- 1Oncology Department, Xiyuan Hospital of China Academy of Chinese Medical Sciences, Beijing, China
- 2National Institute of Complementary Medicine (NICM) Health Research Institute, Western Sydney University, Penrith, NSW, Australia
- 3School of Statistics, Renmin University of China, Beijing, China
- 4School of Beijing University of Chinese Medicine, Beijing, China
Background: Fatigue is a common source of distress for cancer survivors. The severity of cancer-related fatigue varies significantly, which may be due to individual differences in host factors.
Aim: This cross-sectional study aims to explore how demographic, oncological, sociological, psychological, and stress-related hormones levels interact to influence the distinct experiences of fatigue (Cancer-related fatigue [CRF] occurrence and fatigue degree).
Methods: A cross-sectional study carried out at the oncology outpatient and ward department of Xiyuan Hospital of China Academy of Chinese Medical Sciences recruited 306 cancer patients between January 2021 to December 2021. General information, fatigue, psychological factors was evaluated by general information questionnaire, the Revised Piper’s Fatigue Scale-Chinese Version (RPFS-CV), and the self-report Hospital Anxiety and Depression Scale (HADS). Stress-related hormones were measured with chemiluminescent enzyme immunoassay (Zhengzhou Antobio).
Results: 306 patients were included, 229 (74.8%) were diagnosed with CRF, including 94 (41.0%) with mild fatigue, 121 (52.8%) with moderate fatigue, and 14 (6.1%) with severe fatigue. Multivariate regression analysis showed that higher depression scores, aldosterone levels may increase the risk of CRF. Patients who are obese (Body mass index ≥ 28 kg/m2) may help to reduce the risk of CRF. Other contributing factors for increased levels of fatigue (p< 0.05) include being female, having anxiety, depression and high aldosterone levels.
Conclusion: The research suggested that CRF was a common symptom in cancer survivors and pay attention to these influencing factors may help to better identify patients susceptible to fatigue and provide long-term, targeted interventions.
1 Introduction
The number of cancer survivors is predicted to reach 20.6 million in 2040 due to advancements in early detection, diagnosis, treatment, and rehabilitation (1). Cancer survivors report that fatigue is a disruptive symptom month or even years after treatment is completed. More than 30% of tumor-free cancer survivors in China report persistent fatigue, even years after finishing treatment (2–4). The NCCN defines cancer-related fatigue (CRF) as a distressing, persistent, subjective sense of physical, emotional, and/or cognitive tiredness or exhaustion related to cancer or cancer treatment that is not proportional to recent activity and interferes with usual functioning (5). The distressing, persistent, multi-dimensional nature of fatigue leads to treatment interruption, decreased quality of life, which makes it difficult for patients to resume “regular” family, work, and life (6–9).
Numerous variables that influence CRF can be broadly categorized into three categories: oncological variables, demographic variables, and psychosocial variables (10, 11). Tumors and anti-tumor therapy are the direct causes of CRF, nevertheless, susceptibilities to exhaustion and levels of fatigue might vary among individuals with the same types of cancer or receiving the same treatments. For instance, one study found that among 67 breast cancer patients receiving simultaneous chemotherapy, 46.3% displayed higher levels of exhaustion and 56.7% displayed lower levels of fatigue (12), while there were similar differences among tumor patients who received other treatments or did not receive treatment. Female, insomniac, depressed, neurotic, and other factors are widely known as possible influencing factors of CRF. In recent years, some scholars have proposed that the difference experience of fatigue may be related to some congenital factors (e.g., SNP genes, cellular aging) or specific social and psychological factors (e.g., childhood abuse, depression history, trait anxiety, catastrophizing, etc.) (3, 13), broadening the researchers’ horizons.
The specific pathological mechanism of CRF has still not been elucidated (14–18), several studies have shown that some factors, such as anxiety, depression, physical and mental stress, may act as persistent stressors to affect the HPA axis and autonomic nervous system, causing neuroendocrine rhythm dysregulation(e.g., diurnal cortisol, aldosterone, adrenocortical hormone, etc.) (19–21). Cortisol has long been considered as a potential predictor of CRF, many clinical studies have demonstrated that CRF is associated with a flat cortisol secretion rhythm (22, 23). In fact, there is evidence that high salivary aldosterone concentrations as a marker of depression and prolonged chronicity, which also has been implicated in CRF (24, 25). Therefore, we conducted a 1-year cross-sectional study to further explore CRF specific or persistent influencing factors and potential predictors, so as to help clinicians identify susceptible populations for CRF and give patients long-term, more targeted intervention strategies.
2 Methods
2.1 Study design and setting
A cross-sectional survey was carried out in the outpatient department and ward of the Oncology Department of Xiyuan Hospital, China Academy of Chinese Medical Sciences from January 2021 to December 2021. Recruitment of study participants occurred between April 26, 2021, and December 31, 2021. Study protocol registered with the China Clinical Trials Registry (Registration number: ChiCTR2100045404; Registration date: April 14, 2021). Ethical approval was obtained from the Medical Ethics Committee of Xiyuan Hospital, China Academy of Chinese Medical Sciences (Approval number:2021XLA027; Approval date: March 26, 2021), and all subjects signed an informed consent form before being investigated.
2.2 Study population
Included patients were aged 18 and above, with clear diagnosis of tumour by biopsy, pathology, or cytology and KPS ≥ 60 points. Exclusion criteria included major traumatic damage such as surgical treatment in the last month, severe combined heart, liver, kidney and other systemic diseases, poor compliance, severe cognitive impairment or psychiatric disorders, inability to complete the scale, and those who are unaware of their disease.
2.2 Data measurement
2.2.1 General information
A questionnaire was created to gather general information of patients (including demographic characteristics, oncological characteristics, and sociological characteristics). Demographic characteristics include gender, age, exercise (intensity, frequency, and time), dietary habits (dietary structure, dietary taste), and BMI. Oncological characteristics include tumor type, pathological type, whether tumor-free, metastatic site, tumor stage, stage of disease treatment, whether surgery was performed, previous treatment, current treatment, comorbidities, and KPS. Sociological characteristics include marital status, educational level, fatigue cognition, work nature, work status, and family income.
2.2.2 Fatigue screening, diagnosis, evaluation
2.2.2.1 Filter criteria
Patients who met the inclusion and exclusion criteria were initially assessed using the Visual Analogue Fatigue Scale (VAFS) (26); a score of 0 was defined as non-CRF, and a score of 1 or above was then further diagnosed in accordance with the CRF diagnostic criteria in the ICD-10.
2.2.2.2 Diagnostic criteria
Patients with a VAFS scale screening score of 1 or higher were referred to the 10th International Conference on the Revision of the International Classification of Diseases diagnostic criteria for CRF (ICD-10) (27).
2.2.2.3 Evaluation criteria
The Revised Piper’s Fatigue Scale-Chinese Version (RPFS-CV) scale was used to measure fatigue in patients who satisfied the diagnostic standards for CRF. Patients were classified into three categories of fatigue: mild fatigue (1-3 points), moderate fatigue (4-6 points), and severe fatigue (7–10 points) (28).
2.2.3 Assessment tools
2.2.3.1 The visual analogue fatigue scale
VAFS (26) is mainly used to screen whether the patient is tired. To start, mark a horizontal line on the paper that is 10 cm long with a 0 on the left side and a 10 on the right. A score of 0 indicates no fatigue, a score of 10 complete exhaustion, a score of 1-3 indicates mild fatigue, a score of 4-6 indicates moderate fatigue, and a score of 4-6 indicates severe fatigue. The scale is simple and easy to fill out, and can be used for measurement multiple times. Fatigue is assessed and recorded at 7 a.m., 12 a.m., 1 p.m., and 7 p.m. every day to understand the dynamic changes of CRF and influencing factors of patients in the wakeful state, to help patients manage time reasonably, allocate energy, and improve self-management ability. VAFS score is especially suitable for patients with cancer fatigue and pain.
2.2.3.2 The revised Piper’s fatigue scale-Chinese version
RPFS-CV is a Chinese translation of the original scale that has been validated in China and is extensively utilized in local clinical research (28). It comprises of 24 questions assessing total CRF. Item 1 asks the patient if fatigue is present and, if so, continues with the following questions, item 2 records the duration of the patient ‘s fatigue. Items 3-24 respectively evaluated the four dimensions of fatigue, namely, behavioural/severity (items 3-8), emotion (items 9-13), feeling (items 14-18) and cognition/emotion (items 19-24). Patients can be categorized into four categories of weariness according to the Likert 11 scale: none (0 points), mild (1-3 points), moderate (4-6 points), and severe (7-10 points).
2.2.3.3 Anxiety/depression assessment
The self-report Hospital Anxiety and Depression Scale (HADS) (29) was used to assess the level of anxiety (HADS-A) and depression (HADS-D) during the previous week. The scale is composed of 7 questions each for anxiety (HADS-A) and depression (HADS-D) questions, symptoms were reported on a scale from 0 (not at all) to 3 (most of the time). Scores of 0 to 7 were considered asymptomatic, 8 to 10 indicated a suspicious presence, and 11 to 21 indicated a confirmed presence. In our study, all scores of 8 and above were considered as the presence of anxiety/depressive states.
2.2.3.4 Karnofsky performance Status, body mass index
KPS Index is an assessment tool for functional impairment and prognosis in cancer survivors (30). Ranging from 0 (dead) to 100 (normal activity, healthy), with a high score considered to be between 80 and 100. Based on Chinese Adult Body Weight Standard (31), we divided patients into four groups according to their body weight: lean (BMI< 18.5 kg/m2), normal weight (BMI 18.5-23.9 kg/m2), overweight (BMI 24.0-27.9 kg/m2) and obesity (BMI ≥ 28.0 kg/m2).
2.2.3.5 Stress-related hormones
Test items include adrenocortical hormone (ACTH), aldosterone (ALD), renin (Renin), and cortisol (COR). The detection time is the early morning of the day or early morning the following day. A tube of venous blood is collected from the patient in a supine position on an empty stomach. More than 3ml of EDTA-K2 (dipotassium ethylenediaminetetraacetate) is used for anticoagulation (long-headed purple tube). Within half an hour after the sample is collected, the sample is sent to the laboratory. Plasma was separated by centrifugation at 3000g for 10 min at 4°C, and then aliquoted into siliconized polypropylene tubes and stored at −80°C until batched assay. The detection kit was Zhengzhou Autobio Diagnostics Quantitative Assay Kit (chemiluminescent enzyme immunoassay).
2.2.4 Information collection and quality control
Patients who met the inclusion and exclusion criteria were screened by two trained investigators (Shanshan GU and Jinghui Wang). After the investigators obtained informed consent from the patients and signed the informed consent form, patients completed the scales independently or with the assistance of the investigators under the guidance of the investigators and an attending oncologist to ensure the quality of the study.
2.2.5 Sample size calculations
The purpose of this cross-sectional study was to explore the prevalence of CRF, requiring a two-sided test, α taking 0.05, then Z is 1.96, p is the incidence of CRF, the literature reports the incidence of CRF is 60%-100% (5), this study takes 60% incidence, δ for the allowable error, δ take 0.1p. Using a 0.05 significance level, 80% power, the sample size required for this investigation is about 256, considering 10-15% incomplete data or other reasons for exclusion, a total of at least 300 cases are proposed to be included. The calculated sample size is sufficient to build a multi-factor logistic regression model.
2.2.6 Data analysis
Data were entered into Epidata software using double entry, and IBM SPSS Statistics 26 software was used for data statistics and analysis. Shapiro-Wilk (S-W) normality test revealed that the distributions of anxiety, depression, and stress-related endocrine hormones differ from a normal distribution. The mean value and standard deviation were calculated for continuous variables and number (n) and proportion (%) of participants were reported for categorical measures. The χ2 test was used to compare the differences between the non-CRF and CRF groups on demographic, oncological, and sociological characteristics, the Mann-Whitney U rank sum test was used to compare the differences between groups on anxiety, depression, and serum hormone levels. The Kruskal-Wallis H rank sum test was used to compare the differences between the three groups of mild, moderate, and severe fatigue on demographic, oncological, and sociological characteristics, anxiety and depression, and serum hormone levels. Binary logistic regression and orthogonal logistic regression were established to investigate the specific degree of influence of the factors significant in the analysis of variance on whether suffering from CRF and the degree of fatigue of patients, respectively. Analysis was adjusted by controlling for age, gender, BMI, stage of disease treatment, and previous and current treatment modalities. A two-tailed test with a test level of 0.05 and 95% confidence interval was used, and missing values were filled in using the mean.
3 Results
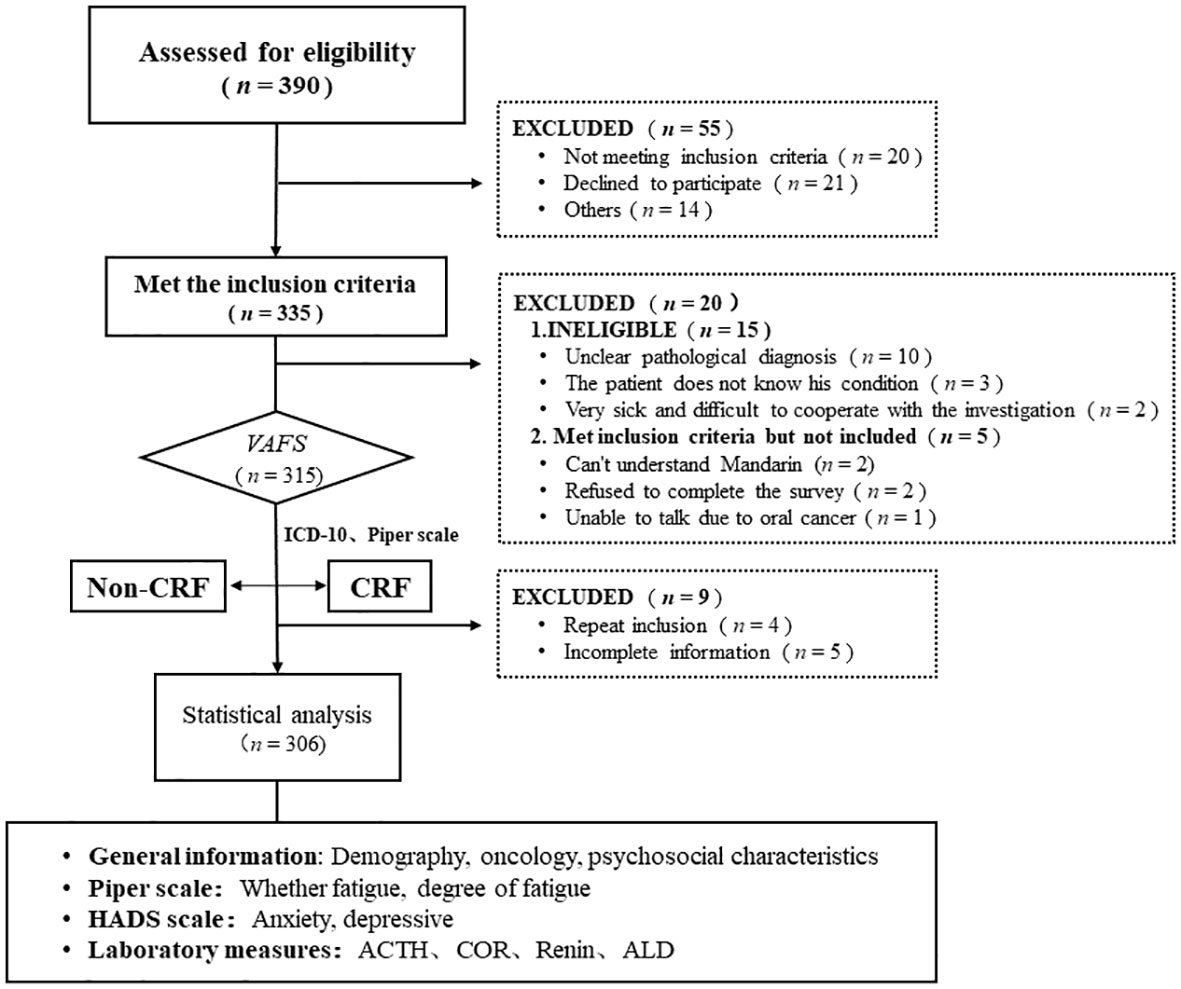
A total of 390 tumor patients were screened in this study, with 335 tumor patients who met the inclusion criteria, 315 who patients entered fatigue screening or evaluation, and 306 patients who were included in the final statistical analysis (Figure 1).
3.1 General information
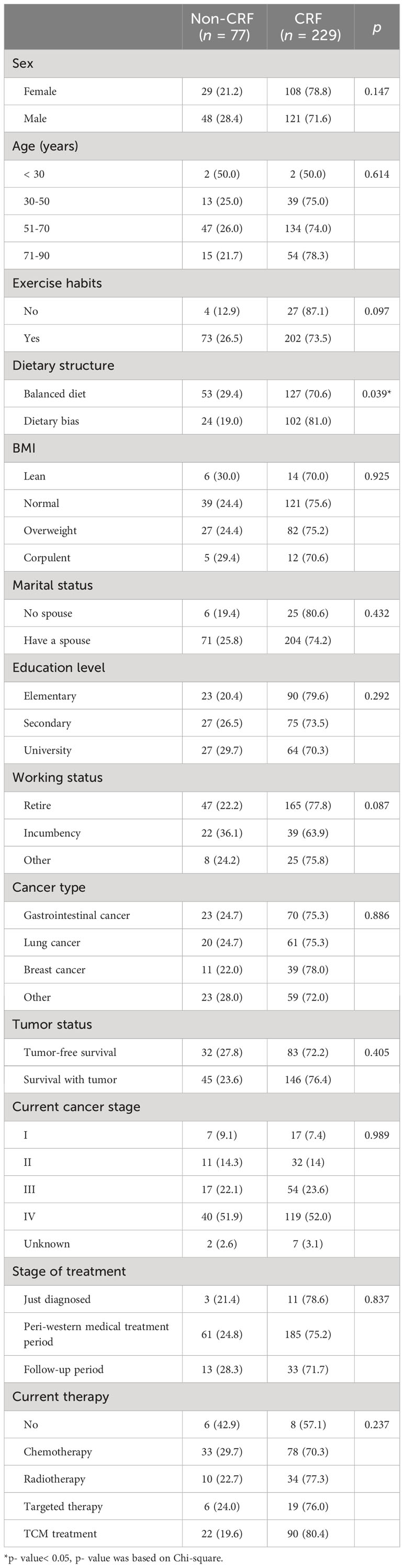
Of the 306 patients, 229 (75.0%) were experiencing CRF. Among CRF patients, those with mild, moderate, and severe fatigue comprised 41.0%, 52.8%, 6.1%, respectively. The median age was 63 (22-89) years, which consisted of 169 males (55.2%) and 137 females (44.8%). Most of the patients (90%) had exercise habits, whereas 41.2% had dietary bias. There were 93 cases (30.4%) of gastrointestinal cancer, 81 cases (26.5%) of lung cancer, 50 cases (16.3%) of breast cancer, 191 cases (62.4%) of patients with tumor survival, 230 cases (75.2%) of III-IV patients, about 60% of patients received anti-tumor therapy, and 111 patients (36.2%) patients were receiving chemotherapy. Thirty-one (10.1%) had no spouse, 32 (10.5%) had heard of CRF, and 14 (4.6%) had actively intervened for fatigue. The total mean score of anxiety and depression were 3.11 ± 3.69, 5.65 ± 4.83 points, respectively. The average level of aldosterone was 162.14 ± 61.56 pg/ml (Table 1).
3.2 Comparison of differences between CRF and non-CRF groups
As shown in Table 2, in the general information, dietary structure (p = 0.039), KPS (p< 0.001), and history of anti-tumor treatment (p = 0.036) were significantly associated with the occurrence of CRF. There was no significant difference in the sociological data between non-fatigue and fatigue groups (p > 0.05).
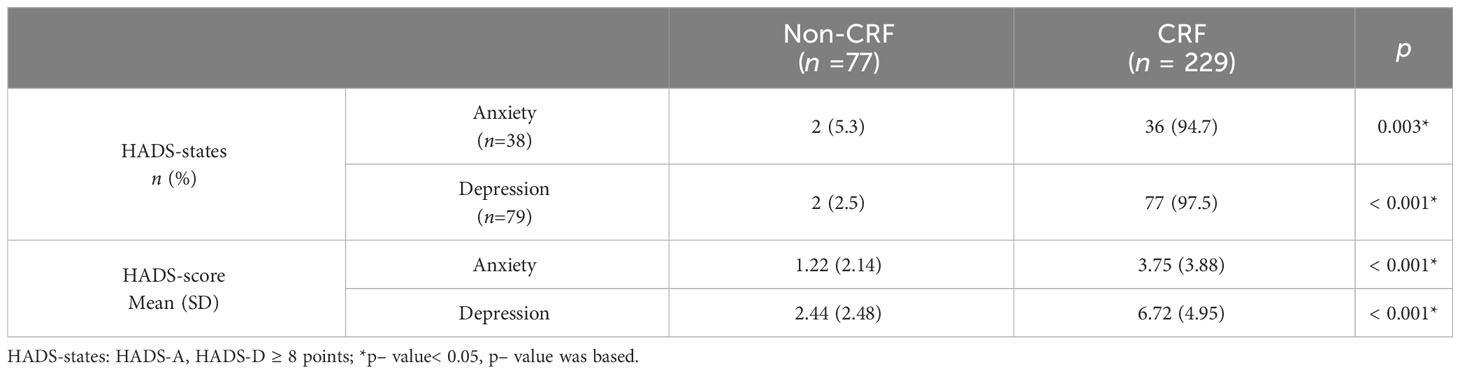
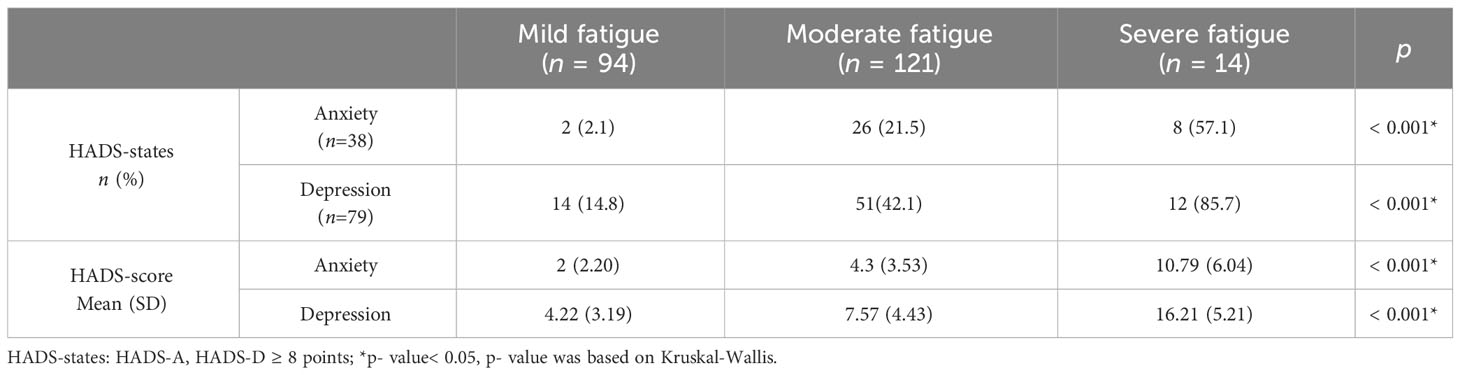
As shown in Table 3, in terms of both anxiety (p = 0.003) and depression (p< 0.001) states and anxiety (p< 0.001) and depression (p< 0.001) scores, the difference comparison results showed a statistically significant association between anxiety, depression, and CRF incidence.
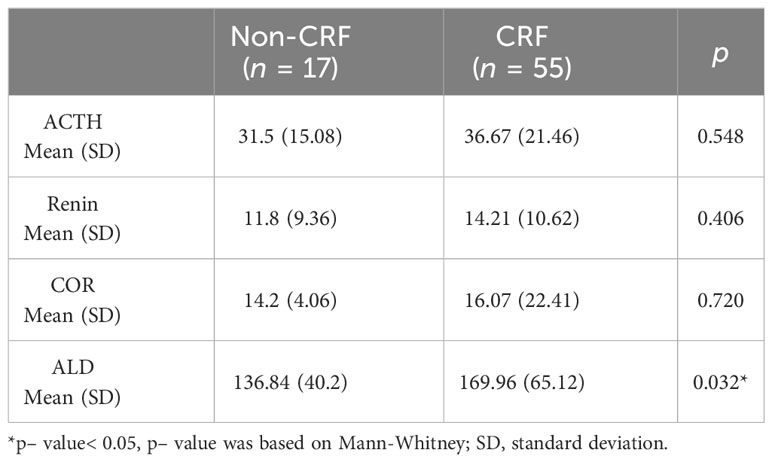
As shown in Table 4, there was a significant correlation between aldosterone levels and CRF occurrence (p< 0.001), however, no significant correlation was found between ACTH, COR, Renin and CRF (p > 0.05).
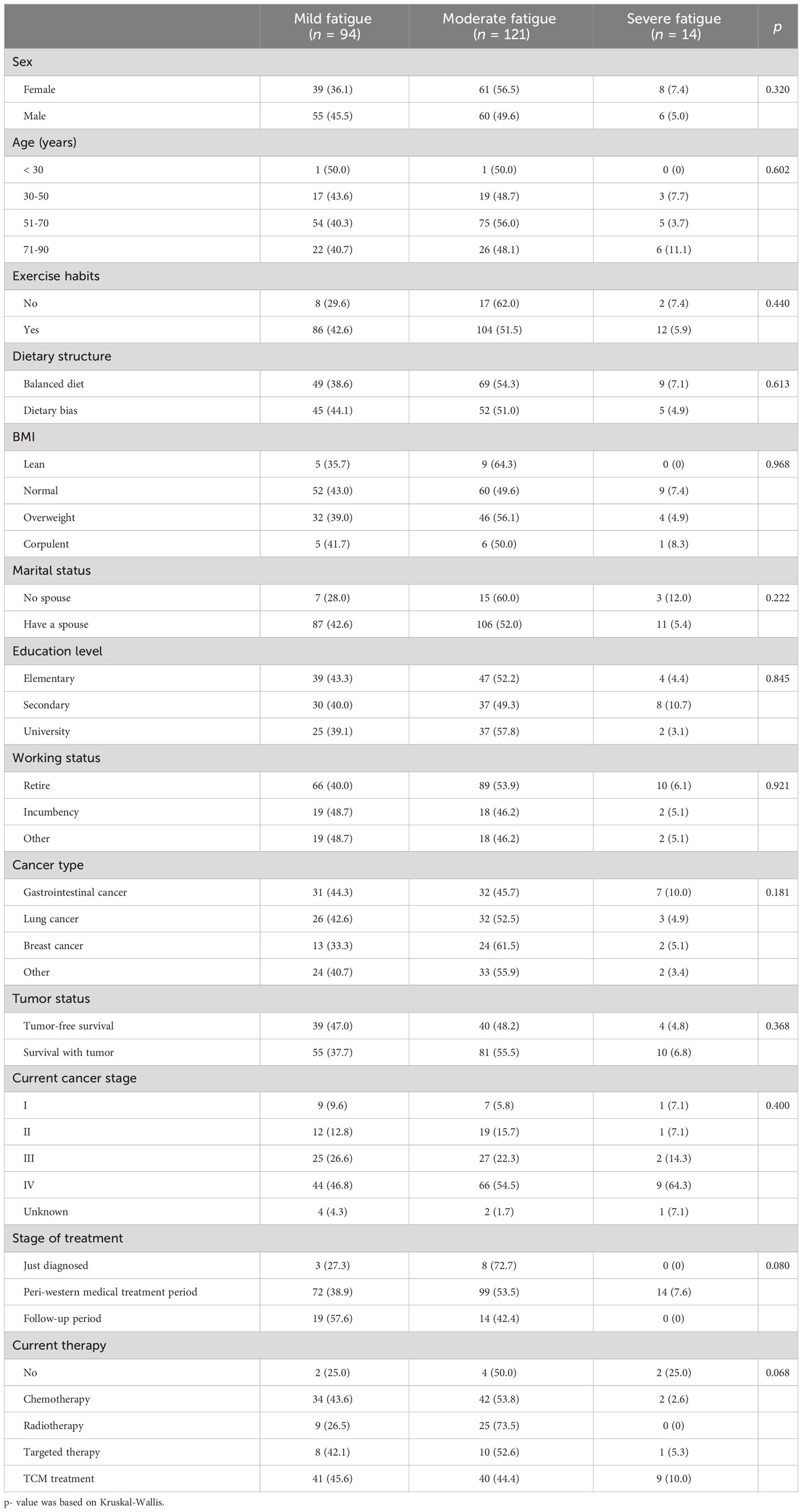
3.3 Comparison of different levels of fatigue
As Table 5 shows, there was no statistically significant difference in demographic, sociological characteristics between groups with different levels of fatigue (p > 0.05), however, KPS were significantly associated with fatigue level (p< 0.001).
Similar to the results of the between-group difference comparison for CRF or not, anxiety and depression (anxiety and depression states and anxiety and depression scores) were significantly correlated with fatigue level (p< 0.001) (Table 6).
In terms of serum hormone levels, there was no significant correlation that was found between ACTH, Renin, COR, ALD and different levels of fatigue (p > 0.05) (Table 7).
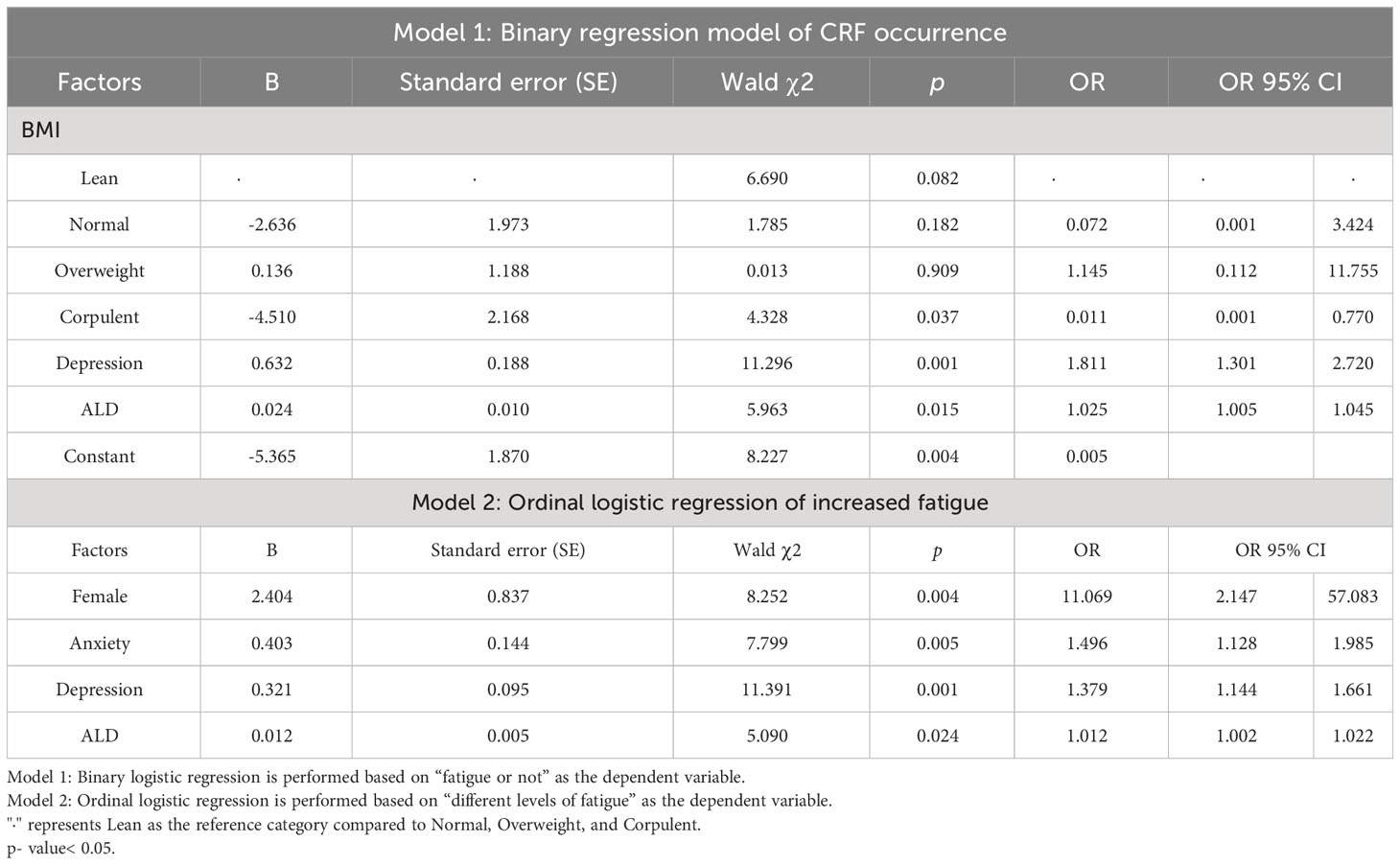
3.4 Multivariable analysis
In addition to including variables with p< 0.05 in the difference comparison into multivariable logistic regression analysis, control variables such as age, gender, BMI, stage of disease treatment, and previous and current treatment modalities were also included. Because of the strong correlation between KPS scores and fatigue, they were excluded from the regression. As model 1 shows, BMI ≥ 28 kg/m2 was negatively associated with the occurrence of CRF (OR = 0.011, 95% CI 0.001-0.770, p = 0.037), and higher depression scores (OR = 1.811, 95% CI 1.301-2.720, p = 0.001) or the higher the level of aldosterone the higher the risk of CRF (OR =1.025, 95% CI 1.005-1.045, p = 0.015). As model 2 shows, relative to male, female had an increased risk of fatigue (OR = 11.069, 95% CI 2.147-57.083, p = 0.004). The model also showed that the higher the anxiety, depression score, and aldosterone levels, the higher the risk of increased fatigue (OR = 1.496, 95% CI 1.128-1.985, p = 0.005; OR = 1.379, 95% CI 1.144-1.661, p = 0.001; OR = 1.012, 95%CI 1.002-1.022, p = 0.024) (Table 8).
4 Discussion
Our study revealed that CRF is a prevalent issue among various types of cancer survivors, yet it has not received sufficient attention in China (30). We included 17 kinds of tumors, among which gastrointestinal tumors, lung cancer, and breast cancer are the most common cancers with a high incidence of CRF. No significant differences were observed across cancer types, aligning with Wang XS’s findings (31). Besides, our study showed that the prevalence of fatigue in 75% of cancer survivors indicates that nearly two-thirds of these individuals will require fatigue management. But the reality is that only 10.5% of patients have heard of CRF, and less than 5% seeking active interventions. These findings emphasize the critical need to integrate CRF education and awareness programs into clinical settings, ensuring that patients are adequately informed about this debilitating condition.
Similar to previously studies (32, 33), our study found that gender, emotion state, and BMI were influential factors for CRF. Specifically, women, as well as individuals with higher depression scores, were found to be at a higher risk of CRF and increased fatigue, which aligns with existing studies (29, 34).Women tend to be more susceptible to anxiety and depression when facing changes in their body function and quality of life after cancer treatment (35). Hence, the NCCN panel recommended that in addition to regular fatigue assessment, emotional assessment be integrated into patient care at all stages—from admission to hospitalization and post-discharge—to prevent or alleviate fatigue in vulnerable populations (36). Due to pharmacologic interventions had limited study data in CRF patients and depression or anxiety, often presents as a cluster of symptoms alongside fatigue. As a result, interventions that affect multiple systems, such as complementary therapies, may be more recommended (37). Indeed, psychosocial, exercise, and mind-body interventions appear to be more beneficial for CRF than pharmacotherapy (38), perhaps because these approaches have effects on a range of biobehavioral processes relevant for fatigue.
Our study introduces some novel insights into the relationship between dietary habits, BMI, and CRF. Patients with dietary biases, such as those following a vegan or meat-based diet and those consuming a high-salt diet, appeared to be more prone to CRF. Furthermore, our multivariate regression analysis suggests that a BMI of ≥28 kg/m² may reduce the risk of CRF. These findings raise intriguing questions about the potential links between nutrition, obesity, and CRF. While previous studies have generally associated obesity with poorer cancer survival rates and increased fatigue, recent research has proposed the concept of an “obesity paradox” in cancer patients (39). Based on this point of view, many studies have proved that early obesity is related to a higher survival rate of cancer patients. For example, a meta-analysis showed that colorectal cancer patients with higher BMI had lower mortality than normal-weight patients (40), and another cross-sectional study showed that CRF development, inflammatory markers and fatty acid levels were mainly associated with class II (35.0-39.9 kg/m2) and class III (≥40.0kg/m2) obesity (41). In addition, a study that intervened breast cancer patients with a Mediterranean diet and exercise for 1 year showed that a traditional Mediterranean diet and weight loss reduced the level of fatigue in the patients (42). Thus, the NCCN guidelines state that cancer and treatment can interfere with dietary intake, nutrition consultation may be helpful in managing the nutritional deficiencies that result from anorexia, diarrhea, nausea, and vomiting (43). Our study contributes to this discussion, highlighting the complex relationship between body weight, diet, and CRF. Large RCTs are needed to determine the impact of nutrition therapy on CRF in the future.
Chronic stressors like anxiety and depression can affect CRF by influencing the hypothalamic-pituitary-adrenal (HPA) axis (19, 44). Through a negative feedback control loop, the HPA axis normally regulates the release of stress-related hormones (i.e., cortisol and ACTH) in response to physical or psychological stress (45). Our study suggests that high aldosterone levels are an important risk factor for CRF and increased fatigue. According to earlier research, patients with depression have dysregulation of the RAAS system, and their plasma aldosterone levels are 2.77 times higher than those of healthy people without psychological problems (46). Recent studies have pointed out that activation of the RAAS system is closely related to tumor proliferation and angiogenesis, and tumor progression is usually the direct cause of fatigue (47). Considering the key roles of the RAAS system and aldosterone in tumor progression and depression, we speculate that aldosterone may be an important predictor of the differential experience of fatigue. Because it was only a cross-sectional survey, the link between these findings and the CRF is exploratory. However, our findings and previous research indicate that CRF is linked to emotional distress (such as anxiety and depression) and stress-related hormones. Identifying the emotional distress and neuroendocrine alterations underlying CRF is an important focus for future research and has significant implications for interventions.
Our study has several limitations, in order to know the overall incidence of CRF in different tumors, so we did not limit the tumor species included, which may make the results lack relevance in some aspects. However, our analysis revealed that CRF is a common symptom among patients with different tumors, which is in line with our original idea to draw attention to CRF by investigating its prevalence. CRF is a multidimensional and subjective symptom, so we used the scale as an assessment tool to better reflect the patient’s immediate fatigue or psychological condition. On the other hand, because of the small sample size and single-center cross-sectional design, all the factors we assessed were obtained at one time point, and the relationship between CRF and these factors was exploratory, and the potential association needs to be confirmed by further prospective studies. In addition, because the scale we used was an examiner-rating scale, we could not exclude that patient modified some of the results; although the scale was a Chinese version, semantic and cultural differences were still found in the specific application, and two of the patients declined to complete the final survey because the scale entries were difficult to comprehend during the survey.
5 Conclusions
In conclusion, our study showed that gender, BMI, emotional distress, and aldosterone may be influential factors in the differential experience of fatigue. This underscores the importance of comprehensive assessments that consider fatigue, emotional health, and nutritional status, which are essential for preventing and reducing CRF and improving quality of life in the growing population of cancer survivors. Furthermore, the identification of neural processes and neuroendocrine alterations that influence fatigue may help in the development of targeted interventions for those most in need.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The Medical Ethics Committee of Xiyuan Hospital, China Academy of Chinese Medical Sciences (Approval number: 2021XLA027; Approval date: March 26, 2021). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YX, SG contributed to the study conception and design. Support in sample size estimation and choice of suitable measuring instruments/questionnaires was provided by DY and KL, SG, LG, JW, XG, LF, JS contributed to the data collection. SG performed the data analysis and wrote the paper YX, XZ, A.L revised the paper. All authors read and approved the final manuscript.
Funding
This work was supported by the National Key Research and Development Program of China (Grant Number: 2018YFC1707406).
Acknowledgments
We thank the patients who participated in this study. The authors would like to thank all the physicians and nurses who helped recruit patients at Xiyuan Hospital, China Academy of Chinese Medical Sciences. We appreciated the support of the National Key Research and Development Program of China (Research Project of Modernization on TCM, Grant number: 2018YFC1707406).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Morrow GR. Cancer-related fatigue: causes, consequences, and management. oncologist (2007) 12 Suppl 1:1–3. doi: 10.1634/theoncologist.12-S1-1
3. Fabi A, Falcicchio C, Giannarelli D, Maggi G, Cognetti F, Pugliese P. The course of cancer related fatigue up to ten years in early breast cancer patients: What impact in clinical practice? Breast (2017) 34:44–52. doi: 10.1016/j.breast.2017.04.012
4. Abrahams HJG, Gielissen MFM, Schmits IC, Verhagen CAHHVM, Rovers MM, Knoop H. Risk factors, prevalence, and course of severe fatigue after breast cancer treatment: a meta-analysis involving 12 327 breast cancer survivors. Ann Oncol (2016) 27(6):965–74. doi: 10.1093/annonc/mdw099
5. Fabi A, Bhargava R, Fatigoni S, Guglielmo M, Horneber M, Roila F, et al. Cancer-related fatigue: ESMO Clinical Practice Guidelines for diagnosis and treatment. Ann Oncol (2016) 27(6):965–74. doi: 10.1016/j.annonc.2020.02.016
6. Dolgoy N, Brose JM, Dao T, Suderman K, Gross D, Ho C, et al. Functional, work-related rehabilitative programming for cancer survivors experiencing cancer-related fatigue. Br J Occup Ther (2020) 84:212–21. doi: 10.1177/0308022620927351
7. Goedendorp MM, Jacobsen PB, Andrykowski MA. Fatigue screening in breast cancer patients: identifying likely cases of cancer-related fatigue. Psycho-Oncol (2016) 25(3):275–81. doi: 10.1002/pon.3907
8. Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J Clin Oncol (2000) 18(4):743–53. doi: 10.1200/JCO.2000.18.4.743
9. Nilsson R, Dahl AA, Bernklev T, Kersten H, Haug ES. Work status and work ability after radical prostatectomy or active surveillance for prostate cancer. Scand J Urol (2020) 54(3):194–200. doi: 10.1080/21681805.2020.1750473
10. Chen LM, Yang QL, Duan YY, Huan XZ, He Y, Wang C. Multidimensional fatigue in patients with nasopharyngeal carcinoma receiving concurrent chemoradiotherapy: incidence, severity, and risk factors. Support Care Cancer (2021) 29(9):5009–19. doi: 10.1007/s00520-021-06054-7
11. Sorensen HL, Schjolberg TK, Smastuen MC, Utne I. Social support in early-stage breast cancer patients with fatigue. BMC Womens Health (2020) 20(1):243. doi: 10.1186/s12905-020-01106-2
12. Hajj A, Chamoun R, Salameh P, Khoury R, Hachem R, Sacre H, et al. Fatigue in breast cancer patients on chemotherapy: a cross-sectional study exploring clinical, biological, and genetic factors. BMC Cancer (2022) 22(1):16. doi: 10.1186/s12885-021-09072-0
13. Hughes A, Suleman S, Rimes KA, Marsden J, Chalder T. Cancer-related fatigue and functional impairment - Towards an understanding of cognitive and behavioural factors. J Psychosom Res (2020) 134:110127. doi: 10.1016/j.jpsychores.2020.110127
14. Cameron B, Webber K, Li H, Bennett BK, Boyle F, de Souza P, et al. Genetic associations of fatigue and other symptoms following breast cancer treatment: A prospective study. Brain Behav Immun Health (2020) 10:100189. doi: 10.1016/j.bbih.2020.100189
15. Bower JE. Cancer-related fatigue–mechanisms, risk factors, and treatments. Nat Rev Clin Oncol (2014) 11(10):597–609. doi: 10.1038/nrclinonc.2014.127
16. Ryan JL, Carroll JK, Ryan EP, Mustian KM, Fiscella K, Morrow GR. Mechanisms of cancer-related fatigue. Oncologist (2007) 12 Suppl 1:22–34. doi: 10.1634/theoncologist.12-S1-22
17. Payne JK. Altered circadian rhythms and cancer-related fatigue outcomes. Integr Cancer Ther (2011) 10(3):221–33. doi: 10.1177/1534735410392581
18. al-Majid S, McCarthy DO. Cancer-induced fatigue and skeletal muscle wasting: the role of exercise. Biol Res Nurs (2001) 2(3):186–97. doi: 10.1177/109980040100200304
19. Aboalela N, Lyon D, Elswick RK Jr, Kelly DL, Brumelle J, Bear HD, et al. Perceived stress levels, chemotherapy, radiation treatment and tumor characteristics are associated with a persistent increased frequency of somatic chromosomal instability in women diagnosed with breast cancer: A one year longitudinal study. PloS One (2015) 10(7):e0133380. doi: 10.1371/journal.pone.0133380
20. Abrahams HJG, Gielissen MFM, de Lugt M, Kleijer EFW, de Roos WK, Balk E, et al. The Distress Thermometer for screening for severe fatigue in newly diagnosed breast and colorectal cancer patients. Psycho-Oncol (2017) 6(5):693–7. doi: 10.1002/pon.4208
21. Daniel LC, Meltzer LJ, Gross JY, Flannery JL, Forrest CB, Barakat LP. Sleep practices in pediatric cancer patients: Indirect effects on sleep disturbances and symptom burden. Psychooncology (2021) 30(6):910–8. doi: 10.1002/pon.5669
22. Lambert M, Brunet J, Couture-Lalande ME, Bielajew C. Aerobic physical activity and salivary cortisol levels among women with a history of breast cancer. Complement Ther Med (2019) 42:12–8. doi: 10.1016/j.ctim.2018.10.018
23. Schmidt ME, Semik J, Habermann N, Wiskemann J, Ulrich CM, Steindorf K. Cancer-related fatigue shows a stable association with diurnal cortisol dysregulation in breast cancer patients. Brain Behav Immun (2016) 52:98–105. doi: 10.1016/j.bbi.2015.10.005
24. Häfner S, Baumert J, Emeny RT, Lacruz ME, Bidlingmaier M, Reincke M, et al. To live alone and to be depressed, an alarming combination for the renin-angiotensin-aldosterone-system (RAAS). Psychoneuroendocrinology (2012) 37(2):230–7. doi: 10.1016/j.psyneuen.2011.06.007
25. Murck H, Schlageter L, Schneider A, Adolf C, Heinrich D, Quinkler M, et al. The potential pathophysiological role of aldosterone and the mineralocorticoid receptor in anxiety and depression - Lessons from primary aldosteronism. J Psychiatr Res (2020) 130:82–8. doi: 10.1016/j.jpsychires.2020.07.006
26. Glaus A. Assessment of fatigue in cancer and non-cancer patients and in healthy individuals. Support Care Cancer (1993) 1(6):305–15. doi: 10.1007/BF00364968
27. Van Belle S, Paridaens R, Evers G, Kerger J, Bron D, Foubert J, et al. Comparison of proposed diagnostic criteria with FACT-F and VAS for cancer-related fatigue: proposal for use as a screening tool. Support Care Cancer (2005) 13(4):246–54. doi: 10.1007/s00520-004-0734-y
28. So WK, Tai JW. Fatigue and fatigue-relieving strategies used by Hong Kong Chinese patients after hemopoietic stem cell transplantation. Nurs Res (2005) 54(1):48–55. doi: 10.1097/00006199-200501000-00007
29. Deng Y, He S, Wang J. Validation of the Hospital Anxiety and Depression Scale and the Perceived Stress Scale and psychological features in patients with periodontitis. J Periodontol (2021) 92(11):1601–12. doi: 10.1002/JPER.20-0756
30. Qiongyang L. Analysis of the symptomatic features and associated factors of cancer-caused fatigue in colorectal cancer patients. Beijing: Beijing University of Traditional Chinese Medicine (2019).
31. Wang XS, Zhao F, Fisch MJ, O'Mara AM, Cella D, Mendoza TR, et al. Prevalence and characteristics of moderate to severe fatigue: a multicenter study in cancer patients and survivors. Cancer (2014) 120(3):425–32. doi: 10.1002/cncr.28434
32. Sharour LA. Cancer-related fatigue, laboratory markers as indicators for nutritional status among patients with colorectal cancer. Nutr Cancer (2020) 72(6):903–8. doi: 10.1080/01635581.2019.1669674
33. Baden M, Lu L, Drummond FJ, Gavin A, Sharp L. Pain, fatigue and depression symptom cluster in survivors of prostate cancer. Support Care Cancer (2020) 28(10):4813–24. doi: 10.1007/s00520-019-05268-0
34. Bekhbat M, Treadway MT, Goldsmith DR, Woolwine BJ, Haroon E, Miller AH, et al. Gene signatures in peripheral blood immune cells related to insulin resistance and low tyrosine metabolism define a sub-type of depression with high CRP and anhedonia. Brain Behav Immun (2020) 88:161–5. doi: 10.1016/j.bbi.2020.03.015
35. Jim HS, Sutton SK, Jacobsen PB, Martin PJ, Flowers ME, Lee SJ. Risk factors for depression and fatigue among survivors of hematopoietic cell transplantation. Cancer (2016) 122(8):1290–7. doi: 10.1002/cncr.29877
36. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN guidelines®) cancer-related fatigue (2023). American: NCCN.
37. van Waart H, Stuiver MM, van Harten WH, Geleijn E, Kieffer JM, Buffart LM, et al. Effect of low-intensity physical activity and moderate- to high-intensity physical exercise during adjuvant chemotherapy on physical fitness, fatigue, and chemotherapy completion rates: results of the PACES randomized clinical trial. J Clin Oncol (2015) 33(17):1918–27. doi: 10.1200/JCO.2014.59.1081
38. Mustian KM, Alfano CM, Heckler C, Kleckner AS, Kleckner IR, Leach CR, et al. Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue a meta-analysis. JAMA Oncol (2017) 3(7):961–8. doi: 10.1001/jamaoncol
39. Lennon H, Sperrin M, Badrick E, Renehan AG. The obesity paradox in cancer: a review. Curr Oncol Rep (2016) 18(9):56. doi: 10.1007/s11912-016-0539-4
40. Li Y, Li C, Wu G, Yang W, Wang X, Duan L, et al. The obesity paradox in patients with colorectal cancer: a systematic review and meta-analysis. Nutr Rev (2022) 80(7):1755–68. doi: 10.1093/nutrit/nuac005
41. Inglis JE, Kleckner AS, Lin PJ, Gilmore NJ, Culakova E, VanderWoude AC, et al. Excess body weight and cancer-related fatigue, systemic inflammation, and serum lipids in breast cancer survivors. Nutr Cancer (2021) 73(9):1676–86. doi: 10.1080/01635581.2020.1807574
42. Montagnese C, Porciello G, Vitale S, Palumbo E, Crispo A, Grimaldi M, et al. Quality of life in women diagnosed with breast cancer after a 12-month treatment of lifestyle modifications. Nutrients (2020) 13(1):136. doi: 10.3390/nu13010136
43. Brown JK. A systematic review of the evidence on symptom management of cancer-related anorexia and cachexia. Oncol Nurs Forum (2002) 29(3):517–32. doi: 10.1188/02
44. Brown LF, Kroenke K. Cancer-related fatigue and its associations with depression and anxiety: a systematic review. Psychosomatics (2009) 50(5):440–7. doi: 10.1016/j.bbi.2020.03.015
45. Hsiao FH, Jow GM, Kuo WH, Wang MY, Chang KJ, Lai YM, et al. A longitudinal study of diurnal cortisol patterns and associated factors in breast cancer patients from the transition stage of the end of active cancer treatment to post-treatment survivorship. Breast (2017) 36:96–101. doi: 10.1016/j.breast.2017.06.016
46. Emanuele E, Geroldi D, Minoretti P, Coen E, Politi P. Increased plasma aldosterone in patients with clinical depression. Arch Med Res (2005) 36(5):544–8. doi: 10.1016/j.arcmed.2005.03.046
Keywords: cancer-related fatigue, cancer survivors, influencing factors, anxiety, depression, stress-related hormones
Citation: Gu S, Xu Y, Zhu X, Lam A, Yi D, Gong L, Wang J, Guo X, Fu L, Shi J, Wang F and Liu K (2023) Characteristics of cancer-related fatigue and its correlation with anxiety, depression, and stress-related hormones among Chinese cancer survivors: a cross-sectional study. Front. Oncol. 13:1194673. doi: 10.3389/fonc.2023.1194673
Received: 27 April 2023; Accepted: 05 October 2023;
Published: 26 October 2023.
Edited by:
Maya Bizri, American University of Beirut, LebanonReviewed by:
Pritanjali Singh, All India Institute of Medical Sciences (Patna), IndiaSemra Bulbuloglu, Istanbul Aydın University, Türkiye
Copyright © 2023 Gu, Xu, Zhu, Lam, Yi, Gong, Wang, Guo, Fu, Shi, Wang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yun Xu, eHl4aWFvNzhAMTYzLmNvbQ==
 Shanshan Gu
Shanshan Gu Yun Xu
Yun Xu Xiaoshu Zhu
Xiaoshu Zhu Anderson Lam2
Anderson Lam2