
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 09 June 2023
Sec. Cancer Genetics
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1187521
This article is part of the Research Topic Predictive Biomarkers of Immune-checkpoint Inhibitors Immunotherapies in Hepatocellular Carcinomas and Gastric Cancers View all 12 articles
Introduction: Gastric cancer is one of the most common cancers in the world. This study aimed to identify genes, biomarkers, and metabolic pathways affecting gastric cancer using bioinformatic analysis and meta-analysis.
Methods: Datasets containing gene expression profiles of tumor lesions and adjacent non-tumor mucosa samples were downloaded. Common differentially expressed genes between data sets were selected to identify hub genes and further analysis. Gene Expression Profiling and Interactive Analyses (GEPIA) and the Kaplan-Meier method were used to further validate the expression level of genes and plot the overall survivalcurve, respectively.
Results and disscussion: KEGG pathway analysis showed that the most important pathway was enriched in ECM-receptor interaction. Hub genes includingCOL1A2, FN1, BGN, THBS2, COL5A2, COL6A3, SPARC and COL12A1 wereidentified. The top interactive miRNAs including miR-29a-3p, miR-101-3p,miR-183-5p, and miR-15a-5p targeted the most hub genes. The survival chart showed an increase in mortality in patients with gastric cancer, which shows the importance of the role of these genes in the development of the disease and can be considered candidate genes in the prevention and early diagnosis of gastric cancer.
Gastric cancer (GC) is the fifth most common cancer in the world and the fourth leading cause of cancer death (1). In addition to non-genetic factors such as smoking, alcohol consumption, poor diet, physical inactivity, viral infections, and stress, genetic factors are also other contributing factors to this cancer (2).
To date, the only treatment for GC is surgery and chemotherapy (3). Because early-stage GC is usually asymptomatic and diagnosis is often made in the advanced stage of the disease. Using the bioinformatics technique is an effective way to identify genes and their protein products as biomarkers. In this way, markers can be examined in the blood of patients in the early stages, and then diagnostic tests can be performed (1, 4).
Microarray allows the simultaneous examination of tens to thousands of RNAs in an organism or a cell (5). Analysis of the expression level of this number of genes reveals the processes that take place simultaneously in the cell. Also, comparing the expression level of genes in both healthy and sick conditions provide valuable studies of how the disease originated and progressed (6).
Despite being a high-throughput method for gene expression analysis, microarray has some limitations that are generally related to reproducibility and sensitivity to technical and computational errors (7). Integrating already existing information can increase the reproducibility and reliability of results. The statistical technique of integrating different but related studies is called meta-analysis, which makes it possible to identify common genes and understand common molecular mechanisms. Integrated analysis of biological information helps identify and screen cancer-related genes and develop new treatment strategies for disease management (8). In this study, two gene expression profiles in the GEO database containing samples from GC tumor tissue and adjacent tissue were used to identify common genes and biomarkers and metabolic pathways affecting GC.
Gene expression data were downloaded from the public database of the Gene Expression Omnibus (GEO) database of NCBI (http://www.ncbi.nlm.nih.gov/geo/). The first dataset (GEO accession number GSE79973) contained gene expression profiling of 10 samples of tumor lesions and 10 samples of adjacent non-tumor mucosa. The second dataset (GEO accession number GSE19826) contained gene expression profiling of 12 samples of tumor lesions and 12 samples of adjacent non-tumor mucosa numbers. The gene expression profiling was generated by the Affymetrix platform.
We applied the GEO2R analysis to identify differentially expressed genes (DEGs) with Benjamini–Hochberg correction to control the false discovery rate. Cut-off criteria for sorting significant DEGs were p-value < 0.05 and |log2 fold change (FC)| ≥ 1. Finally, the mentioned analysis was applied to both selected datasets, and differentially expressed genes with different expression were obtained for both datasets. To integrate the data, common differentially expressed genes in both datasets were selected for further analyses.
Database for Annotation, Visualization, and Integrated Discovery (DAVID) was used to interpret the list of DEGs. Pathway analysis and gene ontology (GO) analysis were performed using DAVID. Biological pathways with a p-value less than 0.05 were considered significant. The outcomes of DAVID were then imported into the GO plot in R Studio. The GO Bubble plot was used to visualize the functional enrichment of DEGs, which facilitates the combination of expression data with functional assessment outcomes. DEGs were exposed to Clue GO v2.5.7 to perform and visualize GO analysis. The p-values <0.05 were considered significant.
The Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) database was used to study the protein products of the genes. This database provides networks containing regulatory connections between genes as output. Cytohubba is a simple Cytoscape plugin that ranks the importance of nodes in the PPI network with different algorithms for identifying key biological elements. In this study, using the degree algorithm, 8 genes with a rank higher than 7 were selected as hub genes.
Using the Kaplan-Meier plotter (http://kmplot.com/analysis/), Kaplan-Meier diagrams of the top 8 hub genes were drawn. The Kaplan-Mahir estimator is an estimator for estimating the survival function from survival information.
Hub genes and their protein products were evaluated by Gene Expression Profiling Interactive Analysis (GEPIA) web tool. GEPIA is a web-based tool for providing fast and customizable functions based on TCGA and GTEx data (http://gepia.cancer-pku.cn). This database was also used to plot survival and gene expression diagrams in a box plot.
The Encyclopedia RNA Interactomes (ENCORI, http://starbase.sysu.edu.cn/) is a public platform that detects more than 2.5 million miRNA-mRNA interactions. The target miRNAs of the hub genes were screened based on criteria of SLIP-DATA ≥ 5, Degradome-Data ≥ 0, pan-Cancer ≥ 6, and programNum ≥ 3 using the Encyclopedia RNA Interactomes. In addition, the miRNA-hub gene network was created by Cytoscape software.
Interactions between hub genes and related therapeutic drugs were examined through the drug-gene interaction database (http://www.dgidb.org/search interactions). The drug-gene interaction database is a web-based source of information on gene-drug interactions. We also mapped the interactive network between hub genes and effective drugs using the STITCH online tool (http://stitch.embl.de/cgi).
To obtain the DEGs, we analyzed the GSE79973 and GSE19826 datasets separately using GEO2R. We investigated common DEGs between two datasets. Finally, 38 common genes were found between these two datasets (Figure 1).
To understand the functions and biological significance of the mutual DEGs, we performed an enrichment analysis of DEGs in terms of biological process (BP), molecular function (MF), and cellular component (CC) (Table 1). Sixteen significantly enriched BP terms were found, the most significant of which were “collagen catabolic process”, “extracellular matrix organization”, and “skeletal system development”. Ten significant CC terms were identified, the most significant of which were “proteinaceous extracellular matrix”, “collagen trimer”, and “extracellular matrix”. The significantly enriched MF terms were “extracellular matrix structural constituent”, “integrin binding”, “calcium ion binding”, and “heparin binding”. KEGG pathway enrichment analysis revealed the association of the DEGs in 5 pathways including “Protein digestion and absorption”, “ECM-receptor interaction”, “Focal adhesion”, “Amoebiasis”, and “PI3K-Akt signaling pathway” (Table 2).
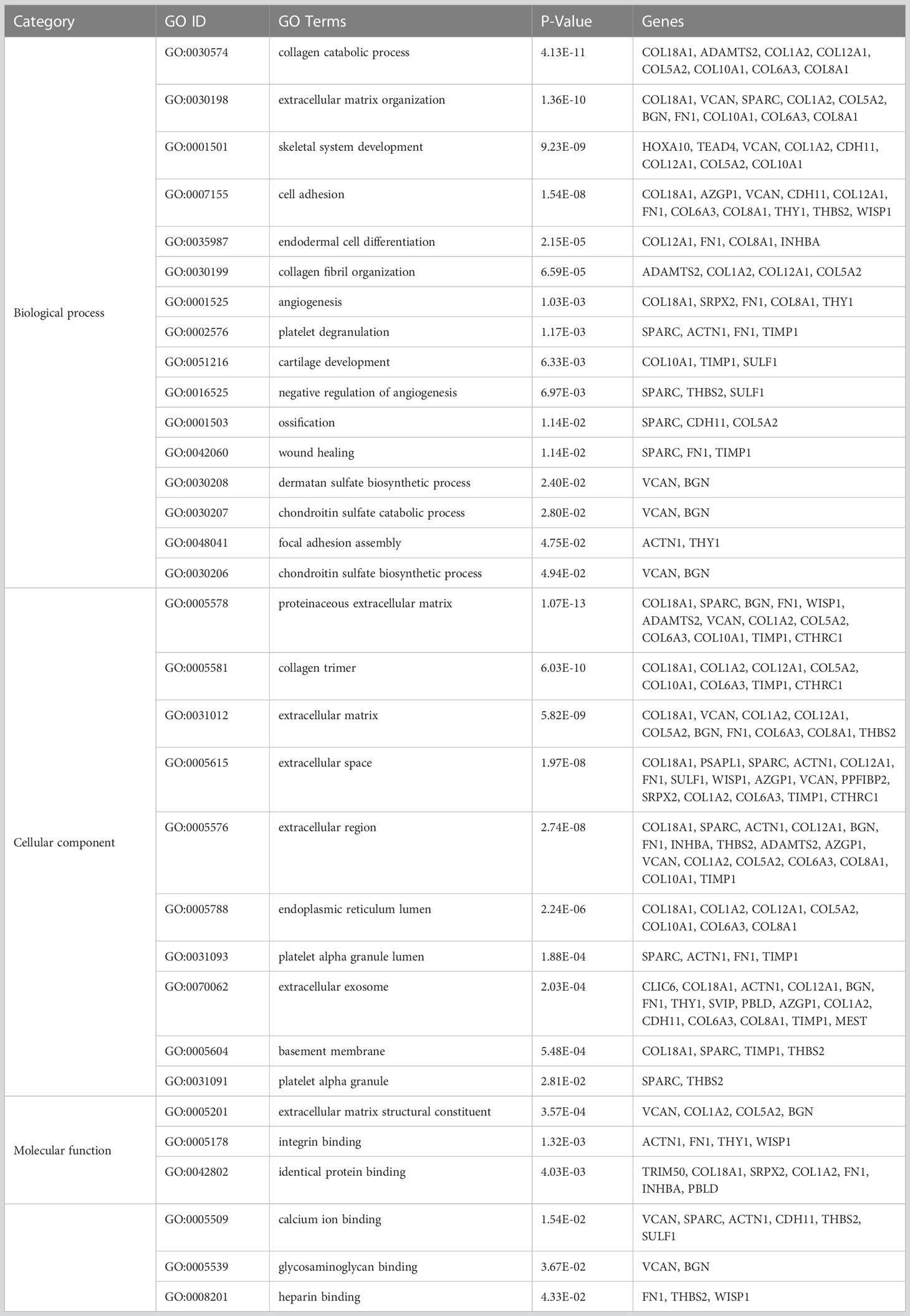
Table 1 The significant Gene Ontology terms enriched by the mutually differentially expressed genes in GSE79973 and GSE19826.
Biomolecules in biological systems work by interacting with each other. Therefore, to gain insight into the functional interaction of DEGs, a substantial protein-protein interaction (PPI) network was formed using the STRING dataset for the common proteins of datasets (Figure 2). PPI network using cyto-Hubba plugin in Cytoscape software showed that 8 genes including COL1A2, FN1, BGN, THBS2, COL5A2, COL6A3, SPARC and COL12A1, which were known as hub genes, had the greatest effect compared to other genes.
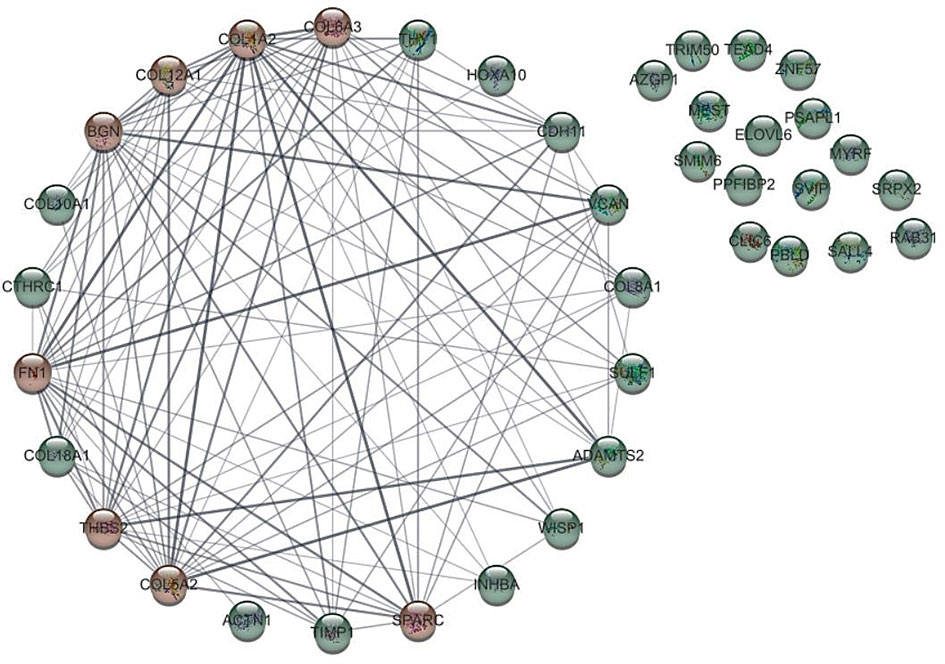
Figure 2 Protein-protein interaction of genes with different expression (DEGs) with other genes. The hub gene is marked in red.
To identify the prognostic value of the eight hub genes, overall survival curves based on expression were plotted using the Kaplan-Meier method (Figure 3). The curves showed that overexpression of seven key genes (COL1A2, FN1, BGN, THBS2, COL6A3, SPARC, COL12A1) was associated with reduced overall survival time in GC patients. This difference was not significant for the COL5A2 gene. For more accurate validation of the hub genes, and in particular the links between protein networks, the final genes were examined in the GEPIA web tool. Gene expression diagrams were plotted as box diagrams (Figure 4). The expression of all hub genes was higher in GC patients than in healthy subjects, but among them, BGN and THBS2 showed the highest increase in expression, while the difference in expression was the lowest in COL6A3 and FN1.
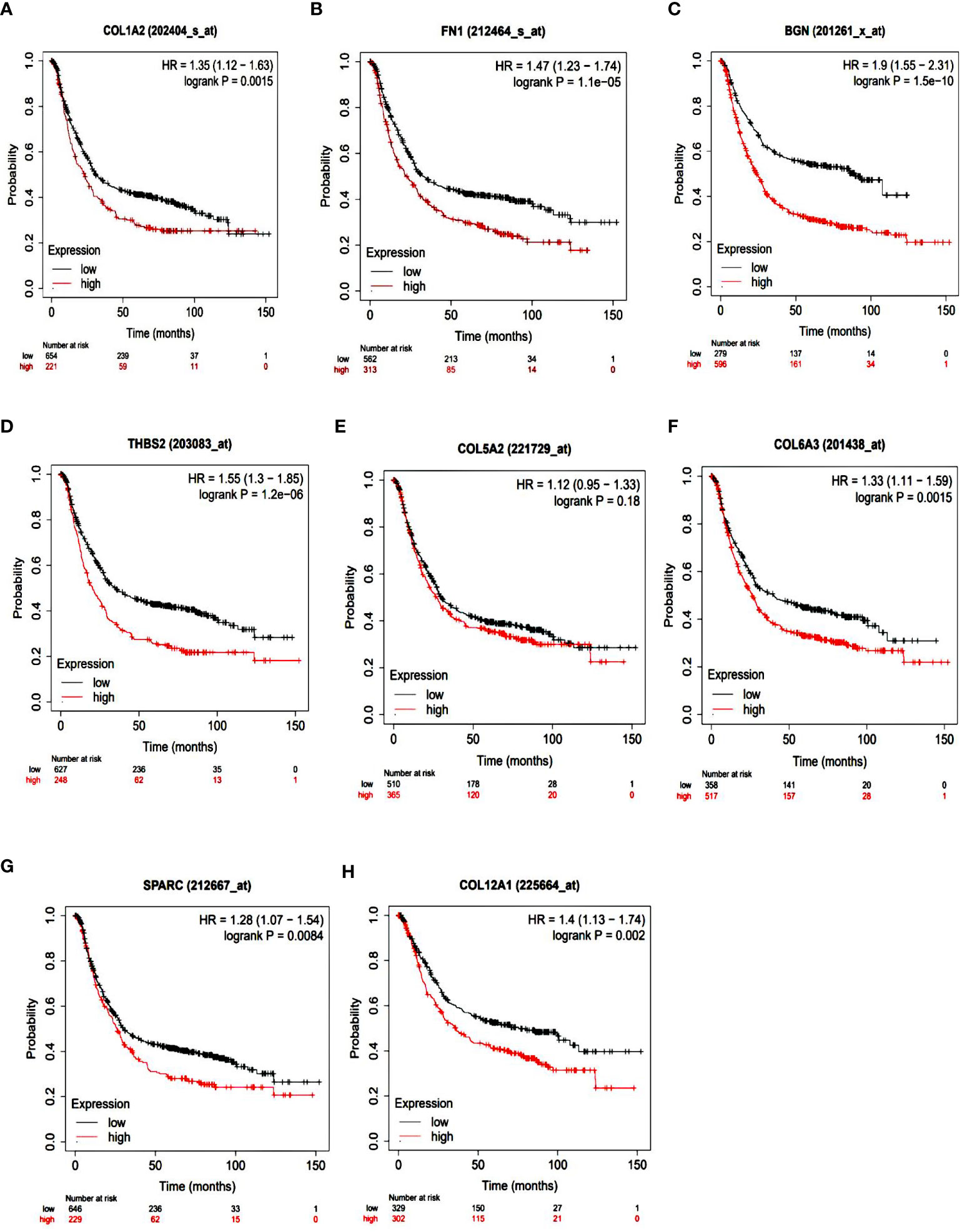
Figure 3 Kaplan-Meier overall survival analyses of patients with gastric cancer based on expression of the eight key genes. (A) COL1A2; (B) FN1; (C) BGN; (D) THBS2; (E) COL5A2; (F) COL6A3; (G) SPARC; (H) COL12A1.
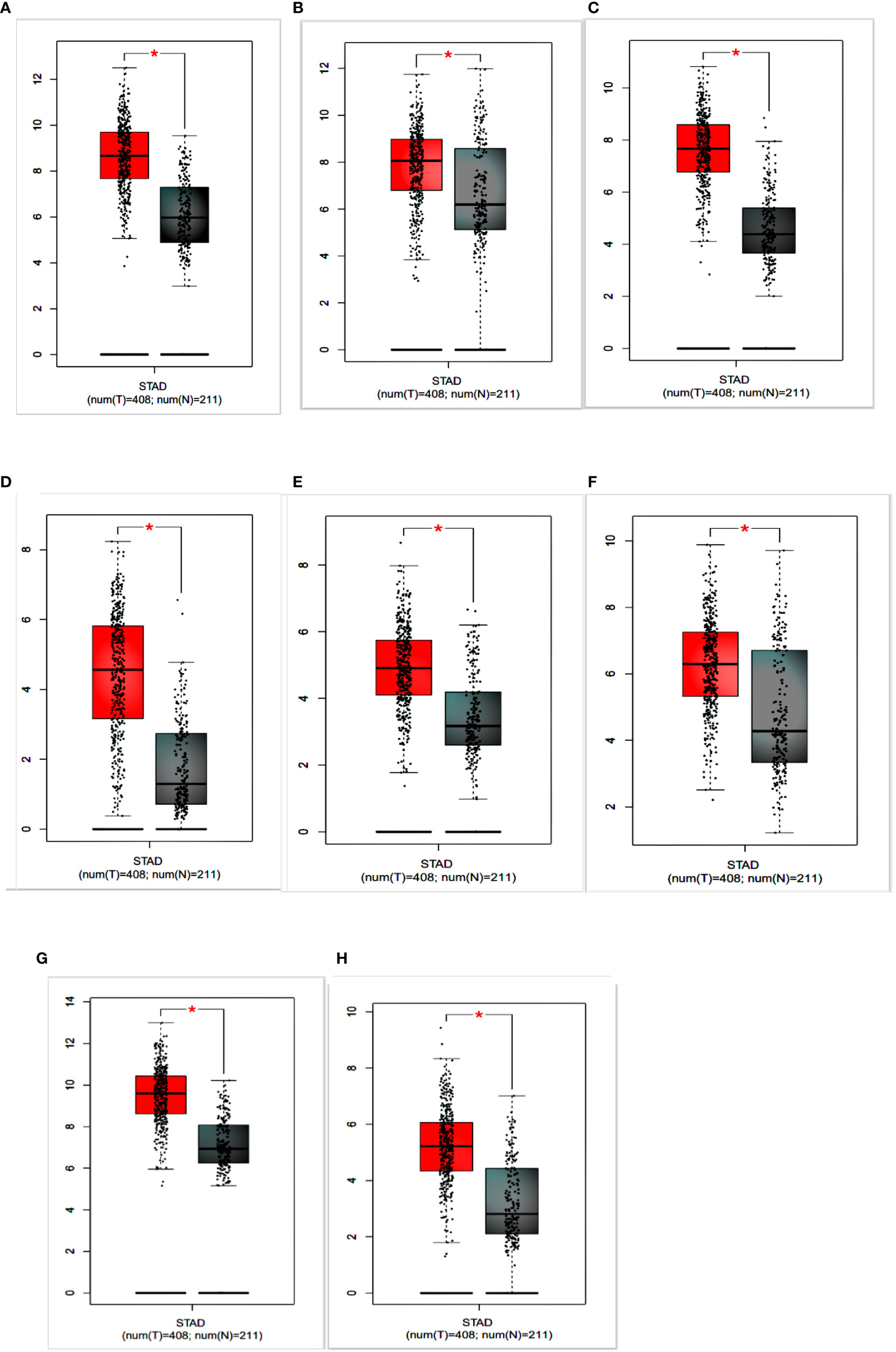
Figure 4 Expression of hub genes in gastric cancer patients and healthy individuals is shown in a box plot. (A) COL1A2; (B) FN1; (C) BGN; (D) THBS2; (E) COL5A2; (F) COL6A3; (G) SPARC; (H) COL12A1.
The exact relationship between hub genes and miRNA was determined (Table 3) and the miRNA-hub gene interaction network consisting of 8 hub genes and 98 miRNA was formed by cytoscape software (Figure 5). The four interactive hub genes that most miRNAs target were: COL12A1 (grade = 47), FN1 (grade = 34), COL1A2 (grade = 26), and COL5A2 (grade = 22). In addition, hsa-hsa-miR-29a-3p (grade = 9), hsa-miR-101-3p (grade = 5), hsa-miR-183-5p (grade = 4), hsa-miR-15a-5p (degree, score = 3) were the top four interactive miRNAs that targeted the most hub genes.
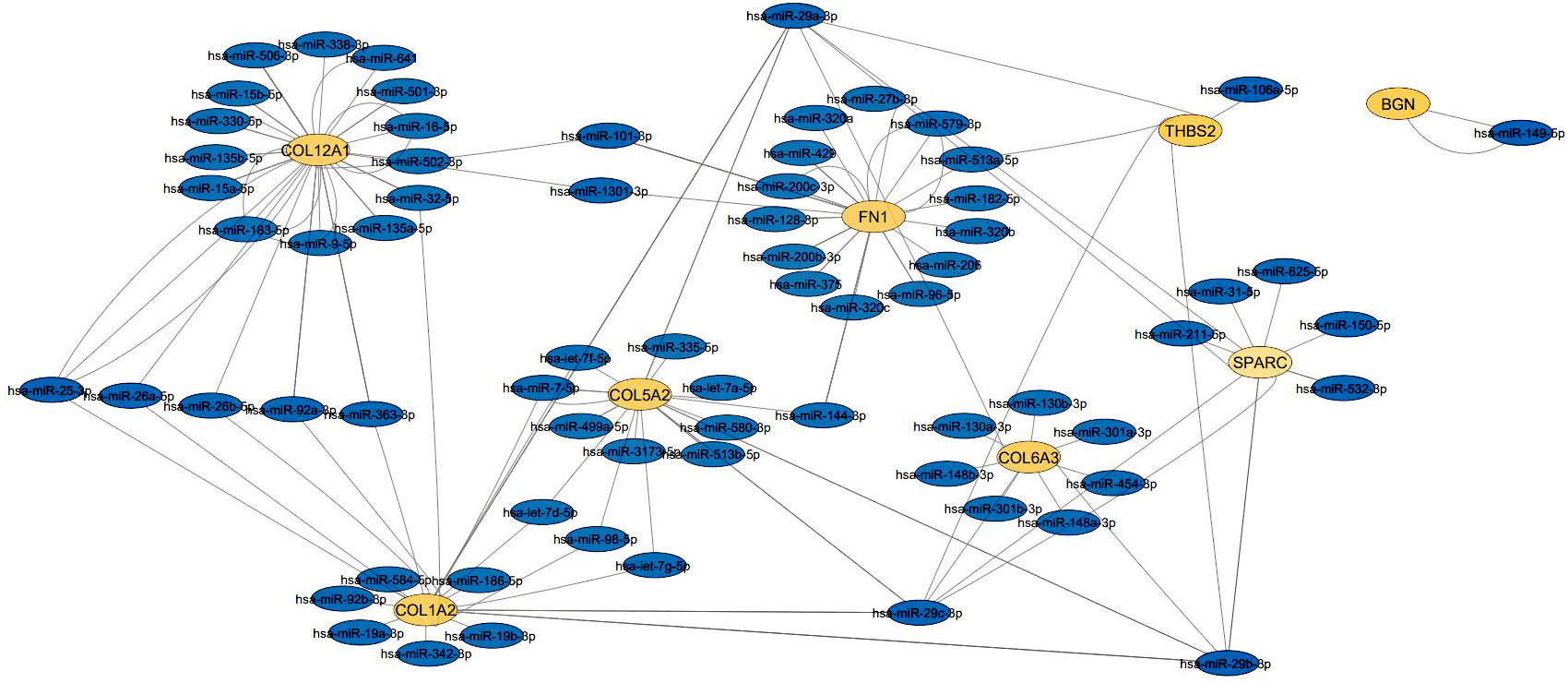
Figure 5 The interaction network between hub genes and target miRNAs. Hub genes are presented in orang circles, whereas target miRNAs are shown in blue circles.
Drugs were ranked based on the highest total score in the drug-gene interaction database (Table 4). A total of 6 drugs related to 5 key genes (FN1, COL1A2, THBD2, COL6A3, COL15A2) were found and the network of drugs and hub genes was plotted using Cytoscape (Figure 6). No associated drugs were found for BGN and SPARC genes.
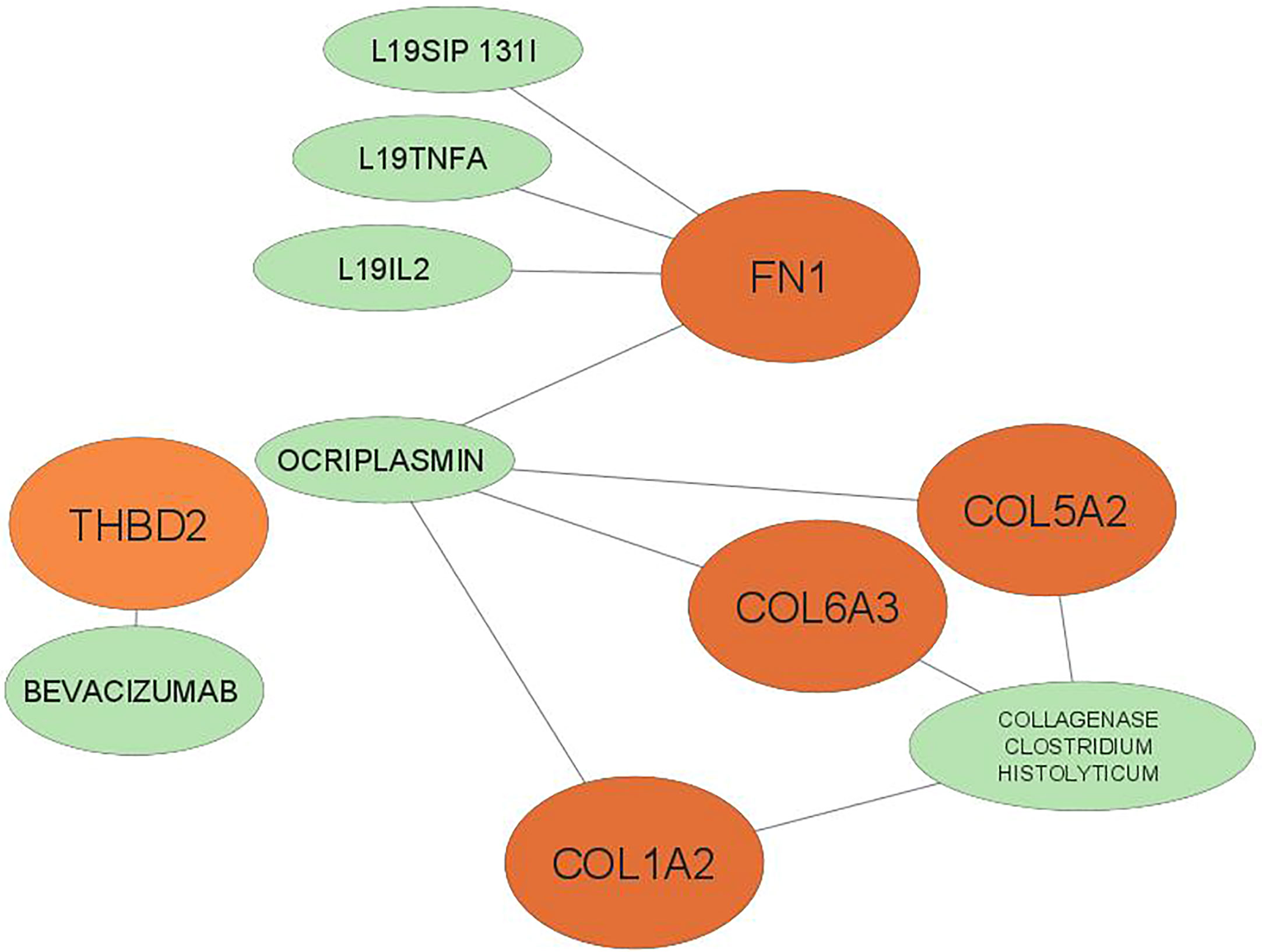
Figure 6 The 6 predicted drugs associated with the hub genes. orange represents hub genes and green represents potential drugs. BGN, SPARC No interactions found.
Today, early detection of cancer and its effective treatment are critical in the recovery, diagnosis and management of cancer. Therefore, it is necessary to identify sensitive and specific methods of diagnosis in the early stages. As a result, the knowledge of cancer at the molecular level has greatly increased, and this has led to the use of targeted therapies for cancer. The discovery of biomarkers from microarray data is an important goal in molecular medicine and has wide clinical applications. Biomarkers have a wide range of applications in the early diagnosis of the disease, the diagnosis of the disease stage, the study of the treatment process and the prediction of cancer recurrence.
In the present study, two datasets with the Affymetrix Human Genome joint platform were used. Each data set included a comparison of gastric tumor tissue samples with adjacent normal tissue. Using commonalities over a set of data, which is a meta-analysis approach to data analysis, provides highly reliable results for future analysis. Meta-analysis showed that 38 genes in both datasets were differently expressed between the two GC and adjacent normal tissues, and further analyzes were performed on these common differentially expressed genes. The results of GO analysis in biological process enrichment showed that these common DEGs were more significantly enriched in skeletal system development, negative regulation of angiogenesis, collagen fibril organization, and cartilage development. This was similar to the results reported by Wu et al. (2019) who reported that DEGs were riched in the biological processes of collagen fibril organization and skeletal system development through the integration of five microarray datasets related to GC (9).
KEGG pathway analysis of DEGs showed that the most important pathway was enriched in ECM-receptor interaction and most genes (6 genes) were involved in protein digestion and absorption and focal adhesion. The extracellular matrix (ECM) consists of proteoglycans (collagen and elastin), fibrous proteins (lumican and decorin) and binding proteins (fibronectin and vitronectin) and also acts as a reservoir of CTGF, β-TGF and other growth factors (10). As a key component, ECM plays a role in processes such as cell division, cell migration, differentiation, carcinogenesis, and apoptosis (11–13).
Cao et al. (2018) showed that most DEGs were enriched in three mRNA expression profiles associated with GC in ECM, collagen catabolic process, fibrillar collagen organization, and cell adhesion. They also reported that three KEGG pathways, including receptor interaction - ECM, protein digestion and absorption, and focal adhesion pathways were significantly enriched (14). Li et al. (2020) who analyzed four GEO datasets from the GC population of China, found similar results (13).
Using identified DEGs between gastric cancerous tissue and healthy gastric tissue, Jiang et al. (2014) reported that the ECM receptor interaction pathway identified in multiple cancers also plays a key role in GC biology (15).
Gene network analysis of 38 differentially expressed genes with 38 nodes and 107 edges showed that 16 genes had no relationship with each other and other genes in the network, while 8 genes including COL1A2, FN1, BGN, COL5A2, THBS2, COL6A3, SPARC, COL12A1, and COL1A2, showed the highest degree of connection, respectively. Since the higher the degree of binding of a gene in the network, the greater its association with GC (16), these 8 genes were identified as hub genes.
Hub gene survival analysis was performed and showed that increasing the expression of seven hub genes COL1A2, FN1, BGN, THBS2, COL6A3, SPARC, and COL12A1 significantly reduced the survival of GC patients, but this relationship was not significant for COL5A2 gene. This was in contrast to the results of Liu et al. (2018) who reported that down expression of the COL5A2 gene indicated better overall survival in patients with the disease (17). The results of the study of the expression of hub genes in tumors and normal tissues using GEPIA confirms the increased expression of these genes in GC.
Different signaling pathways of intracellular messengers are involved in the proliferation, invasion and metastasis of cancers. The binding of growth factors and cytokines to the TGFβ receptor activates PI3K and activates the Akt pathway. Akt can activate β-catenin by stimulating nuclear transfer or degradation complex degradation, thereby, directly and indirectly, affecting the Wnt pathway (18, 19). Akt also inhibits TSC1/2 through phosphorylation and activates the mTOR pathway (19, 20). Phosphatase and tensin homolog (PTEN) can inhibit the activation of the AKT pathway by acting on Phosphatidylinositol-3,4,5-triphosphate (PIP3) and converting it to Phosphatidylinositol 4,5-bisphosphate (PIP2) (21). Integrins are also other receptors that can send extracellular signals to Focal adhesion kinase (FAK). Activation of the Wnt pathway also leads to the activation of the disheveled protein, resulting in the degradation of the degradation complex, as well as allowing the production of dephosphorylated beta-catenin and its migration to the nucleus (22). Hub genes can affect the proliferation and invasion of GC cells and transcriptional activation by acting on either of these pathways (Figure 7).
The COL1A2, COL5A2, COL6A3, and COL12A1 genes provide instructions for making a component of type I, V, VI, and XII collagen, respectively, and participate in the formation of collagen in extracellular matrix proteins (13). Some collagen proteins are associated with the progression and prognosis of cancer. These collagens stimulate the transfer of tumor cells (23, 24).
A study reported that COL1A2 gene mRNA expression in malignant gastric tissues was significantly higher than in normal malignant tissues (25). Increased COL1A2 expression has also been reported in many types of cancer, including breast cancer, cervical cancer, and colon cancer (26). The expression of COL1A2, COL6A3, and THBS2 genes leads to the development, migration, and invasion of gastric cancer cells, and reduces apoptosis through the PI3K-Akt pathway (16, 27). mir 129-5p can reduce the expression of COL1A1 and thus inhibit the proliferation, invasion, and migration of GC cells (28).
A bioinformatic analysis study has shown that COL5A2 is a candidate biomarker of GC (29). Also Alpha 2 collagen chain V plays an important role in the development of colorectal cancer, breast tumors, and osteosarcoma (30). Research shows that increased expression of COL5A2 is associated with increased expression of cytokines such as VEGF, which lead to the growth of tumor cells and unrestricted angiogenesis (30).
Through microarray meta-analysis, Xie et al. (2014) showed that COL6A3 is overexpressed in GC (31). Collagen VI can directly affect tumor cells and increase tumorigenesis by activating the Akt – GSK-3b – β-catenin – TCF/LEF pathway and positively regulating transcription factors (TFs), protein kinases, angiogenic factors, and growth factors (32). Collagen VI also promotes resistance to chemotherapy through 1F/1E (MT1F/1E). The conversion of tumor cells from an epithelial phenotype to a mesenchymal phenotype is the epithelial-mesenchymal transfer (EMT), which is a sign of tumor migration and proliferation. Endotrophin (ETP), a peptide isolated from collagen VI, targets tumor cells via the TGF-b-dependent pathway to induce EMT and fibrosis. It causes tumor inflammation through macrophages and increases the expression of TNF-a and IL-6, and also increases angiogenesis with high expression of CD31, VEGFR2, and HIF1a (33).
Wu et al. (2020) reported that there is a negative association between COL12A1 methylation and colorectal cancer (34). COL12A1 alters the structural components of the extracellular matrix by using some of the kinases, miRNAs, and transcription factors associated with cancer and by integrin binding and collagen binding (34, 35).
Fibronectin 1 (FN1) is part of the extracellular matrix (ECM) and acts as a mediator of interaction between cells and the extracellular matrix and plays an important role in cell adhesion, migration, growth, and differentiation, thereby normalizing the body’s normal function (36). In the EMT process, which it happens tumor migration and proliferation., the expression of Vimentin and N-cad factors increases, and E- cadherin decreases (37). In a study to determine the expression of EMT markers in GC, Western blot was used and it was found that after overexpression of FN1, N-cad and Vimentin proteins are significantly less expressed, while E-cadherin showed a large increase, which indicates the relationship between FN1 and EMT (38). Sun et al. (2020) reported about the relationship between FN1 expression and clinical pathology and prognosis of GC that FN1 is a potential biomarker for poor prognosis in patients with GC (39). Zang et al. (2017) reported that increased expression of miR-200c can prevent the proliferation, migration, and progression of GC by reducing the expression of the FN1 gene (40). Low FN1 expression leads to increased apoptosis and decreased cell proliferation by inactivating the PI3K/AKT signaling pathway (28).
Biglin (BGN) is an important member of the leucine-rich proteoglycan family that is involved in the development of various types of human cancer and their metastasis, and BGN is found in the extracellular matrix of various tissues (41). BGN can participate in the regeneration of blood vessels, the organization of the extracellular matrix, and the metabolic process of carbohydrates (41). BGN is an essential component of the extracellular matrix (ECM) and by encoding one of the ECM proteoglycans, it binds to TGF-beta, causing cancer (42). BGN has a significant negative correlation with B cells and a positive correlation with dendritic cells, CD8 + T and CD4 + T, macrophages, and neutrophils in various types of cancers including GC (43). Hu et al. (2014) reported that BGN regulates GC metastasis and plays an oncogenic role by activating the FAK signaling pathway (22). Chen et al. (2020) reported that BGN expression is significantly higher in GC tissues and is associated with lymph node metastasis, and depth of tumor invasion (44).
Thrombospondins (THBS2) play an important role in ECM receptor interaction pathways (27). THBS2 are alglycoproteins released from various cells, including stromal fibroblasts, endothelial cells, and immune cells (45). The difference in THBS2 expression in GC tissue was consistent with results that reported a vital role for the ECM receptor interaction pathway in cancer progression. Overexpression of THBS2 is associated with cancer progression and metastasis in GC and can be used as a biomarker in predicting the clinical outcome of GC patients (46).
SPARC is a cysteine-rich acidic glycoprotein that acts as a mediator in tissue regeneration, expression of proteins involved in ECM, and formation of collagen and matrix metalloproteinases (13, 45). SPARC expression in gastric cancer is significantly associated with metastasis and can be used as a useful marker in tumor prediction (47, 48).
The exact relationship between the hub gene and miRNA showed that the four genes COL12A1, FN1, COL1A2, and COL5A2 target more miRNAs than the other hub genes, SPARC, COL6A3, THBS2, and BGN. Among these, the highest interaction was related to COL12A1 (grade = 47) and the lowest interaction was related to BGN (grade =2). In cancer, miRNAs can generally act as tumor suppressors or oncogenes, and sometimes can even depending on the type of tumor play both roles (49). Also, miR-29a-3p, miR-101-3p, miR-183-5p, and miR-15a-5p were the top four interactive miRNAs, respectively, that targeted the most hub genes. miR-29a-3p reduces the proliferation and invasion of GC cells by regulating HAS3 expression (50, 51). On the other hand, MIAT uses the MIAT/miR-29a-3p/HDAC4 axis and by increasing MIAT expression causes high expression of HDAC4 as the downstream target of miR-29a-3p, thereby increasing cell proliferation, migration, and invasion of GC cells (52). By increasing EZH2 expression, LINC01303 can inhibit the activity of miR-101-3p, thereby inducing the proliferation, migration, and invasion of gastric cancer cells (53). One study showed that PHLDA1 expression is increased through the circ_0027599/miR-101 pathway, suppressing GC cells and their metastasis (54). miR-183-5p acts as an oncogene by reducing TPM1 expression and inactivating Bcl-2/P53 signaling pathways in GC, by increasing proliferation, migration, and cell invasion (55). Increased expression of miR-15a-5p reduces Gc cell metastasis. Studies have shown that LINC_00355 increases PHF19 expression, which targets miR-15a-5p downstream, resulting in the proliferation, transmission, and attack of cancer cells (56, 57).
Using the DGIdb database, a total of 6 drugs affecting GC were identified. The three drugs L19IL2, L19TNFA, and L19SIP 131I had the highest overall score on the website, with a large difference from the other drugs. All three of them belonged to the FN1 gene. Excess B domain of fibronectin (ED-B) indicates tumor angiogenesis (58). L19, a human antibody, can target this marker and act as an immune cytokine when paired with IL2 (58). The results show that radiotherapy in combination with L19-IL2 will give a better response this response depends on the expression of ED-B (58, 59).
Intralesional administration of L19-IL2 and L19-TNF is a simple and effective way to eradicate non-surgical melanoma lesions or make them suitable for surgical resection. SIP, which is a small immunoprotein in combination with L19, can target ED-B (60). Tijink et al.(2006) Showed that radioimmunotherapy with L19-SIP-131I, alone or in combination with cetuximab, appeared to be effective in treating head and neck cancer (60).
As a result of this bioinformatics meta-analysis, eight hub genes including COL1A2, FN1, BGN, THBS2, COL5A2, COL6A3, SPARC, and COL12A1 were identified that may play an important role in GC. They can be considered candidate genes in the GC prevention and early detection program. Further experimental studies are needed to confirm the findings of the present analysis. Also, miR-29a-3p, miR-101-3p, miR-183-5p, and miR-15a-5p were the top four interactive miRNAs, respectively, that targeted the most hub genes, and the three drugs of L19IL2, L19TNFA and L19SIP 131I had the highest overall score.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
ED and ZP: data analysis and writing the manuscript. SM and SG: designing, project administration, supervision, validation, review, and proofread. All authors have read and agreed to the published version of the manuscript. All authors contributed to the article.
Special thanks should be given to R&D Center of BioGenTAC Inc. for providing research infrastructure.
Author ZP was employed by BioGenTac Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Wang FH, Zhang XT, Li YF, Tang L, Qu XJ, Ying JE, et al. The Chinese society of clinical oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun (2021) 41(8):747–95. doi: 10.1002/cac2.12193
2. Joshi SS, Badgwell BD. Current treatment and recent progress in gastric cancer. CA Cancer J Clin (2021) 71(3):264–79. doi: 10.3322/caac.21657
3. Orditura M, Galizia G, Sforza V, Gambardella V, Fabozzi A, Maddalena Laterza M, et al. Treatment of gastric cancer. World J Gastroenterol (2014) 20(7):1635–49. doi: 10.3748/wjg.v20.i7.1635
4. Tutunchi S, Akhavan S, Bereimipour A, Hossein Ghaderian SM. Evaluation of important molecular pathways and candidate diagnostic biomarkers of noninvasive to invasive stages in gastric cancer by in silico analysis. J Oncol (2021) 2021:1–12. doi: 10.1155/2021/5571413
5. Kalisky T, Blainey P, Quake SR. Genomic analysis at the single-cell level. Annu Rev Genet (2011) 45: 431–45. doi: 10.1146/annurev-genet-102209-163607
6. DeRisi J, Penland L, Bittner ML, Meltzer PS, Ray M, Chen Y, et al. Use of a cDNA microarray to analyse gene expression. Nat Genet (1996) 14:457–60. doi: 10.1038/ng1296-457
7. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Reprint–preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Phys Ther (2009) 89(9):873–80. doi: 10.1016/j.jclinepi.2009.06.005
8. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PloS Med (2009) 6(7):e1000097. doi: 10.7326/0003-4819-151-4-200908180-00135
9. Wu Q, Zhang B, Wang Z, Hu X, Sun Y, Xu R, et al. Integrated bioinformatics analysis reveals novel key biomarkers and potential candidate small molecule drugs in gastric cancer. Pathol Pract (2019) 215(5):1038–48. doi: 10.1016/j.prp.2019.02.012
10. Christner PJ, Ayitey S. Extracellular matrix containing mutated fibrillin-1 (Fbn1) down regulates Col1a1, Col1a2, Col3a1, Col5a1, and Col5a2 mRNA levels in tsk/+ and Tsk/Tsk embryonic fibroblasts. Amino Acids (2006) 30(4):445–51. doi: 10.1007/s00726-005-0265-y
11. Fischer H, Stenling R, Rubio C, Lindblom A. Colorectal carcinogenesis is associated with stromal expression of COL11A1 and COL5A2. Carcinogenesis (2001) 22(6):875–8. doi: 10.1093/carcin/22.6.875
12. Januchowski R, Zawierucha P, Ruciński M, Nowicki M, Zabel M. Extracellular matrix proteins expression profiling in chemoresistant variants of the A2780 ovarian cancer cell line. BioMed Res Int (2014) 2014. doi: 10.1155/2014/365867
13. Li M, Wang X, Liu J, Mao X, Li D, Wang Z, et al. Identification of core prognosis-related candidate genes in Chinese gastric cancer population based on integrated bioinformatics. BioMed Res Int (2020) 2020. doi: 10.1155/2020/8859826
14. Cao L, Chen Y, Zhang M, Xu Dq, Liu Y, Liu T, et al. Identification of hub genes and potential molecular mechanisms in gastric cancer by integrated bioinformatics analysis. PeerJ (2018) 7):1–12. doi: 10.7717/peerj.5180
15. Jiang HB, Yang TJ, Lu P, Ma YJ. Gene expression profiling of gastric cancer. Eur Rev Med Pharmacol Sci (2014) 18(15):2109–15.
16. Zhang S, Xiang X, Liu L, Yang H, Cen D, Tang G. Bioinformatics analysis of hub genes and potential therapeutic agents associated with gastric cancer. Cancer Manag Res (2021) 13:8929. doi: 10.2147/CMAR.S341485
17. Liu L, Chen F, Xiu A, Du B, Ai H, Xie W. Identification of key candidate genes and pathways in endometrial cancer by integrated bioinformatical analysis. Asian Pacific J Cancer Prev (2018) 19(4):969–75. doi: 10.22034/APJCP.2018.19.4.969
18. Zhan T, Rindtorff N, Boutros M. Wnt signaling in cancer. Oncogene (2017) 36(11):1461–73. doi: 10.1038/onc.2016.304
19. Qin W, Cao L, Massey IY. Role of PI3K/Akt signaling pathway in cardiac fibrosis. Mol Cell Biochem (2021) 476(11):4045–59. doi: 10.1007/s11010-021-04219-w
20. Pópulo H, Lopes JM, Soares P. The mTOR signalling pathway in human cancer. Int J Mol Sci (2012) 13(2):1886–918. doi: 10.3390/ijms13021886
21. Jamaspishvili T, Berman DM, Ross AE, Scher HI, De Marzo AM, Squire JA, et al. Clinical implications of PTEN loss in prostate cancer. Nat Rev Urol (2018) 15(4):222–34. doi: 10.1038/nrurol.2018.9
22. Hu L, Duan Y, Li J, Su L, Yan M, Zhu Z, et al. Biglycan enhances gastric cancer invasion by activating FAK signaling pathway. Oncotarget (2014) 5(7):1885. doi: 10.18632/oncotarget.1871
23. Sang L, Wang X-M, Xu D-Y, Zhao W-J. Bioinformatics analysis of aberrantly methylated-differentially expressed genes and pathways in hepatocellular carcinoma. World J Gastroenterol (2018) 24(24):2605. doi: 10.3748/wjg.v24.i24.2605
24. Wang J, Jiang Y-H, Yang P-Y, Liu F. Increased collagen type V α2 (COL5A2) in colorectal cancer is associated with poor prognosis and tumor progression. Onco Targets Ther (2021) 14:2991. doi: 10.2147/OTT.S288422
25. Li J, Ding Y, Li A. Identification of COL1A1 and COL1A2 as candidate prognostic factors in gastric cancer. World J Surg Oncol (2016) 14(1):1–5. doi: 10.1186/s12957-016-1056-5
26. Zhuo C, Li X, Zhuang H, Tian S, Cui H, Jiang R, et al. Elevated THBS2, COL1A2, and SPP1 expression levels as predictors of gastric cancer prognosis. Cell Physiol Biochem (2016) 40(6):1316–24. doi: 10.1159/000453184
27. Ao R, Guan L, Wang Y, Wang JN. Silencing of COL1A2, COL6A3, and THBS2 inhibits gastric cancer cell proliferation, migration, and invasion while promoting apoptosis through the PI3k-akt signaling pathway. J Cell Biochem (2018) 119(6):4420–34. doi: 10.1002/jcb.26524
28. Liu X, Wu J, Zhang D, Bing Z, Tian J, Ni M, et al. Identification of potential key genes associated with the pathogenesis and prognosis of gastric cancer based on integrated bioinformatics analysis. Front Genet (2018) 9(JUL):1–14. doi: 10.3389/fgene.2018.00265
29. Li Z, Liu Z, Shao Z, Li C, Li Y, Liu Q, et al. Identifying multiple collagen gene family members as potential gastric cancer biomarkers using integrated bioinformatics analysis. PeerJ (2020) 8:e9123. doi: 10.7717/peerj.9123
30. Ding Y-L, Sun S-F, Zhao G-L. COL5A2 as a potential clinical biomarker for gastric cancer and renal metastasis. Med (Baltimore) (2021) 100(7): e24561. doi: 10.1097/MD.0000000000024561
31. Xie X, Liu X, Zhang Q, Yu J. Overexpression of collagen VI α3 in gastric cancer. Oncol Lett (2014) 7(5):1537–43. doi: 10.3892/ol.2014.1910
32. Sun H. Identification of key genes associated with gastric cancer based on DNA microarray data. Oncol Lett (2016) 11:525–30. doi: 10.3892/ol.2015.3929
33. Chen P, Cescon M, Bonaldo P. Collagen VI in cancer and its biological mechanisms. Trends Mol Med (2013) 19(7):410–7. doi: 10.1016/j.molmed.2013.04.001
34. Wu Y, Xu Y. Integrated bioinformatics analysis of expression and gene regulation network of COL12A1 in colorectal cancer. Cancer Med (2020) 9(13):4743–55. doi: 10.1002/cam4.2899
35. Jiang X, Wu M, Xu X, Zhang L, Huang Y, Xu Z, et al. COL12A1, a novel potential prognostic factor and therapeutic target in gastric cancer. Mol Med Rep (2019) 20(4):3103–12. doi: 10.3892/mmr.2019.10548
36. Wang R, Fu L, Li J, Zhao D, Zhao Y, Yin L. Microarray analysis for differentially expressed genes between stromal and epithelial cells in development and metastasis of invasive breast cancer. J Comput Biol (2020) 27(0):1–13. doi: 10.1089/cmb.2019.0154
37. Satelli A, Li S. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell Mol Life Sci (2011) 68(18):3033–46. doi: 10.1007/s00018-011-0735-1
38. Zhou Y, Cao G, Cai H, Huang H, Zhu X. The effect and clinical significance of FN1 expression on biological functions of gastric cancer cells. Cell Mol Biol (2020) 66(5):191–8. doi: 10.14715/cmb/2020.66.5.32
39. Sun Y, Zhao C, Ye Y, Wang Z, He Y, Li Y, et al. High expression of fibronectin 1 indicates poor prognosis in gastric cancer. Oncol Lett (2020) 19(1):93–102. doi: 10.3892/ol.2019.11088
40. Zhang H, Sun Z, Li Y, Fan D, Jiang H. MicroRNA-200c binding to FN1 suppresses the proliferation, migration and invasion of gastric cancer cells. BioMed Pharmacother (2017) 88:285–92. doi: 10.1016/j.biopha.2017.01.023
41. Sun C, Yuan Q, Wu D, Meng X, Wang B. Identification of core genes and outcome in gastric cancer using bioinformatics analysis. Oncotarget (2017) 8(41):70271. doi: 10.18632/oncotarget.20082
42. Zhao S, Yin X, Zhao W, Liu L, Wang Z. Biglycan as a potential diagnostic and prognostic biomarker in multiple human cancers. Oncol Lett (2020) 19(3):1673–82. doi: 10.3892/ol.2020.11266
43. Chen W, Yang Z. Identification of differentially expressed genes reveals BGN predicting overall survival and tumor immune infiltration of gastric cancer. Comput Math Methods Med (2021) 2021:1–21. doi: 10.1155/2021/5494840
44. Chen X, Li X, Hu X, Jiang F, Shen Y, Xu R, et al. LUM expression and its prognostic significance in gastric cancer. Front Oncol (2020) 10:605. doi: 10.3389/fonc.2020.00605
45. Ng K, Shea QT, Wong T, Luk ST, Tong M, Lo C, et al. Chemotherapy-enriched THBS2-deficient cancer stem cells drive hepatocarcinogenesis through matrix softness induced histone H3 modifications. Adv Sci (2021) 8(5):2002483. doi: 10.1002/advs.202002483
46. Wang X, Zhang L, Li H, Sun W, Zhang H, Lai M. THBS2 is a potential prognostic biomarker in colorectal cancer. Sci Rep (2016) 6(1):1–14. doi: 10.1038/srep33366
47. Wang L, Yang M, Shan L, Qi L, Chai C, Zhou Q, et al. The role of SPARC protein expression in the progress of gastric cancer. Pathol Oncol Res (2012) 18(3):697–702. doi: 10.1007/s12253-012-9497-9
48. Ma J, Ma Y, Chen S, Guo S, Hu J, Yue T, et al. SPARC Enhances 5-FU chemosensitivity in gastric cancer by modulating epithelial-mesenchymal transition and apoptosis. Biochem Biophys Res Commun (2021) 558:134–40. doi: 10.1016/j.bbrc.2021.04.009
49. Shenouda SK, Alahari SK. MicroRNA function in cancer: oncogene or a tumor suppressor? Cancer Metastasis Rev (2009) 28(3):369–78. doi: 10.1007/s10555-009-9188-5
50. Bai F, Jiu M, You Y, Feng Y, Xin R, Liu X, et al. MiR-29a-3p represses proliferation and metastasis of gastric cancer cells via attenuating HAS3 levels. Mol Med Rep (2018) 17(6):8145–52. doi: 10.3892/mmr.2018.8896
51. Zhao Z, Wang L, Song W, Cui H, Chen G, Qiao F, et al. Reduced miR-29a-3p expression is linked to the cell proliferation and cell migration in gastric cancer. World J Surg Oncol (2015) 13(1):1–7. doi: 10.1186/s12957-015-0513-x
52. Li Y, Wang K, Wei Y, Yao Q, Zhang Q, Qu H, et al. lncRNA-MIAT regulates cell biological behaviors in gastric cancer through a mechanism involving the miR-29a-3p/HDAC4 axis. Oncol Rep (2017) 38(6):3465–72. doi: 10.3892/or.2017.6020
53. Cao C, Xu Y, Du K, Mi C, Yang C, Xiang L, et al. LINC01303 functions as a competing endogenous RNA to regulate EZH2 expression by sponging miR-101-3p in gastric cancer. J Cell Mol Med (2019) 23(11):7342–8. doi: 10.1111/jcmm.14593
54. Wang L, Shen J, Jiang Y. Circ-0027599/PHDLA1 suppresses gastric cancer progression by sponging miR-101-3p.1 11 medical and health sciences 1112 oncology and carcinogenesis 06 biological sciences 0601 biochemistry and cell biology. Cell Biosci (2018) 8(1):1–10. doi: 10.1186/s13578-018-0252-0
55. Lin J, Shen J, Yue H, Cao Z. miRNA-183-5p. 1 promotes the migration and invasion of gastric cancer AGS cells by targeting TPM1. Oncol Rep (2019) 42(6):2371. doi: 10.3892/or.2019.7354
56. Zhang J, Lv W, Liu Y, Fu W, Chen B, Ma Q, et al. LINC_00355 promotes gastric cancer progression by upregulating PHF19 expression through sponging miR-15a-5p. BMC Cancer (2021) 21(1):1–11. doi: 10.1186/s12885-021-08227-3
57. Zare A, Alipoor B, Omrani MD, Zali MR, Alamdari NM, Ghaedi H. Decreased miR-155-5p, miR-15a, and miR-186 expression in gastric cancer is associated with advanced tumor grade and metastasis. Iran BioMed J (2019) 23(5):338. doi: 10.29252/ibj.23.5.5
58. Zegers CML, Rekers NH, Quaden DHF, Lieuwes NG, Yaromina A, Germeraad WTV, et al. Radiotherapy combined with the immunocytokine L19-IL2 provides long-lasting antitumor effects. Clin Cancer Res (2015) 21(5):1151–60. doi: 10.1158/1078-0432.CCR-14-2676
59. Danielli R, Patuzzo R, Di Giacomo AM, Gallino G, Maurichi A, Di Florio A, et al. Intralesional administration of L19-IL2/L19-TNF in stage III or stage IVM1a melanoma patients: results of a phase II study. Cancer Immunol Immunother (2015) 64(8):999–1009. doi: 10.1007/s00262-015-1704-6
Keywords: bioinformatic analysis, biomarker, gastric cancer, hub genes, meta-analysis
Citation: Darang E, Pezeshkian Z, Mirhoseini SZ and Ghovvati S (2023) Bioinformatics and pathway enrichment analysis identified hub genes and potential biomarker for gastric cancer prognosis. Front. Oncol. 13:1187521. doi: 10.3389/fonc.2023.1187521
Received: 16 March 2023; Accepted: 23 May 2023;
Published: 09 June 2023.
Edited by:
Zhanjun Guo, Fourth Hospital of Hebei Medical University, ChinaCopyright © 2023 Darang, Pezeshkian, Mirhoseini and Ghovvati. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shahrokh Ghovvati, R2hvdnZhdGlAZ3VpbGFuLmFjLmly
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.