
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 05 June 2023
Sec. Breast Cancer
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1196592
Background: The rate of breast-conserving surgery is very low in China, compared with that in developed countries; most breast cancer patients receive mastectomy. It is great important to explore the possibility of omitting axillary lymph node dissection (ALND) in early-stage breast cancer patients with 1 or 2 positive sentinel lymph nodes (SLNs) in China. The aim of this study was to develop a nomogram based on elastography for the prediction of the risk of non-SLN (NSLN) metastasis in early-stage breast cancer patients with 1 or 2 positive SLNs.
Methods: A total of 601 breast cancer patients were initially recruited. According to the inclusion and exclusion criteria, 118 early-stage breast cancer patients with 1 or 2 positive SLNs were finally enrolled and were assigned to the training cohort (n=82) and the validation cohort (n=36), respectively. In the training cohort, the independent predictors were screened by logistic regression analysis and then were used to conducted the nomogram for the prediction of NSLN metastasis in early-stage breast cancer patients with 1 or 2 positive SLNs. The calibration curves, concordance index (C-index), the area under the receiver operating characteristic (ROC) curve (AUC), and Decision curve analysis (DCA) were used to verified the performance of the nomogram.
Results: The multivariable analysis showed that the enrolled patients with positive HER2 expression (OR=6.179, P=0.013), Ki67≥14% (OR=8.976, P=0.015), larger lesion size (OR=1.038, P=0.045), and higher Emean (OR=2.237, P=0.006) were observed to be the independent factors of NSLN metastasis. Based on the above four independent predictors, a nomogram was conducted to predict the risk of the NSLN metastasis in early-stage breast cancer patients with 1 or 2 positive SLNs. The nomogram showed good discrimination in the prediction of NSLN metastasis, with bias-corrected C-index of 0.855 (95% CI, 0.754-0.956) and 0.853 (95% CI, 0.724-0.983) in the training and validation cohorts, respectively. Furthermore, the AUC was 0.877 (95%CI: 0.776- 0.978) and 0.861 (95%CI: 0.732-0.991), respectively, indicating a good performance of the nomogram. The calibration curve suggested a satisfactory agreement between the predictive and actual risk in both the training (χ2 = 11.484, P=0.176, HL test) and validation (χ2 = 6.247, p = 0.620, HL test) cohorts, and the obvious clinical nets were revealed by DCA.
Conclusions: We conducted a satisfactory nomogram model to evaluate the risk of NSLN metastasis in early-stage breast cancer patients with 1 or 2 SLN metastases. This model could be considered as an ancillary tool to help such patients to be selectively exempted from ALND.
Breast cancer, the primary factor of tumor-related morbidity in women worldwide, has grown rapidly in recent years, presenting a great threat to human health (1). According to the published data, breast cancer has become the leading cancer after surpassing lung cancer in the number of new cases per year, and is also a significant cause of tumor-related deaths (2). It is well known that axillary lymph node (ALN) status is a crucial factor in evaluating the staging, prognosis, and treatment of breast cancer. Sentinel lymph node biopsy (SLNB) can be used for accurately assessing the axillary lymph node status of patients with early-stage breast cancer and has been widely used in clinical practice (3). Patients with positive sentinel lymph node (SLN) unavoidably receive axillary lymph node dissection (ALND), despite postoperative complications and poorer quality of life (4). Unfortunately, several studies have reported that more than half of breast cancer patients with positive SLN who receive ALND do not have non-SLN (NSLN) metastasis, implying that ALND is unnecessary for all these patients (5–7), which have led to a debate on the need for routine ALND in early-stage breast cancer patients with 1 or 2 positive SLNs. The National Comprehensive Cancer Network guidelines advise that early-stage breast cancer patients with 1 or 2 positive SLNs who receive breast-conserving surgery and radiotherapy are exempt from ALND. Meanwhile, the nomograms were developed by Kimberly Van Zee (8), Tenon (9), and Helsinki University (10) for the prediction of the probability of NSLN metastasis in patients with early-stage breast cancer who had SLN metastasis, which are all conducted in developed countries. However, few nomogram models have been conducted in developing countries represented by China. The rate of breast-conserving surgery is very low in China, compared with that in developed countries; most breast cancer patients receive mastectomy (11). It is great important to explore the possibility of omitting ALND in early-stage breast cancer patients with 1 or 2 positive SLNs in China.
The stiffness of malignant lesion is greater compared with the benign lesion, because tumor stiffness is associated with the degree of tumor malignancy (12). Ultrasound elastography has been reported to be able to quantitatively and qualitatively measure tumor stiffness, which is important for distinguishing benign or malignant lesion (13, 14). Recent studies showed the use of ultrasound elastography in the examination of many organs, such as lymph nodes, liver, and thyroid (15, 16). Shear wave elastography (SWE), a novel elastography technique, provides quantitative information on measuring tissue stiffness (17, 18). Recently, SWE was reported to be associated with the axillary lymph node metastasis in breast cancer (19). However, no nomogram based on SWE was developed for the prediction of the axillary lymph node metastasis in breast cancer.
This study aimed to develop a nomogram based on elastography for the prediction of the risk of NSLN metastasis in early-stage breast cancer patients with 1 or 2 positive SLNs.
Clinical data of early-stage breast cancer patients diagnosed in the Hulunbuir People’s Hospital and Wuxi Huishan District People’s Hospital from October 2019 to November 2022 were retrospectively collected. Inclusion criteria: (1) 18 years of age or older; (2) 1 or 2 SLN metastases; (3) successful SLNB and ALND; (4) first-time diagnosis of breast cancer; (5) receiving mastectomy. Exclusion criteria: (1) Treated with neoadjuvant chemotherapy; (2) Presence of other tumors; (3) Stage IV breast cancer patients; (4) Missing clinical data. A total of 118 early-stage breast cancer patients with 1 or 2 positive SLNs were eventually enrolled in this study. In addition, those patients enrolled from Hulunbuir People’s Hospital were assigned to the training cohort (n=82), and those enrolled from Wuxi Huishan District People’s Hospital were assigned to the validation cohort (n=36).
The Ethics Committee of the Hulunbuir People’s Hospital (No. 2021SYY-021) and Wuxi Huishan District People’s Hospital (No. HYLL20220510001) approved this study. Informed consents were provided by participants.
The clinical data of enrolled patients were collected, including ultrasound characteristics (Tumor size, Tumor shape, Tumor margin, Inner echo, Multifocality, Calcification, color doppler flow imaging [CDFI], and mean stiffness [Emean]) and clinicopathologic characteristics (age, body mass index [BMI], location of primary tumor, menstrual status, estrogen receptor, progesterone receptor, carbohydrate antigen [CA] 153, carcinoembryonic antigen [CEA], CA125, the number of negative SLNs, HER2 status, histological grade, and the Ki67 index).
In the training cohort, the independent predictors were screened by logistic regression analysis and then were used to conducted the nomogram for the prediction of NSLN metastasis in early-stage breast cancer patients with 1 or 2 positive SLNs.
The calibration curves and concordance index (C-index) were used to internally and externally assess the calibration and discrimination of the nomogram, respectively. Furthermore, the area under the receiver operating characteristic (ROC) curve (AUC) was calculated to explore the performance of the nomogram, and Hosmer-Lemeshow (HL) test was applied to the assessment of the calibration of model and an insignificant test statistic represents a perfect calibration. The clinical usefulness of the model was determined by decision curve analysis (DCA).
Mann-Whitney U test and chi-square test were used to compare the continuous data that were skewed distributed (age, BMI, tumor size, and Emean) and categorical data (location of primary tumor, menstrual status, estrogen receptor, progesterone receptor, CA153, CEA, CA125, the number of negative SLNs, HER2 status, histological grade, Ki67 index, tumor shape, tumor margin, inner echo, multifocality, CDFI), respectively. Following the univariate logistic regression, multivariable analysis was performed to find the independent predictors, which was the basis of the nomogram. The statistical analyses were conducted with Medcalc software (version 18.0, Ostend, Belgium) and R package version 3.7.
A total of 601 breast cancer patients were initially recruited. According to the inclusion and exclusion criteria, 118 early-stage breast cancer patients with 1 or 2 positive SLNs were finally enrolled and were assigned to the training cohort (n=82) and the validation cohort (n=36), respectively. No significant differences were observed in baseline characteristics, including clinicopathologic and ultrasound characteristics, between the training and validation cohorts (all P>0.05) (Table 1).
The univariate analysis based on clinicopathologic and ultrasound characteristics were performed to explore the potential predictors of NSLN metastasis in the training cohort. As shown in Table 2, only HER2 status (P=0.001), Ki67 index (P=0.023), tumor size (P=0.002), and Emean (P<0.001) were detected to be significantly associated with NSLN metastasis, which was confirmed in Supplementary Table 1. Furthermore, multivariable analysis showed that the enrolled patients with positive HER2 expression (OR=6.179, P=0.013), Ki67≥14% (OR=8.976, P=0.015), larger lesion size (OR=1.038, P=0.045), and higher Emean (OR=2.237, P=0.006) were observed to be the independent factors of NSLN metastasis (Table 2). To further observe the performance of the above independent factors for NSLN metastasis, we applied ROC curve. As shown in Figures 1A, B, the AUC of HER2 status, Ki67 index, tumor size, and Emean in the training cohort were 0.681, 0.636, 0.778, and 0.770, respectively, and the AUC in the validation cohort were 0.688, 0.604, 0.753, and 0.740, respectively, which further verified the performance of these four independent factors for NSLN metastasis.
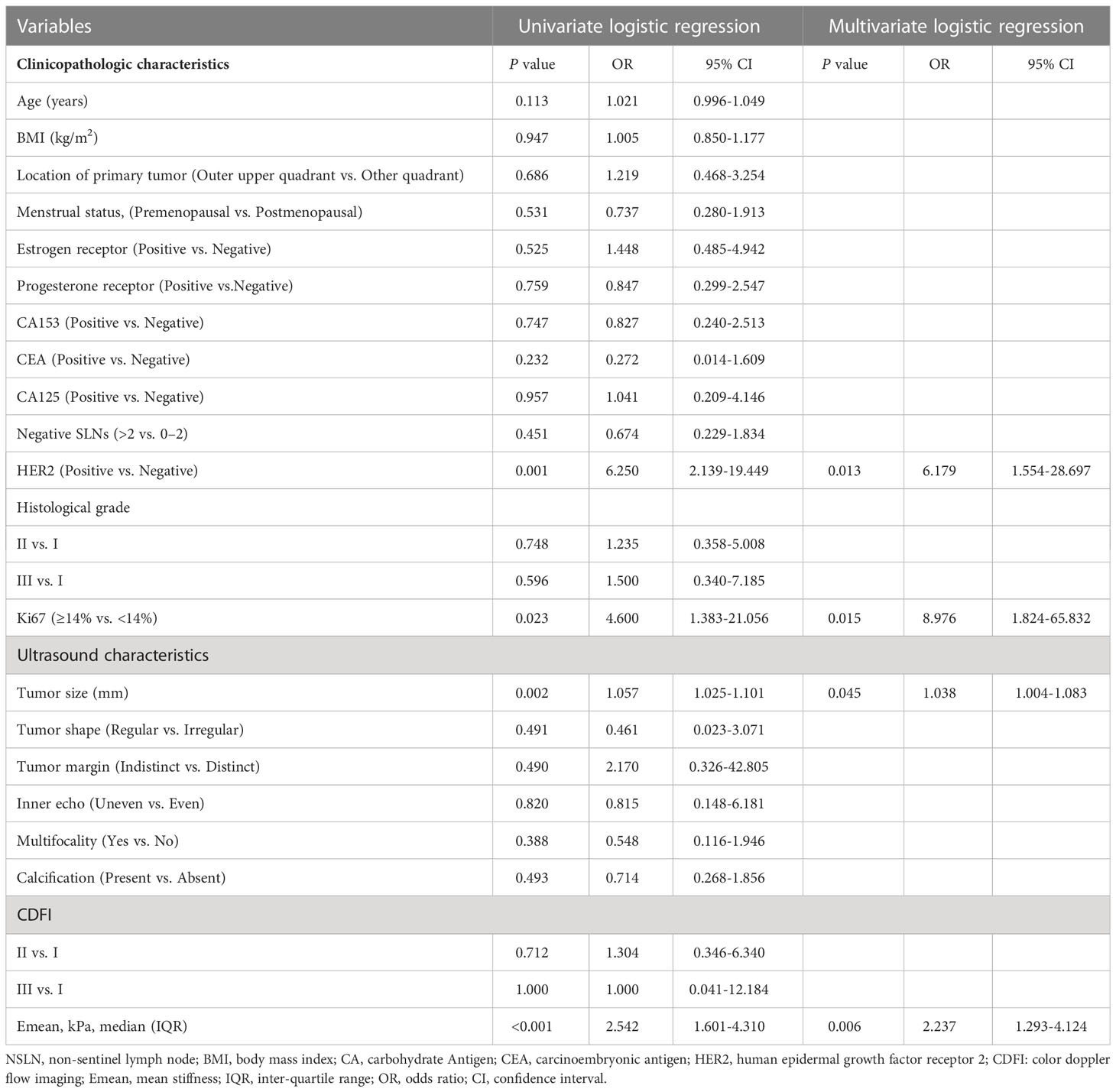
Table 2 Univariate and multivariate logistic analyses to determine the independent predictors associated with NSLN metastasis in the training cohort.
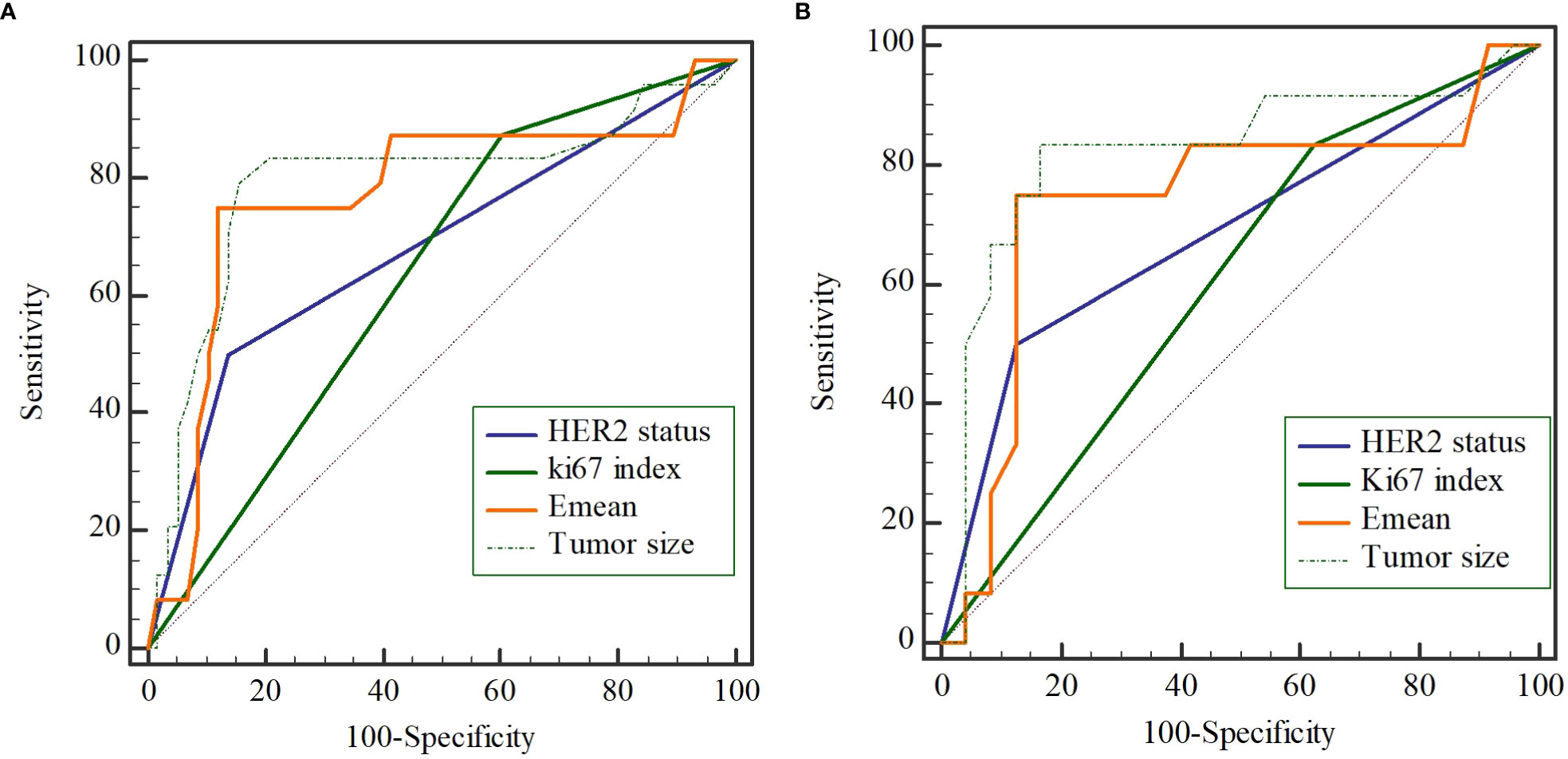
Figure 1 The performance of 4 independent predictors on non-sentinel lymph node (NSLN) metastasis verified by the receiver operating characteristic (ROC) curve in the training cohort (A) and the validation cohort (B).
Based on the above four independent predictors, a nomogram was conducted to predict the risk of the NSLN metastasis in early-stage breast cancer patients with 1 or 2 positive SLNs. As shown in Figure 2, Emean had the greatest impact on the NSLN metastasis, with a maximum score of 100. The nomogram showed good discrimination in the prediction of NSLN metastasis, with bias-corrected C-index of 0.855 (95% CI, 0.754-0.956) and 0.853 (95% CI, 0.724-0.983) in the training and validation cohorts, respectively. Furthermore, as shown in Figures 3A, D, the AUC was 0.877 (95%CI: 0.776- 0.978) and 0.861 (95%CI: 0.732-0.991), respectively, indicating a good performance of the nomogram. The calibration curve suggested a satisfactory agreement between the predictive and actual risk in both the training (χ2 = 11.484, P=0.176, HL test) and validation (χ2 = 6.247, p = 0.620, HL test) cohorts (Figure 3B, E), and the obvious clinical nets were revealed by DCA (Figure 3C, F).
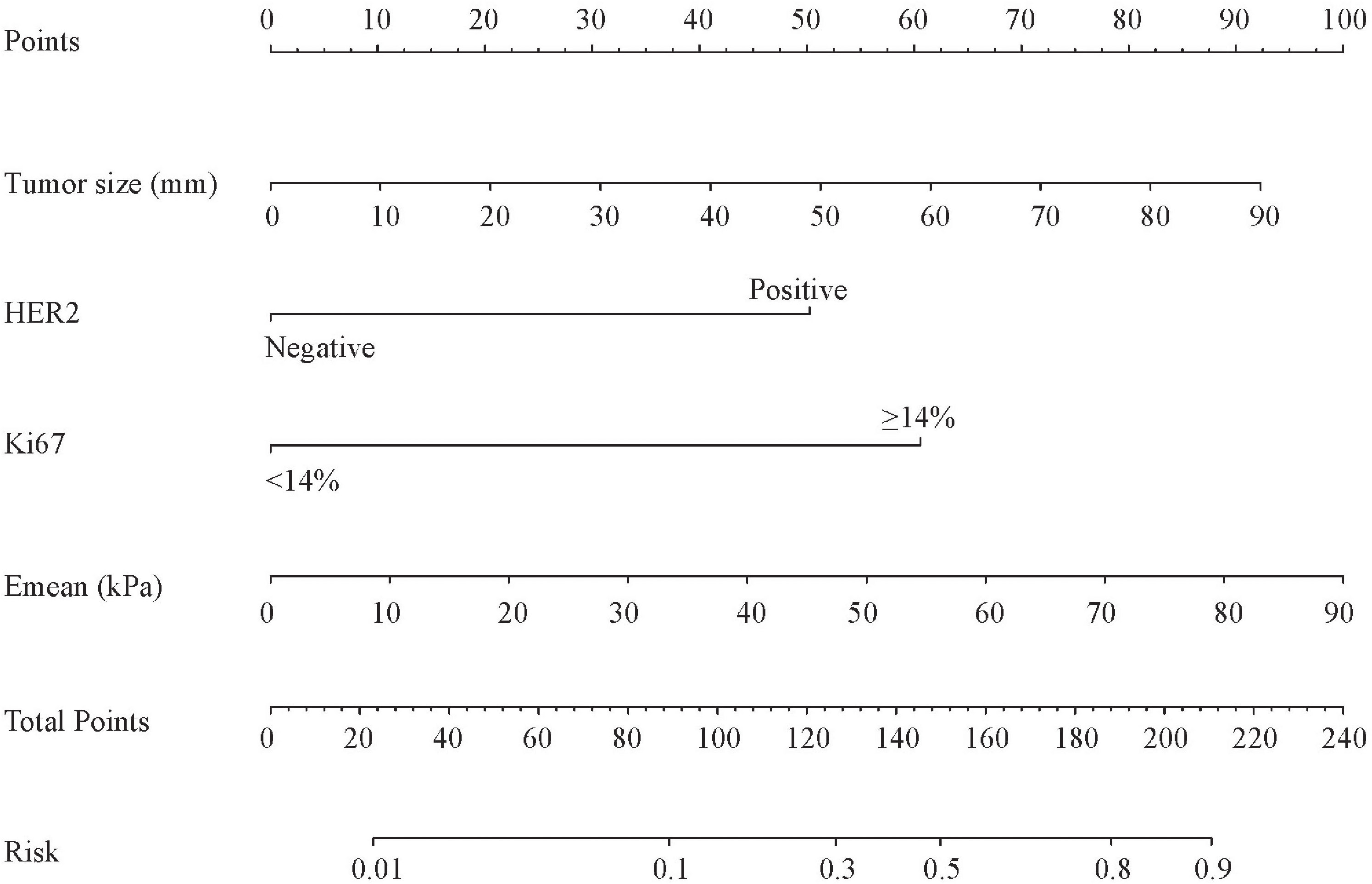
Figure 2 The nomogram predicting the risk of non-sentinel lymph node metastasis in breast cancer patients with 1 or 2 sentinel lymph node metastases. HER2, human epidermal growth factor receptor 2; Emean, mean stiffness.
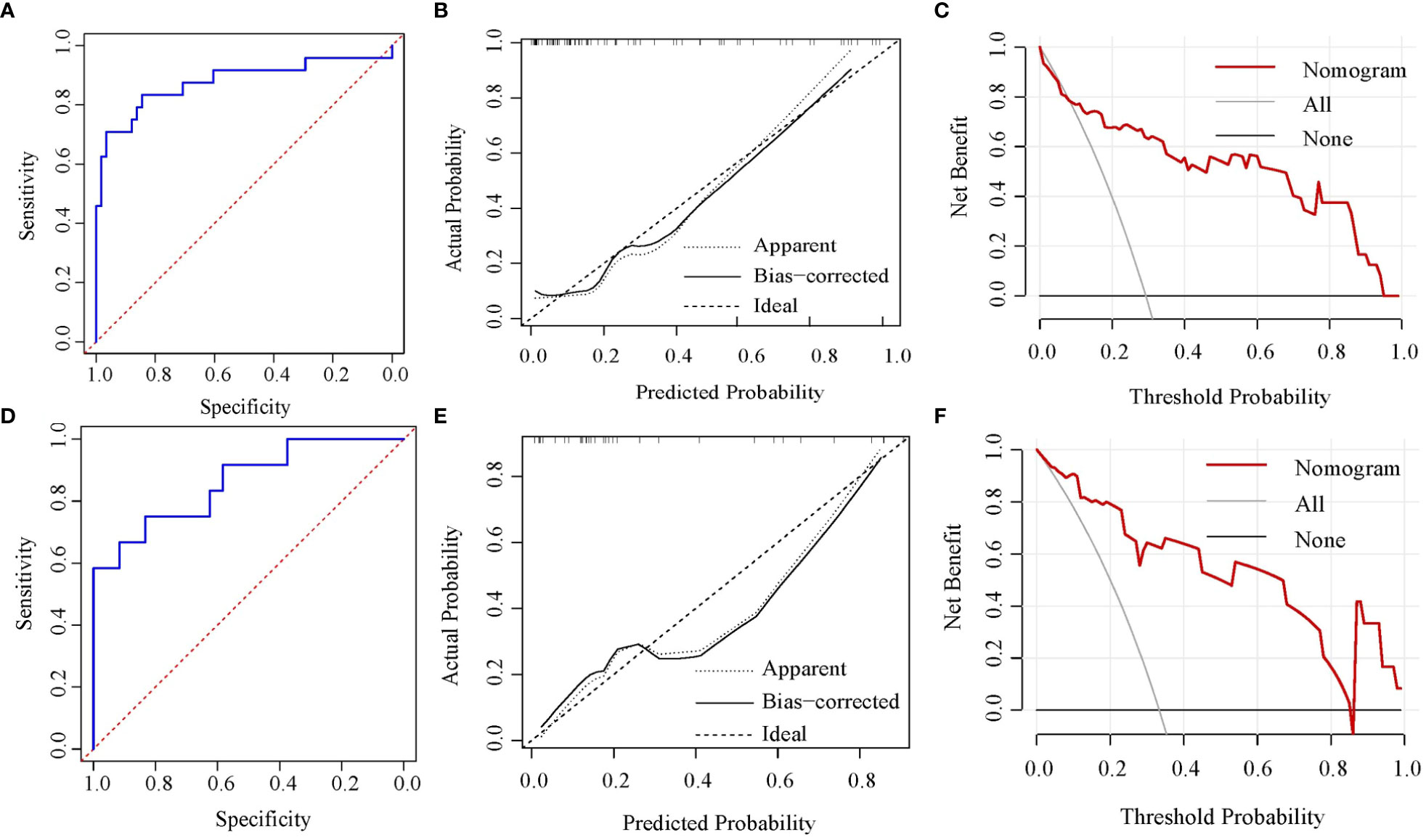
Figure 3 Nomogram verification [(A-C) for training cohort and (D-F) for validation cohort]. (A, D) ROC curves; (B, E) calibration plots; (C, F) DCA plots. ROC, receiver operating characteristic; DCA, decision curve analysis.
The nomogram can be used to calculate the scores of each independent predictor, and the predicted probability that corresponds to the total points calculated by the sum of the scores of each independent predictor was the risk of NSLN metastasis in early-stage breast cancer patients with 1 or 2 positive SLNs. For example, when a patient with 50mm in lesion size, positive HER-2 expression, Ki67<14%, and 60kPa in Emean, the total scores were about 169. It showed that the risk of a patient with NSLN metastasis was about 60%.
The routine ALND therapy for breast cancer patients with 1 or 2 SLN metastases is overtreatment because there is no further NSLN metastasis in approximately 60% of such patients, while positive lymph nodes other than SLN, if not removed, can cause residual tumor, leading to axillary recurrence (5–7). Thus, the development of a model that accurately predicts NSLN metastasis could improve the management of patients with positive SLNs. Medical nomograms graphically present statistical prediction models using biological and clinical variables to give the risk of a clinical event, such as death or lymph node metastasis, for a given individual (20). Preoperative nomograms can be used to assess the probability of lymph node metastases, helping clinicians find patients who may benefit more from more extensive surgery (21, 22). Our retrospective study aimed to develop a nomogram based on elastography for the prediction of the risk of NSLN metastasis in early-stage breast cancer patients with 1 or 2 positive SLNs. A prediction model was conducted after we discovered the independent risk factors of NSLN metastasis. This satisfactory model was then verified using data from the training and validation cohorts, respectively.
As the tumor grows, it tends to become stiffer due to an increase in the fibrous component and a decrease in the necrotic component, which is well understood (23). SWE is a promising elastic imaging technology, allowing quantitative measurement of tissue stiffness (24). The lymph node metastasis of breast cancer has been reported to be associated with elastic modulus of breast masses (25). Moreover, the increased tumor stiffness is thought to be an easier infiltration of tumor cells into the tissue interstices, which is an independent predictor for the prediction of tumor recurrence (26–28). In this study, we found that the higher Emean was associated with a higher risk of NSLN metastasis, which may be interpreted by the histopathologic composition of the tumor. In addition, we visualized the effect of Emean on NSLN metastasis through the nomogram model.
The present study showed that tumor size and HER2 status were the independent risk factors of NSLN metastasis and that larger tumor size and positive HER2 expression leaded to a higher risk of NSLN metastasis. Other studies have also reported that these two factors are associated with NSLN metastasis (10, 29). In addition, we also found that Ki67 was the independent risk factor of NSLN metastasis, which was consistent with the conclusion that the high expression of Ki67 significantly improves the probability of lymph node metastasis (30).
The main strength of this study is the development of the nomogram with good performance for the prediction of the risk of NSLN metastasis in early-stage breast cancer patients with 1 or 2 positive SLNs in China. The risk of NSLN metastasis could be calculated repeatedly depending on the variable clinical status, and the increased risk of NSLN metastasis is dynamically evaluated, which would be very helpful for clinicians to adopt individualized treatment strategies. The C-index of the nomogram conducted by Liu et al. was 0.740 and 0.689 in the training and validation cohorts, respectively (31). Compared to previous results, our nomogram including elastography with greater C-index showed a better performance.
Two limitations exist in this study. First, due to the retrospective design of this study, clinical data for some patients were partially missing. Second, the patients enrolled in this study were from only 2 hospitals, which leaded to the nomogram conducted in this study was not confirmed in other populations. The research with a larger sample size will be performed to verify this nomogram model.
We conducted a satisfactory nomogram model to evaluate the risk of NSLN metastasis in early-stage breast cancer patients with 1 or 2 SLN metastases. This model could be considered as an ancillary tool to help such patients to be selectively exempted from ALND.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by Hulunbuir People’s Hospital (No. 2021SYY-021) and Wuxi Huishan District People’s Hospital (No. HYLL20220510001). The patients/participants provided their written informed consent to participate in this study.
Study design: HD, XZ, WW. Data collection and analysis: HD, JZ, GZ, XZ, WW. Supervision: HD, XZ, WW. Statistics: HD, JZ, GZ. Manuscript writing: HD. Manuscript revision: XZ, WW. Approval of the manuscript: all authors.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1196592/full#supplementary-material
Supplementary Table 1 | Baseline characteristics of patients between the term delivery and preterm delivery group in the training cohort.
1. Kashyap D, Pal D. Global increase in breast cancer incidence: risk factors and preventive measures. Biomed Res Int (2022) 2022:9605439. doi: 10.1155/2022/9605439
2. Sung H, Ferlay J, Siegel RL. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
3. Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Costantino JP, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP b-32 randomised phase 3 trial. Lancet Oncol (2010) 11(10):927–33. doi: 10.1016/s1470-2045(10)70207-2
4. Fleissig A, Fallowfield LJ, Langridge CI, Johnson L, Newcombe RG, Dixon JM, et al. Post-operative arm morbidity and quality of life. results of the ALMANAC randomised trial comparing sentinel node biopsy with standard axillary treatment in the management of patients with early breast cancer. Breast Cancer Res Treat (2006) 95(3):279–93. doi: 10.1007/s10549-005-9025-7
5. Dingemans SA, de Rooij PD, van der Vuurst de Vries RM, Budel LM, Contant CM, van der Pool AE. Validation of six nomograms for predicting non-sentinel lymph node metastases in a Dutch breast cancer population. Ann Surg Oncol (2016) 23(2):477–81. doi: 10.1245/s10434-015-4858-8
6. Meretoja T, Leidenius M. Lymphovascular invasion. J Natl Cancer Inst (2013) 105(19):1514. doi: 10.1093/jnci/djt231
7. Mittendorf EA, Hunt KK, Boughey JC, Bassett R, Degnim AC, Harrell R, et al. Incorporation of sentinel lymph node metastasis size into a nomogram predicting nonsentinel lymph node involvement in breast cancer patients with a positive sentinel lymph node. Ann Surg (2012) 255(1):109–15. doi: 10.1097/SLA.0b013e318238f461
8. Van Zee KJ, Manasseh DM, Bevilacqua JL, Boolbol SK, Fey JV, Tan LK, et al. A nomogram for predicting the likelihood of additional nodal metastases in breast cancer patients with a positive sentinel node biopsy. Ann Surg Oncol (2003) 10(10):1140–51. doi: 10.1245/aso.2003.03.015
9. Barranger E, Coutant C, Flahault A, Delpech Y, Darai E, Uzan S. An axilla scoring system to predict non-sentinel lymph node status in breast cancer patients with sentinel lymph node involvement. Breast Cancer Res Treat (2005) 91(2):113–9. doi: 10.1007/s10549-004-5781-z
10. Meretoja TJ, Leidenius MH, Heikkilä PS, Boross G, Sejben I, Regitnig P, et al. International multicenter tool to predict the risk of nonsentinel node metastases in breast cancer. J Natl Cancer Inst (2012) 104(24):1888–96. doi: 10.1093/jnci/djs455
11. Chiu AS, Thomas P, Killelea BK, Horowitz N, Chagpar AB, Lannin DR. Regional variation in breast cancer surgery: results from the national cancer database (NCDB). Am J Surg (2017) 214(5):907–13. doi: 10.1016/j.amjsurg.2017.07.008
12. Evans A, Whelehan P, Thomson K, Brauer K, Jordan L, Purdie C, et al. Differentiating benign from malignant solid breast masses: value of shear wave elastography according to lesion stiffness combined with greyscale ultrasound according to BI-RADS classification. Br J Cancer (2012) 107(2):224–9. doi: 10.1038/bjc.2012.253
13. Kim HJ, Kim SM, Kim B, La Yun B, Jang M, Ko Y, et al. Comparison of strain and shear wave elastography for qualitative and quantitative assessment of breast masses in the same population. Sci Rep (2018) 8(1):6197. doi: 10.1038/s41598-018-24377-0
14. Yıldız MS, Goya C, Adin ME. Contribution of sonoelastography to diagnosis in distinguishing benign and malignant breast masses. J Ultrasound Med (2020) 39(7):1395–403. doi: 10.1002/jum.15236
15. Liu Y, Xiong W, Xu JM, Liu YX, Zhang J. Correlations between the expression of c-erB-2, CD34 and ER in breast cancer patients and the signs of conventional ultrasonography and ultrasound elastography. Eur Rev Med Pharmacol Sci (2018) 22(17):5539–45. doi: 10.26355/eurrev_201809_15815
16. Bavu E, Gennisson JL, Couade M, Bercoff J, Mallet V, Fink M, et al. Noninvasive in vivo liver fibrosis evaluation using supersonic shear imaging: a clinical study on 113 hepatitis c virus patients. Ultrasound Med Biol (2011) 37(9):1361–73. doi: 10.1016/j.ultrasmedbio.2011.05.016
17. Hu X, Liu Y, Qian L. Diagnostic potential of real-time elastography (RTE) and shear wave elastography (SWE) to differentiate benign and malignant thyroid nodules: a systematic review and meta-analysis. Med (Baltimore) (2017) 96(43):e8282. doi: 10.1097/MD.0000000000008282
18. Zhang H, Dong Y, Jia X, Zhang J, Li Z, Chuan Z, et al. Comprehensive risk system based on shear wave elastography and BI-RADS categories in assessing axillary lymph node metastasis of invasive breast cancer-a multicenter study. Front Oncol (2022) 12. doi: 10.3389/fonc.2022.830910
19. Cheng Y, Li G, Jing H, Yuan S, Zhang L, Cheng W. Effectiveness of quantitative shear wave elastography for the prediction of axillary lymph node metastasis. Evid Based Complement Alternat Med (2022) 2022. doi: 10.1155/2022/8769889
20. Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol (2015) 16(4):e173–180. doi: 10.1016/S1470-2045(14)71116-7
21. Kim DY, Shim SH, Kim SO, Lee SW, Park JY, Suh DS, et al. Preoperative nomogram for the identification of lymph node metastasis in early cervical cancer. Br J Cancer (2014) 110(1):34–41. doi: 10.1038/bjc.2013.718
22. Thompson AM, Turner RM, Hayen A, Aniss A, Jalaty S, Learoyd DL, et al. A preoperative nomogram for the prediction of ipsilateral central compartment lymph node metastases in papillary thyroid cancer. Thyroid (2014) 24(4):675–82. doi: 10.1089/thy.2013.0224
23. Chamming’s F, Latorre-Ossa H, Le Frère-Belda MA, Fitoussi V, Quibel T, Assayag F, et al. Shear wave elastography of tumour growth in a human breast cancer model with pathological correlation. Eur Radiol (2013) 23(8):2079–86. doi: 10.1007/s00330-013-2828-8
24. Li T, Li H, Xue J, Miao J, Kang C. Shear wave elastography combined with gray-scale ultrasound for predicting central lymph node metastasis of papillary thyroid carcinoma. Surg Oncol (2021) 36:1–6. doi: 10.1016/j.suronc.2020.11.004
25. Evans A, Rauchhaus P, Whelehan P, Thomson K, Purdie CA, Jordan LB, et al. Does shear wave ultrasound independently predict axillary lymph node metastasis in women with invasive breast cancer? Breast Cancer Res Treat (2014) 143(1):153–7. doi: 10.1007/s10549-013-2747-z
26. Zhou J, Zhan W, Chang C, Zhang X, Jia Y, Dong Y, et al. Breast lesions: evaluation with shear wave elastography, with special emphasis on the “stiff rim” sign. Radiology (2014) 272(1):63–72. doi: 10.1148/radiol.14130818
27. Colleoni M, Rotmensz N, Maisonneuve P, Sonzogni A, Pruneri G, Casadio C, et al. Prognostic role of the extent of peritumoral vascular invasion in operable breast cancer. Ann Oncol (2007) 18(10):1632–40. doi: 10.1093/annonc/mdm268
28. de Mascarel I, Bonichon F, Durand M, Mauriac L, MacGrogan G, Soubeyran I, et al. Obvious peritumoral emboli: an elusive prognostic factor reappraised. multivariate analysis of 1320 node-negative breast cancers. Eur J Cancer (1998) 34(1):58–65. doi: 10.1016/s0959-8049(97)00344-4
29. Houvenaeghel G, Bannier M, Nos C, Giard S, Mignotte H, Jacquemier J, et al. Non sentinel node involvement prediction for sentinel node micrometastases in breast cancer: nomogram validation and comparison with other models. Breast (2012) 21(2):204–9. doi: 10.1016/j.breast.2011.09.013
30. Wei D, Xin Y, Rong Y, Hao Y. Correlation between the expression of VEGF and Ki67 and lymph node metastasis in non-small-Cell lung cancer: a systematic review and meta-analysis. Evid Based Complement Alternat Med (2022) 2022:9693746. doi: 10.1155/2022/9693746
Keywords: nomogram, elastography, non-sentinel lymph node metastasis, breast cancer, axillary lymph node dissection
Citation: Duan H, Zhang J, Zhang G, Zhu X and Wang W (2023) An improved nomogram including elastography for the prediction of non-sentinel lymph node metastasis in breast cancer patients with 1 or 2 sentinel lymph node metastases. Front. Oncol. 13:1196592. doi: 10.3389/fonc.2023.1196592
Received: 30 March 2023; Accepted: 23 May 2023;
Published: 05 June 2023.
Edited by:
Nosheen Masood, Fatima Jinnah Women University, PakistanCopyright © 2023 Duan, Zhang, Zhang, Zhu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xingmeng Zhu, emh1LnhpbmdtZW5nQDE2My5jb20=; Wenjia Wang, MzA4MDU4MDU3QHFxLmNvbQ==
†ORCID: Xingmeng Zhu, orcid.org/0009-0005-7460-5082
Wenjia Wang, orcid.org/0009-0004-3339-3557
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.