- 1Evidence Generation Platform, Pfizer Inc., New York, NY, United States
- 2Statistical Research and Data Science Center, Global Biometrics and Data Management, Pfizer Inc., New York, NY, United States
Background: There is heterogeneity in the literature regarding the strength of association between Eastern Cooperative Oncology Group performance status (ECOG PS) and mortality. We conducted a systematic review and meta-analysis of studies reporting the prognostic value of ECOG PS on overall survival (OS) in metastatic prostate cancer (mPC).
Methods: PubMed was searched from inception to March 21, 2022. A meta-analysis pooling the effect of ECOG PS categories (≥2 vs. <2, 2 vs. <2, and ≥1 vs. <1) on OS was performed separately for studies including patients with metastatic castration-resistant prostate cancer (mCRPC) and metastatic castration-sensitive prostate cancer (mCSPC) using a random-effects model. Analyses were stratified by prior chemotherapy and study type.
Results: Overall, 75 studies, comprising 32,298 patients, were included. Most studies (72/75) included patients with mCRPC. Higher ECOG PS was associated with a significant increase in mortality risk, with the highest estimate observed among patients with mCRPC with an ECOG PS of ≥2 versus <2 (hazard ratio [HR]: 2.10, 95% confidence interval [CI]: 1.87–2.37). When stratifying by study type, there was a higher risk estimate of mortality among patients with mCRPC with an ECOG PS of ≥1 versus <1 in real-world data studies (HR: 1.98, 95% CI: 1.72–2.26) compared with clinical trials (HR: 1.32, 95% CI: 1.13–1.54; p < 0.001). There were no significant differences in the HR of OS stratified by previous chemotherapy.
Conclusion: ECOG PS was a significant predictor of OS regardless of category, previous chemotherapy, and mPC population. Additional studies are needed to better characterize the effect of ECOG PS on OS in mCSPC.
1 Introduction
While the direction of the association between the Eastern Cooperative Oncology Group performance status (ECOG PS) and overall survival (OS) in oncology patients is known, there is heterogeneity in the literature regarding the magnitude of that association (1–6). Knowing the strength of that association in a specific population is an important parameter for understanding the impact of bias (residual confounding) in real-world data (RWD) studies. There are numerous studies that have reported on the association between ECOG PS and OS in prostate cancer; however, there is heterogeneity in defining ECOG PS categories, as well as heterogeneity in study populations (1, 2, 7–9).
Chen et al. recently assessed the prognostic value of ECOG PS on OS in castration-resistant prostate cancer (CRPC) using a systematic literature review and meta-analysis approach (7). In their analysis of 20 studies, patients with ECOG PS ≥2 had a significantly increased mortality risk (hazard ratio [HR]: 2.10, 95% confidence interval [CI]: 1.68–2.62) compared with those with a lower ECOG PS (7). However, Chen et al. included studies in both the non-metastatic and metastatic settings, and did not differentiate between CRPC and castration-sensitive prostate cancer (CSPC) due to limited studies in CSPC (7). Furthermore, the last search described in Chen et al. was performed in May 2019 (7), and since then, the literature and guidelines have evolved, including more studies in the metastatic CSPC (mCSPC) setting (9–12).
To date, a systematic review approach of the prognostic value of ECOG PS on OS has not been studied in the context of metastatic prostate cancer (mPC) alone. Thus, more recent studies, and newly indicated treatments may yield different findings from the Chen et al. study (7), particularly with a less heterogeneous prostate cancer population. Therefore, we performed a systematic review of the literature to summarize the evidence on the association between ECOG PS and OS both in patients with mCRPC and patients with mCSPC.
2 Materials and methods
This systematic review and meta-analysis was conducted in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (13).
2.1 Literature search
A systematic literature search of published articles using the PubMed databases from inception to March 21, 2022 was performed on available Food and Drug Administration approved treatments for mPC, including the following treatments: “docetaxel,” “cabazitaxel,” “apalutamide,” “abiraterone,” “enzalutamide,” “darolutamide,” “sipuleucel-T,” “radium-223,” “olaparib,” “rucaparib camsylate,” and “mitoxantrone hydrochloride”. Each agent was searched separately using the following combined search terms: name of drug (Title/Abstract), with prostate cancer (Title/Abstract) and “metastatic” or “advanced”. In addition, review articles were screened for relevant references that may not have been captured in the search.
2.2 Study selection and data extraction
Studies that reported the multivariate HR, and the corresponding 95% CI or p-value of OS according to ECOG PS were included. Both clinical trials and observational studies were considered for inclusion. Reviews, case reports, editorials, preclinical studies, studies on combination therapies, non-English language articles, studies without HRs of OS according to ECOG PS, and studies reporting locally advanced prostate cancer were excluded.
For each included study, the following information was extracted: 1. study characteristics: title, authors, and publication year; 2. trial characteristics: study design, geographic location, sample size, and intended treatment; 3. patient population characteristics: patient type, demographics, background therapy, ECOG PS categorization strategy (≥2 vs. <2, 2 vs. <2, and ≥1 vs. <1), and metastatic population; and 4. HR, 95% CI, and p-values associated with OS according to the stratified criteria listed above.
2.3 Quality assessment
Publication bias in the included studies was assessed using Egger’s test as well as a contour-enhanced funnel plot using R software (version 4.0.3) (14–17). The trim and fill method was performed as a sensitivity analysis to detect and adjust for publication bias (18), assessing the robustness of conclusions to publication bias.
2.4 Statistical analysis
HRs on the log scale from multivariate models reported in each study were collected. If the multivariate HR reported in a study was for a reverse comparison ECOG PS category (e.g., ≥2 vs. <2), then the HR point estimates were inverted, and the 95% CI was transformed accordingly. Meta-analysis was performed using R software (version 1.4.1717). HRs and their 95% CIs were pooled together using the generic inverse variance method under the fixed effect(s) meta-analysis from the “metafor” package in R1 (19, 20). I2 and Chi-square statistics were calculated to quantify and test between-study heterogeneity (21, 22). Typically, I2 ≥50% indicates substantial study heterogeneity, in which case the random-effects model was used for pooling of HRs (23, 24). Subgroup analyses were performed, stratified by ECOG PS (≥2 vs. <2, 2 vs. <2, or ≥1 vs. <1), and the following: study type, metastatic population, or prior chemotherapy history of patients.
3 Results
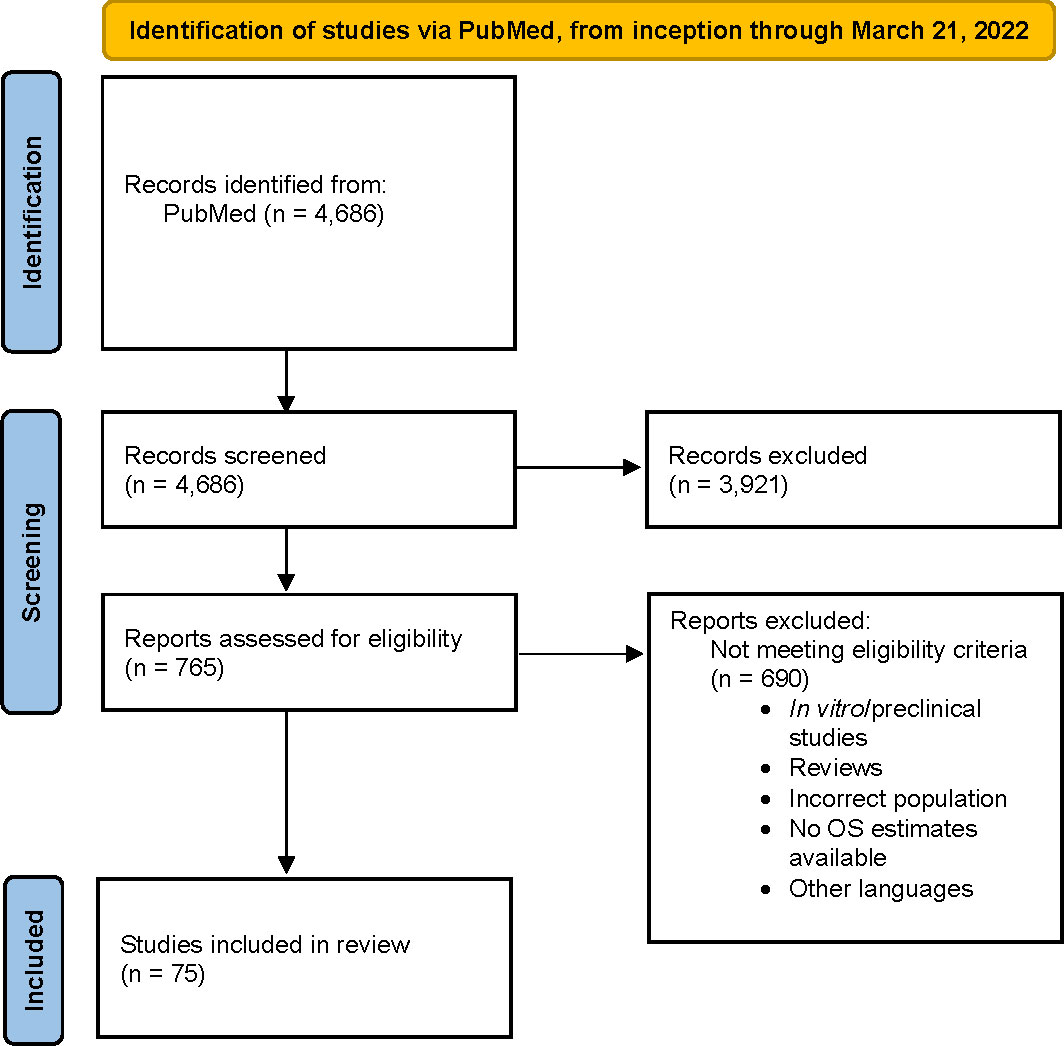
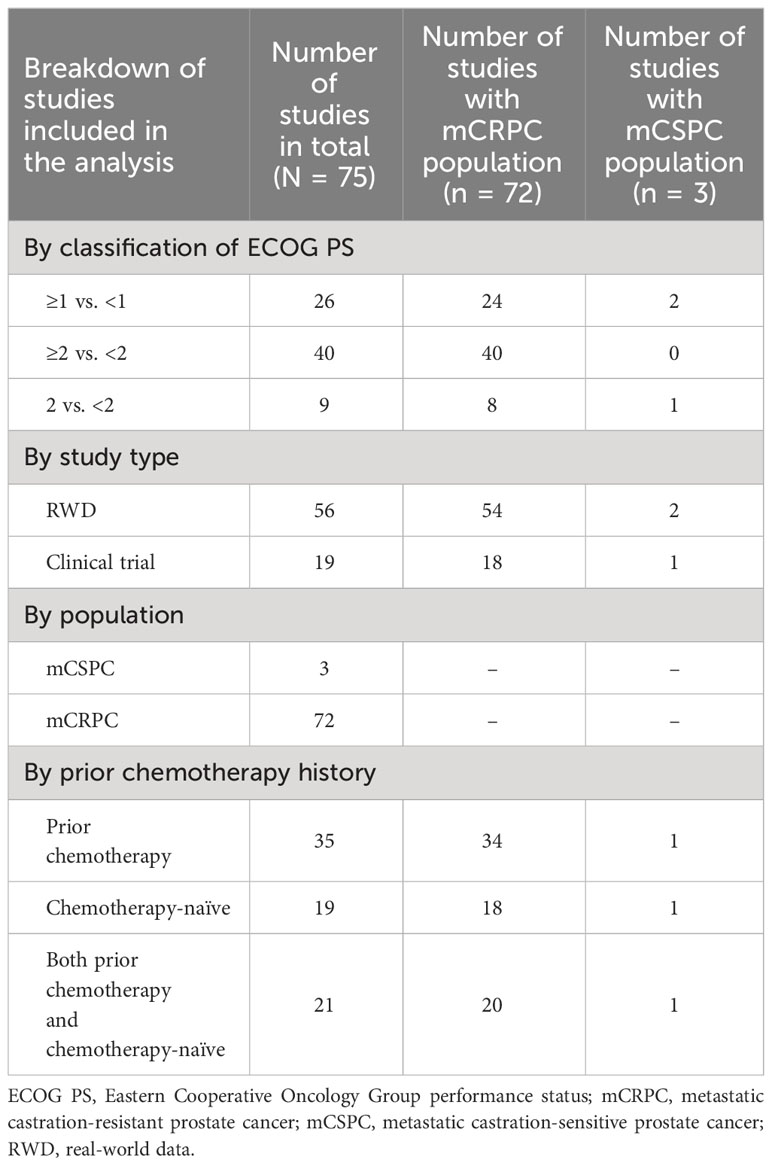
A total of 4,686 studies were identified. After applying the inclusion and exclusion criteria, 75 studies were selected for analysis (1–6, 8–12, 25–88). Most studies classified ECOG PS as ≥2 versus <2 (n = 40), were RWD studies (n = 56), in the mCRPC population (n = 72), and included patients with prior chemotherapy history (n = 35). A flow chart for final study selection is shown in Figure 1.
The total number of patients enrolled in the included studies was 32,298, with individual study populations ranging from 31 to 4,436 across the 75 included studies. The breakdown of study characteristics is shown in Table 1.
Meta-analysis results are presented in Figure 2. In addition, the HRs for OS are shown by ECOG PS classification (Supplementary Figures 1, 2), study type (Supplementary Figures 3, 4), and prior chemotherapy history (Supplementary Figure 5), using a random-effects model. In all subpopulations, higher ECOG PS was associated with a statistically significant increase in mortality risk when compared with lower ECOG PS. Across all studies, the highest mortality estimate was observed when comparing patients with mCRPC with ECOG PS ≥2 versus <2 (HR: 2.10, 95% CI: 1.87–2.37) (Figure 2). When comparing across all three ECOG PS categories, there was a significant difference between the pooled HRs of OS (p = 0.046). For ECOG PS ≥1 compared with <1, patients with mCRPC had an HR for OS of 1.68 (95% CI: 1.44–1.94). Among patients with mCSPC, the same comparison yielded a numerically higher risk estimate of OS (HR: 2.16, 95% CI: 1.43–3.25); however, the difference did not reach statistical significance (p = 0.247).
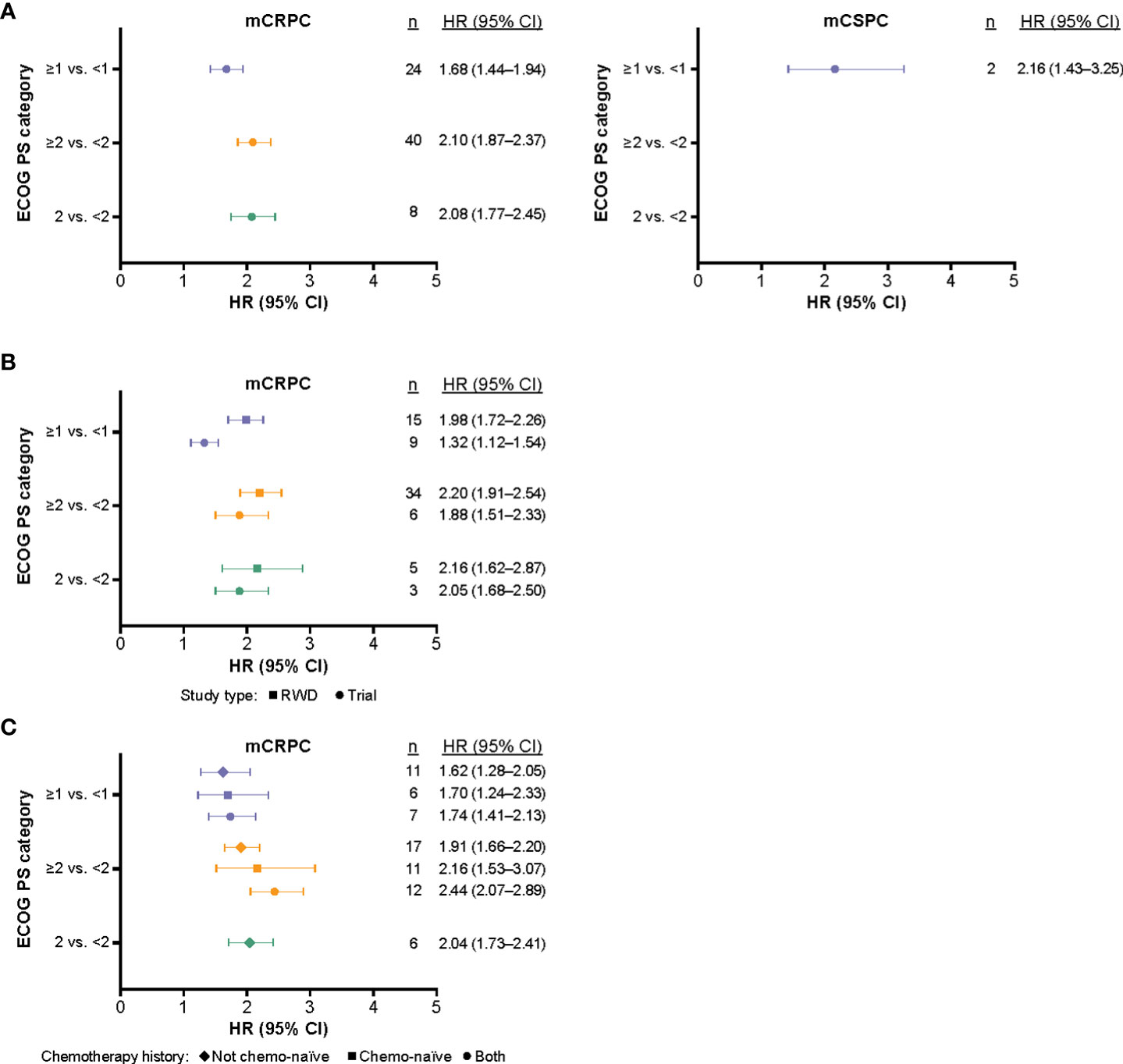
Figure 2 The synthesized HRs (95% CI) of OS according to ECOG PS (based on multivariate results) and mCRPC versus mCSPC* (A), and stratified by study type (B), and chemotherapy history (C). *Further subgroup analyses stratified by study type and prior chemotherapy status were not performed for mCSPC studies due to the small sample size. CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; mCRPC, metastatic castration-resistant prostate cancer; mCSPC, metastatic castration-sensitive prostate cancer; OS, overall survival; RWD, real-world data.
Subgroup analysis stratified by study type indicated that patients with mCRPC from RWD studies in the ≥1 versus <1 ECOG PS category had a statistically significant higher risk estimate of OS (HR: 1.98, 95% CI: 1.72–2.26) compared with patients included in clinical trials with the same ECOG PS category (HR: 1.32, 95% CI: 1.12–1.54; p < 0.001; Figure 2). Patients with mCSPC from RWD studies in the ≥1 versus <1 ECOG PS category, had a numerically higher risk estimate of OS (HR: 2.16, 95% CI: 1.43–3.25), compared with patients with mCRPC from RWD studies in the same ECOG PS category (HR: 1.88, 95% CI: 1.51–2.33); however, this difference did not reach statistical significance (p = 0.684).
Subgroup analysis stratified by chemotherapy history indicated that prior chemotherapy status did not have a significant impact on the risk estimate of OS for each ECOG PS category (ECOG PS ≥1 vs. <1, p = 0.905; ECOG PS ≥2 vs. <2, p = 0.091). Subgroup analysis stratified by prior chemotherapy status for mCSPC studies was not performed due to small sample size (n = 3 studies).
For studies that reported multivariate HRs of OS stratified by ECOG PS, both the Egger’s test and the contour-enhanced funnel plot indicated the presence of publication bias. The Egger’s test for patients with mCRPC in the ≥2 versus <2 ECOG PS category indicated presence of publication bias for the included studies (p = 0.037), and the funnel plot appeared to have studies missing in areas of statistical non-significance (Supplementary Figure 6).
The Egger’s test for patients with mCRPC in the ≥1 versus <1 ECOG PS category indicated presence of publication bias for the included studies (p = 0.031; Supplementary Figure 7). The Egger’s test for patients with mCSPC in the ≥2 versus <2 ECOG PS category was underpowered due to the number of studies being less than 10, leading to insufficient evidence to suggest publication bias.
4 Discussion
To our knowledge, this is the first systematic review and meta-analysis of the prognostic value of ECOG PS on OS in the context of mPC alone. Overall, higher ECOG PS scores were found to be associated with higher mortality risk, compared with lower ECOG PS scores, but the highest mortality estimate was observed among patients with mCRPC with an ECOG PS of ≥2 versus <2 (HR: 2.10, 95% CI: 1.87–2.37). Our pooled HR value is consistent with the finding from Chen et al. (7), who found that patients with CRPC with a higher ECOG PS (≥2) had a significantly increased mortality risk (HR: 2.10, 95% CI: 1.68–2.62) compared with those with a lower ECOG PS (<2). Furthermore, although limited to only two studies, we were able to analyze the estimates for patients with mCSPC, which were also found to be associated with OS. Lastly, we had consistent findings with Chen et al. (7) when stratifying by prior chemotherapy history, as there were no significant differences in the pooled HR of OS across all three ECOG PS categories analyzed.
Interestingly, the prognostic value of ECOG PS on OS was significantly higher for patients with mCRPC from RWD studies in the ≥1 versus <1 ECOG PS category compared with patients from clinical trials, indicating evidence of heterogeneity between study type (RWD vs. clinical trial). These data corroborate the recent suggestion that the strict inclusion and exclusion criteria used in clinical trials do not reflect the heterogeneity of higher risk populations, including older individuals and individuals with concomitant conditions or multimorbidity, observed in the real-world (89), and highlight that the prognostic value of ECOG PS on OS may be underestimated in clinical trials compared with RWD studies.
Finally, the results of this study provide clinical trial as well as real-world estimates of the association of ECOG PS with mortality. This can be useful when assessing the impact of key clinical characteristics, such as ECOG PS, that are often not available or partly missing in administrative claims databases and electronic health records. These missing data often lead to limitations of residual confounding (90, 91). Several methodological approaches have been developed to deal with residual confounding due to missing or partly missing data (92). Many of these approaches involve imputations as well as sensitivity analyses, such as tipping point analysis and the E-value (93, 94), and require assumptions on the minimum strength of association required between the missing confounder and both the treatment and outcome, to nullify the results (91, 95). However, understanding the plausibility of these imputed estimates has traditionally been obtained from a small number of specific studies (95). A more transparent approach would be one where the estimates are obtained from a systematic search of the literature via a pooled estimate, accounting for heterogeneity. This study provides estimates via a systematic process which can be replicated in order to improve internal validity of future RWD studies in mCRPC and other settings. Moreover, while ECOG PS is only one of the risk factors in mPC, understanding the prognostic value of this important risk factor helps assess the impact of residual confounding for a given real-world study.
The results of this study should be interpreted in the context of several limitations. We were limited in the mCSPC analyses due to the low number of studies in this setting. Furthermore, the Egger’s test and the contour-enhanced funnel plot indicated the presence of publication bias. However, the inclusion of studies only reporting multivariate HR on ECOG PS may have contributed to this. Sensitivity analysis using the trim and fill method showed that while the adjusted pooled HR effect estimate was different from the unadjusted pooled HR estimate, indicating publication bias, it did not change the conclusion that ECOG PS is significantly associated with OS. Additionally, we did not have patient-level data and thus were unable to adequately adjust confounders with ECOG PS in the analysis due to ecological bias.
Despite these limitations, our study has multiple strengths; namely the high number of studies included in the mCPRC analysis. Furthermore, we were able to stratify by several characteristics, which resulted in numerous estimates that can be better incorporated in future studies to address residual confounding. Moreover, our inclusion of both clinical trials and RWD studies highlighted differences that further the idea that real-world patients may have different clinical profiles, and that higher-risk patients are often excluded from clinical trials. Finally, unlike the previous study on this topic (7), our inclusion criteria limited our search to an mPC population to reduce heterogeneity between studies.
5 Conclusions
In conclusion, higher ECOG PS scores were significantly associated with higher mortality risk, compared with lower ECOG PS scores, within both the mCRPC and mCSPC settings. Subgroup analyses showed that there were significant differences in pooled HRs for patients in RWD studies, compared with clinical trials. Future studies can incorporate these estimates in sensitivity analyses to better capture the effect of residual confounding when ECOG PS data are missing in the context of mPC and mortality. Additional studies are needed to better characterize the risk of ECOG PS on OS in the mCSPC setting, and to understand its role in other cancer populations and outcomes.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, if requested, without undue reservation.
Author contributions
JA and JW contributed to the conception and design of the study. JA and CK conducted the study selection and data extraction. CK and HC conducted the statistical analysis. JA, CK, and JW wrote the first draft of the manuscript. All authors critically revised the manuscript and approved the submitted version.
Funding
The authors declare that this study received funding from Pfizer Inc. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Acknowledgments
The authors would like to thank everyone involved in this study. Medical writing and editorial assistance were provided by Megan Christian of Onyx (a division of Prime, London, UK), funded by Pfizer Inc. The sponsor was involved in the study design, collection, analysis and interpretation of data, as well as data checking of information provided in the manuscript. However, ultimate responsibility for opinions, conclusions, and data interpretation lies with the authors.
Conflict of interest
JA, CK, HC, and JW are employees of Pfizer Inc.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1194718/full#supplementary-material
Footnotes
References
1. Poon DM, Chan K, Lee SH, Chan TW, Sze H, Lee EKC, et al. Abiraterone acetate in metastatic castration-resistant prostate cancer - the unanticipated real-world clinical experience. BMC Urol (2016) 16:12. doi: 10.1186/s12894-016-0132-z
2. Choi SY, Ryu J, You D, Jeong IG, Hong JH, Ahn H, et al. Prognostic factors of oncologic outcomes in metastatic chemotherapy-naïve castration-resistant prostate cancer treated with enzalutamide in actual clinical practice in east asia. Urol Oncol (2018) 36(9):401.e11–.e18. doi: 10.1016/j.urolonc.2018.06.004
3. Fan L, Wang R, Chi C, Cai W, Zhang Y, Qian H, et al. Systemic immune-inflammation index predicts the combined clinical outcome after sequential therapy with abiraterone and docetaxel for metastatic castration-resistant prostate cancer patients. Prostate (2018) 78(4):250–6. doi: 10.1002/pros.23465
4. Kosaka T, Hongo H, Watanabe K, Mizuno R, Kikuchi E, Oya M. No significant impact of patient age and prior treatment profile with docetaxel on the efficacy of cabazitaxel in patient with castration-resistant prostate cancer. Cancer Chemother Pharmacol (2018) 82(6):1061–6. doi: 10.1007/s00280-018-3698-1
5. Miyake H, Matsushita Y, Watanabe H, Tamura K, Suzuki T, Motoyama D, et al. Significance of de ritis (Aspartate Transaminase/Alanine transaminase) ratio as a significant prognostic but not predictive biomarker in japanese patients with metastatic castration-resistant prostate cancer treated with cabazitaxel. Anticancer Res (2018) 38(7):4179–85. doi: 10.21873/anticanres.12711
6. Sonpavde G, Pond GR, Templeton AJ, Kwon ED, De Bono JS. Impact of single-agent daily prednisone on outcomes in men with metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis (2017) 20(1):67–71. doi: 10.1038/pcan.2016.44
7. Chen W-J, Kong D-M, Li L. Prognostic value of ECOG performance status and gleason score in the survival of castration-resistant prostate cancer: a systematic review. Asian J Androl (2021) 23(2):163–9. doi: 10.4103/aja.aja_53_20
8. Van Praet C, Rottey S, Van Hende F, Pelgrims G, Demey W, Van Aelst F, et al. Which factors predict overall survival in patients with metastatic castration-resistant prostate cancer treated with abiraterone acetate post-docetaxel? Clin Genitourin Cancer (2017) 15(4):502–8. doi: 10.1016/j.clgc.2017.01.019
9. Fizazi K, Scher HI, Molina A, Logothetis CJ, Chi KN, Jones RJ, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol (2012) 13(10):983–92. doi: 10.1016/s1470-2045(12)70379-0
10. Miyazawa Y, Sekine Y, Arai S, Oka D, Nakayama H, Syuto T, et al. Prognostic factors in hormone-sensitive prostate cancer patients treated with combined androgen blockade: A consecutive 15-year study at a single japanese institute. In Vivo (2021) 35(1):373–84. doi: 10.21873/invivo.12268
11. Iacovelli R, Ciccarese C, Mosillo C, Bimbatti D, Fantinel E, Stefani L, et al. Comparison between prognostic classifications in De novo metastatic hormone sensitive prostate cancer. Target Oncol (2018) 13(5):649–55. doi: 10.1007/s11523-018-0588-8
12. Abdel-Rahman O, Cheung WY. Impact of prior local treatment on the outcomes of metastatic hormone-sensitive prostate cancer: Secondary analysis of a randomized controlled trial. Clin Genitourin Cancer (2018) 16(6):466–72. doi: 10.1016/j.clgc.2018.07.007
13. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (2021) 372:n71. doi: 10.1136/bmj.n71
14. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (1997) 315(7109):629–34. doi: 10.1136/bmj.315.7109.629
15. Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol (2008) 61(10):991–6. doi: 10.1016/j.jclinepi.2007.11.010
16. Lin L, Chu H, Murad MH, Hong C, Qu Z, Cole SR, et al. Empirical comparison of publication bias tests in meta-analysis. J Gen Intern Med (2018) 33(8):1260–7. doi: 10.1007/s11606-018-4425-7
17. Lin L, Shi L, Chu H, Murad MH. The magnitude of small-study effects in the cochrane database of systematic reviews: an empirical study of nearly 30 000 meta-analyses. BMJ Evid Based Med (2020) 25(1):27–32. doi: 10.1136/bmjebm-2019-111191
18. Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics (2000) 56(2):455–63. doi: 10.1111/j.0006-341x.2000.00455.x
19. Viechtbauer W. Conducting meta-analyses in r with the metafor package. J Stat Softw (2010) 36(3):1–48. doi: 10.18637/jss.v036.i03
20. Rice K, Higgins JPT, Lumley T. A re-evaluation of fixed effect(s) meta-analysis. J R Stat Soc Ser A Stat Soc (2018) 181(1):205–27. doi: 10.1111/rssa.12275
21. Lin L, Chu H, Hodges JS. Alternative measures of between-study heterogeneity in meta-analysis: Reducing the impact of outlying studies. Biometrics (2017) 73(1):156–66. doi: 10.1111/biom.12543
22. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
23. Higgins JPT, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc (2009) 172(1):137–59. doi: 10.1111/j.1467-985X.2008.00552.x
24. Veroniki AA, Jackson D, Bender R, Kuss O, Langan D, Higgins JPT, et al. Methods to calculate uncertainty in the estimated overall effect size from a random-effects meta-analysis. Res Synth Methods (2019) 10(1):23–43. doi: 10.1002/jrsm.1319
25. Sonpavde G, Pond GR, Plets M, Tangen CM, Hussain MHA, Lara PN Jr., et al. Validation of the association of RECIST changes with survival in men with metastatic castration-resistant prostate cancer treated on SWOG study S0421. Clin Genitourin Cancer (2017) 15(6):635–41. doi: 10.1016/j.clgc.2017.05.014
26. Rathkopf DE, Smith MR, de Bono JS, Logothetis CJ, Shore ND, de Souza P, et al. Updated interim efficacy analysis and long-term safety of abiraterone acetate in metastatic castration-resistant prostate cancer patients without prior chemotherapy (COU-AA-302). Eur Urol (2014) 66(5):815–25. doi: 10.1016/j.eururo.2014.02.056
27. Goyal J, Pond GR, Galsky MD, Hendricks R, Small A, Tsao C-K, et al. Association of the charlson comorbidity index and hypertension with survival in men with metastatic castration-resistant prostate cancer. Urol Oncol (2014) 32(1):36.e27–34. doi: 10.1016/j.urolonc.2013.02.015
28. Fuerea A, Baciarello G, Patrikidou A, Albigès L, Massard C, Di Palma M, et al. Early PSA response is an independent prognostic factor in patients with metastatic castration-resistant prostate cancer treated with next-generation androgen pathway inhibitors. Eur J Cancer (2016) 61:44–51. doi: 10.1016/j.ejca.2016.03.070
29. Caffo O, De Giorgi U, Fratino L, Alesini D, Zagonel V, Facchini G, et al. Clinical outcomes of castration-resistant prostate cancer treatments administered as third or fourth line following failure of docetaxel and other second-line treatment: Results of an italian multicentre study. Eur Urol (2015) 68(1):147–53. doi: 10.1016/j.eururo.2014.10.014
30. Ryan CJ, Dutta S, Kelly WK, Middleberg R, Russell C, Morris MJ, et al. Androgens and overall survival in patients with metastatic castration-resistant prostate cancer treated with docetaxel. Clin Genitourin Cancer (2020) 18(3):222–9.e2. doi: 10.1016/j.clgc.2019.10.002
31. Rescigno P, Lorente D, Dolling D, Ferraldeschi R, Rodrigues DN, Riisnaes R, et al. Docetaxel treatment in PTEN- and ERG-aberrant metastatic prostate cancers. Eur Urol Oncol (2018) 1(1):71–7. doi: 10.1016/j.euo.2018.02.006
32. Schellhammer PF, Chodak G, Whitmore JB, Sims R, Frohlich MW, Kantoff PW. Lower baseline prostate-specific antigen is associated with a greater overall survival benefit from sipuleucel-t in the immunotherapy for prostate adenocarcinoma treatment (IMPACT) trial. Urology (2013) 81(6):1297–302. doi: 10.1016/j.urology.2013.01.061
33. Park JC, Pratz CF, Tesfaye A, Brodsky RA, Antonarakis ES. The effect of therapeutic anticoagulation on overall survival in men receiving first-line docetaxel chemotherapy for metastatic castration-resistant prostate cancer. Clin Genitourin Cancer (2015) 13(1):32–8. doi: 10.1016/j.clgc.2014.04.008
34. Buttigliero C, Pisano C, Tucci M, Vignani F, Bertaglia V, Iaconis D, et al. Prognostic impact of pretreatment neutrophil-to-lymphocyte ratio in castration-resistant prostate cancer patients treated with first-line docetaxel. Acta Oncol (2017) 56(4):555–62. doi: 10.1080/0284186x.2016.1260772
35. Sharova E, Maruzzo M, Del Bianco P, Cavallari I, Pierantoni F, Basso U, et al. Prognostic stratification of metastatic prostate cancer patients treated with abiraterone and enzalutamide through an integrated analysis of circulating free microRNAs and clinical parameters. Front Oncol (2021) 11:626104. doi: 10.3389/fonc.2021.626104
36. Yang Z, Ni Y, Zhao D, Zhang Y, Wang J, Jiang L, et al. Corticosteroid switch from prednisone to dexamethasone in metastatic castration-resistant prostate cancer patients with biochemical progression on abiraterone acetate plus prednisone. BMC Cancer (2021) 21(1):919. doi: 10.1186/s12885-021-08670-2
37. Kelly R, Anton A, Wong S, Shapiro J, Weickhardt A, Azad A, et al. Real-world use of first-generation antiandrogens: impact on patient outcomes and subsequent therapies in metastatic castration-resistant prostate cancer. BJU Int (2021) 128(Suppl 1):18–26. doi: 10.1111/bju.15364
38. Nadal R, Tsai H-L, Sinibaldi VJ, Paller CJ, Antonarakis ES, Denmeade SR, et al. Prognostic factors for clinical outcomes in patients with metastatic castration resistant prostate cancer treated with sequential novel androgen receptor-directed therapies. Prostate (2016) 76(5):512–20. doi: 10.1002/pros.23141
39. Beer TM, Ryan CW, Venner PM, Petrylak DP, Chatta GS, Ruether JD, et al. Intermittent chemotherapy in patients with metastatic androgen-independent prostate cancer: results from ASCENT, a double-blinded, randomized comparison of high-dose calcitriol plus docetaxel with placebo plus docetaxel. Cancer (2008) 112(2):326–30. doi: 10.1002/cncr.23163
40. Qu Y-Y, Dai B, Kong Y-Y, Ye D-W, Yao X-D, Zhang S-L, et al. Prognostic factors in chinese patients with metastatic castration-resistant prostate cancer treated with docetaxel-based chemotherapy. Asian J Androl (2013) 15(1):110–5. doi: 10.1038/aja.2012.110
41. Oudard S, Banu E, Scotte F, Banu A, Medioni J, Beuzeboc P, et al. Prostate-specific antigen doubling time before onset of chemotherapy as a predictor of survival for hormone-refractory prostate cancer patients. Ann Oncol (2007) 18(11):1828–33. doi: 10.1093/annonc/mdm332
42. Miyake H, Sakai I, Terakawa T, Harada K, Fujisawa M. Oncological outcome of docetaxel-based chemotherapy for japanese men with metastatic castration-resistant prostate cancer. Urol Oncol (2013) 31(6):733–8. doi: 10.1016/j.urolonc.2011.06.006
43. van Soest RJ, Nieuweboer AJM, de Morrée ES, Chitu D, Bergman AM, Goey SH, et al. The influence of prior novel androgen receptor targeted therapy on the efficacy of cabazitaxel in men with metastatic castration-resistant prostate cancer. Eur J Cancer (2015) 51(17):2562–9. doi: 10.1016/j.ejca.2015.07.037
44. Francini E, Gray KP, Shaw GK, Evan CP, Hamid AA, Perry CE, et al. Impact of new systemic therapies on overall survival of patients with metastatic castration-resistant prostate cancer in a hospital-based registry. Prostate Cancer Prostatic Dis (2019) 22(3):420–7. doi: 10.1038/s41391-018-0121-2
45. Uchimoto T, Komura K, Fukuokaya W, Kimura T, Takahashi K, Nishimura K, et al. Early prostate-specific antigen (PSA) change at four weeks of the first-line treatment using abiraterone and enzalutamide could predict early/primary resistance in metastatic castration-resistant prostate cancer. Cancers (Basel) (2021) 13(3):526. doi: 10.3390/cancers13030526
46. Wei XX, Perry J, Chang E, Zhang L, Hiatt RA, Ryan CJ, et al. Clinical variables associated with overall survival in metastatic castration-resistant prostate cancer patients treated with sipuleucel-t immunotherapy. Clin Genitourin Cancer (2018) 16(3):184–90.e2. doi: 10.1016/j.clgc.2017.12.004
47. Kongsted P, Svane IM, Lindberg H, Sengeløv L. Clinical impact of the number of treatment cycles in first-line docetaxel for patients with metastatic castration-resistant prostate cancer. Clin Genitourin Cancer (2017) 15(2):e281–7. doi: 10.1016/j.clgc.2016.08.019
48. Matsubara N, Yamada Y, Tabata K-I, Satoh T, Kamiya N, Suzuki H, et al. Comparison of sequential treatment with androgen receptor-targeted agent followed by another androgen receptor-targeted agent versus androgen receptor-targeted agent followed by docetaxel in chemotherapy-naive patients with metastatic castration-resistant prostate cancer. Clin Genitourin Cancer (2017) 15(6):e1073–80. doi: 10.1016/j.clgc.2017.07.016
49. Mehra N, Dolling D, Sumanasuriya S, Christova R, Pope L, Carreira S, et al. Plasma cell-free DNA concentration and outcomes from taxane therapy in metastatic castration-resistant prostate cancer from two phase III trials (FIRSTANA and PROSELICA). Eur Urol (2018) 74(3):283–91. doi: 10.1016/j.eururo.2018.02.013
50. Matsubara N, Yamada Y, Tabata K-I, Satoh T, Kamiya N, Suzuki H, et al. Abiraterone followed by enzalutamide versus enzalutamide followed by abiraterone in chemotherapy-naive patients with metastatic castration-resistant prostate cancer. Clin Genitourin Cancer (2018) 16(2):142–8. doi: 10.1016/j.clgc.2017.09.008
51. Scher HI, Fizazi K, Saad F, Taplin M-E, Sternberg CN, Miller K, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med (2012) 367(13):1187–97. doi: 10.1056/NEJMoa1207506
52. Kumano Y, Hasegawa Y, Kawahara T, Yasui M, Miyoshi Y, Matsubara N, et al. Pretreatment neutrophil to lymphocyte ratio (NLR) predicts prognosis for castration resistant prostate cancer patients underwent enzalutamide. BioMed Res Int (2019) 2019:9450838. doi: 10.1155/2019/9450838
53. Raju R, Sahu A, Klevansky M, Torres J. Real-world data on outcomes in metastatic castrate-resistant prostate cancer patients treated with abiraterone or enzalutamide: A regional experience. Front Oncol (2021) 11:656146. doi: 10.3389/fonc.2021.656146
54. Shore ND, Ionescu-Ittu R, Laliberté F, Yang L, Lejeune D, Yu L, et al. Beyond frontline therapy with abiraterone and enzalutamide in metastatic castration-resistant prostate cancer: A real-world US study. Clin Genitourin Cancer (2021) 19(6):480–90. doi: 10.1016/j.clgc.2021.07.009
55. de Morrée ES, Vogelzang NJ, Petrylak DP, Budnik N, Wiechno PJ, Sternberg CN, et al. Association of survival benefit with docetaxel in prostate cancer and total number of cycles administered: A post hoc analysis of the mainsail study. JAMA Oncol (2017) 3(1):68–75. doi: 10.1001/jamaoncol.2016.3000
56. Montgomery B, Kheoh T, Molina A, Li J, Bellmunt J, Tran N, et al. Impact of baseline corticosteroids on survival and steroid androgens in metastatic castration-resistant prostate cancer: exploratory analysis from COU-AA-301. Eur Urol (2015) 67(5):866–73. doi: 10.1016/j.eururo.2014.06.042
57. Quinn DI, Tangen CM, Hussain M, Lara PN Jr., Goldkorn A, Moinpour CM, et al. Docetaxel and atrasentan versus docetaxel and placebo for men with advanced castration-resistant prostate cancer (SWOG S0421): a randomised phase 3 trial. Lancet Oncol (2013) 14(9):893–900. doi: 10.1016/s1470-2045(13)70294-8
58. Delanoy N, Hardy-Bessard A-C, Efstathiou E, Le Moulec S, Basso U, Birtle A, et al. Sequencing of taxanes and new androgen-targeted therapies in metastatic castration-resistant prostate cancer: Results of the international multicentre retrospective CATS database. Eur Urol Oncol (2018) 1(6):467–75. doi: 10.1016/j.euo.2018.05.009
59. Azad AA, Eigl BJ, Murray RN, Kollmannsberger C, Chi KN. Efficacy of enzalutamide following abiraterone acetate in chemotherapy-naive metastatic castration-resistant prostate cancer patients. Eur Urol (2015) 67(1):23–9. doi: 10.1016/j.eururo.2014.06.045
60. Khalaf DJ, Avilés CM, Azad AA, Sunderland K, Todenhöfer T, Eigl BJ, et al. A prognostic model for stratifying clinical outcomes in chemotherapy-naive metastatic castration-resistant prostate cancer patients treated with abiraterone acetate. Can Urol Assoc J (2018) 12(2):E47–52. doi: 10.5489/cuaj.4600
61. Kuppen MC, Westgeest HM, van der Doelen MJ, van den Eertwegh AJ, Coenen JL, Aben KK, et al. Real-world outcomes of radium-223 dichloride for metastatic castration resistant prostate cancer. Future Oncol (2020) 16(19):1371–84. doi: 10.2217/fon-2020-0039
62. Jeong CW, Kang M, Il Jung S, Kim T-H, Park SW, Joung JY, et al. Importance of androgen-deprivation therapy during enzalutamide treatment in men with metastatic castration-resistant prostate cancer following chemotherapy: results from retrospective, multicenter data. Prostate Cancer Prostatic Dis (2019) 22(1):150–8. doi: 10.1038/s41391-018-0088-z
63. Conteduca V, Caffo O, Galli L, Maugeri A, Scarpi E, Maines F, et al. Association among metabolic syndrome, inflammation, and survival in prostate cancer. Urol Oncol (2018) 36(5):240.e1–11. doi: 10.1016/j.urolonc.2018.01.007
64. de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med (2011) 364(21):1995–2005. doi: 10.1056/NEJMoa1014618
65. Ahmed ME, Andrews JR, Alamiri J, Higa J, Haloi R, Alom M, et al. Adding carboplatin to chemotherapy regimens for metastatic castrate-resistant prostate cancer in postsecond generation hormone therapy setting: Impact on treatment response and survival outcomes. Prostate (2020) 80(14):1216–22. doi: 10.1002/pros.24048
66. Caffo O, Maines F, Donner D, Veccia A, Chierichetti F, Galligioni E. Impact of enzalutamide administration on primary prostate cancer volume: a metabolic evaluation by choline positron emission tomography in castration-resistant prostate cancer patients. Clin Genitourin Cancer (2014) 12(5):312–6. doi: 10.1016/j.clgc.2014.03.004
67. Caffo O, Frantellizzi V, Monari F, Galli L, Costa RP, Pinto C, et al. Sequencing life-prolonging agents in castration-resistant prostate cancer patients: Comparison of sequences with and without (223)Ra. Cancer Biother Radiopharm (2021) 36(5):391–6. doi: 10.1089/cbr.2020.4442
68. Zhao J-G, Liu J-D, Shen P-F, Tang X, Sun G-X, Zhang X-M, et al. Prior switching to a second-line nonsteroidal antiandrogen does not impact the therapeutic efficacy of abiraterone acetate in patients with metastatic castration-resistant prostate cancer: a real-world retrospective study. Asian J Androl (2018) 20(6):545–50. doi: 10.4103/aja.aja_58_18
69. Boegemann M, Khaksar S, Bera G, Birtle A, Dopchie C, Dourthe L-M, et al. Abiraterone acetate plus prednisone for the management of metastatic castration-resistant prostate cancer (mCRPC) without prior use of chemotherapy: report from a large, international, real-world retrospective cohort study. BMC Cancer (2019) 19(1):60. doi: 10.1186/s12885-019-5280-6
70. Alvim CM, Mansinho A, Paiva RS, Brás R, Semedo PM, Lobo-Martins S, et al. Prognostic factors for patients treated with abiraterone. Future Sci OA (2019) 6(2):FSO436. doi: 10.2144/fsoa-2019-0079
71. Al-Ezzi EM, Alqaisi HA, Iafolla MAJ, Wang L, Sridhar SS, Sacher AG, et al. Clinicopathologic factors that influence prognosis and survival outcomes in men with metastatic castration-resistant prostate cancer treated with radium-223. Cancer Med (2021) 10(17):5775–82. doi: 10.1002/cam4.4125
72. Maughan BL, Luber B, Nadal R, Antonarakis ES. Comparing sequencing of abiraterone and enzalutamide in men with metastatic castration-resistant prostate cancer: A retrospective study. Prostate (2017) 77(1):33–40. doi: 10.1002/pros.23246
73. Hung J, Taylor AR, Divine GW, Hafron JM, Hwang C. The effect of time to castration resistance on outcomes with abiraterone and enzalutamide in metastatic prostate cancer. Clin Genitourin Cancer (2016) 14(5):381–8. doi: 10.1016/j.clgc.2016.03.021
74. Zhao J, Shen P, Sun G, Chen N, Liu J, Tang X, et al. The prognostic implication of intraductal carcinoma of the prostate in metastatic castration-resistant prostate cancer and its potential predictive value in those treated with docetaxel or abiraterone as first-line therapy. Oncotarget (2017) 8(33):55374–83. doi: 10.18632/oncotarget.19520
75. Fujiwara M, Yuasa T, Komai Y, Numao N, Yamamoto S, Fukui I, et al. Efficacy, prognostic factors, and safety profile of enzalutamide for non-metastatic and metastatic castration-resistant prostate cancer: A retrospective single-center analysis in japan. Target Oncol (2020) 15(5):635–43. doi: 10.1007/s11523-020-00759-1
76. van der Doelen MJ, Stockhaus A, Ma Y, Mehra N, Yachnin J, Gerritsen WR, et al. Early alkaline phosphatase dynamics as biomarker of survival in metastatic castration-resistant prostate cancer patients treated with radium-223. Eur J Nucl Med Mol Imaging (2021) 48(10):3325–34. doi: 10.1007/s00259-021-05283-6
77. Ahmed ME, Joshi VB, Badawy M, Pagliaro LC, Karnes RJ, Lowe V, et al. Radium-223 in the third-line setting in metastatic castration-resistant prostate cancer: Impact of concomitant use of enzalutamide on overall survival (OS) and predictors of improved OS. Clin Genitourin Cancer (2021) 19(3):223–9. doi: 10.1016/j.clgc.2020.12.009
78. Miyake H, Matsushita Y, Tamura K, Motoyama D, Ito T, Sugiyama T, et al. Impact of prior androgen receptor-axis-targeted agents on the clinical activity of subsequent docetaxel in patients with metastatic castration-resistant prostate cancer: comparative assessment between abiraterone acetate and enzalutamide. Med Oncol (2017) 34(12):200. doi: 10.1007/s12032-017-1060-9
79. Miyake H, Matsushita Y, Tamura K, Motoyama D, Ito T, Sugiyama T, et al. No significant impact of response to prior androgen receptor-axis-targeted agents on the efficacy of subsequent docetaxel in patients with metastatic castration-resistant prostate cancer. Int J Clin Oncol (2018) 23(3):576–83. doi: 10.1007/s10147-017-1230-y
80. Miyake H, Sato R, Watanabe K, Matsushita Y, Watanabe H, Motoyama D, et al. Prognostic significance of third-line treatment for patients with metastatic castration-resistant prostate cancer: comparative assessments between cabazitaxel and other agents. Int J Clin Oncol (2021) 26(9):1745–51. doi: 10.1007/s10147-021-01956-2
81. Kongsted P, Svane IM, Lindberg H, Sengeløv L. Predictors of chemotherapy-induced toxicity and treatment outcomes in elderly versus younger patients with metastatic castration-resistant prostate cancer. Clin Genitourin Cancer (2016) 14(6):e559–68. doi: 10.1016/j.clgc.2016.03.018
82. Antoun S, Bayar A, Ileana E, Laplanche A, Fizazi K, di Palma M, et al. High subcutaneous adipose tissue predicts the prognosis in metastatic castration-resistant prostate cancer patients in post chemotherapy setting. Eur J Cancer (2015) 51(17):2570–7. doi: 10.1016/j.ejca.2015.07.042
83. Fan L, Yang Y, Chi C, Ma X, Wang R, Gong Y, et al. Neuroendocrine differentiation markers guide treatment sequence selection in metastatic castration-resistant prostate cancer. Prostate (2019) 79(6):567–73. doi: 10.1002/pros.23762
84. Xu XS, Ryan CJ, Stuyckens K, Smith MR, Saad F, Griffin TW, et al. Correlation between prostate-specific antigen kinetics and overall survival in abiraterone acetate-treated castration-resistant prostate cancer patients. Clin Cancer Res (2015) 21(14):3170–7. doi: 10.1158/1078-0432.CCR-14-1549
85. Caffo O, Wissing M, Bianchini D, Bergman A, Thomsen FB, Schmid S, et al. Survival outcomes from a cumulative analysis of worldwide observational studies on sequential use of new agents in metastatic castration-resistant prostate cancer. Clin Genitourin Cancer (2020) 18(1):69–76.e4. doi: 10.1016/j.clgc.2019.09.010
86. Chi KN, Kheoh T, Ryan CJ, Molina A, Bellmunt J, Vogelzang NJ, et al. A prognostic index model for predicting overall survival in patients with metastatic castration-resistant prostate cancer treated with abiraterone acetate after docetaxel. Ann Oncol (2016) 27(3):454–60. doi: 10.1093/annonc/mdv594
87. Fan L, Chi C, Guo S, Wang Y, Cai W, Shao X, et al. Serum pre-albumin predicts the clinical outcome in metastatic castration-resistant prostate cancer patients treated with abiraterone. J Cancer (2017) 8(17):3448–55. doi: 10.7150/jca.21134
88. Miyake H, Sugiyama T, Aki R, Matsushita Y, Tamura K, Motoyama D, et al. Comparison of alternative androgen receptor-axis-targeted agent (ARATA) and docetaxel as second-line therapy for patients with metastatic castration-resistant prostate cancer with progression after initial ARATA in real-world clinical practice in japan. Clin Genitourin Cancer (2018) 16(3):219–25. doi: 10.1016/j.clgc.2017.11.007
89. Tan YY, Papez V, Chang WH, Mueller SH, Denaxas S, Lai AG. Comparing clinical trial population representativeness to real-world populations: an external validity analysis encompassing 43 895 trials and 5 685 738 individuals across 989 unique drugs and 286 conditions in england. Lancet Healthy Longev (2022) 3(10):e674–89. doi: 10.1016/S2666-7568(22)00186-6
90. US Food and Drug Administration. Framework for FDA’s real world evidence program (2018). Available at: https://www.fda.gov/media/120060/download (Accessed November 22, 2022).
91. Schneeweiss S. Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiol Drug Saf (2006) 15(5):291–303. doi: 10.1002/pds.1200
92. Lee KJ, Simpson JA. Introduction to multiple imputation for dealing with missing data. Respirology (2014) 19(2):162–7. doi: 10.1111/resp.12226
93. Lin DY, Psaty BM, Kronmal RA. Assessing the sensitivity of regression results to unmeasured confounders in observational studies. Biometrics (1998) 54(3):948–63.
94. VanderWeele TJ, Ding P. Sensitivity analysis in observational research: Introducing the e-value. Ann Intern Med (2017) 167(4):268–74. doi: 10.7326/M16-2607
Keywords: prognostic, Eastern Cooperative Oncology Group, survival, meta-analysis, metastatic, prostate cancer, real-world data, publication bias
Citation: Assayag J, Kim C, Chu H and Webster J (2023) The prognostic value of Eastern Cooperative Oncology Group performance status on overall survival among patients with metastatic prostate cancer: a systematic review and meta-analysis. Front. Oncol. 13:1194718. doi: 10.3389/fonc.2023.1194718
Received: 27 March 2023; Accepted: 15 November 2023;
Published: 15 December 2023.
Edited by:
Ronald M Bukowski, Cleveland Clinic, United StatesCopyright © 2023 Assayag, Kim, Chu and Webster. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jonathan Assayag, Sm9uYXRoYW4uQXNzYXlhZ0BwZml6ZXIuY29t
†ORCID: Haitao Chu, orcid.org/0000-0003-0932-598X
 Jonathan Assayag
Jonathan Assayag Chai Kim1
Chai Kim1