
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 19 May 2023
Sec. Hematologic Malignancies
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1193874
This article is part of the Research TopicNovelties in Acute Myeloid Leukemia: From Biology to Clinical ApplicationsView all 13 articles
 Baohang Zhang1,2†
Baohang Zhang1,2† Qingguo Liu1,2*†
Qingguo Liu1,2*† Junfan Li1,2
Junfan Li1,2 Yimin Hu1,2
Yimin Hu1,2 Xin Zhao1,2
Xin Zhao1,2 Pingping Huang1,2
Pingping Huang1,2 Shangzhu Li1,2
Shangzhu Li1,2 Ying Wang1,2
Ying Wang1,2Background: The efficacy of induction chemotherapy (IC) for acute myeloid leukemia (AML) has improved significantly with the application of targeting drugs. Our previous study showed that a 4-day IC regimen of cyclophosphamide (CTX) and Ara-C [CA (4 + 3)] achieved similar complete remission (CR) rate (80%) compared with the traditional 7-day regimen, and the survival rate appeared to be better.
Methods: In this pilot study, we further shortened the CA regimen to 3 days, added low-dose venetoclax (VEN, 200 mg/day) (VCA), and reported the efficacy and safety here.
Results: Twenty-five newly diagnosed adult AML patients were enrolled in this study and evaluated for the remission rate after one cycle of the VCA regimen. The CR/Cri was 92%, and all these patients had undetectable minimal residual disease (MRD−). The estimated overall survival at 12 months was 79.3%. The median time for both platelet recovery and absolute neutrophil count recovery was 16 days, faster than that of traditional IC. Compared with the previous CA (4 + 3) regimen, a higher CR rate (92% vs. 80%, P < 0.01) and a deeper degree of remission (CRMRD− rate, 92% vs. 45%, P < 0.01) were found in the VCA group.
Conclusions: This study showed that the 3-day CTX and Ara-C regimen is highly effective in newly diagnosed AML patients, and the addition of VEN to the CA regimen achieves higher and deeper one-course remission.
Acute myeloid leukemia (AML) is a malignant disorder of hematopoietic stem cells. It accounts for approximately 80% of adult acute leukemia. Optimization of induction chemotherapy (IC), consolidation chemotherapy, or intensive chemotherapy to enhance the clearance of leukemia cells has the potential to improve survival. So far, anthracycline combined with cytarabine (Ara-C) is the first-line IC regimen for AML, and the complete remission (CR) rate after one or two courses of treatment is up to 80% (1, 2). Venetoclax (VEN), a selective BCL-2 inhibitor, may partly overcome the difficulty of treatment caused by the genetic heterogeneity of AML and improve the CR rate. The combination of VEN with hypomethylating agents or low-dose Ara-C in older or unfit newly diagnosed AML has shown significant improvement in overall survival (OS) (3–5). Furthermore, VEN combined with cytotoxic drugs as IC showed that CR rates could exceed 90% (6). These studies suggest that the combined chemotherapy regimens with VEN have a synergistic function.
We previously reported a 4-day IC regimen in AML that includes 4-day cyclophosphamide (CTX) and 3-day Ara-C [CA (4 + 3)] (7). The CR rate was 80%. Among the patients who completed three courses of consolidation chemotherapy, the actual 5-year disease-free survival (DFS) rate was 64%. Since the addition of VEN to IC may improve the CR rate, we further explored a 3-day CA regimen combined with a 7-day VEN (VCA) to explore whether the CR rate could be increased and preliminarily evaluated the survival rate. Correspondingly, a comparison between VCA and historical CA (4 + 3) was reported here.
This study was carried out in the Institute of Hematology and Blood Diseases Hospital, CAMS & PUMC, between April 2021 and July 2022. Patients with newly diagnosed AML [defined by the World Health Organization (8)] were enrolled and classified into three risk groups according to the 2017 European Leukemia Net (ELN) criteria (9). The primary endpoint was CR rate, including CR and CR with incomplete blood count recovery (CRi) according to the modified International Working Group criteria (10). The secondary endpoints included overall survival (OS), minimal residual disease (MRD), response rates, event-free survival (EFS), disease-free survival (DFS), durable remissions, and adverse events. The historical CA (4 + 3) set was used for control. This study was approved by the Ethical Committee of the Institute of Hematology and Blood Diseases Hospital. Informed consent was obtained from the patients and their legal guardians in accordance with the Declaration of Helsinki.
A combined regimen of VEN, CTX, and Ara-C was used as induction chemotherapy. VEN was given orally at the dosage of 200 mg per day from day 1 to day 7. Ara-C (1 g/m2) was administered intravenously every 12 h from day 1 to day 3. CTX was administered at 20 mg/kg/day from day 1 to day 3. Posaconazole was used concomitantly to prevent invasive fungal infections and act synergistically with VEN. Patients with intermediate or poor prognosis were recommended for allogeneic hematopoietic stem cell transplantation (allo-HSCT) once the first CR was achieved. Patients unable to perform allo-HSCT were given consolidation therapy according to the NCCN guidelines. Patients remained on study for OS assessment and follow-up even if they accepted other kinds of treatment.
Adverse events (AEs) were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0 (https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50). Treatment-emergent AEs, including clinical tumor lysis syndrome (TLS), were defined as those that occurred between the first dose of the study drug and 30 days after the last dose of the study drug. Clinical and laboratory TLS was defined according to the criterion reported by Howard et al. (11).
Response assessment was performed between 28 and 35 days after chemotherapy, including bone marrow morphology, cytogenetics, and genetic detection. Flow cytometry was used to quantify the MRD of the marrow. Responses were defined according to the European Leukemia Net recommendations (9): CR as <5% of bone marrow blasts with normal peripheral blood counts (neutrophils ≥ 1.0 × 109/L, platelets ≥ 100 × 109/L) and CR with incomplete hematologic recovery (CRi, neutrophils < 1.0 × 109/L, and/or platelets < 100 × 109/L). Neutrophil recovery was defined as days from the start of induction therapy to neutrophil count recovered to >0.5 × 109/L. Platelet recovery was defined as days from the start of chemotherapy to platelets recovered to >20 × 109/L for twice evaluation without platelet transfusion.
SPSS (version 25.0, Chicago, IL, USA) was used for the statistical analysis. Comparisons between categorical variables were performed with the χ2 test or Fisher’s exact test. The differences between continuous variables were compared using the t-test or the Mann–Whitney U test. Overall survival was evaluated by the Kaplan–Meier method, and the statistical differences between the two groups were evaluated using the log-rank test. P <0.05 was defined as statistically significant.
Twenty-five patients at a median age of 47.4 (range 27–68) years were enrolled in this study, consisting of 16 men (64%) and 9 women (36%). The clinical characteristics of the patients are shown in Table 1. All patients were diagnosed with de novo AML. Twenty-three patients had complications at admission. Infections were the most common, including six pulmonary infections (two hemoptysis), three invasive fungal infections, four pharyngeal or gingival infections, and three neutropenic fevers. Other comorbidities included cardiac insufficiency, vomiting, hypokalemia, and hypoproteinemia.
The ELN risk stratification showed favorable prognosis in 44% of patients, intermediate prognosis in 32% of patients, and adverse prognosis in 24% of patients. All patients had gene mutations related to AML at diagnosis detected by next-generation sequencing (NGS) (Figure 1). Recurrent mutations in RUNX1/RUNXT1, CBFβ-MYH11, NPM1, and CEBPA were found in 11 patients. Three of them also carried mutations in TP53, ASXL1, and/or RUNX1. Another patient had mutations in TP53, ASXL1, or KMT2A. Mutations in the class II gene WT1 were found in 18 patients (76%).
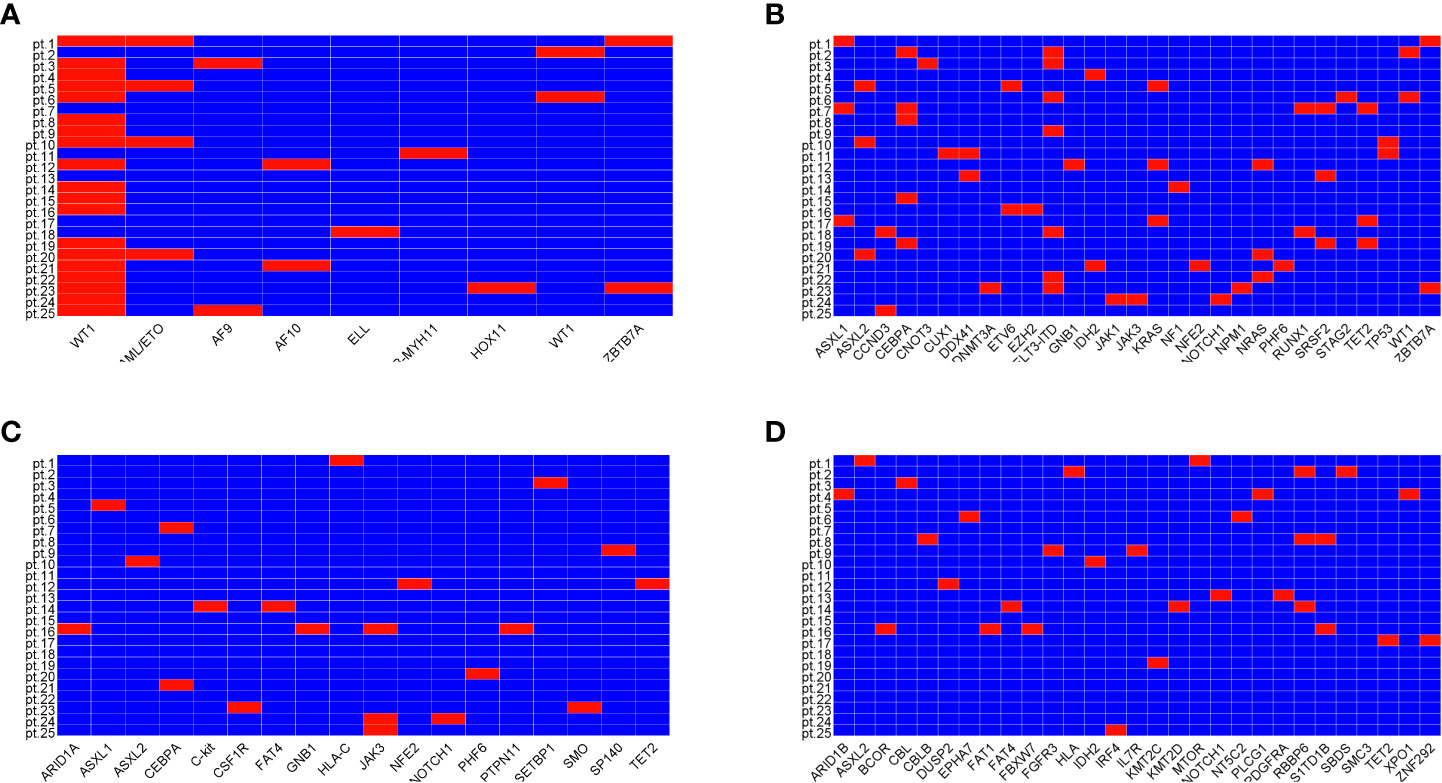
Figure 1 Mutations of genes related to diseases. Mutations of class II genes (A), mutations of class I genes highly related to disease (B), mutation of class I genes moderately related to disease (C), and mutations of class I genes with unknown correlation with disease (D).
Two patients were discharged shortly after chemotherapy due to financial reasons although their hematopoiesis had not yet recovered, and they were confirmed to have died at a later follow-up. Therefore, these two patients were evaluated as having no remission. The other 23 patients all achieved CR (including one CRi) after one course of VCA regimen and the CR rate was 92%. All those 23 patients showed MRD negativity, including the one with CRi (Table 2).
Compared with the previous CA (4 + 3) regimen, patients who received VCA achieved a higher CRMRD− rate (92% vs. 45%, P < 0.01). The time required for platelet recovery (≥20 × 109/L) and neutrophil recovery (≥0.5 × 109/L) was assessed among patients who achieved CR/CRi. The median time for both platelet recovery and neutrophil recovery was 16 (9–20) days, and it had no difference compared with that of the CA (4 + 3) group. The plasma concentration of VEN was detected in five patients and the mean level was 1,880 ng/ml.
The last follow-up of the patients in the VCA group was in December 2022, and the median time of follow-up was 18.6 (1–20.3) months. Two patients died 1 month after IC. Three patients with adverse prognosis stratification relapsed (12%, 3/25) and finally died of the disease. Another patient died of infection during consolidation therapy. Three patients accepted allo-HSCT. By the end of follow-up, 19 patients were alive and remained CR. The median duration of OS was not reached. The estimated OS at 12 months was 79.3%.
The overall survival rate of VCA and CA (4 + 3) is shown in Figure 2. We further combined the data of patients in the VCA group and the CA (4 + 3) group to analyze the predictors for OS. Multivariate analysis showed that VCA regimen use (odds ratio 0.08; 95% CI, 0.01 to 0.59, P = 0.013), younger age (odds ratio 1.08; 95% CI, 1.01 to 1.16, P = 0.025), and the ELN risk stratification of favorable prognosis (odds ratio 0.05; 95% CI, 0 to 0.6, P = 0.018) were associated with better survival.
A summary of treatment-related AEs in patients who received the VCA regimen is shown in Table 2. Patients who received VCA had a lower count of white blood cells compared with those who received CA (4 + 3) (0.02 × 109/L vs. 0.11 × 109/L, P = 0.002). However, the infection rate and infection severity were similar between the two groups. The most common origins of infections were the intestines and lungs. Laboratory-defined TLS was found in one patient (elevations of potassium, phosphorus, and uric acid) without clinical symptoms.
Previous studies of VEN have shown significant improvement in OS when combined with hypomethylating agents (HMAs). A 25% reduction in the risk of death was reported with the combination of VEN, showing that VEN plus LDAC was associated with improvement in median OS (7.2 vs. 4.1 months) (12). Higher response rates and OS were reported using VEN and azacitidine (AZA) than AZA alone (response rate, 66.4% vs. 28.3%; OS, 14.7 vs. 9.6 months) (13). VEN combined with intensive chemotherapy such as CLIA and FLAG-IDA for newly diagnosed AML or high-risk myelodysplastic syndrome also showed encouraging results (3, 14, 15). In our study, the median OS was 23.5 months in the CA (4 + 3) regimen, and it was not reached in the VCA regimen (P = 0.089), demonstrating improved survival. The estimated OS at 12 months of the VCA regimen is 79.3%. This cohort of the study showed that the addition of VEN to the CA regimen leads to a one-course CR rate of up to 92% in adult AML, higher than that of the previous CA (4 + 3) regimen, supporting the role of VEN in improving the inducible remission rate.
A meta-analysis suggested that MRD negativity is associated with better DFS and OS in AML patients (16). Patients who achieved morphological remission but had detectable minimal diseases (MRD+) are at high risk of relapse (17). For patients under 40 years old, consolidation therapy containing high-dose Ara-C reduces relapse rate (18). In the current study, all the patients with CR/CRi achieved MRD negativity (100%). Compared with the one-course MRD-negative rate of 45% in the historical CA (4 + 3) group, patients who received VCA achieved deeper remission regardless of their prognostic stratification. Our results herald the ability of VEN in clearing leukemic cells (19). However, the sample size of this study is small, and further prospective study with a large sample size is required to validate the results. Nevertheless, this study showed that the combination of low-dose VEN with CA achieved deeper remission in newly diagnosed AML.
The hematologic toxicity of IC is highly associated with the duration of agranulocytosis which most likely causes life-threatening infections. The median time to ANC recovery of the VCA regimen was 16 days, much shorter than 29 days in traditional induction chemotherapy (20, 21). This result may be attributed to the short chemotherapy duration of CTX and Ara-C (3 days) and the low total dose but high blood concentration of VEN. VEN is a substrate of cytochrome P450 (CYP) 3A enzyme (CYP3A4). Posaconazole, which is used to prevent invasive fungal infections, also functions as a potent inhibitor of CYP3A4. Hence, the concomitant use of posaconazole and VEN can increase the blood concentration of VEN. The total dosage of VEN in our study is 1,400 mg, much lower than that used in previous reports (4,800, 5,600, 2,800, and 2,700 mg, respectively) (3, 6, 14, 15), but the blood concentration is high. Thus, the fast ANC recovery of VCA results in a lower percentage of severe infection and death caused by infections.
In conclusion, we demonstrate that the VCA regimen could achieve a high CR and CRMRD− rate and long-term survival than traditional IC regimens in newly diagnosed AML. Patients also benefit from the shorter ANC recovery time. A prospective study with a large sample size is required to validate the results.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
This study was approved by the Ethical Committee of the Institute of Hematology and Blood Diseases Hospital. Informed consent was obtained from the patients and/or their legal guardians in accordance with the Declaration of Helsinki.
QL designed the study, enrolled the patients, analyzed the data, and wrote the manuscript. BZ performed the statistical analysis and drafted the manuscript. JL, YH, PH, and SL enrolled the patients and edited the manuscript. XZ and YW analyzed the data and edited the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by the National Natural Science Foundation of China (grant no. 81900127).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Willemze R, Suciu S, Meloni G, Labar B, Marie JP, Halkes CJ, et al. High-dose cytarabine in induction treatment improves the outcome of adult patients younger than age 46 years with acute myeloid leukemia: results of the EORTC-GIMEMA AML-12 trial. J Clin Oncol (2014) 32(3):219–28. doi: 10.1200/jco.2013.51.8571
2. Bassan R, Intermesoli T, Masciulli A, Pavoni C, Boschini C, Gianfaldoni G, et al. Randomized trial comparing standard vs sequential high-dose chemotherapy for inducing early CR in adult AML. Blood Adv (2019) 3(7):1103–17. doi: 10.1182/bloodadvances.2018026625
3. Kadia TM, Reville PK, Borthakur G, Yilmaz M, Kornblau S, Alvarado Y, et al. Venetoclax plus intensive chemotherapy with cladribine, idarubicin, and cytarabine in patients with newly diagnosed acute myeloid leukaemia or high-risk myelodysplastic syndrome: a cohort from a single-centre, single-arm, phase 2 trial. Lancet Haematol (2021) 8(8):e552–61. doi: 10.1016/s2352-3026(21)00192-7
4. Cherry EM, Abbott D, Amaya M, McMahon C, Schwartz M, Rosser J, et al. Venetoclax and azacitidine compared with induction chemotherapy for newly diagnosed patients with acute myeloid leukemia. Blood Adv (2021) 5(24):5565–73. doi: 10.1182/bloodadvances.2021005538
5. Shimony S, Stone RM, Stahl M. Venetoclax combination therapy in acute myeloid leukemia and myelodysplastic syndromes. Curr Opin Hematol (2022) 29(2):63–73. doi: 10.1097/moh.0000000000000698
6. Wang H, Mao L, Yang M, Qian P, Lu H, Tong H, et al. Venetoclax plus 3 + 7 daunorubicin and cytarabine chemotherapy as first-line treatment for adults with acute myeloid leukaemia: a multicentre, single-arm, phase 2 trial. Lancet Haematol. (2022) 9:e415–24. doi: 10.1016/s2352-3026(22)00106-5
7. Liu Q, Gao H, Li J, Hu Y, Wu L, Zhao X, et al. Combination of cyclophosphamide and cytarabine as induction regimen for newly diagnosed adult acute myeloid leukemia. EJHaem (2020) 1(1):79–85. doi: 10.1002/jha2.76
8. Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the world health organization classification of myeloid neoplasms and acute leukemia. Blood (2016) 127(20):2391–405. doi: 10.1182/blood-2016-06-721662
9. Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood (2017) 129(4):424–47. doi: 10.1182/blood-2016-08-733196
10. Cheson BD, Bennett JM, Kopecky KJ, Büchner T, Willman CL, Estey EH, et al. Revised recommendations of the international working group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol (2003) 21(24):4642–9. doi: 10.1200/jco.2003.04.036
11. Howard SC, Jones DP, Pui CH. The tumor lysis syndrome. N Engl J Med (2011) 364(19):1844–54. doi: 10.1056/NEJMra0904569
12. Wei AH, Montesinos P, Ivanov V, DiNardo CD, Novak J, Laribi K, et al. Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: a phase 3 randomized placebo-controlled trial. Blood (2020) 135(24):2137–45. doi: 10.1182/blood.2020004856
13. DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med (2020) 383(7):617–29. doi: 10.1056/NEJMoa2012971
14. Chua CC, Roberts AW, Reynolds J, Fong CY, Ting SB, Salmon JM, et al. Chemotherapy and venetoclax in elderly acute myeloid leukemia trial (CAVEAT): a phase ib dose-escalation study of venetoclax combined with modified intensive chemotherapy. J Clin Oncol (2020) 38(30):3506–17. doi: 10.1200/jco.20.00572
15. DiNardo CD, Lachowiez CA, Takahashi K, Loghavi S, Xiao L, Kadia T, et al. Venetoclax combined with FLAG-IDA induction and consolidation in newly diagnosed and relapsed or refractory acute myeloid leukemia. J Clin Oncol (2021) 39(25):2768–78. doi: 10.1200/jco.20.03736
16. Short NJ, Zhou S, Fu C, Berry DA, Walter RB, Freeman SD, et al. Association of measurable residual disease with survival outcomes in patients with acute myeloid leukemia: a systematic review and meta-analysis. JAMA Oncol (2020) 6(12):1890–9. doi: 10.1001/jamaoncol.2020.4600
17. Cluzeau T, Lemoli RM, McCloskey J, Cooper T. Measurable residual disease in high-risk acute myeloid leukemia. Cancers (Basel) (2022) 14(5):1278. doi: 10.3390/cancers14051278
18. Kim DS, Kang KW, Lee SR, Park Y, Sung HJ, Kim SJ, et al. Comparison of consolidation strategies in acute myeloid leukemia: high-dose cytarabine alone versus intermediate-dose cytarabine combined with anthracyclines. Ann Hematol (2015) 94(9):1485–92. doi: 10.1007/s00277-015-2389-9
19. Montero J, Letai A. Why do BCL-2 inhibitors work and where should we use them in the clinic? Cell Death Differ (2018) 25(1):56–64. doi: 10.1038/cdd.2017.183
20. Lee JH, Joo YD, Kim H, Bae SH, Kim MK, Zang DY, et al. A randomized trial comparing standard versus high-dose daunorubicin induction in patients with acute myeloid leukemia. Blood (2011) 118(14):3832–41. doi: 10.1182/blood-2011-06-361410
Keywords: venetoclax, cyclophosphamide, acute myeloid leukemia, induction chemotherapy, cytarabine
Citation: Zhang B, Liu Q, Li J, Hu Y, Zhao X, Huang P, Li S and Wang Y (2023) Venetoclax plus cyclophosphamide and cytarabine as induction regimen for adult acute myeloid leukemia. Front. Oncol. 13:1193874. doi: 10.3389/fonc.2023.1193874
Received: 25 March 2023; Accepted: 24 April 2023;
Published: 19 May 2023.
Edited by:
Anna Maria Testi, Sapienza University of Rome, ItalyReviewed by:
Weiming Li, Huazhong University of Science and Technology, ChinaCopyright © 2023 Zhang, Liu, Li, Hu, Zhao, Huang, Li and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qingguo Liu, bGl1cWluZ2d1b0BpaGNhbXMuYWMuY24=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.