
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 14 September 2023
Sec. Gastrointestinal Cancers: Colorectal Cancer
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1189324
 Yun-Zhou Xiao1
Yun-Zhou Xiao1 Xiao-Ting Wen2
Xiao-Ting Wen2 Ying-Ying Ying1
Ying-Ying Ying1 Xiao-Yan Zhang1
Xiao-Yan Zhang1 Lu-Yao Li1
Lu-Yao Li1 Zhong-Chu Wang1
Zhong-Chu Wang1 Miao-Guang Su1
Miao-Guang Su1 Xiang-Wu Zheng3*†
Xiang-Wu Zheng3*† Shou-Liang Miao3*†
Shou-Liang Miao3*†Background: Muscle depletion that impairs normal physiological function in elderly patients leads to poor prognosis. This study aimed to evaluate the association between total abdominal muscle area (TAMA), total psoas area (TPA), psoas muscle density (PMD), and short-term postoperative complications in elderly patients with rectal cancer.
Methods: All elderly patients underwent rectal cancer resection with perioperative abdominal computed tomography (CT). Complications were assessed according to the Clavien-Dindo classification. Severe complications were defined as grade III-V following the Clavien-Dindo classification. Univariate and multivariate analyses were performed to evaluate risk factors of short-term severe postoperative complications.
Results: The cohort consisted of 191 patients with a mean age of 73.60 ± 8.81 years. Among them, 138 (72.25%) patients had Clavien-Dindo 0- II, 53 (27.75%) patients had severe postoperative complications (Clavien-Dindo III-V), and 1(0.52%) patient died within 30 days of surgery. PMD was significantly higher in the Clavien-Dindo 0-II cohort compared to the Clavien-Dindo III-V cohort (p=0.004). Nevertheless, TAMA and TPA failed to exhibit significant differences. Moreover, the multivariate regression analysis implied that advanced age [OR 1.07 95%CI (1.02–1.13) p=0.013], male [OR 5.03 95%CI (1.76-14.41) p=0.003], high charlson comorbidity index (CCI) score [OR 3.60 95%CI (1.44-9.00) p=0.006], and low PMD [OR 0.94 95%CI (0.88-0.99) p=0.04] were independent risk factors of Clavien-Dindo III-V.
Conclusion: Preoperative assessment of the PMD on CT can be a simple and practical method for identifying elderly patients with rectal cancer at risk for severe postoperative complications.
Rectal cancer is one of the most common cancers worldwide and a major disease of the elderly. According to the GLOBOCAN project of the WHO cancer research center, the number of new cases of colorectal cancer worldwide in 2018 was about 1.8 million, with approximately 880000 deaths (1). Surgery is the cornerstone of curative therapy for patients with rectal cancer (2). However, the patient will become more vulnerable to postoperative complications since incidence and comorbidity rise steeply with age. Therefore, the prospective identification of patients with an increased risk for postoperative complications could optimize outcomes and guide the therapeutic protocol.
With aging, there are gradual changes in body composition and progressive, as well as systematic loss of skeletal muscle mass. The assessment of skeletal muscle on a single CT slice at the level of the third lumbar vertebra (L3) is strongly correlated with the volume of skeletal muscle in the entire body (3, 4), which can reflect sarcopenia. Numerous studies have revealed that sarcopenia is associated with poor prognosis in various malignancies, comprising gastric cancer (5), colorectal cancer (6), vascular (7), acute mesenteric ischemia (8), and emergency laparotomy surgery (9). Similarly, sarcopenia is commonly observed in elderly patients with rectal cancer, while previous studies have presented conflicting data on its prognostic role in rectal cancer. Chai et al. (10) revealed that sarcopenia defined by total abdominal muscle area (TAMA) was significantly associated with complications. Benedek et al. (11) discovered that total psoas area (TPA) was related to postoperative complications instead of psoas muscle density (PMD), consistent with Wu et al. (9). However, Pekařová et al. (12) and Cuijpers et al. (13) unveiled that PMD was correlated with postoperative complications rather than TPA. Although the indicators of sarcopenia are controversial, the number of cases in our study can tackle this difficulty.
Additionally, there are few studies on the relationship between TAMA, TPA, PMD, and postoperative complications in elderly patients with rectal cancer. Accordingly, this study aimed to investigate the relationship between TAMA, TPA, PMD, and short-term postoperative complications in elderly patients with rectal cancer.
This retrospective study was approved by the ethics committee of PingYang Affiliated Hospital of Wenzhou Medical University. The requirement of patient informed consent was waived owing to the retrospective nature of the study. Our research team reviewed the medical records of 230 consecutive patients with rectal cancer who underwent curative or palliative surgeries from June 2012 to July 2022. Inclusion criteria were patients who were 60 years old or above and had abdominal non-contrast CT scans before surgery. Exclusion criteria were patients who underwent emergency surgeries and had CT scans or clinical data not available, corrupt, or incomplete. Finally, a total of 191 patients were included in our study.
Patients underwent abdominal CT scans before the operation, and the CT films were stored in the picture archiving and communication system (PACS) automatically. Total abdominal muscle area (TAMA), psoas muscle area (PMA), density (PMD), and visceral fat area (VFA) were measured at PACS using preoperative abdominal non-contrast CT images by professional imaging software (INFINITT PACS software version 3.0.11.3 BN17 32 bit, INFINITT Healthcare Co., Ltd, Seoul, Korea). As illuminated in Figure 1, TAMA, TPA, PMD, and VFA were assessed at the cross sections in the third lumbar vertebra (L3) level where both transverse processes were visible. Predefined Hounsfield unit (HU) thresholds were employed for specific tissue demarcation. The Hounsfield unit threshold ranges of -29 – 150 and -150 – -50 were identified as skeletal muscle and visceral fat, respectively. Tissue boundaries were outlined manually as needed.
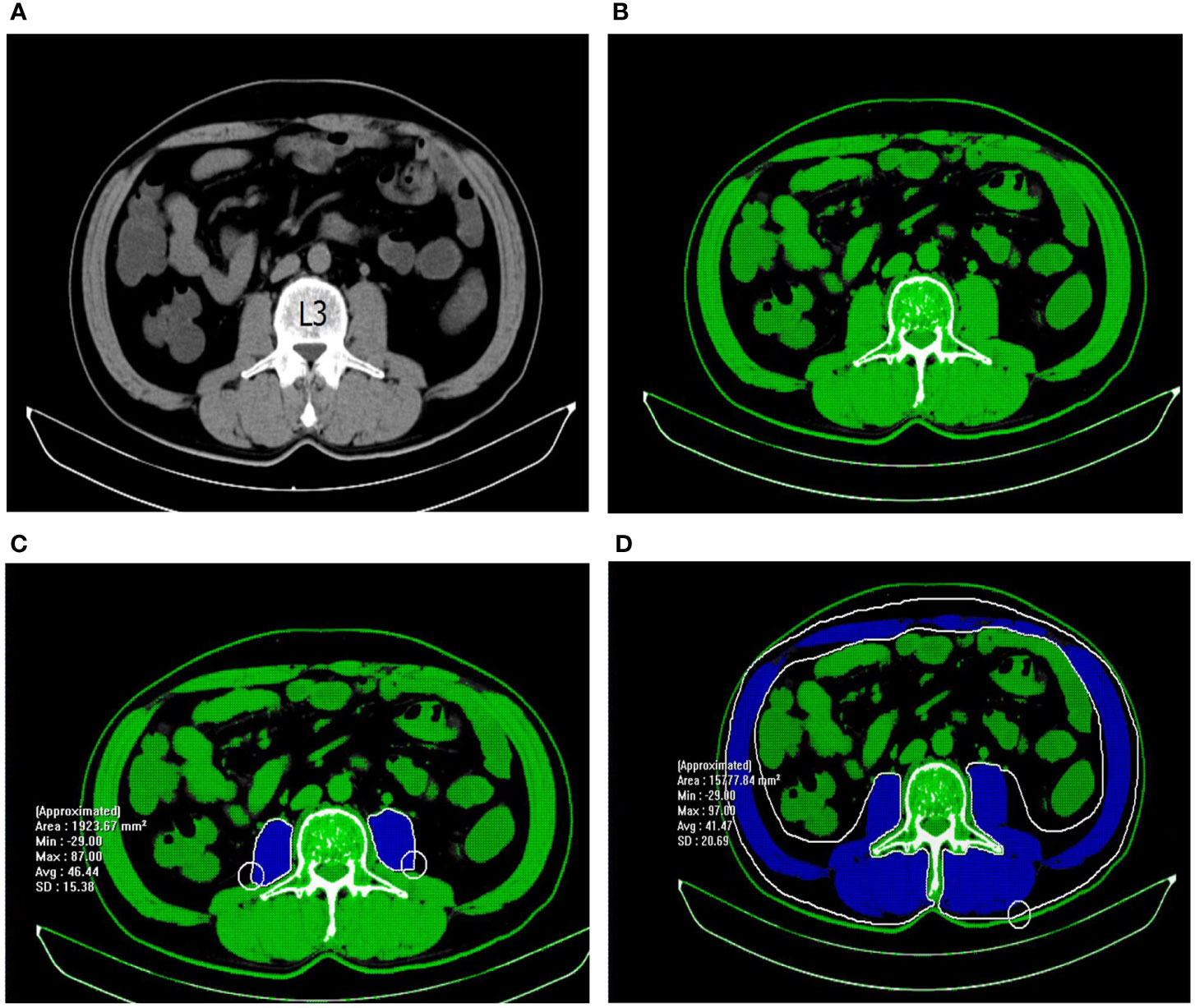
Figure 1 Measurement of abdominal muscle density and area with abdominal non-contrast CT images. (A) The third lumbar vertebra was chosen as a landmark. (B) Tissues with HU thresholds of − 29 to 150 are automatically outlined in green. (C) The selected region of psoas muscle was manually outlined in blue. PMD = 46.44 HU; PMA = 1923.67 mm2. (D) The selected region of total abdominal muscle was manually outlined in blue. TAMA=15777.84 mm2.
TAMA is composed of the psoas, quadratus lumborum, erector spinae, external and internal obliques, transversus, and rectus abdominis. PMA was measured by summing the left and right psoas muscle areas. PMD was quantified as the mean muscle attenuation for the cross-sectional psoas muscle. TAMA and TPA were normalized for height (m2) and reported as L3 skeletal muscle index (cm2/m2) and psoas muscle index (cm2/m2), respectively. According to the cutoff levels of VFA for metabolic syndrome in the Japanese population (14), visceral obesity was defined as men with a VFA > 130 cm2 and women with a VFA > 90 cm2. CT scans were executed by a single operator, skilled in radiological anatomy and body composition analysis, and blinded to the patient outcomes to avoid bias and possible inter-observer variation.
Preoperative patient details and postoperative outcome data were retrieved from electronic patient records. The following data were collected and analyzed retrospectively (1): the patient demographic and clinicopathological features, including age, gender, body mass index (BMI), hemoglobin concentration (a hemoglobin concentration < 120 g/L in men and < 110 g/L in women was defined as anemia), plasma albumin concentration (a plasma albumin concentration < 35g/L was defined as hypoalbuminemia), white blood cell (WBC) count, charlson comorbidity index (CCI) score (15), American Society of Anesthesiology (ASA) grade, nutritional risk screening 2002 (NRS 2002) scores, previous abdominal surgery, previous radiotherapy and chemotherapy, tumor location, histologic type, tumor size, and tumor node metastasis (TNM) stage of tumor (2); operative details, consisting of epidural anesthesia, laparoscopy-assisted operation, combined resection, surgical duration, estimated blood loss, and operation mode (3); postoperative short-term outcomes, composed of postoperative complications within 30 days after surgery. According to the Clavien-Dindo classification, complications were divided into Clavien-Dindo 0-II and Clavien-Dindo III-V, among which Clavien-Dindo III-V complications were defined to be significant (16).
Normally distributed continuous data were presented as mean ± SD, and the differences between groups were compared using Student’s t-test. Non-normally distributed variables were presented as median with interquartile range (IQR), and the Mann-Whitney U-test was used. Categorical data were compared using the chi-squared test or Fisher’s exact probability test. Variables with a value of P < 0.2 in the univariate analyses were included in the subsequent multivariate forward logistic regression analysis. Results are represented as odds ratio (OR) [95% confidence interval]. All tests were 2-sided, and a P-value <0.05 indicated statistical significance. The statistical analyses were performed using the SPSS statistics version 25.0 (IBM, Armonk, NY, USA) software programs.
A total of 191 patients satisfying our inclusion criteria were included in our analysis. The baseline characteristics are detailed in Table 1. The tumor location was mostly located in the upper (115, 60.21%), and 168 (87.96%) patients underwent Dixon surgery. Among them, 88 (46.07%) resections were performed via laparotomy. Most patients had an ASA score ≤ 2 (141, 73.82%), an NRS2002 score < 3 (172, 90.05%) was the most common, and only 75 (39.27%) patients presented a CCI score of 0. There were 82 (42.93%) patients with anemia and 28 (14.66%) patients with hypoalbuminemia.
According to the Clavien-Dindo classification, 138 (72.25%) and 53 (27.75%) had Clavien-Dindo 0-II and severe postoperative complications (Clavien-Dindo III-V), respectively, and 1(0.52%) patient died within 30 days of surgery. There was significant association in advanced age (p<0.01), high ASA score (p<0.01), high CCI score (p<0.01), high NRS2002 score (p=0.044), PMD (p=0.004), and hypoalbuminemia (p=0.017) between Clavien-Dindo 0-II and Clavien-Dindo III-V. TAMA (p=0.123), TPA (p=0.543), and other factors were not significantly associated between the two groups.
The results of the multivariate analysis of factors associated with severe postoperative complications (grade III-V) are shown in Table 2.The multivariate regression analysis suggested that advanced age [OR 1.07 95%CI (1.02-1.13) p=0.013], male [OR 5.03 95%CI (1.76-14.41) p=0.003], high CCI score [OR 3.60 95%CI (1.44-9.00) p=0.006], and low PMD [OR 0.94 95%CI (0.88-0.99) p=0.04] were independent risk factors of Clavien-Dindo III-V.
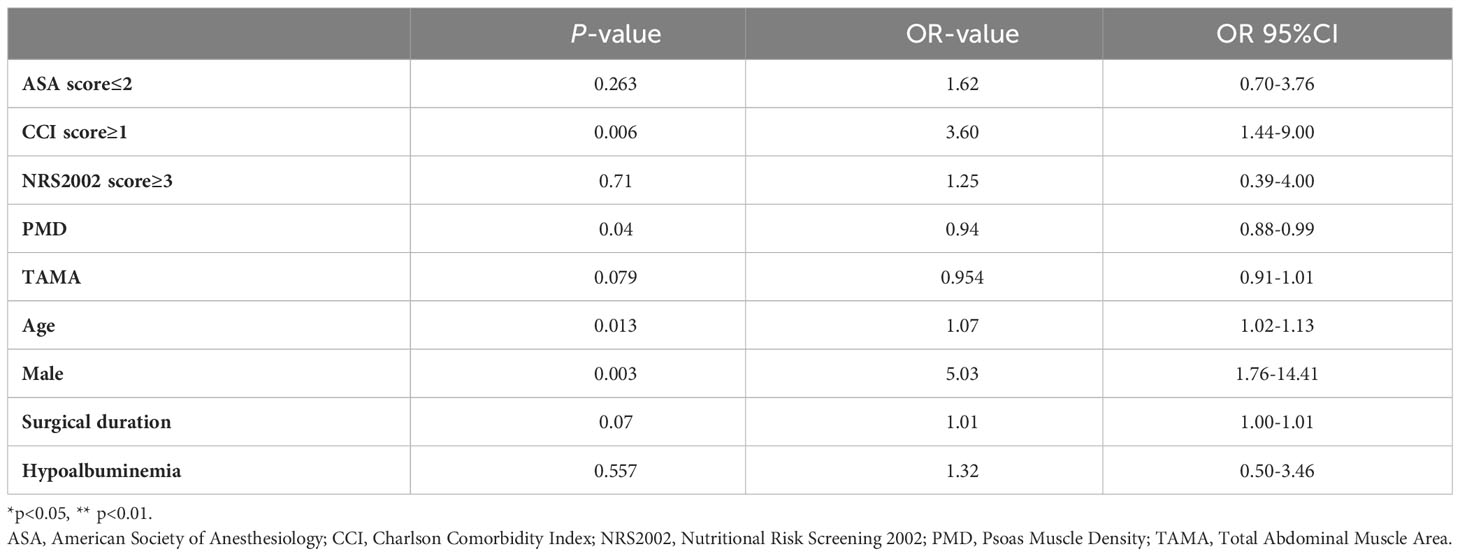
Table 2 Multivariate logistic regression analyses of factors influencing Clavien-Dindo III-IV in rectal cancer resection.
The main finding of the present study was that low PMD, but not TAMA and TPA, is an independent risk factor for severe postoperative complications in elderly patients with rectal cancer. To our knowledge, this is the first study to demonstrate the utility of low PMD based on CT for identifying elderly patients with rectal cancer at risk for postoperative complications.
Low muscle density is an indicator of intramuscular fat deposition (17) and is correlated with physical performance (18). It could reflect the decline in muscle quality. A growing body of evidence revealed that low muscle mass was associated with postoperative complications in various surgical procedures. Moreover, low muscle area (TAMA, TPA) was related to severe postoperative complications in colorectal cancer (19, 20). Similarly, Herrod et al. (21) discovered that psoas density was highly predictive of postoperative morbidity in colorectal cancer patients of all ages. Additionally, Margadant et al. (22) unveiled that low psoas density was ascribed to major postoperative complications in elderly patients who underwent surgery for colorectal cancer. Colorectal cancer develops from the colon or rectum. However, colon cancer and rectal cancer are two separate tumor entities requiring distinct treatment approaches (23, 24) since they have different molecular developmental mechanisms and metastatic patterns (25, 26). Therefore, colon cancer and rectal cancer should be considered independently in evaluating postoperative complications. Our study emphasized rectal cancer, especially among the elderly. Elderly people represent almost all patients diagnosed with and treated for rectal cancer. This trend is likely to become more apparent in the future. Surgical management and treatment decisions for this disease become increasingly complex, while only a few reports specifically wrestle from older patients (2). Frailty is crucial in cancer. The incidence of frailty in elderly patients with cancer is particularly high (27). Frailty is a state of extreme vulnerability to stressors, leading to adverse health outcomes (28–30). Muscle tissue assessment is a quick and easy way to quantify a patient’s level of frailty (31). Age is an independent risk factor for decreased muscle quality (32). This was one of the main reasons to emphasize elderly patients in this study. Additionally, our study also verified that advanced age is an independent risk factor for severe postoperative complications of elderly rectal cancer. With increasing age, various physiological systems decline in the human body, forming a complex, multidimensional, and periodic state of physiological reserve reduction. As a result, resilience and adaptability decreased, and vulnerability to stressors increased (32–35). Consequently, our study lays a little foundation for the preoperative preparation of the elderly for rectal cancer.
Interestingly, our findings suggested that TAMA and TPA were not associated with the short-term postoperative complications of elderly rectal cancer, inconsistent with the studies of Uehara et al. (36) and Jochum et al. (37). Psoas muscle density, instead of area, was associated with the short-term postoperative complications of elderly rectal cancer. Its possible reasons are described as follows. Firstly, intramuscular fat infiltration does not affect its area, implying that PMD decreases while TAMA and TPA remain unchanged. It was assumed in our study that muscle quality rather than quantity was more critical in the estimation of complications risk. Secondly, it can be speculated that muscle fat infiltration can lead to a decrease in muscle strength, TAMA and TPA reflect muscle mass, and PMD reflects muscle strength. Moreover, Zhang et al. (38) uncovered that muscle strength is a better predictor of postoperative complications and overall survival of gastric cancer compared with muscle mass. Similarly, low muscle density, but not area (TAMA, TPA), is an independent risk factor for severe postoperative complications of rectal cancer (39).
Our study also revealed that high CCI scores and males were independent risk factors for severe postoperative complications of elderly rectal cancer, consistent with some previous studies (12, 36, 39). Increasing age is considered a decisive risk factor in association with concomitant diseases (40). It may induce poorer surgical outcomes (41, 42). Portale et al. (43) demonstrated that higher CCI was an independent predictor of short-term results of patients who underwent laparoscopic curative resection for rectal cancer, in line with our study. Research of 196 patients who underwent rectal cancer resection suggested that male patients were an independent risk factor for severe postoperative complications (44). The male patients influence the postoperative complications since the male pelvis is technically more challenging (45).
The present study had several limitations. First, it was a retrospective, single-institution analysis. Second, potential bias and certain limitations with respect to their internal validity and generalization exist due to the limited number of patients in our study. Third, the association of PMD with postoperative complications according to age stratification was not evaluated although we concluded that age had an effect on PMD and postoperative complications. In the future, further studies with a larger study population should be conducted to verify the relationship between PMD and postoperative complications according to age stratification. Finally, the long-term outcome was not analyzed in this study.
To summarize, this study demonstrated that preoperative assessment of the PMD on CT can be a simple and practical method for identifying elderly patients with rectal cancer at risk for severe postoperative complications. PMD should be employed to augment existing methods of patient-risk stratification before surgery.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethics Committee of Pingyang County People’s Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Y-ZX, S-LM, X-TW, and X-WZ conceived and designed the experiments and were responsible for data analysis and writing the manuscript. Y-YY, L-YL, X-YZ, and Z-CW were responsible for providing the clinical samples. M-GS was responsible for data collection. All authors contributed to the article and approved the submitted version.
The authors would like to acknowledge that this work was presented in preprint at Research square on November 15th, 2022.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
2. Manceau G, Karoui M, Werner A, Mortensen NJ, Hannoun L. Comparative outcomes of rectal cancer surgery between elderly and non-elderly patients: a systematic review. Lancet Oncol (2012) 13(12):e525–36. doi: 10.1016/S1470-2045(12)70378-9
3. Shen W, Punyanitya M, Wang Z, Gallagher D, St-Onge MP, Albu J, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol (2004) 97(6):2333–8. doi: 10.1152/japplphysiol.00744.2004
4. Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab (2008) 33(5):997–1006. doi: 10.1139/H08-075
5. Zhuang CL, Huang DD, Pang WY, Zhou CJ, Wang SL, Lou N, et al. Sarcopenia is an independent predictor of severe postoperative complications and long-term survival after radical gastrectomy for gastric cancer: analysis from a large-scale cohort. Med (Baltimore) (2016) 95(13):e3164. doi: 10.1097/MD.0000000000003164
6. van Vugt JLA, Coebergh van den Braak RRJ, Lalmahomed ZS, Vrijland WW, Dekker JWT, Zimmerman DDE, et al. Impact of low skeletal muscle mass and density on short and long-term outcome after resection of stage I-III colorectal cancer. Eur J Surg Oncol (2018) 44(9):1354–60. doi: 10.1016/j.ejso.2018.05.029
7. Drudi LM, Phung K, Ades M, Zuckerman J, Mullie L, Steinmetz OK, et al. Psoas muscle area predicts all-cause mortality after endovascular and open aortic aneurysm repair. Eur J Vasc Endovasc Surg (2016) 52(6):764–9. doi: 10.1016/j.ejvs.2016.09.011
8. Miao SL, Ye XN, Lin TT, Qiu YH, Huang JY, Zheng XW, et al. The psoas muscle density as a predictor of postoperative complications and 30-day mortality for acute mesenteric ischemia patients. Abdom Radiol (NY) (2020) 47(5):1644–53. doi: 10.1007/s00261-020-02714-0
9. Wu XL, Shen J, Danzeng CD, Xu XS, Cao ZX, Jiang W, et al. CT psoas calculations on the prognosis prediction of emergency laparotomy: a single-center, retrospective cohort study in eastern Asian population. World J Emerg Surg (2022) 17(1):31. doi: 10.1186/s13017-022-00435-x
10. Chai VW, Chia M, Cocco A, Bhamidipaty M, D’Souza B. Sarcopenia is a strong predictive factor of clinical and oncological outcomes following curative colorectal cancer resection. ANZ J Surg (2021) 91(5):E292–7. doi: 10.1111/ans.16706
11. Benedek Z, Todor-Boér S, Kocsis L, Bauer O, Suciu N, Coros MF. Psoas muscle index defined by computer tomography predicts the presence of postoperative complications in colorectal cancer surgery. Medicina (Kaunas) (2021) 57(5):472. doi: 10.3390/medicina57050472
12. Pekařová A, Pekař M, Soltes M, Havrlentová L, Chovancová T. Psoas density - an optimal sarcopaenic indicator associated with postoperative complications after colorectal resection for cancer? Wideochir Inne Tech Maloinwazyjne (2021) 16(1):91–7. doi: 10.5114/wiitm.2020.100880
13. Cuijpers ACM, Bongers BC, Heldens AFJM, Bours MJL, van Meeteren NLU, Stassen LPS, et al. Aerobic fitness and muscle density play a vital role in postoperative complications in colorectal cancer surgery. J Surg Oncol (2022) 125(6):1013–23. doi: 10.1002/jso.26817
14. Oka R, Kobayashi J, Yagi K, Tanii H, Miyamoto S, Asano A, et al. Reassessment of the cutoff values of waist circumference and visceral fat area for identifying Japanese subjects at risk for the metabolic syndrome. Diabetes Res Clin Pract (2008) 79(3):474–81. doi: 10.1016/j.diabres.2007.10.016
15. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis (1987) 40(5):373–83. doi: 10.1016/0021-9681(87)90171-8
16. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg (2009) 250(2):187–96. doi: 10.1097/SLA.0b013e3181b13ca2
17. Aubrey J, Esfandiari N, Baracos VE, Buteau FA, Frenette J, Putman CT, et al. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol (Oxf) (2014) 210(3):489–97. doi: 10.1111/apha.12224
18. Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci (2005) 60(3):324–33. doi: 10.1093/gerona/60.3.324
19. Richards SJG, Senadeera SC, Frizelle FA. Sarcopenia, as assessed by psoas cross-sectional area, is predictive of adverse postoperative outcomes in patients undergoing colorectal cancer surgery. Dis Colon Rectum (2020) 63(6):807–15. doi: 10.1097/DCR.0000000000001633
20. Chen WZ, Chen XD, Ma LL, Zhang FM, Lin J, Zhuang CL, et al. Impact of visceral obesity and sarcopenia on short-term outcomes after colorectal cancer surgery. Dig Dis Sci (2018) 63(6):1620–30. doi: 10.1007/s10620-018-5019-2
21. Herrod PJJ, Boyd-Carson H, Doleman B, Trotter J, Schlichtemeier S, Sathanapally G, et al. Quick and simple; psoas density measurement is an independent predictor of anastomotic leak and other complications after colorectal resection. Tech Coloproctol (2019) 23(2):129–34. doi: 10.1007/s10151-019-1928-0
22. Margadant CC, Bruns ER, Sloothaak DA, van Duijvendijk P, van Raamt AF, van der Zaag HJ, et al. Lower muscle density is associated with major postoperative complications in older patients after surgery for colorectal cancer. Eur J Surg Oncol (2016) 42(11):1654–9. doi: 10.1016/j.ejso.2016.05.040
23. Tamas K, Walenkamp AM, de Vries EG, van Vugt MA, Beets-Tan RG, van Etten B, et al. Rectal and colon cancer: Not just a different anatomic site. Cancer Treat Rev (2015) 41(8):671–9. doi: 10.1016/j.ctrv.2015.06.007
24. Doumouras AG, Tsao MW, Saleh F, Hong D. A population- based comparison of 30-day readmission after surgery for colon and rectal cancer: How are they different? J Surg Oncol (2016) 114(3):354–60. doi: 10.1002/jso.24334
25. Kalady MF, Sanchez JA, Manilich E, Hammel J, Casey G, Church JM. Divergent oncogenic changes influence survival differences between colon and rectal adenocarcinomas. Dis Colon Rectum (2009) 52(6):1039–45. doi: 10.1007/DCR.0b013e31819edbd4
26. Paschke S, Jafarov S, Staib L, Kreuser ED, Maulbecker-Armstrong C, Roitman M, et al. Are colon and rectal cancer two different tumor entities? A proposal to abandon the term colorectal cancer. Int J Mol Sci (2018) 19(9):2577. doi: 10.3390/ijms19092577
27. Mohile SG, Xian Y, Dale W, Fisher SG, Rodin M, Morrow GR, et al. Association of a cancer diagnosis with vulnerability and frailty in older Medicare beneficiaries. J Natl Cancer Inst (2009) 101(17):1206–15. doi: 10.1093/jnci/djp239
28. Handforth C, Clegg A, Young C, Simpkins S, Seymour MT, Selby PJ, et al. The prevalence and outcomes of frailty in older cancer patients: a systematic review. Ann Oncol (2015) 26(6):1091–101. doi: 10.1093/annonc/mdu540
29. Makary MA, Segev DL, Pronovost PJ, Syin D, Bandeen-Roche K, Patel P, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg (2010) 210(6):901–8. doi: 10.1016/j.jamcollsurg.2010.01.028
30. Tan KY, Kawamura YJ, Tokomitsu A, Tang T. Assessment for frailty is useful for predicting morbidity in elderly patients undergoing colorectal cancer resection whose comorbidities are already optimized. Am J Surg (2012) 204(2):139–43. doi: 10.1016/j.amjsurg.2011.08.012
31. Buettner S, Wagner D, Kim Y, Margonis GA, Makary MA, Wilson A, et al. Inclusion of sarcopenia outperforms the modified frailty index in predicting 1-year mortality among 1,326 patients undergoing gastrointestinal surgery for a Malignant indication. J Am Coll Surg (2016) 222(4):397–407.e2. doi: 10.1016/j.jamcollsurg.2015.12.020
32. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci (2001) 56(3):M146–56. doi: 10.1093/gerona/56.3.m146
33. Robinson TN, Walston JD, Brummel NE, Deiner S, Brown CH4, Kennedy M, et al. Frailty for surgeons: review of a National Institute on Aging conference on frailty for specialists. J Am Coll Surg (2015) 221(6):1083–92. doi: 10.1016/j.jamcollsurg.2015.08.428
34. Rodríguez-Mañas L, Féart C, Mann G, Viña J, Chatterji S, Chodzko-Zajko W, et al. Searching for an operational definition of frailty: a Delphi method based consensus statement: the frailty operative definition-consensus conference project. J Gerontol A Biol Sci Med Sci (2013) 68(1):62–7. doi: 10.1093/gerona/gls119
35. . Bortz WM 2nd. A conceptual framework of frailty: a review. J Gerontol A Biol Sci Med Sci (2002) 57(5):M283–8. doi: 10.1093/gerona/57.5.m283
36. Uehara H, Yamazaki T, Iwaya A, Kameyama H, Utsumi S. Is radiological psoas muscle area measurement a predictor of postoperative complications after rectal resection for rectal cancer? A retrospective study. Surg Today (2022) 52(2):306–15. doi: 10.1007/s00595-021-02346-x
37. Jochum SB, Kistner M, Wood EH, Hoscheit M, Nowak L, Poirier J, et al. Is sarcopenia a better predictor of complications than body mass index? Sarcopenia and surgical outcomes in patients with rectal cancer. Colorectal Dis (2019) 21(12):1372–8. doi: 10.1111/codi.14751
38. Zhang FM, Zhang XZ, Shi HP, Zhang Z, Wang SL. Comparisons and impacts of the basic components of sarcopenia definition and their pairwise combinations in gastric cancer: A large-scale study in a Chinese population. Front Nutr (2021) 8:709211. doi: 10.3389/fnut.2021.709211
39. Berkel AEM, Klaase JM, de Graaff F, Brusse-Keizer MGJ, Bongers BC, van Meeteren NLU. Patient’s skeletal muscle radiation attenuation and sarcopenic obesity are associated with postoperative morbidity after neoadjuvant chemoradiation and resection for rectal cancer. Dig Surg (2019) 36(5):376–83. doi: 10.1159/000490069
40. Damhuis RAM, Meurs CJC, Meijer WS. Postoperative mortality after cancer surgery in octogenarians and nonagenarians: results from a series of 5,390 patients. World J Surg Oncol (2005) 3:71. doi: 10.1186/1477-7819-3-71
41. Sarikaya H, Benhidjeb T, Iosivan SI, Kolokotronis T, Förster C, Eckert S, et al. Impact of ASA-score, age and learning curve on early outcome in the initiation phase of an oncological robotic colorectal program. Sci Rep (2020) 10:15136. doi: 10.1038/s41598-020-72025-3
42. Cai H, Zhang Y, Liu X, Jiang W, Chen Z, Li S, et al. Association of age and cause-special mortality in patients with stage I/II colon cancer: a population based competing risk analysis. PloS One (2020) 15:e0240715. doi: 10.1371/journal.pone.0240715
43. Portale G, Valdegamberi A, Cavallin F, Frigo F, Fiscon V. Effect of age and comorbidities on short- and long-term results in patients undergoing laparoscopic curative resection for rectal cancer. J Laparoendosc Adv Surg Tech A (2019) 29(3):353–9. doi: 10.1089/lap.2018.0340
44. Ciorogar G, Bartos A, Bartos D, Vesa SC, Pop M, Herdean A, et al. Rectal cancer: factors predicting short outcomes after radical anterior resection. Ann Ital Chir (2017) 88:505–13.
Keywords: rectal cancer, postoperative complications, elderly patient, psoas density, sarcopenia
Citation: Xiao Y-Z, Wen X-T, Ying Y-Y, Zhang X-Y, Li L-Y, Wang Z-C, Su M-G, Zheng X-W and Miao S-L (2023) The psoas muscle density as a predictor of postoperative complications in elderly patients undergoing rectal cancer resection. Front. Oncol. 13:1189324. doi: 10.3389/fonc.2023.1189324
Received: 18 March 2023; Accepted: 29 August 2023;
Published: 14 September 2023.
Edited by:
Sharon R. Pine, University of Colorado Anschutz Medical Campus, United StatesReviewed by:
Giacomo Deiro, University of Turin, ItalyCopyright © 2023 Xiao, Wen, Ying, Zhang, Li, Wang, Su, Zheng and Miao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shou-Liang Miao, bWlhb3Nob3VsaWFuZzlAMTYzLmNvbQ==; Xiang-Wu Zheng, enh3MTExQHNpbmEuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.