- 1Department of Surgery, Division of Surgical Oncology, The Ohio State University Wexner Medical Center, Columbus, OH, United States
- 2Department of Internal Medicine, Division of Medical Oncology at the Arthur G. James Cancer Hospital and Richard J. Solove Research Institute, The Ohio State University Comprehensive Cancer Center, Columbus, OH, United States
- 3Department of Radiation Oncology, The Ohio State University, Comprehensive Cancer Center-James Hospital and Solove Research Institute, Columbus, OH, United States
Hepatocellular Carcinoma (HCC) is one of the most common cancers and a leading cause of cancer related death worldwide. Until recently, systemic therapy for advanced HCC, defined as Barcelona Clinic Liver Cancer (BCLC) stage B or C, was limited and ineffective in terms of long-term survival. However, over the past decade, immune check point inhibitors (ICI) combinations have emerged as a potential therapeutic option for patients with nonresectable disease. ICI modulate the tumor microenvironment to prevent progression of the tumor. Radiotherapy is a crucial tool in treating unresectable HCC and may enhance the efficacy of ICI by manipulating the tumor microenvironment and decreasing tumor resistance to certain therapies. We herein review developments in the field of ICI combined with radiotherapy for the treatment of HCC, as well as look at challenges associated with these treatment modalities, and review future directions of combination therapy.
Introduction
Hepatocellular Carcinoma (HCC) is one of the most common cancers and a leading cause of cancer-related death worldwide (1). Risk factors for HCC include hepatitis B virus (HBV), hepatitis C virus (HCV), non-alcoholic fatty liver disease (NAFLD), alcoholic cirrhosis, tobacco use, and inherited disorders such as hemochromatosis, Wilson’s disease, and alpha-1 antitrypsin deficiency (2). Treatment options for HCC are various and depend on extent of tumor burden, underlying liver disease, and performance status. Options for treatment include resection, transplantation, locoregional and systemic therapies. Given various treatment options, a multi-disciplinary approach to care is essential, with surgical resection or transplant offering the best chance for cure. Unfortunately, many patients are not eligible for surgery given the advanced stage of disease at diagnosis. Consequently, HCC has a poor prognosis with five-year survival of 20-40% (3, 4).
Until recently, systemic therapy for advanced HCC was limited to tyrosine-kinase inhibitors (TKI) and ramucirumab (for AFP > 400ng/ml) and were ineffective in improving long-term survival. First line systemic therapy consisted of sorafenib, a tyrosine kinase inhibitor (5). However, over the past decade, immune check point inhibitors (ICI) combinations have emerged as a potential therapeutic option for patients with advanced stage disease, defined as Barcelona Clinic Liver Cancer (BCLC) stage B or C. ICI have a proven benefit in a multitude of other malignancies such as melanoma, breast, and colon cancer among others, but only recently has been this success been extrapolated to HCC (6–8). The IMbrave 150 trial demonstrated that, compared to sorafenib, the combination of atezolizumab (PD-L1 inhibitor) and bevacizumab (VEGF inhibitor) had improved overall (OS) and progression free survival (PFS) in patients with unresectable HCC (9). Additionally, the CheckMate 040 trial showed promise in the use of nivolumab (PD-1 inhibitor) and ipilimumab (anti-CTLA-4) with combination therapy having favorable objective response rates (ORR) among patients who had previously been treated with sorafenib (10).
The tumor microenvironment and its interaction with host immune cells plays an integral role in preventing cancer progression. Cirrhosis and chronic inflammation from viral hepatitis, alcohol or NAFLD can lead to changes in the hepatic immune response that favor carcinogenesis, which is part of the pathophysiology of HCC. ICI modulate the tumor microenvironment to prevent progression of tumor disease. Immunotherapy has demonstrated improvement in survival after chemoradiation, and it is now considered standard of care in certain cancers, such as non-small cell lung cancer (11). Recently, radiotherapy, in addition to ICI, has demonstrated potential in the treatment of advanced HCC. Radiotherapy is a crucial tool in the treatment of unresectable HCC, and the combination of radiotherapy with sorafenib has demonstrated improved OS and PFS (12). Radiotherapy may enhance the efficacy of ICI by manipulating the tumor microenvironment and decreasing tumor resistance to certain therapies (13–15). Given this, the objective of the current review is to highlight developments in the field of ICI combined with radiotherapy for the treatment of HCC, characterize challenges with these treatment modalities, as well as highlight future directions of combination therapy.
Methods
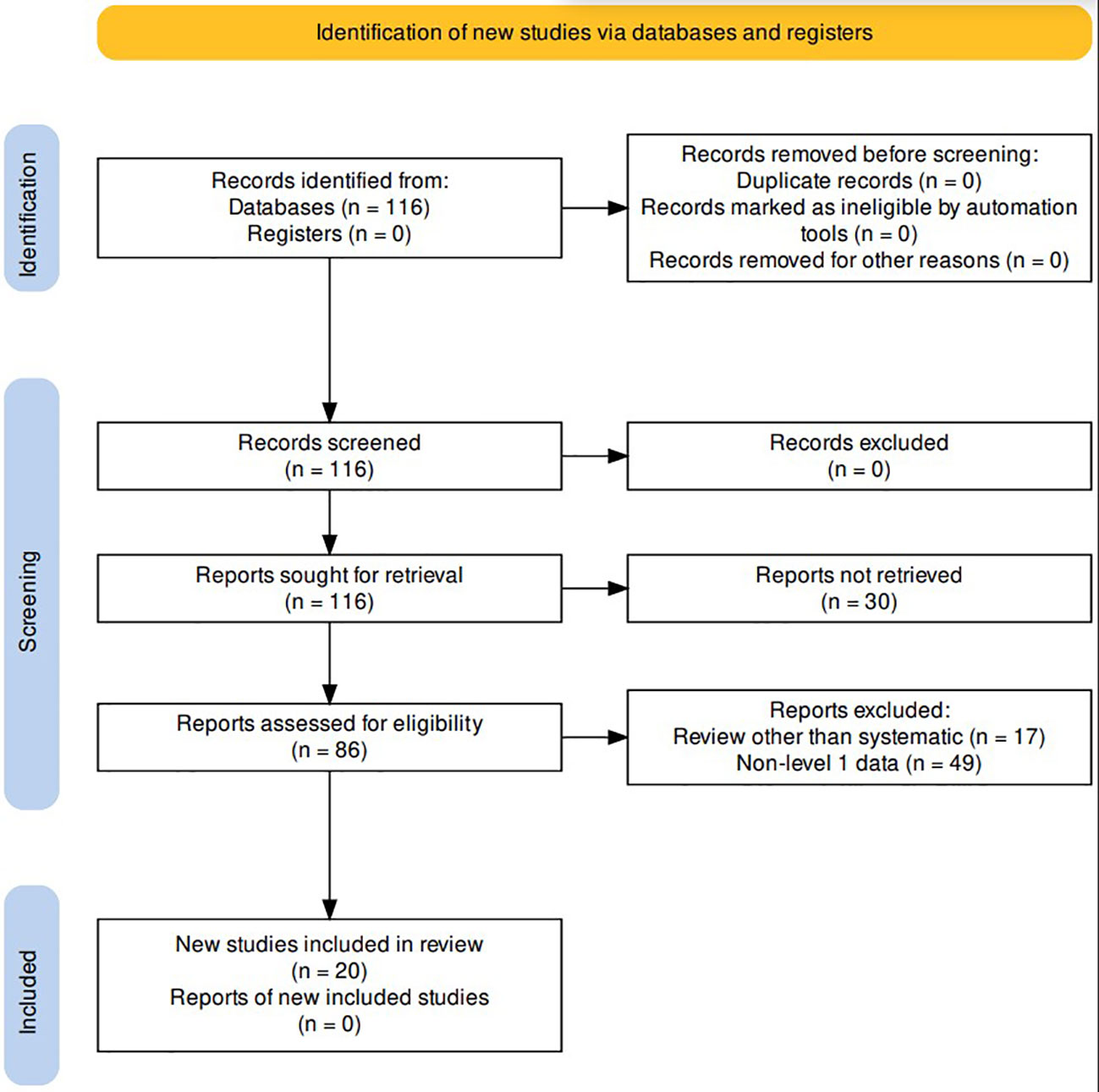
A comprehensive review of the literature was conducted in the PubMed database for studies published between January 2010 through February 2023. The following keywords and MESH terms were included in the search: “radiotherapy,” “immune checkpoint inhibitor,” “hepatocellular carcinoma.” Records were excluded if not written in English or if the full report was not available; reviews that were not systematic in nature (n=17), as well as reports that did not include level 1 data (n=49) were also excluded. A total of 20 studies were included in the final analysis (Figure 1).
Immune checkpoint inhibition for HCC
ICI target inhibitory and stimulatory immunoreceptors, as well as act as regulators of the immune system. ICI therapy has less systemic side effects than cytotoxic chemotherapy with may have more durable responses compared with targeted therapies (16). Tumors cells are unique in their ability to downregulate stimulatory immunoreceptors while upregulating inhibitory immunoreceptors, thereby evading host immune cells and allowing propagation of malignant cells (17). The tumor microenvironment contributes to the suppression of many host innate and adaptive immune cells (18). Among the most prominent and well-studied immunoreceptors responsible for this evasion are PD-1 and PD-L1 and CTLA-4 (19–21). By blocking these immunoreceptors, ICI enhance the antitumor function of host immune cells to curb the spread of malignant cells (16).
Recent trials have demonstrated the efficacy of ICI compared to systemic cytotoxic chemotherapy among patients with advanced HCC. Initially, single agent monotherapy was utilized for treatment; however, the CheckMate 459 study noted no improvement in OS or PFS among patient treated with nivolumab (PD-1 inhibitor) versus sorafenib (22). In turn, focus shifted to combination ICI therapy to address tumor heterogeneity. As noted, the IMbrave 150 trial was a landmark phase III multi-center global trial that compared atezolizumab plus bevacizumab (Atezo-Bev) versus sorafenib among patients with unresectable HCC. Importantly, Atezo-Bev was associated with an improved one-year OS (67.2% Atezo-Bev versus 54.6% sorafenib) and PFS (6.8 months Atezo-Bev versus 4.3 months sorafenib) (23). A recent update of the study from 2022 noted continued improvement in OS and PFS in the Atezo-Bev group (9). Consequently Atezo-Bev was recommended as first line treatment for advanced HCC according to the 2022 BCLC guidelines (5).
Other studies of ICI have investigated tremelimumab, an anti-CTLA-4 monoclonal antibody in combination with durvalumab, an anti-PD-L1 monoclonal antibody. The phase III HIMALAYA trial evaluated 1000 patients with unresectable HCC who had no prior treatment who had preserved liver function with good performance status (24). This study noted that combination therapy with tremlimumab and durvalumab was associated with improvement in ORR and OS versus sorafenib (ORR 20 vs 5% and OS 16.4 vs 13.3 months, respectively) (24). Additionally, the trial demonstrated the inferiority of durvalumab monotherapy versus sorafenib. The safety profiles were similar and main side effects were rash and transaminitis (25). Overall, the HIMALAYA trial demonstrated that tremelimumab and durvalumab may be an acceptable first line alternative to Atezo-Bev.
Another combination therapy regimen of nivolumab, a PD-1 inhibitor, and ipilimumab, another CTLA-4 inhibitor was evaluated in the CheckMate 040 trial (10). In this study, combination therapy with these two drugs was compared with nivolumab monotherapy among 148 patients who had advanced HCC and had previously been treated with sorafenib (10). Of note, there was improved ORR and OS among patients in the combination group; however, there were more side effects including hypothyroidism and adrenal insufficiency. Nivolumab monotherapy has also demonstrated to improve outcomes among patients with HCC, although its impact seemed to be augmented by the addition of ipilimumab (22). Another study assessed pembrolizumab monotherapy as second line treatment for advanced HCC among patients previously treated with sorafenib; OS, ORR, and PFS) were all improved versus placebo (26). As such, pembrolizumab, a anti PD-1 monoclonal antibody, has been approved in the United States as second line therapy for advanced HCC previously treated with sorafenib (27).
Radiotherapy for HCC
Radiotherapy (RT), including external beam radiotherapy (EBRT) and stereotactic body radiotherapy (SBRT) and radioembolization (RE), has evolved over the years to play a crucial role in the treatment of certain cancers, including lung and rectal cancer (28, 29). RT’s role in the treatment of unresectable HCC compared to or in combination with other locoregional therapies continues to emerge, with SBRT and RE options being used in select patients with intermediate and advanced stage HCC (30, 31). Early RT techniques, such as EBRT damaged not only the HCC but also surrounding healthy liver parenchyma, leading to liver insufficiency and radiation induced hepatitis (32). Additional adverse effects from EBRT included ulcers, gastrointestinal bleeding, and pneumonitis (33). More modern techniques have allowed radiation oncologists to target more focal areas of the liver limiting damage to surrounding healthy tissue/viscera. Techniques such as SBRT, in which there is a limited number of high dose RT fractions delivered to a focused area of tumor, minimizes the amount of extraneous radiation to other healthy tissue (34). RE or selective internal radiation therapy is another recent technique that provides focused radiation via radio-labeled Yttrium-90 microspheres directly into the hepatic artery (35). HCC is a hypervascular tumor, with tumor being preferentially supplied by the hepatic arteries and normal hepatocytes receiving their blood supply from the portal vein (36). Consequently, radiation preferentially travels to the tumor via the hepatic arteries (37).
Study on SBRT has increased over the past decade. Wahl et al. compared 224 patients with unresectable HCC without metastases treated with SBRT versus radiofrequency ablation (RFA), demonstrating lower local progression in the SBRT group at one and two years and equivalent OS over the same time (38). Another phase III trial compared proton beam radiotherapy to radiofrequency ablation in patients with recurrent HCC and showed proton beam radiotherapy was non inferior in terms of 2 year PFS (39). Local control rates for advanced HCC treated with SBRT range from 68-95% three years after treatment (40–42). SBRT has been used as bridge to transplant, with comparable outcomes to RFA and Transarterial chemoembolization (TACE) (43). Data have suggested a benefit of SBRT among patients with advanced HCC with portal vein tumor thrombus or inferior vena cava tumor thrombus (44, 45). Additionally, the recent NRG/RTOG 112 phase III clinical trial, showed improved OS, PFS, and quality of life at 6 months without increase in adverse effects for patients with unresectable HCC treated with SBRT followed by sorafenib compared to sorafenib alone (12). Of note, only patients with a limited burden of extrahepatic disease were eligible for enrollment. Although the National Comprehensive Cancer Network (NCCN) includes radiation as an option for patients who are not eligible for transplant there is still a paucity of data directly comparing SBRT to other locoregional therapies (37, 46). SBRT is contraindicated in patients without adequate residual normal liver volume outside the radiation field and in patients with Child-Pugh class B and C cirrhosis (47).
RE has also been studied among patients with advanced HCC. In fact, several randomized trials have compared RE versus sorafenib. Neither The SIRveNIB nor the SARAH trial showed improvement is OS, however the SIRveNIB trial demonstrated superiority of RE over sorafenib in terms of PFS and time to progression, although the SARAH trial noted no difference in PFS (48, 49). Additionally, a different recent randomized controlled trial noted that RE and sorafenib was not associated with improvement in OS versus sorafenib alone (50). The STOP-HCC trial is an ongoing phase III clinical trial that is investigating RE plus sorafenib versus sorafenib alone, which is still enrolling patients (51).
Challenges to monotherapy with ICI or radiotherapy for HCC
While ICI and RT have a proven benefit in the treatment of advanced HCC, these treatments can be associated with clinical challenges. For example, while ICI has demonstrated initial success relative to many cancer types, patients can develop resistance with use (52, 53). Although there are limited data on ICI resistance, several mechanisms for resistance have been proposed. The most studied is Beta-catenin activation secondary to mutation in CTNNB1 gene, which may lead to increased apoptosis in liver cells via nuclear factor κB and decreased recruitment of dendritic cells leading to tumorgenesis (54, 55). Other pathways of resistance include downregulation of antigen processing and presentation via HLA deletion, downregulation of cytokines and signaling pathways (example loss of JAK1/2 function), tumor infiltrating lymphocyte (TIL) exclusion via deletion of PTEN gene and VEGF upregulation, and expression of other coinhibitory checkpoint receptors (56–62). Although these mechanisms have been validated in other types of cancer such as lung and colorectal, these mechanisms have yet to be fully elucidated in HCC.
RT also can have several challenges in the treatment of HCC. For example, there are no consensus guidelines regarding use of RT for treatment of HCC, although most recent NCCN guidelines recommend consideration of EBRT or SBRT as an alternative to ablation or embolization if these therapies have failed or are contraindicated (63). While some clinicians recommend RT for patients with well compensated liver function and patients who have adequate liver volume outside radiation field, there are several relative contraindications to RT. Contraindications may include patients with Child-Pugh Class B or C.
Complications of RT vary from transient to life threatening. Common short term adverse effects include fatigue and nausea while longer term effects include sequela of hepatic injury such as ascites, transaminitis, and, thrombocytopenia (64). In certain rare cases, biliary stenosis can occur as well as radiation induced hepatitis. Additionally, radiation injury to nearby structures such as the stomach, small bowel, colon, ribs, diaphragm can also occur (65, 66). In addition, there are adverse effects associated with ICI therapy. In first line therapy of HCC, atezolizumab has been associated with adverse effects including skin rash, electrolyte abnormalities, anemia, and transamnitis; bevacizumab has been associated with hypertension, abdominal pain, and diarrhea. Serious adverse effects of acute coronary syndromes, vasculitis, and immune mediated myocarditis, as well as immune mediated rashes such as Stevens-Johnson syndrome, and toxic epidermal necrolysis may be associated with nivolumab (67–69).
Combination of ICI and radiotherapy for HCC
Given the potential benefits of both ICI and RT alone in the treatment of HCC, combination therapy with the two modalities is actively being explored. The basis for this combination approach is a hypothetical synergistic effect, which may augment the efficacy of each treatment. RT for cancer is thought to cause irreversible damage to tumor cell DNA thereby initiating cell apoptosis (70). Additionally, the immune system’s role in controlling tumor growth is well established, as it has been demonstrated that cancer survival has been associated with T cell infiltration into the tumor, as well as increased risk of cancer developing in immunosuppression patients (71–73). However, recently, RT’s role in inducing an immune response has become an area of interest with data suggesting that RT augments the efficacy of ICI by impacting the tumor microenvironment (13). The mechanism by which this synergistic effect occurs, however has not been well elucidated.
One proposed mechanisms involves RT-induced direct tumor cell death that stimulates a tumor-specific immune response and modification of the local tumor microenvironment and increased immune cell migration into the tumor (74–76). In addition, by eliminating tumor cells, intracellular contents including antigens and damage-associated molecular patterns are released, which further induce an immune response and lymphocyte infiltration that can augmentate the effect of ICI (14, 77). In particular, CD8+ T-cells and dendritic cells seem crucial to this immune response whereas CD4+ T-cells and macrophages are not as integral (78, 79). Du et al. noted that cyclic guanosine monophosphate-adenosine monophosphate synthase (cGAS) stimulates the interferon gene (STING) pathway and is crucial in RT-induced antitumor immune responses via increased immune check point PD-L1 expression (80). In a study by Kim et al. that compared combination of RT and anti-PD-L1 immunotherapy versus RT or anti-PD-L1 alone in a murine HCC model, there was less tumor growth and longer survival. The authors noted that radiation upregulated PD-L1 expression through IFN-gamma/STAT3 signaling, which might augment the action of immunotherapy (81). In a separate study, Yoo et al. also reported that combination therapy decreased tumor size in a murine HCC model (82). A propensity score matching study compared the combination of anti-PD-1, antiangiogenic therapy and RT (n=54) versus anti-PD-1 and anti-angiogenic therapy alone (n=143) (83). The data demonstrated that the addition of RT improved ORR (42.6% vs 24.5%), median OS (20.1 vs 13.3 months), and PFS (8.7 vs 5.4 months) (83). Similar findings using combination therapy have been reported in other cancer types such as breast and colon cancer (84, 85). This finding has been attributed this to the abscopal effect, which is the deterioration of tumors outside the radiation field during or after RT (86).
The abscopal effect was first described for melanoma in the 1970s and has been linked to mechanisms involving the immune system (87). The abscopal effect likely also plays a role in the treatment of HCC, as demonstrated in murine models. For example, Park et al. reported that RT increased antitumor immune response and the addition of PD-L1 augmented this effect (88). RT causes cellular death and expression of tumor antigens and damage associated molecular patterns (DAMPs), which attract antigen presenting cells such as dendritic cells and actives CD8+ T-cells (89). These cells infiltrate into the tumor microenvironment and promote tumor cell death (15). RT also upregulates immune checkpoint molecules (PD-1. PD-L1, and CTLA-4), which dampens anti-tumor activity. ICI therapy is proposed to block these molecules to restore cytotoxic and anti-tumor activities of T-cells and (Anti-PD-1/PD-L1) dampen the effects of regulatory T cells (90). This proposed synergist mechanism transforms “cold” tumors with low immune cell presence to “hot” tumor with more immune cell infiltration. The use of triple therapy or combination fo PD-1/PD-L1 with RT has been demonstrated to be safe and well tolerated in patients with minimal treatment related adverse effects (91). Figure 2 demonstrates the abscopal effect (92).
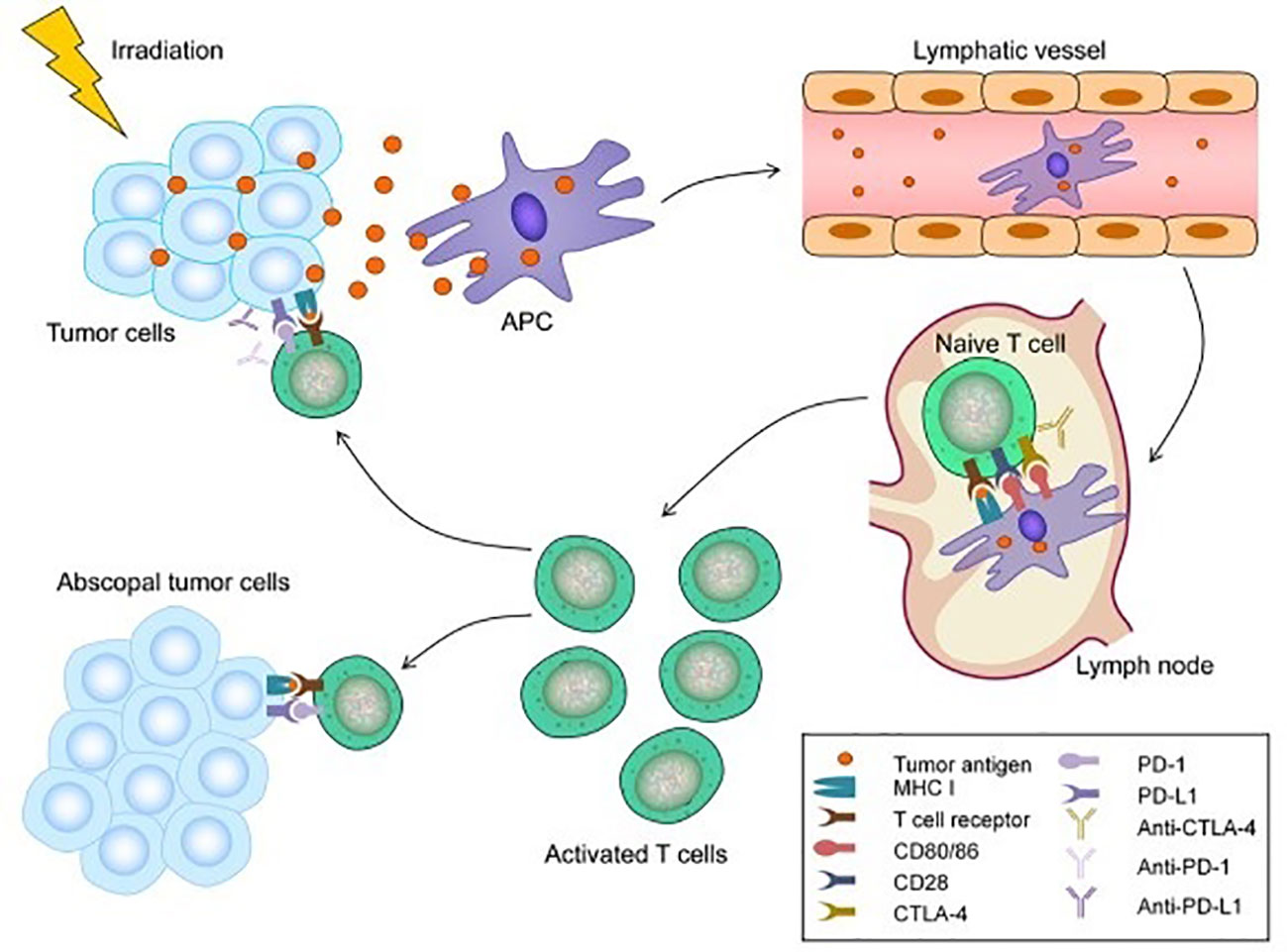
Figure 2 Mechanism of the abscopal effect. Radiotherapy (RT) can lead to immunogenic cell death and the release of tumor antigens by irradiated tumor cells. These neoantigens are taken up by antigen-presenting cells (APCs), such as dendritic cells (DCs) and phagocytic cells. The APCs interact with tumor antigens and then migrate to the lymph nodes where they present antigens to T cells, a process that is mediated by the MHC pathway and other co-stimulatory signals, such as CD80 and CD28. After activation by multiple signals, T cells, especially the CD8+ T cells, are activated and begin to propagate. As a result, activated effector T cells exit the lymph nodes and home to tumors, including primary tumors and non-irradiated tumor metastases, to exert their effect of killing tumor cells. However, cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) competitively combines with CD80/86 and inhibits the activation of T cells. Following T cell activation, programmed cell death 1 (PD-1) receptors that are expressed on the T cell surface bind primarily to programmed death-ligand 1 (PD-L1) and inhibit immune responses. The administration of immune checkpoint blockades of CTLA-1, PD-1, and PD-L1 can enhance the anti-tumor immunity of RT (92).
Radiation induced DNA damage activates DNA damage repair pathways that causes upregulation of CTLA-4 and PD-L1 expression causing immunosuppression within the tumor microenvironment and blunting the effects of ICI (81, 93). Ataxia telangiectasia and Rad3-related protein (ATR), a kinase in the DNA damage repair pathway, may be important in controlling immunosuppression in the tumor microenvironment. Sheng et al. assessed AZD6738, an ATR inhibitor, in a HCC murine model and demonstrated that AZD6738 increase radiotherapy stimulated CD8+T-cell infiltration and activation and reversed the immunosuppressive effects of radiation (94). As such, balancing the immune activation and suppression of RT relative to its effect on ICI is a key concept of ongoing research in the treatment of HCC. In particular, the synergistic effects of RT and ICI in the treatment of HCC works may work in several ways. For example, RT increases diffusion of immune cells into the tumor microenvironment allowing anti-tumor effects, while simultaneous anti-PD-L1, anti-PD1 and anti-CTLA-4 therapy can offset the immunosuppressive consequences induced by RT (90).
Currently, prospective clinical data on the combination RT and ICI in the treatment of HCC are lacking. A 2022 small retrospective study by Su et al. reported on 29 patients with advanced HCC (Child-Pugh A) who were treated with proton beam radiotherapy, as well as anti-PD1 or anti-PD-L1 therapy. The authors reported a median progression free survival of 27.2 months and concluded that combination therapy with RT and ICI was safe and effective for treatment of advanced HCC (95). There are other clinical trials investigating the use of RT and ICI. For example, a phase 2 trial by Tai et al. evaluated the safety and efficacy of sequential RE followed by nivolumab among patients with advanced HCC. Forty patients with unresectable HCC and Child-Pugh A cirrhosis were treated with RE with Y90, with nivolumab started 3 weeks later. The primary outcome was ORR, which was 30.6%. Serious adverse effects (14%) including Steven-Johns syndrome, hepatitis, fever, liver abscess, and ascites (96). Similarly, another phase 2 trial from 2022 assessed SBRT and camrelizumab, an anti-PD1 monoclonal antibody among patients with unresectable HCC. The study enrolled 21 patients with advanced HCC and Child-Pugh A/B liver function and reported an ORR, PFS, and OS of 52.4%, 5.8 months, and 14.2 months, respectively. No severe adverse events were noted (97). The START-FIT trial another single arm phase 2 study treated patients with advanced HCC with sequential transarterial chemoembolization (TACE) then SBRT followed by avelumab, an anti-PD-L1 monoclonal antibody. In this study, 33 patients were enrolled; 4 (12%) subsequently qualified for resection or ablation, and 14 (42%) had complete radiographic response. Adverse events included transaminitis, as well as hepatitis and dermatitis. TACE has been compared with combination RT and immunotherapy; of note, combined therapy with RT and immunotherapy has been noted to have an improved 1- and 2-year PFS and OS (98). Two recent clinical trials are focused on investigating nivolumab and RT for advanced HCC. NASIR-HCC is a single arm phase 2 trial that investigated patients with advanced HCC who underwent RE followed by nivolumab treatment; ORR was 41.5% and OS was 20.9%. A separate phase 1 trial compared SBRT plus nivolumab and ipilimumab versus SBRT and nivolumab alone. Preliminary data have suggested a favorable ORR, PFS and median OS in the SBRT plus nivolumab and ipilimumab; however the trial was stopped prematurely due to poor accural (99). A prospective study from Yu et al. demonstrated that concurrent application of RT during nivolumab treatment resulted in prolonged PFS and OS versus nivolumab alone in a cohort of 76 patient with advanced HCC (100). Smith et al. reported on combination nivolumab and upfront RT among patients with advanced HCC and demonstrated an ORR 35%, which was higher than nivolumab alone. Of note, there is an ongoing phase Ib clinical trial assessing the safety and tolerability of neoadjuvant SBRT and tislelizumab, a PD-1 inhibitor, prior to hepatic resection in patients with resectable HCC (101). There are several other ongoing clinical trials currently enrolling; a summary of these trials is provided (Tables 1, 2).
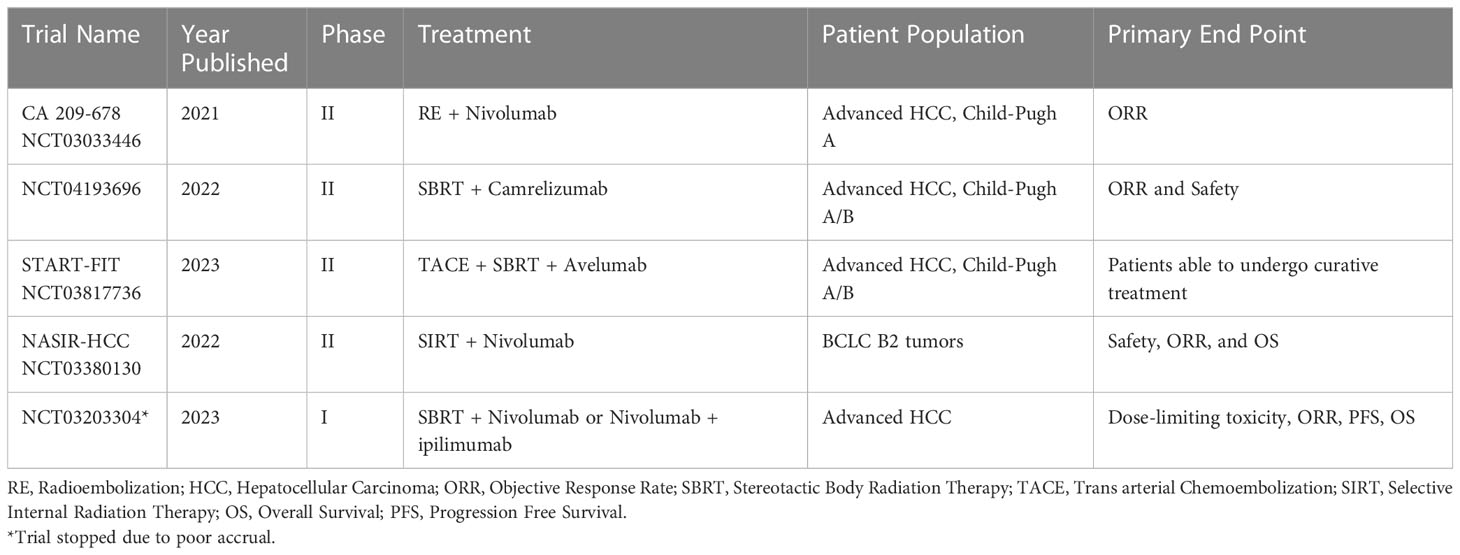
Table 1 Recently published clinical trials using combined RT and ICI for treatment of advanced hepatocellular carcinoma.
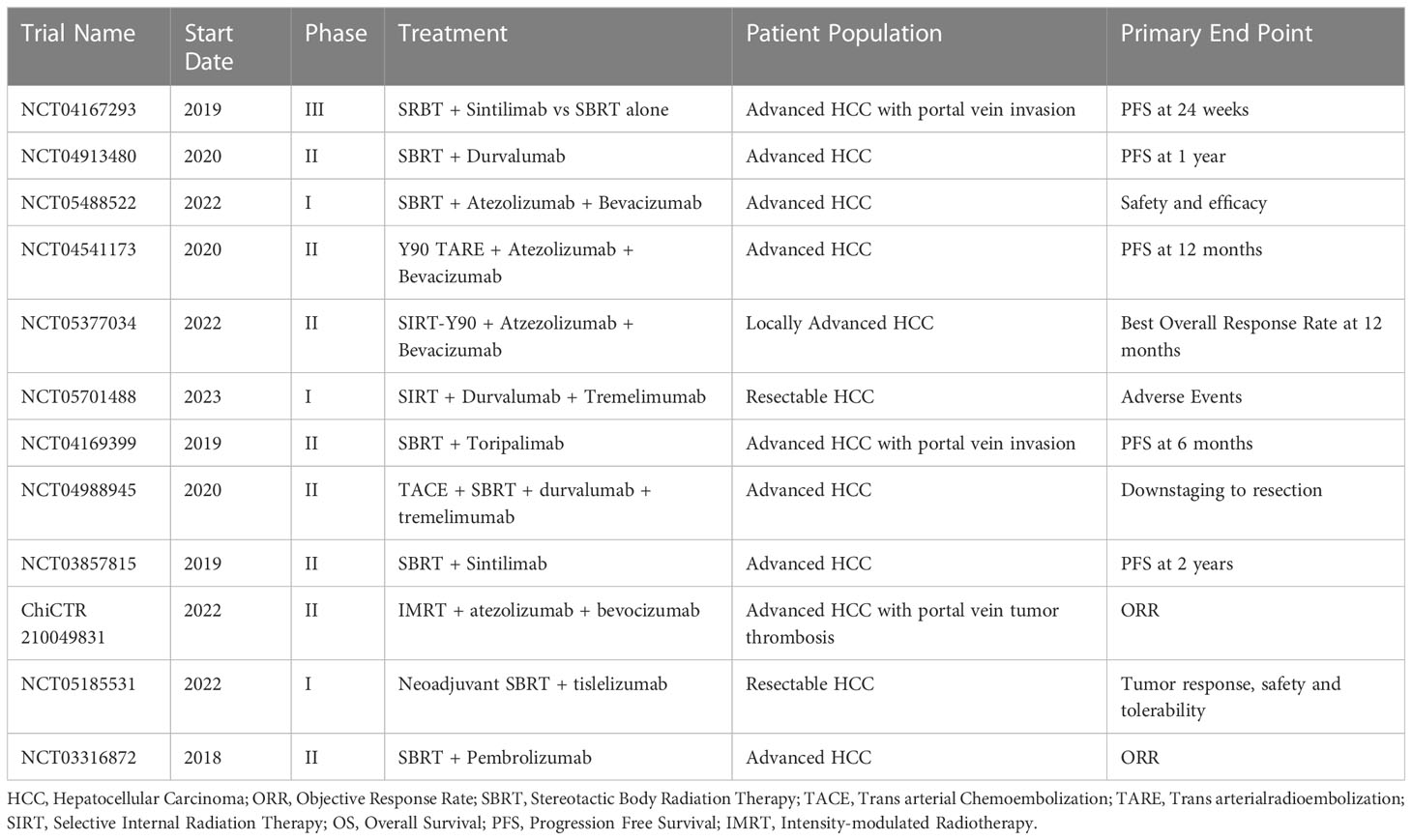
Table 2 Ongoing clinical trials using combined RT and ICI for treatment of advanced hepatocellular carcinoma.
Future directions and challenges to progress
ICI and RT in combination can be effective for the treatment of advanced HCC. The main targets for ICI, which are also impacted by RT, are PD-1/PD-L1 and CTLA-4. Combined ICI and RT may not be effective in all patients with HCC. The reasons for this may be related to the heterogeneity associated with HCC tumors and underlying etiology driving changes at the molecular level affecting the tumor microenvironment. As a result, identifying novel targets for therapy is paramount. Radiation damage to tumor cells induces apoptosis which releases numerous antigens that present potential targets for intervention. Among these are T-cell immunoglobulin mucin-3 (TIM-3), lymphocyte activation gene-3 (LAG-3), and B and T lymphocyte attenuator (BTLA). There are several ongoing clinical trials looking at inhibitors of these antigens including cobolimab (anti-TIM-3) and dostarlimab (anti-PD-1) as well as relatlimab (anti-LAG-3) (NCT03680508, NCT04567615, NCT05337137, NCT04658147) (102).
Recent study has demonstrated that erythroid progenitor cells (EPCs) in the spleen play a role in tumor progression and the subsequent immune response. The proposed mechanism involves creation of reactive oxygen species, and expression of PD-L1 leading to T-cell suppression (103). Among patients with HCC, EPCs produce artemin, a glia cell derived neurotrophic factor that stimulates HCC growth in animal models (104, 105). Combination RT and ICI therapy, particularly anti-PD-L1 could disrupt this pathway by inhibiting accumulation of splenic EPCs. Future research should focus on EPCs as a mechanism by which combination RT and ICI therapy may work.
Chimeric Antigen Receptor T Cell (CAR-T) has also been studied in the treatment of HCC. This technique takes T cells from the patient and engineers the cells to attack certain tumor cells and antigens. A number of potential antigens have been identified as targets for CAR-T therapy, including AFP, GPC-3, MAGE, NY-ESO-1, hTERT, NKG2DL, EpCAM, CD133, CD147, and MUC1 (106). As RT causes the release of innumerable tumor antigens, the combined use of RT and CAR-T therapy may hold promise for the future. Given the number of antigens released from HCC cells, biomarkers to predict which patients may benefit from certain therapies has also been an emerging researched field. Unfortunately, to date, studies have not been able to identify reliable predictive biomarkers for HCC. Markers that have been studied are PD-L1, PD-1, TIM-3, and cytolytic T cell infiltrates, as well as radiosensitive gene signatures (14, 107–110).
Further emerging areas in the treatment of HCC are use of anti-vascular endothelial growth factor (VEGF) therapy. VEGF overexpression can occur in patients with HCC and be responsible for angiogenesis and hypervascularity of HCC tumors, as well as be associated with poor prognosis (111–114). Angiogenesis has long been known to potentiate tumor formation and provides a novel target for drug therapy (115). The landmark IMbrave 150 trial demonstrated that anti-VEGF therapy increased OS and PFS when used with atezolizumab versus sorafenib (9). The mechanisms by which anti-VEGF therapy work has been studied in animal models. Mice treated with VEGF inhibitors had augmented PD-1 targets on T-cells. Combining anti-VEGF and anti-PD-1 therapy allowed for T-cells to function properly and decrease inhibitor immune cells such as Tregs (116). Radiation increases VEGF expression in HCC cells and thus RT may play a role in strengthening the effect of anti-VEGF therapy, similar to ICI therapy (114). A multicenter prospective study of 30 patients from China assessed the efficacy and safety of intensity modulated RT and systemic atezolizumab and bevacizumab in patients with HCC and extrahepatic portal vein tumor thrombus. The authors reported an ORR and median OS of 76.6% and 9.8 months, respectively, with an acceptable safety profile (117). An ongoing phase II trials is currently assessing atezolizumab and bevacizumab plus RT in patients with unresectable HCC and portal vein tumor thrombus with the results expected in the upcoming years (118). Currently, combination therapy with RT and anti-VEGF should be used with caution, however, as adverse effects with anti-VEGF after RT can be severe (119).
Using ICI and RT may help convert unresectable HCC into resectable disease allowing for R1 or R0 resection, known as conversion therapy. Although hepatectomy after conversion therapy may be more challenging, it has been proven to be safe and effective (120). Multiple studies have reported that combination therapy with RT plus either locoregional therapy such as hepatic artery infusion pump or targeted therapy can convert unresectable disease with portal vein tumor thrombus into candidates for resection (121, 122). The role of RT plus ICI in conversion therapy will be an area of active future research. Despite these exciting future directions and progress with ICI and RT, many challenges remain in the treatment of patients with advanced HCC. Most studies on ICI and RT involve Child-Pugh A patients with preserved liver function and functional status. Applying data to patients with worse liver function (i.e., Child-Pugh B/C patients) is challenging. To this point, there are relatively few studies on patients with poor liver function. The CELESTIAL trial did include patients with Child-Pugh B cirrhosis who were treated with cabozantinib, a tyrosine kinase inhibitor (123). Certain trials have advised caution, however, when using certain ICI in patients with poor liver function (124). The data have demonstrated modest efficacy and safety in select subsets of patients with advanced HCC and compromised liver function.
Another challenge with combined RT and ICI is the development of treatment resistance. HCC is a heterogenous tumor with a varied tumor microenvironment, which complex including the extracellular matrix, immune cells, cancer-associated fibroblasts, among others. This heterogeneity makes development of treatment that universally covers all tumors difficult and makes HCC relatively chemo-resistant (125–127). Elucidating mechanisms of resistance in HCC will be crucial to the development of future treatments. An additional challenge is identification of patients who will benefit most from combination therapy, as well as patient selection for combined RT and ICI. For example, SBRT combined with ICI therapy may be of benefit to patients with tumor thrombus in the portal vein, hepatic veins, or vena cava (128–130). Patients with vascular invasion may benefit from combination therapy, however further study is necessary. The timing and sequence of ICI and RT (concurrent or sequential) to maximize benefit is unclear and will also need to be defined in the future (131).
Conclusion
Although HCC remains a leading cause of cancer death, great strides have been made in recent years in the treatment of advanced disease. Among these, ICI therapy has given the opportunity to increase survival in patients who have failed standard first line therapy. The addition of RT helps to augment the effects of ICI, although the mechanisms behind this effect continue to be studied. Despite this great progress, work remains to better identify which tumors respond best to which drugs, given the heterogeneity of HCC. Further research and progress into new drug therapy, predictive biomarkers, and mechanisms of resistance to certain drugs as well as patient selection and sequence of therapy will be crucial as we move into the next generation of treatments for this lethal disease.
Author contributions
All authors equally contributed to the literature review, writing, and editing of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin (2021) 71(1):7–33. doi: 10.3322/caac.21654
2. Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, Allen C, et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: Results from the global burden of disease study 2015. JAMA Oncol (2017) 3(12):1683–91.
3. Jemal A, Ward EM, Johnson CJ, Cronin KA, Ma J, Ryerson B, et al. Annual report to the nation on the status of cancer, 1975-2014, featuring survival. J Natl Cancer Inst (2017) 109(9). doi: 10.1093/jnci/djx030
4. McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of hepatocellular carcinoma. Hepatology (2021) 73 Suppl 1(Suppl 1):4–13. doi: 10.1002/hep.31288
5. Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol (2022) 76(3):681–93. doi: 10.1016/j.jhep.2021.11.018
6. Carlino MS, Larkin J, Long GV. Immune checkpoint inhibitors in melanoma. Lancet (2021) 398(10304):1002–14. doi: 10.1016/S0140-6736(21)01206-X
7. Cen S, Liu K, Zheng Y, Shan J, Jing C, Gao J, et al. BRAF mutation as a potential therapeutic target for checkpoint inhibitors: A comprehensive analysis of immune microenvironment in BRAF mutated colon cancer. Front Cell Dev Biol (2021) 9:705060. doi: 10.3389/fcell.2021.705060
8. Rizzo A, Cusmai A, Acquafredda S, Giovannelli F, Rinaldi L, Misino A, et al. KEYNOTE-522, IMpassion031 and GeparNUEVO: changing the paradigm of neoadjuvant immune checkpoint inhibitors in early triple-negative breast cancer. Future Oncol (2022) 18(18):2301–9. doi: 10.2217/fon-2021-1647
9. Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol (2022) 76(4):862–73.
10. Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: The CheckMate 040 randomized clinical trial. JAMA Oncol (2020) 6(11):e204564. doi: 10.1001/jamaoncol.2020.4564
11. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non-Small-Cell lung cancer. N Engl J Med (2017) 377(20):1919–29. doi: 10.1056/NEJMoa1709937
12. Dawson LA, Winter KA, Knox JJ, Zhu AX, Krishnan S, Guha C, et al. NRG/RTOG 1112: Randomized phase III study of sorafenib vs. stereotactic body radiation therapy (SBRT) followed by sorafenib in hepatocellular carcinoma (HCC). J Clin Oncol (2023) 41(4_suppl):489–9.
13. Burnette BC, Liang H, Lee Y, Chlewicki L, Khodarev NN, Weichselbaum RR, et al. The efficacy of radiotherapy relies upon induction of type i interferon-dependent innate and adaptive immunity. Cancer Res (2011) 71(7):2488–96. doi: 10.1158/0008-5472.CAN-10-2820
14. Chew V, Lee YH, Pan L, Nasir NJM, Lim CJ, Chua C, et al. Immune activation underlies a sustained clinical response to yttrium-90 radioembolisation in hepatocellular carcinoma. Gut (2019) 68(2):335–46. doi: 10.1136/gutjnl-2017-315485
15. Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med (2000) 191(3):423–34. doi: 10.1084/jem.191.3.423
16. He X, Xu C. Immune checkpoint signaling and cancer immunotherapy. Cell Res (2020) 30(8):660–9. doi: 10.1038/s41422-020-0343-4
17. Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med (2018) 24(5):541–50. doi: 10.1038/s41591-018-0014-x
18. Thorsson V, Gibbs DL, Brown SD, Wolf D, Bortone DS, Ou Yang TH, et al. The immune landscape of cancer. Immunity (2018) 48(4):812–30.e14. doi: 10.1016/j.immuni.2018.03.023
19. Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA (2002) 99(19):12293–7. doi: 10.1073/pnas.192461099
20. Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science (2018) 359(6382):1350–5. doi: 10.1126/science.aar4060
21. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-Small-Cell lung cancer. N Engl J Med (2015) 373(17):1627–39. doi: 10.1056/NEJMoa1507643
22. Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol (2022) 23(1):77–90. doi: 10.1016/S1470-2045(21)00604-5
23. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med (2020) 382(20):1894–905. doi: 10.1056/NEJMoa1915745
24. Abou-Alfa GK CS, Kudo M, et al. Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA (abstraact 379). J Clin Oncol (2022) 40:4:379. doi: 10.1200/JCO.2022.40.4_suppl.379
25. Kelley RK, Sangro B, Harris W, Ikeda M, Okusaka T, Kang YK, et al. Safety, efficacy, and pharmacodynamics of tremelimumab plus durvalumab for patients with unresectable hepatocellular carcinoma: Randomized expansion of a phase I/II study. J Clin Oncol (2021) 39(27):2991–3001. doi: 10.1200/JCO.20.03555
26. Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: A randomized, double-blind, phase III trial. J Clin Oncol (2020) 38(3):193–202. doi: 10.1200/JCO.19.01307
27. Vogel A, Martinelli E. Updated treatment recommendations for hepatocellular carcinoma (HCC) from the ESMO clinical practice guidelines. Ann Oncol (2021) 32(6):801–5. doi: 10.1016/j.annonc.2021.02.014
28. Vendrely V, Rivin Del Campo E, Modesto A, Jolnerowski M, Meillan N, Chiavassa S, et al. Rectal cancer radiotherapy. Cancer Radiother (2022) 26(1-2):272–8. doi: 10.1016/j.canrad.2021.11.002
29. Merie R, Gee H, Hau E, Vinod S. An overview of the role of radiotherapy in the treatment of small cell lung cancer - a mainstay of treatment or a modality in decline? Clin Oncol (R Coll Radiol) (2022) 34(11):741–52. doi: 10.1016/j.clon.2022.08.024
30. Berman ZT, Newton I. Diagnosis, staging, and patient selection for locoregional therapy to treat hepatocellular carcinoma. Semin Intervent Radiol (2020) 37(5):441–7. doi: 10.1055/s-0040-1719185
31. Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. (2021) 7(1):6. doi: 10.1038/s41572-020-00240-3
32. Russell AH, Clyde C, Wasserman TH, Turner SS, Rotman M. Accelerated hyperfractionated hepatic irradiation in the management of patients with liver metastases: results of the RTOG dose escalating protocol. Int J Radiat Oncol Biol Phys (1993) 27(1):117–23. doi: 10.1016/0360-3016(93)90428-X
33. Rim CH, Kim CY, Yang DS, Yoon WS. Comparison of radiation therapy modalities for hepatocellular carcinoma with portal vein thrombosis: A meta-analysis and systematic review. Radiother Oncol (2018) 129(1):112–22. doi: 10.1016/j.radonc.2017.11.013
34. Vogel A, Cervantes A, Chau I, Daniele B, Llovet JM, Meyer T, et al. Hepatocellular carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol (2018) 29(Suppl 4):iv238–iv55. doi: 10.1093/annonc/mdy308
35. Sangro B, Salem R. Transarterial chemoembolization and radioembolization. Semin Liver Dis (2014) 34(4):435–43.
36. Memon K, Lewandowski RJ, Kulik L, Riaz A, Mulcahy MF, Salem R. Radioembolization for primary and metastatic liver cancer. Semin Radiat Oncol (2011) 21(4):294–302. doi: 10.1016/j.semradonc.2011.05.004
37. Clinical Practice Guidelines EASL. Management of hepatocellular carcinoma. J Hepatol (2018) 69(1):182–236.
38. Wahl DR, Stenmark MH, Tao Y, Pollom EL, Caoili EM, Lawrence TS, et al. Outcomes after stereotactic body radiotherapy or radiofrequency ablation for hepatocellular carcinoma. J Clin Oncol (2016) 34(5):452–9. doi: 10.1200/JCO.2015.61.4925
39. Kim TH, Koh YH, Kim BH, Kim MJ, Lee JH, Park B, et al. Proton beam radiotherapy vs. radiofrequency ablation for recurrent hepatocellular carcinoma: A randomized phase III trial. J Hepatol (2021) 74(3):603–12.
40. Kwon JH, Bae SH, Kim JY, Choi BO, Jang HS, Jang JW, et al. Long-term effect of stereotactic body radiation therapy for primary hepatocellular carcinoma ineligible for local ablation therapy or surgical resection. stereotactic radiotherapy for liver cancer. BMC Cancer. (2010) 10:475. doi: 10.1186/1471-2407-10-475
41. Bujold A, Massey CA, Kim JJ, Brierley J, Cho C, Wong RK, et al. Sequential phase i and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol (2013) 31(13):1631–9. doi: 10.1200/JCO.2012.44.1659
42. Dewas S, Bibault JE, Mirabel X, Fumagalli I, Kramar A, Jarraya H, et al. Prognostic factors affecting local control of hepatic tumors treated by stereotactic body radiation therapy. Radiat Oncol (2012) 7:166. doi: 10.1186/1748-717X-7-166
43. Sapisochin G, Barry A, Doherty M, Fischer S, Goldaracena N, Rosales R, et al. Stereotactic body radiotherapy vs. TACE or RFA as a bridge to transplant in patients with hepatocellular carcinoma. an intention-to-treat analysis. J Hepatol (2017) 67(1):92–9.
44. Xi M, Zhang L, Zhao L, Li QQ, Guo SP, Feng ZZ, et al. Effectiveness of stereotactic body radiotherapy for hepatocellular carcinoma with portal vein and/or inferior vena cava tumor thrombosis. PloS One (2013) 8(5):e63864. doi: 10.1371/journal.pone.0063864
45. Choi HS, Kang KM, Jeong BK, Jeong H, Lee YH, Ha IB, et al. Effectiveness of stereotactic body radiotherapy for portal vein tumor thrombosis in patients with hepatocellular carcinoma and underlying chronic liver disease. Asia Pac J Clin Oncol (2021) 17(3):209–15. doi: 10.1111/ajco.13361
46. Schwarz RE, Abou-Alfa GK, Geschwind JF, Krishnan S, Salem R, Venook AP. Nonoperative therapies for combined modality treatment of hepatocellular cancer: expert consensus statement. HPB (Oxford). (2010) 12(5):313–20. doi: 10.1111/j.1477-2574.2010.00183.x
47. Culleton S, Jiang H, Haddad CR, Kim J, Brierley J, Brade A, et al. Outcomes following definitive stereotactic body radiotherapy for patients with child-pugh b or c hepatocellular carcinoma. Radiother Oncol (2014) 111(3):412–7. doi: 10.1016/j.radonc.2014.05.002
48. Vilgrain V, Pereira H, Assenat E, Guiu B, Ilonca AD, Pageaux GP, et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol (2017) 18(12):1624–36. doi: 10.1016/S1470-2045(17)30683-6
49. Golfieri R, Bilbao JI, Carpanese L, Cianni R, Gasparini D, Ezziddin S, et al. Comparison of the survival and tolerability of radioembolization in elderly vs. younger patients with unresectable hepatocellular carcinoma. J Hepatol (2013) 59(4):753–61.
50. Ricke J, Klümpen HJ, Amthauer H, Bargellini I, Bartenstein P, de Toni EN, et al. Impact of combined selective internal radiation therapy and sorafenib on survival in advanced hepatocellular carcinoma. J Hepatol (2019) 71(6):1164–74. doi: 10.1016/j.jhep.2019.08.006
51. Chauhan N, Bukovcan J, Boucher E, Cosgrove D, Edeline J, Hamilton B, et al. Intra-arterial TheraSphere yttrium-90 glass microspheres in the treatment of patients with unresectable hepatocellular carcinoma: Protocol for the STOP-HCC phase 3 randomized controlled trial. JMIR Res Protoc (2018) 7(8):e11234. doi: 10.2196/11234
52. Wang Q, Wu X. Primary and acquired resistance to PD-1/PD-L1 blockade in cancer treatment. Int Immunopharmacol. (2017) 46:210–9. doi: 10.1016/j.intimp.2017.03.015
53. Flynn MJ, Larkin JMG. Novel combination strategies for enhancing efficacy of immune checkpoint inhibitors in the treatment of metastatic solid malignancies. Expert Opin Pharmacother. (2017) 18(14):1477–90. doi: 10.1080/14656566.2017.1369956
54. Ruiz de Galarreta M, Bresnahan E, Molina-Sánchez P, Lindblad KE, Maier B, Sia D, et al. β-catenin activation promotes immune escape and resistance to anti-PD-1 therapy in hepatocellular carcinoma. Cancer Discov (2019) 9(8):1124–41. doi: 10.1158/2159-8290.CD-19-0074
55. Nejak-Bowen K, Kikuchi A, Monga SP. Beta-catenin-NF-κB interactions in murine hepatocytes: a complex to die for. Hepatology (2013) 57(2):763–74. doi: 10.1002/hep.26042
56. Fares CM, Van Allen EM, Drake CG, Allison JP, Hu-Lieskovan S. Mechanisms of resistance to immune checkpoint blockade: Why does checkpoint inhibitor immunotherapy not work for all patients? Am Soc Clin Oncol Educ Book (2019) 39:147–64. doi: 10.1200/EDBK_240837
57. George S, Miao D, Demetri GD, Adeegbe D, Rodig SJ, Shukla S, et al. Loss of PTEN is associated with resistance to anti-PD-1 checkpoint blockade therapy in metastatic uterine leiomyosarcoma. Immunity (2017) 46(2):197–204. doi: 10.1016/j.immuni.2017.02.001
58. Gao J, Shi LZ, Zhao H, Chen J, Xiong L, He Q, et al. Loss of IFN-γ pathway genes in tumor cells as a mechanism of resistance to anti-CTLA-4 therapy. Cell (2016) 167(2):397–404.e9. doi: 10.1016/j.cell.2016.08.069
59. Shin DS, Zaretsky JM, Escuin-Ordinas H, Garcia-Diaz A, Hu-Lieskovan S, Kalbasi A, et al. Primary resistance to PD-1 blockade mediated by JAK1/2 mutations. Cancer Discov (2017) 7(2):188–201. doi: 10.1158/2159-8290.CD-16-1223
60. Ozcan M, Janikovits J, von Knebel Doeberitz M, Kloor M. Complex pattern of immune evasion in MSI colorectal cancer. Oncoimmunology (2018) 7(7):e1445453. doi: 10.1080/2162402X.2018.1445453
61. Gettinger S, Choi J, Hastings K, Truini A, Datar I, Sowell R, et al. Impaired HLA class i antigen processing and presentation as a mechanism of acquired resistance to immune checkpoint inhibitors in lung cancer. Cancer Discov (2017) 7(12):1420–35. doi: 10.1158/2159-8290.CD-17-0593
62. Onuma AE, Zhang H, Huang H, Williams TM, Noonan A, Tsung A. Immune checkpoint inhibitors in hepatocellular cancer: Current understanding on mechanisms of resistance and biomarkers of response to treatment. Gene Expr. (2020) 20(1):53–65. doi: 10.3727/105221620X15880179864121
63. Benson AB, D'Angelica MI, Abbott DE, Anaya DA, Anders R, Are C, et al. Hepatobiliary cancers, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw (2021) 19(5):541–65.
64. Sanuki N, Takeda A, Oku Y, Eriguchi T, Nishimura S, Aoki Y, et al. Influence of liver toxicities on prognosis after stereotactic body radiation therapy for hepatocellular carcinoma. Hepatol Res (2015) 45(5):540–7. doi: 10.1111/hepr.12383
65. Takahashi S, Kimura T, Kenjo M, Nishibuchi I, Takahashi I, Takeuchi Y, et al. Case reports of portal vein thrombosis and bile duct stenosis after stereotactic body radiation therapy for hepatocellular carcinoma. Hepatol Res (2014) 44(10):E273–8. doi: 10.1111/hepr.12241
66. Osmundson EC, Wu Y, Luxton G, Bazan JG, Koong AC, Chang DT. Predictors of toxicity associated with stereotactic body radiation therapy to the central hepatobiliary tract. Int J Radiat Oncol Biol Phys (2015) 91(5):986–94. doi: 10.1016/j.ijrobp.2014.11.028
67. Tomita Y, Sueta D, Kakiuchi Y, Saeki S, Saruwatari K, Sakata S, et al. Acute coronary syndrome as a possible immune-related adverse event in a lung cancer patient achieving a complete response to anti-PD-1 immune checkpoint antibody. Ann Oncol (2017) 28(11):2893–5. doi: 10.1093/annonc/mdx326
68. Griffin LL, Cove-Smith L, Alachkar H, Radford JA, Brooke R, Linton KM. Toxic epidermal necrolysis (TEN) associated with the use of nivolumab (PD-1 inhibitor) for lymphoma. JAAD Case Rep (2018) 4(3):229–31. doi: 10.1016/j.jdcr.2017.09.028
69. Dasanu CA. Late-onset stevens-johnson syndrome due to nivolumab use for hepatocellular carcinoma. J Oncol Pharm Pract (2019) 25(8):2052–5. doi: 10.1177/1078155219830166
70. Prise KM, O'Sullivan JM. Radiation-induced bystander signalling in cancer therapy. Nat Rev Cancer. (2009) 9(5):351–60. doi: 10.1038/nrc2603
71. Talmadge JE, Donkor M, Scholar E. Inflammatory cell infiltration of tumors: Jekyll or hyde. Cancer Metastasis Rev (2007) 26(3-4):373–400. doi: 10.1007/s10555-007-9072-0
72. Whiteside TL. Immune responses to malignancies. J Allergy Clin Immunol (2010) 125(2 Suppl 2):S272–83. doi: 10.1016/j.jaci.2009.09.045
73. Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol (2006) 6(11):836–48. doi: 10.1038/nri1961
74. Kalbasi A, June CH, Haas N, Vapiwala N. Radiation and immunotherapy: a synergistic combination. J Clin Invest. (2013) 123(7):2756–63. doi: 10.1172/JCI69219
75. Hodge JW, Ardiani A, Farsaci B, Kwilas AR, Gameiro SR. The tipping point for combination therapy: cancer vaccines with radiation, chemotherapy, or targeted small molecule inhibitors. Semin Oncol (2012) 39(3):323–39. doi: 10.1053/j.seminoncol.2012.02.006
76. Park B, Yee C, Lee KM. The effect of radiation on the immune response to cancers. Int J Mol Sci (2014) 15(1):927–43. doi: 10.3390/ijms15010927
77. Barber GN. STING: infection, inflammation and cancer. Nat Rev Immunol (2015) 15(12):760–70. doi: 10.1038/nri3921
78. Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, et al. Radiation modulates the peptide repertoire, enhances MHC class i expression, and induces successful antitumor immunotherapy. J Exp Med (2006) 203(5):1259–71. doi: 10.1084/jem.20052494
79. Gupta A, Probst HC, Vuong V, Landshammer A, Muth S, Yagita H, et al. Radiotherapy promotes tumor-specific effector CD8+ t cells via dendritic cell activation. J Immunol (2012) 189(2):558–66. doi: 10.4049/jimmunol.1200563
80. Lee Y, Auh SL, Wang Y, Burnette B, Wang Y, Meng Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ t cells: changing strategies for cancer treatment. Blood (2009) 114(3):589–95. doi: 10.1182/blood-2009-02-206870
81. Du SS, Chen GW, Yang P, Chen YX, Hu Y, Zhao QQ, et al. Radiation therapy promotes hepatocellular carcinoma immune cloaking via PD-L1 upregulation induced by cGAS-STING activation. Int J Radiat Oncol Biol Phys (2022) 112(5):1243–55. doi: 10.1016/j.ijrobp.2021.12.162
82. Kim KJ, Kim JH, Lee SJ, Lee EJ, Shin EC, Seong J. Radiation improves antitumor effect of immune checkpoint inhibitor in murine hepatocellular carcinoma model. Oncotarget (2017) 8(25):41242–55. doi: 10.18632/oncotarget.17168
83. Yoo GS, Ahn WG, Kim SY, Kang W, Choi C, Park HC. Radiation-induced abscopal effect and its enhancement by programmed cell death 1 blockade in the hepatocellular carcinoma: A murine model study. Clin Mol Hepatol (2021) 27(1):144–56. doi: 10.3350/cmh.2020.0095
84. Su K, Guo L, Ma W, Wang J, Xie Y, Rao M, et al. PD-1 inhibitors plus anti-angiogenic therapy with or without intensity-modulated radiotherapy for advanced hepatocellular carcinoma: A propensity score matching study. Front Immunol (2022) 13:972503. doi: 10.3389/fimmu.2022.972503
85. Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. (2014) 124(2):687–95. doi: 10.1172/JCI67313
86. Dovedi SJ, Adlard AL, Lipowska-Bhalla G, McKenna C, Jones S, Cheadle EJ, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res (2014) 74(19):5458–68. doi: 10.1158/0008-5472.CAN-14-1258
87. Reynders K, Illidge T, Siva S, Chang JY, De Ruysscher D. The abscopal effect of local radiotherapy: using immunotherapy to make a rare event clinically relevant. Cancer Treat Rev (2015) 41(6):503–10. doi: 10.1016/j.ctrv.2015.03.011
88. Ngwa W, Irabor OC, Schoenfeld JD, Hesser J, Demaria S, Formenti SC. Using immunotherapy to boost the abscopal effect. Nat Rev Cancer. (2018) 18(5):313–22. doi: 10.1038/nrc.2018.6
89. Park JH, Kim HY, Lee A, Seo YK, Kim IH, Park ET, et al. Enlightening the immune mechanism of the abscopal effect in a murine HCC model and overcoming the late resistance with anti-PD-L1. Int J Radiat Oncol Biol Phys (2021) 110(2):510–20. doi: 10.1016/j.ijrobp.2020.12.031
90. Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med (2007) 13(9):1050–9. doi: 10.1038/nm1622
91. Lee YH, Tai D, Yip C, Choo SP, Chew V. Combinational immunotherapy for hepatocellular carcinoma: Radiotherapy, immune checkpoint blockade and beyond. Front Immunol (2020) 11:568759. doi: 10.3389/fimmu.2020.568759
92. Zhong L, Wu D, Peng W, Sheng H, Xiao Y, Zhang X, et al. Safety of PD-1/PD-L1 inhibitors combined with palliative radiotherapy and anti-angiogenic therapy in advanced hepatocellular carcinoma. Front Oncol (2021) 11:686621. doi: 10.3389/fonc.2021.686621
93. Liu Y, Dong Y, Kong L, Shi F, Zhu H, Yu J. Abscopal effect of radiotherapy combined with immune checkpoint inhibitors. J Hematol Oncol (2018) 11(1):104. doi: 10.1186/s13045-018-0647-8
94. Fu J, Xu D, Liu Z, Shi M, Zhao P, Fu B, et al. Increased regulatory t cells correlate with CD8 t-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology (2007) 132(7):2328–39. doi: 10.1053/j.gastro.2007.03.102
95. Farhood B, Najafi M, Mortezaee K. CD8(+) cytotoxic t lymphocytes in cancer immunotherapy: A review. J Cell Physiol (2019) 234(6):8509–21. doi: 10.1002/jcp.27782
96. Sato H, Niimi A, Yasuhara T, Permata TBM, Hagiwara Y, Isono M, et al. DNA double-strand break repair pathway regulates PD-L1 expression in cancer cells. Nat Commun (2017) 8(1):1751. doi: 10.1038/s41467-017-01883-9
97. Sheng H, Huang Y, Xiao Y, Zhu Z, Shen M, Zhou P, et al. ATR inhibitor AZD6738 enhances the antitumor activity of radiotherapy and immune checkpoint inhibitors by potentiating the tumor immune microenvironment in hepatocellular carcinoma. J Immunother Cancer (2020) 8(1). doi: 10.1136/jitc-2019-000340
98. Su CW, Hou MM, Huang PW, Chou YC, Huang BS, Tseng JH, et al. Proton beam radiotherapy combined with anti-PD1/PDL1 immune checkpoint inhibitors for advanced hepatocellular carcinoma. Am J Cancer Res (2022) 12(4):1606–20.
99. Tai D, Loke K, Gogna A, Kaya NA, Tan SH, Hennedige T, et al. Radioembolisation with Y90-resin microspheres followed by nivolumab for advanced hepatocellular carcinoma (CA 209-678): a single arm, single centre, phase 2 trial. Lancet Gastroenterol Hepatol (2021) 6(12):1025–35. doi: 10.1016/S2468-1253(21)00305-8
100. Li JX, Su TS, Gong WF, Zhong JH, Yan LY, Zhang J, et al. Combining stereotactic body radiotherapy with camrelizumab for unresectable hepatocellular carcinoma: a single-arm trial. Hepatol Int (2022) 16(5):1179–87. doi: 10.1007/s12072-022-10396-7
101. Chiang CL, Chiu KW, Lee FA, Kong FS, Chan AC. Combined stereotactic body radiotherapy and immunotherapy versus transarterial chemoembolization in locally advanced hepatocellular carcinoma: A propensity score matching analysis. Front Oncol (2021) 11:798832. doi: 10.3389/fonc.2021.798832
102. Juloori A, Katipally RR, Lemons JM, Singh AK, Iyer R, Robbins JR, et al. Phase 1 randomized trial of stereotactic body radiation therapy followed by nivolumab plus ipilimumab or nivolumab alone in Advanced/Unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys (2023) 115(1):202–13. doi: 10.1016/j.ijrobp.2022.09.052
103. Yu JI, Lee SJ, Lee J, Lim HY, Paik SW, Yoo GS, et al. Clinical significance of radiotherapy before and/or during nivolumab treatment in hepatocellular carcinoma. Cancer Med (2019) 8(16):6986–94. doi: 10.1002/cam4.2570
104. Zhang B, Yue J, Shi X, Cui K, Li L, Zhang C, et al. Protocol of notable-HCC: a phase ib study of neoadjuvant tislelizumab with stereotactic body radiotherapy in patients with resectable hepatocellular carcinoma. BMJ Open (2022) 12(9):e060955. doi: 10.1136/bmjopen-2022-060955
105. Ruff SM, Shannon AH, Pawlik TM. Advances in targeted immunotherapy for hepatobiliary cancers. Int J Mol Sci (2022) 23(22). doi: 10.3390/ijms232213961
106. Grzywa TM, Justyniarska M, Nowis D, Golab J. Tumor immune evasion induced by dysregulation of erythroid progenitor cells development. Cancers (Basel). (2021) 13(4). doi: 10.3390/cancers13040870
107. Han Y, Liu Q, Hou J, Gu Y, Zhang Y, Chen Z, et al. Tumor-induced generation of splenic erythroblast-like ter-cells promotes tumor progression. Cell (2018) 173(3):634–48.e12. doi: 10.1016/j.cell.2018.02.061
108. Steenbrugge J, De Jaeghere EA, Meyer E, Denys H, De Wever O. Splenic hematopoietic and stromal cells in cancer progression. Cancer Res (2021) 81(1):27–34. doi: 10.1158/0008-5472.CAN-20-2339
109. Rochigneux P, Chanez B, De Rauglaudre B, Mitry E, Chabannon C, Gilabert M. Adoptive cell therapy in hepatocellular carcinoma: Biological rationale and first results in early phase clinical trials. Cancers (Basel). (2021) 13(2). doi: 10.3390/cancers13020271
110. Friemel J, Rechsteiner M, Frick L, Böhm F, Struckmann K, Egger M, et al. Intratumor heterogeneity in hepatocellular carcinoma. Clin Cancer Res (2015) 21(8):1951–61. doi: 10.1158/1078-0432.CCR-14-0122
111. Ma L, Hernandez MO, Zhao Y, Mehta M, Tran B, Kelly M, et al. Tumor cell biodiversity drives microenvironmental reprogramming in liver cancer. Cancer Cell (2019) 36(4):418–30.e6. doi: 10.1016/j.ccell.2019.08.007
112. Forker LJ, Choudhury A, Kiltie AE. Biomarkers of tumour radiosensitivity and predicting benefit from radiotherapy. Clin Oncol (R Coll Radiol). (2015) 27(10):561–9. doi: 10.1016/j.clon.2015.06.002
113. Kim HJ, Park S, Kim KJ, Seong J. Clinical significance of soluble programmed cell death ligand-1 (sPD-L1) in hepatocellular carcinoma patients treated with radiotherapy. Radiother Oncol (2018) 129(1):130–5. doi: 10.1016/j.radonc.2017.11.027
114. LeCouter J, Moritz DR, Li B, Phillips GL, Liang XH, Gerber HP, et al. Angiogenesis-independent endothelial protection of liver: role of VEGFR-1. Science (2003) 299(5608):890–3. doi: 10.1126/science.1079562
115. Zhu AX, Duda DG, Sahani DV, Jain RK. HCC and angiogenesis: possible targets and future directions. Nat Rev Clin Oncol (2011) 8(5):292–301. doi: 10.1038/nrclinonc.2011.30
116. Chao Y, Li CP, Chau GY, Chen CP, King KL, Lui WY, et al. Prognostic significance of vascular endothelial growth factor, basic fibroblast growth factor, and angiogenin in patients with resectable hepatocellular carcinoma after surgery. Ann Surg Oncol (2003) 10(4):355–62. doi: 10.1245/ASO.2003.10.002
117. Suh YG, Lee EJ, Cha H, Yang SH, Seong J. Prognostic values of vascular endothelial growth factor and matrix metalloproteinase-2 in hepatocellular carcinoma after radiotherapy. Dig Dis (2014) 32(6):725–32. doi: 10.1159/000368010
118. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell (2011) 144(5):646–74. doi: 10.1016/j.cell.2011.02.013
119. Shigeta K, Datta M, Hato T, Kitahara S, Chen IX, Matsui A, et al. Dual programmed death receptor-1 and vascular endothelial growth factor receptor-2 blockade promotes vascular normalization and enhances antitumor immune responses in hepatocellular carcinoma. Hepatology (2020) 71(4):1247–61. doi: 10.1002/hep.30889
120. Wang K, Xiang YJ, Yu HM, Cheng YQ, Liu ZH, Zhong JY, et al. Intensity-modulated radiotherapy combined with systemic atezolizumab and bevacizumab in treatment of hepatocellular carcinoma with extrahepatic portal vein tumor thrombus: A preliminary multicenter single-arm prospective study. Front Immunol (2023) 14:1107542. doi: 10.3389/fimmu.2023.1107542
121. Wang K, Yu HM, Xiang YJ, Cheng YQ, Ni QZ, Guo WX, et al. Efficacy and safety of radiotherapy combined with atezolizumab plus bevacizumab in treating hepatocellular carcinoma with portal vein tumour thrombus: a study protocol. BMJ Open (2022) 12(12):e064688. doi: 10.1136/bmjopen-2022-064688
122. Pollom EL, Deng L, Pai RK, Brown JM, Giaccia A, Loo BW Jr., et al. Gastrointestinal toxicities with combined antiangiogenic and stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys (2015) 92(3):568–76. doi: 10.1016/j.ijrobp.2015.02.016
123. Luo L, He Y, Zhu G, Xiao Y, Song S, Ge X, et al. Hepatectomy after conversion therapy for initially unresectable HCC: What is the difference? J Hepatocell Carcinoma (2022) 9:1353–68. doi: 10.2147/JHC.S388965
124. He M, Li Q, Zou R, Shen J, Fang W, Tan G, et al. Sorafenib plus hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin vs sorafenib alone for hepatocellular carcinoma with portal vein invasion: A randomized clinical trial. JAMA Oncol (2019) 5(7):953–60. doi: 10.1001/jamaoncol.2019.0250
125. Hamaoka M, Kobayashi T, Kuroda S, Iwako H, Okimoto S, Kimura T, et al. Hepatectomy after down-staging of hepatocellular carcinoma with portal vein tumor thrombus using chemoradiotherapy: A retrospective cohort study. Int J Surg (2017) 44:223–8. doi: 10.1016/j.ijsu.2017.06.082
126. Kelley RK, Ryoo BY, Merle P, Park JW, Bolondi L, Chan SL, et al. Second-line cabozantinib after sorafenib treatment for advanced hepatocellular carcinoma: a subgroup analysis of the phase 3 CELESTIAL trial. ESMO Open (2020) 5(4). doi: 10.1136/esmoopen-2020-000714
127. Masatoshi Kudo AM, Santoro A, Melero I, Gracian AC, Acosta-Rivera M, Choo SuP, et al. Checkmate-040: Nivolumab (NIVO) in patients (pts) with advanced hepatocellular carcinoma (aHCC) and child-pugh b (CPB) status. J Clin Oncol (2019) 37(4_suppl):327. doi: 10.1200/JCO.2019.37.4_suppl.327
128. Kim DW, Talati C, Kim R. Hepatocellular carcinoma (HCC): beyond sorafenib-chemotherapy. J Gastrointest Oncol (2017) 8(2):256–65. doi: 10.21037/jgo.2016.09.07
129. Tang W, Chen Z, Zhang W, Cheng Y, Zhang B, Wu F, et al. The mechanisms of sorafenib resistance in hepatocellular carcinoma: theoretical basis and therapeutic aspects. Signal Transduct Target Ther (2020) 5(1):87. doi: 10.1038/s41392-020-0187-x
130. Cabral LKD, Tiribelli C, Sukowati CHC. Sorafenib resistance in hepatocellular carcinoma: The relevance of genetic heterogeneity. Cancers (Basel). (2020) 12(6). doi: 10.3390/cancers12061576
Keywords: radiotherapy, immune checkpoint inhibitors, hepatocellular carcinoma, combination therapy, stereotactic body radiotherapy
Citation: Shannon AH, Manne A, Diaz Pardo DA and Pawlik TM (2023) Combined radiotherapy and immune checkpoint inhibition for the treatment of advanced hepatocellular carcinoma. Front. Oncol. 13:1193762. doi: 10.3389/fonc.2023.1193762
Received: 25 March 2023; Accepted: 03 July 2023;
Published: 24 July 2023.
Edited by:
Ying-Hong Feng, Uniformed Services University of the Health Sciences, United StatesReviewed by:
Hou Yuzhu, Xi’an Jiaotong University, ChinaDan Duda, Massachusetts General Hospital and Harvard Medical School, United States
Copyright © 2023 Shannon, Manne, Diaz Pardo and Pawlik. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Timothy M. Pawlik, VGltLlBhd2xpa0Bvc3VtYy5lZHU=
†ORCID: Timothy M. Pawlik, orcid.org/0000-0002-7994-9870
 Alexander H. Shannon
Alexander H. Shannon Ashish Manne
Ashish Manne Dayssy A. Diaz Pardo
Dayssy A. Diaz Pardo Timothy M. Pawlik
Timothy M. Pawlik