
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 16 May 2023
Sec. Neuro-Oncology and Neurosurgical Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1190366
 Xinyu Ji1
Xinyu Ji1 Akram Alakel2
Akram Alakel2 Feras M. Ghazawi3*
Feras M. Ghazawi3* Matthew Tsang4
Matthew Tsang4 Andrei Zubarev5
Andrei Zubarev5 Oliver J. Lasry6
Oliver J. Lasry6 Ivan V. Litvinov5,7,8*
Ivan V. Litvinov5,7,8*Background: Gliomas account for over two-thirds of all malignant brain tumors and have few established risk factors beyond family history and exposure to ionizing radiation. Importantly, recent studies highlighted the exposure to ultrafine particles (UFP) as a putative risk factor for malignant brain tumors.
Methods: Clinical and geographic data encompassing all provinces and territories from 1992 to 2010 was obtained from the Canadian Cancer Registry and Le Registre Québécois du Cancer. Linear regression and joinpoint analyses were performed to assess incidence trends. Significantly higher and lower incidence postal codes were then interrogated using Standard Industrial Classification codes to detect significant industrial activity.
Results: In Canada, between 1992 and 2010, there were ~32,360 cases of glioma. Of these, 17,115 (52.9%) were glioblastoma. The overall crude incidence rates of 5.45 and 2.87 cases per 100,000 individuals per year for gliomas and glioblastomas, respectively, were identified. Our findings further revealed increasing crude incidence of gliomas/glioblastomas over time. A male predominance was observed. Provinces leading in glioma incidence included Quebec, Nova Scotia, and New Brunswick. Significantly lower crude incidence of glioma was found in Nunavut, Northwest Territories, Ontario, and Alberta. A putative regional clustering of gliomas was observed, with higher incidence rates in postal code areas correlating with industrial activity related to airport operations.
Conclusion: This study describes the geographic distribution of the glioma disease burden and, potentially, identifies industrial activity related to airport operations as potentially being associated with higher incidence of this cancer.
In Canada, cancer is the leading cause of mortality (1). Latest Cancer Statistics data indicate that brain and central nervous system cancers account for 1-2% of all new malignancies (1, 2). Gliomas, which represent over two-thirds of all malignant brain tumors, originate in the glial cells of the central nervous system (CNS) (3). The World Health Organization (WHO) classifies gliomas as astrocytoma, oligodendroglioma, and ependymoma (3). They present with distinctive genetic mutations, prognostic factors, and response to treatment. Many gliomas, particularly higher-grade tumors such as glioblastoma, are highly invasive and are associated with poor survival (3). As such, determining risk factors associated with the development of such tumors and understanding their epidemiologic distribution is necessary in order to develop effective strategies to prevent and treat this disease.
Established risk factors for CNS malignancies include exposure to ionizing radiation, concurrent diagnosis of a hereditary cancer syndrome (4, 5) and, the absence of allergies (6–9). Ionizing radiation is known to cause a multitude of cancers (10), and in the CNS, exposure to high or moderate doses of medical and environmental ionizing radiation is the only well-established external risk factor for various brain tumors (4, 5). However, even low doses of radiation averaging 1.5 Gy for tinea capitis of the scalp were associated with relative risks of 18, 10, and 3 for nerve sheath tumors, meningiomas, and gliomas, respectively (4, 5). Early age of radiation exposure is associated with a higher risk of CNS malignancy later in life and may explain the obstacles in recognizing causal environmental relations in epidemiologic investigations (11). Interestingly, allergic conditions and higher IgE levels are associated with lower tumor incidences and are hypothesized to enhance tumor surveillance and destruction (6, 8, 9).
Notably, exposure to ultrafine particles (UFPs) may be emerging as a novel risk factor for brain cancers (12, 13). Specifically, a Canadian study recently highlighted a possible association of UFPs and risk of malignant brain tumors (12). UFPs are a subtype of particulate matter that are smaller than 100 nm (≤0.1 µm) in diameter that may be absorbed via alveoli and carried systemically to the CNS or may cross the blood brain barrier though the nose and olfactory circulation (13). Notably, it was documented that increased levels of UFP may be found in the proximity of airports and may be associated with brain cancers (13). Other suspected associations include environmental pollution, cellphone use, malnutrition, and socio-economic status. However, these risk factors remain to be ascertained (14).
Importantly, up to 5% of grade II gliomas can be asymptomatic (4, 15). Hence, medical imaging likely contributes to the discovery of these tumors. Medical imaging is more likely to occur in metropolitan areas in comparison to remote rural communities.
As highlighted above, associations have also been established between gliomas and several rare inheritable syndromes, such as Phakomatoses (neurocutaneous syndromes), Li-Fraumeni, enchondromatosis, and familial polyposis syndromes, which account for <5% of all gliomas (16). Variability in glioma incidence was noted between different ethnic origins, with Caucasian individuals being at higher risk of developing a malignant CNS tumor compared to African-Americans, Asian and Asian/Pacific Islanders (17).
In the United States, Europe, and Australia, glioblastoma is the most commonly reported malignant tumor (14), accounting for 14.2% of all primary brain and other CNS tumors with a particularly low five-year survival rate of ~5 (17). Epidemiologic studies in Canada report similar trends (2, 18, 19), though no detailed analysis by individual cities and specific regions is yet available to demonstrate local trends of CNS malignancies that can help identify putative environmental risk factors.
The primary objective of this study is to describe the geographic distribution of the glioma disease burden in Canada by province, city, and forward sortation area (FSA: first three entries of a postal code). This comprehensive approach to mapping the geographic distribution of CNS cancers may help establish risk factors and identify putative etiologic agents that trigger glioma development.
This study was conducted in accordance with the CISS-RDC-668035 and 13-SSH-MCG-3749 protocols approved by the Social Sciences and Humanities Research Council of Canada (SSHRC) and the Quebec Inter-University Centre for Social Statistics (QICSS), respectively as previously described (20–24). In accordance with the institutional policy, this study received McGill University Research Ethics Board exemption.
Clinical and geographic data was obtained from the Canadian Cancer Registry (CCR) and Le Registre Québécois du Cancer (LRQC). The CCR is a dynamic database of cancer patients from 12 provinces and territories (excluding Quebec), who were diagnosed with primary tumors (20–24).
The LRQC database was used to obtain corresponding data for patients residing in Quebec. As data from the LRQC, unfortunately, only spans the years 1992 to 2010, we decided to analyze the data during this time period to encompass all provinces and territories. Geographic and clinical information, including patients’ sex, year of diagnosis, age at the time of diagnosis, and FSA (the first 3 digits of a postal code, which defines a geographical region of residence), as well as the ICD-O-3 code of the tumor, were acquired from the CCR/LRQC databases.
Gliomas comprise ~81% of all malignant brain tumors (14). Consistent with the updated 2016 WHO classification of central nervous system neoplasms, glioma cases were defined based on the International Classification of Diseases for Oncology ICD-O-3 codes according to cancer morphology: Mixed glioma (ICD-O-3 code 9382), Ependymoma, not specified (NOS) (9392), Astrocytoma, NOS (9400), Glioblastoma (9440), Oligodendroglioma, NOS (9450), Oligodendroglioma, anaplastic (9451), Protolasmic astrocytoma (9410), Gemistocytic astrocytoma (9411), Gliomatosis cerebri (9381), Pleomorhic xanthoastrocytoma (9424), Giant cell Glioblastoma (9441), Gliosarcoma (9442), Astroblastoma (9430), Ganglioglioma, NOS (9505), and Pilocytic astrocytoma (9421). For a summary of ICD diagnostic definitions, please refer to Supplementary Table 1.
Population counts for incidence were obtained on national, provincial, city and FSA postal code levels from the Canadian Census of Population for the years 1996, 2001, 2006, and 2011 according to Statistics Canada. In Canada, postal codes consist of 6 letters and numbers (e.g., H3G 1A4), where the first 3 entries (i.e., FSA) define a region in the country. There are more than 1,600 FSAs across Canada (23). Annual population counts for Canada from the census data was used to calculate annual incidence of glioma and glioblastoma.
To better elucidate the impact of pollution and industrial risk factors on the incidence of gliomas, significantly higher and lower incidence FSAs were searched using Standard Industrial Classification codes to detect significant industrial activity.
In accordance with the CCR, LRQC, and CVS data publication rules, to preserve patient privacy and confidentiality, rounding of variables as absolute numbers for glioma cases was conducted for all values presented in this study. With regards to the rounding of tabular data, SSHRC/Statistics Canada required rounding of each cell count, independent of other cells, to a lower or higher multiple of 5 using an unbiased, random rounding scheme in which counts are more likely to be rounded to their nearest multiple of 5. Counts ≥1 and <5 were restricted from publication, according to the SSHRC regulation. Therefore, if the number of cases were ≥1 but <5 per ICD-O-3 code, then its respective clinical and geographic information could not be released in order to protect patient confidentiality. In order to assess low incidence regional clusters, we were able to review communities with zero recorded glioma cases.
Unless otherwise specified, analyses of the complete data on all patients with glioma across Canada for the period from 1992 through 2010 are presented throughout this report. Incidence rates and 95% confidence intervals (CIs) were calculated and are reported overall, by the year of diagnosis, and for specific regions identified by the mapping analysis. Unless otherwise specified, Canadian Census averages from the years 1996, 2001, 2006, and 2011 were used for all population analyses. CIs were based on the exact Poisson distribution. Trends over time were assessed using simple regression models and joinpoint regression analyses (25). The coefficient of determination was calculated to establish how closely the incidence rates correspond to the regression line. The joinpoint regression analysis determines the best-fitting regression line and determines whether there are points in time (joinpoints) where significant changes take place.
Geographic maps of Canada divided by city and FSA were generated using ArcGIS Pro mapping software. In mapping the CCR/LRQC results, only regions with populations of at least 10,000 individuals based on 1996, 2001, 2006, and 2010 census data were selected to reduce erroneous false-positive hits, in which a few cases of gliomas occurring within scarcely populated cities and postal codes (<10,000 residents) might have artificially inflated the incidence rate.
In Canada, between 1992 and 2010, there were ~32,360 cases of glioma. Of these, 17,115 (52.9%) were glioblastoma. These patients’ clinical characteristics were studied using the demographic information available in two population-based health registries: the CCR and LRQC.
Both total glioma cases and glioblastoma subset cases were analyzed by age and sex to determine the demographic distribution for these malignancies. Our data illustrates that gliomas more commonly affected males (58% males vs. 42% females), which is consistent with the international trends (14). The average age at diagnosis of gliomas was 54.62 years of age (SD = 19.57). However, the highest incidence age group was notably 70-79 years, at 17.55 new glioma cases per 100,000 individuals per year. Studying glioblastoma specifically, male patients were also more affected than female patients (59% males vs. 41% females). The average age of diagnosis was 62.34 years (SD = 13.96). The highest incidence age group remained at 70-79 years, with 12.39 new glioblastoma cases per 100,000 individuals per year.
The overall crude incidence rate of gliomas in Canada during this time period was analyzed, yielding a mean glioma incidence of approximately 5.45 cases per 100,000 individuals per year. Linear regression analysis of the glioma crude incidence rate revealed trend of annual increase of 0.053 ± 0.0050 cases per 100,000 individuals per year.
More specific analysis for glioblastoma incidence in Canada demonstrated a similar consistently increasing trend. The national average for glioblastoma crude incidence was 2.87 cases per 100,000 individuals per year. Linear regression of this incidence from 1992 to 2010 shows an average annual increase of 0.085 ± 0.0064 cases per 100,000 individuals per year (Figure 1). Joinpoint analysis of incidence trend was performed for gliomas and glioblastomas. While there were no changes in incidence trends in gliomas, this analysis detected an increase in incidence slope for glioblastomas following 2005 year (Figure 1).
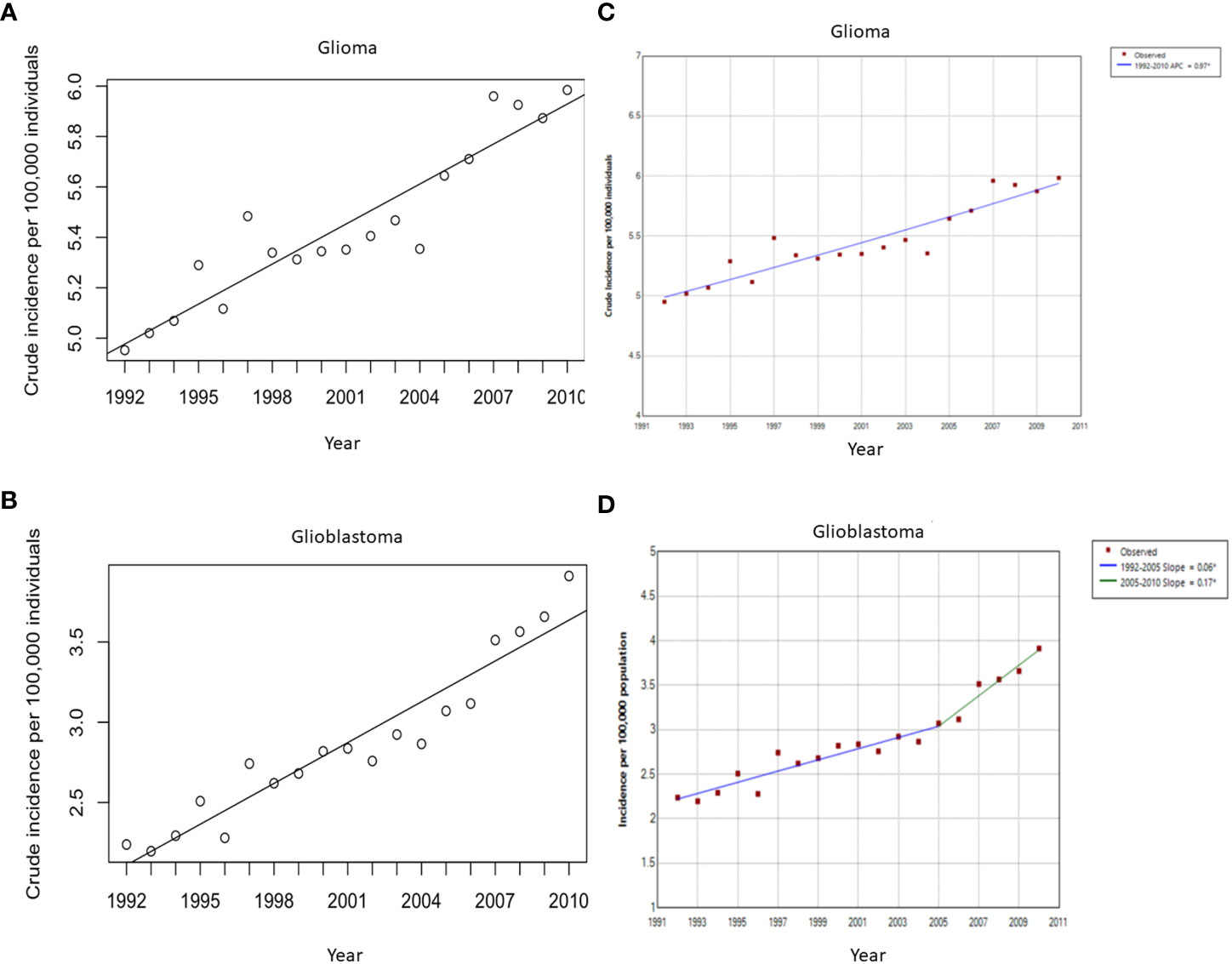
Figure 1 Crude incidence rates (per 100,000 individuals per year) of all glioma (A) and glioblastoma multiforme (B) cases between 1992 and 2010 with the line of best fit, and linear regression analysis of the incidence rate over time. (A) The slope of the line is 0.053 cases per 100,000 individuals per year, p < 0.0001. (B) The slope of the line is 0.085 cases per 100,000 individuals per year, p < 0.0001. Joinpoint analysis for incidence for all gliomas (C) and Glioblastoma multiforme (D). Change in slope in glioblastoma multiforme incidence was detected after 2005 from 0.06 to 0.17 cases per 100,000 per year.
The crude glioma incidence rates were analyzed on provincial, city, and FSA postal code levels, revealing distinct areas of higher incidence. As demonstrated by mapping, Quebec (6.33 cases per 100,000/year), Nova scotia (6.21 cases per 100,000/year), and New Brunswick (5.93 cases per 100,000/year) had significantly higher incidences compared to the national average of 5.45 cases per 100,000 individuals per year. On the other hand, provinces and territories with significantly lower glioma incidences included Alberta (5.11 cases per 100,000/year), Ontario (4.95 cases per 100,000/year), Northwest Territories (2.51 cases per 100,000/year), and Nunavut (1.88 cases per 100,000/year) (Figure 2; Table 1). As mentioned earlier, variability in glioma incidence was noted between different ethnic origins (2, 17). Canadian provinces have different ethnic composition, as detailed in Supplementary Table 2, which could in part explain the observed epidemiologic trends.

Table 1 Provinces/territories with significantly higher or lower incidence of (A) glioma and (B) glioblastoma.
Analyses of glioma incidence by city demonstrated a total of 33 cities, where glioma incidences were significantly higher than the national average of 5.45 cases per 100,000 individuals per year. Meanwhile, 73 cities demonstrated significantly lower incidences. Supplementary Tables 3 and 5 provide detailed incidence of cases.
To better understand the characteristics of regions with higher/lower incidences, we analyzed the incidence of gliomas on a finer level using FSA postal codes across Canada. These analyses revealed 95 FSAs with statistically significant higher incidences. Similarly, glioblastoma incidences by province, city, and FSA were also documented. Full detailed incidences by city and FSA can be found in Supplementary Tables 4 and 6.
Standard Industrial Classification codes for significantly higher and lower glioma incidence FSAs were searched to further elucidate correlations with potentially carcinogenic industrial activity. A T-test comparing the means of higher and lower incidence areas, assuming unequal variance, revealed that pests and farms (p = 0.0018), as well as industrial land use (p = 0.0004), were significantly correlated with increased glioma incidences. However, once FSAs were adjusted for land area, only airport operations (p = 0.0010) were found to be significantly related to increased glioma incidence (Tables 2, 3).
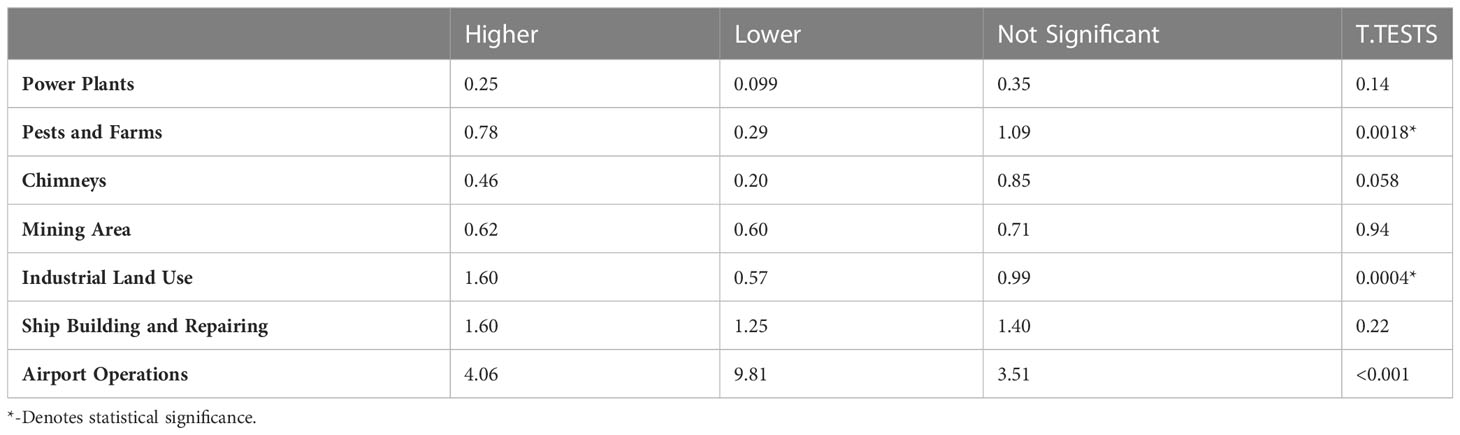
Table 2 Average number of facilities in higher vs. lower incidence FSAs; P-values are the result of a t-test comparing the means assuming unequal variance.
The present study investigated Canadian crude incidence rates of gliomas, with specific subset analysis for glioblastoma. Our findings are consistent with a recent study documenting epidemiology of gliomas/glioblastomas during 2010-2015 years conducted in 4 Canadian Provinces (Manitoba, Ontario, Alberta and British Columbia) (2). Overall, men were found to be more likely than women to have a glioblastoma, as well as to be diagnosed with any glioma. The highest incidence age group for glioblastoma and all gliomas was 70-79, though the average age of diagnosis for both analysis groups was younger. The national average for glioma incidence was 5.45 cases per 100,000/year with an increasing incidence of 0.053 cases per 100,000/year. The incidence of glioblastoma specifically, was 2.87 cases per 100,000/year with an average annual increase of 0.085 cases per 100,000/year. Joinpoint analysis documented a change in incidence slope for glioblastoma after 2005 from 0.06 to 0.17 cases per 100,000 per year. Provincially, Quebec led in glioma/glioblastoma incidence, while the lowest glioma incidence was documented in Nunavut and Northwest Territories. Based on available international data, both glioma and specific glioblastoma subtype findings are consistent with tendencies of incidence increase in the US and Europe (2, 14, 26), suggesting Canada is congruent with international trends in the glioma disease burden.
Currently, the only well-established external risk factors associated with developing gliomas is exposure to ionizing radiation and an inverse relationship to the presence of personal history of atopic dermatitis, rhinitis and other allergies, while exposure to UFPs may be emerging as a novel risk factor for these cancers (12, 13). There is a growing body of literature surrounding “the Cancer Hygiene Hypothesis” (27). In particular, depending on the cancer type, chronic allergic disorders have been connected to both pro and antitumor effects (6, 28, 29). Further research distinguishing the pro and antitumor effects of allergic disorders will help elucidate this relationship. Also, as highlighted earlier, since a number of gliomas may be asymptomatic, increased medical imaging can contribute to higher incidences of gliomas in select urban locations and in specific populations (i.e., individuals who are more likely to seek/receive imaging investigation). Notably, incidence of gliomas is known to vary by ethnicity (with higher incidences being reported in Caucasians) and level of urbanization and socioeconomic status of a given community (26).
According to the Government of Canada’s 2017 Report on Occupational Radiation Exposures (30), approximately 86,000 workers were occupationally exposed to ionizing radiation, with a slight predominance of 53% in male workers. Most exposed workers were nurses, aircrew members, ward aids, and orderlies. However, 80% of all documented ionizing radiation exposures were in low doses (0-1 millisievert or mSv), while the remaining were exposed to moderate doses (1-20mSV), and almost none were exposed to high dose radiation. On a geographic level, the total number of workers with documented ionizing radiation were most concentrated in Ontario (36,000 workers), Quebec (16,000 workers) Alberta (10,000 workers), and British Columbia (9,600 workers). However, once adjusted per-capita of 10,000 workers, the leading provinces were Saskatchewan (111/10,000 workers), New Brunswick (58/10,000 workers), and Ontario (52/10,000 workers). However, the report did not include further analysis on a more regional level and did not permit epidemiological comparison by city or FSA levels. It thus remains possible that pockets of higher ionizing radiation exposure exist within each province. This study heightens the need to investigate environmental and occupational exposures as the cardinal component in the pathogenesis of gliomas.
To better elucidate the impact of pollution and industrial risk factors on the incidence of gliomas, Standard Industrial Classification codes were searched in FSAs with significantly higher and lower glioma incidence. These codes included power plants, pests and farms, chimneys, mining areas, industrial land use, ship building and repairing, and airport operations. This analysis revealed that the presence of pests and farms, as well as industrial land use, was significantly correlated with increased glioma incidences. Different facilities occupy variable areas, and their impact may stretch for miles away from the facility generating pollution, where, for instance, UPSs may impact areas of at least 10 km away from a given major airport (13). Once FSAs were adjusted for land area, only the presence of airport operations was significantly related to increased glioma incidence. Though the risk of increased ionizing radiation exposure for residential areas neighboring airports is yet to be quantified in literature, carcinogenic environmental toxins released by jet fuel combustion, while limited, have previously been established. Though not specifically studied for neurotoxicity, oxidative DNA damage effects were previously demonstrated in peripheral blood lymphocytes and exfoliated buccal cells in airport personnel with long-term jet fuel exposure (31, 32). Geographic findings from this study may also be connected with the outcomes from the Canadian Government’s 2017 Report on Occupational Radiation Exposures findings of predominance of aircrew workers, who may choose to live in the proximity to their workplace. Importantly, as highlighted by a recent study by Wu et al. pollution from UFPs generated at airports may be an important contributor to the increased incidence of gliomas/glioblastomas (13). Other confounding factors must also be accounted for, when discussing living in the proximity to airport operations, such as socioeconomic status and housing standards. Further follow up studies will be necessary to evaluate this important risk factor.
This study had several limitations. Epidemiologic studies are limited by the accuracy of the databases. In particular, this study combined clinical and geographic data from the Canadian Cancer Registry (CCR) and Le Registre Québécois du Cancer (LRQC) in order to determine coherent incidence across time. Although measures have been taken to ensure the quality of data extraction in this study, the risk of data misclassification remains. Importantly, these databases did not provide information on the patients’ ethnicities or family history, both of which are known contributors to developing CNS malignancy. However, studies reveal that monogenic Mendelian disorders comprise only a small percentage of adult glioma incidence on a population level (3, 4), and thus the data analyzed in the present study remains largely reflective of Canadian epidemiology.
Additionally, while mapping by FSA revealed putative clusters, where multiple high-incidence FSAs were located side by side, mapping also revealed instances of low/zero-incidence FSAs located contiguously with reported high-incidence FSAs. This suggests the possible existence of some clusters or sparing by chance. Had these contiguous FSAs been combined, their overall incidence may likely be closer to the national average. Also, considering that glioma and glioblastoma are rare cancers any interpretation of higher/lower incidences in smaller population centers should be made with caution. This is an important limitation of the study. Also, as a number of gliomas may be asymptomatic, increased medical imaging in urban centers leading to incidental diagnosis of early-stage disease, might be affecting the reported numbers. Future investigations for possible glioma incidences should thus focus on true cluster areas of combined high incidence. Finally, this study reports crude incidence and not age-adjusted incidence, which was not possible to calculate due to low number of cases in select areas.
In conclusion, this epidemiologic study reveals a national glioma incidence rate of 5.45 cases per 100,000 individuals per year with demonstrated crude incidence increase over time. A male predominance was observed in incidence of gliomas/glioblastomas. Provincially, Quebec led in incidence, while the lowest glioma incidence rates were documented in Nunavut and Northwest Territories. A putative regional clustering of gliomas was observed, with higher incidence rates in FSAs with industrial activity related to airport operations.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
The presented study was conducted in accordance with protocols approved by the Social Sciences and Humanities Research Council of Canada (SSHRC) and the Québec Inter-University Centre for Social Statistics (QICSS), respectively, protocol numbers: CISS-RDC-668035 and 13-SSH-MCG-3749-S001. Further, in accordance with the institutional policy, this study received an exemption from the McGill University Research Ethics Board review.
Conceptualization, IL and OL. Methodology, XJ, AA, FG, MT, AZ, OL, and IL. Software, XJ and AA. Validation, XJ, AA, FG, AZ, and IL. Formal analysis, XJ, AA, and FG. Investigation, XJ, AA, FG, MT, AZ, OL, and IL. Data curation, XJ. Writing—original draft preparation, XJ. Writing—review and editing, XJ, AA, FG, MT, AZ, OL, and IL. Visualization, XJ and AA. Supervision, OL and IL. Project administration, IL. All authors contributed to the article and approved the submitted version.
This research was supported by the Mach-Gaensslen Foundation of Canada. We also thank our colleagues from the Centre inter universitaire québécois de la statistique sociale (CIQSS) who provided insight and expertise in data extraction that greatly assisted the research.
We thank for the support the employees of the Research Data Centre at McGill University.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1190366/full#supplementary-material
1. Public Health Agency of C, Statistics C, Canadian Cancer S, provincial/territorial cancer r. Release notice - Canadian cancer statistics 2019. Health Promot Chronic Dis Prev Can (2019) 39(8-9):255.
2. Walker EV, Davis FG, Yasmin F, Smith TR, Yuan Y. Incidence and survival of primary central nervous system tumors diagnosed in 4 Canadian provinces from 2010 to 2015. Neurooncol Pract (2023) 10(2):203–13. doi: 10.1093/nop/npac089
3. Wesseling P, Capper D. WHO 2016 classification of gliomas. Neuropathol Appl Neurobiol (2018) 44(2):139–50. doi: 10.1111/nan.12432
4. Barnholtz-Sloan JS, Ostrom QT, Cote D. Epidemiology of brain tumors. Neurol Clin (2018) 36(3):395–419. doi: 10.1016/j.ncl.2018.04.001
5. Yamanaka R, Hayano A, Kanayama T. Radiation-induced gliomas: a comprehensive review and meta-analysis. Neurosurg Rev (2018) 41(3):719–31. doi: 10.1007/s10143-016-0786-8
6. Amirian ES, Zhou R, Wrensch MR, Olson SH, Scheurer ME, Il'yasova D, et al. Approaching a scientific consensus on the association between allergies and glioma risk: a report from the glioma international case-control study. Cancer Epidemiol Biomarkers Prev (2016) 25(2):282–90. doi: 10.1158/1055-9965.EPI-15-0847
7. Braganza MZ, Kitahara CM, Berrington de Gonzalez A, Inskip PD, Johnson KJ, Rajaraman P. Ionizing radiation and the risk of brain and central nervous system tumors: a systematic review. Neuro Oncol (2012) 14(11):1316–24. doi: 10.1093/neuonc/nos208
8. Linos E, Raine T, Alonso A, Michaud D. Atopy and risk of brain tumors: a meta-analysis. J Natl Cancer Inst (2007) 99(20):1544–50. doi: 10.1093/jnci/djm170
9. McCarthy BJ, Rankin K, Il'yasova D, Erdal S, Vick N, Ali-Osman F, et al. Assessment of type of allergy and antihistamine use in the development of glioma. Cancer Epidemiol Biomarkers Prev (2011) 20(2):370–8. doi: 10.1158/1055-9965.EPI-10-0948
10. Gilbert ES. Ionising radiation and cancer risks: what have we learned from epidemiology? Int J Radiat Biol (2009) 85(6):467–82. doi: 10.1080/09553000902883836
11. Wolf A, Naylor K, Tam M, Habibi A, Novotny J, Liscak R, et al. Risk of radiation-associated intracranial malignancy after stereotactic radiosurgery: a retrospective, multicentre, cohort study. Lancet Oncol (2019) 20(1):159–64. doi: 10.1016/S1470-2045(18)30659-4
12. Weichenthal S, Olaniyan T, Christidis T, Lavigne E, Hatzopoulou M, Van Ryswyk K, et al. Within-city spatial variations in ambient ultrafine particle concentrations and incident brain tumors in adults. Epidemiol (2020) 31(2):177–83. doi: 10.1097/EDE.0000000000001137
13. Wu AH, Fruin S, Larson TV, Tseng CC, Wu J, Yang J, et al. Association between airport-related ultrafine particles and risk of malignant brain cancer: a multiethnic cohort study. Cancer Res (2021) 81(16):4360–9. doi: 10.1158/0008-5472.CAN-21-1138
14. Ostrom QT, Bauchet L, Davis FG, Deltour I, Fisher JL, Langer CE, et al. The epidemiology of glioma in adults: a "state of the science" review. Neuro Oncol (2014) 16(7):896–913. doi: 10.1093/neuonc/nou087
15. Warren PP, Lobbous M, Peeri NC, Thompson ZJ, Thompson RC, Olson JJ, et al. Prevalence of asymptomatic glioma and implications for survival. medRxiv (2020). doi: 10.1101/2020.04.27.20080564
16. Ranger AM, Patel YK, Chaudhary N, Anantha RV. Familial syndromes associated with intracranial tumours: a review. Childs Nerv Syst (2014) 30(1):47–64. doi: 10.1007/s00381-013-2309-z
17. Ostrom QT, Price M, Neff C, Cioffi G, Waite KA, Kruchko C, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the united states in 2015-2019. Neuro Oncol (2022) 24(Suppl 5):v1–v95. doi: 10.1093/neuonc/noac202
18. Collaborators GBDN. Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol (2019) 18(5):459–80. doi: 10.1016/S1474-4422(18)30499-X
19. Davis ME. Epidemiology and overview of gliomas. Semin Oncol Nurs (2018) 34(5):420–9. doi: 10.1016/j.soncn.2018.10.001
20. Conte S, Lagace F, Ghazawi FM, Cattelan L, Nath S, Dhillon J, et al. Uveal melanoma incidence trends in Canada: 1992-2010 vs. 2011-2017. Front Med (Lausanne) (2022) 9:1001799. doi: 10.3389/fmed.2022.1001799
21. Darwich R, Ghazawi FM, Rahme E, Alghazawi N, Burnier JV, Sasseville D, et al. Retinoblastoma incidence trends in Canada: a national comprehensive population-based study. J Pediatr Ophthalmol Strabismus (2019) 56(2):124–30. doi: 10.3928/01913913-20190128-02
22. Ghazawi FM, Le M, Cyr J, Netchiporouk E, Rahme E, Alakel A, et al. Analysis of acute myeloid leukemia incidence and geographic distribution in Canada from 1992 to 2010 reveals disease clusters in sarnia and other industrial US border cities in Ontario. Cancer (2019) 125(11):1886–97. doi: 10.1002/cncr.32034
23. Ghazawi FM, Netchiporouk E, Rahme E, Tsang M, Moreau L, Glassman S, et al. Comprehensive analysis of cutaneous T-cell lymphoma (CTCL) incidence and mortality in Canada reveals changing trends and geographic clustering for this malignancy. Cancer (2017) 123(18):3550–67. doi: 10.1002/cncr.30758
24. Lagace F, Ghazawi FM, Le M, Rahme E, Savin E, Zubarev A, et al. Analysis of incidence, mortality trends, and geographic distribution of breast cancer patients in Canada. Breast Cancer Res Treat (2019) 178(3):683–91. doi: 10.1007/s10549-019-05418-2
25. Lagace F, Ghazawi FM, Le M, Savin E, Zubarev A, Powell M, et al. Incidence and mortality of prostate cancer in Canada during 1992-2010. Curr Oncol (2021) 28(1):978–90. doi: 10.3390/curroncol28010096
26. Cote DJ, Ostrom QT, Gittleman H, Duncan KR, CreveCoeur TS, Kruchko C, et al. Glioma incidence and survival variations by county-level socioeconomic measures. Cancer (2019) 125(19):3390–400. doi: 10.1002/cncr.32328
27. Oikonomopoulou K, Brinc D, Kyriacou K, Diamandis EP. Infection and cancer: revaluation of the hygiene hypothesis. Clin Cancer Res (2013) 19(11):2834–41. doi: 10.1158/1078-0432.CCR-12-3661
28. Engkilde K, Thyssen JP, Menne T, Johansen JD. Association between cancer and contact allergy: a linkage study. BMJ Open (2011) 1(1):e000084. doi: 10.1136/bmjopen-2011-000084
29. Merrill RM, Isakson RT, Beck RE. The association between allergies and cancer: what is currently known? Ann Allergy Asthma Immunol (2007) 99(2):102–16; quiz 17-9, 50. doi: 10.1016/S1081-1206(10)60632-1
30. Canada H. 2017 Report on occupational radiation exposures in Canada. Ottawa, Ontario, Canada (2018).
31. Cavallo D, Ursini CL, Carelli G, Iavicoli I, Ciervo A, Perniconi B, et al. Occupational exposure in airport personnel: characterization and evaluation of genotoxic and oxidative effects. Toxicology (2006) 223(1-2):26–35. doi: 10.1016/j.tox.2006.03.003
Keywords: glioblastoma, epidemiology, public health, airport operations, ultrafine particles (UFPs), glioma
Citation: Ji X, Alakel A, Ghazawi FM, Tsang M, Zubarev A, Lasry OJ and Litvinov IV (2023) Investigation of incidence and geographic distribution of gliomas in Canada from 1992 to 2010: a national population-based study highlighting the importance of exposure to airport operations. Front. Oncol. 13:1190366. doi: 10.3389/fonc.2023.1190366
Received: 20 March 2023; Accepted: 02 May 2023;
Published: 16 May 2023.
Edited by:
Supriya Mallick, AIIMS, IndiaReviewed by:
Prashanth Giridhar, Tata Memorial Centre, IndiaCopyright © 2023 Ji, Alakel, Ghazawi, Tsang, Zubarev, Lasry and Litvinov. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ivan V. Litvinov, aXZhbi5saXR2aW5vdkBtY2dpbGwuY2E=; Feras M. Ghazawi, ZmVhbEB0b2guY2E=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.