898/j.cnki.issn.1000-2020.07.020
91. Chon YE, Cheon J, Kim H, Kang B, Ha Y, Kim DY, et al. Predictive biomarkers of survival in patients with advanced hepatocellular carcinoma receiving atezolizumab plus bevacizumab treatment. Cancer Med (2023) 12:2731–8. doi: 10.1002/cam4.5161
92. D’Alessio A, Fulgenzi CAM, Nishida N, Schönlein M, Felden JV, Schulze K, et al. Preliminary evidence of safety and tolerability of atezolizumab plus bevacizumab in patients with hepatocellular carcinoma and Child-Pugh A and B cirrhosis: a real-world study. Hepatology (2022) 76:1000–12. doi: 10.1002/hep.32468
93. Fulgenzi CAM, Cheon J, D’Alessio A, Nishida N, Ang C, Marron TU, et al. Reproducible safety and efficacy of atezolizumab plus bevacizumab for HCC in clinical practice: Results of the AB-real study. Eur J Cancer (2022) 175:204–13. doi: 10.1016/j.ejca.2022.08.024
94. Hiraoka A, Kumada T, Tada T, Hirooka M, Kariyama K, Tani J, et al. Atezolizumab plus bevacizumab treatment for unresectable hepatocellular carcinoma: Early clinical experience. Cancer Rep (Hoboken) (2022) 5:e1464. doi: 10.1002/cnr2.1464
95. Kim BK, Cheon J, Kim H, Kang B, Ha Y, Kim DY, et al. Atezolizumab/bevacizumab vs. Lenvatinib as first-line therapy for unresectable hepatocellular carcinoma: A real-world, multi-center study. Cancers (Basel) (2022) 14:1747. doi: 10.3390/cancers14071747
96. Matsumoto H, Tsuchiya K, Nakanishi H, Hayakawa Y, Yasui Y, Uchihara N, et al. Clinical usefulness of monitoring muscle volume during atezolizumab plus bevacizumab therapy in patients with unresectable hepatocellular carcinoma. Cancers (Basel) (2022) 14:3551. doi: 10.3390/cancers14143551
97. Persano M, Rimini M, Tada T, Suda G, Shimose S, Kudo M, et al. Clinical outcomes with atezolizumab plus bevacizumab or lenvatinib in patients with hepatocellular carcinoma: a multicenter real-world study. J Cancer Res Clin Oncol (2023) 149:5591–602. doi: 10.1007/s00432-022-04512-1
98. Sasaki R, Nagata K, Fukushima M, Haraguchi M, Miuma S, Miyaaki H, et al. Evaluating the role of hepatobiliary phase of gadoxetic acid-enhanced magnetic resonance imaging in predicting treatment impact of lenvatinib and atezolizumab plus bevacizumab on unresectable hepatocellular carcinoma. Cancers (Basel) (2022) 14:827. doi: 10.3390/cancers14030827
99. Fan Y, Ge C, Chen C. Efficacy of Erika combined with Renvastinib in the treatment of advanced primary liver cancer. Mod Pract Med (2022) 34:1462–4. doi: 10.3969/j.issn.1671-0800.2022.11.025
100. Fu H, Ke C, Zhang B, Peng E. Analysis of efficacy of PD-1 inhibitor combined with renvatinib in the treatment of advanced liver cancer. Shanghai Med Pharm J (2022) 43:29–30. Available at: http://qikan.cqvip.com/Qikan/Article/Detail?id=7106570386
101. Gu X, Jiang Z, Yang M, Shi L. Efficacy of sorafenib with thymosinα1for patients with ER hepatocellular carcinoma. Jiangsu Med J (2010) 36:2491–3. doi: CNKI:SUN:YIYA.0.2010-21-004
102. Wang J, Xu L, Yuan G, Xu X, Zhou X, Luo R, et al. Efficacy and safety of sintilimab in combination with lenvatinib therapy as second⁃line regimen for patients with unresectable hepatocellular carcinoma. Mod Pract Med (2022) 38:1130–5. doi: 10.3969/j.issn.1006⁃5725.2022.09.016
103. Wu D, Shi J, Zhang Q, Liu L. Clinical observation of patients with advanced primary liver cancer treated by Sorafenib combined with arsenic trioxide. Mod Oncol (2019) 27:2728–31. doi: 10.3969/j.issn.1672-4992.2019.15.025
104. Yan L, Li Y, Jiang L, Wen H. Efficacy of camrelizumab combined with apatinib in the treatment of advanced liver cancer. Henan Med Res (2022) 31:1203–7. doi: 10.3969/j.issn.1004-437X.2022.07.012
105. Zhao M, Liu P, Zhang Y, Da J, Dai Y, Du Y, et al. Efficacy of PD-1 antibody combined with anti-angiogenic drug in the treatment of patients with advanced hepatocellular carcinoma. Chin J Cancer Prev Treat (2021) 28:1247–52. doi: 10.16073/j.cnki.cjcpt.2021.16.10
106. Zhu D, Yang S, Li Y, Gu J, Ren X, Zhang H, et al. Efficacy of carrelizumab combined with sorafenib onadvanced hepatocellular carcinoma. J Chin Pract Diagn (2021) 35:1063–7. doi: 10.13507/j.issn.1674-3474.2021.10.023
107. Zong H, Qi C, Wu F, Li M, Li J, Huang Z, et al. Effect and adverse reaction of oxaliplatin combined with epirubicin in the treatment of primary liver cancer. Chin Gen Pract (2017) 20:163–4. doi: CNKI:SUN:QKYX.0.2017-S3-068
108. Feng Z, Rong P, Wang W. Meta-analysis of the efficacy and safety of PD-1/PD-L1 inhibitors administered alone or in combination with anti-VEGF agents in advanced hepatocellular carcinoma. Gut (2020) 69:1904–6. doi: 10.1136/gutjnl-2019-320116
109. Sonbol MB, Riaz IB, Naqvi SAA, Almquist DR, Mina S, Almasri J, et al. Systemic therapy and sequencing options in advanced hepatocellular carcinoma: A systematic review and network meta-analysis. JAMA Oncol (2020) 6:e204930. doi: 10.1001/jamaoncol.2020.4930
110. Han Y, Zhi WH, Xu F, Zhang CB, Huang XQ, Luo JF, et al. Selection of first-line systemic therapies for advanced hepatocellular carcinoma: A network meta-analysis of randomized controlled trials. World J Gastroenterol (2021) 27:2415–33. doi: 10.3748/wjg.v27.i19.2415
111. Ma L, Hernandez MO, Zhao Y, Mehta M, Tran B, Kelly M, et al. Tumor cell biodiversity drives microenvironmental reprogramming in liver cancer. Cancer Cell (2019) 36:418–430.e6. doi: 10.1016/j.ccell.2019.08.007
112. Yi M, Jiao D, Qin S, Chu Q, Wu K, Li A, et al. Synergistic effect of immune checkpoint blockade and anti-angiogenesis in cancer treatment. Mol Cancer (2019) 18:60. doi: 10.1186/s12943-019-0974-6
113. Lin YY, Tan CT, Chen CW, Ou DL, Cheng AL, Hsu C, et al. Immunomodulatory Effects of Current targeted Therapies on Hepatocellular Carcinoma: Implication for the Future of immunotherapy. Semin Liver Dis (2018) 38:379–88. doi: 10.1055/s-0038-1673621
114. Chen WM, Zhang XP, Yan YY, Sun X, Li L. Targeting the interactions between lymphocytes and liver cancer stem cells in combination with ICI therapy is a promising therapeutic strategy. Hepatoma Res (2023) 9:2. doi: 10.20517/2394-5079.2022.52
115. Kwee SA, Tiirikainen M. Beta-catenin activation and ICI therapy resistance in hepatocellular carcinoma: mechanisms and biomarkers. Hepatoma Res (2021) 7:8. doi: 10.20517/2394-5079.2020.124
Keywords: advanced hepatocellular carcinoma, systemic combination therapy, targeted therapy plus ICI therapy, efficacy, safety
Citation: Li P, Hu M, Liu M, Ren X, Liu D, Liu J, Yin J, Tan X and Cao G (2023) The efficacy and safety of different systemic combination therapies on advanced hepatocellular carcinoma: a systematic review and meta-analysis. Front. Oncol. 13:1197782. doi: 10.3389/fonc.2023.1197782
Received: 31 March 2023; Accepted: 04 September 2023;
Published: 25 September 2023.
Edited by:
Guanghua Rong, Fifth Medical Center of the PLA General Hospital, ChinaReviewed by:
Zhi-De Hu, Inner Mongolia Medical University, ChinaCopyright © 2023 Li, Hu, Liu, Ren, Liu, Liu, Yin, Tan and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guangwen Cao, Z2Nhb0BzbW11LmVkdS5jbg==
† These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.